Abstract
Purpose
This study aims to investigate the impact of the Chinese New Year (CNY) holiday season on the outcomes of In Vitro Fertilization (IVF) fresh embryo transfer cycles.
Participants and Methods
This retrospective study analyzed 4688 patients who received their first IVF fresh cycle attempt between January 2017 and October 2021. Of these, 4449 women underwent IVF during non-holiday seasons, while 239 women were treated during the CNY holiday season. The study included women who underwent IVF treatment during the specified time frame. The primary outcome was the live birth rate (LBR).
Results
The study found that the LBR of IVF performed during the CNY holiday season was 32.22%, which is significantly lower than that of the non-holiday season (43.38%, p<0.001). Multivariate logistic regression analysis showed that the CNY holiday season (OR=0.62, 95% CI 0.47–0.82, p=0.001) was an independent factor associated with the live birth rate. Propensity score matching (PSM) data analysis showed that the LBR in the CNY holiday season group was 31.78% compared to 42.64% in the non-holiday season group (p=0.005). Inverse probability of treatment weighting (IPTW) data also indicated that the CNY holiday season had a lower LBR than the non-holiday season (OR=0.64, 95% CI 0.47–0.87, p=0.005).
Conclusion
IVF performed during the CNY holiday season results in a lower live birth rate, potentially indicating that certain lifestyle adjustments during this period, such as unhealthy dietary, tobacco and alcohol usage, sleep disruption, and emotional stress experienced could have some influence on the outcomes.
Keywords: Chinese New Year holiday, live birth rate, culture, stress, smoking, alcohol
Background
The Chinese New Year (CNY) is the biggest holiday celebration in Chinese culture, which marks the beginning of a new year according to the traditional lunar calendar. The festivity typically lasts over a month, and during this time, people engage in long-distance travel, frequent and massive shopping, thorough residence cleaning, extensive food preparation, family gatherings, and social outings. These activities bring abrupt changes to daily work and life routines, causing significant amounts of psychological stress amid festival joys. It is highly likely that such behavioral and psychological changes associated with the CNY holiday may affect fertility1 and result in unusual demand for cyclical treatment for some pregnant women receiving in vitro fertilization (IVF).
Several studies have implicated that environmental and social factors may affect pregnancy and IVF outcomes.2–5 However, there has not been any study to investigate whether the CNY holiday season impacts the IVF outcomes. Therefore, this retrospective study aimed to determine whether the CNY holiday season impacts the IVF outcomes differently compared to other months. The study examined the monthly IVF outcomes at our center to investigate how significantly the CNY holiday season affects the IVF outcomes. The findings from this study will provide new insights into the effects of cultural and social factors on IVF outcomes and help physicians advise patients on the best timing for their IVF treatment.
Materials and Methods
Inclusion Criteria involve patients who had their initial IV cycle from January 2017 to October 2021, underwent a fresh embryo transfer, and experienced a live birth from the cycle.
Exclusion criteria pertain to patients lacking complete data for relevant variables.
Implementation Procedure
All patients undergoing their first IVF cycle during January 2017 and October 2021 were included except patients with incomplete data recording. In fresh embryo transfer cycles, controlled ovarian hyperstimulation for IVF comprised various protocols, including GnRH antagonist, luteal phase GnRH agonist, follicular phase long-acting GnRH agonist regimens, and natural cycle protocols. These approaches were carefully designed to prevent the premature rise of progesterone levels before the hCG administration day. On the third day after oocyte retrieval (day 3), two cleavage-stage embryos were transferred as fresh cycles, and in some cases, the transfer was performed with blastocysts on day 5. Embryo quality is determined through an assessment of visual morphology, and embryos are subsequently transferred based on their ranking in terms of quality. However, in certain circumstances, the transfer is canceled. These situations include cases where oocyte retrieval exceeds twenty, patients are identified to be at high risk for ovarian hyperstimulation syndrome (OHSS), or the progesterone (P) level surpasses 2 ng/mL on the hCG triggering day. The implementation process remained the same during the CNY holiday season.
Then the luteal-phase support was administered [90 mg progesterone gel (Merck Serono) plus 20 mg/day dydrogesterone. Based on the embryo transfer dates, participants were divided into the CNY holiday season (the entire January and February) group (CNY group) and the non-holiday season (months other than Jan and Feb) group (N-CNY group).
Data Retrieval
Data were collected retrospectively from the Hospital Information System (HIS) database of the hospital, which included patients’ age, classification of infertility, duration of infertility, and education level. All subjects included in the study had undergone testing for their baseline serum levels of follicle-stimulating hormone (FSH) (μIU/mL), luteinizing hormone (LH) (μIU/mL), estrogen (E2) (pg/mL), progesterone (P) (ng/mL), testosterone (T) (ng/mL), prolactin (PRL) (ng/mL), and anti-Müllerian hormone (AMH) (ng/mL). The tests were performed using commercial kits (Siemens Healthcare Diagnostics) on an automated chemiluminescence immunoassay analyzer. Treatment protocols for superovulation and numbers of retrieved oocytes were also obtained from the HIS database.
Outcomes
Live birth, defined as the delivery of at least one live born neonate in a given embryo transfer cycle, was the primary outcome. Clinical pregnancy was used as the secondary outcome and was defined by the evidence of fetal cardiac activity by sonograph 30 days after the embryo transfer, with ectopic pregnancy included. The outcomes also included miscarriage and pregnancy loss before 20 weeks.
Statistical Analysis
Continuous variables with normal distribution were presented as the mean, followed by standard deviation. The abnormal distribution of continuous variables was introduced as the median and the interquartile range from the first to the third quartile (Q1-Q3). Student’s t-test and Wilcoxon rank-sum test were used for normally and abnormally distributed quantitative data. Chi-square or Fisher’s exact test analyzed categorical variables. Propensity score matching (PSM) was performed to match patients in the N-CNY group with patients in the CNY group. The propensity score data set was constructed using the multivariable logistic regression model, including age, infertility years, education, FSH, E2, P, PRL, LH, T, AMH, and classification of infertility. We used caliper matching with the caliper 0.02 of the pooled standard deviation of the logit of the propensity score. Patients in the CNY group were matched 1:2 to patients in the N-CNY group. The propensity score data generated the inverse probability of treatment weighting (IPTW) data. To balance those observable characteristics, each patient was weighted by the inverse probability in the two groups. Multivariate logistic regression was used to assess the association between the live birth rate and the two treatment groups for the primary endpoint. The model was adjusted by those variates whose p-value was no more than 0.10. Those variates with clinical meanings related to the primary endpoint were adjusted, ignoring the p-value. The results were expressed as adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs). We analyzed the PSM data set and the IPTW data set for sensitivity analysis. All hypothesis tests were two-sided, and a p-value<0.05 was considered statistically significant. Sample size was calculated based on the EPV (events per variable) principle of multivariable analysis. A minimum of five6 and a maximum of ten to fifteen7 endpoint events for each multivariate was requested. Ten variants were assumed to be analyzed leads to 150 live births. As studies reported 40%8 to 50%9 of live birth occurred, 375 samples were needed. The sample size was also determined using a conventional approach. For further comprehensive details, kindly refer to Supplementary Material 1 in the supplementary document.
