Abstract
PURPOSE
The phase III POLO study demonstrated significant progression-free survival (PFS) benefit for active olaparib maintenance therapy versus placebo for patients with metastatic pancreatic adenocarcinoma and a germline BRCA mutation. Here, we report the final analysis of overall survival (OS) and other secondary end points.
PATIENTS AND METHODS
Patients with a deleterious or suspected deleterious germline BRCA mutation whose disease had not progressed after ≥ 16 weeks of first-line platinum-based chemotherapy were randomly assigned 3:2 to active maintenance olaparib (300 mg twice daily) or placebo. The primary end point was PFS; secondary end points included OS, time to second disease progression or death, time to first and second subsequent cancer therapies or death, time to discontinuation of study treatment or death, and safety and tolerability.
RESULTS
In total, 154 patients were randomly assigned (olaparib, n = 92; placebo, n = 62). No statistically significant OS benefit was observed (median 19.0 v 19.2 months; hazard ratio [HR], 0.83; 95% CI, 0.56 to 1.22; P = .3487). Kaplan-Meier OS curves separated at approximately 24 months, and the estimated 3-year survival after random assignment was 33.9% versus 17.8%, respectively. Median time to first subsequent cancer therapy or death (HR, 0.44; 95% CI, 0.30 to 0.66; P < .0001), time to second subsequent cancer therapy or death (HR, 0.61; 95% CI, 0.42 to 0.89; P = .0111), and time to discontinuation of study treatment or death (HR, 0.43; 95% CI, 0.29 to 0.63; P < .0001) significantly favored olaparib. The HR for second disease progression or death favored olaparib without reaching statistical significance (HR, 0.66; 95% CI, 0.43 to 1.02; P = .0613). Olaparib was well tolerated with no new safety signals.
CONCLUSION
Although no statistically significant OS benefit was observed, the HR numerically favored olaparib, which also conferred clinically meaningful benefits including increased time off chemotherapy and long-term survival in a subset of patients.
INTRODUCTION
Pancreatic cancer represents the seventh most common cause of cancer-related deaths worldwide, with increasing incidence and a 5-year survival rate of 9%-11%.1-4 For patients diagnosed with metastatic disease, the 5-year survival rate is 3%.4 Current first-line chemotherapies are associated with toxicities and have a median progression-free survival (PFS) of only 6 months.5-10
CONTEXT
Key Objective
To present the final overall survival (OS) results of the POLO study of active maintenance therapy with olaparib relative to placebo in patients with metastatic pancreatic cancer and a germline BRCA mutation. We have previously reported that olaparib confers a significant progression-free survival benefit relative to placebo.
Knowledge Generated
No statistically significant OS benefit for olaparib relative to placebo was observed. Kaplan-Meier OS curves separated from approximately 24 months, and estimated 3-year survival rates were 33.9% for olaparib and 17.8% for placebo. Statistically significant benefits were demonstrated for other key secondary end points, including time to treatment discontinuation and time to first and second subsequent therapies.
Relevance
Active maintenance therapy with olaparib confers a significant benefit for multiple clinically relevant end points relative to placebo, including increasing time free from subsequent chemotherapy use. The results also indicate a durable response to olaparib in a subset of patients.
In common with breast, ovarian, and prostate cancers,11,12 the risk of pancreatic cancer is increased in patients with loss-of-function BRCA1 and BRCA2 mutations. Estimates of the prevalence of such BRCA mutations among all patients with pancreatic cancer range from 4% to 8%,13-17 with results from the screening phase of POLO indicating a prevalence of approximately 6%.16 As a result of the deficiency in DNA double-strand break repair in cells with deleterious BRCA mutations,18,19 patients with BRCA-mutated cancers are sensitive to platinum-based chemotherapy20,21 and to inhibitors of the single-strand break repair protein poly(adenosine diphosphate-ribose) polymerase (PARP).22-24
The phase III POLO trial investigated the PARP inhibitor olaparib as active maintenance therapy for patients with metastatic pancreatic adenocarcinoma and a germline BRCA mutation (gBRCAm) whose disease had not progressed after at least 16 weeks of first-line platinum-based chemotherapy.25 Active maintenance therapy after cessation of initial treatment aims to extend PFS and overall survival (OS) without compromising health-related quality of life (HRQoL).26-30 A significant PFS benefit was demonstrated in POLO for active maintenance olaparib versus placebo (median PFS by blinded independent central review [BICR]: 7.4 months v 3.8 months; hazard ratio [HR], 0.53; 95% CI, 0.35 to 0.82; P = .004).25 The incidence of grade 3 or higher adverse events (AEs) was similar to that observed among patients receiving olaparib for the treatment of other tumor types.31-33 Active maintenance olaparib in patients with gBRCAm metastatic pancreatic cancer has been approved in multiple countries, including Europe and the United States,34,35 and is recommended in the National Comprehensive Cancer Network clinical guidelines.8
At the time of the data cutoff (DCO) for the primary PFS analysis (DCO1, January 15, 2019), an interim OS analysis (OS data maturity: 46.1%) showed no significant OS difference between the olaparib and placebo arms (median OS: 18.9 months v 18.1 months; HR, 0.91; 95% CI, 0.56 to 1.46; P = .68).25 Here, we report the results of the preplanned final analysis of OS (OS data maturity: 70.1%) and other key secondary end points from POLO at the second DCO (DCO2, July 21, 2020).
PATIENTS AND METHODS
Patients, Trial Design, and Interventions
POLO was a randomized, double-blind, placebo-controlled phase III trial. The full trial Protocol (online only) has been published previously.25 Eligible patients were age 18 years or older with histologically or cytologically confirmed metastatic pancreatic adenocarcinoma and a documented deleterious or suspected deleterious germline mutation in BRCA1 or BRCA2. Patients had received at least 16 weeks of continuous first-line platinum-based chemotherapy; the maximum duration was unlimited if no evidence of disease progression was noted by the investigator at random assignment. At any time after the minimum 16-week period, patients were permitted to discontinue the platinum component of first-line therapy while continuing other elements of their treatment regimen. All patients provided written informed consent for participation in the trial.
Patients were randomly assigned in a 3:2 ratio to receive active maintenance olaparib tablets (300 mg twice daily) or matching placebo until objective radiologic disease progression (according to modified RECIST, version 1.1) or unacceptable toxic effects. No stratification factors were used.
