Abstract
Background
Surgery is a common treatment option in oral cavity cancer (and less frequently in oropharyngeal cancer) to remove the primary tumour and sometimes neck lymph nodes. People with early‐stage disease may undergo surgery alone or surgery plus radiotherapy, chemotherapy, immunotherapy/biotherapy, or a combination of these. Timing and extent of surgery varies. This is the third update of a review originally published in 2007.
Objectives
To evaluate the relative benefits and harms of different surgical treatment modalities for oral cavity and oropharyngeal cancers.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 9 February 2022.
Selection criteria
Randomised controlled trials (RCTs) that compared two or more surgical treatment modalities, or surgery versus other treatment modalities, for primary tumours of the oral cavity or oropharynx.
Data collection and analysis
Our primary outcomes were overall survival, disease‐free survival, locoregional recurrence, and recurrence; and our secondary outcomes were adverse effects of treatment, quality of life, direct and indirect costs to patients and health services, and participant satisfaction. We used standard Cochrane methods. We reported survival data as hazard ratios (HRs). For overall survival, we reported the HR of mortality, and for disease‐free survival, we reported the combined HR of new disease, progression, and mortality; therefore, HRs below 1 indicated improvement in these outcomes. We used GRADE to assess certainty of evidence for each outcome.
Main results
We identified four new trials, bringing the total number of included trials to 15 (2820 participants randomised, 2583 participants analysed). For objective outcomes, we assessed four trials at high risk of bias, three at low risk, and eight at unclear risk. The trials evaluated nine comparisons; none compared different surgical approaches for excision of the primary tumour.
Five trials evaluated elective neck dissection (ND) versus therapeutic (delayed) ND in people with oral cavity cancer and clinically negative neck nodes. Elective ND compared with therapeutic ND probably improves overall survival (HR 0.64, 95% confidence interval (CI) 0.50 to 0.83; I2 = 0%; 4 trials, 883 participants; moderate certainty) and disease‐free survival (HR 0.56, 95% CI 0.45 to 0.70; I2 = 12%; 5 trials, 954 participants; moderate certainty), and probably reduces locoregional recurrence (HR 0.58, 95% CI 0.43 to 0.78; I2 = 0%; 4 trials, 458 participants; moderate certainty) and recurrence (RR 0.58, 95% CI 0.48 to 0.70; I2 = 0%; 3 trials, 633 participants; moderate certainty). Elective ND is probably associated with more adverse events (risk ratio (RR) 1.31, 95% CI 1.11 to 1.54; I2 = 0%; 2 trials, 746 participants; moderate certainty).
Two trials evaluated elective radical ND versus elective selective ND in people with oral cavity cancer, but we were unable to pool the data as the trials used different surgical procedures. Neither study found evidence of a difference in overall survival (pooled measure not estimable; very low certainty). We are unsure if there is a difference in effect on disease‐free survival (HR 0.57, 95% CI 0.29 to 1.11; 1 trial, 104 participants; very low certainty) or recurrence (RR 1.21, 95% CI 0.63 to 2.33; 1 trial, 143 participants; very low certainty). There may be no difference between the interventions in terms of adverse events (1 trial, 148 participants; low certainty).
Two trials evaluated superselective ND versus selective ND, but we were unable to use the data.
One trial evaluated supraomohyoid ND versus modified radical ND in 332 participants. We were unable to use any of the primary outcome data. The evidence on adverse events was very uncertain, with more complications, pain, and poorer shoulder function in the modified radical ND group.
One trial evaluated sentinel node biopsy versus elective ND in 279 participants. There may be little or no difference between the interventions in overall survival (HR 1.00, 95% CI 0.90 to 1.11; low certainty), disease‐free survival (HR 0.98, 95% CI 0.90 to 1.07; low certainty), or locoregional recurrence (HR 1.04, 95% CI 0.91 to 1.19; low certainty). The trial provided no usable data for recurrence, and reported no adverse events (very low certainty).
One trial evaluated positron emission tomography‐computed tomography (PET‐CT) following chemoradiotherapy (with ND only if no or incomplete response) versus planned ND (before or after chemoradiotherapy) in 564 participants. There is probably no difference between the interventions in overall survival (HR 0.92, 95% CI 0.65 to 1.31; moderate certainty) or locoregional recurrence (HR 1.00, 95% CI 0.94 to 1.06; moderate certainty).
One trial evaluated surgery plus radiotherapy versus radiotherapy alone and provided very low‐certainty evidence of better overall survival in the surgery plus radiotherapy group (HR 0.24, 95% CI 0.10 to 0.59; 35 participants). The data were unreliable because the trial stopped early and had multiple protocol violations. In terms of adverse events, subcutaneous fibrosis was more frequent in the surgery plus radiotherapy group, but there were no differences in other adverse events (very low certainty).
One trial evaluated surgery versus radiotherapy alone for oropharyngeal cancer in 68 participants. There may be little or no difference between the interventions for overall survival (HR 0.83, 95% CI 0.09 to 7.46; low certainty) or disease‐free survival (HR 1.07, 95% CI 0.27 to 4.22; low certainty). For adverse events, there were too many outcomes to draw reliable conclusions.
One trial evaluated surgery plus adjuvant radiotherapy versus chemotherapy. We were unable to use the data for any of the outcomes reported (very low certainty).
Authors' conclusions
We found moderate‐certainty evidence based on five trials that elective neck dissection of clinically negative neck nodes at the time of removal of the primary oral cavity tumour is superior to therapeutic neck dissection, with increased survival and disease‐free survival, and reduced locoregional recurrence.
There was moderate‐certainty evidence from one trial of no difference between positron emission tomography (PET‐CT) following chemoradiotherapy versus planned neck dissection in terms of overall survival or locoregional recurrence.
The evidence for each of the other seven comparisons came from only one or two studies and was assessed as low or very low‐certainty.
Keywords: Humans; Immunotherapy; Mouth; Neck; Neoplasm Recurrence, Local; Oropharyngeal Neoplasms; Oropharyngeal Neoplasms/surgery; Randomized Controlled Trials as Topic
Plain language summary
Surgical treatments for oral cavity (mouth) and oropharyngeal (throat) cancers
Key messages
• In people with mouth cancer, elective removal of neck lymph nodes at the same time as primary tumour removal, compared with the removal of neck lymph nodes only when they become cancerous, probably increases survival and reduces recurrence, but may increase the risk of unwanted effects. • Future studies of surgical treatment of mouth and throat cancers should report findings according to primary tumour location and measure quality of life and illness or disability associated with treatment.
What is the background to the review?
Oral cavity (mouth) and oropharyngeal (throat) cancers are becoming more common and are very difficult to cure. Treatment can involve surgery, chemotherapy, radiotherapy, or a combination of these. For people with mouth cancer, the removal of the lymph nodes (small glands that filter cancer cells and other foreign substances) is sometimes part of the treatment; this is known as neck dissection. Surgeons sometimes remove lymph nodes that appear cancer free while removing the original tumour (elective neck dissection). Other surgeons adopt a 'watch and wait' approach, removing lymph nodes when they become cancerous. The type of dissection can be radical neck dissection, where all the lymph nodes are removed, or selective neck dissection, where only diseased nodes are removed. One way to determine whether the lymph node is diseased is to perform a lymph node biopsy.
What did we want to find out?
We wanted to know which surgical treatments are most likely to result in people with mouth and throat cancers living longer (overall survival), living longer without symptoms (disease‐free survival), and not having the cancer come back at the same site (locoregional recurrence) or spread to other sites (recurrence). We also wanted to know if the different treatments have unwanted effects.
What did we do?
We searched for studies that randomly allocated people with mouth or throat cancer to different types of surgical treatment. We summarised the characteristics and findings of relevant studies and assessed our confidence in the results.
What did we find?
We included 15 studies (four new studies in this update) that evaluated nine comparisons of different treatments. No studies compared different approaches to cutting out the original (primary) tumour. The studies involved 2820 participants.
Main results
Five studies evaluated removal of the primary tumour, comparing elective neck dissection with the 'watch and wait approach' in people with mouth cancer. The results show that elective neck dissection probably leads to longer overall and disease‐free survival and less locoregional recurrence, but more unwanted effects.
Two studies compared radical neck dissection versus selective neck dissection in people with mouth cancer. It is unclear which treatment provides better outcomes.
Two trials evaluated a more limited neck dissection (superselective) versus selective neck dissection; we were unable to use the data reported.
One study compared a more selective neck dissection (supraomohyoid) and a modified radical neck dissection. We were unable to use the data reported. The modified radical neck dissection group had more complications, more pain, and poorer shoulder function, but we are very uncertain about the results.
In one study, all the people in one group had a lymph node biopsy and only had neck lymph nodes removed if the biopsy was positive, while all people in the other group had neck lymph nodes removed without a biopsy. There may be no difference between these two approaches in terms of overall survival, disease‐free survival, and locoregional recurrence. No unwanted effects were reported.
One study evaluated using a special scan (positron emission tomography‐computed tomography (PET‐CT)) after combined chemotherapy and radiotherapy to guide decisions about neck dissection, versus a planned neck dissection before or after chemoradiotherapy. There is probably no difference between these approaches in terms of overall survival or locoregional recurrence. There may be no difference in unwanted effects, but we are very uncertain about the results.
One trial suggested that surgery plus radiotherapy may result in better overall survival than radiotherapy alone, but we are very uncertain about the results. Surgery may result in more thickened scar tissue. There may be no difference with regard to other unwanted effects.
One study compared surgery versus radiotherapy in people with throat cancer. There may be no difference in overall survival, disease‐free survival, or unwanted effects, but we are very uncertain about the results.
One study compared surgery followed by radiotherapy versus chemotherapy. People receiving surgery and radiotherapy may live longer without symptoms, but we are very uncertain about the results.
What are the limitations of the evidence?
We are moderately confident that elective neck dissection at the same time as removal of the main tumour improves survival and reduces recurrence. Not all studies provided information about everything that we were interested in.
We are moderately confident that PET‐CT does not improve survival or reduce recurrence. There are too few studies to be certain about the results.
We have little confidence in results from other comparisons due to too few studies and limited information within them.
How up to date is this evidence?
The evidence is current to 9 February 2022.
Summary of findings
Summary of findings 1. Elective neck dissection versus therapeutic (delayed) neck dissection.
| Elective neck dissection versus therapeutic (delayed) neck dissection | ||||||
|
Population: adults with oral cancer Setting: inpatient Intervention: elective ND Comparison: therapeutic (delayed) ND | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (therapeutic ND) | Corresponding risk (elective ND) | |||||
|
Mortality Follow‐up: 3 years |
350a per 1000 | 241 per 1000 (194 to 301) |
HR 0.64 (0.50 to 0.83) |
883 (4 RCTs) |
⊕⊕⊕⊝ Moderatec | We calculated mortality in place of overall survival. |
| 250b per 1000 | 168 per 1000 (134 to 212) | |||||
|
New disease, progression, and mortality Follow‐up: 3 years |
550a per 1000 | 361 per 1000 (302 to 428) |
HR 0.56 (0.45 to 0.70) |
954 (5 RCTs) |
⊕⊕⊕⊝ Moderatec | We calculated new disease, progression, and mortality in place of disease‐free survival. |
| 450b per 1000 | 285 per 1000 (236 to 342) |
|||||
|
Locoregional recurrence Follow‐up: 3 years |
300b per 1000 | 187 per 1000 (142 to 243) |
HR 0.58 (0.43 to 0.78) |
458 (4 RCTs) |
⊕⊕⊕⊝ Moderatec | — |
|
Recurrence Follow‐up: 3 years |
600a per 1000 | 348 per 1000 (288 to 420) |
RR 0.58 (0.48 to 0.70) | 633 (3 RCTs) |
⊕⊕⊕⊝ Moderatec | — |
| Adverse events associated with treatment | — | — |
RR 1.31 (1.11 to 1.54) |
746 (2 RCTs) |
⊕⊕⊕⊝ Moderated | Risk not calculated as event rate (in the therapeutic groups) in 1 study was 3.6% compared with 60.5% in the other study. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; ND: neck dissection; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Based on data from D'Cruz 2015 (lower‐middle income country). b Based on data from Hutchison 2019 (high‐income country). c Downgraded once due to risk of bias. d Downgraded once for risk of bias as only 2/5 trials provided data on adverse events (selective reporting of subjective outcomes).
Summary of findings 2. Elective radical neck dissection versus elective selective neck dissection.
| Elective radical neck dissection versus elective selective neck dissection | ||||||
|
Population: adults with oral cancer Setting: inpatient Intervention: elective radical ND Comparison: elective selective ND | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (elective selective ND) | Corresponding risk (elective radical ND) | |||||
| Total mortality | — | — | — | 252 (2 RCTs) | ⊕⊝⊝⊝ Very lowa |
2 trials reported HR for mortality but used different surgical procedures, so we could not pool data. Neither trial indicated any difference in mortality between the 2 interventions. |
|
New disease, progression, and mortality Follow‐up: 5 years |
300b per 1000 | 184 per 1000 (142 to 243) |
HR 0.57 (0.29 to 1.11) |
104 (1 RCT) | ⊕⊝⊝⊝ Very lowc | These data were calculated from the reported HR for disease‐free survival. |
| Locoregional recurrence | — | — | — | — | — | Not reported. |
|
Recurrence Follow‐up: 5 years |
250d per 1000 | 303 per 1000 (158 to 583) |
RR 1.21 (0.63 to 2.33) |
143 (1 RCT) | ⊕⊝⊝⊝ Very lowe | — |
| Adverse events associated with treatment | 1 trial reported the following adverse events: flap necrosis, wound infection, fistula, vascular rupture, haematoma, seroma, and chyle fistula. There were no complications in 45 participants (59%) in the modified radical neck dissection group and no complications in 54 participants (75%) in the elective neck dissection group. There were 2 postoperative deaths in the modified radical neck dissection group and 1 postoperative death in the elective neck dissection group. | 148 (1 RCT) |
⊕⊕⊝⊝ Lowf | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; ND: neck dissection; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice for very serious inconsistency and once for unclear/high risk of bias. b Based on data from D'Cruz 2015 and Hutchison 2019. c Downgraded twice for very serious imprecision and once for high risk of bias. d Based on data from BHNCSG 1998. e Downgraded twice for very serious imprecision and once for unclear risk of bias. f Downgraded twice for very serious risk of bias.
Summary of findings 3. Superselective neck dissection versus selective neck dissection.
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: superselective ND Comparison: selective ND | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (selective ND) | Corresponding risk (superselective ND) | |||||
| Total mortality | — | — | — | — | — | Not reported. |
| New disease, progression, and mortality | — | — | 32 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
One study reported no clear difference between the interventions, but we were unable to use the data. | |
| Locoregional recurrence | — | — | — | 72 (2 RCTs) |
⊕⊝⊝⊝ Very lowa |
Data not presented in a useable way and inconsistent. 1 study reported no evidence of a difference. The other study reported lower recurrence in the superselective ND group. |
| Recurrence | — | — | — | — | — | Outcome not reported in a usable way in either study. |
| Adverse events associated with treatment | No adverse events reported. | 72 (2 RCTs) |
⊕⊝⊝⊝ Very lowb |
|||
| CI: confidence interval; ND: neck dissection; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice for very serious imprecision and once for unclear risk of bias. We were unable to use the data presented. b Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 4. Supraomohyoid neck dissection versus modified radical neck dissection.
| Supraomohyoid neck dissection versus modified radical neck dissection | ||||||
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: supraomohyoid ND Comparison: modified radical ND | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (modified radical ND) | Corresponding risk (supraomohyoid ND) | |||||
| Total mortality | — | — | — | — | — | Not reported. |
| New disease, progression, and mortality | — | — | — | — | — | Not reported. |
| Locoregional recurrence | — | — | — | — | — | Not reported. |
| Recurrence | — | — | — | — | — | Not reported. |
| Adverse events associated with treatment | Significant difference in complication rates, with lower rates for the supraomohyoid procedure. UW‐QOL scores for all disease‐free survivors were assessed at 1 year after treatment. Scores from 9 disease‐specific domains appeared to show that supraomohyoid ND was superior to modified radical ND in the domains of pain relief (78.8% vs 75.2%, P = 0.013) and shoulder function (81.1% vs 68.1%, P < 0.001), but not in any other domain. |
332 (1 RCT) |
⊕⊝⊝⊝ Very lowa |
— | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; ND: neck dissection; RCT: randomised controlled trial; UW‐QOL: University of Washington Quality of Life Questionnaire. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 5. Sentinel node biopsy versus elective neck dissection.
| Sentinel node biopsy versus elective neck dissection | ||||||
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: sentinel node biopsy Comparison: elective ND | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (elective ND) | Corresponding risk (sentinel node biopsy) | |||||
|
Total mortality Follow‐up: 3 years |
200a per 1000 | 200 per 1000 (182 to 219) |
HR 1.00 (0.90 to 1.11) |
279 (1 RCT) |
⊕⊕⊝⊝ Lowb | We calculated total mortality in place of overall survival. |
|
New disease, progression, and mortality Follow‐up: 3 years |
300a per 1000 | 295 per 1000 (275 to 317) |
HR 0.98 (0.90 to 1.07) |
279 (1 RCT) |
⊕⊕⊝⊝ Lowb | We calculated new disease, progression, and mortality in place of disease‐free survival. |
|
Locoregional recurrence Follow‐up: 3 years |
200c per 1000 | 207 per 1000 (184 to 233) |
HR 1.04 (0.91 to 1.19) |
279 (1 RCT) |
⊕⊕⊝⊝ Lowb | |
| Recurrence | — | — | — | 279 (1 RCT) |
⊕⊝⊝⊝ Very lowd | "[...] the number of patients with recurrences in both arms was almost identical." |
| Adverse events associated with treatment | No adverse events were reported. | ⊕⊝⊝⊝ Verylowd | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; ND: neck dissection; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Based on data from D'Cruz 2015 and Hutchison 2019 for elective neck dissection. b Downgraded once for imprecision and once for unclear risk of bias. c Based on data from Hutchison 2019 for elective neck dissection. d Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 6. PET‐CT following chemoradiotherapy versus planned neck dissection before or after chemoradiotherapy.
| PET‐CT following chemoradiotherapy versus planned neck dissection before or after chemoradiotherapy | ||||||
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: PET‐CT following chemoradiotherapy Comparison: planned ND before or after chemoradiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (planned ND) | Corresponding risk (PET‐CT) | |||||
|
Total mortality Follow‐up: 3 years |
200a per 1000 | 186 per 1000 (135 to 253) |
HR 0.92 (0.65 to 1.31) |
564 (1 RCT) | ⊕⊕⊕⊝ Moderateb | These data were calculated from the reported HR for overall survival. |
| New disease, progression, and mortality | — | — | — | — | — | Outcome not reported in a usable way. |
|
Locoregional recurrence Follow‐up: 3 years |
200c per 1000 | 200 per 1000 (189 to 211) | HR 1.00 (0.94 to 1.06) | 564 (1 RCT) | ⊕⊕⊕⊝ Moderateb | |
| Recurrence | — | — | — | — | — | Outcome not reported in a usable way. |
| Adverse events associated with treatment | 22 surgical complications in PET‐CT group compared with 83 in planned surgery group. | ⊕⊝⊝⊝ Very lowd |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; ND: neck dissection; PET‐CT: positron emission tomography–computed tomography; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Based on data from D'Cruz 2015 and Hutchison 2019 for elective ND. b Downgraded once due to imprecision. c Based on data from Hutchison 2019 for elective ND. d Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 7. Surgery plus radiotherapy versus radiotherapy alone.
| Surgery plus radiotherapy versus radiotherapy alone | ||||||
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: surgery + radiotherapy Comparison: radiotherapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (radiotherapy alone) | Corresponding risk (surgery + radiotherapy) | |||||
|
Total mortality Follow‐up: 3 years |
500a per 1000 | 153 per 1000 (67 to 336) |
HR 0.24 (0.10 to 0.59) |
35 (1 RCT) | ⊕⊝⊝⊝ Very lowb | These data were calculated from the reported HR for overall survival. |
| New disease, progression, and mortality | — | — | — | — | — | Not reported. |
| Locoregional recurrence | — | — | — | — | — | Not reported. |
| Recurrence | — | — | — | — | — | Not reported. |
| Adverse events associated with treatment | Both groups reported the following severe acute adverse effects: subcutaneous fibrosis; telangiectasia (1–4 cm²); and moderate‐to‐severe oedema, xerostomia, trismus, and dysphagia. Subcutaneous fibrosis was more prevalent in the surgery + radiotherapy group (P = 0.042), but the prevalence of other adverse effects appeared to be similar in both groups. | 35 (1 RCT) | ⊕⊝⊝⊝ Very lowc | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Based on data presented by Warnakulasuriya 2009. b Downgraded twice for very serious imprecision and once for high risk of bias. c Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 8. Surgery versus radiotherapy alone.
| Surgery versus radiotherapy alone | ||||||
|
Population: adults with oropharyngeal cancer Setting: inpatient Intervention: surgery Comparison: radiotherapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk (radiotherapy alone) | Corresponding risk (surgery) | |||||
|
Total mortality Follow‐up: 3 years |
500a per 1000 | 437 per 1000 (60 to 994) |
HR 0.83 (0.09 to 7.46) |
68 (1 RCT) |
⊕⊕⊝⊝ Lowb | We calculated mortality in place of overall survival. |
|
New disease, progression, and mortality Follow‐up: 3 years |
600c per 1000 | 625 per 1000 (219 to 979) |
HR 1.07 (0.27 to 4.22) |
68 (1 RCT) |
⊕⊕⊝⊝ Lowb | We calculated new disease, progression, and mortality in place of disease‐free survival. |
| Locoregional recurrence | — | — | — | — | — | Not reported. |
| Recurrence | — | — | — | — | — | Not reported. |
| Adverse events associated with treatment | Both groups reported the following severe acute adverse effects: subcutaneous fibrosis, telangiectasia (1–4 cm²), and moderate‐to‐severe oedema, xerostomia, trismus, and dysphagia. Subcutaneous fibrosis was more prevalent in the surgery + radiotherapy group (P = 0.042), but the prevalence of other adverse effects appeared to be similar in both groups. | 68 (1 RCT) |
⊕⊝⊝⊝ Very lowd | — | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Based on data presented by Warnakulasuriya 2009. b Downgraded twice for very serious imprecision. c For illustrative purposes only. d Downgraded twice for very serious imprecision and once for high risk of bias for subjective outcomes.
