Abstract
The use of catalysis methods to enable Smiles rearrangement opens up new substrate classes for arylation under mild conditions. Here, we describe an N-heterocyclic carbene (NHC) catalysis system that accesses indole and pyrrole aldehyde substrates in a desulfonylative Smiles process. The reaction proceeds under mild, transition-metal-free conditions and captures acyl anion reactivity for the synthesis of a diverse array of 2-aroyl indoles and pyrroles from readily available sulfonamide starting materials.
The Smiles–Truce rearrangement is a powerful approach to transition-metal-free arylation, interchanging easily formed carbon–heteroatom bonds for synthetically more challenging carbon–carbon bonds, often under very simple conditions (Scheme 1A).1 Advances over the past decade exhibit vast scope in terms of the leaving group, nucleophile, and substrate structures accessible to Smiles reactivity.2 A new frontier for Smiles–Truce rearrangement lies in the integration of both the polar and radical manifolds with new catalytic methods. The catalysis approach has been very successful in the radical regime, with metallophotoredox catalysis in particular being instrumental in the development of new single electron Smiles systems.3 Catalysis systems in the polar regime, in contrast, are less developed. Smiles–Truce systems that exploit carbanion rearrangements frequently require strongly basic conditions, with benzylic deprotonation with n-BuLi being the canonical example.4 The introduction of new catalysis approaches to carbanion reactivity could substantially enhance the applications of this arylation technology.
Scheme 1. Smiles Rearrangements.
We have developed a number of carbanion-based Smiles–Truce systems that harness desulfonylation of sulfonamides.5 This powerful class of arylation uses readily accessible sulfonamides and is driven to irreversible completion by SO2 extrusion.6 We wondered whether N-heterocyclic carbene (NHC) catalysis could be utilized for this substrate class, as it would open up new arylation pathways in the absence of strong bases. NHC chemistry is foundational to organocatalysis, driven by the innate ability of NHCs to enable acyl anion umpolung reactivity with aldehyde substrates.7
Notable examples exist for aldehyde arylation using aryl electrophiles such as aryl fluorides,8 iodoniums,9 and arynes.10 Smiles–Truce applications, in contrast, are limited: Glorius established NHC catalysis for the salicylaldehyde-derived phenolic ether system, demonstrating aryl transfer via Stetter-like NHC catalytic cycles to afford aroyl ketones (Scheme 1B).11 The group of Ye subsequently described a radical NHC approach for the same class of aldehyde substrates.12 Recently, the groups of Tobisu and Zhou described an NHC-catalyzed Smiles system on acrylamides, with the reaction hypothesized to proceed through a deoxy-Breslow intermediate arising from NHC addition to the Michael acceptor (Scheme 1C).13 Sulfonamide applications, in contrast, have yet to be developed.
We selected 2-aroyl pyrrole and 2-aroyl indole derivatives as our target structures, which are fundamental building blocks for biologically active heterocycles in the pharmaceutical industry. Our planned Smiles rearrangement would proceed through a favorable 5-membered transition state, delivering aroylated N–H products for further functionalization. The pyrrole products are typically accessed through classical Friedel–Crafts methods with strong Lewis acids as stoichiometric activators.14 However, competing functionalization of the N and C-3 positions often leads to reduced regioselectivity, yielding mixtures of mono- and disubstituted side-products. The indole series can be synthesized de novo, e.g. through a Cadogan cyclization of 2-nitrostyrenes,15 or via organometallic methods that require a directing group on nitrogen.16 The development of a metal-free transformation under mild conditions is thus highly desirable.
