Abstract
INTRODUCTION:
Frailty is common in patients with cirrhosis and increases the vulnerability to internal and external stressors. This study aimed to investigate the impact of frailty, as defined by the Clinical Frailty Scale (CFS), on the risk of acute kidney injury (AKI) and hepatorenal syndrome (HRS-AKI) in hospitalized patients with liver cirrhosis.
METHODS:
We analyzed data of 201 nonelectively hospitalized patients with cirrhosis and without higher-grade chronic kidney disease. Patient characteristics were captured within the first 24 hours of hospital admission, and frailty was assessed using the CFS. Patients were followed for the development of AKI and/or HRS-AKI during the hospital stay.
RESULTS:
In the total cohort, median CFS was 3 (interquartile range 3–4), and 34 (16.9%) patients were frail (CFS >4). During the hospital stay, 110 (54.7%) and 49 (24.3%) patients developed AKI or HRS-AKI, respectively. Patients with AKI or HRS-AKI had a significantly higher CFS than patients without kidney injury (P < 0.001 each). In multivariable analyses, a higher CFS was independently associated with the development of AKI (odds ratio [OR] 1.467, 95% confidence interval (CI) 1.065–2.021) in the total cohort and HRS-AKI (OR 1.809, 95% CI 1.263–2.591) in the subcohort of patients with a history of ascites. In addition, there was a strong association between frailty (OR 3.717, 95% CI 1.456–9.491) and HRS-AKI.
DISCUSSION:
Frailty in patients with cirrhosis is associated with AKI and HRS-AKI. In this context, CFS appears to be a reliable tool to identify patients at high risk for developing AKI or HRS-AKI on hospital admission.
INTRODUCTION
Frailty is a multidimensional syndrome characterized by loss of individual reserve capacity and increased vulnerability to internal and external stressors (1). It has previously been observed that frailty is a robust predictor for hospitalization or death in patients with liver cirrhosis and is independently associated with waitlist mortality in patients awaiting liver transplantation (2,3). However, there is only a small body of literature regarding the relationship between frailty and renal dysfunction or acute kidney injury (AKI) (4). AKI is a frequent, serious, and prognostically relevant complication of liver cirrhosis. Approximately 20%–50% of patients hospitalized for decompensated cirrhosis develop AKI (5,6). It has been demonstrated that frailty represents a clinical parameter that may identify patients with liver cirrhosis most vulnerable to AKI in an outpatient setting using the Liver Frailty Index (LFI) (3) or the more time-consuming Fried Frailty Criteria (7). Frailty as a predictor could both influence treatment decisions and identify those patients who may benefit from early treatment. The latter is particularly important in the treatment of hepatorenal syndrome (HRS-AKI), a functional kidney disease that occurs in patients with decompensated liver cirrhosis and is associated with high mortality (8). However, data on the impact of frailty and the prognostic value of established assessment tools for the prediction of AKI or HRS-AKI in hospitalized patients with liver cirrhosis are currently lacking. For use in an inpatient setting, pragmatic applicability is of particular importance. The validated Clinical Frailty Scale (CFS) allows health professionals to assess the graduation of frailty within a short amount of time and is independent of acute-illness derived frailty (2). Therefore, this study aimed to evaluate the impact of frailty, as defined by the CFS, on the risk of AKI and HRS-AKI in nonelectively hospitalized patients with liver cirrhosis.
METHODS
Patient cohort
Data of 231 consecutive, nonelectively hospitalized patients with liver cirrhosis prospectively enrolled within the first 24 hours of hospital admission between January 2019 and June 2021 at the Cirrhosis Centre Mainz of the University Medical Centre of the Johannes Gutenberg-University in Mainz (Germany) were retrospectively (post hoc) analyzed. A subset of this cohort was previously used to analyze the predictive ability of the CFS for short-term mortality in hospitalized patients with cirrhosis (9). The leading etiology of the underlying liver disease was determined according to clinical, serological, and histological findings. Diagnosis of liver cirrhosis was established by histology or a combination of conclusive appearance in ultrasound, radiological imaging, endoscopic features of portal hypertension, and medical history. Blood biochemistry was assessed in all patients. The Model for End-Stage Liver Disease (MELD) score on the first day of hospitalization was calculated to determine the severity of the underlying liver disease (10). Patients were followed retrospectively via electronic chart review for the development of AKI and HRS-AKI during the hospital stay. AKI and HRS-AKI were defined according to the AKI Network criteria, which are explained in detail below.
Patients were excluded if they fulfilled one or more of the following criteria: preexisting dialysis requirement or a glomerular filtration rate < 60 mL/min/1.73 m2 due to either functional or structural chronic kidney disease such as HRS–non-AKI. The flowchart of the study is displayed in Figure 1.
Figure 1.