Summary of findings 9. Surgery plus adjuvant radiotherapy versus chemotherapy.
| Surgery plus adjuvant radiotherapy versus chemotherapy | |||||||
|
Population: adults with oral or oropharyngeal cancer Setting: inpatient Intervention: surgery + adjuvant radiotherapy Comparison: chemotherapy |
|||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk (chemotherapy) | Corresponding risk (surgery + adjuvant radiotherapy) | ||||||
| Total mortality | — | — | — | 32 (1 RCT) |
⊕⊝⊝⊝ Very lowb | Study reported that survival was significantly better in participants who underwent surgery and RT compared with the CRT group, but there were no useable data. | |
|
New disease, progression, and mortality Follow‐up: 3 years |
— | — | — | — | — | — | |
| Locoregional recurrence | — | — | — | 32 (1 RCT) |
⊕⊝⊝⊝ Very lowb | Reported difference between groups not "statistically significant" (P = 0.355), but there were no useable data. | |
| Recurrence | — | — | — | — | — | Not reported for the oral cancer subgroup. | |
| Adverse events associated with treatment | — | — | — | — | — | Not reported for any participants. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. |
|||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Estimated from chemotherapy arm of Iyer 2015. b Downgraded twice for very serious imprecision and once for high risk of bias.
Background
Description of the condition
Head and neck cancer (HNC) comprises laryngeal, pharyngeal (including oropharyngeal), and oral cavity cancers. Oral cavity and oropharyngeal cancer were historically grouped under the term oral cancer, but they are now considered two distinct cancers owing to differences in risk factors, diagnosis, and clinical management. HNC is the seventh most common cancer in the world, accounting for more than 660,000 new cases and 325,000 deaths annually (Sung 2021). The different types of HNC generally have common risk factors and aetiology, dominated by tobacco smoking and alcohol consumption (Bravi 2021; Gormley 2022). Nasopharyngeal cancer is distinctly associated with Epstein Barr virus (EBV), and oropharyngeal (mid‐throat) cancer is increasingly associated with human papillomavirus (HPV): over 50% of cases in the UK and upwards of 70% in the USA are HPV‐positive (Schache 2016). Worldwide, the association of HPV and oropharyngeal cancer varies considerably (Anantharaman 2017; Mehanna 2016). Oropharyngeal and oral cavity cancers frequently overlap, although modern pathology and sequencing techniques help to determine tumour origin and HPV status (Helliwell 2016). Most cases of HNC are diagnosed at an advanced stage, with associated implications for treatment and prognosis (Creaney 2022).
HNC is treated by multidisciplinary HNC teams in centralised units (Hughes 2012; Lo Nigro 2017). Clinical trials often recruit people with HNC as a single disease entity (Adelstein 2009; Kaanders 2022), which influences the evidence base available for systematic reviews. However, HPV‐positive oropharyngeal cancer is increasingly managed and evaluated as a distinct disease entity (Bteich 2022). Another factor that limits the evidence base is the presence of single‐centre trials, which may show larger intervention effects than multicentre trials; this is particularly important in surgical trials, where outcomes depend on surgical technique and experience of the surgeon (Bafeta 2012).
In 2020, there were an estimated 377,713 incident oral cavity cancer cases and 177,757 associated deaths worldwide; the corresponding figures for oropharyngeal cancer were 98,412 incident cases and 48,143 deaths (Ferlay 2020). There are wide geographical variations in incidence and mortality rates of oral cancers, with the greatest burden in low‐ and middle‐income countries (WHO 2022). However, these rates are increasing across the world, largely owing to oropharyngeal cancer and HPV (Chaturvedi 2013; Gillison 2015; Gormley 2022; Louie 2015; Purkayastha 2016; Simard 2014). From a global perspective, survival following a diagnosis of oral cavity or oropharyngeal cancer remains poor, with a five‐year survival of around 50% and limited improvement since the late 1980s (Gormley 2022; Warnakulasuriya 2009). However, in high‐income countries (e.g. the USA), five‐year survival for oral cavity and pharyngeal cancer is approaching 70% (National Cancer Institute 2022).
There is overwhelming evidence that tobacco use, alcohol consumption, and betel quid chewing are the main risk factors for HNC (Bouvard 2022; Bravi 2021; Winn 2015). There is also strong evidence that low socioeconomic status (educational attainment and income) is associated with substantially increased risk not explained by tobacco and alcohol (Conway 2015). There is a higher incidence of both oral and oropharyngeal cancers in men compared with women (Ferlay 2020; Purkayastha 2016). The risk of HNC increases with age across populations, with most cases diagnosed in people aged over 50 years (Warnakulasuriya 2009).
Oropharyngeal cancer is classified into two distinct types according to HPV status. HPV‐negative oropharyngeal cancer has the same main risk factors as other types of HNC, namely tobacco and alcohol consumption. HPV‐positive oropharyngeal cancer starts with exposure to high‐risk HPV, most often HPV 16, and can develop independently of tobacco or alcohol exposure (Gillison 2000), although smoking may interact with HPV and increase risk (Anantharaman 2016). Studies have associated HPV‐positive cases with a nearly 60% reduction in risk of mortality after adjusting for prognostic factors such as age, ethnicity, staging, smoking status, and treatment regimen (Ang 2010). This may be related to certain characteristics of people with HPV‐positive oropharyngeal cancer, including fewer comorbidities, enhanced anti‐tumour immunity, and greater radiosensitivity and thus better response to radiotherapy (Elrefaey 2014).
Before the mid‐2000s, management of oropharyngeal cancer generally involved primary surgery with adjunctive radiotherapy or chemotherapy. More recent trials have adopted a different approach for HPV‐positive oropharyngeal cancers, focusing on de‐escalation of overall radiotherapy dose, omission/substitution of platinum chemotherapy, or both (O Leary 2022). Surgery remains the main treatment approach for oral cavity cancer; people with advanced oral cancer usually receive adjunctive radiotherapy, chemoradiotherapy, or immune therapy in addition to upfront chemoradiotherapy.
Description of the intervention
Surgery can be combined with one or more other treatments, namely radiotherapy, chemotherapy, or immunotherapy/biotherapy. The sequence of these combination therapies is considered important. Radiotherapy is typically administered postoperatively. Chemotherapy can be administered at the following time points in the therapy sequence.
Before surgery (induction/neoadjuvant; e.g. to shrink a tumour prior to surgery or radiation)
After surgery (adjuvant; e.g. when surgery is considered the primary therapy) and before radiotherapy
At the same time as radiotherapy (concomitant/concurrent; this combination is also called chemoradiotherapy)
Alternating with radiotherapy
From the mid‐2000s, clinicians have used a form of radiotherapy called intensity‐modulated radiotherapy (IMRT) to treat oral and oropharyngeal cancers. IMRT uses higher radiation doses than traditional therapies and improves locoregional control; however, there is limited evidence on the associated side effects (Brennan 2017; Studer 2007).
Locoregional control of the primary tumour is the main criterion of successful cancer treatment. Typically, surgeons aim to cut out mouth cancers with a margin of normal tissue measuring at least 1 cm. Even with this approach, there may be histopathological evidence of cancer cells at the margins, which has prognostic implications (Batsakis 1999; Sutton 2003). Margins that are histologically free of tumour may demonstrate molecular changes; the presence of tumour clonogen repopulation at the margins may be predictive of disease progression (Partridge 2000).
Spread of the tumour to the regional lymph nodes within the neck (cervical nodes) is an early and consistent event in the natural history of oral and oropharyngeal cancers (Haddadin 2000). The extent of cervical involvement is reflected in the staging of the tumour and has prognostic implications (Shah 1990). Therefore, surgical dissection of the cervical lymph nodes at risk of metastasis may be undertaken as part of the management of the primary tumour. Classic radical neck dissection involves removal of all cervical lymph nodes from levels I to V, as well as the sternocleidomastoid muscle, internal jugular vein, submandibular gland, and the spinal accessory nerve; this results in significant postoperative morbidity, particularly in relation to loss of the accessory nerve. In one study of 100 people who underwent radical neck dissection, almost half of participants experienced shoulder pain, shoulder droop, and reduced range of motion (Ewing 1952). Another study (published almost 60 years later) that compared radical neck dissection with accessory nerve‐sparing surgery found that all participants in the radical neck dissection group had severe shoulder dysfunction compared with only 7% of the nerve‐sparing surgery group (Umeda 2010). Radical neck dissection is currently reserved for advanced neck disease.
Modifications to neck dissection procedures to preserve some or all of the associated structures have reduced morbidity (Carew 2003; Robbins 2002). Clinicians increasingly use selective neck dissection in the clinically N0 neck (no palpable nodes on clinical examination). In addition to the extent of neck disease at presentation, spread of the tumour outside the capsule of the lymph nodes (extracapsular spread) is another indicator of poor prognosis (Woolgar 2003). Distant metastasis is uncommon in HNC; one study reported 13.8% in 1022 cases (Duprez 2017). Locoregional disease recurrence remains the dominant mode of treatment failure for people with advanced tumours (Brizel 1998). Historically, clinicians treating oral and oropharyngeal cancers did not focus on distant metastatic disease because locoregional recurrence was the main cause of death, and there were fewer effective chemotherapeutic agents to deal with distant metastases. With improvements in locoregional control, clinicians are increasingly targeting distant metastasis in the management of oral and oropharyngeal cancers.
There is controversy surrounding the management of people with early stage tumours (T1, less than 2 cm; or T2, 2 cm to 4 cm) and clinically negative neck nodes (Woolgar 2003). To date, imaging of the head and neck region is not sufficiently sensitive to identify nodal micrometastases: reported rates of occult metastases range from 23% to 43% (Ebrahimi 2012). Some studies have demonstrated that performing neck dissection at the same time as primary tumour resection, compared with a 'watch and wait' approach (waiting for neck disease to present before performing neck dissection) may improve treatment outcomes (Haddadin 2000; Hughes 1993). One current clinical guideline recommends prophylactic neck treatment in T1 and T2 oral cancer with a clinically negative neck (Paleri 2016), and one study found improved overall and disease‐free survival in people with early‐stage oral squamous‐cell carcinoma (SCC) who had elective neck dissection compared with those who had therapeutic neck dissection (D'Cruz 2015). However, this approach implies over‐treatment and treatment‐associated morbidity in most people (Dias 2001).
Some experts advocate sentinel node biopsy for small tumours with a clinically negative neck. One UK guideline recommends offering biopsy to people with early‐stage oral cancer (T1‐T2N0), as occurs in the Netherlands and Denmark (Holden 2018; NICE 2018). One European study reported that sentinel node biopsy had a sensitivity of 86% and negative predictive value of 95%, and was a reliable and safe oncological technique for staging the clinically N0 neck in people with T1 and T2 oral cancer (Schilling 2015). A larger study, including a meta‐analysis of cT1/T2N0 people with tongue SCC, also showed a high sensitivity and negative predictive value for sentinel node biopsy (Yang 2017). The widespread introduction of this diagnostic technique in oral SCC management will help to ensure timely treatment of people at high risk while sparing those at low risk from unnecessary surgery (Schilling 2017).
How the intervention might work
Surgery is an important part of oral cavity cancer management; it works by removing the primary tumour. It may also involve the removal of the neck lymph nodes as well as tissue surrounding the primary tumour to prevent recurrence. Locoregional control of the primary tumour is the main criterion of successful treatment. The treatment of oropharyngeal cancer has changed from primary surgery with adjunctive radiotherapy or chemoradiotherapy, to being primarily treated with radiotherapy, with surgery being used for early‐stage oropharyngeal treatment, using advanced surgical techniques.
Why it is important to do this review
Surgical treatment for oral cavity and oropharyngeal cancer was identified as a key priority when Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 and again in 2020.
This is an update of a Cochrane Review first published in 2007, and previously updated in 2011 and 2018 (Oliver 2007; Bessell 2011; Bulsara 2018). All the evidence for the seven comparisons included in the 2018 version was of very low certainty. The comparison of elective neck dissection with therapeutic (delayed) neck dissection in people with oral cavity cancer and clinically negative neck nodes provided no evidence of either intervention leading to improved overall survival or disease‐free survival. For the other comparisons, single trials contributed data to effect measures. We knew important studies had been published since 2018 and could add valuable evidence to our review, especially for the comparison elective neck dissection versus therapeutic (delayed) neck dissection.
The management of advanced oral cavity and oropharyngeal cancers is problematic and has traditionally relied on surgery and radiotherapy, both of which are associated with substantial adverse effects. Oropharyngeal cancers have relatively 'silent' symptoms, which may not be present during the early stages of the disease. This could partly explain why the disease stage at diagnosis has not altered since the 1960s despite public education (McGurk 2005). Tumour recurrence and the development of multiple primary tumours are the major causes of treatment failure (Day 1992; Partridge 2000; Woolgar 2003). Surgical treatment may be disfiguring and result in a substantially reduced quality of life due to difficulties with altered appearance, speech, and eating and drinking, with associated social isolation. Developments in surgical delivery aim to improve its efficacy and reduce the impact on people's quality of life.
This review was undertaken as part of a series of reviews looking at the different treatment modalities for oral and oropharyngeal cancer: surgery (Bulsara 2018), chemotherapy (Parmar 2021), radiotherapy (Glenny 2010), and immunotherapy (Chan 2015). In this update of our surgical review, we aimed to answer the following two broad questions.
Does surgery, alone or combined with other treatment modalities, improve outcomes for people with oral cavity and oropharyngeal cancers?
Which type of surgery is most effective for treating people with oral cavity and oropharyngeal cancers?
Objectives
To evaluate the relative benefits and harms of different surgical treatment modalities for oral cavity and oropharyngeal cancers.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs.
Types of participants
Cancer diagnoses were based on the International Classification of Diseases for Oncology (ICD‐O) codes (WHO 1990). We included people with oral cancer (C01–C06) and oropharyngeal cancer (C09, C10). We excluded cancers of the hypopharynx (C13), nasopharynx (C11), larynx (C32), and lip (C00).
We included RCTs of HNC where more than 50% of participants had primary tumours in the oral cavity or oropharynx, or where separate data could be extracted for participants with these cancers.
Cancers were primary SCCs arising from the oral mucosa. We included histological variants of SCCs (e.g. adenosquamous, verrucous, basaloid, papillary). Although these variants are known to have differing natural histories to most conventional SCCs, they have a common aetiology, incidence is low, and they are generally managed in the same way. We also included carcinoma in situ.
We excluded epithelial malignancies of the salivary glands, odontogenic tumours, sarcomas, and lymphomas, as these have a different aetiology and are managed differently.
Types of interventions
Surgical treatment of the primary tumour is typically a main therapeutic intervention. For this review, eligible surgical treatment could involve traditional scalpel‐based surgery, laser cutting or ablation, or harmonic scalpel. We included RCTs of the following comparisons.
One surgical treatment modality versus another surgical treatment modality
Other treatment interventions (radiotherapy, chemotherapy, immunotherapy/biotherapy, or any combination of these) with versus without surgery
Surgery versus no surgery
We anticipated that there would be no studies comparing surgery with placebo (although such studies would have been eligible). We did not consider salvage or palliative surgery.
We included studies that carried out surgical treatment of the neck lymph nodes (cervical lymph nodes) before, after, or at the same time as surgical treatment of the primary tumour. We did not consider studies that investigated surgical treatment of the cervical lymph nodes without treatment of the primary tumour. We included studies concerned with cervical lymph node management in the surgical treatment of the primary tumour.
The treatments evaluated must have been the primary treatment for the tumour, and participants should not have received any prior intervention other than diagnostic biopsy.
Types of outcome measures
We excluded studies that did not measure any of our primary outcomes (e.g. short‐term studies focusing on postsurgical outcomes such as wound closure). As we did not expect to find many data, we planned to report dichotomous outcomes at all available time points; this was not necessary for time‐to‐event data, as hazard ratios (HRs) summarise all time points of measurement.
Primary outcomes
Overall survival (or total mortality; we also planned to evaluate disease‐related mortality, if possible)
Disease‐free survival (i.e absence of new disease, progression, and mortality)
Locoregional recurrence
Recurrence (including loco‐regional recurrence and distant metastasis)
Secondary outcomes
Adverse events associated with treatment
Quality of life
Direct and indirect costs to patients and health services
Participant satisfaction
Search methods for identification of studies
In the original version of this review, searches were conducted as part of a series of Cochrane Reviews on the treatment modalities for oral cavity and oropharyngeal cancer (Oliver 2007). The reviews were divided into four themes: surgery, chemotherapy, radiotherapy, and immunotherapy/targeted therapies. A search strategy was developed that would encompass three of the four broad themes (surgery, chemotherapy, radiotherapy; see Bessell 2011 for details of the search strategy). From 2011 onwards, we conducted a more specific search for the surgery theme.
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year, or publication status restrictions.
Cochrane Oral Health's Trials Register (searched 9 February 2022; Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 1) in the Cochrane Library (searched 9 February 2022; Appendix 2)
MEDLINE Ovid (1946 to 9 February 2022; Appendix 3)
Embase Ovid (1980 to 9 February 2022; Appendix 4)
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, we combined strategies with subject strategy adaptations of the highly sensitive search strategies designed by Cochrane for identifying RCTs and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022).
Searching other resources
Cochrane Oral Health's information specialist searched the following trials registries for ongoing trials.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 9 February 2022; Appendix 5)
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; searched 9 February 2022; Appendix 6)
Where necessary, we contacted authors of key papers and abstracts to request further information about their trials.
We searched the reference lists of included studies and relevant systematic reviews for further studies.
There was no separate search for adverse events associated with interventions; we considered adverse events described in included studies only.
We checked that no included studies had been retracted due to error or fraud.
Data collection and analysis
Selection of studies
At least two review authors (from HW, VB, AMG, DC, MM) independently scanned the titles and abstracts (when available) of all reports identified through the electronic searches. The search was designed to be sensitive and include controlled clinical trials; these were filtered out early in the selection process if they were not randomised. As HNC studies often include people with oral cavity or oropharyngeal cancer, we undertook a broad search to include all possible studies. We obtained the full‐text articles of studies that appeared to meet our inclusion criteria or that provided insufficient information in the title and abstract to make a decision. We excluded studies reported as conference abstracts only. Two review authors independently assessed the full reports of potentially eligible studies to establish whether they met our inclusion criteria. We resolved disagreements by discussion or by consulting a third review author if necessary. We listed studies rejected at this or subsequent stages in the Characteristics of excluded studies table, and recorded our reasons for exclusion.
Data extraction and management
At least two review authors independently extracted data from included studies. The data extraction forms were piloted on several papers and modified as required before use. We discussed any disagreements, consulting a third review author where necessary. However, group discussion was often required following data extraction due to the complexity of the data presented. Where necessary, we contacted study authors for clarification or missing information. Had any trials required translation, an experienced review author would have completed a data extraction sheet in their native language, when available. A second review author would have checked the numerical data from these non‐English language papers.
For each trial, we recorded the following data.
Year of publication, country of origin, and source of study funding
Details of the participants, including demographic characteristics, inclusion and exclusion criteria, and proportion with oral cavity and oropharyngeal cancer
Details of the type of intervention, timing, and duration
Details of the outcomes reported, including method of assessment and time intervals
We planned to include HNC trials with combined data (i.e. no outcome data available by primary tumour site) if more than 50% of participants had oral/oropharyngeal cancer. Where separate 'pure' oral/oropharyngeal cancer data were available, we extracted and analysed these 'pure' data and ignored the combined head and neck data.
