Abstract
Introduction:
Creatinine has limitations in identifying and predicting acute kidney injury (AKI). Our study examined the utility of neutrophil gelatinase-associated lipocalin (NGAL) in predicting AKI in patients presenting to the emergency department (ED), and in predicting the need for renal replacement therapy (RRT), occurrence of major adverse cardiac events (MACE) and all-cause mortality at three months post visit.
Methods:
This is a single-centre prospective cohort study conducted at Singapore General Hospital (SGH). Patients presenting to SGH ED from July 2011 to August 2012 were recruited. They were aged ≥21 years, with an estimated glomerular filtration rate <60 mL/min/1.73 m2, and had congestive cardiac failure, systemic inflammatory response syndrome or required hospital admission. AKI was diagnosed by researchers blinded to experimental measurements. Serum NGAL was measured as a point-of-care test.
Results:
A total of 784 patients were enrolled, of whom 107 (13.6%) had AKI. Mean serum NGAL levels were raised (P < 0.001) in patients with AKI (670.0 ± 431.9 ng/dL) compared with patients without AKI (490.3 ± 391.6 ng/dL). The sensitivity and specificity of NGAL levels >490 ng/dL for AKI were 59% (95% confidence interval [CI] 49%–68%) and 65% (95% CI 61%–68%), respectively. Need for RRT increased 21% per 100 ng/dL increase in NGAL (P < 0.001), whereas odds of death in three months increased 10% per 100 ng/dL increase in NGAL (P = 0.028). No clear relationship was observed between NGAL levels and MACE.
Conclusion:
Serum NGAL identifies AKI and predicts three-month mortality.
Keywords: Acute kidney injury, congestive cardiac failure, emergency department, neutrophil gelatinase-associated lipocalin, systemic inflammatory response syndrome
INTRODUCTION
Acute kidney injury (AKI) is the rapid deterioration of renal function over a period of hours to days, resulting in the failure of the kidney to excrete waste and maintain fluid balance.[1,2,3] AKI is commonly encountered in the emergency department (ED) and has an incidence of about 10%–20%.[4,5,6,7,8,9,10] Without prompt treatment, AKI results in severe consequences such as fluid overload, electrolyte imbalance and uraemia in the short run, as well as progressive chronic kidney disease (CKD) leading to end-stage renal failure in the long run.[3,11]
Various criteria exist to define the presence of AKI, including the RIFLE (Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease) criteria, KDIGO (Kidney Disease Improving Global Outcomes) criteria and AKIN (Acute Kidney Injury Network) criteria.[8] The common feature of all these criteria is the use of serum creatinine level and estimated glomerular filtration rate (eGFR). However, using creatinine to diagnose AKI has its limitations. Creatinine levels are affected by many factors, such as age, gender, muscle mass and liver function.[11] Moreover, creatinine poorly differentiates between prerenal azotaemia, AKI and CKD, all of which result in elevated creatinine levels.[1,2,12] Furthermore, accumulation of serum creatinine lags behind the precipitating insult[1,2,3] and is elevated only when as much as 50% of renal function is lost.[2] Such limitations are especially crucial in the ED because they severely limit our ability to identify and appropriately manage patients with AKI.
To overcome the limitations of creatinine as a marker for AKI, other biomarkers have been explored as alternatives.[13,14,15,16] Among these, neutrophil gelatinase-associated lipocalin (NGAL) shows promise because of its ability to differentiate between prerenal azotaemia, AKI and CKD.[12,17,18,19,20] NGAL is a 25-kDa glycoprotein secreted by renal tubular cells, immune cells and cancer cells, and plays an important role in renal recovery, bacterial defence and inflammation.[21,22,23,24,25,26] NGAL can be detected in urine or serum. Obtaining urinary NGAL is non-invasive but severely limited by patients' poor hydration status and low urine output,[3] whereas obtaining serum NGAL is minimally invasive and convenient in terms of sampling during routine blood investigations. Urinary NGAL performs marginally better than serum NGAL in mild AKI but is similar to serum NGAL in moderate-to-severe cases of AKI.[18] NGAL performs well in both paediatric and adult settings, and has been extensively studied in cases of cardiac surgery,[19,27,28] critical care[29,30] and kidney transplantation.[31,32]
In the ED, where the exact time of renal insult is less clear, NGAL continues to be useful. In one single-centre prospective cohort study (n = 635), urinary NGAL was able to differentiate AKI from other conditions with an area under the receiver operating characteristic curve (AUC) of 0.95, 90% sensitivity and 99% specificity.[12] In a similar multicentre prospective cohort study (n = 1,635), urinary NGAL with a cut-off of 104 ng/mL was able to predict AKI with an AUC of 0.81 and 81% specificity.[33] Furthermore, a United States-based multicentre cohort study (n = 661) showed that plasma NGAL >150 ng/dL was able to predict AKI with an AUC of 0.82 and 96% sensitivity.[34] In another single-centre cohort study (n = 616), plasma NGAL similarly predicted AKI with an AUC of 0.82.[35] These studies conducted in the ED demonstrate the usefulness of NGAL even when the onset of renal insult is uncertain.
Nevertheless, the use of NGAL levels to predict AKI has been inadequately explored in Asian populations. Most available studies were limited by small sample sizes (n = 76–151)[36,37,38,39] or focused on patients with sepsis[40] or acute decompensated heart failure alone.[41] However, the limited studies[35,40] on the long-term prognostic value of NGAL in patients in the ED can be further supplemented. Our study examines the usefulness of serum NGAL as a biomarker of AKI in a multiethnic Asian population and as a predictor of the necessity of renal replacement therapy (RRT), major adverse cardiac events (MACE) and all-cause mortality at three months.
