Abstract
Background:
Glutamine synthetase (GS) and arginase 1 (Arg1) are widely used pathological markers that discriminate hepatocellular carcinoma (HCC) from intrahepatic cholangiocarcinoma; however, their clinical significance in HCC remains unclear.
Methods:
We retrospectively analyzed 431 HCC patients: 251 received hepatectomy alone, and the other 180 received sorafenib as adjuvant treatment after hepatectomy. Expression of GS and Arg1 in tumor specimens was evaluated using immunostaining. mRNA sequencing and immunostaining to detect progenitor markers (cytokeratin 19 [CK19] and epithelial cell adhesion molecule [EpCAM]) and mutant TP53 were also conducted.
Results:
Up to 72.4% (312/431) of HCC tumors were GS positive (GS+). Of the patients receiving hepatectomy alone, GS negative (GS−) patients had significantly better overall survival (OS) and recurrence-free survival (RFS) than GS+ patients; negative expression of Arg1, which is exclusively expressed in GS− hepatocytes in the healthy liver, had a negative effect on prognosis. Of the patients with a high risk of recurrence who received additional sorafenib treatment, GS− patients tended to have better RFS than GS+ patients, regardless of the expression status of Arg1. GS+ HCC tumors exhibit many features of the established proliferation molecular stratification subtype, including poor differentiation, high alpha-fetoprotein levels, increased progenitor tumor cells, TP53 mutation, and upregulation of multiple tumor-related signaling pathways.
Conclusions:
GS− HCC patients have a better prognosis and are more likely to benefit from sorafenib treatment after hepatectomy. Immunostaining of GS may provide a simple and applicable approach for HCC molecular stratification to predict prognosis and guide targeted therapy.
Keywords: Hepatocellular carcinoma, Glutamine synthetase, Molecular stratification, Targeted therapy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies, and cases in China account for approximately half of the HCC cases worldwide.[1,2] For early HCC, surgical resection is the main treatment. However, because most patients present with intermediate or advanced disease at the time of initial diagnosis, surgical resection is only an option for <20% of cases.[3] Sorafenib acts as a multikinase inhibitor targeting the serine-threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptor (PDGFR)-β. It is the first approved systemic targeted drug for advanced HCC, and it has been reported to be able to prolong the median survival time by almost 3 months in patients with advanced HCC.[4] Despite its wide use in advanced HCC since 2007, one major challenge regarding sorafenib treatment is the lack of reliable biomarkers for candidate screening.[5] Currently, partial response after sorafenib treatment is achieved in only 3.3% (vs. 1.3% in the placebo group) of patients with advanced HCC, with 54% (vs. 27.6% in the placebo group) showing stable disease.[6] Several studies have investigated the effect of sorafenib treatment as an adjuvant therapy after surgery in HCC patients, but the results are inconclusive.[7,8] The only phase III trial of sorafenib as adjuvant therapy, the STORM trial, found no difference in recurrence-free survival (RFS) after curative resection or ablation between sorafenib and placebo treatment in early HCC.[9] This result is, in part, because clinically meaningful molecular classification schemes that can predict responders/non-responders to sorafenib are not available.[5] Although expression of certain markers, such as high extracellular signal-regulated kinase (ERK) activity or high tumor necrosis factor-α (TNF-α) levels in HCC tissues,[5,10] has been reported to be able to predict the sensitivity of HCC to sorafenib, such markers are not routinely analyzed in pathological diagnosis. Hence, the key to improving HCC patient treatment is to find novel biomarkers for predicting those who might respond to targeted therapy.
Multiple molecular classifications of HCC based on various gene signatures or immunological microenvironment features have been proposed to predict prognosis in HCC patients, such as G1–G6 subtyping, S1–S3 subtyping, proliferative and non-proliferative subtyping, mutational signature 1–6 subtyping, integrating data of multiple genomes (iC1–iC3) subtyping, subtyping based on immunological features, and immune microenvironment subtyping.[11–15] All these classifications are helpful for predicting patient outcome to some extent. For example, an approved molecular classification divides HCC into two subtypes: a proliferation subtype and a non-proliferation subtype.[14] Compared with the non-proliferation subtype, the main molecular characteristics of the proliferation subtype include chromosomal instability, a high frequency of TP53 mutation, overexpression of cell cycle genes, and overactivation of multiple signaling pathways, such as Wnt-β-catenin, mitogen-activated protein kinase (MAPK), and KRAS signaling. Patients with the proliferation subtype of HCC usually have higher serum levels of alpha-fetoprotein (AFP) and worse clinical outcomes. However, these classification methods lack practicability in the clinic due to their complex parameters and high cost. In addition, these subtyping methods have little reference value for systematic treatment, making them inappropriate for evaluating patient response before targeted therapy. New molecular classifications that more practically, simply and effectively predict patient prognosis and guide systematic therapy are urgently needed.
One of the vital functions of the liver is ammonia metabolism, whereby toxic ammonia is converted to non-toxic urea or other metabolites, a process catalyzed by several critical enzymes, including glutamine synthetase (GS) and arginase 1 (Arg1). GS is a cytosolic enzyme that catalyzes adenosine triphosphate (ATP)-dependent production of glutamine from glutamate and ammonia; Arg1 catalyzes the last step of the urea cycle that generates urea. Interestingly, in normal liver tissue, GS and Arg1 are mutually and exclusively expressed in spatially distributed hepatocytes. GS is restricted to a small subpopulation (approximately 7%) of hepatocytes around the central vein,[16] whereas Arg1 is expressed in other hepatocytes. Immunostaining of both GS and Arg1 is widely used in clinical pathology to discriminate HCC from intrahepatic cholangiocarcinoma, though the role of GS and Arg1 immunostaining in the molecular classification of HCC is still undefined. High GS expression appears to be associated with poor prognosis in HCC, but this conclusion is controversial.[17–20] It has been reported that GS positive (GS+) HCC tumors have a higher differentiation grade[17] and that patients with GS+ HCC tumors experience better overall survival (OS) after radiofrequency thermal ablation.[18] In contrast, reports from other groups have shown that GS may enhance metastasis and recurrence in HCC,[19] and patients with higher GS expression have worse OS when receiving postoperative adjuvant transcatheter arterial chemoembolization.[20] Overall, the association between GS expression and sensitivity to systemic chemotherapy, such as sorafenib adjuvant therapy, remains unclear. In the current study, we assessed GS and Arg1 expression in a large cohort of HCC patients and retrospectively analyzed the relationship between GS/Arg1 expression and patient outcome. The correlation between GS/Arg1 expression and response to adjuvant treatment with sorafenib after radical hepatectomy was further evaluated. Our results show that compared to GS+ patients, GS negative (GS−) patients, who accounted for <30% of all patients and had many molecular signatures and clinical features of the non-proliferation subtype of HCC, had a better prognosis and response to sorafenib administration after surgery.
