Abstract
We report the cobalt-catalyzed aminocyclization of unsaturated N-acyl sulfonamides in the presence of oxygen to provide γ- and δ-lactam aldehydes. Use of an optically active cobalt catalyst resulted in the formation of enantiomerically enriched γ-and δ-lactam alcohols. The γ-lactam aldehydes and alcohols obtained were elaborated into useful building blocks.
Lactams, in particular γ- and δ-lactams, constitute integral parts of numerous natural products and pharmaceuticals.1 For example, they are a key structural feature of antibiotics,2 HIV-1 integrase inhibitors,3 antitumor agents,4 and antidepressants,5 as well as drugs for the treatment of type 2 diabetes (Scheme 1).6 Herein, we report novel cobalt-catalyzed aerobic cyclization reactions of unsaturated N-acyl sulfonamides 1, which provide formyl (2) and hydroxymethyl (3) γ- and δ-lactams. The transformation proceeds with cobalt complexes under an oxygen atmosphere in toluene over 2 h in the presence of molecular sieves. Depending on the workup conditions, aldehydes or primary alcohols may be isolated.
Scheme 1. Biologically Active γ- and δ-Lactams and Aminocyclization of Unsaturated Amides.
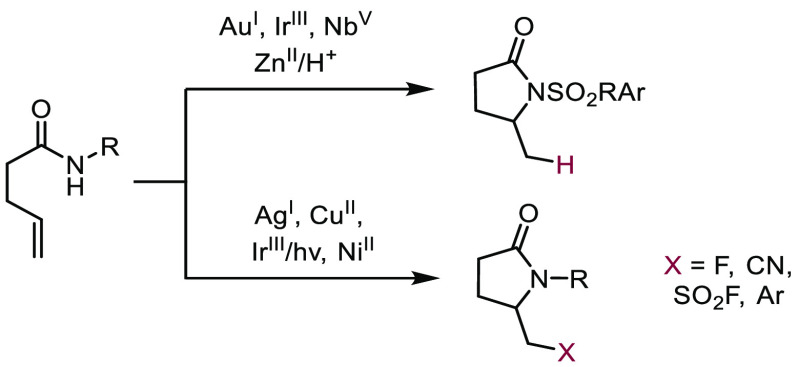
Cycloisomerization reactions of alkenyl N-acyl sulfonamides mediated by gold,7 niobium,8 iridium,9 and zinc catalysts10 provide access to N-sulfonyl lactams (Scheme 2). Oxidative cyclizations of γ-unsaturated N-aryl carboxamides have also been described.11 For example, aminofluorination of alkenyl N-aryl amides leads to 5-fluoromethyl-substituted γ-lactams.12 Additionally, photoredox catalysis has been employed to effect aminofluorosulfonylation and amidoarylation of unactivated olefins.13 Recently, aminocyanation of N-aryl-pent-4-enamides with trimethylsilyl cyanide to provide cyano-containing γ-lactams has been reported.14
Scheme 2. Prior Work: Cyclization of Unsaturated N-Acyl Sulfonamides and Unsaturated N-Aryl Amides.
In previous studies, we disclosed manganese- and cobalt-catalyzed cyclization reactions of unsaturated hydrazones to access a range of complex and highly functionalized pyrazolines.15 We subsequently became interested in expanding the transformation beyond hydrazones. On the basis of reactivity observed for N-acyl sulfonamides in related transformations,161a was subjected to a collection of cobalt catalysts in the presence of oxygen and 4 Å molecular sieves (Table 1). Initially, N-acyl sulfonamide 1a was heated to 55 °C for 12 h in the presence of salcomine under an oxygen atmosphere (1 atm) in toluene (0.1 M). Aldehyde 2a was obtained in 8% yield in addition to unreacted starting material (Entry 1). When the reaction temperature was increased to 105 °C after 2 h, 45% of 2a could be isolated (Entry 2). The use of related cobalt catalysts C3 and C4 did not lead to an increased yield of product (Entries 3 and 4). Elevating the temperature to 110 °C decreased the yield to 41% (Entry 5). Finally, when cobalt salen catalyst C1 was used, aldehyde 2a was obtained in 52% yield (Entry 6). Reducing the catalyst loading of C1 from 20 to 5 mol % increased the yield of product 2a to 63% (Entry 7). In the absence of a catalyst, no product formation was observed (Entry 8).
