Abstract
Herein, a straightforward synthetic approach for the construction of phenanthridin-6(5H)-one skeletons is disclosed. The developed protocol relies on palladium catalysis, providing controlled access to a range of functionalized phenanthridin-6(5H)-ones in 59–88% yields. Furthermore, plausible reaction pathways are proposed based on mechanistic experiments.
Introduction
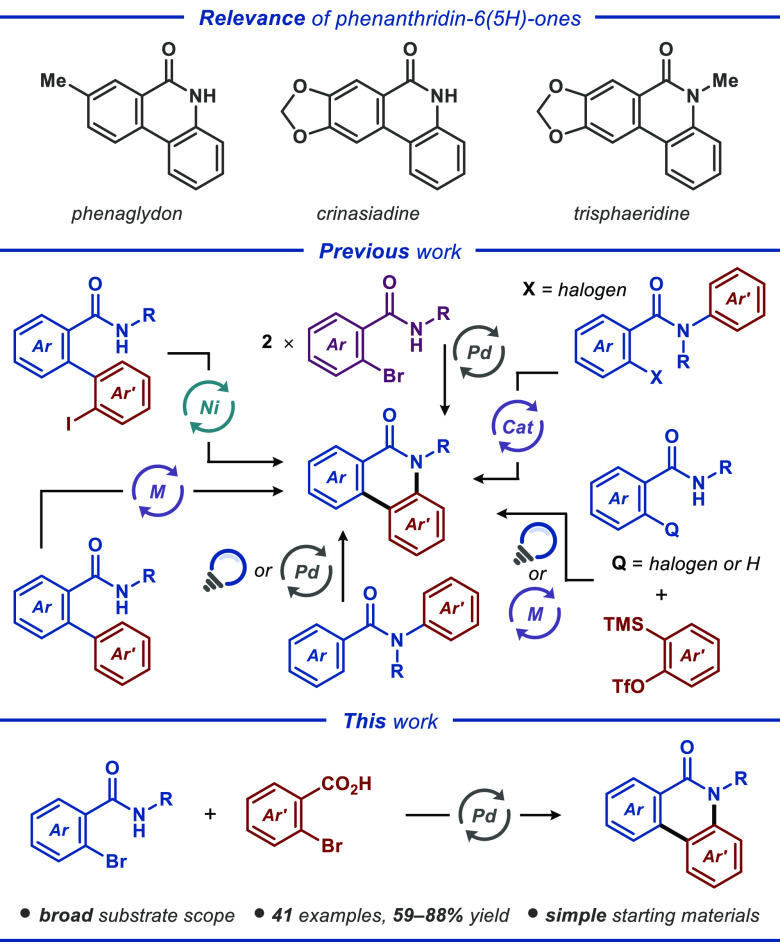
Phenanthridin-6(5H)-one represents a class of tricyclic N-heterocycles that is frequently encountered in alkaloids, such as phenaglydon, crinasiadine, and trisphaeridine (Figure 1, top). These compounds have been documented to possess biological and pharmaceutical activities, including antimycobacterial,1 antagonistic,2 antiproliferative,3 and antitubercular activities.4 Significant attention has been devoted to developing novel synthetic methods for the construction of phenanthridin-6(5H)-one derivatives (Figure 1, middle).6 The Yamada group demonstrated the synthesis of phenanthridin-6(5H)-ones through nickel-catalyzed amidation of aryl iodides.7 At the same time, Chaudhary and co-workers disclosed an organocatalytic protocol proceeding through direct C(sp2)–H bond arylation.8 Similarly, phenanthridin-6(5H)-one derivatives have also been accessed in high yields using the free radical initiator AIBN9 or microwave irradiation.10 Furthermore, phenanthridin-6(5H)-one derivatives have been efficiently assembled from 2-bromophenylbenzamides through a palladium-catalyzed process involving aryl–aryl coupling and deamidation.11 Various strategies have utilized the oxidative coupling of benzamides to construct phenanthridin-6(5H)-one scaffolds. These annulation approaches do not require ortho-halogenation and have been realized with transition-metal-catalyzed12 or photoinduced13 manifolds. In recent years, a direct ortho-C–H/N–H annulation was developed to yield phenanthridin-6(5H)-one derivatives from benzamide and the aryne precursor 2-(trimethylsilyl)phenyl trifluoromethanesulfonate using O2 or K2S2O8 as oxidizing agents.14
Figure 1.
Relevance and synthetic approaches to phenanthridin-6(5H)-one derivatives.
It has been demonstrated that 2-bromobenzoic acid can be easily converted to the corresponding aryne in the presence of a Pd catalyst.15 However, the generated aryne quickly undergoes a trimerization reaction to yield triphenylenes. In this context, we recently reported that 2-(2-bromophenyl)-1H-benzo[d]-imidazole derivatives can be harnessed as an effective coupling partner in combination with 2-bromobenzoic acids to give the corresponding N-fused (benzo)imidazophenanthridine scaffolds in high yields.16 In continuation of our previous studies directed to transition-metal-assisted synthesis of heterocycles,17 we envisaged that phenanthridin-6(5H)-one derivatives could be directly assembled from N-substituted 2-bromobenzamides 1 and 2-bromobenzoic acids 2 in the presence of a metal catalyst (Figure 1, bottom).
