Abstract
Immunotherapy (IO) agents have led to significant improvements in patient outcomes across many tumor types. There have been great efforts to introduce immune checkpoint inhibitors into the treatment paradigm of esophagogastric cancers as well. A number of randomized phase III trials, which will be reviewed here, established the role of these agents in both early-stage and advanced-stage disease. Adjuvant nivolumab is US Food and Drug Administration–approved after neoadjuvant chemoradiation and resection of esophageal and gastroesophageal junction cancers on the basis of the phase III CheckMate 577 trial. In the advanced setting, patients with programmed death receptor ligand-1–positive tumors should be recommended IO in combination with chemotherapy in the first-line setting on the basis of the results from KEYNOTE 590, CheckMate 649, and CheckMate 648. Across trials, chemotherapy continues to play a critical role in the first-line setting and should be offered to all patients who are eligible for systemic therapy, including those with biomarker select tumors. In the later lines of treatment, IO has modest activity, and prior studies have grown largely irrelevant because of the enrollment of IO-naive patients. Similar to other disease types, patients with microsatellite unstable (microsatellite instability high) tumors represent a unique cohort that is more sensitive to IO. However, there are no randomized studies evaluating how best to apply IO in early or advanced stages specifically for the treatment of patients with microsatellite instability high upper GI tumors. Questions remain how to best select patients who benefit from IO treatments, how to augment IO activity in programmed death receptor ligand-1–negative tumors, and how to incorporate IO in late-line settings or for recurrent disease that has been treated with IO-containing regimens during early stages.
INTRODUCTION
Esophagogastric cancers are aggressive tumors that significantly contribute to cancer-related mortality globally. In 2018, they accounted for more than 1.2 million of cancer-related deaths globally and represented about 9% of all new cancer diagnosis.1 About 50% of upper GI cancers are diagnosed in advanced stages, and a significant percentage of patients with early-stage disease develop recurrences after definitive treatments. As such, systemic therapy plays a critical role in the management of these patients. Combination chemotherapy has an established role in treating advanced upper GI cancers, but even with the most active regimens, overall survival (OS) remained about a year.2 Recently, immunotherapy (IO) demonstrated promising activity against upper GI cancers, especially when used in early stages or in combination with chemotherapy. A number of practice-changing studies have led to IO approvals for the management of esophagogastric cancers. Here, we will review these pivotal studies, discuss biomarker selection for IO use, and highlight some of the existing questions in the field.
KEY POINTS
Immune checkpoint inhibitors play a role in the management of patients with upper GI cancers.
Nivolumab is recommended in the adjuvant setting after resection of esophageal and gastroesophageal junction tumors without pathologic complete response after neoadjuvant chemoradiation.
In advanced esophagogastric adenocarcinoma, immunotherapy (IO) in combination with chemotherapy is recommended in the first-line setting to IO-naive patients with programmed death receptor ligand-1 combined positive score ≥ 5 tumors.
In advanced esophageal squamous cell carcinoma, IO in combination with chemotherapy should be offered in the first-line setting to IO-naive patients regardless of tumor programmed death receptor ligand-1 expression. IO only regimens may be used in select patients with contraindications to chemotherapy.
Approaches to microsatellite instability (MSI) high upper GI tumors have not been fully elucidated, but all tumors should be tested for MMR protein expression or MSI status. MSI high/mismatch repair deficient tumors represent a unique subtype of upper GI cancers that is more responsive to IO.
CONTEXT
Key Objective
Upper GI cancers have a poor prognosis. Many recent studies evaluated immunotherapy (IO) activity in the management of these diseases. The results from these studies led to new standard practices that are reviewed here.
Knowledge Generated
This review summarizes recent IO trials in the treatment of upper GI cancers. Data regarding IO activity in upper GI cancers have been mixed across studies, and inconsistencies between regulatory approvals and practice guidelines confound uniform incorporation of these agents into standard practice. Complex study designs, heterogeneous patient selection, and variable biomarker interpretations complicate interpretation of the results.
Relevance
Summary of available data and their applicability to clinical practice provides a roadmap for incorporating IO into the management of upper GI cancers.
IO BIOMARKERS
There has been a significant effort to identify patients with upper GI cancers who benefit from IO treatments. Similarly to its role in predicting IO response in other cancers, programmed death receptor ligand-1 (PD-L1) has emerged as a preferred, but imperfect, biomarker for upper GI tumors. Higher PD-L1 expression on tumor and immune cells has been shown to be predictive of IO response in esophagogastric cancers across many studies.3 However, the predictive value of PD-L1 is not as strong as in other diseases, and its incorporation into clinical practice in upper GI cancers is still evolving. Different scoring systems, antibodies, and thresholds of positivity have been used across studies, making it challenging to contextualize all available data. Going forward, a uniform and simplified approach to PD-L1 testing is much needed.
There are two accepted scoring systems for PD-L1 expression. Tumor positive score (TPS) is determined by the percentage of tumor cells showing partial or complete staining relative to all tumor cells in the sample. Combined positive score (CPS) is determined by the number of PD-L1–staining cells, including tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells and multiplied by 100. Esophagogastric adenocarcinoma (EGA) and esophageal squamous cell carcinoma (ESCC) have different patterns of PD-L1 expression. While PD-L1 is frequently expressed by tumor cells in ESCC, most PD-L1 expression occurs on cells present in tumor microenvironment in EGA.4-6 This suggests that TPS may be a useful biomarker in ESCC, but it should not be used for EGA. For simplification purposes, however, it is reasonable to apply CPS analysis across all upper GI tumors, since TPS-positive tumors will be captured with CPS scoring.