Table 1. Selected Optimization Results for the Cobalt-Catalyzed Cyclization of N-Acyl Sulfonamide 1a.
| entry | catalyst | temperature, time | yielda |
|---|---|---|---|
| 1 | salcomine | 55 °C, 12 h | 8% |
| 2 | salcomine | 105 °C, 2 h | 45% |
| 3 | C3 | 105 °C, 2 h | 45% |
| 4 | C4 | 105 °C, 2 h | 38% |
| 5 | C3 | 110 °C, 2 h | 41% |
| 6 | C1 | 105 °C, 2 h | 52% |
| 7 | C1 (5 mol %) | 105 °C, 2 h | 63% |
| 8 | none | 105 °C, 2 h | 0% |
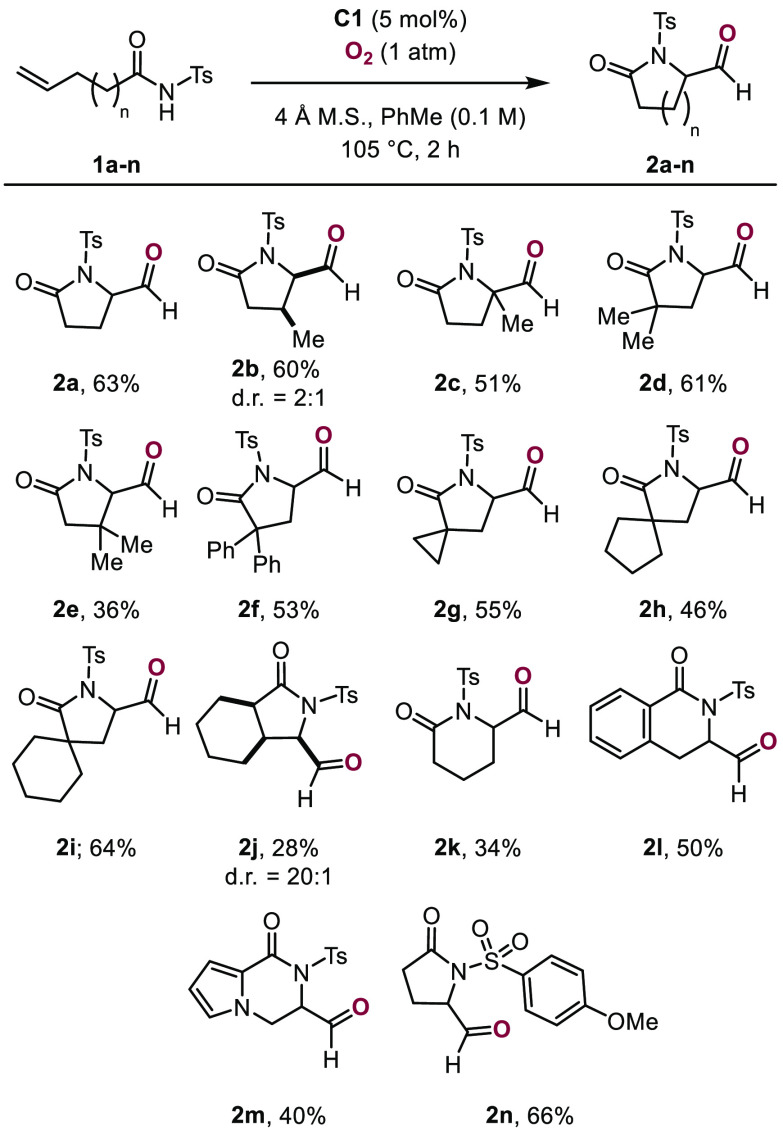
With the optimized reaction parameters in hand, the scope of the cyclization reaction was investigated (Scheme 3). Various unsaturated N-acyl sulfonamides 1a–n were subjected to the optimized reaction conditions, and γ- and δ-lactam aldehydes 2a–n were obtained. Substrates incorporating methyl and phenyl substituents in the α- and β-position led to products 2b and 2f in 36–61% yield. We next evaluated the formation of spiro-γ-lactams. Gratifyingly, 2g–2i were obtained in 46–64% yield. Fused cyclohexane-γ-lactam 2j was produced in 28% yield. We then extended the transformation to δ-lactams, furnishing 2k and dihydroisoquinolinone 2l in 34% and 50% yield, respectively. Pyrrole containing δ-lactam 2m was accessed from the corresponding N-acyl sulfonamide in 40% yield. To expand the scope beyond N-tosyl sulfonamides, N-methoxyphenyl sulfonyl protected amide 1n was subjected to the reaction conditions, giving rise to γ-lactam 2n in 66% yield.