METHODS
This study was approved by the Centralised Institutional Review Board of SingHealth Research. Informed consent was obtained from the patients before enrolment. We recruited adult patients who presented to Singapore General Hospital (SGH) Accident and ED from July 2011 to August 2012. Patients enrolled were aged 21 years and above, had an eGFR of less than 60 mL/min/1.73 m2 and met at least one of the following criteria: being diagnosed with congestive cardiac failure (CCF), meeting at least two of the four diagnostic criteria for systemic inflammatory response syndrome (SIRS) or requiring hospital admission. The detailed inclusion and exclusion criteria for the study can be found in Appendix 1. GFR was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation,[42] which has been validated in a multiethnic Asian population similar to that in Singapore.[43]
The Singapore Renal Registry was screened for study patients requiring RRT within three months after discharge. We also screened the Registry of Deaths for cases of mortality within three months of discharge when the patient was lost to follow-up.
AKI was defined in accordance with the AKIN criteria: (a) new-onset increase in serum creatinine of ≥0.3 mg/dL (≥26.4 mM) within 48 hours; or (b) serum creatinine increase × 1.5 from baseline within 48 hours.[44,45,46]
Urine output was not recorded because it is not a routinely monitored parameter in the ED. Serum NGAL was measured using the Triage® NGAL Test (Alere Inc, San Diego, CA, USA) in conjunction with the Alere Triage® MeterPro, using blood samples obtained during other routine blood investigations. The results were compared between patients who developed AKI and those who did not.
Sample size was estimated using the method for diagnostic test studies with a binary outcome based on sensitivity and specificity.[47] From epidemiological data,[4,5,6,7,8,9,10] the prevalence of AKI in patients presenting to the ED and fulfilling our inclusion criteria was estimated to be about 10%–15%. From other studies using NGAL levels to predict AKI in the ED,[12,34,35] we estimated the sensitivity to be 70%–85%. Assuming a margin of error of 0.1, the required total sample size was about 327–807.
Statistical analysis was performed using R version 3.5.3 (R Core Team, Vienna, Austria). One-way analysis of variance was used to compare continuous variables (age, body mass index [BMI], NGAL), and the chi-square test was used for categorical variables across subgroups partitioned by CCF and SIRS. To determine the performance of diagnostic test characteristics of NGAL, we plotted the receiver operating characteristic (ROC) curve and calculated the AUC. The optimal NGAL cut-offs were derived using the Youden index to maximise the sum of sensitivity and specificity. The negative predictive value (NPV), positive predictive value (PPV) and likelihood ratios of positive and negative tests of NGAL in the diagnosis of AKI were estimated with 95% confidence intervals. We also performed multivariable logistic regression to determine the odds ratio of NGAL for AKI adjusted by patients' demographics (age, gender, race and BMI), metabolic comorbidities (diabetes mellitus, hypertension and dyslipidaemia), atheropathic tendencies (previous myocardial infarction, ischaemic heart disease [IHD], peripheral vascular disease [PVD]) and laboratory values (leukocyte count and platelets).
Secondary analyses were also performed through multiple logistic regression models to determine the association of NGAL with three-month postadmission outcomes of mortality, need for RRT, and presence of MACE, defined as atrial arrhythmia, ventricular fibrillation, cardiogenic shock requiring an intra-arterial balloon pump, acute pulmonary oedema requiring intubation, acute myocardial infarction (AMI), coronary artery bypass graft, and percutaneous coronary intervention.
For patients with serum NGAL beyond the detection limit of our equipment (i.e. >1,300 ng/dL), we arbitrarily compared the incidence of AKI in patients having serum NGAL >1,300 ng/dL (22%) with patients having serum NGAL >1,000 ng/dL (21%). We found no significant difference in our study outcomes. Thus, to simplify our analysis, data points 'greater than' 1,300 were treated as 'equal to' 1,300. To address the possible underestimation of the NGAL standard deviation, we ran a logistic model omitting data points 'greater than' 1,300. We found no difference in the estimation of the coefficient and standard error for NGAL.
RESULTS
From July 2011 to August 2012, 784 patients (476 male) were enrolled. Of these, 213 (27.2%) patients had CCF, 183 (23.3%) had SIRS, 105 (13.4%) had both CCF and SIRS, and 283 (36.1%) required admission but had neither CCF nor SIRS. Our study flow is illustrated in Figure 1. Patient demographics are summarised in Table 1, along with relevant baseline physiologic and laboratory values. Mean serum NGAL levels were significantly raised (P < 0.001) in patients with AKI (670.0 ± 431.9 ng/dL) when compared with those of patients without AKI (490.3 ± 391.6 ng/dL). The serum NGAL levels of patients with and without AKI across each patient group are illustrated in Figure 2.
Figure 1.
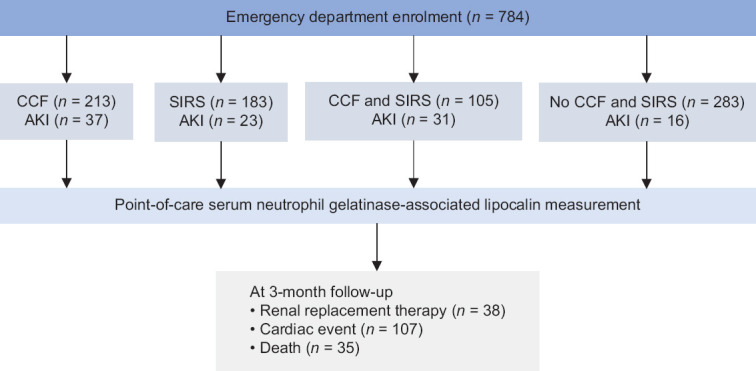
Flowchart shows the enrolment process for the study, with a total of 107 patients having acute kidney injury (AKI). CCF: congestive cardiac failure, SIRS: systemic inflammatory response syndrome
Table 1.