Methods
Ethical approval
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2021607A). Written informed consent forms were obtained from all participants.
Patients and the database
We retrospectively included HCC patients who underwent curative resection between January 2010 and December 2019 at West China Hospital, Sichuan University. Our inclusion criteria were as follows: (1) patients with pathologically confirmed HCC who underwent radical hepatectomy; (2) patients who received no anticancer treatments before hepatectomy; (3) patients with no history or concurrence of other malignant tumors; (4) patients with Child-Pugh liver function grades A–B; and (5) patients with complete clinicopathological and follow-up data. Patients who received adjuvant sorafenib but discontinued sorafenib therapy within 3 months due to severe adverse effects were excluded. Demographic and clinicopathological data were retrieved from the electronic database of the hospital. In our center, all patients receive comprehensive assessment before beginning sorafenib treatment, including their general condition and tumor features and blood tests. The final cohort included 251 HCC patients who received only curative resection and 180 patients who received sorafenib after resection.
Sorafenib therapy generally began at a dose of 400 mg twice a day, and therapy interruption and dose reduction were performed when adverse effects occurred. An initial dose of 200 mg twice a day due to an unsatisfactory general condition and dose reduction to 400 mg every other day were acceptable, but patients who required further dose reduction were excluded from the study.
As administration of sorafenib is recommended for only Barcelona Clinic Liver Cancer (BCLC) stage B/C, only patients who simultaneously met both criteria were included to assess the outcome of sorafenib-based adjuvant therapy. A total of 164 patients who underwent hepatectomy alone (control group) and 146 patients who received hepatectomy combined with administration of sorafenib (sorafenib group) were included. The BCLC staging classification aims to combine tumor stage and liver function parameters of HCC to provide accurate stratification of patients to assess prognosis and guide the treatment strategy through a well-defined schedule. Stage A is defined as solitary HCC irrespective of size or as a multifocal HCC up to three nodules (none of them >3 cm), without macrovascular invasion, extrahepatic spread or cancer-related symptoms, and liver function must be preserved. BCLC B includes asymptomatic patients with multinodular tumors without vascular invasion or extrahepatic spread; liver function belongs to Child-Pugh class A. Stage C comprises patients with either symptomatic tumors or with an invasive tumoral pattern reflected by the presence of vascular invasion or extrahepatic spread.[21]
Procedures
Full patient assessments, including tumor assessment, complete blood count, and a chemistry panel, were performed at the initial visit, at 1 and 3 months after the initial visit, and every 6 months thereafter. After treatment initiation, an independent data safety monitoring committee assessed safety and treatment adherence at 1 and 3 months and at every 6 months thereafter.
Outcomes and assessments
The primary endpoint of the study was RFS, defined as the time from randomization to the first instance of disease recurrence documented by independent radiological assessment or death by any cause, whichever occurred first. The primary endpoint was to identify biomarkers predicting sorafenib efficacy in preventing HCC recurrence in terms of RFS. The secondary endpoint was OS, as defined as the time from randomization to death by any cause.
Immunostaining
Pathologic slides from paraffin blocks prepared using surgical tissue specimens were stained with monoclonal antibodies against the following: Arg1 (1:500; catalog No. 93668; D4E3M; monoclonal rabbit anti-human; Cell Signaling Technology, Inc., USA); GS (1:1000; catalog No. ab176562; EPR13022(B); monoclonal rabbit anti-human; Abcam plc, USA); cytokeratin 19 (CK19) (1:500; catalog No. ab52625; EP1580Y; monoclonal rabbit anti-human; Abcam plc); EpCAM (1:500; catalog No. EM1111; 3-E1-E2; monoclonal mouse anti-human; HUABIO, China); and TP53 (catalog no. MAB-0674; MX008; monoclonal mouse anti-human; Maxim, China).
The immunostaining results were interpreted by three experienced pathologists using a blinded method. GS staining was recorded as positive (GS+) if it was equal to or greater than that of the hepatocytes surrounding the central vein in corresponding adjacent normal tissues.[18] Similarly, tumor specimens were recorded as Arg1+ if the staining was equal to or greater than that of adjacent normal tissues. We identified expression of GS and Arg1 in each HCC patient and divided all patients into four subgroups: GS−Arg1−, GS−Arg1+, GS+Arg1−, and GS+Arg1+. CK19 and EpCAM were regarded as positive if >5% of tumor cells showed membrane staining.[22,23] Cases with moderate or strong nuclear staining in >5% of the cells were considered TP53 positive.
mRNA sequencing
Twenty primary resected HCC tissues (from at least four patients in each subgroup) were collected for mRNA sequencing.
Follow-up
Outpatient follow-up was conducted every 2 to 3 months for the first year after surgery and every 3 to 6 months thereafter. At each visit, patients underwent assessment of liver function, measurement of tumor markers, and computed tomography and/or magnetic resonance imaging. The mean follow-up of patients who underwent only hepatectomy was 33.5 months (range, 6.0–117.0 months) and that of patients who received sorafenib after hepatectomy was 41.3 months (range, 6.0–84.9 months).