Trial intervention was initiated 4-8 weeks after the last dose of first-line chemotherapy. The Protocol did not allow crossover to olaparib, but after discontinuation of study drug, subsequent therapies, which could include PARP inhibitors, were administered at the investigators' discretion. POLO was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and the AstraZeneca bioethics policy, and the trial Protocol was approved by the institutional review boards at each participating center.36
End Points and Assessments
The primary end point was PFS assessed by BICR according to modified RECIST v1.1.25 BICR assessment was discontinued after the primary PFS analysis at DCO1, and a sensitivity analysis of investigator-assessed PFS was performed at both DCO1 and DCO2. Key secondary end points included OS (time from date of randomization until death from any cause), time to second disease progression (PFS2; investigator-assessed objective radiologic or symptomatic progression, or death), time to first subsequent cancer therapy or death (TFST), time to second subsequent cancer therapy or death (TSST), time to discontinuation of study treatment or death (TDT), and safety and tolerability. All end points were assessed from random assignment. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Statistical Analysis
Data on efficacy were analyzed in the intention-to-treat population (all patients who underwent random assignment). Data on safety were summarized in the safety population (patients who received at least one dose of study treatment). To control strongly the overall one-sided type I error rate at 2.5%, a multiple testing plan was used across the primary end point (PFS by BICR) and key secondary end point (OS). PFS was tested first (reported in the study by Golan et al),25 followed by OS when a significant benefit for PFS was observed. The alpha-spending plan for OS at DCO2 was observed to be two-sided P < .046 (on the basis of 106 events). All other secondary end points (PFS2, TFST, TSST, and TDT) were tested at a two-sided significance level of 5% without adjustment for multiplicity. Secondary end points were analyzed using the same methodology as reported previously for the primary analysis of PFS, comprising a log-rank test with the calculation of an HR and accompanying 95% CI.25
Data on patients with no incidence of the relevant end point at the time of the analysis were censored at the date of the last tumor assessment for which data could be evaluated. For OS, patients who were not known to have died before DCO2 were censored at the last recorded date on which they were known to be alive. Time-to-event curves were generated using the Kaplan-Meier method, which was also used to calculate medians for each trial group.37 A sensitivity analysis for OS was conducted to control for subsequent PARP inhibitor use after study treatment discontinuation among patients in the placebo arm, using rank preserving structural failure time models.
Subgroup analyses were conducted using a Cox proportional hazards model containing the treatment group, subgroups, and treatment-by-factor interaction terms. A global interaction test between subgroups was also performed, comparing the fit of a Cox proportional hazards model including treatment, all prespecified baseline factors, and qualifying covariate-by-treatment interaction terms with a model excluding interaction terms. All reported P values are two-sided and coincide with the reported two-sided CIs.
RESULTS
Patients and Treatment
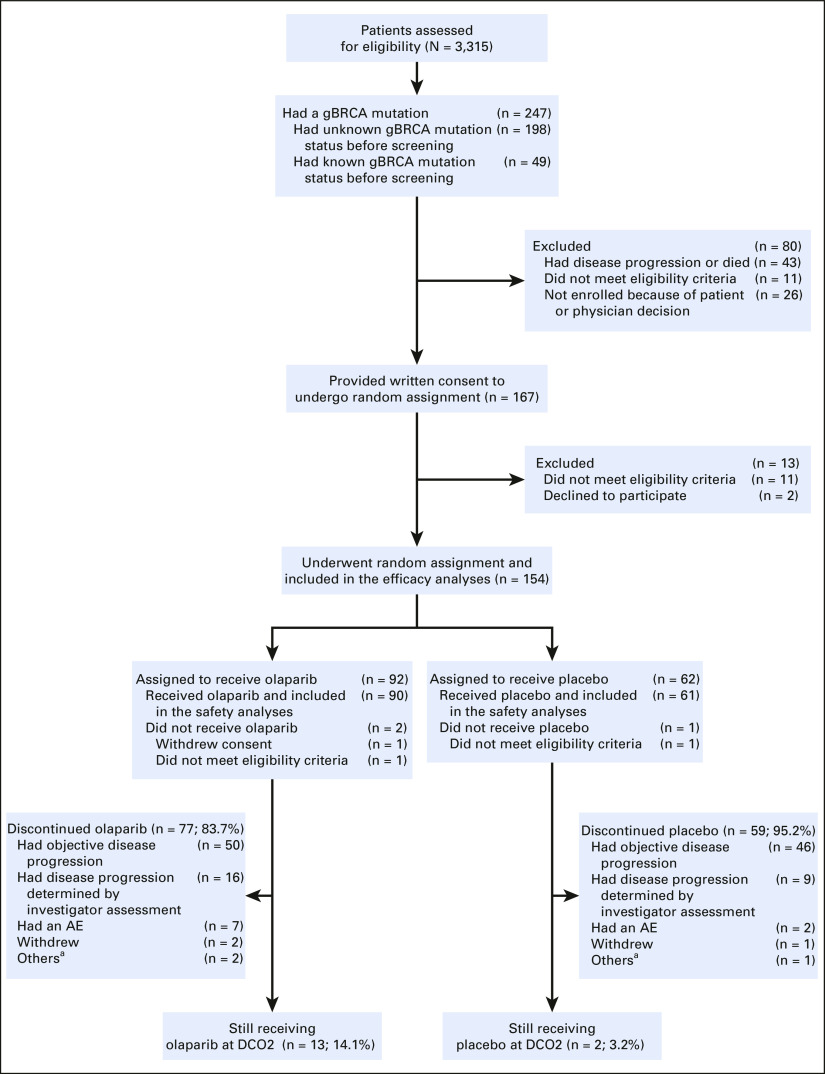
As previously reported, 3,315 patients were screened for trial entry, of whom 154 underwent random assignment (3:2 ratio; olaparib: n = 92; placebo: n = 62).16,25 In total, 90 patients who were randomly assigned to olaparib (97.8%) and 61 who were randomly assigned to placebo (98.4%) received ≥ 1 dose of study treatment and were included in the safety analysis set. At the DCO for the final OS analysis (DCO2), a higher proportion of patients in the olaparib arm (n = 13; 14.1%; treatment duration range: 20.0-57.5 months) than in the placebo arm (n = 2; 3.2%; treatment duration range: 45.8-48.2 months) were still receiving study treatment. Most patients who discontinued study treatment did so as a result of objective or investigator-assessed disease progression (66 [71.7%] and 55 [88.7%] patients in the olaparib and placebo arms, respectively). Full patient disposition is given in Appendix Figure A1 (online only), and a CONSORT flow diagram is given in Appendix Figure A2 (online only).
Baseline characteristics of the included patients have been previously published.25 The median duration of the complete first-line chemotherapy regimen was 4.6 months in the olaparib arm and 4.8 months in the placebo arm (Appendix Table A1, online only). For patients for whom specific data on the duration of the platinum component of therapy were available (olaparib: n = 42; placebo: n = 28), the median duration of the platinum component was 4.5 months in the olaparib arm and 4.8 months in the placebo arm.
Final OS
The preplanned final OS analysis was performed after 108 of the 154 randomly assigned patients (70.1%) had died. The median duration of follow-up for OS in censored patients (time from random assignment to death or date last known to be alive) was 31.3 months in the olaparib arm (range, 0.3-63.5 months) and 23.9 months in the placebo arm (range, 3.9-50.6 months). The HR for OS numerically favored olaparib but did not reach statistical significance (HR, 0.83; 95% CI, 0.56 to 1.22; P = .3487; Fig 1). The median OS was 19.0 months in the olaparib arm and 19.2 months in the placebo arm. At the time of the analysis, 26 patients (28.3%) in the olaparib arm were alive and in follow-up, compared with 11 patients (17.7%) in the placebo arm.
