Abstract
Objectives
The aim of this study was to compare the efficacy of the corticosteroid (CS) injection and shock wave therapy (SWT) in the treatment of greater trochanteric pain syndrome (GTPS).
Patients and methods
Between 2020 September and 2021 October, a total of 60 patients with GTPS (12 males, 48 females; mean age: 50.8±8.5 years; range, 34 to 65 years) were included. The patients were randomly assigned to two groups as the SWT group (n=32) receiving one session of SWT per week for a total of three weeks and CS injection group (n=28) receiving CS and local anesthetic. Both groups were evaluated using the Short Form-36 (SF-36) at baseline and three months and using the Visual Analog Scale (VAS) and Western Ontario and McMaster University Osteoarthritis Index (WOMAC) at baseline, three weeks, and three months.
Results
The mean VAS, greater trochanter tenderness, and WOMAC scores of both groups were similar at baseline, while the third-week and three-month scores were significantly lower in both groups compared to baseline (p<0.05). There was no significant difference in the treatment efficacy between the groups (p>0.05). There was a similar improvement in SF-36 physical function, physical role difficulty, and pain subscales in both groups (p<0.05).
Conclusion
Our study results show that both CS injection and SWT are effective modalities and none of the treatments is superior to each other.
Keywords: Corticosteroid injection, greater trochanteric pain syndrome, shock wave therapy.
Introduction
Lateral hip pain is experienced by 10 to 25% of the general population.[1] Greater trochanteric pain syndrome (GTPS) is a significant and common cause of lateral hip pain.[2] It was previously known as trochanteric bursitis, as the pain was often attributed to inflammation of the peritrochanteric bursa. Greater trochanteric pain syndrome is a preferred term for this clinical condition, as it has been understood that gluteus medius and/or gluteus minimus tendinopathy also contribute to pain. Evaluations based on magnetic resonance imaging have shown that the underlying cause is abductor tendinopathy rather than trochanteric bursa inflammation.[3] In studies performed using ultrasonography, only 20.2% of the patients presented bursa inflammation, whereas gluteal tendinosis and iliotibial band thickening were detected in 49.9% and 28.5%, respectively.[4] As imaging modalities have a limited role in primary diagnosis, this condition is usually diagnosed based on clinical findings. On the contrary, imaging methods may provide helpful findings in the differential diagnosis.[5]
Despite its high prevalence, there is still limited evidence on optimal conservative treatment approaches for GTPS. Frequently used treatments include rest, cold therapy, and glucocorticoid injection. New treatment options have been proposed, after it has been shown that bursitis is not the only cause of pain. The recommended interventions are structured exercise programs, platelet-rich plasma injections, shock wave therapy (SWT), and dry needling.[5-8] The effectiveness of corticosteroid (CS) injection in GTPS management has been well established.[9-11] Since 1990s, SWT has been successfully applied in the treatment of several musculoskeletal diseases. The most common indications are tendon pathologies, such as plantar fasciitis, shoulder calcific tendinitis, and lateral epicondylitis. Considering that GTPS is also a tendinopathy, SWT is expected to be effective in the treatment of GTPS, and its effectiveness has been demonstrated in the literature.[12] However, the number of studies comparing the SWT method with the CS injection is limited.[13] It is crucial to better understand the short- and long-term effects of both approaches in terms of clinical decision-making. In the present study, we, therefore, aimed to compare the efficacy of the CS injection and SWT in the treatment of GTPS.
