Abstract
Background
Basal, reflex, and emotional tears differ in chemical components. It is not yet known whether chemical differences exist in tears of different emotions. We investigated the biochemical basis of emotional tears by performing non-targeted metabolomics analyses of positive and negative emotional tears of humans.
Methods
Samples of reflex, negative, and positive emotional tears were obtained from 12 healthy college participants (11 females and one male). Untargeted metabolomics was performed to identify metabolites in different types of tears. The differentially altered metabolites were screened and assessed using univariate and multivariate analyses.
Results
The orthogonal partial least squares discriminant analysis model showed that reflex, negative, and positive emotional tears were clearly separated. A total of 133 significantly differentially expressed metabolites of electrospray ionization source (ESI-) mode were identified between negative and positive emotional tears. The top 50 differentially expressed metabolites between negative and positive emotional tears were highly correlated. Pathway analysis revealed that secretion of negative emotional tears was associated with some synapses in the brain, regulation of a series of endocrine hormones, including the estrogen signaling pathway, and inflammation activities, while secretion of positive emotional tears was correlated with biotin and caffeine metabolism.
Conclusions
It is indicated that metabolic profiles of reflex, positive, and negative emotional tears of humans are distinct, and secretion of the tears involves distinct biological activities. Therefore, we present a chemical method for detecting human emotions, which may become a powerful tool for the diagnosis of mental diseases and the identification of fake tears.
Keywords: tears, types of tears, metabolomics, emotional evaluation, emotional tears, metabolic profile
Introduction
Three types of tears are produced in humans: basal, reflex, and emotional [1]. Basal and reflex tearing serve the human eyes and their physiological functions are fully understood. Basal tear is the small quantity of tear produced to maintain a lacrimal film on the corneal surface. Reflex tear is a lacrimal flow produced in response to irritations, such as inflammation and foreign body invasion. However, the origin and mechanisms of emotional tearing are poorly studied. Emotional crying is a unique human behavior, resulting from cognitive and psychogenic brain processes [2]. Even though emotional tears also originate from the eyes, they do not serve the organ itself. Emotional tears are communicating signals; they express the need for help or empathic responses. This mysterious behavior has fascinated both scientists and laypeople, but research on this topic is still at the primary stage. Efforts should be aimed at revealing the neurophysiological and biochemical underpinnings of the behavior.
Human newborns exhibit vocal crying when they are separated from their mothers. This behavior is consistent across different species of mammals and birds and requires no previous learning. In this period, the shedding of visible tears is triggered by strong contractions of the orbicularis oculi muscles during the production of distress vocalizations, which stimulate the sensitive corneal sensory nerves [3]. Real emotional tearing appears later than reflex tearing, several months after birth [4]. It is reported that most children exhibit emotional tearing nearly in the sixth week of life while Vignat et al. found that emotional tearing occurs at about four months of age [2].
Tears evolve with increasing age to serve as emotional signals that convey complex information. In infants and children, physical pain and discomfort are important triggers of tears. However, adults and elderly people seldom cry because of physical conditions, and their weeping is usually associated with different emotional states [5]. As an essential additional feature of crying in humans, what is the role of emotional tears? Tearful crying results in tension reduction and has health benefits. The biochemical hypothesis suggests that in negative situations, the (endogenous) release of endorphins or oxytocin while crying is cathartic to the criers [6]. Adults cry in negative situations, such as losses, failures, and helplessness, as well as in positive situations, such as when witnessing the intensification of relationships, prosocial behaviors, and happiness [5].
Tears are composed of proteins, lipids, metabolites, and electrolytes. Chemical components of tears differ among basal, reflex, and emotional tears [7,8]. However, chemical variations in tears of different emotions are yet to be established. Tears of mice contain a chemosignal or pheromone [9]. Gelstein et al. reported that human tears also contain a similar chemosignal [10]. We hypothesized that different emotional tears (positive or negative emotions) contain distinct substances, which form the basis of chemosignals for tearing. Therefore, we investigated the biochemical basis of emotional tears via non-targeted metabolomics analysis of negative and positive emotional tears of humans.
This article was previously posted to the medRxiv preprint server on February 02, 2022 (https://doi.org/10.1101/2022.01.28.22270049).
Materials and methods
Study population
Ethics Statement
This is a diagnostic accuracy test design study. The protocol for this study was approved by the Ethical Committee of Jili Hospital (No. 2021-04; date: May 27, 2021) and was conducted in accordance with the Declaration of Helsinki. The study is registered in the China Clinical Trial Registration Center (Registration No. ChiCTR2100047025, http://www.chictr.org.cn/showproj.aspx?proj=127637). Moreover, written informed consent was obtained from each participant.
Inclusion and Exclusion Criteria
The inclusion criteria for study participants were as follows: aged between 17 and 35 years; no physical diseases during the recent routine health check.
The exclusion criteria were as follows: refractive measurement of ≤ -6.0 D or > +5.0 D; catarrhal inflammation in the recent seven days due to upper respiratory tract infections and conjunctivitis among others; eye surgery within the recent six months; febrile illnesses within the recent seven days; a history of mental illness, such as depression, autism, and schizophrenia, or recent psychic trauma; long duration of insomnia (>one month); smokers (>10 cigarettes per day) and drinkers (drink every day).
All volunteers were subjected to ophthalmic examinations between June 1st and September 30th, 2021. The examination included visual acuity, refraction, slit-lamp, and tear film breakup time to confirm the healthy states of their ocular surfaces. Fifty healthy students (30 females and 20 males) from the Hunan University of Chinese Medicine met the inclusion criteria and were finally recruited into this study.
Study design and sample collection
Tear Types and Identification
Essential balm composition was daubed on the bags beneath the eyes to stimulate the production of reflex tears. Negative-themed (sadness, loss, betrayal, torture, persecution, and discrimination) movies were selected to help produce tears of negative emotions (distressed, upset, guilty, scared, nervous, and afraid emotions) while positive-themed (happiness, love, friendship, faith, and loyalty) movies were selected to help produce tears of positive emotions (excited, strong, enthusiastic, inspired, and determined).
After watching several of the selected movies, the research team voted for the movies that could induce negative or positive emotional tears as follows.
Negative: (1) The Chinese medical documentary Life Matters (S2EP01, https://www.youtube.com/watch?v=knJ3t-GeUyc). It is a story documenting the end stage of children with cancer in a hospital. (2) The movie Manchester by the Sea.
Positive: (1) The movie The Pursuit of Happyness. (2) The movie Hachi: A Dog’s Tale (2009).
Essential balm-induced reflex tears were labeled the C samples (control). Emotional tears were induced by watching different movies. After emotional tears had been successfully induced and collected, participants were immediately required to finish the Positive and Negative Affect Schedule (PANAS-SF), which is a scale consisting of different words that describe feelings and emotions [11]. The participants described emotions according to the scale at that moment to shed tears. If the positive affect score of PANAS-SF ranged from 10 to 50, the tear sample was labeled M (positive, induced by moving scenes), or else the sample was classified as S (negative, induced by sad scenes) when the negative affect score ranged from 10 to 50. To avoid individual variations, the three types of tears were all from the same participants.
Collection of Tears
Each participant was requested to watch a movie and collect tear samples in a private room between 16:00 and 21:00. Sleeping time of the participants during the past 24 hours should have exceeded six hours. The use of contact lenses within the past 24 hours was also forbidden. Before the sample collection process, the face was washed to clean any makeup on the eyelids, eyelash, and face.
