Abstract
Objectives
In this study, we aimed to clarify the impact of a Pilates-based therapeutic exercise on disability, pain, mood, and sleep quality in patients with pregnancy-related lumbopelvic pain (LPP),
Patients and methods
In the single-blinded randomized controlled study conducted between January 2018 and June 2018, 34 pregnant women (mean age: 29.7±6.2 years; range, 18 to 40 years) in the second trimester (week 14-24) with LPP were randomly assigned to a control group and a Pilates group. All patients underwent usual prenatal care. In addition, the selected Pilates exercise was carried out twice a week for 60-min per session for 12 weeks in the Pilates group. The control group was not prescribed an exercise regimen; however, they were not discouraged from exercising. The primary outcome was disability; secondary outcomes were LPP, mood, and sleep quality. Disability [Roland-Morris Disability Questionnaire (RMDQ)], LPP [Visual Analog Scale (VAS)], mood [Hospital Anxiety and Depression Scale, Anxiety (HADS-A) and Depression (HADS-D) subscales], and sleep quality [Pittsburgh Sleep Quality Index (PSQI)] were measured before and after 12 weeks. Adverse effects and adherence were recorded to determine exercise safety and compliance. The intention-to-treat analysis was applied.
Results
The between-group effect sizes were moderate for the RMDQ and VAS scales (d=0.4 and d=0.7, respectively) and small for the HADS-A and HADS-D scales (both d=0.2). The intention-to-treat analysis demonstrated that there was a statistically significant difference in disability, pain, and mood in favor of the Pilates group (p0.05).
Conclusion
Adding Pilates to usual prenatal care should be considered a promising treatment option for pregnancy-related LPP.
Keywords: Exercise, low back pain, lumbopelvic pain, pelvic pain, pilates, pregnancy.
Introduction
Low back pain (LBP) is a common musculoskeletal problem affecting approximately two-thirds of pregnant women, with a frequency up to 76%, and it is a major cause of disability during the second and third trimesters of pregnancy.[1,2] Pain can become highly severe, leading to difficulties in activities of daily living, higher rates of functional disability, increased sick leave, and poor maternal health in conjunction with depression, anxiety, and sleep disturbances.[3,4] Moreover, insufficient recovery may result in postpartum depression, chronic back pain, and long-term impairment.[5,6]
Lumbopelvic pain (LPP) may develop from the 12th rib to the gluteal fold or around the pubic symphysis. As there is no commonly accepted terminology, Wu et al.[7] recommended using LPP due to its encompassing definition for both LBP and pelvic girdle pain, which are related conditions. Despite its high prevalence, the most effective treatment for this common disease is still under debate. A variety of treatment modalities have been proposed for overcoming disability and pain in pregnancy, such as electrotherapy, taping, patient education, exercise, manual therapy, and medication.[8,9]
Currently, Pilates is commonly used in women’s health, including for breast cancer rehabilitation, postmenopausal osteoporosis, sexual dysfunctions, and pelvic floor rehabilitation.[10,11] It is based on a core-strengthening exercise that activates the muscles around the lumbar spine and pelvis;[12] therefore, the most widely researched reason for adopting this exercise method is LPP. Exercise prescription is effective in reducing pain and functional disability and in preventing and treating musculoskeletal dysfunctions in pregnancy, but evidence of the efficacy and safety of a Pilates program as a treatment option for pregnancy-related LPP is lacking.[13] Moreover, recent systematic reviews reported that given the popularity of Pilates among women, evidence must be strengthened by rigorous methodologic approaches, such as intention-to-treat analysis, exact exercise prescription (according to the Consensus on Exercise Reporting Template), and different types of Pilates intervention.[14-17] We hypothesized that Pilates has positive biomechanical effects, contributing to both muscle strength and neuromotor control in pregnant women, particularly in the lumbopelvic area. Thus, the primary purpose of the present controlled trial was to determine the effect of a Pilates-based therapeutic exercise on disability in patients with pregnancy-related LPP. Second, we evaluated pain, depression, anxiety, and sleep quality to answer the hypothesis that contextual benefits reduce disability.
