Abstract
INTRODUCTION:
Periodontal disease is associated with nonalcoholic fatty liver disease (NAFLD). We evaluated periodontal treatment efficacy in patients with NAFLD and periodontal disease.
METHODS:
This multicenter, 2-arm, randomized study recruited adult patients with NAFLD and periodontitis, alanine aminotransferase levels ≥40 U/L, and equivalent steatosis grade ≥1. Forty eligible patients (18 men and 22 women) were randomly assigned to 2 groups (scaling and root planning [SRP; n = 20] and tooth brushing [n = 20] groups) stratified by age and sex. The primary and secondary endpoints were changes in alanine aminotransferase levels and serum Porphyromonas gingivalis IgG antibody titers from baseline to 12 weeks, respectively. Efficacy analysis was performed using an intention-to-treat approach (t test). This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000022079).
RESULTS:
We observed a significantly higher decrease in absolute alanine aminotransferase levels and P. gingivalis IgG antibody titers in the SRP group than in the tooth brushing group (−12 vs 1 U/L; mean difference [δ], −12; 95% confidence interval [CI], −20 to −5; P = 0.002). The decrease in P. gingivalis IgG antibody titer was significantly higher in the SRP group than in the tooth brushing group (FDC381, −1.6 [2.5]; δ, −1.6; 95% CI, −2.7 to −0.4; P = 0.0092; SU63, −1.7 [2.0]; δ, −1.7; 95% CI, −2.7 to −0.7). No life-threatening events or treatment-related deaths occurred.
DISCUSSION:
Periodontal treatment induced significant short-term and mid-term reductions in liver enzyme levels and antibody titers. Further research is warranted to clearly define SRP efficacy and tolerability in patients with NAFLD and periodontitis.
INTRODUCTION
Fatty liver disease in people who consume limited or no alcohol (≤30 and ≤20 g/d of ethanol in men and women, respectively) is defined as nonalcoholic fatty liver disease (NAFLD) and includes nonalcoholic steatohepatitis (NASH). NASH plays a role in increasing the global prevalence of chronic liver disease and is associated with an increased incidence of hepatocellular carcinoma and liver-related mortality (1). In addition, NASH progresses to cirrhosis in 15%–20% of cases and is a rapidly growing indication for liver transplantation. NAFLD is a pandemic with an estimated prevalence of 24%, and cases of the resultant liver cirrhosis and liver malignancy have been rapidly proliferating in recent years (2). NASH is frequently associated with a remarkable increase in serum alanine aminotransferase (ALT) levels, small intestinal bacterial overgrowth, and impaired quality of life (3–5). However, the treatment response is low in stereotypical trials for NASH; therefore, more treatment options to prevent fatty liver disease advancement are needed.
Periodontitis is an infectious disease of the gingiva and periodontal tissues and induces tooth loss due to periodontal apparatus destruction. The prevalence of periodontitis is >47% in adults in the United States (6). More than 700 bacterial species or strains have been detected in the oral cavity (7). Some species such as Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans are involved in periodontitis progression (8,9). Among them, P. gingivalis—a Gram-negative anaerobic bacterium—exerts a predominant influence in periodontal disease progression and bone and tissue destruction (10,11). The lipopolysaccharide (LPS) cell wall integrant of P. gingivalis is a pathogenic factor that causes a wide range of host responses, including the production of proinflammatory cytokines, anti-inflammatory cytokines, and chemokines (12). These cytokines and inflammatory mediators are important in periodontitis progression because host immune and inflammatory responses trigger periodontal tissue destruction under the influence of multiple behavioral, environmental, and genetic factors (13).
In recent years, several researchers have reported a relationship between NAFLD and periodontal disease (14,15). Yoneda et al. (16) reported a significantly higher detection frequency of P. gingivalis in the saliva of patients with NAFLD and NASH than in control participants. Furthermore, they found that a 3-month nonsurgical periodontal treatment (tooth brushing instructions, scaling and root planing [SRP], and minocycline hydrochloride application) in 10 patients with NAFLD improved liver function parameters such as serum levels of aspartate aminotransferase (AST) and ALT. Therefore, infection with periodontal pathogens, primarily P. gingivalis, may be associated with fibrosis severity in patients with NAFLD. The prevention or elimination of P. gingivalis infection using periodontal treatment has a beneficial effect on the management of NAFLD.
Consequently, we aimed to evaluate the efficacy of periodontal treatment in NAFLD patients with periodontal disease. We hypothesized that periodontal treatment of oral infections, including P. gingivalis infection, in these patients would normalize the levels of NAFLD-related clinical markers (see Supplementary Figure 1, Supplementary Digital Content 5, http://links.lww.com/CTG/A865, which outlines the study hypothesis of periodontal treatment for patients with NAFLD by periodontal endotoxin suppression; and see Supplementary Figure 2, Supplementary Digital Content 6, http://links.lww.com/CTG/A866, which shows the relative hepatic fat mass decrease and hepatic fibrosis improvement assessed by magnetic resonance imaging-proton density fat fractionation).
METHODS
Study design and patients
The periodontal treatment in NAFLD trial, also known as the PERION trial, was designed as a prospective, multicenter, 2-arm, randomized comparison study to test the efficacy of a 12-week SRP vs a tooth brushing (no SRP) treatment in patients with NAFLD and moderate periodontitis. Between August 2015 and March 2020, 164 patients (mean age, 57 years; men/women, 92/72) were screened for study inclusion. Finally, 40 adults, in whom the efficacy and safety of periodontal treatment were evaluated for 60 weeks, were included. On August 21, 2015—before research initiation—the Institutional Review Board of Kanagawa Dental University Hospital approved the supporting data associated with the study (approval no. 480). Patients with NAFLD and periodontitis were enrolled at Kanagawa Dental University Yokohama Clinic, Kanagawa Dental University, Iwasaki Internal Medicine Clinic, and the Yokohama City University medical institution in Japan. The research protocol complied with the principles of the Declaration of Helsinki and the Ethics Guidelines for Clinical Research produced by the Ministry of Health, Labour and Welfare. All participants provided written informed consent before to their participation in the study. See Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A861, for patient inclusion and exclusion criteria.
Randomization and masking
We randomly assigned eligible patients to the SRP or no SRP group. Randomization was performed using a computer-generated centrally administered procedure and a permuted block method (block size: 4); the permuted block was stratified by age (65 years or older/younger than 65 years) and sex (male/female). Moreover, randomization was performed using a validated allocation system (Densuke Systems, Tokyo, Japan). Masking was not performed because of the open-label nature of the study.