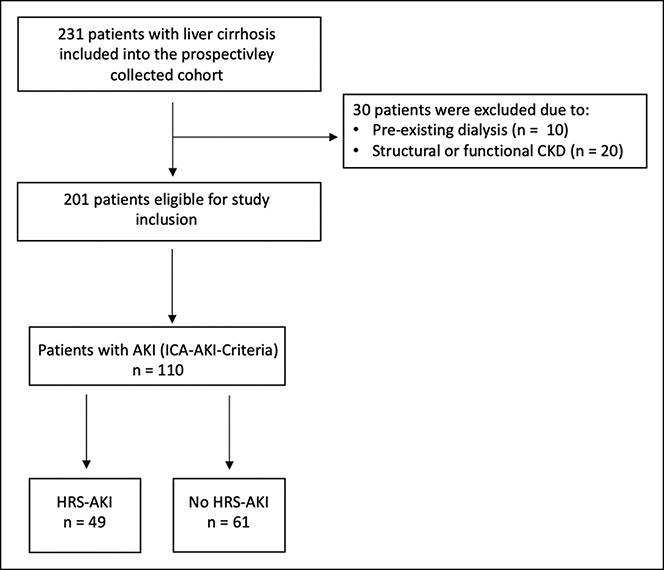
Flowchart of the study. Acute kidney injury (AKI), chronic kidney disease (CKD), hepatorenal syndrome (HRS-AKI), and International Club of Ascites (ICA).
Assessment of frailty
The CFS was used to assess frailty and was performed for each patient at enrollment into the study. CFS was assessed by V.K. under supervision of C.L. For assessment of CFS, disease symptoms, loss of function, cognitive impairment, and life expectancy are considered in a structured manner, which is described in detail elsewhere (9,11). The CFS is based on clinical assessment and divided into 9 categories: (i) very fit, (ii) well, (iii) well with treated comorbid diseases, (iv) apparently vulnerable, (v) mildly frail, (vi) moderately frail, (vii) severely frail, (viii) very severely frail, and (ix) terminally ill (11). According to accepted definitions, frailty was defined as a CFS > 4 (CFS 5–9).
Diagnosis of AKI
The diagnosis of AKI in patients with liver cirrhosis was defined in 2015 by the International Club of Ascites (ICA) according to the AKI Network criteria (12). According to the ICA-AKI criteria, AKI in patients with cirrhosis is defined as an increase in serum creatinine (SCr) of at least 0.3 mg/dL (26.5 mmol/L) within 48 hours or a percentage increase in SCr of at least 50% from baseline, which is already known or presumed, to have occurred within the prior 7 days (12). These criteria classify AKI into different stages (AKI stage 1A and B, AKI stage 2, and AKI stage 3) based on prognostic relevance (13–15):
Stage 1A: Increase in SCr ≥ 0.3 mg/dL from baseline to a value < 1.5- mg/dL.
Stage 1B: Increase in SCr ≥ 0.3 mg/dL from baseline to a value ≥ 1.5- mg/dL.
Stage 2: Increase in SCr > 2-fold to 3-fold from baseline.
Stage 3: Increase in SCr > 3-fold from baseline or SCr ≥ 4.0 mg/dL with an acute increase ≥0.3 mg/dL or initiation of renal replacement therapy.
In the absence of baseline values and an existing AKI on hospital admission, we used the lowest SCr value of the previous 3 months as a baseline to make a prompt diagnosis (12,16).
Diagnosis of HRS-AKI
HRS-AKI is a diagnosis of exclusion. Therefore, it was diagnosed according to the ICA-AKI criteria in the presence of decompensated cirrhosis with ascites and the following criteria (12):
No response after 48 hours of diuretic withdrawal and plasma volume expansion with albumin.
No current or recent use of nephrotoxic drugs (e.g., NSAIDs).
Absence of shock.
Exclusion of signs of structural kidney injury indicated by proteinuria, microhematuria, and/or abnormal renal ultrasonography.
Patients with preexisting structural or functional renal insufficiency, such as HRS-non-AKI, were excluded as previously mentioned.
Ethics
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study was approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz (Nr. 837.052.12 [8153]). Written informed consent was obtained from all participants.
Statistical analysis
Data were analyzed using IBM SPSS Statistic Version 27.0 (IBM, Armonk, NY) and GraphPad Prism Version 8.0.2 (GraphPad Software, CA). Quantitative data are expressed as medians with interquartile ranges (IQR), and pairwise comparisons for quantitative variables were performed with an unpaired t test or with the Mann-Whitney U test. Categorical variables are expressed as frequencies and percentages. For comparison of 2 or more patient groups, a χ2 test was applied.
To identify variables associated with the presence or development of AKI or HRS-AKI during the hospital stay, different multivariable analyses using logistic regression models were conducted. These models were based on a stepwise variable selection procedure. All available clinical and laboratory parameters were included into the models under consideration of potential collinearity (e.g., bilirubin or the International Normalized Ratio are part of the MELD score).
Our complete data analysis is exploratory. Hence, no adjustments for multiple testing were performed. For all tests, we used a 0.05 level to define statistically relevant deviations from the respective null hypotheses.
RESULTS
Demographics and baseline characteristics
A total of 231 patients with liver cirrhosis were recruited. Of these, 30 were excluded from further analysis. Median age of the total cohort was 58 years (IQR 50–65 years), and most patients were male (62.7%). At hospital admission, the median MELD score was 17. Frailty was detected in 34 (17%) patients according to CFS, and median CFS was 3. Additional baseline characteristics are displayed in Table 1.
Table 1.
