Abstract
Progress in the overall treatment of small-cell lung cancer (SCLC) has moved at a slower pace than non–small-cell lung cancer. In fact, the standard treatment regimen for limited stage SCLC has not appreciably shifted in more than 20 years, consisting of four to six cycles of cisplatin and etoposide chemotherapy concurrent with thoracic radiotherapy (TRT) followed by prophylactic cranial irradiation (PCI) for responsive disease. Nevertheless, long-term outcomes have improved with median survival approaching 25-30 months, and approximately one third of patients now survive 5 years. This is likely attributable in part to improvements in staging, including use of brain magnetic resonance imaging and fluorodeoxyglucose–positron emission tomography imaging, advances in radiation treatment planning, and supportive care. The CONVERT and CALGB 30610 phase III trials failed to demonstrate a survival advantage for high-dose, once-daily TRT compared with standard 45 Gy twice-daily TRT, although high-dose, once-daily TRT remains common in practice. A phase III comparison of high-dose 60 Gy twice-daily TRT versus 45 Gy twice-daily TRT aims to confirm the provocative outcomes reported with 60 Gy twice daily in the phase II setting. Efforts over time have shifted from intensifying PCI, to attempting to reduce treatment-related neurotoxicity, to more recently questioning whether careful magnetic resonance imaging surveillance may obviate the routine need for PCI. The addition of immunotherapy has resulted in mixed success in extensive-stage SCLC with modest benefit observed with programmed death-ligand 1 inhibitors, and several ongoing trials assess programmed death-ligand 1 inhibition concurrent or adjuvant to chemoradiotherapy in limited-stage SCLC. Major advances in future treatment will likely depend on a better understanding and exploiting of molecular characteristics of SCLC with increasing personalization of therapy.
INTRODUCTION
Small-cell lung cancer (SCLC) is an aggressive smoking-related malignancy accounting for approximately one seventh of all lung cancers.1 Tumors typically metastasize early resulting in bulky hilar and mediastinal lymph node involvement and distant disease. Approximately one third of patients present with limited-stage disease, with tumor confined to one hemithorax, with ipsilateral supraclavicular lymph node involvement permitted if all sites of disease can be encompassed within a single radiation portal.2 Limited-stage small-cell lung cancer (LS-SCLC) trials generally exclude patients with contralateral hilar and, or contralateral supraclavicular nodal spread. TNM staging is prognostic, although most clinical decisions are still based on the original Veterans Administration Lung Study Group classification.3
CONTEXT
Key Objective
Although standard treatment for limited-stage small-cell lung cancer (LS-SCLC) has not appreciably shifted in more than 20 years, outcomes have improved with median survival approaching 25-30 months, and approximately one third of patients now survive 5 years. This review examines current management, recent advances, and ongoing research in LS-SCLC.
Knowledge Generated
Recently completed and ongoing phase III studies of thoracic radiotherapy, prophylactic cranial irradiation (PCI), and systemic therapy aim to define best practices in LS-SCLC. The role of high-dose daily thoracic radiotherapy remains unsettled. Ongoing studies are geared toward reducing PCI-related neurotoxicity and questioning whether magnetic resonance imaging surveillance may obviate PCI. Trials integrating immune checkpoint inhibitors assess whether the benefits seen in extensive disease apply to LS-SCLC.
Relevance
Outcomes in LS-SCLC are promising with state-of-the-art combined modality therapy. Future progress may depend on refinement of local and systemic therapy with increasing personalization of treatment.
Progress in the treatment of SCLC has moved at a slower pace than non–small-cell lung cancer (NSCLC), with only modest benefit from immune checkpoint inhibitors (ICIs) in extensive-stage disease.4-6 In fact, the treatment regimen for LS-SCLC has not appreciably shifted in more than 20 years, with standard therapy consisting of 4-6 cycles of cisplatin and etoposide chemotherapy (PE) concurrent with thoracic radiotherapy (TRT) followed prophylactic cranial irradiation (PCI) in responsive disease. Nevertheless, long-term outcomes have improved for this population of patients with median survival approaching 25-30 months, and approximately one third of patients now survive 5 years.7,8 This is likely attributable in part to improvements in staging with increased use of brain magnetic resonance imaging (MRI) and 18-fluorodeoxyglucose positron emission tomography (PET), advances in radiation treatment planning, and better supportive care.
INTEGRATION OF TRT
Although SCLC is very responsive to systemic chemotherapy upon initial diagnosis, there is a preponderance of both local and systemic relapse even in cases presenting with limited disease. The value of including TRT was addressed in two meta-analyses published in 1992, which demonstrated improvements in overall survival (OS) and/or local tumor control, albeit at the expense of increased toxicity, with the addition of radiotherapy to chemotherapy.9,10 The impact of local TRT may have been underestimated as included studies used antiquated staging and treatment techniques and used systemic therapy that would currently be considered suboptimal.