Scheme 3. Substrate Scope for the Cobalt-Catalyzed Aminocyclization of Unsaturated N-Acyl Sulfonamides.
Cobalt-salen catalysts have previously been employed in enantioselective transformations.17 Consequently, we sought to examine whether the use of a chiral salen ligand would render the transformation we developed enantioselective. To this end, 1a was subjected to cobalt catalyst C2 under conditions otherwise identical to those described above (O2, 4 Å molecular sieves, PhMe, 105 °C). Under these conditions, formation of product 2a was observed in 40% yield and 70:30 e.r.18
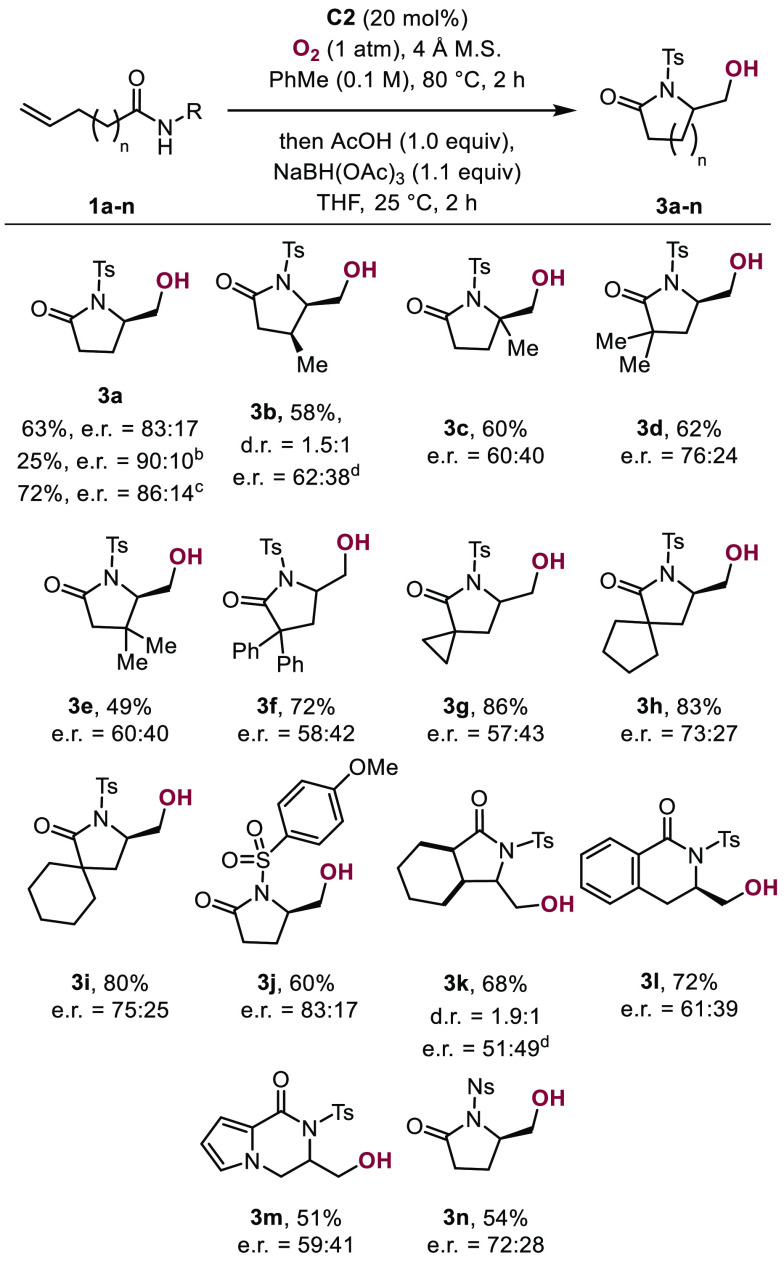
We reasoned that at high temperatures, epimerization of the product aldehyde may readily occur, prompting us to conduct the reaction at lower temperatures in an attempt to abate the erosion of e.r. When the reaction with 1a was carried out at 80 °C, a mixture of aldehyde 2a and alcohol 3a was obtained. This mixture was subsequently reduced in situ by the addition of acetic acid (1.0 equiv) and sodium triacetoxyborohydride (1.1 equiv). Gratifyingly, the alcohol product was thus obtained in 63% yield and 83:17 e.r. (Scheme 4). We demonstrated that when the reaction temperature was lowered further to 55 °C and the reaction was conducted for 2 h the enantiomeric ratio could be improved to 90:10, albeit at a diminished yield of 25%. Conducting the reaction at 55 °C for 48 h led to formation of 3a in 72% yield and 86:14 e.r., which does not constitute a marked benefit over the original conditions (80 °C, 2 h).
