Abstract
PURPOSE
Cisplatin-based combination chemotherapy remains the standard of care for locally advanced or metastatic urothelial cancer (la/mUC); however, toxicity is substantial, responses are rarely durable, and many patients with la/mUC are ineligible. Each enfortumab vedotin and pembrolizumab have shown a survival benefit versus chemotherapy in UC, are not restricted by cisplatin eligibility, and warrant investigation as a first-line (1L) combination therapy in patients ineligible for cisplatin.
METHODS
In this ongoing phase Ib/II, multicenter, open-label study, 1L cisplatin-ineligible patients with la/mUC received enfortumab vedotin 1.25 mg/kg once daily on days 1 and 8 and pembrolizumab 200 mg (day 1) intravenously once daily in 3-week cycles. The primary end point was safety. Key secondary end points included confirmed objective response rate, duration of response (DOR), and overall survival (OS).
RESULTS
Forty-five patients received enfortumab vedotin plus pembrolizumab. The most common treatment-related adverse events (TRAEs) were peripheral sensory neuropathy (55.6%), fatigue (51.1%), and alopecia (48.9%). Twenty-nine patients (64.4%) had grade 3 or higher TRAEs; the most common were increased lipase (17.8%), maculopapular rash (11.1%), and fatigue (11.1%). One death (2.2%) was classified as a TRAE. The confirmed objective response rate after a median of nine cycles was 73.3% with a complete response rate of 15.6%. The median DOR and median OS were 25.6 months and 26.1 months, respectively.
CONCLUSION
Enfortumab vedotin plus pembrolizumab showed a manageable safety profile. Most patients experienced tumor shrinkage. The median DOR and median OS exceeding 2 years in a cisplatin-ineligible patient population make this a promising combination currently under investigation in a phase III study (ClinicalTrials.gov identifier: NCT04223856).
INTRODUCTION
Locally advanced or metastatic urothelial carcinoma (la/mUC) is a lethal malignancy. Approximately half of all patients with la/mUC are ineligible for cisplatin chemotherapy because of impaired renal function, poor performance status, and other comorbidities.1-6 Historically, first-line (1L) carboplatin-based regimens have shown limited activity and have been poorly tolerated.7,8 Programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors are restricted to a limited number of patients who are either cisplatin-ineligible with a high level of PD-L1 expression or are not eligible for any platinum-based therapy. Although maintenance therapy with avelumab after 1L platinum-gemcitabine treatment has shown a survival benefit, only patients who did not progress with 1L therapy were eligible. These limitations highlight the continuous unmet need for more effective and tolerable 1L treatment options for cisplatin-ineligible patients.
CONTEXT
Key Objective
To assess the safety and tolerability of first-line enfortumab vedotin in combination with pembrolizumab in patients with advanced urothelial cancer (aUC) who are cisplatin-ineligible.
Knowledge Generated
Enfortumab vedotin plus pembrolizumab showed a tolerable and manageable safety profile and a confirmed objective response rate of 73.3%. With the median duration of response and the overall survival exceeding 2 years, this combination offers a potential first-line treatment option for patients with aUC.
Relevance
The antitumor activity of enfortumab vedotin plus pembrolizumab appears to be higher than that of conventional carboplatin-based chemotherapy in patients with aUC. Given this potential clinical benefit, enfortumab vedotin plus pembrolizumab received Breakthrough Therapy designation by the US Food and Drug Administration and is under further investigation in phase II and III studies.
Both enfortumab vedotin and pembrolizumab are effective monotherapy treatments in patients with la/mUC.9-12 Preclinical studies of vedotin antibody–drug conjugates (ADCs), including enfortumab vedotin, show that these ADCs induce hallmarks of immunogenic cell death, including the release of damage-associated molecular patterns.13-16 Damage-associated molecular patterns are recognized by innate and adaptive immune cells, which ultimately leads to engulfment of tumor cells by antigen-presenting cells and subsequent cross-presentation of tumor antigens to cytotoxic T cells. These T cells mount antigen-specific responses that are further augmented by PD-1/PD-L1 inhibitors, such as pembrolizumab. Thus, combining enfortumab vedotin with pembrolizumab may enhance antitumor activity versus either agent alone on the basis of their distinct and complementary engagement of the immune system. Here, we present safety and efficacy results from the EV-103 Dose Escalation Phase and Dose Expansion Cohort A to evaluate enfortumab vedotin plus pembrolizumab in 1L cisplatin-ineligible patients with la/mUC.
METHODS
Trial Participants
Eligible patients (age ≥ 18 years) had histologically documented la/mUC (including squamous differentiation and mixed cell types), an Eastern Cooperative Oncology Group performance status score of 0 or 1 (on a 5-point scale; higher scores indicate greater disability), and an investigator-assessed life expectancy of 3 or more months. Patients had measurable disease according to RECIST v1.117 and adequate organ function and were eligible for pembrolizumab therapy. Patients with pre-existing grade 2 or higher sensory or motor neuropathy, active CNS metastases, or uncontrolled diabetes (defined as hemoglobin A1c [HbA1c] ≥ 8% or HbA1c 7% to < 8% with associated diabetes symptoms) were excluded.
During the Dose Escalation Phase, investigators determined if patients were either ineligible for 1L cisplatin-based chemotherapy and had not received prior systemic therapy for la/mUC or had disease progression during or after treatment with at least one platinum-containing regimen in the neoadjuvant or adjuvant setting. Patients in Dose Expansion Cohort A were all ineligible for cisplatin-based chemotherapy at enrollment on the basis of investigator assessment or if they had an Eastern Cooperative Oncology Group performance status of 2, impaired renal function (defined as creatinine clearance, calculated or measured) ≥ 30 and <60 mL/min, hearing loss/dysfunction, age, and/or allergy to cisplatin. Previous adjuvant or neoadjuvant platinum-based therapy was not permitted within 12 months of the study. Full eligibility criteria are provided in the trial protocol, available with the full text of this article in the Protocol (online only).
Treatment
The recommended doses of enfortumab vedotin were determined in the Dose Escalation phase. The enfortumab vedotin dose was escalated from 1 mg/kg to 1.25 mg/kg (maximum total dose 125 mg) intravenously (IV) over 30 minutes once on days 1 and 8 of every 3-week cycle in cohorts of three patients. Pembrolizumab was given as 200 mg IV once on day 1 of each 3-week cycle and was administered 30 minutes after enfortumab vedotin. Patients were permitted to continue study treatment until radiographically confirmed disease progression, unacceptable toxicity, investigator decision, consent withdrawal, the start of subsequent anticancer therapy, or pregnancy.
Trial Oversight
This study was designed by the sponsors in collaboration with an advisory committee. The study protocol was approved by independent review boards or ethics committees, and the trial was conducted in agreement with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines. Written informed consent was obtained from patients before any study procedures. Aggregated safety data were generated by the sponsor biostatisticians and analyzed by sponsors and authors. Safety parameters were evaluated throughout the treatment cycle and study by the safety monitoring committee.