Patients and Methods
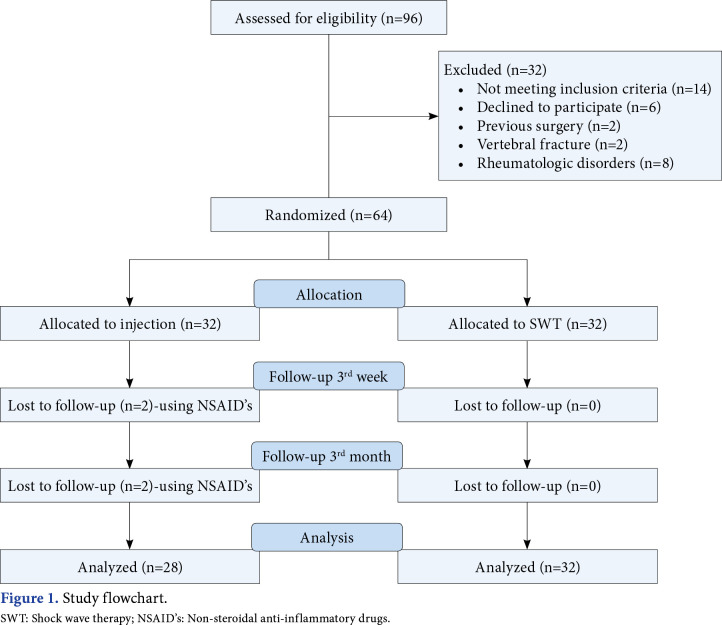
This single-blind, randomized study was conducted at Göztepe Prof. Dr. Süleyman Yalçın City Hospital, Department of Physical Medicine and Rehabilitation between 2020 September and 2021 October. Patients aged between 40 and 70 years who were diagnosed with GTPS in our clinic were screened. The first examination of the patients was done by a specialist physician. Inclusion criteria were as follows: (i) lateral hip pain for at least six weeks, (ii) increased pain and tenderness in the greater trochanter along with manual palpation, (iii) increased pain during active abduction and passive adduction, and (iv) pain while sleeping on the affected side.[5,6] Patients with previous hip or back surgery, a history of vertebral fracture, spondyloarthropathy, diseases such as fibromyalgia that may cause diffuse chronic pain, previous treatment for GTPS, and contraindications for SWT such as pregnancy, bleeding disorder, presence of a pacemaker, and anticoagulant use were excluded. Demographic and clinical data, accompanying musculoskeletal diseases, comorbidities, and previous treatments were recorded. Initially, a total of 96 patients with GTPS were included and only 64 of them were found to be eligible for the study. The patients were randomly assigned to two groups as the SWT group (n=32) and CS injection group (n=28). As four patients in the CS injection group did not attend to follow-up visits, they were excluded. Finally, the study was completed with 60 patients (12 males, 48 females; mean age: 50.8±8.5 years; range, 34 to 65 years). The study flowchart is shown in Figure 1.
Figure 1. Study flowchart. SWT: Shock wave therapy; NSAID’s: Non-steroidal anti-inflammatory drugs.
Randomization and assessments
The first researcher did the pre-treatment examination and randomized patients to the groups using the sealed envelope method.[14] After randomization, the treatments were applied to the patients. The primary outcomes were Visual Analog Scale (VAS) scores for overall pain level and tenderness due to compression of the greater trochanter major, and the Western Ontario and McMaster University Osteoarthritis Index (WOMAC) score, which was used to investigate the impact of lateral hip pain on quality of life. The Short Form Health Survey (SF-36) was used to determine secondary outcomes. The second researcher who was blinded to the group allocation made the follow-up assessments at three weeks and three months. The data were collected and analyzed by an independent clinician.
Outcome measures
The VAS is a popular tool for the measurement of pain. A numerical rating scale (NRS) with 100 mm in length was used in the study where 0 indicates “no pain” and 10 indicates “worst pain imaginable”. The lateral hip pain and tenderness intensity at grater trochanter were measured by VAS.