Demographics and clinical characteristics of the cohort at the time of study inclusion (hospital admission)
| Variable | Total cohort, n = 201 |
| Age, y (IQR) | 58 (50–65) |
| Male sex, n (%) | 126 (62.7) |
| Etiology | |
| Alcohol, n (%) | 128 (63.7) |
| Viral hepatitis, n (%) | 10 (5.0) |
| NAFLD/cryptogenic, n (%) | 34 (16.9) |
| Cholestatic/autoimmune, n (%) | 17 (8.5) |
| Other/mixed, n (%) | 12 (6.0) |
| Median MELD score (IQR) | 17 (12–24) |
| Child-Pugh A/B/C, n (%) | 11 (5.5)/116 (57.7)/74 (36.8) |
| ACLF, n (%) | 76 (37.8) |
| Infection, n (%) | 67 (33.3) |
| History of ascites, n (%) | 184 (91.5) |
| History of overt HE, n (%) | 60 (29.9) |
| History of HRS-AKI, n (%) | 52 (25.9) |
| Complications at admission (multiple complications possible) | |
| Overt HE | 50 (24.9) |
| Ascites with need for paracentesis | 171 (85.1) |
| Variceal bleeding | 4 (2.0) |
| Nonvariceal bleeding | 14 (7.0) |
| SBP | 23 (11.4) |
| Infection | 67 (33.3) |
| Sodium, mmol/L (IQR) | 136 (133–139) |
| Albumin, g/L (IQR) | 25 (21–31) |
| Bilirubin, mg/dL (IQR) | 2.8 (1.3–8.9) |
| INR (IQR) | 1.5 (1.3–1.9) |
| Hemoglobin, g/dL (IQR) | 10.2 (8.6–12.2) |
| Platelets, nL (IQR) | 106 (72–159) |
| WBC, nL (IQR) | 7.6 (4.6–11.1) |
| CRP, mg/L (IQR) | 23 (8–51) |
| SCr, mg/dL (IQR) | 1.02 (0.78–1.62) |
| AKI at admission, n (%) | 44 (20.4) |
| CFS (IQR) Frail (CFS > 4), n (%) |
3 (3–4) 34 (17.0) |
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; CFS, Clinical Frailty Scale; CRP, C-reactive protein; HE, hepatic encephalopathy; HRS-AKI, hepatorenal syndrome; INR, International Normalized Ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; SBP, spontaneous bacterial peritonitis; SCr, serum creatinine; WBC, white blood cell count.
On the day of study inclusion, 20.4% had AKI based on the ICA-AKI criteria. The median SCr level was 1.02 mg/dL (IQR 0.78–1.62). No patient died during the hospital stay without an episode of AKI.
Variables associated with AKI during the hospital stay in the total cohort
In total, 110 (54.7%) patients had AKI at hospital admission or during the hospital stay. Of those, 25 (22.7%) patients progressed to AKI stage 1A, 23 (20.9%) to stage 1B, 19 (17.3%) to stage 2, and 43 (39.1%) to stage 3. Triggers of AKI were infections (n = 24), bleeding (n = 11), refractory ascites (n = 46), volume depletion (n = 25), or a combination/other (n = 4). Patients with AKI differed significantly from patients without AKI in terms of the presence of infections at admission, liver functions tests such as MELD, inflammation parameters (white blood cell count and C-reactive protein [CRP]), hemoglobin levels, and CFS (Table 2, Figure 2). The frequency of the different stages of AKI did not differ significantly between frail and robust patients (CFS cutoff > 4, see Supplementary Figure 1, http://links.lww.com/CTG/A834). To identify variables associated with AKI during the hospital stay, a multivariable logistic regression analysis with a stepwise variable selection procedure was conducted (Table 3). Here, a higher CFS (odds ratio [OR] 1.467, 95% confidence interval [CI] 1.065–2.021, P = 0.019), a higher MELD score (OR 1.100, 95% CI 1.053–1.149, P < 0.001), a higher CRP (OR 1.014, 95% CI 1.004–1.025, P = 0.007), and lower hemoglobin levels (OR 0.801, 95% CI 0.692–0.927, P = 0.003) were independently associated with AKI during the hospital stay (Table 3). This multivariable logistic regression analysis was repeated including Child-Pugh class instead of the MELD score. Here, CFS remained independently associated with AKI during the hospital stay (OR 1.440, 95% CI 1.055–1.964, P = 0.021) (see Supplementary Table 1, http://links.lww.com/CTG/A835).
Table 2.