FIG 1.
Kaplan-Meier estimates for OS. Circles indicate censored observations. DCO, data cutoff; HR, hazard ratio; OS, overall survival.
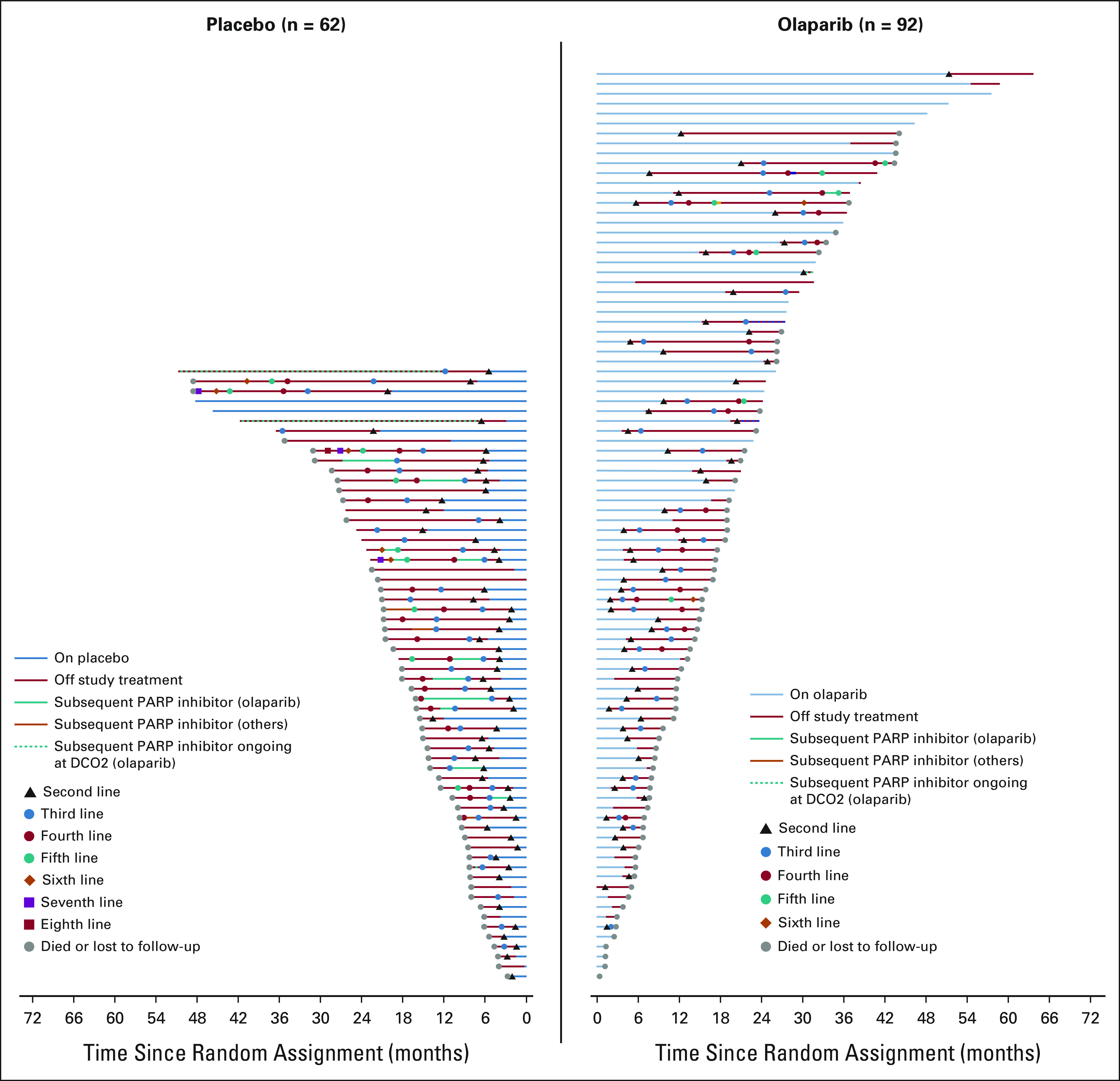
The Kaplan-Meier OS curves separated from approximately 24 months (Fig 1). In total, 34 patients (37.0%) in the olaparib arm and 17 patients (27.4%) in the placebo arm survived for more than 2 years after random assignment (Fig 2). For this subset of patients, the median duration of study treatment was 25.9 months in the olaparib arm and 7.3 months in the placebo arm. The greatest point of separation in the curves was at 36 months, at which point survival rates were 33.9% in the olaparib arm and 17.8% in the placebo arm.
FIG 2.

Swimmer plot for patients who survived more than 2 years. Each bar represents an individual patient. aPatients who received subsequent olaparib and had ongoing olaparib treatment at second data cutoff. OS, overall survival.
Final OS outcomes for prespecified subgroups were generally consistent with the overall population, and the result of the global interaction test for subgroup factors was not statistically significant (P = .2947; Fig 3). Median OS was longer for patients who had received > 6 months of first-line chemotherapy (olaparib: 32.5 months; 95% CI, 17.2 to 43.6 months; placebo: 20.6 months; 95% CI, 16.1 to 27.2 months) than for patients who had received ≤ 6 months of first-line chemotherapy (olaparib: 17.0 months; 95% CI, 11.7 to 19.2 months; placebo: 15.0 months; 95% CI, 10.6 to 21.1 months).
FIG 3.

OS subgroup analyses. The central dashed line indicates an HR of 1 (no treatment effect); outer dashed lines indicate 95% CI result in all patients; the size of circles is proportional to the overall number of events. Subgroups in which fewer than five OS events occurred per group were not included in the analysis. The prespecified gemcitabine-cisplatin subgroup included two patients in the olaparib group and three patients in the placebo group; this subgroup did not meet the threshold for inclusion in the subgroup analysis. Patients who received gemcitabine–cisplatin are not included in the others subcategory of the previous chemotherapy subgroup, but are included in the doublet chemotherapy subgroup. Race was determined from patient records. BICR, blinded independent central review; CR, complete response; ECOG, Eastern Cooperative Oncology Group; FOLFIRINOX, folinic acid–fluorouracil-irinotecan-oxaliplatin; gBRCA, germline BRCA; HR, hazard ratio; OS, overall survival; PR, partial response; SD, stable disease.