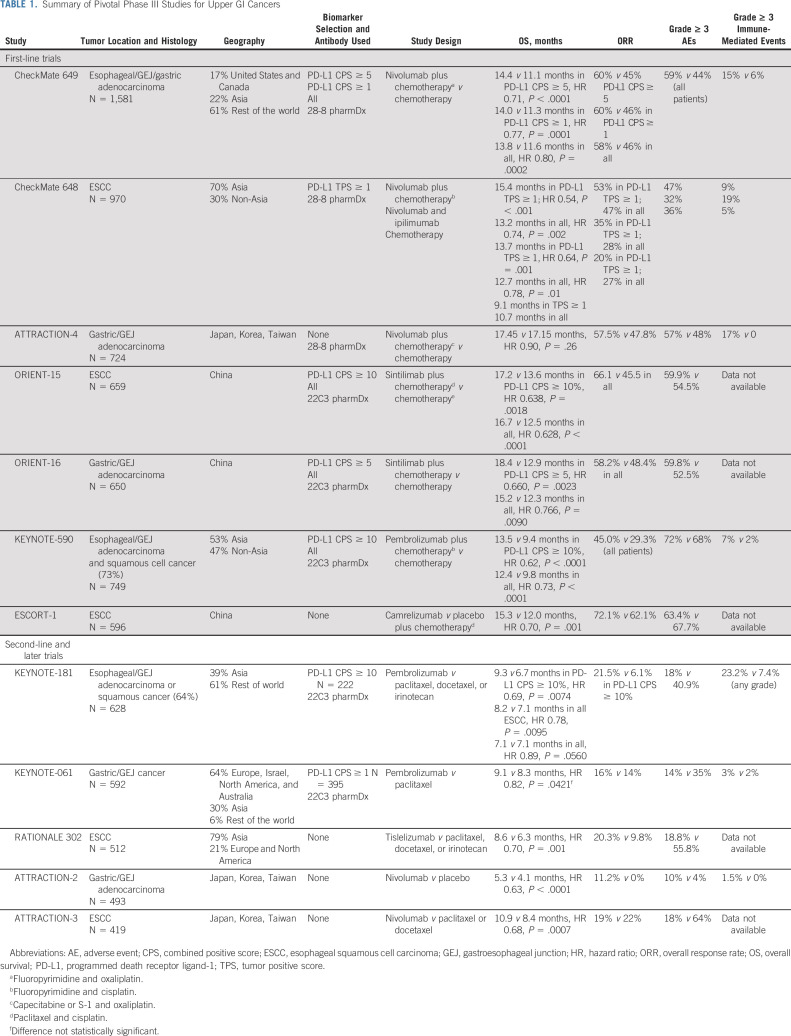
Different assays have been applied to determine PD-L1 status in upper GI cancers depending on the IO agent evaluated (Table 1). Fortunately, there are growing data on concordance between the different antibodies, but this information is still emerging.7,8 To add to the complexity, the threshold for PD-L1 positivity has not been consistent across trials. Although CPS ≥ 1 and 10 have been used in studies with pembrolizumab, nivolumab studies used a cutoff of 5, albeit with a different antibody. Spatial and temporal tumor heterogeneity further complicates assessing PD-L1 status. The most robust biomarker evaluation may require more than one biopsy and repeat testing over the course of disease progression. However, how to interpret discordant results (making treatment decision on the basis of lowest v highest CPS) is not clear at present time.9-12
TABLE 1.
Summary of Pivotal Phase III Studies for Upper GI Cancers
All of these issues underscore the complex biology of upper GI cancers, as well as underline the need for continued optimization of PD-L1 as a biomarker and for identification of new predictive biomarkers. At present time, DAKO 22C3 clone is the most commonly used antibody to determine PD-L1 CPS in advanced upper GI cancers. The PD-L1 CPS treatment threshold depends on histology and line of treatment and will be addressed in subsequent sections. PD-L1 status does not guide therapy selection in early-stage disease.
In addition to PD-L1, upper GI tumors should be tested for microsatellite instability (MSI) high (MSI-H) and tumor mutational burden. Pembrolizumab is US Food and Drug Administration (FDA)–approved for patients with mismatch repair deficiency and/or high tumor mutational burden. MSI-H status is a strong predictive biomarker of IO response in upper GI tumors, similarly to other diseases.13 As such, evaluation of MSI status or MMR protein expression should be performed in all patients.