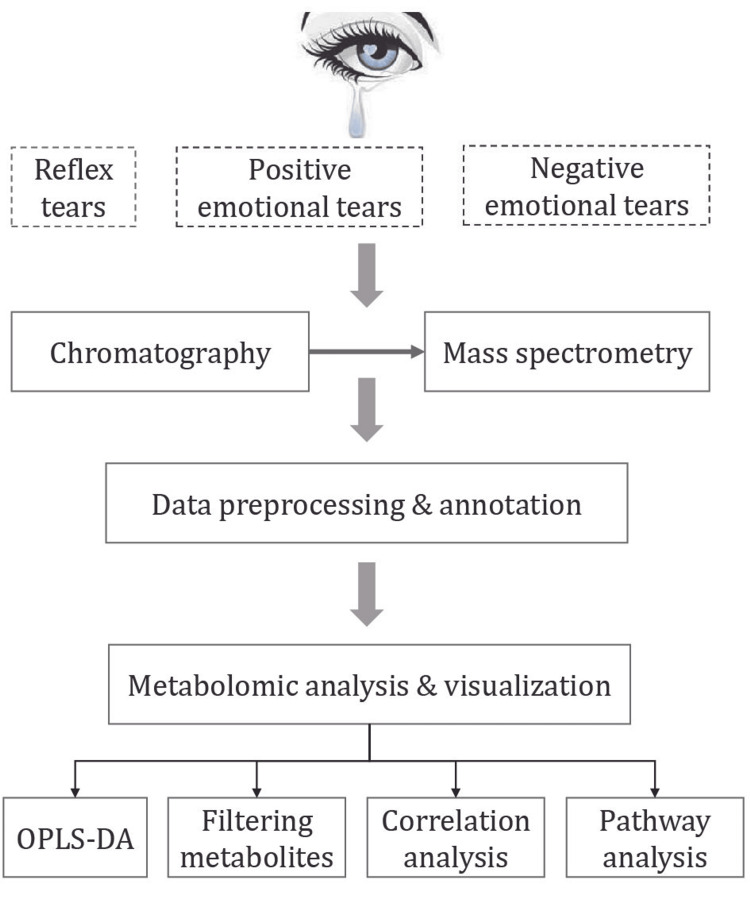
The Schirmer tear test strips were used for collecting tears from both eyes. The Schirmer strip was bent at the preformed notch at 90° and placed into a conjunctival sac of the participant at the junction of the mid and temporal thirds of the lower lid when the first drop of tears flowed out of the eyelid. The volume of a 35 mm Schirmer strip is 35 μL. The length of the moistened area was measured using the millimeter scale on the strip. Three Schirmer strips (wetting of more than 25 mm for each strip) collecting each type of tear from every participant were placed in a 2.0 mL sterile cryogenic vial, frozen, and immediately stored in a -80°C refrigerator for metabolomic analyses. The collecting interval between different tear types for each participant was more than 24 hours. Finally, different tear types (reflex, positive, and negative emotional tears) were obtained from the 12 participants. The workflow for this study was as shown in Figure 1.
Figure 1. Workflow of tears collection and analysis.
OPLS-DA: orthogonal partial least squares discriminant analysis.
Metabolomics analyses
Sample Preparation
Samples were taken out from the -80°C refrigerator and thawed on ice. The redundant part was cut off to keep each of the Schirmer strips at 24 mm length. Then, 70% methanol-water internal standard extractant (300 μL) was mixed in the corresponding Eppendorf tube with sample number, vortexed for five minutes, sonicated in an ice water bath for 10 minutes, and allowed to stand still at -20°C for 30 minutes. The mixture was centrifuged (12,000 rpm, 4°C) for 10 minutes, and 200 μL of the supernatant was transferred to a new centrifugal tube. The supernatant was centrifuged (12,000 rpm, 4°C) for three minutes and 150 μL of the supernatant was subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses. Moreover, 20 μL of each tear sample from each group was pipetted into a centrifuge tube to prepare a quality control (QC) sample for LC-MS/MS analysis. The researchers who performed the metabolomics analyses were blinded to the types of samples.
Chromatography
Chromatographic separation was performed using a Shimadzu LC-20AD UPLC system (Shimadzu Scientific Instruments, Kyoto, Japan) equipped with a Waters ACQUITY UPLC HSS T3 C18 column (1.8 µm, 2.1 mm * 100 mm; Waters Corporation, New Delhi, India). The column temperature was set at 40°C while the flow rate was 0.40 mL/min. The gradient system consisted of 0.1% formic acid in ultrapure Water in mobile phase A and 0.1% formic acid in acetonitrile in mobile phase B (0 minutes, 5% B; 11 minutes, 90% B; 12 minutes, 90% B; 12.1 minutes, 5% B; 14 minutes, 5% B).
Mass Spectrometry
Tandem mass spectrometry (MS/MS) was performed using an AB Sciex TripleTOF® 6600 quadrupole time-of-flight (QTOF) mass analyzer (AB Sciex LLC, Framingham, MA). Mass calibration was conducted daily, as instructed by the manufacturer. An electrospray ionization (ESI) source was used for mass spectrometric detection in positive and negative ionization modes. The QC samples were used for MS/MS data acquisition and source parameters are as shown in Table S1.
Data analysis
Data Preprocessing and Annotation
