Abstract
PURPOSE
Palbociclib is a cyclin-dependent kinase 4 and 6 inhibitor approved for advanced breast cancer. In the adjuvant setting, the potential value of adding palbociclib to endocrine therapy for hormone receptor–positive breast cancer has not been confirmed.
PATIENTS AND METHODS
In the prospective, randomized, phase III PALLAS trial, patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative early breast cancer were randomly assigned to receive 2 years of palbociclib (125 mg orally once daily, days 1-21 of a 28-day cycle) with adjuvant endocrine therapy or adjuvant endocrine therapy alone (for at least 5 years). The primary end point of the study was invasive disease-free survival (iDFS); secondary end points were invasive breast cancer–free survival, distant recurrence-free survival, locoregional cancer-free survival, and overall survival.
RESULTS
Among 5,796 patients enrolled at 406 centers in 21 countries worldwide over 3 years, 5,761 were included in the intention-to-treat population. At the final protocol-defined analysis, at a median follow-up of 31 months, iDFS events occurred in 253 of 2,884 (8.8%) patients who received palbociclib plus endocrine therapy and in 263 of 2,877 (9.1%) patients who received endocrine therapy alone, with similar results between the two treatment groups (iDFS at 4 years: 84.2% v 84.5%; hazard ratio, 0.96; CI, 0.81 to 1.14; P = .65). No significant differences were observed for secondary time-to-event end points, and subgroup analyses did not show any differences by subgroup. There were no new safety signals for palbociclib in this trial.
CONCLUSION
At this final analysis of the PALLAS trial, the addition of adjuvant palbociclib to standard endocrine therapy did not improve outcomes over endocrine therapy alone in patients with early hormone receptor–positive breast cancer.
INTRODUCTION
Breast cancer is the most frequent malignancy in women worldwide and is curable in about three of four patients with early-stage, nonmetastatic disease.1,2 The most prevalent subtype of early breast cancer is hormone receptor–positive,3 and—after surgery, and radiotherapy and chemotherapy if indicated—adjuvant endocrine treatment (ET) for at least 5 years constitutes the standard of care.4 Despite outcome improvements over the years, a sizable proportion of patients still experience disease recurrence. Thus, novel agents targeting new pathways, aimed at overcoming resistance to endocrine therapy, are being investigated.5
CONTEXT
Key Objective
The addition of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) such as palbociclib to endocrine treatment (ET) has improved outcomes for patients with metastatic hormone receptor-positive human epidermal growth factor receptor 2–negative breast cancer. Large clinical trials are conducted to assess the usefulness of CDK4/6i in the curative early disease setting. PALLAS investigates the efficacy and safety of the addition of two years of adjuvant palbociclib to ET in patients with HR+ human epidermal growth factor receptor 2 early breast cancer.
Knowledge Generated
Two years of adjuvant palbociclib added to ET did not improve invasive disease-free survival or any other efficacy end point (4-year invasive disease-free survival 84.2% v 84.5%; hazard ratio 0.96). 44.9% of patients did not complete two years of palbociclib treatment, mainly because of protocol-defined reasons (neutropenia).
Relevance
PALLAS is the largest adjuvant CDK4/6i trial in the curative breast cancer setting. The results of this final protocol-defined analysis do not support the addition of palbociclib to ET. Translational research on the basis of the sizable trans-PALLAS program will be needed to identify reasons for the observed palbociclib efficacy difference compared with the metastatic setting.
Cyclin-dependent kinases 4 and 6 (CDK4/6) trigger the phosphorylation of the retinoblastoma protein involved in cancer cell S-phase entry, and their blockade by specific inhibitors (CDK4/6i) can arrest cell cycle progression through G1 phase, thus promoting transient cell cycle withdrawal into a quiescent state and eventually permanent senescence, either of which can prohibit tumor progression.6 Clinically, CDK4/6i have improved outcomes in patients with advanced breast cancer.7-9 Palbociclib is a first-in-class oral CDK4/6i that, in combination with endocrine therapy, provides significant increases in objective response rates and progression-free survival in patients with advanced breast cancer, established in three randomized trials (PALOMA-1-3).7,10,11 All these studies met their primary end point by markedly prolonging progression-free survival versus ET alone, with overall survival (OS) improvements in some settings.12
The addition of palbociclib to ET has been proven to be clinically safe in these trials,13 which is a precondition for using a drug in the early—curative—breast cancer setting. Typically, reversible neutropenia without associated infection is the most common side effect, and the feasibility of using this drug with its well-defined toxicity profile in the adjuvant setting has been previously established.14 Experimental data suggest that palbociclib not only is active against proliferating tumor cells but also can exert anticancer stem-cell activity.15
Here, we report the final results of the global phase III Palbociclib CoLlaborative Adjuvant Study (PALLAS) that was designed to determine whether the addition of palbociclib to standard adjuvant endocrine therapy improves outcomes compared with endocrine therapy alone in patients with early hormone receptor–positive breast cancer.
PATIENTS AND METHODS
Trial Design and Oversight
This prospective, multicenter, randomized open-label phase III trial was conducted in 406 centers in 21 countries on five continents worldwide (listed in the Data Supplement [online only] available together with the Protocol [online only] and the statistical analysis plan). The trial was cosponsored and led by the academic groups Austrian Breast and Colorectal Cancer Study Group (ABCSG) and the Alliance Foundation Trials (AFT), in collaboration with PrECOG LLC, the NSABP Foundation, the Breast International Group, and the German Breast Group and in agreement with Pfizer, Inc, which provided funding and drug for the study. A global steering committee oversaw the trial design and conduct, and an international Independent Data Monitoring Committee monitored the trial regularly.
Data collection and monitoring were controlled by the study sponsors. Separate but harmonized databases were held for sites inside and outside the United States, with the data being merged on a monthly basis. Data quality was ensured by reviews following respective policies, and all data analyses were performed by ABCSG's and AFT's statistical teams. The funder had no role in data collection, data analysis, or data interpretation.
All analyzed patients provided written informed consent, and the trial was performed in strict accordance with ICH-GCP guidelines (ClinicalTrials.gov identifier: NCT02513394, EudraCT 2014-005181-30). The trial was approved by institutional review boards and ethics committees. The first author wrote the first draft of the article, with input from the other authors. All the authors contributed to the interpretation of the data and to revisions in the article and made the decision to submit the article for publication. All the authors vouch for the integrity, accuracy, and completeness of the data and for the fidelity of the trial to the Protocol and analysis plans.
Trial Participants and Random Assignment
We enrolled patients with histologically confirmed stage II or III hormone receptor–positive breast cancer and age 18 years or older. Before random assignment, patients had completed definitive breast surgery (and (neo)adjuvant chemotherapy and/or radiotherapy, if indicated). Trial inclusion was possible irrespective of eventual neoadjuvant treatment response. Standard adjuvant endocrine therapy commenced within 12 months of the histologic diagnosis, and enrollment into the study had to occur within 6 months of starting endocrine therapy.16
A permuted block design with random block size (4 or 6) was used to randomly assign the patients 1:1 to receive 2 years of palbociclib in addition to ongoing standard adjuvant endocrine therapy or ongoing standard adjuvant endocrine therapy alone, stratified by anatomic stage (IIA v IIB or III), previous adjuvant or neoadjuvant chemotherapy (yes v no), age (≤ 50 years v > 50 years), and geographical region (United States v Europe v others). PALLAS statistical staff at the Mayo Clinic (Rochester, MN) provided the random assignment schedule created using SAS software (version 9.4) to Oracle Corporation (Redwood City, CA) who designed the telephone-based and web-based interactive response technology. Enrollment of patients with stage IIA disease was capped at 1,000 patients.
Trial Procedures
Lack of metastatic disease had to be proven per institutional practice before random assignment (Data Supplement). Also, the receipt of a tumor tissue block at a central biorepository was mandatory before random assignment.
Patients randomly assigned to arm A received palbociclib at a starting dose of 125 mg orally once daily, days 1-21, followed by 7 days off, in a 28-day cycle for a total duration of 2 years, in addition to standard adjuvant endocrine therapy (tamoxifen and aromatase inhibitor, with or without ovarian function suppression) for a duration of at least 5 years. The Protocol allowed for palbociclib dose reductions (to 100 mg and 75 mg, once daily, respectively) and interruptions, with discontinuation required for repeated severe neutropenia (grade 3 or higher). Patients randomly assigned to arm B received endocrine therapy alone for a duration of at least 5 years. Patients in both arms were evaluated by physical examination and laboratory testing at least quarterly for the first 2 years, followed by every 6-month intervals until year 5 and annually until year 10. Any imaging was symptom-directed, as per international guidelines. Follow-up methodology and intensity were similar between study arms, and necessary adaptations occurred during the COVID-19 pandemic.
Trial End Points
All time-to-event end points comply with the original Standardized Definitions for Efficacy End Points (STEEP) criteria,17 except for the secondary end point invasive breast cancer–free survival, which is defined in the updated STEEP criteria,18 and for locoregional recurrence-free survival (for details, see the Data Supplement). The primary end point was invasive disease-free survival (iDFS) defined as the time from random assignment to the date of first event: local or regional invasive ipsilateral recurrence, contralateral invasive breast cancer, distant recurrence, second primary invasive cancer of nonbreast origin, or death from any cause.
Statistical Analysis
Sample size was estimated for the primary end point of iDFS on the basis of a hazard ratio (HR) of 0.75 as the target effect size of palbociclib plus endocrine therapy versus endocrine therapy alone. Originally, the required sample size was estimated to be 4,600 patients, but was increased in 2018 to 5,600 because of lower event rates observed in comparable clinical trials as anticipated for this trial. Using a group sequential design, 469 events had to occur to achieve a power of 85% in the final analysis. The design included two interim analyses (IA) for nonbinding futility (IA1 and IA2) and for binding superiority (IA2 only) using O'Brien-Fleming boundaries on the basis of Lan-DeMets spending functions to control the overall one-sided type I error rate at 0.025. They were scheduled to occur when 33% and 67% of the 469 events were observed. At the second interim analysis (IA2), the test statistic crossed the predefined futility boundary and palbociclib was discontinued in the approximately 350 patients who still were on active palbociclib treatment on May 29, 2020.16
According to the intention-to-treat principle, all randomly assigned patients were included in the final analyses. Only patients who withdrew their informed consent and explicitly prohibited the use of any collected data were excluded. Stratified log-rank tests with stratification factors of (neo)adjuvant chemotherapy (yes v no) and age (≤ 50 v > 50) at random assignment were used for the comparisons of time-to-event end points between the two treatment groups. Results were summarized using Kaplan-Meier curves with yearly estimates of the survival rates. HRs with two-sided 95% CIs were calculated via stratified Cox proportional hazards regression models. For post hoc comparisons between treatment arms within subgroups on the basis of patient characteristics and clinicopathologic factors, unstratified Cox models were used. Further post hoc analyses investigated treatment effect heterogeneity and the possible impact of drug exposure in relation to iDFS, as well as palbociclib dose reductions and early discontinuations over time (for details, see the Data Supplement).
Safety analyses were performed in all randomly assigned patients excluding those who did not start palbociclib or ET (safety population), according to actual treatment received. Incidences of treatment-emergent toxicities were reported per treatment arm and maximum grade within each adverse event term.
Analyses were carried out using SAS software (version 9.4). Two-sided P values < .05 were considered statistically significant. All data obtained through November 20, 2020, the date when 469 events were documented in the clinical database, were included in statistical analysis.
RESULTS
Patients and Follow-Up
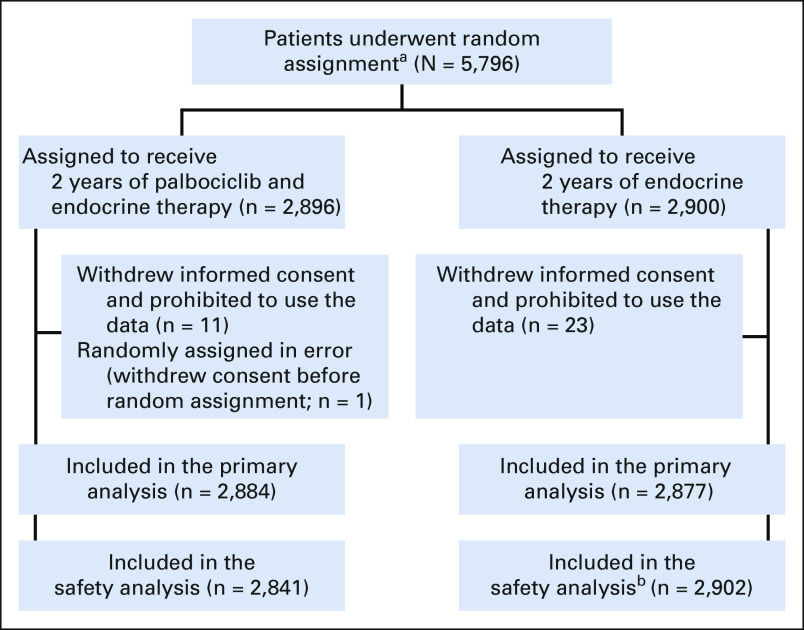
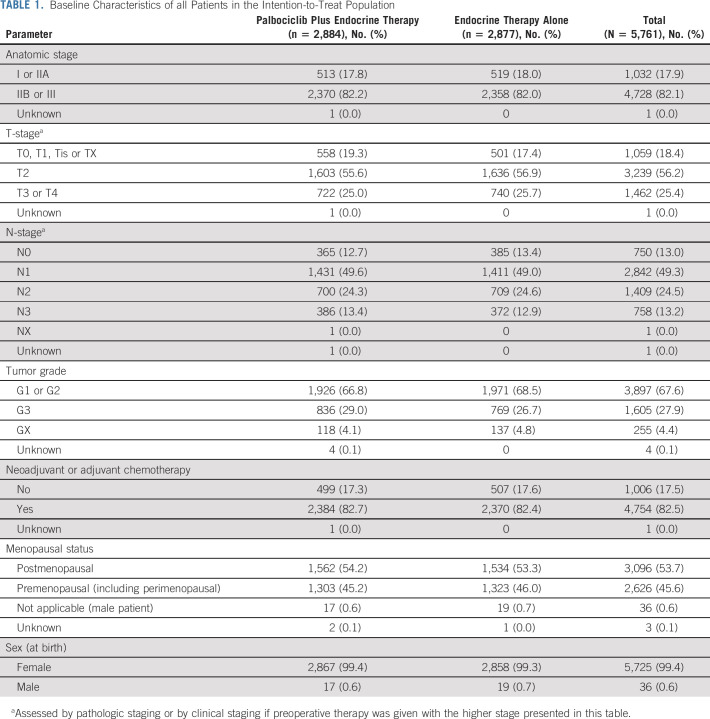
Of the 5,796 patients randomly assigned between September 1, 2015, and November 30, 2018, 35 withdrew consent for use of all data, resulting in 5,761 patients included in the intention-to-treat population (Fig 1). Among these, 2,884 were randomly assigned to receive palbociclib plus endocrine therapy and 2,877 to receive endocrine therapy alone. Treatment arms in the trial were well balanced (Table 1, Data Supplement): the median age was 52 years (interquartile range, 45-61 years), and about half of the 5,761 patients were postmenopausal. One third had stage IIB disease, and half of the patients had stage III disease. Neoadjuvant chemotherapy was given to 1,939 (33.7%) patients and 2,875 (49.9%) received adjuvant chemotherapy before random assignment. An aromatase inhibitor was initiated in 3,872 (67.2%) patients and tamoxifen in 1,872 (32.5%) patients; 1,243 (21.6%) patients received a concurrent luteinizing hormone releasing-hormone agonist for ovarian function suppression. The median follow-up for this final analysis was 31 months (interquartile range, 24.5-37.3 months).
FIG 1.
CONSORT diagram. aOf 6,688 patients who were screened for eligibility, 892 were not randomly assigned because of the following reasons: not meeting inclusion criteria (n = 466), declined to participate (n = 259), and other reasons (n = 167), leading to 5,796 randomly assigned patients. bIncludes 36 patients from the palbociclib plus endocrine therapy group who received endocrine therapy only.
TABLE 1.
Baseline Characteristics of all Patients in the Intention-to-Treat Population
Efficacy
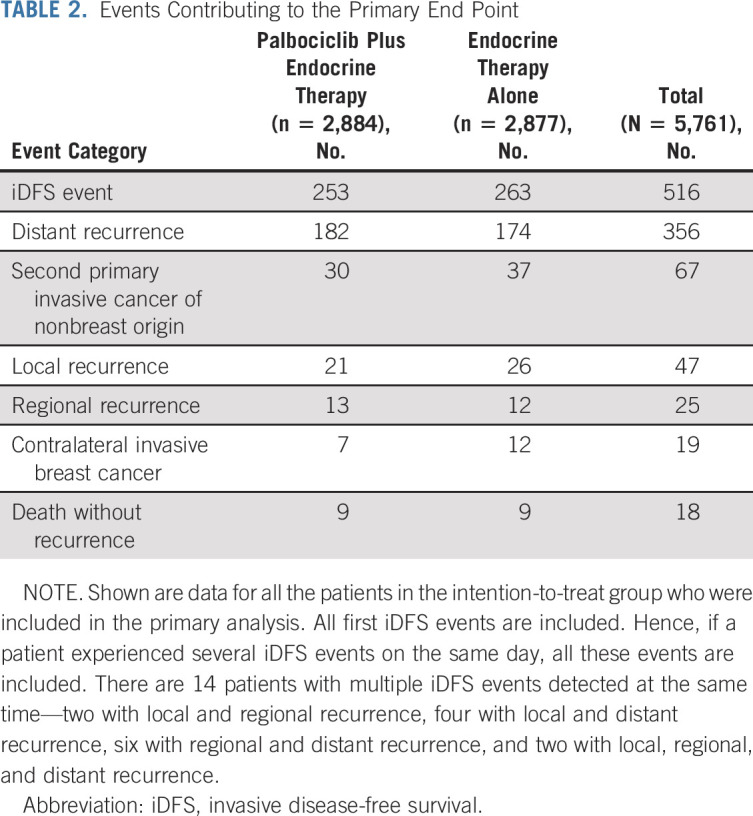
Primary end point events occurred in 516 (9.0%) patients, 253 (8.8%) in patients treated with palbociclib plus endocrine therapy and 263 (9.1%) in patients receiving endocrine therapy alone. Distant metastasis was the first event in 356 (6.2%) patients, and second primary invasive cancer of nonbreast origin in 67 (1.2%) patients. Local or regional recurrences, contralateral invasive breast cancers, and deaths without prior cancer recurrence were first events in < 1% of patients (Table 2). One hundred seventy-six (3.1%) patients died during the observational period, of whom, 100 (3.5%) patients were treated with palbociclib plus endocrine therapy, and 76 (2.6%) patients were treated with endocrine therapy alone (Data Supplement).