Stata SE 13 (Serial number 401306302851), R software version 4.2.0 (http://cran.r-project.org/), and easy-R (www.empowerstats.com) were used for statistical analysis. GraphPad was used to generate figures.
Results
Patient and Clinical Characteristics
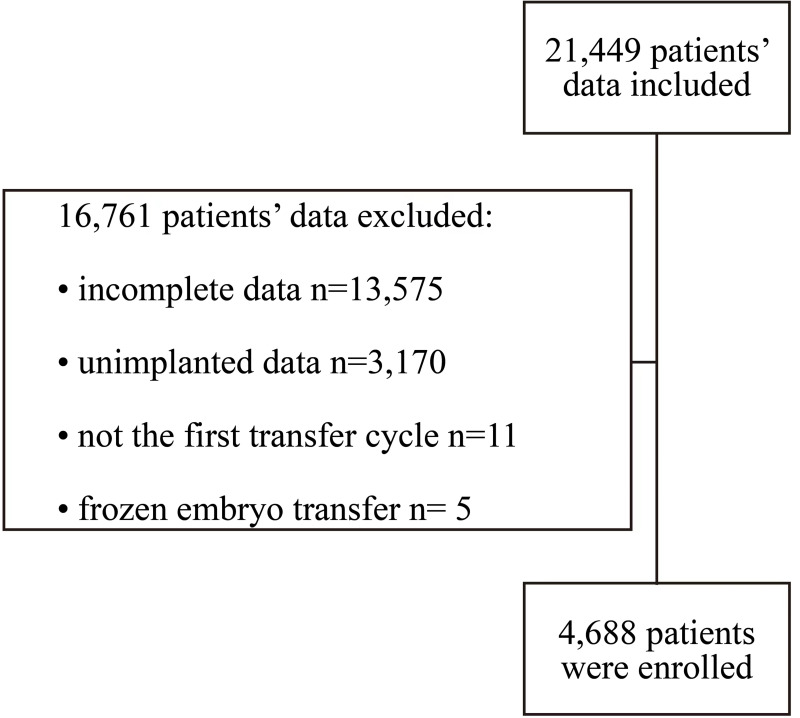
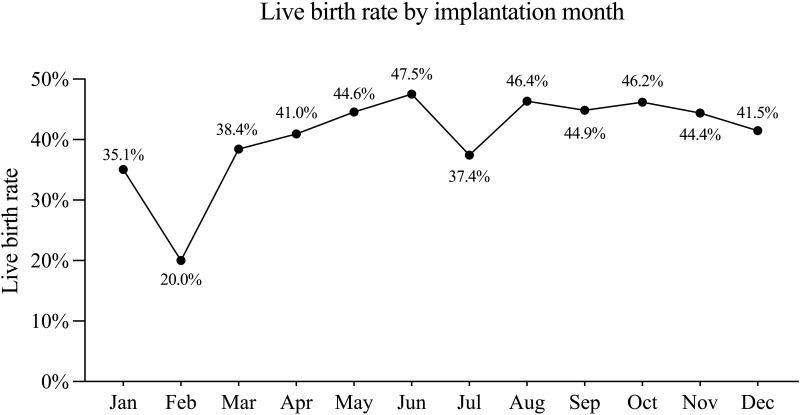
A total of 4688 women (Figure 1) receiving IVF with the first fresh embryo transfer (ET) cycle were included in the analysis. The lowest LBR by the month when ET was performed appeared in Jan (35.1%) and Feb (20.0%), while the LBR with ET in all other months ranged between 37.4% and 47.5% (Figure 2).
Figure 1.
Flow diagram for participants enrolled in this study.
Figure 2.
Live birth rate by implantation month.
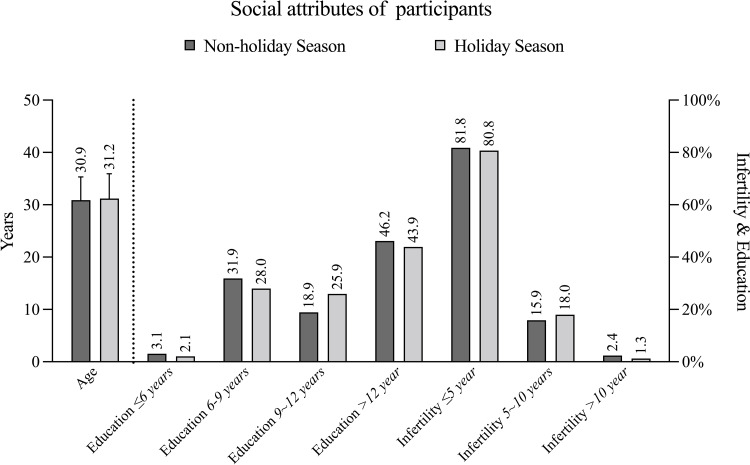
General information of participants is shown in Table 1 and Figure 3. The percentages of patients by years of education at ≤6, 6~9, 9~12, and above 12 were 3.06%, 31.87%, 18.88%, and 46.19%, respectively, in the N-CNY group, versus 2.09%, 28.03%, 25.94%, 43.93%, respectively, in the CNY group (p=0.048). The average baseline E2 level in the N-CNY group was 49.75±52.11 (median 40) pg/mL, versus 45.69±48.82 (median 39) pg/mL in the CNY group (p=0.047). The average baseline LH level in the N-CNY group (7.26±110.64 μIU/mL, median 4.44) was significantly higher than that of the CNY group (4.56±2.42 μIU/mL, median 4.11) (p=0.002). The serum baseline FSH, P, PRL, T, and AMH levels were comparable between the two groups. There were no statistical differences between these two groups for age, infertility years, and classification of infertility.
Table 1.