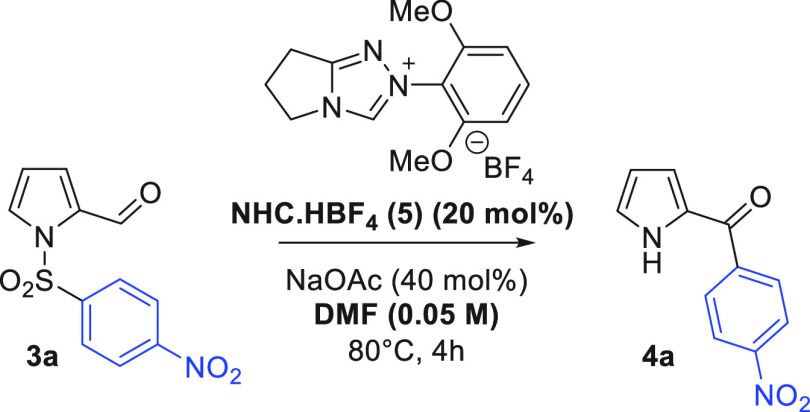
We began work with nosylated indole substrate 1a, prepared in one step by facile sulfonamide formation on commercially available indol-2-carbaldehyde. A screen of various NHC catalysts was immediately successful, with Smiles reactivity observed when using triazolium based NHCs (1–5), with NHC 1 delivering the highest yield of ketone 2a when used with NaOAc in DMF (75%, Scheme 2). Imidazolium and thioazolium NHCs proved ineffective for our process, failing to induce the desired reactivity (see Supporting Information (SI) for details). Further variation in base, solvent, and temperature did not afford improvements, and control experiments established the requirement for NHC and base for reaction (Table 1, entries 9 and 10).
Scheme 2. Substrate Scope.
0.1 mmol scale.
Table 1. Reaction Optimizationa.
| entry | deviation | yield (%)b |
|---|---|---|
| 1 | – | 75% |
| 2 | DMSO | 64% |
| 3 | toluene | 0% |
| 4 | K3PO4 | 64% |
| 5 | DBU | 15% |
| 6 | 30 °Cc | 60% |
| 7 | 50 °Cc | 69% |
| 8 | NHC 1 (10 mol %) | 65% |
| 9 | No NHC | 0% |
| 10 | No Base | 0% |
0.1 mmol scale.
Isolated yield.
16 h reaction time.
With optimal conditions in hand, the scope of the reaction was examined, beginning with the migrating ring. The reaction proved successful with both ortho- and para-nitro groups due to their unique ability to stabilize the anionic Meisenheimer intermediate (Scheme 2, entries 2a–2f). However, weaker electron-withdrawing functionalities such as p-CN, p-CF3, and p-CO2Me, which have been used successfully in anionic Smiles systems,17 were unsuccessful under the NHC catalysis conditions (SI Table 8). The 2-pyridyl substrate did deliver the expected product 2g, but in a low yield. Instability of the starting sulfonamide 1g could account for the reduced yields as high levels of degradation were observed in each case. Turning to the indole heteroarene structure, a selection of alkyl, methoxy, and halogen groups were all well-tolerated around the indole arene ring, and we could successfully substitute the 3-position with a Me group without penalty (2h–2m). The parent example (2a) could be scaled up to 1 mmol without penalty (62% yield) and to 1 g with a small reduction in efficiency (48% yield).
Turning to pyrrole sulfonamides, a brief optimization established comparable reactivity, but using NHC 5 as the optimal catalyst under more dilute conditions (Table 2). In comparison to the indole reaction, the aroylated pyrrole products appear to be less stable as there is a significant decrease in product yield when left heating for 16 h (Table 2, entry 3).
Table 2. Pyrrole Reaction Optimizationa.
0.1 mmol scale.
Isolated yield.
16 h reaction time at 50 °C.
The pyrrole series worked well for the cardinal p- and o-nosyl substrates, giving good yields of ketone 4a–4c (Scheme 3). Production of 4a was investigated on a 1 mmol scale and gave a very similar yield (65%). Further exemplifications were more challenging, with N–S bond fragmentation observed in a number of cases, eroding yields.18 Halogen substitution was tolerated in the migrating ring (4d and 4e), and an iodopyrrole substrate was successfully transformed into 4g, but in diminished yield.