IO IN THE TREATMENT OF EARLY-STAGE DISEASE
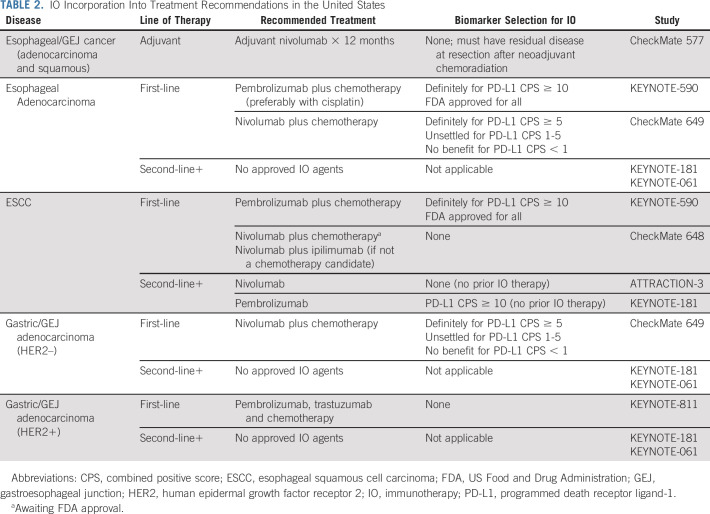
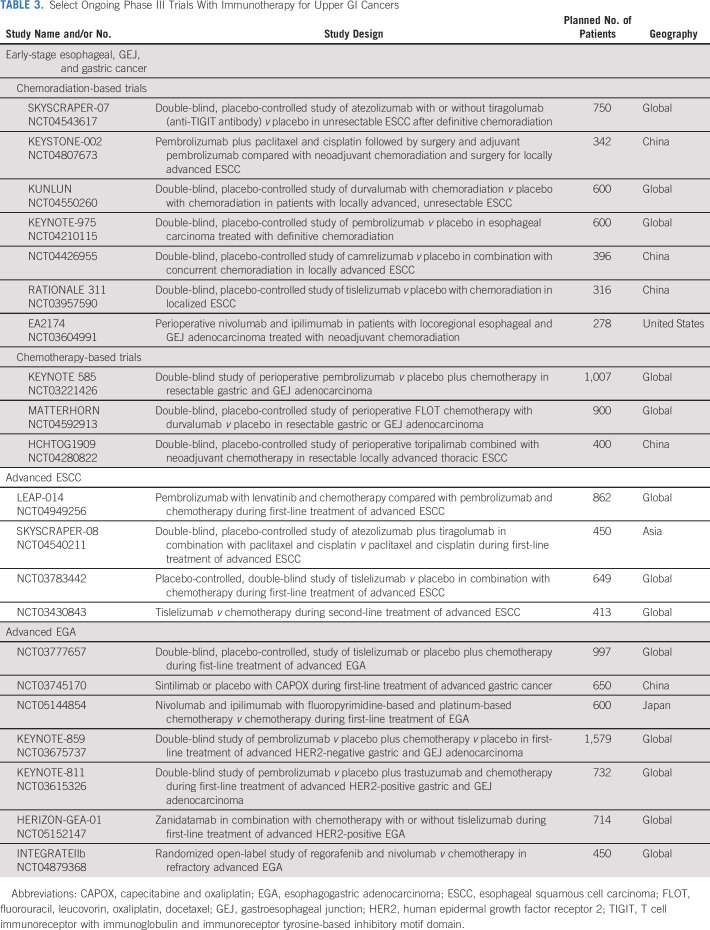
A multidisciplinary approach is essential for optimal outcomes in patients with early-stage upper GI cancers. Patients with esophageal and gastroesophageal junction (GEJ) tumors are typically treated with neoadjuvant chemoradiation followed by surgical resection.14 This approach offers a 13% absolute benefit in long-term survival over 10 years (38% v 25% with surgery alone, P = .004).15 However, distant recurrence rates remain significant, especially in those with residual disease at the time of resection.15,16 Until recently, no adjuvant systemic therapy was recommended in this setting. These standards were updated on the basis of the results of CheckMate 577, a global phase III study of adjuvant nivolumab.17 This study enrolled 1,085 patients with tumors of either adeno- or squamous (29%) histology who had residual disease at the time of resection after completion of neoadjuvant chemoradiation (> 50% had lymph node–positive disease). Patients were randomly assigned 2:1 to either 1 year of adjuvant nivolumab or placebo. Nivolumab significantly improved disease-free survival (22.4 v 11.0 months [months], hazard ratio [HR] 0.69), with the greatest benefit seen in ESCC (HR 0.61 for ESCC v 0.75 for adenocarcinoma). Disease-free survival improved regardless of PD-L1 CPS, although the benefit was greater in those with a CPS ≥ 5 (29.4 v 10.2 months) compared with those with a CPS < 5 (16.3 v 11.1 months) tumors. Notably, there was a low rate of grade ≥ 3 adverse events (AEs) related to nivolumab versus placebo (13% v 6%). On the basis of these results, the FDA approved 1 year of adjuvant nivolumab for patients with esophageal and GEJ tumors who have residual pathologic disease at the time of resection after completion of neoadjuvant chemoradiation (Table 2). This recommendation is endorsed by the National Comprehensive Cancer Network (NCCN) and ASCO guidelines, and adjuvant IO should be offered to all patients regardless of tumor histology or PD-L1 expression.18 An ongoing phase II/III EA2174 study is evaluating whether the addition of nivolumab to chemoradiation in the preoperative setting and ipilimumab to adjuvant nivolumab can improve upon the efficacy seen in CheckMate 577 with acceptable toxicities (ClinicalTrials.gov identifier: NCT03604991; Table 3).
TABLE 2.
IO Incorporation Into Treatment Recommendations in the United States
TABLE 3.
