Abstract
Since 2017, the number of agents for acute myeloid leukemia (AML) has rapidly expanded. Given the increased therapeutic options, better identification of high-risk subsets of AML and more refined approaches to patient fitness assessment, the decisions surrounding selection of intensive chemotherapy versus lower-intensity treatment have grown increasingly more nuanced. In this review, we present available data for both standard and investigational approaches in the initial treatment of AML using an intensive chemotherapy backbone or a lower-intensity approach. We summarize management strategies in newly diagnosed secondary AML, considerations around allogeneic stem-cell transplantation, and the role of maintenance therapy. Finally, we highlight important areas of future investigation and novel agents that may hold promise in combination with standard therapies.
INTRODUCTION
Acute myeloid leukemia (AML), characterized by the clonal expansion of myeloid blasts in the blood and bone marrow, is the most common form of leukemia in adults with approximately 20,000 new cases diagnosed annually in the United States.1 Since 2017, the armamentarium for AML has expanded to include new frontline strategies for older or unfit patients, mutation-targeted agents for specific disease subsets, and maintenance agents. Here, we summarize the current evidence informing treatment decisions in the frontline therapy of AML. We review factors influencing the selection of intensive versus lower-intensity regimens, novel combination approaches, and pressing management questions in need of prospective data.
FACTORS IN THE ASSESSMENT OF INTENSIVE VERSUS LOWER-INTENSITY TREATMENT
The median age of diagnosis for patients with AML in the United States is 68 years.2 Patients older than age 60 years suffer worse survival because of higher treatment-related mortality and inherent resistance of disease to intensive chemotherapy (IC).3-5 Owing to these considerations and the recent development of lower-intensity treatment regimens with considerable efficacy, initial assessment of a patient with AML requires careful determination of their candidacy for IC.
Several patient-related factors play into this determination. An analysis of 1,127 patients with AML on Southwestern Oncology Group protocols and 2,238 patients treated on MD Anderson protocols identified age and performance status as the most significant predictors of treatment-related mortality; however, age may primarily serve as a surrogate of patient-specific comorbidities and disease-specific factors.6 In an analysis of 998 patients with AML or myelodysplastic syndrome (MDS) treated with IC, patients with an Eastern Cooperative Oncology Group performance status of 0-1 (n = 629) had an 8-week mortality of 23% and 1-year overall survival (OS) of 35%, compared with 72% and 7%, respectively, in those with an Eastern Cooperative Oncology Group of 3-4 (n = 120).7 Physicians routinely gauge patient fitness on the basis of intuition, a subjective process prone to bias. Consensus-based criteria have been developed to help add objectivity to the identification of patients at risk of significant toxicity from IC including the Ferrara criteria.8 Subsequent analysis of patients treated with IC found a median OS of 4.8 months in patients with criteria defining unfitness for IC compared to a median OS of 36.8 months in patients meeting no unfitness criteria.9 Geriatric assessment (GA) is a valuable tool in predicting outcomes as well. A GA composed of cognitive testing, psychological function, physical function, and comorbid conditions in patients older than 60 years (n = 74) ultimately treated with IC identified impaired cognition and physical function as risk factors for poorer OS.10 A separate prospective study of GA in patients older than 60 years treated with IC (n = 105) also confirmed the negative impact of impairment in a GA domain on OS.11 These data highlight that age alone should not guide decision making around the use of IC in frontline management of AML.
Even in patients who are considered candidates for IC, disease-specific factors should be taken into consideration as well. Higher-risk disease as defined by cytogenetics and molecular mutations may not benefit from IC.12 Given the advancements of effective lower-intensity regimens in AML, it is becoming increasingly complex to identify the patients most likely to benefit from IC.13 The Acute Leukemia French Association 1,200 investigators investigated outcomes of patients 60 years or older with newly diagnosed AML treated with IC by integrating the European Leukemia Net (ELN) 2017 risk classification with mutation status in seven genes. On the basis of this, patients were stratified into those whose predicted outcome was favorable (2-year OS 66.1%), intermediate (2-year OS 39%), and adverse (2-year OS 2.8%), reflecting marked chemoresistance in the latter subset.14 A single-center prospective study integrating GA along with genetic markers in the selection of therapy for adults 60 years or older with AML (n = 28) demonstrated feasibility of this approach with a 30-day mortality of 4% and 1-year OS of 66%.15 Finally, a propensity-matched analysis of patients receiving IC compared with hypomethylating agent (HMA) with venetoclax (VEN) suggested benefit of HMA-VEN over IC in certain high-risk populations such as those with RUNX1 mutations.16 Treatment approaches in high-risk subsets of AML such as secondary AML (sAML) and TP53-mutated AML that have poor outcomes with IC are discussed later in this review. Given the importance of genetic and cytogenetic markers in identifying patients who are most likely to benefit from IC, obtaining this information before initiating therapy should be considered and can be safely done.17 In summary, treatment recommendations in newly diagnosed AML should go beyond age and patient-related factors but also need to incorporate disease-related factors that may predict for those patients most likely to benefit from IC.