Statistical analysis
Comparisons between groups were performed using the Mann–Whitney U test, chi-squared test, or Fisher's exact test, as appropriate. Risk factors associated with the prognosis of HCC patients were evaluated using Cox regression analysis. Kaplan–Meier estimates, hazard ratios (HRs), and 95% confidence intervals (CIs) were calculated; and time-to-event curves were generated for RFS and OS. Statistical significance was set at P < 0.05. The statistical analysis was performed using SPSS (version 25.0; Chicago, IL, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics of patients
We retrospectively analyzed 251 HCC patients who received only curative resection at West China Hospital, Sichuan University, from 2010 to 2019 for clinical information analysis. The clinical and pathological characteristics are summarized in Supplementary Table 1. The median age of the patients was 51 years (23–83 years), and the patients were mostly male (85.7%, 215/251). Hepatitis B virus (HBV) infection was a dominant etiological factor. Patients with a history of liver cirrhosis accounted for 59.8% (150/251) of the total. There were 104 patients with microvascular invasion (MVI). The percentages of patients with BCLC stage A and stage B/C disease were 35% (87/251) and 65.3% (164/251), respectively. Most patients had preserved liver function with Child-Pugh status A. RFS and OS curves for all patients are shown in Supplementary Figure 1.
The clinical and pathological characteristics of 180 patients who received sorafenib treatment after hepatectomy are also summarized in Supplementary Table 1. Compared with patients who received surgery only, those who received sorafenib after hepatectomy showed significant differences in multiple clinical characteristics, such as tumor number, MVI, satellite lesions, and liver cirrhosis.
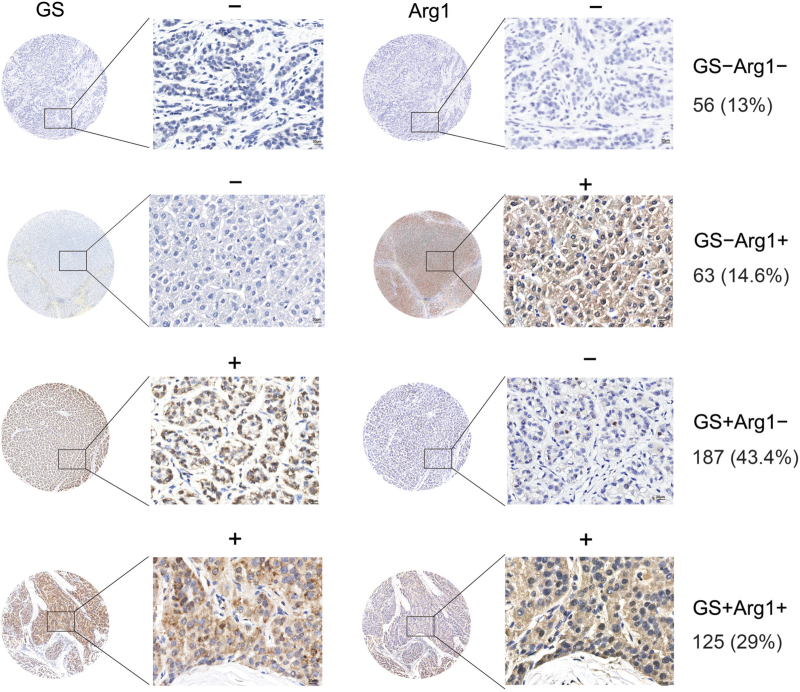
All resected specimens were subjected to immunostaining for GS and Arg1. Overall, up to 72.4% (312/431) of patients were GS+, and 43.6% (188/431) of patients were Arg1+. The percentages of patients with GS+Arg1+, GS+Arg1−, GS−Arg1+, and GS−Arg1− staining were 29.0% (125/431), 43.4% (187/431), 14.6% (63/431), and 13.0% (56/431), respectively [Figure 1]. No significant differences in the distribution of the four subgroups were observed between those who did or did not undergo adjuvant treatment with sorafenib [Supplementary Table 2]. Among 431 patients, GS− HCC tumors accounted for 24.8% (30/121) of BCLC stage A tumors; GS− HCC tumors accounted for 28.7% (89/310) of BCLC stage B/C tumors [Figure 2A and Supplementary Table 2].
Figure 1.
Subgrouping of HCC tumors based on immunostaining of GS and Arg1. Arg1: Arginase 1; GS: Glutamine synthetase; HCC: Hepatocellular carcinoma. Scale bar = 20 μm.
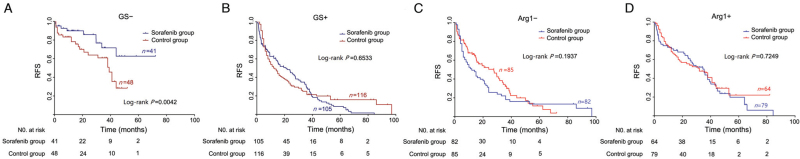
Figure 2.
Kaplan–Meier curves showing RFS according to immunostaining of GS and Arg1 in patients who received surgery alone or surgery combined with adjuvant sorafenib. Arg1: Arginase 1; GS: Glutamine synthetase; RFS: Recurrence-free survival.
GS positivity and Arg1 negativity predict worse prognosis in HCC patients
In 251 patients who received only hepatectomy, bivariate analysis showed AFP level, tumor size, tumor number, tumor differentiation, and presence of MVI to be closely related to HCC recurrence [Supplementary Table 3]. In contrast to the much lower proportion of GS+ cells in the healthy liver, 74.1% of patients (186/251) were identified to have GS+ cells, far exceeding the percentage of GS− patients. In addition, 101 (40.2%) cases were identified as Arg1+. There were no significant differences in patient characteristics between the GS+ and GS− or Arg1+ and Arg1− groups [Supplementary Tables 4 and 5].