Subsequent Therapy After Study Treatment Discontinuation
At DCO2, the majority of patients had discontinued study treatment (77 patients [83.7%] in the olaparib arm and 59 patients [95.2%] in the placebo arm). Patients in both arms received multiple lines of subsequent therapy (range, 2-6 in the olaparib arm and 2-8 in the placebo arm; Appendix Fig A3, online only and Appendix Table A2, online only); 57 patients (62.0%) in the olaparib arm and 54 patients (87.1%) in the placebo arm received any subsequent therapy. The most common second-line subsequent therapies were platinum-based chemotherapies, most often folinic acid–fluorouracil-irinotecan-oxaliplatin (Appendix Table A3, online only). Of patients who discontinued study treatment, six patients (7.8%) in the olaparib arm and 16 patients (27.1%) in the placebo arm received a subsequent PARP inhibitor. Among the 17 patients in the placebo arm who survived for more than 2 years, four (23.5%) received a subsequent PARP inhibitor (olaparib in all cases) and two (11.8%) had ongoing olaparib treatment at DCO2. The response rate to second-line therapy was 5.3% in the olaparib arm and 5.6% in the placebo arm, and stable disease was observed in 28.1% and 24.1%, respectively. In the OS sensitivity analysis adjusted for subsequent PARP inhibitor use among patients in the placebo arm, the treatment effect was consistent with the unadjusted analysis (rank preserving structural failure time model–adjusted median OS: 19.0 months in the olaparib arm v 18.1 months in the placebo arm; HR, 0.81; 95% CI, 0.56 to 1.24).
Other Secondary End Points
At DCO2, median investigator-assessed PFS, TFST, TSST, and TDT were all significantly longer in the olaparib arm than in the placebo arm, whereas the HR for PFS2 did not reach statistical significance (Fig 4). The median investigator-assessed PFS was 6.7 months in the olaparib arm and 3.7 months in the placebo arm (HR, 0.49; 95% CI, 0.33 to 0.73; P = .0004), with estimated 3-year progression-free rates of 23.1% and 5.4%. The median PFS2 was 16.9 months in the olaparib arm and 9.3 months in the placebo arm (HR, 0.66; 95% CI, 0.43 to 1.02; P = .0613), and estimated 3-year PFS2 rates were 31.2% and 13.1%. The median TFST was 9.0 months in the olaparib arm and 5.4 months in the placebo arm (HR, 0.44; 95% CI, 0.30 to 0.66; P < .0001), with estimated 3-year first subsequent chemotherapy-free rates of 21.5% and 3.6%. The median TSST was 14.9 months in the olaparib arm and 9.6 months in the placebo arm (HR, 0.61; 95% CI, 0.42 to 0.89; P = .0111), with estimated 3-year second subsequent chemotherapy-free rates of 23.4% and 5.9%. The median TDT was 7.5 months in the olaparib arm and 3.8 months in the placebo arm (HR, 0.43; 95% CI, 0.29 to 0.63; P < .0001), with estimated 3-year study treatment discontinuation–free rates of 17.2% and 3.3%. Appendix Table A4 (online only) summarizes the data for key primary and secondary end points tested at DCO1 and DCO2.25
FIG 4.

Kaplan-Meier estimates for other secondary end points: (A) investigator-assessed PFS, (B) PFS2, (C) TFST, (D) TSST, and (E) TDT. HR, hazard ratio; PFS, progression-free survival; PFS2, second progression-free survival; TDT, time to discontinuation of treatment; TFST, time to first subsequent cancer therapy or death; TSST, time to second subsequent cancer therapy or death.
Safety
In the 151 patients in the safety population, the median total duration of treatment was 7.5 months (range, 0.8-57.5 months) in the olaparib arm and 3.7 months (range, 0.1-48.2 months) in the placebo arm. Along with fatigue and anemia, gastrointestinal AEs were frequently reported in both arms (Table 1). Serious AEs occurred in 28 patients (31.1%) who received olaparib and in 10 patients (16.4%) who received placebo. Grade ≥ 3 AEs were reported in 44 patients (48.9%) and 15 patients (24.6%) in the olaparib and placebo arms, respectively. One AE that occurred in the olaparib arm during the 30-day follow-up period after study treatment discontinuation resulted in death (reported previously and not causally related to study treatment).25 AEs leading to treatment discontinuation were reported in eight patients (8.9%) and one patient (1.6%) in the olaparib and placebo arms, respectively. There were no reports of myelodysplastic syndrome or acute myeloid leukemia in either treatment arm. No new primary malignancies were reported since DCO1, and there was one new case of grade 1 pneumonitis in the olaparib arm.25 No new safety signals were observed in the time between DCO1 and DCO2 (Appendix Table A5, online only).25 AEs were commonly managed through dose reduction or interruption (Table 1 and Appendix Table A6, online only).
TABLE 1.
Summary of AEs Occurring in at least 15% of the Study Population

DISCUSSION
POLO is the first randomized phase III study to investigate active maintenance therapy in patients with gBRCAm pancreatic cancer who had previously received first-line platinum-based chemotherapy.25 In this final OS analysis, a higher proportion of olaparib-treated than placebo-treated patients remained alive and in follow-up (28.3% v 17.7%, respectively). No statistically significant difference in OS between the two trial arms was demonstrated although the HR estimate did move in favor of olaparib with a slight tightening of the CI between DCO1 (HR, 0.91; 95% CI, 0.56 to 1.46; P = .68) and DCO2 (HR, 0.83; 95% CI, 0.56 to 1.22; P = .35).
The Kaplan-Meier OS curves separate at approximately 24 months, indicative of a subset of long-term survivors in the olaparib arm (37.0% of olaparib-treated patients survived for more than 2 years after random assignment v 27.4% in the placebo arm). Among those who survived for more than 2 years, patients in the olaparib arm remained on study treatment more than 3 times longer than patients in the placebo arm (25.9 months v 7.3 months). The tail on the curve may reflect a distinct biologic subgroup of patients who have a unique deficiency in homologous recombination, and further research is required to explore this interesting hypothesis.20,38
The Kaplan-Meier OS curves cross at approximately 12 months, indicating that the HR decreases over time, with the reported HR representing an average over the observed extent of follow-up. The greatest point of separation of the Kaplan-Meier OS curves is at 3 years, when nearly twice as many patients in the olaparib arm than in the placebo arm were alive (33.9% v 17.8%). Although comparing studies is challenging, the POLO OS data are comparable with those from prospective phase II studies of patients with BRCA-mutated or PALB2-mutated pancreatic cancer who have previously been treated with platinum-based chemotherapy or PARP inhibitors.38,39
Our results do not suggest an effect of the duration of prior platinum therapy on olaparib efficacy. Results of the subgroup analyses of PFS25 and OS comparing patients who had received ≤ 6 or > 6 months of first-line chemotherapy were comparable, with a trend toward increased benefits of olaparib for patients who had received > 6 months of first-line chemotherapy. Although several possible PARP inhibitor resistance mechanisms have been detected in the laboratory, only BRCA reversions have been observed in the clinical setting, mostly in ovarian cancer where platinum is often rechallenged over multiple lines.40 Patients in the POLO trial received only first-line platinum-based chemotherapy, during which their disease did not progress, and it is therefore unlikely that these patients had developed resistance to olaparib therapy. It should be noted that the study was not powered to detect differences between the subgroups, so definitive conclusions cannot be drawn.
