Abstract
According to current research, the primary active ingredients of Radix Astragali (RA), such as saponins, flavonoids, and polysaccharides, play an important role in anti-inflammatory effects. However, the exact molecular mechanism underlying the action was not elucidated to date. Our research attempted to determine the active components in RA and to investigate the interaction between the active components and targets involved in the anti-inflammation activity by network pharmacology and molecular docking. The active components and targets of RA were screened out by TCMSP. Thereafter, through the “anti-inflammation effect” and “inflammation” as the keywords, disease targets were obtained from the GeneCards database. The PPI network was constructed with Cytoscape 3.8.0 software to screen core targets. The GO function and KEGG analysis were enriched and analyzed through the Metascape platform, obtaining the 3-dimensional view of the core targets from the PDB database, and then, performing molecular docking in AutoDock Vina, a heatmap was constructed using the binding free energies in GraphPad Prism 8. The Discovery Studio software was used for docking analysis, and eventually, the docking results were visualized. We also explored the targets and signaling pathways of Astragaloside IV acting on anti-inflammatory effects via constructing compound-disease-target-pathway network. 18 active components and 45 targets of RA were screened out. The main anti-inflammatory active components of RA were quercetin, Astragaloside IV, kaempferol, 7-O-methylisomucronulatol, and formononetin, and the strongly interacting core proteins were TNF, IL6, IL1B, TLR4, CXCL8, CCL2, IL10, VEGFA, and MMP9. The signal pathways mainly involved include Lipid and atherosclerosis, IL-17 signaling pathway, Chagas disease, leishmaniasis, and TNF signaling pathway. Moreover, molecular docking showed that the 2 most active compounds, Astragaloside IV and kaempferol, could efficiently bind with the targets TNF, TLR4, and IL10. Astragaloside IV may play a part in anti-inflammatory effects through pathways such as HIF-1 signaling pathway, Inflammatory bowel disease and Hepatitis B ect. RA exhibits the characteristic of multicomponent and multitarget synergistic effects in exerting anti-inflammatory effects and the effective component of RA is Astragaloside IV, targeting TNF, TLR4, and IL10.
Keywords: anti-inflammatory, molecular docking, network pharmacology, radix astragali
1. Introduction
The inflammatory response is a dynamic defense response of an organism against invading pathogens. This response involves a series of complex processes, including phagocytosis and the release of inflammatory cytokines and chemokines, which can help the organism to destroy and eliminate pathogens.[1] However, excessive and persistent inflammation can cause irreversible damage, even life-threatening, to the organism itself.[2] Currently, several studies have considered inflammation resolution as a new method to treat inflammatory diseases.[3] The therapeutic effects of contemporary anti-inflammatory drugs have been adequately confirmed; however, the long-term use of such drugs generally leads to serious and life-threatening side effects. Therefore, product-based anti-inflammatory compounds with superior efficacy and minimal toxicity can be used as possible therapeutic alternatives.[4] Hence, it is particularly important to search for anti-inflammatory drugs and determine the drug components primarily responsible for the anti-inflammatory effects.
Radix Astragali (RA), called Huangqi in Chinese, is the dried root of the Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or the Astragalus membranaceus (Fisch.) Bge, which is distributed in provinces and regions, such as Inner Mongolia, Shaanxi, Gansu, Qinghai, Sichuan, and Tibet. In Shen Nong’s Herbal Classic written in 200 AD, it was first described that RA demonstrated a wide range of therapeutic effects and low toxicity, nourishing qi and blood, and was often used in the Pharmacopoeia of the People’s Republic of China to treat the “qi deficiency” syndrome.[5,6] Clinically, RA is used as the primary component of multiple polyhydroxy formulations for treating patients with cancer and inflammation-related diseases. As a traditional Chinese medicine (TCM), it exhibits various pharmacological effects, such as immunity enhancement, metabolism regulation, and antioxidant and antitumor activities.[7,8] Recent studies have shown that RA exhibits strong anti-inflammatory activity, but its anti-inflammatory mechanism still needs to be elucidated.
In 2007, the concept of network pharmacology was first described, and in 2008, it was introduced into the activity exploration of TCM. Since then, network pharmacology has been successfully applied in the field of TCM research.[9] Network pharmacology maintains systematic characteristics by establishing a data analysis database and extracting relevant information through the network platform, as well as using relevant software to analyze data, build a drug-target-disease information network, and then ultimately discover the pharmacological and toxic activities of drugs and the mechanisms of their action. Network pharmacology has emerged as a novel and effective method for scientifically interpreting the complex pharmacological mechanisms underlying the activities of TCM owing to its advantages in predicting drug targets and improving drug discovery efficiency.[10] Simultaneously, molecular docking studies the pharmacological effects from the molecular level, thereby revealing the molecular mechanism of drug actions.[11]
Through network pharmacology and molecular docking, the current study determined the effective ingredients and target genes of RA and then constructed protein–protein interaction (PPI) by searching the RA-anti-inflammation intersection targets, eventually determining the core targets. Next, by the molecular docking of core targets and primary active components, the underlying molecular mechanism of the anti-inflammatory effect of RA was confirmed. These findings will provide new methods for the research and treatment of inflammatory diseases. The research flowchart is described in Figure 1.
Figure 1.
A flowchart of the anti-inflammatory effect of RA. DL = drug-likeness, GO = gene ontology, KEGG = Kyoto encyclopedia of genes and genomes, OB = oral bioavailability, RA = Radix Astragali, TCMSP = Traditional Chinese Medicine System Pharmacology.
2. Materials and methods
2.1. Screening active ingredients and gene targets
Using the Traditional Chinese Medicine System Pharmacology (TCMSP) database (http://tcmspw.com/tcmsp.php), the active ingredients of RA were detected. Based on the absorption of exogenous chemicals by ADME, the compounds with oral bioavailability (OB) ≥ 30% and druglike (DL) ≥ 0.18 were selected. Then, the targets were determined according to MOL.ID of the effective compounds. Furthermore, through reference consultation, we learned that several studies are available on the anti-inflammatory activity of Astragaloside IV, despite the compound does not meet the OB and DL standards, it was still added.[12–16] In the PubChem database (https://pubchem.ncbi.nlm.nih.gov), we searched the Canonical SMILES number of Astragaloside IV and predicted its targets through the Super-PRED database (https://prediction.charite.de). Thereafter, all protein targets were imported into the UniProt database (https://www.uniprot.org/) for normalization, and the duplicated targets were removed.
The drug components were numbered, and then the “network” files and “type” files were prepared, which contain the drug, drug active ingredients, genes, and classification information, importing them into the Cytoscape 3.8.0 software for constructing a drug-component-target network, to perform network topology analysis. The shape, color, transparency, and size of the target point were adjusted according to the degree value (number of gene connections). The size of the drug components or genes represented the degree value of the connection. The larger the shape of the drug component or gene, the greater the degree value of connectivity of the drug component or gene in the network.
2.2. Constructing the Venny diagram
Disease-related targets with the keywords “inflammation” and “anti-inflammatory” were obtained from the Gene Cards database. Using the Relevance score ≥ 10 as the screening criterion, the disease targets were retained after removing duplicates. The online tool (http://www.bioinformatics.com.cn/) was used to create the Venny diagram to obtain the cross targets of the active components of RA, as well as the inflammatory targets. The drug–disease intersection genes are the potential targets for anti-inflammatory drug components.
2.3. PPI network construction and cluster analysis
First, we imported the data of the intersection genes into the String platform (https://string-db.org/). The species was limited to “Homo sapiens,” a “Medium confidence < 0.400>” was set in the “Required score” and a “Medium 5 percent” in the “FDR string.” Finally, the “.tsv” file was downloaded, and the results were imported into the Cytoscape 3.8.0 software to construct a PPI network diagram and screen core genes.