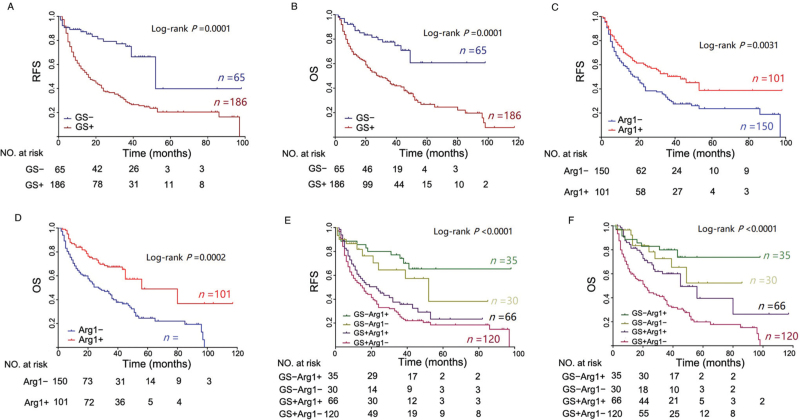
In the 186 GS+ patients, there were 133 recurrences (71.5%) during the follow-up period; recurrence occurred in 32.3% of GS− patients (21/65, P < 0.0001; Table 1). Moreover, Kaplan–Meier curves showed that GS+ patients had significantly shorter RFS than GS− patients [Figure 3A]. The median RFS was 16.0 months in the GS+ group compared with 52.0 months in the GS− group (HR in the GS− subgroup, 0.29; 95% CI, 0.21 to 0.41; P = 0.0001). The 1-year, 3-year, and 5-year RFS rates were 56.4%, 28.9%, and 20.4%, respectively, for GS+ patients and 87.3%, 74.9% and 39.8%, respectively, for GS− patients. We further analyzed the association between GS expression and OS in these cohorts; 20.0% of GS− patients and 62.4% of GS+ patients died during the follow-up period (P < 0.0001; Table 1). According to Kaplan–Meier curve analysis [Figure 3B], 1-, 3-, and 5-year OS rates were 90.4%, 77.4%, and 60.7%, respectively, for the GS− group and 67.4%, 43.5% and 26.8%, respectively, for the GS+ group (P < 0.0001). Collectively, GS+ patients had shorter RFS and OS than GS− patients.
Table 1.
The relationship between HCC recurrence/HCC-induced death and the immunostaining of GS and Arg1.
| Recurrence | Death | |||||
| Variables | Yes, n (%) | No, n (%) | P value | Yes, n (%) | No, n (%) | P value |
| GS+ | 133 (71.51) | 53 (28.49) | <0.0001 | 116 (62.37) | 70 (37.63) | <0.0001 |
| GS− | 21 (32.31) | 44 (67.69) | 13 (20.00) | 52 (80.00) | ||
| Arg1+ | 52 (51.49) | 49 (48.51) | 0.0084 | 37 (36.63) | 64 (63.37) | 0.0002 |
| Arg1− | 102 (68.00) | 48 (32.00) | 92 (61.33) | 58 (38.67) | ||
| GS−Arg1− | 10 (33.33) | 20 (66.67) | <0.0001 | 5 (16.67) | 25 (83.33) | <0.0001 |
| GS−Arg1+ | 11 (31.43) | 24 (68.57) | 8 (22.86) | 27 (77.14) | ||
| GS+Arg1− | 92 (76.67) | 28 (23.33) | 87 (72.50) | 33 (27.50) | ||
| GS+Arg1+ | 41 (60.12) | 25 (39.88) | 29 (43.94) | 37 (56.06) | ||
Arg1: Arginase 1; GS: Glutamine synthetase; GS: Glutamine synthetase; GS−: GS negative; GS+: GS positive; HCC: Hepatocellular carcinoma.
Figure 3.
Kaplan–Meier curves showing the RFS (A,C,E) and OS (B,D,F) of patients grouped by single or combined immunostaining of GS and Arg1. Arg1: Arginase 1; GS: Glutamine synthetase; RFS: Recurrence-free survival; OS: Overall survival.
The recurrence rates of Arg1− and Arg1+ patients were 68.00% and 51.49% during the follow-up period, respectively [Table 1], and Arg1− patients had significantly shorter median RFS times than Arg1+ patients (18.0 months vs. 43.0 months; HR in the Arg1+ subgroup, 0.61; 95% CI, 0.45 to 0.83; P = 0.0031; Figure 3C). Overall, 1-, 3-, and 5-year RFS rates were 70.1%, 50.3%, and 38.7%, respectively, for Arg1+ patients and 59.3%, 31.3% and 23.6%, respectively, for Arg1− patients (P = 0.0031; Figure 3D); 1-, 3-, and 5-year OS rates of Arg1− patients were 66%, 41.1%, and 24.6%, respectively, for Arg1− patients and 83.9%, 67.4% and 49%, respectively, for Arg1+ patients (P = 0.0002; Figure 3D). The median OS was 27.0 months in the Arg1+ group compared with 56.0 months in the Arg1− group (HR in the Arg1+ subgroup, 0.45; 95% CI, 0.32–0.63; P = 0.0001). Similarly, 36.6% of patients in the Arg1+ group died from HCC, as compared with 61.3% of those in the Arg1− group (P = 0.0002; Table 1). Collectively, both RFS and OS were significantly higher in Arg1+ patients than in Arg1− patients.
Furthermore, patients were divided into four subgroups according to combined immunostaining of GS and Arg1: GS−Arg1− (11.9%), GS−Arg1+ (14.0%), GS+Arg1− (47.8%), and GS+Arg1+ (26.3%), with total recurrence rates of 33.3%, 31.4%, 76.7%, and 60.1% (P < 0.0001) and rates of mortality of 16.7%, 22.9%, 72.5%, and 43.9%, respectively [Table 1]. Kaplan–Meier curves revealed significant differences in RFS and OS between the four subgroups (P < 0.0001). GS+Arg1− subgroup patients experienced the worst postoperative outcome, with 1-, 3-, and 5-year RFS rates of 54.0%, 25.2%, and 18.4% and 1-, 3-, and 5-year OS rates of 59.8%, 34.3%, and 19.9%, respectively [Figure 3E]. In contrast, GS−Arg1+ patients achieved the best outcome; 1-, 3-, and 5-year RFS rates were 85.7%, 74.2%, and 65.4% and 1-, 3-, and 5-year OS rates 88.6%, 79.9%, and 73.8%, respectively [Figure 3F].
In univariate analysis for RFS and OS, high GS expression and low Arg1 expression were found to have a significant correlation with unfavorable RFS and OS. In multivariate analysis, high GS expression and low Arg1 expression continued to be prognostic indicators for OS. Therefore, GS+ was identified as an independent risk factor for RFS and OS, and Arg1+ was found to be associated with a reduced risk of RFS and OS [Supplementary Figure 2].
