Abstract
Study Objectives:
Philips Respironics issued a voluntary recall of positive airway pressure devices used to treat obstructive sleep apnea in June 2021. We surveyed sleep medicine clinicians from the American Academy of Sleep Medicine membership to assess the impact of the recall on clinicians and patients.
Methods:
One hundred thirty-six clinicians participated between June 2022 and November 2022. Participants reported their treatment recommendations for patients affected by the recall, their patients’ behaviors regarding the recall, the recall’s impact on them as clinicians and on their patients, and the approximate time their patients were waiting for a replacement device.
Results:
Clinicians most commonly reported first learning about the recall from Philips (25.0%), and patients most commonly first heard about the recall from news sources (34.5%). Most clinicians (62.4%) reported that they recommended patients continue using a recalled device. In comparison, only 9.3% of clinicians reported encouraging patients to stop using their recalled device. Clinicians reported that 59.9% of patients continued treatment with their recalled device, whereas 26.5% stopped treatment. Clinicians reported that over one-third of their patients were still waiting for a replacement machine. Most (86.8%) clinicians reported their stress levels were affected due to the recall, and 91.5% of clinicians reported the recall affected their patients’ health and well-being. Most (83.3%) clinicians reported the recall affected their patients’ trust in medicine.
Conclusions:
Clinicians reported that the Philips recall impaired the vast majority of their patients’ health and trust in medicine and that many patients were still waiting for replacement devices.
Citation:
Robbins R, Epstein LJ, Pavlova MK, et al. Quantifying the impact of the Philips recall on patients with sleep apnea and clinicians. J Clin Sleep Med. 2023;19(9):1677–1683.
Keywords: OSA, sleep apnea, CPAP, Philips recall
BRIEF SUMMARY
Current Knowledge/Study Rationale: Philips Respironics issued a voluntary recall of millions of positive airway pressure devices used in obstructive sleep apnea treatment due to concerns regarding the degradation of sound abatement foam present in their devices. We surveyed clinician members of the American Academy of Sleep Medicine to quantify the recall’s impact.
Study Impact: Our results demonstrate the pervasive impact of the recall on sleep apnea care. Clinicians report that the Philips recall eroded patients’ trust in medicine and impaired patients’ and clinicians’ health and well-being. Clinicians’ responses also revealed that many patients were still waiting for replacement devices more than 1 year after the recall was initiated.
INTRODUCTION
Philips Respironics (Philips), one of the largest manufacturers of positive airway pressure (PAP) devices used to treat obstructive sleep apnea (OSA), including continuous PAP and bilevel PAP devices, issued a voluntary recall of these devices in June 2021, affecting more than 10 million devices in the United States.1 Philips stated that they issued the recall after receiving several complaints related to particulates in the air circuits of their devices. This was subsequently found to be caused by the degradation of polyester-based polyurethane sound abatement foam in their devices leading to the release of toxic particles that may cause serious health concerns if inhaled or swallowed.1 Public communication from Philips urged patients to stop using their devices and to speak to their health care clinician to determine next steps and to register for a replacement device.2 Unfortunately, as of November 2022 only 2 million (10% of the needed) replacement devices had been shipped to patients.3
The Philips recall of millions of continuous PAP and bilevel PAP devices triggered a public health crisis due to the sheer number of patients affected.4 The recall also represents a challenging case of medical decision-making in several ways. First, some patients were forced to make a difficult decision between terminating use of a life-sustaining treatment and continuing such treatment despite potential health concerns. Second, clinicians were ill-equipped to assist patients in this decision because these key stakeholders were given opaque information about replacement devices. Moreover, the recall was initiated in the midst of the coronavirus disease 2019 pandemic, a time when sleep laboratories across the United States observed an increase in patient volume, clinic wait times, and clinician burden, as well as reduced public trust in medical information.5
Our objective was to understand the impact of the Philips recall on patients with sleep apnea and sleep medicine clinicians. To do so, we conducted a survey of the membership of the largest national organization of sleep medicine clinicians in the United States. We sought to understand the impact of the Philips recall on sleep medicine specialists (eg, increased stress, work/life balance) and patients (eg, increased worry or fear, access to PAP devices, wait times for a new machine) in order to inform future measures to support patients’ decision-making and patients’ and clinicians’ health and well-being.