Patient characteristics.
| Characteristic | n (%)/Mean±standard deviation | P | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| All patients (n=784) | CCF (n=213) | SIRS (n=183) | Both CCF and SIRS (n=105) | No CCF or SIRS (n=283) | ||
| Age (yr) | 69.9±12.7 | 70.0±11.4 | 68.9±13.9 | 70.7±12.3 | 70.0±13.0 | 0.707 |
|
| ||||||
| Male gender | 476 (60.7) | 138 (64.8) | 114 (62.3) | 76 (72.4) | 148 (52.3) | 0.001 |
|
| ||||||
| Body mass index | 25.5±5.9 | 25.7±6.2 | 25.5±7.5 | 25.2±4.7 | 25.5±5.1 | 0.921 |
|
| ||||||
| Ethnicity | 0.534 | |||||
|
| ||||||
| Chinese | 560 (71.4) | 152 (71.4) | 131 (71.6) | 71 (67.6) | 206 (72.8) | – |
|
| ||||||
| Malay | 111 (14.2) | 28 (13.1) | 28 (15.3) | 17 (16.2) | 38 (13.4) | – |
|
| ||||||
| Indian | 99 (12.6) | 28 (13.1) | 23 (12.6) | 17 (16.2) | 31 (11.0) | – |
|
| ||||||
| Others | 14 (1.8) | 5 (2.3) | 1 (0.5) | 0 (0) | 8 (2.8) | – |
|
| ||||||
| Comorbidities | ||||||
|
| ||||||
| Hypertension | 656 (83.7) | 181 (85.0) | 142 (77.6) | 89 (84.4) | 244 (86.2) | 0.084 |
|
| ||||||
| Diabetes mellitus | 471 (60.1) | 145 (68.1) | 106 (57.9) | 65 (61.9) | 155 (54.8) | 0.023 |
|
| ||||||
| Dyslipidaemia | 567 (72.3) | 169 (79.3) | 110 (60.1) | 84 (80.0) | 204 (72.1) | <0.001 |
|
| ||||||
| Previous myocardial infarction | 197 (25.1) | 85 (39.9) | 23 (12.6) | 45 (42.9) | 44 (15.5) | <0.001 |
|
| ||||||
| History of IHD/CAD | 374 (47.7) | 147 (69.0) | 62 (33.9) | 68 (64.8) | 97 (34.3) | <0.001 |
|
| ||||||
| Prior congestive cardiac failure | 208 (26.5) | 109 (51.2) | 21 (11.5) | 41 (39.0) | 37 (13.1) | <0.001 |
|
| ||||||
| Atrial arrhythmia | 150 (19.1) | 63 (29.6) | 21 (11.5) | 31 (29.5) | 35 (12.4) | <0.001 |
|
| ||||||
| Ventricular tachycardia | 13 (1.7) | 6 (2.8) | 1 (0.5) | 4 (3.8) | 2 (0.7) | 0.044 |
|
| ||||||
| History of CVA/TIA | 143 (18.2) | 44 (20.7) | 26 (14.2) | 20 (19.0) | 53 (18.7) | 0.404 |
|
| ||||||
| Peripheral vascular disease | 75 (9.6) | 27 (12.7) | 17 (9.3) | 12 (11.4) | 19 (6.7) | 0.14 |
|
| ||||||
| NGAL (ng/dL) | 514.8±401.8 | 405.5±359.1 | 667.7±404.9 | 579±403.2 | 474.5±397.8 | <0.001 |
|
| ||||||
| Acute kidney injury | 107 (13.6) | 37 (17.4) | 23 (12.6) | 31 (29.5) | 16 (5.7) | <0.001 |
|
| ||||||
| Adverse outcome | ||||||
|
| ||||||
| Death at index visit | 25 (3.2) | 6 (2.8) | 13 (7.1) | 6 (5.7) | 0 (0) | <0.001 |
|
| ||||||
| Need for RRT within/at 3 mth | 38 (4.8) | 13 (6.1) | 4 (2.2) | 11 (10.5) | 10 (3.5) | 0.008 |
|
| ||||||
| Cardiac events within/at 3 mth | 107 (13.6) | 38 (17.8) | 16 (8.7) | 21 (20) | 32 (11.3) | 0.008 |
CAD: coronary artery disease, CVA: cerebral vascular accident, IHD: ischaemic heart disease, NGAL: neutrophil gelatinase-associated lipocalin, RRT: renal replacement therapy, SIRS: systemic inflammatory response syndrome, TIA: transient ischaemic attack
Figure 2.