Assessment of risk of bias in included studies
At least two review authors independently conducted assessment of risk of bias of included studies using the Cochrane risk of bias tool (RoB 1; Higgins 2011). We resolved any disagreements through discussion or by consulting a third review author where necessary. We assessed six domains for each included study: sequence generation, allocation concealment, blinding (of outcome assessors), completeness of outcome data, selective outcome reporting, and other potential sources of bias. We made an overall risk of bias assessment for each study at an outcome level (objective or subjective), according to the most severe assessment of the domains.
We assigned a 'low', 'high', or 'unclear' risk of bias judgement for each domain according to the following criteria.
Sequence generation: low risk if use of a random number table, computerised system, central randomisation by statistical co‐ordinating centre, randomisation by an independent service using minimisation technique, permuted block allocation, or Zelan technique. If the paper merely stated that participants were randomised or randomly allocated with no further information, we assigned an unclear risk of bias judgement. Otherwise, we judged the study at high risk of bias.
Allocation concealment: low risk if centralised allocation including access by telephone call or fax; or pharmacy‐controlled randomisation or sequentially numbered, sealed, opaque envelopes. If it was clear that the investigators had not used any of these methods, we judged the study at high risk of bias. If this information was unavailable, we judged the study at unclear risk.
Blinding of outcome assessment (objective outcomes): as mortality is the primary outcome that is most frequently and reliably reported, we decided to assess all trials as being at low risk of bias for this domain.
Blinding of outcome assessment (subjective outcomes): given the nature of the interventions, all subjective outcome measurements were considered to be at high risk of bias for this domain.
Outcome data: outcome data were considered complete if all randomised participants were included in the analysis of the outcome(s). However, in trials of treatment for cancer, this is rarely the case. We considered trials with less than 10% attrition (less than 10% of randomised participants excluded from analyses) at low risk of bias if they provided reasons for exclusions for each group, and the numbers and reasons were similar in each group. Where postrandomisation exclusions were greater than 10%, or there were no reasons provided for exclusions for each group, or where rates and reasons were different for each group, we assigned an unclear risk of bias judgement.
Selective outcome reporting: we judged trials at low risk of reporting bias if the outcomes of interest described in the methods section were systematically reported in the results section. Where reported outcomes did not include prespecified outcomes or outcomes expected in trials of treatments for oral and oropharyngeal cancer, or where trials reported additional analyses, we considered them at unclear risk of bias.
Other bias: we noted examples of potential sources of bias such as baseline imbalance in potentially important prognostic factors between the treatment groups, or the use of a co‐intervention in only one group (e.g. nasogastric feeding). If there was no information about the intervention groups at baseline, we assessed studies at unclear risk of bias. If there was a major problem with the conduct of the study such as early termination, we judged it at high risk of other bias.
Measures of treatment effect
The primary outcome most frequently and reliably reported in cancer studies is total mortality, expressed as an HR along with a 95% confidence interval (CI). An HR provides an estimate of the ratio of the hazard rates for a particular event between the experimental group and a control group over the duration of the entire study. For overall survival, the event of interest is death (total mortality). It is preferable to express the outcome in terms of overall survival; however, statistically, the estimate of effect is the HR of death. Similarly, for disease‐free survival, we used the HR for the combined outcome of new disease, progression, and mortality.
We entered these data into the meta‐analysis using the inverse variance method. If studies did not quote HRs, we calculated the log HR and the standard error (SE) from the available summary statistics or Kaplan‐Meier curves, according to the methods proposed by Parmar and colleagues (Parmar 1998), or we requested these data from study authors.
For dichotomous outcomes, we expressed the estimates of effect of an intervention as risk ratios (RRs) together with 95% CIs. We used dichotomous data for primary outcomes only where HRs were unavailable or could not be calculated. We planned to combine data from similar follow‐up periods.
Unit of analysis issues
The participant was the unit of analysis. We analysed cluster‐RCTs as described in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). To include studies with more than two intervention groups in a meta‐analysis, we omitted groups that were irrelevant to the comparison, and combined multiple relevant groups to create a single pair‐wise comparison. Most of the trials included time‐to‐event data presented as HRs. For data presented for all participants at different time points, we used the latest time point. We took care to analyse the data for events that may re‐occur (e.g. adverse events) to avoid unit‐of‐analysis errors.
Dealing with missing data
We attempted to contact trial authors to retrieve data missing from the trial report, or to clarify areas where data or trial design and conduct were unclear.
The primary method of analysis was using HRs, which accounts for missing data by censoring.
Assessment of heterogeneity
We conducted meta‐analyses only if there were studies of similar comparisons reporting the same outcome measures. We assessed the significance of any discrepancies in the estimates of the treatment effects between trials using the Chi2 test and the I2 statistic.
Assessment of reporting biases
For the four primary outcomes, had there been sufficient studies (more than 10 per comparison), we would have generated funnel plots and investigated possible publication bias through a visual inspection of funnel plot asymmetry and through the Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test (Begg 1994; Egger 1997).
Data synthesis
We conducted meta‐analyses only if there were studies of similar comparisons reporting the same outcome measures. We combined RRs for dichotomous data and HRs for survival data using random‐effects models. If studies reported RRs over a fixed time point such as three years, we used these data to calculate the HR over that period so that we could include the outcome in a meta‐analysis of HRs.
Subgroup analysis and investigation of heterogeneity
Due to the different natural history and treatment regimens for oral cavity and oropharyngeal cancers, we planned to analyse these cancer types separately, if possible, for overall survival and disease‐free survival, using a formal statistical test to compare subgroups.
Sensitivity analysis
Had there been sufficient data, we would have performed sensitivity analyses to examine the effects of randomisation, allocation concealment, and quality of follow‐up/completeness of data on overall survival and disease‐free survival.
Summary of findings and assessment of the certainty of the evidence
We developed summary of findings tables for all comparisons; each table included our four primary outcomes and adverse events associated with treatment. We assessed the certainty of the body of evidence using the GRADE approach, which considers the overall risk of bias of the included studies, directness of the evidence, consistency of the results, precision of the estimates, and risk of publication bias. We rated the certainty of the body of evidence for each outcome as high, moderate, low, or very low (GRADEpro GDT; Schünemann 2013; Schünemann 2022). We formatted the summary of findings tables for HRs as outlined by Skoetz 2020.
Results
Description of studies
Results of the search
We identified 3374 research papers through the electronic searching for this update, after the removal of duplicates (Figure 1). In the title and abstract screen, we identified 22 potentially eligible records. After retrieving and reading the full‐text articles, we included four new trials in this review update (Garrel 2020; Hutchison 2019; Nichols 2019; Pandey 2018). We identified eight new ongoing trials (see Characteristics of ongoing studies), and we excluded five new trials (see Characteristics of excluded studies). We also excluded a trial that had been included in previous versions of this review, as the primary site delivered interstitial radiotherapy rather than surgery (Vandenbrouck 1980).
1.
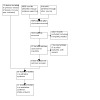
PRISMA diagram showing study selection process.
Included studies
We included 15 RCTs in this review update. Eight were multicentre trials (BHNCSG 1998; Bier 1994; Garrel 2020; Hutchison 2019; Mehanna 2017; Nichols 2019; Robertson 1998; Yuen 2009), with the number of centres ranging from three to 38. Four trials were undertaken in India (D'Cruz 2015; Fakih 1989; Pandey 2018; Rastogi 2018), three in the UK (Hutchison 2019; Mehanna 2017; Robertson 1998), two in Brazil (BHNCSG 1998; Kligerman 1994), two in China (Guo 2014; Yuen 2009), one in France (Garrel 2020), one in Canada and Australia (Nichols 2019), one in centres across Europe (Austria, Germany, and Switzerland; Bier 1994), and one in Singapore (Iyer 2015). We wrote to the authors of 12 of the included studies to request missing data and details about the randomisation sequence generation and allocation concealment, as appropriate. We received replies from the authors of five trials (D'Cruz 2015; Kligerman 1994; Mehanna 2017; Pandey 2018; Robertson 1998); based on these replies, we made changes to the risk of bias assessments for three trials.
Participants
Participants were recruited over periods ranging from two years to 10 years, with the earliest recruitment commencing in 1985 (Fakih 1989). A total of 2820 participants were randomly allocated to treatments, and 2583 were included in the outcome evaluations.
Fourteen included trials reported tumour extent using the TNM system (where T defines tumour size and any spread of cancer into nearby tissue, N defines spread of cancer to nearby lymph nodes, and M defines metastasis). Seven trials included people with T1 to T2 tumours (D'Cruz 2015; Fakih 1989; Garrel 2020; Hutchison 2019; Kligerman 1994; Nichols 2019; Yuen 2009), two included people with T2 to T4 tumours (BHNCSG 1998; Robertson 1998), one included people with T1 to T3 tumours (Rastogi 2018), and four included people with T1 to T4 tumours (Guo 2014; Iyer 2015; Mehanna 2017; Pandey 2018). One trial did not record tumour stage at trial entry (Bier 1994).
Nine trials included people with clinically negative neck nodes (BHNCSG 1998; D'Cruz 2015; Fakih 1989; Garrel 2020; Hutchison 2019; Kligerman 1994; Pandey 2018; Rastogi 2018; Yuen 2009), three trials included people with neck nodes clinically staged as N0 to N2 (Guo 2014; Nichols 2019; Robertson 1998), one trial included people with neck nodes clinically staged as N0 to N3 (Iyer 2015), one trial included people with clinically staged N2 to N3 nodes (Mehanna 2017), and one trial included clinically negative or positive neck nodes without specifying the stage (Bier 1994).
Of the 15 included trials, nine recruited people with oral cavity cancer only (BHNCSG 1998; Bier 1994; D'Cruz 2015; Fakih 1989; Hutchison 2019; Kligerman 1994; Pandey 2018; Rastogi 2018; Yuen 2009); three included people with oral cavity or oropharyngeal cancer (Garrel 2020; Guo 2014; Robertson 1998); one included people with cancer of the oral cavity, oropharynx, hypopharynx, larynx, and maxillary sinus (Iyer 2015); one included people with cancer of the oral cavity, tonsil, base of tongue, supraglottis, and glottis or subglottis (Mehanna 2017); and one included people with oropharyngeal cancer (Nichols 2019). Table 10 shows the cancer type, stage, and nodal status of participants in the 15 included trials.
1. Stage of cancer.
| Study | Cancer types | TNM stage | Nodal status |
| BHNCSG 1998 | Oral cavity | T2–T4 | Negative neck |
| Bier 1994 | Oral cavity | Not reported | Negative or positive neck, range unspecified |
| D'Cruz 2015 | Oral cavity | T1–T2 | Negative neck |
| Fakih 1989 | Oral cavity | T1–T2 | Negative neck |
| Garrel 2020 | Oral cavity/oropharyngeal | T1–T2 | Negative neck |
| Guo 2014 | Oral cavity/oropharyngeal | T1–T4 | N0‐2 |
| Hutchison 2019 | Oral cavity | T1–T2 | Negative neck |
| Iyer 2015 | Oral cavity (other cancer types included in the study but only data for oral cavity cancer included in the review) | T1–T4 | N0‐3 |
| Kligerman 1994 | Oral cavity | T1–T2 | Negative neck |
| Mehanna 2017 | Oral cavity, tonsil, base of tongue, supraglottis and glottis or subglottis | T1–T4 | N2‐3 |
| Nichols 2019 | Oropharyngeal | T1–T2 | N0‐2 |
| Pandey 2018 | Oral cavity | T1–T4 | Negative neck |
| Rastogi 2018 | Oral cavity | T1–T3 | Negative neck |
| Robertson 1998 | Oral cavity/oropharyngeal | T2–T4 | N0‐2 |
| Yuen 2009 | Oral cavity | T1–T2 | Negative neck |
In the TNM cancer staging system, T defines tumour size and any spread of cancer into nearby tissue, N defines spread of cancer to nearby lymph nodes; and M defines metastasis.
Interventions
No included trials compared different surgical approaches to the excision of the primary tumour.
Eleven trials of participants with oral cavity cancers compared either different surgical techniques for management of the lymph nodes in the neck or different timing of removal of the lymph nodes in the neck (BHNCSG 1998; Bier 1994; D'Cruz 2015; Fakih 1989; Garrel 2020; Guo 2014; Hutchison 2019; Kligerman 1994; Pandey 2018; Rastogi 2018; Yuen 2009).
Five trials compared the timing of neck dissection, evaluating elective neck dissection at the same time as primary tumour excision versus therapeutic neck dissection (D'Cruz 2015; Fakih 1989; Hutchison 2019; Kligerman 1994; Yuen 2009). Kligerman 1994 evaluated the supraomohyoid approach to elective neck dissection in a group of participants with clinically negative neck nodes versus therapeutic neck dissection if the nodes became clinically positive. Yuen 2009 evaluated elective selective neck dissection at the time of glossectomy versus glossectomy plus therapeutic neck dissection if nodes became clinically positive. Fakih 1989 used elective radical neck dissection at the same time as primary tumour resection in a group with clinically negative neck nodes versus the same procedure if the neck nodes become positive in the other group. D'Cruz 2015 and Hutchison 2019 evaluated selective neck dissection versus modified therapeutic neck dissection. Garrel 2020 evaluated treatment on the basis of sentinel node biopsy versus radical neck dissection.
Four trials compared different types of neck dissection surgery at the time of removal of the primary tumour (BHNCSG 1998; Bier 1994; Guo 2014; Rastogi 2018). In Bier 1994, both groups had radical resection of the primary tumour; in addition, one group had radical neck dissection while the other had selective neck dissection. The Brazilian Study group compared modified radical neck dissection with supraomohyoid neck dissection in conjunction with resection of the primary tumour (BHNCSG 1998). Pandey 2018 and Rastogi 2018 compared superselective neck dissection with supraomohyoid neck dissection in conjunction with resection of the primary tumour. Guo 2014 compared supraomohyoid neck dissection with modified radical neck dissection in conjunction with resection of the primary tumour.
Robertson 1998 compared surgery followed by radiotherapy with radiotherapy alone in a group of participants with oral cavity or oropharyngeal cancer. Nichols 2019 compared robotic surgery with radiotherapy alone. Iyer 2015 compared surgery and adjuvant radiotherapy with concurrent chemoradiotherapy. Mehanna 2017 evaluated a positron emission tomography‐computed tomography (PET‐CT)‐guided 'watch and wait' policy (with neck dissection undertaken only if no/incomplete response to chemoradiotherapy identified) versus planned neck dissection before or after radical chemoradiotherapy for locally advanced head and neck SCC.
Outcome measures
The duration of follow‐up in the included trials ranged from approximately 15 months (Bier 1994) to 122 months (Yuen 2009). Three trials did not report total mortality or overall survival (Pandey 2018; Rastogi 2018; Yuen 2009); not all the remaining trials provided data in a form suitable for inclusion in meta‐analysis. Five trials did not report disease‐free survival (BHNCSG 1998; Mehanna 2017; Pandey 2018; Rastogi 2018; Robertson 1998), and four trials did not report locoregional recurrence (BHNCSG 1998; Guo 2014; Robertson 1998; Yuen 2009).
Five trials did not mention adverse events (Bier 1994; Fakih 1989; Iyer 2015; Kligerman 1994; Yuen 2009), and nine trials did not report quality of life measures (BHNCSG 1998; Bier 1994; D'Cruz 2015; Fakih 1989; Garrel 2020; Iyer 2015; Kligerman 1994; Robertson 1998; Yuen 2009).
Excluded studies
We excluded 26 studies (five from the searches for this update) for the following reasons.
Not an RCT (Jinyun 2015)
Short‐term outcome measures (Batra 2016; Dean 2013; Dziegielewski 2019; Fan 2017; Fritz 2016; Funahara 2017; Lin 2016; Minkovich 2011; Oswal 2017; Verma 2017; Walen 2011)
Less than 50% of participants with oral cancer (Aladashi 2020; Hintz 1979a; Hintz 1979b)
Abstract only or insufficient information (Christensen 2019; Gundale 2017; Kramer 1987; McCaul 2012; McCaul 2017; Zhang 2010)
Full‐text article unavailable (Uppal 2012)
Focus of the trial was evaluation of presurgical chemotherapy (Chaukar 2021; Zhong 2013)
Inappropriate intervention (diagnostic aid) and same treatment for both groups (Durham 2020)
Primary site delivered interstitial radiotherapy rather than surgery (Vandenbrouck 1980)
Risk of bias in included studies
Figure 2 and Figure 3 present the risk of bias assessment results visually.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Eight trials reported adequate sequence generation methods (D'Cruz 2015; Fakih 1989; Garrel 2020; Hutchison 2019; Mehanna 2017; Nichols 2019; Pandey 2018; Robertson 1998). Three trials reported adequate allocation concealment (Hutchison 2019; Nichols 2019: Robertson 1998), and the authors of another two trials confirmed adequate allocation concealment (Mehanna 2017; Pandey 2018). Therefore, we judged five trials at overall low risk of selection bias (Hutchison 2019; Mehanna 2017; Nichols 2019; Pandey 2018; Robertson 1998), and the other 10 studies at unclear risk.
Blinding
Blinding of participants and clinicians is not feasible in surgical trials, but blinding of outcome assessment is both possible and desirable. As mortality is the primary outcome that is most frequently and reliably reported, we decided to judge all trials at low risk of bias for the objective outcomes.
We assessed six trials at high risk of bias for blinding of subjective participant‐reported outcomes (Garrel 2020; Guo 2014; Mehanna 2017; Nichols 2019; Pandey 2018; Rastogi 2018).
Incomplete outcome data
We judged 12 trials at low risk of bias with regard to incomplete outcome data because all the randomised participants were adequately accounted for in the outcome evaluation. Of the remaining trials, we assessed two at high risk of attrition bias (Bier 1994; Fakih 1989), and one at unclear risk (D'Cruz 2015). Both Bier 1994 and Fakih 1989 presented an interim analysis of a subgroup of participants and have not published the final analysis as far as we are aware. In both trials, it was unclear how many participants were randomly allocated to each intervention group, and how many in each group were subsequently excluded from the analysis or analysed in a group other than that to which they were originally allocated (or both). It is likely that those excluded from the analysis (because they refused surgery or had extracapsular rupture during surgery) had a different outcome from those included in the analysis.
Selective reporting
We considered 14 trials at low risk of reporting bias because they reported expected, clinically important outcomes. Pandey 2018 presented insufficient data on survival so was at unclear risk.
Other potential sources of bias
We judged nine trials at low risk of other bias because the intervention groups appeared to be similar at baseline, and we identified no other sources of bias (BHNCSG 1998; D'Cruz 2015; Garrel 2020; Guo 2014; Hutchison 2019; Mehanna 2017; Nichols 2019; Rastogi 2018; Yuen 2009).
Three trials provided no information regarding the baseline characteristics of participants in each group, so these trials were at unclear risk of other bias (Bier 1994; Fakih 1989; Kligerman 1994). Pandey 2018 was also at unclear risk because it provided insufficient details of the trial, and the report was published nine years after recruitment ended.
We assessed Robertson 1998 at high risk of other bias because it was stopped with only 35 people recruited (the planned sample size was 350 participants) because clinicians felt it was unethical to continue. While the investigators followed appropriate procedures and conducted and reported an interim analysis, it is unclear from this report whether a priori stopping rules were in place. Additionally, more than half of the participants in this trial did not receive radiotherapy as planned owing to problems with faulty equipment. This likely had a greater effect on the outcomes of the radiotherapy‐only arm of the trial. We assessed Iyer 2015 at high risk of other bias because it was stopped early due to poor accrual.
Overall risk of bias
We assessed four studies at high overall risk of bias (Bier 1994; Fakih 1989; Iyer 2015; Robertson 1998), and three trials at low overall risk of bias (Hutchison 2019; Mehanna 2017; Nichols 2019), for the objective outcomes. The remaining eight trials were at unclear overall risk of bias for the objective outcomes. When considering subjective outcomes, six trials were at high risk of bias (Garrel 2020; Guo 2014; Mehanna 2017; Nichols 2019; Pandey 2018; Rastogi 2018), and only Hutchison 2019 was at low risk of bias overall.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Comparison 1: elective neck dissection versus therapeutic (delayed) neck dissection
See Table 1.
Five trials compared the timing of the neck dissection: one group had neck dissection at the same time as primary tumour resection, while the other group had a separate procedure for neck dissection after primary tumour resection only when there was clinical evidence of disease in the neck nodes (D'Cruz 2015; Fakih 1989; Hutchison 2019; Kligerman 1994; Yuen 2009). All participants had oral cavity cancers (specifically tongue or floor of mouth tumours) and clinically negative neck nodes on study entry.