Endpoints
The primary endpoint was the absolute change in the ALT level from baseline after the 12-week SRP treatment. See Supplementary Table 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A862, which presents the secondary and tertiary endpoints.
Assessment of liver stiffness and steatosis
Ultrasonic diagnosis of fatty liver was performed in B mode first, presenting with bright liver, hepatorenal echo contrast, attenuation, and vascular blurring of the intrahepatic portal vein and hepatic venous branch. The diagnostic tools, i.e., vibration-controlled transient elastography and magnetic resonance elastography (MRE), were then used to confirm these qualitative findings. All patients (40 adults) underwent vibration-controlled transient elastography and MRE at baseline and at 12 and 60 weeks.
Statistical analyses
A previous pilot study showed that nonsurgical periodontal treatments administered to 10 patients with NAFLD for 3 months ameliorated their liver function parameters, such as serum levels of AST and ALT; in that study, a mean ALT change of −25.0 (IU/L), with an SD of 25, was observed (16). Based on these data, the sample size was determined to guarantee the power of the analysis of variance F test. We predicted the average changes in ALT levels in the SRP and tooth brushing groups to be −25 U/L and 0, respectively, with a common SD of 25. Hence, the minimum required number of patients per group (using a power of 80% and a bilateral significance level of 5%) was computed to be 32. However, we recruited 40 patients to compensate for potential study dropouts.
Intention-to-treat (ITT) (patients who underwent randomization) and per-protocol (patients without protocol deviations) populations were used to assess the primary endpoint. The primary efficacy analysis was performed on the ITT population. Summary statistics (mean ± SD) were computed for ALT levels at baseline and at 12 weeks. In the primary analysis, differences between the baseline and posttreatment ALT levels were determined for each participant and compared using the Wilcoxon signed-rank test. The significance level was 5% on both sides. In addition to P values, we provided point estimates with 95% confidence intervals (CIs). For sensitivity analysis, the 2 groups were compared for the primary endpoints using analysis of covariance, adjusting for baseline ALT values as a covariate. Secondary and tertiary endpoints were analyzed using the same approach as for primary endpoints. Moreover, we computed the mean difference (δ) and 95% CI between groups for factors that were determined to have significant changes by t test analysis. The Student t test was used to compare endpoints between the 2 groups. Continuous variables were summarized at each time point, and the SRP and no-SRP groups were compared using the same procedure as that for the primary endpoints. All analyses were performed using SPSS (version 26, IBM, Armonk, NY) and SAS (version 9.4, SAS Institute, Cary, NC) software.
Concomitant treatment
Thiazolidinedione and bariatric surgery were prohibited from the time of screening to the end of follow-up. Combined oral and injectable antibiotics were prohibited from 3 months before screening to the end of the study. However, a 5-day course of oral antibiotics only was permitted from before the screening to the end of the study. The following restricted concomitant medications were permitted only if they were continuously used with a stable dose and regimen from 12 weeks before screening and kept stable until the end of the study: antihypertensive drugs (angiotensin II receptor blockers only); vitamin E; antidyslipidemic drugs; and antidiabetic drugs (dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 receptor agonists).
RESULTS
The 40 eligible patients (mean age, 58 years; men: 18, women: 22) were randomly assigned to the SRP (n = 20) and no-SRP (n = 20) groups and were included in the efficacy and safety analyses (Figure 1). The 2 groups had similar baseline demographic and clinical characteristics (Table 1). There were no significant differences in liver stiffness between the 2 groups (Table 1). Included patients had no ongoing periodontal treatment before SRP and no antibiotic use. By assessing the periodontal pocket depth (PPD) (mean [SD]: SRP, 2.8 [0.5]; no SRP, 2.8 [0.5]) and bleeding on probing (SRP, 37 [32]; no SRP, 42 [51]), both study groups were shown to have periodontal disease–induced inflammation sites (Table 1). Although there were no changes in body weight and body mass index at 12 weeks after baseline, the ITT population in the SRP group