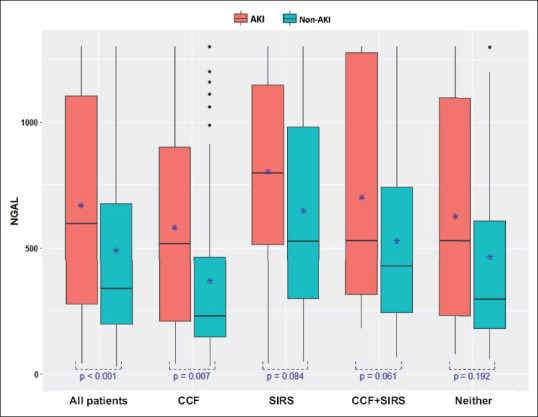
Box plot shows neutrophil gelatinase-associated lipocalin (NGAL) levels in patients with and without acute kidney injury (AKI) according to patient groups. Mean serum NGAL levels were significantly increased in patients with AKI vs. those without AKI (670.0 ± 431.9 ng/dL vs. 490.3 ± 391.6 ng/dL; P < 0.001). *Mean NGAL values. CCF: congestive cardiac failure; SIRS: systemic inflammatory response syndrome
The ROCs for the four different patient groups are summarised in Table 2, whereas the various AUCs are illustrated in Supplementary Figure 1, Appendix 2. Considering all patients, the predictive ability of serum NGAL for AKI was maximised at a cut-off of 490 ng/dL with an AUC of 0.62, sensitivity of 59% and specificity of 65%. Within patient subgroups, predicting AKI was maximised with increasing serum NGAL cut-offs—from patients with neither CCF nor SIRS (NGAL = 521 ng/dL, AUC = 0.60, sensitivity = 56%, specificity = 69%), those with CCF only (NGAL = 513.5 ng/dL, AUC = 0.65, sensitivity = 51%, specificity = 0.78%) and SIRS only (NGAL = 647.5 ng/dL, AUC = 0.63, sensitivity = 70%, specificity = 61%), to those with both CCF and SIRS (NGAL = 840.5 ng/dL, AUC = 0.61, sensitivity = 39%, specificity = 81%). Across all patient groups, NGAL had an NPV for AKI ranging from 0.85 to 0.99 and a PPV for AKI ranging from 0.18 to 0.67.
Table 2.
Receiver operating characteristics (ROC) in different patient groups.
| ROC | All patients (n=784) | CCF (n=213) | SIRS (n=183) | Both CCF and SIRS (n=105) | No CCF or SIRS (n=283) |
|---|---|---|---|---|---|
| AKI, n (%) | 107 (13.6) | 37 (17.4) | 23 (12.6) | 31 (29.5) | 16 (5.7) |
|
| |||||
| NGAL AKI cut-off (ng/dL) | 490 | 513.5 | 647.5 | 840.5 | 521 |
|
| |||||
| AUC | 0.624 | 0.646 | 0.625 | 0.612 | 0.595 |
|
| |||||
| ROCa | |||||
|
| |||||
| Sensitivity | 0.59 (0.49–0.68) | 0.51 (0.34–0.68) | 0.70 (0.47–0.87) | 0.39 (0.22–0.58) | 0.56 (0.30–0.80) |
|
| |||||
| Specificity | 0.65 (0.61–0.68) | 0.78 (0.72–0.84) | 0.61 (0.53–0.68) | 0.81 (0.70–0.89) | 0.69 (0.63–0.75) |
|
| |||||
| Youden’s index | 0.23 (0.10–0.36) | 0.30 (0.66–0.52) | 0.30 (0.00–0.55) | 0.20 (−0.08–0.47) | 0.26 (−0.07–0.55) |
|
| |||||
| PPV | 0.21 (0.16–0.26) | 0.33 (0.21–0.47) | 0.20 (0.12–0.31) | 0.46 (0.27–0.67) | 0.10 (0.05–0.18) |
|
| |||||
| NPV | 0.91 (0.88–0.93) | 0.88 (0.82–0.93) | 0.93 (0.87–0.97) | 0.76 (0.65–0.85) | 0.96 (0.93–0.99) |
|
| |||||
| LR+ | 1.66 (1.38–2.00) | 2.38 (1.56–3.63) | 1.77 (1.27–2.46) | 2.05 (1.07–3.91) | 1.83 (1.15–2.93) |
|
| |||||
| LR− | 0.64 (0.50–0.80) | 0.62 (0.44–0.87) | 0.50 (0.27–0.94) | 0.76 (0.56–1.02) | 0.63 (0.36–1.11) |
Among all patients, the predictive ability of neutrophil gelatinase-associated lipocalin (NGAL) for acute kidney injury (AKI) was maximised at serum NGAL cut-off of 490 ng/dL (area under the curve [AUC] = 0.624, sensitivity 59% and specificity 65%). Across all patient groups, NGAL had a negative predictive value (NPV) for AKI ranging from 0.85 to 0.99, and a positive predictive value (PPV) for AKI ranging from 0.18 to 0.67. aData is expressed as point estimation (95% confidence interval). CCF: congestive cardiac failure, LR+: positive likelihood ratio, LR−: negative likelihood ratio, SIRS: systemic inflammatory response syndrome
As illustrated in Figure 3, when the metabolic comorbidities (hypertension, diabetes mellitus and dyslipidaemia) and atheropathic tendencies (myocardial infarction, ischaemic heart disease or peripheral vascular disease) of all patients were incorporated into a multivariate model with NGAL, the predictive value was improved by 20.2% to an AUC of 0.75 at an NGAL cut-off of 490 ng/dL. As summarised in Table 3, the odds of developing AKI increased by 12% per 100 ng/dL rise in NGAL (P < 0.001) from the baseline or when compared with another similar patient from the same subgroup.
Figure 3.
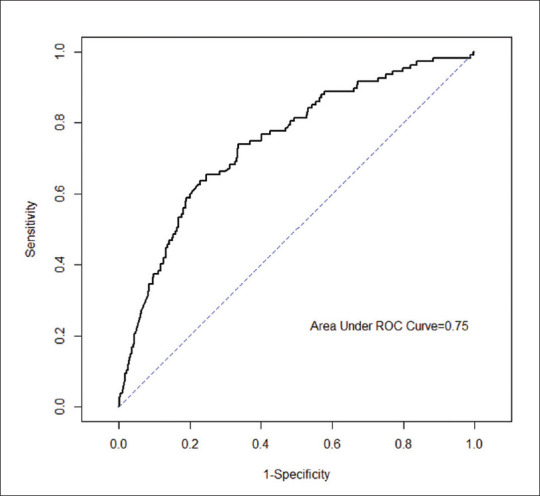
Receiver operating characteristics (ROC) curve shows all patients (area under curve 0.75), incorporating clinical variables such as metabolic comorbidities and atheropathic tendencies.
