Abstract
INTRODUCTION:
There is increased risk of several malignancies in inflammatory bowel disease (IBD). However, evidence regarding risk of cervical cancer in IBD is conflicting. We aimed to investigate the risk of cervical cancer in IBD by undertaking a systematic review and meta-analysis of unselected, population-based studies.
METHODS:
MEDLINE, EMBASE, and Cochrane Library were searched using Medical Subject Heading terms, and 2 reviewers independently screened results. Pooled hazard ratios (HRs) were calculated using random effects model meta-analysis for risk of cervical cancer in IBD. Subgroup meta-analysis was undertaken to assess risk of cervical cancer by IBD subtype (Crohn's disease and ulcerative colitis), treatment exposure, and grade of lesion.
RESULTS:
We screened 1,393 articles to identify 5 population-based studies, including 74,310 patients with IBD and 2,029,087 reference patients, across 5 different countries. Pooled random effects model meta-analysis of these studies did not show statistically significant increased risk for cervical cancer in IBD compared with reference populations (HR: 1.24; 95% confidence interval [CI]: 0.94–1.63). Meta-analysis by grade of lesion showed increased risk of low-grade cervical lesions (HR: 1.15; 95% CI: 1.04–1.28). Meta-analysis by disease subtype indicated no statistically significant increased risk in Crohn's disease (HR: 1.36; 95% CI: 0.83–2.23) or ulcerative colitis (HR: 0.95; 95% CI: 0.72–1.25) or in patients treated with antitumor necrosis factor (HR: 1.19; 95% CI: 0.64–2.21) or thiopurines (HR: 0.96; 95% CI: 0.60–1.50).
DISCUSSION:
This meta-analysis of high-quality, unselected population-based studies shows no statistically significant increased risk of cervical cancer in patients with IBD. There is, however, increased risk of low-grade cervical lesions compared with the general population.
INTRODUCTION
Crohn's disease (CD) and ulcerative colitis (UC) are associated with an increased risk of gastrointestinal and extraintestinal malignancies (1,2) due to chronic inflammation and exposure to long-term immunosuppressive treatments, which can predispose to abnormal cell proliferation (3,4). Immune dysregulation in the context of chronic infection can also promote persistence of oncogenic infectious agents, such as human papillomavirus (HPV) (3,5). The evidence for the increased risk of persistent HPV infection in inflammatory bowel disease (IBD) patients on immunosuppressive therapies is supported by that seen in other immunosuppressive states, including human immunodeficiency virus infection, and immunosuppression after organ transplantation (5–7). There is, however, conflicting evidence for risk of cervical cancer in patients with IBD (8–10).
Population screening for cervical cancer entails cervical Papanikolaou (Pap) smear tests for abnormal cytology and/or HPV colonization (11). An increased risk of cervical cancer in IBD due to predisposition to persistent HPV infection is assumed, with increased surveillance for HPV infection in patients with IBD and targeted efforts to increase HPV vaccination uptake in this patient group. The European Crohn's and Colitis guidelines recommend 2 smears within the first year of IBD diagnosis and annual screening thereafter (12). This is supported by recent literature, advising reduced interval screening in immunosuppressed, high-risk groups including patients with IBD, organ transplant recipients, and human immunodeficiency virus-positive patients (13–15). On the other hand, the British Society of Gastroenterology states that there is insufficient evidence to support increased screening for patients with IBD and instead encourage participation in routine screening with the general public (16).
A 2015 meta-analysis exploring the risk of cervical cancer in patients with IBD treated with immunosuppressive therapy found an increased risk in those treated with thiopurines (odds ratio [OR]: 1.34; 95% confidence interval [CI]: 1.23–1.46) (17). This article, however, only identified 8 studies of mixed methodologies and used highly selected study populations (only those with severe IBD expression) (18,19). In addition, although the authors included good quality studies and adjusted for confounders, only patients with immunosuppressant exposure were included in their meta-analysis. We have, therefore, undertaken a systematic review and meta-analysis of high-quality, population-based studies exploring the risk of cervical cancer in unselected IBD patient cohorts.
METHODS
Search strategy
In May 2021, a systematic search of the literature was performed on MEDLINE, EMBASE, and Cochrane Library. Along with subject headings, search terms were used as follows: (inflammatory bowel disease OR IBD OR Crohn's disease OR CD or Crohn* or ulcerative colitis OR UC) AND (cervical ca* OR (cervical or cervix) adj (cancer or dysplas*) OR cervical intraepithelial neoplasia OR CIN). See Table 1 (Supplementary Digital Content 1, http://links.lww.com/CTG/A847) for full search strategy. Reference lists of included articles were also manually searched for relevant articles.