Results and Discussion
We commenced our investigation by utilizing 2-bromo-N-methylbenzamide (1a) and 2-bromobenzoic acid (2a) as the model substrates, CuI as the catalyst precursor, and K2CO3 as the base in DMF at 100 °C. To our disappointment, the desired product 3a was not detected under these reaction conditions (Table 1, entry 1). A similar outcome was observed with AgOTf as the metal catalyst (Table 1, entry 2). Gratifyingly, formation of the desired annulation product 3a could be promoted by palladium-based catalysts, including Pd(OAc)2, PdCl2, Pd(PPh3)2Cl2, and Pd(PPh3)4 (Table 1, entries 3–6), with Pd(OAc)2 displaying the best reactivity and furnishing the desired product in 54% yield (Table 1, entry 3). Notably, the addition of auxiliary phosphine-based ligands, such as PPh3, Xantphos, P(4-MeOC6H4)3, and P(4-MeC6H4)3, promoted the desired reactivity (Table 1, entries 7–10) with PPh3 providing product 3a in 70% yield (Table 1, entry 7). Apart from K2CO3, other common bases, such as Na2CO3, Cs2CO3, and tBuOK, were evaluated and found less critical for the desired transformation (Table 1, entries 11–13). Carrying out the reaction under the optimized conditions for our previously disclosed protocol16 for the synthesis of N-fused (benzo)imidazophenanthridine scaffolds did not afford the desired annulation product 3a (Table 1, entry 14). Instead, the trimerization product (triphenylene) was afforded under these reaction conditions. Next, the effect of the reaction temperature was examined (Table 1, entries 15–18) with 120 °C being the most suitable for the developed protocol. The use of polar aprotic solvents, such as DMF, DMSO, and DMA, was revealed to be beneficial (Table 1, entries 17, 19–20), while the nonpolar solvents xylene and toluene resulted in slightly diminished yields (Table 1, entries 21–22). Finally, a control experiment conducted in the absence of Pd(OAc)2 highlights the critical role of the palladium precursor in achieving effective coupling (Table 1, entry 23).
Table 1. Optimization of Reaction Conditionsa.
| entry | [M] | ligand | base | solvent | temp (°C) | yield (%)b |
|---|---|---|---|---|---|---|
| 1 | CuI | – | K2CO3 | DMF | 100 | 0 |
| 2 | AgOTf | – | K2CO3 | DMF | 100 | 0 |
| 3 | Pd(OAc)2 | – | K2CO3 | DMF | 100 | 54 |
| 4 | PdCl2 | – | K2CO3 | DMF | 100 | 31 |
| 5 | Pd(PPh3)2Cl2 | – | K2CO3 | DMF | 100 | 40 |
| 6 | Pd(PPh3)4 | – | K2CO3 | DMF | 100 | 48 |
| 7 | Pd(OAc)2 | PPh3 | K2CO3 | DMF | 100 | 70 |
| 8 | Pd(OAc)2 | Xantphos | K2CO3 | DMF | 100 | 68 |
| 9 | Pd(OAc)2 | P(4-MeOC6H4)3 | K2CO3 | DMF | 100 | 64 |
| 10 | Pd(OAc)2 | P(4-MeC6H4)3 | K2CO3 | DMF | 100 | 69 |
| 11 | Pd(OAc)2 | PPh3 | Na2CO3 | DMF | 100 | 64 |
| 12 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMF | 100 | 72 |
| 13 | Pd(OAc)2 | PPh3 | tBuOK | DMF | 100 | 67 |
| 14c | Pd(OAc)2/CuI | PPh3 | Cs2CO3 | DMF | 110 | 0 |
| 15 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMF | 80 | 57 |
| 16 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMF | 110 | 73 |
| 17 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMF | 120 | 75 |
| 18 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMF | 130 | 73 |
| 19 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMSO | 120 | 73 |
| 20 | Pd(OAc)2 | PPh3 | Cs2CO3 | DMA | 120 | 72 |
| 21 | Pd(OAc)2 | PPh3 | Cs2CO3 | xylene | 120 | 65 |
| 22 | Pd(OAc)2 | PPh3 | Cs2CO3 | toluene | 120 | 70 |
| 23 | – | PPh3 | Cs2CO3 | DMF | 120 | 0 |
Reaction conditions: Reactions were carried out with 1a (107 mg, 0.50 mmol), 2a (121 mg, 0.60 mmol), catalyst (10 mol %), ligand (20 mol %), and base (1.0 mmol) in solvent (5.0 mL) under argon for 10 h.
Isolated yields of 3a after purification by column chromatography.
Reaction was carried out with 1a (107 mg, 0.50 mmol), 2a (121 mg, 0.60 mmol), Pd(OAc)2 (5 mol %), CuI (10 mol %), PPh3 (20 mol %), and Cs2CO3 (0.5 mmol) in DMF (5.0 mL) under argon for 8 h.