Table 3.
Adjusted odds ratio (OR) of developing acute kidney injury, incorporating clinical variables.a
| Variable | Adjusted OR (95% CI) | P |
|---|---|---|
| NGAL | 1.12 (1.06–1.17) | <0.001 |
|
| ||
| Metabolic comorbidities | 2.32 (0.87–8.10) | 0.129 |
|
| ||
| Atheropathic tendencies | 0.40 (0.24–0.65) | <0.001 |
|
| ||
| Patients with CCF | 5.38 (2.81–10.77) | <0.001 |
|
| ||
| Patients with SIRS | 1.99 (1.01–4.00) | 0.05 |
|
| ||
| Patient with both CCF and SIRS | 9.12 (4.58–18.88) | <0.001 |
aIncorporates clinical variables such as metabolic comorbidities and atheropathic tendencies in all patients (adjusted OR 1.12), patients with congestive cardiac failure (CCF) (adjusted OR 5.38), and patients with both CCF and systemic inflammatory response syndrome (SIRS) (adjusted OR 9.12), P<0.001.
On exploring patient subgroups, we observed that patients with both CCF and SIRS (adjusted odds ratio [OR] = 9.12, P < 0.001) or CCF alone (adjusted OR = 5.38, P < 0.001) had higher odds of developing AKI than patients with neither CCF nor SIRS. Interestingly, the odds of developing AKI are 0.4 (P < 0.001) in patients with atheropathic tendencies (past AMI, IHD and PVD). Factors such as patient age, gender, race, BMI, white blood cell count and platelet count do not affect the ability of NGAL to predict AKI [Supplementary Table 1, Appendix 2].
In the secondary analysis of our clinical model, shown in Table 4, the odds of requiring RRT increased by 21% per 100 ng/dL increase in NGAL (P < 0.001) from the baseline or when compared with similar patients from the same subgroup. Similarly, the odds of death within three months after discharge also increased by 10% per 100 ng/dL increase in NGAL (p = 0.028). However, our results showed a negative relationship between NGAL levels and MACE (adjusted OR = 0.93, P = 0.016). From our model, metabolic comorbidities did not show statistically significant correlation with need for RRT, MACE or death within three months from admission.
Table 4.
Use of neutrophil gelatinase-associated lipocalin (NGAL) to predict three-month adverse outcomes.a
| Variable | Need for RRT | All-cause death | MACE | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| NGAL | 1.21 (1.12–1.31) | <0.001 | 1.10 (1.01–1.19) | 0.022 | 0.93 (0.87–0.98) | 0.016 |
|
| ||||||
| Age | 0.97 (0.94–0.99) | 0.014 | – | – | 0.98 (0.97–1.00) | 0.071 |
|
| ||||||
| Metabolic comorbidities | 0.80 (0.24–3.70) | 0.744 | – | – | 2.28 (0.79–9.69) | 0.183 |
|
| ||||||
| Atheropathic tendencies | 0.40 (0.19–0.88) | 0.021 | 0.85 (0.38–2.04) | 0.696 | 1.84 (1.10–3.16) | 0.023 |
|
| ||||||
| Patients with CCF | 3.17 (1.24–8.47) | 0.017 | 4.55 (1.77–13.37) | 0.003 | 1.37 (0.81–2.34) | 0.24 |
|
| ||||||
| Patients with SIRS | 0.37 (0.10–1.18) | 0.112 | 0.21 (0.01–1.24) | 0.149 | 0.89 (0.46–1.69) | 0.737 |
|
| ||||||
| Patients with both CCF and SIRS | 4.34 (1.63–11.82) | 0.003 | 5.13 (1.83–15.75) | 0.002 | 1.88 (1.00–3.49) | 0.048 |
aIncorporating clinical variables such as patient age, metabolic comorbidities and atheropathic tendencies, NGAL was able to predict three-month adverse outcomes of renal replacement therapy necessity (adjusted odds ratio [OR] 1.21, P<0.001), all-cause death (adjusted OR 1.10, P=0.022) and major adverse cardiac events (MACE) (atrial arrhythmia, ventricular fibrillation, cardiogenic shock requiring intra-arterial balloon pump, acute pulmonary oedema requiring intubation, acute myocardial infarction, coronary artery bypass graft and percutaneous coronary intervention) (adjusted OR 0.93, P=0.016). Patient ‘metabolic comorbidities’ was removed from the model predicting all-cause death, because all patients who died in three months had at least one of these comorbidities. CCF: congestive cardiac failure, SIRS: systemic inflammatory response syndrome
DISCUSSION
Most studies on NGAL have focused on patient populations with a clear renal insult such as cardiac surgery,[19,27,28] severe illness necessitating critical care[29,30] and kidney transplantation.[31,32] In addition, the few studies performed in the ED setting have largely been in Western populations.[12,33,34,35] This study is a single-centre prospective cohort study that evaluates the performance of serum NGAL in predicting AKI in a broad cohort of multiethnic Asian patients presenting to the ED with CCF or SIRS. The use of point-of-care (POC) instruments, the Triage® NGAL Test (Alere Inc, San Diego, CA, USA) and Alere Triage® MeterPro was demonstrated to be convenient and practical for the ED setting.