2.4. GO and KEGG analysis
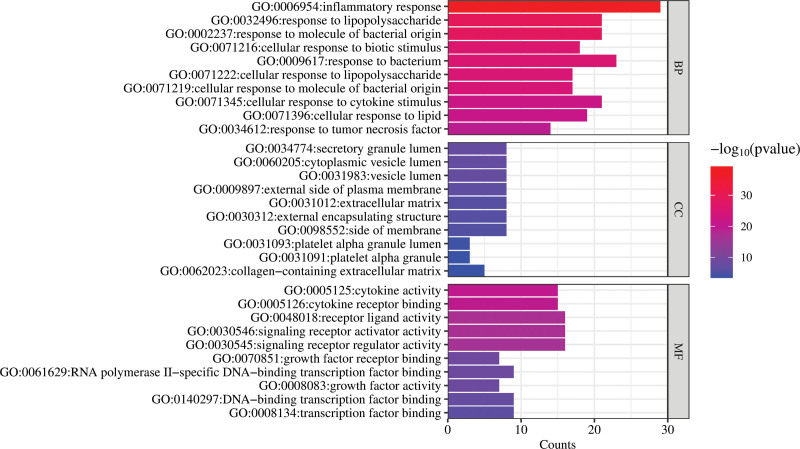
In the Metascape database (http://metascape.org/), Gene ontology (GO) functional analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed. GO is the primary bioinformatics tool for annotating genes and analyzing processes such as molecular function (MF), cellular components (CC), and biological processes (BP).[17] KEGG is used to focus on advanced functions and biological systems using large-scale molecular data generated from high-throughput experimental techniques.[13,18] In the Metascape database (https://metascape.org/gp/index.html), the intersection genes were copied to the website, “Homo sapiens” was set as the species screening conditions, and the custom analysis was performed. The obtained report page was finally downloaded. Thereafter, the results of the GO functional analysis and KEGG pathway enrichment analysis of the intersection genes were secured. In the BP, CC, and MF analysis of GO, the top 10 entries were selected to create the GO functional analysis diagram using the online tool (http://www.bioinformatics.com.cn/). The top 20 related pathways in the KEGG pathway were screened out and constructed a KEGG enrichment analysis diagram was constructed. The GO functional analysis and KEGG pathway enrichment analysis were visualized using the 2 aforementioned diagrams.
2.5. Molecular docking
AutoDock Tools 1.5.7, Discovery Studio 2019 Client, and PyMOL software were used for revealing the interactions between the active RA components and target proteins. The 3D structures of the compounds were downloaded as mol2 files from the TCMSP database. During the docking process, the torsion centers and torsion bonds were detected. The 3D molecular structure files were obtained from the PDB database (https://www1.rcsb.org/), and the Autodock software was used to delete water molecules and hydrogenation for target proteins and hydrogenate the drug ligand molecules. Molecular docking was performed at the site where the original inhibitor of the protein was located, and in the absence of an auto-ligand inhibitor, an active pocket site was established on the entire protein. Finally, AutoDock Vina was used to dock and determine the optimal structure of the active ligands. The docking mode was semiflexible docking, the exhaustiveness was set to 8 and the maximum number of conformation output was set to 10. PyMOL was used for protein dehydration, and the protein-ligand complexes were derived. All target proteins were docked with the ligands, and the lowest binding energy was used to construct a heat map. The Autodock Vina results and core proteins were imported into PyMoL and saved in PDB format. Thereafter, the Discovery Studio software was used for analysis, and eventually, the docking results were visualized.
3. Results
3.1. Prediction of active drug components and targets of RA
The findings from the TCMSP database were collected, and from then, screened a total of 87 compounds were screened out. Among these 87 compounds, 17 met the OB ≥ 30% and DL ≥ 0.18 criteria, and had corresponding targets. The chemical structural formulas and active targets of the 17 compounds were downloaded in the mol2 format. By consulting references, Astragaloside IV was included despite the fact that it did not meet the screening criteria. Its targets were predicted using the Super-PRED database. Ultimately, a total of 279 gene targets were obtained.
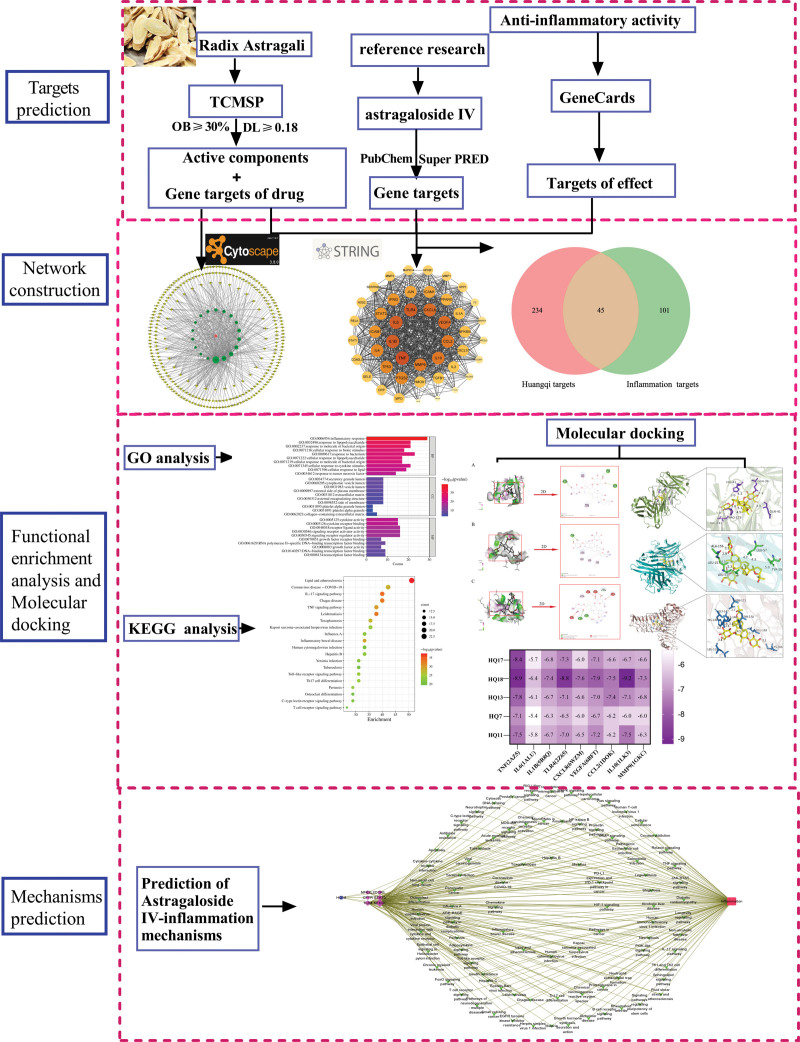
The active ingredients of Huangqi were numbered (Table 1), and the Network and Type files containing the active ingredients of the drugs, related genes, and classification information were prepared. These were imported into Cytoscape 3.8.0 software, and a drug-component-target map was constructed (Fig. 2). The green, brown-yellow, and red circles represented the active ingredients, targets, and Huangqi respectively, which were involved in the anti-inflammatory effect. The top 5 components of higher degree are HQ17, HQ18, HQ13, HQ7, and HQ11, which are the main active components responsible for the anti-inflammatory effect of Huangqi.
Table 1.