METHODS
Design and participants
Participants were recruited from the membership of the American Academy of Sleep Medicine, which is a professional association composed of sleep specialists and sleep researchers. There were 3 methods of recruitment of sleep clinicians: (1) direct mail through the American Academy of Sleep Medicine membership directory, (2) email blasts to all American Academy of Sleep Medicine members, and (3) posts to the members-only discussion board. Interested participants provided consent to participate in the study then were screened for eligibility. Eligible participants were health care professionals (eg, physicians, nurse practitioners, respiratory therapists, and sleep technologists) who see/treat patients with OSA. There were 182 participants who provided consent and met eligibility criteria. Among those who consented and met eligibility criteria, 46 did not respond to any questions after the eligibility screener and were removed from the analysis, leaving 136 respondents. Participants who completed the survey were entered into a drawing for 1 of 3 $50 gift certificates.
Questionnaire
Participants reported how they and their patients first heard about the recall from a list of options (eg, Philips Respironics, social media). Participants reported the extent to which Philips could have done more to support patients and themselves (clinicians) affected by the recall on 5-point Likert scales of agreement. We also asked participants to report whether they recommended patients continue or stop treatment with a recalled device or try alternative therapies on a scale from 1 (“never”) to 4 (“all of the time”). Participants reported the proportion of their patients who “continued using their recalled machine,” “stopped using their recalled machine,” or “started using an alternative therapy” on a scale from 0% (none of my patients) to 100% (all of my patients). Participants reported the average time patients waited for a new machine from 1 month to 12 months or “still waiting,” given that our survey started 1 year after the recall. Participants reported the extent to which the recall affected them as clinicians (eg, their stress levels, their work/life balance) and their patients’ health and well-being, as well as their relationship with their patient and their patient’s trust in medicine on a 5-point Likert scale from “significantly disrupted” to “significantly improved.” Clinicians reported the strategies and styles they enacted to support their patients, such as shared decision-making or empathic communication. Finally, participants were asked to respond to several demographic questions about themselves as clinicians, their patients, and their practice.
Analysis
Responses were analyzed using descriptive statistics, including means and standard deviations for continuous variables and percentages for ordinal variables. We compare the reported sources where providers and patients first heard about the recall using a chi-square test. Data were analyzed in Stata Statistical Software Version 16 (StataCorp, LP, College Station, Texas).
RESULTS
One hundred thirty-six clinicians agreed to participate and contributed data to the survey. Among the sample, participants were most commonly physicians (61.9%), followed by nurse practitioners (20.0%). Clinicians were more likely to identify as female (54.3%) and White or Caucasian (76.4%). Among participants, 11.6% reported being Hispanic or Latino/a. Telemedicine was reported to be common and used with 29.5% of patients; 44.3% reported 15 or more years practicing practice and 32.1% reported working in a public hospital. Clinicians reported patients with moderate/severe OSA comprised on average 51.2% of their patients, and on average 39.2% reported seeing patients that use a Philips device (Table 1).
Table 1.
Demographic characteristics summarizing the study sample (n = 136).