Demographics and clinical characteristics of the patients with and without acute kidney injury (AKI) during the hospital stay (n = 201)
| Variable | Patients with AKI n = 110 | Patients without AKI n = 91 | P value |
| Age, y (IQR) | 59 (50–65) | 57 (49–65) | 0.504 |
| Male sex, n (%) | 66 (60.0) | 60 (65.9) | 0.387 |
| Etiology | |||
| Alcohol, n (%) | 71 (64.5) | 57 (62.6) | 0.957 |
| Viral hepatitis, n (%) | 6 (5.5) | 4 (4.4) | |
| NAFLD, n (%) | 18 (16.4) | 16 (17.6) | |
| Cholestatic/autoimmune, n (%) | 8 (7.3) | 9 (9.9) | |
| Other/mixed, n (%) | 7 (6.4) | 5 (5.5) | |
| Median MELD score (IQR) | 21 (15–27) | 15 (10–18) | <0.001 |
| Child-Pugh A/B/C, n (%) | 3 (2.7)/61 (55.5)/46 (41.8) | 8 (8.8)/55 (60.4)/28 (30.8) | 0.074 |
| ACLF, n (%) | 64 (58.1) | 12 (13.2) | <0.001 |
| Infection, n (%) | 50 (45.5) | 17 (18.7) | <0.001 |
| History of ascites, n (%) | 105 (95.4) | 79 (86.8) | 0.028 |
| History of HE, n (%) | 38 (34.5) | 22 (24.2) | 0.110 |
| History of HRS-AKI, n (%) | 34 (30.9) | 18 (19.8) | 0.073 |
| Sodium, mmol/L (IQR) | 136 (132–139) | 137 (134–139) | 0.149 |
| Albumin, g/L (IQR) | 24 (20–29) | 28 (22–34) | 0.014 |
| Bilirubin, mg/dL (IQR) | 3.3 (1.2–13.5) | 2.8 (1.5–5.2) | 0.264 |
| INR (IQR) | 1.6 (1.3–2.1) | 1.4 (1.3–1.8) | 0.049 |
| Hemoglobin, g/dL (IQR) | 9.7 (8.5–11.4) | 11.0 (9.1–12.6) | 0.004 |
| Platelets, nL (IQR) | 110 (72–162) | 106 (69–152) | 0.338 |
| WBC, nL (IQR) | 8.8 (5.5–12.7) | 6.1 (4.1–9.3) | <0.001 |
| CRP, mg/L (IQR) | 34 (14–54) | 13 (6–33) | <0.001 |
| SCr, mg/dL (IQR) | 1.41 (0.96–2.27) | 0.81 (0.68–1.03) | <0.001 |
| CFS (IQR) Frail (CFS > 4), n (%) |
4 (3–4) 24 (21.8) |
3 (3–4) 10 (11.0) |
<0.001 0.041 |
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
ACLF, acute-on-chronic liver failure; CFS, Clinical Frailty Scale; CRP, C-reactive protein; HE, hepatic encephalopathy; HRS-AKI, hepatorenal syndrome; INR, International Normalized Ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; SCr, serum creatinine; WBC, white blood cell count.
Figure 2.
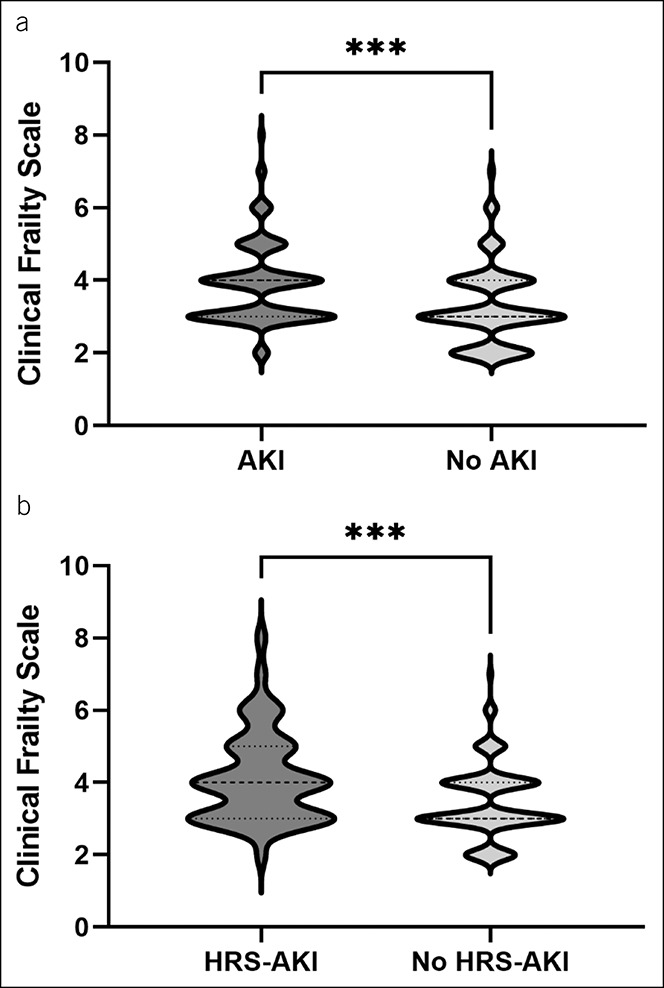
Violin plots displaying the median Clinical Frailty Scale of patients with and without acute kidney injury (AKI) or hepatorenal syndrome (HRS-AKI). (a) Displays the median Clinical Frailty Scale of patients with (n = 110) and without (n = 91) AKI during the hospital stay (***P < 0.001). (b) Displays the median Clinical Frailty Scale of patients with a history of ascites with (n = 49) and without (n = 135) HRS-AKI during the hospital stay (***P = 0.001).
Table 3.
Logistic regression analyses of variables associated with the development of an acute kidney injury (AKI) during the hospital stay (total cohort, n = 201)
| Total cohort | OR (95% CI) | P value |
| CFS MELD CRP Hemoglobin |
1.467 (1.065–2.021) 1.100 (1.053–1.149) 1.014 (1.004–1.025) 0.801 (0.692–0.927) |
0.019 <0.001 0.007 0.003 |
Logistic regression analyses were built based on a stepwise variable selection procedure. Not significant were a history of ascites, albumin, sodium, white blood cell count, platelets, infection at study inclusion, sex, age, a history of hepatic encephalopathy, a history of hepatorenal syndrome, and alcoholic etiology of liver cirrhosis.
CFS, Clinical Frailty Scale; 95% CI, 95% confidence interval; CRP, C-reactive protein; MELD, model for end-stage liver disease; OR, odds ratio.