Fakih 1989 performed classical radical neck dissection procedures and pooled data after one year of follow‐up. D'Cruz 2015, Hutchison 2019, and Yuen 2009 performed elective selective neck dissection of level I to III nodes; D'Cruz 2015 reported data at three years, Yuen 2009 presented disease‐free survival data at five years, and Hutchison 2019 reported data at five years. Kligerman 1994 used a supraomohyoid elective neck dissection procedure, and reported data after 3.5 years of follow‐up.
Primary outcomes
Overall survival (or total mortality)
Four trials presented overall survival data, two as HRs (D'Cruz 2015; Hutchison 2019), and two as binary data over fixed time periods (Fakih 1989 at one year, Kligerman 1994 at three years), which we entered as HR data. The pooled HR for mortality showed evidence of a benefit for elective neck dissection over therapeutic neck dissection (HR 0.64, 95% CI 0.50 to 0.83; P < 0.001, I2 = 0%; 4 trials, 883 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Elective neck dissection (ND) versus therapeutic (delayed) ND, Outcome 1: Total mortality
Disease‐free survival (or new disease, progression, and mortality)
Five trials provided data for disease‐free survival, two as HRs (D'Cruz 2015; Hutchison 2019), and three as binary data over fixed time periods (Fakih 1989 at one year, Kligerman 1994 at three years, Yuen 2009 at five years), which we entered as HR data. The pooled HR for new disease, progression, and mortality showed evidence of benefit for elective neck dissection over therapeutic neck dissection (HR 0.56, 95% CI 0.45 to 0.70; P < 0.001, I2 = 12%; 5 trials, 954 participants; moderate‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Elective neck dissection (ND) versus therapeutic (delayed) ND, Outcome 2: New disease, progression, and mortality
Locoregional recurrence
Four trials reported data for locoregional recurrence; one trial presented HR data (Hutchison 2019), and three trials presented binary data over fixed time periods, which we entered as HR data (D'Cruz 2015; Fakih 1989; Kligerman 1994). The pooled HR showed evidence of a benefit for elective neck dissection over therapeutic neck dissection (HR 0.58, 95% CI 0.43 to 0.78; P < 0.001, I2 = 0%; 4 trials, 458 participants; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Elective neck dissection (ND) versus therapeutic (delayed) ND, Outcome 3: Locoregional recurrence
Recurrence
All five trials reported recurrence (as distinct from locoregional recurrence); however, we were unable to use the data from Hutchison 2019 (Table 2 in supplemental data) or Yuen 2009, as the time points of measurement differed amongst participants. The remaining three trials reported recurrence over fixed time periods (D'Cruz 2015; Fakih 1989; Kligerman 1994). There was evidence of a reduction in disease recurrence in the elective neck dissection group (RR 0.58, 95% CI 0.48 to 0.70; P < 0.001, I2 = 0%; 3 trials, 633 participants; moderate‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Elective neck dissection (ND) versus therapeutic (delayed) ND, Outcome 4: Recurrence
Secondary outcomes
Adverse events associated with treatment
In D'Cruz 2015, 6.6% of elective surgery participants and 3.6% of therapeutic surgery participants reported adverse events. These included neck haematoma, chyle leak, oral bleeding, postoperative infection, and anaphylaxis. Hutchison 2019 found that adverse events of any grade were more frequent in the elective neck dissection arm, and most were low‐grade events. Neck sensory and motor nerve abnormalities and problems with swallowing were more common in the elective neck dissection arm. A meta‐analysis of total adverse events showed they were more common in the elective arms (RR 1.31, 95% CI 1.11 to 1.54; P = 0.002, I2 = 0; 2 trials, 746 participants; moderate‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Elective neck dissection (ND) versus therapeutic (delayed) ND, Outcome 5: Total adverse events
The other three trials did not report adverse events (Fakih 1989; Kligerman 1994; Yuen 2009).
Quality of life
Only Hutchison 2019 reported quality of life, finding similar scores in the two groups for many measures, such as emotional functioning and problems swallowing. The only statistically significant differences were for dry mouth (elective group worse) and nausea (elective group better).
Direct and indirect costs to patients and health services
Only Hutchison 2019 reported resource use. The participants who had elective neck dissection spent a median of six days in hospital for the procedure, compared with two days for those who had primary tumour resection only (Wilcoxon P < 0.001).
Participant satisfaction
No trials reported participant satisfaction.
Comparison 2: elective radical neck dissection versus elective selective neck dissection
See Table 2.
Two trials compared neck dissection surgery of differing extent (BHNCSG 1998; Bier 1994). Owing to differences between two studies with regard to participant characteristics at baseline and surgical procedures, we did not meta‐analyse their results.
BHNCSG 1998 evaluated a modified classical neck dissection procedure with accessory nerve preservation versus a supraomohyoid neck dissection to achieve compartmental excision of levels I to III neck nodes in 148 participants with T2 to T4 primary lesions in the oral cavity and clinically negative nodes. Following intraoperative frozen sections of the nodes, three participants in the supraomohyoid neck dissection group with histologically positive nodes underwent the modified classical neck dissection instead.
In Bier 1994, 104 participants with either clinically negative or positive but movable neck nodes were randomised to either a radical neck dissection or a selective neck dissection where the platysma, sternocleidomastoid muscle, internal jugular vein, and accessory nerve were left in place. Primary tumours were in the oral cavity.
Primary outcomes
Overall survival (or total mortality)
There was very low‐certainty evidence of little or no difference in the HR for mortality between radical and selective neck dissection in BHNCSG 1998 (HR 1.14, 95% CI 0.70 to 1.86; P = 0.60; 148 participants; Analysis 2.1) and in Bier 1994 (HR 0.87, 95% CI 0.41 to 1.83; P = 0.71; 104 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2: Elective radical neck dissection (ND) versus elective selective ND, Outcome 1: Total mortality
Disease‐free survival (or new disease, progression, and mortality)
Only Bier 1994 reported disease‐free survival. There was very low‐certainty evidence of little or no difference between radical and selective neck dissection in the HR for new disease, progression, and mortality (HR 0.57, 95% CI 0.29 to 1.11; P = 0.10; 104 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Elective radical neck dissection (ND) versus elective selective ND, Outcome 2: New disease, progression, and mortality
Locoregional recurrence
Neither trial reported locoregional recurrence.
Recurrence
Only BHNCSG 1998 provided binary data for recurrence at a fixed time point (five years). There was very low‐certainty evidence of little or no difference between radical and selective neck dissection in recurrence (RR 1.21, 95% CI 0.63 to 2.33; P = 0.56; 143 participants; Analysis 2.3). Bier 1994 reported recurrence at variable follow‐up times, so we were unable to use the data.
2.3. Analysis.

Comparison 2: Elective radical neck dissection (ND) versus elective selective ND, Outcome 3: Recurrence
Secondary outcomes
Adverse events associated with treatment
BHNCSG 1998 reported the following adverse events: flap necrosis, wound infection, fistula, vascular rupture, haematoma, seroma, and chyle fistula. There were no complications in 45/76 participants in the modified radical neck dissection group and in 54/72 participants in the supraomohyoid neck dissection group. There were two postoperative deaths in the modified radical neck dissection group and one in the supraomohyoid neck dissection group.
Quality of life
Neither trial reported quality of life.
Direct and indirect costs to patients and health services
Neither trial reported costs.
Participant satisfaction
Neither trial reported participant satisfaction.
Comparison 3: superselective neck dissection versus selective neck dissection
See Table 3.
Two trials evaluated superselective neck dissection versus selective neck dissection in participants with oral cavity cancer and clinically negative neck nodes (Pandey 2018; Rastogi 2018). Both were small studies: Pandey 2018 included 32 people and Rastogi 2018 included 40 people.
Primary outcomes
Overall survival (or total mortality)
Neither study reported overall survival.
Disease‐free survival (or new disease, progression, and mortality)
Only Pandey 2018 reported disease‐free survival. At a median of 36 months of follow‐up, disease‐free survival was 83% in the superselective neck dissection group and 91% in the selective neck dissection group; the difference in survival between the two groups was not statistically significant (P = 0.69; very low‐certainty evidence). Due to variable follow‐up, we were unable to analyse these data.
Locoregional recurrence
Pandey 2018 found no difference in locoregional failure between the groups: three of 12 participants in the superselective neck dissection group and three of 20 participants in the selective neck dissection group had locoregional failure.
Rastogi 2018 investigated locoregional recurrence for 2.5 years using a survival analysis regression model (Kaplan‐Meier); however, we were unable to calculate HRs from the data provided. The study report stated "the P value by Kaplan‐Meier survival analysis was less than.05. Therefore, the SSND (super selective) group showed a lower rate of recurrence compared with the SND (selective group (P < .5)."
The findings from both studies were inconclusive (very low‐certainty evidence).
Recurrence
Only Pandey 2018 reported recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Secondary outcomes
Adverse events associated with treatment
Neither study reported adverse events.
Quality of life
Both studies investigated shoulder function. Pandey 2018 reported significant differences in shoulder abduction between one and six months favouring the superselective group, but not at 12 months. There were no reported differences for shoulder flexion. Rastogi 2018 analysed data for shoulder morbidity subjectively and objectively. The results for both measures showed less shoulder morbidity and improved quality of life for superselective neck dissection compared with selective neck dissection. Only P values were presented, so we were unable to use the data.
Rastogi 2018 performed subjective analysis measuring shoulder morbidity using the Neck Dissection Quality of Life (ND‐QOL) questionnaire. Data showed that the mean score for the superselective neck dissection group (30.4) was significantly higher (P = 0.01) than for the selective neck dissection group (19.4).
Pandey 2018 found no significant difference in quality of life between the two groups. Rastogi 2018 stated that quality of life measured by the ND‐QOL was significantly better in the superselective neck dissection group than in the selective neck dissection group. There were no other data presented to confirm this position, other than the scores on the ND‐QOL questionnaire.
The findings from both studies were inconclusive (very low‐certainty evidence).
Direct and indirect costs to patients and health services
Neither study reported costs.
Participant satisfaction
Neither study reported participant satisfaction.
Comparison 4: supraomohyoid neck dissection versus modified radical neck dissection
See Table 4.
One trial evaluated supraomohyoid neck dissection versus modified radical neck dissection (Guo 2014). Participants had oral cavity or oropharyngeal cancer, T1 to T4 tumours, and neck nodes clinically staged as N0 to N2.
Primary outcomes
Overall survival (or total mortality)
Guo 2014 reported overall survival during the follow‐up period but with different follow‐up times, so we could not analyse the data. The study report stated "During the follow‐up period 113 (35.1%) of the 322 patients died (SOND [supraomohyoid neck dissection]: 53 cases, MRND [modified radical neck dissection]: 60 cases)".
Disease‐free survival (or new disease, progression, and mortality)
Guo 2014 did not report disease‐free survival. It did report three‐year disease‐specific survival rates, but disease‐specific survival was not an outcome of this review.
Locoregional recurrence
Guo 2014 reported locoregional recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Recurrence
Guo 2014 reported recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Secondary outcomes
Adverse events associated with treatment
Guo 2014 provided limited information on adverse events. The study report stated "There was a significant difference in the complication rates between both groups (SOND [supraomohyoid neck dissection] group vs. MRND [modified radical neck dissection] group: 13.0% vs. 21.9%, P = 0.040). The most frequent complication was wound infection." The report summarised other significant complications.
Quality of life
Guo 2014 assessed University of Washington Quality of Life Questionnaire (UW‐QOL) scores for all disease‐free survivors at one year after treatment (Deleyiannis 1997); scores from nine disease‐specific domains appeared to show that supraomohyoid neck dissection was superior to modified radical neck dissection in the domains of pain relief (78.8% versus 75.2%; P = 0.013) and shoulder function (81.1% versus 68.1%; P < 0.001), but not in any of the other domains (very low‐certainty evidence).
Direct and indirect costs to patients and health services
Guo 2014 did not report costs.
Participant satisfaction
Guo 2014 did not report participant satisfaction.
Comparison 5: sentinel node biopsy versus elective neck dissection
See Table 5.
One large multicentre trial evaluated sentinel node biopsy versus elective neck dissection in people with unclear nodal status (Garrel 2020). In the biopsy group, the participants with a positive biopsy received a neck dissection.
Primary outcomes
Overall survival (or total mortality)
Garrel 2020 provided very low‐certainty evidence of little or no difference between sentinel node biopsy and elective neck dissection in total mortality at two years (HR 0.95, 95% 0.89 to 1.02; P = 0.15; 279 participants; Analysis 3.1), and low‐certainty evidence of little or no difference at five years (HR 1.00, 95% CI 0.90 to 1.11; P = 1.00; 279 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Sentinel node biopsy versus elective neck dissection (ND), Outcome 1: Total mortality
Disease‐free survival (or new disease, progression, and mortality)
Garrel 2020 provided very low‐certainty evidence of little or no difference between sentinel node biopsy and elective neck dissection in new disease, progression, and mortality at two years (HR 0.97, 95% 0.92 to 1.03; P = 0.32; 279 participants; Analysis 3.2), and low‐certainty evidence of little or no difference at five years (HR 0.98, 95% CI 0.90 to 1.07; P = 0.66; 279 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Sentinel node biopsy versus elective neck dissection (ND), Outcome 2: New disease, progression, and mortality
Locoregional recurrence
Garrel 2020 provided very low‐certainty evidence of little or no difference between sentinel node biopsy and elective neck dissection in locoregional recurrence at two years (HR 0.98, 95% 0.88 to 1.09; P = 0.72; 279 participants; Analysis 3.3), and low‐certainty evidence of little or no difference at five years (HR 1.04, 95% CI 0.91 to 1.19; P = 0.58; 279 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Sentinel node biopsy versus elective neck dissection (ND), Outcome 3: Locoregional recurrence
Recurrence
Garrel 2020 reported recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Secondary outcomes
Adverse events associated with treatment
Garrel 2020 did not report adverse events.
Quality of life
Garrel 2020 reported neck‐shoulder functional morbidity, assessed by a self‐report neck and shoulder impairment questionnaire and arm abduction test. Up to 12 months after surgery, the scores of some items on the neck‐shoulder questionnaire favoured the sentinel biopsy (limited in ability to do your work or leisure activities, limited in ability to reach objects above your head); however, there were no statistically significant differences at 24 months (data not presented). The arm abduction test showed significant benefit for the sentinel biopsy participants at two, four, and six months; however, this benefit was not apparent at 12 and 18 months.
Direct and indirect costs to patients and health services
Garrel 2020 did not report costs.
Participant satisfaction
Garrel 2020 did not report participant satisfaction.
Comparison 6: PET‐CT following chemoradiotherapy versus planned neck dissection before or after chemoradiotherapy
See Table 6.
Mehanna 2017 evaluated PET‐CT‐guided surveillance (with neck dissection only if no response or incomplete response to chemoradiotherapy) versus planned neck dissection (either before or after chemoradiotherapy) in 564 people with nodal disease (stage N2 or N3).
Primary outcomes
Overall survival (or total mortality)
There was moderate‐certainty evidence of little or no difference between PET‐CT following chemoradiotherapy and planned neck dissection before or after chemoradiotherapy in terms of their effect on total mortality (HR 0.92, 95% CI 0.65 to 1.31; P = 0.66, 564 participants; Analysis 4.1).
4.1. Analysis.

Comparison 4: PET‐CT following chemoradiotherapy versus planned neck dissection (ND) before or after chemoradiotherapy, Outcome 1: Total mortality
Disease‐free survival (or new disease, progression, and mortality)
Mehanna 2017 provided limited data that we were unable to analyse. The study report stated "Disease‐specific mortality and mortality from other causes did not differ significantly between the two groups (P = 0.80 and 0.41, respectively, according to Gray's test for differences)."
Locoregional recurrence
We calculated HRs from the two‐year locoregional recurrence rates provided in Mehanna 2017. There was moderate‐certainty evidence of little or no difference between PET‐CT following chemoradiotherapy and planned neck dissection before or after chemoradiotherapy in terms of their effect on locoregional recurrence (HR 1.00, 95% CI 0.94 to 1.06; P = 1.00; 564 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: PET‐CT following chemoradiotherapy versus planned neck dissection (ND) before or after chemoradiotherapy, Outcome 2: Locoregional recurrence
Recurrence
Mehanna 2017 provided limited data that we were unable to analyse. The study report stated "Documented recurrence in the nodes only (without concurrent disease in the primary site) occurred in 1 patient in the planned‐surgery group and in 3 patients in the surveillance group. Distant metastases were identified in 23 patients in the planned‐surgery group and in 21 patients in the surveillance group."
Secondary outcomes
Adverse events associated with treatment
There were 22 surgical complications after neck dissection in the surveillance group compared with 83 in the planned‐surgery group.
Quality of life
Mehanna 2017 assessed quality of life using the European Organisation for Research and Treatment of Cancer 30‐item Quality of Life Questionnaire (EORTC QLQ‐C30). There was a small difference in mean change in global health status scores in favour of the PET‐CT group at six months after randomisation relative to the planned surgery group (difference in mean change 4.94; P = 0.09). This difference narrowed at 12 months (difference in mean change 3.03; P = 0.09) and was no longer apparent at 24 months (difference in mean change –0.81; P = 0.85).
Direct and indirect costs to patients and health services
Mehanna 2017 undertook an economic evaluation consisting of two components: a within‐trial analysis and a decision analytic model. The primary analysis was conducted from a UK National Health Service (NHS) secondary care perspective (i.e. including NHS hospital costs). Compared with planned neck dissection, PET‐CT surveillance produced an incremental net health benefit of 0.16 quality‐of‐life years (QALYs; 95% CI 0.03 to 0.28) over the trial period, and 0.21 QALYs (95% CI 0.41 to 0.85) over the modelled lifetime horizon.
Participant satisfaction
Mehanna 2017 did not report participant satisfaction.
Comparison 7: surgery plus radiotherapy versus radiotherapy alone
See Table 7.
One trial evaluated surgery plus postoperative radiotherapy versus radiotherapy alone (Robertson 1998). Participants in the surgery group had wide local excision of the primary tumour together with either a radical neck dissection or a more selective neck dissection at the discretion of the surgeon. The investigators intended to recruit 175 people with oral cavity or oropharyngeal cancer (neck nodes clinically staged as N0 to N2) to each arm of the trial, but after recruiting 35 participants, the trial was stopped due to the high death rate in the radiotherapy alone arm.
Primary outcomes
Overall survival (or total mortality)
Robertson 1998 provided data from an interim analysis of 35 participants after 23 months. The evidence for total mortality favoured the surgery group (HR 0.24, 95% CI 0.10 to 0.59; P = 0.002; 35 participants; very low‐certainty evidence; Analysis 5.1). This estimate should be interpreted with extreme caution for several reasons. First, the authors stated that "the difference in survival is likely to be inflated" due to the small number of participants in the analysis. Second, only 41% of participants in the radiotherapy only arm received their radiotherapy as planned due to problems with faulty machines, and there were several other protocol violations in the trial. Finally, in the surgery plus radiotherapy arm, 50% of participants received radiotherapy as planned, but 12% received neither surgery to the mandible nor neck dissection.
5.1. Analysis.

Comparison 5: Surgery plus radiotherapy (RT) versus RT alone, Outcome 1: Total mortality
Disease‐free survival (or new disease, progression, and mortality)
Robertson 1998 did not report disease‐free survival.
Locoregional recurrence
Robertson 1998 did not report locoregional recurrence.
Recurrence
Robertson 1998 did not report recurrence.
Secondary outcomes
Adverse events associated with treatment
Robertson 1998 reported the following severe acute adverse events in both groups: subcutaneous fibrosis, telangiectasia (1 cm² to 4 cm²), and moderate‐to‐severe oedema, xerostomia, trismus, and dysphagia. Subcutaneous fibrosis was more prevalent in the surgery plus radiotherapy group (P = 0.042), but the prevalence of other adverse events appeared to be similar in both groups (very low‐certainty evidence).
Quality of life
Robertson 1998 did not report quality of life.
Direct and indirect costs to patients and health services
Robertson 1998 did not report costs.
Participant satisfaction
Robertson 1998 did not report participant satisfaction.
Comparison 8: surgery versus radiotherapy alone
See Table 8.
One trial compared transoral robotic surgery (TORS) with radiotherapy in people with oropharyngeal cancer and nodal status of N0 to N2 (Nichols 2019). Concurrent chemotherapy was recommended for participants in the radiotherapy group with node‐positive disease. Participants in the TORS group underwent selective neck dissections at the time of surgery or within two weeks.
Primary outcomes
Overall survival (or total mortality)
Nichols 2019 provided low‐certainty evidence of little or no difference between TORS and radiotherapy in terms of their effect on total mortality (HR 0.83, 95% CI 0.09 to 7.46; P = 0.87; 68 participants; Analysis 6.1).