showed a larger decrease in absolute ALT levels (−12 [15], δ = −12, 95% CI: −20 to −5, P = 0.002) than those in the no-SRP group (Table 2). The decrease in P. gingivalis IgG antibody titer was significantly higher in the SRP group (FDC381; −1.6 [2.5], δ = −1.6, 95% CI: −2.7 to −0.4, P = 0.0092 SU63; −1.7 [2.0], δ = −1.7, 95% CI: −2.7 to −0.7). Therefore, the secondary endpoint was also met (Table 2). The decreased ALT levels and decreased P. gingivalis IgG antibody titers in the SRP group were sustained until week 60 from baseline (Table 3). Post hoc analysis indicated that the decrease in ALT levels from baseline to week 12 was significantly higher in endotoxin activity assay (EAA) responders (EAA < −0.04), than nonresponders (EAA > −0.04), in the SRP group (EAA responders/nonresponders: SRP, −18 [11]/4 [5], δ = −23, 95% CI: −33 to −13, P = 0.0002; no SRP, −6 [4]/3 [8], δ = −9, 95% CI: −16 to −2, P = 0.02) (Figure 2A). Similarly, the SRP group had significantly lower AST levels from baseline to weeks 12 and 60 than the no-SRP group (SRP, −9.6 [13], no SRP, 0.6 [4], δ = −10, 95% CI: −16.4 to −4, P = 0.001) (Tables 2 and 3). The decrease in AST levels from baseline to week 12 was significantly higher in EAA responders than in nonresponders in the SRP group (EAA responders/nonresponders: SRP, −15 [11]/3[4], δ = −18, 95% CI: −27 to −9, P = 0.0009; no SRP: −3 [3]/2 [4], δ = −5, 95% CI: −8 to −1, P = 0.03) (Figure 2B). In addition, the decrease in gamma-glutamyl transferase (GGT) levels from baseline to weeks 12 and 60 was significantly higher in the SRP group than in the no-SRP group (SRP, −27 [53], no SRP, 1 [11], δ = −27, 95% CI: −52 to −3, P = 0.03) (Tables 2 and 3). The decrease in GGT levels from baseline to week 12 was significantly higher in EAA responders than in nonresponders in the SRP group (EAA responders/nonresponders: −42 [58]/8 [6], δ = −50, 95% CI: 100–0.06, P = 0.0497) (Figure 2C). There was no significant change in alkaline phosphatase levels from baseline between the 2 groups (Table 2). In addition, compared with the no-SRP group, the SRP group exhibited a significant reduction in liver fat content (LFC) from baseline to weeks 12 and 60 (SRP −2.2 [2.9], no SRP 0.08 [1.2], δ = −2.2, 95% CI: −3.7 to −0.8, P < 0.001) (Tables 2 and 3). The decrease in LFC from baseline to week 12 was significantly higher in EAA responders than in nonresponders in the SRP group (EAA responders/nonresponders: SRP, −3.4 [2.5]/0.7 [1.1], δ = −2.7, 95% CI: −6.4 to −1.9, P = 0.001; no SRP, −1.1 [1.1]/0.5 [1.0], δ = −1.6, 95% CI: −2.7 to −0.5, P = 0.007) (Figure 2D). The reduction in liver stiffness evaluated by MRE from baseline to week 12 was significantly higher in the SRP group than in the no-SRP group (SRP −0.3 [0.4], no SRP 0.03 [0.3], δ = −0.3, 95% CI: −0.5 to −0.1, P = 0.001) (Table 2). However, this reduction was not observed at 60 weeks from baseline (Table 3). The reduction in liver stiffness from baseline to week 12 was significantly higher in EAA responders than in nonresponders in the SRP group (EAA responders/nonresponders: SRP, −0.5 [0.3]/0.1 [0.1], δ = −0.6, 95% CI: −0.8 to −0.3, P = 0.0005; no SRP, −0.2 [0.1]/0.1 [0.3], δ = −0.4, 95% CI: −0.6 to −0.1, P = 0.01) (Figure 2E). Similarly, compared with the no-SRP group, the SRP group had a significant reduction in both controlled attenuation parameter (CAP) and liver stiffness measurement from baseline to week 12 (CAP: SRP −35.7 [68.6], no SRP 6.3 [27.2], δ = −29, 95% CI: −62.8 to 4, P = 0.04; liver stiffness measurement: SRP −0.8 [1.7], δ = −0.8, 95% CI: −1.7 to 0.1, P = 0.04) (Table 2). However, there was no significant change in CAP from baseline to week 60 (Table 3).
Figure 1.
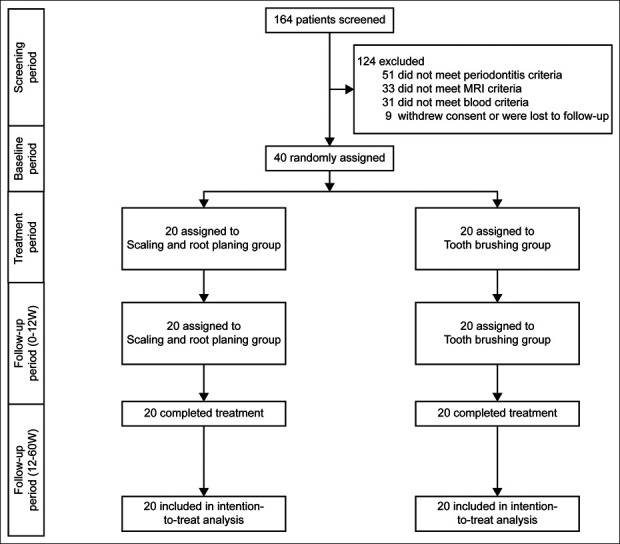
Of 164 patients, 40 met the inclusion criteria and were randomly assigned to the test group (n = 20) and to the control group (n = 20). The patients were then followed up to 12 and 60 weeks.
Table 1.
Baseline characteristics of the study population
| Variables | Normal range | SRP | No SRP (tooth brushing) |
| (n = 20) | (n = 20) | ||
| Demographics | |||
| Age (yr) | 61 ± 10 | 54 ± 15 | |
| Male, n (%) | 8 (40) | 10 (50) | |
| Comorbidities | |||
| Type 2 diabetes, n (%) | 5 (25) | 9 (45) | |
| Dyslipidemia, n (%) | 6 (30) | 8 (40) | |
| Hypertension, n (%) | 6 (30) | 10 (50) | |
| Hyperuricemia, n (%) | 2 (10) | 4 (20) | |
| Cardiovascular disease, n (%) | 1 (5) | 1 (5) | |
| Thyroid disease (hypothyroidism), n (%) | 0 (0) | 0 (0) | |
| Concomitant drug use | |||
| Antidiabetic, n (%) | 5 (25) | 10 (50) | |
| DPP4-inhibitor, n (%) | 3 (15) | 4 (20) | |