| n | % | |
|---|---|---|
| Role | ||
| Physician | 65 | 61.9 |
| Nurse practitioner | 21 | 20.0 |
| Physician assistant | 5 | 4.8 |
| Sleep technologist | 8 | 7.6 |
| Respiratory therapist | 3 | 2.9 |
| Dentist | 1 | 1.0 |
| Retired medical doctor | 1 | 1.0 |
| Clinic director | 1 | 1.0 |
| Sex | ||
| Male | 48 | 45.7 |
| Female | 57 | 54.3 |
| Race | ||
| White or Caucasian | 81 | 76.4 |
| American Indian/Alaska Native | 1 | 0.9 |
| Asian | 10 | 9.4 |
| Other | 8 | 7.6 |
| Prefer not to say | 6 | 5.7 |
| Hispanic or Latino/a | 12 | 11.6 |
| Age | ||
| <34 years old | 8 | 9.4 |
| 35–44 years old | 30 | 28.3 |
| 45–54 years old | 27 | 25.5 |
| 55–64 years old | 25 | 23.6 |
| 65+ years old | 14 | 13.2 |
| Years practicing medicine | ||
| Less than 5 years | 17 | 16.0 |
| 5 years up to 10 years | 24 | 22.6 |
| 10 years up to 15 years | 18 | 17.0 |
| 15 years up to 20 years | 17 | 16.0 |
| More than 20 years | 30 | 28.3 |
| Type of practice | ||
| Hospital (private) | 19 | 17.9 |
| Hospital (public) | 34 | 32.1 |
| Group practice | 23 | 21.7 |
| Individual practice | 14 | 13.2 |
| Academic | 15 | 14.2 |
| n | Mean (SD) | |
| Percent of patients using telemedicine | 113 | 29.5 (27.6) |
| Proportion of your OSA patients using ResMed devices | 113 | 56.4 (25.1) |
| Proportion of your OSA patients using Philips CPAP | 112 | 39.2 (24.1) |
| Proportion of your OSA patients using oral appliances | 112 | 8.5 (11.6) |
| Proportion of your patients | ||
| Race/ethnic minority | 109 | 34.9 (19.4) |
| Non-English-speaking | 110 | 10.2 (13.6) |
| Low English proficiency | 107 | 12.7 (12.6) |
| No. of patients/year | 135 | 2,236.0 (3,576.7) |
| Proportion of patients that have moderate/severe sleep apnea | 113 | 51.2 (17.5) |
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, SD = standard deviation.
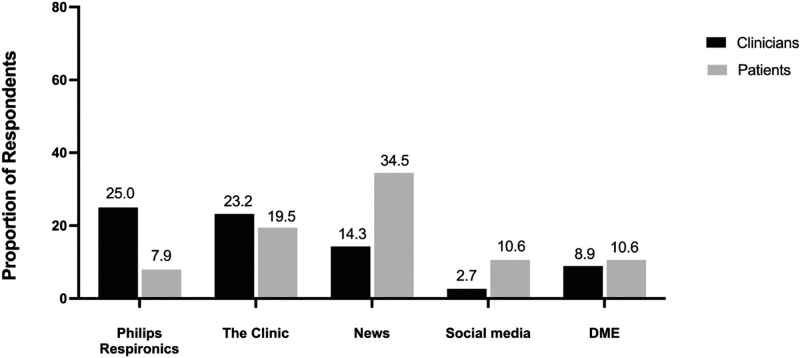
Clinicians most commonly reported first hearing about the Philips recall from Philips Respironics (25%), followed by their clinic/institution (23.2%). They reported that their patients first heard about the Philips recall from the news (34.5%) or their clinic (19.4%). Responses as to where clinicians first heard about the Philips recall did differ from the places the patients first heard about the recall (Χ = 48.1, P < .001; Figure 1).
Figure 1. How clinicians and patients first heard news about the Philips recall.
Clinicians selected the source where they and their patients reported first hearing about the Philips recall. DME = durable medical equipment.
Clinicians reported the patient characteristics they considered most important in their treatment recommendations were “OSA symptom severity,” with 99.1% indicating it as somewhat/very important; “medical comorbidities,” with 96.3% designating it as somewhat/very important; and “patient risk of accidents,” with 94.4% selecting it as somewhat/very important. “Patient’s age” was rated as less important, with only 42.5% designating it as somewhat/very important.