Patients and Methods
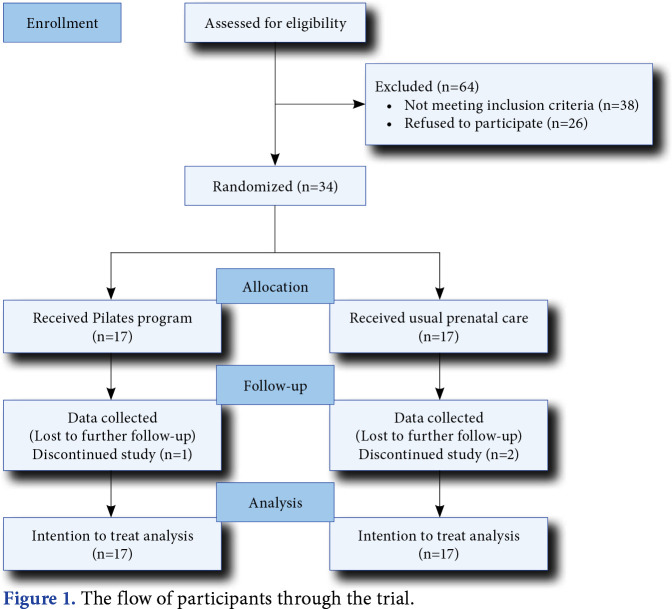
The single-blinded randomized controlled study was conducted at the University of Health Sciences, Kocaeli Derince Training and Research Hospital, Department of Physical Medicine and Rehabilitation between January 2018 and June 2018. Seventy-four potentially eligible patients who were willing and able to attend a 12-week exercise program were contacted via advertisements posted in the outpatient clinic. Initially, all participants provided a detailed history and underwent a physical examination of the cardiovascular, neurological, and musculoskeletal system. To be eligible to participate, patients had to (i) be aged between 18 and 40 years, (ii) be 14-24 weeks pregnant, (iii) have a singleton pregnancy, (iv) have LPP that began after pregnancy week 12, (v) have a VAS score ≥2, (vi) not be in any exercise program in the three months prior to the intervention, and (vii) have no knowledge of Pilates exercise. Patients were excluded if they (i) had any other condition that causes LPP (e.g., disc herniation, prior lumbar surgery, and ankylosing spondylitis), (ii) had an unstable medical condition that interrupts the exercise program (e.g., neurologic disorder, rheumatoid arthritis, and severe vascular disease), (iii) had pregnancy-related risks/previous pregnancy-related complications that preclude exercise, and (iv) had a musculoskeletal disorder requiring modification of the exercise program (e.g., previous nerve injuries/operation on the lower extremities). Consequently, 34 pregnant women (mean age: 29.7±6.2 years; range, 18 to 40 years) were found eligible and diagnosed with pregnancy-related LPP. These patients were randomized into one of two groups (control, Pilates; n=17 each; Figure 1).
Figure 1. The flow of participants through the trial.
All the clinical outcomes were assessed at baseline and after the treatment program by an assessor blinded to the group allocation of the subjects. The primary outcome was disability, while the secondary outcomes were the LPP intensity, mood, and sleep quality. Disability was assessed via the Roland-Morris Disability Questionnaire (RMDQ), one of the most comprehensively validated outcome measures for LBP.[18,19] It consists of 24 items representing “physical functions likely to be affected by lower back pain.” Each item scores 0 or 1 (yes or no), and the score ranges from 0 to 24, with higher scores reflective of higher related disability. The patients’ pain was measured with the Visual Analog Scale (VAS). Participants were asked to score their perception of pain on a 10-cm scale. The scale ranged from 0 (no pain) to 10 (the most pain). Mood was assessed via the Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire used to determine anxiety and depression levels.[20,21] Each item of the anxiety subscale (HADS-A) and the depression subscale (HADS-D) was scored with values ranging from 0 to 3. The lowest possible score for depression and anxiety was 0, indicating no distress, and the highest possible score was 21, indicating maximum distress. We anticipated that the effects of exercise on mood would be observable after the Pilates exercise program. Overall sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI).[22,23] The seven components of the PSQI provide a measure of global sleep quality, including sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction, with a possible range of 0 to 21 points. A higher global PSQI score indicates worse sleep quality. A score of 5 or higher provided a sensitive and specific measure of poor sleep quality.[22]
Intervention
All subjects received usual prenatal care and education about preventing LBP, the benefits of regular exercise, and using hot compresses when LBP exacerbates. The Pilates group also received a supervised exercise program. According to current guidelines, we did not ask the controls not to exercise, although they were not given any exercise prescription. The Pilates exercise program was carried out twice a week for 60-min per session for 12 weeks by an experienced Pilates instructor. The exercise program was performed based on traditional Pilates principles, including centering, control, precision, concentration, breath, and flow.