TABLE 2.
Events Contributing to the Primary End Point
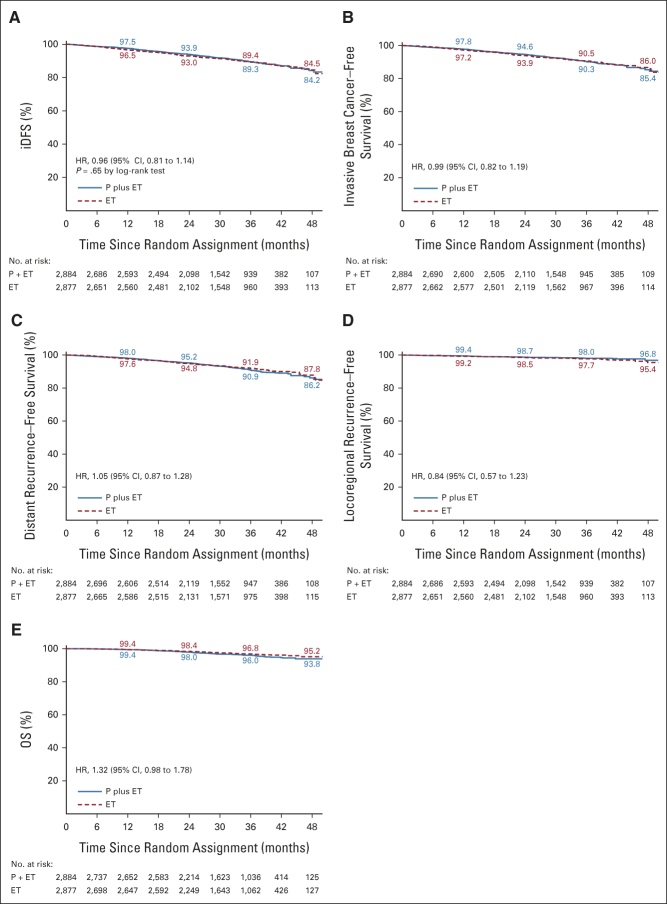
The primary end point of iDFS was not significantly different in patients receiving palbociclib and endocrine therapy versus those receiving endocrine therapy alone, with a HR of 0.96 (CI, 0.81 to 1.14; P = .65) and iDFS rates at year 4 of 84.2% versus 84.5% (Fig 2A). The two treatment groups also did not differ with regard to secondary end points: 4-year survival rates for invasive breast cancer–free survival: 85.4% versus 86.0%; distant recurrence-free survival: 86.2% versus 87.8%; locoregional recurrence-free survival: 96.8% versus 95.4%; and OS of 93.8% versus 95.2%, respectively (Figs 2B-2E). Similar results were obtained for iDFS within subgroups of patients (Fig 3).
FIG 2.
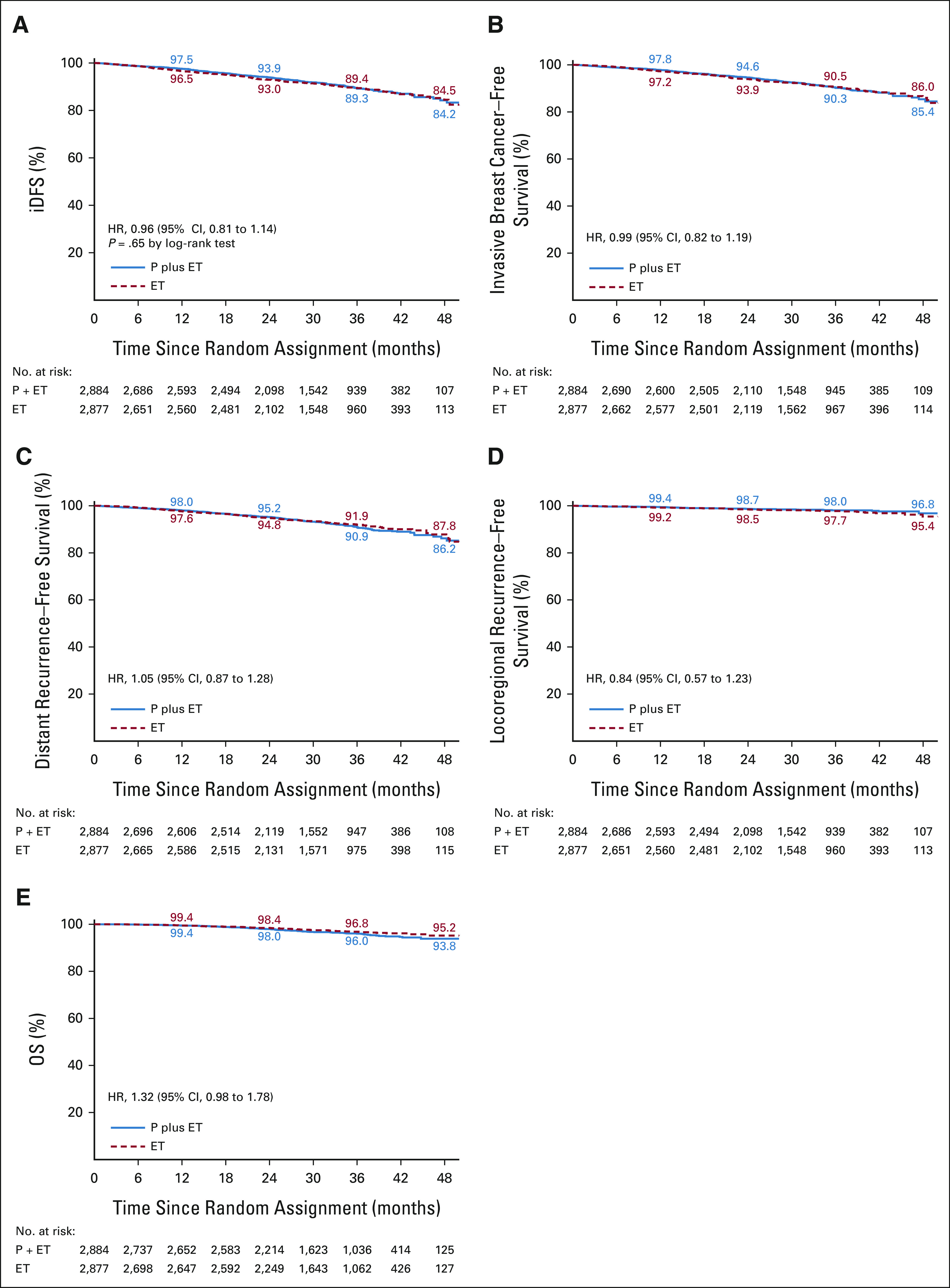
Kaplan-Meier estimates of survival for the primary end point of (A) iDFS and for the secondary end points of (B) invasive breast cancer–free survival, (C) distant recurrence-free survival, (D) locoregional recurrence-free survival, and (E) OS are shown for women with stage II-III histologically confirmed hormone receptor–positive, HER2-negative breast cancer. The HRs with 95% CIs are given for each end point. In addition, the P value of a stratified log-rank test is shown for the primary end point. The numbers along the curves indicate the event-free rates in yearly intervals. ET, endocrine treatment; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; iDFS, invasive disease-free survival; OS, overall survival; P, palbociclib.
FIG 3.
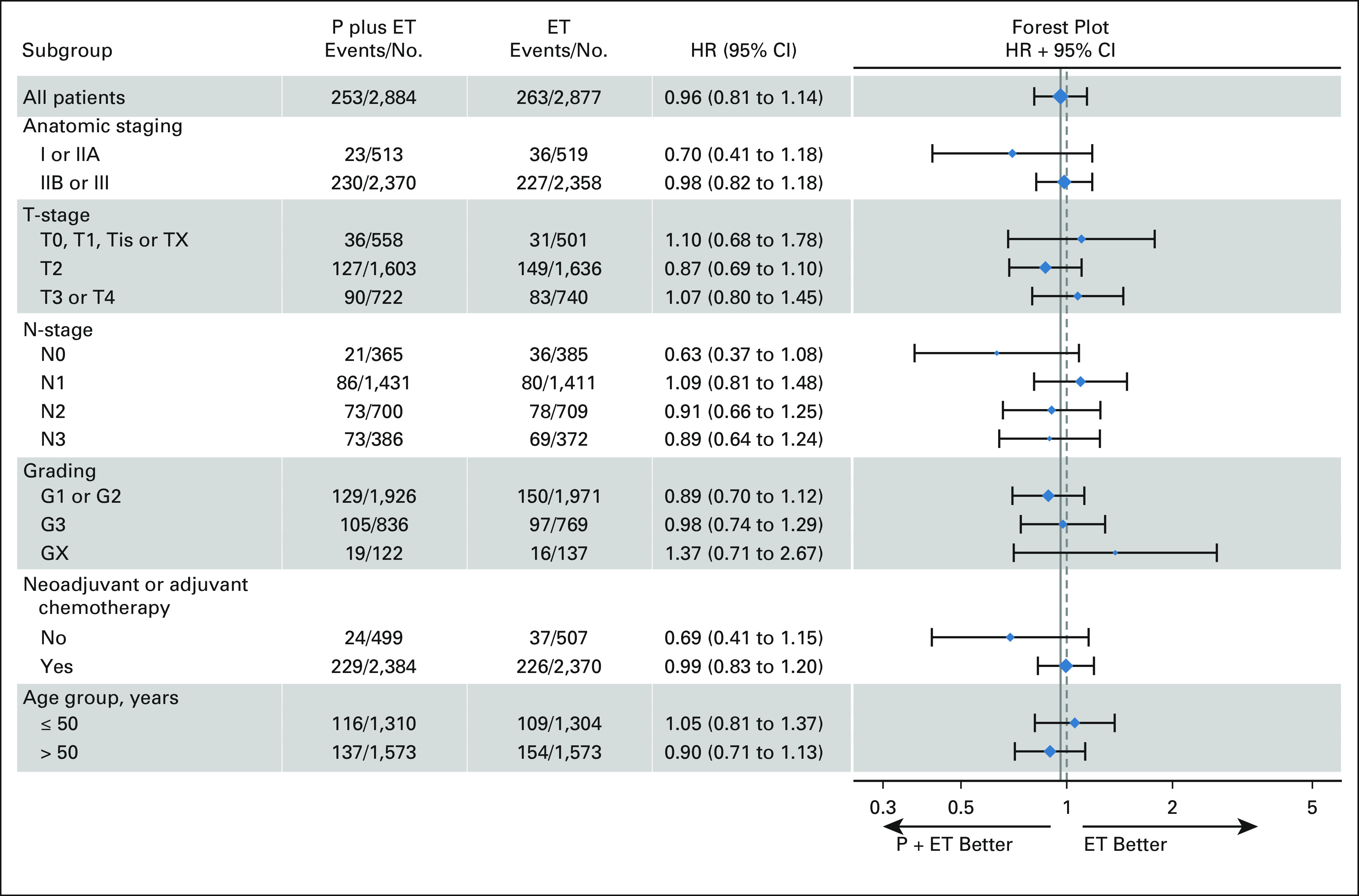
The forest plot shows the HRs (diamonds) and 95% CIs (horizontal lines) with regard to invasive disease-free survival within subgroups according to anatomic stage, tumor stage (T-stage), nodal status (N-stage), histologic grade, prior neoadjuvant or adjuvant chemotherapy, and age group. The solid vertical line indicates the overall HR estimate, and the dashed vertical line indicates a HR of 1.00. No significant interactions between subgroups and treatment groups were observed. ET, endocrine treatment; HR, hazard ratio; P, palbociclib.
Treatment effect heterogeneity analysis to detect a relationship between a higher baseline risk and the HR effect size showed no significant differential palbociclib effect trend from low- to high-risk patients with regard to iDFS (Data Supplement).
Disease Recurrences
The majority of first distant recurrences in both arms occurred in nonvisceral locations (n = 256, 67.7% of patients with distant recurrences), particularly in bone (Data Supplement). Characteristic of the enrolled hormone receptor–positive breast cancer population, visceral metastases were less frequent, and if occurring, predominantly in liver and lung. Disease recurrence in the central nervous system was rare (n = 19, 5.0%).
Treatment Exposure
Over the 2-year treatment period shown, a total of 55.2% (95% CI, 53.3 to 57.0) and 33.4% (31.7 to 35.1) of patients required reductions to a 100 mg and to a 75 mg daily dose, respectively (Data Supplement). Furthermore, cumulative incidence of early palbociclib discontinuations was 44.9% (CI, 43.1 to 46.7, Data Supplement), but landmark analysis did not show significant iDFS benefit for patients who remained on palbociclib for a longer duration (Data Supplement): the greatest difference was observed at the 24-month landmark comparing patients who did receive the full preplanned palbociclib treatment versus those who discontinued early (unadjusted HR = 0.89, 95% CI, 0.58 to 1.34; adjusted HR = 0.79, 95% CI, 0.52 to 1.20).
Safety and Side Effects
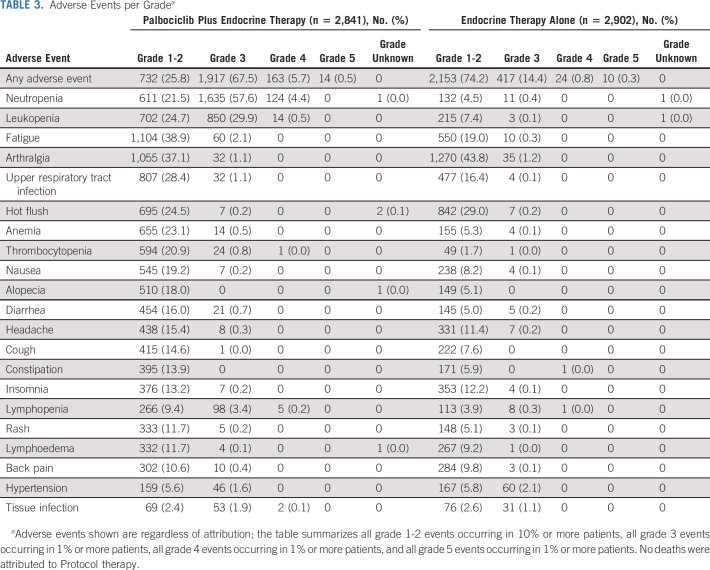
Of 5,761 patients, 5,743 (2,841 in the palbociclib plus endocrine therapy group and 2,902 in the endocrine therapy–alone group) initiated treatment and were included in the safety population. There were no new safety signals with respect to palbociclib treatment: overall, 2,826 (99.5%) patients receiving palbociclib and endocrine therapy and 2,604 (89.7%) patients receiving endocrine therapy alone experienced at least one adverse event. The respective frequencies of serious adverse events are 369 (13.0%) for the palbociclib and endocrine therapy group and 229 (7.9%) for the group of patients receiving endocrine therapy alone.
Table 3 summarizes all-grade toxicities in ≥ 10% of patients for grade 1-2 and ≥ 1% of patients for grade 3, 4, and 5; the Data Supplement shows serious adverse events per grade. The most common adverse events in patients who received palbociclib and endocrine therapy were neutropenia (83.5%), leukopenia (55.1%), and fatigue (41.0%), which were less common in patients who received endocrine therapy alone (5%, 7.5%, and 19.3%, respectively). Other adverse events more common in patients who received palbociclib and endocrine therapy compared with those receiving endocrine therapy alone were upper respiratory tract infection (29.5% v 16.6%), anemia (23.5% v 5.5%), thrombocytopenia (21.8% v 1.7%), and alopecia (18.0% v 5.1%). None of the 176 deaths in the trial were considered related to study treatment.
TABLE 3.
Adverse Events per Gradea
DISCUSSION
This final Protocol-defined analysis of the PALLAS trial, with 5,796 recruited patients and a 31-month median follow-up and exceeding the predefined number of events, did not show significant improvements in survival end points for the addition of palbociclib to adjuvant endocrine therapy. These definitive findings from the PALLAS trial, already indicated by an interim analysis,16 are surprising given the established efficacy of palbociclib and other CDK4/6i in advanced breast cancer. The combination of CDK4/6i with endocrine therapy became the approved standard of care for hormone receptor–positive breast cancer in the metastatic setting on the basis of large phase III studies: five studies evaluated CDK4/6i in first-line metastatic patients and showed consistent improvement in progression-free survival8,10,19-21 and partly in OS22,23 across studies. Three trials evaluated CDK4/6i in the pretreated metastatic setting and showed significant improvements in progression-free survival and OS,24,25 with the OS benefit in PALOMA-3 confined to patients who had documented sensitivity to previous endocrine therapy.12
Overall, CDK4/6i are considered safe with manageable toxicity.13,26 There were no new safety signals in this large adjuvant trial, with the majority of early discontinuations resulting from neutropenia (Protocol-mandated) and certain demographic factors associated with early discontinuation (unpublished data).27 However, landmark analyses did not reveal suboptimal drug exposure as a reason for the observed lack of palbociclib efficacy.
A smaller trial of 1 year of adjuvant palbociclib in the specific situation of high-risk disease after limited response to neoadjuvant chemotherapy, PENELOPE-B, also showed no significant iDFS benefit.28 By contrast, the monarchE trial investigating 2 years of adjuvant abemaciclib in high-risk patients reported an interim result, suggesting a significant iDFS benefit for CDK4/6i,29 and an updated analysis confirmed a relative 30% hazard rate reduction at a median follow-up of 27 months (HR = 0.70, P < .0001).30 Ribociclib is currently being studied in the adjuvant setting in the NATALEE study (NCT03701334).
There are several possible explanations for the observed outcome differences seen in adjuvant CDK4/6i studies, including differences in study populations and procedures, drug regimens, follow-up durations, or efficacy differences between the drugs. The latter is unlikely given the almost identical magnitude of benefits in advanced breast cancer trials.31 Preclinically, palbociclib has similar potency against cyclin D1-CDK4 and cyclin D2-CDK6 complexes, whereas ribociclib and abemaciclib have greater potency against CDK4 than CDK6.32,33 Subtle differences have been reported from CDK4/6i trials in the metastatic setting: although palbociclib appears to be more effective in patients with bone-only disease,34 abemaciclib appears to be most effective in patients with aggressive and poor prognosis disease,35 which is in line with the reported benefit of adjuvant abemaciclib in the monarchE study of high-risk patients. A sizable fraction of higher risk patients enrolled in PALLAS and, in line with PENELOPE-B, did not derive greater benefit from palbociclib than the general study population.