After the optimal reaction conditions were identified, the scope and limitations of the developed protocol were evaluated. Initially, a series of N-substituted 2-bromobenzamides 1 were engaged in a reaction with 2-bromobenzoic acid 2a. Aliphatic groups, such as methyl, ethyl, npropyl, and nbutyl, all furnished the corresponding products 3b–3f and 3h–3i in moderate to high yields (61–75%). However, N-tbutyl-2-bromobenzamide failed to produce the desired annulation product 3g, presumably due to ample steric hindrance. The use of 2-bromobenzamides 1 bearing various N-substituted aromatic and heteroaromatic moieties demonstrated that various functional groups, such as halogens, ethers, nitrile, furan, and thiophene, were compatible with the developed protocol, furnishing products 3j–3t in moderate to high yields (66–82%). The structure of product 3j was confirmed by single-crystal X-ray analysis (CCDC 2210960).
The synthetic versatility of the developed protocol was further explored by employing 2-bromobenzamides 1 with a range of substituents at the aromatic core (Scheme 1). The reactions with 2-bromobenzamides 1 substituted with various aliphatic, chloro, and fluoro groups all provided the expected annulation products 3u–3af in moderate to high yields (62–79%). Next, the scope of compatible 2-bromobenzoic acid annulation partners 2 was evaluated (Schemes 1 and 2). Here, 4,5-dimethoxy-2-bromobenzoic acid (2b) underwent effective annulation with N-substituted 2-bromobenzamides to produce 3ag–3aj in high yields (84–88%, Scheme 1). Finally, the disclosed protocol was successfully applied to access quinolone-derived alkaloid phenaglydon (4). Thus, subjecting annulation product 3u to refluxing trifluoroacetic acid afforded the debenzylated product phenaglydone (4) in an excellent yield of 92% (Scheme 1).
Scheme 1. Reaction Scope and Synthetic Application,
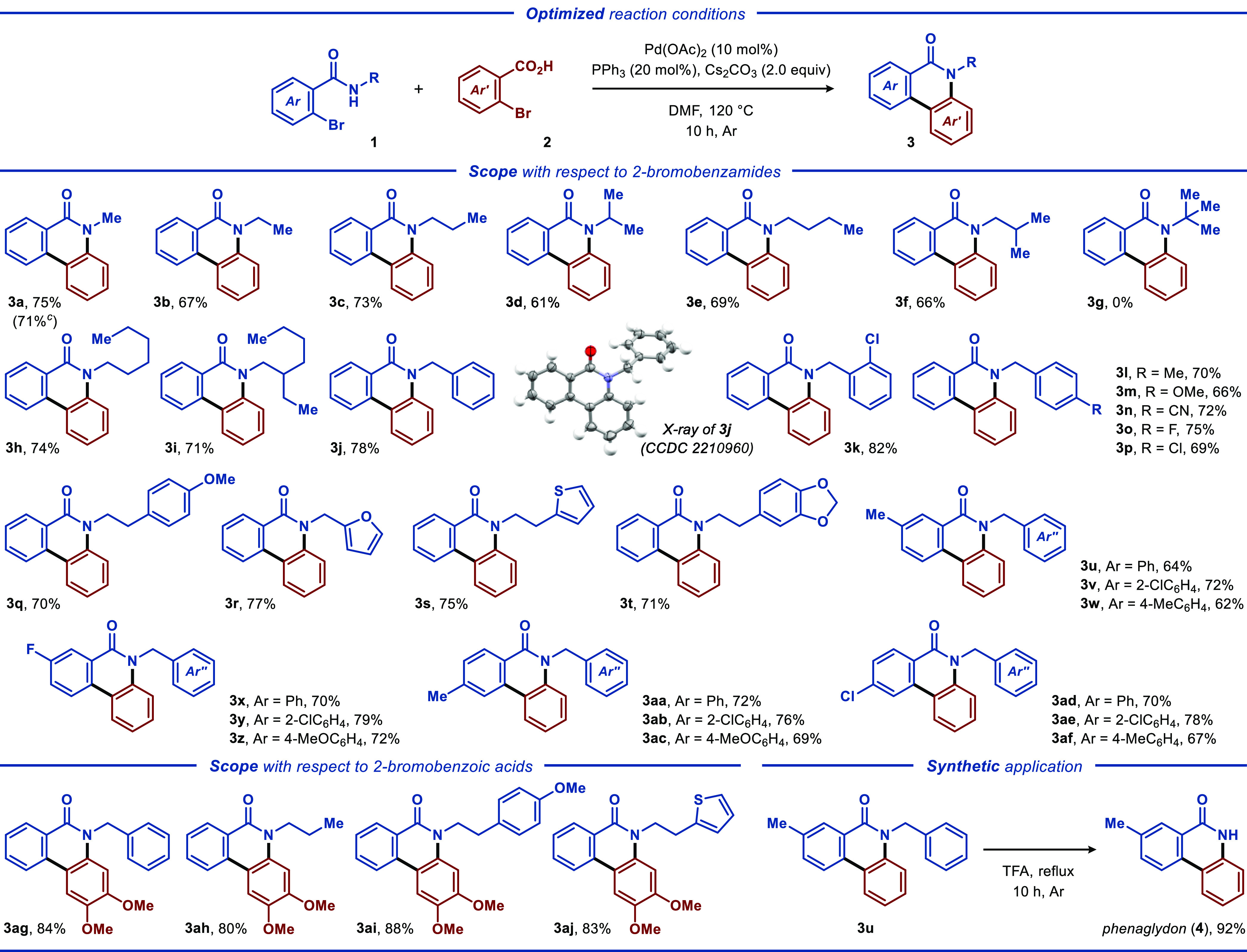
Reaction conditions: Reactions were carried out with 1 (0.50 mmol), 2 (0.60 mmol), Pd(OAc)2 (12 mg, 0.05 mmol), PPh3 (26 mg, 0.10 mmol), and Cs2CO3 (326 mg, 1.0 mmol) in DMF (5.0 mL) under argon at 120 °C for 10 h.