Scheme 3. Pyrrole Smiles–Truce Optimization.
0.1 mmol scale.
A diverse array of functionalizations were shown to be possible on both the 2-aroyl indole and pyrrole products (Scheme 4). Bromination in the 3-position of the indole proceeded smoothly, yielding bromoindole 5, a substrate for downstream cross-coupling reactions. The 2-aroyl indole could be reduced using NaBH4, revealing secondary alcohol 6, for further reactions. Functionalization at both indole and pyrrole nitrogen centers was successful through alkylation with iodomethane and K2CO3 in DMF (7a and 7b). Importantly, conversion of the nitro group to the aniline can be achieved with zinc reduction (8a and 8b). The aniline substrates can subsequently be utilized in a range of pharmaceutically useful chemistries including metal coupling, amide couplings, and SNAr. Subjection of the ortho-fluoro product 4e to SNAr conditions gave the tricyclic 9H-pyrrolo[1,2-a]indol-9-one product 9, representing a new entry into this heterocycle.
Scheme 4. Product Derivatization.
Reaction conditions: (a) 0.1 mmol scale, NBS (1.2 equiv), DMF (0.1 M), rt 3 h; (b) 0.1 mmol scale, NaBH4 (3 equiv), MeOH (0.1 M), 0 °C, 16 h; (c) 0.1 mmol scale, MeI (2 equiv), K2CO3 (2.5 equiv), DMF (0.1 M), rt, 16 h; (d) 0.1 mmol scale, Zn (4.2 equiv), AcOH, EtOH:H2O (3 mL, 1:2 EtOH:H2O), 80 °C, 3 h; (e) 0.1 mmol scale, Cs2CO3 (2 equiv), DMF (0.1 M), substrate 4e, 70 °C, 16 h.
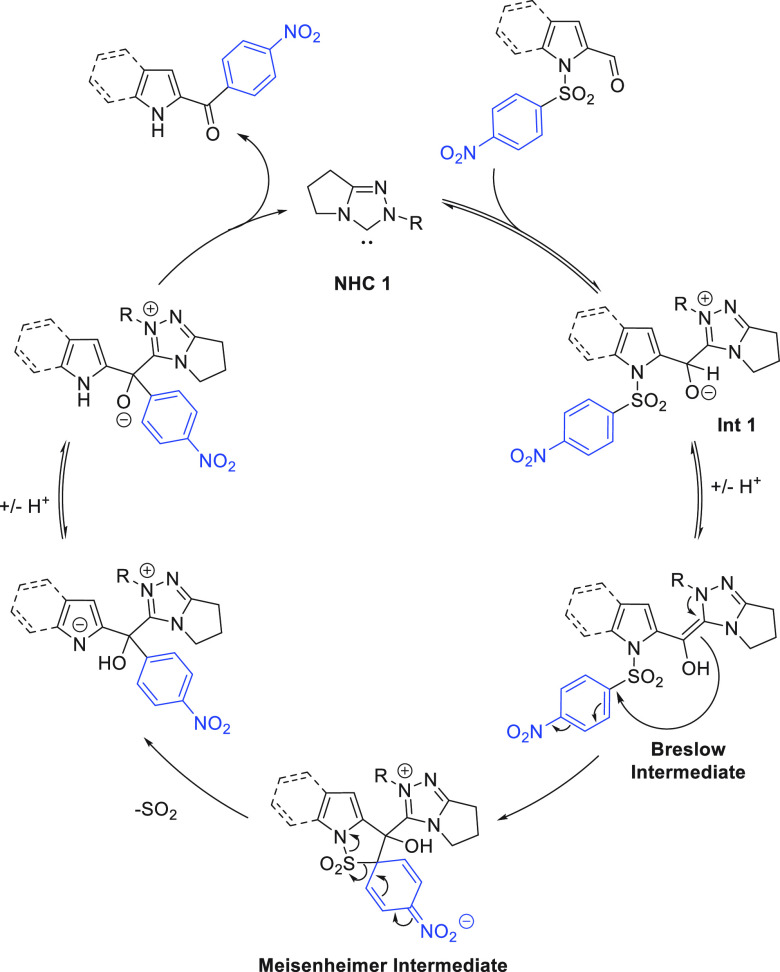
Turning to the mechanism of the reaction, we carried out a crossover experiment to probe any intermolecularity in the proposed aryl transfer step (Scheme 5). Using substrates 1c and 1h, we observed no crossover product formation, supporting the Smiles–Truce reaction pathway. An outline mechanism is set out in Scheme 6: Based on control experiments with no base (Table 1, entry 10) and previous literature, NHC 1 must first be generated via deprotonation. Addition of the carbene to the aldehyde forms the Breslow intermediate, which reacts via the Smiles rearrangement through a Meisenheimer intermediate. Following loss of sulfur dioxide and catalyst regeneration, product 2-aroyl indole or pyrrole is formed.