Variables associated with HRS-AKI during the hospital stay in the total cohort
In total, 49 (24.3%) patients had HRS-AKI at hospital admission or during the hospital stay. Triggers of HRS-AKI were infections (n = 14), bleeding (n = 4), refractory ascites (n = 19), volume depletion (n = 1), or a combination/other (n = 11). To identify variables associated with HRS-AKI during the hospital stay, we conducted univariable and multivariable analyses. Patients without a history of ascites (n = 17) were excluded from these analyses because ascites is a prerequisite for HRS-AKI. Patients with HRS-AKI differed significantly from patients without HRS-AKI in terms of the presence of infections at admission, liver functions tests such as MELD, history of HRS-AKI, hemoglobin levels, inflammation parameters (white blood cell count and CRP), and CFS (Table 4).
Table 4.
Demographics and clinical characteristics of the subgroup of patients with a history of ascites with and without hepatorenal syndrome (HRS-AKI) during the hospital stay (n = 184)
| Variable | Patients with HRS-AKI, n = 49 | Patients without HRS-AKI, n = 135 | P value |
| Age, y (IQR) | 57 (50–63) | 58 (49–66) | 0.363 |
| Male sex, n (%) | 28 (57.1) | 91 | 0.198 |
| Etiology | |||
| Alcohol, n (%) | 39 (79.6) | 82 (60.7) | 0.192 |
| Viral hepatitis, n (%) | 1 (2.0) | 8 (5.9) | |
| NAFLD, n (%) | 6 (12.2) | 25 (18.5) | |
| Cholestatic/autoimmune, n (%) | 12 (24.5) | 12 (8.9) | |
| Other/mixed, n (%) | 8 (16.3) | 8 (5.9) | |
| Median MELD score (IQR) | 25 (18–30) | 16 (11–21) | <0.001 |
| Child-Pugh A/B/C, n (%) | 1 (2.0)/23 (46.9)/25 (51.0) | 6 (4.4)/81 (60.0)/48 (35.6) | 0.150 |
| Infection, n (%) | 26 (53.1) | 37 (27.4) | 0.001 |
| History of HE, n (%) | 20 (40.8) | 38 (28.1) | 0.102 |
| History of HRS-AKI, n (%) | 22 (44.9) | 28 (20.7) | 0.001 |
| Sodium, mmol/L (IQR) | 137 (131–140) | 136 (132–138) | 0.300 |
| Albumin, g/L (IQR) | 25 (21–31) | 25 (20–31) | 0.992 |
| Bilirubin, mg/dL (IQR) | 4.4 (1.5–16.2) | 2.8 (1.3–7.2) | 0.128 |
| INR (IQR) | 1.7 (1.3–2.3) | 1.5 (1.3–1.8) | 0.080 |
| Hemoglobin, g/dL (IQR) | 9.6 (8.4–11.0) | 10.6 (9–12.5) | 0.009 |
| Platelets, nL (IQR) | 116 (75–164) | 103 (69–151) | 0.238 |
| WBC, nL (IQR) | 9.2 (7.3–14.3) | 7.1 (4.5–10.8) | 0.238 |
| CRP, mg/L (IQR) | 40 (22–54) | 17 (7–43) | <0.001 |
| SCr, mg/dL (IQR) | 2.05 (1.21–3.13) | 0.95 (0.75–1.30) | <0.001 |
| CFS (IQR) Frail (CFS > 4), n (%) |
4 (3–5) 15 (30.6) |
3 (3–4) 17 (12.6) |
0.001 0.004 |
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
CFS, Clinical Frailty Scale; CRP, C-reactive protein; HE, hepatic encephalopathy; HRS-AKI, hepatorenal syndrome; INR, International Normalized Ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; SCr, serum creatinine; WBC, white blood cell count.
In multivariable logistic regression analysis, a higher CFS (OR 1.809, 95% CI 1.263–2.591, P = 0.001), a higher MELD score (OR 1.120, 95% CI 1.069–1.173, P < 0.001), and lower hemoglobin levels (OR 0.776, 95% CI 0.645–0.933, P = 0.007) were independently associated with HRS-AKI during the hospital stay (Table 5). In a second multivariable logistic regression analysis including frailty (CFS > 4) as a dichotomous variable, higher MELD scores, lower hemoglobin levels, a history of HRS-AKI, and frailty (OR 3.717, 95% CI 1.456–9.491, P = 0.006) were independently associated with HRS-AKI (Table 5). The multivariable logistic regression analysis was repeated including the Child-Pugh class instead of the MELD score. Here, CFS remained independently associated with HRS-AKI (OR 1.622, 95% CI 1.146–2.295, P = 0.006) (see Supplementary Table 2, http://links.lww.com/CTG/A835).
Table 5.
Logistic regression analyses of variables associated with the development of a hepatorenal syndrome (HRS-AKI) in patients with a history of ascites during the hospital stay (total cohort, n = 184)
| Total cohort | OR (95% CI) | P value |
| Model 1a | ||
| CFS | 1.809 (1.263–2.591) | 0.001 |
| MELD | 1.120 (1.069–1.173) | <0.001 |
| Hemoglobin | 0.776 (0.645–0.933) | 0.007 |
| Model 2b | ||
| Frailty (CFS > 4) | 3.717 (1.456–9.491) | 0.006 |
| MELD | 1.117 (1.065–1.171) | <0.001 |
| Hemoglobin | 0.778 (0.646–0.938) | 0.008 |
| History of HRS-AKI | 2.253 (1.018–4.990) | 0.045 |
Logistic regression analyses were built based on a stepwise variable selection procedure.