TRT TIMING AND SEQUENCING
Alternating or sequencing chemotherapy and TRT were common in early European trials, but administering TRT concurrent with chemotherapy has been adopted as standard practice, given the relatively favorable outcomes with this approach. The JCOG-9104 phase III trial strongly supported concurrent over sequential therapy in the setting of modern chemoradiotherapy, although OS differences did not quite reach statistical significance (median survival of 27 months compared with 19.7 months, P = .08).11
Although the long-standing debate regarding the optimal timing for TRT in LS-SCLC has not been completely settled, most guidelines and practice patterns are in line with TRT starting with the first or second cycle of chemotherapy.12,13 Earlier TRT should result in better outcomes by reducing the time for resistant tumor clones to develop after initiating systemic therapy. The majority of studies directly addressing TRT timing are underpowered and further hampered by outdated staging and treatment methods. The primary trial supporting late TRT, CALGB 8083, was conducted before the routine utilization of cisplatin chemotherapy.14 However, the classic National Cancer Institute of Canada study supporting early TRT used hypofractionated TRT and antiquated techniques including spinal cord (and potentially tumor) blocking.15 While cycle 2 TRT bested cycle 6 TRT in the National Cancer Institute of Canada study, the benefit was attributed to fewer brain metastases with cycle 2 TRT, as local tumor control appeared similar in both cohorts.
Meta-analyses assessing TRT timing suggest benefit from early TRT with some caveats. The most comprehensive report, published in 2016, did not find an OS benefit for earlier or shorter TRT when data from nine studies including 2,305 patients were analyzed with median 10 years of follow-up.16 However, the importance of maintaining chemotherapy intensity was emphasized as earlier TRT was beneficial among trials with a similar proportion of patients who were compliant with chemotherapy. This analysis is somewhat confounded by the inclusion of JCOG 9104, where early TRT was concurrent but late TRT was sequential following chemotherapy,11 and the Intergroup (INT) 0096 trial, where TRT was initiated with cycle 1 in both arms and thus studied intensity of TRT, not simply timing.17
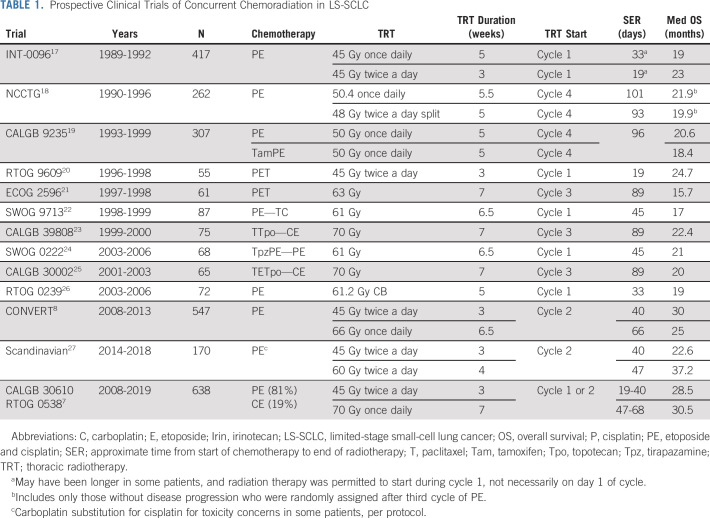
As a matter of clinical practice, it has become challenging to routinely initiate TRT with initial chemotherapy because of the complexity of modern radiotherapy treatment planning. There was a consensus to mandate cycle 1 TRT when CALGB 30610 (C30610) initiated, but the trial was amended early because of slow accrual and < 50% of patients received TRT with cycle 1 chemotherapy.7 TRT was started with the second chemotherapy cycle in CONVERT,8 and begins with cycle 2 chemotherapy in the ongoing NRG LU-005 assessing atezolizumab (NCT03811002). Early TRT is favored in our practice, but some clinical circumstances may dictate consideration of delaying TRT. Outcomes from select cooperative group studies dating back to INT-0096, including several initiating TRT with chemotherapy cycle 3 or 4, are detailed in Table 1.
TABLE 1.