Scheme 5. Crossover Experiment.
0.1 mmol scale, standard conditions, NMR yields.
Scheme 6. Mechanistic Pathway.
The extrusion of SO2 could in principle impact the catalytic cycle by sequestering the NHC catalyst – NHC–SO2 adducts have been reported in the literature for some classes of NHC.19 Recent work from Maulide and co-workers identified this phenomenon for an amine-mediated desulfonylative Smiles reaction, where DABCO was used to set up a Smiles reaction via a Morita–Baylis–Hilman process.2c We varied the NHC stoichiometry (Table 1) to investigate the possibility that the Lewis base catalyst is deactivated in this manner. While the reaction did start to lose efficiency at low loadings, higher loadings (>20 mol %) did not noticeably improve the reaction, suggesting that SO2 capture by NHC is not significant for the reaction at hand.
In conclusion, we have demonstrated the use of NHC catalysis for desulfonylative Smiles rearrangements. Extrusion of the Lewis acidic SO2 byproduct was found to be feasible under catalysis conditions, establishing a mild and direct route to the aroylated indole and pyrrole heterocycles.
Acknowledgments
We acknowledge the EPSRC (grant EP/S023755/1) and BBSRC for funding studentships for C.S. and A.T. Professor Anthony Green (University of Manchester) is thanked for helpful discussions.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c01089.
Preparative procedures and spectroscopic data for all starting materials and Smiles rearrangement products, plus NMR spectra for synthesized compounds. (PDF)
Author Contributions
† C.S. and A.T. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Wales S. M.; Saunthwal R. K.; Clayden J. C(Sp3)-Arylation by Conformationally Accelerated Intramolecular Nucleophilic Aromatic Substitution (SNAr). Acc. Chem. Res. 2022, 55, 1731–1747. 10.1021/acs.accounts.2c00184. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huynh M.; De Abreu M.; Belmont P.; Brachet E. Spotlight on Photoinduced Aryl Migration Reactions. Chem.—Eur. J. 2021, 27, 3581–3607. 10.1002/chem.202003507. [DOI] [PubMed] [Google Scholar]; c Whalley D. M.; Greaney M. F. Recent Advances in The Smiles Rearrangement: New Opportunities for Arylation. Synthesis 2022, 54, 1908–1918. 10.1055/a-1710-6289. [DOI] [Google Scholar]
- Selected recent examples:; a Saunthwal R. K.; Schwarz M.; Mallick R. K.; Terry-Wright W.; Clayden J. Enantioselective Intramolecular A-Arylation of Benzylamine Derivatives: Synthesis of a Precursor to Levocetirizine. Angew. Chem., Int. Ed. 2023, 62, e202216758 10.1002/anie.202216758. [DOI] [PubMed] [Google Scholar]; b Zhen G.; Zeng G.; Jiang K.; Wang F.; Cao X.; Yin B. Visible-Light-Induced Diradical-Mediated Ipso-Cyclization towards Double Dearomative [2 + 2]-Cycloaddition or Smiles-Type Rearrangement. Chem.