INTENSIVE CHEMOTHERAPY APPROACHES
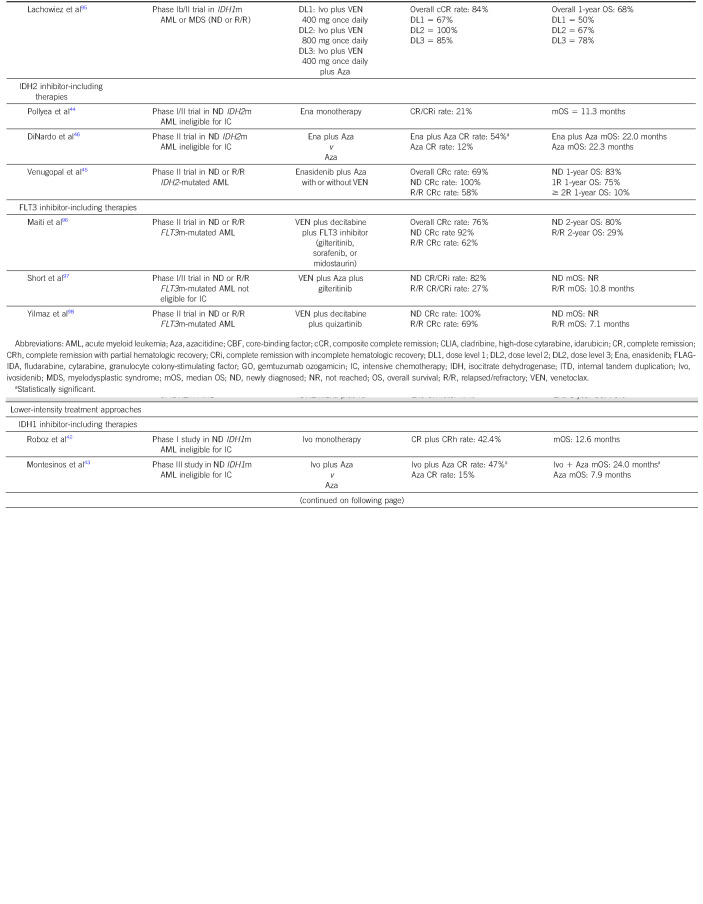
IC is considered the treatment of choice in young and/or fit patients. For decades, the 7 + 3 (7 days of continuous cytarabine combined with 3 days of an anthracycline) induction regimen followed by high-dose cytarabine (HIDAC) consolidation was considered the standard-of-care intensive approach. In recent years, alternative IC backbones have emerged, leading to numerous options without a clear consensus on the favored regimen among them.18 Given the variation in IC regimens used across institutions, we will focus instead on the addition of novel agents to an IC backbone (Table 1).
TABLE 1.
Select Novel Approaches in Frontline Treatment of AML
Midostaurin and FLT3-Mutated AML
Patients with FLT3-mutated AML are candidates for midostaurin, an oral multitargeted kinase inhibitor, in combination with IC. The phase III RATIFY trial randomly assigned patients with FLT3 internal tandem duplication or a point mutation in the tyrosine kinase domain of the protein to 7 + 3 induction, followed by consolidation and maintenance with or without midostaurin. Patients in the midostaurin arm enjoyed a significantly longer median OS of 75 months compared with 26 months in the control arm. Notably, 57% of patients on trial underwent allogeneic hematopoietic cellular transplantation (allo-HCT); 28.1% of patients in the midostaurin group underwent allo-HCT in first complete remission (CR1), and 22.7% of patients in the placebo group underwent allo-HCT in CR1.When data were censored at the time of allo-HCT, the 4-year OS in the midostaurin group was 64% compared with 56% in the control group (P = .08).19
Although the inclusion of midostaurin to IC in FLT3-mutated AML is the current standard of care, investigation of second-generation FLT3 inhibitors in the frontline setting is ongoing.20 The phase III QuANTUM-First trial (NCT02668653) investigating quizartinib combined with IC versus IC alone in patients with FLT3-internal tandem duplication–mutated AML demonstrated superior median OS in the quizartinib arm (31.9 months v 15.1 months) and similar rates of CR/CR with incomplete hematologic recovery (CRi) (71.6% v 64.7%).21 Phase III trials comparing IC with gilteritinib and IC with crenolanib against IC with midostaurin are also underway (NCT03836209 and NCT03258931).
Gemtuzumab Ozogamicin
Intensive regimens have been combined with gemtuzumab ozogamicin (GO). GO is an antibody-drug conjugate targeting CD33, a transmembrane receptor expressed in most cases of AML. Although initial data on the addition of GO to standard chemotherapy demonstrated increased mortality and led to the drug being withdrawn from market, more recent studies using a lower and fractionated dose have demonstrated benefit in specific disease subsets. The benefit of GO combined with IC appears to be most well defined in core-binding factor (CBF) AML, which is characterized by the presence of either t(8;21)(q22;q22) or inv(16)(p13q22)/t(16;16) and is classified as favorable risk.12,22,23 A meta-analysis of five randomized trials reported significantly longer 5-year OS in favorable risk AML treated on GO arms compared with non-GO containing arms (76% v 55%), with the primary driver of improved survival being reduced rates of relapse.24 Another approach being investigated in CBF AML is the addition of dasatinib, a multikinase inhibitor targeting receptor tyrosine kinase, to standard IC. Early-phase studies have demonstrated high CR rates and durable OS with this approach.25,26
GO has also been prospectively investigated in NPM1-mutated AML. The AMLSG 09-09 study randomly assigned patients age 18 years or older with newly diagnosed NPM1-mutated AML to receive IC with or without GO with the primary end point of event-free survival (EFS). There was no significant difference in EFS with a 2-year EFS of 52.6% in the standard arm and 58.1% in the GO arm. Two-year cumulative incidence of relapse in patients achieving a CR/CRi was lower in the GO arm. However, cumulative incidence of death at 2 years was similar in the two arms (8.3% GO arm, 7.1% standard arm), and the early death rate was 10.7% in the GO arm compared with 5.7% in the standard arm (P = .05).27 Of note, the spleen tyrosine kinase inhibitor entospletinib has demonstrated favorable efficacy in early-phase trials combining it with IC for NPM1-mutated AML, and a randomized phase III trial is currently underway (NCT05020665).28
Venetoclax
Given the efficacy of VEN, an oral selective B-cell leukemia/lymphoma-2 inhibitor, as part of non-IC regimens for AML, there is much interest in exploring its role in combination with IC as well. Most recently, Wang et al29 evaluated VEN combined with 7 + 3; CR rate was 91% with nearly all patients testing negative for measurable residual disease (MRD) at the end of induction. A propensity score–matched, cohort study of two such ongoing trials30,31 found improved CR rates and EFS in patients treated with VEN-containing IC regimens as compared with IC alone but no significant difference in OS,32 highlighting that these promising single-arm results must be evaluated prospectively against current standards of care.
IDH1- and IDH2-Mutated AML
Other agents being investigated in combination with IC include the isocitrate dehydrogenase (IDH)1 and IDH2 inhibitors, ivosidenib and enasidenib, respectively. A phase I study investigated ivosidenib with 7 + 3 for patients with newly diagnosed IDH1-mutated AML and enasidenib with 7 + 3 for patients with newly diagnosed IDH2-mutated AML. Among patients treated with IC plus ivosidenib, the CR rate was 55% with no dose-limiting toxicities while CR rate among patients treated with IC plus enasidenib was 47% with one dose-limiting toxicity observed. Median OS was not reached for the IDH1-mutated cohort and was 26 months for the IDH2-mutated cohort.33 Although the combination of an IDH inhibitor with IC appears to be safe and efficacious, randomized, prospective analyses of these regimens are needed to identify the benefit of IDH inhibition in the frontline setting versus at the time of relapse or progression. The HOVON150AML study is a randomized trial currently seeking to answer this question (NCT03839771).