| Metformin, n (%) | 3 (15) | 3 (15) | |
| Sulfonylurea, n (%) | 2 (10) | 2 (10) | |
| Antilipidemic, n (%) | 4 (20) | 8 (40) | |
| Antihypertensive, n (%) | 5 (25) | 9 (45) | |
| Antiplatelet, n (%) | 0 (0) | 0 (0) | |
| Laxatives of regular use, n (%) | 0 (0) | 0 (0) | |
| Liver image | |||
| CAP by VCTE (dB/m) | 313 ± 58 | 322 ± 62 | |
| LSM by VCTE (kPa) | 9.8 ± 5.2 | 10.0 ± 6.4 | |
| Liver fat content by MRI-PDFF (%) | 13.9 ± 7.5 | 11.8 ± 5.5 | |
| Liver stiffness by MRE (kPa) | 3.7 ± 1.2 | 3.8 ± 2.1 | |
| Blood endotoxin levels | |||
| EAA (×102) | 3.0 ± 0.1 | 2.5 ± 0.1 | |
| Metabolic factors | |||
| Weight (kg) | 76 ± 15 | 79 ± 22 | |
| BMI (kg/m2) | 28.2 ± 4.3 | 29.3 ± 5.2 | |
| Glucose (mg/dL) | 73–109 | 107 ± 42 | 124 ± 48 |
| Insulin (μU/mL) | 1.8–12.2 | 19 ± 22 | 25 ± 29 |
| HOMA-IR | <1.6 | 7 ± 14 | 9 ± 13 |
| Liver function | |||
| Platelet counts | 16–35 | 20 ± 4 | 21 ± 6 |
| AST (U/L) | 13–30 | 38 ± 21 | 36 ± 11 |
| ALT (U/L) | 10–42 | 46 ± 6 | 47 ± 6 |
| GGT (U/L) | 13–64 | 117 ± 213 | 63 ± 49 |
| ALP (U/L) | 106–332 | 240 ± 120 | 230 ± 80 |
| T.Bil (mg/dL) | 0.3–1.2 | 0.7 ± 0.2 | 0.8 ± 0.3 |
| Lipids | |||
| Tcho (mg/dL) | 150–219 | 210 ± 53 | 182 ± 42 |
| LDL-C (mg/dL) | 70–139 | 118 ± 43 | 100 ± 34 |
| HDL-C (mg/dL) | 40–96 | 49 ± 23 | 23 ± 11 |
| TG (mg/dL) | 50–149 | 222 ± 158 | 164 ± 88 |
| Inflammation marker | |||
| HsCRP (mg/L) | <0.3 | 0.2 ± 0.3 | 0.2 ± 0.3 |
| Ferritin (ng/mL) | 25–280 | 265 ± 168 | 220 ± 186 |
| CK-18 M30 (U/L) | 205 ± 170 | 255 ± 196 | |
| TNF-α (pg/mL) | 0.8–1.7 | 7.7 ± 5.8 | 9.3 ± 7.8 |
| IL-6 (pg/mL) | <4 | 2.7 ± 1.5 | 2.8 ± 1.4 |
| Fibrosis marker | |||
| Type IV collagen 7s (ng/mL) | <6 | 4.3 ± 1.0 | 4.5 ± 1.2 |
| WFA-M2BPGi (C.O.I.) | <1 | 4.6 ± 2.6 | 3.8 ± 1.6 |
| Renal function | |||
| BUN (mg/dL) | 8–22 | 13 ± 3 | 15 ± 3 |
| Creatinine (mg/dL) | 0.61–1.04 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| eGFR (mL/min) | 93 ± 25 | 90 ± 23 | |
| Periodontal assessment | |||
| PPD | 2.8 ± 0.5 | 2.8 ± 0.5 | |
| BOP | 37 ± 32 | 42 ± 51 | |
| PPD ≥4 mm, n (%) | 20 (100) | 20 (100) | |
| Oral bacteria (Porphyromonas gingivalis) (cell/mL) × 106 | 6.8 ± 1.2 | 3.8 ± 6.8 | |
| Antibody titer (Porphyromonas gingivalis) FDC381 | 1.7 ± 3.4 | −0.18 ± 0.6 | |
| Antibody titer (Porphyromonas gingivalis) SU63 | 2.1 ± 2.5 | 0.5 ± 1.9 | |
| IMT mean | 1.0 ± 0.3 | 1.1 ± 0.3 | |
| IMT max | 1.2 ± 0.5 | 1.4 ± 0.5 | |
| SF-8 quality of life questionnaire score | |||
| Physical component | 47 ± 9 | 45 ± 10 | |
| Mental component | 48 ± 8 | 48 ± 8 |
Data are reported as either mean ± SD or number (percentage).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BOP, bleeding on probing; BUN, blood urea nitrogen; CAP, controlled attenuation parameter; CI, confidence interval; CK-18, cytokeratin-18 fragment; DPP4, dipeptidyl peptidase-4; EAA, endotoxin activity assay; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HDL-C, HDL, cholesterol; HOMA-IR, homeostasis model assessment-estimated insulin resistance; HsCRP, high-sensitivity C-reactive protein; IL, interleukin; IMT, intima media thickness; LDL-C, LDL-cholesterol; LSM, liver stiffness measurement; MRE, magnetic resonance elastography; PDFF, proton density fat fraction; PPD, periodontal pocket depth; SF-8, Short Form-8; SRP, scaling and root planing; T.Bil, total bilirubin; Tcho, total cholesterol; TG, triglyceride; TNF, tumor necrosis factor; VCTE, vibration-controlled transient elastography; WFA-M2BPGi, Wisteria floribunda agglutinin-positive Mac-2, binding protein glycosylation isomer.
Table 2.
Changes in SRP-related factors from baseline to 12 weeks posttreatment (intention-to-treat population)
| SRP | No SRP (tooth brushing) | SRP vs brushing | |||
| δ | 95% CIs | P value | |||
| (n = 20) | (n = 20) | for t test | |||
| Liver image | |||||
| CAP by VCTE (dB/m) | −35.7 ± 68.6 | −6.3 ± 27.2 | −29 | −62.8 to 4 | 0.04 |
| LSM by VCTE (kPa) | −0.8 ± 1.7 | −0.06 ± 0.9 | −0.8 | −1.7 to 0.1 | 0.04 |
| Liver fat content by MRI-PDFF (%) | −2.2 ± 2.9 | 0.08 ± 1.2 | −2.2 | −3.7 to −0.8 | 0.001 |
| Liver stiffness by MRE (kPa) | −0.3 ± 0.4 | 0.03 ± 0.3 | −0.3 | −0.5 to −0.1 | 0.001 |
| Blood endotoxin levels | |||||
| EAA (×10−2) | −0.04 ± 0.09 | 0.0003 ± 0.03 | −0.04 | −0.07 to −0.02 | 0.002 |
| Metabolic factors | |||||
| Weight (kg) | −0.05 ± 0.4 | −0.1 ± 0.9 | 0.82 | ||
| BMI (kg/m2) | −0.02 ± 0.1 | −0.03 ± 0.3 | 0.85 | ||
| Glucose (mg/dL) | −3.9 ± 11 | −0.5 ± 11 | 0.35 | ||
| Insulin (μU/mL) | −1.8 ± 4 | −0.9 ± 4 | 0.51 | ||
| HOMA-IR | −0.7 ± 2 | −0.5 ± 2 | 0.77 | ||
| Liver function | |||||
| Platelet count | 0.2 ± 1.2 | −1.5 ± 4.2 | 0.08 | ||