The original data file obtained through LC-MS analysis was first converted into the mzML format using the ProteoWizard (Palo Alto, CA) software. Peak extraction, alignment, and retention time correction were performed using the XCMS program. Then, the support vector regression (SVR) method was used to correct the peak area [12]. Peaks with deletion rates > 50% were filtered in samples of each group. Then, the metabolic identification information was obtained by searching the self-built database of the lab, public databases (Metlin and Human Metabolome Database (HMDB)), as well as MetDNA.
Statistical Analysis and Visualization
Principal component analysis (PCA) was used to reduce the dimensionality of the multidimensional dataset. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to establish metabolite differences among the tear types (Q2 > 0.50). A validation plot was used to assess the validity of the OPLS-DA model by comparing 200 random permutations of the Y variable and goodness of fit (R2Y and Q2) [13]. The corresponding variable importance in the projection (VIP) values were calculated using the OPLS-DA model and the VIP value > 1 indicated significant differences. The T-test (p < 0.05) and fold change (FC; FC ≥ 2 or FC ≤ 0.5) were used to screen the differentially expressed metabolites. Pearson correlation and cluster analyses were conducted to determine the correlations between the differentially expressed metabolites. Further, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed to identify the pathways that were associated with emotional tears. Statistical analyses and visualization were performed using the R software (version 4.1, The R Foundation, Vienna, Austria).
Results
Demographic information
Thirty-six tear samples (12 samples for each tear type, i.e., reflex, positive, and negative emotion) were obtained from the 12 participants (11 females and one male). Ophthalmic examinations of the participants did not reveal abnormal outcomes (Table S2).
Metabolomic analysis
QC Samples Analysis
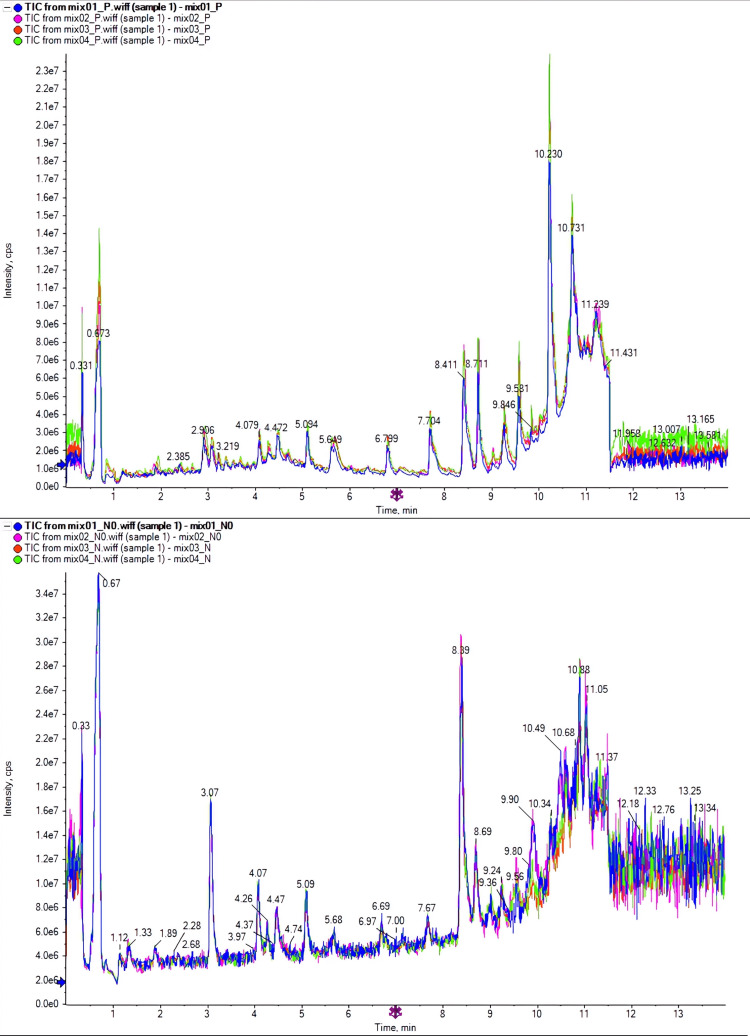
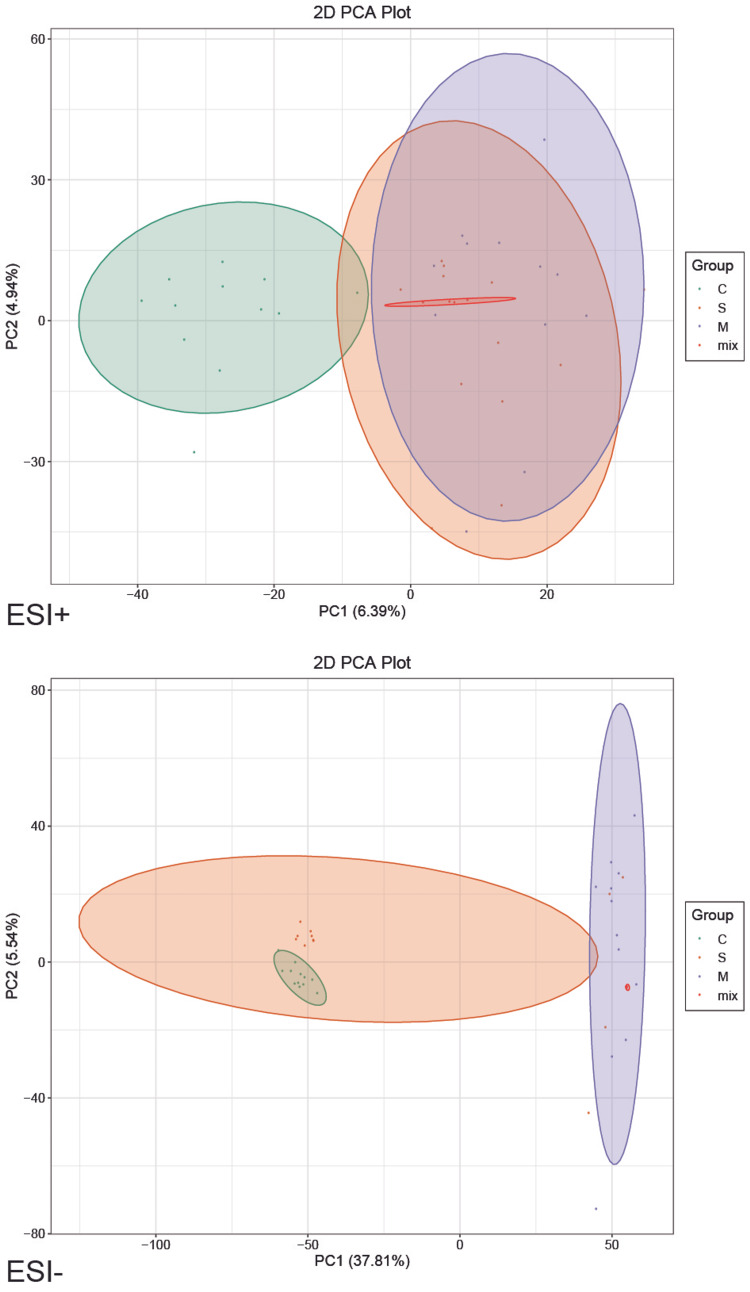
In ESI+ and ESI- modes, the total ion chromatogram (TIC) of tears in QC samples was as shown in Figure S1. The overlap of the TIC curves was high, exhibiting good stability of mass spectrometry (MS) for the same sample at different times. The PCA plot for all samples revealed that the QC samples were tightly clustered (Figure S2), indicating good analytical reproducibility of the current metabolomics study.
Metabolic Profiles of Different Tears Types
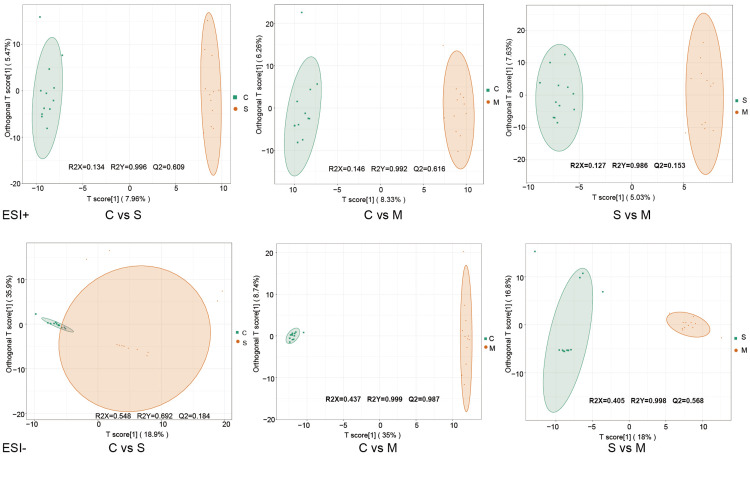
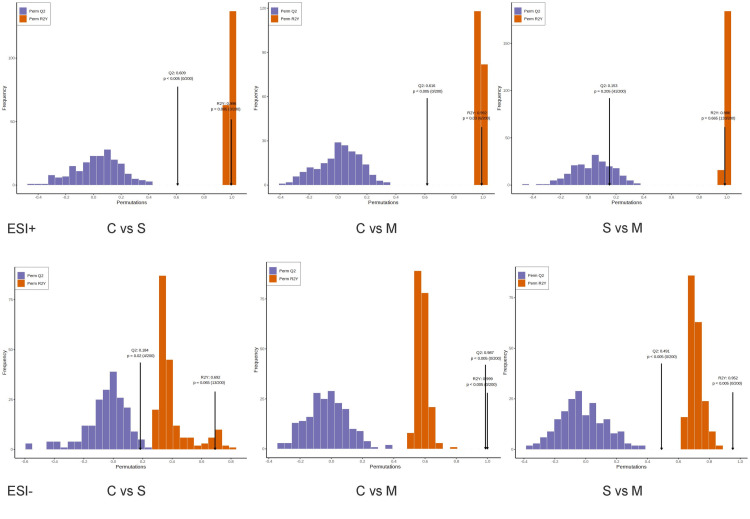
The OPLS-DA plot (Figure 2) revealed a remarkable separation between reflex (C), negative emotion (S), and positive emotion (M) tears. Specifically, the OPLS-DA score between C and S resulted in an R2Y = 0.996 and Q2 = 0.609 for ESI+ mode. The OPLS-DA score between C and M revealed R2Y = 0.992 and Q2 = 0.616 for the ESI+ mode, as well as R2Y = 0.999 and Q2 = 0.987 for the ESI- mode. The OPLS-DA score between S and M resulted in an R2Y = 0.952 and Q2 = 0.568 for ESI- mode. Moreover, validation analysis as presented in Figure S3 supports the reliability and good fitting of the OPLS-DA model in C vs. S and C vs. M of the ESI+ mode, as well as C vs. M and S vs. M of the ESI- mode, because the p-values for permuted R2Y and Q2 were all under 0.05.
Figure 2. OPLS-DA score plots of multivariate statistical analysis between reflex tears, negative emotional tears, and positive emotional tears.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; OPLS-DA: orthogonal partial least squares discriminant analysis; ESI: electrospray ionization.