Prospective Clinical Trials of Concurrent Chemoradiation in LS-SCLC
TRT DOSE AND FRACTIONATION
Traditionally, modest doses of TRT (40-50 Gy) with conventional fractionation were thought to be effective for LS-SCLC, given the high tumor response rate. However, ultimate local tumor control is suboptimal following conventional TRT as best demonstrated by the outcomes after 45 Gy once-daily radiotherapy from INT-0096, as the majority of patients had a component of thoracic tumor relapse.17
An accelerated twice-daily regimen was developed in the 1980s in an effort to enhance TRT efficacy. INT-0096 tested both fractionation and TRT intensity in comparing 45 Gy once-daily TRT over 5 weeks with 45 Gy twice-daily (twice a day) TRT over weeks. OS was significantly improved on the twice a day arm as 5-year OS increased from 16% to 26% with twice a day TRT, creating a paradigm shift toward more routine consideration of accelerated hyperfractionation.17 The main tradeoff in the experimental arm was a doubling of severe acute esophageal toxicity. INT-0096 was one of the few trials (in any disease) to demonstrate altering the intensity of radiotherapy in a chemotherapy-sensitive disease could ultimately affect OS. Nevertheless, the impact on clinical practice was muted in part because of concerns about treatment-related toxicity and logistical considerations with treating patients twice a day. Only 25% of recently surveyed radiation oncologists favored twice a day radiation therapy (RT).28
One criticism articulated regarding INT-0096 was the relatively low dose of once-daily TRT used, and the (unproven) widely held assumption that higher-dose once-daily TRT would be as effective as twice a day TRT. The advent of advanced radiotherapy planning facilitated the utilization of high-dose daily radiotherapy in multiple prospective trials in NSCLC and LS-SCLC. Even before conformal radiotherapy, phase I data from the CALGB noted the maximum tolerated dose for daily fractionation to be at least 70 Gy in 35 fractions.29 A subsequent phase II CALGB trial using 70 Gy daily RT suggested promising outcomes, particularly since TRT was initiated with the third cycle of chemotherapy and a carboplatin backbone was used.23 Two major phase III trials have now been reported assessing whether high-dose once-daily TRT would improve OS compared with standard 45 Gy twice a day TRT.7,8 In the CONVERT trial, conducted in Europe and Canada, high-dose once-daily (66 Gy in 33 fractions) was not superior to 45 Gy twice a day. Median OS was 30 and 25 months, and 5-year OS was 34% and 31% in the twice a day and once-daily arms, respectively. Although the hazard ratio favored twice a day TRT, the difference did not reach statistical significance (P = .14). CONVERT investigators concluded twice a day regimen should remain the standard of care and noted that more patients assigned to twice a day completed TRT. Regardless, the subsequently designed LU-005 trial permits either 66 Gy once-daily or 45 Gy twice a day. Initial results of the C30610/RTOG 0538 were recently presented. Once again, once-daily TRT, this time 70 Gy once-daily in 35 fractions over 7 weeks, was not superior to 45 Gy twice a day in 3 weeks.6 The median and 5-year OS were 30.5 months and 34% with 70 Gy once-daily TRT and 28.5 months and 29% with 45 Gy twice a day, respectively. While not designed to assess noninferiority, the favorable outcomes for the 70 Gy cohort provides the strongest evidence supporting high-dose once-daily TRT in LS-SCLC. In contrast to the INT-0096 trial, most toxicities were comparable between treatment arms in both the CONVERT and CALGB trials with similar rates of esophageal toxicity.
The lack of an OS benefit with high-dose once-daily TRT in the CONVERT and CALGB trials coupled with the outright negative results of high-dose once-daily TRT in NSCLC have called into question the role of conventional dose escalation in the face of chemoradiotherapy for lung cancer.7,8,30 Although outcomes with 70 Gy cohort in the initial report of the CALGB study seem favorable, direct evidence supporting a dose response for once-daily TRT is lacking. Further dosimetric analysis from recent randomized trials may provide insight regarding the therapeutic index for high-dose once-daily TRT, with particular attention given to the impact of high TRT doses to normal structures.
Alternative approaches to improve TRT efficacy have included studying whether accelerating the treatment course may be just as effective using hypofractionated TRT (larger once-daily fractions). In a Norwegian phase II study, 45 Gy twice a day resulted in more complete responses and numerically longer median OS compared with 42 Gy in 15 fractions, both completed over 3 weeks.31 This group more recently reported provocative results with higher-dose twice a day TRT, 60 Gy in 40 fractions, as 2 years OS reached 74% in a phase II setting. A phase III comparison with 45 Gy twice a day is ongoing to determine whether the benefit of 60 Gy twice a day TRT will hold up.27 A hybrid concomitant boost approach, mixing once-daily and twice a day TRT, was studied in RTOG-0239 and initially included in the C30610 trial.26 This arm was dropped from the study during a planned interim analysis,32 but long-term outcomes from this cohort will soon be available.
TRT PLANNING
Advances in radiotherapy planning coupled in part with a shift in treatment philosophy away from treating clinically uninvolved regional nodal regions have contributed to an improved therapeutic ratio. Seminal trials such as INT-0096 electively targeted the bilateral mediastinal lymph nodes (at a minimum). Implementation of fluorodeoxyglucose-PET imaging for treatment planning may identify unsuspected regional nodal metastasis up to 25% of patients. Several reports suggest treatment of clinically uninvolved mediastinal nodal regions may safely be omitted in PET staged patients.33-35 CONVERT and C30610 did not include elective mediastinal irradiation, although the ipsilateral hilum was included in the C30610.
Many treatment planning considerations for LS-SCLC mimic the NSCLC setting. Accounting for tumor movement is critical, and four-dimensional computed tomography should be routinely used to encompass target motion, with expansions for clinical target volume and planning target volume. If chemotherapy has been initiated before TRT, target volumes should be designed to account for response to chemotherapy though initially involved nodal regions should be targeted. Image guidance preferably with cone-beam computed tomography is preferable and may be particularly helpful in detecting rapid changes in tumor volume during therapy in patients with bulky SCLC. Adaptive replanning should be considered in cases of major response and/or significant anatomic changes during therapy, and was included as an option on the 70 Gy arm of C30610. Intensity-modulated radiotherapy (IMRT) often results in superior dosimetric plans compared with 3D conformal radiation therapy, with the suggestion of improved outcomes from a post hoc analysis of RTOG-0617.36 Cardiac dosimetry was independently associated with OS, underscoring the importance of heart avoidance. Approximately half of C30610 patients were treated with IMRT, and analysis is planned with particular focus on cardiac dose and other organs at risk.