| AST (U/L) | −9.6 ± 13 | 0.6 ± 4 | −10 | −16 to −4 | 0.0013 |
| ALT (U/L) | −12 ± 15 | 1 ± 8 | −12 | −20 to −5 | 0.002 |
| GGT (U/L) | −27 ± 53 | 1 ± 11 | −27 | −52 to −3 | 0.03 |
| ALP (U/L) | −8 ± 33 | 0 ± 10 | 0.32 | ||
| T.Bil (mg/dL) | 0 ± 0.2 | 0 ± 0.1 | 0.15 | ||
| Lipids | |||||
| Tcho (mg/dL) | −12.4 ± 19 | −0.8 ± 11 | −12 | −22 to −2 | 0.02 |
| LDL-C (mg/dL) | −8.2 ± 15 | 0.6 ± 6 | −9 | −16 to −1 | 0.02 |
| HDL-C (mg/dL) | −0.7 ± 7 | −1.2 ± 8 | 0.84 | ||
| TG (mg/dL) | −18 ± 34 | −1 ± 11 | −17 | −33 to −1 | 0.04 |
| Inflammation markers | |||||
| HsCRP (mg/L) | −0.01 ± 0.06 | 0.01 ± 0.03 | 0.11 | ||
| Ferritin (ng/mL) | −35 ± 42 | 17 ± 63 | −52 | −86 to −18 | 0.004 |
| CK-18 M30 (U/L) | 11 ± 68 | 42 ± 88 | 0.21 | ||
| TNF-α (pg/mL) | −0.2 ± 2.2 | −0.05 ± 6.9 | 0.94 | ||
| IL-6 (pg/mL) | −0.3 ± 1 | −0.1 ± 0.7 | 0.51 | ||
| Fibrosis marker | |||||
| Type IV collagen 7S (ng/mL) | −0.06 ± 0.2 | 0.01 ± 0.2 | 0.26 | ||
| WFA-M2BPGi (C.O.I.) | −0.007 ± 1.3 | 0.2 ± 0.6 | 0.5 | ||
| Renal function | |||||
| BUN (mg/dL) | −1.2 ± 2.9 | −0.3 ± 2.2 | 0.27 | ||
| Creatinine (mg/dL) | −0.007 ± 0.05 | −0.01 ± 0.05 | 0.77 | ||
| eGFR | 0.2 ± 8.8 | 3.6 ± 17.3 | 0.44 | ||
| Periodontal assessment | |||||
| PPD | −0.4 ± 0.3 | −0.1 ± 0.3 | −0.3 | −0.5 to −0.1 | 0.002 |
| PPD ≧ 4 mm (%) | 18 ± 90 | 17 ± 85 | 0.64 | ||
| BOP | −20 ± 6 | −9 ± 6 | 0.22 | ||
| Oral bacteria (Porphyromonas gingivalis) (cell/mL) × 106 | −0.5 ± 1.2 | 1.4 ± 0.7 | −0.6 | −1.3 to −0.003 | 0.049 |
| Antibody titer (Porphyromonas gingivalis) FDC381 | −1.6 ± 2.5 | −0.03 ± 0.3 | −1.6 | −2.7 to −0.4 | 0.0092 |
| Antibody titer (Porphyromonas gingivalis) SU63 | −1.7 ± 2.0 | 0.005 ± 0.6 | −1.7 | −2.7 to −0.7 | 0.0013 |
| IMT mean | −0.03 ± 0.08 | 0.02 ± 0.04 | −0.05 | 0.09 to −0.009 | 0.016 |
| IMT max | −0.03 ± 0.2 | 0.005 ± 0.07 | 0.42 | ||
| SF-8 quality of life questionnaire score | |||||
| Physical component | −0.09 ± 6.6 | 0.26 ± 2.1 | 0.82 | ||
| Mental component | 1.4 ± 3.0 | 0.1 ± 4.2 | 0.28 | ||
Data are reported as either mean ± SD or number (percentage).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BOP, bleeding on probing; BUN, blood urea nitrogen; CAP, controlled attenuation parameter; CI, confidence interval; CK-18, cytokeratin-18 fragment; EAA, endotoxin activity assay; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HDL-C, HDL, cholesterol; HOMA-IR, homeostasis model assessment-estimated insulin resistance; HsCRP, high-sensitivity C-reactive protein; IL, interleukin; IMT, intima media thickness; LDL-C, LDL-cholesterol; LSM, liver stiffness measurement; MRE, magnetic resonance elastography; PDFF, proton density fat fraction; PPD, periodontal pocket depth; SF-8, Short Form-8; SRP, scaling and root planing; T.Bil, total bilirubin; Tcho, total cholesterol; TG, triglyceride; TNF, tumor necrosis factor; VCTE, vibration-controlled transient elastography; WFA-M2BPGi, Wisteria floribunda agglutinin-positive Mac-2, binding protein glycosylation isomer.
Table 3.
Changes in SRP-related factors from baseline to 60 weeks (intention-to-treat population)
| SRP | No SRP (brushing) | SRP vs brushing | |||
| δ | 95% CIs | P value | |||
| (n = 20) | (n = 20) | for t test | |||
| Liver image | |||||
| CAP by VCTE (dB/m) | −44.8 ± 110 | −8.8 ± 29 | 0.16 | ||
| LSM by VCTE (kPa) | −2.0 ± 3.0 | −0.18 ± 1.2 | −1.9 | −3.3 to 0.4 | 0.014 |
| Liver fat content by MRI-PDFF (%) | −3.3 ± 4.2 | 0.17 ± 1.7 | −3.4 | −5.5 to −1.4 | 0.0016 |
| Liver stiffness by MRE (kPa) | 3.1 ± 1.7 | 3.4 ± 2.3 | 0.7 | ||
| Blood endotoxin levels | |||||
| EAA (×10−2) | −0.06 ± 0.07 | 0.003 ± 0.04 | −0.06 | −0.1 to −0.002 | 0.0023 |
| Metabolic factors | |||||
| Weight (kg) | 0.15 ± 0.6 | 0.05 ± 1.4 | 0.77 | ||
| BMI (kg/m2) | 0.07 ± 0.2 | 0.03 ± 0.5 | 0.73 | ||
| Glucose (mg/dL) | −5.2 ± 18 | −1.4 ± 15 | 0.47 | ||
| Insulin (μU/mL) | −3.3 ± 5 | −1.0 ± 4 | 0.12 | ||
| HOMA-IR | −1.1 ± 2 | −0.7 ± 3 | 0.6 | ||
| Liver function | |||||
| Platelet count | −0.3 ± 3.3 | −1.5 ± 3.8 | 0.28 | ||
| AST (U/L) | −11 ± 14 | −1 ± 12 | −10 | −19 to −1 | 0.044 |
| ALT (U/L) | −15 ± 23 | 1 ± 10 | −16 | −20 to −5 | 0.0057 |
| GGT (U/L) | −36 ± 75 | 2 ± 16 | −37 | −72 to −2 | 0.0373 |
| ALP (U/L) | −12 ± 38 | −8 ± 26 | 0.64 | ||
| T.Bil (mg/dL) | 0 ± 0.2 | 0.1 ± 0.2 | 0.07 | ||
| Lipids | |||||
| Tcho (mg/dL) | −17.4 ± 29 | 1.1 ± 23 | −18 | −35 to −2 | 0.03 |
| LDL-C (mg/dL) | −10 ± 18 | −3 ± 25 | −7 | 0.3 | |
| HDL-C (mg/dL) | −1.1 ± 16 | 4.6 ± 33 | 0.49 | ||
| TG (mg/dL) | −29 ± 54 | −2 ± 26 | 0.052 | ||
| Inflammation marker | |||||
| HsCRP (mg/L) | −0.03 ± 0.09 | −0.01 ± 0.07 | 0.27 | ||