Baseline Characters of All Participants
| Non-CNY Holiday Season (n=4449) | CNY Holiday Season (n=239) | p-value | |
|---|---|---|---|
| Age | |||
| Mean±sd | 30.88±4.45 | 31.19±4.72 | 0.293 |
| Min-max | 20–48 | 21–48 | |
| Infertility years | |||
| ≤5 year | 3637 (81.75%) | 193 (80.75%) | 0.396 |
| 5~10 years | 707 (15.89%) | 43 (17.99%) | |
| >10 year | 105 (2.36%) | 3 (1.26%) | |
| Education | |||
| ≤6 years | 136 (3.06%) | 5 (2.09%) | 0.048 |
| 6–9 years | 1418 (31.87%) | 67 (28.03%) | |
| 9~12 years | 840 (18.88%) | 62 (25.94%) | |
| >12 year | 2055 (46.19%) | 105 (43.93%) | |
| FSH | |||
| Mean±sd | 7.99±3.21 | 7.64±2.31 | 0.207# |
| Median (q1-q3) | 7.53(6.36–8.96) | 7.34(6.16–8.77) | |
| E2 | |||
| Mean±sd | 49.75±52.11 | 45.69±48.82 | 0.047# |
| Median (q1-q3) | 40(27–58) | 39(24.5–53) | |
| P | |||
| Mean±SD | 1.04±3.88 | 0.7±0.56 | 0.544# |
| Median (Q1-Q3) | 0.6(0.38–0.9) | 0.59(0.38–0.86) | |
| PRL | |||
| Mean±SD | 18.09±97.21 | 15.76±17.11 | 0.921# |
| Median (Q1-Q3) | 12.5(9.23–17.28) | 12.41(9.34–17.06) | |
| LH | |||
| Mean±SD | 7.26±110.64 | 4.56±2.42 | 0.002# |
| Median (Q1-Q3) | 4.44(3.25–6.24) | 4.11(3.02–5.61) | |
| T | |||
| Mean±SD | 0.43±0.17 | 0.44±0.16 | 0.776# |
| Median (Q1-Q3) | 0.44(0.32–0.56) | 0.44(0.32–0.57) | |
| AMH | |||
| Mean±SD | 3.03±2.05 | 3.01±2.16 | 0.582# |
| Median (Q1-Q3) | 2.59(1.56–4.01) | 2.56(1.59–3.62) | |
| Classification of infertility | 0.307 | ||
| Primary | 2232 (50.17%) | 128 (53.56%) | |
| Secondary | 2217 (49.83%) | 111 (46.44%) |
Note: #Mann–Whitney test.
Abbreviations: CNY, Chinese New Year; AMH, anti-Müllerian hormone; E2, estrogen; FSH, Follicle-Stimulating Hormone; LH, luteinizing hormone; P, progesterone; PRL, prolactin; T, testosterone.
Figure 3.
Social attributes of participants.
Outcomes of Assisted Reproductive Technology (ART) for All Participants
There were significant differences in use of ovarian stimulation treatment protocols between N-CNY group and CNY group (p<0.001). In the N-CNY group, there were 0.36% (16/4449) treated by natural cycles or short cycles, 24.79% (1103/4449) by antagonist protocol, 44.35% (1973/4449) by GnRH agonist protocol, and 30.50% (1357/4449) by depot GnRHa protocol, respectively. In contrast, there were none treated by natural cycles or short cycles, 35.56% (85/239) by antagonist protocol, 42.68% (102/239) by GnRH agonist protocol, and 21.76% (52/239) by depot GnRHa protocol in the CNY group.
In the N-CNY group, 50.80% (2264/4449) had clinical pregnancy, versus 41.42% (99/239) in the CNY group (p=0.004). Of 4449 women in the N-CNY group, 1930 had live birth (43.38%), while 77 of 239 women (32.22%) in the CNY group had live birth (p<0.001). There was no statistical difference in the number of oocytes retrieved and pregnancy loss between the two groups (Table 2).
Table 2.
Outcome of ART for All Participants
| Non-CNY Holiday Season (n=4449) | CNY Holiday Season (n=239) | p-value | |
|---|---|---|---|
| Treatment regimen | |||
| Natural cycles or short cycle | 16 (0.36%) | 0 (0.00%) | <0.001 |
| Antagonist protocol | 1103 (24.79%) | 85 (35.56%) | |
| GnRH agonist protocol | 1973 (44.35%) | 102 (42.68%) | |
| Depot GnRHa protocol | 1357 (30.50%) | 52 (21.76%) | |
| Number of oocytes | |||
| Mean±SD | 10.19±5.01 | 9.97±4.96 | 0.511(t-test) |
| Median (Q1-Q3) | 10(6–14) | 10(6–14) | 0.578# |
| 0–5 | 877 (19.74%) | 55 (23.11%) | 0.458 |
| 6~10 | 1578 (35.52%) | 76 (31.93%) | |
| 11~15 | 1291 (29.06%) | 73 (30.67%) | |
| 16 and more | 697 (15.69%) | 34 (14.29%) | |
| Clinical pregnancy | |||
| No | 2185 (49.11%) | 140 (58.58%) | 0.004 |
| Yes | 2264 (50.89%) | 99 (41.42%) | |
| Pregnancy loss | |||
| No | 4115 (92.49%) | 217 (90.79%) | 0.334 |
| Yes | 334 (7.51%) | 22 (9.21%) | |
| Live birth | |||
| No | 2519 (56.62%) | 162 (67.78%) | <0.001 |
| Yes | 1930 (43.38%) | 77 (32.22%) |
Note: #Mann–Whitney test.
Live Birth results for All Participants
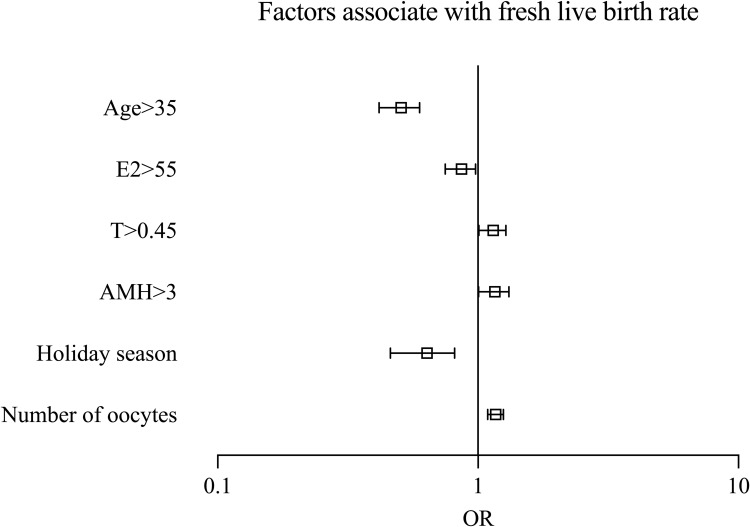
Table 3 and Figure 4 present the live birth results after adjustment by logistic regression, indicating that the age (OR=0.5, 95% CI 0.42–0.60, p<0.001), serum baseline level of T (OR=1.14, 95% CI 1.01–1.28, p=0.036), AMH (OR=1.15, 95% CI 1.01–1.32, p=0.036), the CNY holiday season (OR=0.62, 95% CI=0.47–0.82, p=0.001), number of oocytes retrieved (OR=1.17, 95% CI 1.09–1.25, p<0.001) were independent factors associate with the outcome of live birth.
Table 3.