Significant differences in favor of the olaparib arm were observed for multiple secondary end points, including the significantly longer duration of study treatment and median time to first and second subsequent cancer therapies relative to the placebo arm. Substantially more patients in the olaparib arm than in the placebo arm remained free of subsequent cancer therapy at 3 years. This is unique to a phase III trial in the active maintenance setting and is clinically meaningful for patients because it extends time free from the potentially toxic effects of subsequent chemotherapy. This is also particularly relevant in the subset of the study population with an extended OS. Patients in the olaparib arm also had a numerically longer PFS2, with an HR that favored olaparib, although the result did not reach statistical significance. Increasing PFS2 is also clinically meaningful, suggesting preservation of treatment benefits.41 The strength of the association between olaparib and each of the end points appears to decrease in a logical order (TDT, TFST, PFS, TSST, PFS2, OS), suggesting internal validity of the results.
Although OS is considered to be the most compelling end point for demonstrating the clinical benefit of anticancer therapies, it is likely that the POLO trial was not adequately powered to detect a statistically significant OS benefit. OS is longer among patients with gBRCAm pancreatic cancer exposed to platinum-based chemotherapy than the overall population of patients with pancreatic cancer,20,21 and when the trial was designed, no information was available about patient survival on active maintenance therapy after stopping platinum-based chemotherapy. Patients with gBRCAm pancreatic cancer are rare, representing only approximately 6% of all patients with pancreatic cancer.16 Random assignment of 2,200 patients would have been needed to show a 3-month improvement in OS relative to placebo with 80% power (18 months v 21 months), which would require the screening of 37,000 patients assuming a gBRCAm prevalence of 6%. PFS is a direct measure of the biologic effect of a drug on tumor growth, and extended PFS delays the time to starting a subsequent cytotoxic chemotherapy and therefore preserves HRQoL. For these reasons, PFS was considered a more practical and clinically relevant end point than OS to measure benefits in this biomarker-selected subset of patients with pancreatic cancer. PFS is generally accepted as a surrogate end point for OS in other tumors, especially in diseases that have extended OS and for which patients receive multiple subsequent additional lines of treatment, although there remains an incomplete understanding of how PFS reliably predicts OS in gBRCAm pancreatic cancer.42 The data from POLO will be useful in the design of future trials in biomarker-selected patient populations.
The use of subsequent therapies after discontinuation of study treatment might have also confounded the OS outcome. Partly because patients with gBRCAm pancreatic cancer treated with platinum-based chemotherapy have a better prognosis than a general population of patients with pancreatic cancer,20,43 a higher proportion of patients in POLO (more than 70%) received subsequent therapy than in other positive randomized controlled trials of first-line treatment of patients with metastatic pancreatic cancer.9,10 The most common treatments at second line were platinum-based systemic chemotherapies, of which approximately two thirds were folinic acid–fluorouracil-irinotecan-oxaliplatin. Because disease had not progressed during first-line platinum-based chemotherapy, these patients were still likely to be sensitive to subsequent platinum-based regimens, as reflected in the proportion of patients in both arms who responded or had stable disease during second-line chemotherapy. The similarity of the response rates to second-line therapy between the trial arms is also reassuring in that it indicates that exposure to olaparib does not reduce subsequent sensitivity to platinum. Although crossover to olaparib was not permitted in the Protocol, 27.1% of patients in the placebo arm received a PARP inhibitor, mostly olaparib, at the investigators' discretion after discontinuation of study treatment. In the sensitivity analysis conducted to control for this, the HR moved slightly in favor of olaparib although the difference was small and the result did not reach statistical significance.
Finally, active maintenance olaparib was generally well tolerated, and no new safety signals were observed between DCO1 and DCO2.25 The safety profile of active maintenance olaparib remains consistent with previous experience in other tumor types.31-33 A small group of patients have received olaparib for an extended period of time in the POLO trial; it is therefore reassuring that there were no reports of myelodysplastic syndrome or acute myeloid leukemia in either treatment arm and no new primary malignancies on olaparib. HRQoL has also been demonstrated to be preserved on active maintenance olaparib during the trial.44
In conclusion, although no statistically significant OS benefit for active maintenance olaparib compared with placebo was demonstrated, benefits for multiple other key secondary end points were observed. Active maintenance olaparib significantly prolonged TFST, TSST, and TDT, whereas PFS2 was extended with an HR favoring olaparib without reaching statistical significance. With a generally well-tolerated safety profile, active maintenance olaparib conferred clinically meaningful benefit to patients, including increased time without chemotherapy and durable response in a subset of patients.
ACKNOWLEDGMENT
We thank the patients who participated in this trial, their families, and our coinvestigators. Medical writing support was provided by Martin Guppy, PhD, and Stephen Sweet, PhD, of Oxford PharmaGenesis Ltd, Oxford, United Kingdom.
APPENDIX
FIG A1.
Full patient disposition, showing screening, enrollment, random assignment, and patients still receiving study treatment at DCO2 (July 21, 2020). One patient in the placebo arm was found not to have met the eligibility criteria after initiation of trial intervention, and the intervention was discontinued on day 3. After random assignment, one patient in each trial arm was found not to have met the eligibility criteria and both were included in the intention-to-treat efficacy analyses. Because neither patient received a trial intervention, they were not included in the safety analyses. aAny reason not specifically recorded. AE, adverse event; DCO, data cutoff; gBRCA, germline BRCA.
FIG A2.

CONSORT diagram showing patient disposition through enrollment, allocation, follow-up, and analysis. AE, adverse event.
FIG A3.
Swimmer plot for all patients, showing the use of subsequent therapy. Each horizontal line represents an individual patient. DCO, data cutoff; PARP, poly(adenosine diphosphate-ribose) polymerase.
TABLE A1.
Duration of First-Line Chemotherapy (complete regimen and platinum component)
TABLE A2.
Use of Subsequent Therapies
TABLE A3.
Second-Line Therapies

TABLE A4.
Summary of Efficacy Results at Primary PFS (DCO1) and Final OS (DCO2) DCOs

TABLE A5.
Summary of AEs at Primary Progression-Free Survival (DCO1) and Final Overall Survival (DCO2) DCOs
TABLE A6.