Study eligibility
Studies comparing the relative risk of cervical cancer or dysplasia in patients with IBD with the general population were included. Eligible studies included patients diagnosed with IBD while living in a specific region who could be followed up for a specific time period and where the study population was representative of the general IBD population. Studies were excluded if they used selected populations from secondary and tertiary centers that are not population-representative or selected IBD patients directly from cervical screening databases, which do not account for patients who are not engaged with screening programs, for example, only patients receiving treatment for IBD. Outcomes could include relative or absolute risk of cervical cancer, dysplasia, or intraepithelial neoplasia in IBD populations compared with reference populations. Studies were excluded if the outcome was not clearly reported or if the sole outcome was HPV infection without abnormal cytology. Author names and institutions were cross-referenced, and where multiple studies used the same cohort, articles were excluded to avoid duplicated data with articles reporting the most recent data used.
Study selection and data extraction
Articles were screened independently by authors SM and RE, and any discrepancies were resolved by K.A. or T.J. See Figure 1 for a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart detailing the screening process.
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the article screening process.
We extracted data from all studies included in the final analysis, including primary outcome of interest, for example, relative risk estimates for cervical cancer in patients with IBD. Other data variables extracted include author, average patient age, Pap screening ratio, publication year, study start and end dates, follow-up time (in years, and where available, person-years), and number of patients with IBD. We also extracted data on treatment exposure, number of patients with CD and UC, number of cancer events by grade of lesion, number of cancer events in CD and UC, and relative risk estimates for cancer in CD and UC, compared with the reference population, where available.
Study quality was assessed using the Newcastle-Ottawa Scale with each study assigned a rating out of 9 (20) (Table 2). There was a maximum of 4 points allocated to cohort selection, assessing the representativeness of the IBD and non-IBD cohorts, ascertainment of IBD, and demonstration that cervical cancer was not present before IBD diagnosis. Up to 2 points were allocated for comparability of the cohorts, assessing whether controls were matched by age, region, and socioeconomic categories. Up to 3 points were allocated for the method of identifying cervical cancer cases and follow-up.
Table 2.
Newcastle-Ottawa assessment of study quality
| Study | Assessment of quality of a cohort study—Newcastle-Ottawa Scale domain | Total NOS score (max. 9) | |||||||||
| Selection | Comparability | Outcome | |||||||||
| 1. Representativeness of the exposed cohort | 2. Selection of the nonexposed cohort | 3. Ascertainment of exposure | 4. Demonstration that outcome of interest was not present at the start of the study | Total (max. 4) | Comparability of cohorts on the basis of the design or analysis (max. 2) | 1. Assessment of outcome | 2. Was follow-up long enough for outcomes to occur? | 3. Adequacy of follow-up of cohorts | Total (max. 3) | ||
| Bernstein et al. (24) | ★ (a) Patients from the University of Manitoba IBD Database | ★ (a) 10:1 matching with non-IBD patients from the Manitoba Health database | ★ (a) At least 5 separate physician contacts and/or hospitalizations for an IBD diagnosis | ★ (a) Exclusion of cancer diagnoses that occurred before IBD diagnosis | 4 | ★ (a) Study controls for age and sex ★ (b) Study controls for postal area of residence |
★ (a) ICD-9-CM codes used to identify diagnoses in the Manitoba Health database | ★ (a) 13 yr follow-up (1984–1997) | ★ (a) Complete follow-up | 3 | 9 |
| Goetgebuer et al. (25) | (b) Patients from a Dutch nationwide IBD biobank (PSI) with cervical screening history | ★ (a) 4:1 matching with non-IBD patients from a cervical screening registry (PALGA) | ★ (a) Confirmed IBD diagnosis according to the biobank registry | (b) No demonstration that cervical cancer was not present before IBD diagnosis | 2 | ★ (a) Study controls for age and sex ★ (b) Study controls for urbanization level |
★ (a) Coded diagnoses identified from national screening | ★ (a) Median follow-up of 13 yr | ★ (a) Complete follow-up | 7 | |