As bulky mediastinal adenopathy is common in SCLC, meeting traditional metrics used for planning NSCLC, particularly volume of lung receiving 20 Gy (V20), can be challenging. V20 lung was predictive of pulmonary toxicity in a review 100 patients treated to 70 Gy on phase II CALGB SCLC trials. Although V20 lung exceeded 40% in 30 patients, only three patients developed grade 3 toxicity, with no grade 4-5 events.37 These data support consideration of aggressive treatment of LS-SCLC even when traditional metrics cannot be strictly met (ie, V20 < 35%-40%). Otherwise, organ at risk constraints parallel those for NSCLC when conventional fractionation is used. With 45 Gy twice a day TRT, less data are available to guide normal tissue constraints, although particular attention should be given to spinal cord dosing.
CURRENT STATE OF PCI
PCI has been a mainstay of treatment in SCLC for decades. A meta-analysis from the late 1990s demonstrated a significant reduction in intracranial metastases (IM) and a 5.4% improvement in survival at 3 years after complete tumor response in patients with mostly LS-SCLC.38 The observation that higher doses of PCI resulted in a lower rate of IM led to development of trials comparing high-dose PCI, 36 Gy either once-daily or twice a day, to standard 25 Gy in 10 fractions.39,40 Unfortunately, higher-dose PCI was associated with both higher late neurotoxicity and reduced OS. Concerns over the late neurocognitive effects of PCI have underpinned the design of recent trials. NRG CC003, which builds upon the experience with hippocampal-avoidant whole-brain RT in the IM population, compares standard whole-brain radiotherapy (WBRT) with hippocampal-avoidant-IMRT (NCT02635009) with primary end points of neurocognitive decline and intracranial relapse. More recently, the benefit of PCI as a routine component of initial therapy has been directly challenged in large part to results of a Japanese trial in extensive-stage (ES)-SCLC, which suggested that with strict MRI staging and surveillance, PCI does not improve OS.41 The substantial reduction in incidence of IM (69% v 48%) with PCI did not lead to an OS difference. Although this trial did not include patients with LS-SCLC, it was noted that trials included in the classic meta-analysis demonstrating an OS benefit with PCI were performed before routine availability of MRI. A recently activated phase III noninferiority study (SWOG 1827 aka MAVERICK) compares PCI with MRI surveillance in both LS-SCLC and ES-SCLC (NCT04155034). A key secondary end point is the rate of cognitive failure–free survival. Moreover, recent data challenge the long-held axiom that, given the concern of subsequent rapid intracranial relapse, WBRT is necessary in treating brain metastases in SCLC.42 Radiosurgery is currently being studied as an alternative to WBRT (NCT04804644), and if radiosurgery is ultimately deemed an acceptable strategy for managing SCLC BM, it may provide further ammunition to modify routine practice of PCI.
Although the role of PCI has been questioned in LS-SCLC, the majority of prospective data emanate from trials including PCI. For the time being, there are insufficient data to abandon the practice, and PCI remains a standard recommendation on the active LU-005 trial.
SYSTEMIC THERAPY
Systemic chemotherapy for lung cancer dates back to the first half of the 20th century including a Veterans Affairs comparison of various agents including nitrogen mustard, diethylstilbestrol, testosterone, progesterone, and cortisone to treat inoperable bronchogenic malignancies, including SCLC.43 The observation that using multiple non–cross-resistant chemotherapy agents lead to better outcomes than using single-agent chemotherapy led to the use of multidrug regimens such as cyclophosphamide, doxorubicin, and vincristine; cyclophosphamide, epirubicin, and vincristine; and etoposide and cisplatin (PE).44 Etoposide was noted to particularly be active in SCLC, and synergistic when combined with cisplatin, with complete response rate of 52% and partial response rate of 48% in patients with limited-stage disease.45 PE also became the preferred regimen for SCLC because of toxicity profile and the ability to safely integrate with TRT. Various PE dosing regimens are used in LS-SCLC, including cisplatin 60 mg/m2 on day 1 and etoposide 120 mg/m2 on days 1-3 as used in INT-0096, with alternative regimens of cisplatin 75 mg/m2 on day 1 and etoposide 100 mg/m2 on days 1-3, or cisplatin 25 mg/m2 on days 1-3 and etoposide 100 mg/m2 on days 1-3, both of which were permitted on CONVERT.8,17 Four cycles of chemotherapy are recommended, and the use of myeloid growth factors is not recommended during concurrent chemoradiation because of potential severe toxicity.12,46
Attempts to improve outcomes with the inclusion of more intensive systemic therapy, newer-generation chemotherapy, and systemic targeted therapy have been unsuccessful in improving the therapeutic index. For example, the addition to paclitaxel to PE with 45 Gy twice a day increased acute toxicity without improving outcomes in the phase II RTOG 9609 study and a pilot SWOG 0222 trial demonstrated the hypoxic cell sensitizer tirapazamine increased toxicity without clear benefit.20,24 Although there was substantial initial enthusiasm for anti–vascular endothelial growth factor agents in SCLC, the addition of bevacizumab to chemoradiotherapy resulted in severe local toxicity including tracheoesophageal fistula.47 Treatment with irinotecan and cisplatin resulted in improved OS compared with PE in ES-SCLC in a Japanese experience, but the JCOG 0202 study did not show a benefit for including consolidation irinotecan and cisplatin in LS-SCLC.48,49
Carboplatin is often substituted for cisplatin in clinical practice, given underlying comorbidities in many patients with LS-SCLC. Carboplatin is generally better tolerated, although hematologic toxicity may be greater. A meta-analysis of four randomized studies comparing first-line cisplatin versus carboplatin included two trials of patients with LS-SCLC.50 Response rate, progression-free survival (PFS), and OS were similar in both chemotherapy groups. Although most LS-SCLC prospective studies have been restricted to PE, C30610 was amended to allow carboplatin to enhance accrual, and the active LU005 trial allows either carboplatin or cisplatin. When used in LS-SCLC, the recommended dosing is carboplatin area under the curve 5-6 on day 1 and etoposide 100 mg/m2 on days 1-3.