LOWER-INTENSITY REGIMENS IN AML
As discussed, IC may be associated with significant morbidity and mortality; thus, the development of efficacious lower-intensity regimens has been an active area of investigation.
Hypomethylating Agents With Venetoclax
HMAs are often used as a backbone for lower-intensity therapy. Azacitidine and decitabine monotherapy have demonstrated clinical benefit over low-dose cytarabine (LoDAC) in AML,34-36 although survival with this approach is quite limited.37 This has led to the exploration of combination therapy. VIALE-A, a randomized phase III trial comparing azacitidine with and without VEN in patients with newly diagnosed AML ineligible for IC, demonstrated that the combination led to significantly improved OS (14.7 v 9.6 months). Significantly improved median OS was also seen in sAML (16.4 v 10.6 months) and intermediate-risk AML (20.8 v 12.4 months).38 A single-center propensity-matched cohort analysis sought to identify subgroups that may benefit from azacitidine-VEN in comparison with IC. Factors that favored azacitidine-VEN included age 65 years or older, high-risk disease by ELN 2017 criteria, and RUNX1 mutations although ELN 2017 intermediate-risk disease had more favorable outcomes with IC.16 Although this doublet has become the standard lower-intensity regimen for newly-diagnosed AML in patients who are not candidates for IC, its impact in other subsets is still being ascertained. HMA-VEN is being investigated in younger patients with adverse-risk AML, and a phase II study of HMA-VEN in patients age 18-59 years with ELN 2017 adverse-risk disease is ongoing. Interim analysis of 14 patients demonstrated a CR/CR with partial hematologic recovery rate of 64% compared with a historical CR/CR with partial hematologic recovery rate of 38%.39 Use of HMA-VEN in other high-risk AML subsets such as sAML and TP53-mutated AML is discussed later in this review.
Targeted Therapy
Targeted therapies (with FDA-approved drugs available against mutated FLT3, IDH1, and IDH2) represent an opportunity for delivering treatment efficacy with tolerable toxicity in patients with AML ineligible for IC. The phase III LACEWING trial evaluated gilteritinib plus azacitidine versus azacitidine monotherapy in patients with FLT3-mutated AML ineligible for IC; the primary end point of median OS was not significantly different across arms (approximately 9 months) despite higher response rates in the gilteritinib-containing arm.40 In contrast, pooled analyses of patients with newly diagnosed FLT3-mutated AML treated with HMA-VEN demonstrate a median OS of 12 months41 Given these data, there is much interest in the investigation of FLT3 inhibitors combined with the HMA-VEN backbone (Table 1).
Ivosidenib monotherapy was evaluated as up-front treatment in patients with IDH1-mutated AML ineligible for IC; composite CR rate (CR + CRi) was 42.4% with a median OS of 12.6 months, results that supported its approval as first-line treatment in this population.42 A phase III trial randomly assigned a similar population to azacitidine with and without ivosidenib; the combination arm showed significantly improved 12-month EFS (37% v 12%) and OS (24.0 v 7.9 months).43 An early-phase trial of enasidenib monotherapy in patients with IDH2-mutated, newly diagnosed AML ineligible for IC demonstrated an overall response rate of 31% and a median OS of 11.3 months.44 Enasidenib combined with azacitidine is also under investigation in the frontline setting: a phase II trial demonstrated promising response rates, with a composite CR rate of 100% in the seven patients with newly-diagnosed AML.45 A randomized phase II study of azacitidine with or without enasidenib demonstrated a significantly higher response rate in the combination arm (74% v 36%); however, median OS was similar in both arms (approximately 22 months).46 Using these data in practice presents a challenge, as HMA-VEN has been shown to be especially beneficial in patients with IDH1 or IDH2 mutations.47 A direct comparison of these targeted strategies with HMA-VEN would provide more clarity on the optimal sequence of these therapies. Trials are also incorporating targeted therapy into VEN-based regimens in newly diagnosed AML patients. Available data for these combination approaches are summarized in Table 1.
Low-Dose Cytarabine Combination Therapy
In unfit patients with newly diagnosed AML, other low intensity chemotherapy backbones can be considered. Although LoDAC as a single agent has shown limited antileukemia activity,48 it has been used in combination with other agents with more favorable results. Although LoDAC with glasdegib and LoDAC with VEN have demonstrated improved OS compared with LoDAC alone,49,50 the efficacy of azacitidine-VEN has established it as the standard of care lower-intensity regimen in the frontline setting.
Another lower-intensity regimen investigated in the frontline setting is the combination of adenosine nucleoside analogues with LoDAC alternating with decitabine. A combined analysis of two phase II trials of clofarabine or cladribine combined with LoDAC alternating with decitabine in patients with newly diagnosed AML age 60 years or older and unfit (n = 248) showed an overall response rate of 66%. With a median follow-up of 60 months, median OS was 12.5 months and survival among older adults compared favorably with historical controls when stratified by age. The 4- and 8-week mortality rates were 2% and 11%, respectively.30,51,52 In addition, a phase II study investigating alternating cycles of LoDAC plus cladribine plus VEN with cycles of azacitidine plus VEN demonstrated a composite CR rate of 93% with median OS not reached at a median follow-up of 22 months.53
SECONDARY AML AND THERAPY-RELATED AML
Although the 2022 WHO and International Consensus Classification criteria do not have a specific diagnosis for sAML, we will define it as AML arising from an underlying hematologic condition (eg, MDS or myeloproliferative neoplasm).22,23,54 Cases of AML that develop in patients with previous exposure to cytotoxic chemotherapy or radiotherapy will be defined as therapy-related AML (t-AML).22,23,55 Because of the particularly poor OS in these subsets of AML and TP53-mutated AML, there is significant interest in the development of novel therapeutic strategies for such patients in the frontline setting.56-59
CPX-351, a liposomal encapsulation of cytarabine and daunorubicin, has been prospectively investigated in t-AML, AML arising from MDS or chronic myelomonocytic leukemia, and AML with MDS-related cytogenetic abnormalities. Lancet et al conducted a phase III trial of CPX-351 compared with 7 + 3 in this patient population (age 60-75 years) and demonstrated an improved CR rate (37% v 26%) and improved OS (9.5 v 5.9 months). Median time to count recovery after induction was longer in the CPX-351 arm with similar rates of neutropenic fever in both arms. Long-term follow-up demonstrated a 5-year OS rate of 18% in the CPX-351 group and 8% in the 7 + 3 group.60 Thirty-five percent of patients had TP53 mutations, and median OS among these patients was not significantly different in the CPX-351 and 7 + 3 arms (4.5 v 5.1 months).61 Of note, both reinduction and consolidation therapy in the 7 + 3 group was 5 days of continuous cytarabine with 2 days of daunorubicin (5 + 2) as opposed to HIDAC.