Scheme 4. Substrate Scope of the Cobalt-Catalyzed Aminocyclization with Subsequent Reduction.
Enantiomeric ratios were determined via chiral HPLC or SFC by comparison with racemic samples (see SI). For the enantioenriched reaction it was found, that catalyst C2 required higher catalyst loadings of 20 mol %. Reaction conditions: (b) 55 °C, 2 h; (c) 55 °C, 48 h. (d) e.r. of the major diastereomer.
We next examined the substrate scope of the aminocyclization reaction. Subjecting 1b–1f to reaction conditions at 80 °C followed by reductive workup resulted in the formation of γ-lactams 3b to 3f bearing methyl and phenyl groups in 49–72% yield and up to 76:24 e.r. N-Methoxyphenyl sulfonyl protected aminoalcohol 3j was accessed from the corresponding sulfonamide in 60% yield (e.r. = 83:17). Spiro-fused γ-lactam alcohols 3g to 3i were obtained in 80–86% yield (e.r.= 57:43 to 75:25). Bicyclic γ-lactam 3k and fused δ-lactams 3l and 3m were isolated in 68% (d.r. = 1.9:1), 72% (e.r. = 61:39), and 51% yields (e.r. = 59:41), respectively. Finally, product 3n bearing an N-nosyl group was obtained in 54% yield with 72:28 e.r.
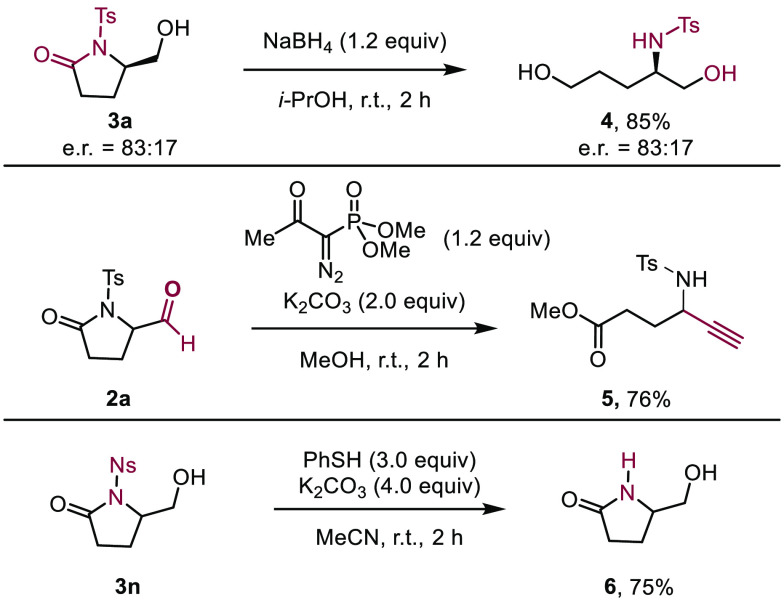
To showcase the versatility of the lactams, they were subjected to a variety of product derivatization reactions (Scheme 5). Treatment of 3a with sodium borohydride resulted in a reductive ring opening, providing diol 4 in 85% yield. When aldehyde 2a was subjected to the Ohira–Bestmann reagent in the presence of K2CO3 in methanol, ester 5 was obtained in 76% yield. Removal of N-protecting groups can be challenging, which led us to showcase said reaction for one of the products obtained in this transformation. To this end, when γ-lactam 3n was subjected to a combination of thiophenol (3.0 equiv) and K2CO3 (4.0 equiv), removal of the N-nosyl group was effected and free amide 6 obtained in 75% yield
Scheme 5. Selected Derivatization of γ-Lactam Aldehydes and Alcohols.