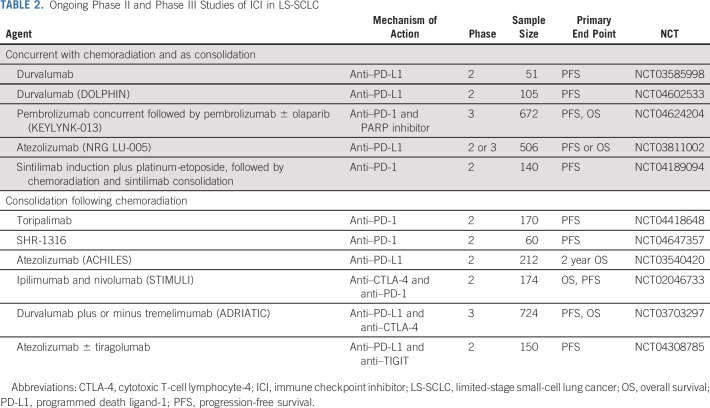
The addition of ICIs to first-line chemotherapy has resulted in mixed success in ES-SCLC. Although programmed death ligand-1 (PD-L1) inhibitors modestly improve survival, improved outcomes have not been observed with cytotoxic T-cell lymphocyte-4 inhibition in the front-line setting.4-6 Consolidation ipilimumab and nivolumab did not improve 2-year PFS in the STIMULI phase II trial, although long-term OS may be a better metric for ultimate efficacy of ICIs.51 Ongoing phase 2 or 3 clinical trials evaluating the role of ICIs concurrent or adjuvant to chemoradiation LS-SCLC are listed in Table 2.
TABLE 2.
Ongoing Phase II and Phase III Studies of ICI in LS-SCLC
SPECIAL CONSIDERATIONS
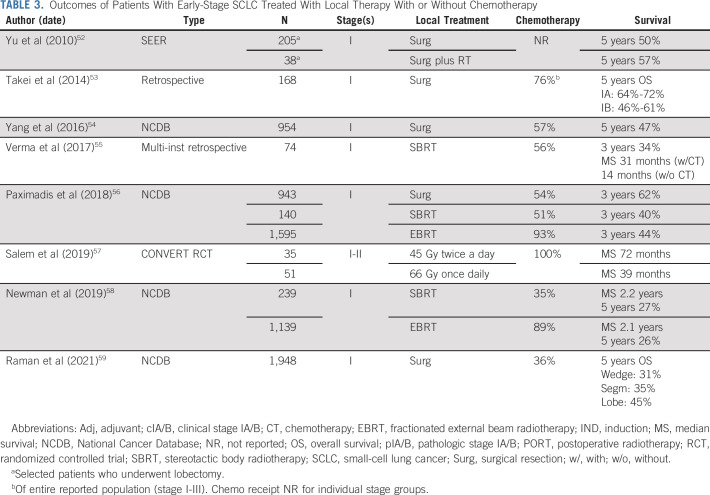
Although early-stage (node-negative) disease only represents approximately 5% of SCLC, debate regarding optimal local management has grown in recent years with increased utilization of stereotactic body radiotherapy (SBRT) as well as advances in thoracic surgical techniques. There is limited prospective surgical evidence in SCLC, although National Comprehensive Cancer Network guidelines include surgical resection for patients with T1-2 N0 patients following mediastinal staging.12 This is supported by contemporary database studies describing encouraging outcomes. SBRT has also been proposed as an attractive option for this population, as underlying lung disease renders many patients with SCLC medically inoperable, and is included in updated guidelines.13 Analogous to surgery, supporting data are limited to retrospective or database series. Table 3 summarizes select reports in early-stage SCLC, recognizing limitations in the data regarding the relative value of surgery and radiotherapy. The CONVERT study provides particularly provocative prospective data for chemoradiotherapy in early-stage SCLC with median OS of 50 months for all stage I-II patients, reaching 72 months for those in the twice a day TRT cohort.57 As such, treatment with chemoradiation should not be dismissed as a consideration for appropriate patients with early disease. Regardless of local therapy, adjuvant chemotherapy should be considered for node-negative patients, best illustrated by a National Cancer Database analysis of 954 patients, where adjuvant chemotherapy was associated with improved median OS from 42 to 66 months after R0 resection.54 Prospectively, the addition of atezolizumab to neoadjuvant chemotherapy is being evaluated in surgical patients (NCT04696939). Striking improvements in OS have also been reported with the use of chemotherapy in patients treated with SBRT.55,60 Current guidelines suggest consideration of adjuvant mediastinal radiation therapy for pathologic N2 disease, although data are limited.12,61 The use of PCI in early-stage disease is controversial and has been particularly questioned in surgical patients with N0 disease.62
TABLE 3.