The authors attest to the accuracy and completeness of the data and the fidelity of the study to the protocol. All the authors had access to the data used in the preparation of the manuscript. The authors, with writing and editorial support funded by the trial sponsors, developed and approved the manuscript.
End Points and Assessments
The primary end point was safety as assessed by adverse events (AEs), laboratory abnormalities, and dose-limiting toxicities (only during the Dose Escalation Phase). Adverse events, including AEs of special interest (AESIs; preidentified on the basis of enfortumab vedotin), were classified by the system organ class and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA Version 23.0; Data Supplement, online only) and graded according to the National Cancer Institute common terminology criteria for AEs version 4.03. Treatment-related adverse events (TRAEs) were determined by the investigator and assessed for the treatment combination. Immune-mediated (im) AEs were evaluated using previously described criteria for pembrolizumab monotherapy.9
Key secondary end points included disease control rate, confirmed objective response rate, duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Investigators assessed and confirmed antitumor efficacy by reviewing computed tomography (CT) scans with IV contrast of the chest, abdomen, and pelvis. CT without contrast or magnetic resonance imaging was permitted if contrast was contraindicated; the same imaging modality was recommended throughout the study. Response assessment time points were calculated from cycle 1 day 1, and objective response rates were confirmed by the investigators per RECIST v1.1, with repeat scans 4-5 weeks after the first documented response. Subsequent response assessments after confirmation of response were performed every 9 weeks (± 7 days) until 1 year after the first dose and then every 12 weeks (± 7 days).
In addition, Nectin-4 and PD-L1 expression levels were assessed retrospectively on baseline archival or fresh tumor specimens. Briefly, formalin-fixed, paraffin-embedded tumor biopsies were collected at screening and assessed centrally for Nectin-4 protein expression levels. Immunostained slides were scored by a pathologist to generate an H-score (range, 0-300). PD-L1 expression status was assessed using the combined positive score (low, < 10; high, ≥ 10).
Statistical Analysis
Safety and efficacy end points were assessed in all patients who received any dose of enfortumab vedotin or pembrolizumab. Patients who received the recommended dose of enfortumab vedotin in combination with pembrolizumab in the 1L setting in the Dose Escalation Phase (N = 5) were pooled for analysis with Dose Expansion Cohort A (N = 40). Time-to-event end points, such as DOR, PFS, and OS, were estimated using the Kaplan-Meier method, with 95% CIs by the complementary log-log transformation. Objective response rate and disease control rate were summarized with 95% CIs using the Clopper-Pearson method. The sample size of Dose Expansion Cohort A was determined using Simon's two-stage minimax design. With a target sample size of 39 patients and assuming at least a 55% objective response rate, Dose Expansion Cohort A had a power of 80% to detect a historical objective response rate of ≥ 35% for the corresponding population.
RESULTS
Patient Disposition and Baseline Characteristics
We report data on 45 cisplatin-ineligible patients with la/mUC who received enfortumab vedotin 1.25 mg/kg IV once daily on days 1 and 8 and pembrolizumab 200 mg IV once on day 1 in the 1L setting at 18 US clinical sites. At data cutoff (October 13, 2020), 21 (46.7%) patients remained on study, with 7 (15.6%) patients still receiving treatment and 14 (31.1%) patients in long-term follow-up (Fig 1). Patients received a median of 9 (range, 1-34) cycles of study treatment.
FIG 1.
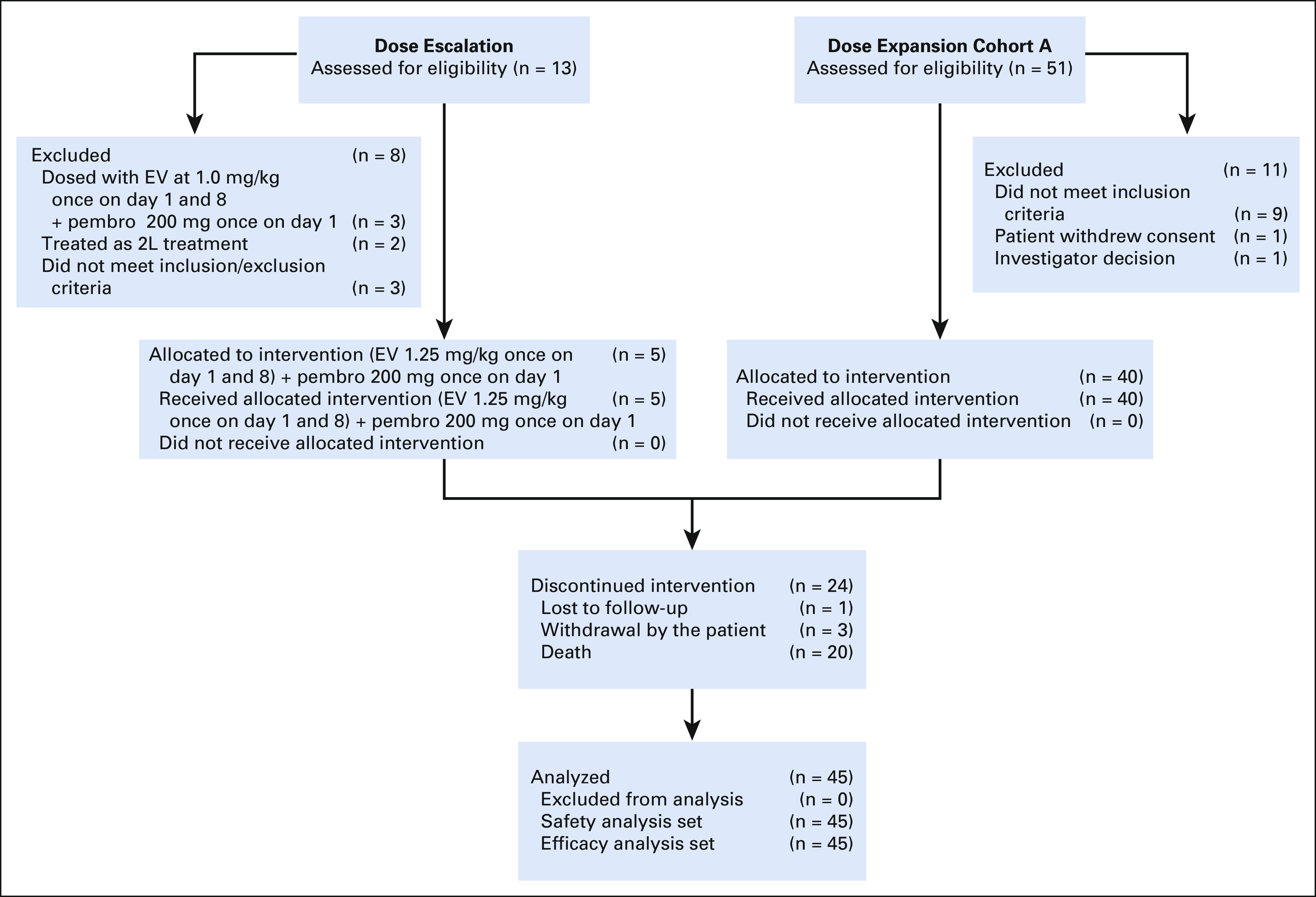
Screening, allocation, follow-up, and analyses. 2L, second-line; EV, enfortumab vedotin; pembro, pembrolizumab.