| Ferritin (ng/mL) | −59 ± 73 | 10 ± 55 | −69 | −110 to −27 | 0.0018 |
| CK-18 M30 (U/L) | 27 (62) | 40 (85) | 0.57 | ||
| TNF-α (pg/mL) | −0.2 (2.3) | −0.2 (6.4) | 0.99 | ||
| IL-6 (pg/mL) | −0.07 (1.1) | −0.03 (0.7) | 0.91 | ||
| Fibrosis marker | |||||
| Type IV collagen 7S (ng/mL) | −0.23 (0.5) | 0.09 (0.4) | −0.3 | −0.62 to −0.02 | 0.035 |
| WFA-M2BPGi (C.O.I.) | −0.05 (1.4) | 0.07 (0.6) | 0.73 | ||
| Renal function | |||||
| BUN (mg/dL) | −0.2 ± 3.6 | 0 ± 2.1 | 0.83 | ||
| Creatinine (mg/dL) | −0.007 ± 0.06 | −0.02 ± 0.05 | 0.62 | ||
| eGFR | −1.2 ± 10.4 | 4.4 ± 17.3 | 0.22 | ||
| Periodontal assessment | |||||
| PPD | −0.5 ± 0.3 | −0.2 ± 0.5 | −0.3 | −0.6 to −0.01 | 0.038 |
| PPD ≧4 mm (%) | 18 ± 90 | 17 ± 85 | 0.22 | ||
| BOP | −22 ± 27 | −14 ± 36 | 0.37 | ||
| Oral bacteria (Porphyromonas gingivalis) (cell/mL) × 106 | −0.4 ± 1.2 | 0.2 ± 0.7 | 0.07 | ||
| Antibody titer (Porphyromonas gingivalis) FDC381 | −1.4 ± 2.6 | −0.02 ± 0.5 | −1.4 | −2.6 to −0.2 | 0.0023 |
| Antibody titer (Porphyromonas gingivalis) SU63 | 2.1 ± 2.5 | 0.5 ± 1.9 | 0.06 | ||
| IMT mean | −0.09 ± 0.17 | 0.05 ± 0.012 | −0.14 | −0.23 to −0.005 | 0.0046 |
| IMT max | −0.1 ± 0.2 | 0.05 ± 0.2 | −0.2 | −0.3 to −0.02 | 0.028 |
| SF-8 quality of life questionnaire score | |||||
| Physical component | 0.17 ± 8.2 | 0.01 ± 6.0 | 0.94 | ||
| Mental component | 2.2 ± 4.6 | 0.2 ± 6.3 | 0.26 | ||
Data are reported as either mean ± SD or number (percentage).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BOP, bleeding on probing; BUN; blood urea nitrogen; CAP, controlled attenuation parameter; CI, confidence interval; CK-18, cytokeratin-18 fragment; DPP4, dipeptidyl peptidase-4; EAA, endotoxin activity assay; eGFR, estimated glomerular filtration rate; GGT, gamma-glutamyl transferase; HDL-C, HDL-cholesterol; HOMA-IR, homeostasis model assessment-estimated insulin resistance; HsCRP, high-sensitivity C-reactive protein; IL, interleukin; IMT, intima media thickness; LDL-C, LDL-cholesterol; LSM, liver stiffness measurement; MRE, magnetic resonance elastography; PDFF, proton density fat fraction; PPD, periodontal pocket depth; SF-8, Short Form-8; SRP, scaling and root planing; T.Bil, total bilirubin; Tcho, total cholesterol; TG, triglyceride; TNF, tumor necrosis factor; VCTE, vibration-controlled transient elastography; WFA-M2BPGi, Wisteria floribunda agglutinin-positive Mac-2, binding protein glycosylation isomer.
Figure 2.
EAA responders showed absolute changes in ALT, AST, GGT, liver fat content, and liver stiffness and improvement in intraoral parameters by periodontal treatment. SRP group EAA responders (n = 14); SRP group EAA nonresponders (n = 6); No-SRP group EAA responders (n = 4); No-SRP group EAA nonresponders (n = 16). ALT, alanine aminotransferase; AST, aspartate aminotransferase; EAA, endotoxin activity assay; GGT, γ-glutamyl transpeptidase; SRP, scaling and root planing.
The decrease in endotoxin levels (evaluated using an activity assay) from baseline to weeks 12 and 60 was significantly higher in the SRP group than in the no-SRP group (SRP −0.04 [0.09], no SRP 0.0003 [0.03], δ = −0.04, 95% CI: −0.07 to −0.02, P = 0.002; and SRP −0.06 [0.07], no SRP 0.003 [0.04], δ = −0.06, 95% CI: −0.1 to −0.002, P = 0.0023) (Tables 2 and 3). There was no significant change in platelet counts at week 12 from baseline (Table 2). Levels of noninvasive inflammatory and fibrosis markers showed less significant changes between the 2 groups (Table 2), while ferritin showed a significant decrease only in the SRP group (SRP −35 [42], no SRP 17 [63], δ = −52, 95% CI: −86 to −18, P = 0.004). The reduction in PPD was significantly higher in the SRP group than in the no-SRP group (PPD −0.4 [0.3], δ = −0.3, 95% CI: −0.5 to −0.1, P = 0.002). Similarly, the decrease in oral P. gingivalis bacterial counts was significantly higher in the SRP group (bacterial count −0.5 [1.2], δ = −0.6, 95% CI: −1.3 to −0.003, P = 0.049). Nevertheless, no significant change was observed in the proportion of patients with bleeding on probing and PPD ≥4 mm between the SRP and no-SRP groups (Table 2). See Supplementary Table 3, Supplementary Digital Content 3, http://links.lww.com/CTG/A863, which shows the change in the genus levels of oral microbiota between the non-SRP and SRP groups during the 12 weeks of treatment. None of the 40 patients in the study experienced a serious adverse event. During the study period, no life-threatening events or treatment-related deaths occurred (see Supplementary Table 4, Supplementary Digital Content 4, http://links.lww.com/CTG/A864, which provides details of treatment-related adverse events).