6.1. Analysis.

Comparison 6: Surgery versus radiotherapy (RT) alone, Outcome 1: Mortality
Disease‐free survival (or new disease, progression, and mortality)
Nichols 2019 provided low‐certainty evidence of little or no difference between TORS and radiotherapy in terms of their effect on new disease, progression, and mortality (HR 1.07, 95% CI 0.27 to 4.22; P = 0.92; 68 participants; Analysis 6.2).
6.2. Analysis.

Comparison 6: Surgery versus radiotherapy (RT) alone, Outcome 2: New disease, progression, and mortality
Locoregional recurrence
Nichols 2019 reported locoregional recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Recurrence
Nichols 2019 reported recurrence, but the participants had variable follow‐up times, so we were unable to use the data.
Secondary outcomes
Adverse events associated with treatment
Nichols 2019 provided some detailed information on over 60 adverse events in the text. The incidence of treatment‐related grade 2 or higher adverse events was similar: 31/34 participants in the radiotherapy group compared with 33/34 participants in the TORS group (RR 0.94, 95% CI 0.83 to 1.06). Participants in the radiotherapy group had more hearing loss, neutropenia, constipation, and tinnitus; whereas oral bleeding and trismus were more common in the TORS plus neck dissection group. There were too many outcomes measured to draw any sensible conclusions (very low‐certainty evidence).
Quality of life
Nichols 2019 did not report quality of life.
Direct and indirect costs to patients and health services
Nichols 2019 did not report costs.
Participant satisfaction
Nichols 2019 did not report participant satisfaction.
Comparison 9: surgery plus adjuvant radiotherapy versus chemotherapy
See Table 9.
One trial evaluated neck dissection surgery plus adjuvant radiotherapy versus chemotherapy in 119 participants with histologically confirmed resectable stage III/IV head and neck SCC (excluding nasopharynx and salivary gland SCC), with and without nodal disease (Iyer 2015). The median follow‐up for surviving participants was 13 years. As 48% of participants had oral cavity and oropharyngeal cancer, we were only able to use data from the oral cavity cancer subgroup (32 participants) and the oropharyngeal cancer subgroup (25 participants). The text stated "there were no differences noted between the treatment arms with regard to cancers of the oropharynx, larynx, and hypopharynx"; however, it is unclear which outcomes this statement relates to, and the study provided no other information on the oropharynx subgroup.
Primary outcomes
Overall survival (or total mortality)
Iyer 2015 did not report overall survival data for the oral cavity cancer subgroup; however, the text stated "For the oral cavity, survival was significantly better in patients who underwent surgery and RT compared with the CRT group".
Disease‐free survival (or new disease, progression, and mortality)
Iyer 2015 did not report disease free survival.
Locoregional recurrence
Iyer 2015 reported that locoregional recurrence‐free survival in the oral cavity cancer subgroup was not statistically significant between the groups (P = 0.355), but there were no useable data (very low‐certainty evidence).
Recurrence
Iyer 2015 did not report recurrence for the oral cavity cancer subgroup.
Secondary outcomes
Adverse events associated with treatment
Iyer 2015 did not report adverse events.
Quality of life
Iyer 2015 did not report quality of life.
Direct and indirect costs to patients and health services
Iyer 2015 did not report costs.
Participant satisfaction
Iyer 2015 did not report participant satisfaction.
Discussion
Summary of main results
We undertook this systematic review to evaluate whether treatment with surgery improves outcomes for people with oral cavity and oropharyngeal cancers, and to determine which type of surgery is most effective for treating people with oral cavity and oropharyngeal cancers. We included 15 RCTs with a combined total of 2820 randomised participants. Approximately 2074 of these participants had oral cavity cancers. Most trials were at high or unclear risk of bias overall.
No trials compared different surgical approaches to the removal of the primary tumour. Five trials evaluated the timing of neck dissection surgery in the course of treatment, and five trials evaluated the extent of neck dissection.
Five trials evaluated elective neck dissection versus therapeutic (delayed) neck dissection (Table 1). All participants had oral cavity cancers, specifically tongue or floor of mouth tumours, and clinically negative neck nodes. All the evidence was graded as moderate‐certainty. Elective neck dissection compared with therapeutic neck dissection probably improves overall survival, disease‐free survival, locoregional recurrence, and recurrence; but is probably associated with more adverse events (although these were generally of lower grade in the included trials). The results for this comparison may reflect the likely improvements in background standards of care associated with oral cancer surgery and the increasing use of adjuvant chemoradiation after surgery in the newer trials (D'Cruz 2015; Hutchison 2019). When we combined only these two recent trials, the pooled HR for total mortality was 0.66 (95% CI 0.49 to 0.89; P = 0.006), and the pooled HR for new disease, progression, and mortality was 0.53 (95% CI 0.37 to 0.77; P < 0.001); both results clearly favour elective neck dissection.
Two trials compared elective radical (comprehensive) neck dissection with a selective neck dissection in people with oral cavity cancers (Table 2). One trial included only participants with clinically negative neck nodes and the other included those with movable positive neck nodes as well. There may be no difference between the interventions in terms of overall survival, disease‐free survival, and recurrence, but the evidence is very uncertain.
Two trials evaluated superselective neck dissection versus selective neck dissection but provided no usable data (Table 3).
One trial evaluated supraomohyoid neck dissection versus modified radical neck dissection (Table 4). We were unable to use the data for the primary outcomes. The evidence was very uncertain for adverse events, with more complications, pain, and poorer shoulder function in the modified radical neck dissection group.
One large multicentre trial evaluated sentinel node biopsy versus elective neck dissection (Table 5). There may be no difference between the interventions in overall survival, disease‐free survival, and locoregional recurrence. The study did not fully report recurrence data and stated "no adverse events were judged to be related to the interventions".
One trial evaluated PET‐CT (with neck dissection only if no/incomplete response to chemoradiotherapy identified) versus planned neck dissection before or after chemoradiotherapy (Table 6). There is probably no difference in overall survival and locoregional recurrence between the interventions. The trial did not provide usable data for the other outcomes.
One trial evaluated surgery plus postoperative radiotherapy versus radiotherapy alone (Table 7). It was stopped early due to an unacceptably high death rate in the radiotherapy alone group. Surgery plus radiotherapy compared with radiotherapy alone may improve overall survival, but the evidence is very uncertain. Subcutaneous fibrosis was more prevalent in the surgery plus radiotherapy group, and both groups had similar prevalences of other adverse events, but the evidence was very uncertain. The trial did not report other outcomes of interest.
One small trial evaluated surgery versus radiotherapy in people with oropharyngeal cancer (Table 8). There may be no difference between the interventions in overall survival or disease‐free survival. The trial did not report locoregional recurrence or recurrence. Over 60 adverse events were described, but there were too many outcomes measured to draw any reliable conclusions.
One trial compared surgery plus adjunctive radiotherapy versus chemoradiotherapy (Table 9). The study reported that survival was significantly better in participants who underwent surgery and radiotherapy compared with the chemoradiotherapy group, but there were no useable data for this outcome (or for any other outcomes).
Overall completeness and applicability of evidence
This review included only studies that directly compared different surgical treatment modalities against one another, or compared surgery to a different treatment regimen such as radiotherapy, chemotherapy, or immunotherapy.
For trials to be included, they had to have more than 50% participants with oral cavity or oropharyngeal cancer, or provide separate data for participants with oral cavity or oropharyngeal cancer. In this version of the review, 2074 of 2820 participants had oral cavity cancers, most commonly in the tongue or floor of mouth. Participant numbers ranged from 20 to 596 in the 15 included trials, and people were recruited between 1966 and 2020. This update includes two new large multicentre clinical trials published since 2017. An important factor to consider when interpreting results is whether the trials are single‐centre or multicentre; multicentre trials may reflect a wider range of surgical experience and provide more generalisable findings.
For the five trials in the primary comparison that compared elective neck dissection with therapeutic (delayed) neck dissection, the reporting was generally good. Four of these trials reported overall survival and locoregional control, and five reported disease‐free survival. Two of these trials provided information on adverse events. Only two other studies (included in the other eight comparisons) reported harms or adverse events of treatment, but neither presented outcomes per person (BHNCSG 1998; Robertson 1998). Incorporation of quality of life outcomes into randomised trials is essential if the true benefits and harms of different types of surgery are to be evaluated. It is noteworthy that while some trials included in this review reported participant dropouts due to refusal to undergo the allocated surgical treatment, there was no information on the quality of life of these people compared with those who stayed in the trials.
Only one of the included studies, published in 2019, focused purely on people with oropharyngeal cancer. Since the late 2000s, the percentage of people with oropharyngeal cancer who test positive for HPV has increased steadily. It is now recognised that HPV status is an important factor in the prognosis of people with oropharyngeal cancer (Adelstein 2009; Brizel 2011). If future updates of this review include sufficient data, we will undertake a subgroup analysis for the surgical management of HPV‐related oropharyngeal cancer and the surgical management of non‐HPV related oropharyngeal cancer. In addition, robotic surgery is now being used for oropharyngeal cancer. We anticipate further trials of this technique, and will consider restructuring the review to reflect newly emerging comparisons. It may be appropriate to split the review in the future to cover oral cavity cancer and oropharyngeal cancer separately.
Quality of the evidence
The overall certainty of the evidence included in this systematic review was moderate for the first comparison (elective neck dissection versus therapeutic neck dissection) and for some outcomes in two other comparisons (supraomohyoid neck dissection versus modified radical neck dissection; PET‐CT following chemoradiotherapy versus planned neck dissection before or after chemoradiotherapy). The evidence for the other comparisons was low‐ or very low‐certainty. Trials at low risk of selection bias, attrition bias, reporting bias, and other bias were judged at overall low risk of bias for objective outcomes such as total mortality. Only three of the included studies met these criteria. No trials reported blinding of participants or outcome assessors. We recognise that blinding is difficult to maintain in trials of surgery, and it may not be possible (or ethical) to blind trial participants. The clinicians treating participants likely performed many of the outcome assessments.
Participants were recruited over five decades (1966 to 2020). Surgical and non‐surgical treatments for oral cavity and oropharyngeal cancers have advanced substantially since the 2000s. Further objective assessments of current surgical treatments for these cancers are needed to inform both patients and clinicians about the benefits and risks of different treatments.
Potential biases in the review process
To minimise risk of bias related to study selection, we used a comprehensive search strategy with no language restrictions, and we clearly specified inclusion criteria for the review in line with the other reviews in this series (Chan 2015; Glenny 2010; Parmar 2021).
Figure 3 shows the review authors' judgements about each risk of bias item presented as percentages across all included studies. We applied the low risk of bias judgement for objective outcomes (based on overall survival) to all 15 studies, although not all studies evaluated overall survival. This represents a source of bias in the review process.
Agreements and disagreements with other studies or reviews
One of the newly included studies reported a meta‐analysis of overall survival, disease‐free survival, and locoregional control for the primary comparison of elective neck dissection versus therapeutic neck dissection (Hutchison 2019 (SEND)). The findings favour elective neck dissection, in line with this review.
We found two reviews that evaluated treatment of neck dissection in the surgical treatment of oral cavity cancer (Fasunla 2011; Kowalski 2007). Kowalski 2007 investigated dichotomous outcomes (percentages in each group) in three RCTs (Fakih 1989; Kligerman 1994; Vandenbrouck 1980). This previous review included no meta‐analysis and only noted the summary outcome estimates, without examining their variance. The conclusions were based on "vote‐counting" and "suggest that elective neck dissection offers advantages in terms of overall, cancer specific and disease‐free survival". Fasunla 2011 reviewed the same RCTs plus one other (Yuen 2009), and reported the dichotomous outcome of disease‐specific death after approximately three years of follow‐up. The RR of disease‐specific death favoured elective neck dissection (RR 0.57, 95% CI 0.36 to 0.89).
We chose to evaluate overall survival/total mortality because we believe it is the most important outcome for patients, and we used HRs where possible, as they have the advantage of incorporating all available information, including data from participants who did not complete the trial. We look forward to adding data from ongoing trials to the next update of this review, especially trials on oropharyngeal SCC induced by HPV
Authors' conclusions
Implications for practice.
We found moderate‐certainty evidence based on five trials that elective neck dissection of clinically negative neck nodes at the time of removal of the primary oral cavity tumour is superior to therapeutic neck dissection, with increased survival and disease‐free survival, and reduced locoregional recurrence.
There was moderate‐certainty evidence from one trial of no difference between positron emission tomography–computed tomography (PET‐CT) following chemoradiotherapy versus planned neck dissection for overall survival or locoregional recurrence.
The evidence for each of the other seven comparisons came from only one or two studies and was assessed as low‐ or very low‐certainty.
Implications for research.
This review identified the following areas for improvement in future research into the surgical treatment of oral or oropharyngeal tumours.
Trial authors should follow the CONSORT guidelines when reporting on their trials. Ideally, trials should report hazard ratios with 95% confidence intervals for survival data, or present data that allow for the calculation of this estimate of effect.
Health‐related quality of life, adverse events, health economics, and resource use are important outcome measures that should be integral to all trials of oral cavity and oropharyngeal cancers.
There should be standardised and consistent reporting of adverse events and morbidity associated with treatment, with results reported per participant.
Future trials of oral cavity and oropharyngeal cancers should report data based on the location of the primary tumour.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2023 | New search has been performed | Updated search run |
| 31 August 2023 | New citation required and conclusions have changed | Review updated with four new studies (662 randomised participants, 629 analysed participants); one study in previous version has now been excluded due to inappropriate intervention (no surgery of primary tumour). Conclusions of review changed, supporting elective neck dissection over therapeutic (delayed) neck dissection. Search strategy for ClinicalTrial.gov amended to include oropharyngeal cancer. Protocol change: we excluded studies that did not measure any of our primary outcomes. Risk of bias assessment amended to differentiate between objective and subjective outcomes. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 6 April 2020 | Amended | Minor edit to description of GRADE in 'Summary of findings' tables |
| 4 December 2018 | New citation required and conclusions have changed | Conclusions for comparisons already included remain the same, and have low‐ to very low‐certainty evidence, but new comparisons have been added. |
| 20 December 2017 | New search has been performed | Search updated and five new studies included. New comparisons added. New lead author and byline. |
| 4 July 2011 | New search has been performed | Searches updated to 17 February 2011. |
| 4 July 2011 | New citation required and conclusions have changed | Two new trials added. New comparisons, and conclusions. Twenty‐four previously included trials now moved to other oral cancer reviews on chemotherapy and radiotherapy. |
| 28 April 2009 | Amended | Minor changes to the data. |
| 20 June 2008 | Amended | Converted to new review format. |
Acknowledgements
Cochrane Oral Health supported the authors in the development of this systematic review. The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Nicole Skoetz, University of Cologne and University Hospital Cologne, Germany
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Marwah Anas El‐Wegoud, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Julia Turner, Cochrane Central Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Iain Hutchison, Professor of Oral and Maxillofacial Surgery at Saint Bartholomews and the Royal London School of Medicine and Dentistry, Queen, Mary University, London (clinical review); Allan Hackshaw, UCL Cancer Institute, UCL (clinical review); Brian Stafford, Community representative, Director of Patient Care, ISCOM Switzerland (consumer review); Jennifer Hilgart, Cochrane (methods review). One additional peer reviewer provided search peer‐review but chose not to be publicly acknowledged.
We would also like to thank Anne Littlewood for carrying out the searches for the review and Laura MacDonald for supporting the development of the review. We would like to recognise the support of peer reviewers on previous versions of this review.
We would like to thank all trialists who provided additional information for this and previous versions of the review.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see https://oralhealth.cochrane.org/trials.
#1 MESH DESCRIPTOR Head and Neck Neoplasms AND INREGISTER #2 MESH DESCRIPTOR Mouth Neoplasms AND INREGISTER #3 MESH DESCRIPTOR Gingival Neoplasms AND INREGISTER #4 MESH DESCRIPTOR Palatal Neoplasms AND INREGISTER #5 MESH DESCRIPTOR Tongue Neoplasms AND INREGISTER #6 ((cancer* or tumour* or tumor* or neoplas* or malignan* or carcinoma* or metatasta*) AND (oral* or intra‐oral* or intraoral* or "intra oral*" or gingiva* or oropharyn* or mouth* or tongue* or cheek* or gum* or palatal* or palate* or "head and neck")) AND INREGISTER #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MESH DESCRIPTOR Surgical Procedures, Operative EXPLODE ALL AND INREGISTER #9 (surgery or surgical or operat*):ti,ab AND INREGISTER #10 (dissect* NEAR2 neck*):ti,ab AND INREGISTER #11 (excision or excise or resect*):ti,ab AND INREGISTER #12 MESH DESCRIPTOR Lymph Node Excision EXPLODE ALL AND INREGISTER #13 MESH DESCRIPTOR Oral Surgical Procedures AND INREGISTER #14 (lymphadenectom* or glossectom* or maxillectom* or micrographic or mandibulectom* or hemi‐mandibulectom* or hemimandibulectom* or oropharyngectom*):ti,ab AND INREGISTER #15 ("transoral robotic surg*" or "trans oral robotic surg*" or "trans‐oral robotic surg*" or TORS) #16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #7 and #16
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MESH DESCRIPTOR Head and Neck Neoplasms AND CENTRAL:TARGET #2 MESH DESCRIPTOR Mouth Neoplasms AND CENTRAL:TARGET #3 MESH DESCRIPTOR Gingival Neoplasms AND CENTRAL:TARGET #4 MESH DESCRIPTOR Palatal Neoplasms AND CENTRAL:TARGET #5 MESH DESCRIPTOR Tongue Neoplasms AND CENTRAL:TARGET #6 ((cancer* or tumour* or tumor* or neoplas* or malignan* or carcinoma* or metatasta*) NEAR5 (oral* or intra‐oral* or intraoral* or "intra oral*" or gingiva* or oropharyn* or mouth* or tongue* or cheek* or gum* or palatal* or palate* or "head and neck")) AND CENTRAL:TARGET #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MESH DESCRIPTOR Surgical Procedures, Operative EXPLODE ALL AND CENTRAL:TARGET #9 (surgery or surgical or operat*):ti,ab AND CENTRAL:TARGET #10 (dissect* NEAR2 neck*):ti,ab AND CENTRAL:TARGET #11 (excision or excise or resect*):ti,ab AND CENTRAL:TARGET #12 MESH DESCRIPTOR Lymph Node Excision EXPLODE ALL AND CENTRAL:TARGET #13 MESH DESCRIPTOR Oral Surgical Procedures AND CENTRAL:TARGET #14 (lymphadenectom* or glossectom* or maxillectom* or micrographic or mandibulectom* or hemi‐mandibulectom* or hemimandibulectom* or oropharyngectom*):ti,ab AND CENTRAL:TARGET #15 ("transoral robotic surg*" or "trans oral robotic surg*" or "trans‐oral robotic surg*" or TORS):ti,ab AND CENTRAL:TARGET #16 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 #17 #7 and #16
Appendix 3. MEDLINE Ovid search strategy
The subject search (1 to 17) was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in MEDLINE (as described in Lefebvre 2022, box 3b) (lines 18 to 28).
1. "Head and neck neoplasms"/ 2. "Mouth neoplasms"/ 3. "Gingival neoplasms"/ 4. "Palatal neoplasms"/ 5. "Tongue neoplasms"/ 6. ((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).ti,ab. 7. or/1‐6 8. exp Surgical procedures, operative/ 9. (surgery or surgical or operat$).ti,ab. 10. (dissect$ adj2 neck$).ti,ab. 11. (excision or excise or resect$).ti,ab. 12. exp Lymph node excision/ 13. Oral surgical procedures/ 14. (lymphadenectom$ or glossectom$ or maxillectom$ or micrographic or mandibulectom$ or hemi‐mandibulectom$ or hemimandibulectom$ or oropharyngectom$).ti,ab. 15. ("transoral robotic surg*" or "trans oral robotic surg*" or "trans‐oral robotic surg*" or TORS).ti,ab. 16. or/8‐15 17. 7 and 16
18. randomized controlled trial.pt. 19. controlled clinical trial.pt. 20. randomized.ab. 21. placebo.ab. 22. drug therapy.fs. 23. randomly.ab. 24. trial.ab. 25. groups.ab. 26. or/18‐25 27. exp animals/ not humans.sh. 28. 26 not 27
29. 17 and 28
Appendix 4. Embase Ovid search strategy
The subject search (lines 1 to 16) was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in Embase (as described in Lefebvre 2022, box 3e) (lines 17 to 51).