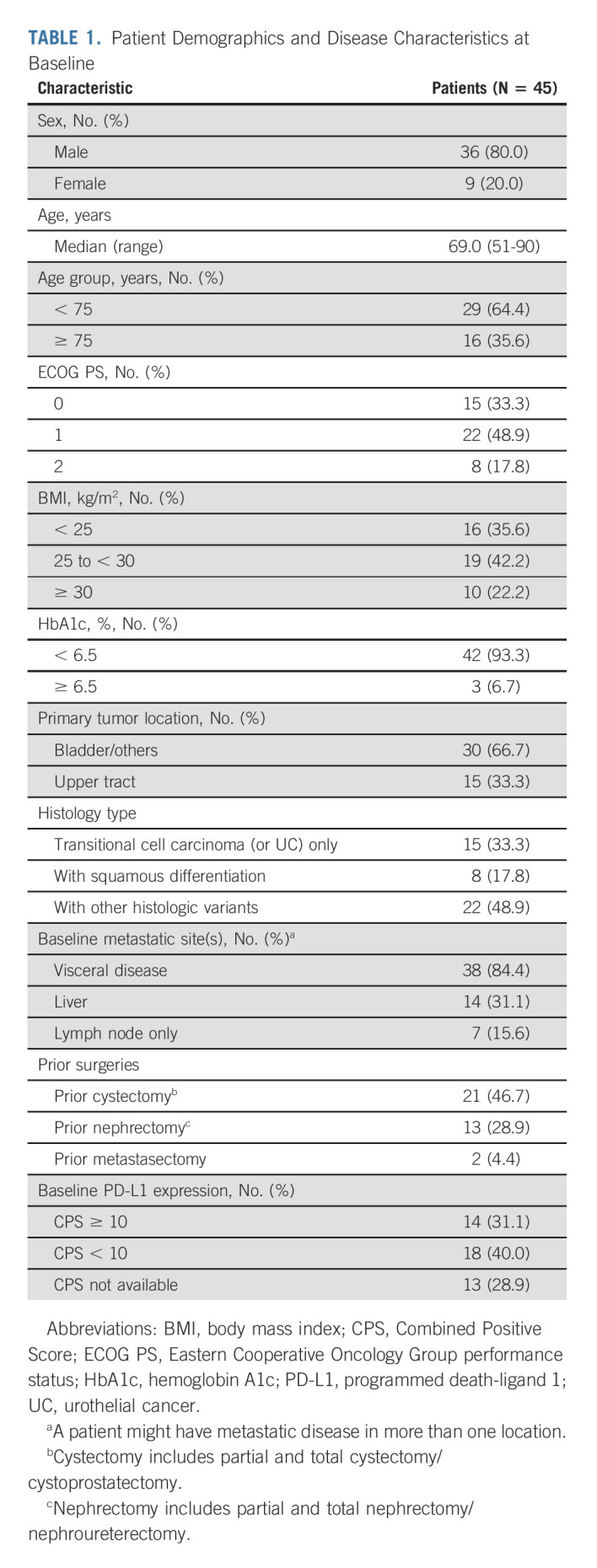
At baseline, patients were predominately male (80.0%) and the median age was 69 years; 35.6% were age ≥ 75 years. Visceral metastases were present in 84.4% of patients, including 31.1% with liver metastases. Disease originated in upper tract in 33.3% of patients (Table 1).
TABLE 1.
Patient Demographics and Disease Characteristics at Baseline
Safety
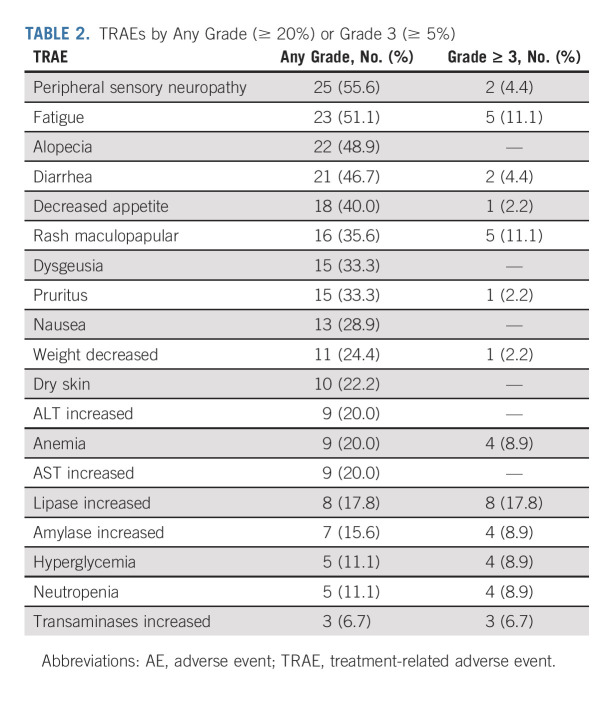
The most common TRAEs were peripheral sensory neuropathy, fatigue, and alopecia; the most common grade 3 or higher events were asymptomatic lipase elevation, fatigue, and maculopapular rash (Table 2). Seven patients (15.6%) experienced a serious TRAE, with no serious TRAE occurring more than once. TRAEs led to dose reductions in 14 (31.1%) patients and discontinuations in 11 (24.4%) patients and were not mutually exclusive. Peripheral sensory neuropathy was the most common TRAE leading to either dose reduction (six patients, 13.3%) or treatment discontinuation (four patients, 8.9%). No patients discontinued therapy because of a skin reaction or hyperglycemia. One patient (2.2%) died because of a TRAE (multiple organ dysfunction syndrome).
TABLE 2.
TRAEs by Any Grade (≥ 20%) or Grade 3 (≥ 5%)
Treatment-related AESIs prespecified for analysis (as composite terms defined by MedDRA) were peripheral neuropathy, skin reactions, and hyperglycemia (Data Supplement). Treatment-related peripheral neuropathy occurred in 28 (62.2%) patients, and the median time to first onset was 2.4 (interquartile range [IQR], 1.9-4.6) months. The median time (IQR) to resolution for peripheral neuropathy was 5.2 (3.5-8.6) months. Most peripheral neuropathies (57.8%) were grade ≤ 2 (53.3%). Seven of eight (87.5%) patients who had peripheral neuropathy at baseline developed treatment-related peripheral neuropathy. Of the 37 patients without peripheral neuropathy at baseline, 21 (56.8%) developed treatment-related peripheral neuropathy. Among patients who had treatment-related peripheral neuropathy, 19 (67.8%) resolved or improved at last follow-up.