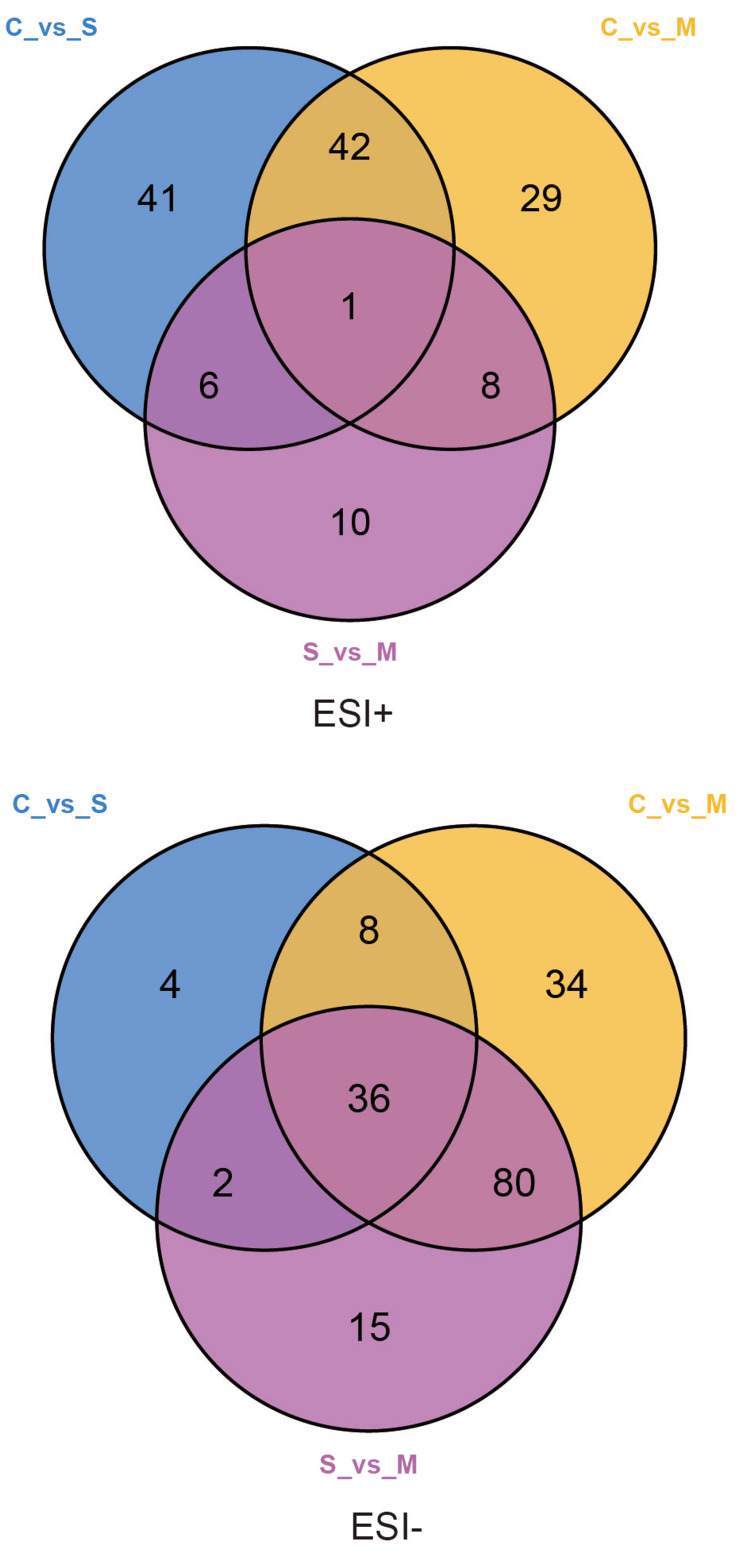
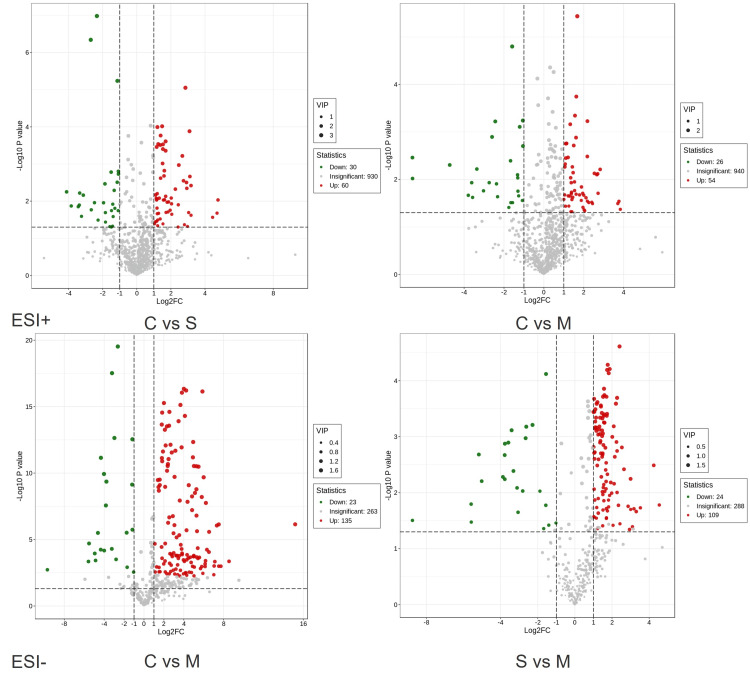
The criteria for screening the differentially expressed metabolites were VIP value > 1, p-value < 0.05, and FC ≥ 2 or FC ≤ 0.5. There were 90 and 80 differentially expressed metabolites in C vs. S and C vs. M of the ESI+ mode, respectively. The top five differentially expressed metabolites (based on VIP value) between C and S were γ-dodecalactone, 24-epibrassinolide, PA(18:3(6Z,9Z,12Z)/16:0), 12-ketodeoxycholic acid, and 2-(2,6-dimethoxy-4-prop-2-enylphenoxy)-1-(3,4,5-trimethoxyphenyl) propan-1-ol. The top five differentially expressed metabolites between C and M were 2-phenylacetamide, (S)-cotinine N-oxide, γ-dodecalactone, lauric acid, and brinzolamide. Moreover, 158 and 133 differentially expressed metabolites were identified between C vs. M and S vs. M of ESI- modes, respectively. The top five differentially expressed metabolites between C and M of the ESI- mode were alprazolam, dethiobiotin, (-)-threo-Iso(homo)2-citrate, 1,3-diacetoxy-4,6,12-tetradecatriene-8,10-diyne, and maculine. The top five differentially expressed metabolites between S and M were 1,3-diacetoxy-4,6,12-tetradecatriene-8,10-diyne, indole-3-acetic acid, free fatty acids (FFA) (18:0), nopaline, and N6-acetyl-L-lysine. These metabolites are displayed in a volcano (Figure S4) and Venn (Figure 3) plots. A total of 133 significantly differentially expressed metabolites were identified between negative and positive emotional tears in the ESI- mode (Table S3).
Figure 3. Venn plots of screened metabolites between reflex tears, negative emotional tears, and positive emotional tears.
ESI: electrospray ionization.
Correlation Analysis of Differentially Expressed Metabolites
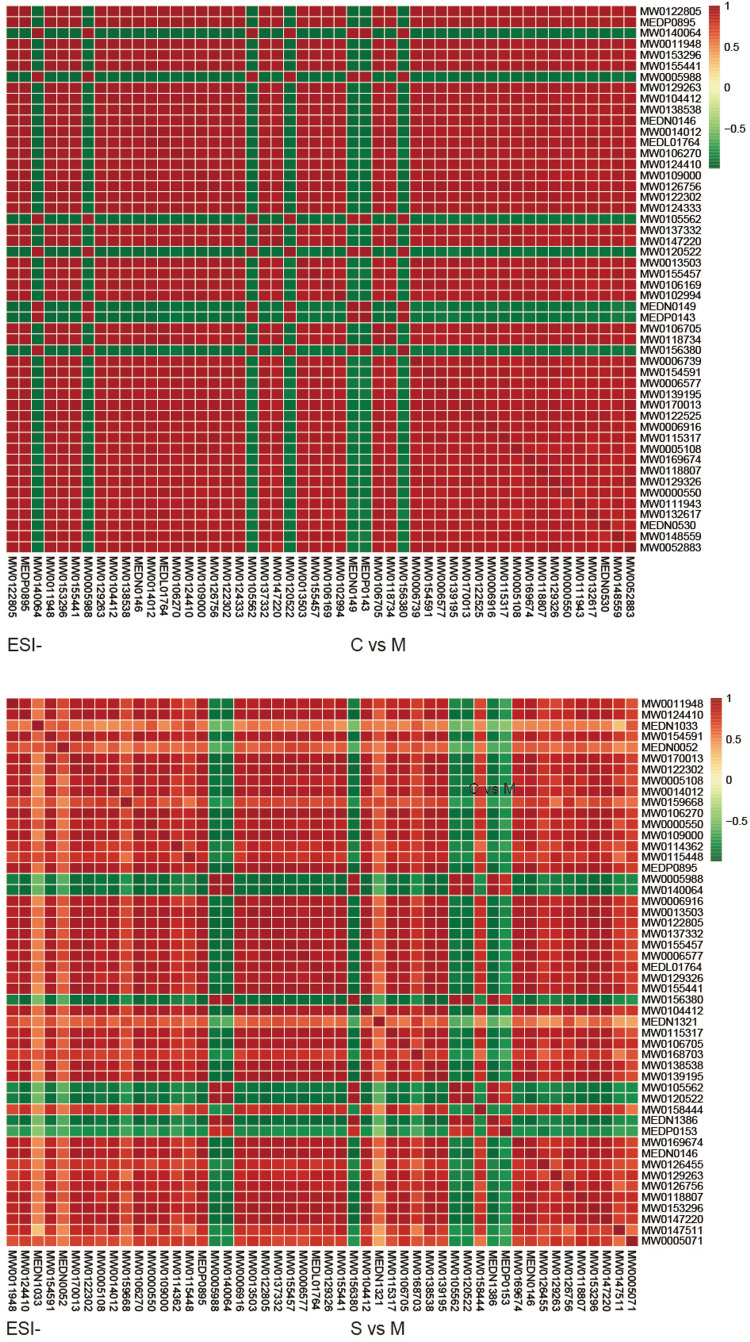
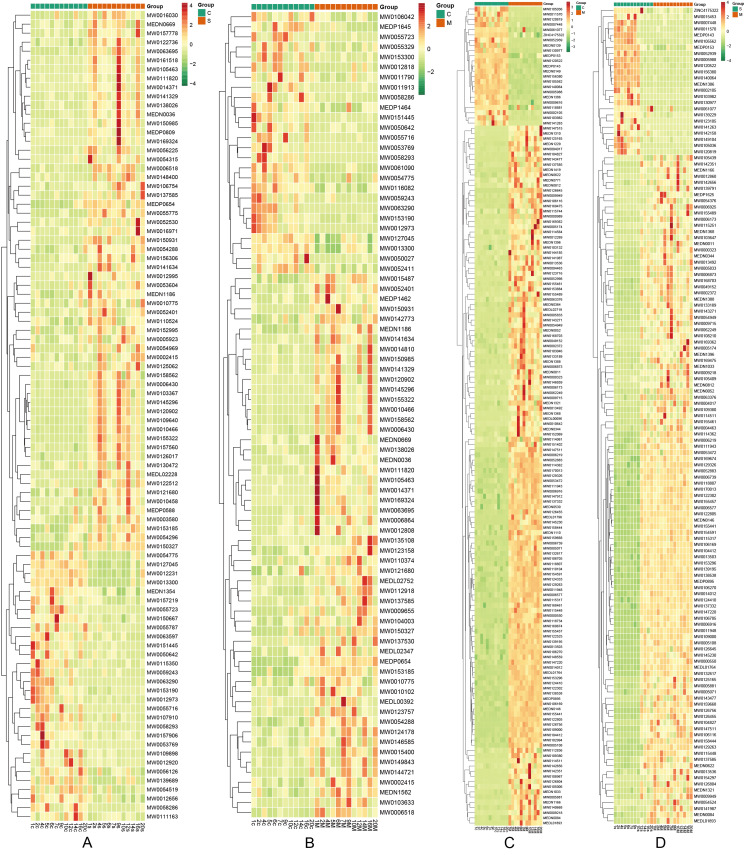
The relationships between the differentially expressed metabolites and the different classifications of tears were assessed via cluster and Pearson correlation analyses. Heatmaps of cluster analysis show relative intensity distributions and relationships of the metabolites. Metabolites in different groups were divided using color. The downregulated and upregulated metabolites are closely clustered (Figure S5). Pearson correlation analysis was also performed to assess the relations among the top 50 differentially expressed metabolites (VIP value) in each mode. Heatmaps of correlations showed that metabolites in C vs. M and S vs. M of the ESI- mode were highly correlated (Figure 4).