1. "Head and neck tumor"/ 2. "Mouth tumor"/ 3. "Gingiva tumor"/ 4. "Jaw tumor"/ 5. "Tongue tumor"/ 6. ((cancer$ or tumour$ or tumor$ or neoplas$ or malignan$ or carcinoma$ or metatasta$) adj5 (oral$ or intra‐oral$ or intraoral$ or "intra oral$" or gingiva$ or oropharyn$ or mouth$ or tongue$ or cheek$ or gum$ or palatal$ or palate$ or "head and neck")).ti,ab. 7. or/1‐6 8. exp Oral surgery/ 9. (surgery or surgical or operat$).ti,ab. 10. (dissect$ adj2 neck$).ti,ab. 11. (excision or excise or resect$).ti,ab. 12. "Lymph node dissection"/ 13. (lymphadenectom$ or glossectom$ or maxillectom$ or micrographic or mandibulectom$ or hemi‐mandibulectom$ or hemimandibulectom$ or oropharyngectom$).ti,ab. 14. ("transoral robotic surg*" or "trans oral robotic surg*" or "trans‐oral robotic surg*" or TORS).ti,ab. 15. or/8‐14 16. 7 and 15
17. Randomized controlled trial/
18. Controlled clinical study/
19. random$.ti,ab.
20. randomization/
21. intermethod comparison/
22. placebo.ti,ab.
23. (compare or compared or comparison).ti.
24. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
25. (open adj label).ti,ab.
26. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
27. double blind procedure/
28. parallel group$1.ti,ab.
29. (crossover or cross over).ti,ab.
30. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
31. (assigned or allocated).ti,ab.
32. (controlled adj7 (study or design or trial)).ti,ab.
33. (volunteer or volunteers).ti,ab.
34. human experiment/
35. trial.ti.
36. or/17‐35
37. random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
38. Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
39. (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
40. (Systematic review not (trial or study)).ti.
41. (nonrandom$ not random$).ti,ab.
42. "Random field$".ti,ab.
43. (random cluster adj3 sampl$).ti,ab.
44. (review.ab. and review.pt.) not trial.ti.
45. "we searched".ab. and (review.ti. or review.pt.)
46. "update review".ab.
47. (databases adj4 searched).ab.
48. (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
49. Animal experiment/ not (human experiment/ or human/)
50. or/37‐49
51. 36 not 50
Appendix 5. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
Advanced search:
“oral cancer” AND surgery in "Condition or Diesease"
Limited to interventional studies under "Study Type"
We also searched for:
"oropharyngeal cancer" AND surgery in "Condition or Diesease"
Limited to interventional studies under "Study Type"
Appendix 6. World Health Organization International Clinical Trials Registry Platform search strategy
Advanced search: oral cancer
Data and analyses
Comparison 1. Elective neck dissection (ND) versus therapeutic (delayed) ND.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total mortality | 4 | Hazard Ratio (IV, Random, 95% CI) | 0.64 [0.50, 0.83] | |
| 1.2 New disease, progression, and mortality | 5 | Hazard Ratio (IV, Random, 95% CI) | 0.56 [0.45, 0.70] | |
| 1.3 Locoregional recurrence | 4 | Hazard Ratio (IV, Random, 95% CI) | 0.58 [0.43, 0.78] | |
| 1.4 Recurrence | 3 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.70] |
| 1.5 Total adverse events | 2 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.11, 1.54] |
Comparison 2. Elective radical neck dissection (ND) versus elective selective ND.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total mortality | 2 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.2 New disease, progression, and mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.3 Recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Sentinel node biopsy versus elective neck dissection (ND).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1.1 2 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1.2 5 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.2 New disease, progression, and mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.2.1 2 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.2.2 5 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Locoregional recurrence | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 2 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3.2 5 years | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 4. PET‐CT following chemoradiotherapy versus planned neck dissection (ND) before or after chemoradiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Total mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.92 [0.65, 1.31] | |
| 4.2 Locoregional recurrence | 1 | Hazard Ratio (IV, Random, 95% CI) | 1.00 [0.94, 1.06] |
Comparison 5. Surgery plus radiotherapy (RT) versus RT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Total mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.24 [0.10, 0.59] |
Comparison 6. Surgery versus radiotherapy (RT) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.09, 7.46] | |
| 6.2 New disease, progression, and mortality | 1 | Hazard Ratio (IV, Random, 95% CI) | 1.07 [0.27, 4.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BHNCSG 1998.
| Study characteristics | ||
| Methods | Location of trial: Brazil Number of centres: multicentre (8) Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: May 1990 to December 1993 Number randomised: 148 (all OC: 42% tongue, 33% FOM, 8% inferior gingiva, 17% RMT) Number analysed: 148 |
|
| Interventions |
MRND vs SOH Group 1 (n = 76): MRND: surgery conducted centripetally toward the submandibular triangle. Group 2 (n = 72): SOH: dissection performed to achieve a compartmental excision of levels I, II and III lymph nodes. Where a positive node was confirmed during the procedure, the operation was converted to an MRND. For both groups, PORT was indicated in cases with positive margins or positive lymph nodes (or both) in the specimen. RT was over 5 consecutive weeks to deliver a total dose of 50 Gy. All participants had primary tumour resection. |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: 5 years |
|
| Notes | HR data taken from Kaplan‐Meier graph (no numbers at risk). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were stratified by institution and laterality (unilateral or bilateral) and subsequently randomised." Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Groups appeared similar at baseline. No evidence of other potential sources of bias. |
Bier 1994.
| Study characteristics | ||
| Methods | Location of trial: Germany, Austria, and Switzerland Number of centres: multicentre Funding: not stated Trial ID: not stated (part of the German‐Austrian‐Swiss Association for Head and Neck Tumours (DOSAK)) |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: uncertain Number randomised: 167 (all OC: 37% tongue, 21% FOM, 16% RMT, 14% mandible, 8% maxilla, 3% cheek, 1% other) Number analysed: 104 |
|
| Interventions |
Radical ND vs selective ND Group 1 (n = 48): radical ND (ipsilateral) on the draining lymph nodes. Radical dissection designated as removal of:
Group 2 (n = 56): selective ND (ipsilateral) on the draining lymph nodes. Selective dissection designated as retention of the platysma, sternocleidomastoid muscle, internal jugular vein, and the accessory nerve. All participants underwent radical resection of the primary tumour. |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: 4 years |
|
| Notes | Preliminary report. ND was followed by RT or chemotherapy (or both) in participants not undergoing radical resection of the primary tumour and in participants with capsular rupture in ≥ 1 lymph node. These participants were not included in the analysis. HR data taken from Kaplan‐Meier graph (no numbers at risk). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized according to the treatment‐dependant prognostic index (TPI) of the DOSAK." Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis of 104/167 participants randomised published in 1994. No subsequent publication identified. Participants who did not have radical surgery at the primary site and participants who had extracapsular rupture of ≥ 1 lymph node were not included in the evaluation. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | No information about comparability of groups at baseline. |
D'Cruz 2015.
| Study characteristics | ||
| Methods | Location of trial: India Number of centres: 1 Funding: Tata Memorial Centre Trial ID: NCT00193765 |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: 2004 to 2014 Number randomised: 596 Number analysed: 496 |
|
| Interventions |
Elective vs therapeutic ND in node‐negative OC Group 1 (n = 298): elective surgery (ipsilateral selective ND with clearance of the submandibular (level I), upper jugular (level II), and midjugular (level III) nodes). Participants with metastatic nodal disease that was discovered during surgery (operative findings or frozen section) had a modified ND performed with nodal clearance extended to include the lower jugular (level IV) and posterior triangle (level V) nodes. Group 2 (n = 298): therapeutic surgery (the same surgical procedure for the primary tumour followed by monitoring, with modified ND (levels I–V) only at the time of nodal relapse). All participants underwent oral excision of the primary tumour with adequate margins (i.e. ≥ 5 mm). All participants underwent secondary randomisation for follow‐up (to receive either physical examination or physical examination + ultrasonography of the neck). |
|
| Outcomes | Primary outcomes
Secondary outcomes: none noted Duration of follow‐up: median 39 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator (i.e. prepared computerised block design). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was confirmed by the trial authors; however, the methods are unclear. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45 participants excluded from elective surgery group (1 withdrew consent, 1 had previous chemotherapy, 43 did not complete 9‐month follow‐up). 55 participants excluded from therapeutic surgery group (2 had lesion crossing midline, 53 did not complete 9‐month follow‐up). |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all prespecified (primary and secondary) outcomes of interest in the review were reported as per the protocol. |
| Other bias | Low risk | No other apparent bias |
Fakih 1989.
| Study characteristics | ||
| Methods | Location of trial: India Number of centres: 1 Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria: not stated Recruitment period: July 1985 to September 1988 Number randomised: 100 (all OC; 100% tongue) Number analysed: 70 |
|
| Interventions |
Elective radical ND vs therapeutic radical ND Group 1 (n = 30): radical ND (ipsilateral) Group 2 (n = 40): only participants developing neck node metastasis underwent radical ND All participants underwent resection of the primary tumour (standard anterior two‐thirds hemiglossectomy). |
|
| Outcomes | Primary outcomes
Secondary: none noted Duration of follow‐up: 1 year |
|
| Notes | No data available for calculation of HR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised from previously generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Interim analysis, no final analysis reported. 73 participants entered into protocol, 12 refused treatment, and 2 were declared unfit for surgery. Of the remaining 59 who completed initial treatment, 35 who completed a median of 22 months' follow‐up were included in the analysis (approximately 48%). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Unclear risk | No information about comparability of groups at baseline. |
Garrel 2020.
| Study characteristics | ||
| Methods | Location of trial: France Number of centres: 10 Funding: French National Institute of Cancer STIC 2007 Trial ID: NCT02855723 (Senti‐MERORL) |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: 2008 to 2013 Number randomised: 307 Number analysed: 279 |
|
| Interventions |
Sentinal node biopsy vs ND Group 1 (n = 140): sentinal node biopsy alone if negative, or followed by ND if positive, during primary tumour surgery Group 2 (n = 139): ND |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: mean follow‐up was 4.95 (SD 2.45) years in the ND arm and 4.74 (SD 2.55) years in the sentinal biopsy arm. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2‐arm random assignment was performed using small balanced blocks at each centre, with a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if investigators were able to conceal treatment arm from participants or surgeons. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | Neck‐shoulder functional morbidity, assessed using a self‐report neck and shoulder impairment questionnaire. Subjective outcome; unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small numbers of participants not analysed relative to those randomised. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | Groups appeared similar at baseline. No evidence of other potential sources of bias. |
Guo 2014.
| Study characteristics | ||
| Methods | Location of trial: China Number of centres: 1 Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria:
Exclusion criteria: not stated Recruitment period: June 1999–May 2010 Number randomised: 332 Number analysed: 322 |
|
| Interventions |
SOH vs MRND Group 1 (n = 166): SOH (109 participants received surgery alone, 57 received surgery + PORT) Group 2 (n = 166): MRND (114 participants received surgery alone, 52 received surgery + PORT) |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: median 76 months (1 year for QoL) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | QoL assessments. Subjective outcome; unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (3%) participants lost to follow‐up soon after randomisation were unable to be included in the analysis (4 in SOH ND treatment arm, 6 in MRND treatment arm). |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No other apparent bias. |
Hutchison 2019.
| Study characteristics | ||
| Methods | Location of trial: UK Number of centres: multicentre (27 hospitals; 25 recruiting to the RCT) Funding: Cancer Research UK Trial ID: UKCRN 2069, ISCRTN 65018995, NCT00571883 |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: 18 June 2007–10 July 2015 Number randomised: 255 (61% tongue, 19% FOM, 10% buccal mucosa, 6% gingivae, < 1% palate, < 1% tonsil, < 1% other) Number analysed: 250 |
|
| Interventions |
Elective vs therapeutic ND in node‐negative OC Group 1: elective ND (END; n = 126): resection of primary tumour with END. Standard END harvesting lymph nodes from levels I to IV including levels Ia/b and IIa/b on the same side as tumour Group 2: therapeutic ND (n = 124): resection of primary tumour through open mouth without neck incisions or neck surgery |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: 5 years (median follow‐up was 57 months (25th to 75th centile 43 to 61 months) |
|
| Notes | IDMEC recommended early termination of accrual because preliminary data were consistent with Mumbai trial (D'Cruz 2015). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A minimisation computer program stratified patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was achieved using a central randomisation system." |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We considered all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "5 patients were found to be ineligible and so excluded from all analyses." Considered a small number unlikely to introduce bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in full. |
| Other bias | Low risk | The study was well conducted and there was no evidence of any other bias. |
Iyer 2015.
| Study characteristics | ||
| Methods | Location of trial: Singapore Number of centres: not stated Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: August 1996–February 2002 Number randomised: 57 of 119 participants had oral cavity or oropharyngeal cancer Number analysed: 57 of 118 participants had oral cavity or oropharyngeal cancer |
|
| Interventions |
Surgery and adjuvant RT vs concurrent CRT For participants with oral cavity and oropharyngeal cancer: Group 1 (n = 13): radical surgery + adjuvant RT Group 2 (n = 19): combination chemotherapy with cisplatin and 5‐fluorouracil and concurrent RT Randomisation was stratified according to primary tumour site (oral cavity/oropharynx, larynx/hypopharynx, others) and lymph node status (lymph‐node positive vs lymph‐node negative). |
|
| Outcomes | Primary outcomes
Secondary outcomes: none reported Duration of follow‐up: 10 years |
|
| Notes | Although only 48% of participants had oral or oropharyngeal cancer, there were narrative results for the oral cavity subgroup, and information in the text for the oropharyngeal subgroup. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant missing from analysis (excluded) after histopathological assessment confirmed adenocarcinoma. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | High risk | Quote: "The trial was halted prematurely due to poor accrual." |
Kligerman 1994.
| Study characteristics | ||
| Methods | Location of trial: Brazil Number of centres: 1 Funding: government (personal communication) Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria: not reported Recruitment period: 1987–1992 Number randomised: 67 (61% tongue, 39% FOM) Number analysed: 67 |
|
| Interventions |
Elective ND vs therapeutic ND Group 1 (n = 34): elective SOH. Dissection of levels 1–3 + resection of submandibular gland, preserving the sternocleidomastoid muscle, spinal accessory nerve and internal jugular vein Group 2 (n = 33): therapeutic ND All participants underwent resection of the primary tumour. |
|
| Outcomes | Primary outcomes
Secondary outcomes: not reported Duration of follow‐up: 3.5 years |
|
| Notes | Paper reported that overall survival assessed by Kaplan‐Meier actuarial method, but not presented. HR data taken from Kaplan‐Meier graph (no numbers at risk) for DFS. Locoregional failure data unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All 67 patients were stratified by stage [...] and those in each stage were randomised." Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Unclear risk | No information about comparability of groups at baseline. |
Mehanna 2017.
| Study characteristics | ||
| Methods | Location of trial: UK Number of centres: 38 Funding: Health Technology programme of National Institute for Health Research Technology Assessment Programme and Cancer Research UK Trial ID: ISRCTN13735240 |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: 2 October 2007 to 23 August 2012 Number randomised: 564 (84.4% OP cancer) Number analysed: 564 (personal communication) |
|
| Interventions |
PET‐CT surveillance (following CRT) vs planned ND (either before or after CRT) Group 1 (n = 282): PET‐CT 12 weeks after completion of CRT (surveillance group), with ND performed only if PET‐CT showed an incomplete or equivocal response Group 2 (n = 282): planned ND (either before or after CRT) |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: 36 months (median) |
|
| Notes | Before randomisation, each participating centre had to specify on a per‐participant basis whether planned ND would be performed within 4 weeks before or within 4 to 8 weeks after completion of CRT. In addition, before randomisation, clinicians selected CRT regimens from a list of the approved study regimens. ITT analysis was carried out for all 564 participants. Kaplan‐Meier analysis was used to estimate survival rate due to the loss of some participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation algorithm used; Table 1 listed variables for comparison. |
| Allocation concealment (selection bias) | Low risk | Undertaken by a trials unit and probably centralised, and confirmed by trial author. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | QoL assessments; subjective outcome with unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants assessed as part of the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Study protocol was published and outcomes were published according to protocol. |
| Other bias | Low risk | No other potential sources of bias identified. |
Nichols 2019.
| Study characteristics | ||
| Methods | Location of trial: Canada and Australia (ORATOR trial) Number of centres: multicentre (6) Funding: Canadian Cancer Society Research Institute Grant (701842) Trial ID: NCT01590355 (ORATOR trial) |
|
| Participants | Inclusion criteria
Exclusion criteria
Number randomised: 68 Number analysed: 68 (34 per group) Recruitment period: August 2012–June 2017 |
|
| Interventions |
Surgery with ND vs RT Group 1 (n = 34): Transoral Robotic Surgery (TORS) plus ND: a surgical robot was used to excise the primary tumour with 1‐cm margins, with selective ND done at time of surgery or within 2 weeks. Group 1 (n = 34): RT: 70 Gy in 35 fractions over 7 weeks to areas of gross disease and 56 Gy to low risk nodal areas. Concurrent chemotherapy was recommended for patients with node‐positive disease. High dose cisplatin (100 mg/m2 intravenously) every 3 weeks was preferred. There were no planned NDs. |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: every 3 months for the first 2 years and every 6 months thereafter until 5 years |
|
| Notes | The primary outcome for the study was MDADI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Following stratification by p16 status, patients were randomly assigned using computer‐generated randomisation list with permutated blocks of four." |
| Allocation concealment (selection bias) | Low risk | Quote "On receipt of these documents, enrolment and assignment were done by a trial coordinator who was not involved in clinical management. The co‐ordinator was the only person able to access the locked, concealed randomisation list, and treatment group was communicated by email." |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | QoL using MD Anderson Dysphagia Index (MDADI); other QoL tools included EORTC, QLQ‐C30, H&N35, VHI‐10, NDII, PNQ, FOIS). Subjective outcomes; unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants withdrew consent after randomisation to the radiotherapy group, and did not receive allocated intervention. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported as per the protocol. |
| Other bias | Low risk | No other apparent biases. |
Pandey 2018.
| Study characteristics | ||
| Methods | Location of trial: India Number of centres: unclear but probably 1 Funding: none Trial ID: NCT00847717 |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: December 2007–August 2009 Number randomised: 32 Number analysed: 32 |
|
| Interventions |
Superselective ND vs selective ND in node‐negative OC Group 1 (n = 12): superselective ND: IIb preserving superselective neck dissection Group 2 (n = 20): selective ND: conventional supraomohyoid neck dissection |
|
| Outcomes | Primary outcome
Secondary outcomes
Duration of follow‐up: median 36 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomisation." |
| Allocation concealment (selection bias) | Low risk | Allocation concealment confirmed by trial author. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | Quality of life measured by FACT‐HN questionnaire at 1 year. Subjective outcome; unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomised is the same as number analysed. No attrition. |
| Selective reporting (reporting bias) | Unclear risk | The reporting of DFS is simply by % at median follow‐up 36 months. No HR provided and we were unable to use these data. |
| Other bias | Unclear risk | Insufficient detail in the trial report. Recruitment was 2007 to 2009, but the report was published in 2018. |
Rastogi 2018.
| Study characteristics | ||
| Methods | Location of trial: India Number of centres: 1 Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: August 2014–March 2017 Number randomised: 20 Number analysed: 20 |
|
| Interventions |
Superselective ND vs selective ND in node‐negative OC Group 1 (n = 10): superselective ND of levels I, IIa, IIb, and III Group 2 (n = 10): selective ND of levels I, IIa, and III |
|
| Outcomes | Primary outcome
Secondary
Duration of follow‐up period for all participants: 2.5 years |
|
| Notes | Small sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the randomisation occurred using the "slot method". |
| Allocation concealment (selection bias) | Unclear risk | Unclear if the investigators utilised appropriate allocation concealment. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Blinding of outcome assessment ‐ subjective outcomes (detection bias) | High risk | QoL assessed by subjective questionnaire (Neck Dissection Quality of Life Questionnaire). Subjective outcome; unblinded assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial with analysis undertaken for all. |
| Selective reporting (reporting bias) | Low risk | Outcomes clearly stated in methods section and appropriately measured in results section. |
| Other bias | Low risk | No other sources of bias noted. |
Robertson 1998.
| Study characteristics | ||
| Methods | Location of trial: UK Number of centres: multicentre (4) Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: December 1991–December 1993 Number randomised: 35 (33/35 OC: 40% tongue, 43% FOM, 11% RMT, 6% tonsil) Number analysed: 35 |
|
| Interventions |
Surgery + RT vs RT alone Group 1 (n = 17): surgery: radical resection and ND + PORT. Radical surgery involved wide local excision of the primary tumour with 1‐cm margin. A radical or functional ND was carried out at the same time at the discretion of the surgeon. Reconstruction of the oral cavity was carried out immediately. PORT comprised 60 Gy in 30 fractions over 6 weeks, commencing within 6 to 8 weeks of surgery. Group 2 (n = 18): RT alone; 66 Gy in 33 fractions over 6.5 weeks, receiving 2 Gy per day |
|
| Outcomes | Primary outcome
Secondary outcome
Duration of follow‐up: 3 years |
|
| Notes | The intended sample size was 350 people, but trial was stopped early due to concerns about the number of deaths in the RT alone arm. HR data taken from Kaplan‐Meier graph (no numbers at risk). Data presented in Kaplan‐Meier estimates for DFS, but not used as graph started at 50% for RT alone arm. Authors provided additional information relating to allocation concealment and the characteristics of tumours. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random permuted blocks of four were used for randomization." This was following stratification according to institution and site of primary disease. |
| Allocation concealment (selection bias) | Low risk | Randomisation via a telephone call to the West of Scotland Clinical Trials Office. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting of outcomes. |
| Other bias | High risk | Anticipated enrolment of 350 participants, but trial stopped after 35 participants recruited because clinicians felt it was unethical to continue. Appropriate procedures and analysis were conducted. More than half of participants had either delays or interruptions to the planned RT schedule. It is likely that this would have had a greater effect in the outcomes of the RT alone arm of this trial. |
Yuen 2009.