Ten of 45 (22.2%) patients had baseline hyperglycemia/diabetes mellitus. Hyperglycemia occurred in five (11.1%) patients (one grade 2; four grade 3) with a median time to first onset of 0.5 months (IQR, 0.5-0.5), of which three (30%) were considered treatment-related (grade 3). The median (IQR) time to resolution was 1.6 (0.7-1.6) months. At last follow-up, treatment-related hyperglycemia experienced by three patients had resolved; the two remaining patients with hyperglycemia unrelated to treatment improved to grade 2. Hyperglycemia occurred more frequently in patients with a body mass index of ≥ 30 kg/m2 or with baseline hyperglycemia or diabetes mellitus.
Skin reactions occurred in 30 (66.7%) patients with a median time to first onset of 0.7 (IQR: 0.33-4.1) months. Twenty-eight (62.2%) patients experienced grade ≤ 3, and two (4.4%) grade 4 (dermatitis bullous and toxic epidermal necrolysis), and there were no grade 5 events. Twenty-two (73.3%) patients had all events resolved, and five (16.7%) patients showed improvement at last follow-up. The median time to resolution was 1.0 (0.4, 2.2) months. Eight (26.7%) patients had ongoing skin reactions at last follow-up; seven (23.3%) patients had a grade 1 event, and one (3.3%) patient had a grade 4 event.
Twenty (44.4%) patients had treatment-emergent imAEs of any grade: eight (17.8%) grade 1-2, 12 (26.7%) grade 3-4, and no grade 5 events. Treatment-emergent imAEs and the most common imAEs requiring systemic steroids are described in the Data Supplement.
Efficacy
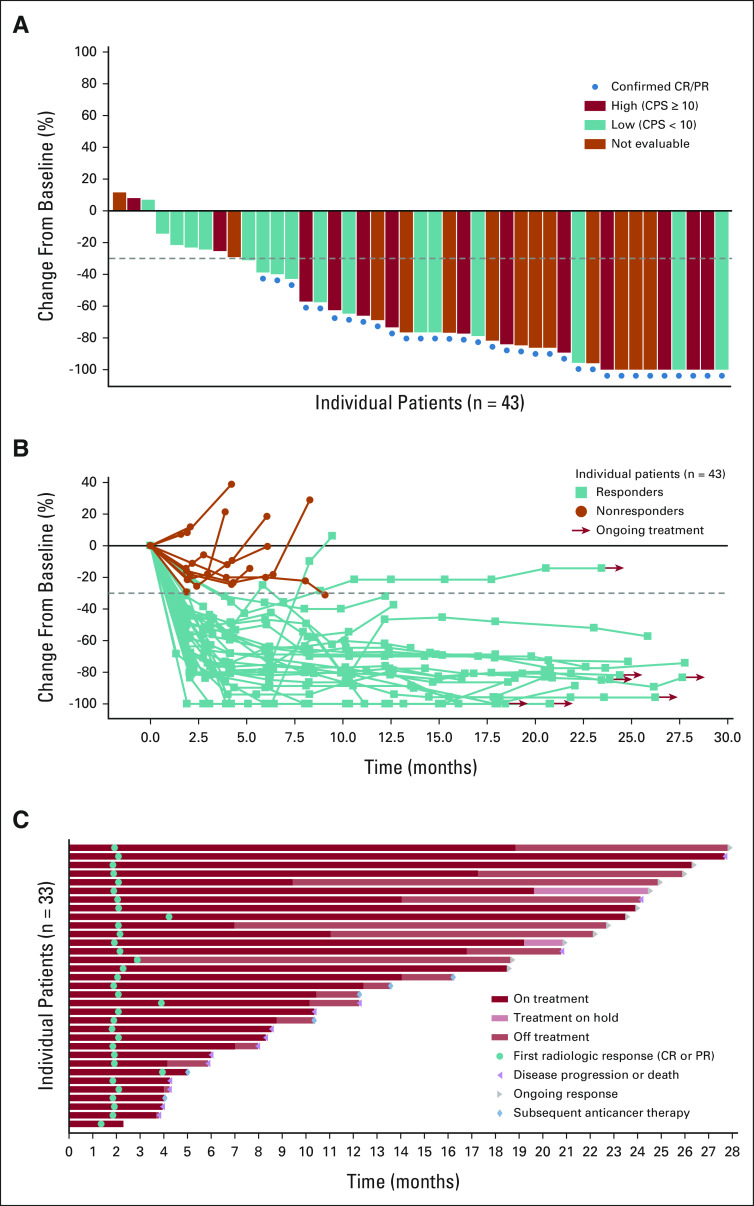
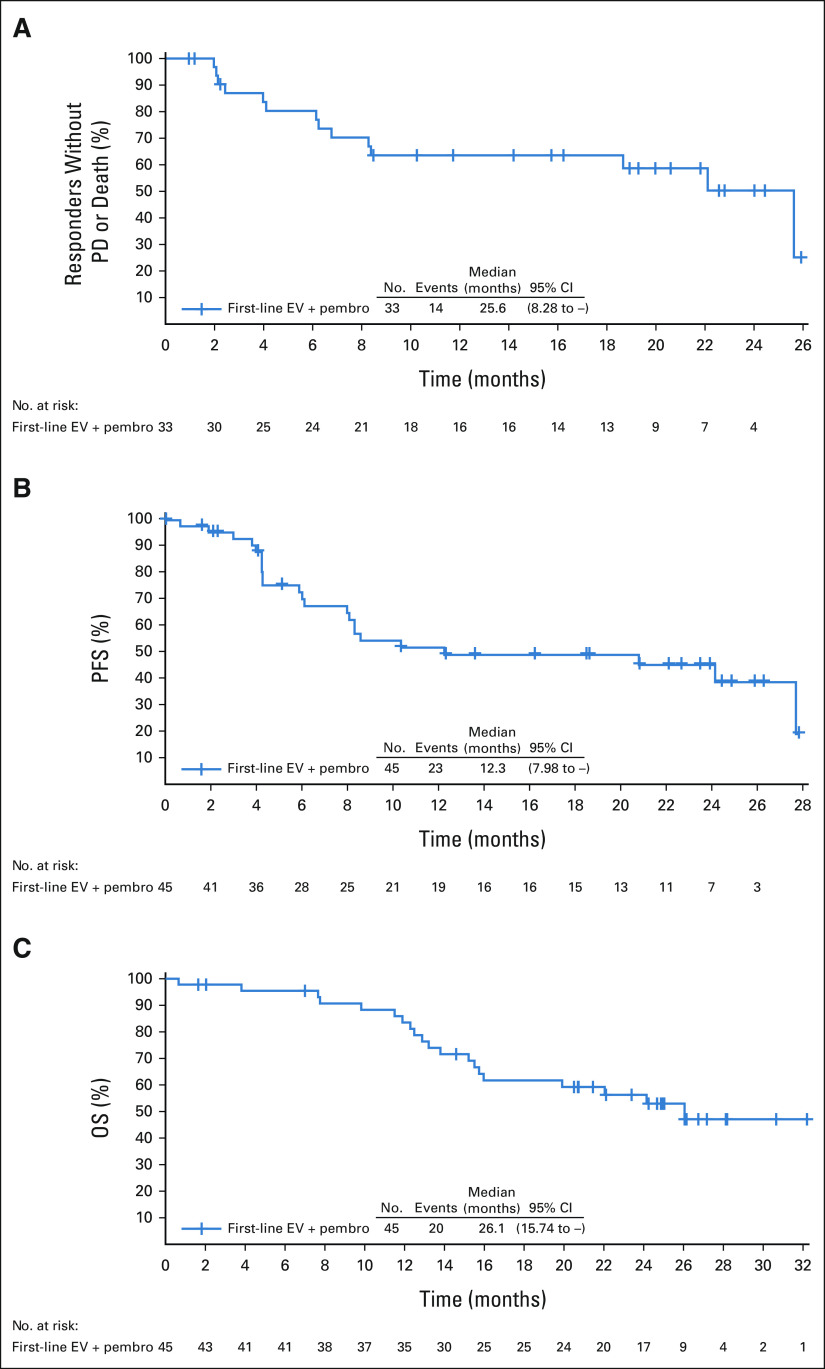
The disease control rate was 93.3% with an investigator-confirmed objective response rate (RECIST version 1.1) of 73.3% (95% CI, 58.1 to 85.4; 33 of 45 patients); seven patients (15.6%) achieved a complete response; 26 patients (57.8%) achieved a partial response (Fig 2A and Data Supplement). Twenty-nine of the 33 (87.9%) responses were observed at the first tumor assessment (week 9 ± 1 week; Fig 2B), and the median time to response was 2.1 months (Fig 2C). The median DOR was 25.6 months with a median follow-up of 20.0 months (Fig 3A). The median PFS was 12.3 months (Fig 3B); the median OS was 26.1 months with a median follow-up of 24.9 months (Fig 3C).
FIG 2.
Change in target lesions from baseline. (A) Waterfall plot of change from baseline in the sum of the diameters of target lesions by investigator per RECIST v1.1. (B) Change from baseline of the sum of diameters of target lesions (the dotted horizontal line indicates threshold for partial response (–30%) but is not necessarily indicative of response) and (C) Swimmer plot of time to response and duration of response in patients achieving confirmed objective response per RECIST v1.1. PD-L1 expression status was assessed using the combined positive score (low, < 10; high ≥ 10) with a validated PD-L1 IHC assay using the 22C3 antibody. Two patients did not have any post-baseline response assessments before the end of the study and did not have change from baseline in sum of the diameters of target lesions. CPS, combined positive score; CR, complete response; IHC, immunohistochemistry; PR, partial response.
FIG 3.
(A) DOR, (B) PFS, and (C) OS by the investigator per RECIST v1.1. DOR, duration of response; EV, enfortumab vedotin; OS, overall survival; PD, progressive disease; pembro, pembrolizumab; PFS, progression-free survival.