The WOMAC is a valid and reliable index that is widely used for the evaluation of patients with osteoarthritis. The Turkish validity and reliability study of WOMAC was conducted by Tüzün et al.,[15] and it consists of three sections and 24 questions in which pain, stiffness, and physical function are investigated. Each question is scored on a Likert scale as follows: 0= none, 1= mild, 2= moderate, 3= severe, and 4= very severe. The total score is the sum of all the three section scores and ranges from 0 to 96. The total score is obtained by calculating the percentage of the total points. High scores indicate increased pain and stiffness and impaired physical function. The WOMAC was previously used in randomizedcontrolled trials on GTPS.[16]
The Turkish validity and reliability of the SF-36, which is not specific to any age, disease group, or treatment type, was carried out by Koçyiğit et al.[17] The fourth and fifth items in the 36-item questionnaire provide a Yes/No response, while the remaining items are Likert types (three and six points). Each item is scored on a scale of 0-100, with 0 representing poor condition and increasing scores toward 100 representing well-being. The scale evaluates the last four weeks and consists of eight subscales. These parameters are physical function (10 items), physical role difficulty (4 items), emotional role difficulty (3 items), energy-vitality (4 items), mental (spiritual) health (5 items), social functionality (2 items), pain (2 items), and general health perception (5 items).
Treatments
Corticosteroid injection was administered by the first clinician. After cleansing the skin with chlorhexidine, a mixture of 1 mL of 40 mg triamcinolone acetonide and 2 mL of 2% prilocaine hydrochloride (20 mg/mL) was applied vertically to the most sensitive point on the greater trochanter using a 5-mL syringe and a 5-cm-long 23-gauge needle.[18]
Shock wave therapy was performed with a Modus®-brand radial extracorporeal SWT (ESWT) device (Inceler Medical Ltd., Ankara, Türkiye) by a same physiotherapist experienced in the field of SWT applications. For three weeks, the patients received one session per week for the greater trochanter area in the lateral decubitus position. In each session, 2,000 beats were applied with a 20-mm applicator at a frequency of 12 Hz and a pressure of 2 bar. The application settings were determined according to manufacturer’s instructions and also previous studies about GTPS.[19-21]
Paracetamol 1000 mg daily was allowed for pharmacological pain management during the study. Nevertheless, whenever possible, the patients were reminded to avoid this treatment. The need for paracetamol per month was investigated at three weeks and three months. In addition, the pain caused by the treatments during the application was questioned.
Statistical analysis
Study power analysis and sample size calculation were performed using the G*Power version 3.1.7 software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The clinical significance of efficacy was determined as two-unit decrease in VAS. According to the literature, the initial mean VAS values were 5.9±1.6 and decreased to 2.5±1.5 after SWT.[21] The sample size was calculated for a significance level of 0.05 and 80% power. The resulting sample size was 23 per group. Assuming a dropout rate of 5%, the target sample size was 50 in total. The null hypothesis was there would be no significant difference between the groups after three months.
Statistical analysis was performed using the IBM SPSS for Windows version 25.0 software (IBM Corp., Armonk, NY, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (min-max) or number and frequency, where applicable. The conformity of the data to the normal distribution was evaluated using the Shapiro-Wilk test. The Pearson chi-square test was used to compare categorical data. The general linear model for repeated measures was used to analyze for quantitative variables. A p value of <0.05 was considered statistically significant.
Results
A total of 32 patients in the SWT group and 28 patients in the CS injection group completed the study. Demographic data of the patients are summarized in Table 1. There was no significant difference in the age, sex, body mass index, education status, occupation, concomitant disease, and smoking between the groups (p>0.05).
Table 1. Demographic and clinical characteristics of patients.