Figure 4. Correlation plots of the top 50 differentially expressed metabolites between reflex tears, negative emotional tears, and positive emotional tears.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; ESI: electrospray ionization.
The Enriched Metabolic Pathways
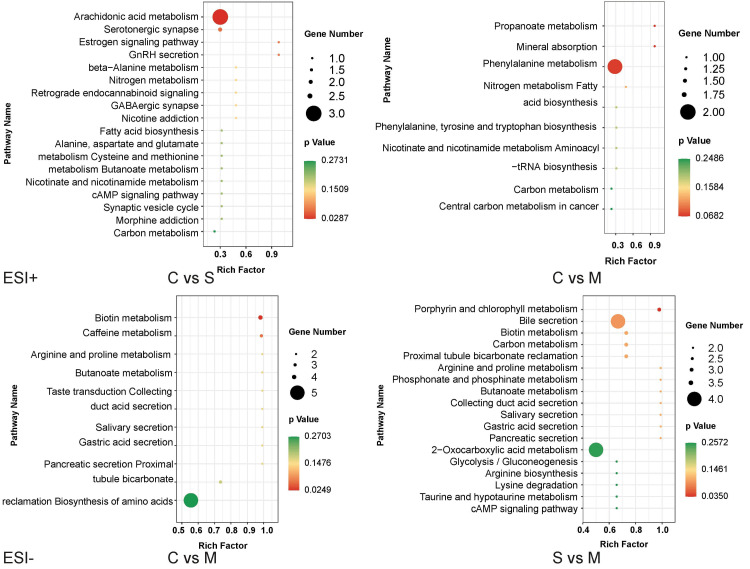
The ClusterProfiler package was used to analyze the relationships between the differentially expressed metabolites after which all the annotated metabolites were mapped into biochemical pathways for mechanistic interpretations. The KEGG enrichment scatter plot was used to show the significantly related pathways (p < 0.30 and Rich factor > 0.20). The most enriched pathways in C vs. S (Figure 5) were arachidonic acid metabolism, serotonergic synapse, estrogen signaling pathway, gonadotropin-releasing hormone (GnRH) secretion, and beta-alanine secretion and metabolism. The most enriched pathways in C vs. M (Figure 5) were propanoate metabolism, mineral absorption, phenylalanine metabolism, and nitrogen metabolism of the ESI+ mode, as well as biotin metabolism and caffeine metabolism of the ESI- mode. The most enriched pathways of S vs. M (Figure 5) were porphyrin & chlorophyll metabolism, bile secretion, biotin metabolism, arginine & proline metabolism, as well as phosphonate & phosphinate metabolism.
Figure 5. KEGG enrichment scatter plots of the identified differentially expressed metabolites between reflex tears, negative emotional tears, and positive emotional tears.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; KEGG: Kyoto Encyclopedia of Genes and Genomes; ESI: electrospray ionization; GnRH: gonadotropin-releasing hormone.
Re-evaluation of Screened Metabolites
The second-level MS spectra for the top five differentially expressed metabolites between the groups were re-evaluated since some of the predicted metabolites were not endogenous from human beings. Then, we predicted other substances using different methods as references for future work (Table 1).
Table 1. Re-evaluation of the screened metabolites.
Endo: endogenous human metabolite; RT: retention time; Score: prediction score from second-level mass spectrometry spectrums.
| ID | Group | Formula | RT | Name | Score | Endo |
| MW0010466 | C vs. S + | C23H30O7 | 614.976 | 2-(2,6-dimethoxy-4-prop-2-enylphenoxy)-1-(3,4,5-trimethoxyphenyl)propan-1-ol | 0.6549 | No |
| C23H29F3O6 | 614.976 | 5-trans fluprostenol | 0.6573 | Yes | ||
| C17H24N4O3 | 614.976 | Lys-Trp-OH | 0.6067 | Yes | ||
| MW0127045 | C vs. S + | C12H22O2 | 11.1875 | γ-dodecalactone | 0.6256 | No |
| C12H22O2 | 11.1875 | δ-dodecalactone | 0.6061 | No | ||
| C12H22O2 | 11.1875 | cis-5-dodecenoic acid | 0.5820 | Yes | ||
| MW0153296 | C vs. M - | C13H9NO4 | 3.1094 | Maculine | 0.5779 | No |
| C3H7NO2S | 3.1094 | D-cysteine | 0.5405 | Yes | ||
| MW0122805 | C vs. M - | C17H13ClN4 | 5.1059 | Alprazolam | 0.7589 | No |
| C15H16O7 | 5.1059 | 4-[3-(3,4-dihydroxyphenyl)-2-hydroxypropyl]benzene-1,2,3,5-tetrol | 0.7258 | Yes |
Discussion
The untargeted metabolomics analysis based on the LC-MS/MS technique in the present study showed that different tears types revealed distinct metabolomic profiles. Visual separation was explicit from OPLS-DA score plots and Q2 values in C vs. S and C vs. M of ESI+ mode, as well as C vs. M and S vs. M of ESI- mode are all greater than 0.50, which is acceptable for classification in human metabolomic studies [14,15]. Specifically, 133 differentially expressed metabolites were identified between positive and negative emotional tears in ESI- mode. Correlation analysis revealed significant positive or negative correlations between the top 50 differentially expressed metabolites based on the VIP score. Moreover, some metabolites, such as indole-3-acetic acid (MW0124410) and free fatty acids 18:0 (FFA 18:0, MEDN1033) were only up-regulated in positive emotional tears while some metabolites were up-regulated in both positive and negative emotional tears. Various metabolites, such as 1,3-diacetoxy-4,6,12-tetradecatriene-8,10-diyne (MW0011948) and nopaline (MW0154591), were abundant in positive emotional tears. Some metabolites, such as (-) threo-iso(homo)2citrate (MW0140064) and aloe-emodin (MW0005988), were down-regulated in positive emotional tears. These results indicate that metabolite abundances significantly differed between positive and negative emotional tears. Therefore, the filtered metabolites are potential biomarkers for the detection of real emotions in humans.