Univariate Analysis and Multivariate Logistic Analysis for All Participants
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| n (%) | OR (95% CI) | Crude P | OR (95% CI) | Adjusted P | |
| Age | |||||
| ≤35 | 3950 (84.26%) | 1.0 | |||
| >35 | 738 (15.74%) | 0.43 (0.36, 0.52) | <0.001 | 0.51(0.42, 0.61) | <0.001 |
| Education | |||||
| ≤6 years | 141 (3.01%) | 1.0 | |||
| 6–9 years | 1485 (31.68%) | 1.05 (0.74, 1.49) | 0.786 | ||
| 9~12 years | 902 (19.24%) | 0.93 (0.65, 1.33) | 0.682 | ||
| >12 year | 2160 (46.08%) | 1.09 (0.77, 1.54) | 0.635 | ||
| Infertility years | |||||
| ≤5 year | 3830 (81.7%) | 1.0 | |||
| 5~10 years | 750 (16%) | 1.07 (0.92, 1.26) | 0.380 | ||
| >10 year | 108 (2.3%) | 0.79 (0.53, 1.17) | 0.245 | ||
| FSH | |||||
| ≤10 | 3968 (84.64%) | 1.0 | |||
| >10 | 720 (15.36%) | 0.79 (0.67, 0.93) | 0.004 | 0.98(0.83, 1.17) | 0.845 |
| E2 | |||||
| ≤55 | 3411 (72.76%) | 1.0 | |||
| >55 | 1277 (27.24%) | 0.84 (0.74, 0.96) | 0.010 | 0.86(0.76, 0.99) | 0.031 |
| P | |||||
| ≤1 | 3761 (80.23%) | 1.0 | |||
| >1 | 927 (19.77%) | 0.97 (0.84, 1.12) | 0.664 | ||
| PRL | |||||
| ≤10 | 1452 (30.97%) | 1.0 | |||
| >10 | 3236 (69.03%) | 1.01 (0.89, 1.14) | 0.918 | ||
| LH | |||||
| ≤5 | 2822 (60.2%) | 1.0 | |||
| >5 | 1866 (39.8%) | 1.03 (0.92, 1.16) | 0.624 | ||
| T | |||||
| ≤0.45 | 2530 (53.97%) | 1.0 | |||
| >0.45 | 2158 (46.03%) | 1.23 (1.10, 1.38) | 0.001 | 1.13(1.00, 1.28) | 0.042 |
| AMH | |||||
| ≤3 | 2740 (58.45%) | 1.0 | |||
| >3 | 1948 (41.55%) | 1.47 (1.31, 1.66) | <0.001 | 1.15(1.01, 1.32) | 0.036 |
| Reason of infertility | |||||
| Primary | 2360 (50.34%) | 1.0 | |||
| Secondary | 2328 (49.66%) | 0.86 (0.76, 0.96) | 0.010 | 0.99(0.88, 1.12) | 0.857 |
| Treatment regimen | |||||
| Natural cycles or short cycle | 16 (0.34%) | 1.0 | |||
| Antagonist protocol | 1188 (25.34%) | 1.65 (0.53, 5.14) | 0.3903 | ||
| GnRH agonist protocol | 2075 (44.26%) | 2.40 (0.77, 7.48) | 0.1299 | ||
| Depot GnRHa protocol | 1409 (30.06%) | 2.64 (0.85, 8.21) | 0.0946 | ||
| Oocyte retrieval season | |||||
| Non-summer time | 2573 (55.14%) | 1.0 | |||
| Summer time (May-August) | 2093 (44.86%) | 1.10 (0.98, 1.23) | 0.120 | ||
| Holiday season | |||||
| Non-CNY Holiday Season (Mar. ~ Dec.) | 4449 (94.9%) | 1.0 | |||
| CNY Holiday Season (Jan ~ Feb) | 239 (5.1%) | 0.62 (0.47, 0.82) | 0.001 | 0.62(0.47, 0.82) | 0.001 |
| Number of oocytes retrieval | |||||
| 0–5 | 932 (19.91%) | 1.0 | |||
| 6–10 | 1654 (35.33%) | 1.62 (1.37, 1.92) | <0.001 | 1.44 (1.20, 1.71) | <0.001 |
| 11–15 | 1364 (29.14%) | 2.09 (1.76, 2.49) | <0.001 | 1.71 (1.41, 2.07) | <0.001 |
| 16 and more | 731 (15.62%) | 2.06 (1.68, 2.51) | <0.001 | 1.59 (1.27, 1.99) | <0.001 |
Abbreviations: AMH, anti-Müllerian hormone; E2, estrogen; FSH, Follicle-Stimulating Hormone; LH, luteinizing hormone; P, progesterone; PRL, prolactin; T, testosterone; CNY, Chinese New Year.
Figure 4.
Factors associate with fresh live birth rage.
Multivariate Logistic Analysis for Participants Receiving IVF During Regular Wintertime
The CNY group had a lower live birth rate than others whose embryo transfer took place from Nov. to Dec., known as regular wintertime in north China (OR=0.61, 95% CI 0.45–0.84, p=0.002). Meanwhile, T level (OR=1.35, 95% CI 1.04–1.76, p=0.024) and number of oocytes retrieved (OR=1.25, 95% CI=1.07–1.46, p=0.004) were independent factors associated with live birth rate (Table 4). The comparative data between the CNY group and the non-winter time group can be found in Tables S1 and S2 within the supplementary document.
Table 4.
Multivariate Logistic Analysis for Participants Implanted During Wintertime (Jan., Feb., Nov. and Dec.)
| Multivariate Analysis | ||
|---|---|---|
| OR (95% CI) | Adjusted P | |
| Age | ||
| ≤35 | ||
| >35 | 0.71(0.48, 1.07) | 0.103 |
| FSH | ||
| ≤10 | ||
| >10 | 0.88(0.58, 1.32) | 0.525 |
| E2 | ||
| ≤55 | ||
| >55 | 0.89(0.67, 1.19) | 0.427 |
| T | ||
| ≤0.45 | ||
| >0.45 | 1.35(1.04, 1.76) | 0.024 |
| AMH | ||
| ≤3 | ||
| >3 | 1.25(0.93, 1.66) | 0.138 |
| Reason of infertility | ||
| Primary | ||
| Secondary | 0.79(0.61, 1.04) | 0.092 |
| Holiday season | ||
| Non-CNY Holiday Season Wintertime (Nov. ~ Dec.) | ||
| CNY Holiday Season (Jan. ~ Feb.) | 0.61(0.45, 0.84) | 0.002 |
| Number of oocytes | ||
| 0–5 | ||
| 6–10 | 1.25(1.07, 1.46) | 0.004 |
| 11–15 | ||
| 16 and more | ||
Abbreviations: AMH, anti-Müllerian hormone; E2, estrogen; FSH, Follicle-Stimulating Hormone; LH, luteinizing hormone; P, progesterone; PRL, prolactin; T, testosterone.
Outcome of ART for Matched Participants
The general characters of participants before and after matching are shown in Table 5. All variables were comparable. As shown in Table 6, 97 of 236 women from the CNY group had clinical pregnancy (41.10%). In contrast, 232 of 469 from the N-CNY group had clinical pregnancy (49.47%). The CNY group had a significantly lower clinical pregnancy rate than that of the N-CNY group (p=0.036). For live birth, 75 of 236 (31.78%) women had live birth in the CNY group, versus 200 of all 469 (42.64%) women in the N-CNY group (p=0.005). In addition, there was no difference in pregnancy loss between the two groups (p=0.239).