| Goldacre et al. (26) | (b) Patients from the Oxford Record Linkage Study (ORLS) with ICD codes for IBD or celiac disease | ★ (a) Reference cohort from ORLS coded under unrelated hospital admissions | ★ (a) Confirmed IBD diagnosis according to NHS statistics data | ★ (a) Exclusion of cancer diagnoses that occurred before IBD diagnosis | 4 | ★ (a) Study controls for age ★ (b) Study controls for urbanization level |
★ (a) Cancer diagnosis coded in ORLS data | ★ (a) Mean follow-up of 8.5 yr for the UC cohort and 10.0 yr for the CD cohort | ★ (a) Complete follow-up | 4 | 8 |
| Hemminki et al. (27) | ★ (a) Patients identified from the Swedish Hospital Discharge Register with confirmed autoimmune disease | (b) Expected numbers of cancer cases calculated from Swedish Cancer Registry data | ★ (a) Confirmed IBD diagnosis according to Swedish hospital records | ★ (a) Only includes cancer diagnoses made after IBD diagnosis | 3 | ★ (a) Study controls for age and sex ★ (b) Study controls for region, socioeconomic status, comorbidity, parity, and age at first childbirth |
(b) Observed number of cancer diagnoses in AI patients according to the Swedish Cancer registry | ★ (a) Mean follow- up of 32 yr | ★ (a) Complete follow-up | 2 | 7 |
| Rungoe et al. (28) | ★ (a) Patients with IBD identified from the Danish National Patient Register (DNPR) | ★ (a) 50:1 matching of controls from DNPR | ★ (a) Confirmed IBD diagnosis according to DNPR | ★ (a) Patients with IBD followed from the date of IBD diagnosis to the date of cervical cancer diagnosis | 4 | ★ (a) Study controls for age and sex ★ (b) Study controls for municipality |
★ (a) Confirmed cervical dysplasia or cancer diagnosis according to the Danish Cancer Register | ★ (a) Mean follow-up of 7.8 yr for the UC cohort and 8.3 yr for the CD cohort | ★ (a) Complete follow-up | 3 | 9 |
(a, b, c, d), NOS assessment form description; CD, Crohn's Disease; DNPR, Danish National Patient Register; IBD, inflammatory bowel disease; ICD, implantable cardioverter defibrillator; NHS, National Health Service; NOS, Newcastle-Ottawa Scale; ORLS, Oxford Record Linkage Study; UC, ulcerative colitis.
★Newcastle-Ottawa Scale score: selection (max.★★★★), comparability (max.★★), and outcome (max.★★★).
Statistical analysis
Analyses were performed using R version 3.6.2 (R Studio using “meta” and “metfor” packages). For articles eligible for meta-analysis, we calculated a pooled hazard ratio (HR) for risk of cervical cancer in IBD. We used the overall HR or analogous relative risk estimate (21) and 95% CI presented for each study and calculated standard errors from 95% CIs to undertake a random effects model (REM) meta-analysis using the generic inverse variance method with χ2 used to calculate variance. Where available, we used adjusted relative risk estimates presented for each study. Where the relative risk estimate of cervical cancer in IBD was not reported, we assumed participants with both CD and UC to represent the same population in a given study and combined the relative risk estimates presented for CD and UC, along with their respective standard errors to calculate a pooled IBD risk estimate with a 95% CI. We undertook REM meta-analysis of study estimates because of an a priori assumption of the presence of both interstudy and intrastudy heterogeneity. Subgroup meta-analysis was undertaken for risk of cervical cancer by IBD subtype (CD or UC), risk of low-grade and high-grade lesions, and treatment exposure, where these data were available. Meta-regression was undertaken for the year of publication, ratio of Pap screening in IBD and non-IBD populations, duration of study follow-up (in years), and average age of study participants. Sensitivity analyses were undertaken to exclude low-quality studies, using a Hartung-Knapp-Sidik-Jonkman estimator in the place of DerSimonian-Laird for variance. Publication bias was evaluated using visual inspection of a funnel plot for the degree of asymmetry. Analyses were performed in R, using the “metagen” and “metabin” functions in “meta”x and “metafor”y packages (22,23).
RESULTS
Search results and study selection
Our initial search yielded 1,738 articles or 1,393 after removal of duplicate records. After title and abstract screening, 46 articles were included and a further 3 identified from reference lists. Forty-nine articles underwent full article screening, and according to the eligibility criteria, 5 articles were identified for inclusion (24–28). The included articles were unselected, population-based studies with a pooled total of 74,310 patients with IBD and a total reference population of 2,029,087 patients. The data in each study were obtained from regional or national patient databases within their respective countries: Canada, Denmark, Sweden, the Netherlands, and the United Kingdom (Table 1).
Table 1.