—Eur. J. 2023, 29, e202203217 10.1002/chem.202203217. [DOI] [PubMed] [Google Scholar]; c Lemmerer M.; Zhang H.; Fernandes A. J.; Fischer T.; Mießkes M.; Xiao Y.; Maulide N. Synthesis of α-Aryl Acrylamides via Lewis-Base-Mediated Aryl/Hydrogen Exchange. Angew. Chem. Int. Ed 2022, 61, e202207475 10.1002/anie.202207475. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Allen A. R.; Poon J.-F.; McAtee R. C.; Watson N. B.; Pratt D. A.; Stephenson C. R. J. Mechanism of Visible Light-Mediated Alkene Aminoarylation with Arylsulfonylacetamides. ACS Catal. 2022, 12, 8511–8526. 10.1021/acscatal.2c02577. [DOI] [PMC free article] [PubMed] [Google Scholar]; d1 Huang J.; Liu F.; Zeng L.-H.; Li S.; Chen Z.; Wu J. Accessing Chiral Sulfones Bearing Quaternary Carbon Stereocenters via Photoinduced Radical Sulfur Dioxide Insertion and Truce–Smiles Rearrangement. Nat. Commun. 2022, 13, 7081. 10.1038/s41467-022-34836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chen X.; Wang Q.; Zhang Z.; Niu Z.-J.; Shi W.-Y.; Gong X.-P.; Jiao R.-Q.; Gao M.-H.; Liu X.-Y.; Liang Y.-M. Copper-Catalyzed Hydrogen Atom Transfer and Aryl Migration Strategy for the Arylalkylation of Activated Alkenes. Org. Lett. 2022, 24, 4338–4343. 10.1021/acs.orglett.2c01427. [DOI] [PubMed] [Google Scholar]; f Noten E. A.; McAtee R. C.; Stephenson C. R. J. Catalytic Intramolecular Aminoarylation of Unactivated Alkenes with Aryl Sulfonamides. Chem. Sci. 2022, 13, 6942–6949. 10.1039/D2SC01228F. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Radhoff N.; Studer A. 1,4-Aryl Migration in Ketene-Derived Enolates by a Polar-Radical-Crossover Cascade. Nat. Commun. 2022, 13, 3083. 10.1038/s41467-022-30817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Shi Z.; Li Y.; Li N.; Wang W.; Lu H.; Yan H.; Yuan Y.; Zhu J.; Ye K. Electrochemical Migratory Cyclization of N-Acylsulfonamides. Angew. Chem., Int. Ed. 2022, 61, e202206058 10.1002/anie.202206058. [DOI] [PubMed] [Google Scholar]; i Horst B.; Verdoorn D. S.; Hennig S.; van der Heijden G.; Ruijter E. Enantioselective Total Synthesis of (−)-Limaspermidine and (−)-Kopsinine by a Nitroaryl Transfer Cascade Strategy. Angew. Chem., Int. Ed. 2022, 61, e202210592 10.1002/anie.202210592. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Abe M.; Nitta S.; Miura E.; Kimachi T.; Inamoto K. Nitrile Synthesis via Desulfonylative–Smiles Rearrangement. J. Org. Chem. 2022, 87, 4460–4467. 10.1021/acs.joc.1c03011. [DOI] [PubMed] [Google Scholar]; k Cheibas C.; Fincias N.; Casaretto N.; Garrec J.; El Kaïm L. Passerini–Smiles Reaction of A-Ketophosphonates: Platform for Phospha-Brook/Smiles Embedded Cascades. Angew. Chem., Int. Ed. 2022, 61, e202116249 10.1002/anie.202116249. [DOI] [PubMed] [Google Scholar]; l Sephton T.; Large J. M.; Butterworth S.; Greaney M. F. Diarylamine Synthesis via Desulfinylative Smiles Rearrangement. Org. Lett. 2022, 24, 1132–1135. 