Isolated product yields are reported.
Reaction carried out on a 1 mmol scale.
Scheme 2. Investigations for Probing the Reaction Mechanism and Proposed Reaction Mechanism,
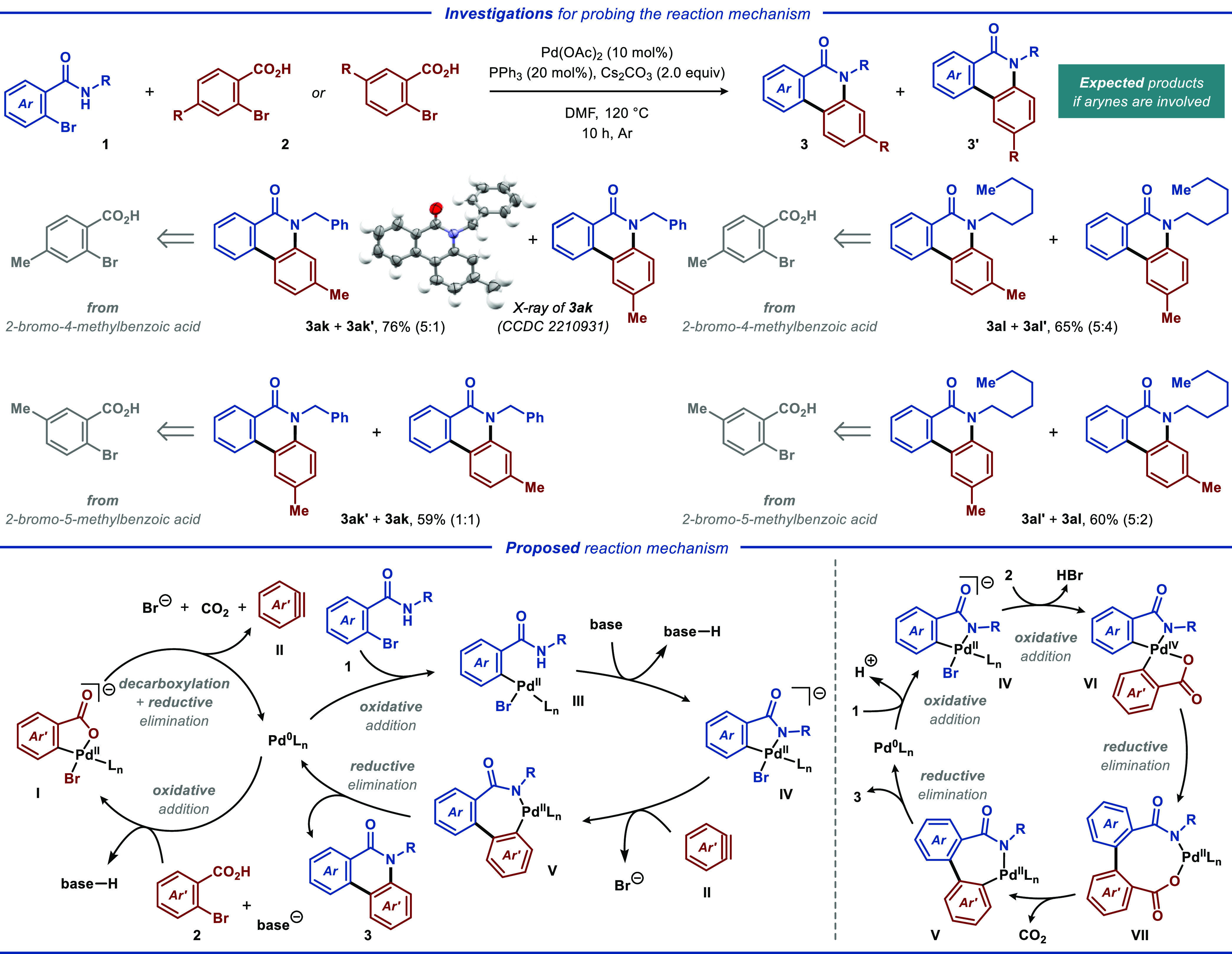
Reaction conditions: Reactions were carried out with 1 (0.50 mmol), 2 (0.60 mmol), Pd(OAc)2 (12 mg, 0.05 mmol), PPh3 (26 mg, 0.10 mmol), and Cs2CO3 (326 mg, 1.0 mmol) in DMF (5.0 mL) under argon at 120 °C for 10 h.