| Treatment | ESWT (n=32) | Injection (n=28) | p | ||||
| n | % | Mean±SD | n | % | Mean±SD | ||
| Age (year) | 50.0±9.1 | 51.7±7.7 | 0.45* | ||||
| Body mass index (kg/m2) | 27.9±4.2 | 27.4±3.3 | 0.618* | ||||
| Sex | |||||||
| Male | 5 | 41.7 | 7 | 58.3 | 0.52 | ||
| Female | 27 | 56.3 | 21 | 43.8 | 0.82** | ||
| Education | |||||||
| Primary-Secondary School | 17 | 50 | 17 | 50 | 1.339** | ||
| High School | 8 | 50 | 8 | 50 | 0.512 | ||
| College-University | 7 | 70 | 3 | 30 | |||
| Occupation | |||||||
| Not working | 18 | 48.6 | 19 | 51.4 | 1.019** | ||
| Employee | 5 | 55.6 | 4 | 44.4 | |||
| Desk job | |||||||
| Comorbidity | 9 | 64.3 | 5 | 35.7 | 0.601 | ||
| No | 16 | 55.2 | 13 | 44.8 | 3.201** | ||
| At least one | 13 | 46.4 | 15 | 53.6 | 0.202 | ||
| More than one | 3 | 100 | 0 | 0 | |||
| Smoking | |||||||
| No | 21 | 50 | 21 | 50 | 0.625** | ||
| Yes | 11 | 61.1 | 7 | 38.9 | 0.574 | ||
| ESWT: Extracorporeal shock wave therapy; SD: Standard deviation; * Independent samples t-test; ** Chi-square test was used. | |||||||
The baseline, third week, and third month values of the groups are shown in Table 2. There Was a significant improvement in the primary endpoints (i.e., VAS, trochanteric tenderness, and WOMAC scores) at three weeks in both groups and this improvement sustained up to three months. However, there was no significant difference in any time period between the groups (change over time p=0.001, the difference between the groups p>0.05). The SF-36 subscales, which were considered as secondary endpoint measures, were evaluated prior to treatment and at three months, and physical function, physical role difficulty, and pain subscales were found to be improved in both groups, with no significant difference between the groups (p>0.05).
Table 2. General linear model analysis for repeated measurements.
| ESWT (n=32) | Injection (n=28) | |||
| Treatment | Mean±SD | Mean±SD | f | p |
| VAS-before treatment (cm) | 6.84±0.95 | 6.96±0.99 | ||
| VAS-3rd week (cm) | 1.09±0.96 | 0.78±0.73 | 1399.5* 0.789** |
0.001* 0.378** |
| VAS-3rd month (cm) | 1.03±1.03 | 0.78±0.83 | ||
| Trochanteric tenderness-before treatment (cm) | 6.84±1.01 | 7.00±1.05 | ||
| Trochanteric tenderness-3rd week (cm) | 1.18±1.06 | 0.78±0.73 | 982.5* 1.711** |
0.001* 0.185** |
| Trochanteric tenderness-3rd month (cm) | 1.03±0.99 | 0.78±0.73 | ||
| WOMAC-before treatment | 47.00±8.80 | 46.78±9.56 | ||
| WOMAC-3rd week | 7.59±7.59 | 5.10±3.83 | 1.580* 1.153** |
0.001* 0.287** |
| WOMAC-3rd month | 6.73±6.54 | 4.27±4.35 | ||
| SF-36 Physical function before treatment | 58.90±13.42 | 58.75±11.98 | 1.537* | 0.001* |
| SF-36 Physical function-3rd month | 70.90±13.42 | 70.75±11.98 | 0.002** | 0.962** |
| SF-36 Physical role difficulty before treatment | 36.71±16.78 | 36.60±14.40 | 402.1* | 0.001* |
| SF-36 Physical role difficulty -3rd month | 43.81±16.90 | 45.42±14.67 | 0.34** | 0.853** |
| SF-36 Emotional role limitation-before treatment | 58.50±19.67 | 59.22±19.83 | 43.81* | 0.069* |
| SF-36 Emotional role limitation-3rd month | 59.21±18.60 | 60.92±18.24 | 0.62** | 0.805** |
| SF-36 Energy-before treatment | 59.06±15.78 | 54.82±12.58 | 1.495* | 0.226* |
| SF-36 Energy-3rd month | 61.25±13.55 | 57.32±10.49 | 0.32** | 0.859** |
| SF-36 Mental Health-before treatment | 63.84±13.72 | 64.28±11.77 | 0.12* | 0.687* |
| SF-36 Mental Health-3rd month | 63.96±14.18 | 64.35±11.93 | 0.15** | 0.902** |
| SF-36 Social function-before treatment | 53.64±15.80 | 56.62±12.64 | 1.19* | 0.279* |
| SF-36 Social function-3td month | 59.89±13.09 | 60.91±10.24 | 0.369** | 0.546** |
| SF-36 Pain-before treatment | 46.18±11.06 | 45.75±12.40 | 25.21* | 0,001* |
| SF-36 Pain-3rd month | 53.34±10.77 | 49.64±9.40 | 0.626** | 0.432** |
| General health-before treatment (cm) | 55.93±15.26 | 56.07±13.28 | 0.77* | 0.783* |
| General health-3rd month | 56.28±14.76 | 56.03±12.50 | 1.153** | 0.287** |
| SD: Standard deviation; VAS: Visual Analog Scale; WOMAC: Western Ontario and McMaster University Osteoarthritis Index; SF-36: Short Form Health Survey; * Change over time; ** Difference between groups. | ||||