The active components of Huangqi.
| Number | ID | Name | Degree |
|---|---|---|---|
| HQ1 | MOL000211 | Mairin | 1 |
| HQ2 | MOL000239 | Jaranol | 13 |
| HQ3 | MOL000296 | hederagenin | 23 |
| HQ4 | MOL000033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | 1 |
| HQ5 | MOL000354 | isorhamnetin | 36 |
| HQ6 | MOL000371 | 3,9-di-O-methylnissolin | 23 |
| HQ7 | MOL000378 | 7-O-methylisomucronulatol | 45 |
| HQ8 | MOL000379 | 9,10-dimethoxypterocarpan-3-O-β-D-glucoside | 3 |
| HQ9 | MOL000380 | (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-3-ol | 22 |
| HQ10 | MOL000387 | Bifendate | 7 |
| HQ11 | MOL000392 | formononetin | 38 |
| HQ12 | MOL000417 | Calycosin | 22 |
| HQ13 | MOL000422 | kaempferol | 62 |
| HQ14 | MOL000433 | FA | 3 |
| HQ15 | MOL000439 | isomucronulatol-7,2’-di-O-glucosiole | 1 |
| HQ16 | MOL000442 | 1,7-Dihydroxy-3,9-dimethoxy pterocarpene | 4 |
| HQ17 | MOL000098 | quercetin | 151 |
| HQ18 | MOL000409 | Astragaloside IV | 80 |
Figure 2.
Drug-component-target network.
3.2. Overlapping targets between RA and anti-inflammatory effect
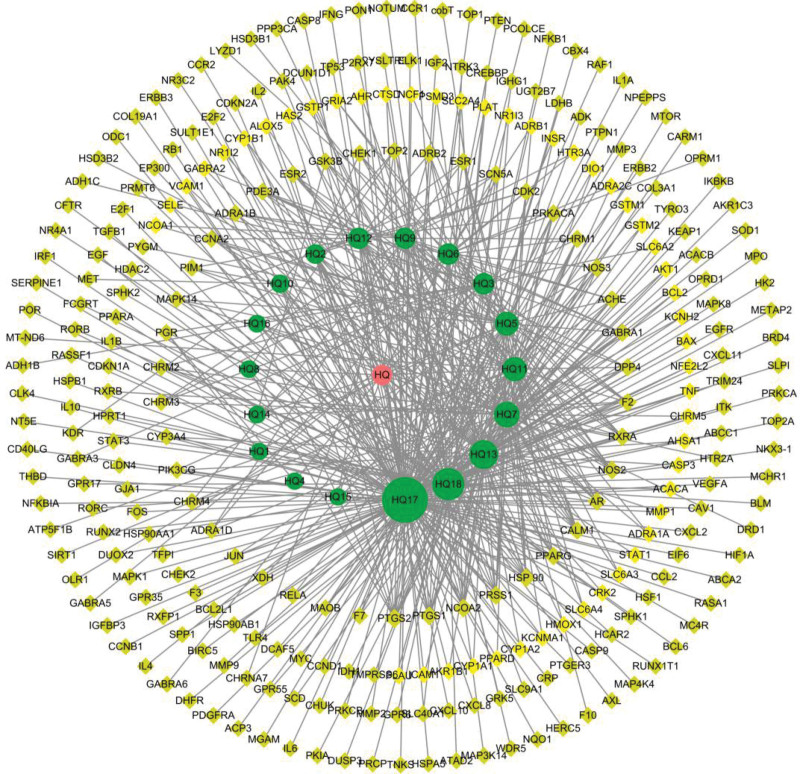
Using “inflammation” and “anti-inflammatory” as the keywords, 11,448 and 3961 targets were obtained from the Gene Cards database. Using the Relevance score ≥ 10 as the screening criterion, 132 and 50 targets were selected, respectively. After removing duplication, 146 relevant targets were retained. The online tool (http://www.bioinformatics.com.cn/) was used to create the Venny diagram (Fig. 3). The drug-disease intersection genes and 45 intersection targets were obtained.
Figure 3.
Venn diagram of the potential gene targets.
3.3. PPI network
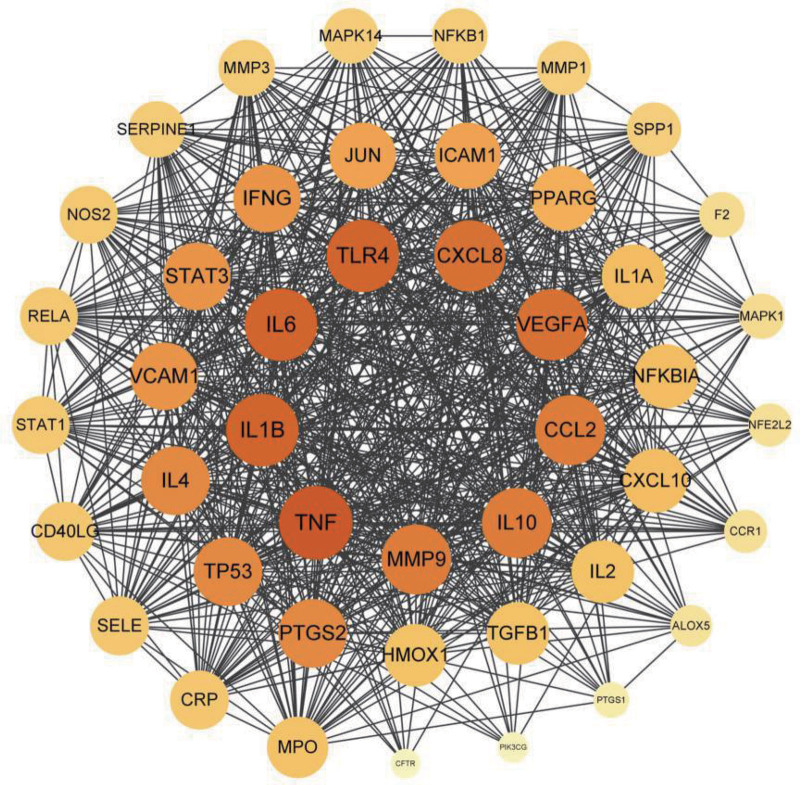
The intersection genes of the “Huangqi-anti-inflammatory effect” were copied into the String platform and downloaded as the “.tsv” file. Subsequently, the “.tsv” file was imported into the Cytoscape 3.80 software to construct the PPI network and screen the core genes (Fig. 4). detailed information on the core proteins is provided on account of degree value in Table 2. The PPI network involves 45 nodes and 734 edges. The top 9 active targets included TNF (tumor necrosis factor, degree = 44), IL1B (Interleukin-1 beta, degree = 43), IL6 (Interleukin-6, degree = 43), TLR4 (Toll-like receptor 4, degree = 43), CXCL8 (Interleukin-8, degree = 42), VEGFA (vascular endothelial growth factor A, degree = 42), CCL2 (C–C motif chemokine 2, degree = 41), IL10 (Interleukin-10, degree = 41), MMP9 (Matrix metalloproteinase-9, degree = 41). These 9 inner circle genes may be the core targets for Huangqi to exert its anti-inflammatory effect.
Figure 4.
PPI network analysis. PPI = protein–protein interaction.
Table 2.