Isolated product yields are reported. Regioisomeric ratios were determined by 1H NMR analysis.
To probe the reaction mechanism, a set of control reactions were carried out under the optimized reaction conditions. When 4- or 5-substituted 2-bromobenzoic acids were used as the coupling partners, the respective annulated products were obtained as mixtures of two regioisomers (Scheme 2, top). Such poor regioselectivity indicates that the reaction proceeds through arynes as the key intermediates, as has been proposed for related transformations featuring palladium catalysis.18 Based on the literature precedents,19 a plausible mechanism that does not contradict the above control reactions is proposed (Scheme 2, bottom left). Initially, base-assisted oxidative addition of 2-bromobenzoic acid 2 to Pd0 provides the key aryl-PdII species I. This species undergoes extrusion of CO2 to afford aryne intermediate II while regenerating Pd0 and completing the first of the catalytic cycles. Meanwhile, the second of the catalytic cycles is onset by oxidative addition of the Pd0 catalyst to 2-bromobenzamide 1 to give aryl-PdII species III, which in the presence of a base furnishes the five-membered palladacycle IV. Insertion of previously produced aryne II into the PdII–C bond of IV results in C–C bond formation, while subsequent reductive elimination from the seven-membered palladacycle V forges the desired C–N bond. Thereby, the latter step regenerates the Pd0 catalyst, concluding the second of the catalytic cycles, and furnishes the desired annulation product 3. An alternative mechanism proceeding without formation of an aryne intermediate features a single catalytic cycle and PdIV species as the key intermediate (Scheme 2, bottom right).20 Here, the reaction is onset by oxidative addition of 2-bromobenzamide 1 to the Pd0 catalyst, furnishing aryl-PdII intermediate IV. In the key step of the reaction, this intermediate undergoes a second oxidative addition reaction to 2-bromobenzoic acid 2, producing diaryl-PdIV species VI. Subsequently, this species undergoes reductive elimination to produce the biaryl PdII-metallacycle VII, which eliminates CO2 to furnish the PdII intermediate V. Finally, the latter intermediate undergoes reductive elimination, concluding the catalytic cycle and furnishing desired product 3.
Conclusions
In conclusion, we disclosed a simple procedure for accessing phenanthridin-6(5H)-one derivatives through palladium-mediated annulation of 2-bromobenzamides and 2-bromobenzoic acids. The annulation reaction delivers the phenanthridin-6(5H)-one derivatives in high yields and is compatible with a variety of functional groups, providing a modular method for accessing a range of structurally diversified phenanthridin-6(5H)-one motifs.
Experimental Section
General Information
All reagents were purchased from commercial sources and used without treatment, unless otherwise indicated. The products were purified by column chromatography over silica gel. 1H NMR and 13C NMR spectra were recorded at 25 °C on a Varian spectrometer at 400 and 101 MHz, respectively, with TMS as the internal standard. High-resolution mass spectra (HRMS) were recorded on a BRUKER AutoflexIII Smartbeam mass spectrometer. High-resolution mass spectra (HRMS) were recorded on a Bruker microTof using electrospray ionization (ESI).
General Procedure for the Synthesis of Phenanthridinones 3
To a 10 mL Schlenk tube equipped with a magnetic stir bar were added 2-bromobenzamide 1 (0.500 mmol, 1.00 equiv), o-bromobenzoic acid 2 (0.750 mmol, 1.50 equiv), DMF (4.0 mL), Cs2CO3 (163 mg, 0.500 mmol, 1.00 equiv), PPh3 (26 mg, 0.100 mmol, 0.200 equiv), and Pd(OAc)2 (11 mg, 0.05 mmol, 0.100 equiv). The reaction mixture was stirred at 120 °C in an oil bath for about 10 h. The resulting mixture was concentrated, and the residue was taken up in ethyl acetate. The organic layer was washed with brine, dried over Na2SO4, and concentrated. Purification of the crude product by column chromatography (silica gel; petroleum ether/ethyl acetate 30:1) afforded 3.
Acknowledgments
Financial support from the Outstanding Youth Fund of Jiangsu Province (BK20211607), the NSFC of China (No. 21702078), the 2021 National and Provincial College Students Innovation and Entrepreneurship Training Program (202110320010Z), the Priority Academic Program Development of Jiangsu Higher Education Institutions, FORMAS (Grant No. 2019-01269), the Swedish Research Council (Grant No. 2020-04764), the Magnus Bergvall Foundation, KTH Royal Institute of Technology, and the Ministry of Education and Science of the Russian Federation (Program No. 075-03-2021-287/6) is gratefully acknowledged. The Olle Engkvist Foundation and the Wenner-Gren Foundations are kindly acknowledged for postdoctoral fellowships to E.V.S. and J.-Q. L., respectively.
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c01429.
The authors declare no competing financial interest.