In total, 12 patients in the SWT group reported the use of paracetamol after the procedure. The dose was an average of 1000 mg/day. Post-procedural pain in the ESWT group was usually relieved within the first 24 h and was well tolerated. No patients discontinued treatment in the SWT group. The patients in the injection group did not experience pain, and the treatment was well tolerated. In this group, four patients who started using non-steroidal anti-inflammatory drugs for other diseases were excluded from the study.
Discussion
In the present study, we compared the efficacy of the CS injection and SWT in the treatment of GTPS. Our study showed that both treatment methods had similar effects in the short-term and mid-term. As this was a randomized-controlled trial, inclusion and exclusion criteria were strictly followed. The clinical status of all patients included in the study was similar, and the pain experienced was mostly due to GTPS. However, GTPS is considered an additional finding in many diseases in daily clinical practice, which should be kept in mind while interpreting the results of our study.
Shock wave therapy, as many other tendinopathies, has been shown to be an effective method in the treatment of GTPS. There have been studies with focused ESWT (fESWT) and radial ESWT (rESWT). In the fESWT method, compressed and concentrated shock waves are transmitted to deeper tissues. On the contrary, shock waves aimed at a wider and more superficial area are used in the rESWT modality. Carlisi et al.[19] compared the effectiveness of fESWT and ultrasound therapy and found that fESWT was superior to ultrasound in the short-term and mid-term. In a multi-center study by Ramon et al.,[20] fESWT was compared with exercise and sham fESWT. In this study, the level of pain decreased from 6.3 to 2, and the fESWT method had a success rate of 86.8% when combined with exercise. Similarly, the pain level decreased in this study. Seo et al.[21] confirmed the favorable effect of electrohydraulic fESWT in GTPS and reported a success rate of 83.3%. Nevertheless, further studies are needed to decide which technique and application method should be used to provide the optimal SWT treatment. Wheeler et al.[22] compared the single-dose rESWT method to three generally accepted doses and found no significant difference in their efficacy. We applied rESWT in three sessions over three weeks, as recommended by the manufacturer’s instructions and previous studies. On the contrary, the difference between applications is not specific to SWT. For CS injections, there are differences in the fluid to be injected and the method of administration. It should be noted that the clinical response may vary depending on the amount of local anesthetics and CSs used; it may also vary depending on whether the injection was guided or not. According to a recent review, image- and palpation-guided CS-anesthetic injections are accepted both feasible, safe, and effective to treat GTPS providing clinical improvement up to three to six months.[23] In our study, we preferred palpation guidance to administer local anesthetic and CS injections. The results are satisfactory. We believe that using sonographic guidance may increase the success rate, but palpation-based injections also seem to be effective.
In a systematic review of GTPS therapy, adverse events were reported, such as short-term pain and minor skin irritations at the injection site and skin irritation and local swelling following SWT.[7] In our study, we did not observe such adverse reactions in the injection group. In the SWT group, local skin irritations were managed well and were not reported as a complaint by the patients. Some patients, however, reported an increase in pain severity that lasted up to 24 h after the application. However, this side effect did not cause patients to discontinue the treatment. As a result of this transient effect, the need for analgesics was higher in the SWT group.