Characteristics of included studies
| Study, country | Year | Study period | Source population | IBD cohort (n) | Mean age at inclusion (yr) | Reference cohort (n) | Reported relative risk estimate (95% CI) | Overall incidence (%) and incidence rate (per 100,000 pyrs) of cervical cancer | Adjusted variables | NOS study qualitya | ||||||
| Overall IBD | CD | UC | IBD | CD | UC | Estimate type | Patients with IBD | Non-IBD patients | ||||||||
| Bernstein et al., Canada (24) | 2001 | 1998–1997 | Patients in the Manitoba region of Canada | 5,529 | 2,857 | 2,672 | 38.9 | 55,290 | 0.91 (0.28, 2.97) | Not reported | Not reported | IRR | 0.054% 13.5 per 100,000 pyrs |
Not reported | Age, area of residence, and time period | 9 |
| Goetgebuer et al., Netherlands (25) | 2021 | 2000–2016 | Patients in the Netherlands identified from Dutch nationwide IBD bank | 2,098 | 1,382 | 716 | 42 | 8,392 | 1.46 (1.07, 2.00) | Not reported | Not reported | HR | 0.095% 8.55 per 100,000 pyrs |
0.083% 7.50 per 100,000 pyrs |
Age and year of record | 7 |
| Goldacre et al., UK (26) | 2008 | 1963–1999 | Patients from the Oxford region of the United Kingdom | 12,117 | 5,127 | 6,990 | 45.2 | 457,071 | 2.30 (1.27, 4.15) | 2.63 (1.12, 5.29) | 1.91 (0.69, 4.24) | RR | 0.15% 3.21 per 100,000 pyrs |
0.052% (expected incidence) 1.44 per 100,000 pyrs (expected) |
Age, date of earliest record, and urbanization level | 8 |
| Hemminki et al., Sweden (27) | 2012 | 1964–2008 | Patients in Sweden identified from a national hospital discharge register | 27,158 | 12,886 | 14,272 | Not reported | [calculated expected number of cases] | 0.94 (0.71, 1.23) | 0.89 (0.58, 1.31) | 0.98 (0.67, 1.40) | SIR | 0.21% CD: 6.63 per 100,000 pyrs UC: 6.37 per 100,000 pyrs |
Not reported | Age groups, time period, region, and socioeconomic status | 7 |
| Rungoe et al., Denmark (28) | 2015 | 1979–2011 | Patient in Denmark identified from a national register | 27,408 | 8,717 | 18,691 | 44.5 | 1,508,334 | 1.03 (1.08, 0.82) | 1.53 (1.04, 2.27) | 0.78 (0.53, 1.13) | IRR | 0.20% 6.16 per 100,000 pyrs |
0.20% 5.92 per 100,000 pyrs |
Age, area of residence, and time period; no history of IBD or hysterectomy | 9 |
Maximum score = 9.
CD, Crohn's disease; CI, confidence interval; ERR, expected rate ratio; HR, hazard ratio; IBD, inflammatory bowel disease; IRR, incidence rate ratio; NOS, Newcastle-Ottawa Scale; pyrs, person years; SIR, standardized incidence rate; UC, ulcerative colitis.
Study quality assessment
All 5 included studies scored between 7 and 9 on the Newcastle-Ottawa Scale for quality assessment, indicating high-quality methodology, the details of which are summarized in Table 2. Rungoe et al. and Bernstein et al. both met all criteria in the quality assessment, achieving a score of 9. Goldacre et al. scored 8, with 1 point deducted for representativeness of the exposed cohort because this point was awarded to other studies with a wide source population identified from national databases, whereas the authors used data limited to a region in Oxford, United Kingdom. Goetgebuer et al. scored 7, with 1 point deducted for representativeness of the exposed cohort, because the authors used the Dutch nationwide IBD biobank but only included patients with data regarding cervical screening history, and 1 point for lack of demonstration that cervical cancer was not present before IBD diagnosis. Hemminki et al. scored 7, with 1 point deducted for the use of an expected number of cancer cases in lieu of a matched, nonexposed cohort and 1 point for the assessment of outcome using the Swedish cancer registry, rather than direct diagnoses from the hospital records which were used for identifying IBD cases.
Overall risk of cancer
All 5 studies identified for full article inclusion were judged eligible for meta-analysis and their relative risk estimates were pooled to present an overall HR of 1.24 (95% CI: 0.94–1.63; Figure 2), indicating no significantly increased risk of cervical cancer in patients with IBD. Tests for model heterogeneity showed χ2 = 0.0531, I2 = 60%, and P = 0.04, which suggests mild-moderate heterogeneity among the studies included. A Danish population study and a Swedish population study had the greatest impact on the pooled estimate, contributing a weight of 27.8% each to the pooled REM HR. Both studies reported no increased risk of cervical cancer in IBD (Rungoe et al. (28) HR: 1.08; 95% CI: 0.82–1.42; Hemminki et al. (27) HR: 0.94; 95% CI: 0.71–1.23). Where absolute risk was not directly reported by the authors, it was calculated by the overall number of cervical cancer cases in the IBD population for each study: This ranged from 0.12% in the study by Goldacre et al. to 7.29% in that by Goetgebuer et al.
Figure 2.

Forest plot of REM meta-analysis of HRs for cervical cancer in patients with IBD across included studies. HR, hazard ratio; IBD, inflammatory bowel disease; REM, random effects model.