Select Ongoing Phase III Trials With Immunotherapy for Upper GI Cancers
At present time, IO is not indicated for upper GI cancers treated with perioperative chemotherapy.19 However, the benefit of IO is being explored in a number of ongoing trials (Table 3). KEYNOTE 585 is a global phase III trial evaluating the benefit of pembrolizumab with perioperative FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) chemotherapy (ClinicalTrials.gov identifier: NCT03221426).20 The MATTERHORN trial is a phase III study of perioperative durvalumab with FLOT chemotherapy (ClinicalTrials.gov identifier: NCT04592913).6 HCHTOG1909 is a phase III study conducted in China comparing neoadjuvant toripalimab plus chemotherapy versus chemotherapy for patients with locoregional ESCC.21
The best approach to MSI-H upper GI cancers is under investigation. Similar to MSI-H colorectal cancers, resectable MSI-H gastric and GEJ tumors may derive no benefit from perioperative chemotherapy. In the exploratory analysis of patients with MSI-H tumors enrolled in the perioperative MAGIC trial, patients with MSI-H tumors had a better prognosis when treated with surgery alone and potentially experienced detrimental effects from chemotherapy.22 Similar results were seen in a retrospective analysis of patients with MSI-H tumors treated with adjuvant chemoradiotherapy.23 IO, however, has promising activity in this setting. The prospective phase II GERCOR-NEONIPIGA study that enrolled 32 patients with MSI-H gastric and GEJ cancers demonstrated an unprecedented pathologic complete response rate of 59% with preoperative nivolumab and ipilimumab.24 These results certainly support further investigation of this approach in a larger study. However, in the absence of prospective randomized data, it is difficult to recommend deviating from the established standard of care with FLOT, especially for patients undergoing curative-intent treatment. For patients with MSI-H tumors who are not candidates for combination chemotherapy or whose tumors are progressing on chemotherapy, neoadjuvant IO is emerging as a viable option.
IO IN THE TREATMENT OF ADVANCED EGA
First-Line Setting: Human Epidermal Growth Factor Receptor 2–Negative
A series of landmark studies for advanced EGA demonstrated that IO incorporation into first-line treatment improves patient outcomes (Table 1). Although the best biomarker to predict response is still under debate, clinicians should feel comfortable offering combination IO-chemotherapy to patients with PD-L1 positive tumors in the first-line setting.
CheckMate 649 was a pivotal trial that resulted in the nivolumab FDA approval for advanced EGA. In this global phase III trial, patients with untreated advanced EGA (no known human epidermal growth factor receptor 2 [HER2]–positive status) were randomly assigned to chemotherapy alone, chemotherapy plus nivolumab, or nivolumab plus ipilimumab.25 PD-L1 positivity was not required for enrollment. During accrual, the primary study population was amended to include patients whose tumors had a PD-L1 CPS ≥ 5% on the basis of emerging data from CheckMate 032.26 With nearly 1,600 patients randomly assigned in a 1:1 fashion to either chemotherapy (fluoropyrimidine and oxaliplatin doublet) or chemotherapy plus nivolumab, the study demonstrated benefit with the addition of nivolumab. Nivolumab prolonged OS (14.4 v 11.1 months, HR 0.71) and progression-free survival (PFS; 7.7 v 6.0 months, HR 0.68) in patients with PD-L1 CPS ≥ 5%. The study also met all formally tested secondary end points, including improving OS in all treated patients, but the benefit was driven by the PD-L1 CPS ≥ 5 group (about 60% of patients), with the HR decreasing with the inclusion of tumors with lower or negative PD-L1 expression (17% with CPS < 1). This study also demonstrated that chemotherapy plays an indispensable role in the first-line setting, with the ipilimumab and nivolumab cohort experiencing increased deaths early during treatment and no OS improvement regardless of biomarker selection. On the basis of the results of this trial, the FDA approved nivolumab in combination with chemotherapy in the first-line setting for all patients with EGA. However, per NCCN category 1 recommendations, nivolumab should be reserved for those with PD-L1 CPS ≥ 5 tumors (Table 2).
The role of pembrolizumab in upper GI adenocarcinomas was evaluated in two large trials, KEYNOTE 062 and KEYNOTE 590.27,28 KEYNOTE-062 enrolled patients with gastric and GEJ adenocarcinoma. Although the design was similar design to CheckMate 649, addition of pembrolizumab did not improve OS.27 There are a number of potential explanations for discordant results between the two studies. Biomarker selection differed, including the PD-L1 CPS threshold and antibodies selected (22C3 clone in KEYNOTE-062 and 28-8 clone in CheckMate 649). The chemotherapy backbones were slightly different, with KEYNOTE-062 using cisplatin-based chemotherapy and CheckMate 649 oxaliplatin-based chemotherapy. The ongoing KEYNOTE-859 study uses oxaliplatin-based chemotherapy and will address whether chemotherapy selection contributed to differing results.29 In addition, 13% of patients in CheckMate 649 had esophageal adenocarcinoma, whereas KEYNOTE-062 enrolled patients with gastric and GEJ cancers only. KEYNOTE 590, however, enrolled patients with esophageal cancer, but both adenocarcinomas and squamous cell tumors were eligible. With 73% of enrolled patients having ESCC, the results of this study are certainly more relevant for this disease. Multiple primary end points and subgroup analysis of KEYNOTE 590 complicate the interpretation of study results. However, this study ultimately resulted in the FDA approval of pembrolizumab in addition to chemotherapy for the first-line treatment of esophageal cancer on the basis of a significant OS improvement in patients with PD-L1 CPS ≥ 10 tumors (17.4 v 10.8 months, HR 0.69) and among all randomly assigned patients (12.4 v 9.8 months, HR 0.73). In a subgroup of patients with adenocarcinoma, OS was longer with pembrolizumab treatment, although the effect was less pronounced (11.6 v 9.9 months, HR 0.73). Despite broad regulatory approval, the NCCN guidelines give a stronger recommendation for pembrolizumab for PD-L1 CPS ≥ 10 esophageal tumors, favoring cisplatin-based chemotherapy (category 1) over oxaliplatin-based chemotherapy (category 2a).