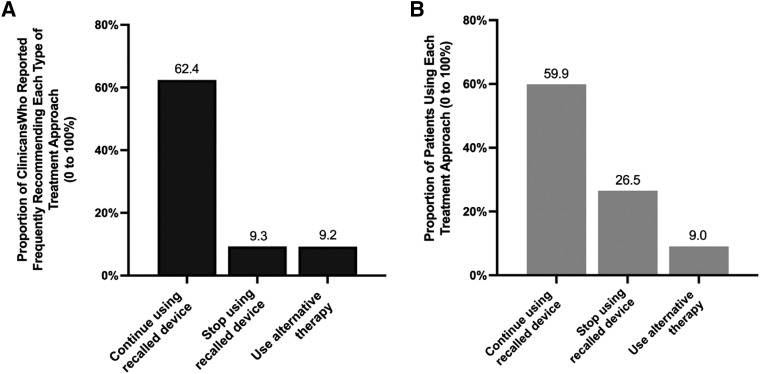
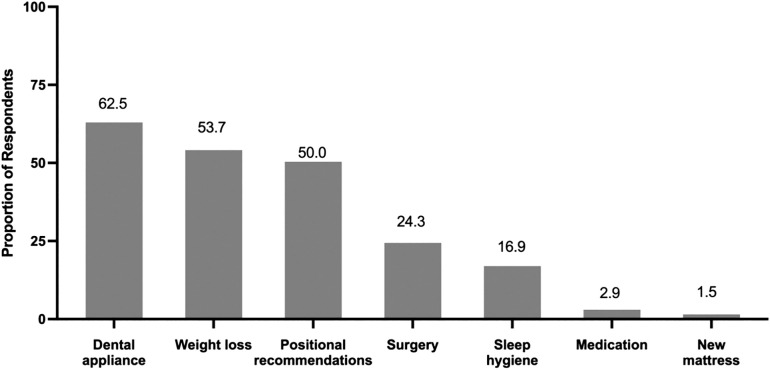
Regarding clinician treatment recommendations, the most common advice (ie, “most of the time” or “all of the time”) was to continue treatment with a recalled device (62.4%), whereas only 9.3% reported advising their patients to stop treatment with a recalled device and 9.2% reported advising their patients to use alternative treatments (Figure 2). Full responses to the questions assessing frequency of each treatment recommendation can be found in the supplemental material. The most commonly recommended alternative treatment was dental appliances (62.5%), followed by weight loss (53.7%) (Figure 3). Clinicians were asked to report the communication style they employed to assist their patients affected by the recall. The most common communication style clinicians employed to assist their patients was a shared decision-making model (69.9%), followed by patient-centered communication (59.6%) and empathetic communication (57.4%).
Figure 2. Most frequent treatment approaches that clinicians reported providing to their patients and the proportion of patients utilizing each treatment approach.
(A) Clinician recommendations to patients affected by the recall by treatment approach. The responses clinicians made represent the proportion of clinicians that reported frequently making a treatment recommendation (eg, to continue using a recalled device or stop using the recalled device) “most of the time” or “all the time.” (B) Patients’ behavior by treatment approach. The responses illustrating patient responses are the average proportion of patients that clinicians estimated were behaving. The questions assessing patients’ behavior asked clinicians to estimate out of 100% (all patients) the number behaving certain ways (eg, continuing to use a recalled device). The bars representing patient behavior shown on the graph depict average responses to these questions.
Figure 3. Alternative treatments clinicians reported recommending for their patients.
Clinicians used check boxes to indicate the alternative therapies they recommended to their patients affected by the recall. These responses were divided by the total population for an estimate of the proportion of clinicians recommending which alternative treatments.
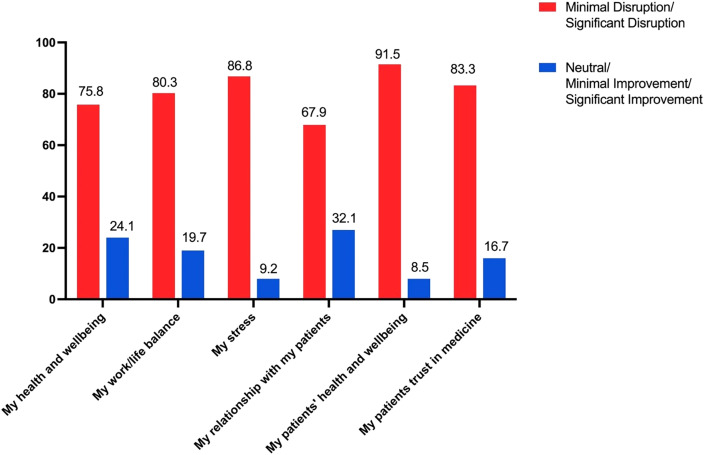
Among the clinicians, 86.8% reported the recall increased their stress, 91.5% reported the recall impaired their patients’ health and well-being, and 83.3% reported the recall impaired their patients’ trust in medicine (Figure 4).
Figure 4. Impacts of the Philips recall reported on patients’ and clinicians’ health, well-being, and stress and patients’ trust in medicine.