Table 5.
Characters for Matched Participants by Propensity Score
| Non-CNY Holiday Season (n=469) | CNY Holiday Season (n=236) | p-value | SMD | |
|---|---|---|---|---|
| Age | ||||
| Mean±sd | 31.21±4.54 | 31.19±4.74 | 0.96 | 0.004 |
| Min-max | 20–46 | 21–48 | ||
| Infertility years | ||||
| ≤5 year | 387(82.52%) | 190 (80.51%) | 0.307 | 0.126 |
| 5~10 years | 70(14.93%) | 43 (18.22%) | ||
| >10 year | 12(2.56%) | 3 (1.27%) | ||
| Education | ||||
| ≤6 years | 7(1.49%) | 5 (2.12%) | 0.889 | 0.062 |
| 6–9 years | 139(29.64%) | 67 (28.39%) | ||
| 9~12 years | 110(23.45%) | 59 (25%) | ||
| >12 year | 213(45.42%) | 105 (44.49%) | ||
| FSH | ||||
| Mean±sd | 7.66±2.41 | 7.66±2.32 | 0.891# | 0.003 |
| Median (q1-q3) | 7.35(6.26–8.7) | 7.36(6.16–8.84) | ||
| E2 | ||||
| Mean±sd | 47.58±42.15 | 46.03±49.01 | 0.116# | 0.034 |
| Median (q1-q3) | 40(28–59) | 39(25–53.25) | ||
| P | ||||
| Mean±SD | 0.68±0.61 | 0.7±0.56 | 0.449# | 0.036 |
| Median (Q1-Q3) | 0.57(0.36–0.84) | 0.59(0.39–0.86) | ||
| PRL | ||||
| Mean±SD | 14.46±10.5 | 15.73±17.2 | 0.745# | 0.089 |
| Median (Q1-Q3) | 12.74(9.4–16.52) | 12.4(9.33–16.91) | ||
| LH | ||||
| Mean±SD | 4.58±2.74 | 4.57±2.43 | 0.646# | 0.001 |
| Median (Q1-Q3) | 3.95(2.98–5.5) | 4.12(3.02–5.62) | ||
| T | ||||
| Mean±SD | 0.43±0.16 | 0.43±0.16 | 0.602# | 0.030 |
| Median (Q1-Q3) | 0.44(0.32–0.55) | 0.44(0.32–0.57) | ||
| AMH | ||||
| Mean±SD | 2.95±2.04 | 2.97±2.13 | 0.948# | 0.011 |
| Median (Q1-Q3) | 2.41(1.46–3.98) | 2.54(1.55–3.6) | ||
| Reason of infertility | 0.474 | |||
| Primary | 239 (50.96%) | 127 (53.81%) | 0.057 | |
| Secondary | 230 (49.04%) | 109 (46.19%) |
Notes: #Mann–Whitney test.
Abbreviations: AMH, anti-Müllerian hormone; E2, estrogen; FSH, Follicle-Stimulating Hormone; LH, luteinizing hormone; P, progesterone; PRL, prolactin; T, testosterone; SMD, standardized mean difference, A larger value of the standardized mean difference indicates a more significant difference between the two groups of data.
Table 6.
Outcome of ART for Matched Participants by Propensity Score
| Non-CNY Holiday Season (n=469) | CNY Holiday Season (n=236) | p-value | SMD | |
|---|---|---|---|---|
| Treatment regimen | ||||
| Natural cycles or short cycle | 0.915 | 0.033 | ||
| Antagonist protocol | 167 (35.61%) | 82 (34.75%) | ||
| GnRH agonist protocol | 205 (43.71%) | 102 (43.22%) | ||
| Depot GnRHa protocol | 97 (20.68%) | 52 (22.03%) | ||
| Number of oocytes | ||||
| 0–5 | 101 (21.54%) | 55 (23.40%) | 0.412 | 0.136 |
| 6~10 | 177 (37.74%) | 75 (31.91%) | ||
| 11~15 | 121 (25.80%) | 71 (30.21%) | ||
| 16 and more | 70 (14.93%) | 34 (14.47%) | ||
| Clinical pregnancy | ||||
| No | 237 (50.53%) | 139 (58.90%) | 0.036 | 0.169 |
| Yes | 232 (49.47%) | 97 (41.10%) | ||
| Pregnancy loss | ||||
| No | 437 (93.18%) | 214 (90.68%) | 0.239 | 0.092 |
| Yes | 32 (6.82%) | 22 (9.32%) | ||
| Live birth | ||||
| No | 269 (57.36%) | 161 (68.22%) | 0.005 | |
| Yes | 200 (42.64%) | 75 (31.78%) |
Abbreviations: SMD, standardized mean difference, A larger value of the standardized mean difference indicates a more significant difference between the two groups of data.
Comparison of Live Birth for IPTW Data
Based on the IPTW data analysis, the CNY group also showed a lower live birth rate than that of the N-CNY group (OR=0.64, 95% CI 0.47–0.87, p=0.005) (Table 7). The baseline characteristics of the IPTW dataset are presented in Table S3 in the supplementary documents.
Table 7.
Comparison of Live Birth for IPTW Data
| Multivariate Analysis | ||
|---|---|---|
| OR (95% CI) | Adjusted P | |
| Non-CNY Holiday Season | ||
| CNY Holiday Season | 0.64(0.47, 0.87) | 0.005 |
Abbreviations: IPTW, Inverse probability of treatment weighting; CNY, Chinese New Year.
Discussion
It has been reported in various medical specialties that there is a higher incidence of morbidity during off-hours such as holidays and nighttime. Studies have indicated that obstetrics, in particular, are more likely to encounter severe adverse neonatal events during nighttime hours10 and on weekends.11 In this retrospective study, we investigated the monthly outcomes of fresh embryo transfer cycles in IVF and observed a decreased live birth rate during the CNY holiday season, particularly in the month of February. The reduction in live birth rate was significant, with a 15.1% lower rate in February compared to January. While the exact reasons for this decline are unknown, we have several assumptions that may explain this trend.