Information of 9 core targets.
| Uniprot ID | Gene symbol | Protein name | Degree |
|---|---|---|---|
| P01375 | TNF | Tumor necrosis factor | 44 |
| P05231 | IL6 | Interleukin-6 | 43 |
| P01584 | IL1B | Interleukin-1 beta | 43 |
| O00206 | TLR4 | Toll-like receptor 4 | 43 |
| P10145 | CXCL8 | Interleukin-8 | 42 |
| P15692 | VEGFA | Vascular endothelial growth factor A | 42 |
| P13500 | CCL2 | C-C motif chemokine 2 | 41 |
| P22301 | IL10 | Interleukin-10 | 41 |
| P14780 | MMP9 | Matrix metalloproteinase-9 | 41 |
CCL2 = C–C motif chemokine 2, CXCL8 = interleukin-8, IL10 = interleukin-10, IL1B = interleukin-1B, IL-6 = interleukin-6, MMP9 = matrix metalloproteinase-9, TLR4 = toll-like receptor 4, TNF = tumor necrosis factor, VEGFA = vascular endothelial growth factor A.
3.4. GO functional analysis and KEGG enrichment analysis of RA for anti-inflammatory effect
GO is the primary bioinformatics tool for annotating genes and analyzing them. The enrichment of BP indicates the involvement of intersecting proteins in the biological processes. Further, the CC analysis suggests that the intersection proteins are involved in the cellular environment. MF, that is, the protein function at the molecular level, can catalyze biochemical reactions.
A total of 45 intersection genes related to the Huangqi-anti-inflammatory effects were introduced into the Metascape platform and GO functional analysis was performed from the targets exerting the anti-inflammatory effects at the BP, CC, and MF levels. The top 10 items were separately selected for visual analysis (Fig. 5). BP had a total of 974 items, mainly including inflammatory response, response to lipopolysaccharide, response to the molecules of bacterial origin, and cellular response to a biotic stimulus. CC included 21 items, mainly involving secretory granule lumen, cytoplasmic vesicle lumen, vesicle lumen, and platelet alpha granule lumen. MF had 60 items, mainly including cytokine activity, cytokine receptor binding, receptor–ligand activity, and signaling receptor activator activity molecular function. The length of the bars in Figure 5 represents the count of the targets, and the color scale (blue to red) indicates the size of the logarithmic P value of each item; the closer to red, the smaller the P value, the more significant the entry. The results of GO functional analysis showed that Huangqi mainly regulated the inflammatory response, lipopolysaccharide response, and other related inflammatory processes in the body by regulating the combination of substances released in the vesicle lumen and some related receptors.
Figure 5.
GO functional analysis. GO = gene ontology.
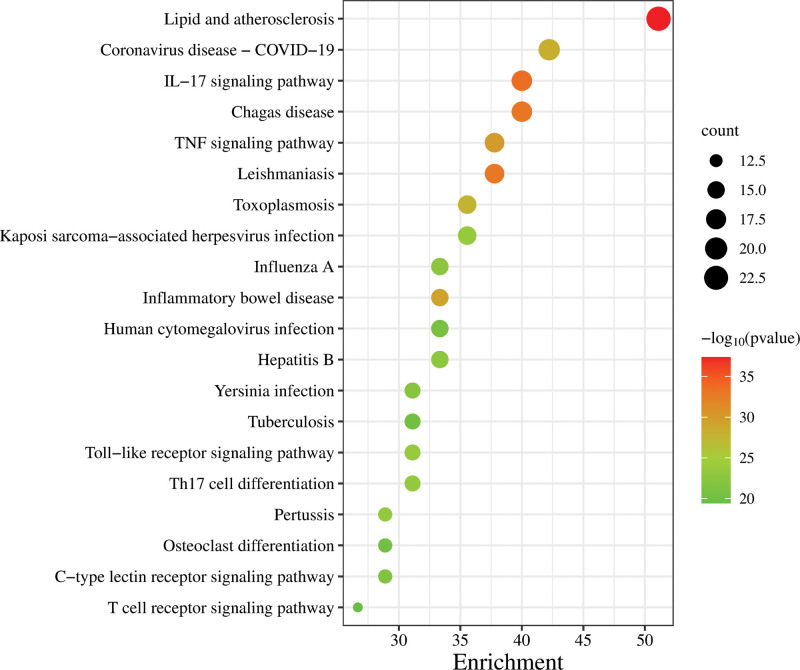
A total of 128 signaling pathways were involved in the KEGG analysis. In the KEGG enrichment analysis, the top 20 signaling pathways were selected based on the logarithmic P value and the number of genes involved in each pathway. These pathways were strongly linked to the anti-inflammatory effects of the drugs. The size of the circle indicates the number of targets, and the color scale (green to red) demonstrates the size of the logarithmic P value of the pathway; the closer to the red, the more significant the pathway in exerting the anti-inflammatory effect. As shown in Figure 6, the anti-inflammatory activity pathways were mainly enriched in Lipid and atherosclerosis, IL-17 signaling pathway, Chagas disease, Leishmaniasis, TNF signaling pathway, and inflammatory bowel disease signaling pathways, which suggested that the active components of Huangqi probably exert the anti-inflammatory effects through multiple signaling pathways. Therefore, Huangqi can target various functions and biological factors to exhibit its anti-inflammatory effects. However, this conclusion needs to be verified further.