Subgroup analysis
Four studies presented risk estimates for cervical cancer by CD and UC (Figure 3). In CD, despite increased risk for cervical cancer according to Goldacre et al. and Rungoe et al. (HR: 2.63; 95% CI: 1.12–5.29; HR: 1.53; 95% CI: 1.04–2.27 (26,28)), overall pooled risk was not increased (HR: 1.36; 95% CI: 0.83–2.23). There were no included studies that showed an increased risk of cervical cancer in UC, with an overall HR of 0.95 (95% CI: 0.72–1.25).
Figure 3.
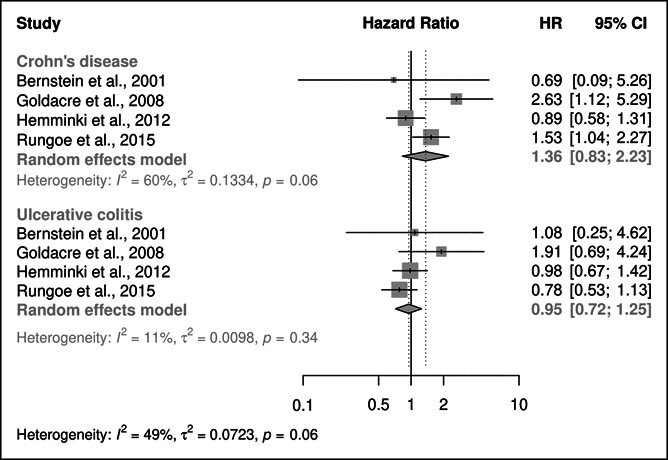
Forest plot of REM subgroup meta-analysis of HRs for risk of cervical cancer in patients with IBD by IBD subtype. CD, Crohn's disease; HR, hazard ratio; IBD, inflammatory bowel disease; REM, random effects model; UC, ulcerative colitis.
Data on risk of cervical cancer by medical treatment and grade of cervical cancer lesion were only available for 2 studies: Goetgebuer et al. and Rungoe et al. (25,28). Although Rungoe et al. showed an increased risk for cervical cancer in treatment with tumor necrosis factor alpha (TNF-alpha) inhibitors (HR: 1.65; 95% CIs: 1.05–2.58), subgroup meta-analysis of the 2 studies showed no increased risk in patients with IBD treated with TNF-alpha inhibitors (HR: 1.19; 95% CIs: 0.64–2.21) or immunomodulators (HR: 0.96; 95% CIs: 0.61–1.50; see Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A847). Subgroup meta-analysis by grade of lesion showed an increased risk of low-grade squamous intraepithelial lesions (LSIL) with a HR of 1.15 (95% CIs: 1.04–1.28; Figure 4), largely because of the study by Rungoe et al. which contributed a weight of 39.5% to the pooled estimate. However, there was no statistically increased risk of high-grade squamous intraepithelial lesions with a HR of 1.36 (95% CI: 0.97–1.90; Figure 4).
Figure 4.

Forest plot of REM subgroup meta-analysis of HRs risk of cervical cancer in patients with IBD by grade of lesion. HR, hazard ratio; IBD, inflammatory bowel disease; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; REM, random effects model.
Meta-regression and Pap screening ratio
Meta-regression was undertaken for the year of publication, ratio of Pap screening in patients with IBD compared with non-IBD patients, duration of follow-up, and average age of population. Year of publication, duration of follow-up, nor average age of population was a predictor of individual study effect size for risk of cervical cancer in IBD. The Pap screening ratio was found to be a contributor to the variability seen in effect size, contributing 31% to heterogeneity seen; however, this was not to a statistically significant level (P = 0.21). Only 2 of the included studies reported a Pap screening ratio: Rungoe et al. reported that IRR for uptake of Pap screening compared with the reference population was 1.06 (95% CI: 1.04–1.08) in patients with UC and 0.99 (95% CI: 0.96–1.02) in patients with CD (28). Goetgebuer et al. provided the raw number of screening episodes in the IBD cohort and the control cohort, giving a Pap screening ratio of 1.14 when compared with the reference population (25).
Sensitivity analysis and publication bias
The 2 studies with the lowest quality assessment score were by Goetgebuer et al. (HR: 1.46; 95% CI: 1.07–2.00) and Hemminki et al. (HR: 0.94; 95% CI: 0.71–1.23), which contributed weights of 25.6% and 27.8%, respectively, to the pooled summary estimate (25,27). Exclusion of these 2 resulted in a pooled HR for cervical cancer in IBD of 1.36 (95% CI: 0.78–2.40; see Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A847). The use of Sidik-Jonkman variance estimator for meta-analysis gave a HR of 1.25 (95% CI: 0.90–1.74; see Figure 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A847). Visual inspection of the funnel plot did not show evidence for publication bias (see Figure 4, Supplementary Digital Content 1, http://links.lww.com/CTG/A847).