Responses were observed to be independent of Nectin-4 and PD-L1 expression levels. Thirty-eight of 39 patients had detectable Nectin-4 expression (H-score ≥ 0). The distribution of expression was similar among responders and nonresponders (Data Supplement). The confirmed objective response rate in PD-L1 High (combined positive score ≥ 10; n = 14 patients) and PD-L1 Low (combined positive score < 10; n = 18 patients) subgroups was 78.6% (95% CI, 49.2 to 95.3) and 61.1% (95% CI, 35.7 to 82.7), respectively (Data Supplement). The confirmed objective response rate in the PD-L1–not evaluable subgroup (n = 13) was 84.6% (95% CI, 54.6 to 98.1). The objective response rate was 57.1% (95% CI, 28.9 to 82.3) in eight patients with liver metastases and 73.3% (95% CI, 44.9 to 92.2) in 11 patients with primary upper tract disease. Two of six (18.2%) patients were censored because starting a new antitumor treatment achieved complete response and they went on to receive potentially curative therapy (see the Data Supplement for pharmacokinetic/pharmacodynamic data).
DISCUSSION
Enfortumab vedotin plus pembrolizumab is a 1L platinum-free regimen that showed promising antitumor activity and a manageable safety profile in cisplatin-ineligible patients, including those with impaired performance status and/or liver metastases. Most patients experienced rapid responses to the combination. Our results suggest that responses were durable with both the median DOR and the median OS exceeding 2 years. This activity was observed independent of the PD-L1 expression level and disease site of origin (upper or lower tract) and in prespecified patient subgroups with poor prognostic characteristics, including those with liver metastases. The safety profile of the combination, including AESIs, was manageable and consistent with enfortumab vedotin or pembrolizumab as monotherapy.9,10,12,18,19
Approximately 50% of la/mUC patients never receive any 1L treatment.20 Recent data suggest that treatment rates may be increasing.21 Among patients who receive treatment, platinum-based regimens, particularly cisplatin/carboplatin plus gemcitabine, have been common 1L options in la/mUC.1,22 Recent analyses suggest that many patients with la/mUC never receive additional therapy after initial treatment, further emphasizing the importance of achieving disease control with 1L therapy.23,24 Moreover, half of all patients with la/mUC are ineligible to receive cisplatin because of comorbidities,3,25,26 and survival is poor among cisplatin-ineligible patients who receive 1L therapy, likely because of the activity of carboplatin and underlying patient comorbidities. Some improvements in outcomes with carboplatin/gemcitabine have been observed likely because of better experience and supportive care measures. However, survival in more contemporary trials has been significantly influenced by the use of checkpoint inhibitors in later lines. Furthermore, fewer patients who receive carboplatin/gemcitabine may be eligible to benefit from avelumab because of lower response rates and decreased durability compared with cisplatin/gemcitabine.27,28 This finding, combined with the historically lower rate of disease control seen with carboplatin-gemcitabine, underscores the need for effective 1L treatment options. To our knowledge, the confirmed response and disease control rates observed with the combination of enfortumab vedotin plus pembrolizumab, the median DOR, and OS exceeding 2 years are the highest reported to date for 1L treatment in la/mUC. Results suggest that enfortumab vedotin plus pembrolizumab is an active and durable treatment option that does not require the use of 1L platinum therapy.