| Study characteristics | ||
| Methods | Location of trial: Hong Kong, China Number of centres: 3 Funding: not stated Trial ID: not stated |
|
| Participants | Inclusion criteria
Exclusion criteria
Recruitment period: 1996–2004 Numbers randomised: 72 (100% tongue) Numbers analysed: 71 |
|
| Interventions |
Elective selective ND vs therapeutic radical ND Group 1 (n = 36): elective ipsilateral selective ND of level I, II, or III neck nodes Group 2 (n = 36): therapeutic (delayed) dissection. Participants received ultrasound examinations every 3 months for the first 3 years. If nodal recurrence was detected, these participants underwent either radical or modified radical ND followed by RT. All participants in the trial had transoral glossectomy with 1.5 resection margins. |
|
| Outcomes | Primary outcomes
Secondary outcomes
Duration of follow‐up: 34–122 months (median follow‐up duration was 93 months in the elective selective ND arm and 92 months in the therapeutic ND arm) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by tumour stage. Method of sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes; insufficient information to determine whether allocation was concealed from investigators. |
| Blinding of outcome assessment ‐ objective outcomes (detection bias) | Low risk | We judged all trials at low risk of bias for this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant allocated to observation group was subsequently found to have T3 tumour and was withdrawn. All other randomised participants were included in the outcome evaluations. |
| Selective reporting (reporting bias) | Low risk | Reported nodal and local recurrence, DFS and disease‐specific death in full. No reporting of mortality in each group. |
| Other bias | Low risk | Groups appeared similar at baseline. |
AJCC: American Joint Committee on Cancer; cm: centimetre; CRT: chemoradiotherapy; CT: computer tomography; DFS: disease‐free survival; DSS: disease‐specific survival; ECOG: Eastern Cooperative Oncology Group; FOM: floor of mouth; Gy: Gray; HNSCC: head and neck squamous‐cell carcinoma; HNC: head and neck cancer; HR: hazard ratio; ITT: intention‐to‐treat; MRI: magnetic resonance imaging; MRND: modified radical classical neck dissection; n: number of participants; NCR: neck control rate; ND: neck dissection; OC: oral cancer; OP: oropharyngeal cancer; PET‐CT: positron emission tomography–computed tomography; PORT: postoperative radiotherapy; QoL: quality of life; RCT: randomised controlled trial; RMT: retromolar trigone; RT: radiotherapy; SCC: squamous‐cell carcinoma; SD: standard deviation; SE: standard error; SOH: supraomohyoid neck dissection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aladashi 2020 | < 50% of participants had oral cancer. |
| Batra 2016 | Short‐term outcomes only (wound closure). |
| Chaukar 2021 | Ineligible comparison as looking at the effect of chemotherapy on the cancer to provide bone sparing surgery. The purpose of the study "was to explore the potential role and safety of neoadjuvant chemotherapy (NACT)". This trial would meet the criteria for the Cochrane Oral Health review "Interventions for the treatment of oral and oropharyngeal cancers: chemotherapy". |
| Christensen 2019 | This study evaluates the impact of additional use of optical imaging on lymph node yield. |
| Dean 2013 | Short‐term outcomes only (e.g. operative time, blood loss during surgery, time drains are kept in place, amount of drainage). |
| Durham 2020 | Ineligible interventions for the review; the intervention was a diagnostic aid and the treatment did not differ between the 2 groups. |
| Dziegielewski 2019 | No appropriate outcomes (shoulder function). |
| Fan 2017 | Short‐term outcomes only (e.g. postoperative immune response and surgical stress). |
| Fritz 2016 | Short‐term outcomes only (e.g. blood loss and operating time). |
| Funahara 2017 | Short‐term outcomes only (e.g. surgical wound infections). |
| Gundale 2017 | Abstract; insufficient information. |
| Hintz 1979a | HNC study; < 50% or participants had oral cancer/oropharyngeal cancer. |
| Hintz 1979b | HNC study; < 50% of participants had oral cancer/oropharyngeal cancer. |
| Jinyun 2015 | Not an RCT. |
| Kramer 1987 | Insufficient detail in published report to establish what the surgical procedures involved and whether these were the same in all groups. Insufficient information to enable risk of bias assessment. |
| Lin 2016 | Short‐term study only looking at immediate postsurgical outcomes. |
| McCaul 2012 | Abstract; insufficient information. |
| McCaul 2017 | Abstract; insufficient information. |
| Minkovich 2011 | Short‐term outcomes only (e.g. malposition of peripherally inserted central venous catheters). |
| Oswal 2017 | Short‐term outcomes only (e.g. wound closure). |
| Uppal 2012 | Original article unavailable. |
| Vandenbrouck 1980 | Primary site not treated with surgery, but with interstitial radiotherapy. |
| Verma 2017 | Short‐term study only investigating immediate postsurgical outcomes. |
| Walen 2011 | Short‐term study on postoperative pain. |
| Zhang 2010 | Abstract; insufficient information. |
| Zhong 2013 | Surgery was not the comparison, mainly chemotherapy. The aim of the study was "to evaluate induction chemotherapy with docetaxel, cisplatin, and fluorouracil (TPF) followed by surgery and postoperative radiotherapy versus up‐front surgery and postoperative radiotherapy in patients with locally advanced resectable oral squamous cell carcinoma (OSCC)". This trial would meet the criteria for the Cochrane Oral Health review "Interventions for the treatment of oral and oropharyngeal cancers: chemotherapy". |
HNC: head and neck cancer; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Bußmann 2020.
| Study name | TopROC |
| Methods | 2‐arm, multicentre, open‐label RCT Setting: around 20 centres in Germany |
| Participants | Patients with locoregionally advanced, but transorally resectable oropharyngeal cancer (OPSCC) |
| Interventions | Group 1: transoral surgical resection with neck dissection followed by risk‐adapted adjuvant radio(chemo)therapy 56‐66 Gy (+chemo) Group 2: primary radio(chemo)therapy 70–72 Gy (+chemo), residual tumour may be subject to salvage surgery |
| Outcomes |
|
| Starting date | 2018 recruitment |
| Contact information | Chia‐Jung Busch, cjbusch@uke.de |
| Notes | Funding: German Cancer Aid (Deutsche Krebschilfe project number 1120027) |
Channir 2018.
| Study name | Transoral robotic surgery for head and neck cancer |
| Methods | 2‐arm RCT Setting: Denmark |
| Participants | People with early‐stage HPV‐induced OPSCC |
| Interventions | Group 1: newly introduced transoral robotic surgery Group 2: traditional radiation therapy |
| Outcomes | Long‐term functional outcomes |
| Starting date | Not stated |
| Contact information | Hani Channir, Righospitalet, Copenhagen, Denmark |
| Notes |
Lai 2021.
| Study name | NRG Oncology HN006: randomized phase II/III trial of sentinel lymph node biopsy versus elective neck dissection for early stage oral cavity cancer |
| Methods | International multi‐institutional phase II/III prospective 2‐arm parallel RCT (72 locations) |
| Participants | People with early‐stage oral cavity cancer; "pathologically (histologically or cytologically) proven diagnosis of squamous cell carcinoma of the oral cavity, including the oral (mobile) tongue, floor of mouth (FOM), mucosal lip, buccal mucosa, lower alveolar ridge, upper alveolar ridge, retromolar gingiva (retromolar trigone; RMT), or hard palate prior to registration" |
| Interventions | Group 1: SLN biopsy: participants receive an imaging agent via injection and undergo planar imaging and SPECT/CT over 1–2 hours, then undergo SLN biopsy. Group 2: standard elective ND |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 8 July 2020 |
| Contact information | Principal Investigator: Stephen Y Lai, NRG Oncology, National Cancer Institute (NCI) |
| Notes |
NCT01334320.
| Study name | Survival benefit of elective neck dissection in T1, 2 N0 M0 oral squamous cell carcinoma |
| Methods | RCT |
| Participants | Histologically confirmed T1 or T2 N0 M0 (clinical) squamous‐cell carcinoma of oral tongue, buccal mucosa, gingiva, floor of mouth or hard palate |
| Interventions | Group 1; elective superior omohyoid ND Group 2: watch and wait (resection of primary tumour and therapeutic dissection of neck when clinical evidence of disease) |
| Outcomes |
|
| Starting date | April 2011 |
| Contact information | Dr Guiqing Lao, Hospital of Stomatology, Sun Yat‐sen University, Guangdong, China (drliaoguiqing@hotmail.com) |
| Notes | Planned enrolment of 448 participants |
NCT0274382.
| Study name | A study on tumor budding guiding individualized surgical planning of early‐stage oral squamous cell carcinoma |
| Methods | 4‐arm RCT |
| Participants | Oral squamous cell carcinoma confirmed by pathology, including primary two‐thirds prior to the tongue, buccal mucosa, gingiva, mouth floor, hard palate mucosa. Primary lesion should measure no more than 4 cm. Clinical examination (including physical examination and MRI) should reveal no cervical lymph node metastases or distant metastasis. |
| Interventions | Group 1: high‐level tumour budding group with CLND. Resection of primary lesion and CLND. Group 2: high‐level group without CLND. Resection of primary lesion only. Group 3: low‐level tumour budding group with CLND. Resection of primary lesion and cervical lymph node dissection. Group 4: low‐level group without CLND. Resection of primary lesion only. |
| Outcomes | Primary outcomes
Secondary outcomes
Outcomes reviewed every 3 months in the first year and every 6 months in the following 4 years. Time from first operation to death or discovery of the first cervical lymphatic metastases will be recorded. |
| Starting date | 4 September 2022 |
| Contact information | Guanghua School of Stomatolagy, Hospital of Stomatolagy Sun Yat‐sen University, Guangzhou, Guangdong, China, 510055 Jinsong Hou, houjsgz@aliyun.com; Cheng Wang, drwangcheng@outlook.com |
| Notes |
NCT03385720.
| Study name | Submandibular gland preservation during neck dissection for oral squamous cell carcinoma |
| Methods | RCT, parallel design, double blind |
| Participants | People with primary oral squamous cell carcinoma of early stage (T1N0 and T2N0) |
| Interventions | Group 1: modified neck dissection with preservation of submandibular gland during neck dissection Group 2: traditional neck dissection without preservation of submandibular gland |
| Outcomes |
|
| Starting date | 5 December 2017 |
| Contact information | Dr Jia‐Zeng Su, sujiazeng@163.com |
| Notes |
NCT04738786.
| Study name | Clinical study evaluating the proper surgical safety margin for early stage oral tongue cancers |
| Methods | Prospective, double‐blind, 2‐arm multicentre randomised non‐inferiority clinical trial, comparing 1.5 cm surgical safety margin versus 1.0 cm surgical safety margin in curative resection for cT1‐2N0 oral tongue cancer |
| Participants | T1‐2N0 oral tongue cancer patients |
| Interventions | Wide vs narrow surgical safety margin (i.e. margin of apparently non‐tumorous tissue around a tumour that has been surgically removed (resected normal‐looking tissues from the gross tumour border). The surgical safety margin is applied to all directions of 3‐dimensional tumours (mucosal and deep side). Group 1: wide (1.5 cm) safety margin surgery for cT1‐2N0 oral tongue cancer (surgical resection including 1.5 cm normal tissue around the gross tumours) Group 2: narrow (1.0 cm) safety margin surgery for cT1‐2N0 oral tongue cancer (surgical resection including 1.0 cm normal tissue around the gross tumours) |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 18 January 2021 |
| Contact information | Han‐Sin Jeong, hansin.jeong@gmail.com; Jeon‐Yeob Jang, manup1377@gmail.com |
| Notes |
Tankara 2018 (RESPOND).
| Study name | JCOG1601, RESPOND |
| Methods | Unblinded, 2‐arm, multicentre RCT in 28 institutions in Japan. |
| Participants | People with histologically proven squamous cell carcinoma using biopsied tongue specimen |
| Interventions | Group 1: partial glossectomy with prophylactic neck dissection Group 2: partial glossectomy alone |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Enrolment began November 2017 |
| Contact information | N Hanai, hanai@aichi‐cc.jp |
| Notes | Funding: AMED Grant number JP18ck0106438 and National Cancer Center Research and Development Fund |
Wang 2019.
| Study name | ChiCTR1800019128 |
| Methods | Parallel‐group, randomised, non‐inferiority trial |
| Participants | People with early oral carcinoma (plan to enrol 522 people) |
| Interventions | Group 1: Ib neck dissection group: resection of primary + ipsilateral or bilateral neck dissection (level I to III) Group 2: IIb neck retention group: resection of primary + ipsilateral or bilateral neck dissection (level I to III, except level IIb) |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Unclear but registered on 26 October 2018 |
| Contact information | Lei Wang, LeiW1993@163.com |
| Notes | Supported by Shanghai Ninth People's Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China |
CLND: cervical lymph node dissection; FOM: floor of mouth; Gy: Gray; HPV: human papillomavirus; SLN: sentinel lymph node; M: distant metastasis; MRI: magnetic resonance imaging; N: lymph node; ND: neck dissection; OPSCC: oropharyngeal squamous cell carcinoma; QoL: quality of life; RCT: randomised controlled trial; SPECT/CT: single photon emission computed tomography/chemotherapy; T: primary tumour; vs: versus
Differences between protocol and review
This section includes changes made since previous iterations of the review (Bessell 2011; Bulsara 2018; Oliver 2007), as well as changes from the protocol (Oliver 2006).
Types of interventions: the intervention under evaluation must have been surgery. We excluded trials where all participants received the same surgical regimen and were randomised to other treatments.
Outcomes: local regional control was renamed locoregional recurrence; studies that did not measure any of our primary outcomes were excluded.
Search methods: search strategy for ClinicalTrial.gov amended to include oropharyngeal cancer.
Statistical analysis: we considered it more appropriate to use random‐effects models for any pooling of studies.
Risk of bias: the original quality assessment approach was replaced by the Cochrane risk of bias tool (RoB 1; Higgins 2011); we amended the risk of bias assessment to differentiate between objective and subjective outcomes.
We updated the data synthesis section. The primary outcome that was most reliably and frequently reported was total mortality expressed as a hazard ratio. For dichotomous outcomes, we expressed the estimates of effect as risk ratios with 95% confidence intervals. We used dichotomous data over a fixed time period (e.g. five years) to calculate hazard ratios so that we could include more studies in meta‐analyses.
We performed no subgroup analyses for this update.
Contributions of authors
All review authors contributed to the previous version of this review
HW, VB, and AMG co‐ordinated and managed the review update.
The trials search strategy was refined with input from VB. (It was designed by Cochrane Oral Health Information Specialist Anne Littlewood.)
HW, VB, AMG, DC, and MM screened the titles and abstracts.
HW organised retrieval of papers.
HW and VB screened retrieved papers against the inclusion criteria.
HW, VB, AMG, JC, DC, and MM extracted data, appraised the risk of bias in the included studies, and assessed the certainty of the body of evidence for each main comparison and outcome.
HW and AMG provided a methodological perspective.
VB, JC, DC, and MM provided a clinical perspective and updated the background.
All review authors helped to address the substantial peer reviewer comments.
Sources of support
Internal sources
-
The School of Dentistry, The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre, UK
Support to Cochrane Oral Health.
-
The University of Dundee, UK
Support to Cochrane Oral Health.
-
The University of Glasgow, UK
Support to Cochrane Oral Health.
External sources
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships-alliances). Contributors over recent years have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the National Health Service, or the Department of Health and Social Care.
-
National Institutes of Health (NIH), National Institute of Dental & Craniofacial Research (NIDCR), USA
From 2005 to 2007 we had a grant from NIDCR, NIH USA for the update of systematic reviews and undertaking new reviews in field of oral cancer (£130,000), which supported the publication of the first version of this review
Declarations of interest
HW: none known. I am Emeritus Co‐ordinating Editor of Cochrane Oral Health, and was not involved in the editorial process. VB: none known. AMG: none known. I am joint Co‐ordinating Editor of Cochrane Oral Health, and was not involved in the editorial process. JC: none known. I am joint Co‐ordinating Editor of Cochrane Oral Health, and was not involved in the editorial process. DC: none known. MM: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
BHNCSG 1998 {published data only}
- Brazilian Head and Neck Cancer Study Group. Results of a prospective trial on elective modified radical classical versus supraomohyoid neck dissection in the management of oral squamous carcinoma. American Journal of Surgery 1998;176(5):422-7. [DOI] [PubMed] [Google Scholar]
Bier 1994 {published data only}
- Bier J, Howaldt HP, Pitz H, 4th German-Austrian-Swiss Study Group therapy study. Prospective, randomized, clinical study of squamous cell cancer of the mouth: "Radical neck dissection versus conservative neck dissection". Fortschritte der Kiefer- und Gesichts-Chirurgie 1992;37:108-10. [PubMed] [Google Scholar]
- Bier J, Schlums D, Metelmann H, Howaldt HP, Pitz H. A comparison of radical and conservative neck dissection. International Journal of Oral & Maxillofacial Surgery 1993;22(2):102-7. [DOI] [PubMed] [Google Scholar]
- Bier J. Radical neck dissection versus conservative neck dissection for squamous cell carcinoma of the oral cavity. Recent Results in Cancer Research 1994;134:57-62. [DOI] [PubMed] [Google Scholar]
D'Cruz 2015 {published data only}
- D'Cruz A. Re: Cochrane review on “Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment" [personal communication]. Email to: H Worthington 6 February 2023.
- D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. New England Journal of Medicine 2015;373(6):521-9. [DOI: 10.1056/NEJMoa1506007] [DOI] [PubMed] [Google Scholar]
- NCT00193765. Elective vs therapeutic neck dissection in treatment of early node negative squamous carcinoma of oral cavity. www.clinicaltrials.gov/ct2/show/NCT00193765 (first received 19 September 2005).
Fakih 1989 {published data only}
- Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. American Journal of Surgery 1989;158(4):309-13. [DOI] [PubMed] [Google Scholar]
- Fakih AR, Rao RS, Patel AR. Prophylactic neck dissection in squamous cell carcinoma of oral tongue: a prospective randomized study. Seminars in Surgical Oncology 1989;5(5):327-30. [DOI] [PubMed] [Google Scholar]
Garrel 2020 {published data only}
- Garrel R, Perriard F, Favier V, Richard F, Pierre Daures J, De Boutray M. Equivalence randomized trial comparing treatment based on sentinel node biopsy versus neck dissection in operable T1-T2N0 oral and oropharyngeal cancer. Journal of Clinical Oncology Conference: 2020 Annual Meeting of the American Society of Clinical Oncology, ASCO 2020. United States 2020;38(Suppl 15):6501. [Google Scholar]
- Garrel R, Poissonnet G, Moyà Plana A, Fakhry N, Dolivet G, Lallemant B, et al. Equivalence randomized trial to compare treatment on the basis of sentinel node biopsy versus neck node dissection in operable T1-T2N0 oral and oropharyngeal cancer. Journal of Clinical Oncology 2020;38(34):4010-18. [DOI: 10.1200/JCO.20.01661] [DOI] [PubMed] [Google Scholar]
- NCT02855723. Randomized, open-label economic and medical study on the lymph node management of squamous cell carcinoma of the oral cavity and oropharynx tumor Stage 1 or 2, nodes 0 (T1-T2 N0) operable (SentiMERORL). clinicaltrials.gov/ct2/show/NCT02855723 (first received 4 August 2016).
Guo 2014 {published data only}
- Guo CB, Feng Z, Zhang JG, Peng X, Cai ZG, Mao C, et al. Supraomohyoid neck dissection and modified radical neck dissection for clinically node-negative oral squamous cell carcinoma: a prospective study of prognosis, complications and quality of life. Journal of Craniofacial Surgery 2014;42(8):1885-90. [DOI: 10.1016/j.jcms.2014.07.007] [DOI] [PubMed] [Google Scholar]
Hutchison 2019 {published data only}65018995
- Hutchison IL, Ridout F, Cheung SM, Shah N, Hardee P, Surwald C, et al. Nationwide randomised trial evaluating elective neck dissection for early stage oral cancer (SEND study) with meta-analysis and concurrent real-world cohort. British Journal of Cancer 2019;121(10):827-36. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN65018995. The role of selective neck dissection in patients with early oral squamous cell carcinoma (1-3cm primary size) and no clinical evidence of lymph node metastases in the neck (N0). www.isrctn.com/ISRCTN65018995 (first received 23 April 2010).