Outcomes of Patients With Early-Stage SCLC Treated With Local Therapy With or Without Chemotherapy
TREATMENT OF OLDER PATIENTS
Relatively little prospective evidence is available to guide therapy for elderly patients with LS-SCLC, although the median age at diagnosis approaches 70 years. Given the abysmal prognosis without therapy, undertreatment is a major concern in elderly patients, and 40% of patients age 70 years or older in a National Cancer Database analysis did not receive TRT.63 Multivariate analysis suggested treatment with combined therapy had the greatest impact on OS, even for patients > 80 years, and the benefit held for the population with defined comorbidity. Evidence from prospective clinical trials also suggests patients benefit from aggressive treatment regardless of age. Similar response rates and event-free survival were observed with older and younger patients on INT-0096, with a suggestion of improved outcomes with twice a day TRT.64 The recent CONVERT trial provides even more promising evidence for treatment of older patients with modern therapy as median OS (29 months v 30 months) and PFS (18 months v 16 months) were essentially equivalent in patients older and younger than 70 years, respectively.65 Older patients may be at increased risk for treatment-related toxicities, often meriting careful patient selection including the use of formal geriatric assessment tools, using measures to mitigate toxicity, and ensuring close monitoring. In the CONVERT trial, chemotherapy compliance was similar regardless of age, although older patients were less likely to complete optimal TRT. Deciding whether older patients should receive PCI is particularly complex, as studies suggest neurotoxicity may be directly related to age, yet clinical trial analysis suggest that fit older patients may still benefit from PCI.
FUTURE DIRECTIONS
There is ample opportunity to move beyond the standard treatment approach for LS-SCLC defined last century by INT-0096—through optimization or intensification of local therapy, challenging traditional thinking about the role of PCI (with critical attention toward quality of life), and particularly developing more effective systemic therapy. Radiotherapy advances such as adapting TRT intensity to metabolic tumor response and treatment with proton therapy are of interest but may not be widely applicable to patients with LS-SCLC, although tailoring TRT according to disease extent, underlying patient characteristics, and tumor biology should be an area of focus in the future.
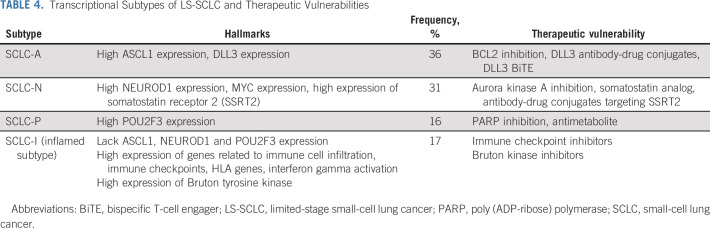
In contrast to NSCLC, identifying actionable therapeutic targets and valid biomarkers to direct systemic therapy in SCLC has remained challenging. PD-L1 expression and tumor mutational burden do not necessarily correlate with the OS benefit observed with ICIs in ES-SCLC, and treatment benefit might depend on other factors related to the tumor microenvironment. Although active phase III trials will help determine whether ICIs have magnified benefit in LS-SCLC, perhaps because of lower (micro)metastatic tumor burden or interrelationship between ICIs and TRT, additional strategies will be needed for the majority of patients with LS-SCLC. To that extent, other agents including DNA damage response inhibitors and TIGIT targeting antibodies (NCT04624204 and NCT04308785) are being studied in combination with PD(L)-1 inhibitors (Table 2). Gay et al, using tumor expression data mostly derived from resected LS-SCLC, recently suggested a framework for a personalized approach with the classification of four distinct SCLC subtypes. Hallmarks of these subtypes and potential therapeutic vulnerability are shown in Table 4.66 Of particular note is an inflamed subtype, most likely to respond to ICIs, which was seen in only 17% of patients. Additional molecular changes inherent to SCLC may potentially be exploited including loss of tumor suppressor genes TP53 and Rb1.67 The novel p53 reactivator eprenetapopt has shown promise in hematologic malignancies and is being studied in solid tumors along with pembrolizumab (NCT04383938).68,69 FGFR1 alterations may also be present in SCLC, and a phase I trial is evaluating anlotinib, an FGFR 1-3 multikinase inhibitor, concurrent with chemoradiation in LS-SCLC (NCT04882033).67
TABLE 4.
Transcriptional Subtypes of LS-SCLC and Therapeutic Vulnerabilities
In the end, it will also be essential to gain a better understanding of the interdependence of local and systemic therapy to optimize management of LS-SCLC. Both the sequence and intensity of TRT may ultimately affect response to novel therapeutics, while newer systemic agents may affect thoracic and CNS relapse as demonstrated in trials assessing ICIs in NSCLC,70 potentially influencing decisions regarding TRT and/or shifting the therapeutic ratio of PCI.