The red bars indicate responses from clinicians that reported the recall disrupted either minimally or significantly. The blue bars indicate responses that the recall did not disrupt (ie, either responses of “neutral,” “minimally improved,” or “significantly improved”).
Clinicians reported that 35.5% of their patients were still waiting for a new device. Among the patients who received a new device, clinicians reported the average wait time was 7.1 months (standard deviation = 3.1 months). The majority of clinicians indicated they either “somewhat” or “strongly” agreed with statements that Philips could have done more to support patients (92.0%) and clinicians (91.97%) affected by the recall.
DISCUSSION
The results of this study indicate that notice of the worldwide recall of PAP devices by one of the largest manufacturers of such equipment was poorly disseminated to both medical clinicians and their patients, and receipt of replacement devices was delayed for a large portion of patients. This has resulted in adverse consequences to the medical care of patients with OSA as well as reported impairment in the health and well-being of clinicians and patients.
The small number of patients and clinicians who were informed of the recall directly by Philips highlights the communication breakdown between the device manufacturer and the patients using their devices. Future policy efforts should be directed toward requiring device manufacturers to develop communication channels with patients so that vital information, such as that pertaining to a recalled device, is better transmitted to patients, as others have urged.4
Although adverse consequences for continued use were potentially severe, we found that over 60% of clinicians reported advising their patients to continue use of their recalled device most or all of the time, whereas only 9% reported advising their patients to stop using their recalled device. In addition, clinicians reported that the majority of patients (> 60%) continued using their device. We believe that multiple factors contributed to the continued use of potentially harmful therapy, including the lack of readily available alternate treatments, the proven improvement in symptoms experienced by patients who were using PAP therapy, the relatively small percentage of PAP users who had reported adverse consequences from using a recalled device, and the unknown true risks and potential long-term adverse outcomes. Nevertheless, our findings underscore a stark difference between the device manufacturer’s recommendations to cease use and the majority of providers who, according to our study, recommended continued use of recalled devices. The contrast we uncovered between industry recommendations to cease use and medical recommendations to continue was likely extremely confusing to patients and may have inspired mistrust in medicine. Given that the majority of clinicians recommended continued use of recalled devices and that many patients reported continued use of recalled devices, future research is needed to explore adherence to recalled devices. Should the recalled polyester-based polyurethane foam be associated with serious adverse outcomes, adherence information might be useful to ascertain the potential exposure to the foam and toxic particles.
In the process of deciding whether to continue use or discontinue use of PAP therapy, clinicians commonly reported employing shared decision-making and empathic communication to assist their patients in negotiating the challenging decision of whether to terminate therapy or continue their treatment despite the (unknown) adverse consequences associated with continued use. These findings are consistent with a report from Morgenthaler et al detailing their institutional response to the Philips recall, which emphasized empathic communication to attenuate patient anxiety and worry.6 Although this represents an advancement in patient involvement and utilization of patient-centered approaches, it is not clear whether this represents a change in clinicians’ approaches or the lack of information on which to provide informed input. Future research may consider employing objective methods for measuring clinician–patient communication styles in medical decision-making contexts such as the Philips recall. Future research may also explore how different objectively measured communication styles relate to treatment decision-making and possibly also patient outcomes.
Finally, we found that clinicians reported profound impacts of the recall on their health and well-being, with more than 75% of clinicians reporting a negative impact on their health and well-being, work/life balance, stress levels, and patient relationships. Most concerning, 83% of clinicians reported the recall impaired their patients’ trust in medicine. The primary driver of the stress was lack of information and pathways to resolution. Particularly given the current coronavirus disease 2019–related skepticism regarding the medical community, better methods for informing and supporting patients and clinicians need to be developed. Surveys such as this one should be the first step in reviewing and improving the response to device or other medical-related hazard development.
Although this study is the first to our knowledge to explore clinician-reported effects on patients and clinicians of the Philips recall, there are several notable limitations of the present work. Our study sample was relatively small and not randomly selected. It is a limitation that we did not ask participants to report the location of their clinical practice. We did not exclude trainees who may have been in our sample and not offered as much perspective as more-seasoned clinicians could offer. Our recruitment efforts were limited to direct emails and posts to a newsletter. Therefore, we are unable to determine the number of clinicians that were contacted. Additionally, our sample is not necessarily a representative sample of sleep medicine clinicians. Moreover, it is possible that the clinicians who responded to our survey were particularly upset about the recall, therefore biasing our results. Therefore, future research will be needed to understand the patient and clinician-reported impacts of the Philips recall more fully. In addition, clinicians were asked to report on their perceptions about their patients’ behaviors. Therefore, our findings relating to patients’ behavior need to be confirmed in future research with actual patients.