10.1021/acs.orglett.1c04122. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Hervieu C.; Kirillova M. S.; Suárez T.; Müller M.; Merino E.; Nevado C. Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres. Nat. Chem. 2021, 13, 327–334. 10.1038/s41557-021-00668-4. [DOI] [PubMed] [Google Scholar]; n Kang L.; Wang F.; Zhang J.; Yang H.; Xia C.; Qian J.; Jiang G. High Chemo-/Stereoselectivity for Synthesis of Polysubstituted Monofluorinated Pyrimidyl Enol Ether Derivatives. Org. Lett. 2021, 23, 1669–1674. 10.1021/acs.orglett.1c00092. [DOI] [PubMed] [Google Scholar]; o Chen F.; Shao Y.; Li M.; Yang C.; Su S. J.; Jiang H.; Ke Z.; Zeng W. Photocatalyzed cycloaromatization of vinylsilanes with arylsulfonylazides. Nat. Commun. 2021, 12, 3304. 10.1038/s41467-021-23326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; p Radhoff N.; Studer A. Functionalization of α-C(sp3)–H Bonds in Amides Using Radical Translocating Arylating Group. Angew. Chem., Int. Ed. 2021, 60, 3561–3565. 10.1002/anie.202013275. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Gillaizeau-Simonian N.; Barde E.; Guérinot A.; Cossy J. Cobalt-Catalyzed 1,4-Aryl Migration/Desulfonylation Cascade: Synthesis of α-Aryl Amides. Chem.—Eur. J. 2021, 27, 4004–4008. 10.1002/chem.202005129. [DOI] [PubMed] [Google Scholar]; r Wang Z. S.; Chen Y. B.; Zhang H. W.; Sun Z.; Zhu C.; Ye L. W. Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl–Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines. J. Am. Chem. Soc. 2020, 142, 3636–3644. 10.1021/jacs.9b13975. [DOI] [PubMed] [Google Scholar]; s Yan J.; Cheo H. W.; Teo W. K.; Shi X.; Wu H.; Idres S. B.; Deng L. W.; Wu J. A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrogen Atom Transfer Photocatalyst. J. Am. Chem. Soc. 2020, 142, 11357–11362. 10.1021/jacs.0c02052. [DOI] [PubMed] [Google Scholar]; t Ruzi R.; Ma J.; Yuan X.; Wang W.; Wang S.; Zhang M.; Dai J.; Xie J.; Zhu C. Deoxygenative Arylation of Carboxylic Acids by Aryl Migration. Chem.—Eur. J. 2019, 25, 12724–12729. 10.1002/chem.201903816. [DOI] [PubMed] [Google Scholar]
- a) Review:; Allen A. R.; Noten E. A.; Stephenson C. R. J. Aryl Transfer Strategies Mediated by Photoinduced Electron Transfer. Chem. Rev. 2022, 122, 2695–2751. 10.1021/acs.chemrev.1c00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Truce W. E.; Ray W. J. Jr; Norman O. L.; Eickemeyer D. B. Rearrangements of Aryl Sulfones. I. The metalation and rearrangement of mesityl phenyl sulfone. J. Am. Chem. Soc. 1958, 80, 3625–3629. 10.1021/ja01547a038. [DOI] [Google Scholar]; See also:; b Naito T.; Dohmori R. Rearrangement of sulfonamide derivatives IV. Rearrangement reaction of the sulfonamide derivatives of pyridine and quinoline 1-oxides. Chem. Pharm. Bull. 1955, 3, 38–42. 10.1248/cpb1953.3.38. [DOI] [PubMed] [Google Scholar]