Occurrence, Resolution, and Management of Fatigue/Asthenia, Nausea, Anemia, and Vomiting
Hedy L. Kindler
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Deciphera, Novocure, Seattle Genetics, Inhibrx
Research Funding: Aduro Biotech (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Merck (Inst), Verastem (Inst), Bristol Myers Squibb (Inst), Polaris (Inst), Deciphera (Inst), Inhibrx (Inst), Roche/Genentech (Inst), Tesaro (Inst), MacroGenics (Inst), Leap Therapeutics (Inst), FibroGen (Inst), Vivace Therapeutics (Inst), Constellation Pharmaceuticals (Inst), Harpoon Therapeutics (Inst), Bayer (Inst), Seattle Genetics (Inst), Blueprint Medicines (Inst)
Pascal Hammel
Consulting or Advisory Role: Vect-Horus, ERYTECH Pharma (Inst), AstraZeneca (Inst), Rafael Pharmaceuticals, Mylan, Ipsen (Inst)
Speakers' Bureau: AstraZeneca, Servier, Mylan
Research Funding: ERYTECH Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Halozyme (Inst)
Travel, Accommodations, Expenses: Ipsen, Halozyme, Shire, Pfizer/EMD Serono, Vect-Horus
Michele Reni
Consulting or Advisory Role: Celgene, Lilly, AstraZeneca, Panavance Therapeutics, Viatris, Sotio, Servier, MSD/AstraZeneca
Research Funding: Celgene (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Celgene
Other Relationship: Celgene, AstraZeneca
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GlaxoSmithKline, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte, Astellas Pharma
Research Funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol Myers Squibb (Inst)
Teresa Macarulla
Consulting or Advisory Role: Sanofi/Aventis, Celgene, Roche, QED Therapeutics, Baxter, Incyte, Servier, Lilly, Ipsen, AstraZeneca, MSD, Eisai, Genzyme, Menarini, Prime Oncology, Ability Pharma, Advance Medical, BioLineRX, Zymeworks, Aptitude Health, Swedish Orphan Biovitrum, Basilea, Medscape, Novocure, Paraxel, PPD, Ellipses Pharma, Hirslanden/GITZ, Imedex, Janssen, MFAR, Marketing Farmacéutico & Investigación Clínica, Polaris Consulting, Scilink Comunicación Científica SC, Surface Oncology, ANGEM
Research Funding: Celgene (Inst), Agios (Inst), ASLAN Pharmaceuticals (Inst), Bayer (Inst), Roche (Inst), Genentech (Inst), AstraZeneca (Inst), Immunomedics (Inst), Lilly (Inst), Merrimack (Inst), Millennium (Inst), Novocure (Inst), Pfizer (Inst), Pharmacyclics (Inst), AbbVie (Inst), Ability Pharma (Inst), Amc Medical Research (Inst), Amgen (Inst), ARMO Biosciences (Inst), Basilea Pharmaceutica International (Inst), BeiGene (Inst), Keralty Group (Inst), BioLineRx (Inst), Blueprint Medicines (Inst), Boston Biomedical (Inst), Bristol Myers Squibb (BMS) (Inst), Cantargia AB (Inst), Eisai (Inst), ERYTECH Pharma (Inst), F. Hoffmann-la Roche (Inst), FibroGen (Inst), Halozyme (Inst), Incyte (Inst), Ipsen (Inst), Loxo (Inst), Medimmune (Inst), Merck Sharp & Dohme (Inst), Nelum Corp (Inst), Novartis (Inst), OncoMed (Inst), VCN Biosciences (Inst), Zymeworks (Inst)
Travel, Accommodations, Expenses: Merck, H3 Biomedicine, Sanofi, Celgene, Servier, Prime Oncology, Incyte
Michael J. Hall
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: I share a patent with several Fox Chase investigators for a novel method to investigate hereditary CRC genes (Inst)
Travel, Accommodations, Expenses: GRAIL
Other Relationship: Myriad Genetics, Invitae, Caris Life Sciences
Joon Oh Park
Consulting or Advisory Role: Celgene, Merck Serono, Servier, AstraZeneca, MediRama
Research Funding: Celgene, Medpacto, Servier
Daniel Hochhauser
Stock and Other Ownership Interests: Roche, Novartis
Research Funding: Merck Serono
Travel, Accommodations, Expenses: Celgene
Dirk Arnold
Employment: Asklepios Kliniken
Honoraria: Bayer, Merck Serono, Roche/Genentech, Servier, Bristol Myers Squibb, Merck Sharp and Dome, AstraZeneca, Amgen, Boston Scientific, Pierre Fabre, Ipsen
Consulting or Advisory Role: Bayer, Merck Serono, Biocompatibles, Terumo, Bristol Myers Squibb, MSD Oncology, AstraZeneca
Research Funding: Roche/Genentech (Inst), Sanofi (Inst), Oncolytics (Inst)
Travel, Accommodations, Expenses: Boston Scientific
Uncompensated Relationships: ESMO Council, ESMO Journals (Ann Oncol, ESMO Open), German Society for Hematology and Medical Oncology, German Cancer Society, European Organisation for Research and Treatment of Cancer (EORTC)
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Anke Reinacher-Schick
Honoraria: Amgen, Roche, Merck Serono, Bristol Myers Squibb, MSD, MCI Group, AstraZeneca
Consulting or Advisory Role: Amgen, Roche, Merck Serono, Bristol Myers Squibb, MSD, AstraZeneca, Pierre Fabre
Research Funding: Roche (Inst), Celgene (Inst), Ipsen (Inst), Amgen (Inst), Alexion Pharmaceuticals (Inst), AstraZeneca (Inst), Lilly (Inst), Servier (Inst), AIO-Studien (Inst), Rafael Pharmaceuticals (Inst), ERYTECH Pharma (Inst), BioNTech (Inst)
Travel, Accommodations, Expenses: Roche
Giampaolo Tortora
Consulting or Advisory Role: Celgene, Merck Serono, MSD Oncology, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: Merck Serono, Roche
Hana Algül
Consulting or Advisory Role: Pfizer, Servier/Pfizer, AstraZeneca
Research Funding: Chugai Pharma (Inst)
Eileen M. O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Adicet Bio (I), AstraZeneca (I), Alnylam (I), Autem Medical (I), BeiGene (I), Berry Genomics (I), CytomX Therapeutics, Eisai (I), Exelixis (I), Genentech/Roche (I), Genoscience Pharma (I), Helio Health (I), Incyte (I), Ipsen (I), Legend Biotech (I), Merck, Nerviano Medical Sciences (I), QED Therapeutics (I), RedHill Biopharma (I), Yiviva (I), Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, BioSapien, Thetis Pharma, Cend Therapeutics, Flatiron Health (I)
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Sonal Bordia
Employment: Merck
David McGuinness
Employment: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Karen Cui
Employment: AstraZeneca
Stock and Other Ownership Interests: Bristol Myers Squibb, AstraZeneca
Gershon Y. Locker
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Talia Golan
Honoraria: MSD, AstraZeneca/MedImmune, AbbVie, BioLineRx
Consulting or Advisory Role: AbbVie, AstraZeneca
Speakers' Bureau: AstraZeneca, MSD Oncology
Research Funding: AstraZeneca (Inst), MSD (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as an abstract at the ASCO Gastrointestinal Cancers Symposium 2021.