Figure 6.
KEGG enrichment analysis. IL-17 = interleukin-17, KEGG = Kyoto encyclopedia of genes and genomes, TNF = tumor necrosis factor.
3.5. Visualization of molecular docking results
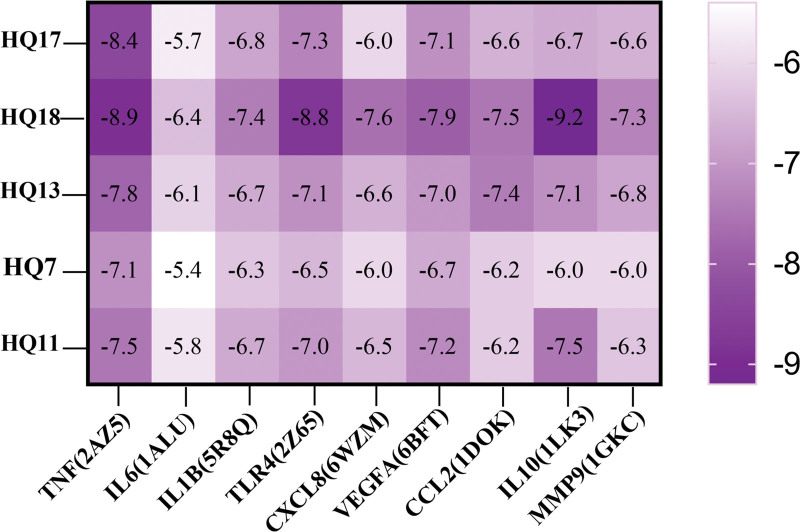
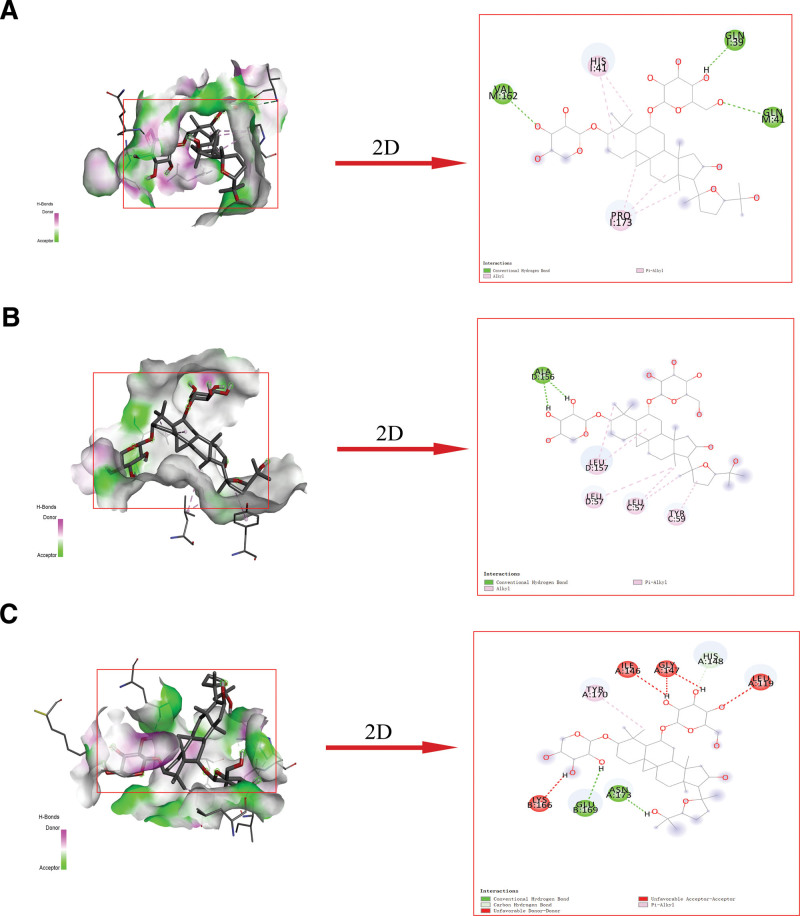
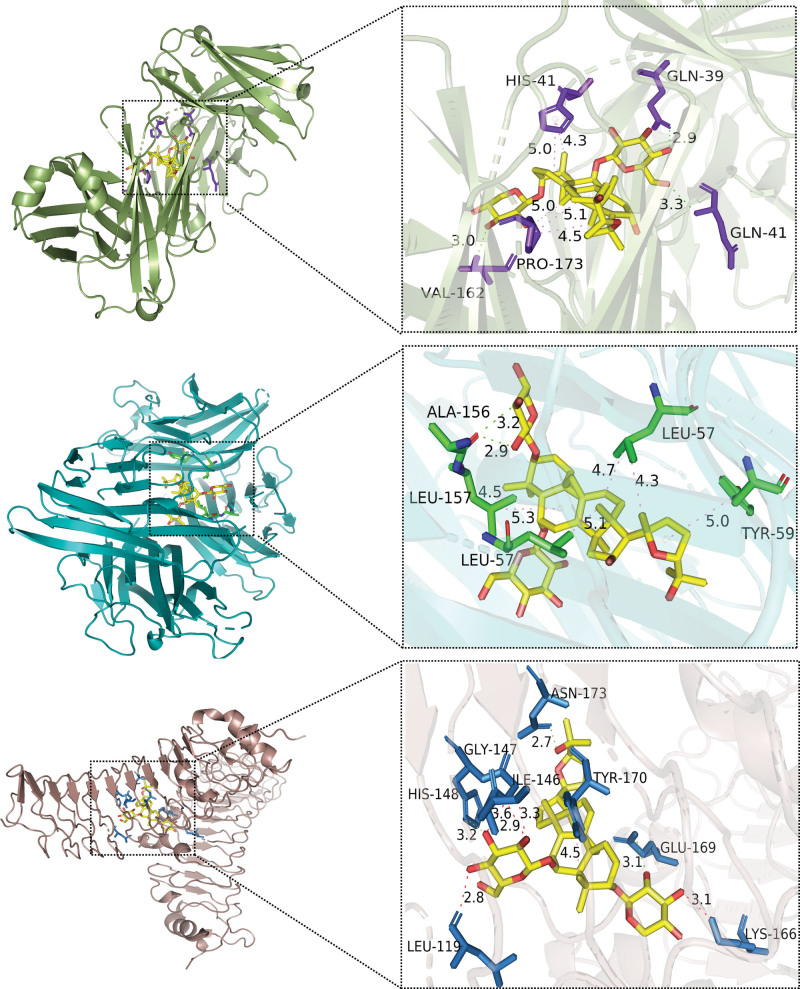
Molecular docking was conducted based on related targets and the main active ingredients screened from the drug-ingredient targets. The interactions between the potential active compounds and core targets were analyzed using AutoDock Vina 1.5.7, Discovery Studio 4.5 Client, and PyMoL. The first 5 active compounds were selected, namely HQ17 (quercetin, MOL000098), HQ18 (Astragaloside IV, MOL000409), HQ13 (kaempferol, MOL000422), HQ7 (7-O-methylisomucronulatol, MOL000378), and HQ11 (formononetin, MOL000392). The protein structures of the core targets were obtained from the PDB database, including TNF (PDB ID:2AZ5), IL6 (PDB ID:1ALU), IL1B (PDB ID:5R8Q), TLR4 (PDB ID:2Z65), CXCL8 (PDB ID: 6WZM), CCL2 (PDB ID: 1DOK), IL10 (PDB ID: 1LK3), VEGFA (PDB ID: 6BFT), and MMP9 (PDB ID: 1GKC). The binding energies were obtained from the docking analysis of AutoDock Vina 1.5.7, and a heat map was constructed (Fig. 7). Lower binding energy values express stronger binding capacity. Despite the combination of IL 6 and HQ17, HQ7, and HQ11, all docking binding energies were ≤−6 kcal/mol, which indicates stable binding between the active components and protein targets. The findings showed that the intermolecular forces such as hydrogen bonding and hydrophobic effects (pi-sigma, pi-alkyl, and alkyl interactions) are primarily functional between the residues of active protein targets and potential active components. The first 3 docking results with the lowest docking binding energies are shown in Figure 8. The Astragaloside IV-IL 10 complex was stabilized by 3 hydrogen bonds with the residues GLN 41, VAL 162 on the B chain, GLN 39 on the A chain, and the pi-alkyl and alkyl interactions were stabilized by HIS 41, PRO 173 on the I chain (Fig. 8A). Meanwhile, the Astragaloside IV-TNF complex exhibited 1 hydrogen bond of ALA 156 on the D chain, 4 pi-alkyl, and alkyl interactions, including LEU 57, TYR 59 on the C chain and LEU 57, LEU 157 on the D chain (Fig. 8B). In addition, the Astragaloside IV-TLR4 complex formed 2 hydrogen bonds with residues ASN 173 on the A chain and GLU 169 on the B chain and 1 pi-alkyl interaction with TYR 170 on the A chain. The red band indicates that this amino acid might have an adverse effect on binding (Fig. 8C). More detailed information related to the visualization of molecular docking with the top 3 binding energies, including bond length and 2 binding positions, is displayed in Figure 9.
Figure 7.
Heat map of molecular docking. CCL2 = C–C motif chemokine 2, CXCL8 = interleukin-8, IL10 = interleukin-10, IL1B = interleukin-1B, IL-6 = interleukin-6, MMP9 = matrix metalloproteinase-9, TLR4 = toll-like receptor 4, TNF = tumor necrosis factor, VEGFA = vascular endothelial growth factor A.
Figure 8.
Molecular docking diagrams with 2D and 3D plots. (A) Astragaloside IV–IL10, (B) Astragaloside IV–TNF, and (C) Astragaloside IV–TLR4 complexes. IL10 = interleukin-10, TLR4 = toll-like receptor 4, TNF = tumor necrosis factor.
Figure 9.
More detailed information of molecular docking. (A) Astragaloside IV–IL10, (B) Astragaloside IV–TNF, and (C) Astragaloside IV–TLR4 complexes. IL10 = interleukin-10, TLR4 = toll-like receptor 4, TNF = tumor necrosis factor.