- a Rabet P. T. G.; Boyd S.; Greaney M. F. Metal-Free Intermolecular Aminoarylation of Alkynes. Angew. Chem., Int. Ed. 2017, 56, 4183–4186. 10.1002/anie.201612445. [DOI] [PubMed] [Google Scholar]; b Barlow H. L.; Rabet P. T. G.; Durie A.; Evans T.; Greaney M. F. Arylation Using Sulfonamides: Phenylacetamide Synthesis through Tandem Acylation-Smiles Rearrangement. Org. Lett. 2019, 21, 9033–9035. 10.1021/acs.orglett.9b03429. [DOI] [PubMed] [Google Scholar]; c Johnson S.; Kovacs E.; Greaney M. F. Arylation and alkenylation of activated alkyl halides using sulfonamides. Chem. Commun. 2020, 56, 3222–3224. 10.1039/D0CC00220H. [DOI] [PubMed] [Google Scholar]
- Chu X.-Q.; Ge D.; Cui Y.-Y.; Shen Z.-L.; Li C.-J. Desulfonylation via Radical Process: Recent Developments in Organic Synthesis. Chem. Rev. 2021, 121, 12548–12680. 10.1021/acs.chemrev.1c00084. [DOI] [PubMed] [Google Scholar]
- Reviews:; a Enders D.; Niemeier O.; Henseler A. Organocatalysis by N-Heterocyclic Carbenes. Chem. Rev. 2007, 107, 5606–5655. 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; b Hopkinson M. N.; Richter C.; Schedler M.; Glorius F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]; c Ohmiya H. N-Heterocyclic Carbene-Based Catalysis Enabling Cross-Coupling Reactions. ACS Catal. 2020, 10, 6862–6869. 10.1021/acscatal.0c01795. [DOI] [Google Scholar]; d Das T. K.; Biju A. T. Imines as acceptors and donors in N-heterocyclic carbene (NHC) organocatalysis. Chem. Commun. 2020, 56, 8537–8552. 10.1039/D0CC03290E. [DOI] [PubMed] [Google Scholar]
- Suzuki Y.; Ota S.; Fukuta Y.; Ueda Y.; Sato M. N-Heterocyclic Carbene-Catalyzed Nucleophilic Aroylation of Fluorobenzenes. J. Org. Chem. 2008, 73, 2420–2423. 10.1021/jo7023569. [DOI] [PubMed] [Google Scholar]
- Toh Q. Y.; McNally A.; Vera S.; Erdmann N.; Gaunt M. J. Organocatalytic C-H Bond Arylation of Aldehydes to Bis-heteroaryl Ketones. J. Am. Chem. Soc. 2013, 135, 3772–3775. 10.1021/ja400051d. [DOI] [PubMed] [Google Scholar]
- Biju A. T.; Glorius F. Intermolecular N-heterocyclic carbene catalyzed hydroacylation of arynes. Angew. Chem., Int. Ed. 2010, 49, 9761–9764. 10.1002/anie.201005490. [DOI] [PubMed] [Google Scholar]
- Janssen-Muller D.; Singha S.; Lied F.; Gottschalk K.; Glorius F. NHC-Organocatalyzed C-Ar-O Bond Cleavage: Mild Access to 2-Hydroxybenzophenones. Angew. Chem., Int. Ed. 2017, 56, 6276–6279. 10.1002/anie.201610203. [DOI] [PubMed] [Google Scholar]
- Xia Z. H.; Dai L.; Gao Z. H.; Ye S. N-Heterocyclic carbene/photo-cocatalyzed oxidative Smiles rearrangement: synthesis of aryl salicylates from O-aryl salicylaldehydes. Chem. Commun. 2020, 56, 1525. 10.1039/C9CC09272B. [DOI] [PubMed] [Google Scholar]