GS− patients have a better response to adjuvant treatment with sorafenib
Compared with patients who received only hepatectomy, those who received adjuvant therapy with sorafenib did not have significantly improved RFS [Supplementary Figure 3A], consistent with the results of the STORM trial. Considering that adjuvant therapy with sorafenib is often prescribed for patients with a high risk of recurrence after surgery, patients who met the criterion of BCLC stage B/C in each group were selected for further analysis: 164 patients who only received hepatectomy were assigned to the control group, and 146 patients who received sorafenib after surgery were assigned to the sorafenib group. However, no significant differences were observed between the sorafenib group and the control group with respect to the baseline features, such as Child-Pugh status, HBV infection, liver cirrhosis, MVI, tumor size, tumor number, and satellites. In the sorafenib group, adverse events after sorafenib therapy were mainly hand-foot skin reactions, diarrhea, and alopecia and were gastrointestinal, constitutional, or dermatological in nature. In particular, there were no significant differences in the distribution of the four subgroups between the two groups [Table 2]. In the sorafenib group, GS+ HCC tumors still accounted for a relatively high proportion (71.9%). In general, the median RFS time did not differ significantly between the sorafenib and control groups (HR in the control group, 0.88; 95% CI, 0.68–1.15; P = 0.3252; Supplementary Figure 3B).
Table 2.
Baseline patient demographics and disease characteristics in the two groups of patients with a high risk of recurrence.
| Variable | Control group (n=164) | Sorafenib group (n=146) | P value |
| Age (years) | 51.0 (24.0–82.0) | 52.7 (21.0–74.0) | 0.0627 |
| Female/male | 25/139 | 22/124 | 0.6630 |
| PLT (109/L) | 160.83 (38.00–476.00) | 147.50 (30.00–458.00) | 0.1707 |
| TBIL (μmol/L) | 17.61 (0.00–391.00) | 17.23 (3.80–71.20) | 0.1373 |
| ALT (U/L) | 48.65 (8.00–279.00) | 60.73 (8.00–781.00) | 0.4658 |
| AST (U/L) | 42.89 (13.00–345.00) | 65.95 (30.00–1412.00) | 0.0352 |
| Neutrophil (109/L) | 2.63 (0.86–40.00) | 3.56 (1.29–58.40) | 0.5128 |
| Lymphocyte (109/L) | 1.50 (0.30–7.00) | 1.44 (0.46–30.60) | 0.4170 |
| TP (g/L) | 69.18 (26.00–87.00) | 66.54 (26.00–84.90) | 0.3404 |
| ALB (g/L) | 41.19 (20.40–73.10) | 41.10 (5.40–73.10) | 0.0070 |
| GGT (U/L) | 90.14 (12.00–625.00) | 99.10 (14.00–1319.00) | 0.0652 |
| Differentiation | 0.8405 | ||
| Poor | 85 (51.83) | 74 (50.69) | |
| Moderate-well | 79 (48.17) | 72 (49.31) | |
| Etiology | 0.9563 | ||
| Hep B | 143 (84.62) | 127 (86.99) | |
| Other | 21 (15.38) | 19 (13.01) | |
| AFP | 0.4660 | ||
| Low (serum AFP ≤500) | 91 (55.49) | 87 (59.59) | |
| High (serum AFP >500) | 73 (44.51) | 59 (40.41) | |
| Satellites | 37 (25.34) | 43 (29.45) | 0.1662 |
| Cirrhosis | 0.5322 | ||
| Yes | 88 (53.6) | 80 (54.79) | |
| No | 76 (46.4) | 66 (45.21) | |
| Tumor size (cm), median (range) | 8.69 (5.00–20.00) | 8.96 (5.00–25.00) | 0.5331 |
| Tumor number | 0.9908 | ||
| One | 143 (87.19) | 128 (87.68) | |
| Two | 13 (7.93) | 11 (7.53) | |
| More | 8 (4.88) | 7 (4.79) | |
| MVI (≥1) | 82 (50) | 79 (54.11) | 0.4953 |
| Child–Pugh class, n (%) | 0.1815 | ||
| A | 159 (96.95) | 144 (98.63) | |
| B | 5 (3.05) | 2 (1.37) | |
| BCLC stage, n (%) | 0.2837 | ||
| B | 134 (81.71) | 126 (86.30) | |
| C | 30 (18.29) | 20 (13.7) | |
| GS and Arg1 in HCC | 0.6122 | ||
| GS−Arg1− | 19 (17.68) | 22 (15.07) | |
| GS−Arg1+ | 29 (17.68) | 19 (13.01) | |
| GS+Arg1− | 66 (40.24) | 60 (41.10) | |
| GS+Arg1+ | 50 (30.48) | 45 (30.82) |
Data are presented as n (%) or median (interquartile range). ALB: Albumins; ALT: Alanine aminotransferase; AST: Aminotransferase; AFP: Alpha-fetoprotein; Arg1: Arginase 1; BCLC: Barcelona Clinic Liver Cancer; GGT: Glutamyltransferase; GS: Glutamine synthetase; GS−: GS negative; GS+: GS positive; HCC: Hepatocellular carcinoma; MVI: Microvascular invasion; PLT: Platelet count; TBIL: Total bilirubin; TP: Total protein.
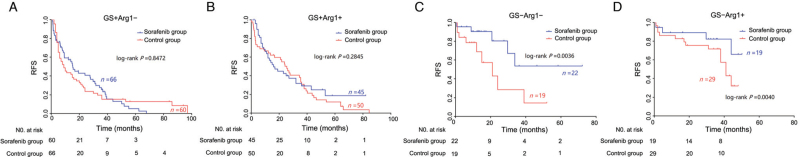
Notably, for GS− patients, Kaplan-Meier curves showed significant differences in RFS between the sorafenib group and the control group (HR in the sorafenib group, 0.46; 95% CI, 0.23–0.93; P = 0.0042). The 1- and 3-year RFS rates of the sorafenib group were 89.9% and 71.7%, respectively, and they were 78.2% and 60.9% in the control group, respectively [Figure 2A]. Among GS− patients, nine cases of recurrence (22.0%) occurred in the sorafenib group during the follow-up period; recurrence occurred in 41.7% of GS− patients (20/48) in the control group. In contrast, GS+ patients did not show any effective response to sorafenib treatment (P = 0.6533; Figure 2B). When the patients were stratified according to Arg1 expression, Kaplan-Meier curves indicated no significant therapeutic effect of sorafenib across both subgroups [Figure 2C,D]. We further analyzed the sorafenib response in the four subgroups stratified according to combined immunostaining of GS and Arg1. Interestingly, only GS− HCC patients exhibited a good response to sorafenib, regardless of the Arg1 expression status [Figure 4].