CFS, Clinical Frailty Scale; 95% CI, 95% confidence interval; HRS-AKI, hepatorenal syndrome; MELD, model for end-stage liver disease; OR, odds ratio.
Model 1 included CFS as a metric variable. Not significant were albumin, sodium, white blood cell count, platelets, infection at study inclusion, sex, age, a history of hepatic encephalopathy, a history of hepatorenal syndrome, C-reactive protein, and alcoholic etiology of liver cirrhosis.
Model 2 included frailty (CFS > 4) as a dichotomous variable. Not significant were albumin, sodium, white blood cell count, platelets, infection at study inclusion, sex, age, a history of hepatic encephalopathy, C-reactive protein, and alcoholic etiology of liver cirrhosis.
DISCUSSION
AKI and HRS-AKI are common in hospitalized patients with liver cirrhosis, and both are associated with a poor prognosis (13–15,17,18). Therefore, the identification of prognostic factors is of pivotal importance to detect patients at high risk in whom preventive measures would be useful. In this study, we found a robust association between a higher CFS stage and the presence or development of AKI and HRS-AKI in nonelectively hospitalized German patients with liver cirrhosis. In addition, frailty, as defined by a CFS > 4, was associated with a 3.7-fold risk increase for HRS-AKI during the hospital stay in multivariable analysis.
In hepatology, many efforts have been made to quantify frailty in patients with liver cirrhosis such as the Fried frailty criteria, the Short Physical Performance Battery, walking speed, 6-minute walk distance, the LFI, or the CFS (2,3,19–21). In the current study, the CFS was chosen because it is a simple assessment tool focusing on the clinical assessment of the patients' activity level, mobility, and independence in daily physical and cognitive activities in the period before the assessment (11). This is of pivotal importance in nonelectively hospitalized patients with liver cirrhosis due to concurrent acute-illness frailty (e.g., caused by hepatic encephalopathy or infections) and may be an advantage of the CFS over more physical-based tests such as the LFI. In addition, the CFS assessment is inexpensive and easy to perform.
A strong relationship between frailty and AKI in the elderly population has been previously reported (22). In our current study in patients with liver cirrhosis, a higher CFS stage, as well as frailty, was independently associated with the occurrence of HRS-AKI and AKI during the hospital stay. Other independent predictors for AKI or HRS-AKI included well-established factors such as a higher MELD score and lower hemoglobin levels, likely reflecting a more advanced disease stage of liver cirrhosis, or higher CRP values, likely reflecting systemic inflammation e.g., caused by infections. Currently, there is no comparable study investigating the impact of frailty on the risk of AKI and HRS-AKI in hospitalized patients with liver cirrhosis available. However, Cullaro et al. (4) analyzed data of more than 1,000 outpatients with cirrhosis and identified frailty as a risk factor for AKI but not HRS-AKI captured by the LFI. A bidirectional relationship between frailty and AKI as well as HRS-AKI seems plausible. Frailty is a consequence of multiple dysregulations in physiological circuits including the cardiovascular, musculoskeletal, neurological, and endocrinological systems. Studies conducted in recent years suggest that the immune system may play a particularly significant role in the development of frailty (23–27). A recent systematic review concluded that frailty is associated with higher levels of several inflammatory markers such as interleukin 6, CRP, and tumor necrosis factor–α (24). In a study investigating inflammation as a key marker for frailty, frail patients showed upregulated monocytic expression of a potent proinflammatory chemokine, CXCL-10, compared with matched nonfrail controls (28). Frail individuals and patients with liver cirrhosis, especially those who develop HRS-AKI, share common risk factors, such as systemic inflammation, and might be vulnerable to AKI and HRS-AKI through the same inflammatory response. A well-studied example regarding the interplay of cirrhosis and inflammation is the altered vascular tone mediated by cirrhosis-induced high proinflammatory cytokine levels (29). Proinflammatory cytokines worsen splanchnic and systemic vasodilation through nitric oxide overproduction (30). Splanchnic vasodilation leads to decreased systemic vascular resistance with the development of effective arterial hypovolemia. The activation of vasoconstrictor systems leads to marked renal vasoconstriction, low glomerular filtration rate, and development of HRS-AKI (29,31). Inflammatory mediators lead to further systemic vasodilatation and also could cause direct kidney tissue damage (32–34). Other factors associated with decompensation and HRS-AKI, such as ascites, abnormal renal autoregulation, and decreased cardiac output may lead to increased energy expenditure, decreased food intake, and physical inactivity, thereby increasing the risk of developing and worsening frailty (29,31,34,35). This may lead to a vicious cycle finally culminating in HRS-AKI. However, further research is needed to investigate this relationship, including the pathophysiological mechanisms of HRS-AKI, in detail.
Interestingly, only 17% of all patients included in this study were frail (defined as a CFS >4). Compared with a recently published study by Tandon et al., who screened 300 outpatients with liver cirrhosis with the CFS and reported that 18% of their patients were frail, the frequency of frailty according to the CFS in our study seems to be moderate. This may be explained by differences in the prevalence of frailty between countries or to some degree by subjectivity in the assessment of CFS. However, the reliability of CFS has been proven in previous studies. It also has to be mentioned that the frequency of frailty in our study differs from other studies that used tests relying on active patient participation and physical tasks. Those tests may be influenced by acute decompensation and may therefore overestimate the prevalence of frailty (36–38). All in all, the CFS seems to be a valuable tool to predict outcomes in hospitalized patients because it is independent of the acute deterioration of the patient's condition (9,39–42). In the United Kingdom, the CFS is already routinely applied in some hospitals in all patients over 75 years of age who present to the hospital via the emergency department (43). By regularly screening patients with liver cirrhosis on the first day of hospital admission, patients at high risk of developing AKI or HRS-AKI could be identified early and renal protective measurements could be initiated; existing diuretic treatment should be used cautiously, and the potential precipitating factors of AKI identified and treated (12). However, it has to be acknowledged that it is impossible to improve frailty in the short-term setting in nonelectively hospitalized patients with acute decompensation.