In conclusion, we have reported the cobalt-catalyzed aminocyclization of unsaturated N-acyl sulfonamides to give access to a range of formyl and hydroxymethylated γ- and δ-lactams. Aerobic cobalt catalysis led to the formation of γ- and δ-lactam aldehydes, whereas the use of chiral cobalt catalyst C2 with reductive workup gave rise to enantiomerically enriched γ- and δ-lactam alcohols. Finally, derivatizations of the prepared products, including ring opening and N-nosyl deprotection, were carried out, demonstrating the versatility of the products derived from the cyclization protocol.
Acknowledgments
This work was funded by the European Research Council (OLECAT, Grant ID 833540). M.B. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c02390.
Experimental procedures and characterization data for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Di Maso M. J.; Nepomuceno G. M.; St. Peter M. A.; Gitre H. H.; Martin K. S.; Shaw J. T. Synthesis of (±)-Bisavenanthramide B-6 by an Anionic Anhydride Mannich Reaction. Org. Lett. 2016, 18, 1740–1743. 10.1021/acs.orglett.6b00413. [DOI] [PubMed] [Google Scholar]; b Shen D.-Y.; Nguyen T. N.; Wu S.-J.; Shiao Y.-J.; Hung H.-Y.; Kuo P.-C.; Kuo D.-H.; Thang T. D.; Wu T.-S. γ- and δ-Lactams from the Leaves of Clausena lansium. J. Nat. Prod. 2015, 78, 2521–2530. 10.1021/acs.jnatprod.5b00148. [DOI] [PubMed] [Google Scholar]; c Fu T.-h.; McElroy W. T.; Shamszad M.; Martin S. F. Formal Syntheses of Naturally Occurring Welwitindolinones. Org. Lett. 2012, 14, 3834–3837. 10.1021/ol301424h. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Shenvi R. A.; Corey E. J. A Short and Efficient Synthesis of (−)-7-Methylomuralide, a Potent Proteasome Inhibitor. J. Am. Chem. Soc. 2009, 131, 5746–5747. 10.1021/ja901400q. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Okazaki Y.; Ishizuka A.; Ishihara A.; Nishioka T.; Iwamura H. New Dimeric Compounds of Avenanthramide Phytoalexin in Oats. J. Org. Chem. 2007, 72, 3830–3839. 10.1021/jo0701740. [DOI] [PubMed] [Google Scholar]; f Masse C. E.; Morgan A. J.; Adams J.; Panek J. S. Syntheses and Biological Evaluation of (+)-Lactacystin and Analogs. Eur. J. Org. Chem. 2000, 2000, 2513–2528. . [DOI] [Google Scholar]; g Caruano J.; Muccioli G. G.; Robiette R. Biologically active γ-lactams: synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. 10.1039/C6OB01349J. [DOI] [PubMed] [Google Scholar]
- a Fong A.; Ross M.; Boudreau J.; Nokhbeh R.; Tilbe K.; Lee H. Raja 42, a novel gamma lactam compound, is effective against Clostridioides difficile. PLoS One 2021, 16 (9), e0257143 10.1371/journal.pone.0257143. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Davies G. M; Hitchcock P. B; Loakes D.; Young D. W Synthesis of Reactive γ-Lactams Related to Penicillins and Cephalosporins. Tetrahedron Lett. 1996, 37, 5601–5604. 10.1016/0040-4039(96)01135-5. [DOI] [Google Scholar]
- a Velthuisen E. J.; Johns B. A.; Temelkoff D. P.; Brown K. W.; Danehower S. C. The design of 8-hydroxyquinoline tetracyclic lactams as HIV-1 integrase strand transfer inhibitors. Eur. J. Med. Chem. 2016, 117, 99–112. 10.1016/j.ejmech.2016.03.038. [DOI] [PubMed] [Google Scholar]; b Metífiot M.; Maddali K.; Johnson B. C.; Hare S.; Smith S. J.; Zhao X. Z.; Marchand C.; Burke T. R. Jr.; Hughes S. H.; Cherepanov P.; Pommier Y. Activities, Crystal Structures, and Molecular Dynamics of Dihydro-1H-isoindole Derivatives, Inhibitors of HIV-1 Integrase. ACS Chem. Biol. 2013, 8, 209–217. 10.1021/cb300471n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a O̅mura S.; Crump A. Lactacystin: first-in-class proteasome inhibitor still excelling and an exemplar for future antibiotic research. J. Antibiot. 2019, 72, 189–201. 10.1038/s41429-019-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fenical W.; Jensen P. R.; Palladino M. A.; Lam K. S.; Lloyd G. K.; Potts B. C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. 10.1016/j.bmc.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Mix E.; Winblad B. The Antidepressant and Antiinflammatory Effects of Rolipram in the Central Nervous System. CNS Drug Reviews 2001, 7, 387–398. 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C.H. Boehringer Sohn AG & Co. KG . Polynucleotides Having Promoter Activity. Patent WO2011/161128 A1, 2011.