It was demonstrated in the neoadjuvant setting that palbociclib may preferentially work in the most luminal tumors,36 and it might be that any antiproliferative effect of adjuvant palbociclib can only be shown in the absence of cytotoxic chemotherapy.37 In the same study, tumor cell proliferation recovered notably between the end of neoadjuvant palbociclib and surgery, indicating that continuous CDK4/6i treatment (as with abemaciclib in the monarchE study) may provide the advantage of uninterrupted cell cycle inhibition, which could better promote tumor cell senescence38 than intermittent therapy.39 Of several described resistance mechanisms against CDK4/6i, reactive resistance by clonal evolution or adaptation is an unlikely reason for palbociclib's inefficiency in the adjuvant setting.40,41 Also, the lack of benefit of palbociclib in the early disease setting may simply reflect the lack of an available sensitive target: successful cessation of the cell cycle requires proliferating cancer cells, and breast cancer stem cells that emerge from dormancy in a stochastic manner over time—the putative target of adjuvant therapy—might not be affected by the CDK4/6i.39 By contrast, in the neoadjuvant setting, with proliferating target cells as in advanced disease, the addition of palbociclib to anastrozole led to higher cell cycle arrest than anastrozole alone36 and abemaciclib led to inhibition of cell cycle progression measured by Ki67 expression.42
The lack of adjuvant palbociclib efficacy does not preclude further integration of CDK4/6i into the breast cancer treatment algorithm. In addition to new combinations being currently investigated in various breast cancer subtypes,43 translational science will help to better understand unique tissue and/or serum biomarkers44 that may predict individual benefit or resistance45 for each of the approved CDK4/6 inhibitors to guide optimal patient selection and treatment combinations.46 The sizable trans-PALLAS biorepository containing both tumor tissue and serial plasma specimens together with the planned clinical long-term follow-up of patients will serve as an excellent resource to support such important research.
ACKNOWLEDGMENT
We are indebted to our patients and their families who have contributed to this and other clinical trials; the academic PALLAS trial is cosponsored by the Austrian Breast and Colorectal Cancer Study Group (https://www.abcsg.com) and the Alliance Foundation (https://acknowledgments.alliancefound.org), in collaboration with the Eastern Cooperative Oncology Group, the NSABP Foundation, Inc, the German Breast Group, and the Breast International Group—at all these organizations and at all 406 study sites in 21 countries around the globe, numerous individuals contributed to the success of the study and gave care—partly under the challenges of the COVID-19 pandemic—to our trial patients, including but not limited to investigators, physicians, study nurses, data management associates, and trial center staff in the centers (a full list of contributors can be found in the Data Supplement); we thank members of the independent data monitoring committee for their service, Pfizer for funding the trial, and Jana Link and Martina Putz (both full-time employees of ABCSG) for assistance in the preparation of the article.
Please see our Appendix (online only) for a full list of the PALLAS groups and investigators and their affiliations.
APPENDIX. Full List of PALLAS Groups and Investigators
Cooperative Groups
Alliance Foundation Trials (AFT)
Austrian Breast & Colorectal Cancer Study Group (ABCSG)
Cancer Trials Ireland
SOLTI—Breast Cancer Research Group
GBG Forschungs GmbH
GEICAM Spanish Breast Cancer Group
Canadian Cancer Trials Group (CCTG)
Breast Cancer Trials ANZ
Breast International Group (BIG)
International Breast Cancer Study Group (IBCSG), Switzerland
CCS Associates, Inc (CCSA)
PrECOG, LLC (PreCOG)
The NSABP Foundation, Inc (NSABP)
Australia
Southern Highlands Cancer Centre: Danielle Harward, Emma Eagles, Eunice Dai, Hiren Mandaliya, Howard Chan, Jennifer Jagoe, Jeralyn Jacquet, Joanne Pearson, Melissa Quaggiotto—McMahon, Peey-Sei Kok, Sarah Khan, Sayeda Naher, Shalini Subramaniam, Stephen Della-Fiorentina
St John of God Hospital, Bunbury: Andrew Kiberu, Helen Barry, Sandra Carvalho, Suzanne Webb
Breast Cancer Research Centre—WA: Albert Gan, Alexis Chung, Arlene Chan, Chris Lomma, Claire Beecrof, Cynthia Gregory, Fiona Benino, Frances McGlone, Gabrielle Jones, Jeannette Devoto, Jessica McCann, Julie Uhlmann, Julieanne Barrett, Melanie Cheah, Naomi Brook, Nicola Jones, Nicola O'Neil, Peter Willsher, Preetam Deshmukh, Sarah Hacking, Sally Jackson, Sally Pontre, Silvie Radmil, Sophie Cornell, Tijana Coe, Nakita Stephens, Tracie Ernenwein
Peter MacCallum Cancer Centre: Alice Bergin, Anita Krishnan, Anna Hobinchet, Arvind Sahu, Cath Healy, Deepti Pandey, Felicity Sutton, Geoffrey Lindeman, John Lai, Kelly-Anne Phillips, Kelsey Bumford, Lauren Keller, Lironne Wein, Louisa Lo, Lyndsey Grollman, Marijke Alexander, Marisa Grossi, Michael Green, Peter Savas, Prue Francis, Richard de Boer, Ronan Burder, Sarah-Jane Dawson, Sherene Loi, Sophie Katsabanis, Stephen Luen, Sweta Chauhan, Zhen Rong Siow, Laura Adams, Geness Borgueta, Roshni Thamarassery
Macquarie University: Abhijit Parshionikar, Amy Kelty, Dhanusha Sabanathan, Jenny Gilchrist, Junie McCourt, Kristine Nakhel, Louise Grice, Luke Garcia, Manny Marquez, Natalia Inness, Pirooz Poursoltan, Radhika Butala, Ray Yung, Renuka Chittajallu, Richard Kefford, Tomoko Barnier
South West Healthcare (Warrnambool): Ashlin Keane, Ian Collins, Jane Kelly, Janet Keith, Kaye McDowall, Lucy Leonard, Marcelle Hennig, Sandra Robinson, Susan Shaw, Theresa Hayes
Flinders Medical Centre: Alex Scott-Hoy, Alison Richards, Bogda Koczwara, Christos Karapetis, Dania Ruminski-Smith, Ganessan Kichenadasse, Kelly Mead, Monique Swan, Muhammad Abbas, Myron Klevansky, Sina Vatandoust
Royal Brisbane and Women's Hospital: Alison Hadley, Andrea Junghans, Aneta Suder, Annette Cubitt, Cara Powell, Erin Purdon, Gel Bolanos, Haidar Al-Saig, Jade Allan, Jasmine Brady, Jeffrey Goh, Jenna Peckston, Jenny Campbell, Lourdes Pamposa, Michelle Nottage, Natasha Roberts, Po-ling Inglis, Sarah McGuckin, Wendy Pritchard
The Breast & Endocrine Centre: Gail Walker, Hollie Ritchie, Judith Silcock, Kathryn Rebellato, Katie Frankiewicz, Nicholas Zdenkowski, Rob Paterson, Rosemary Hurley, Victoria Sproule
Royal Hobart Hospital: Christine Storey, Ciara Conduit, Dana Byers, David Boadle, Elizabeth Campbell-Taylor, Ian Byard, Lesley Oliver, Louise Nott, Melanie Wuttke, Paula Davidson, Rosemary Young, Sue Davoren, Tania Campbell
Sunshine Hospital: Angela Baugh, Caitlin Murphy, Catherine Oakman, Christine Muttiah, David Campbell, Frances Barnett, Heike Raunow, Jessica Tanner, Lewis Au, Lisa Magee, Lisa Wilkinson, Maria Hadfield, Michael Green, Nathan Hope, Sally Greenberg, Shannon Uren, Shehara Mendis, Shirley Wong, Siobhan Gallus, Vanessa Wong
Fiona Stanley Hospital: Aesha Gandhi, Afaf Abed, Anna Cannon, Andrew Redfern, Andrisha Inderjeeth, Azim Khan, Bella Nguyen, Caroline Stone, Cassie Riley, Chris Lomma, Claire Savage, Edwin Tan, Hilary Martin, Indunil Weerasena, Jaye Harding, Jordanna Wilson, Kanako Ohara, Kim Kennedy, Gabriela Marsavela, Mihitha Ariyapperuma, Melvin Chin, Muhammad Hakeem, Navin Palayoor, Nicola O'Neill, Ngie Law, Omar Faruque, Piyush Grover, Raj Tota, Sanjana Kondola, Sankha Mitra, Timothy Humphries, Timothy Slattery, Veenoo Agarwal, Wei-Sen Lam, Yasir Khan
The Northern Hospital: Ainsley Campbell, Chrissie Risteski, Elizabeth Buckley, Frances Barnett, Jisha Jose, Jo-An Seah, Josephine Stewart, Karen Matoga, Lynda Harrison, Shane White, Wing Hing Yau, Kira Edwards
Wollongong Hospital: Ali Tafreshi, Asif Khattak, Carla Brown, Carly Leighton, Daniel Brungs, Gary Tincknell, Ho Wai Siu, Jared Millican, Julie Hollis, Kim Bloomfield, Lawrence Kasherman, Maryna Brown, Nathan O'Dea, Surinder Wadhwa, Susan Beck, Wendy Riordan
St Vincent's Hospital, Melbourne: Anthony Dowling, Christopher Hart, Genni Newnham, Jasmine Hee, Melissa Moore, Nadia Ranieri, Pruedence Francis, Sue-Anne McLachlan
Gosford Hospital: Cassandra Rubio, Craig Kukard, Kathy Hall, Leonie Kelly, Malmaruha Arasaratnam, Mamta Bagia, Matthew Chan, Matthew Wong, Michelle Dixon, Namrata Nayar, Richard Clayton, Susan Tiley
Orange Health Service: Alison Coote, Carol Han, Catherine Richards, Jodie Stewart, Kerry Lenton, Lauren Bradbury, Lyn Ley Lam, Lynnette Meadley, Peter Fox, Robert Zielinski
Frankston Hospital: Beng Kong Jun, Diane Canning, Dawson Ellen, Jacqui Thomson, Judi Clarke, Judith Reilly, Nathan Dorembus, Nicole Potasz, Oliver Klein, Ratcliffe Gordon, Sally Heath, Sean Chinnathumby, Theresa de Man, William Poole, Yoland Antill, Zee Wan Wong
Coffs Harbour Health Campus: Amber Carle, Ankit Jain, Annabel Pickett, Harry Gasper, Joanne Smith, Karen Briscoe, Pinky Baghi, William Fox
University Hospital Geelong: Catherine Oakman, Damon Arezzolo, David Campbell, Inger Olesen, Karen White, Lea-Anne Harrison, Mandy McPhee, Michelle Edwards, Seamus Wilson
Royal Hobart Hospital: David Boadle, Louise Nott, Ian Byard, Melanie Wuttke, Rosemary Young, Ciara Conduit
Andrew Love Cancer Centre, Barwon Health, Geelong, VIC, Australia: Louise White, Mandy McPhee, Lea-Anne Harrison, Andrea DOZZI
Barwon Health, Geelong, VIC, Australia: Tracy Shields, YapThrift
Austria
Breast Health Center/Dep Surgery, Comprehensive Cancer Center (CCC), Medical University Vienna/Vienna General Hospital, Vienna, Austria: Florian Fitzal, Ruth Exner, Yelena Devyatko, Michael Gnant, Rupert Bartsch, Stephanie Kacerovsky-Strobl, Michael Bolliger
Department for Medicine I—Division of Oncology, Medical University of Vienna, Vienna, Austria: Maximilian Marhold, Beate Rottenmanner, Heidrun Forstner
Dept of OB/GYN and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria: Christian Singer, Georg Pfeiler, Michael Seifert, Daniela Dittrich, Hanka Sahbegovic, Yvonne Öhler, Ludmilla Strnadova, Ingeborg Brandl
Breast Centre, St Veit an der Glan, Austria: Viktor Wette, Ursula Wette-Tabery, Susanne Grabuschnig
Department of Obstetrics and Gynecology, Innsbruck Medical University, Innsbruck, Austria: Christian Marth, Daniel Egle, Christine Brunner, Magdalena Ritter, Verena Wieser, Johannes Eder, Anna Holzmann, Carmen Albertini
Center for Oncology and Hematology, Klinik Ottakring, Vienna, Austria: Kathrin Strasser-Weippl
Breast Health Center, Department of Surgery, Ordensklinikum Linz Sisters of Charity, Linz, Austria: Ruth Helfgott, Dietmar Heck, Hans-Jörg Fehrer, Markus Acko, Sandra Fuchs, Manuela Binder-Reisinger, Alexandra Gunesch, Farid Moinfar
Klinik Hietzing, Abteilung für Gynäkologie und Geburtshilfe/Karl Landsteiner Institut für gynäkologische Onkologie und Senologie, Vienna, Austria: Paul Sevelda, Christian Peters-Engl, Michael Janauer, Carmen Zwick, Maria Schütz, Tanaz Modarressy-Onghaie, Maria Dechant, Michael Mossig, Barbara Zelger-Hosmann, Petra Hnizdo, Simona Siegl, Ursula Denison
Division of Oncology, Department of Internal Medicine, Medical University of Graz, Austria: Marija Balic, Christoph Suppan, Renate Schaberl-Moser, Michael Halm, Heidi Peinsith, Jasmin Spiegelberg, Carina Kreuter, Sylvia Tripolt
Department of Obstetrics and Gynecology, Medical University of Graz, Graz, Austria: Gunda Pristauz-Telsnigg, Karl Tamussino, Edgar Petru, Vesna Bjelic-Radisic, Jasmin Lerch, Daniela Gold, Elisabeth Trapp, Arnim Bader, Nicole Schreiner, Angelika Boandl
Salzkammergut-Klinikum Vöcklabruck, Vöcklabruck, Austria: Ferdinand Haslbauer, Agnes Linortner
Department of Internal Medicine III with Haematology, Medical Oncology, Haemostaseology, Infectiology and Rheumatology, Oncologic Center, Salzburg Cancer Research Institute – Laboratory for Immunological and Molecular Cancer Research (SCRI-LIMCR), Paracelsus Medical University, Salzburg, Austria, Cancer Cluster Salzburg, Austria: Simon Gampenrieder, Lukas Weiss, Gabriel Rinnerthaler, Patrick Morre, Teresa Magnes, Sibylle Jäger, Claudia Sartori, Florian Huemer, Bianca Radl, Carmen Lehner, Lukas Ratzinger, Volker Hauck, Michael Leisch, Verena Schintl, Brigitte Mlineritsch, Martin Moik, Charlotte Schönlieb, Theresa Westphal, Konstantin Schlick, Michael Grundbichler, Felix Renneberg, Josef Klappacher, Petra Luft, Michaela Schachner
Institute of Pathology, Paracelsus Medical University/Salzburger Landeskliniken (SALK), Salzburg, Austria: Daniel Neureiter, Bettina Neumayer
Department of Hematology and Internal Oncology, Kepler Universitätsklinikum, Linz, Austria: Thomas Kühr, Gerhard Hochreiner, David Kiesl, Riad Ghanem, Jutta Hackl, Isabella Rauscher
Department für Hämato-Onkologie, LKH Hochsteiermark, Leoben, Austria: Christoph Tinchon, Angelika Pichler
Department für Chirurgie, LKH Hochsteiermark, Leoben, Austria: Walter Herz
Department für Hämato-Onkologie, LKH Hochsteiermark, Leoben, Austria: Laurenz Schöffmann, Manuela Maderdonner, Theresia Egger, Kathrin Heinreich
St Josef Krankenhaus Wien, Vinzenz Gruppe, Vienna, Austria: Ulrich Schmidbauer
Department of Internal Medicine IV, Klinikum Wels-Grieskirchen, Wels, Austria: Sonja Heibl, Josef Thaler, Vera Trommet, Christa Reder
Department of Surgery, Breast Health Center, State Hospital Wiener Neustadt, Wiener Neustadt, Austria: Stefan Halper, Werner Kwasny, Eva Kristandl
Institute of Clinical Pathology and Molecular Pathology, Thermenregion Landesklinikum Wiener Neustadt, Wiener Neustadt, Austria: Tanja Würger, Angie Auer, Wolfgang Hulla
Department of Internal Medicine 1, University Hospital of St Pölten, Karl Landsteiner University of Health Sciences, Karl Landsteiner Institute for Nephrology and Hematooncology, St Pölten, Austria: Martin Wiesholzer, Petra Pichler, Maria Gold, Daniela Hochsteger
Abteilung für Gynäkologie und Geburtshilfe, BHS Ried, Ried, Austria: Monika Penzinger
Department of Int.-Oncology, LKH Feldkirch/Coop. Group of Gynaecology, Feldkirch, Austria: Margit Sandholzer, Bernd Hartmann, Klaus Gasser, Michele Atzl, Sonja Wallner, Judith Mathis
Division of Oncology, Department of Internal Medicine II, Pyhrn-Eisenwurzen Klinikum Steyr, Steyr, Austria: Dieter Rossmann, Hanns Hauser, Simone Gruber, Georg Schreil, Regina Neuhauser, Doris Prauhart
Belgium
CHU UCL Namur/Site Sainte-Elisabeth: Donatienne Taylor, Peter Vuylsteke; Grand Hôpital de Charleroi, Belgium: Jean-Luc Canon
University Hospital of Antwerp, Antwerp, Belgium: Konstantinos Papadimitriou, Stefanie Verheyden, Astrid Reymer
CHU St Pierre: Corina MARTINEZ-MENA, Séverine Pascal
Cliniques universitaires Saint-Luc, Bruxelles, Belgique: François Duhoux, Nathalie Blondeel, Elodie Villar, Yves Humblet, Filomena Mazzeo, Jean-François Baurain, Frank Cornelis
GZA Sint Augustinus, Belgium: Annemie Prové
UZ Leuven, Belgium: Patrick Neven
OLV Aalst: Greet Huygh, Adelheid Roelstraete
Medical Oncology Department, Institut Bordet, Université Libre de Bruxelles, Belgium: Michail Ignatiadis, Fanny Bustin
Medical oncology dpt, CHU de Liège, Liège, Belgium: Guy Jerusalem, Maude Piron, Meriem Boukerroucha, Sabrina Uccello, Véronique Loo, Laurence Lousberg, Nathalie Marchal, Andrée Rorive, Elodie Gonne
CHC Montlégia, Liège, Belgium: Marie-Pascale Graas, François Kreutz, Ludivine Collard