This study has several limitations that have to be acknowledged. First, our findings should be interpreted in the context of the study design. Our study was observational and retrospective and based on a single-center analysis. Therefore, we are reluctant to draw a definitive conclusion either on pathomechanisms or on causalitiy between frailty and AKI or HRS-AKI. Frailty has become increasingly important in the scientific context and clinical care in recent years; however, there is still no international consensus regarding its measurement and definition (44). In this study, we assessed frailty using the CFS, and we are therefore unable to analyze the predictive ability of other frailty measures for the prediction of AKI or HRS-AKI. In addition, the CFS captures frailty during the period before hospital admission. Therefore, complications of cirrhosis that may run a subacute course before hospital admission may interfere with the results of CFS. We excluded patients with preexisting dialysis treatment and chronic kidney disease (CKD) to get a purer and more homogenous cohort. However, our findings are therefore not expandable to patients with cirrhosis and CKD. Future studies are needed to delineate the possible connection between frailty and AKI in patients with concurrent CKD.
In conclusion, we found a robust association between a higher CFS stage and frailty and the development of AKI or HRS-AKI in nonelectively hospitalized German patients with liver cirrhosis. By regularly screening for the presence of frailty at hospital admission using CFS, patients at high risk of developing AKI or HRS-AKI could be identified early and preventive measures could be initiated.
CONFLICTS OF INTEREST
Guarantor of the article: Christian Labenz, MD.
Specific author contributions: Performed research: E.M.S., W.M.K., V.K., S.J.G., L.K., J.M.S., P.R.G., M.N., M.A.W., J.W.M., and C.L. Contributed to acquisition of data: E.M.S., W.M.K., V.K., M.N., and C.L. Designed the experiments and analyzed the data: E.M.S., W.M.K., V.K., M.N., and C.L. Contributed reagents/materials/analysis tools: P.R.G., M.A.W., and C.L. Wrote the paper: E.M.S. and C.L. Critical revision of the draft: W.M.K., V.K., S.J.G., L.K., J.M.S., P.R.G., M.N., M.A.W., and J.W.M. Statistical analysis: E.M.S. and C.L. All authors approved the final version of the manuscript and the authorship list.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Frailty is common in patients with liver cirrhosis.
✓ Frailty increases the vulnerability to internal and external stressors.
WHAT IS NEW HERE
✓ A higher Clinical Frailty Scale (CFS), a measure of frailty, was associated with acute kidney injury (AKI) in nonelectively hospitalized patients with cirrhosis.
✓ In the subcohort of patients with a history of ascites, there was an independent association between CFS and the development of hepatorenal syndrome-AKI.
✓ There was a strong association between frailty (CFS > 4) and HRS-AKI.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A834, http://links.lww.com/CTG/A835
Contributor Information
Eva Maria Schleicher, Email: Eva.schleicher@unimedizin-mainz.de.
Wolfgang Maximilian Kremer, Email: Maximilian.kremer@unimedizin-mainz.de.
Vasiliki Kalampoka, Email: bkalampoka@gmail.com.
Simon Johannes Gairing, Email: Simonjohannes.gairing@unimedizin-mainz.de.
Leonard Kaps, Email: Leonard.kaps@unimedizin-mainz.de.
Jörn M. Schattenberg, Email: Joern.schattenberg@unimedizin-mainz.de.
Peter Robert Galle, Email: Peter.galle@unimedizin-mainz.de.
Marcus-Alexander Wörns, Email: Marcus-alexander.woerns@klinikumdo.de.
Michael Nagel, Email: Michael.nagel@klinikumdo.de.
Julia Weinmann-Menke, Email: Julia.weinmann-menke@unimedizin-mainz.de.
ACKNOWLEDGEMENTS
This manuscript contains parts of the medical thesis of V.K. E.M.S. and S.J.G. are supported by the Clinician Scientist Fellowship “Else Kröner Research College: 2018_Kolleg.05.” C.L. is supported by the Clinical Research Fellowship Program by the Mainz Research School of Translational Biomedicine.