Supplementary Material
References
- Nagesh H. N.; Naidu K. M.; Rao D. H.; Sridevi J. P.; Sriram D.; Yogeeswari P.; Chandra Sekhar K. V. G. Design, Synthesis and Evaluation of 6-(4-((Substituted-1H-1,2,3-triazol-4-yl)methyl)piperazin-1-yl)phenanthridine Analogues as Antimycobacterial Agents. Bioorg. Med. Chem. Lett. 2013, 23, 6805–6810. 10.1016/j.bmcl.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y.; Mori S.; Makishima M.; Fujii S.; Kagechika H.; Hashimoto Y.; Ishikawa M. Novel Nonsteroidal Progesterone Receptor (PR) Antagonists with a Phenanthridinone Skeleton. ACS Med. Chem. Lett. 2018, 9, 641–645. 10.1021/acsmedchemlett.8b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama M.; Aoyama H.; Mukai R.; Nakamura M.; Yoshimura K.; Okamoto M.; Ohshima T.; Hashimoto Y.; Baba M. A Novel Tetramethylnaphthalene Derivative Selectively Inhibits Adult T-Cell Leukemia (ATL) Cells in vitro. Anticancer Res. 2014, 34, 1771–1778. [PubMed] [Google Scholar]
- Nagesh H. N.; Suresh N.; Mahalakshmi Naidu K.; Arun B.; Padma Sridevi J.; Sriram D.; Yogeeswari P.; Chandra Sekhar K. V. G. Synthesis and Evaluation of Anti-Tubercular Activity of 6-(4-Substitutedpiperazin-1-yl) Phenanthridine Analogues. Eur. J. Med. Chem. 2014, 74, 333–339. 10.1016/j.ejmech.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Wurz G.; Hofer O.; Greger H. Structure and Synthesis of Phenaglydon, a New Quinolone Derived Phenanthridine Alkaloid from Glycosmis Cyanocarpa. Nat. Prod. Lett. 1993, 3, 177–182. 10.1080/10575639308043859. [DOI] [Google Scholar]
- a Feng M.; Tang B.; Wang N.; Xu H.-X.; Jiang X. Ligand Controlled Regiodivergent C1 Insertion on Arynes for Construction of Phenanthridinone and Acridone Alkaloids. Angew. Chem., Int. Ed. 2015, 54, 14960–14964. 10.1002/anie.201508340. [DOI] [PubMed] [Google Scholar]; b Feng M.; Tang B.; Xu H.-X.; Jiang X. Collective Synthesis of Phenanthridinone through C-H Activation Involving a Pd-Catalyzed Aryne Multicomponent Reaction. Org. Lett. 2016, 18, 4352–4355. 10.1021/acs.orglett.6b02109. [DOI] [PubMed] [Google Scholar]; c Takamatsu K.; Hirano K.; Miura M. Copper-Mediated Decarboxylative Coupling of Benzamides with ortho-Nitrobenzoic Acids by Directed C–H Cleavage. Angew. Chem., Int. Ed. 2017, 56, 5353–5357. 10.1002/anie.201701918. [DOI] [PubMed] [Google Scholar]; d Das R.; Kapur M. Palladium-Catalyzed, ortho-Selective C-H Halogenation of Benzyl Nitriles, Aryl Weinreb Amides, and Anilides. J. Org. Chem. 2017, 82, 1114–1126. 10.1021/acs.joc.6b02731. [DOI] [PubMed] [Google Scholar]; e Thorat V. H.; Upadhyay N. S.; Murakami M.; Cheng C.-H. Nickel-Catalyzed Denitrogenative Annulation of 1,2,3-Benzotriazin-4-(3H)-ones with Benzynes for Construction of Phenanthridinone Scaffolds. Adv. Synth. Catal. 2018, 360, 284–289. 10.1002/adsc.201701143. [DOI] [Google Scholar]; f Ling F.; Zhang C.; Ai C.; Lv Y.; Zhong W. Metal-Oxidant-Free Cobalt-Catalyzed C(sp2)-H Carbonylation of ortho-Arylanilines: An Approach toward Free (NH)-Phenanthridinones. J. Org. Chem. 2018, 83, 5698–5706. 10.1021/acs.joc.8b00730. [DOI] [PubMed] [Google Scholar]; g Meng Y.-Y.; Si X.-J.; Song Y.-Y.; Zhou H.-M.; Xu F. Palladium-Catalyzed Decarbonylative Annulation of Phthalimides with Arynes: Direct Construction of Phenanthridinones. Chem. Commun. 2019, 55, 9507–9510. 10.1039/C9CC04868E. [DOI] [PubMed] [Google Scholar]
- Sen A.; Dhital R. N.; Sato T.; Ohno A.; Yamada Y. M. A. Switching from Biaryl Formation to Amidation with Convoluted Polymeric Nickel Catalysis. ACS. Catal. 2020, 10, 14410–14418. 10.1021/acscatal.0c03888. [DOI] [Google Scholar]