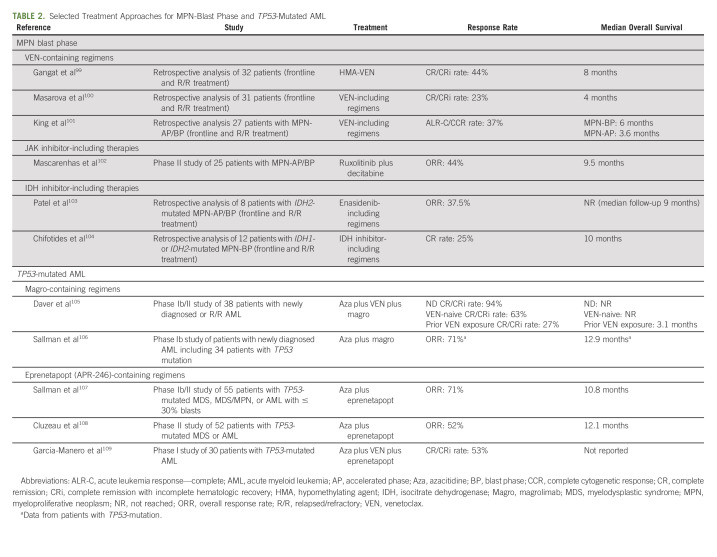
Recent analyses comparing CPX-351 with other treatment strategies support clinical equipoise in this subtype of disease. A retrospective analysis comparing HIDAC and purine analogue–based regimens with CPX-351 in sAML, AML with MDS-related cytogenetics, and t-AML demonstrated similar OS in both cohorts.62 A multicenter retrospective analysis recently compared outcomes of CPX-351 versus HMA-VEN in newly diagnosed AML; however, this was not restricted to the patient population investigated in the phase III CPX-351 trial. Median OS was higher in the CPX-351 group, but when controlling for rates of allo-HCT, survival was similar.63 Another analysis demonstrated a similar median OS in patients treated with CPX-351 compared with HMA-VEN (13 v 11 months, P = .22).64 Prospective analyses of CPX-351 versus HMA-VEN or HIDAC-based regimens are needed for these subsets of disease. Novel therapies in sAML have also had limited success. Similarly poor outcomes are noted in patients with TP53-mutated AML, which is significantly enriched among sAML/t-AML. An analysis of 291 patients with TP53-mutated AML found a median OS of < 10 months across all treatment approaches, including a median OS of 9.2 months for patients treated with 7 + 3 and 6.7 months for those treated with HMA-VEN.56 Table 2 summarizes select outcomes data in sAML and TP53-mutated AML with novel treatment approaches.
TABLE 2.
Selected Treatment Approaches for MPN-Blast Phase and TP53-Mutated AML
TRANSPLANT CONSIDERATION/MAINTENANCE THERAPIES IN FIRST REMISSION
Minimizing time from AML diagnosis to allo-HCT should be the treating physician's goal, as time to allo-HCT affects survival,65,66 and each consolidation cycle confers accumulating toxicity with no proven benefit in those destined for transplant.67,68 Considering the weeks required for donor arrangements and rigorous patient evaluations, consultation with a transplant physician should occur at time of diagnosis which, in most circumstances, means while the patient is still hospitalized.
In appropriate transplant candidates, allograft in first remission is widely recommended in all subtypes of AML except favorable risk disease without MRD at the end of induction.69 Even so, there are notable exceptions: Favorable risk features appear to be less advantageous with increasing age, with older patients faring worse due in part to inherent chemoresistance70-72; and co-occurring mutations influence prognosis and refine existing prognostic schemes.73 Molecular characteristics at diagnosis in patients 60 years or older with AML who undergo allo-HCT in CR1 are predictive of leukemia-free survival and persistence of MRD.74 These data should, therefore, be integrated into the transplant decision.