CHR Verviers, Belgium: Annelore Barbeaux
Canada
Odette Cancer Centre Sunnybrook Health Sciences Centre: Ellen Warner, Debra Burns, Shenur Jamani
Kingston Health Sciences Centre: Mihaela Mates, Julie Holiday, Jessica Ferguson, Marleen Ross-Smith
The Vitalite Health Network—Dr Leon Richard Oncology Centre: Ali Benjelloun, Nathalie Godin, Nancy Halle
Niagara Health System: Rachel Vandermeer, Joanne Haun, Tim Van Helvert
Grand River Regional Cancer Centre at Grand River Hospital: Andrea Molckovsky, Elyse Wellhauser, Anna Granic
BCCA—Abbotsford Centre: Tamana Walia, Cheryl Carrasco, Urmila Shinde-Surabathula
Tom Baker Cancer Centre: Sasha Lupichuk, Yi Wang, GZakhari
The Jewish General Hospital: Mark Basik, Aline Mamo, Marie-Pascale Guay
BCCA—Cancer Centre for the Southern Interior: Simon Baxter, Mohan Elango, Tuong-Van Kim
CancerCare Manitoba: Danielle Desautels, Marshall Pitz, Vallerie Gordon, Susan Green, Rhonda Nichol, Caitlin Kitkowski, Kathleen Marek, Saroj Niraula, Joyce Wei, Rick Prayag
CHUM-Centre Hospitalier de l'Universite de Montreal: Rami Younan, Jean-Pierre Ayoub, Stepanie Viau, Marie Christine Rodrigue, Erica-Julie Patocskai, Sothun Lim, Sabrina Lavoie, Saima Hassan, Valerie Favreau, Nicole Derepentigny, Danielle Charpentier, Nawel Belaiboud, Guylai; ne Beaupre, Andre Robidoux, Lydia Tkalec, Lyne Gauthier
QEII Health Sciences Centre Capital District Health Authority: Kara Bursey, Claudia Harding
Regional Health Authority B, Zone 2 Saint John Regional Hospital: Margot Burnell, Sharon Turnell, Susan Kelly
Saskatoon Cancer Centre: Amer Sami, Dominique Wagner, Shahid Ahmed, Andrea Gallivan, Jolene Crump
Juravinski Cancer Centre at Hamilton Health Sciences: Stephanie Skeldon, Louise Bordeleau, Sandra Turner, Fiona Hellicar
BCCA—Vancouver Cancer Centre: Caroline A. Lohrisch, Kathy Ng, Wendy Won
University Health Network Princess Margaret Cancer Centre: Christine Elser, Vicky Gillman, Soha Ahrari
CHA-Hopital Du St-Sacrement: Julie Lemieux, Isabelle Ouellet, Chantal Gagnon
University Health Network Princess Margaret Cancer Centre: Melissa Lo, Lindsay Muyot, Irene Li
Allan Blair Cancer Centre: Muhammad Salim, Wanda Hawryluk, Leah Phillips
Germany
University Medical Center Hamburg; Department of Gynecology, Germany: Volkmar Müller, Isabell Witzel, Alma Rausch, Tanja Kummernuß, Kerstin Riecke
Department of Obstetrics and Gynecology, University Medical Center Mainz, Germany: Christine Diehl, Marcus Schmidt
Universitätsklinikum Schleswig-Holstein, Campus Lübeck, Frauenklinik, Germany: Ch.Hanker, Achim Rody, H.-G.Cirkel, Kerstin Eckhoff
Klinikum Hoechst, Frankfurt, Germany: Joachim Rom, Volker Moebus, Annette Junker-Stein, Christina Weissmueller, Matthias Bode
Department of Obstetrics and Gynecology, St Josefs-Hospital Wiesbaden, Germany: Antje Lehnert, Carolin Hammerle, Bettina Blau-Schneider, Andrea Lunkenheimer
Universitätsklinikum Halle (Saale), Universitätsklinik und Poliklinik für Gynäkologie, Germany: Prof. Dr med. Christoph Thomssen, Dr med. Susanne Steer
Gynecological Department, Städtisches Klinikum, Karlsruhe, Germany: Gabriele Kaltenecker, Laura Boosz
AGAPLESION Markus Krankenhaus, Gynäkologie und Gynäkologische Onkologie, Frankfurt am Main, germany: Marc Thill, Christiane Brandi, Fariba Khandan, Tina Schnitzbauer, Aynur Koccu-Yüzlek, Madeleine Modrow
Frauenarzt-Zentrum-Zehlendorf, Berlin, Germany: WolfgangGraffunder, Lidia Perlova-Griff, Michelle Pais, Anke Stehling
Helios Klinik Rottweil, Frauenklinik, Germany: Dr Alexander Miller, Dr Levente Márton Urbancsek, Katrin Schick
Elisabeth Krankenhaus, Brustzentrum, Kassel, Germany: Sabine Schmatloch, Ute Pfetzing, Daniela Borries, Christina Trzeja-Höhlein, Gudrun Rau, Ivonne Kurzhals
Praxisklinik Berlin, Dr Peter Klare, Germany: Peter Klare, Simone Diepold, Amely Gärtner, Alena Keil
Helios Klinikum Berlin-Buch, Berlin, Germany: Michael Untch, Kathi Schreiber, Holger Schultz, Antje Sperfeld, Christine Mau, Anett Töpfer, Anne Jülicher
St Vincenz Krankenhaus, Limburg, Germany: Angelika Ober, Dr Peter Scheler, Dr Winfried Obermeier, Christine Neu-Reusch
Erlangen University Hospital, Department of Gynecology and Obstetrics, Comprehensive Cancer Center Erlangen-EMN, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen: Patrik Pöschke, Gianna RobertaTitzmann, AnahíPontones
St Elisabeth Hospital, Leipzig, Germany: Dagmar Dr med. Langanke
Praxis und Tagesklinik für gynäkologische Onkologie, Ebersberg, Germany: Isolde Gröll
Klinikum Oldenburg AöR, Universitätsklinik für Innere Medizin – Onkologie und Hämatologie, Germany: Univ.-Prof. Dr med. Claus-Henning Köhne, Dr med. Ruth Renzelmann, Bernd Thole, Imke Rosien, Carsten Seemann
GP Dres. Wilke/Wagner/Petzoldt, Fuerth, Germany: Jochen Wilke, Harald Wagner, Alexander Petzoldt, Traudl Walter, Annett Beer
Universitätsklinikum Aachen, Klinik für Gynäkologie und Geburtsmedizin, Germany: Elmar Stickeler, Katja Krauß, Sandra Richlowski, Barbara Flege, Cordula Franz
Marienhospital Bottrop gGmbH, Germany: ChristianKolberg, Sarah Wetzig, Katharina Freienstein
Oncologianova GmbH, Recklinghausen, Germany: Till-Oliver Emde, Dr med. Ludger Heflik
MVZ Eggenfelden: Dr Jürgen Terhaag
Studien GbR Braunschweig, Germany: Dr Ralf Lorenz, Dr Janine Kreiss-Sender, Bibiana Kalensee
Centrum für Hämatologie und Onkologie Bethanien; Frankfurt a. M.; Germany: Hans Tesch, Karin Baumbach
Haga Ziekenhuis, Den Haag, the Netherlands: Daniel Houtsma
Hungary
Petz Aladar County Teaching Hospital, Gyor, Hungary: Tamas Pinter, Gabriella Herodek, Adel Ambrus, Marta Janoki
University of Szeged, Department of Oncotherapy, Szeged, Hungary: Zsuzsanna Kahán, Aliz Nikolényi, Györgyi Kelemen, Judit Pepó, Irén Tánczos
Országos Onkológiai Intézet, “B” Belgyógyászati-Onkológiai és Klinikai Farmakológiai Osztály, Budapest, Hungary: Gábor Rubovszky, Erika Hitre, Balázs Madaras, Brigitta Balogh, Petra Nagy, Tímea Burján
University of Debrecen Medical Center, Clinic of Oncology, Debrecen, Hungary: Péter Árkosy, Judit Tóth, Andrea Gonda
Uzsoki utcai Kórház, Fovárosi Onkoradiológiai Központ, Budapest, Hungary: László Landherr
Ireland
Mater Misericordiae University Hospital, Dublin and Cancer Trials Ireland: M.Kelly
Mater Misericordiae University Hospital, Dublin Ireland: JaneHiggins
Clinical Trials Research Unit, Mater Misericordiae University Hospital, Dublin Ireland: Ciara Clancy, Denise Scott, Lorraine Weymes
Cancer Clinical Trials & Research Unit, Beaumont Hospital, Dublin: Patrick Morris, Liam Grogan, Oscar Breathnach, Bryan Hennessy, Keith Egan, Audrey Carthy, Aishling Mason, Lorna Mulvihill, Lisa Keogh, Marieke Amerlynck, Louise Coleman, Karl Browne, Bridget Lynam
Consultant Oncologist, St Vincent's University Hospital, Elm Park, Dublin 4, Ireland: Janice Walshe
Consultant Pathologist, St Vincent's University Hospital, Elm Park, Dublin 4, Ireland: Cecily Quinn
Cancer Trials Cork, Cork University Hospital, Ireland: Seamus O'Reilly, John Waldron, Claire Cronin, Edel Hassett, Sarah Thompson, Claire Brady, Deirdre O'Mahony, Aine Cadogen, Zara Aston, Malgorzata Cwiak, Nessa Gallwey, Deirdre Huggard, Katrina Falvey, Elizabeth Lenihan, Jack Gleeson, Will Mullally, John Greene, Cathy Cogan, Zeeshan Zameer, Erica Kelly, Carolyn Maloney, Eileen McMahon, Muhammad Raheel Khan, BrackenClarke, Hazel O'Sullivan, Mohammed Osman, Anna Cole, Emma Skelton, Susan Potter, Marita Barrett, Lorraine Griffin, Laura Whelan, Abdul Rehman Farooq, Aymen Amasayb, Niamh Peters, Shahid Iqbal, Cian Ronayne, Niamh O'Donovan
University Hospital Waterford, Waterford, Ireland: Miriam O'Connor, Paula Calvert, Emmet Jordan, Anne Horgan, Lucasz Milewski, Grainne Smith Lehane, Eoin Tabb, Darren Walsh, Helena Dwyer, Flordeliza Calacsan, Elaine Shanahan
St James's Hospital, Dublin, Ireland: John Kennedy
Cancer Clinical Trials Unit, Department of Cancer Services, University Hospital Limerick, St Nessans Road, Dooradoyle, Limerick, V94 F858: Grzegorz Korpanty, Linda Coate, Marie Hanrahan, Susan Nagle, Ciara Cantrell
Bon Secours Hospital, Cork, Ireland: Dr Conleth Murphy, Aoife O'Shea, Evelyn O'Sullivan Greene
Sligo University Hospital, Sligo, Ireland: Michael Martin, Asma Patel, Francios Malan, Moira Maxwell, Margaret Burke
Israel
Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel: Bar Avrham, Rinat Yerushalmi
Oncology Department, Sourasky Medical Center, 6 Weitzmann St, Tel Aviv, Israel: Wolf Ido, Giti Fisher, Alina Alkhasov, Mirit Goz, Shulim Shpigel, Amir Sonnenblick, Hana Maik-Notea
Oncology Division, Breast Oncology Institute, Sheba Medical Center, Tel Hashomer, Ramat Gan, Israel: Einav Gal-Yam, Irena Klain, Merav Shtal, Limor Peretz, Lilia Solomon Amar, Bella Kaufman*
Italy
Policlinico di Monza, Italy: Domenico De Toma, Marco Mucciante, Emilio Bajetta
Ospedale Misericordia of Grosseto, Italy: Fausto Petrelli, Veronica Lonati, Carmelo Bengala, Ilaria Pastina, Michele Bindi
IRCCS Ospedale San Raffaele, Milano, Italy: Giampaolo Bianchini, Alessia Rognone, Stefania Zambelli, Patrizia Zucchinelli, Caterina Riccio, Domenica Ceraulo, Elena Gritti
University Hospital of Florence, Florence, Italy: Lorenzo Livi, Icro Meattini
AOU Città della Salute e della Scienza di Torino, Italy: GraziaBaù, Alessandra Surace, Mario Marengo, Aurelia Mondino
IRCCS IRST, Meldola, Italy: Andrea Rocca, Michela Palleschi, Lorenzo Cecconetto, Francesca Mannozzi
Ospedale Santo Stefano, Prato, Italy: Laura Biganzoli, Erica Moretti, Luca Livraghi, Elena Zafarana, Silvia Cappadona
AOU Arcispedale S.Anna, Ferrara, Italy: Antonio Frassoldati, Alessio Schirone, RaffaellaMartella
Ospedale Policlinico San Martino of Genova, Italy: Alberto Ballestrero, Valentina Barbero
Ospedale di Parma, Italy: Antonino Musolino, Alessandro Viansone
AULSS9 Scaligera Mater Salutis Hospital, Department of Medical Oncology: Andrea Bonetti, Filippo Greco, Anna Mercanti, Jacopo Giuliani, Eva Pigozzi, Jessica Insolda, Maddalena Buniotto
Japan
Chiba Cancer Center, Breast Surgery, Japan: Rikiya Nakamura
Hokkaido Cancer Center, Breast Surgery, Japan: Masato Takahashi
Kyoto University Hospital, Breast Surgery, Japan: Masahiro Takada
Nagoya City University Graduate School of Medical Sciences, Breast Surgery, Japan: Tatsuya Toyama
National Hospital Organization Osaka National Hospital, Breast Surgery, Japan: Hiroyuki Yasojima, Norikazu Masuda
Osaka International Cancer Institute, Breast and Endocrine Surgery, Japan: Takahiro Nakayama
Mexico
Centro Oncológico Estatal ISSEMYM, Toluca EDOMEX, México: Saul Campos-Gómez, A.Campos-Gómez, Marcela García-Garcés, JesúsValdés-Andrade, Fabiola Palacios Gutiérrez
Instituto Nacional De Cancerólogía, CDMX México: AnelCabrera-Galeana, MarielyRodriguez Pacheco, RMuñoz Montaño, Diana Flores-Díaz, AntonioMatus-Santos, Abigaíl Sanchez Vázquez
Hospital Zambrano Hellion TecSalud, Monterrey NL, México: MaytéVillarreal-Garza, Omar Peña-Curiel, MargaritaMaldonado-García, NairoviVillaseñor Vázquez, GabrielaTorres Ríos
Poland
Samodzielny Publiczny Zaklad Opieki Zdrowotnej Opolskie Centrum Onkologii im. prof. Tadeusza Koszarowskiego, Opole, Poland: Barbara Radecka, Joanna Hudala-Klecha, Magdalena Derus, Natalia Obrusnik, Patryk Zajac
Instytut MFS Sp. z o. o., Lódz: Ewa Kalinka, Igor Symonowicz, Elzbieta Turska
Uniwersyteckie Centrum Kliniczne, ul. Smoluchowskiego 17, 80-214 Gdansk, Poland: Jacek Jassem, Elzbieta Senkus-Konefka
Narodowy Instytut Onkologii im. Marii Sklodowskiej-Curie, ul. W. K. Roentgena 5, 02-781 Warszawa, Poland: Zbigniew Nowecki, Elzbieta Sierkowska, Joanna Wisniewska, Marta Flejszer, Agnieszka Jagiello-Gruszfeld, Anna Górniak, Roman Dubianski
Portugal
IPO Porto, Porto, Portugal: Susana Sousa, Miguel Abreu, Júlio Oliveira, Sara Alves, Cláudia Vieira, Marta Ferreira, Isabel Pimentel, João Dias, CassianoNeves, Ana Ferreira, Li Bei, Helena Magalhães, SofiaPatrão, Iolanda Vieira, Bordalo eSá, Deolinda Pereira, Inés Pousa, RosárioCouto, Andreia Cruz, Mariana Brandão, Fontes eSousa, RitaLopes, Sarah Lopes, Helena Rocha, Ana Finisterra, Liliana Azevedo, Berta Reis, Cláudia Leite
Fundação Champalimaud, Lisbon, Portugal: Fátima Cardoso, Berta Sousa, Joana Ribeiro, Helena Gouveia, Arlindo Ferreira, Maurício Chumbo, Susana Pedro, Leonor Bastos, Carolina Almeida, Patricia Lourenço, Brigida Maio, Daniela Lopes, Marta Martinho, Manuela Seixas, Tânia Lucas
Hospital CUF Descobertas, Lisbon, Portugal: Ana Parece, FaíscaNoronha, MarisaSalgueiro, CrisóstomoCosta, AzambujaBraga, Li Bei, AlpuimCosta, CassianoNeves, CláudiaMonteiro, PauloFernandes, Catarina Santos, SérgioGomes, CunhaMonteiro, Cristina Teixeira, ReisAlves, Licínio Leite, HenriquesGrãos, PiresPacheco, SantanaLopes
IPO Lisboa, Lisbon, Portugal: Emanuel Gouveia, Catarina Cardoso, Duarte Machado, Elisabete Lopez, Rosete Pais, Rita Conde, Ana Inácio, Humberto Gonçalves
Hospital da Luz, Lisbon, Portugal: NazaréRosado, Miguel Pimenta, PatríciaGomes
South Korea
Severance Hospital, Yonsei University Health System: Joo Hyuk Sohn
Samsung Medical Center: Young-Hyuck Im
Seoul National University Hospital: Seock-Ah Im
Spain
Hospital Universitario Puerta de Hierro de Majadahonda. Oncology Department: Blanca Cantos, Miriam Mendez, CristobalSanchez, Rocio Navarro, Maite Artero
Hospital San Pedro de Alcántara(Cáceres)Oncology: HelenaLopez, Santiago Gonzalez, Sonia Alonso, Alicia Lopez
Institut Oncològic Dr Rosell, Hospital Universitari Quirón Dexeus, Barcelona, Spain: Xavier Gonzàlez Farré, Alejandro Martínez Bueno, JoséGarcía Mosquera, Mónica Tellechea, Elena Ovalle
Hospital Clinico Universitario de Santiago de Compostela. Oncology Unit: Rafael Lopez, Ana Palacios, Carolina Garcia, Cristina Blanco
HOSPITAL SANT JOAN DE REUS, Reus, Spain: Kepa Amillano, Mireia Melé, Maria Masvidal, Jana Repkova, Cinta Albacar, Berta Caballle, Dolors Salsench, Júlia Gurí, Montserrat Boj, Anna Gomez
Hospital 12 de Octubre. Medical Oncology: Luis Manso, Pablo Tolosa, Eva Ciruelos, Elsa Bernal, Ana Sanchez, Rosa Rodriguez
Medical Oncology Department, VHIO, Barcelona, Spain: Meritxell Bellet, Jesús Soberimo, Esther Zamora, Beatriz Rojas, Carolina Ortiz, Analia Azaro, Míriam Arumí, Raúl Sánchez, Santiago Escrivá, Laia Garrigós, Pol González, Núria Clotet
HOSPITAL CLÍNICO UNIVERSITARIO VIRGEN DE LA ARRIXACA—IMIB SERVICIO DE ONCOLOGÍA MÉDICA, Murcia, Spain: Pilar Sánchez Henarejos, Luis Alonso Romero, Jerónimo Martínez García, Ana Puertes Boix, Dolores Jiménez Lucas, Ana Pérez Aranda, Paula Ruiz Carreño
HOSPITAL CLÍNIC BARCELONA: Montserrat Muñoz, Nuria Chic, Olga Martínez, Laura Berenguer Jou, Barbara Adamo, Maria Vidal, Blanca Gonzàlez-Farré, Marga Fabian Espinilla, Ángeles Quintas Vila, Marta Boillos Fernández, Maribel Grande Robles
Hospital Son Espases, Oncology, Baleares, Spain: Antonia Perello Martorell, Jesus Alarcon, Nieves Ferrer, Margarita Oliver, Úrsula Sastre
Institut Català d'Oncologia, Hospital Moisès Broggi, Sant Joan Despí, Spain: Rafael Villanueva Vázquez, Adelaida Piera Sancerni, Sabela Recalde Penabad, Marta Ferrer Cardona
Institut Català d'Oncologia, IDIBELL, Hospitalet, Barcelona, Spain: Miguel Gil Gil, Agostina Stradella, Catalina Falo, Sabela Recalde
Hospital Universitario Son Llatzer, Palma de Mallorca, Spain: Isabel Garau, Maria Galán, Maria Iglesias, Iria González, Sabina Soler, Sergio Ayuga, del MarFiol, Georgina Ponsa