Enfortumab vedotin plus pembrolizumab had a manageable safety profile, and no new or unexpected safety concerns were identified. Most treatment-related peripheral neuropathies were grade ≤ 2 in severity and had resolved or improved at the time of last follow-up, consistent with longer-term clinical experience with other vedotin ADCs, such as brentuximab vedotin.29-32 Peripheral neuropathy can often be managed via dose reductions and/or interruptions. Skin reactions, including severe skin reactions, are known AEs for enfortumab vedotin and pembrolizumab. A total of three (7%) patients experienced serious skin reactions, which did not result in treatment discontinuation. These adverse events were captured in the analyses for both imAEs and AESIs. The contributions of each agent are unknown for skin toxicity at this time. In this study, overall skin events were primarily grade ≤ 2 and the majority completely resolved at last follow-up. Management of skin reactions included dose modifications or the use of topical or systemic steroids per protocol, as well as previously published guidance.33,34 The rate of treatment-related hyperglycemia was low and resolved in all patients by last follow-up. Hyperglycemia was more prevalent in patients with pre-existing hyperglycemia or diabetes mellitus and/or a body mass index ≥ 30 kg/m2. These findings are not entirely unexpected given that la/mUC disproportionately affects older adults who have a history of comorbidities, including diabetes.3,11,22,23 However, no patients in this trial discontinued therapy because of a skin reaction or hyperglycemia.
In addition to the limitations inherent to the single-arm design and a modest sample size, the study was not designed to determine the individual effects of enfortumab vedotin or pembrolizumab on efficacy and safety. There was no central radiology review in the current analysis, and no quality-of-life/patient-reported outcomes were collected. Although comparisons with historical data must be interpreted with caution, the antitumor activity of enfortumab vedotin plus pembrolizumab reported here appears to be higher than that of conventional carboplatin-based chemotherapy in this patient population. Given the potential clinical benefits, this combination received Breakthrough Therapy Designation by the US Food and Drug Administration and is undergoing further evaluation in cisplatin-ineligible patients versus enfortumab vedotin alone in a randomized part of this study (Cohort K). Furthermore, a randomized, phase III study is enrolling an unselected 1L population evaluating this combination compared with cisplatin or carboplatin plus gemcitabine (EV-302/KN-A39, ClinicalTrials.gov identifier: NCT04223856). In addition, enfortumab vedotin plus pembrolizumab is currently under investigation in two randomized phase III studies in muscle-invasive bladder cancer (EV-303/KN-905, ClinicalTrials.gov identifier: NCT03924895 and EV-304/KN-B15, ClinicalTrials.gov identifier: NCT04700124).35,36
On the basis of data from these EV-103 cohorts, enfortumab vedotin plus pembrolizumab could provide a highly active and durable 1L platinum-free option for patients whose disease may not be suitable for treatment with a cisplatin-based chemotherapy, pending prospective validation in randomized studies.
ACKNOWLEDGMENT
We thank the patients who participated in this study, their families, and the investigators and staff at EV-103 clinical study sites; the members of the safety monitoring committee; and Holly Tomlin, MPH, and Sarah Canestaro, MS, for medical writing and editorial assistance (funded by Seagen Inc). We acknowledge the medical monitoring of the study, the entire EV-103 study team, and Ray Liao (Seagen Inc, Safety) and Leah Hogdal (Seagen Inc, Biomarkers).
Christopher J. Hoimes
Honoraria: Seagen Inc
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Prometheus, Seagen Inc, Genentech/Roche, Merck Sharp & Dohme, 2bPrecise
Speakers' Bureau: Bristol Myers Squibb, Genentech/Roche, Astellas Pharma, Seagen Inc, Eisai
Research Funding: Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Novartis (Inst), Alkermes (Inst), Dynavax Technologies (Inst), Nektar (Inst), NanoCarrier (Inst), Seagen Inc (Inst), Astellas Pharma (Inst), Bristol Myers Squibb Foundation (Inst), BioNTech SE (Inst), Crispr Therapeutics (Inst), NeoImmuneTech (Inst), Mirati Therapeutics (Inst)
Uncompensated Relationships: 2bPrecise (Inst)
Thomas W. Flaig
Leadership: Aurora Oncology
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seagen Inc, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, AstraZeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seagen Inc, La Roche-Posay, Merck
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed two patents related in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or licensed at this time
Matthew I. Milowsky
Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences
Consulting or Advisory Role: Loxo/Lilly
Research Funding: Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Incyte (Inst), Seagen Inc (Inst), G1 Therapeutics (Inst), Alliance Foundation Trials (Inst), Alliance for Clinical Trials in Oncology (Inst), Clovis Oncology (Inst), Arvinas (Inst), Regeneron (Inst)
Other Relationship: Elsevier, Medscape
Terence W. Friedlander
Leadership: Med BioGene
Honoraria: EMD Serono, AstraZeneca/MedImmune, Astellas Scientific and Medical Affairs Inc
Consulting or Advisory Role: AbbVie, Dendreon, Dava Oncology, EMD Serono, Merck, Astellas Pharma, Foundation Medicine, Basilea
Research Funding: Janssen, Seagen Inc (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Neon Therapeutics (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Genentech/Roche, Jounce Therapeutics
Mehmet Asim Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seagen Inc
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seagen Inc (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst), NiKang Therapeutics (Inst)
Shilpa Gupta
Stock and Other Ownership Interests: Nektar, Moderna Therapeutics
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Gilead Sciences, Guardant Health, AVEO, EMD Serono, Pfizer, Merck, Loxo/Lilly
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, AstraZeneca, Seagen Inc, Janssen Oncology, Novartis
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Exelixis (Inst), Eisai (Inst), Bayer (Inst), AstraZeneca (Inst), Seagen Inc/Astellas (Inst)
Other Relationship: Pfizer
Jaime R. Merchan
Consulting or Advisory Role: Merck
Research Funding: Corvus Pharmaceuticals (Inst), Genentech/Roche (Inst), Tizona Therapeutics, Inc (Inst), Tocagen (Inst), Vyriad (Inst), Sillajen (Inst), Replimune (Inst), Peloton Therapeutics (Inst), Eisai (Inst), Seagen Inc/Astellas (Inst), Merck (Inst), Rubius Therapeutics (Inst), BioNTech (Inst), Trishula Therapeutics (Inst), Exelixis (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen Inc, UroGen pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen Inc, Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXcel therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Carolyn Sasse
Employment: Astellas Pharma
Blanca Homet Moreno
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Yao Yu
Employment: Seagen Inc
Stock and Other Ownership Interests: Seagen Inc
Anne-Sophie Carret
Employment: Seagen Inc
Stock and Other Ownership Interests: Seagen Inc
Honoraria: Seagen Inc
Travel, Accommodations, Expenses: Seagen Inc
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, PeerView, Research To Practice, IntelliSphere, Clinical Care Options, Physicians' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seagen Inc, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceuticals, Alligator Bioscience
Research Funding: Genentech/Roche (Inst), Seagen Inc (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 7
PRIOR PRESENTATION
Presented in part at the 2021 ASCO Annual Meeting, Chicago, IL, June 4-8, 2021.
SUPPORT
Supported by Astellas Pharma US; Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ; and Seagen Inc, EV-103/KN-869, ClinicalTrials.gov identifier: NCT03288545.