The question of age and its relationship to transplant candidacy is frequently encountered given the median age of AML onset. Allo-HCT can be well tolerated in older adults including carefully selected septuagenarians,75,76 with the GA used to assess physiological age77,78; biological age alone does not, therefore, represent a barrier to transplant referral. Reduced intensity conditioning enables harnessing the graft-versus-leukemia effect while minimizing the toxicity of allo-HCT, expanding the pool of candidates who may benefit from this therapy.79,80 Although there are not randomized data in this regard, an analysis of adults age 60-75 years treated on National Clinical Trials Network protocols demonstrated higher transplant-related mortality in patients who underwent allo-HCT but also improved 5-year OS compared with those who received consolidation alone (29% v 14%).81 Analysis of adults age 60-70 years treated on the National Cancer Research Institute AML16 trial who achieved a CR/CRi demonstrated similar results, with a significantly improved 5-year OS in patients who underwent allo-HCT compared with those treated with chemotherapy (37% v 20%, P < .0001).82
Finally, for those not candidates for allo-HCT, oral azacitidine represents the only approved maintenance strategy in AML, indicated for nontransplant candidates in remission after intensive induction chemotherapy with or without consolidation.83 Oral azacitidine extended survival compared with placebo in favorable risk subgroups such as mutated NPM1 and in patients at high risk of relapse on the basis of FLT3 or MRD status.84,85 Importantly, the majority of patients in this study received 0-1 cycles of consolidation so the added benefit of oral azacitidine after optimal consolidation is unknown. Maintenance FLT3 inhibition remains an unproven strategy as existing studies looking at the addition of a FLT3 inhibitor to frontline therapy did not include a second random assignmet at the time of maintenance.19,86,87 Patients in remission after lower-intensity therapy including HMA with or without VEN or targeted inhibitors should continue therapy indefinitely on the basis of current available evidence.88
SUMMARY
Since 2017, the treatment landscape for AML has markedly changed. Our approach to newly diagnosed AML is summarized in Figure 1. In patients appropriate for IC, utilization of disease-specific factors should determine the addition of additional therapies to the IC backbone (eg, GO for CBF-AML, midostaurin for FLT3-mutated AML). Questions of high clinical relevance include the identification of AML subsets likely to benefit from novel therapies added to IC and whether lower-intensity therapy is the appropriate choice for high-risk subsets unlikely to benefit from IC such as TP53-mutated AML. For patients appropriate for a lower-intensity regimen, our preferred approach is the use of HMA-VEN. Utilization of novel agents in combination with HMA-VEN, incorporation of MRD negativity into clinical management in patients on lower intensity therapy, and identification of patients appropriate for a trial of therapy discontinuation are pressing areas of investigation.16,89,90 There is also much interest in the development of all-oral regimens for the treatment of newly diagnosed AML.91 As treatment paradigms continue to evolve to include indefinite therapies for AML, ensuring quality-of-life preservation alongside treatment efficacy is vital.92,93 Finally, a number of novel agents hold promise and are being investigated in combination with a variety of backbones. Some agents of note include the anti-CD47 antibody magrolimab, TP53 reactivator eprenetapopt, MDM2 inhibitors, and immunotherapeutic agents.94 In summary, treatment selection for newly diagnosed AML has become an increasingly nuanced decision; patient-specific factors and disease biology should be carefully considered and should inform novel combination approaches in the context of prospective trials.
FIG 1.
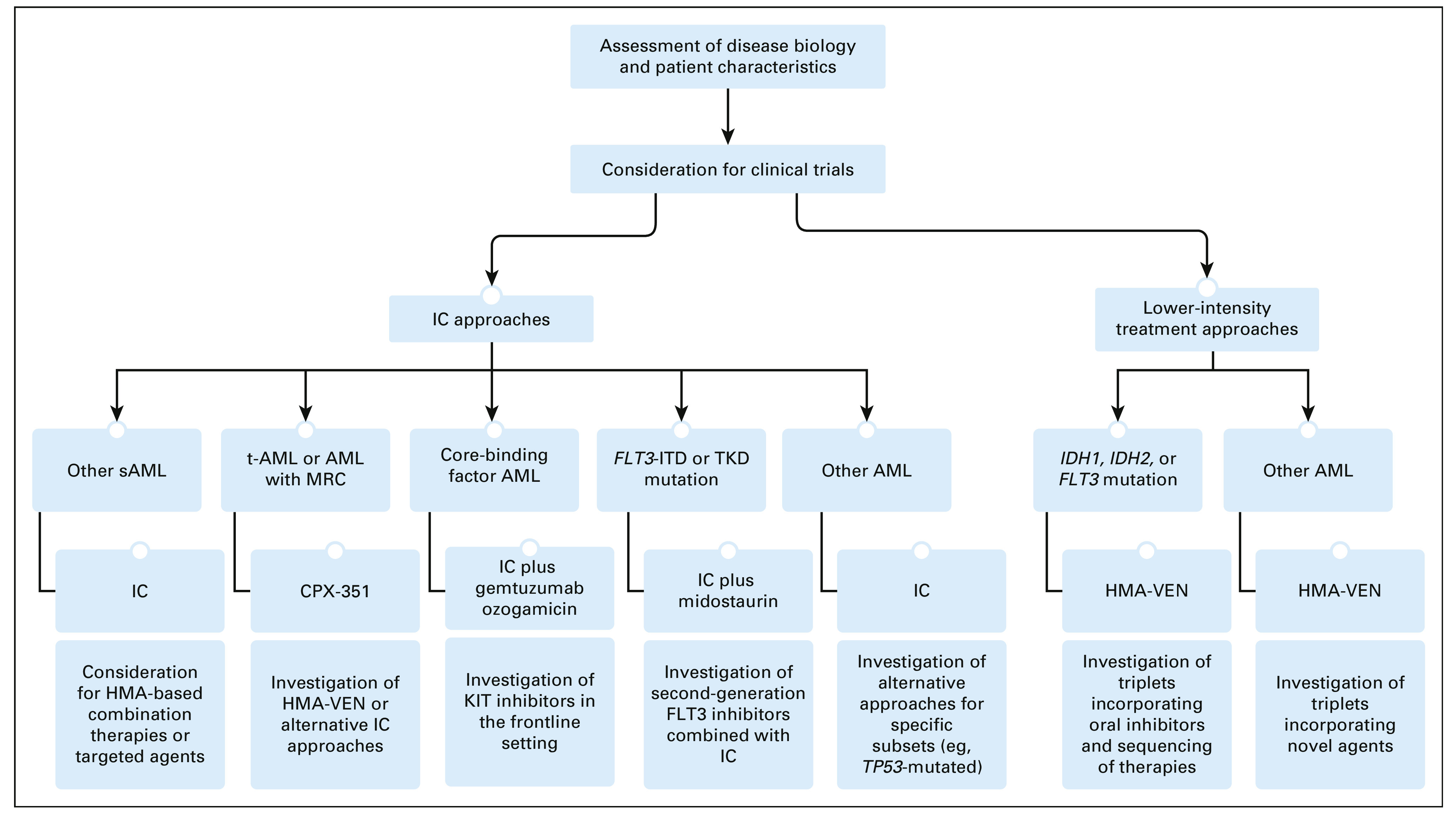
Our treatment approach to newly diagnosed AML and areas of investigation. HMA, hypomethylating agent; IC, intensive chemotherapy; ITD, internal tandem duplication; KIT, receptor tyrosine kinase; MRC, myelodysplasia-related changes; sAML, secondary acute myeloid leukemia; t-AML, therapy-related AML; TKD, tyrosine kinase domain; VEN, venetoclax.
ACKNOWLEDGMENT
The authors would like to acknowledge Dr. Olatoyosi Odenike and Dr. Wendy Stock for their review of the manuscript.