REFERENCES
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: Implications for clinical practice and public health. Lancet 2019;394(10206):1365–75. [DOI] [PubMed] [Google Scholar]
- 2.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: A prospective evaluation of the clinical frailty scale. Am J Gastroenterol 2016;111(12):1759–67. [DOI] [PubMed] [Google Scholar]
- 3.Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019;156(6):1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullaro G, Verna EC, Duarte-Rojo A, et al. Frailty and the risk of acute kidney injury among patients with cirrhosis. Hepatol Commun 2022;6:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48(6):2064–77. [DOI] [PubMed] [Google Scholar]
- 6.Fede G, D'Amico G, Arvaniti V, et al. Renal failure and cirrhosis: A systematic review of mortality and prognosis. J Hepatol 2012;56(4):810–8. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. , Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–M157. [DOI] [PubMed] [Google Scholar]
- 8.Salerno F, Cazzaniga M, Merli M, et al. , Italian Association of the Hospital Gastroenterologists AIGO investigators. Diagnosis, treatment and survival of patients with hepatorenal syndrome: A survey on daily medical practice. J Hepatol 2011;55(6):1241–8. [DOI] [PubMed] [Google Scholar]
- 9.Kremer WM, Nagel M, Reuter M, et al. Validation of the clinical frailty scale for the prediction of mortality in patients with liver cirrhosis. Clin Transl Gastroenterol 2020;11(7):e00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath PS, Kim WR, Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology 2007;45(3):797–805. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ Can Med Assoc J 2005;173(5):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62(4):968–74. [DOI] [PubMed] [Google Scholar]
- 13.Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol 2013;59(3):474–81. [DOI] [PubMed] [Google Scholar]
- 14.Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59(3):482–9. [DOI] [PubMed] [Google Scholar]
- 15.Huelin P, Piano S, Solà E, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2017;15(3):438–45.e5. [DOI] [PubMed] [Google Scholar]
- 16.Angeli P, Garcia-Tsao G, Nadim MK, et al. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the international Club of ascites (ICA) consensus document. J Hepatol 2019;71(4):811–22. [DOI] [PubMed] [Google Scholar]
- 17.Tandon P, James MT, Abraldes JG, et al. Relevance of new definitions to incidence and prognosis of acute kidney injury in hospitalized patients with cirrhosis: A retrospective population-based cohort study. PLoS One 2016;11(8):e0160394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassegoda O, Huelin P, Ariza X, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol 2020;72(6):1132–9. [DOI] [PubMed] [Google Scholar]
- 19.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates: Frailty in liver transplant candidates. Am J Transpl 2014;14(8):1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapper EB, Konerman M, Murphy S, et al. Hepatic encephalopathy impacts the predictive value of the fried frailty index. Am J Transplant 2018;18(10):2566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66(2):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiesisibieke ZL, Tung TH, Xu QY, et al. Association of acute kidney injury with frailty in elderly population: A systematic review and meta-analysis. Ren Fail 2019;41(1):1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulop T, McElhaney J, Pawelec G, et al. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr 2015;41:26–40. [DOI] [PubMed] [Google Scholar]
- 24.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Pothier K, Gana W, Bailly N, et al. Associations between frailty and inflammation, physical, and psycho-social health in older adults: A systematic review. Front Psychol 2022;13:805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang SS, Weiss CO, Xue QL, et al. Association between inflammatory-related disease burden and frailty: Results from the women's health and aging studies (WHAS) I and II. Arch Gerontol Geriatr 2012;54(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X, Li H, Leng SX. Inflammation and immune system Alterations in frailty. Clin Geriatr Med 2011;27(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu T, Yang H, Walston JD, et al. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine 2009;46(3):319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol 2014;61(6):1385–96. [DOI] [PubMed] [Google Scholar]
- 30.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology 2006;43(S1):S121–S131. [DOI] [PubMed] [Google Scholar]
- 31.Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63(5):1272–84. [DOI] [PubMed] [Google Scholar]
- 32.Navasa M, Follo A, Filella X, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality. Hepatology 1998;27(5):1227–32. [DOI] [PubMed] [Google Scholar]
- 33.Clària J, Stauber RE, Coenraad MJ, et al. , CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure EF-CLIF. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64(4):1249–64. [DOI] [PubMed] [Google Scholar]
- 34.Juanola A, Solé C, Toapanta D, et al. Monitoring renal function and therapy of hepatorenal syndrome patients with cirrhosis. Clin Liver Dis 2021;25(2):441–60. [DOI] [PubMed] [Google Scholar]
- 35.Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol 2021;75(Suppl 1):S147–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cron DC, Friedman JF, Winder GS, et al. Depression and frailty in patients with end-stage liver disease referred for transplant evaluation. Am J Transpl 2016;16(6):1805–11. [DOI] [PubMed] [Google Scholar]
- 37.Lai JC, Covinsky KE, McCulloch CE, et al. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhanji RA, Narayanan P, Moynagh MR, et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl 2019;25(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunaga A, Hikoso S, Yamada T, et al. , OCVC-Heart Failure Investigators. Prognostic impact of Clinical Frailty Scale in patients with heart failure with preserved ejection fraction. ESC Heart Fail 2021;8(4):3316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niemeläinen S, Huhtala H, Andersen J, et al. The clinical frailty scale is a useful tool for predicting postoperative complications following elective colon cancer surgery at the age of 80 years and above: A prospective, multicentre observational study. Colorectal Dis 2021;23(7):1824–36. [DOI] [PubMed] [Google Scholar]
- 41.Reichart D, Rosato S, Nammas W, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg 2018;54(6):1102–9. [DOI] [PubMed] [Google Scholar]
- 42.Torsney KM, Romero-Ortuno R. The Clinical Frailty Scale predicts inpatient mortality in older hospitalised patients with idiopathic Parkinson's disease. J R Coll Physicians Edinb 2018;48(2):103–7. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Ortuno R, Forsyth DR, Wilson KJ, et al. The association of geriatric syndromes with hospital outcomes. J Hosp Med 2017;12(2):83–9. [DOI] [PubMed] [Google Scholar]
- 44.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: A call to action. J Am Med Dir Assoc 2013;14(6):392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