Currently, patient preference plays an important role in the treatment selection. Both treatments have similar efficacy, but the outstanding features of CS injection are that it is easy to administer, provides a rapid clinical response, is administered only once, and is cost-effective. However, SWT is a favorable alternative for patients who are allergic to local anesthetics and CSs and are hesitant to receive injections. It can be also used in patients who do not respond to injection therapy.[24] The use of SWT may be more appropriate in patients with dominant tendinopathy rather than bursitis.
The present study is notable for having a very well-homogenized study group, a sufficient sample size, and objective data and treatments that were compared. To avoid a confounding factor that can alter the treatment response, no exercise program was given to our patients. After the final evaluations at three months, the study was terminated and an exercise program was recommended to the patients. Therefore, the study provides no information on long-term efficacy. The main limitations of our study are that the diagnosis of GTPS was made only based on clinical findings, factors such as bursitis and tendinopathy, which may cause GTPS, and was not confirmed by an imaging study. Also, the palpation test of the greater trochanter is known to have poor specificity, and clinical provocative pain tests may be used to rule out false-positive results.
In conclusion, although both CS injection and SWT seem to be effective treatments for GTPS, neither of them is clinically superior to another. Therefore, the choice of treatment should be based on several factors, including the patient’s preferences and the cost of treatment. It is recommended that patients consult with their healthcare provider to identify the most appropriate treatment plan for their specific condition. Further high-quality, randomized clinical trials are needed to elucidate the long-term efficacy of these treatment modalities in patients with GTPS.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Maramara University, Faculty of Medicine Ethics Committee (date: 24.08.2020, no: 09.2020.986). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design, analysis and/or interpretation, writing the article: H.Ç.Y., İ.Y.; Control/ supervision, data collection and/or processing, literature review, critical review, references and fundings, materials. other: H.Ç.Y., İ.Y., F.B.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: Epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88:988–992. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho GW, Howard TM. Greater trochanteric pain syndrome: More than bursitis and iliotibial tract friction. Curr Sports Med Rep. 2012;11:232–238. doi: 10.1249/JSR.0b013e3182698f47. [DOI] [PubMed] [Google Scholar]
- 3.Blankenbaker DG, Ullrick SR, Davis KW, De Smet AA, Haaland B, Fine JP. Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skeletal Radiol. 2008;37:903–909. doi: 10.1007/s00256-008-0514-8. [DOI] [PubMed] [Google Scholar]
- 4.Long SS, Surrey DE, Nazarian LN. Sonography of greater trochanteric pain syndrome and the rarity of primary bursitis. AJR Am J Roentgenol. 2013;201:1083–1086. doi: 10.2214/AJR.12.10038. [DOI] [PubMed] [Google Scholar]
- 5.Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279–289. doi: 10.1016/j.pmr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Williams BS, Cohen SP. Greater trochanteric pain syndrome: A review of anatomy, diagnosis and treatment. Anesth Analg. 2009;108:1662–1670. doi: 10.1213/ane.0b013e31819d6562. [DOI] [PubMed] [Google Scholar]