Due to the long-standing tradition, many Chinese people experience sudden changes in their daily lives during the CNY holiday season. This includes unhealthy dietary choices, frequent tobacco and alcohol consumption due to family and social gatherings, and sleep deprivation. Although these changes only last for two months, they can negatively impact overall health and compromise IVF success rates.12 During the CNY holiday season, people tend to indulge in excessive eating, which can lead to weight gain. Studies have shown that holiday weight gain can result from consuming high amounts of salt, sugar, and fatty foods, which can lead to imbalanced nutrition.13,14 This pattern is also observed during the CNY holiday season. Studies have suggested that fatty meals may have acute adverse effects on oocyte mitochondria,15 and that high-fat diets can have detrimental effects on male fertility.16 Research has shown that certain dietary patterns can have negative effects on IVF outcomes. For example, a prospective study found that certain Chinese food patterns and a Western food style involving “Puffed food-Candy-Bakery” were associated with a lower likelihood of achieving biochemical pregnancy.17 Additionally, a primate study showed that a short-term high-fat Western-style diet before IVF treatment led to a reduction in blastocyst numbers and dysregulation of RNA binding and mitochondrial function.18 Consumption of beverages with high sugar content has also been linked to a lower number of total, mature, and fertilized oocytes, as well as a lower number of top-quality embryos after ovarian stimulation.19 Higher intake of supplemental folic acid, vitamin B12, vitamin D, produce with low pesticide residue, whole grains, dairy, soy foods and seafood were defined as pro-fertility diet, which was associated with an increased probability of live birth of women undergoing IVF.20 However, during the CNY holiday season, people tend to change their diet in order to indulge in more tasty foods, which often contain higher amounts of fat, salt, and sugar. Such a shift in diet pattern could result in a reduced intake of pro-fertility nutrients.
The CNY holiday season is characterized by frequent family and social gatherings, which can create occasions for excessive tobacco exposure and alcohol consumption. This can have a negative impact on IVF success rates as smoking and secondhand smoke exposure have been linked to decreased fertility and adverse pregnancy outcomes.21 Additionally, smoking is deeply ingrained in Chinese culture and cigarette gifting and sharing is a common practice during the CNY holiday season, which can make it difficult for smokers to quit. It is important to note that the negative impacts of tobacco exposure on IVF outcomes are dependent on various factors such as hormonal status, individual health conditions, the amount and duration of exposure, and personal sensitivity to cigarettes. Previous research has shown that women who are exposed to tobacco have a 17% increased risk of implantation failure and a 19% decreased likelihood of live birth following IVF.22 Such negative impacts of tobacco exposure depend on hormonal status, individual health conditions, the amount of tobacco exposure, the total length of exposure time, and personal sensitivity to cigarettes.23 Therefore, the increased exposure to tobacco during the CNY holiday season may have a detrimental effect on IVF outcomes. Compelling evidence has demonstrated that smoking negatively influences IVF outcomes.24–26 Moreover, using of both e-cigarette and regular cigarette by women was associated with small-for-gestational-age birth.27,28 A meta-analysis across twenty different studies with 5865 subjects in total showed that the exposure to smoking had negative impacts on semen,29 which may reduce the efficacy of assisted reproductive technology (ART). Also, smoking induces DNA damage in sperms30,31 thereby influencing IVF outcomes.24,32 Altogether, increased tobacco exposure by both partners may contribute to the LBR decline from IVF performed during the CNY holiday season.
The CNY holiday celebration is widely linked to alcohol abuse.33,34 One study found that female and male alcohol consumption is a primary risk factor for IVF.35 Female alcohol consumption was associated with a decreased number of oocyte retrieval and an increased risk of miscarriage. For males, one additional drink per day increased the risk of not achieving a live birth and drinking one month before IVF increased the risk of spontaneous miscarriage. When both of the couple drink, as few as four alcoholic drinks per week may cause a decrease in IVF live birth rate.36
It is common for people to drastically change their sleep patterns and even experience sleep deprivation due to festival celebrations and holiday travel. The traditional New Year’s Eve celebration often involves staying up late or even staying up all night. A systematic review has shown that both female and male fertility and IVF outcomes may be negatively impacted by decreased sleep duration and changes in chronotype.37
In addition to the aforementioned lifestyle changes, emotional stress is another critical factor for the live birth of IVF performed during the CNY holiday season. During this period, patients often feel stressed about making decisions regarding whether or not to interact with certain relatives or friends due to the financial pressures associated with travel, gift purchasing, entertaining, and alcohol consumption. One study showed that holiday sedentary behavior can indirectly impact anxiety through neurobiological markers.38 Furthermore, stress can be increased by traveling and interacting with visitors during the Chinese New Year holiday season. Patients receiving IVF during this period may experience stress from IVF itself and the CNY preparation and celebration. Several studies have shown that stress has negative impacts on IVF outcomes, including the number of oocyte retrieval, fertilization, pregnancy, spontaneous abortion, live birth, multiple gestations, and low birth weight. A meta-analysis39 including articles showed that psychological stress might diminish IVF success rates, possibly due to hypothalamic dysfunction either via neurotransmission alterations, catecholamine depletion, or interference with hypothalamic receptors for neurotransmitters.40 Elevated glucocorticoid levels due to stress could have inhibitory effects on steroidogenesis, spermatogenesis, and sperm maturation. A study indicated that psychological stress among men at the time of oocyte retrieval had negative impacts on sperm quality.41 Moreover, a cross-sectional study showed a negative association between self-reported stress and sperm parameters.42
Numerous studies have examined the effect of psychological stress on IVF outcomes. Ebbesen’s research showed that stressful life events have a negative impact on the success rate of IVF.43 A meta-analysis also revealed a small but significant association between stress and reduced pregnancy chances in female patients undergoing ART.44 Additionally, a study of 160 infertile women undergoing IVF demonstrated a negative correlation between psychological stress and IVF outcomes.45 Women experiencing excessive stress may face a high risk of not achieving a successful live birth delivery.39 Various methods have been employed to evaluate psychological stress. The PHQ-9 and PHQ-2 are easy-to-use tools to measure depressive symptoms that are suitable for infertility patients.46 Haimovici et al evaluated psychological stress using questionnaires and visual analog scales and analyzed cytokines in serum, semen, cervicovaginal, and follicular fluids. This study suggested that stress-induced cytokines in both partners may indicate IVF failure.47 Another study investigated the influence of psychological stress on embryo cleavage kinetics and found that a stress management program offered to infertile couples could retard the first embryo cleavage and accelerate embryo compaction.48 Collectively, avoiding psychological stress for both partners receiving IVF is beneficial to increase the success rate.
Travel can also have an impact on IVF outcomes. During the Chinese New Year holiday season, many people make travel plans, causing patients to rush to complete their IVF treatment before leaving. Additionally, medical staff may also travel during this period, resulting in changes in work shifts that can affect the IVF outcome.49,50 For example, patients may have follow-up appointments with doctors and nurses who are not familiar with their medical history and treatment plan. Further research is needed to investigate the potential effects of medical staff changes on IVF outcomes during the holiday season.
Furthermore, it is important to acknowledge that medical staff may also experience similar external adverse effects during the festival period, leading to physical and mental exhaustion, reduced attention span, deviations in their performance, and even potential errors. Consequently, these challenges could potentially contribute to a decline in the live birth rate during the fresh cycle of IVF throughout the CNY celebrations.