- Liu X.-Y.; Li C.-H.; Che C.-M. Phosphine Gold(I)-Catalyzed Hydroamination of Alkenes under Thermal and Microwave-Assisted Conditions. Org. Lett. 2006, 8, 2707–2710. 10.1021/ol060719x. [DOI] [PubMed] [Google Scholar]
- Ferrand L.; Tang Y.; Aubert C.; Fensterbank L.; Mouries-Mansuy V.; Petit M.; Amatore M. Niobium-Catalyzed Intramolecular Addition of O–H and N–H Bonds to Alkenes: A Tool for Hydrofunctionalization. Org. Lett. 2017, 19, 2062–2065. 10.1021/acs.orglett.7b00657. [DOI] [PubMed] [Google Scholar]
- Nagamoto M.; Yanagi T.; Nishimura T.; Yorimitsu H. Asymmetric Cyclization of N-Sulfonyl Alkenyl Amides Catalyzed by Iridium/Chiral Diene Complexes. Org. Lett. 2016, 18, 4474–4477. 10.1021/acs.orglett.6b01954. [DOI] [PubMed] [Google Scholar]
- Chou T.-H.; Yu B.-H.; Chein R.-J. ZnI2/Zn(OTf)2-TsOH: a versatile combined-acid system for catalytic intramolecular hydrofunctionalization and polyene cyclization. Chem. Commun. 2019, 55, 13522–13525. 10.1039/C9CC07242J. [DOI] [PubMed] [Google Scholar]
- For an example of a cyclization reaction resulting in the formation of vinyl amides see:; Nishimura T.; Nagamoto M.; Yorimitsu H. Iridium-Catalyzed Intramolecular Oxidative Cyclization of Alkenyl Amides and Alkenoic Acids. Synthesis 2017, 49, 4272–4282. 10.1055/s-0036-1588435. [DOI] [Google Scholar]
- Li Z.; Song L.; Li C. Silver-Catalyzed Radical Aminofluorination of Unactivated Alkenes in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 4640–4643. 10.1021/ja400124t. [DOI] [PubMed] [Google Scholar]
- a Zhong T.; Yi J.-T.; Chen Z.-D.; Zhuang Q.-C.; Li Y.-Z.; Lu G.; Weng J. Photoredox-catalyzed aminofluorosulfonylation of unactivated olefins. Chem. Sci. 2021, 12, 9359–9365. 10.1039/D1SC02503A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zheng S.; Gutierrez-Bonet A.; Molander G. A. Merging Photoredox PCET with Ni-Catalyzed Cross-Coupling: Cascade Amidoarylation of Unactivated Olefins. Chem. 2019, 5, 339–352. 10.1016/j.chempr.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Wang L.; Mu H.; Zhang Q.; Fang Z.; Li D. Synthesis of cyano-substituted γ-lactams through a copper-catalyzed cascade cyclization/cyanation reaction. Org. Biomol. Chem. 2023, 21, 1168–1171. 10.1039/D2OB02086F. [DOI] [PubMed] [Google Scholar]
- Balkenhohl M.; Kölbl S.; Georgiev T.; Carreira E. M. Mn- and Co-Catalyzed Aminocyclizations of Unsaturated Hydrazones Providing a Broad Range of Functionalized Pyrazolines. JACS Au 2021, 1, 919–924. 10.1021/jacsau.1c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fischer D. M.; Balkenhohl M.; Carreira E. M. Cobalt-Catalyzed Cyclization of Unsaturated N-Acyl Sulfonamides: a Diverted Mukaiyama Hydration Reaction. JACS Au 2022, 2, 1071–1077. 10.1021/jacsau.2c00186. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wdowik T.; Chemler S. R. Direct Synthesis of 2- Formylpyrrolidines, 2-Pyrrolidinones and 2-Dihydrofuranones via Aerobic Copper-Catalyzed Aminooxygenation and Dioxygenation of 4-Pentenylsulfonamides and 4-Pentenylalcohols. J. Am. Chem. Soc. 2017, 139, 9515–9518. 10.1021/jacs.7b05680. [DOI] [PMC free article] [PubMed] [Google Scholar]; c de Haro T.; Nevado C. Flexible Gold-Catalyzed Regioselective Oxidative Difunctionalization of Unactivated Alkenes. Angew. Chem., Int. Ed. 2011, 50, 906–910. 10.1002/anie.201005763. [DOI] [PubMed] [Google Scholar]; d Wang H.; Wang Y.; Liang D.; Liu L.; Zhang J.; Zhu Q. Copper-Catalyzed Intramolecular Dehydrogenative Aminooxygenation: Direct Access to Formyl-Substituted Aromatic N-Heterocycles. Angew. Chem., Int. Ed. 2011, 50, 5678–5681. 10.1002/anie.201100362. [DOI] [PubMed] [Google Scholar]; e Toh K. K.; Wang Y.-F.; Ng E. P. J.; Chiba S. Copper Mediated Aerobic Synthesis of 3-Azabicyclo[3.1.0]hex-2-enes and 4- Carbonylpyrroles from N-Allyl/Propargyl Enamine Carboxylates. J. Am. Chem. Soc. 2011, 133, 13942–13945. 10.1021/ja206580j. [DOI] [PubMed] [Google Scholar]; f Peng H.; Akhmedov N. G.; Liang Y.-F.; Jiao N.; Shi X. Synergistic Gold and Iron Dual Catalysis: Preferred Radical Addition toward Vinyl–Gold Intermediate over Alkene. J. Am. Chem. Soc. 2015, 137, 8912–8915. 10.1021/jacs.5b05415. [DOI] [PubMed] [Google Scholar]; g Fischer D. M.; Freis M.; Amberg W. M.; Lindner H.; Carreira E. M. Organophotocatalytic carboheterofunctionalization of unactivated olefins with pendant nucleophiles. Chem. Sci. 2023, 14, 7256–7261. 10.1039/D3SC02250A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hapke M.; Hilt G.. Cobalt Catalysis in Organic Synthesis: Methods and Reactions; Wiley-VCH Verlag GmbH & Co. KGaA, 2019. [Google Scholar]; b Discolo C. A.; Touney E. E.; Pronin S. V. Catalytic Asymmetric Radical–Polar Crossover Hydroalkoxylation. J. Am. Chem. Soc. 2019, 141, 17527–17532. 10.1021/jacs.9b10645. [DOI] [PubMed] [Google Scholar]; c Pellissier H.; Clavier H. Enantioselective Cobalt-Catalyzed Transformations. Chem. Rev. 2014, 114, 2775–2823. 10.1021/cr4004055. [DOI] [PubMed] [Google Scholar]; d Zheng Y.; Zheng C.; Gu Q.; You S.-Li Enantioselective C–H functionalization reactions enabled by cobalt catalysis. Chem. Catalysis 2022, 2, 2965–2985. 10.1016/j.checat.2022.08.020. [DOI] [Google Scholar]; e Ebisawa K.; Izumi K.; Ooka Y.; Kato H.; Kanazawa S.; Komatsu S.; Nishi E.; Shigehisa H. Catalyst- and Silane-Controlled Enantioselective Hydrofunctionalization of Alkenes by Cobalt-Catalyzed Hydrogen Atom Transfer and Radical-Polar Crossover. J. Am. Chem. Soc. 2020, 142, 13481–13490. 10.1021/jacs.0c05017. [DOI] [PubMed] [Google Scholar]
- The absolute configuration of the accessed alcohol has been assigned as R by Mosher ester analysis (see SI).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.