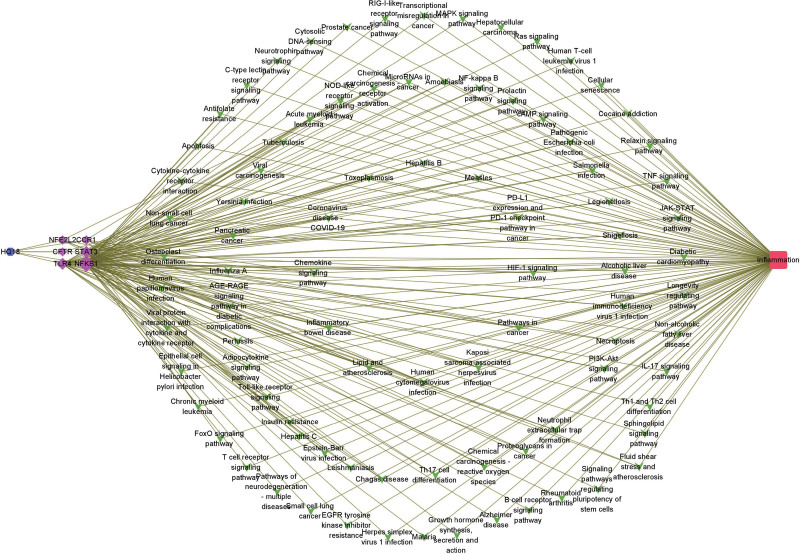
Based on the binding free energy, it can be concluded that the binding effect of Astragaloside IV with the core proteins was relatively good compared to other protein-component complexes. To explore the targets and signaling pathways of Astragaloside IV acting on anti-inflammatory effects, we searched for the intersection targets of Astragaloside IV and the anti-inflammatory effects. 6 proteins were found, namely NFKB1, STAT3, TLR4, CCR1, NFE2L2, and CFTR, in descending order of degree. These 6 genes in the KEGG enrichment pathways were involved in 89 pathways, including Lipid and atherosclerosis, HIF-1 signaling pathway, chemokine signaling pathway, hepatitis B, and inflammatory bowel disease. Thus, we speculated that Astragaloside IV may exert anti-inflammatory effects mainly through these 6 components acting on the related signaling pathways (Fig. 10).
Figure 10.
Astragaloside IV-inflammation-target-pathway. The blue quadrate node represents ingredient HQ18, purple round nodes represents the genes, green oblong nodes represents pathways, and yellow hexagon node represents inflammation.
4. Discussion
Inflammation is an important instrument of the defense system of the body. Nevertheless, the excessive activation of inflammatory cells and cytokine secretion can lead to serious damage to the body. Moreover, inflammatory factors, including biological factors, physical and chemical factors, necrotic tissue, hypersensitivity reaction, and pathological changes, can cause tissue damage. Huangqi is a typical multieffect TCM, originally mentioned in Shen Nong’s Herbal Classic as “sweet and mild in taste, mainly responsible for the long-term failure and pain of carbuncle and gangrene, and expelling pus and pain.” It is currently listed in the Pharmacopoeia of the People’s Republic of China and exhibits the effects of tonifying and expelling evil, including tonifying qi and yang, consolidating the surface and stopping sweat, promoting fluid and blood circulation, promoting stagnation and unblocking obstructions, supporting toxin and purulent discharge, and astringing sores and generating muscles.
Huangqi exerts anti-inflammatory and immunomodulatory activities when used alone or in combination with other drugs, but research on the specific mechanisms of its action is lacking. In the current study, 18 active components of Huangqi were selected by network pharmacology and predicted 279 potentially anti-inflammatory targets were predicted. By constructing the “TCN-component-target” network map, the findings of this study revealed that the anti-inflammatory activity of Huangqi was multitarget and multicomponent. The top 5 active ingredients of Huangqi exerting anti-inflammatory effects included 7-O-methylisomucronulatol, formononetin, kaempferol, quercetin, and Astragaloside IV. Among these, quercetin, kaempferol and formononetin are flavonoids. Flavonoids exhibit a significant inhibitory effect on cyclooxygenase-2 and are expected to function as anti-inflammatory agents, but the specific mechanism of their action is unelucidated.[19–21] Among the top 5 active ingredients of Huangqi, 45 gene targets were obtained for 7-O-methylisomucronulatol, 38 for formononetin, 62 for kaempferol, 151 for quercetin, and 80 for Astragaloside IV, which showed that Huangqi could exert anti-inflammatory effects through 5 main active components on multiple targets. The results of the PPI network analysis suggest that TNF, IL 6, IL 1 B, TLR 4, CXCL 8, VEGF A, CCL 2, IL 10, and MMP 9 were related to inflammation, chemotaxis, adhesion, immunity, and might be the core targets of the anti-inflammatory effects of Huangqi. TNF acts as a core to coordinate inflammatory immune responses, inducing the expression of inflammatory mediators, not only by promoting inflammatory gene activation of MAPK and NF-κB signaling but also contributing to the development of inflammatory diseases by triggering cell death.[22] Results from the study on Prostatitis revealed that blocking TNF can significantly reduce epithelial hyperplasia, NF-κB activation, and macrophage-mediated inflammation within the prostate tissue.[23] TNF can also initiate complex molecular pathways by interacting with its receptor TNFR1, causing inflammation and cell death.[24] IL-6 demonstrates extensive biological activities owing to its trans-signaling mechanism as a key cytokine in the inflammatory response.[25] IL-6-related disorders are closely associated with the regulation of JAK/STAT 3, Ras/MAPK, PI3K-PKB/Akt, as well as CD4+ T cells and VEGF levels, which leads to the development of various inflammatory and immune diseases.[26]IL-1B may affect the healing process of diabetic foot ulcers by regulating inflammation and immune cell infiltration.[27] TLR 4 belongs to the TLR receptor family and can induce pro-inflammatory responses to invasive pathogens.[28] Studies have shown that TLR-4 plays a crucial role in arterial thrombosis, and the inhibition of TLR-4 can alleviate inflammation and early thrombosis.[29] CXCL-8 is a chemokine that participates in tumor angiogenesis and is associated with promoting the metastasis of many malignant tumors.[30] Patients with cardiovascular disease usually exhibit high concentrations of VEGFA, which is related to the severity of the disease and poor prognosis.[31]VEGFA can regulate angiogenesis, vascular permeability, and inflammation by binding to VEGFR-1 and VEGFR-2.[32] CCL2 is also a pro-inflammatory factor that can activate signaling pathways related to CYP19A1 transcription, thereby contributing to the pro-inflammatory environment and aromatase expression in obesity, leading to the deterioration of menopausal-related inflammatory conditions.[33] The results from the study of the pathogenesis of psoriasis reveal that the pharmacological inhibition of MMP-9 can reduce skin vasodilation, vascular permeability, and inflammation.[34]
To date, more than 200 compounds have been isolated and identified from RA, including flavonoids, saponins, and polysaccharides.[35,36] Among them, RA polysaccharides, flavonoids, and Astragaloside IV are 3 classes of beneficial compounds closely related to the pharmacological activity and therapeutic effect of RA.[37] Studies have shown that the RA polysaccharide PG2 improves cancer symptom clusters and the quality of life in patients with metastatic disease by inhibiting NF-κB and CD31/PECAM-1, regulating inflammation-associated macrophage activity, and angiogenesis.[38] In recent years, the protective effects of flavonoids have attracted considerable attention in overcoming various diseases, such as cancer, cardiovascular disease, osteoporosis, and chronic inflammation. Ononin is a main flavonoid component with biological activity in RA membranaceus.[39] In the dextran sodium sulfate (DSS) – induced colitis mouse model, Ononin activated mitochondrial autophagy, inhibited NLRP3 inflammasome formation, and the production of inflammatory cytokines and mediators. Therefore, triggering mitochondrial autophagy is a potential strategy for treating inflammatory diseases.[40] Studies have also found that flavonoids exhibit a strong inhibitory effect on liver fibrosis by inhibiting NF-κB and various downstream inflammatory factors.[41] Astragaloside IV may regulate the differentiation of activated CD4+ subset T cells (including Th 1, Th 17, and Treg) through JAK/STAT and NF-κB signaling, induce the apoptosis of activated CD4 T cells, and ultimately significantly reduce the demyelination and inflammatory infiltration in the central nervous system of mice with autoimmune encephalomyelitis.[15] In addition, Astragaloside IV protects the intestinal epithelium from sepsis-induced barrier dysfunction by inhibiting the RhoA/NLRP 3 inflammasome signaling pathway.[42] Astragaloside IV can restrain TLR4/p38MAPK pathway by reducing the TNF-α, IL-1β, and IL 6 levels and expression of ED1, TLR 4, p38MAPK, and p-p38MAPK, which inhibits the LPS-induced myocardial inflammation and ameliorate LPS-induced myocardial injury.[43,44] The molecular mechanism of Astragaloside IV action in the prevention and treatment of atherosclerosis may involve the regulation of lncRNA-TUG 1 by reducing the expression of ICAM-1, VCAM-1, IL-8, and MCP-1 and mRNA expression of TUG 1, T-p38, and p-p38, to suppress the p38 MAPK signaling pathway and vascular endothelial inflammatory response.[45] High-dose RA polysaccharides effectively improve airway inflammation and control VEGF protein expression in the lung tissue to cut down airway vascular remodeling in asthmatic rats.[46] Astragaloside IV plays a crucial role by regulating MMP-9 to mediate the NLRP 3/Caspase-1 signaling pathway, for alleviating hypoxia-ischemia-induced brain injury and inhibiting the inflammatory response of hypoxic-ischemic brain tissue and HT22 hippocampal neurons in neonatal rats.[47] This finding suggests that these proteins are key targets for the Huangqi components to realize the anti-inflammatory effects.