Tears are made up of water, electrolytes, proteins, lipids, and mucins that form layers on the surface of the eyes [16]. The compositions vary significantly in different tear types (basal, reflex, and emotional). Humans are the only mammals known to produce tears in response to emotional states, such as joy or grief. Secretion of emotional tears may also serve a biological function by excreting stress-inducing hormones built up through times of emotional distress [17] and a form of social signaling, such as eliciting help and support from those around you. Emotional tears contain higher protein content. Therefore, they are more viscous, sticky to the skin, and take longer to roll down the face [18]. On the contrary, reflex tears are more dilute to help wash out any irritants to your eyes from foreign particles or vapors. They contain more antimicrobial compounds, such as lysozyme and defensin peptides, to prevent infections [19]. We found that the levels of some anti-bacterial metabolites, such as 24-epibrassinolide [20], were higher in reflex tears than in the other tear types. Studies on tears are still at the preliminary stage, and a lot of unknown components are yet to be identified. Even though we have proved that metabolites from different emotional tears are not identical, there is a need to confirm the biomarkers for diagnosing different emotions from tears.
The KEGG enrichment analysis of C vs. S revealed that secretion of negative emotional tears is associated with the regulation of some synapses in the brain, including serotonergic synapse and GABAergic synapse, and regulation of a series of endocrine hormones, such as the estrogen signaling pathway and GnRH secretion. Importantly, it also involves arachidonic acid metabolism, which plays a key role in inflammation [21,22]. Therefore, negative emotional crying may induce fluctuations in sex hormones and inflammatory activities.
On the other hand, KEGG enrichment analysis of C vs. M revealed that shedding of positive emotional tears is closely associated with biotin and caffeine metabolism. Brain areas demanding higher levels of biotin metabolism include the centers of auditory and visual activities [23], implying that biotin plays a pivotal role in activating carboxylases and the development of neurological diseases [24]. As the most commonly used stimulant drug for brain activities, caffeine improves vigilance and cognition [25], and the engendering positive emotional tears may be comparable to caffeine stimulation.
Positive emotional tears shedding is also associated with arginine and proline metabolism. The panel of metabolites associated with depression in animal models and patients has reported that levels of arginine, proline, taurine, glycine, and alanine are higher in depressed than in healthy individuals [26,27]. Microbial functions and metabolites, including proline, converging in glutamate/GABA metabolism are linked to depression. For instance, proline supplementation in mice exacerbated depression, along with microbial translocation [28]. Positive emotional tears production is also related to a series of external secretion pathways in the present study, such as salivary secretion, gastric acid secretion, and pancreatic secretion. Brain electrophysiological activities and salivary secretion can improve negative emotions and reduce amino acid (arginine, proline, histidine, and taurine) concentrations in saliva [25]. In summary, the secretion of either positive or negative emotional tears involves different biological activities.
Psycho-emotional weeping demonstrates mental responses to the environment and elicits sympathy as well as social support from observers [29,30]. Fake tears or “crocodile tears” are an insincere tearing display that can sometimes be used to manipulate and deceive; for instance, a disguise to get off charges in court [31]. They originate from ancient Greeks who had an anecdote in which crocodiles would pretend to weep while luring their prey in. “Crocodile tears” are typically related to simulated tears of celebrities and politicians, and conveying fabricated remorse during criminal court proceedings [31,32]. Detection of emotional deception is crucial in the social credit system, such as in commercial negotiations, charitable causes, and court trials. This study provides a convenient and promising way to expose emotional deception via chemical approaches.
The main limitation of this study is the small sample size. Although 50 participants were recruited, only 12 (24%) successfully completed the emotional tear collection process, probably because an adult person crying in front of a collector is rather embarrassing and difficult. Thus, because of the limited sample size, the diagnostic performance of the metabolites cannot be evaluated by the receiver operating characteristic (ROC) curve and area under the curve (AUC). However, the results are satisfactory because, in contrast to other metabolomics studies, tear samples of different types in this study were from the same 12 subjects, without individual variations. Another limitation was confounding factors, such as gender differences with regard to crying, whereby women are definitely better at it than men; thus, only one man (1/20) finished the emotional tears collection process. Furthermore, there are no tear metabolite databases, and the identification of metabolites as well as KEGG enrichment in this study was based on traditional algorithms and metabolite databases of human blood and urine. Therefore, the results of metabolite detection and metabolic pathways analysis in this study are inconsistent with reality. A tear omics database should be established for biomarker exploration and enrichment analyses.
Conclusions
We analyzed the metabolites in 36 tears samples from 12 participants to investigate the metabolic characteristics between reflex, positive, and negative emotional tears using the non-targeted LC-MS/MS metabolomics approach. There were marked variations in metabolites among the three tear types, suggesting that tear metabolomics has great potential for human emotional detection. Moreover, the secretion of positive and negative emotional tears is associated with different biological activities, including biotin metabolism, arginine, and proline metabolism, among others. Therefore, our findings form a basis for the development of chemical methods for detecting human emotions, and for the diagnosis of mental diseases and the identification of fake tears.
Acknowledgments
Hao Liang and Songye Wu contributed equally to this work. Raw and processed datasets of the mass spectrum generated during and/or analyzed during the current study are available in the China National GeneBank DataBase (CNGBdb) Repository (https://db.cngb.org/search/metabolize/METM0000028/). Furthermore, the original data for identified metabolites information and analysis of this work can be downloaded from https://github.com/hao203/tears. This research study was supported by grants from the China Postdoctoral Science Foundation (No. 2020M682578), the Science and Technology Innovation Program of Hunan Province (No. 2022RC1021), the Guidance Project of Liu Liang Academician Workstation (No. 21YS002), and Hunan Province Degree and Graduate Teaching Reform Research Project (No. 2021JGYB117). We wish to thank the staff of Genecreate Biological Engineering Co., Ltd. for the technical assistance in the MS analysis of the samples in the present study. We are also grateful to the participants in the tears collection for their invaluable contribution. This manuscript is available as a preprint on medRxiv (https://doi.org/10.1101/2022.01.28.22270049). Authorship Credit: H.L. conceived and designed the experiments, and drafted the article. S.W. collected the samples and data, performed the data analysis, and drew the figures. D.Y. participated in the design of the study and helped to draft the manuscript. J.H. performed the LC-MS/MS analysis. X.Y. participated in the sample collection and review & editing of the manuscript. J.G. reviewed and validated the design and results of the psychological part. Z.Q. reviewed and validated the LC-MS/MS analysis. L.T. participated in data validation, review & editing of the manuscript, and supervision of the study. Q.P. took the lead in planning the study, the interpretation of the results, and project administration. The authors thank Lunhui Duan, Liu Yang, Qingwen Xu, Rujia Huang, and Meiheriayi Maimaitituersun for sample collection and data curation.
Appendices
The supplementary figures and tables can be downloaded from https://doi.org/10.6084/m9.figshare.21408768.
Figure S1
Figure 6. Total ion chromatogram (TIC) of tears in the quality control (QC) samples.
Figure S2
Figure 7. Principal component analysis (PCA) of whole samples.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; ESI: electrospray ionization.
Figure S3
Figure 8. Validation analysis by comparing the goodness-of-fit (R2 and Q2) of OPLS-DA models with the goodness-of-fit of 200 Y-permutated models.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; OPLS-DA: orthogonal partial least squares discriminant analysis; ESI: electrospray ionization.
Figure S4
Figure 9. Volcano plots of differentially expressed metabolites.
C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; ESI: electrospray ionization.
Figure S5
Figure 10. Heatmaps of cluster analysis of the overall differentially expressed metabolites.
A: ESI+ C vs. S; B: ESI+ C vs. M; C: ESI- C vs. M; D: ESI- S vs. M; C Group: reflex tears; S Group: negative emotional tears; M Group: positive emotional tears; ESI: electrospray ionization.
Table S1
Table 2. The source MS/MS parameters.
MS/MS: tandem mass spectrometry.
| Parameter | ESI+ | ESI- |
| IonSpray Voltage | 5,500 | -4,500 |
| Ion source gas 1 | 50 | 50 |
| Declustering potential (DP) | 60 | -60 |
| Collision energy spread | 15 | 15 |
| Ion release width | 15 | 15 |
| Curtain gas | 35 | 35 |
| Temperature | 550 | 550 |
| Ion source gas 2 | 60 | 60 |
| Collision energy | 30 | -30 |
| Ion release delay | 30 | 30 |
Table S2
Table 3. Ocular surface examinations of participants.