[24] To ensure pregnancy safety, the Pilates program was achieved at a low-moderate intensity following the current American Congress of Obstetricians and Gynecologists exercise prescription.[25] They trained in two groups using a Pilates ball as exercise equipment. Participants were instructed to practice synchronized breathing with each movement. The movements that required 0-degree extension of the knee (e.g., saw, corkscrew, and single leg stretch) were modified and performed with the knee slightly flexed (Figure 2). It was ensured that the patients avoided being in the supine position for a long time and were well hydrated. At the end of each exercise session, stretching exercises were performed. Progression was achieved by adding complex Pilates exercises, and the challenge over time to decrease rest intervals. The Pilates instructor also corrected the body alignment of each patient based on Pilates principles.
Figure 2. 1. Hundred; 2. Seated pelvic tilt; 3. Spine stretch forward; 4. One leg circle; 5. Cat-cow; 6. Mermaid stretch; 7. Saw; 8. Shoulder bridge; 9. Corkscrew; 10. Single leg stretch; 11. Side kick series-circle; 12. Side kick series-front; 13. Side kick series-back; 14. Side kick series-lift; 15. Side kick series-inner thigh; 16. Rolling like a ball.
Adherence and adverse effects
Adherence (percentage of prescribed sessions accomplished) was recorded to monitor exercise compliance. Adverse effects associated with the exercise program were also recorded to determine exercise safety. The patients who performed at least 80% of the prescribed sessions were identified as having completed the Pilates program. Adherence data were essential to count missed exercise periods to get a precise estimate of exercise sessions for generalizing the findings about exercise effects; thus, it is a powerful modulator of health care system effectiveness.[26] Adverse events were defined as an increase in pain within one day after an exercise session. If the accident of aggravation occurred, interruption of or withdrawal from the Pilates exercise was decided based on the type of injury.
Statistical analysis
The sample size was calculated using the G*Power version 3.1.9.7 software (Heinrich- Heine-Universität Düsseldorf, Düsseldorf, Germany). The effect size was considered as 0.89 for between-group comparisons with respect to the postintervention disability levels evaluated by RMDQ.[9] Calculation of the sample size indicated that 34 patients were required (1:1 randomization, 17 per group) to allow 80% power to detect significance at the 5% level. Subjects were randomly allocated to either control or exercise group using the random number generation function in a commercially available software (Microsoft Excel; Microsoft Corp., Redmond, WA, USA).
Data were analyzed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA). In the presentation of the data, the mean ± standard (SD) deviation or median and interquartile range were used for quantitative variables. In the analysis of quantitative variables, normality and homogeneity tests were performed. The Kolmogorov-Smirnov test was used for normality, and Levene’s test was used for homogeneity. In between-group comparisons, the t-test was used for measurements that fit a normal distribution. The Mann-Whitney U test was used to measure the differences between the groups for the qualitative variables. For normally distributed variables, the paired t-test was used, while the Wilcoxon test was used for nonnormally distributed variables to measure repeated variables within groups. The Pearson chi-square test was used for qualitative variables. For two-by-two structures, Fisher exact test was used. The intention-to-treat analysis was applied, and the data of every randomized subject were analyzed during each follow-up period, regardless of whether they were lost to follow-up or withdrew from the study. The missing follow-up measurements were imputed with baseline values. A p-value <0.05 was considered statistically significant.
Results
The mean ages of the patients were 28.8±5.5 and 30.7±7.0 years in the control group and Pilates group, respectively. Their baseline demographic data and clinical characteristics are shown in Table 1. There was no significant difference between the two groups in terms of demographic data, including age, body mass index, and parity. The participants in the two groups also had similar baseline characteristics.