Serum NGAL was significantly raised (P < 0.001) in patients with AKI compared with those without AKI, confirming the utility of serum NGAL in differentiating these two patient groups. Moreover, incorporating variables such as metabolic comorbidities and atheropathic tendencies in our clinical model enhanced its predictive value by 20.2% from AUC 0.62 to 0.75 at an NGAL cut-off of 490 ng/dL. Knowing that incorporating metabolic comorbidities and atheropathic tendencies improves pretest probability for AKI, clinicians could take into account these variables when interpreting NGAL results.
The predictive ability of NGAL for AKI was also maximised at increasing serum NGAL cut-offs across different patient subgroups — from NGAL levels of 521 ng/dL in patients without CCF or SIRS to 840.50 ng/dL in patients with both CCF and SIRS [Table 2]. This is not unexpected given that CCF and SIRS are known to increase serum NGAL independent of AKI.[21,22,23,24,25] However, the rise in serum NGAL is less significant between AKI and non-AKI patient subgroups (0.007 ≤ P ≤ 0.192). This could be because many of our patients with AKI (n = 100, 22%) had very high serum NGAL (>1,300 ng/dL) that exceeded the upper detection limit of our POC testing device. Serum NGAL exceeding 1,300 ng/dL is unexpected, and such values have been treated as outliers elsewhere.[34] In our study, treating patients with NGAL >1,300 ng/dL as outliers was attempted but found to worsen our statistical power, suggesting that a larger initial pool of patients may have been required for our demographics. The independent rise in serum NGAL with CCF and SIRS may have also acted as a confounding factor. Knowing patients' baseline serum NGAL levels may help to distinguish NGAL contributions from AKI, CCF or SIRS and help determine outlier NGAL values. Differences in sample preparation among the different studies may have also partly contributed to this discrepancy. A device with a higher detection limit, such as 2,000 ng/dL,[34,48] may have been more appropriate for our patient demographics as well. Further investigation may be necessary to address this observation.
Interestingly, as shown in Table 3, patients with atheropathic tendencies seem to have a decreased risk of developing AKI (adjusted OR 0.40, P < 0.001). The reason for this is unclear and more research is needed in this area.
As a diagnostic tool, some authors[49,50] have proposed that raised serum NGAL could be used as a single unequivocal biomarker for AKI, analogous to troponin T for AMI (sensitivity 0.85, specificity 0.80).[51,52,53] Looking across patient subgroups, our results show that NGAL had a specificity of 0.61–0.81 and a sensitivity of 0.39–0.70. These values are insufficient for a rule-in or rule-out test. However, taking into consideration the prevalence of AKI in these subgroups, our results reveal that NGAL had a much higher NPV (0.85–0.99) than PPV (0.18–0.67). Given that CCF and SIRS are known risk factors for developing AKI, serum NGAL may be used to rule out AKI in low to medium pretest probability settings. In other words, in a broad cohort of undifferentiated patients presenting to the ED, low serum NGAL may be more suitably used for ruling out AKI, analogous to D-dimer[54] for venous thromboembolism. Nevertheless, more studies are needed to validate the use of NGAL as a rule-out test. Similarly, other authors[13] have also cautioned that more rigorous work needs to be done before the goal for a troponin-like biomarker for AKI can be actualised.
As a prognostication tool, our clinical model showed that the odds of patients needing RRT within three months after discharge increased by 21% for every 100 ng/dL rise in serum NGAL (P < 0.001) [Table 4]. Our result is consistent with that of a previous German study, which observed that raised urinary NGAL predicted the need for long term RRT (n = 145, P = 0.021) over four years.[55] The positive correlation between NGAL levels and the need for RRT is understandable, because AKI can cause permanent kidney damage without prompt treatment. The high odds of RRT with rise in NGAL emphasise the urgency of treating AKI early.
Furthermore, the odds of all-cause death in three months (n = 35) also rise by 10% per 100 ng/dL rise in serum NGAL (P = 0.028) [Table 4]. This is not unexpected, because NGAL levels are known to rise with CCF, SIRS and CKD, which are conditions detrimental to patients' health. Our findings are also supported by studies conducted elsewhere. In patients with CCF, Alvelos et al. followed up with 121 Portuguese patients for three months and showed that high serum NGAL is predictive of all-cause death (n = 27, P = 0.016).[56] A larger study involving 260 Japanese patients was conducted by Nakada et al., who found that high urinary NGAL is strongly associated with all-cause death (n = 99, P = 0.0004) over 18 months.[41] Wang et al. also studied 440 Chinese patients and found that serum NGAL was correlated with all-cause death (n = 137, P = 0.01) over 28 days.[40]
In our study, we have demonstrated the association between NGAL and AKI as well as the need for RRT in patients with CCF or SIRS. However, our results do not show a clear relationship between NGAL levels and MACE; in fact, it is interesting to note that our data seem to suggest a negative correlation between NGAL levels and MACE (adjusted OR = 0.93, P = 0.016) [Table 4]. This may be because of the multiple intricate mechanisms underlying the cardiorenal syndrome.[57,58,59] Because of this complex nature of correlation, other studies[26,60] have also yielded mixed conclusions, and we recommend that more dedicated work be invested in this area.
As seen in Supplementary Table 1 in Appendix 2, factors such as patient age, gender, race, BMI, leukocyte count and platelet count do not significantly affect the ability of NGAL to predict AKI. This observation is consistent with current literature[12,17,19,20] and adds further evidence that NGAL, unlike creatinine, is unaffected by patient age, gender, BMI etc. This reiterates the benefits of using NGAL over creatinine as a marker for AKI. Furthermore, patient metabolic comorbidities such as hypertension, diabetes mellitus and dyslipidaemia did not affect the odds of RRT, MACE or death at three months after discharge [Table 4]. This suggests that prognosticating with NGAL is independent of patient comorbidities.