Oncology, HOSPITAL QUIRON SALUD SAGRADIO CORAZÓN, Sevilla, Spain: Antoniovirizuela, Carlosquero, María Valero, María Alemán, Jakinde Aguinagalde, Nazaret Martinez, Angel Albacete
Hospital Arnau de Vilanova (Valencia) Oncology: Antonio Llombart, Vicente Carañana, Julia Hidalgo, Mara Ocasar, Laura Calabuig, Angela Real
Complejo Asistencial Universitario de Leon Oncology Unit: Ana Lopez, Andres Garcia, AlejandraLopez, Felisa Fernandez
Institut Català d'Oncologia, IDIBELL, Hospitalet, Barcelona, Spain: Adela Fernández Ortega, Rafael Villanueva, Andrea Vethencourt, Silvia Vázquez Fernández, Sonia Pernas, Ariadna Iserte, Marc Andres, Marta Pamies
Department of Hematology and Medical Oncology. Hospital G. Universitario Morales Meseguer, Murcia, Spain: Francisco Ayala de la Peña, Elena García Martínez, Elisa García Garre, Pilar de la Morena Barrio, Gema Marín Zafra, Natalia Andúgar Villaescusa, Antonio López Oliva
Hospital del Mar. Barcelona, Spain: Sonia Servitja Tormo, Joan Albanell Mestres, Ignasi Tusquets Trias de Bes, Maria Martinez Garcia, Tamara Martos Cardenas, David Casadevall Aguilar, Clara Carreras Vila, Marta Macia Valldeperas, Gemma Martinez Peña, Roser Correa Soler
MD Anderson Cancer Center (Madrid), Spain: Laura García Estévez, Isabel Calvo Plaza, Isabel Gallegos Sancho, Raúl Márquez Vázquez, Miriam Delgado Sánchez, María Belén Bautista Caro, María Bernal Alonso, Irene Fernández Bravo, Beatriz Pradillo Rodríguez, Emilie Andress Fernandini, Raquel Sánchez Nieto, David Pérez Anchordoqui
HU Josep Trueta, Instituto Catalán de Oncología (ICO)—Girona. Avda Francia sn, 17007 Girona, Spain: Sonia Del Barco Berrón, Gema Viñas, Helena Plà, Berta Valls, Nadia López, Eugeni López-Bonet
Hospital Universitario Central de Asturias, Servicio de Oncología Médica, Sección de Mama. Oviedo, Spain: Yolanda Fernández Pérez, Maria Muñiz Castrillo, Cecilia Gonzalez, Esther Uriol, MaríaGarcía, Javier Fernández, Manuela Suarez, Eva López, Inmaculada Menéndez, Marta Izquierdo Manuel, Sara Fernández Arrojo, Clara Iglesias Gómez
Hospital Universitario Virgen Macarena. Sevilla, Spain: Fernando Henao Carrasco, Luis de la Cruz Merino, Esteban Nogales Fernández, Eduardo Montilla Burgos, Irene Junco Vicente, Isabel Benítez, Cristina González Maria, LuisaSánchez León
Hospital Clínico Universitario Lozano Blesa. Zaragoza, Spain: Raquel Andrés Conejero, Pilar Bueso Inglan, Laura Murillo Jaso, Belén Martín, Isabel Huercanos, JoséBardina, Marisa De la Rica, Elisa Quílez, María Álvarez, Natalia Alonso, Maitane Ocáriz
Servicio de Oncología, Hospital Teresa Herrera, Complejo hospitalario de A Coruña (CHUAC), A Coruña, Spain: Silvia Antolín Novoa, Cristina Reboredo Rendo, Lourdes Calvo, Maria Quindós Varela, Maria Mateos Salvador, Belén López Cortabitarte, Isabel Santamarina Cainzos, Adrián López Bellas
Department of Medical Oncology, Consorci Sanitari de Terrassa, Terrassa, Spain: Angels Arcusa Lanza, Marta Hernandez Griso, M. Carme Aracil Noëlle, Manuela Gonzalez Navarro, Luis Fernandez Morales, Meritxell Vilanova Vila, Nuria Barragan Guerrero, Conchi Roldan Polo, Marta Andrés Granyo, Sonia Lopez Aviles, Lidia Menchen Palau, Mar Pineda Yuste, Remei Blanco Guerrero, Maria Marin Alcala, Ignasi Roig Quilez
Hospital Provincial de Castellon, Spain: Eduardo Martínez de Dueñas, Santiago Olmos Antón, Carmen Herrero Vicent, María Fonfría Espacia, Sofía Rebolledo Molina, Javier Munarriz Ferrandis, Regina Romero Llorens, Francisco García Piñon, Marta Melia Prades, Ariadna Granados Sánchez, Alba Cebrián Jimenez
Hospital Universitario de Burgos, Spain: AscensiónHernando Fernández de Aránguiz, María García Gonzalez, Clara de Pablo Delgado
Hospital General Universitario de Elche, Alicante, Spain: Álvaro Rodríguez-Lescure, Teresa Quintanar, Elena Asensio, Vicente Boix, Rosana Perea, Miriam López-Cases
Centro Oncológico de Galicia, A Coruña, Spain: Manuel Ramos Vazquez, Ana Gonzalez Quintas, Pilar Togores Torres, CarlosMéndez Méndez, Ana Medina Colmenero, Margarita Amenedo Gancedo, Carolina Pena Álvarez, Alberto de la Cruz, Begoña Uriarte Carbón, Belén Ferreiro Rosende, Rebeca Santiso, Lorena Paris Bouzas
Complejo Hospitalario de Navarra. Servicio de oncología médica. Spain: JuanIllarramendi, Susana de la Cruz, Esteban Salgado, Teresa Prieto Leache, Esther Aznárez Arellano, Laura Miguélez Rivera, Patricia Ochoa Sanz
Hospital General Universitario Gregorio Marañon, Madrid, Spain: Miguel Martín, Sara López-Tarruella, Iván Márquez, IsabelPalomero, Yolanda Gilarranz, Isabel Echavarría, Gloria Marquez Lozano, Isabel Ranz, Salvador Gamez, Inmaculada Aparicio, Blanca Herrero, Ana Ruíz Bolaños, Sara Fernández, Ana García Maldonado, Tatiana Massarrah, Sonia Delgado, Magdalena Esteban, Carolina Ayllón, Inmaculada Gras, Ana Mur
Hospital Universitario Virgen del Rocío. Sevilla, Spain: Manuel Ruiz Borrego, Rosario González Mancha, Álvaro Montaño Periañez, Alejandro Falcón González, Javier Salvador Bofill, Marta Benavent Viñuales, ÁngelesOyarzabal Céspedes, ManuelCuevas Milla, Beatriz Esquinas Chaparro
Hospital Ramón y Cajal. Madrid, Spain: Noelia Martínez Jañez, María Fernández Abad, Belén Pérez Mies, Álvaro Molina Ruano, Isabel Muñoz, Cristina Pueyo
Servicio Oncología Médica, Hospital Universitario Miguel Servet; Instituto Investigación Sanitaria de Aragón. Zaragoza, Spain: Alicia Guerrero Molina, Noemi López Martinez, Rafael Huarte Lacunza, Silvia Bernabé Antolín, Antonio Antón Torres, Juan Lao Romera, de JesúsPuértolas Hernández, Elena Aguirre Ortega, Iñaki Alvarez Bustos, Ana Nuño Alves, PilarFelices Lobera, Ibón Gurruchaga Sotes, Jorge Hernando Cubero, Ricardo Lara López-Doriga, Laura Rodríguez Sastre, Concepción Velazquez Martinez, Pilar Ferrández Antón, Marta Rodriguez Hernández
B-ARGO. ICO Badalona. HGTiP, Badalona, Spain: Eudald Felip, Margarita Romeu, CarlosPardo, Beatriz Ciranqui, Vanesa Quiroga, Mireia Margeli, Judith Rodriguez
Oncology, Hospital General de Granollers, Granollers, Spain: Vanesa Ortega, Laura Jolis, Maria Tudela
Hospital Univerisario la Paz. Oncology: Pilar Zamora, Andres Redondo, Beatriz Castelo, Pilar Boyero, Ana Martinez
Hospital Universitario Fundacion Jimenez Oncology service: Yann Yzarzugaza, LuisArranz, Francisco Lobo, Berta Martin, Javier Sanchez, Sergio Galan
Hospital Univerisario Madrid Norte Sanchinarro, Centro Oncologico Clara Campal, Oncology Department: Natalia Ramirez, Eva Ciruelos, Beatriz Rojas, Elena Sevillano, Raquel Bratos, Estela Vega, Myriam Hernandez, Maria Bielza, JesusSanguesa
UGCI Oncología Médica. Hospitales Regional y Universitario Virgen de la Victoria. Málaga. IBIMA. Spain: Emilio Alba, Nuria Ribelles, Tamara Diaz, Antonia Marquez, Bella Pajares, Alfonso Sanchez, JoseBermejo, Francisco Carabantes, Enrique Saez, Carmen Manzano, Mar Moreno, Estefanía Bellagarza, Margarita Suardiaz, Irene Rojas
Hospital Clínico Universitario Valencia, Spain: Silvia Coret, Begoña Bermejo, MiguelCejalvo, TeresaMartínez, Cristina Hernando, Sara Rodríguez, Ignacio Castaño, Julia Soler
Servicio de Oncología, Hospital Virgen de la Salud, Toledo, Spain: IgnacioChacón López-Muñiz, Carmen Esteban Esteban, DavidCárdenas, Iciar García Carbonero, María Sánchez García, Amalia González Blázquez, Lourdes Méndez-Gómez Chacón, Cristina Blázquez
Hospital Universitario Reina Sofía de Córdoba. Servicio de Oncología. Spain: Juan De la haba, Ignacio Porras, Cristina Morales, Pedro Sánchez, MagdalenaVillatoro, Carlos Rodríguez, Sonia Rodríguez
Hospital Universitario Donostia-BioDonostia—Oncología Medica—San Sebastian, Spain: Isabel Alvarez Lopez, Nerea Ancizar Lizárraga, Cristina Churruca Galaz, Ana Paisan Ruiz, Maria Diez Zubizarreta, Brígida Esteban, PilarETXART
Hospital General Universitario de Alicante, Alicante, Spain: JuanPonce Lorenzo, Inmaculada Lozano Cubo, Bartomeu Massutí Sureda, Natividad Martínez Banaclocha, Elena Peña Zurdo, Natalia Gómez Peral, Jenifer Sánchez Martín, GenaroMontoyo Pujol, Montserrat García Araque
Hopital Universitario Fundacion Hospital Alcorcon, Oncology Unit: Clara Olier, Diana Moreno, Ruth Martinez
Sweden
Karolinska Universitetssjukhuset, Solna, Sweden: Theodoros Foukakis
Gävle Hospital, Sweden: Per Edlund
Universitetssjukhuset Örebro, Sweden: Kenneth Villman
Sahlgrenska University Hospital, Göteborg, Sweden: Barbro Linderholm
Akademiska Sjukhuset Uppsala, Sweden: Henrik Lindman
Taiwan
Taipei Veterans General Hospital, Taipei, Taiwan: Ling-Ming Tseng, Kuang-Liang King, Yi-Fang Tsai, Jen-Hwey Chiu, Yen-Shu Lin, Chun-Yu Liu, Ta-Chung Chao, Hsiang-Chung Tseng, Hsin-I Chen
Changhua Christian Hospital, Changhua, Taiwan: Shou-Tung Chen, Hsin-Shun Tseng, Hung-wen Lai, Chin-Cheng Su, Ya-Wen Yang, Wan-Ju Hung, Ai-Ping Lai
Kaohsiung Medical University Hospital, Kaohsiung, Taiwan: Ming-Feng Hou
Division of Hemato-Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan: Wei-Pang Chung
Clinical Trial Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan: Yi-Hsin Hsu
National Taiwan University Hospital: Chiun-Sheng Huang, Ming-Yang Wang, Po-Han Lin
The Netherlands
Zaandam Medical Center, Zaandam, the Netherlands: D.Bakker
Máxima MC, Dept of Oncology, Eindhoven/Veldhoven, the Netherlands: Wouter Dercksen
St Antonius Hospital, Nieuwegein, the Netherlands: Maartje Los
United Kingdom
Velindre University NHS Trust, Cardiff, UK: Annabel Borley, Clare Boobier, Rachel Williams, Claire Lang, Catherine Sullivan, Anila Parveen
University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK: Jeremy Braybrooke, Alison Markham, Barbara Freitas, Carlotta Clemente, Jessica Nuttall
Guy's and St Thomas' NHS Foundation Trust: Eleni Karapanagiotou
The Christie NHS Foundation Trust: JonHowell, Sally Wood
Royal Cornwall Hospital, Truro, Cornwall, UK: Rebecca Sargent, Madalina Chifu, Duncan Wheatley, Caroline Goddard, Anne Griffiths, SallyAnne Platt, Nicholas Ashley, Anita Steele, Alistair Thomson, Charlotte Thomson
Northern Centre for Cancer Care, Freeman Hospital, Newcastle, UK: Mark Verrill
The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK: Mark Verrill, Nicola Cresti, Katy Lambert, Irena Bibby, Sue Farrell, Hannah Downs
Northern Centre for Cancer Care, Freeman Hospital, Newcastle, UK: David Goodwin, Eleanor Cameron
The Leicester Royal Infirmary: Samreen Ahmed, Vina Bhundia
Royal Liverpool University Hospital, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK: Christopher Holcombe, Margaret Tuson, Karen Janes, Laura Price, Ian Quayle
Nottingham University Hospitals NHS Trust, Nottingham, UK: Kerstie Johnson, Patricia Lawton, Shaymaa Hosni, Anjana Anand Anand, Sarah Khan, Charlotte Kamlow, Michael O'Cathail, Mayuran Sivanandan, Jun Hao Lim
Beatson West of Scotland Cancer Centre: Rosemary Stevens, Shilpa Thapar
Charing Cross Hospital -Imperial College Healthcare NHS Trust: Susan Cleator, Ogegbo, Darwisa Abdulla
Maidstone and Tunbridge wells NHS Trust: Catherina Harper Wynne, Charlotte Abson, Rema Jyothirimayi, Russell Burcombe, Lachhimi Gurung, Tracey Sage
University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK: Paula Byrne, Eve Watson, Elysia Gower
The Royal Marsden NHS Foundation Trust: Alistair Ring, Anne Barrell, Amarjit Bhambra-Mills
United States
New Hampshire Oncology Hematology PA-Hooksett: Hannah Widdison, Douglas Weckstein, Alison Fleury, Jessica Long, Sarah Diianni, Garrick Johnson
Northern Light Cancer Institute: Sarah Sinclair, Zarah Lucas
Washington University School of Medicine: Haeseong Park, Ashley Frith
Cancer Centers of Southwest Oklahoma Research: Jose Eugenio Najera, Susie Mccoy, Aman Garsa, Diandra Stewart, Manal Robin-Hanna, Sandra Fails, Than Aye, Narender Gorukanti, Nadim Nimeh, Monica Rhodes
Lowell General Hospital: Anasuya Gunturi, Melanie Edwards, Gayle Hincks, Caroline Dudman, Nancy Bettez, Stephanie Valcourt-Dexter, Murat Anamur
New England Cancer Specialists (Scarborough): Chiara Battelli
Christiana Care Health System-Christiana Hospital: Michael Guarino, Dhaval Shah, Lexington Medical Center/Lexington Oncology Associates: Steven Madden, Jennifer Peagler, Vijaya Korrapati, Shannon Hallman, Paula Cox, Kate Madden, Chelsea Stillwell, Asheesh Lal, Cindy Frick
Coborn Cancer Center at Saint Cloud Hospital: Donald Jurgens, Dahlia Elkadi, Hilary Ufearo, Stacy St Onge, Katie Greb, Stacey Nistler, Cheryl Kelley, Austin Fournier, Lesley Kurwoski, Valery Hoover, Thomas Bieniek, Hani Alkhatib, Englewood: Michael Schleider, Audrey Ades
Inova Schar Cancer Institute: Katherine Harnden, Mary Wilkinson, Leo Majarucon, Robyn Mayhew, Elizabeth Gebregeorgis, Samiha Islam, Safae Chouraichi, Joshelynn Asis, Kenyatta Scott, Hunfa Asghar, Lauren Mauro, Lubna Saqib, Angela Pennisi, Karina Castillo-Martinez, Stephanie Vanbebber, Stacey Banks, Takada Harris, Claudia Franco, Sara Fritz, Wanda Chestnut
Georgetown University Medical Center: Claudine Isaacs, Princess Alintah, Kathryn Bailey, Julie Castle, Asma Dilawari, Christopher Gallagher, Sana Khawar, Antonella Novielli, Yvonne Ottaviano, Jean Flack
Carle Cancer Center: Maria Grosse Perdekamp
Physicians' Clinic of Iowa- Hematology & Oncology: William Fusselman, Samantha Gage, Amy Ganske, Catherine Fiala, Melissa Coulter, Nicole Hoffmann, Bharat Jenigiri, Rasa Buntinas, Heidi Barnhart, Lesley Vancura, Elizabeth Heineman, Danielle Vanicek
St Luke's Hospital – Center for Cancer Care: Donald Busiek, Rebecca Whitehead, Judith Link, Julie Gill, Karen Estes, Melissa Rooney, Way Huey, David Kuperman, Monica Desai
Palo Alto Medical Foundation Health Care: Natalia Colocci, Tida Tanti, Priyamvadha Chakravarthi, Claudia Chavez, Paula Kushlan, Tanya Pozniansky, Janet Wang, Kathy Welch, Derrick Wong, Glenn Wong, Han-Hsing Wu, Michael Wu, Edith Paredes-Abrego, Helen Chen, Michele Kildare, Sandy Esqueda, Selina Perez
University of Chicago Comprehensive Cancer Center LAPS: Olwen Hahn
Duke University Medical Center: Martin Kowalsky, Kelly Westbrook, Keiana Watkins, Caroline Morales, Heather Sperling, Michelle Parks, Kate Hogan, Felecia Henson, Nancy Outzs, William Morrissey, Cheryl Maxey-Morgan, Sarah Sammons, Sherry Stewart, Carey Anders, Susan Dent, Rachel Pienknagura, Rita Deimler, Paul Marcom, Gretchen Kimmick, Jeremy Force, Jennie Petruney, Dawn Barringer, Matt Hofmeier, Laura Gorski, Rebecca Hickman
Rhode Island Hospital: Mary Anne Lopresti
Sharp Memorial Hospital: Reema Batra, Jackie Carney, Amy King, Thea Flower, Martha Garcia, Galen Steinhoff
Missouri Baptist Medical Center: Bryan Faller, Michael Bolger, Henry Robinson, Christine Joyce, Christopher Sanders, Brenda Gebhart, Atif Shafqat, Jennifer Dill, Belal Firwana, Melissa Rooney, Alan Lyss
University of New Mexico: Zoneddy Dayao, Andrea Yost, Deborah Brown, Kim Hoffman, Christopher Lee, Karwyn Gustafson, Pranshu Bansal, Malcolm Purdy, Priscilla Garcia, Ursa Brown-Glaberman, William Adler, Angela Arenas, Bruce Greenfield, Jennifer Wilson, Leslie Byatt, Bernard Tawfik, Harlie Custer, Jacklyn Nemunaitis, Steven Chavez, Milena Overby, Lizbeth Contreras, Rama Medavarapu, Fred Gentry, Monique Robertson
Monument Health Rapid City Hospital: Angie Dunbar, Jana Isbell, Joshua Lukenbill, Abdel-Ghani Azzouqa, Michael Robinson, Katheryn Arrambide