Table 1. Baseline demographic and clinical characteristics.
| Control group | Pilates group | ||||||||
| n | % | Mean±SD | Min-Max | n | % | Mean±SD | Min-Max | ||
| Age (year) | 28.8±5.6 | 20-40 | 30.8±7.0 | 18-40 | 0.378 | ||||
| Height (cm) | 162.0±4.5 | 155-170 | 159.8±5.6 | 151-171 | 0.210 | ||||
| Weight (kg) | 69.2±13.1 | 56-100 | 64.5±13.1 | 48-90 | 0.303 | ||||
| Body mass index (kg/m2) | 26.4±5.0 | 21.83-40.79 | 25.3±4.9 | 16.42-33.87 | 0.520 | ||||
| Gestastion (weeks) | 17.2±1.8 | 14-23 | 19.1±3.5 | 14-23 | 0.062 | ||||
| Parity | 0.186 | ||||||||
| 0 | 4 | 23.5 | 9 | 52.9 | |||||
| 1 | 5 | 29.4 | 4 | 23.5 | |||||
| >2 | 8 | 47.1 | 4 | 23.5 | |||||
| Region | 0.830 | ||||||||
| a | 13 | 76.5 | 12 | 70.6 | |||||
| b | 3 | 17.6 | 3 | 17.6 | |||||
| c | 1 | 5.9 | 2 | 11.8 | |||||
| Pre-exercise | 0.500 | ||||||||
| Yes | 2 | 11.8 | 3 | 17.6 | |||||
| No | 15 | 88.2 | 14 | 82.4 | |||||
| SD: Standard deviation; a: Low back pain; b: Pelvic girdle pain; c: Both. | |||||||||
One patient had a minor muscle injury that healed spontaneously without any treatment in the Pilates group during the intervention period. The level of adherence to the exercise program was 88%, as collected via participants’ daily exercise diaries. Two patients in the control group and one patient in the Pilates group were lost during the study (Figure 1).
A within-group analysis revealed that the RMDQ score was significantly reduced after intervention [mean difference (MD) 5.9 points; 95% confidence interval (CI): 4.1 to 7.6] in the Pilates group. Concerning the secondary outcome variables, there was also a significant effect of Pilates on the VAS (MD 3.0 points; 95% CI: 1.9 to 3.9), HADS-A (MD 3.6 points; 95% CI: 2.2 to 4.9), and HADS-D (MD 2.6 points; 95% CI: 1.5 to 3.6). In the control group, statistically significant differences were not found in all these outcomes (p>0.05).
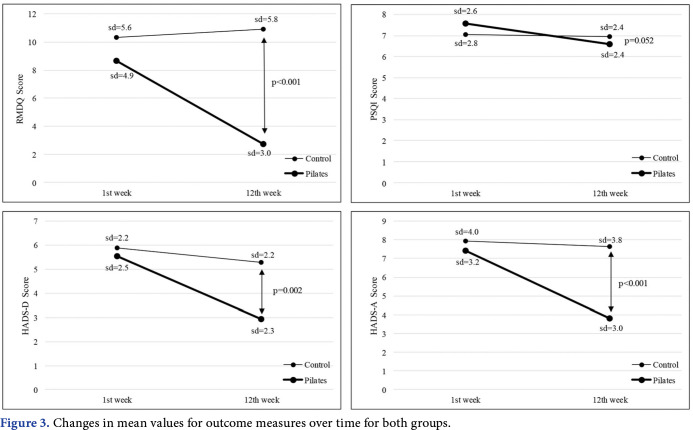
The between-group effect sizes were moderate for the RMDQ and VAS scales (d=0.4 and d=0.7, respectively), small for the HADS-A and HADS-D scales (both d=0.2) after 12 weeks of treatment sessions (Table 2). There were statistically significant differences in terms of RMDQ, VAS, HADS-A, and HADS-D (p<0.05) in the between-group analysis. There was no significant difference between the two groups in terms of PSQI (p>0.05, Figure 3).