DISCUSSION
To our knowledge, this is the first study to suggest that interventional periodontal disease treatment may be a new therapeutic option for patients with NAFLD. We found an improvement in NAFLD after periodontal treatment. Patients in the SRP group met predefined primary (reduction in absolute ALT levels) and secondary (reduction in absolute P. gingivalis IgG antibody titers) endpoints (17,18). In addition, several other parameters (AST, GGT, EAA, and LFC) improved in the SRP group.
NAFLD/NASH is a heterogeneous disease with a correspondingly complex pathophysiology, including redundant pathways that vary between patients. The complexity of NAFLD/NASH pathophysiology enables the use of a variety of potentially practicable therapeutic targets (19). The recent treatment options for NAFLD are based on antimetabolite, anti-inflammatory, and antifibrotic mechanisms. However, the efficacy of monotherapy is limited. Consequently, new treatments based on new theories are needed.
High levels of blood endotoxin constitute an important factor in NASH-induced inflammation and fibrosis. Endotoxin-induced hypersensitivity reportedly plays an important role in NAFLD progression through the leptin pathway (20). The increased endotoxin level in patients with NAFLD is an influential stimulator of fatty liver development, thereby leading to NAFLD progression and increased intestinal permeability (21–23). Previous studies have reported that P. gingivalis endotoxins can adversely affect the brain and liver (24,25).
In this study, periodontal SRP intervention dramatically improved the levels of ALT, AST, and GGT and LFC and liver stiffness. These improvements were correlated with intraoral parameters. Of interest, there was a higher effect of SRP on liver enzymes, LFC, and liver stiffness in patients with NAFLD who were EAA responders. In addition, ALT-level, AST-level, and GGT-level improvements were maintained for up to week 60 in the SRP group participants. Therefore, reductions in oral P. gingivalis bacterial counts, and its serum IgG antibody titers, may be effective for up to 12 weeks post-SRP treatment; these reductions gradually stopped, decreasing in the follow-up period after week 60. We hypothesized that SRP treatment would have a limited effect on imaging findings of the liver. The intraoral P. gingivalis count may return to its previous state when the PPD is at least 4 mm in the SRP and no-SRP groups (26–28). At 12 and 60 weeks, the SRP group had significantly improved oral findings compared with the standard care group. The improvement in PPD by 0.5 mm was significantly effective, which may be of clinical significance (29,30). It has also been reported that patients with NASH are more sensitive to endotoxins than healthy participants, and even trace amounts of endotoxin can cause liver damage (20). Even if the improvement of 0.5 mm in PPD is mild for the treatment of the oral cavity, it may have improved the liver damage by reducing the endotoxin caused by oral P. gingivalis. A decrease in PPD of 0.5 mm may be a weak improvement in the oral cavity but may be sufficient to reduce endotoxin levels.
Furthermore, the oral effects of endotoxin stimulation expand to the mesentery, from whence liver damage can ensue. In our study, periodontal therapy resulted in remarkable improvements in patient NAFLD status (liver enzymes and LFC), which was probably due to SRP improving the liver condition, including liver damage, by reducing the amount of P. gingivalis–derived LPS reduction. It has been suggested that LPS activation may contribute to intracellular lipid accumulation and inflammatory response (25).
This study has several advantages. First, it is the first randomized controlled trial in patients with NAFLD having periodontal disease. Second, we measured absolute changes in liver enzyme levels and LFC using EAA responder/nonresponder subanalysis. Last, we compared colocalized regions of interest for oral examinations of biochemical markers, such as liver enzyme levels on weeks 0, 12, and 60.
Nevertheless, this study has limitations. The sample size was relatively small. Moreover, there was a possibility of selection bias because liver biopsy was not performed. Last, we did not include control participants who had no periodontal disease history. Therefore, future long-term, large-scale studies should include patients with NASH without periodontal disease; a liver biopsy–based comparative histological assessment should also be performed.
In conclusion, SRP treatment significantly reduced liver enzyme levels, endotoxin levels, and LFC in patients with NAFLD and periodontal disease and was generally well tolerated in these patients. SRP-induced oral P. gingivalis–derived endotoxin reduction may be a prospective strategy for the treatment of patients with NAFLD. Further nationwide, large-scale studies are warranted to clearly define the efficacy and tolerability of SRP in patients with NAFLD and periodontitis.
CONFLICTS OF INTEREST
Guarantor of the article: Masato Minabe, DDS, PhD.
Specific author contributions: This study was designed by 2 investigators (Y.K. and T. Kessoku). A.N. and M.M. contributed to the study design. T.S., S.S., T. Kobayashi, T. Kurihashi, T.M., and T.I. were responsible for data collection and study patients. P. gingivalis titer was measured by S.T. and K.H. N.H., T. Kobayashi., H.U., and K.W. served as scientific advisors. T.H. and M.Y. critically reviewed the study proposal. Physicians collected the data, which were validated and analyzed by an independent statistician (M.T.). Y.K. and T. Kessoku. were responsible for the preparation of tables and figures. All authors participated in manuscript writing and reviewing.
Financial support: This investigator-initiated clinical trial was sponsored by the Kanagawa Dental University. This work was supported by the Ministry of Health, Labour and Welfare, Japan (grant number 16K11872). The funders of this trial had no role in the study design, data collection, data analysis, data interpretation, and report writing.
Potential competing interests: None to report.
Ethical approval: The Institutional Review Board of Kanagawa Dental University Hospital approved the supporting data associated with the research (approval no. 480). Patients with NAFLD and periodontitis were enrolled at Kanagawa Dental University Yokohama Clinic, Kanagawa Dental University, Iwasaki Internal Medicine Clinic, and the Yokohama City University medical institution in Japan. All participants provided written informed consent before their participation in the study. See Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A861, for patient inclusion and exclusion criteria.
Clinical trial number: This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000022079) (https://www.umin.ac.jp/ctr/ctr_regist.htm) in August 2015.
Data availability statement: The data that support the study findings are available from the corresponding author on reasonable request.
Study Highlights.
WHAT IS KNOWN
✓ Periodontal disease is associated with nonalcoholic fatty liver disease (NAFLD)
✓ Infection with periodontal pathogens, primarily Porphyromonas gingivalis, may be associated with fibrosis severity in NAFLD.
WHAT IS NEW HERE
✓ Periodontal treatment efficacy in nonalcoholic fatty liver disease was evaluated
✓ Scaling and root planing reduced liver enzyme, endotoxin, and liver fat levels
✓ Periodontal treatment can reduce liver enzyme levels and antibody titers
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A861, http://links.lww.com/CTG/A862, http://links.lww.com/CTG/A863, http://links.lww.com/CTG/A864, http://links.lww.com/CTG/A865, http://links.lww.com/CTG/A866
Yohei Kamata, Takaomi Kessoku, Tomoko Shimizu, and Satsuki Sato contributed equally to this work.