- Yadav L.; Tiwari M. K.; Shyamlal B. R. K.; Chaudhary S. Organocatalyst in Direct C(sp2)-H Arylation of Unactivated Arenes: [1-(2-Hydroxyethyl)-piperazine]-Catalyzed Inter-/Intra-molecular C-H Bond Activation. J. Org. Chem. 2020, 85, 8121–8141. 10.1021/acs.joc.0c01019. [DOI] [PubMed] [Google Scholar]
- Bhakuni B. S.; Kumar A.; Balkrishna S. J.; Sheikh J. A.; Konar S.; Kumar S. KOtBu Mediated Synthesis of Phenanthridinones and Dibenzoazepinones. Org. Lett. 2012, 14, 2838–2841. 10.1021/ol301077y. [DOI] [PubMed] [Google Scholar]
- Dao P. D. Q.; Lim H.-J.; Cho C. S. Weak Base-Promoted Lactamization under Microwave Irradiation: Synthesis of Quinolin-2(1H)-ones and Phenanthridin-6(5H)-ones. ACS Omega 2018, 3, 12114–12121. 10.1021/acsomega.8b01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Furuta T.; Kitamura Y.; Hashimoto A.; Fujii S.; Tanaka K.; Kan T. Efficient Synthesis of Phenanthridinone Derivatives via a Palladium-Catalyzed Coupling Process. Org. Lett. 2007, 9, 183–186. 10.1021/ol062599z. [DOI] [PubMed] [Google Scholar]; b Donati L.; Leproux P.; Prost E.; Michel S.; Tillequin F.; Gandon V.; Porée F.-H. Solvent/Base Effects in the Selective Domino Synthesis of Phenanthridinones That Involves High-Valent Palladium Species: Experimental and Theoretical Studies. Chem. Eur. J. 2011, 17, 12809–12819. 10.1002/chem.201101354. [DOI] [PubMed] [Google Scholar]
- a Thu H.-Y.; Yu W.-Y.; Che C.-M. Intermolecular Amidation of Unactivated sp2 and sp3 C–H Bonds via Palladium-Catalyzed Cascade C–H Activation/Nitrene Insertion. J. Am. Chem. Soc. 2006, 128, 9048–9049. 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]; b Gui Q.; Yang Z.; Chen X.; Liu J.; Tan Z.; Guo R.; Yu W. Synthesis of Phenanthridin-6(5H)-ones via Copper-Catalyzed Cyclization of 2-Phenylbenzamides. Synlett 2013, 24, 1016–1020. 10.1055/s-0032-1316898. [DOI] [Google Scholar]; c Laha J. K.; Jethava K. P.; Dayal N. Palladium-Catalyzed Intramolecular Oxidative Coupling Involving Double C(sp2)-H Bonds for the Synthesis of Annulated Biaryl Sultams. J. Org. Chem. 2014, 79, 8010–8019. 10.1021/jo5011334. [DOI] [PubMed] [Google Scholar]
- Wang N.; Wang D.; He Y.; Xi J.; Wang T.; Liang Y.; Zhang Z. Photoinduced Annulation of N-Phenylbenzamides for the Synthesis of Phenanthridin-6(5H)-Ones. Adv. Synth. Catal. 2022, 364, 1150–1155. 10.1002/adsc.202101389. [DOI] [Google Scholar]
- a Peng X.; Wang W.; Jiang C.; Sun D.; Xu Z.; Tung C.-H. Strain-Promoted Oxidative Annulation of Arynes and Cyclooctynes with Benzamides: Palladium-Catalyzed C-H/N-H Activation for the Synthesis of N-Heterocycles. Org. Lett. 2014, 16, 5354–5357. 10.1021/ol5025426. [DOI] [PubMed] [Google Scholar]; b Pimparkar S.; Jeganmohan M. Palladium-Catalyzed Cyclization of Benzamides with Arynes: Application to the Synthesis of Phenaglydon and N-Methylcrinasiadine. Chem. Commun. 2014, 50, 12116–12119. 10.1039/C4CC05252H. [DOI] [PubMed] [Google Scholar]; c Zhang T.-Y.; Lin J.-B.; Li Q.-Z.; Kang J.-C.; Pan J.-L.; Hou S.-H.; Chen C.; Zhang S.-Y. Copper-Catalyzed Selective ortho-C-H/N-H Annulation of Benzamides with Arynes: Synthesis of Phenanthridinone Alkaloids. Org. Lett. 2017, 19, 1764–1767. 10.1021/acs.orglett.7b00442. [DOI] [PubMed] [Google Scholar]; d Zhao J.; Li H.; Li P.; Wang L. Annulation of Benzamides with Arynes Using Palladium with Photoredox Dual Catalysis. J. Org. Chem. 2019, 84, 9007–9016. 10.1021/acs.joc.9b00893. [DOI] [PubMed] [Google Scholar]; For a related example, see:; e Lu C.; Dubrovskiy A. V.; Larock R. C. Palladium-Catalyzed Annulation of Arynes by o-Halobenzamides: Synthesis of Phenanthridinones. J. Org. Chem. 2012, 77, 8648–8656. 10.1021/jo3016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S.; Gowrisankar S.; Kim E. S.; Kim J. N. A Brand-New Pd-Mediated Generation of Benzyne and Its [2 + 2 + 2] Cycloaddition: δ-Carbon Elimination and Concomitant Decarboxylation. Tetrahedron Lett. 2008, 49, 6569–6572. 10.1016/j.tetlet.2008.09.017. [DOI] [Google Scholar]