DISCUSSION
This systematic review and meta-analysis included all unselected, population-based studies investigating the risk of cervical cancer in IBD. Based on a total of 74,310 patients with IBD and a total reference population of 2,029,087 patients, we found a numerically but nonstatistically significant increased risk for cervical cancer in patients with IBD, pooled HR 1.24 (95% CI: 0.94–1.63). Subgroup meta-analyses of the limited data available also showed no statistically significant increased risk by IBD subtype (CD or UC), nor any evidence of increased risk with exposure to biological or thiopurine therapies. However, analysis by grade of lesion showed a small but significantly increased risk of LSIL in patients with IBD compared with reference populations; HR 1.15 (95% CIs: 1.04–1.28).
Our findings are derived from population-representative cohort studies; however, some smaller studies have provided conflicting evidence for risk of cervical cancer in IBD. In a 2008 study of 40 female patients with IBD, the incidence of abnormal cervical cytology compared with matched, healthy controls was 42.5% versus 7%, respectively (P < 0.001); additionally, patients with IBD were more likely to have higher grade lesions (P < 0.001) (8), but these results are based on a small study population with limited generalizability to the general IBD population. Two studies published the subsequent year indicated a small or statistically insignificant risk of cervical abnormalities in female patients with IBD compared with controls, including 1 study using data from the same Canadian-based IBD cohort as Bernstein et al. (9,10,29). This study did report an increased risk of cervical abnormalities in patients with CD (OR: 1.66; 95% CI: 1.08–2.54); however, this was limited to patients using oral contraceptives, who were less likely to use barrier protection and therefore more likely to be exposed to HPV (9).
An increased risk of cervical cancer in IBD has previously been reported: In a 2013 Danish cohort study, Jess et al. reported a systemic inflammatory response of 1.65 (95% CI: 1.10–2.37) for cervical dysplasia in patients with IBD, associated with young age at diagnosis, smoking, 5-aminosalicylic acid exposure, and thiopurine exposure (1). This study used data from Northern Jutland and provided detailed analysis of cervical dysplasia in IBD, but it was excluded from our meta-analysis because of duplicated data with Rungoe et al., which used a larger cohort with longer follow-up time (28). Our meta-analysis provides the most accurate estimate to date of risk of cervical cancer in patients with IBD by inclusion of only high-quality studies with representative source populations.
A 2017 Korean population-based study found a systemic inflammatory response of 5.7 (95% CI: 2.4–11.1) for cervical cancer in female UC patients with exposure to at least 1 form of medical IBD treatment (30), and higher rates of HPV in patients with IBD may be attributable to immune dysregulation or treatment-induced immunosuppression (31,32). However, findings from our analysis do not provide sufficient evidence for this treatment-associated risk, according to the 2 studies included which reported risk by treatment exposure. Although Rungoe et al. provided a HR of 1.65 (95% CI: 1.05–2.58) for patients on TNF-alpha inhibitors, Goetgebuer et al. found no increased risk in these patients and there was no overall pooled increased risk in patients with exposure to immunomodulators (25,28).
Our subgroup meta-analysis by grade of lesion shows an increased risk for LSIL, which was not seen for high-grade squamous intraepithelial lesions, although only 2 studies provided these data. This observed increased risk of LSIL may also reflect increased participation of patients with IBD in national cervical screening programs, which is supported by the data on screening participation in our included studies. Increased Pap screening frequency in patients with IBD would result in more frequent incidental findings of low-grade lesions, which have not yet developed into higher grade lesions.
Our meta-analysis does not indicate a statistically significant increased risk of cervical cancer in patients with IBD, and therefore, our evidence does not support a need for increased screening of patients with IBD. Recent literature suggests that more targeted prevention practices, particularly HPV vaccination, are required to reduce cervical cancer risk in patients with IBD (33,34). However, our findings indicate that patients with IBD already display greater uptake of health services when compared with the general population, including more frequent cervical screening. The 2 studies which provided a Pap screening ratio (25,28) come from Denmark and the Netherlands, with source populations which are representative of the general IBD population in each country and their participation in screening services. However, the increased or comparable Pap screening ratios found in these studies contradict that described in recent literature, which suggests that patients with IBD are not adequately screened for cervical cancer (35–37). It is likely that IBD populations in Denmark and the Netherlands have a better uptake of cervical screening than other IBD populations, who may have reduced access to comprehensive national screening programs. Given that only 2 of our 5 included studies provided a Pap screening ratio, further data are needed to adequately explore this factor and its contribution to cervical cancer risk in patients with IBD.