Figure 4.
Kaplan–Meier curves showing RFS according to combined immunostaining of GS and Arg1 in patients who received surgery alone or surgery combined with adjuvant sorafenib. Arg1: Arginase 1; GS: Glutamine synthetase; RFS: Recurrence-free survival.
Molecular characteristics and gene signatures of GS+ HCC
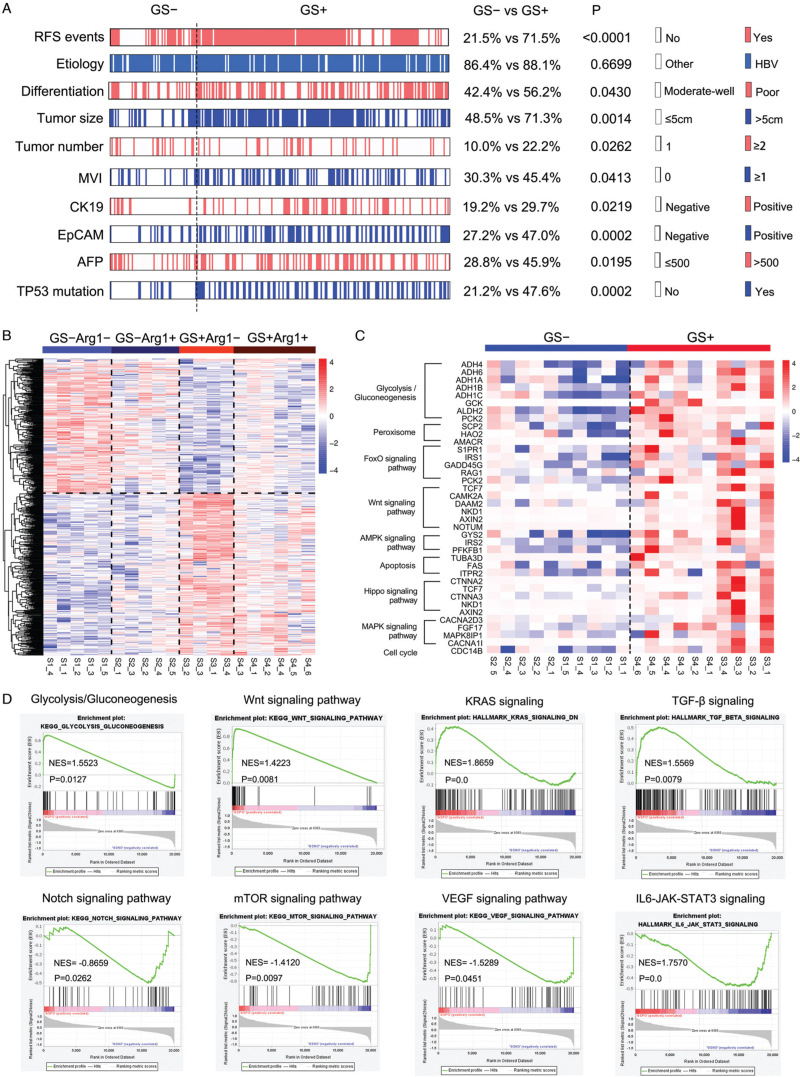
Our data suggest that HCC tumors with different GS and Arg1 expression levels have distinct biological behaviors, leading to different prognoses and responses to targeted therapy. Because GS expression has better significance for guiding prognostication and targeted therapy, we first analyzed expression of the progenitor markers CK19 and EpCAM, both of which have been adopted in molecular classifications of HCC, and high expression of these markers often indicates poor prognosis.[22,23] Indeed, the rates of positive CK19 and EpCAM expression in GS+ patients were significantly higher than those in GS− patients [Figure 5A and Supplementary Figure 4]. Moreover, GS+ patients exhibited poorer differentiation and higher AFP levels as well as much more frequent TP53 mutations [Figure 5A].
Figure 5.
Analysis of gene expression profiles in HCC tumors with different GS and Arg1 expression levels. (A) Heatmap of multiple indicators after grouping according to GS expression in 251 HCC patients who received only hepatectomy. (B) Heatmap of differentially expressed genes. (C) Abnormal activation of multiple signaling pathways in GS+ HCC tumors. (D) GSEA graphs depicting enrichment of Wnt, KRAS, and TGF-β in GS+ tumors and of Notch, mTOR, and JAK-STAT3 in GS− tumors. Arg1: Arginase 1; GSEA: Gene set enrichment analysis; GS: Glutamine synthetase; GS−: GS negative; GS+: GS positive; HCC: Hepatocellular carcinoma.
We next conducted mRNA sequencing to determine gene expression profiles of the four subgroups of HCC tumors. The heatmap showed significant differences in gene expression profiles between the four subgroups, with the GS expression status having a more notable impact on the profiles than the Arg1 expression status [Figure 5B]. Compared with GS− tissues, GS+ HCC tumors displayed aberrant activation of multiple tumor-related signaling pathways, such as the FoxO, Wnt, AMPK, MAPK, Hippo, and cell cycle signaling pathways [Figure 5C]. We then performed gene set enrichment analysis to more comprehensively describe the characteristics of GS− and GS+ HCC tumors. GS+ HCC tumors showed upregulated signatures related to glucose metabolism, TGF-β signaling, Wnt and KRAS signaling, whereas the mTOR, Notch and JAK-STAT3 signaling pathways were downregulated [Figure 5D].