Supported by the National Cancer Institute of the National Institutes of Health under Award No. K12CA076917 (C.J.H.). Supported in part by National Cancer Institute Cancer Center Support grant No. P30 CA008748.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Deidentified patient-level trial data that underlie the results reported in this publication will be made available on a case-by-case basis to researchers who provide a methodologically sound proposal. Additional documentation may also be made available. Data availability will begin after approval of the qualified request and end 30 days after receipt of data sets. All requests can be submitted to CTDR@seagen.com and will be reviewed by an internal review committee.
Please note that the data sharing policy of this clinical study's sponsor, Seagen Inc, requires all requests for clinical trial data be reviewed to determine the qualification of the specific request. This policy is available at https://www.seagen.com/healthcare-professionals/clinical-data-requests and is aligned with BIO's Principles on Clinical Trial Data Sharing (available at https://www.bio.org/blogs/principles-clinical-trial-data-sharing-reaffirm-commitment).
AUTHOR CONTRIBUTIONS
Conception and design: Christopher J. Hoimes, Matthew I. Milowsky, Terence W. Friedlander, Daniel P. Petrylak, Carolyn Sasse, Anne-Sophie Carret, Jonathan E. Rosenberg
Provision of study materials or patients: Matthew I. Milowsky, Terence W. Friedlander, Shilpa Gupta, Sandy Srinivas, Jaime R. Merchan, Jonathan E. Rosenberg
Collection and assembly of data: Christopher J. Hoimes, Thomas W. Flaig, Matthew I. Milowsky, Terence W. Friedlander, Shilpa Gupta, Sandy Srinivas, Anne-Sophie Carret, Jonathan E. Rosenberg
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christopher J. Hoimes
Honoraria: Seagen Inc
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, Prometheus, Seagen Inc, Genentech/Roche, Merck Sharp & Dohme, 2bPrecise
Speakers' Bureau: Bristol Myers Squibb, Genentech/Roche, Astellas Pharma, Seagen Inc, Eisai
Research Funding: Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Novartis (Inst), Alkermes (Inst), Dynavax Technologies (Inst), Nektar (Inst), NanoCarrier (Inst), Seagen Inc (Inst), Astellas Pharma (Inst), Bristol Myers Squibb Foundation (Inst), BioNTech SE (Inst), Crispr Therapeutics (Inst), NeoImmuneTech (Inst), Mirati Therapeutics (Inst)
Uncompensated Relationships: 2bPrecise (Inst)
Thomas W. Flaig
Leadership: Aurora Oncology
Stock and Other Ownership Interests: Aurora Oncology
Consulting or Advisory Role: Seagen Inc, Janssen Oncology
Research Funding: Novartis, Bavarian Nordic, Dendreon, GTx, Janssen Oncology, Medivation, Sanofi, Pfizer, Bristol Myers Squibb, Roche/Genentech, Exelixis, Aragon Pharmaceuticals, Sotio, Tokai Pharmaceuticals, AstraZeneca/MedImmune, Lilly, Astellas Pharma, Agensys, Seagen Inc, La Roche-Posay, Merck
Patents, Royalties, Other Intellectual Property: The University of Colorado has filed two patents related in which I am an inventor. These are related to early-stage bladder cancer treatment and detection. Neither is commercialized or licensed at this time
Matthew I. Milowsky
Stock and Other Ownership Interests: Pfizer, Merck, Gilead Sciences
Consulting or Advisory Role: Loxo/Lilly
Research Funding: Merck (Inst), Roche/Genentech (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Incyte (Inst), Seagen Inc (Inst), G1 Therapeutics (Inst), Alliance Foundation Trials (Inst), Alliance for Clinical Trials in Oncology (Inst), Clovis Oncology (Inst), Arvinas (Inst), Regeneron (Inst)
Other Relationship: Elsevier, Medscape
Terence W. Friedlander
Leadership: Med BioGene
Honoraria: EMD Serono, AstraZeneca/MedImmune, Astellas Scientific and Medical Affairs Inc
Consulting or Advisory Role: AbbVie, Dendreon, Dava Oncology, EMD Serono, Merck, Astellas Pharma, Foundation Medicine, Basilea
Research Funding: Janssen, Seagen Inc (Inst), Incyte (Inst), Bristol Myers Squibb (Inst), Neon Therapeutics (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune, Genentech/Roche, Jounce Therapeutics
Mehmet Asim Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seagen Inc
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seagen Inc (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst), NiKang Therapeutics (Inst)
Shilpa Gupta
Stock and Other Ownership Interests: Nektar, Moderna Therapeutics
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Gilead Sciences, Guardant Health, AVEO, EMD Serono, Pfizer, Merck, Loxo/Lilly
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, AstraZeneca, Seagen Inc, Janssen Oncology, Novartis
Research Funding: Bristol Myers Squibb (Inst), Genentech (Inst), Merck (Inst), Exelixis (Inst), Eisai (Inst), Bayer (Inst), AstraZeneca (Inst), Seagen Inc/Astellas (Inst)
Other Relationship: Pfizer
Jaime R. Merchan
Consulting or Advisory Role: Merck
Research Funding: Corvus Pharmaceuticals (Inst), Genentech/Roche (Inst), Tizona Therapeutics, Inc (Inst), Tocagen (Inst), Vyriad (Inst), Sillajen (Inst), Replimune (Inst), Peloton Therapeutics (Inst), Eisai (Inst), Seagen Inc/Astellas (Inst), Merck (Inst), Rubius Therapeutics (Inst), BioNTech (Inst), Trishula Therapeutics (Inst), Exelixis (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca, Myovant Sciences, Caris Life Sciences, Sorrento Therapeutics, AVEO
Research Funding: Pfizer (Inst), Bayer (Inst), Tempus (Inst)
Daniel P. Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seagen Inc, UroGen pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics, Regeneron, Gilead Sciences
Research Funding: Progenics (Inst), Sanofi (Inst), Endocyte (Inst), Genentech (Inst), Merck (Inst), Astellas Medivation (Inst), Novartis (Inst), AstraZeneca (Inst), Bayer (Inst), Lilly (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Pfizer (Inst), Roche (Inst), Seagen Inc, Clovis Oncology (Inst), Bristol Myers Squibb (Inst), Advanced Accelerator Applications (Inst), Agensys (Inst), BioXcel therapeutics (Inst), Eisai (Inst), Mirati Therapeutics (Inst), Replimune (Inst), Medivation (Inst), Gilead Sciences (Inst)
Expert Testimony: Celgene, Sanofi
Carolyn Sasse
Employment: Astellas Pharma
Blanca Homet Moreno
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Yao Yu
Employment: Seagen Inc
Stock and Other Ownership Interests: Seagen Inc
Anne-Sophie Carret
Employment: Seagen Inc
Stock and Other Ownership Interests: Seagen Inc
Honoraria: Seagen Inc
Travel, Accommodations, Expenses: Seagen Inc
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, PeerView, Research To Practice, IntelliSphere, Clinical Care Options, Physicians' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seagen Inc, Bayer, BioClin Therapeutics, QED Therapeutics, Pharmacyclics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals, Gilead Sciences, Hengrui Pharmaceuticals, Alligator Bioscience
Research Funding: Genentech/Roche (Inst), Seagen Inc (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dash A, Galsky MD, Vickers AJ, et al. : Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107:506-513, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Galsky MD, Hahn NM, Rosenberg J, et al. : Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol 29:2432-2438, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Galsky MD, Pal SK, Lin SW, et al. : Real-world effectiveness of chemotherapy in elderly patients with metastatic bladder cancer in the United States. Bladder Cancer 4:227-238, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivas P, Plimack ER, Balar AV, et al. : Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer (KEYNOTE-052): Outcomes in older patients by age and performance status. Eur Urol Oncol 3:351-359, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternberg CN, de Mulder P, Schornagel JH, et al. : Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42:50-54, 2006 [DOI] [PubMed] [Google Scholar]