- Geng X.; Shatskiy A.; Alvey G. R.; Liu J.-Q.; Kärkäs M. D.; Wang X.-S. Tandem Palladium/Copper-Catalyzed Decarboxylative Approach to Benzoimidazo- and Imidazophenanthridine Skeletons. Org. Lett. 2022, 24, 9194–9199. 10.1021/acs.orglett.2c03647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Chen Y.; Shatskiy A.; Liu J.-Q.; Kärkäs M. D.; Wang X.-S. Silver-Promoted (4 + 1) Annulation of Isocyanoacetates with Alkylpyridinium Salts: Divergent Regioselective Synthesis of 1,2-Disubstituted Indolizines. Org. Lett. 2021, 23, 7555–7560. 10.1021/acs.orglett.1c02754. [DOI] [PubMed] [Google Scholar]; b Wang Y.-C.; Chen X.; Alvey G. R.; Shatskiy A.; Liu J.-Q.; Kärkäs M. D.; Wang X.-S. Copper-Assisted Wittig-Type Olefination of Aldehydes with p-Toluenesulfonylmethyl Isocyanide. Org. Chem. Front. 2022, 9, 4158–4163. 10.1039/D2QO00472K. [DOI] [Google Scholar]; c Xu L.; Liu X.; Alvey G. R.; Shatskiy A.; Liu J.-Q.; Kärkäs M. D.; Wang X.-S. Silver-Catalyzed Controlled Intermolecular Cross-Coupling of Silyl Enol Ethers: Scalable Access to 1,4-Diketones. Org. Lett. 2022, 24, 4513–4518. 10.1021/acs.orglett.2c01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Peng X.; Wang W.; Jiang C.; Sun D.; Xu Z.; Tung C.-H. Strain-Promoted Oxidative Annulation of Arynes and Cyclooctynes with Benzamides: Palladium-Catalyzed C-H/N-H Activation for the Synthesis of N-Heterocycles. Org. Lett. 2014, 16, 5354–5357. 10.1021/ol5025426. [DOI] [PubMed] [Google Scholar]; b Zhao J.; Li H.; Li P.; Wang L. Annulation of Benzamides with Arynes Using Palladium with Photoredox Dual Catalysis. J. Org. Chem. 2019, 84, 9007–9016. 10.1021/acs.joc.9b00893. [DOI] [PubMed] [Google Scholar]
- For selected reviews on Pd-mediated C–N coupling reactions, see:; a Bariwal J.; Van der Eycken E. C-N Bond Forming Cross-Coupling Reactions: An Overview. Chem. Soc. Rev. 2013, 42, 9283–9303. 10.1039/c3cs60228a. [DOI] [PubMed] [Google Scholar]; b Ruiz-Castillo P.; Buchwald S. L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Qiu M.; Fu X.; Fu P.; Huang J. Construction of Aziridine, Azetidine, Indole and Quinoline-Like Heterocycles via Pd-Mediated C-H Activation/Annulation Strategies. Org. Biomol. Chem. 2022, 20, 1339–1359. 10.1039/D1OB02146J. [DOI] [PubMed] [Google Scholar]; For selected examples, see:; d Wang G.-W.; Yuan T.-T.; Li D.-D. One-Pot Formation of C–C and C–N Bonds through Palladium-Catalyzed Dual C–H Activation: Synthesis of Phenanthridinones. Angew. Chem., Int. Ed. 2011, 50, 1380–1383. 10.1002/anie.201005874. [DOI] [PubMed] [Google Scholar]; e Fan Z.; Wu K.; Xing L.; Yao Q.; Zhang A. Palladium-Catalyzed Double C-H Activation: One-Pot Synthesis of Benzo[c]pyrazolo[1,2-a]cinnolin-1-ones from 5-Pyrazolones and Aryl Iodides. Chem. Commun. 2014, 50, 1682–1684. 10.1039/C3CC47989G. [DOI] [PubMed] [Google Scholar]; f Li H.; Zhao J.; Yi S.; Hu K.; Feng P. Consequent Construction of C-C and C-N Bonds via Palladium-Catalyzed Dual C-H Activation: Synthesis of Benzo[c]cinnoline Derivatives. Organometallics 2021, 40, 880–889. 10.1021/acs.organomet.0c00800. [DOI] [Google Scholar]
- a Zhao Q.; Fu W. C.; Kwong F. Y. Palladium-Catalyzed Regioselective Aromatic Extension of Internal Alkynes through a Norbornene-Controlled Reaction Sequence. Angew. Chem., Int. Ed. 2018, 57, 3381–3385. 10.1002/anie.201713207. [DOI] [PubMed] [Google Scholar]; b Gu Y.; Sun X.; Wan B.; Lu Z.; Zhang Y. C(sp3)-H Activation-Enabled Cross-Coupling of Two Aryl Halides: An Approach to 9,10-Dihydrophenanthrenes. Chem. Commun. 2020, 56, 10942–10945. 10.1039/D0CC04602G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.