Table 2. Comparison of variables at baseline and at 12 weeks.
| Control group | Pilates group | pab | d | |||||||
| Mean±SD | Median | Q1-Q3 | 95% CI | Mean±SD | Median | Q1-Q3 | 95% CI | |||
| Visual Analog Scale | ||||||||||
| Preintervention | 4.0 | 3.0-5.0 | 2.0-6.0c | 5.0 | 3.0-5.0 | 2.0-8.0c | 0.394b | 0.035 | ||
| Postintervention | 5.0 | 5.0-7.0 | 4.0-8.0c | 2.0 | 1.0-2.0 | 0.0-4.0c | <0.001b | 0.724 | ||
| RMDQ | ||||||||||
| Preintervention | 10.4±5.6 | 7.48-13.22 | 8.6±4.9 | 6.14-11.16 | 0.350 | 0.027 | ||||
| Postintervention | 10.9±5.8 | 7.94-13.95 | 2.7±3.0 | 1.18-4.24 | <0.001 | 0.456 | ||||
| PSQI | ||||||||||
| Preintervention | 7.1±2.8 | 5.61-8.51 | 7.6±2.6 | 6.23-8.95 | 0.576 | 0.010 | ||||
| Postintervention | 6.9±2.4 | 5.70-8.18 | 6.6±2.4 | 5.37-7.81 | 0.670 | 0.006 | ||||
| HADS-D | ||||||||||
| Preintervention | 5.9±2.2 | 5.88-10.00 | 5.5±2.5 | 5.78-9.05 | 0.660 | 0.006 | ||||
| Postintervention | 5.3±2.2 | 5.68-9.61 | 2.9±2.3 | 2.29 -5.36 | <0.001 | 0.248 | ||||
| HADS-A | ||||||||||
| Preintervention | 7.9±4.0 | 4.76-7.00 | 7.4±3.2 | 4.27-6.79 | 0.673 | 0.006 | ||||
| Postintervention | 7.6±3.8 | 4.16-6.43 | 3.8±3.0 | 1.77-4.11 | <0.001 | 0.227 | ||||
| SD: Standard deviation; RMDQ: Roland-Morris Disability Questionnaire; PSQI: Pittsburgh Sleep Quality Index; HADS-D: Hospital Anxiety and Depression Scale-Depression; HADS-A: Hospital Anxiety and Depression Scale-Anxiety; d: Effect size (Cohen’s d); d<0.2 small, d=0.5 medium, d>0.8 large; a: T-test is used for analyzing the difference of means between groups; b: Mann-Whitney U Test was applied; c: Represents min-max. | ||||||||||
Figure 3. Changes in mean values for outcome measures over time for both groups.
Discussion
This is the first prospective randomized trial examining the effect of a 12-week Pilates exercise on disability in pregnancy-related LPP. Our results demonstrated that a Pilates-based therapeutic exercise program improves symptom severity in patients with pregnancy-related LPP.
To date, there are inconsistent results regarding the etiology of LPP during pregnancy. It has been accepted according to biomechanical theory that the expanding uterus and weight gain lead the body’s center of gravity to move, increasing pressure on the lower back.[27] Another theory of pregnancy-related LPP is that the influence of pregnancy hormones, such as relaxin, on supporting ligaments causes increased laxity, impaired pelvis stability, and inefficient neuromotor control.[28] Similarly, in individuals with LBP, the local musculature exhibits disturbed motor control patterns and decreased activation of the deep abdominals in functional and loaded postures.[29,30] Recent research reported that in contrast to static conditions, lateral trunk lean is the primary factor women use in pregnancy to keep the center of gravity closer to the base of support, and more attention should be given to the design of exercise programs targeting core strength and pelvic stability.[31] It has been shown that Pilates as a motor control exercise improves spinal stability by activating the core stabilizing muscles.[32] According to a meta-analysis of 16 randomized controlled trials, when compared with general exercise, motor control exercise was superior in treating disability in patients with chronic LPP.[33] It is also believed that Pilates exercises have a greater effect, improving self-efficacy and mood due to its principles, including centering, breath, and concentration (greater awareness). We are of the opinion that one of these mechanisms may have a beneficial influence on pregnancy-related LPP, resulting in overcoming the decreased activation of muscles, inefficient neuromotor control, and disordered mood. We did not observe any significant changes in sleep quality, which may be attributed to the fact that sleep might be worsened with the progression of the gestation week, regardless of the exercise program.