SUPPORT
Supported by AstraZeneca, as part of an alliance between AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ, and by a grant (P30-17 CA008748) from the National Institutes of Health National Cancer Institute Cancer Center.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
AUTHOR CONTRIBUTIONS
Conception and design: Hedy L. Kindler, Michele Reni, Eric Van Cutsem, Teresa Macarulla, Anke Reinacher-Schick, Gershon Y. Locker, Talia Golan
Administrative support: Dirk Arnold, Gershon Y. Locker
Provision of study materials or patients: Pascal Hammel, Eric Van Cutsem, Teresa Macarulla, Michael J. Hall, Giampaolo Tortora, Hana Algül, Eileen M. O'Reilly, Talia Golan
Collection and assembly of data: Hedy L. Kindler, Pascal Hammel, Michele Reni, Eric Van Cutsem, Teresa Macarulla, Michael J. Hall, Joon Oh Park, Daniel Hochhauser, Dirk Arnold, Anke Reinacher-Schick, Giampaolo Tortora, Hana Algül, Eileen M. O'Reilly, Karen Cui, Gershon Y. Locker, Talia Golan
Data analysis and interpretation: Hedy L. Kindler, Pascal Hammel, Michele Reni, Eric Van Cutsem, Teresa Macarulla, Michael J. Hall, Joon Oh Park, Dirk Arnold, Do-Youn Oh, Anke Reinacher-Schick, Giampaolo Tortora, Eileen M. O'Reilly, Sonal Bordia, David McGuinness, Karen Cui, Gershon Y. Locker, Talia Golan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hedy L. Kindler
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Deciphera, Novocure, Seattle Genetics, Inhibrx
Research Funding: Aduro Biotech (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Merck (Inst), Verastem (Inst), Bristol Myers Squibb (Inst), Polaris (Inst), Deciphera (Inst), Inhibrx (Inst), Roche/Genentech (Inst), Tesaro (Inst), MacroGenics (Inst), Leap Therapeutics (Inst), FibroGen (Inst), Vivace Therapeutics (Inst), Constellation Pharmaceuticals (Inst), Harpoon Therapeutics (Inst), Bayer (Inst), Seattle Genetics (Inst), Blueprint Medicines (Inst)
Pascal Hammel
Consulting or Advisory Role: Vect-Horus, ERYTECH Pharma (Inst), AstraZeneca (Inst), Rafael Pharmaceuticals, Mylan, Ipsen (Inst)
Speakers' Bureau: AstraZeneca, Servier, Mylan
Research Funding: ERYTECH Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Halozyme (Inst)
Travel, Accommodations, Expenses: Ipsen, Halozyme, Shire, Pfizer/EMD Serono, Vect-Horus
Michele Reni
Consulting or Advisory Role: Celgene, Lilly, AstraZeneca, Panavance Therapeutics, Viatris, Sotio, Servier, MSD/AstraZeneca
Research Funding: Celgene (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Celgene
Other Relationship: Celgene, AstraZeneca
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GlaxoSmithKline, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte, Astellas Pharma
Research Funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol Myers Squibb (Inst)
Teresa Macarulla
Consulting or Advisory Role: Sanofi/Aventis, Celgene, Roche, QED Therapeutics, Baxter, Incyte, Servier, Lilly, Ipsen, AstraZeneca, MSD, Eisai, Genzyme, Menarini, Prime Oncology, Ability Pharma, Advance Medical, BioLineRX, Zymeworks, Aptitude Health, Swedish Orphan Biovitrum, Basilea, Medscape, Novocure, Paraxel, PPD, Ellipses Pharma, Hirslanden/GITZ, Imedex, Janssen, MFAR, Marketing Farmacéutico & Investigación Clínica, Polaris Consulting, Scilink Comunicación Científica SC, Surface Oncology, ANGEM
Research Funding: Celgene (Inst), Agios (Inst), ASLAN Pharmaceuticals (Inst), Bayer (Inst), Roche (Inst), Genentech (Inst), AstraZeneca (Inst), Immunomedics (Inst), Lilly (Inst), Merrimack (Inst), Millennium (Inst), Novocure (Inst), Pfizer (Inst), Pharmacyclics (Inst), AbbVie (Inst), Ability Pharma (Inst), Amc Medical Research (Inst), Amgen (Inst), ARMO Biosciences (Inst), Basilea Pharmaceutica International (Inst), BeiGene (Inst), Keralty Group (Inst), BioLineRx (Inst), Blueprint Medicines (Inst), Boston Biomedical (Inst), Bristol Myers Squibb (BMS) (Inst), Cantargia AB (Inst), Eisai (Inst), ERYTECH Pharma (Inst), F. Hoffmann-la Roche (Inst), FibroGen (Inst), Halozyme (Inst), Incyte (Inst), Ipsen (Inst), Loxo (Inst), Medimmune (Inst), Merck Sharp & Dohme (Inst), Nelum Corp (Inst), Novartis (Inst), OncoMed (Inst), VCN Biosciences (Inst), Zymeworks (Inst)
Travel, Accommodations, Expenses: Merck, H3 Biomedicine, Sanofi, Celgene, Servier, Prime Oncology, Incyte
Michael J. Hall
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: I share a patent with several Fox Chase investigators for a novel method to investigate hereditary CRC genes (Inst)
Travel, Accommodations, Expenses: GRAIL
Other Relationship: Myriad Genetics, Invitae, Caris Life Sciences
Joon Oh Park
Consulting or Advisory Role: Celgene, Merck Serono, Servier, AstraZeneca, MediRama
Research Funding: Celgene, Medpacto, Servier
Daniel Hochhauser
Stock and Other Ownership Interests: Roche, Novartis
Research Funding: Merck Serono
Travel, Accommodations, Expenses: Celgene
Dirk Arnold
Employment: Asklepios Kliniken
Honoraria: Bayer, Merck Serono, Roche/Genentech, Servier, Bristol Myers Squibb, Merck Sharp and Dome, AstraZeneca, Amgen, Boston Scientific, Pierre Fabre, Ipsen
Consulting or Advisory Role: Bayer, Merck Serono, Biocompatibles, Terumo, Bristol Myers Squibb, MSD Oncology, AstraZeneca
Research Funding: Roche/Genentech (Inst), Sanofi (Inst), Oncolytics (Inst)
Travel, Accommodations, Expenses: Boston Scientific
Uncompensated Relationships: ESMO Council, ESMO Journals (Ann Oncol, ESMO Open), German Society for Hematology and Medical Oncology, German Cancer Society, European Organisation for Research and Treatment of Cancer (EORTC)
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho Pharmaceutical, ASLAN Pharmaceuticals, Halozyme, Zymeworks, Celgene, Basilea, BeiGene, Turning Point Therapeutics
Research Funding: AstraZeneca, Novartis, Array BioPharma, Lilly, Servier, BeiGene, MSD, Handok
Anke Reinacher-Schick
Honoraria: Amgen, Roche, Merck Serono, Bristol Myers Squibb, MSD, MCI Group, AstraZeneca
Consulting or Advisory Role: Amgen, Roche, Merck Serono, Bristol Myers Squibb, MSD, AstraZeneca, Pierre Fabre
Research Funding: Roche (Inst), Celgene (Inst), Ipsen (Inst), Amgen (Inst), Alexion Pharmaceuticals (Inst), AstraZeneca (Inst), Lilly (Inst), Servier (Inst), AIO-Studien (Inst), Rafael Pharmaceuticals (Inst), ERYTECH Pharma (Inst), BioNTech (Inst)
Travel, Accommodations, Expenses: Roche
Giampaolo Tortora
Consulting or Advisory Role: Celgene, Merck Serono, MSD Oncology, Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: Merck Serono, Roche
Hana Algül
Consulting or Advisory Role: Pfizer, Servier/Pfizer, AstraZeneca
Research Funding: Chugai Pharma (Inst)
Eileen M. O'Reilly
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Adicet Bio (I), AstraZeneca (I), Alnylam (I), Autem Medical (I), BeiGene (I), Berry Genomics (I), CytomX Therapeutics, Eisai (I), Exelixis (I), Genentech/Roche (I), Genoscience Pharma (I), Helio Health (I), Incyte (I), Ipsen (I), Legend Biotech (I), Merck, Nerviano Medical Sciences (I), QED Therapeutics (I), RedHill Biopharma (I), Yiviva (I), Novartis, Rafael Pharmaceuticals, Seattle Genetics, Boehringer Ingelheim, IDEAYA Biosciences, Noxxon Pharma, BioSapien, Thetis Pharma, Cend Therapeutics, Flatiron Health (I)
Research Funding: AstraZeneca/MedImmune (Inst), Celgene (Inst), Genentech (Inst), Roche (Inst), Silenseed (Inst), Arcus Ventures (Inst), BioNTech (Inst), Elicio Therapeutics (Inst), Parker Institute for Cancer Immunotherapy (Inst)