- 6.von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068-3077, 2000 [DOI] [PubMed] [Google Scholar]
- 7.De Santis M, Bellmunt J, Mead G, et al. : Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30:191-199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Santis M, Bellmunt J, Mead G, et al. : Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer "unfit" for cisplatin-based chemotherapy: Phase II—results of EORTC study 30986. J Clin Oncol 27:5634-5639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balar AV, Castellano D, O'Donnell PH, et al. : First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 18:1483-1492, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Fradet Y, Bellmunt J, Vaughn DJ, et al. : Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann Oncol 30:970-976, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles T, Rosenberg JE, Sonpavde GP, et al. : Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125-1135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu EY, Petrylak DP, O'Donnell PH, et al. : Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV201): A multicentre, single-arm, phase 2 trial. Lancet Oncol 22:872-882, 2021 [DOI] [PubMed] [Google Scholar]
- 13.Challita-Eid PM, Satpayev D, Yang P, et al. : Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 76:3003-3013, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Doronina SO, Senter PD. Chapter 4: Auristatin payloads for antibody-drug conjugates (ADCs), in Cytotoxic Payloads for Antibody-Drug Conjugates. Cambridge, UK, Royal Society of Chemistry 2019, pp 73-99. [Google Scholar]
- 15.Doronina SO, Toki BE, Torgov MY, et al. : Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778-784, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Krysko DV, Garg AD, Kaczmarek A, et al. : Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12:860-875, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Powles T, Csoszi T, Ozguroglu M, et al. : Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol 22:931-945, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg JE, O'Donnell PH, Balar AV, et al. : Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 37:2592-2600, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swami U, Haaland B, Kessel A, et al. : Comparative effectiveness of immune checkpoint inhibitors in patients with platinum refractory advanced urothelial carcinoma. J Urol 205:709-717, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Sonpavde GP, Galsky MD, Wright P, et al. Real world treatment patterns and clinical outcomes with first-line therapy in cisplatin-eligible and ineligible patients with advanced urothelial carcinoma. J Clin Oncol 40 (suppl 16; abstr 4565) [Google Scholar]
- 22.Simeone JC, Nordstrom BL, Patel K, et al. : Treatment patterns and overall survival in metastatic urothelial carcinoma in a real-world, US setting. Cancer Epidemiol 60:121-127, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Morgans AK, Hepp Z, Shah SN, et al. : Real-world burden of illness and unmet need in locally advanced or metastatic urothelial carcinoma following discontinuation of PD-1/L1 inhibitor therapy: A Medicare claims database analysis. Urol Oncol 39:733.e1-733.e10, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Swami U, Grivas P, Pal SK, Agarwal N: Utilization of systemic therapy for treatment of advanced urothelial carcinoma: Lessons from real world experience. Cancer Treat Res Commun 27:100325, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Malangone-Monaco E, Wilson K, Varker H, et al. : A real-world study of chemotherapy treatment and costs in metastatic urothelial cancer (mUC) patients in the United States. J Clin Oncol 35, 2017. (suppl 15; abstr e16009) [Google Scholar]
- 26.Parikh RB, Feld EK, Galsky MD, et al. : First-line immune checkpoint inhibitor use in cisplatin-eligible patients with advanced urothelial carcinoma: A secular trend analysis. Future Oncol 16:4341-4345, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Powles T, Park SH, Voog E, et al. : Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218-1230, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Grivas P, Park SH, Voog E, et al. : Avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone with 1L chemotherapy (CTx) for advanced urothelial carcinoma (UC): Subgroup analyses from JAVELIN Bladder 100. Ann Oncol 3, 2020. (abstr 704MO) [Google Scholar]
- 29.Chen R, Gopal AK, Smith SE, et al. : Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 128:1562-1566, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskowitz CH, Walewski J, Nademanee A, et al. : Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood 132:2639-2642, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Pace A, Brower B, Conway D, Leis D: Enfortumab vedotin: Nursing perspectives on the management of adverse events in patients with locally advanced or metastatic urothelial carcinoma. Clin J Oncol Nurs 25:E1-E9, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Straus DJ, Długosz-Danecka M, Connors JM, et al. : Brentuximab vedotin with chemotherapy for stage III or IV classical Hodgkin lymphoma (ECHELON-1): 5-year update of an international, open-label, randomised, phase 3 trial. Lancet Haematol 8:e410-e421, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Belum VR, Benhuri B, Postow MA, et al. : Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 60:12-25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacouture ME, Patel AB, Rosenberg JE, O'Donnell PH: Management of dermatologic events associated with the Nectin-4-directed antibody-drug conjugate enfortumab vedotin. Oncologist 27:e223-e232, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galsky MD, Necchi A, Shore N, et al. : KEYNOTE-905: A phase III study of cystectomy plus perioperative pembrolizumab versus cystectomy alone in cisplatin (cis)-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). Ann Oncol 30:v401, 2019 [Google Scholar]
- 36.Hoimes CJ, Bedke J, Loriot Y, et al. : KEYNOTE-B15/EV-304: Randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol 39, 2021. (suppl 15; abstr TPS4587) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified patient-level trial data that underlie the results reported in this publication will be made available on a case-by-case basis to researchers who provide a methodologically sound proposal. Additional documentation may also be made available. Data availability will begin after approval of the qualified request and end 30 days after receipt of data sets. All requests can be submitted to CTDR@seagen.com and will be reviewed by an internal review committee.
Please note that the data sharing policy of this clinical study's sponsor, Seagen Inc, requires all requests for clinical trial data be reviewed to determine the qualification of the specific request. This policy is available at https://www.seagen.com/healthcare-professionals/clinical-data-requests and is aligned with BIO's Principles on Clinical Trial Data Sharing (available at https://www.bio.org/blogs/principles-clinical-trial-data-sharing-reaffirm-commitment).