Jeffrey A. Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Saiama N. Waqar
Research Funding: Spectrum Pharmaceuticals, Lilly, Pfizer, Genentech/Roche, Daiichi Sankyo, Newlink Genetics, EMD Serono, Puma Biotechnology, Novartis, Xcovery, Synermore biologics, Celgene, Vertex, Bristol Myers Squibb, Stem CentrRx, Hengrui Therapeutics, Checkpoint Therapeutics, Ignyta, AstraZeneca, ARIAD, Roche, Merck
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey A. Bogart, Saiama N. Waqar, Michael D. Mix
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeffrey A. Bogart
Stock and Other Ownership Interests: Cardan Robotics, Verve Medical
Saiama N. Waqar
Research Funding: Spectrum Pharmaceuticals, Lilly, Pfizer, Genentech/Roche, Daiichi Sankyo, Newlink Genetics, EMD Serono, Puma Biotechnology, Novartis, Xcovery, Synermore biologics, Celgene, Vertex, Bristol Myers Squibb, Stem CentrRx, Hengrui Therapeutics, Checkpoint Therapeutics, Ignyta, AstraZeneca, ARIAD, Roche, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Govindan R, Page N, Morgensztern D, et al. : Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539-4544, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Zelen M: Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3:31-42, 1973 [PubMed] [Google Scholar]
- 3.Nicholson AG, Chansky K, Crowley J, et al. : The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 11:300-311, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Mansfield AS, Szczesna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Dvorkin M, Chen Y, et al. : Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 394:1929-1939, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Rudin CM, Awad MM, Navarro A, et al. : Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38:2369-2379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogart JA, Wang XF, Masters GA, et al. : Phase 3 comparison of high-dose once-daily (QD) thoracic radiotherapy (TRT) with standard twice-daily (BID) TRT in limited stage small cell lung cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol 39, 2021. (suppl 15; abstr 8505) [Google Scholar]
- 8.Faivre-Finn C, Snee M, Ashcroft L, et al. : Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol 18:1116-1125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pignon JP, Arriagada R, Ihde DC, et al. : A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618-1624, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Warde P, Payne D: Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890-895, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Takada M, Fukuoka M, Kawahara M, et al. : Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan clinical Oncology Group Study 9104. J Clin Oncol 20:3054-3060, 2002 [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network panel : NCCN Guidelines: Small Cell Lung Cancer—Version 3.2021, Plymouth Meeting, PA, National Comprehensive Cancer Network, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Simone CB II, Bogart JA, Cabrera AR, et al. : Radiation therapy for small cell lung cancer: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 10:158-173, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry MC, Herndon JE III, Eaton WL, et al. : Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: An update of Cancer and Leukemia Group B Study 8083. J Clin Oncol 16:2466-2467, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Murray N, Coy P, Pater JL, et al. : Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 11:336-344, 1993 [DOI] [PubMed] [Google Scholar]
- 16.De Ruysscher D, Lueza B, Le Pechoux C, et al. : Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: Usefulness of the individual patient data meta-analysis. Ann Oncol 27:1818-1828, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turrisi AT III, Kim K, Blum R, et al. : Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265-271, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Schild SE, Bonner JA, Shanahan TG, et al. : Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 59:943-951, 2004 [DOI] [PubMed] [Google Scholar]
- 19.McClay EF, Bogart J, Herndon JE II, et al. : A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). Am J Clin Oncol 28:81-90, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ettinger DS, Berkey BA, Abrams RA, et al. : Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: A radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol 23:4991-4998, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Horn L, Bernardo P, Sandler A, et al. : A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): A trial of the Eastern Cooperative Oncology Group. J Thorac Oncol 4:527-533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelman MJ, Chansky K, Gaspar LE, et al. : Phase II trial of cisplatin/etoposide and concurrent radiotherapy followed by paclitaxel/carboplatin consolidation for limited small-cell lung cancer: Southwest Oncology Group 9713. J Clin Oncol 22:127-132, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Bogart JA, Herndon JE II, Lyss AP, et al. : 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B Study 39808. Int J Radiat Oncol Biol Phys 59:460-468, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Le QT, Moon J, Redman M, et al. : Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J Clin Oncol 27:3014-3019, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AA, Wang XF, Bogart JA, et al. : Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J Thorac Oncol 2:645-651, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Komaki R, Paulus R, Ettinger DS, et al. : Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys 83:e531-e536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gronberg BH, Killingberg KT, Flotten O, et al. : High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet Oncol 22:321-331, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Farrell MJ, Yahya JB, Degnin C, et al. : Radiation dose and fractionation for limited-stage small-cell lung cancer: Survey of US radiation oncologists on practice patterns. Clin Lung Cancer 20:13-19, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Choi NC, Herndon JE II, Rosenman J, et al. : Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol 16:3528-3536, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Bradley JD, Paulus R, Komaki R, et al. : Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 16:187-199, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gronberg BH, Halvorsen TO, Flotten O, et al. : Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol 55:591-597, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Bogart JA, Wang X, Masters GA, et al. : Short Communication: Interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on CALGB 30610 (Alliance)/RTOG 0538. Lung Cancer 156:68-71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley JD, Dehdashti F, Mintun MA, et al. : Positron emission tomography in limited-stage small-cell lung cancer: A prospective study. J Clin Oncol 22:3248-3254, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Shirvani SM, Komaki R, Heymach JV, et al. : Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 