This study has some limitations. Because an inclusion criterion for this systematic review is population-based studies, studies that recruited patients with IBD from cervical screening registries, secondary/tertiary care settings, or insurance databases were excluded. We therefore only included 5 studies, limiting our ability to undertake subgroup analysis and meta-regression. We also only had data from 4 countries in Europe and 1 Canadian cohort, restricting the generalizability of our findings to other countries, particularly those without free and easily accessible health care. All studies included reported relative risk estimates compared with age-matched reference populations, and several studies reported and adjusted for screening frequency, urbanization, and socioeconomic status, allowing us to provide a highly reliable estimate for risk of cervical cancer in IBD. However, we were unable to control for HPV vaccination within our study population; because many HPV vaccine programs were rolled out in the past 10–15 years and the most recent study period in our included studies ended in 2016 (25), there were insufficient data for us to properly assess the impact of HPV vaccination programs on cervical cancer risk. We were also able to assess the risk of cervical cancer in CD and UC and grade of lesion and explore the impact of medical treatment exposure, but we were limited by availability of data because only 2 studies reported on treatment exposure.
Findings from this meta-analysis of population-based studies indicate no statistically significant increased risk of cervical cancer or high-grade lesions in patients with IBD or in either disease subtype (CD or UC) compared with the general population. However, we do identify increased risk of low-grade intraepithelial lesions in patients with IBD.
CONFLICTS OF INTEREST
Guarantor of the article: Rahma Elmahdi, MD, PhD.
Specific author contributions: All authors were responsible for study concept and design. S.M. and R.E. were responsible for data acquisition. R.E. was responsible for data analysis. All authors were responsible for data interpretation. S.M. was responsible for drafting of the manuscript, and R.E., T.J., and K.A. were responsible for critical revision for important intellectual content. T.J. obtained funding for the work undertaken.
Financial support: This work was supported by the Danish National Research Foundation (Grant No: DNRF148).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Inflammatory bowel disease (IBD) is associated with increased risk of malignancy, which may be due to immunosuppressive treatment and chronic inflammation.
✓ Evidence on the risk for cervical cancer in IBD is, however, conflicting.
WHAT IS NEW HERE
✓ Meta-analysis of high-quality population-based studies shows no significantly increased risk of cervical cancer in patients with IBD.
✓ There seems to be an increased risk of low-grade cervical lesions in patients with IBD.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A847
Contributor Information
Tine Jess, Email: jess@dcm.aau.dk.
Kristine Allin, Email: allin@dcm.aau.dk.
Rahma Elmahdi, Email: rahmae@dcm.aau.dk.
References
- 1.Jess T, Horváth-Puhó E, Fallingborg J, et al. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: A Danish population-based cohort study. Am J Gastroenterol 2013;108(12):1869–76. [DOI] [PubMed] [Google Scholar]
- 2.Chang M, Chang L, Chang HM, et al. Intestinal and extraintestinal cancers associated with inflammatory bowel disease. Clin Colorectal Cancer 2018;17(1):e29–37. [DOI] [PubMed] [Google Scholar]
- 3.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22(20):4794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abreu MT, Peek RM, Jr. Gastrointestinal malignancy and the microbiome. Gastroenterology 2014;146(6):1534–46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugué PA, Rebolj M, Garred P, et al. Immunosuppression and risk of cervical cancer. Expert Rev Anticancer Ther 2013;13(1):29–42. [DOI] [PubMed] [Google Scholar]
- 6.Vajdic CM, van Leeuwen MT. What types of cancers are associated with immune suppression in HIV? Lessons from solid organ transplant recipients. Curr Opin HIV AIDS 2009;4(1):35–41. [DOI] [PubMed] [Google Scholar]
- 7.Clifford G, Franceschi S. Immunity, infection, and cancer. Lancet 2007;370(9581):6–7. [DOI] [PubMed] [Google Scholar]
- 8.Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol 2008;103(3):631–6. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Demers AA, Nugent Z, et al. Risk of cervical abnormalities in women with inflammatory bowel disease: A population-based nested case-control study. Gastroenterology 2009;136(2):451–8. [DOI] [PubMed] [Google Scholar]
- 10.Lees CW, Critchley J, Chee N, et al. Lack of association between cervical dysplasia and IBD: A large case-control study. Inflamm Bowel Dis 2009;15(11):1621–9. [DOI] [PubMed] [Google Scholar]