Our results are consistent with a previous study based on Pilates exercises for the treatment of pregnancy-related LPP. Oktaviani,[34] evaluated pain in pregnant women in the third trimester (gestational age >28 weeks) after Pilates exercise. They used a once-weekly Pilates exercise program for eight weeks without detailing the program, and a significant improvement in VAS was observed. Fontana Carvalho et al.[35] compared the effects of lumbar stabilization and stretching exercises for the treatment of gestational LPP. After the interventions, 20 women in total completed the study, and there was a significant reduction in pain and balance for both interventions, but no change in the disability score. Two possible interpretations of the contrast in disability from our results might be the exercise type and exercise duration. They used six weeks of stretching and lumbar stabilization exercises, such as phasic perineum, tonic perineum, tonic mobility, and pelvic swing. We believe both modalities consist of mobility and balance exercises that are relatively less challenging; therefore, they may be less sufficient than strengthening-type exercises. Further, it has been shown that women who reported higher depression/anxiety symptoms appeared to experience higher levels of functional disability in relation to their LPP.[36] In our setting (12 weeks of a strengthening-based Pilates exercise program), we achieved a significant improvement in mood, which might play a role in encouraging women and decreasing comorbidity. Consequently, compared with other exercises, Pilates as a motor control exercise may therefore be proposed as important in the rehabilitation of pregnancy.
Recommendations for exercise prescription during pregnancy suggest exercise on most or all days of the week at a moderate intensity for 20-30 min per day.[25] After the second trimester, the prescribed exercise program may be interrupted due to the high prevalence of pregnancy-related LPP. Participation in a regular fitness program may not alter the proportion of women reporting pelvic girdle pain or LPP during pregnancy.[37] Adding Pilates to usual prenatal care should be considered in the treatment of pregnant women who tend to decrease their physical activity level due to pain as it is well tolerated by pregnant women suffering from LPP.
The present study has some limitations. At the beginning of the study, the sample included pregnant women who were not in the third trimester. Since the exercise program was planned for 12 weeks, pregnant women whose pain started in the second trimester were included. After 12 weeks, in the third trimester, though it was anticipated that their disability and pain would possibly worsen compared to the second trimester, they had less disability and pain, leading to better mood. Thus, it is also demonstrated that the beneficial effects of Pilates-based therapeutic exercise may last into the third trimester of pregnancy as well if the exercise program is started without delay. Another limitation of the study is that the patients with obstetric or medical comorbidities were not assigned to an exercise program. Future studies should investigate which subgroups of those patients respond to Pilates-based exercise without any adverse effects.
In conclusion, despite a fraction of randomized controlled studies in the LBP rehabilitation area, we carefully chose patients with pregnancy-related LPP and rigorously described the method to allow replication in clinical practice. Ultimately, Pilates-based therapeutic exercise is a promising treatment option for pregnancy-related LPP.
Acknowledgments.