In summary, our findings provide support for the use of NGAL as a biomarker for AKI in the ED. We have also provided additional evidence of the utility of NGAL in a multiethnic Asian population. The point-of-care NGAL meter was found to be a feasible equipment for predicting AKI in the ED, albeit with some limitations. NGAL, as a convenient and early biomarker for AKI, is especially useful in patients presenting to the ED with 'normal' creatinine because NGAL serves as an early predictor for AKI. Efforts can then be taken to closely monitor these patients and avoid nephrotoxic drugs and contrast scans. As a prognostication tool, NGAL is correlated strongly with a need for RRT at three months and weakly with all-cause mortality at three months, regardless of existing patient comorbidities.
Financial support and sponsorship
This study was funded via a research grant from the Venerable Yen Pei-National Kidney Foundation Research Fund.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
Patient inclusion and exclusion criteria for the study
Inclusion criteria:
Patients must meet all of the following inclusion criteria to be eligible for enrolment into the trial:
Age > 21 years
eGFR < 60 mL/min/1.73m2 (calculated via CKD EPI)
One of the following
-
(a)
Primary diagnosis of cardiac failure OR
-
(b)
At least 2 out of 4 of the following SIRS (Systemic Inflammatory Response Syndrome)
- Temperature > 38ºC or < 36ºC
- Respiratory rate > 20 breaths per minute or a PaCO2 < 32 mmHg
- Pulse rate of > 90 beats per minute
- Total white blood count of > 12,000 cells/mm3 or < 4,000 cells/mm3 or > 10% immature forms OR
-
(c)
Requires hospital admission
Exclusion criteria:
The presence of any of the following will exclude a patient from study enrolment:
Patients who are on renal replacement therapy, e.g. haemodialysis or peritoneal dialysis
Women who are nursing or have a positive pregnancy test (pregnant)
Patients who have a known terminal illness
Patients who present with cardiac arrest
Patients who have a ‘Do Not Resuscitate’ order
Patients with congenital heart disease
Patients with critical aortic stenosis
Patients who are not ambulant e.g. wheelchair or bed bound
APPENDIX 2
Supplementary Table 1.
Adjusted OR for developing acute kidney injury, incorporating various clinical variables.
| Variable | Adjusted OR | CI 2.5% | CI 97.5% | p-value |
|---|---|---|---|---|
| NGAL | 1.11 | 1.05 | 1.17 | < 0.001 |
| Age | 1 | 0.98 | 1.02 | 0.833 |
| Gender | 0.97 | 0.6 | 1.57 | 0.914 |
| Race | 0.86 | 0.5 | 1.44 | 0.577 |
| Hypertension, DM, dyslipidaemia | 2.43 | 0.8 | 10.63 | 0.164 |
| Cardiovascular risk factors | 0.64 | 0.4 | 1.03 | 0.066 |
| BMI | 1.01 | 0.97 | 1.05 | 0.496 |
| WBC | 1 | NA | 1 | 0.579 |
| Platelet | 1 | 1 | 1 | 0.579 |
Factors such as patient age, gender, race, BMI, WBC count and platelet count do not significantly confound the ability of NGAL to predict AKI. BMI: body mass index; CI: confidence interval; DM: diabetes mellitus; NGAL: neutrophil gelatinase-associated lipocalin; OR: odds ratio; WBC: white blood cell
Supplementary Figure 1.
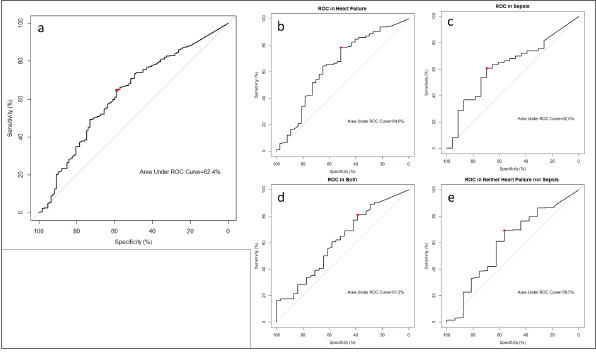
Area under the curve shows (a) all patients, those with (b) congestive cardiac failure (CCF), (c) sepsis, (d) both CCF and sepsis, and (e) neither CCF nor sepsis patients. CCF: congestive cardiac failure
REFERENCES
- 1.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004;30:33–7. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 3.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607–25. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 5.Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–6. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 6.Safari S, Hashemi B, Forouzanfar MM, Shahhoseini M, Heidari M. Epidemiology and outcome of patients with acute kidney injury in emergency department; a cross-sectional study. Emerg (Tehran) 2018;6:e30. [PMC free article] [PubMed] [Google Scholar]
- 7.Scheuermeyer FX, Grafstein E, Rowe B, Cheyne J, Grunau B, Bradford A, et al. The clinical epidemiology and 30-day outcomes of emergency department patients with acute kidney injury. Can J Kidney Health Dis. 2017;4:205435811770398. doi: 10.1177/2054358117703985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X, et al. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144. doi: 10.1186/cc13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–93. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385:2616–43. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 11.Lameire N, Hoste E. Reflections on the definition, classification, and diagnostic evaluation of acute renal failure. Curr Opin Crit Care. 2004;10:468–75. doi: 10.1097/01.ccx.0000144939.24897.71. [DOI] [PubMed] [Google Scholar]
- 12.Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alge JL, Arthur JM. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–55. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo SH, Endre ZH. Biomarkers in acute kidney injury (AKI) Best Pract Res Clin Anaesthesiol. 2017;31:331–44. doi: 10.1016/j.bpa.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Rizvi MS, Kashani KB. Biomarkers for early detection of acute kidney injury. J Appl Lab Med. 2017;2:386–99. doi: 10.1373/jalm.2017.023325. [DOI] [PubMed] [Google Scholar]
- 16.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury-pathophysiological basis and clinical performance. Acta Physiol (Oxf) 2017;219:554–72. doi: 10.1111/apha.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronco C. Biomarkers for acute kidney injury: Is NGAL ready for clinical use? Crit Care. 2014;18:680. doi: 10.1186/s13054-014-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning M, Mao X, Niu Y, Tang B, Shen H. Usefulness and limitations of neutrophil gelatinase-associated lipocalin in the assessment of kidney diseases. J Lab Precis Med. 2018;3:1–11. [Google Scholar]