Sonal Bordia
Employment: Merck
David McGuinness
Employment: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Karen Cui
Employment: AstraZeneca
Stock and Other Ownership Interests: Bristol Myers Squibb, AstraZeneca
Gershon Y. Locker
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Talia Golan
Honoraria: MSD, AstraZeneca/MedImmune, AbbVie, BioLineRx
Consulting or Advisory Role: AbbVie, AstraZeneca
Speakers' Bureau: AstraZeneca, MSD Oncology
Research Funding: AstraZeneca (Inst), MSD (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rawla P, Sunkara T, Gaduputi V: Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J Oncol 10:10-27, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Pancreatic Cancer Collaborators : The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 4:934-947, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2020. CA Cancer J Clin 70:7-30, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Ducreux M, Cuhna AS, Caramella C, et al. : Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v56-68, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 6.European Society for Medical Oncology (ESMO) : Guidelines Committee. eUpdate—Cancer of the pancreas treatment recommendations. 2019. https://www.esmo.org/guidelines/gastrointestinal-cancers/pancreatic-cancer/eupdate-cancer-of-the-pancreas-treatment-recommendations
- 7.Sohal DP, Mangu PB, Laheru D: Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract 13:261-264, 2017 [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network : Clinical practice guidelines in oncology: NCCN guidelines for pancreatic adenocarcinoma V.1.2021. https://www.nccn.org
- 9.Conroy T, Desseigne F, Ychou M, et al. : FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817-1825, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP, et al. : Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691-1703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welcsh PL, King MC: BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet 10:705-713, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Oh M, Alkhushaym N, Fallatah S, et al. : The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 79:880-895, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Ghiorzo P: Genetic predisposition to pancreatic cancer. World J Gastroenterol 20:10778-10789, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holter S, Borgida A, Dodd A, et al. : Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33:3124-3129, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Friedenson B: BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed 7:60, 2005 [PMC free article] [PubMed] [Google Scholar]
- 16.Golan T, Kindler HL, Park JO, et al. : Geographic and ethnic heterogeneity of germline BRCA1 or BRCA2 mutation prevalence among patients with metastatic pancreatic cancer screened for entry into the POLO trial. J Clin Oncol 38:1442-1454, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Peretti U, Cavaliere A, Niger M, et al. : Germinal BRCA1-2 pathogenic variants (gBRCA1-2pv) and pancreatic cancer: Epidemiology of an Italian patient cohort. ESMO Open 6:100032, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh CS: Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol 137:343-350, 2015 [DOI] [PubMed] [Google Scholar]
- 19.O'Connor MJ: Targeting the DNA damage response in cancer. Mol Cell 60:547-560, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Golan T, Kanji ZS, Epelbaum R, et al. : Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111:1132-1138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waddell N, Pajic M, Patch AM, et al. : Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518:495-501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong PC, Boss DS, Yap TA, et al. : Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123-134, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Sachdev E, Tabatabai R, Roy V, et al. : PARP inhibition in cancer: An update on clinical development. Target Oncol 14:657-679, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Wei M, Xu J, et al. : PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol Cancer 19:49, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan T, Hammel P, Reni M, et al. : Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan PS, Bilger M, de Lima Lopes G, et al. : Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations. Cancer Med 6:1847-1860, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammel P, Vitellius C, Boisteau E, et al. : Maintenance therapies in metastatic pancreatic cancer: Present and future with a focus on PARP inhibitors. Ther Adv Med Oncol 12:1758835920937949, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reverdy T, Sajous C, Peron J, et al. : Front-line maintenance therapy in advanced ovarian cancer-current advances and perspectives. Cancers (Basel) 12:2414, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gogineni V, Morand S, Staats H, et al. : Current ovarian cancer maintenance strategies and promising new developments. J Cancer 12:38-53, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín-Richard M, Tobeña M: First-line maintenance treatment in metastatic colorectal cancer (mCRC): Quality and clinical benefit overview. J Clin Med 10:470, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pujade-Lauraine E, Ledermann JA, Selle F, et al. : Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18:1274-1284, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Robson M, Im SA, Senkus E, et al. : Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523-533, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration : Lynparza prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf
- 35.European Medicines Agency : Lynparza. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza#authorisation-details-section
- 36.AstraZeneca : AstraZeneca global policy: Bioethics. 2016. https://www.astrazeneca.com/content/dam/az/our-company/Documents/Bioethics%20policy%207.0.pdf
- 37.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 38.O'Reilly EM, Lee JW, Zalupski M, et al. : Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol 38:1378-1388, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiss KA, Mick R, O'Hara MH, et al. : Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol 39:2497-2505, 2021 [DOI] [PubMed] [Google Scholar]
- 40.Lin KK, Harrell MI, Oza AM, et al. : BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 9:210-219, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Matulonis UA, Oza AM, Ho TW, et al. : Intermediate clinical endpoints: A bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 121:1737-1746, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Pasalic D, McGinnis GJ, Fuller CD, et al. : Progression-free survival is a suboptimal predictor for overall survival among metastatic solid tumour clinical trials. Eur J Cancer 136:176-185, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wattenberg MM, Asch D, Yu S, et al. : Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer 122:333-339, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammel P, Kindler HL, Reni M, et al. : Health-related quality of life in patients with a germline BRCA mutation and metastatic pancreatic cancer receiving maintenance olaparib. Ann Oncol 30:1959-1968, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.