To investigate the anti-inflammatory effect of the Huangqi-anti-inflammatory targets from the aspects of gene function and signaling pathways, 45 intersection targets were subjected to GO functional analysis and KEGG pathway enrichment analysis. The GO analysis revealed 974 BP items, 21 CC items, and 60 MF items. The results showed that Huangqi mainly influenced the inflammatory response of the body, lipopolysaccharide response, and other related inflammatory reactions by regulating the combination of substances released in the vesicle lumen and some related receptors. The results of the KEGG pathway analysis identified 128 pathways closely related to the anti-inflammatory effects of Huangqi, mainly enriched in Lipid and atherosclerosis, IL-17 signaling pathway, Chagas disease, leishmaniasis, TNF signaling pathway, and inflammatory bowel disease. Moreover, the findings from the PPI network, GO functional analysis, and KEGG enrichment analysis reveal that Huangqi exerts anti-inflammatory effects by the multitarget regulation of multiple pathways. Molecular docking results suggest that the binding energies of Astragaloside IV and kaempferol with the core protein are ≤−6 kcal/mol. Particularly, the binding energies of Astragaloside IV with TNF, TLR4, and IL10 are lower; therefore, these bindings are more stable.
5. Conclusion
In summary, Huangqi exerts its anti-inflammatory effect mainly through 18 active ingredients acting on 45 inflammatory genes. The GO functional analysis involved 1055 items, such as inflammatory response, response to lipopolysaccharide, response to bacterial molecules, and cellular response to a biotic stimulus. The KEGG pathway enrichment analysis results suggested enrichments primarily related to atherosclerosis, IL-17 signaling pathway, Chagas disease, leishmaniasis, and TNF signaling pathway. In particular, the combinations of the main active ingredients such as quercetin, Astragaloside IV, kaempferol, 7-O-methylisomucronulatol, formononetin, and proteins such as TNF, IL6, IL1B, TLR4, CXCL8, CCL2, IL10, VEGFA, and MMP9 exert an anti-inflammatory effect via regulating atherosclerosis, IL-17 signaling pathway, Chagas disease, Leishmaniasis, TNF signaling pathway, inflammatory bowel disease, and other pathways. The results from the molecular docking studies demonstrated that Astragaloside IV binds efficiently with the core protein. Through network pharmacology and molecular docking, this study explored the active ingredients and targets and signaling pathways related to the anti-inflammatory activity of Huangqi. The findings provide a theoretical basis for further study on the underlying mechanisms of the anti-inflammatory action of Huangqi, which can guide future experimental research in this field.
Author contributions
Conceptualization: Jianwei Ren.
Formal analysis: Jianwei Ren, Ming Lei.
Writing – original draft: Yuetian Ding.
Writing – review & editing: Shangze Li.
Abbreviations:
- BP
- biological processes
- CC
- cellular components
- CCL2
- C–C motif chemokine 2
- CXCL8
- interleukin-8
- DL
- drug-likeness
- GO
- gene ontology
- IL10
- interleukin-10
- IL-17
- interleukin-17
- IL1B
- interleukin-1B
- IL-6
- interleukin-6
- KEGG
- Kyoto encyclopedia of genes and genomes
- MF
- molecular function
- MMP9
- matrix metalloproteinase-9
- OB
- oral bioavailability
- PPI
- protein–protein interaction
- RA
- Radix Astragali
- TCM
- traditional Chinese medicine
- TCMSP
- Traditional Chinese Medicine System Pharmacology
- TLR4
- toll-like receptor 4
- TNF
- tumor necrosis factor
- VEGFA
- vascular endothelial growth factor A
JR and YD contributed equally to this work.
The present study was supported by the program of the Science and Technology Plan Project of Xizang Autonomous Region of China (no. XZ202101ZR0104G), the Central Guidance on Local Science and Technology Development Fund of Tibet (grand no. XZ202301YD0040C and XZ202101ZD0021G), and Wuhan University of Technology-Tibet University Pathogenic biology team construction research program (no. LZT2021010).
Not applicable. Our data was based on bioinformatics analysis and the data from TCMSP, UniProt, GeneCards, String, Metascape, GO, and KEGG, so our study did not require the approval of an ethics committee.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Ren J, Ding Y, Li S, Lei M. Predicting the anti-inflammatory mechanism of Radix Astragali using network pharmacology and molecular docking. Medicine 2023;102:35(e34945).
Contributor Information
Jianwei Ren, Email: 2467057909@qq.com.
Yuetian Ding, Email: ytding2022@163.com.
Shangze Li, Email: shangze.li@whu.edu.cn.