The authors would like to acknowledge Allan Menezes and Baris Aksu for supporting this research.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Kocaeli University Non-Interventional Clinical Research Ethics Committee (date: 07.02.2018, no: KU-GOKAEK 2018/39). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept, design, control/ supervision, literature review: A.Y.K., P.Y.; Data collection and/or processing: P.Y., G.B.; Analysis and/or interpretation, writing the article, critical review, references and fundings, materials, other: P.Y., A.Y.K., G.B.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Mohseni-Bandpei MA, Fakhri M, Ahmad-Shirvani M, Bagheri-Nessami M, Khalilian AR, Shayesteh-Azar M, et al. Low back pain in 1,100 Iranian pregnant women: Prevalence and risk factors. Spine J. 2009;9:795–801. doi: 10.1016/j.spinee.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Sencan S, Ozcan-Eksi EE, Cuce I, Guzel S, Erdem B. Pregnancy-related low back pain in women in Turkey: Prevalence and risk factors. Ann Phys Rehabil Med. 2018;61:33–37. doi: 10.1016/j.rehab.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Skaggs CD, Prather H, Gross G, George JW, Thompson PA, Nelson DM. Back and pelvic pain in an underserved United States pregnant population: A preliminary descriptive survey. J Manipulative Physiol Ther. 2007;30:130–134. doi: 10.1016/j.jmpt.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Malmqvist S, Kjaermann I, Andersen K, Økland I, Larsen JP, Brønnick K. The association between pelvic girdle pain and sick leave during pregnancy; a retrospective study of a Norwegian population. BMC Pregnancy Childbirth. 2015;15:237–237. doi: 10.1186/s12884-015-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiezer M, Hage-Fransen MAH, Otto A, WiefferPlatvoet MS, Slotman MH, Nijhuis-van der Sanden MWG, et al. Risk factors for pelvic girdle pain postpartum and pregnancy related low back pain postpartum; a systematic review and meta-analysis. Musculoskelet Sci Pract. 2020;48:102154–102154. doi: 10.1016/j.msksp.2020.102154. [DOI] [PubMed] [Google Scholar]
- 6.Demissie Z, Siega-Riz AM, Evenson KR, Herring AH, Dole N, Gaynes BN. Physical activity and depressive symptoms among pregnant women: The PIN3 study. Arch Womens Ment Health. 2011;14:145–157. doi: 10.1007/s00737-010-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu WH, Meijer OG, Uegaki K, Mens JM, van Dieën JH, Wuisman PI, et al. Pregnancy-related pelvic girdle pain (PPP), I: Terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13:575–589. doi: 10.1007/s00586-003-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liddle SD, Pennick V. Interventions for preventing and treating low-back and pelvic pain during pregnancy. CD001139Cochrane Database Syst Rev. 2015;2015 doi: 10.1002/14651858.CD001139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halis F, Yildirim P, Kocaaslan R, Cecen K, Gokce A. Pilates for better sex: Changes in sexual functioning in healthy Turkish women after pilates exercise. J Sex Marital Ther. 2016;42:302–308. doi: 10.1080/0092623X.2015.1033576. [DOI] [PubMed] [Google Scholar]
- 11.Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: A randomized controlled study. Eur J Phys Rehabil Med. 2010;46:481–487. [PubMed] [Google Scholar]
- 12.Stankovic A, Lazovic M, Kocic M, Dimitrijevic L, Stankovic I, Zlatanovic D, et al. Lumbar stabilization exercises in addition to strengthening and stretching exercises reduce pain and increase function in patients with chronic low back pain: Randomized clinical openlabel study. Turk J Phys Med Rehab. 2012;58:177–183. [Google Scholar]
- 13.Shiri R, Coggon D, Falah-Hassani K. Exercise for the prevention of low back and pelvic girdle pain in pregnancy: A meta-analysis of randomized controlled trials. Eur J Pain. 2018;22:19–27. doi: 10.1002/ejp.1096. [DOI] [PubMed] [Google Scholar]
- 14.Mazzarino M, Kerr D, Wajswelner H, Morris ME. Pilates method for women's health: Systematic review of randomized controlled trials. Arch Phys Med Rehabil. 2015;96:2231–2242. doi: 10.1016/j.apmr.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Barros BS, Imoto AM, O'Neil J, Duquette-Laplante F, Perrier MF, Dorion M, et al. The management of lower back pain using pilates method: Assessment of content exercise reporting in RCTs. Disabil Rehabil. 2022;44:2428–2436. doi: 10.1080/09638288.2020.1836269. [DOI] [PubMed] [Google Scholar]