- 7.Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: A systematic review. Br J Sports Med. 2017;51:97–104. doi: 10.1136/bjsports-2015-095858. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan EP, Middleton EF, Brunette M. Evaluation and management of greater trochanter pain syndrome. Phys Ther Sport. 2015;16:205–214. doi: 10.1016/j.ptsp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Brinks A, van Rijn RM, Willemsen SP, Bohnen AM, Verhaar JA, Koes BW, et al. Corticosteroid injections for greater trochanteric pain syndrome: A randomized controlled trial in primary care. Ann Fam Med. 2011;9:226–234. doi: 10.1370/afm.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SP, Strassels SA, Foster L, Marvel J, Williams K, Crooks M, et al. Comparison of fluoroscopically guided and blind corticosteroid injections for greater trochanteric pain syndrome: Multicentre randomised controlled trial. b1088BMJ. 2009;338 doi: 10.1136/bmj.b1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shbeeb MI, O'Duffy JD, Michet CJ Jr, O'Fallon WM, Matteson EL. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23:2104–2106. [PubMed] [Google Scholar]
- 12.Furia JP, Rompe JD, Maffulli N. Low-energy extracorporeal shock wave therapy as a treatment for greater trochanteric pain syndrome. Am J Sports Med. 2009;37:1806–1813. doi: 10.1177/0363546509333014. [DOI] [PubMed] [Google Scholar]
- 13.Rompe JD, Segal NA, Cacchio A, Furia JP, Morral A, Maffulli N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am J Sports Med. 2009;37:1981–1990. doi: 10.1177/0363546509334374. [DOI] [PubMed] [Google Scholar]
- 14.Doig GS, Simpson F. Randomization and allocation concealment: A practical guide for researchers. J Crit Care. 2005;20:187–193. doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Tüzün EH, Eker L, Aytar A, Daşkapan A, Bayramoğlu M. Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthritis Cartilage. 2005;13:28–33. doi: 10.1016/j.joca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Nissen MJ, Brulhart L, Faundez A, Finckh A, Courvoisier DS, Genevay S. Glucocorticoid injections for greater trochanteric pain syndrome: A randomised double-blind placebo-controlled (GLUTEAL) trial. Clin Rheumatol. 2019;38:647–655. doi: 10.1007/s10067-018-4309-6. [DOI] [PubMed] [Google Scholar]
- 17.Koçyiğit H, Aydemir O, Fişek G, Ölmez N, Memiş A. Kısa Form-36 (SF-36)'nın Türkçe versiyonunun güvenilirliği ve geçerliliği. İlaç ve Tedavi Dergisi. 1999;12:102–106. [Google Scholar]
- 18.Brinks A, van Rijn RM, Willemsen SP, Bohnen AM, Verhaar JA, Koes BW, et al. Corticosteroid injections for greater trochanteric pain syndrome: A randomized controlled trial in primary care. Ann Fam Med. 2011;9:226–234. doi: 10.1370/afm.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlisi E, Cecini M, Di Natali G, Manzoni F, Tinelli C, Lisi C. Focused extracorporeal shock wave therapy for greater trochanteric pain syndrome with gluteal tendinopathy: A randomized controlled trial. Clin Rehabil. 2019;33:670–680. doi: 10.1177/0269215518819255. [DOI] [PubMed] [Google Scholar]
- 20.Ramon S, Russo S, Santoboni F, Lucenteforte G, Di Luise C, de Unzurrunzaga R, et al. Focused shockwave treatment for greater trochanteric pain syndrome: A multicenter, randomized, controlled clinical trial. J Bone Joint Surg [Am] 2020;102:1305–1311. doi: 10.2106/JBJS.20.00093. [DOI] [PubMed] [Google Scholar]
- 21.Seo KH, Lee JY, Yoon K, Do JG, Park HJ, Lee SY, et al. Longterm outcome of low-energy extracorporeal shockwave therapy on gluteal tendinopathy documented by magnetic resonance imaging. e0197460PLoS One. 2018;13 doi: 10.1371/journal.pone.0197460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler PC, Dudson C, Calver R, Goodall D, Gregory KM, Singh H, et al. Three sessions of radial extracorporeal shockwave therapy gives no additional benefit over "minimal-dose" radial extracorporeal shockwave therapy for patients with chronic greater trochanteric pain syndrome: A double-blinded, randomized, controlled trial. e7-18Clin J Sport Med. 2022;32 doi: 10.1097/JSM.0000000000000880. [DOI] [PubMed] [Google Scholar]
- 23.Sconfienza LM, Adriaensen M, Alcala-Galiano A, Allen G, Aparisi Gómez MP, Aringhieri G, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: A Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part IV, hip. Eur Radiol. 2022;32:551–560. doi: 10.1007/s00330-021-07997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan J, Lovell ME. Extracorporeal shockwave therapy for refractory greater trochanteric pain syndrome. MOJ Orthop Rheumatol. 2015;2:102–104. [Google Scholar]