Jennifer H. Cooperrider
Employment: AbbVie
Anand Ashwin Patel
Research Funding: Bristol Myers Squibb/Celgene (Inst), Servier (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by Award Number T32CA009566 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
M.T.N. and A.A.P. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Mariam T. Nawas, Anand Ashwin Patel
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The Cup Runneth Over: Treatment Strategies for Newly Diagnosed Acute Myeloid Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jennifer H. Cooperrider
Employment: AbbVie
Anand Ashwin Patel
Research Funding: Bristol Myers Squibb/Celgene (Inst), Servier (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 2.SEER : Cancer State Facts: Leukemia‐Acute Myeloid Leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, et al. : Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113:4179-4187, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Ravandi F, O’Brien S, et al. : Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 116:4422-4429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daver N, Wei AH, Pollyea DA, et al. : New directions for emerging therapies in acute myeloid leukemia: The next chapter. Blood Cancer J 10:107, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter RB, Othus M, Borthakur G, et al. : Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: A novel paradigm for treatment assignment. J Clin Oncol 29:4417-4423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’brien S, Cortes J, et al. : Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090-1098, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ferrara F, Barosi G, Venditti A, et al. : Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 27:997-999, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Palmieri R, Othus M, Halpern AB, et al. : Accuracy of SIE/SIES/GITMO consensus criteria for unfitness to predict early mortality after intensive chemotherapy in adults with AML or other high-grade myeloid neoplasm. J Clin Oncol 38:4163-4174, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klepin HD, Geiger AM, Tooze JA, et al. : Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121:4287-4294, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min G-J, Cho B-S, Park S-S, et al. : Geriatric assessment predicts non-fatal toxicities and survival for intensively treated older adults with AML. Blood 139:1646-1658, 2022 [DOI] [PubMed] [Google Scholar]
- 12.Döhner H, Wei AH, Appelbaum FR, et al. : Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood 140:1345-1377, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Short NJ, Kantarjian H: Choosing between intensive and less intensive front-line treatment approaches for older patients with newly diagnosed acute myeloid leukaemia. Lancet Haematol 9:e535-e545, 2022 [DOI] [PubMed] [Google Scholar]
- 14.Itzykson R, Fournier E, Berthon C, et al. : Genetic identification of patients with AML older than 60 years achieving long-term survival with intensive chemotherapy. Blood 138:507-519, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Bhatt VR, Wichman C, Al-Kadhimi ZS, et al. : Integrating geriatric assessment and genetic profiling to personalize therapy selection in older adults with acute myeloid leukemia. J Geriatr Oncol 13:871-874, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherry EM, Abbott D, Amaya M, et al. : Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv 5:5565-5573, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röllig C, Kramer M, Schliemann C, et al. : Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 136:823-830, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Kadia T, DiNardo C, et al. : Acute myeloid leukemia: Current progress and future directions. Blood Cancer J 11:41, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone RM, Mandrekar SJ, Sanford BL, et al. : Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454-464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambinder AJ, Levis M: Potential targeting of FLT3 acute myeloid leukemia. Haematologica 106:671-681, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erba H, Montesinos P, Vrhovac R, et al. : S100: Quizartinib prolonged survival vs placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients aged 18-75 years with newly diagnosed FLT3-ITD AML. HemaSphere 6:1-2, 2022 [Google Scholar]
- 22.Arber DA, Orazi A, Hasserjian RP, et al. : International consensus classification of myeloid neoplasms and acute leukemia: Integrating morphological, clinical, and genomic data. Blood 140:1200-1228, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury JD, Solary E, Abla O, et al. : The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 36:1703-1719, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hills RK, Castaigne S, Appelbaum FR, et al. : Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: A meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15:986-996, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paschka P, Schlenk RF, Weber D, et al. : Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia—Results of the AMLSG 11-08 trial. Leukemia 32:1621-1630, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Marcucci G, Geyer S, Laumann K, et al. : Combination of dasatinib with chemotherapy in previously untreated core binding factor acute myeloid leukemia: CALGB 10801. Blood Adv 4:696-705, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlenk RF, Paschka P, Krzykalla J, et al. : Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: Early results from the prospective randomized AMLSG 09-09 phase III study. J Clin Oncol 38:623-632, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker AR, Byrd JC, Blachly JS, et al. : Entospletinib in combination with induction chemotherapy in previously untreated acute myeloid leukemia: Response and predictive significance of HOXA9 and MEIS1 expression. Clin Cancer Res 26:5852-5859, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Mao L, Yang M, et al. : Venetoclax plus 3 + 7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: A multicentre, single-arm, phase 2 trial. Lancet Haematol 9:e415-e424, 2022 [DOI] [PubMed] [Google Scholar]
- 30.Kadia TM, Reville PK, Borthakur G, et al. : Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol 8:e552-e561, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNardo CD, Lachowiez CA, Takahashi K, et al. : Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol 39:2768-2778, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachowiez CA, Reville PK, Kantarjian H, et al. : Venetoclax combined with induction chemotherapy in patients with newly diagnosed acute myeloid leukaemia: A post-hoc, propensity score-matched, cohort study. Lancet Haematol 9:e350-e360, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein EM, DiNardo CD, Fathi AT, et al. : Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: A phase 1 study. Blood 137:1792-1803, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. : Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28:562-569, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Dombret H, Seymour JF, Butrym A, et al. : International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126:291-299, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantarjian HM, Thomas XG, Dmoszynska A, et al. : Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 30:2670-2677, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeidan AM, Wang R, Wang X, et al. : Clinical outcomes of older patients with AML receiving hypomethylating agents: A large population-based study in the United States. Blood Adv 4:2192-2201, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiNardo CD, Jonas BA, Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Xie J, Yang X, et al. : Venetoclax plus decitabine for young adults with newly diagnosed ELN adverse-risk acute myeloid leukemia: Interim analysis of a prospective, multicenter, single-arm, phase 2 trial. Blood 138:35, 2021 [Google Scholar]