- a Yasui K.; Kamitani M.; Fujimoto H.; Tobisu M. N-Heterocyclic Carbene-Catalyzed Truce-Smiles Rearrangement of N-Arylacrylamides via the Cleavage of Unactivated C(aryl)-N Bonds. Org. Lett. 2021, 23, 1572–1576. 10.1021/acs.orglett.0c04281. [DOI] [PubMed] [Google Scholar]; b Hu Y.; Wang Z.; Luo H.; Jin H.; Liu Y.; Zhou B. NHC-catalyzed Truce–Smiles rearrangement of N-aryl methacrylamides for the synthesis of trans-cinnamides. Org. Biomol. Chem. 2021, 19, 3834–3837. 10.1039/D1OB00443C. [DOI] [PubMed] [Google Scholar]
- a Taylor J. E.; Jones M. D.; Williams J. M. J.; Bull S. D. Friedel-Crafts Acylation of Pyrroles and Indoles using 1,5-Diazabicyclo 4.3.0 non-5-ene (DBN) as a Nucleophilic Catalyst. Org. Lett. 2010, 12, 5740–5743. 10.1021/ol1025348. [DOI] [PubMed] [Google Scholar]; b Huffman J. W.; Smith V. J.; Padgett L. W. Acylation of N-p-toluenesulfonylpyrrole under Friedel-Crafts conditions: evidence for organoaluminum intermediates. Tetrahedron 2008, 64, 2104–2112. 10.1016/j.tet.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Laha J. K.; Hunjan M. K.; Hegde S.; Gupta A. Aroylation of Electron-Rich Pyrroles under Minisci Reaction Conditions. Org. Lett. 2020, 22, 1442–1447. and references therein 10.1021/acs.orglett.0c00041. [DOI] [PubMed] [Google Scholar]
- Cadogan J. I. G.; Cameron-Wood M.; Mackie R. K.; Searle R. J. G. The Reactivity of Organophosphorus Compounds. Part XIX. Reduction of Nitro-Compounds by Triethyl Phosphite: A Convenient New Route to Carbazoles, Indoles, Indazoles, Triazoles, and Related Compounds. J. Chem. Soc. 1965, 4831–4837. 10.1039/jr9650004831. [DOI] [Google Scholar]
- a Saulnier M. G.; Gribble G. W. Generation and reactions of 3-lithio-1-(phenylsulfonyl)indole. J. Org. Chem. 1982, 47, 757–761. 10.1021/jo00344a001. [DOI] [Google Scholar]; b Zhou B.; Yang Y.; Li Y. Rhodium-Catalyzed Oxidative C2-Acylation of Indoles with Aryl and Alkyl Aldehydes. Chem. Commun. 2012, 48, 5163–5165. 10.1039/c2cc31351k. [DOI] [PubMed] [Google Scholar]
- Holden C. M.; Sohel S. M. A.; Greaney M. F. Metal Free Bi(hetero)aryl Synthesis: A Benzyne Truce–Smiles Rearrangement. Angew. Chem., Int. Ed. 2016, 55, 2450–2453. 10.1002/anie.201510236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ozaki T.; Yorimitsu H.; Perry G. J. P. Primary Sulfonamide Functionalization via Sulfonyl Pyrroles: Seeing the N–Ts Bond in a Different Light. Chem.—Eur. J. 2021, 27, 15387–15391. 10.1002/chem.202102748. [DOI] [PubMed] [Google Scholar]; b Ozaki T.; Yorimitsu H.; Perry G. J. P. Late-stage sulfonic acid/sulfonate formation from sulfonamides via sulfonyl pyrroles. Tetrahedron 2022, 117–118, 132830 10.1016/j.tet.2022.132830. [DOI] [Google Scholar]
- Finger L. H.; Guschlbauer J.; Harms K.; Sundermeyer J. N-Heterocyclic Olefin–Carbon Dioxide and – Sulfur Dioxide Adducts: Structures and Interesting Reactivity Patterns. Chem.—Eur. J. 2016, 22, 16292–16303. 10.1002/chem.201602973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.