Discussion
A clinically applicable molecular stratification that can predict prognosis and guide systematic therapy in HCC is still lacking. In the current study, we found that GS+ HCC tumors had a poorer prognosis than GS− HCC tumors. When patients with a high risk of recurrence were not molecularly stratified, adjuvant treatment with sorafenib after radical hepatectomy did not result in a significant improvement in RFS. Nonetheless, GS− patients, who accounted for <30% of all the HCC patients, were able to benefit from sorafenib treatment. Moreover, GS+ HCC tumors exhibited high AFP levels and immunostaining of CK19, EpCAM, and mutant TP53, as well as poor differentiation and aberrant activation of multiple tumor-related signaling pathways.
HCC can be confidently diagnosed with imaging techniques and serum-based methods. In general, the risk of complications, such as tumor seeding and bleeding, has limited the application of liver biopsy in HCC diagnosis.[24,25] As a result, liver biopsy is not routinely performed for HCC patients according to the current guidelines, which might have hampered the development of molecular stratification schemes for HCC that can define prognosis and guide targeted therapies. In the normal liver, GS+ hepatocytes account for a very small proportion of total hepatocytes; however, GS expression is particularly dramatically upregulated in advanced HCC.[17–19] Expression of GLUL, the gene encoding GS and a downstream target of Wnt/β-catenin signaling, is enhanced in HCC due to the high frequency of activating mutations of CTNNB1.[26] GLUL is also transcriptionally regulated through PI3K-AKT1-FoxO signaling.[27] By catalyzing synthesis of glutamine, which provides carbon and nitrogen for various biosynthetic processes, GS has also been found to play an important role in promoting tumor development. A previous study showed that GS promotes nucleotide synthesis to facilitate repair of damaged DNA and thus promote the growth of cancer cells after radiation.[28] It is speculated that GS expression increases with the progression of HCC; however, we only observed a slight but insignificant increase in the positive rate of GS expression in BCLC stage B/C HCC tumors compared to stage A HCC tumors, which suggests that GS expression might be determined at the initiation of HCC. It would be interesting to identify whether these GS+ HCC tumors primarily originate from pericentral hepatocytes (GS+) or Arg1+ hepatocytes that obtained GS expression with activation of Wnt/β-catenin signaling. Recently, a lineage-tracing strategy targeting GS+ cells has shown the role of GS+ cells in liver repopulation; however, their fate in tumorigenesis requires further examination.[29,30]
As mentioned above, GS promotes nucleotide synthesis to facilitate damaged DNA repair and promote breast cancer cell growth after radiation. In addition, human HCC cell lines that highly express GS exhibit strong drug resistance and tend to be aggressive.[31] The benefit of sorafenib treatment as adjuvant therapy after hepatectomy or ablation is still under debate. In agreement with the STORM trial,[9] we did not observe that adjuvant treatment with sorafenib improved RFS in BCLC stage B/C patients when they were not molecularly stratified. Strikingly, GS− patients had much better RFS with sorafenib treatment after hepatectomy, suggesting that overexpression of GS plays a role in promoting cancer resistance to tyrosine kinase inhibitor (TKI) challenge. Although the mechanism by which GS promotes drug resistance is still incompletely understood, our findings provide a simple way to identify potentially sensitive patients.
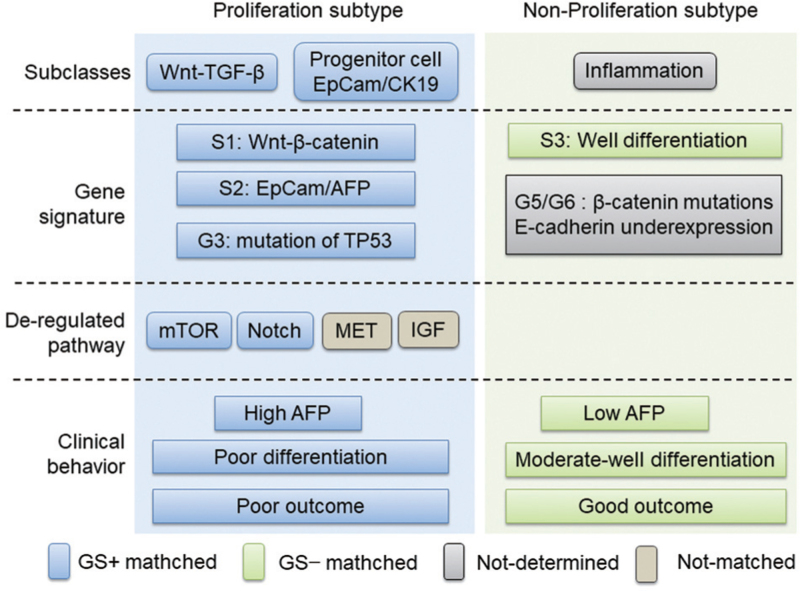
We noted that GS+ HCC tumors share many cellular and molecular characteristics and gene signatures with the well-established proliferation subtype of HCC, such as poor differentiation, high AFP level, high frequency of immunostaining of progenitor markers (CK19 and EpCAM) and mutant TP53, and increased signaling pathway dysregulation, in line with the established S1/S2 class and G3 class. In contrast, GS− HCC tumors tended to share characteristics with the non-proliferation subtype of HCC, as characterized by S3 and G5/G6 signatures, a moderately to well-differentiated status and low AFP [Figure 6]. Collectively, immunostaining of GS might allow for rough grouping of HCC tumors into proliferation or non-proliferation subtypes, and given that GS immunostaining is widely performed in clinical pathology, our work is expected to provide a very simple and applicable approach to predict patient prognosis and guide targeted therapy.
Figure 6.
GS+ HCC tumors share similar features with proliferation subtype HCC tumors. GS: Glutamine synthetase; GS+: GS positive; HCC: Hepatocellular carcinoma.