- 40.Wang ES, Montesinos P, Minden MD, et al. : Phase 3, open-label, randomized study of gilteritinib and azacitidine vs azacitidine for newly diagnosed FLT3-mutated acute myeloid leukemia in patients ineligible for intensive induction chemotherapy. Blood 140:1845-1857, 2022 [DOI] [PubMed] [Google Scholar]
- 41.Konopleva M, Thirman MJ, Pratz KW, et al. : Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naïve acute myeloid leukemia. Clin Cancer Res 28:2744-2752, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roboz GJ, DiNardo CD, Stein EM, et al. : Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 135:463-471, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montesinos P, Recher C, Vives S, et al. : Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med 386:1519-1531, 2022 [DOI] [PubMed] [Google Scholar]
- 44.Pollyea DA, Tallman MS, de Botton S, et al. : Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia 33:2575-2584, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venugopal S, Takahashi K, Daver N, et al. : Efficacy and safety of enasidenib and azacitidine combination in patients with IDH2 mutated acute myeloid leukemia and not eligible for intensive chemotherapy. Blood Cancer J 12:1-7, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiNardo CD, Schuh AC, Stein EM, et al. : Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol 22:1597-1608, 2021 [DOI] [PubMed] [Google Scholar]
- 47.Pollyea DA, DiNardo CD, Arellano ML, et al. : Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res 28:2753-2761, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burnett AK, Milligan D, Prentice AG, et al. : A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109:1114-1124, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Heuser M, Robak T, Montesinos P, et al. : Glasdegib (GLAS) plus low-dose cytarabine (LDAC) in AML or MDS: BRIGHT AML 1003 final report and four-year overall survival (OS) follow-up. J Clin Oncol 38:7509, 2020 [Google Scholar]
- 50.Wei AH, Montesinos P, Ivanov V, et al. : Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 135:2137-2145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadia TM, Cortes J, Ravandi F, et al. : Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: A phase 2 single-arm trial. Lancet Haematol 5:e411-e421, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadia TM, Faderl S, Ravandi F, et al. : Final results of a phase 2 trial of clofarabine and low-dose cytarabine alternating with decitabine in older patients with newly diagnosed acute myeloid leukemia. Cancer 121:2375-2382, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadia TM, Reville PK, Wang X, et al. : Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 40:3848-3857, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soulier J: Introduction to a review series on secondary leukemia. Blood 136:1, 2020 [DOI] [PubMed] [Google Scholar]
- 55.Voso MT, Falconi G, Fabiani E: What's new in the pathogenesis and treatment of therapy-related myeloid neoplasms. Blood 138:749-757, 2021 [DOI] [PubMed] [Google Scholar]
- 56.Badar T, Atallah E, Shallis RM, et al. : Outcomes of TP53 mutated AML with evolving frontline therapies: Impact of allogeneic stem cell transplantation on survival. Am J Hematol 97:e232-e235, 2022 [DOI] [PubMed] [Google Scholar]
- 57.Short NJ, Venugopal S, Qiao W, et al. : Impact of frontline treatment approach on outcomes in patients with secondary AML with prior hypomethylating agent exposure. J Hematol Oncol 15:12, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boddu P, Kantarjian HM, Garcia-Manero G, et al. : Treated secondary acute myeloid leukemia: A distinct high-risk subset of AML with adverse prognosis. Blood Adv 1:1312-1323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martínez-Cuadrón D, Megías-Vericat JE, Serrano J, et al. : Treatment patterns and outcomes of 2310 patients with secondary acute myeloid leukemia: A PETHEMA registry study. Blood Adv 6:1278-1295, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lancet JE, Uy GL, Newell LF, et al. : CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 8:e481-e491, 2021 [DOI] [PubMed] [Google Scholar]
- 61.Lindsley RC, Coleman Lindsley R, Gibson CJ, et al. : Genetic characteristics and outcomes by mutation status in a phase 3 study of CPX-351 versus 7 3 in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML). Blood 134:15, 2019 [Google Scholar]
- 62.Benitez LL, Perissinotti AJ, Rausch CR, et al. : Multicenter comparison of high-dose cytarabine-based regimens versus liposomal daunorubicin and cytarabine (CPX-351) in patients with secondary acute myeloid leukemia. Leuk Lymphoma 62:2184-2192, 2021 [DOI] [PubMed] [Google Scholar]
- 63.Grenet J, Jain AG, Burkart M, et al. : Comparing outcomes between liposomal daunorubicin/cytarabine (CPX-351) and HMA + venetoclax as frontline therapy in acute myeloid leukemia. Blood 138:32, 2021 [Google Scholar]
- 64.Matthews AH, Perl AE, Luger SM, et al. : Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv 6:3997-4005, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pidala J, Lee SJ, Ahn KW, et al. : Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood 124:2596-2606, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SJ, Klein J, Haagenson M, et al. : High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110:4576-4583, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Zhu Y, Gao Q, Du J, et al. : Effects of post-remission chemotherapy before allo-HSCT for acute myeloid leukemia during first complete remission: A meta-analysis. Ann Hematol 97:1519-1526, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Tallman MS, Rowlings PA, Milone G, et al. : Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood 96:1254-1258, 2000 [PubMed] [Google Scholar]
- 69.Döhner H, Estey E, Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ostronoff F, Othus M, Lazenby M, et al. : Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol 33:1157-1164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Büchner T, Berdel WE, Haferlach C, et al. : Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 27:61-69, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Appelbaum FR, Kopecky KJ, Tallman MS, et al. : The clinical spectrum of adult acute myeloid leukaemia associated with core binding factor translocations. Br J Haematol 135:165-173, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Papaemmanuil E, Gerstung M, Bullinger L, et al. : Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374:2209-2221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murdock HM, Kim HT, Denlinger N, et al. : Impact of diagnostic genetics on remission MRD and transplantation outcomes in older patients with AML. Blood 139:3546-3557, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muffly L, Pasquini MC, Martens M, et al. : Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 130:1156-1164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McClune BL, Weisdorf DJ, Pedersen TL, et al. : Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol 28:1878-1887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayani R, Rosko A, Olin R, et al. : Use of geriatric assessment in hematopoietic cell transplant. J Geriatr Oncol 11:225-236, 2020 [DOI] [PubMed] [Google Scholar]