Given different tumor biology depending on geography, studies performed exclusively in Asian countries are not directly applicable to all patients, but they will be reviewed here briefly for completeness. ATTRACTION-4 was a randomized, multicenter phase II/III study conducted in Japan, Korea, and Taiwan, which explored the benefit of adding nivolumab to chemotherapy in 724 treatment-naive gastric and GEJ adenocarcinoma patients.30,31 The study met its primary end point of PFS improvement (10.45 v 8.34 months, HR 0.68), but there was no difference in OS, possibly because of subsequent therapies or lack of biomarker selection (17.45 v 17.15 months, HR 0.90). Notably, OS in this patient population was much higher than seen in other global studies, reflective of different tumor biology. By contrast, sintilimab (a humanized anti–programmed cell death protein-1 [PD-1] monoclonal antibody) significantly improved OS when added to chemotherapy in patients with previously untreated advanced gastric and GEJ adenocarcinoma (15.2 v 12.3 months, HR 0.76) in a phase III trial conducted in China (ORIENT-16, ClinicalTrials.gov identifier: NCT03745170).32 This benefit was observed in those with PD-L1 CPS ≥ 5 tumors and in all randomly assigned patients.
Taken altogether, a subset of patients with HER2-negative EGA benefit from the addition of IO to first-line chemotherapy (Table 2). Despite broad FDA approvals of IO in this setting, IO utilization for these patients should be more nuanced. Accumulating evidence demonstrates that only patients with PD-L1 CPS positive tumors benefit from IO, and the degree of benefit correlates with the level of PD-L1 positivity, as was seen in a subgroup analysis from KEYNOTE 590, KEYNOTE 062, and CheckMate 649.33 Further supporting this, combined subgroup analysis of PD-L1 low subgroups from KEYNOTE 590 (CPS 1-9), KEYNOTE 062 (CPS 1-9), and CheckMate 649 (CPS 1-4) demonstrated no benefit from IO addition in these patients. As such, in the first-line setting, nivolumab should be added to chemotherapy in patients with PD-L1 CPS ≥ 5 EGA, and pembrolizumab should be added to chemotherapy in those with PD-L1 CPS ≥ 10 esophageal adenocarcinoma. Omission of chemotherapy in the first-line setting is not a viable option, even in a biomarker select patient population. It is not known whether patients with esophageal and GEJ cancers who receive nivolumab in the adjuvant setting derive benefit from additional IO at the time of recurrence. Timing of recurrence (while undergoing treatment with nivolumab v > 6 months after treatment completion) will likely play a role, but this question needs to be explored in prospective studies. Furthermore, tumor spatial and temporal heterogeneity continues to be a confounding factor when approaching patients in clinic and deciding on optimal therapy.
First-Line Setting: HER2-Positive
About 20% of EGA overexpress HER2 or harbor HER2 gene amplification.34 Trastuzumab in combination with chemotherapy has been the standard first-line treatment for these tumors for over a decade.35 Recently, the addition of pembrolizumab to standard therapy demonstrated impressive overall response rate (ORR), PFS, and OS in two phase II trials.36,37 On the basis of these results, there is an ongoing phase III study, KEYNOTE-811, evaluating pembrolizumab in this setting.38 At the preplanned interim analysis of KEYNOTE-811 after enrollment of 260 participants with at least 8.5 months of follow-up, pembrolizumab significantly increased ORR compared with placebo (74.4% v 51.9%).39 There were no new safety signals, and the addition of pembrolizumab to standard therapy is now FDA-approved and endorsed by the NCCN guidelines in this setting, regardless of tumor PD-L1 status.
Second-Line and Beyond
There have been a number of studies evaluating activity of IO in EGA in second or later lines of treatment. A few key takeaway points emerge from these data. First, these studies enrolled IO-naive patients, and thus the results have become largely irrelevant at a time when anti–PD-1 antibodies are FDA-approved in first-line and adjuvant settings. Second, clinical activity with these agents has been quite limited in advanced lines and, similar to first-line studies, is limited to a biomarker select patient population. These studies will be briefly reviewed here for completion. KEYNOTE-181 was a global phase III study that enrolled patients with both adeno- and squamous esophageal cancer whose tumors progressed on one prior line of therapy. The study met one of its primary end points of improving OS in patients with PD-L1 CPS ≥ 10 tumors compared with physician's choice chemotherapy.40 However, the signal was primarily seen in patients with ESCC, with a HR of 0.93 for adenocarcinoma, even in biomarker select patient population. KEYNOTE-061 was a phase III study of pembrolizumab versus paclitaxel for patients with gastric and GEJ tumors after progression on one line of standard therapy.41 This study failed to meet its primary end points of OS and PFS in patients with PD-L1 CPS ≥ 1 tumors. Despite paucity of randomized data, pembrolizumab was temporarily FDA approved for PD-L1 CPS ≥ 1 tumors in the third-line setting.42 However, this indication has now been withdrawn as it has not been supported by additional data. Nivolumab is approved in Asia in the third-line setting on the basis of the results of the phase III ATTRACTION-2 trial that demonstrated improved OS compared with placebo (5.25 v 4.14 months, HR 0.62).43
In summary, currently, there are no FDA-approved IO agents in the United States in second or later lines for the treatment of advanced EGA. Future studies should take into consideration the recent IO approvals in earlier lines. There remains a significant unmet need to improve outcomes in patients with PD-L1–negative tumors across therapy lines.