- 19.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann Clin Biochem. 2014;51:335–51. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–12. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 21.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 22.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–69. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coles M, Diercks T, Muehlenweg B, Bartsch S, Zölzer V, Tschesche H, et al. The solution structure and dynamics of human neutrophil gelatinase-associated lipocalin. J Mol Biol. 1999;289:139–57. doi: 10.1006/jmbi.1999.2755. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 26.Brisco MA, Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep. 2014;11:485–99. doi: 10.1007/s11897-014-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 28.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuartero M, Betbesé AJ, Núñez K, Baldirà J, Ordonez-Llanos J. Does whole-blood neutrophil gelatinase-associated lipocalin stratify acute kidney injury in critically ill patients? Dis Markers. 2019;2019:8480925. doi: 10.1155/2019/8480925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 32.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–55. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. The diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med. 2010;56:52–9e1. doi: 10.1016/j.annemergmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Soto K, Papoila AL, Coelho S, Bennett M, Ma Q, Rodrigues B, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8:2053–63. doi: 10.2215/CJN.12181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Han J, Liu J, Liang B, Wang X, Wang C. Clinical significance of novel biomarker NGAL in early diagnosis of acute renal injury. Exp Ther Med. 2017;14:5017–21. doi: 10.3892/etm.2017.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shum HP, Leung NYW, Chang LL, Tam OY, Kwan AM, Chan KC, et al. Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute kidney injury in intensive care unit patients after major non-cardiac surgery. Nephrology (Carlton) 2015;20:375–82. doi: 10.1111/nep.12400. [DOI] [PubMed] [Google Scholar]
- 38.Choi HM, Park KT, Lee JW, Cho E, Jo SK, Cho WY, et al. Urine neutrophil gelatinase-associated lipocalin predicts graft outcome up to 1 year after kidney transplantation. Transplant Proc. 2013;45:122–8. doi: 10.1016/j.transproceed.2012.05.080. [DOI] [PubMed] [Google Scholar]
- 39.Dai X, Zeng Z, Fu C, Zhang S, Cai Y, Chen Z. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19:223. doi: 10.1186/s13054-015-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Zhang Q, Zhao X, Dong G, Li C. Diagnostic and prognostic value of neutrophil gelatinase-associated lipocalin, matrix metalloproteinase-9, and tissue inhibitor of matrix metalloproteinases-1 for sepsis in the emergency department: An observational study. Crit Care. 2014;18:634. doi: 10.1186/s13054-014-0634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakada Y, Kawakami R, Matsui M, Ueda T, Nakano T, Takitsume A, et al. Prognostic value of urinary neutrophil gelatinase-associated lipocalin on the first day of admission for adverse events in patients with acute decompensated heart failure. J Am Heart Assoc. 2017;6:e004582. doi: 10.1161/JAHA.116.004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011;58:56–63. doi: 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 44.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: Beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–8. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 46.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin Kidney J. 2013;6:8–14. doi: 10.1093/ckj/sfs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48:193–204. doi: 10.1016/j.jbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Point-of-care neutrophil gelatinase-associated lipocalin (NGAL) tests. National Institute for Health Research Community Healthcare MedTech and In Vitro Diagnostics Co-operative, University of Oxford. [[Last accessed on 2019 Nov 12]]. Available from: https://www.community.healthcare.mic.nihr.ac.uk/reports-and-resources/horizon-scanning-reports/point-of-care-neut rophil-gelatinase-associated-lipocalin-ngal-tests.
- 49.Devarajan P. Kidney attack: Is NGAL set to take the stage with troponins. In: Rangaswami J, Lerma EV, Ronco C, editors. Cardio-Nephrology. Cham: Springer International Publishing; 2017. 61 pp. [Google Scholar]
- 50.Devarajan P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 51.Ohman EM, Armstrong PW, Christenson RH, Granger CB, Katus HA, Hamm CW, et al. Cardiac troponin T levels for risk stratification in acute myocardial ischemia.GUTSA IIA Investigators. N Engl J Med. 1996;335:1333–41. doi: 10.1056/NEJM199610313351801. [DOI] [PubMed] [Google Scholar]
- 52.Babuin L, Jaffe AS. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173:1191–202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 54.Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev. 2016;2016:CD010864. doi: 10.1002/14651858.CD010864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singer E, Schrezenmeier EV, Elger A, Seelow ER, Krannich A, Luft FC, et al. Urinary NGAL-positive acute kidney injury and poor long-term outcomes in hospitalized patients. Kidney Int Rep. 2016;1:114–24. doi: 10.1016/j.ekir.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvelos M, Lourenço P, Dias C, Amorim M, Rema J, Leite AB, et al. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol. 2013;165:51–5. doi: 10.1016/j.ijcard.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 57.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation. 2019;139:e840–78. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 58.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9:99–111. doi: 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 59.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–44. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 60.Fu S, Zhao S, Ye P, Luo L. Biomarkers in cardiorenal syndromes. Biomed Res Int. 2018;2018:9617363. doi: 10.1155/2018/9617363. [DOI] [PMC free article] [PubMed] [Google Scholar]