Limitations
The limitations of this study should be acknowledged. Firstly, as a retrospective study, some variables that may negatively impact the live birth rate were not recorded, such as smoking, changes in BMI, sleep quantity and quality, travel, and psychological stress. Secondly, this study only focused on the live birth rate of the first fresh implantation cycle, and the cumulative live birth rate during the holiday season compared to the non-holiday season is still unknown. Thirdly, the results of this study are based only on data from one IVF center, and it is unclear whether the holiday season impacts other cities in China as social cultures vary across regions. Therefore, future prospective multi-center studies are needed to provide new insights into the above factors that may affect the live birth rate. Lastly, our hypothesis was centered on investigating whether the live birth rate of patients undergoing IVF during the CNY season differed from those outside this period. However, it is essential to acknowledge that due to limitations in data collection, our study did not encompass a comprehensive analysis of all the patients’ daily life patterns during the CNY season. Consequently, not all the assumptions we mentioned were subjected to direct testing in our research.
Conclusions
This study is the first to demonstrate a previously unknown decline in the live birth rate from IVF fresh cycles with embryo transfer occurring during the CNY holiday season. During the CNY holiday season, IVF procedures yield a reduced live birth rate, suggesting that lifestyle habits like unhealthy diets, tobacco and alcohol consumption, sleep disturbances, and emotional stress might impact the treatment’s outcomes.
Funding Statement
1. Funder name: S&T Program of Hebei under Grant, China. Grant Number: 20377714D, Grant Recipient: Guimin Hao. 2. Funder name: Hebei Province Medical Science Research Key Project, China. Grant Number: 20211494, Grant Recipient: Jiajia Zhai.
Abbreviations
CNY, Chinese New Year; LBR, live birth rate; PSM, Propensity score matching.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
We affirm that this research study has been conducted in strict adherence to the principles delineated in the Declaration of Helsinki. The study analyzed the data of fresh embryo transfer (ET) cycles performed at the reproductive center of the Second Hospital of Hebei Medical University between January 2017 and October 2021. As a retrospective research, informed consents were not obtained from patients prior to this study. Given the retrospective nature of the study, numerous subjects were inaccessible, particularly within the context of the COVID-19 period. Consequently, the approval for waiving informed consent was obtained in conjunction with the study protocol from the Research Ethics Committee of the Second Hospital affiliated with Hebei Medical University (Approval number: 2022-R060). Adhering to the guidelines of the Second Hospital affiliated with Hebei Medical University, all sensitive information was expunged prior to extracting data from the Hospital Information System database.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Malekpour P, Hasanzadeh R, Javedani Masroor M, Chaman R, Motaghi Z. Effectiveness of a mixed lifestyle program in couples undergoing assisted reproductive technology: a study protocol. Reprod Health. 2023;20(1):112. doi: 10.1186/s12978-023-01652-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg CR, Shi M, DeRoo LA, Basso O, Skjærven R. Season and preterm birth in Norway: a cautionary tale. Int J Epidemiol. 2015;44(3):1068–1078. doi: 10.1093/ije/dyv100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hviid A, Laksafoss A, Hedley P, et al. Assessment of seasonality and extremely preterm birth in Denmark. JAMA Netw Open. 2022;5(2):e2145800. doi: 10.1001/jamanetworkopen.2021.45800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia KFB, Farland LV, Missmer SA, Racowsky C. The association between season, day length, and temperature on clinical outcomes after cryopreserved embryo transfer. Fertil Steril. 2022;117(3):539–547. doi: 10.1016/j.fertnstert.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Zhao M, Zhang H, Waters THB, Chung JPW, Li TC, Chan DYL. The effects of daily meteorological perturbation on pregnancy outcome: follow-up of a cohort of young women undergoing IVF treatment. Environ Health. 2019;18(1):103. doi: 10.1186/s12940-019-0538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 7.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: [DOI] [PubMed] [Google Scholar]
- 8.Wei D, Liu JY, Sun Y, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–1318. doi: 10.1016/s0140-6736(18)32843-5 [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi: 10.1056/NEJMoa1705334 [DOI] [PubMed] [Google Scholar]
- 10.Einerson BD. Coverage and capacity: addressing the ‘night & weekend effect’ in obstetrics. BJOG. 2018;125(7):892. doi: 10.1111/1471-0528.15032 [DOI] [PubMed] [Google Scholar]
- 11.Snowden JM, Caughey AB. Is there a weekend effect in obstetrics? BMJ. 2015;351:h6192. doi: 10.1136/bmj.h6192 [DOI] [PubMed] [Google Scholar]
- 12.Boedt T, Matthys C, Lie Fong S, et al. Systematic development of a mobile preconception lifestyle programme for couples undergoing IVF: the PreLiFe-programme. Hum Reprod. 2021;36(9):2493–2505. doi: 10.1093/humrep/deab166 [DOI] [PubMed] [Google Scholar]
- 13.Helander EE, Wansink B, Chieh A. Weight gain over the holidays in three countries. N Engl J Med. 2016;375(12):1200–1202. doi: 10.1056/NEJMc1602012 [DOI] [PubMed] [Google Scholar]
- 14.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000;342(12):861–867. doi: 10.1056/nejm200003233421206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabozzi G, Iussig B, Cimadomo D, et al. The impact of unbalanced maternal nutritional intakes on oocyte mitochondrial activity: implications for reproductive function. Antioxidants. 2021;10(1):91. doi: 10.3390/antiox10010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crean AJ, Senior AM. High-fat diets reduce male reproductive success in animal models: a systematic review and meta-analysis. Obes Rev. 2019;20(6):921–933. doi: 10.1111/obr.12827 [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Zhang X, Zhao X, et al. Preconception dietary patterns and associations with IVF outcomes: an ongoing prospective cohort study. Front Nutri. 2022;9:808355. doi: 10.3389/fnut.2022.808355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravisankar S, Ting AY, Murphy MJ, et al. Short-term Western-style diet negatively impacts reproductive outcomes in primates. JCI Insight. 2021;6(4). doi: 10.1172/jci.insight.138312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machtinger R, Gaskins AJ, Mansur A, et al. Association between preconception maternal beverage intake and in vitro fertilization outcomes. Fertil Steril. 2017;108(6):1026–1033. doi: 10.1016/j.fertnstert.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskins AJ, Nassan FL, Chiu YH, et al. Dietary patterns and outcomes of assisted reproduction. Am J Obstet Gynecol. 2019;220(6):567.e1–567.e18. doi: 10.1016/j.ajog.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich ZC, Hu M, Xiao S. Gifting and sharing cigarettes in a rural Chinese village: a cross-sectional study. Tob Control. 2014;23(6):496–500. doi: 10.1136/tobaccocontrol-2012-050956 [DOI] [PubMed] [Google Scholar]