Dana-Farber/Brigham and Women's Cancer Center at Milford Regional, Dana-Farber/Brigham and Women's Cancer Center at South Shore: Erica Mayer, Christine Agius, Caroline Block, Dnielle Bowes, Craig Bunnell, Harold Burstein, Brittany Bychkovsky, Margaret Campbell, Wendy Chen, Lauren Czapla, Meredith Faggen, Judy Garber, Michael Hassett, Christina Herold, Allie Hershey, Rinath Jeselsohn, Sheheryar Kabraji, Elizabeth Kasparian, Anne Kelly, Ian Krop, PLeone, Jennifer Ligibel, Nancy Lin, Morgan Rutter, Rachel Freedman, Jennifer McKenna, Kelly O'Reilly, Beth Overmoyer, Leroy Parker, Heather Parsons, Ann Partridge, Kathleen Roche, Elahe Salehi, Rochelle Scheib, Susan Schumer, Lindsay Shaw, Sarah Tonaley, Erica Winer, Adrienne Waks, Philip Poorvu, Jolivette Ritzer, Otto Metzger, Kimberly Huff, Ryan Tamargo, Margaret Turgeon, Sharyn Kurtz, Jaclyn Lehnus, Kelly Marchetti, Daniel Morganstern, Linda Morse, Holly O'Kane, Natalie Sinclair, Jill Gormley, Amanda Livengood, Amy Carrier, Kailee Paulson, Britt Selland, Lisa Caradonna, Brittany Hill, Isaac Klein, Nikita Patel, Allison Casella, Colleen Macauley, Mona Kaddis, Emily Dinuovo, Naeem Tahir, Michael Constantine, Humberto Rossi, Alexandra Bailey, Kerry Hennessey, France Fuller, Lauren McGovern, Kathryn Pasquale, Meghan Seaward, Daniel Roberts, DojaChi, Nicle Hixon, PO'Connor, Gillian Serino, Rajitha Sunkara, John Doney, Gillian Milton, Melissa Yee, Reve Shields, Amanda Freeman, Stefani Freeman, Charles Cronis, Therese Mulvey, Katherine Harris, Steven Come, Janine Morrissey, Thao-Mary Tran, Jillian Alberti, Anne Cropp, Elleen Dunlea, Wafa Osmani, Laura Steere, Madeline Tenenbaum, Hailey Mullen, Nithya Swaminathan, Marie-Claire Remolano, Erica Porter, Jeff Kimmel, Catherine Callahan, Keith Jones, Sonia Ribeiro, Stacy Degrazia, Elaine Danielczyk, Olivia Lawton, Rebecca Modiste, Melissa Austin, Brandee Decotis, Helen Dinan, Shannon Peabody, Lorraine Haley, Saida Hussein, Rebeka Lovato, Meegan Petersen
Katmai Oncology Group: Jeanne Anderson, Shannon Smiley, Kathleen Wilsack Shue, Ellen Chirichella, Theodore Kim
Huntsman Cancer Institute/University of Utah: Adam Cohen, Anne Fitzgerald, Mei Wei, Marica Maryott, Elyse D'Astous, Saundra Buys, Anna Beck, Christos Vaklavas, Namita Chittoria, Michelae Fonger
Southern Cancer Center PC-Providence: Brian Heller, Deana Waldrup, Karla Childers, Connie Uzel, William Mcevoy, J. Reece Jones, Jeffrey George, Sherri Arledge, Nicole Angel, Michael Meshad
State University of New York Upstate Medical University: Sam Benjamin, Erinn McDowell, Melissa Reale, Abirami Sivapiragasam
Northwell Health Cancer Institute: Ruby Sharma, Shirley Lilavois, Melissa Ramgadoo, Sarah Davis, Chrystal Landry
Cone Health Cancer Center: Sherry Patterson, Vinay Gudena, Sharon Brookbank, Cameo Windham, Yan Feng, Vivian Sheidler, Nikki Eldreth, Mira Leonetti, Kaye Shoffner, Gustav Magrinat, Govinda Brahmanday, Connie Holt, Cindy Shaw, Christine Mccarty, Anne Marley, Anna Hurd, Janak Choski, Susan Cowrad
University of Illinois: Kent Hoskins, David Chan, Michael Pacini, Meredith Russell, Oana Danciu, Chiung-Mi Pan, Ayesha Zaidi
University of Oklahoma Health Sciences Center: Wajeeha Razaq, Sobia Nabeel, Erin Hartsburg, Wajeeha Razaq, Sobia Nabeel, Erin Hartsburg
Mayo Clinic Rochester: Matthew Goetz, Mitchell Perrizo, Kimberly Putz, Stacey Rud, Jean Jensen, Jackie Heim
Mercy Hospital Saint Louis: Bethany Sleckman, Alesia Bell, Carol Antinora, Gina Threlkeld, Heide Rodgers, John Finnie, Kavitha Kosuri, Laura Boekemeier, Laura Hooper, Michelle Nobs, Somasekhara Bandi, Syed Huq
Mount Sinai Medical Center: Michael Schwartz
Cancer Research for the Ozarks NCORP: Kelby Hutchings, Jay Carlson, K. Tummala, Heather White, Srikant Nannapaneni, Nicole Holman, Kristina Gardner, Michelle Baker, Roger Holden, Lavanya Tiriveedhi, Jiantao Ding, Erin Mccaig, Brooke Gillett, Robert Ellis, Lisa Bogart, Patricia Hanson, Nic Perry, Richard Schwab
UC San Diego Moores Cancer Center: Richard Schwab, Jillian Mccarthy, Haifa Mshaiel, Jay Flores, Cristal Martinez, Teresa Helsten, Sarah Boles, Barbara Parker, Angelique E. Richardson, Rebecca A. Shatsky, Millicent Abanilla, Sauntee Braddock, Katharine Nelson, Sara Neugroschl
OSF Saint Anthony Medical Center: Shylendra Sreenivasappa, Karen Blatter
FirstHealth of the Carolinas-Moore Regional Hospital: Charles Kuzma, Anne Krembel, Julie Williams, Pamela Mason, Michael Batalo, Lori DeSpain
Dartmouth Hitchcock Medical Center: Mary Chamberlin
Morton Plant Hospital: Vijaya Gadiyaram, Trang Doan
Norris Cotton Cancer Center-Nashua: Mary Chamberlin
University of Wisconsin Hospital and Clinics: Ruth O'Regan, Joel Pollen, Amy Stella, Madeline Mead, Loyda Braithwaite, Amanda Parkes, Molly Monson, Tamara Koehn, Mark Burkard, Kathryn Kernien, Kari Wisinski, Lyndsey Deverman, Caitlin Stark, Amye Tevaarwerk, Alex Dennee, Erin Clements
AdventHealth Orlando: Carlos Alemany, Darshana Ullah, Jamie Hild, Yecenia Feliz
Massachusetts General Hospital: Therese Mulvey, Meegan Petersen, Katherine Harris, Doris Stocker, Elizabeth Walsh, Hannah Lyons, Gayle Calistro, MHaley, Meredith Faggen, Nicole Hixon, Rebeka Lovato, Nicole DiRuzza, Jennifer Faig, Amanda Freeman, Jingjing Hu, Hannah Kavanaugh, Gillian Serino, Sara Cahill, P.O'Connor, Rajitha Sunkara, Magen Crepeau, Leanne Homan, Maria Shellock, Karleen Habin
Hematology Oncology Associates of Central New York-East Syracuse: Jeffrey Kirshner, Deborah Appleton, Kelly Cohn
Mayo Clinic in Arizona: Donald Northfelt, Nicholas Schroeder, Karen Anderson, Brenda Ernst, Ashley Heeney, Vicki Davis, Patricia Dyer
Roswell Park Cancer Institute: Ellis Levine, Jody Skipper, Heather Cameron, Lindsey Hacherl
Memorial Sloan-Kettering Cancer Center LAPS: Diana Lake
UCSF Helen Diller Comprehensive Cancer Center: Hope Rugo, Mark Moasser, Laura Quintal, Sarah Donahue, Michelle Melisko, John Park, Amy Jo Chien, Amy Deluca, Melanie Majure, Ivy Wong, Micah Viss, Amelia Gliwa, Gretchen Fulgencio, Melody Gawliu, Madeline Tait
Hartford Hospital: Brian Byrne
Queens Hospital Center: Margaret Kemeny
Carolinas Medical Center/Levine Cancer Institute: Geetha Vallabhaneni, Antoinette Tan, Jenna Gregory, Tia Riley, Wendy Vandermolen, Kelry Preston, Jessica Masterson
Cedars-Sinai Medical Center: Heather McArthur, Reva Basho, Alice Chung, Armando Giuliano, Philomena McAndrew, Monica Mita, Dorothy Park, Alyssa Ahorro, Tierra Manigault, Lauren Wong, Yankamma Curry, Amit Gupta, Ryan Constantino, Parisa Mirzadehgan, Suwicha Limvorasak, Ashenafi Abebe
Essentia Health Duluth Clinic: Bret Friday, Karin Bohline, Tammie Mlodozyniec, Marsha Erickson, Amy Nos
Mayo Clinic in Florida: Alvaro Moreno-Aspitia, Sara Chumsri, Paula Fuqua, Brenda Ernst, Morgan Weidner.
Ohio State University Comprehensive Cancer Center: Sagar Sardesai, Maryam Lustberg, Bhuvaneswari Ramaswamy, Raquel Reinbolt, Daniel Stover, Jeffrey VanDeusen, Robert Wesolowski, Nicole Williams, Evelyn Nguyen, Claire Limbert, Jeremy Thompson, Shayna Phillips, Anna Gackowski
Phelps County Regional Medical Center: Thomas Guerrero-Garcia, Amy Weckman, Stephen Toothaker, Tezo Keredan, Linda Schumacher, Janette Richards, Kayla Smith
MD Anderson Cancer Center: Vicente Valero
Gundersen Lutheran Medical Center: Kurt Oettel, Christine Meyer, Lori Meyer, Leah Dietrich
Marin Cancer Care: Bobbie Head, Jaime Chang, Kristin Anderson, Peter Eisenberg, Jennifer Lucas, Alex Metzger, Barbara Galligan, Melissa Chafoya
Mary Imogene Bassett Hospital: Eric Bravin, Brittany Halstead, Katelyn Tessier, Tiffany Shanker, Danielle Knight, Jeffrey Allerton, Anush Patel, Diana Gaetano, Bryan Lee, Frederika Foreman, James Leonardo, Bharatsinh Gharia, Marcy Canary
Medical Oncology and Hematology Associates-Des Moines: Seema Harichand-Herdt, Aaron Anderson, Joshua Lukenbill, Cortney Bax, Tracy Sarin, Thomas Buroker, Steven Heddinger, Robert Behrens, Margaret Wilson, Melissa Stravers, Matthew Hill, Daniel Buroker, Amy Hughes, Andrea Zarling
Wheaton Franciscan Healthcare—All Saints: Young Choi, Syed Hassan, Subarna Paul, Michelle Czechowicz, David Fucile, Sharon Olesen
Metro Minnesota Community Oncology Research Consortium: Daniel Anderson, Nicole Hoese
Anne Arundel Medical Center: Julie Oda, Jay Rhee, Stanley Watkins, Carol Tweed, Jeanine Werner, David Weng
Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins: Antonio Wolff, Judith Cunningham, Chamberlain Ruth, Ashley Bruns, Maureen Ross, Ambika Shivarajpur, Emam-Ahmed Marwa, Leisha Emens, Ben Park, Roisin Connolly, Vered Stearns, Anne Delisa, Deborah Armstrong, Ann Folmer, Amy Deery, Ar'Lena Smith, Neal Kellie, Antonio Wolff, Mary Kate Jones, Jeffrey Reynolds, Jason Freeman, Hayden Chae, Meghan Tolan, Katie Papathakis, Angellina Song, Christine Hunter, Tyrell Schaffer, Ameha Aynalem, Janice Powers, Maria Raquel Nunes, Cesar Santa-Maria, Andrew Phan, John Grouse, Lucy Chung, Kathryn Estopinal, Brittany Wood, Rita Denbow, Pauline Newman, Nelli Zafman, Kathryn Gaffney, Justin Mahosky, John Fetting, Fred Nyachieo, Danijela Jelovac, Carol Riley, Christine Collins, Catherine Bishop, Bruce Kressel, Katherine Devine
Greater Baltimore Medical Center: Melissa Loomis, Karen Oakjones-Burgess, Judy Bosley, Pamela Nickoles, Kathryn Tower, Robert Donegan, Rina Patel, Melissa Wolf, Sandra Woodring, Bishal Bista, Paul Celano
Reading Hospital/McGlinn Cancer Institute: Terrence Cescon, Janet Knoblauch, Leah Ernst, Patrick Colarusso, Nick Leasure, Barbara Miller, Nicole Agostino
Lancaster General Hospital: Elizabeth Horenkamp, Krista Budzik, Brenda Groff, Susan Tollett, Drew Mace, Shyam Balepur, Sivendran Shanthi, Randall Oyer, Raymond Powell, Matthew Brennan, Ami Jhaveri, Nandi Reddy, Jocelyn Wozney, Caitlyn Mcnaughton, Samuel Kerr, Missy Heisey, Cassie Burlingame, Kimberlee Young
Stanford Women's Cancer Center: Melinda Telli, Sharon Yuan, Sinyoung Park, Pei Jen Chang, Scott Mayeda, Diem Tran, Allison Kurian, Annabel Castaneda, Shruti Sheth, Joshua Gruber, George Sledge, Frank Stockdale, Jessica Foran, Wyatt Gross, Kaitlin Zablotsky, Kathryn Bucknell, Mehak Rahman, Amanda Selin
Ochsner Medical Center: John Cole, Chris Theodossiou, Zoe Larned
Indiana University Health Ball Memorial Hospital: Angela Ippel, Maitri Kalra, Cathleen Carlos, Rachael Maddox, Elizabeth Koselke, Stacey Cadogan, Haley Edwards, Cathy Spears, Jonathan Berkowitz
George Washington University Medical Faculty Associates: Sharad Goyal, Lauren Mauro, Holly Dushkin, Paula Brooke Burgess, Monica Rengifo-Pardo, Anonglack Vongprachith, Imad Tabbara, Robert Siegel, Arley Hunter, Maureen Mcmahon, La Keisha Queen, John Pittman, Nicole Dornbush, John Pittman, Victoria Bera, Naja Foushee, Erin Collins, Hiwot Guebre-Xabiher, Donna Embersit, Jessica Mncube, Melanie Bossi, Cynthia Parker, Anonglack (Toukie) Vongprachith, Richard Lush
WellSpan Health-York Cancer Center: Debi Oxenberg, Amir Tabatabai, Jill Messina, Joyce Huang, Jill Daugherty, Jiajoyce Conway, Christina Patterson, Diane Noll, Cindy Strobeck, Robin Miller, Dan Sotirescu, Merry-Jo Keesee, Nicole Imamovic, Erica Zahos, Joan Moore, Tonnet Fitzgerald, Brian Couch, Robert Rice, Giridhar Adiga, Ikechukwu Akunyili, Chanh Huynh
Orchard Healthcare Research Inc: Ira Oliff, Angel Sanchez-Rivera, Ofelia Hernandez, Joy Jardinico, Jessica Garcia, Adi Gidron, Kelley Kozma, Shawna Colon
Covenant Medical Center: Mohammed Masri, Mukund Nadipuram, Kimberly Maxfield, Dana Mcdougall
ThedaCare Regional Cancer Center: Rachel Kopesky, Jane Klemp, Noreen Wynn, Hanna Huss, Yao Xin, Daisy Boehm, Krystal Schepp, Crystal Guttman, Kristin Wenzel, Nicole Gunderson, Molly Schumacher, Amelia Antrim
UW Cancer Center at ProHealth Care: Amelia Antrim, Julie Bergman, Timothy Wassenaar, Tabraiz Mohammed, Christopher Hake, Rebecca Mlodzik, Julie Buska, Debra Detroye, Chanda Miller, Jonathan Kapke, Derek Serna
Tufts Medical Center: Rachel Buchsbaum, Michelle Dinh, Jaime Chisholm, John Erban, Kellie Sprague, Robin Kallfelz
CAMC Cancer Center: Steven Jubelirer, Megan Ware, Augusta Kosowicz, Cohen Justin, James Frame, Deng Zhang
Columbia Saint Mary's Water Tower Medical Commons: Ranveer Nand, Sharon Moore, Sharon Olesen, Bobby Paul, Rebecka Snyder, Peter Johnson, Kari Kruchko, Varsha Shah, Michael Keefe, Doug Puffer, Cathy Bares, Michelle Czechowicz, Crystal Stair
HSHS Saint Vincent Hospital: Burnette Brian, Callaway Warren Ruth, Cimino Teresa, Koffarnus Amy, Sweney Carol
Illinois Cancer Care, PC: Hattiangadi Trupti, Demont Schlappi Caitlin, Le-Lindqwister Nguyet, Todd-Hitchcock Ashton
Fox Chase Cancer Center: Lori Goldstein, Elias Obeid, Kathy Alpaugh, Jason Incorvati, Shauna Souchuck, Abeerah Wasti, Neha Mohan, Alexandra Hunt, Jennifer Winn, Benjamin Young, Noele Myers, Jing Chien, Sarin Chhab, Matthew Zibelman
Hematology & Oncology Associates of Northeastern PA: Liptock Kristin, Haefele Lee Ann
Beth Israel Deaconess Medical Center: Steven Come, Mary Tran, Janine Morrissey
Indiana University—Melvin and Bren Simon Cancer Center LAPS: Kathy Miller, Alesha Arnold, Alicia Hedges, Abby Koons, Angela Patterson, Bamidele Adesunloye, Lauren Roland, Carol Bain, Cathleen Carlos, Carol Lesch, Elizabeth Koselke, Emily Nelson, Hannah Meckes, Heather Wallace, Joseph Spahr, Vijaya Kakani, Tori Alston, Robert Orr, Lisa Nickol, Kayla Ackerman, Jessica Lenig, Josh Hein, Lina Sego, Lorie King, Stephanie Griebenow, Nicky Young, Elizabeth Day, Rachael Custer, Susan Hunter, Jacob Realey, Christina Glendenning, Erin Conder, Christina Cooper, Amber Whitaker, Monica Breedlove, Latrice Sterling, Shola Jhanji, Latrice Vaughn
Penn State Milton S Hershey Medical Center: Cristina Truica, Leah Cream, Monali Vasekar
Beth Israel Deaconess Hospital-Plymouth Jordan Club Cancer Center: Koomey James Matthew, Halloran Laurie, Debra White, Irina Gurevich
Hackensack University Medical Center: Stanley Waintraub, Terri Mizerak, Puneet Kaur, Melanie Marcincak, Burcu Sat, Claire Coleman, Michael Tortoriello, Greg Eskinazi, Donna Mcnamara, Deena Graham, Aretha Disalvo
Missouri Valley Cancer Consortium: Erin Smith, Jen Elmer, Kevin Sherbeck, Heather Murray-Wollen, Timothy Huyck, Khaliqullah Nasrati, Mary Beth Wilwerding, Swetha Mereddy, Kristina Benson, Gamini Soori, Steven Dunder
Laura and Isaac Perlmutter Cancer Center at NYU Langone: Yelena Novik, Kevin Simpson
NorthShore University HealthSystem-Evanston Hospital: Jamie Feld, Angeline Uy, Honey Bronson, Poornima Saha, Elaine Wade, Michele Britto, Susan Greve, Teresa Law
Cancer Research Consortium of West Michigan NCORP: Abby Dalman, Suzanne Jonas, Amy Vanderwoude, Angela Gorham, Anthony Hicks, Beth Guiles, Brooke Werlein, Connie Greene, Cori Beene, Hemasri Tokala, Jacquelyn Godley, Kathleen Yost, Marianne Melnik, Shauna Schrader, Sunil Nagpal, Thomas Gribbin, Wendi Mitchell
McFarland Clinic PC-William R Bliss Cancer Center:
Joseph Merchant, Rondi Leedom, Debra Prow, Venkatesh Rudrapatna, Erin Hackett, Catherine Lawrence, Kristi Elliott, Sreenath Kodali
Vanderbilt University/Ingram Cancer Center: Ingrid Mayer, Lynda O'Rear, Heather Cucullu, Tammy Lewis-Burgan, Ellen Heimann-Nichols, Jennifer Joyner, Andrea Koontz, Mark Miller, Terra Taylor, Freda Stevenson, Nithida Nhanthavong, Allison Walker, Ben Ho Park, Chelsea Henrichs, Richard Berry, Matthew Price, Jennifer Luther, Kathryn Williford, Kimberly Brown, Adam Stater, Brent Rexer, Elizabeth Blackmon, Leah Van Hoozer, Denise King, Jannine Hewitt, Vandana Abramson, Tatiana Frazier, Margaret Whitson, Tacora Wright