In conclusion, large numbers of both clinicians and patients were not provided timely information regarding the Philips recall of PAP devices. This has resulted in negative consequences for patient care and detrimental impacts on the health and well-being of both patients and clinicians.
DISCLOSURE STATEMENT
All authors approved the final manuscript. Work for this study was performed at the Division of Sleep and Circadian Disorders, Brigham & Women’s Hospital, Boston, Massachusetts and the Departments of Molecular and Cellular Biology and Statistics, Harvard University, Cambridge, Massachusetts. Dr. Rebecca Robbins is supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (K01HL150339). Dr. Robbins has received funding from Bryte Labs and consulting fees from Sonesta Hotels, Oura Ring, Savoir Beds, and byNacht. Dr. Quan receives support from the NHLBI and has served as a consultant for Best Doctors, Bryte Foundation, Jazz Pharmaceuticals, and Whispersom. Dr. Pavlova has received research support from Vanda, Lundbeck, and Jazz Pharmaceuticals; honoraria for didactic presentations from Oakstone, Audiodigest, and Sanofi; and consulting honoraria from Massmedical, Neurodiem, and Pear Therapeutics. Dr. Bertisch receives research support from NIH, the American Academy of Sleep Medicine Foundation, and Harvard Catalyst and serves as a consultant for ResMed and Idorsia Pharmaceuticals. Dr. Epstein is a consultant to the American Academy of Sleep Medicine, AIM Specialty Health, eviCore Healthcare and Somnoware Healthcare Systems. The remaining authors report no conflicts of interest.
ACKNOWLEDGMENTS
R.R., S.F.Q., L.J.E., and S.M.B. conceptualized the study. All authors reviewed the protocol, assisted in data analysis and interpretation, and provided critical feedback. R.R. and S.F.Q. drafted the manuscript.
ABBREVIATIONS
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
REFERENCES
- 1. US Food & Drug Administration . Philips Respironics Recalls Certain Ventilators and BiPAP Machines Due to Potential Health Risks from PE-PUR Sound Abatement Foam. FDA. July 22, 2021. Accessed November 21, 2022. . https://www.fda.gov/medical-devices/medical-device-recalls/philips-respironics-recalls-certain-ventilators-and-bipap-machines-due-potential-health-risks-pe-pur .
- 2. US Food & Drug Administration . Update: Certain Philips Respironics Ventilators, BiPAP Machines, and CPAP Machines Recalled Due to Potential Health Risks: FDA Safety Communication. November 17, 2022. . Accessed November 21, 2022. https://www.fda.gov/medical-devices/safety-communications/update-certain-philips-respironics-ventilators-bipap-machines-and-cpap-machines-recalled-due .
- 3. Philips . Voluntary Recall Information. Accessed November 21, 2022. . https://www.usa.philips.com/healthcare/e/sleep/communications/src-update .
- 4. Kadakia KT , Ross JS , Rathi VK . The Philips Respironics recall of ventilators and positive airway pressure machines–breakdowns in medical device surveillance . JAMA Intern Med. 2023. ; 183 ( 1 ): 5 – 8 . [DOI] [PubMed] [Google Scholar]
- 5. Batool-Anwar S , Omobomi OS , Quan SF . Impact of the novel coronavirus disease on treatment adherence and sleep duration in patients with obstructive sleep apnea treated with positive airway pressure . J Clin Sleep Med. 2020. ; 16 ( 11 ): 1917 – 1920 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgenthaler TI , Linginfelter EA , Gay PC , Anderson SE , Herold D , Brown V , Nienow JM . Rapid response to medical device recalls: an organized patient-centered team effort . J Clin Sleep Med. 2022. ; 18 ( 2 ): 663 – 667 . [DOI] [PMC free article] [PubMed] [Google Scholar]