- 22.Benedict MD, Missmer SA, Vahratian A, et al. Secondhand tobacco smoke exposure is associated with increased risk of failed implantation and reduced IVF success. Hum Reprod. 2011;26(9):2525–2531. doi: 10.1093/humrep/der226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dechanet C, Brunet C, Anahory T, Hamamah S, Hedon B, Dechaud H. [Effects of cigarette smoking on female reproduction: from oocyte to embryo (Part I)] Effets du tabagisme sur la reproduction: de l’ovocyte à l’embryon (Partie I). Gynecol Obstet Fertil. 2011;39(10):559–566. doi: 10.1016/j.gyobfe.2011.07.033 [DOI] [PubMed] [Google Scholar]
- 24.Joesbury KA, Edirisinghe WR, Phillips MR, Yovich JL. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13(6):1506–1513. doi: 10.1093/humrep/13.6.1506 [DOI] [PubMed] [Google Scholar]
- 25.Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382–1390. doi: 10.1093/humrep/16.7.1382 [DOI] [PubMed] [Google Scholar]
- 26.Zitzmann M, Rolf C, Nordhoff V, et al. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2003;79(Suppl 3):1550–1554. doi: 10.1016/s0015-0282(03)00339-x [DOI] [PubMed] [Google Scholar]
- 27.Shittu AAT, Kumar BP, Okafor U, Berkelhamer SK, Goniewicz ML, Wen X. Changes in e-cigarette and cigarette use during pregnancy and their association with small-for-gestational-age birth. Am J Obstet Gynecol. 2022;226(5):730.e1–730.e10. doi: 10.1016/j.ajog.2021.11.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111(2):230–246. doi: 10.2105/ajph.2020.305999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016;70(4):635–645. doi: 10.1016/j.eururo.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 30.Rubes J, Lowe X, Moore D, et al. Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril. 1998;70(4):715–723. doi: 10.1016/s0015-0282(98)00261-1 [DOI] [PubMed] [Google Scholar]
- 31.Zenzes MT, Bielecki R, Reed TE. Detection of benzo(a)pyrene diol epoxide-DNA adducts in sperm of men exposed to cigarette smoke. Fertil Steril. 1999;72(2):330–335. doi: 10.1016/s0015-0282(99)00230-7 [DOI] [PubMed] [Google Scholar]
- 32.Hughes EG, YoungLai EV, Ward SM. Cigarette smoking and outcomes of in-vitro fertilization and embryo transfer: a prospective cohort study. Hum Reprod. 1992;7(3):358–361. doi: 10.1093/oxfordjournals.humrep.a137650 [DOI] [PubMed] [Google Scholar]
- 33.Ceballos NA, Sharma S, Patterson TL, Graham R, Howard K. Stress, immune function and collegiate holiday drinking: a pilot study. Neuropsychobiology. 2015;72(1):8–15. doi: 10.1159/000438757 [DOI] [PubMed] [Google Scholar]
- 34.Chan RHW, Wong TY, Dong D, Kim JH. Alcohol social media marketing in Hong Kong: a content analysis of Facebook posts. J Adolesc Health. 2023;73:461–469. doi: 10.1016/j.jadohealth.2023.05.007 [DOI] [PubMed] [Google Scholar]
- 35.Klonoff-Cohen H, Lam-Kruglick P, Gonzalez CJF. Effects of maternal and paternal alcohol consumption on the success rates of in vitro fertilization and gamete intrafallopian transfer. Fertil Steril. 2003;79(2):330–339. doi: 10.1016/s0015-0282(02)04582-x [DOI] [PubMed] [Google Scholar]
- 36.Rossi BV, Berry KF, Hornstein MD, Cramer DW, Ehrlich S, Missmer SA. Effect of alcohol consumption on in vitro fertilization. Obstet Gynecol. 2011;117(1):136–142. doi: 10.1097/AOG.0b013e31820090e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caetano G, Bozinovic I, Dupont C, Léger D, Lévy R, Sermondade N. Impact of sleep on female and male reproductive functions: a systematic review. Fertil Steril. 2021;115(3):715–731. doi: 10.1016/j.fertnstert.2020.08.1429 [DOI] [PubMed] [Google Scholar]
- 38.Pineda JCD, Kokubun K, Ikaga T, Yamakawa Y. Housing quality and behavior affect brain health and anxiety in healthy Japanese adults. Sci Rep. 2021;11(1):11999. doi: 10.1038/s41598-021-91363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klonoff-Cohen H, Natarajan L. The concerns during assisted reproductive technologies (CART) scale and pregnancy outcomes. Fertil Steril. 2004;81(4):982–988. doi: 10.1016/j.fertnstert.2003.08.050 [DOI] [PubMed] [Google Scholar]
- 40.Klonoff-Cohen HJH. Female and male lifestyle habits and IVF: what is known and unknown. Hum Reprod. 2005;11(2):179–203. doi: 10.1093/humupd/dmh059 [DOI] [PubMed] [Google Scholar]
- 41.Clarke RN, Klock SC, Geoghegan A, Travassos DE. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Hum Reprod. 1999;14(3):753–758. doi: 10.1093/humrep/14.3.753 [DOI] [PubMed] [Google Scholar]
- 42.Nordkap L, Jensen TK, Hansen ÅM, et al. Psychological stress and testicular function: a cross-sectional study of 1215 Danish men. Fertil Steril. 2016;105(1):174–87.e1–2. doi: 10.1016/j.fertnstert.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 43.Ebbesen SM, Zachariae R, Mehlsen MY, et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24(9):2173–2182. doi: 10.1093/humrep/dep185 [DOI] [PubMed] [Google Scholar]
- 44.Matthiesen SM, Frederiksen Y, Ingerslev HJ, Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): a meta-analysis. Hum Reprod. 2011;26(10):2763–2776. doi: 10.1093/humrep/der246 [DOI] [PubMed] [Google Scholar]
- 45.Gourounti K, Anagnostopoulos F, Vaslamatzis G. The relation of psychological stress to pregnancy outcome among women undergoing in-vitro fertilization and intracytoplasmic sperm injection. Women Health. 2011;51(4):321–339. doi: 10.1080/03630242.2011.574791 [DOI] [PubMed] [Google Scholar]
- 46.Maroufizadeh S, Omani-Samani R, Almasi-Hashiani A, Amini P, Sepidarkish M. The reliability and validity of the Patient Health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with infertility. Reprod Health. 2019;16(1):137. doi: 10.1186/s12978-019-0802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haimovici F, Anderson JL, Bates GW, et al. Stress, anxiety, and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol. 2018;79(4):e12832. doi: 10.1111/aji.12832 [DOI] [PubMed] [Google Scholar]
- 48.Raad G, Tanios J, Kerbaj S, et al. Stress management during the intracytoplasmic sperm injection cycle may slow down first embryo cleavage and accelerate embryo compaction: a pilot randomized controlled trial. Psychother Psychosom. 2021;90(2):119–126. doi: 10.1159/000512530 [DOI] [PubMed] [Google Scholar]
- 49.Sachs L. Firm but fair policies for staff vacations and holidays. J Med Pract Manage. 2002;18(1):42–44. [PubMed] [Google Scholar]
- 50.Latham J, Fagan J. Control of Christmas holiday census and staffing. J Nurs Adm. 1979;9(10):19–23. doi: 10.1097/00005110-197910000-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.