BUT: tear break-up time; L: left eye; R: right eye.
| No. | Birth | Sex | BUT(L)-first | BUT(L)-avg. | BUT(R)-first | BUT(R)-avg. | Red-eye scan | Other |
| 1 | 2001.9 | Female | 18.16 | 20.29 | 20.86 | 20.69 | Negative | Eyestone |
| 2 | 2002.2 | Female | 5.61 | 12.02 | 19.30 | 22.14 | Negative | |
| 4 | 2002.6 | Female | 5.68 | 15.73 | 8.03 | 15.00 | Negative | |
| 5 | 2002.5 | Female | 20.27 | 22.94 | 20.33 | 22.35 | Negative | |
| 6 | 2001.3 | Female | 6.75 | 17.34 | 3.63 | 10.60 | Negative | Conjunctival pigmentation |
| 7 | 2002.5 | Female | 10.90 | 16.70 | 4.69 | 12.36 | Negative | |
| 9 | 2001.12 | Female | 5.86 | 11.30 | 8.98 | 17.75 | Negative | |
| 10 | 2002.7 | Female | 22.94 | 23.40 | 7.07 | 13.13 | Negative | |
| 12 | 1986.12 | Male | 5.42 | 12.17 | 6.31 | 15.66 | Negative | |
| 14 | 1989.6 | Female | 7.91 | 10.23 | 11.28 | 14.53 | Negative | |
| 16 | 2000.7 | Female | 19.18 | 19.66 | 10.32 | 19.37 | Negative | |
| 20 | 1993.9 | Female | 7.33 | 12.71 | 19.69 | 21.35 | Negative |
Table S3
Table 4. Significantly differentially expressed metabolites (top 10) of ESI- mode between negative and positive emotional tears.
ESI: electrospray ionization; FFA: free fatty acids
| Index | Compounds | Formula | Precursor (g/mol) | Mass (g/mol) | Score | RT (min) | MS_level | CAS_ID | HMDB_ID | cpd_ID | Metlin_ID | Pubchem.CID | Class.I | Class.II | Source | 1s | 2s | 4s | 5s | 6s | 7s | 9s | 10s | 12s | 14s | 16s | 20s | 1M | 2M | 4M | 5M | 6M | 7M | 9M | 10M | 12M | 14M | 16M | 20M | VIP | p-value | Fold_Change | Log2FC | Type |
| MW0011948 | 1,3-diacetoxy-4,6,12-tetradecatriene-8,10-diyne | C18H20O4 | 335.1034 | 300.1362 | 0.5077 | 4.0852 | MS2-Insilico | 29576-66-7 | HMDB0030922 | -- | -- | 131751099 | Lipids and lipid-like molecules | Fatty acyls | rp | 4078.7 | 3781.91 | 4743.45 | 3366.38 | 3676.65 | 3323.08 | 3080.38 | 3.36 | 30517.07 | 28279.7 | 3319.95 | 26017.29 | 22900.88 | 27070.26 | 27122.4 | 22703.63 | 26853.0 | 29263.75 | 24935.77 | 27783.71 | 30617.02 | 29340.11 | 30117.96 | 28707.28 | 1.9083621044205163 | 0.00019274884831816985 | 2.8673415716828887 | 1.5197137756703256 | up |
| MW0124410 | Indole-3-acetic acid | C10H9NO2 | 174.055 | 175.0633 | 0.6363 | 4.0853 | MS2-Search-DB | 87-51-4 | HMDB0000197 | C00954 | -- | 802 | Organoheterocyclic compounds | Indoles and derivatives | rp | 42326.27 | 36927.66 | 20.9 | 45723.61 | 8.67 | 37404.05 | 38556.47 | 51951.01 | 143231.71 | 130676.78 | 122531.05 | 161411.07 | 148706.98 | 146886.6 | 144230.73 | 145499.84 | 177103.72 | 134494.27 | 156837.65 | 143132.48 | 169676.01 | 156432.96 | 150456.97 | 154431.26 | 1.8026757658684305 | 0.0002575232676103954 | 2.254512575557102 | 1.172815557277837 | up |
| MEDN1033 | FFA (18:0) | C18H36O2 | 283.2563 | 284.2715 | 0.8577 | 11.4088 | MS2-Search-Local | 57-11-4 | HMDB0000827 | C01530 | -- | -- | FA | FFA | rp | 75470.79 | 94787.27 | 71648.32 | 60859.92 | 68215.24 | 19.21 | 124063.32 | 83906.84 | 56310.7 | 189641.99 | 86508.19 | 66825.87 | 103876.87 | 321012.73 | 556786.25 | 257220.01 | 527847.98 | 393178.25 | 497127.27 | 421123.94 | 6950.69 | 427442.09 | 715732.81 | 300150.61 | 1.7967367095449402 | 0.0002568037808020118 | 4.629096898663692 | 2.2107307621887657 | up |
| MW0154591 | Nopaline; D-nopaline; N2-(D-1,3-dicarboxypropyl)-L-arginine; N-[(1S)-4-carbamimidamido-1-carboxybutyl]-D-glutamic acid | C11H20N4O6 | 341.0919 | 304.1383 | 0.6495 | 6.02 | MS2-MetDNA | 22350-70-5 | -- | C01682 | -- | 108012 | Organic acids and derivatives | Carboxylic acids and derivatives | rp | 514.51 | 6.19 | 9.28 | 8.89 | 6.96 | 722.66 | 1004.85 | 1895.76 | 7977.08 | 6715.72 | 5904.04 | 8301.61 | 5285.09 | 6279.56 | 8355.69 | 6967.58 | 8055.53 | 10815.43 | 8912.82 | 7807.61 | 9338.57 | 6819.43 | 10186.63 | 9318.21 | 1.783167004139699 | 0.0001389048904649927 | 2.9679292841471474 | 1.5694567177587069 | up |
| MEDN0052 | N6-acetyl-L-lysine | C8H16N2O3 | 223.0941 | 188.116 | 0.5834 | 6.26 | MS2-MetDNA | 692-04-6 | HMDB0000206 | C02727 | -- | 92832 | Amino acid and its metabolomics | Amino acid derivatives | rp | 370.15 | 415.44 | 13.34 | 503.75 | 358.88 | 519.94 | 538.95 | 383.32 | 3924.21 | 147.34 | 72.21 | 4119.9 | 532.95 | 6409.84 | 5273.44 | 4606.9 | 5452.68 | 8780.54 | 4596.74 | 4694.33 | 4181.44 | 4824.13 | 5401.36 | 196.33 | 1.7758084775539775 | 0.00020214900681613798 | 4.834046042069317 | 2.27323121417688 | up |
| MW0170013 | Vulpinic acid | C19H14O5 | 321.0636 | 322.0841 | 0.5953 | 5.1052 | MS2-Search-DB | 521-52-8 | -- | -- | 44073 | 54690323 | -- | -- | rp | 11743.48 | 14.05 | 9841.35 | 10165.96 | 10337.24 | 11344.66 | 12214.86 | 9116.26 | 49591.63 | 47083.03 | 42764.51 | 54355.07 | 47200.12 | 40685.86 | 42500.84 | 55636.01 | 59274.58 | 64657.99 | 55603.51 | 54153.84 | 60810.3 | 50371.66 | 48219.09 | 44471.63 | 1.7725980410170212 | 0.00024157323803183721 | 2.321854838979924 | 1.2152777785643052 | up |
| MW0122302 | 6-Quinoxalinecarbonitrile, 1,2,3,4-tetrahydro-7-nitro-2,3-dioxo- | C9H4N4O4 | 266.9898 | 232.0233 | 0.6222 | 5.6002 | MS2-Search-DB | 115066-14-3 | -- | C13668 | -- | 3721046 | Organoheterocyclic compounds | Diazanaphthalenes | rp | 492.64 | 60.22 | 72.64 | 50.43 | 108.39 | 438.73 | 1330.73 | 13.93 | 6451.32 | 5268.16 | 6716.86 | 7020.77 | 4862.41 | 5738.94 | 6947.75 | 7693.81 | 7703.85 | 7783.95 | 6323.42 | 7800.87 | 8762.17 | 6843.76 | 6399.51 | 7521.55 | 1.771034976231105 | 0.00018132456196628678 | 3.0109734870732447 | 1.5902300045633035 | up |
| MW0005108 | 4-hydroxymandelonitrile | C8H7NO2 | 165.0716 | 149.0477 | 0.5356 | 4.2807 | MS2-Search-DB | - | -- | C00650 | -- | 166768 | Benzenoids | Phenols | rp | 65.88 | 3.15 | 5.44 | 3.15 | 155.96 | 145.45 | 310.98 | 354.21 | 2766.3 | 3718.76 | 1928.86 | 2186.74 | 3941.9 | 4244.89 | 3094.09 | 3320.11 | 2908.38 | 2933.55 | 3590.01 | 2587.11 | 3596.83 | 2226.74 | 2264.56 | 4114.48 | 1.7678814114042791 | 6.405247396614681e-05 | 3.3338814998522963 | 1.7372028257959218 | up |
| MW0014012 | 3-dehydroepiandrosterone sulfate | C19H28O5S | 404.1346 | 368.1657 | 0.8469 | 7.6549 | MS2-MetDNA | 651-48-9 | -- | C04555 | 34483 | 12594 | Lipids and lipid-like molecules | Steroids and steroid derivatives | rp | 3602.97 | 18.9 | 10.76 | 9.13 | 3673.89 | 4286.74 | 9.13 | 4396.03 | 21632.45 | 17621.07 | 17730.13 | 30444.26 | 26195.71 | 25981.82 | 26430.57 | 21314.63 | 22765.09 | 22928.19 | 18122.94 | 22374.94 | 25218.89 | 26664.72 | 19845.72 | 25064.3 | 1.7666660885883936 | 0.00037596016108426203 | 2.7351115371846366 | 1.4515996669710634 | up |
| MW0159668 | HCO3- | HCO3 | 59.9856 | 60.9926 | 0.6009 | 9.7594 | MS2-Insilico | 71-52-3 | HMDB00595 | C00288 | -- | -- | -- | -- | rp | 257.89 | 193.64 | 121.03 | 174.49 | 309.28 | 241.87 | 243.29 | 44.65 | 146704.54 | 28789.54 | 67189.61 | 8552.42 | 163433.31 | 118119.98 | 50637.72 | 128718.1 | 79778.86 | 98425.22 | 125205.04 | 133063.01 | 45336.17 | 100981.3 | 131118.66 | 164174.77 | 1.765757024695546 | 2.438918855627368e-05 | 5.29617998415883 | 2.404952151193692 | up |