- 11.Arbyn M, Anttila A, Jordan J, et al. European guidelines for quality assurance in cervical cancer screening. Second edition—summary document. Ann Oncol 2010;21(3):448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: Results of the 3rd ECCO pathogenesis scientific workshop (III). J Crohns Colitis 2014;8(1):31–44. [DOI] [PubMed] [Google Scholar]
- 13.Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Genit Tract Dis 2019;23(2):87–101. [DOI] [PubMed] [Google Scholar]
- 14.Parian A, Lazarev M. Who and how to screen for cancer in at-risk inflammatory bowel disease patients. Expert Rev Gastroenterol Hepatol 2015;9(6):731–46. [DOI] [PubMed] [Google Scholar]
- 15.Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: Preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112(2):241–58. [DOI] [PubMed] [Google Scholar]
- 16.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 3):s1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis 2015;21(5):1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SC, Glynn RJ, Giovannucci E, et al. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: A population-based cohort study. Ann Rheum Dis 2015;74(7):1360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia J, Bratcher JM, Korelitz B, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World J Gastroenterol 2006;12(38):6167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells GA, Shea DOC, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Clinical Epidemiology, Ottawa Hospital Research Institute, 2013. [Google Scholar]
- 21.Harrer MC P, Furukawa TA, Ebert DD. Pooling effect sizes. In: Doing Meta-Analysis with R: A Hands-On Guide. Chapman & Hall/CRC Press (Taylor & Francis), 2021. [Google Scholar]
- 22.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health 2019;22(4):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36(3):1–48. [Google Scholar]
- 24.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease. Cancer 2001;91(4):854–62. [DOI] [PubMed] [Google Scholar]
- 25.Goetgebuer RL, Kreijne JE, Aitken CA, et al. Increased risk of high-grade cervical neoplasia in women with inflammatory bowel disease: A case-controlled cohort study. J Crohns Colitis 2021;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldacre MJ, Wotton CJ, Yeates D, et al. Cancer in patients with ulcerative colitis, Crohn's disease and coeliac disease: Record linkage study. Euro J Gastroenterol Hepatol 2008;20(4):297–304. [DOI] [PubMed] [Google Scholar]
- 27.Hemminki K, Liu X, Ji J, et al. Effect of autoimmune diseases on risk and survival in female cancers. Gynecol Oncol 2012;127(1):180–5. [DOI] [PubMed] [Google Scholar]
- 28.Rungoe C, Simonsen J, Riis L, et al. Inflammatory bowel disease and cervical neoplasia: A population-based nationwide cohort study. Clin Gastroenterol Hepatol 2015;13(4):693–700.e1. [DOI] [PubMed] [Google Scholar]
- 29.Singh H, Demers AA, Nugent Z, et al. Risk of cervical abnormalities in women with inflammatory bowel disease: A population-based nested case-control study. Gastroenterology 2009;136(2):451–8. [DOI] [PubMed] [Google Scholar]
- 30.Jung YS, Han M, Park S, et al. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: A nationwide population-based study. J Crohns Colitis 2017;11(8):954–62. [DOI] [PubMed] [Google Scholar]
- 31.Ricci C, Scaldaferri F, Colombo F, et al. Prevalence of cervical HPV and attitude towards cervical screening in IBD patients under immunomodulatory treatment: A multidisciplinary management experience. Eur Rev Med Pharmacol Sci 2020;24(2):564–70. [DOI] [PubMed] [Google Scholar]
- 32.Gaidos JKJ, Bickston SJ. HPV infection and vaccination in patients with inflammatory bowel disease. Prac Gastroenterol 2012;36(10):27–31. [Google Scholar]
- 33.Reich J, Wasan SK, Farraye FA. Vaccination and health maintenance issues to consider in patients with inflammatory bowel disease. Gastroenterol Hepatol 2017;13(12):717–2450. [PMC free article] [PubMed] [Google Scholar]
- 34.Reich JS, Farraye FA, Wasan SK. Preventative care in the patient with inflammatory bowel disease: What is new?. Dig Dis Sci 2016;61(8):2205–16. [DOI] [PubMed] [Google Scholar]
- 35.McMullan S, Pandya S, Vermani S, et al. Increased awareness of the cervical cancer risk and need for Pap smears in women with inflammatory bowel disease is necessary. Inflamm Bowel Dis 2012;18:S53. [Google Scholar]
- 36.Long MD, Porter CQ, Sandler RS, et al. Suboptimal rates of cervical testing among women with inflammatory bowel disease. Clin Gastroenterol Hepatol 2009;7(5):549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Moron JM, Cabello Fernandez A, Pallares-Manrique H, et al. Patients with inflammatory bowel disease who are on immunosuppressive therapy perform regular gynecologic screening for uterine cervical cancer?. J Crohns Colitis 2017;11(Suppl 1):S434–5. [Google Scholar]