Contributor Information
Yohei Kamata, Email: kamata@kdu.ac.jp.
Takaomi Kessoku, Email: takaomi0027@gmail.com.
Tomoko Shimizu, Email: shimizu@kdu.ac.jp.
Satsuki Sato, Email: ssato528@gmail.com.
Takashi Kobayashi, Email: tkhkcb@gmail.com.
Takeo Kurihashi, Email: kurihashi@kdu.ac.jp.
Toshiya Morozumi, Email: morozumi@kdu.ac.jp.
Tomoyuki Iwasaki, Email: toiwasaki-dm@umin.ac.jp.
Shogo Takashiba, Email: takashi@okayama-u.ac.jp.
Kazu Hatanaka, Email: kazu_t@md.okayama-u.ac.jp.
Nobushiro Hamada, Email: hamada@kdu.ac.jp.
Toshiro Kodama, Email: kodama@kdu.ac.jp.
Takuma Higurashi, Email: takuma_h@yokohama-cu.ac.jp.
Masataka Taguri, Email: masataka.taguri@gmail.com.
Masato Yoneda, Email: dryoneda@yahoo.co.jp.
Haruki Usuda, Email: h-usuda@med.shimane-u.ac.jp.
Koichiro Wada, Email: koiwada@med.shimane-u.ac.jp.
Atsushi Nakajima, Email: nakajima-tky@umin.ac.jp.
ACKNOWLEDGMENTS
We thank the patients, their families, the study coordinators, investigators, and the PERION study team. We thank Editage (https://editage.jp) for editing the manuscript draft and providing help in drafting the abstract.
REFERENCES
- 1.Page JM, Harrison SA. NASH and HCC. Clin Liver Dis 2009;13:631–47. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 3.Maevskaya EA, Mayev IV, Kucheryavyy YA, et al. Clinical feature of nonalcoholic steatohepatitis, associated with chronic constipation [in Russian]. Eksp Klin Gastroenterol 2015(9):36–44. [PubMed] [Google Scholar]
- 4.Augustyn M, Grys I, Kukla M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clin Exp Hepatol 2019;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49:1877–87. [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012;91:914–20. [DOI] [PubMed] [Google Scholar]
- 7.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005;43:5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodet C, Chandad F, Grenier D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis [in French]. Pathol Biol (Paris) 2007;55:154–62. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Chaparro PJ, Gonçalves C, Figueiredo LC, et al. Newly identified pathogens associated with periodontitis: A systematic review. J Dent Res 2014;93:846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt SC, Kesavalu L, Walker S, et al. Virulence factors of Porphyromonas gingivalis. Periodontol 1999;20:168–238. [DOI] [PubMed] [Google Scholar]
- 11.How KY, Song KP, Chan KG. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front Microbiol 2016;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Li H, Yang MF, et al. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. Plos One 2012;7:e39224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang S, Krauss JL, Domon H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol 2011;186:869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuraji R, Fujita M, Ito H, et al. Effects of experimental periodontitis on the metabolic system in rats with diet-induced obesity (DIO): An analysis of serum biochemical parameters. Odontology 2018;106:162–70. [DOI] [PubMed] [Google Scholar]
- 15.Nakahara T, Hyogo H, Ono A, et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol 2018;53:269–80. [DOI] [PubMed] [Google Scholar]
- 16.Yoneda M, Naka S, Nakano K, et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol 2012;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alazawi W, Bernabe E, Tai D, et al. Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. Plos One 2017;12:e0185902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JY, Park YM, Lee GN, et al. Association between toothbrushing and non-alcoholic fatty liver disease. Plos One 2021;16:e0243686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: Current and emerging. J Hepatol 2018;68:362–75. [DOI] [PubMed] [Google Scholar]
- 20.Imajo K, Fujita K, Yoneda M, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab 2012;16:44–54. [DOI] [PubMed] [Google Scholar]
- 21.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis 2001;21:27–41. [DOI] [PubMed] [Google Scholar]
- 22.Zhao LF, Jia JM, Han DW. The role of enterogenous endotoxemia in the pathogenesis of non-alcoholic steatohepatitis [in Chinese]. Zhonghua Gan Zang Bing Za Zhi 2004;12:632. [PubMed] [Google Scholar]
- 23.Kessoku T, Imajo K, Honda Y, et al. Characteristics of fecal microbiota in Japanese patients with nonalcoholic fatty liver disease: A connection among gut-permeability, endotoxin and NAFLD. Gastroenterology 2017;152:S1200. [Google Scholar]
- 24.Hayashi K, Hasegawa Y, Takemoto Y, et al. Continuous intracerebroventricular injection of Porphyromonas gingivalis lipopolysaccharide induces systemic organ dysfunction in a mouse model of Alzheimer's disease. Exp Gerontol 2019;120:1–5. [DOI] [PubMed] [Google Scholar]
- 25.Ding LY, Liang LZ, Zhao YX, et al. Porphyromonas gingivalis-derived lipopolysaccharide causes excessive hepatic lipid accumulation via activating NF- κB and JNK signaling pathways. Oral Dis 2019;25:1789–97. [DOI] [PubMed] [Google Scholar]
- 26.Mdala I, Olsen I, Haffajee AD, et al. Multilevel analysis of bacterial counts from chronic periodontitis after root planing/scaling, surgery, and systemic and local antibiotics: 2-year results. J Oral Microbiol 2013;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preus HR, Dahlen G, Gjermo P, et al. Microbiologic observations after four treatment strategies among patients with periodontitis maintaining a high standard of oral hygiene: Secondary analysis of a randomized controlled clinical trial. J Periodontol 2015;86:856–65. [DOI] [PubMed] [Google Scholar]
- 28.Theodoro LH, Silva SP, Pires JR, et al. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci 2012;27:687–93. [DOI] [PubMed] [Google Scholar]
- 29.Fang H, Han M, Li QL, et al. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: A systematic review and meta-analysis. J Periodontal Res 2016;51:417–30. [DOI] [PubMed] [Google Scholar]
- 30.Goodson JM, Haffajee AD, Socransky SS, et al. Control of periodontal infections: A randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol 2012;39:526–36. [DOI] [PubMed] [Google Scholar]