Emory University Hospital: Autumn Lunceford, Joan Kramer, Mckenzie Duncan, Florence Nwokeji, Geraldine Dodd, Sheryl Jefferson, Vanessa Falero, Ashley Trumbull, Luisa Hernandez-Encarnacion, Angela Lewis, Jane Meisel, Carolyn Simms, J. William Eley, Sarah Friend, Manali Bhave, Angie Cox, Elisavet Paplomata, La-Urshalar Brock, Zoila Freeman, Keerthi Gogineni, Chi-Cobi Speaks, Darien Middleton, Mylin Torres, Shabnam Montazeri, Suchita Pakkala, William Read, Kandra Horne, Susan Rogers, Kelly Steely, Felicia Williams, Kim Nguyen
University of South Alabama Mitchell Cancer Institute: William Russell, Sachcin Pai, Lakeyla Bates, Daniel Cameron, Wilma Baliem, Vanessa Jones, Teja Poosarla, Letoya Craig, Franciso Pamela, Payne Csierra, Rodgers Lisa, Layne Bailey, Jong Fuh Tzer, Barton Kristen, Tarver Kimberly, Blount Winona, Claudia Mccalla, Katie Ollis, Kristen Barton, Lori Russell, Grant Turner, Winona Blount, Csierra Payne, Anthony Tolbert, Megan Smith, Fuh Tzer Jong, Lisa Rodgers, Jennie Berry, Connor Best, Kimberly Tarver, Pamela Francisco
Thomas Jefferson University: Allison Zibelli, Gerome Dominguez, Courtney Porado, Melissa Furio, Hasan Alhasani, Braden Rall, Rita Murphy, Suzanne Jorfi, Rebecca Jaslow, Frederick Fellin, Melissa Furio, Daniel Silver, Hasan Alhasani, Noelle Sowers, David Britton, Maysa Abu-Khalaf
Ingalls Memorial Hospital: Danielle Sterrenberg, Kimberly Kruczek, James Wallace, Mark Kozloff, Alan Fisher, Stacy Chandler, Toya Williams, Lynne Muir, Jessica Seibert, Joy Vlamakis, Amanda Sarsfield, Robin Muhammad, Margaret Marriott
University of Pennsylvania/Abramson Cancer Center: Jane Lee, Gina Dang, Maria Snyder, Jessica Mcgraw, Stacey Calesnick, David Phuong, Heather Bergmann, Allison Tafun, Valena Davis, Arielle Swenson, Parvaiz Syed, Alexis Super, Kelly Logan, David Mintzer, Charlotte Roberts, Joanna Kosmaoglou, Marissa Ranieri, Desire Fenderson, Rhamoana Green, Marco Georeno, Lydia M Rost, Courtney Neuman, Teng (Outhayvanh) Chan, Laetitia Simeral, Patricia Locantore-Ford, Chandana Kakani, Mehrin (Mir) Masud-Elias, Arthur Staddon, Susan Kruse-Sullivan, Erinn Kober, Douglas Beach, David Henry, Brook Batzel, Sarah Kraus, Ingrid Kohut, Lee Hartner, Tyler Salovin, James Colyar, Margaret Crowley, Dorothea Taylor, Tara Perloff
University of Miami Miller School of Medicine-Sylvester Cancer Center: Carmen Calfa, Halyna Hailes, Onaidy Torres, Moreisby Izquierdo, Anna Dellarole, Donald Cantrell, Angie Denis, Daney Chica, Debora Geary, Yudeisy Mendez, Lilibet Zamora Cabezas, Reshma Mahtani, Frances Valdes-Albini, Deborah Conte, Charles Vogel, Alejandra Perez, Mauricio Escobar, Daney Chica
Hospital of the University of Pennsylvania Perelman Center for Advanced Medicine: Angela Demichele, Dusanka Lalic, Ryan Bowe, Robin Holmes, Jason Dewitt, Aileen Duffy, Susan Domchek, Catherine Rudloff, Desiree Johnson, Amy Clark, John Glick, Donna Li, Angela Bradbury, Natalya Kopylova, Payal Shah, Eric Hamscher, Kenneth Rockwell, Kevin Fox, Jessica Savage, Jennifer Matro, Susan Carney, Ayesha Fraser, Shannon Deluca
Western States Cancer Research NCORP: Sami Diab, Jessica Truong, Gwen Boone, Terah Hardcastle, Kimberly Reyna, Haley Metz, Kevin Sewell, Heather Hensley, Chanel Chan, Anthony Hopf, Nicole Steiner, Candice Fortuna, Vijitha Bonigala, Katie Hesketh, Kayla Clancy, Jessica Davis, Katherine Albert, Jennifer Buchanan, Theresa Funderburg, Samuel Shelanski, Patrick Moran, Pamela Lemme, Michele Basche, Mandy Garza, Lori Jensen, Joan Letourneau, Ginger Licht, David Andorsky, Yating Lee, Mira Morgan
Wheaton Franciscan Healthcare—Wauwatosa: Luis Hernandez, Kelly Flugaur, Betsy Karli, Francis Cuevas, Michelle Czechowicz, Terry Cannestra, Leena Varkey Maramattom, Jonathan Treisman
Beth Israel Medical Center—New York: Paula Klein, Christopher Hickson, Gargi Atul Joshi, Heather Ferreri, Indra Nandallal, Jean Hum, Kiev Gimpel-Tetra, Mohamed Shahin, Nicholas Shuman, Varun Mangela, Theresa Shao, Tiffany Xing, Takhir Mirzoyev, Stephen Malamud, Shiela Pierre, Paul Dantas, Neha Kumarley, Mary Burke, Julie Fasano, Ivy Cohen, Hanna Irie, Grace Jiang, Elizabeth Roy, Deborah Lehrer, Dana Ostrowski, Charles Shapiro, Amy Tiersten, Anupama Goel, Aarti Bhardwaj, Parita Patel
Montefiore Medical Center-Einstein Campus: Janice Simpson, Karen Fehn, Moreen Bozier, Pragna Patel, Della Makower, Prena Etchen, Kathy Lora, Robyn-Anne Chisholm, Jesus Anampa Mesias, Joseph Sparano, Sun Young Oh
Rutgers Cancer Institute of New Jersey: Nancy Chan, Yue (Joy) Wang, Shruti Desai, Nayana Patel, Coral Omene, Maryann Trotta, Deborah Toppmeyer
Nebraska Hematology and Oncology: Hutchins Mark, Kent Disney, Kristina Benson, Swetha Mereddy
Southeast Nebraska Cancer Center: Steven Dunder, Bronson Riley, Lindsay Clements, Sarah Mayo, Karri Donahue, Candice Toombs
Holy Cross Hospital: Cheryl Aylesworth, Stella Carolan, Melin Vranesic, Kennan Bradley, Lyudmila Kalnitskaya, Linda Burrell, Beatrix Lam, Stacy Tam, Erica Redmond, Ibukun Agbede, Parisa Navabi, Susie Choi, Kimberly Chen
University of Kentucky-Markey Cancer Center: Mara Chambers
Women and Infant's Hospital of Rhode Island: William Sikov, MD, JSakr
Rush University Medical Center: Melody Cobleigh
Mercy Medical Center: David Riseberg, Lisa McConnell, Alexandra Cline
DuPage Medical Group Ltd: Nafisa Burhani, Ali Lakhani, Arvind Kumar, Jennifer Crocker, Sanjiv Modi, Karen Sceniak, Jason Jung-Gon Suh, Tonya Finger, Kulumani Sivarajan, Ellen Gustafson, Worood Abboud
Avera Cancer Institute: Heidi Nickles, Melissa Hoogeveen, Jason Jones, Melissa Nietert
Kaiser Permanente Northern California: Jennifer Suga
Memorial Health University: Lester Robertson
Frederick Regional Health System James M Stockman Cancer Institute:
Hema DiMaggio, Mark Goldstein, Brianna Lucas, Patrick Mansky, Brian O'Connor, Elhamy Eskander
Peninsula Regional Medical Center: Walid El Ayass
Long Beach Memorial Medical Center-Todd Cancer Institute: Amol Rao, Kristina Alejo, Laura Macias, Javier Zuniga
Baylor College of Medicine: Mothafar Rimawi, KentOsborne, Mothaffar Rimawi, C.Pavlick, S.Coronel, R.Nangia, Valentina Hoyos, Maryam Nemati Shafaee, LizMichael, J. Ellis
Cancer Care Specialists of Central Illinois: James Wade, III
VCU Massey Cancer Center Dalton Oncology Clinic: Harry Bear
Cleveland Clinic Cancer Center Hillcrest Hospital: Jame Abraham, GBud, Aneel Chowdhary, Hamed Daw, Donald Eicher, Abdo Haddad, Kevin Kerwin, Megan Kruse, Vinit Makkar, Seema Misbah, Alberto Montero, Halle Moore, Brenna Elliott, Traci Stafford, Michelle Hartman, Alycia Gatta, Carol Chapman, Chapman, Stacey Thompson, Megan Nottingham, Janet Szucs, Nicholas Link, Edwin Soeder, Bernadette Clark, Kelly Thunberg, Jessica Zebrowski, Randall Voytilla, Kelly Fargo, Anthony Salatino, Stephanie Elsbury, Janelle Finnie, Cindy Jones, Sandra Downing, Sarah Viviani, Kate McCaffrey, Lisa Mirossay
Kaiser Permanente Irvine Medical Center: Allen I Joo, Zanmei Lopez, Sergio Hernandez
Saint Joseph Mercy Hospital: Philip Stella, Elie Dib, MD, Li Ding, MD
Harry and Jeanette Weinberg Cancer Center: Yvonne Ottaviano
MedStar Good Samaritan Hospital: Barbara Rector, Mahsa Mohebtash, Lois Kamugisha, Corinne Denney, Jae Lee
Advocate Lutheran General Hospital: Sherri Velez, Jackie Kessler, Yelizaveta Yanovskaya, Sigrun Hallmeyer
Norton Cancer Institute—Brownsboro: Joey Quesenberry, Laila Agrawal, Sandy Stencavage, Jeffrey Hargis
Providence Cancer Center Oncology & Hematology Care: Katherine Conlin, Marie Moxon, Lee Mellinger
Magee-Womens Hospital of UPMC: Adam Brufsky
Allegheny General Hospital: Jane Raymond, Ashley Young, Diane Buchbarker, Erin Baldauf, Thomas Julian, Sarah Miller, Christie Hilton, Donna Creese, Maricel Castaner, Helen Analo, Larisa Greenberg, Samantha Cavolo, Casey Moffa, Elizabeth Masteller, Noelle Brunsing, Sara Ohm
Houston Methodist: Charles Geyer, Polly Niravath, MD, Lacey Burey
Maryland Oncology Hematology: Carolyn Hendricks
University of Iowa Hospitals and Clinic: Mark Karwal
University of Florida Health Cancer Center at Orlando Health: Nikita Shah, Maria Jarquin-Castillo, Gillian Jobson, Beth Vance
CCS Associates, Inc (CCSA): Donya Bagheri, Kisha Thomas, Robin Bravo, Shukurat Sulaiman, Crystal Oparah, Mark Rogers, Rachael Motoma, Kay Salinas, Rebekah Castillo, Renee Henderson, Maria Bagoly, Sharanya Ramasubramanian, Susan Carlson, Sandra Hotchkiss, Anni Ngo
PrECOG, LLC (PreCOG): Peter O'Dwyer, Donna Marinucci, Sherri Janousky, Lisa Johnson, Karen Padilla, Soumya Chappidi, Nancy Cooper, Gary Chan, Christine Mullin, Fernanda Almestica, Maria de Alvare, Helen Alexander
The NSABP Foundation, Inc (NSABP): Gloria Gero, CCRC, Linda Robitaille, BScN, Pam Williams, RN MSN, CCRP, Anita Hades, RN, Bridget Dimitt, Senior CRA, Donna Houpt, RN, CCRC, Jordan Brenner, Aaron Forbes, Tracy Murphy, Gerrie Nagy
Alliance Foundation Trials, LLC (AFT): M. Bertagnolli, MD, Otto Metzger, MD, Trinidad Ajazi, Sheilah Hurley, Carter DuFrane, Kadine Solomon, Majagamy Ramos-Bordonaro, MSRA, CCRP, Jennifer Wang, BA, BS, Silvy Saltzman, Jhansi Yerrabelli, Stephanie Moine, MPH, Shruti Singh, MBS, Kelsey Norris
Alliance Foundation Trials—SDC at Mayo Clinic Rochester: Scott Wickman, MBA, PMP, Michelle M. Mahoney, MS, MBA, Erin Green, MBA, David Zahrieh, PhD, Sumithra J. Mandrekar, PhD, Zack Coffey, Aaron Johnson, Lori Vine, Alex Zoroufy, Leigh Gomez-Dahl, Carla Hilton, Kathleen S. Tenner, Erica N. Heying, Marcia Wilson, Jane Emerson, Katie Allen-Zeigler, MBA, Garth Nelson, MS, Kathy Welsh, Allison Booth, Kristie Stolzenberg, Sophie Fangel, Patrick O'Brien
Alliance Foundation Trials—Biorepository at Washington University: Mark Watson MD, PhD, Patsy Alldredge, Jacqueline Snider
*Deceased
Michael Gnant
Employment: Sandoz (I)
Honoraria: Amgen, Novartis, AstraZeneca, Lilly
Consulting or Advisory Role: Daiichi Sankyo, Veracyte, Tolmar¸ LifeBrain, Lilly
Amylou C. Dueck
Patents, Royalties, Other Intellectual Property: Royalties from licensing fees for a patient symptom questionnaire (MPN-SAF)
Miguel Martin
Honoraria: Roche/Genentech, Lilly, Pfizer, Novartis, Pierre Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, Taiho Pharmaceutical, PharmaMar
Speakers' Bureau: Lilly/ImClone, Roche/Genentech, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst)¸ Puma Biotechnology (Inst)
Other Relationship: Roche, Novartis
Hal J. Burstein
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD¸ Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Antonio C. Wolff
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: A.C.W. has been named as an inventor on one or more issued patents or pending patent applications related to methylation in breast cancer, has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Arlene Chan
Honoraria: Novartis, Lilly
Consulting or Advisory Role: Lilly
Research Funding: Eisai
Eric P. Winer
Honoraria: Genomic Health, Genentech/Roche
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Georg Pfeiler
Honoraria: Amgen, Roche/Genentech, Pfizer¸ Lilly, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, Novartis, UCB, Accord Healthcare
Consulting or Advisory Role: Pfizer¸ Amgen¸ Lilly, Novartis, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, UCB, Roche/Genentech
Speakers' Bureau: Roche/Genentech, Pfizer, Lilly, Novartis, UCB, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, Accord Healthcare, Amgen, Accord Healthcare
Research Funding: Pfizer, Roche/Genentech
Travel, Accommodations, Expenses: Novartis, Lilly, Roche/Genentech, Lilly, AstraZeneca/Daiichi Sankyo
Kathy Miller
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Seattle Genetics (Inst), Pfizer (Inst), Astex Pharmaceuticals (Inst), British Biotech (Inst), CytomX Therapeutics (Inst), Alphamab (Inst)
Marco Colleoni
Research Funding: Roche (Inst)
Gabor Rubovsky
Consulting or Advisory Role: Pfizer, Novartis, Roche, Gilead Sciences
Travel, Accommodations, Expenses: Amgen
Judith M. Bliss
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Puma Biotechnology (Inst), Pfizer (Inst), Roche (Inst), GlaxoSmithKline/Novartis (Inst), Lilly (Inst), Janssen-Cilag (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Ingrid A. Mayer
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, Genentech, GlaxoSmithKline, Immunomedics, MacroGenics, Pfizer, AbbVie, Seattle Genetics, Puma Biotechnology, Cyclacel, Blueprint Medicines, Sanofi
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst)
Christian Singer
Honoraria: Novartis, AstraZeneca/MedImmune, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: AstraZeneca/MedImmune, Daiichi-Sankyo, Gilead Sciences, Sanofi/Aventis, Novartis
Speakers' Bureau: Novartis, AstraZeneca/MedImmune
Research Funding: Novartis, Sanofi, Myriad Genetics, Roche, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Novartis
Zbigniew Nowecki
Travel, Accommodations, Expenses: Roche/Genentech
Olwen Hahn
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical
Honoraria: Cardinal Health (I)
Consulting or Advisory Role: Pfizer, hmpglobal.com
Travel, Accommodations, Expenses: Cardinal Health (I)
Jacqui Thomson
Honoraria: MSD Oncology, Roche/Genentech
Norman Wolmark
Open Payments Link: https://openpaymentsdata.cms.gov/physician/159597
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan
Consulting or Advisory Role: Samsung
Research Funding: MacroGenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Mylan, AstraZeneca Spain, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/summary
Guenther G. Steger
Honoraria: Pfizer, Lilly, Novartis, Roche, AstraZeneca/Daiichi Sankyo, Teva, Pfizer, Lilly, Novartis
Travel, Accommodations, Expenses: Roche, Teva
Blanca Hernando Fernández de Aránguiz
Honoraria: GlaxoSmithKline, PharmaMar, Clovis Oncology
Travel, Accommodations, Expenses: Pfizer, MSD Oncology, Roche
Tufia C. Haddad
Research Funding: Takeda (Inst)
Meritxell Bellet Ezquerra
Consulting or Advisory Role: Pfizer, Lilly, Novartis
Speakers' Bureau: Lilly, Pfizer, Novartis
Hannes Fohler
Employment: Takeda (I)
Research Funding: Pfizer (Inst)
Otto Metzger
Honoraria: Grupo Oncoclinicas, Roche
Research Funding: Susan G. Komen for the Cure (Inst), Pfizer (Inst), Roche/Genentech (Inst), Eisai (Inst), Cascadian Therapeutics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Grupo Oncoclinicas
Anita Jallitsch-Halper
Research Funding: Pfizer (Inst)
Kadine Solomon
Employment: Alliance Foundation Trials
Stock and Other Ownership Interests: Pfizer, Merck, Moderna Therapeutics
Céline Schurmans
Research Funding: Pfizer (Inst), Roche/Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Servier (Inst), Pfizer (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: My organization receives royalties from Agendia
Kathy P. Theall
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Travel, Accommodations, Expenses: Pfizer
Dongrui R. Lu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Kathleen Tenner
Stock and Other Ownership Interests: Merck
Christian Fesl
Research Funding: Pfizer (Inst)
Angela DeMichele
Research Funding: Pfizer (Inst), Genentech (Inst), Calithera Biosciences (Inst), Novartis (Inst)
Erica L. Mayer
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Gilead Sciences
Research Funding: Pfizer (Inst)
No other potential conflicts of interest were reported.