82:e91-7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Loon J, De Ruysscher D, Wanders R, et al. : Selective nodal irradiation on basis of 18FDG-PET scans in limited-disease small-cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys 77:329-336, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Chun SG, Hu C, Choy H, et al. : Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol 35:56-62, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salama JK, Pang H, Bogart JA, et al. : Predictors of pulmonary toxicity in limited stage small cell lung cancer patients treated with induction chemotherapy followed by concurrent platinum-based chemotherapy and 70 Gy daily radiotherapy: CALGB 30904. Lung Cancer 82:436-440, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auperin A, Arriagada R, Pignon JP, et al. : Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 341:476-484, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Le Pechoux C, Dunant A, Senan S, et al. : Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A randomised clinical trial. Lancet Oncol 10:467-474, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Wolfson AH, Bae K, Komaki R, et al. : Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 81:77-84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi T, Yamanaka T, Seto T, et al. : Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 18:663-671, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Rusthoven CG, Yamamoto M, Bernhardt D, et al. : Evaluation of first-line radiosurgery vs whole-brain radiotherapy for small cell lung cancer brain metastases: The FIRE-SCLC Cohort Study. JAMA Oncol 6:1028-1037, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf J, Spear P, Yesner R, et al. : Nitrogen mustard and the steroid hormones in the treatment of inoperable bronchogenic carcinoma. Am J Med 29:1008-1016, 1960 [DOI] [PubMed] [Google Scholar]
- 44.Sierocki JS, Hilaris BS, Hopfan S, et al. : cis-Dichlorodiammineplatinum(II) and VP-16-213: An active induction regimen for small cell carcinoma of the lung. Cancer Treat Rep 63:1593-1597, 1979 [PubMed] [Google Scholar]
- 45.Evans WK, Shepherd FA, Feld R, et al. : VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 3:1471-1477, 1985 [DOI] [PubMed] [Google Scholar]
- 46.Bunn PA Jr., Crowley J, Kelly K, et al. : Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: A prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 13:1632-1641, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Spigel DR, Hainsworth JD, Yardley DA, et al. : Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 28:43-48, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Noda K, Nishiwaki Y, Kawahara M, et al. : Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85-91, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Kubota K, Hida T, Ishikura S, et al. : Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): A randomised phase 3 study. Lancet Oncol 15:106-113, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Rossi A, Di Maio M, Chiodini P, et al. : Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J Clin Oncol 30:1692-1698, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Peters S, Pujol J, Dafni U, et al. : Consolidation ipilimumab and nivolumab vs observation in limited stage SCLC after chemo-radiotherapy: Results from the ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 31:S1142-S1215, 2020. (suppl 4) [DOI] [PubMed] [Google Scholar]
- 52.Yu JB, Decker RH, Detterbeck FC, et al. : Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 5:215-219, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Takei H, Kondo H, Miyaoka E, et al. : Surgery for small cell lung cancer: A retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 9:1140-1145, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Yang CF, Chan DY, Speicher PJ, et al. : Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 34:1057-1064, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verma V, Simone CB II, Allen PK, et al. : Multi-institutional experience of stereotactic ablative radiation therapy for stage I small cell lung cancer. Int J Radiat Oncol Biol Phys 97:362-371, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paximadis P, Beebe-Dimmer JL, George J, et al. : Comparing treatment strategies for stage I small-cell lung cancer. Clin Lung Cancer 19:e559-e565, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salem A, Mistry H, Hatton M, et al. : Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: Secondary analysis of a randomized clinical trial. JAMA Oncol 5:e185335, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman NB, Sherry AD, Byrne DW, et al. : Stereotactic body radiotherapy versus conventional radiotherapy for early-stage small cell lung cancer. J Radiat Oncol 8:239-248, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raman V, Jawitz OK, Yang CJ, et al. : The effect of extent of resection on outcomes in patients with limited stage small cell lung cancer. J Thorac Cardiovasc Surg 161:1484-1492.e5, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verma V, Simone CB II, Allen PK, et al. : Outcomes of stereotactic body radiotherapy for T1-T2N0 small cell carcinoma according to addition of chemotherapy and prophylactic cranial irradiation: A multicenter analysis. Clin Lung Cancer 18:675-681.e1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong AT, Rineer J, Schwartz D, et al. : Assessing the impact of postoperative radiation therapy for completely resected limited-stage small cell lung cancer using the National Cancer Database. J Thorac Oncol 11:242-248, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Zhang D, Zhou X, et al. : Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer 9:433-439, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corso CD, Rutter CE, Park HS, et al. : Role of chemoradiotherapy in elderly patients with limited-stage small-cell lung cancer. J Clin Oncol 33:4240-4246, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuen AR, Zou G, Turrisi AT, et al. : Similar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer 89:1953-1960, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Christodoulou M, Blackhall F, Mistry H, et al. : Compliance and outcome of elderly patients treated in the Concurrent Once-daily Versus Twice-Daily Radiotherapy (CONVERT) trial. J Thorac Oncol 14:63-71, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gay CM, Stewart CA, Park EM, et al. : Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39:346-360.e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peifer M, Fernandez-Cuesta L, Sos ML, et al. : Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 44:1104-1110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cluzeau T, Sebert M, Rahme R, et al. : Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: A phase II study by the Groupe Francophone des Myelodysplasies (GFM). J Clin Oncol 39:1575-1583, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallman DA, DeZern AE, Garcia-Manero G, et al. : Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 39:1584-1594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rittmeyer A, Barlesi F, Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]