References
- [1].Hou C, Chen L, Yang L, et al. An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromol. 2020;153:248–55. [DOI] [PubMed] [Google Scholar]
- [2].Zhang Z, Luo D, Xie J, et al. Curcumin’s metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB Pathway. Front Pharmacol. 2018;9:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gilroy DW, Lawrence T, Perretti M, et al. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discovery. 2004;3:401–16. [DOI] [PubMed] [Google Scholar]
- [4].Jalil J, Attiq A, Hui CC, et al. Modulation of inflammatory pathways, medicinal uses and toxicities of Uvaria species: potential role in the prevention and treatment of inflammation. Inflammopharmacology. 2020;28:1195–218. [DOI] [PubMed] [Google Scholar]
- [5].Cao L, Yang S. Discussion on the bidirectional function of Astragali Radix. Tradit Chin Med Res. 2018;31:11–3. [Google Scholar]
- [6].Liu Y, Chen Y, Xu H, et al. Excavating and sorting out the application of astragalus in ancient Chinese Medicine Books. Chin J Ethnomed Ethnopharm. 2023;32:78–81. [Google Scholar]
- [7].Zhang Y, Liu H, Wang R, et al. Chemical constituents and pharmacological effects of Astragali Radix and predictive analysis on quality markers. Chin J New Drugs. 2023;32:410–9. [Google Scholar]
- [8].Wang X. Progress in modern pharmacology and clinical research of astragali radix. Technol Wind. 2017;309:182. [Google Scholar]
- [9].Xie J, Gao S, Li L, et al. Research progress and application strategy on network pharmacology in Chinese materia medica. Chin Tradit Herbal Drugs. 2019;50:2257–65. [Google Scholar]
- [10].Zhang R, Zhu X, Bai H, et al. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu R, Shen Y, Ma F, et al. Application of molecular docking in screening of anti-inflammatory constituents of traditional Chinese medicine and their mechanisms. Chin J Pharmacol Toxicol. 2018;32:497–506. [Google Scholar]
- [12].Tan YQ, Chen HW, Li J. Astragaloside IV: an effective drug for the treatment of cardiovascular diseases. Drug Des Devel Ther. 2020;14:3731–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu Y, Fan Z, Chen Z, et al. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi H, Zhou P, Gao G, et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem. 2021;45:e13757. [DOI] [PubMed] [Google Scholar]
- [15].Yang L, Xing F, Han X, et al. Astragaloside IV regulates differentiation and induces apoptosis of activated CD4+ T cells in the pathogenesis of experimental autoimmune encephalomyelitis. Toxicol Appl Pharmacol. 2019;362:105–15. [DOI] [PubMed] [Google Scholar]
- [16].Ding Q, Gao J, Zheng J, et al. Astragaloside IV attenuates inflammatory injury and promotes odontoblastic differentiation in lipopolysaccharide-stimulated MDPC-23 cells and rat pulpitis. J Oral Pathol Med. 2019;48:951–8. [DOI] [PubMed] [Google Scholar]
- [17].Hassan H, Shanak S. GOTrapper: a tool to navigate through branches of gene ontology hierarchy. BMC Bioinf. 2019;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kanehisa M, Goto S, Sato Y, et al. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kiruthiga N, Alagumuthu M, Selvinthanuja C, et al. Molecular modelling, synthesis and evaluation of flavone and flavanone scaffolds as anti-inflammatory agents. Antiinflamm Antiallergy Agents Med Chem. 2021;20:20–38. [DOI] [PubMed] [Google Scholar]
- [20].Rathee P, Chaudhary H, Rathee S, et al. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets. 2009;8:229–35. [DOI] [PubMed] [Google Scholar]
- [21].Sinyeue C, Matsui M, Oelgemöller M, et al. Synthesis and investigation of flavanone derivatives as potential new anti-inflammatory agents. Molecules. 2022;27:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. 2023;23:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vickman RE, Aaron-Brooks L, Zhang R, et al. TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease. Nat Commun. 2022;13:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Choksi S, Choudhary G, Liu ZG. Transition from TNF-induced inflammation to death signaling. Methods Mol Biol. 2021;2248:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33:127–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaur S, Bansal Y, Kumar R, et al. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020;28:115327. [DOI] [PubMed] [Google Scholar]
- [27].Gan MS, Yang B, Fang DL, et al. IL-1B can serve as a healing process and is a critical regulator of diabetic foot ulcer. Ann Transl Med. 2022;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78:1233–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rai V, Radwan MM, Nooti S, et al. TLR-4 Inhibition Attenuates Inflammation, thrombosis, and stenosis in arteriovenous fistula in yucatan miniswine. Cardiol Cardiovasc Med. 2022;6:432–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. 2018;51:140–5. [DOI] [PubMed] [Google Scholar]
- [31].Braile M, Marcella S, Cristinziano L, et al. VEGF-A in cardiomyocytes and heart diseases. Int J Mol Sci. 2020;21:5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Y, Zhu X, Cui H, et al. The Role of the VEGF family in coronary heart disease. Front Cardiovasc Med. 2021;8:738325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Martínez-Chacón G, Yatkin E, Polari L, et al. CC chemokine ligand 2 (CCL2) stimulates aromatase gene expression in mammary adipose tissue. FASEB J. 2021;35:e21536. [DOI] [PubMed] [Google Scholar]
- [34].Alves-Filho JC, Marcel Silva Melo B, Ryffel B. MMP-9 Mediates cross-talk between neutrophils and endothelial cells in psoriasis. J Invest Dermatol. 2021;141:716–8. [DOI] [PubMed] [Google Scholar]
- [35].Wu X, Zhou W, Wei Q, et al. Cytoprotective effects of the medicinal herb Astragalus membranaceus on lipopolysaccharide-exposed cells. Mol Med Rep. 2018;18:4321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gong AGW, Duan R, Wang HY, et al. Evaluation of the pharmaceutical properties and value of astragali radix. Medicines (Basel). 2018;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hu XJ. Protective capability of Astragalus (Huangqi) on auditory function in a rat model of estrogen deficiency. Chin Med J (Engl). 2019;132:106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bamodu OA, Kuo KT, Wang CH, et al. Astragalus polysaccharides (PG2) Enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T Cell-mediated anticancer immune responses in patients with lung cancer. Nutrient. 2019;11:2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luo LY, Fan MX, Zhao HY, et al. Correction to “pharmacokinetics and bioavailability of the isoflavones formononetin and ononin and their in vitro absorption in ussing chamber and Caco-2 Cell Models”. J Agric Food Chem. 2018;66:12453. [DOI] [PubMed] [Google Scholar]
- [40].Yu T, Lu X, Liang Y, et al. Ononin alleviates DSS-induced colitis through inhibiting NLRP3 inflammasome via triggering mitophagy. Immun Inflammation Dis. 2023;11:e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].An L, Lin Y, Li L, et al. integrating network pharmacology and experimental validation to investigate the effects and mechanism of astragalus flavonoids against hepatic fibrosis. Front Pharmacol. 2020;11:618262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xie S, Yang T, Wang Z, et al. Astragaloside IV attenuates sepsis-induced intestinal barrier dysfunction via suppressing RhoA/NLRP3 inflammasome signaling. Int Immunopharmacol. 2020;78:106066. [DOI] [PubMed] [Google Scholar]
- [43].Li M, Wang H, Lu M, et al. Inhibitory effect of astragaloside IV on myocardial injury induced by lipopolysaccharide-in mice by TLR4/p38 MAPK signaling pathway. Pharmacol Clinics Chin Materia Medica. 2017;33:35–8. [Google Scholar]
- [44].Wang G, Zhao Y. Effects of Astragaloside IV on myocardial injury and inflammatory reaction in exhaustive exercise rats. J Domestic Anim Ecol. 2022;43:43–7. [Google Scholar]
- [45].Liu J, Qin H. Mechanism of astragaloside A in preventing and treating AS by regulating long chain non coding RNA. Inform Tradit Chin Med. 2023;7. [Google Scholar]
- [46].Yan W, Chang J. Effect of astragalus polysaccharides on airway inflammation and lung tissue vascular endothelial growth factor expression of asthma rats. Chin J Clin Pharmacol. 2020;36:953–5. [Google Scholar]
- [47].Li N, Liu C. The molecular mechanism of astragaloside IV regulating MMP-9 mediated NLRP3/Caspase-1 signaling pathway to improve hypoxic ischemic brain injury. Liaoning Universi Tradit Chin Med. 2022;1–112. [Google Scholar]