IO FOR ADVANCED ESCC
First-Line Setting
For advanced (unresectable or metastatic) ESCC, IO has demonstrated the most promising activity in early line. Pembrolizumab in combination with chemotherapy is now FDA-approved in the first-line setting on the basis of the results of KEYNOTE 590 trial discussed earlier. CheckMate 648 was a global phase III trial of the addition of nivolumab to chemotherapy (fluorouracil and cisplatin) for ESCC.44 This study randomly assigned 970 patients to chemotherapy, chemotherapy plus nivolumab, or nivolumab and ipilimumab. Among patients with PD-L1 TPS ≥ 1% (49% of patients enrolled), OS of 15.2 months was longest in the nivolumab/chemotherapy arm versus 13.7 months in the nivolumab/ipilimumab arm and 9.1 months in the chemotherapy alone arm (P < .0001). This survival benefit was diminished, but still significant, across the analysis of all patients (P = .0021). Nivolumab with chemotherapy will likely be FDA-approved in the near future for the first-line ESCC treatment. Although CheckMate 648 suggests that a chemotherapy-free option with ipilimumab and nivolumab is a possible alternative for some patients, especially those who are not fit for chemotherapy, a higher number of deaths early on during treatment in the IO cohort is reflective of a critical role that chemotherapy plays in the management of these patients. In addition, the contribution of ipilimumab is not clear from the study design, as there was no nivolumab only arm. Taken altogether, ideally, patients who are candidates for systemic therapy should receive IO in combination chemotherapy, which can be modified to account for anticipated tolerance issues.45 In patients who are not candidates for any cytotoxic therapy, IO only regimen may be a viable option if there is low volume of disease with limited associated symptoms.
In addition to the global CheckMate 648 and KEYNOTE 590 studies, three recent phase III studies for patients with ESCC conducted in China evaluated IO addition to chemotherapy in the first-line setting (ORIENT-15, ESCORT-1, and JUPITER-06).46-48 These trials had similar designs, but they used paclitaxel and cisplatin as the chemotherapy backbone. All three studies demonstrated OS improvement with IO addition, regardless of PD-L1 status. The degree of OS improvement appeared to be larger compared with global trials in similar patient population (KEYNOTE-590 and CheckMate 648), and control arms had better outcomes. This is likely reflective of biological differences in ESCC between Western and Asian patients, and possibly differential activity of various chemotherapy agents.49 The results from these trials are not directly applicable to patients outside of China, but they do raise questions about the most appropriate chemotherapy backbone for the treatment of ESCC.
Second-Line and Beyond
Patients who did not receive IO with the first-line treatment can be considered for IO in later lines, although the efficacy is expected to diminish. KEYNOTE 181 established the efficacy of pembrolizumab against IO-naive tumors with PD-L1 CPS ≥ 10.40 Improved survival was also seen with tislelizumab in the second-line (RATIONALE 302), independent of PD-L1 status, compared with investigator-chosen standard chemotherapy.50 Nivolumab also showed similar activity (independent of PD-L1) with improved OS compared with chemotherapy in the ATTRACTION-3 trial, and it is now FDA-approved on the basis of these results.51
In summary, single-agent IO use in second and later lines is supported by high-quality phase III data. However, similar to advanced EGA, IO efficacy is reduced in later lines, and its relevance is diminishing with increased utilization of IO in earlier lines.
IO FOR MISMATCH REPAIR DEFICIENT AND MICROSATELLITE UNSTABLE (MSI-H) UPPER GI CANCERS
About 4%-22% of gastroesophageal tumors are mismatch repair deficient (dMMR)/MSI-H, which is associated with improved clinical prognosis.52-55 These tumors have differential response to IO compared with MSS tumors and should be approached differently. Evaluation of microsatellite status or MMR protein expression should be performed in all patients, regardless of tumor stage.
The utility of IO in metastatic dMMR/MSI-H EGA was summarized in post hoc analysis of trials with pembrolizumab.56 The initial signal of activity, specifically in MSI-H gastric and GEJ cancer, was reported from KEYNOTE-059 where late-line treatment with pembrolizumab resulted in 57.1% ORR in MSI-H tumors (n = 7).42 Significant activity of anti–PD-1 therapy has been seen in earlier lines as well. In KEYNOTE-061, median OS in the dMMR/MSI-H cohort (n = 27; 5.3%) was not reached with pembrolizumab versus 8.1 months with chemotherapy.41,56 Pembrolizumab treatment resulted in 47% ORR versus 17% with paclitaxel. In the first-line KEYNOTE-062 trial, OS was not reached for patients with dMMR/MSI-H cancers treated with either pembrolizumab monotherapy or pembrolizumab with chemotherapy versus 8.5 months for chemotherapy alone (n = 50; 7.3%).27,56 Similarly, in CheckMate 649, OS was not reached in patients with MSI-H tumors treated with nivolumab plus chemotherapy versus 12.3 months in those treated with chemotherapy alone, HR 0.37 (n = 44; 3%).6 These results suggest moving IO to earlier lines of treatment. The contribution of chemotherapy to IO in a first-line setting is unknown. Given the rarity of these tumors, conducting a randomized trial to answer this question will be challenging. It is possible that IO alone may be sufficient as the first-line therapy for patients with MSI-H tumors. However, those with symptomatic disease should probably be started on combination chemotherapy and IO, regardless of MSI status, to ensure rapid disease response. Chemotherapy can subsequently be de-escalated and patients may be continued on IO alone. This approach, however, is not supported by prospective randomized data.