Listen to the podcast at jcopodcast.libsynpro.com
PRIOR PRESENTATION
Presented in part at SABCS 2021, December 7-10, 2021, San Antonio, TX. Preliminary data were presented in part at European Society of Medical Oncology 2020, September 19-21, 2020, Madrid, Spain and at SABCS 2020, December 8-11, 2020, San Antonio, TX.
SUPPORT
The academic PALLAS trial is legally cosponsored by the Austrian Breast and Colorectal Cancer Study Group (https://www.abcsg.com) and the Alliance Foundation (https://acknowledgments.alliancefound.org), in collaboration with the Eastern Cooperative Oncology Group, the NSABP Foundation, Inc, the German Breast Group, and the Breast International Group. The trial was funded by Pfizer, who provided study drug and financial support. In addition, the academic organizations ABCSG and AFT supported the trial by providing human resources.
CLINICAL TRIAL INFORMATION
A.D. and E.L.M. are shared last authors.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02554.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Gnant, Amylou C. Dueck, Sophie Frantal, Hal J. Burstein, Richard Greil, Antonio C. Wolff, Eric P. Winer, Georg Pfeiler, Kathy Miller, Hannes Fohler, Otto Metzger, Anita Jallitsch-Halper, Christian Fesl, Angela DeMichele, Erica L. Mayer
Administrative support: Michael Gnant, Hal J. Burstein, Richard Greil, Georg Pfeiler, Norman Wolmark, Hannes Fohler, Otto Metzger, Kadine Solomon, Céline Schurmans
Provision of study materials or patients: Michael Gnant, Miguel Martin, Hal J. Burstein, Richard Greil, Arlene Chan, Georg Pfeiler, Kathy Miller, Ingrid A. Mayer, Christian Singer, Jacqui Thomson, Hope S. Rugo, Guenther G. Steger, Tufia C. Haddad, Antonia Perelló Martorell, Meritxell Bellet Ezquerra, Otto Metzger, Céline Schurmans, Angela DeMichele, Erica L. Mayer
Collection and assembly of data: Michael Gnant, Amylou C. Dueck, Sophie Frantal, Miguel Martin, Hal J. Burstein, Richard Greil, Peter Fox, Antonio C. Wolff, Arlene Chan, Georg Pfeiler, Kathy Miller, Marco Colleoni, Jennifer M. Suga, Gabor Rubovsky, Ingrid A. Mayer, Christian Singer, Zbigniew Nowecki, Olwen Hahn, Jacqui Thomson, Norman Wolmark, Kepa Amillano, Guenther G. Steger, Blanca Hernando Fernández de Aránguiz, Antonia Perelló Martorell, Meritxell Bellet Ezquerra, Hannes Fohler, Otto Metzger, Kadine Solomon, Céline Schurmans, Kathleen Tenner, Christian Fesl, Angela DeMichele, Erica L. Mayer
Data analysis and interpretation: Michael Gnant, Amylou C. Dueck, Sophie Frantal, Miguel Martin, Hal J. Burstein, Richard Greil, Peter Fox, Antonio C. Wolff, Arlene Chan, Georg Pfeiler, Kathy Miller, Judith M. Bliss, Ingrid A. Mayer, Christian Singer, Hope S. Rugo, Guenther G. Steger, Tufia C. Haddad, Otto Metzger, Kathy P. Theall, Dongrui R. Lu, Christian Fesl, Angela DeMichele, Erica L. Mayer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael Gnant
Employment: Sandoz (I)
Honoraria: Amgen, Novartis, AstraZeneca, Lilly
Consulting or Advisory Role: Daiichi Sankyo, Veracyte, Tolmar¸ LifeBrain, Lilly
Amylou C. Dueck
Patents, Royalties, Other Intellectual Property: Royalties from licensing fees for a patient symptom questionnaire (MPN-SAF)
Miguel Martin
Honoraria: Roche/Genentech, Lilly, Pfizer, Novartis, Pierre Fabre
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, AstraZeneca, Taiho Pharmaceutical, PharmaMar
Speakers' Bureau: Lilly/ImClone, Roche/Genentech, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst)¸ Puma Biotechnology (Inst)
Other Relationship: Roche, Novartis
Hal J. Burstein
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD¸ Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Antonio C. Wolff
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Ionis Pharmaceuticals
Patents, Royalties, Other Intellectual Property: A.C.W. has been named as an inventor on one or more issued patents or pending patent applications related to methylation in breast cancer, has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Arlene Chan
Honoraria: Novartis, Lilly
Consulting or Advisory Role: Lilly
Research Funding: Eisai
Eric P. Winer
Honoraria: Genomic Health, Genentech/Roche
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Georg Pfeiler
Honoraria: Amgen, Roche/Genentech, Pfizer¸ Lilly, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, Novartis, UCB, Accord Healthcare
Consulting or Advisory Role: Pfizer¸ Amgen¸ Lilly, Novartis, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, UCB, Roche/Genentech
Speakers' Bureau: Roche/Genentech, Pfizer, Lilly, Novartis, UCB, AstraZeneca/Merck, AstraZeneca/Daiichi Sankyo, Accord Healthcare, Amgen, Accord Healthcare
Research Funding: Pfizer, Roche/Genentech
Travel, Accommodations, Expenses: Novartis, Lilly, Roche/Genentech, Lilly, AstraZeneca/Daiichi Sankyo
Kathy Miller
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Genentech/Roche, Athenex, AstraZeneca, Bristol Myers Squibb/Celgene
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Seattle Genetics (Inst), Pfizer (Inst), Astex Pharmaceuticals (Inst), British Biotech (Inst), CytomX Therapeutics (Inst), Alphamab (Inst)
Marco Colleoni
Research Funding: Roche (Inst)
Gabor Rubovsky
Consulting or Advisory Role: Pfizer, Novartis, Roche, Gilead Sciences
Travel, Accommodations, Expenses: Amgen
Judith M. Bliss
Research Funding: AstraZeneca (Inst), Merck Sharp & Dohme (Inst), Puma Biotechnology (Inst), Pfizer (Inst), Roche (Inst), GlaxoSmithKline/Novartis (Inst), Lilly (Inst), Janssen-Cilag (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Pfizer
Ingrid A. Mayer
Consulting or Advisory Role: Novartis, AstraZeneca, Lilly, Genentech, GlaxoSmithKline, Immunomedics, MacroGenics, Pfizer, AbbVie, Seattle Genetics, Puma Biotechnology, Cyclacel, Blueprint Medicines, Sanofi
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst)
Christian Singer
Honoraria: Novartis, AstraZeneca/MedImmune, Daiichi Sankyo Europe GmbH
Consulting or Advisory Role: AstraZeneca/MedImmune, Daiichi-Sankyo, Gilead Sciences, Sanofi/Aventis, Novartis
Speakers' Bureau: Novartis, AstraZeneca/MedImmune
Research Funding: Novartis, Sanofi, Myriad Genetics, Roche, AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Roche, Novartis
Zbigniew Nowecki
Travel, Accommodations, Expenses: Roche/Genentech
Olwen Hahn
Leadership: Via Oncology
Stock and Other Ownership Interests: Teleflex Medical
Honoraria: Cardinal Health (I)
Consulting or Advisory Role: Pfizer, hmpglobal.com
Travel, Accommodations, Expenses: Cardinal Health (I)
Jacqui Thomson
Honoraria: MSD Oncology, Roche/Genentech
Norman Wolmark
Open Payments Link: https://openpaymentsdata.cms.gov/physician/159597
Hope S. Rugo
Honoraria: Puma Biotechnology, Mylan
Consulting or Advisory Role: Samsung
Research Funding: MacroGenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst), Sermonix Pharmaceuticals (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Mylan, AstraZeneca Spain, Merck
Open Payments Link: https://openpaymentsdata.cms.gov/summary
Guenther G. Steger
Honoraria: Pfizer, Lilly, Novartis, Roche, AstraZeneca/Daiichi Sankyo, Teva, Pfizer, Lilly, Novartis
Travel, Accommodations, Expenses: Roche, Teva
Blanca Hernando Fernández de Aránguiz
Honoraria: GlaxoSmithKline, PharmaMar, Clovis Oncology
Travel, Accommodations, Expenses: Pfizer, MSD Oncology, Roche
Tufia C. Haddad
Research Funding: Takeda (Inst)
Meritxell Bellet Ezquerra
Consulting or Advisory Role: Pfizer, Lilly, Novartis
Speakers' Bureau: Lilly, Pfizer, Novartis
Hannes Fohler
Employment: Takeda (I)
Research Funding: Pfizer (Inst)
Otto Metzger
Honoraria: Grupo Oncoclinicas, Roche
Research Funding: Susan G. Komen for the Cure (Inst), Pfizer (Inst), Roche/Genentech (Inst), Eisai (Inst), Cascadian Therapeutics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Grupo Oncoclinicas
Anita Jallitsch-Halper
Research Funding: Pfizer (Inst)
Kadine Solomon
Employment: Alliance Foundation Trials
Stock and Other Ownership Interests: Pfizer, Merck, Moderna Therapeutics
Céline Schurmans
Research Funding: Pfizer (Inst), Roche/Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Servier (Inst), Pfizer (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: My organization receives royalties from Agendia
Kathy P. Theall
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Honoraria: Pfizer
Travel, Accommodations, Expenses: Pfizer
Dongrui R. Lu
Employment: Pfizer
Stock and Other Ownership Interests: Pfizer
Kathleen Tenner
Stock and Other Ownership Interests: Merck
Christian Fesl
Research Funding: Pfizer (Inst)
Angela DeMichele
Research Funding: Pfizer (Inst), Genentech (Inst), Calithera Biosciences (Inst), Novartis (Inst)
Erica L. Mayer
Consulting or Advisory Role: Lilly, Novartis, AstraZeneca, Gilead Sciences
Research Funding: Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yi M, Li T, Niu M, et al. : Epidemiological trends of women's cancers from 1990 to 2019 at the global, regional, and national levels: A population-based study. Biomark Res 9:1-12, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dafni U, Tsouri Z, Alatsathianos B: Breast cancer in the European Union: Incidence and survival across European countries. Breast Care (Basel) 14:344-353, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbeck N, Gnant M: Breast cancer. Lancet 389:1134-1150, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Curigliano G, Thürlimann B, et al. : Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32:1216-1236, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ: Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med 383:2557-2570, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ: A new cell-cycle target in cancer—Inhibiting cyclin D–dependent kinases 4 and 6. N Engl J Med 375:1920-1923, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Crown JP, Lang I, et al. : The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol 16:25-35, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Hortobagyi GN, Stemmer SM, Burris HA, et al. : Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738-1748, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Sledge GW Jr, Toi M, Neven P, et al. : MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR1/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875-2884, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Martin M, Rugo HS, et al. : Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925-1936, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Turner NC, Ro J, André F, et al. : Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373:209-219, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Turner NC, Slamon DJ, Ro J, et al. : Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379:1926-1936, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Finn RS, Rugo HS, Gelmon KA, et al. : Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Updated analysis with up to 5Yearsof follow -up. Oncologist 26:e74955, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer EL, DeMichele A, Rugo HS, et al. : A phase II feasibility study of palbociclib in combination with adjuvant endocrine therapy for hormone receptor-positive invasive breast carcinoma. Ann Oncol 30:1514-1520, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Kishino E, Ogata R, Saitoh W, et al. : Anti-cell growth and anti-cancer stem cell activity of the CDK4/6 inhibitor palbociclib in breast cancer cells. Breast Cancer 27:415-425, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Mayer EL, Dueck AC, Martin M, et al. : Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomized, phase 3 study. Lancet Oncol 22:212-222, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA, Barlow WE, Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol 25:2127-2132, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Tolaney SM, Garrett-Mayer E, White J, et al. : Updated standardized definitions for efficacy end points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J Clin Oncol 39:2720-2731, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slamon DJ, Neven P, Chia S, et al. : Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465-2472, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Johnston S, Martin M, Di Leo A, et al. : MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5:1-8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathy D, Im SA, Colleoni M, et al. : Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol 19:904-915, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Im SA, Lu YS, Bardia A, et al. : Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381:307-316, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Slamon DJ, Neven P, Chia S, et al. : Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann Oncol 32:1015-1024, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Sledge GW Jr, Toi M, Neven P, et al. : The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol 6:116-124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slamon DJ, Neven P, Chia S, et al. : Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 382:514-524, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Darden C, Mitra D, McSorley D, et al. : Treatment satisfaction in women receiving palbociclib combination therapies for advanced/metastatic breast cancer. Future Oncol 15:141-150, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Mayer EL, Fesl C, Dueck A, et al. : Treatment exposure and discontinuation in the PALLAS trial: PALbociclib CoLlaborative adjuvant study of palbociclib with adjuvant endocrine therapy for HR+/HER2- early breast cancer. Cancer Res 81, 2021 (suppl; abstr PD2-03)
- 28.Loibl S, Marme F, Martin M, et al. : Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the penelope-B trial. J Clin Oncol 39:1518-1530, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Johnston SRD, Harbeck N, Hegg R, et al. : Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 38:3987-3998, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbeck N, Rastogi P, Martin M, et al. : Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 32:1571-1581, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Petrelli F, Ghidini A, Pedersini R, et al. : Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: An adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat 174:597-604, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Wells CI, Vasta JD, Corona CR, et al. : Quantifying CDK inhibitor selectivity in live cells. Nat Commun 11:1-11, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George MA, Qureshi S, Omene C, et al. : Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol 11:2471, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serra F, Lapidari P, Quaquarini E, et al. : Palbociclib in metastatic breast cancer: Current evidence and real-life data. Drugs Context 8:212579, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiLeo A, O’Shaugnessy J, Sledge GW, et al. : Prognostic characteristics in hormone receptor-positive advanced breast cancer and characterization of abemaciclib efficacy. NPJ Breast Cancer 4:1-8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma CX, Gao F, Luo J, et al. : NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 23:4055-4065, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prat A, Saura C, Pascual T, et al. : Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomized, phase 2 trial. Lancet Oncol 21:33-43, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Wagner V, Gil J: Senescence as a therapeutically relevant response to CDK4/6 inhibitors. Oncogene 39:5165-5176, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris-Hanon O, Marazita MC, Romorini L, et al. : Palbociclib effectively halts proliferation but Fails to induce senescence in patient-derived glioma stem cells. Mol Neurobiol 56:7810-7821, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Ono M, Oba T, Shibata T, et al. : The mechanisms involved in the resistance of estrogen receptor-positive breast cancer cells to palbociclib are multiple and change over time. J Cancer Res Clin Oncol 147:3211-3224, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera-Abreu MT, Palafox M, Asghar U, et al. : Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res 76:2301-2313, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurvitz SA, Martin M, Press MF, et al. : Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR(+)/HER2(-) breast cancer. Clin Cancer Res 26:566-580, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldner M, Pandolfi N, Maciel D, et al. : Combined endocrine and targeted therapy in luminal breast cancer. Expert Rev Anticancer Ther 21:1237-1251, 2021 [DOI] [PubMed] [Google Scholar]
- 44.Servetto A, Napolitano F, De Angelis C, et al. : A review of the use of next generation sequencing methodologies to identify biomarkers of resistance to CDK4/6 inhibitors in ER+/HER2- breast cancer. Crit Rev Oncol Hematol 157:103191, 2021 [DOI] [PubMed] [Google Scholar]
- 45.O’Leary B, Cutts RJ, Liu Y, et al. : The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 8:1390-1403, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey C, Black JRM, Reading JL, et al. : Tracking cancer evolution through the disease course. Cancer Discov 11:916-932, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.02554.