There were some limitations that may confine our results. This study was retrospective and performed at a single center. The lack of control by randomization and blinding might limit the validity of our conclusions. In addition, the limited number of patients, especially GS− patients after subgrouping, is likely to lead to statistical bias. Most importantly, sorafenib was used as an adjuvant treatment following surgery in our study, and it is not clear whether a similar response can be achieved in GS− patients who have not undergone surgery. Therefore, a prospective controlled trial to address this is urgently needed. In addition, whether other TKIs used in HCC treatment, such as regorafenib and lenvatinib, can achieve better outcomes in GS− patients requires further analysis. Our work also highlights the requirement for routine pathological examination before targeted therapy, regardless of whether a patient is subjected to hepatectomy.
Acknowledgments
We thank Changli Lu for the pathological evaluation and guidance. We thank Jie Chen for discussion. We would also like to acknowledge West China Biobanks for their help in providing HCC tissues. We are grateful to Shanghai Oe Biotech Co., Ltd. for providing sequencing services.
Funding
The project was supported by grants from the Natural Science Foundation of China (No. 82072689), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. 2020HXFH010), the China Postdoctoral Science Foundation Grant (No. 2021M692304), and the PostDoctor Research Project, West China Hospital, Sichuan University (Nos. 2020HXBH069, 2021HXBH031).
Conflicts of interests
None.
Supplementary Material
Footnotes
How to cite this article: Shao M, Tao Q, Xu Y, Xu Q, Shu Y, Chen Y, Shen J, Zhou Y, Wu Z, Chen M, Yang J, Shi Y, Wen T, Bu H. Glutamine synthetase-negative hepatocellular carcinoma has better prognosis and response to sorafenib treatment after hepatectomy. Chin Med J 2023;136:2066–2076. doi: 10.1097/CM9.0000000000002380
Supplemental digital content is available for this article.
References
- 1.Bae SDW, George J, Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin Med J 2022; 135:547–556. doi: 10.1097/CM9.0000000000002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J 2021; 134:2911–2921. doi: 10.1097/CM9.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei ZQ, Zhang YW. Transcatheter arterial chemoembolization followed by surgical resection for hepatocellular carcinoma: a focus on its controversies and screening of patients most likely to benefit. Chin Med J 2021; 134:2275–2286. doi: 10.1097/CM9.0000000000001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Pinyol R, Montal R, Bassaganyas L, Sia D, Takayama T, Chau GY, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut 2019; 68:1065–1075. doi: 10.1136/gutjnl-2018-316408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Zhao G, Wei K, Zhang Q, Ma W, Song T, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends 2014; 8:333–338. doi: 10.5582/bst.2014.01120. [DOI] [PubMed] [Google Scholar]
- 8.Liao Y, Zheng Y, He W, Li Q, Shen J, Hong J, et al. Sorafenib therapy following resection prolongs disease-free survival in patients with advanced hepatocellular carcinoma at a high risk of recurrence. Oncol Lett 2017; 13:984–992. doi: 10.3892/ol.2016.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015; 16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 10.Tan W, Luo X, Li W, Zhong J, Cao J, Zhu S, et al. TNF- α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2019; 40:446–456. doi: 10.1016/j.ebiom.2018.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017; 67:727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 2011; 140:1501.e–1512.e. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018; 15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019; 380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Electronic address: wheeler@bcm.edu, Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 2017; 169:1327.e–1341.e. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebhardt R, Coffer PJ. Hepatic autophagy is differentially regulated in periportal and pericentral zones-a general mechanism relevant for other tissues? Cell Commun Signal 2013; 11:21.doi: 10.1186/1478-811X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, et al. Gadoxetic acid-enhanced magnetic resonance imaging reflects co-activation of β-catenin and hepatocyte nuclear factor 4α in hepatocellular carcinoma. Hepatol Res 2018; 48:205–216. doi: 10.1111/hepr.12911. [DOI] [PubMed] [Google Scholar]
- 18.Dal Bello B, Rosa L, Campanini N, Tinelli C, Torello Viera F, D’Ambrosio G, et al. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res 2010; 16:2157–2166. doi: 10.1158/1078-0432.CCR-09-1978. [DOI] [PubMed] [Google Scholar]
- 19.Osada T, Nagashima I, Tsuno NH, Kitayama J, Nagawa H. Prognostic significance of glutamine synthetase expression in unifocal advanced hepatocellular carcinoma. J Hepatol 2000; 33:247–253. doi: 10.1016/s0168-8278(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 20.Ying L, Cheng M, Lu Y, Tao Q, Chen X, Shen B, et al. Glutamine metabolism scoring predicts prognosis and therapeutic resistance in hepatocellular carcinoma. Pathol Oncol Res 2021; 27:1610075.doi: 10.3389/pore.2021.1610075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022; 76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 2006; 12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 23.Rhee H, Kim H, Park YN. Clinico-radio-pathological and molecular features of hepatocellular carcinomas with keratin 19 expression. Liver Cancer 2020; 9:663–681. doi: 10.1159/000510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shioga T, Kondo R, Ogasawara S, Akiba J, Mizuochi S, Kusano H, et al. Usefulness of tumor tissue biopsy for predicting the biological behavior of hepatocellular carcinoma. Anticancer Res 2020; 40:4105–4113. doi: 10.21873/anticanres.14409. [DOI] [PubMed] [Google Scholar]
- 25.Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol 2018; 24:4000–4013. doi: 10.3748/wjg.v24.i35.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goessling W. Position is destiny: metabolism and cell identity. Cell Metab 2019; 29:1017–1019. doi: 10.1016/j.cmet.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Vos KE, Coffer PJ. Glutamine metabolism links growth factor signaling to the regulation of autophagy. Autophagy 2012; 8:1862–1864. doi: 10.4161/auto.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu S, Li Z, Xiao L, Hu W, Zhang L, Xie B, et al. Glutamine synthetase promotes radiation resistance via facilitating nucleotide metabolism and subsequent DNA damage repair. Cell Rep 2019; 28:1136.e–1143.e. doi: 10.1016/j.celrep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 29.He L, Pu W, Liu X, Zhang Z, Han M, Li Y, et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science 2021; 371:eabc4346.doi: 10.1126/science.abc4346. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Wang YG, Jia Y, Li L, Yoon J, Zhang S, et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021; 371:eabb1625.doi: 10.1126/science.abb1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadoret A, Ovejero C, Terris B, Souil E, Lévy L, Lamers WH, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 2002; 21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.