- 78.Kennedy VE, Olin RL: Haematopoietic stem-cell transplantation in older adults: Geriatric assessment, donor considerations, and optimisation of care. Lancet Haematol 8:e853-e861, 2021 [DOI] [PubMed] [Google Scholar]
- 79.Giralt S, Estey E, Albitar M, et al. : Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: Harnessing graft-versus-leukemia without myeloablative therapy. Blood 89:4531-4536, 1997 [PubMed] [Google Scholar]
- 80.Gratwohl A, Baldomero H, Passweg J, et al. : Increasing use of reduced intensity conditioning transplants: Report of the 2001 EBMT activity survey. Bone Marrow Transpl 30:813-831, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Ustun C, Le-Rademacher J, Wang H-L, et al. : Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): An alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia 33:2599-2609, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russell NH, Hills RK, Thomas A, et al. : Outcomes of older patients aged 60 to 70 years undergoing reduced intensity transplant for acute myeloblastic leukemia: Results of the NCRI acute myeloid leukemia 16 trial. Haematologica 107:1518-1527, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei AH, Döhner H, Pocock C, et al. : Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med 383:2526-2537, 2020 [DOI] [PubMed] [Google Scholar]
- 84.Döhner H, Wei AH, Roboz GJ, et al. : Prognostic impact of NPM1 and FLT3 mutations at diagnosis and presence of measurable residual disease (MRD) after intensive chemotherapy (IC) for patients with acute myeloid leukemia (AML) in remission: Outcomes from the QUAZAR AML-001 trial of oral azacitidine (Oral-AZA) maintenance. Blood 138:804, 2021 [Google Scholar]
- 85.Roboz GJ, Ravandi F, Wei AH, et al. : Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status. Blood 139:2145-2155, 2022 [DOI] [PubMed] [Google Scholar]
- 86.Röllig C, Serve H, Noppeney R, et al. : Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: Long-term follow-up of the randomized controlled SORAML trial. Leukemia 35:2517-2525, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larson RA, Mandrekar SJ, Huebner LJ, et al. : Midostaurin reduces relapse in FLT3-mutant acute myeloid leukemia: The alliance CALGB 10603/RATIFY trial. Leukemia 35:2539-2551, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sekeres MA, Guyatt G, Abel G, et al. : American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv 4:3528-3549, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pratz KW, Jonas BA, Pullarkat V, et al. : Measurable residual disease response and prognosis in treatment-naïve acute myeloid leukemia with venetoclax and azacitidine. J Clin Oncol 40:855-865, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chua CC, Hammond D, Kent A, et al. : Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia. Blood Adv 6:3879-3883, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel AA, Cahill K, Saygin C, et al. : Cedazuridine/decitabine: From preclinical to clinical development in myeloid malignancies. Blood Adv 5:2264-2271, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pratz KW, Panayiotidis P, Recher C, et al. : Venetoclax combinations delay the time to deterioration of HRQoL in unfit patients with acute myeloid leukemia. Blood Cancer J 12:71, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gangat N, Tefferi A: To live is well but to live well is better: Venetoclax combination therapy and quality-of-life in acute myeloid leukemia. Blood Cancer J 12:75, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu H: Emerging agents and regimens for AML. J Hematol Oncol 14:49, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lachowiez CA, Borthakur G, Loghavi S, et al. : A phase Ib/II study of ivosidenib with venetoclax +/- azacitidine in IDH1-mutated myeloid malignancies. J Clin Oncol 39:7012, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maiti A, DiNardo CD, Daver NG, et al. : Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J 11:25-26, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Short NJ, DiNardo CD, Daver N, et al. : A triplet combination of azacitidine, venetoclax and gilteritinib for patients with FLT3-mutated acute myeloid leukemia: Results from a phase I/II study. Blood 138:696, 2021 [Google Scholar]
- 98.Yilmaz M, Kantarjian HM, Muftuoglu M, et al. : Quizartinib with decitabine and venetoclax (triplet) is highly active in patients with FLT3-ITD mutated acute myeloid leukemia (AML). J Clin Oncol 39:e19019, 2021 [Google Scholar]
- 99.Gangat N, Guglielmelli P, Szuber N, et al. : Venetoclax with azacitidine or decitabine in blast-phase myeloproliferative neoplasm: A multicenter series of 32 consecutive cases. Am J Hematol 96:781-789, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Masarova L, DiNardo CD, Bose P, et al. : Single-center experience with venetoclax combinations in patients with newly diagnosed and relapsed AML evolving from MPNs. Blood Adv 5:2156-2164, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.King AC, Weis TM, Derkach A, et al. : Multicenter evaluation of efficacy and toxicity of venetoclax based combinations in patients with accelerated and blast phase myeloproliferative neoplasms. Am J Hematol 97:e7-e10, 2022 [DOI] [PubMed] [Google Scholar]
- 102.Mascarenhas JO, Rampal RK, Kosiorek HE, et al. : Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv 4:5246-5256, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patel AA, Cahill K, Charnot-Katsikas A, et al. : Clinical outcomes of IDH2-mutated advanced-phase Ph-negative myeloproliferative neoplasms treated with enasidenib. Br J Haematol 190:e48-e51, 2020 [DOI] [PubMed] [Google Scholar]
- 104.Chifotides HT, Masarova L, Alfayez M, et al. : Outcome of patients with IDH1/2-mutated post–myeloproliferative neoplasm AML in the era of IDH inhibitors. Blood Adv 4:5336-5342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daver N, Konopleva M, Maiti A, et al. : Phase I/II study of azacitidine (AZA) with venetoclax (VEN) and magrolimab (magro) in patients (pts) with newly diagnosed older/unfit or high-risk acute myeloid leukemia (AML) and relapsed/refractory (R/R) AML. Blood 138:371, 2021 [Google Scholar]
- 106.Sallman D, Asch A, Kambhampati S, et al. : AML-196: The first-in-class anti-CD47 antibody magrolimab in combination with azacitidine is well tolerated and effective in AML patients: Phase 1b results. Clin Lymphoma Myeloma Leuk 21:S290, 2021 [Google Scholar]
- 107.Sallman DA, DeZern AE, Garcia-Manero G, et al. : Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 39:1584-1594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cluzeau T, Sebert M, Rahmé R, et al. : Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: A phase II study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol 39:1575-1583, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia-Manero G, Goldberg AD, Winer ES, et al. : Phase I and expansion study of eprenetapopt (APR-246) in combination with venetoclax (VEN) and azacitidine (AZA) in TP53-mutant acute myeloid leukemia (AML). Blood 138:3409, 2021 [Google Scholar]