- 16.Hu X, Ma M, Zhao X, Sun W, Liu Y, Zheng Z, et al. Effects of exercise therapy for pregnancy-related low back pain and pelvic pain: A protocol for systematic review and metaanalysis. e17318Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on Exercise Reporting Template (CERT): Explanation and elaboration statement. Br J Sports Med. 2016;50:1428–1437. doi: 10.1136/bjsports-2016-096651. [DOI] [PubMed] [Google Scholar]
- 18.Roland M, Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Küçükdeveci AA, Tennant A, Elhan AH, Niyazoglu H. Validation of the Turkish version of the Roland-Morris Disability Questionnaire for use in low back pain. Spine (Phila Pa 1976) 2001;26:2738–2743. doi: 10.1097/00007632-200112150-00024. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Aydemir Ö, Güvenir T, Küey L, Kültür S. Hastane anksiyete ve depresyon ölçeği Türkçe formunun geçerlilik ve güvenilirlik çalışması. Turk Psikiyatri Derg. 1997;8:280–287. [Google Scholar]
- 22.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Agargün MY, Kara H, Anlar Ö. Reliability and Validity of the Turkish version of the Pittsburgh Sleep Quality Index. Turk Psikiyatri Derg. 1996;7:107–111. [Google Scholar]
- 24.Pilates J, Miller W. Return to life through contrology. Incline Village: Presentation Dynamics; 1945. [Google Scholar]
- 25.Physical activity and exercise during pregnancy and the postpartum period: ACOG Committee opinion, number 804. e178-e188Obstet Gynecol. 2020;135 doi: 10.1097/AOG.0000000000003772. [DOI] [PubMed] [Google Scholar]
- 26.Hecksteden A, Faude O, Meyer T, Donath L. How to construct, conduct and analyze an exercise training study. Front Physiol. 2018;9:1007–1007. doi: 10.3389/fphys.2018.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katonis P, Kampouroglou A, Aggelopoulos A, Kakavelakis K, Lykoudis S, Makrigiannakis A, et al. Pregnancy-related low back pain. Hippokratia. 2011;15:205–210. [PMC free article] [PubMed] [Google Scholar]
- 28.Dehghan F, Haerian BS, Muniandy S, Yusof A, Dragoo JL, Salleh N. The effect of relaxin on the musculoskeletal system. e220-9Scand J Med Sci Sports. 2014;24 doi: 10.1111/sms.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976) 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 30.Yildirim P, Gultekin A. The effect of a stretch and strengthbased yoga exercise program on patients with neuropathic pain due to lumbar disc herniation. Spine (Phila Pa 1976) 2022;47:711–719. doi: 10.1097/BRS.0000000000004316. [DOI] [PubMed] [Google Scholar]
- 31.Krkeljas Z. Changes in gait and posture as factors of dynamic stability during walking in pregnancy. Hum Mov Sci. 2018;58:315–320. doi: 10.1016/j.humov.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Phrompaet S, Paungmali A, Pirunsan U, Sitilertpisan P. Effects of pilates training on lumbo-pelvic stability and flexibility. Asian J Sports Med. 2011;2:16–22. doi: 10.5812/asjsm.34822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byström MG, Rasmussen-Barr E, Grooten WJ. Motor control exercises reduces pain and disability in chronic and recurrent low back pain: A meta-analysis. E350-8Spine (Phila Pa 1976) 2013;38 doi: 10.1097/BRS.0b013e31828435fb. [DOI] [PubMed] [Google Scholar]
- 34.Oktaviani I. Pilates workouts can reduce pain in pregnant women. Complement Ther Clin Pract. 2018;31:349–351. doi: 10.1016/j.ctcp.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Fontana Carvalho AP, Dufresne SS, Rogerio de Oliveira M, Couto Furlanetto K, Dubois M, Dallaire M, et al. Effects of lumbar stabilization and muscular stretching on pain, disabilities, postural control and muscle activation in pregnant woman with low back pain. Eur J Phys Rehabil Med. 2020;56:297–306. doi: 10.23736/S1973-9087.20.06086-4. [DOI] [PubMed] [Google Scholar]
- 36.Virgara R, Maher C, Van Kessel G. The comorbidity of low back pelvic pain and risk of depression and anxiety in pregnancy in primiparous women. BMC Pregnancy Childbirth. 2018;18:288–288. doi: 10.1186/s12884-018-1929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haakstad LA, Bø K. Effect of a regular exercise programme on pelvic girdle and low back pain in previously inactive pregnant women: A randomized controlled trial. J Rehabil Med. 2015;47:229–234. doi: 10.2340/16501977-1906. [DOI] [PubMed] [Google Scholar]