TOXICITIES OF IO
Addition of immune checkpoint inhibitors to chemotherapy is associated with increased toxicities. Higher rates of grade ≥ 3 treatment-related AEs were observed across a number of trials (Table 1). For example, in CheckMate 649, 59% of patients had grade ≥ 3 AEs with nivolumab addition versus 44% with chemotherapy alone.6 Although up to a third of patients developed immune-mediated toxicities, most were mild, with grade ≥ 3 observed in ≤ 5% of patients.6 Treatment-related deaths were rare in all studies. Given that IO will be used most frequently in combination with other agents, determining toxicity contributions may be challenging in practice. It is recommended to refer to ASCO guidelines for immune-mediated toxicity management on how best to approach individual patients.57
SUMMARY AND FUTURE DIRECTIONS
Upper GI cancers are heterogeneous tumors with a complicated biology. Immune checkpoint inhibitors are now part of standard treatment for management of EGA and ESCC in early and advanced stages. These practice changes must be incorporated into future IO trial designs, some of which should focus on investigating mechanisms and ways to overcome secondary IO resistance. Furthermore, a substantial proportion of patients have immunologically cold tumors and derive no benefit from IO. Research efforts to improve the activity of existing agents, find novel combinations, and better identify patients who are most likely to respond to current treatments are of great importance. Emerging immune targets, such as TIGIT and DKK1, are being evaluated in ongoing trials after promising activity in earlier studies (ClinicalTrials.gov identifiers: NCT04363801, NCT04047862). Combination of IO with small-molecule inhibitors and antiangiogenic agents, such as cabozantinib and lenvatinib, are other approaches under active investigation (ClinicalTrials.gov identifiers: NCT03321630, NCT03539822). Finally, as new biomarkers continue to define subsets of upper GI cancers, for example, claudin-18.2 and FGFR2b, it will be critical to establish how to prioritize available therapies or combine agents against these targets to optimize patient outcomes.
Monica A. Patel
Honoraria: OncLive/MJH Life Sciences
Sam J. Lubner
Stock and Other Ownership Interests: Elephas Bio
Consulting or Advisory Role: Elephas Bio
Research Funding: Agios (Inst), Bristol Myers Squibb Foundation (Inst), AstraZeneca/MedImmune/Spirogen (Inst), Incyte (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1046782
Noelle K. LoConte
Consulting or Advisory Role: AbbVie, Personal Genome Diagnostics
Nataliya V. Uboha
Stock and Other Ownership Interests: Natera, Exact Sciences
Consulting or Advisory Role: Gerson Lehrman Group, Lilly, LEK, M3, Ipsen, AstraZeneca, Taiho Pharmaceutical, Incyte, Guidepoint Global, Taiho Pharmaceutical, QED Therapeutics, Astellas Pharma, Pfizer, Helsinn Therapeutics
Research Funding: EMD Serono (Inst), Taiho Pharmaceutical (Inst), Lilly (Inst), Ipsen (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by University of Wisconsin, Carbone Cancer Center (P30 CA014520).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Esophagogastric Cancers: Integrating Immunotherapy Therapy Into Current Practice
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Monica A. Patel
Honoraria: OncLive/MJH Life Sciences
Sam J. Lubner
Stock and Other Ownership Interests: Elephas Bio
Consulting or Advisory Role: Elephas Bio
Research Funding: Agios (Inst), Bristol Myers Squibb Foundation (Inst), AstraZeneca/MedImmune/Spirogen (Inst), Incyte (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1046782
Noelle K. LoConte
Consulting or Advisory Role: AbbVie, Personal Genome Diagnostics
Nataliya V. Uboha
Stock and Other Ownership Interests: Natera, Exact Sciences
Consulting or Advisory Role: Gerson Lehrman Group, Lilly, LEK, M3, Ipsen, AstraZeneca, Taiho Pharmaceutical, Incyte, Guidepoint Global, Taiho Pharmaceutical, QED Therapeutics, Astellas Pharma, Pfizer, Helsinn Therapeutics
Research Funding: EMD Serono (Inst), Taiho Pharmaceutical (Inst), Lilly (Inst), Ipsen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs CS, Shitara K, Di Bartolomeo M, et al. : Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 20:420-435, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Doi T, Piha-Paul SA, Jalal SI, et al. : Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 36:61-67, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Thompson ED, Zahurak M, Murphy A, et al. : Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 66:794-801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doki Y, Ajani JA, Kato K, et al. : Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 386:449-462, 2022 [DOI] [PubMed] [Google Scholar]
- 6.Janjigian YY, Shitara K, Moehler M, et al. : First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince EA, Sanzari JK, Pandya D, et al. : Analytical concordance of PD-L1 assays utilizing antibodies from FDA-approved diagnostics in advanced cancers: A systematic literature review. JCO Precis Oncol 5:953-973, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn S, Kim KM: PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod Pathol 34:1719-1727, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Schoemig-Markiefka B, Eschbach J, Scheel AH, et al. : Optimized PD-L1 scoring of gastric cancer. Gastric Cancer 24:1115-1122, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye M, Huang D, Zhang Q, et al. : Heterogeneous programmed death-ligand 1 expression in gastric cancer: Comparison of tissue microarrays and whole sections. Cancer Cell Int 20:186, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalpakoff M, Hund S, Musser J, et al. : Intrapatient tumor heterogeneity in IHC interpretation using PD-L1 IHC 22C3 pharmDx. Appl Immunohistochem Mol Morphol 29:667-673, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou KI, Peterson B, Serritella A, et al. : Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin Cancer Res 26:6453-6463, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Hagen P, Hulshof MC, van Lanschot JJ, et al. : Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074-2084, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Eyck BM, van Lanschot JJB, Hulshof M, et al. : Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol 39:1995-2004, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Liu H, Chen Y, et al. : Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: The NEOCRTEC5010 randomized clinical trial. JAMA Surg 156:721-729, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly RJ, Ajani JA, Kuzdzal J, et al. : Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384:1191-1203, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Shah MA, Hofstetter WL, Kennedy EB, et al. : Immunotherapy in patients with locally advanced esophageal carcinoma: ASCO treatment of locally advanced esophageal carcinoma guideline rapid recommendation update. J Clin Oncol 39:3182-3184, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Al-Batran