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethical Committee of Jili Hospital issued approval 2021-04. The protocol for this study was approved by the Ethical Committee of Jili Hospital and was conducted in accordance with the Declaration of Helsinki (No. 2021-04; date: May 27, 2021). The study is registered in the China Clinical Trial Registration Center (Registration No.: ChiCTR2100047025; http://www.chictr.org.cn/showproj.aspx?proj=127637). Moreover, written informed consent was obtained from each participant.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Origin and types of emotional tearing. Murube J, Murube L, Murube A. Eur J Ophthalmol. 1999;9:77–84. doi: 10.1177/112067219900900201. [DOI] [PubMed] [Google Scholar]
- 2.Basal, reflex, and psycho-emotional tears. Murube J. Ocul Surf. 2009;7:60–66. doi: 10.1016/s1542-0124(12)70296-3. [DOI] [PubMed] [Google Scholar]
- 3.The neurobiology of human crying. Bylsma LM, Gračanin A, Vingerhoets AJ. Clin Auton Res. 2019;29:63–73. doi: 10.1007/s10286-018-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natural selection and the origin and evolution of weeping in man. Montagu A. JAMA. 1960;174:392–397. doi: 10.1001/jama.1960.63030040004012. [DOI] [PubMed] [Google Scholar]
- 5.Why only humans shed emotional tears: Evolutionary and cultural perspectives. Gračanin A, Bylsma LM, Vingerhoets AJ. Hum Nat. 2018;29:104–133. doi: 10.1007/s12110-018-9312-8. [DOI] [PubMed] [Google Scholar]
- 6.Vingerhoets A. Oxford, UK: Oxford University Press; 2013. Why Only Humans Weep: Unravelling the Mysteries of Tears. [Google Scholar]
- 7.Threads for tear film collection and support in quantitative amino acid analysis. Avilov V, Zeng Q, Shippy SA. Anal Bioanal Chem. 2016;408:5309–5317. doi: 10.1007/s00216-016-9624-7. [DOI] [PubMed] [Google Scholar]
- 8.Emotional tears and NGF: a biographical appreciation and research beginning. Provine RR. Arch Ital Biol. 2011;149:269–274. [Google Scholar]
- 9.Chemosensory cues from the lacrimal and preputial glands stimulate production of IP3 in the vomeronasal organ and aggression in male mice. Thompson RN, Napier A, Wekesa KS. Physiol Behav. 2007;90:797–802. doi: 10.1016/j.physbeh.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Human tears contain a chemosignal. Gelstein S, Yeshurun Y, Rozenkrantz L, Shushan S, Frumin I, Roth Y, Sobel N. Science. 2011;331:226–230. doi: 10.1126/science.1198331. [DOI] [PubMed] [Google Scholar]
- 11.Development and validation of brief measures of positive and negative affect: the PANAS scales. Watson D, Clark LA, Tellegen A. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 12.pseudoQC: a regression-based simulation software for correction and normalization of complex metabolomics and proteomics datasets. Wang S, Yang H. Proteomics. 2019;19:0. doi: 10.1002/pmic.201900264. [DOI] [PubMed] [Google Scholar]
- 13.PLS/OPLS models in metabolomics: the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Triba MN, Le Moyec L, Amathieu R, et al. Mol Biosyst. 2015;11:13–19. doi: 10.1039/c4mb00414k. [DOI] [PubMed] [Google Scholar]
- 14.Assessment of PLSDA cross validation. Westerhuis JA, Hoefsloot HCJ, Smit S, et al. Metabolomics. 2008;4:81–89. [Google Scholar]
- 15.An untargeted metabolomics analysis of antipsychotic use in bipolar disorder. Burghardt KJ, Evans SJ, Wiese KM, Ellingrod VL. Clin Transl Sci. 2015;8:432–440. doi: 10.1111/cts.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Complexity of the tear film: importance in homeostasis and dysfunction during disease. Dartt DA, Willcox MD. Exp Eye Res. 2013;117:1–3. doi: 10.1016/j.exer.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tears: symbol of healing towards the individuation. Kyoung SE. JSST. 2015;6:59–75. [Google Scholar]
- 18.Viewpoint: why do we cry? Are tears 'purposeless'? Sidebotham C. Br J Gen Pract. 2020;70:179. doi: 10.3399/bjgp20X709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antimicrobial compounds in tears. McDermott AM. Exp Eye Res. 2013;117:53–61. doi: 10.1016/j.exer.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacterial infection and pre-treatment with 24-epibrassinolide markedly affect the heat emission and membrane permeability of rape cotyledons. Janeczko A, Tóbias I, Skoczowski A, Dubert F, Gullner G, Barna B. Thermochim Acta. 2007;458:88–91. [Google Scholar]
- 21.Diverse ways of perturbing the human arachidonic acid metabolic network to control inflammation. Meng H, Liu Y, Lai L. Acc Chem Res. 2015;48:2242–2250. doi: 10.1021/acs.accounts.5b00226. [DOI] [PubMed] [Google Scholar]
- 22.Arachidonic acid metabolites in cardiovascular and metabolic diseases. Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Int J Mol Sci. 2018;19:3285. doi: 10.3390/ijms19113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Localization of biotinidase in the brain: implications for its role in hearing loss in biotinidase deficiency. Heller AJ, Stanley C, Shaia WT, Sismanis A, Spencer RF, Wolf B. Hear Res. 2002;173:62–68. doi: 10.1016/s0378-5955(02)00609-3. [DOI] [PubMed] [Google Scholar]
- 24.Biotin in metabolism, gene expression, and human disease. León-Del-Río A. J Inherit Metab Dis. 2019;42:647–654. doi: 10.1002/jimd.12073. [DOI] [PubMed] [Google Scholar]
- 25.Does acute caffeine ingestion alter brain metabolism in young adults? Xu F, Liu P, Pekar JJ, Lu H. Neuroimage. 2015;110:39–47. doi: 10.1016/j.neuroimage.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Effect of coriander plants on human emotions, brain electrophysiology, and salivary secretion. Zhang W, Li Z, Wang L, Liu H, Liu H. Biology (Basel) 2021;10:1283. doi: 10.3390/biology10121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plasma metabonomics as a novel diagnostic approach for major depressive disorder. Zheng P, Gao HC, Li Q, et al. J Proteome Res. 2012;11:1741–1748. doi: 10.1021/pr2010082. [DOI] [PubMed] [Google Scholar]
- 28.Microbiota alterations in proline metabolism impact on depression through GABA and ECM homeostasis. [PREPRINT] Mayneris-Perxachs J, Arnoriaga-Rodrguez M, Martn M, et al. Research Square. 2021 doi: 10.1016/j.cmet.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 29.If I cry, do you care? Individual differences in empathy moderate the facilitation of caregiving words after exposure to crying faces. Lockwood P, Millings A, Hepper E, Rowe AC. J Individ Differ. 2013;34:41–47. [Google Scholar]
- 30.The social impact of emotional tears. Vingerhoets AJ, van de Ven N, van der Velden Y. Motiv Emot. 2016;40:455–463. doi: 10.1007/s11031-016-9543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A call for the empirical investigation of tear stimuli. Krivan SJ, Thomas NA. Front Psychol. 2020;11:52. doi: 10.3389/fpsyg.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crocodile tears: facial, verbal and body language behaviours associated with genuine and fabricated remorse. Ten Brinke L, Macdonald S, Porter S, O'Connor B. Law Hum Behav. 2012;36:51–59. doi: 10.1037/h0093950. [DOI] [PubMed] [Google Scholar]