SE, Homann N, Pauligk C, et al. : Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 393:1948-1957, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Bang YJ, Van Cutsem E, Fuchs CS, et al. : KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 15:943-952, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Liu XB, Sun HB, et al. : A phase III study on neoadjuvant chemotherapy versus neoadjuvant toripalimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: Henan Cancer Hospital Thoracic Oncology Group 1909 (HCHTOG1909). Ann Transl Med 9:73, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smyth EC, Wotherspoon A, Peckitt C, et al. : Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol 3:1197-1203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SM, An JY, Byeon SJ, et al. : Prognostic value of mismatch repair deficiency in patients with advanced gastric cancer, treated by surgery and adjuvant 5-fluorouracil and leucovorin chemoradiotherapy. Eur J Surg Oncol 46:189-194, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Andre T, Tougeron D, Piessen G, et al. : Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): The GERCOR NEONIPIGA phase II study. J Clin Oncol 40, 2022. (abstr 244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moehler M, Shitara K, Garrido M, et al. : Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 31:S1142-S1215, 2020 [Google Scholar]
- 26.Janjigian YY, Bendell J, Calvo E, et al. : CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol 36:2836-2844, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shitara K, Van Cutsem E, Bang YJ, et al. : Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JM, Shen L, Shah MA, et al. : Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398:759-771, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Tabernero J, Bang YJ, Van Cutsem E, et al. : KEYNOTE-859: A phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol 17:2847-2855, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boku N, Ryu MH, Kato K, et al. : Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: Interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 30:250-258, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boku N, Ryu MH, Oh DY, et al. : LBA7_PR Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol 31:S1192, 2020 [Google Scholar]
- 32.Xu J, Jiang H, Pan Y, et al. : Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ)adenocarcinoma (ORIENT-16): First results of a randomized, double-blind, phase III study, ESMO Congress 2021. Ann Oncol 32:S1283-S1346, 2021 [Google Scholar]
- 33.Zhao JJ, Yap DWT, Chan YH, et al. : Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol 40:392-402, 2022 [DOI] [PubMed] [Google Scholar]
- 34.Van Cutsem E, Bang YJ, Feng-Yi F, et al. : HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18:476-484, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Janjigian YY, Maron SB, Chatila WK, et al. : First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol 21:821-831, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rha SY, Lee C-K, Kim HS, et al. : Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol 38, 2020. (abstr 3081) [Google Scholar]
- 38.Chung HC, Bang YJ, Fuchs CS, et al. : First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol 17:491-501, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janjigian YY, Kawazoe A, Yanez P, et al. : The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600:727-730, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima T, Shah MA, Muro K, et al. : Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38:4138-4148, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Shitara K, Ozguroglu M, Bang YJ, et al. : Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 392:123-133, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Fuchs CS, Doi T, Jang RW, et al. : Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 4:e180013, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang YK, Boku N, Satoh T, et al. : Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461-2471, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Chau I, Doki Y, Ajani JA, et al. : Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol 39, 2021. (abstr LBA4001) [Google Scholar]
- 45.Hall PS, Swinson D, Cairns DA, et al. : Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of Life and cancer control among older and frail patients with advanced gastroesophageal cancer: The GO2 phase 3 randomized clinical trial. JAMA Oncol 7:869-877, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen L: Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: First results of the phase III ORIENT-15 study. Ann Oncol 32:S1283-S1346, 2021 [Google Scholar]
- 47.Xu R: JUPITER-06: A randomized, double-blind, phase III study of toripalimab versus placebo in combination with first-line chemotherapy for treatment naive advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol 32:S1040-S1075, 2021 [Google Scholar]
- 48.Xu R-H, Luo H, Lu J, et al. : ESCORT-1st: A randomized, double-blind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy versus chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC). J Clin Oncol 39, 2021. (abstr 4000) [Google Scholar]
- 49.Li J, Yao X, Kortmansky JS, et al. : Phase II study of modified FOLFOX6 with bevacizumab in metastatic gastroesophageal adenocarcinoma. Am J Clin Oncol 40:146-151, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Shen L, Kato K, Kim S-B, et al. : RATIONALE 302: Randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol 39, 2021: (abstr 4012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato K, Cho BC, Takahashi M, et al. : Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506-1517, 2019 [DOI] [PubMed] [Google Scholar]
- 52.Amonkar M, Lorenzi M, Zhang J, et al. : Structured literature review (SLR) and meta-analyses of the prevalence of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in gastric, colorectal, and esophageal cancers. J Clin Oncol 37, 2019. (suppl 15; abstr e15074) [Google Scholar]
- 53.Zang Ys, Dai C, Xu X, et al. : Comprehensive analysis of potential immunotherapy genomic biomarkers in 1000 Chinese patients with cancer. Cancer Med 8:4699-4708, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hause RJ, Pritchard CC, Shendure J, et al. : Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 22:1342-1350, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Fang W-L, Chen M-H, Huang K-H, et al. : The clinicopathological features and genetic mutations in gastric cancer patients according to EMAST and MSI status. Cancers (Basel) 12:551, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao J, Fuchs CS, Shitara K, et al. : Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 7:895-902, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brahmer JR, Lacchetti C, Schneider BJ, et al. : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36:1714-1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]