- NCT00571883. Neck surgery in treating patients with early-stage oral cancer. clinicaltrials.gov/ct2/show/NCT00571883 (first received 12 December 2007).
Iyer 2015 {published data only}
- Iyer NG, Tan DS, Tan VK, Wang W, Hwang J, Tan N-C, et al. Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10-year update and subset analysis. Cancer 2015;121(10):1599-607. [DOI] [PubMed] [Google Scholar]
- Soo KC, Tan EH, Wee J, Lim D, Tai BC, Khoo ML et al. Surgery and adjuvant radiotherapy vs concurrent chemoradiotherapy in stage III/IV nonmetastatic squamous cell head and neck cancer: a randomised comparison. British Journal of Cancer 2005;8(93):279-86. [DOI: 10.1038/sj.bjc.6602696] [PMCID: PMC2361563] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kligerman 1994 {published data only}
- Dr Kilgerman. Personal communcation 2006.
- Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. American Journal of Surgery 1994;168(5):391-4. [DOI] [PubMed] [Google Scholar]
Mehanna 2017 {published data only}
- ISRCTN13735240. A multicentre randomised phase III trial comparing positron emission tomography–computed tomography guided watch and wait policy versus planned NECK dissection for the management of locally advanced (N2/N3) nodal metastases in patients with head and neck squamous cancer. www.isrctn.com/ISRCTN13735240 (first received 21 May 2007). [DOI] [PMC free article] [PubMed]
- Mehanna H, McConkey CC, Rahman JK, Wong W-L, Smith AF, Nutting C, et al. PET-NECK a multicentre randomised phase III non-inferiority trial comparing a positron emission tomography-computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in patients with squamous cell head and neck cancer. Health Technology Assessment 2017;21:17. [DOI: 10.3310/hta21170] [DOI] [PMC free article] [PubMed]
- Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AG, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. New England Journal of Medicine 2016;374(15):1444-54. [DOI: 10.1056/NEJMoa1514493] [DOI] [PubMed] [Google Scholar]
- Mehanna H. Re: Cochrane review on “Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment" [personal communication]. Email to: H Worthington 31 January 2023.
Nichols 2019 {published data only}
- Fabian A, Krug D. Radiotherapy versus transoral robotic surgery for primary treatment of oropharyngeal squamous cell carcinoma: randomisation decides. Strahlentherapie und Onkologie 2019;196(2):202-4. [DOI] [PubMed] [Google Scholar]
- NCT01590355. A phase II randomized trial for early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs trans-oral robotic surgery (ORATOR). clinicaltrials.gov/ct2/show/NCT01590355 (first received 2 May 2012).
- Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncology 2019;20(10):1349-59. [DOI: 10.1016/S1470-2045(19)30410-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nichols AC, Theurer J, Prisman E, Read N, Berthelet E, Tran E, et al. Randomized trial of radiotherapy versus transoral robotic surgery for oropharyngeal squamous cell carcinoma: long-term results of the ORATOR trial. Journal of Clinical Oncology 2022;40(8):866-75. [DOI: 10.1200/JCO.21.01961] [DOI] [PubMed] [Google Scholar]
- Nichols AC, Yoo J, Hammond JA, Fung K, Winquist E, Read N, et al. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. trans-oral robotic surgery (ORATOR) – study protocol for a randomized phase II trial. BMC Cancer 2013;13:133. [DOI: 10.1186/1471-2407-13-133] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma DA, Theurer J, Prisman E, Read N, Berthelet E, Tran E, et al. Radiotherapy versus trans-oral robotic surgery for oropharyngeal squamous cell carcinoma: results of a randomized trial. Radiotherapy and Oncology 2019;139(Supp 1):S39-40. [Google Scholar]
Pandey 2018 {published data only}
- NCT00847717. Trial of IIb preserving neck dissection. clinicaltrials.gov/ct2/show/NCT00847717 (first received 19 February 2009).
- Pandey M, Karthikeyan S, Joshi D, Kumar M, Shukla M. Results of a randomized controlled trial of level IIb preserving neck dissection in clinically node-negative squamous carcinoma of the oral cavity. World Journal of Surgical Oncology 2018;16(1):219. [DOI: 10.1186/s12957-018-1518-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M. Re: Cochrane review on “Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment" [personal communication]. Email to: H Worthington 2 February 2023.
Rastogi 2018 {published data only}
- Rastogi S, Sharma A, Choudhury R, Tripathi S, Al Wayli H, Amrithraj A, et al. Is superselective neck dissection safer than supraomohyoid neck dissection for oral carcinoma patients with N0 neck in terms of shoulder morbidity and recurrence rate? Journal of Oral and Maxillofacial Surgery 2018;76(3):647-55. [DOI: 10.1016/j.joms.2017.08.002] [DOI] [PubMed] [Google Scholar]
Robertson 1998 {published data only}
- Dr Robertson. Personal communication 2006.
- Robertson AG, Soutar DS, Paul J, Webster M, Leonard AG, Moore KP, et al. Early closure of a randomized trial: surgery and postoperative radiotherapy versus radiotherapy in the management of intra-oral tumours. Clinical Oncology (Royal College of Radiologists (Great Britain)) 1998;10(3):155-60. [DOI] [PubMed] [Google Scholar]
Yuen 2009 {published data only}
- Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head & Neck 2009;31(6):765-72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aladashi 2020 {published data only}
- Aladashi OQ, Shindy MI, Noaman SA, Alqutaibi AY, Refahee SM. Effect of submental flap reconstruction versus obturator rehabilitation after maxillectomy on quality of life: a randomized clinical trial. International Journal of Oral and Maxillofacial Surgery 2021;50(9):1156-60. [DOI: 10.1016/j.ijom.2020.12.008] [DOI] [PubMed] [Google Scholar]
Batra 2016 {published data only}
- Batra J, Bekal RK, Byadgi S, Attresh G, Sambyal S, Vakade CD. Comparison of skin staples and standard sutures for closing incisions after head and neck cancer surgery: a double-blind, randomized and prospective study. Journal of Maxillofacial and Oral Surgery 2016;15(2):243-50. [DOI: 10.1007/s12663-015-0809-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaukar 2021 {published data only}
- Chaukar D, Pai PS, Chaturvedi P, Pantvaidya G, Deshmukh A, Nair D, et al. A prospective phase II open-label randomized controlled trial to compare mandibular preservation in upfront surgery to neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. Journal of Clinical Oncology Conference: 2020 Annual Meeting of the American Society of Clinical Oncology, ASCO 2020. United States 2020;38(Suppl 15):6518. [Google Scholar]
- Chaukar D, Prabash K, Rane P, Patil VM, Thiagarajan S, Ghosh-Laskar S, et al. Prospective phase II open-label randomized controlled trial to compare mandibular preservation in upfront surgery with neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. Journal of Clinical Oncology 2021;40(3):272-81. [DOI] [PubMed] [Google Scholar]
Christensen 2019 {published data only}
- Christensen A, Juhl K, Kiss K, Lelkaitis G, Charabi BW, Mortensen J, et al. Near-infrared fluorescence imaging improves the nodal yield in neck dissection in oral cavity cancer – a randomized study. European Journal of Surgical Oncology 2019;45(11):2151-8. [DOI] [PubMed] [Google Scholar]
Dean 2013 {published data only}
- Dean A, Alamillos F, Centella I, Garcia-Alvarez S. Neck dissection with the harmonic scalpel in patients with squamous cell carcinoma of the oral cavity. Journal of Cranio-maxillo-facial Surgery 2013;42(1):84-7. [DOI: 10.1016/j.jcms.2013.02.007] [DOI] [PubMed] [Google Scholar]
Durham 2020 {published data only}
- Durham JS, Brasher P, Anderson DW, Yoo J, Hart R, Dort JC, et al. Effect of fluorescence visualization-guided surgery on local recurrence of oral squamous cell carcinoma: a randomized clinical trial. JAMA Otolaryngology – Head & Neck Surgery 2020;146(12):1149-55. [DOI: 10.1001/jamaoto.2020.3147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh CF, Durham JS, Brasher PM, Anderson DW, Berean KW, MacAulay CE, et al. Canadian Optically-guided approach for Oral Lesions Surgical (COOLS) trial: study protocol for a randomized controlled trial. BMC Cancer 2011;11:462. [DOI: 10.1186/1471-2407-11-462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dziegielewski 2019 {published data only}
- Dziegielewski PT, McNeely ML, Ashworth N, O'Connell DA, Barber B, Courneya KS, et al. 2b or not 2b? Shoulder function after level 2b neck dissection: a double-blind randomized controlled clinical trial. Cancer 2020;126(7):1492-501. [DOI] [PubMed] [Google Scholar]
Fan 2017 {published data only}
- Fan S, Zhong JL, Chen WX, Chen WL, Li QX, Wang YY, et al. Postoperative immune response and surgical stress in selective neck dissection: comparison between endoscopically assisted dissection and open techniques in cT1-2N0 oral squamous cell carcinoma. Journal of Cranio-maxillo-facial Surgery 2017;45(8):1112-6. [DOI: 10.1016/j.jcms.2016.11.021] [DOI] [PubMed] [Google Scholar]
Fritz 2016 {published data only}
- Fritz DK, Matthews TW, Chandarana SP, Nakoneshny SC, Dort JC. Harmonic scalpel impact on blood loss and operating time in major head and neck surgery: a randomized clinical trial. Journal of Otolaryngology – Head & Neck Surgery 2016;45(58):1-6. [DOI: 10.1186/s40463-016-0173-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Funahara 2017 {published data only}
- Funahara M, Yanamoto S, Ueda M, Suzuki T, Ota Y, Nishimaki F, et al. Prevention of surgical site infection after oral cancer surgery by topical tetracycline: results of a multicenter randomized controlled trial. Medicine 2017;96(48):1-6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gundale 2017 {published data only}
- Gundale A, Rajdeep, Vasanthan L, Tirkey AK, Rajinikanth J. A randomised controlled trial of intraoperative brief electrical stimulation vs no stimulation of spinal accessory nerve for prevention of shoulder dysfunction after oncologic neck dissection in oral cavity squamous cell carcinomas-an interim report. Head & Neck 2017;39(S1):224.
Hintz 1979a {published data only}
- Hintz B, Charyulu K, Chandler JR, Sudarsanam A, Garciga C. Randomized study of local control and survival following radical surgery or radiation therapy in oral and laryngeal carcinomas. Journal of Surgical Oncology 1979;12(1):61-74. [DOI] [PubMed] [Google Scholar]
Hintz 1979b {published data only}
- Hintz B, Charyulu K, Chandler JR, Sudarsanam A, Garciga C. Randomized study of control of the primary tumor and survival using preoperative radiation, radiation alone, or surgery alone in head and neck carcinomas. Journal of Surgical Oncology 1979;12(1):75-85. [DOI] [PubMed] [Google Scholar]
Jinyun 2015 {published data only}
- Jinyun L, Wenxiao H, Jie C, Ronghua B. Clinical application of the combined radical operation without breaking lower lip and mandible for tongue and lingual root carcinoma. Chinese Journal of Otorhinolaryngology Head and Neck Surgery 2015;50(3):225-9. [DOI: 10.3760/cma. j. issn. 1673-0860. 2015. 03. 010] [PMID: ] [PubMed] [Google Scholar]
Kramer 1987 {published data only}
- Kramer S, Gelber RD, Snow JB, Marcial VA, Lowry LD, Davis LW, et al. Combined radiation therapy and surgery in the management of advanced head and neck cancer: final report of study 73-03 of the Radiation Therapy Oncology Group. Head & Neck Surgery 1987;10(1):19-30. [DOI] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin WJ, Wang CC, Jiang RS, Huang YC, Ho HC, Liu SA. A prospective randomised trial of LigaSure Small Jaw® versus conventional neck dissection in head and neck cancer patients. Clinical Otolaryngology 2016;42(2):245-51. [DOI] [PubMed] [Google Scholar]
McCaul 2012 {published data only}
- McCaul JA, Kulkami R, Gouldesbrough D, Boye T, Sutton D, Abdel-Galil K, et al. LIHNCS; randomised control trial in oral and maxillofacial surgery interim data and recruitment report. British Journal of Oral & Maxillofacial Surgery 2012;50(Suppl 1):S22. [DOI: 10.1016/j.bjoms.2012.04.203] [DOI] [Google Scholar]
McCaul 2017 {published data only}
- McCaul JA, McMahon JM, Quantrill J, Gilbert K, Mehanna HM, Shaw R, et al. LIHNCS: Lugol's iodine in head and neck cancer surgery – a multi-centre, randomised, controlled trial assessing the effectiveness of Lugol's iodine to assist excision of moderate dysplasia, severe dysplasia and carcinoma in-situ at mucosal resection margin of oral and oropharyngeal squamous cell carcinoma. Journal of Clinical Oncology 2017;35(Suppl 15):6065. [DOI: 10.1200/JCO.2017.35.15_suppl.6065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minkovich 2011 {published data only}
- Minkovich L, Djaiani G, McCluskey SA, Mitsakakis N, Gilbert RW, Beattle WS. Frequent malpositions of peripherally inserted central venous catheters in patients undergoing head and neck surgery [Mauvais positionnements frequents des catheters veineux centraux inseres par voie peripherique chez les patients subissantune chirurgie au niveau de la tete et du cou]. Canadian Journal of Anesthesia 2011;58:709-13. [DOI] [PubMed] [Google Scholar]
Oswal 2017 {published data only}
- Oswal S, Borle R, Bhola N, Jadhav A, Surana S, Oswal R. Surgical staples: a superior alternative to sutures for skin closure after neck dissection – a single-blinded prospective randomized clinical study. Journal of Oral and Maxillofacial Surgery 2017;75(12):2707.e1-6. [DOI: 10.1016/j.joms.2017.08.004] [DOI] [PubMed] [Google Scholar]
Uppal 2012 {published data only}
- Uppal A, Kakar FD, Uppal SA, Majeed R. Comparison of the complication between the radical neck dissection & selective neck dissection for the patient of oral squamous cell carcinoma. Medical Forum Monthly 2012;23(1):31-4. [Google Scholar]
Vandenbrouck 1980 {published data only}
- Vandenbrouck C, Sancho-Garnier H, Chassagne D, Saravane D, Cachin Y, Micheau C. Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer 1980;46(2):386-90. [DOI] [PubMed] [Google Scholar]
Verma 2017 {published data only}
- Verma RK, Mathiazhagan A, Panda NK. Neck dissection with harmonic scalpel and electrocautery? A randomised study. Auris Nasus Larynx 2017;44(5):590-5. [DOI: 10.1016/j.anl.2016.11.004] [DOI] [PubMed] [Google Scholar]
Walen 2011 {published data only}
- Walen SG, Rudmik LR, Dixon E, Matthews TW, Nakoneshny SC, Dort JC. The utility of the harmonic scalpel in selective neck dissection: a prospective, randomized trial. Otolaryngology and Head and Neck Surgery 2011;144(6):894-9. [DOI: 10.1177/0194599811403874] [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang F, Yang K, Li Y, Chen DR, Xiang L. Comparison of surgical effects on patients with oral squamous cell carcinoma in cN0 stage operated in two kinds of neck dissection. Stomatology 2010;30(2):98-100.
Zhong 2013 {published data only}
- Zhong LP, Zhang CP, Ren GX, Guo W, William WN Jr, Hong CS, et al. Long-term results of a randomized phase III trial of TPF induction chemotherapy followed by surgery and radiation in locally advanced oral squamous cell carcinoma. Oncotarget 2015;6(21):18707-14. [DOI: 10.18632/oncotarget.4531] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong LP, Zhang CP, Ren GX, Guo W, William WN Jr, Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. Journal of Clinical Oncology 2013;31(6):744-51. [DOI: 10.1200/jco.2012.43.8820] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Bußmann 2020 {published data only}
- Betz CS, Laban S, Wittekindt C, Stromberger C, Tribius S, Klußmann J-P, et al. Trial in progress: comparative effectiveness trial of transoral head and neck surgery followed by adjuvant radio(chemo)therapy versus primary radiochemotherapy for oropharyngeal cancer (TopROC). Laryngo-Rhino-Otologie 2021;100 (Suppl 2):S110-1. [DOI: 10.1055/s-0041-1727925] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bußmann L, Laban S, Wittekindt C, Stomberger C, Tribius S, Mockelmann N, et al. Comparative effectiveness trial of transoral head and neck surgery followed by adjuvant radio(chemo)therapy versus primary radiochemotherapy for oropharyngeal cancer (TopROC). BMC Cancer 2020;20(1):701. [DOI: 10.1186/s12885-020-07127-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03691441. Comparative effectiveness trial of transoral head and neck surgery followed by adjuvant radio(chemo)therapy versus primary radiochemotherapy for oropharyngeal cancer. clinicaltrials.gov/ct2/show/NCT03691441 (first received 1 October 2018). [DOI] [PMC free article] [PubMed]
Channir 2018 {published data only}
- Channir H, Isenberg AL, Rubek N, Lomholt AF, Scott S, Tvedskov JF, et al. Transoral robotic surgery for head and neck cancer. Ugeskrift for Laeger 2018;180(47):V05180341. [PubMed] [Google Scholar]
Lai 2021 {published data only}
- Lai SY, Torres-Saavedra PA, Dunlap NE, Beadle BM, Chang SS, Subramaniam RM, et al. NRG Oncology HN006: randomized phase II/III trial of sentinel lymph node biopsy versus elective neck dissection for early stage oral cavity cancer. Journal of Clinical Oncology 2021;39(Suppl 15):TPS6093. [Google Scholar]
- NCT04333537. Comparing sentinel lymph node (SLN) biopsy with standard neck dissection for patients with early-stage oral cavity cancer. www.clinicaltrials.gov/ct2/show/NCT04333537 (first received 3 April 2020).
NCT01334320 {unpublished data only}
- NCT01334320. Survival benefit of elective neck dissection in T1,2 N0 M0 oral squamous cell carcinoma. clinicaltrials.gov/ct2/show/NCT01334320 (first received 13 April 2011).
NCT0274382 {unpublished data only}
- NCT02743832. A study on tumor budding guiding individualized surgical planning of early-stage oral squamous cell carcinoma. clinicaltrials.gov/ct2/show/NCT02743832 (first received 19 April 2016).
NCT03385720 {unpublished data only}
- NCT03385720. Submandibular gland preservation during neck dissection for oral squamous cell carcinoma. clinicaltrials.gov/ct2/show/NCT03385720 (first received 28 December 2017).
NCT04738786 {unpublished data only}
- NCT04738786. Clinical study evaluating the proper surgical safety margin for early stage oral tongue cancers. www.clinicaltrials.gov/ct2/show/NCT04738786 (first received 4 February 2021).
Tankara 2018 (RESPOND) {published data only}
- Hanai N, Asakage T, Kiyota N, Homma A, Monden N, Fukushima H, et al. A randomized phase III study to evaluate the value of the omission of prophylactic neck dissection for stage I/II tongue cancer (RESPOND: jCOG1601). Annals of Oncology : Official Journal of the European Society for Medical Oncology 2018;29(Suppl 8):viii 396. [Google Scholar]
- Tanaka K, Hanai N, Eba J, Mizusawa J, Asakage T, Homma A, et al. Randomized phase III study to evaluate the value of omission of prophylactic neck dissection for stage I/II tongue cancer: Japan Clinical Oncology Group study (JCOG1601, RESPOND). Japanese Journal of Clinical Oncology 2018;48(12):1105-8. [DOI: 10.1093/jjco/hyy125] [PMID: ] [DOI] [PubMed] [Google Scholar]
- UMIN000030098. Randomized phase III study to evaluate the value of omission of prophylactic neck dissection for stage I/II tongue cancer (JCOG1601, RESPOND). center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000034135 (first received 24 November 2017). [UMIN000030098]
Wang 2019 {published data only}
- ChiCTR1800019128. IIb neck dissection or not in cT1-T2N0 oral cancer: a clinical randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=32298 (first received 26 October 2018).
- Wang L, Wang L, Song X, Cui C, Ma C, Guo B, et al. The necessity of IIb dissection in T1-T2N0M0 oral squamous cell carcinoma: protocol for a randomized controlled trial. Trials 2019;20(1):600. [DOI: 10.1186/s13063-019-3683-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adelstein 2009
Anantharaman 2016
- Anantharaman D, Muller DC, Lagiou P, Ahrens W, Holcátová I, Merletti F. Combined effects of smoking and HPV16 in oropharyngeal cancer. International Journal of Epidemiology 2016;45(3):752-61. [DOI: 10.1093/ije/dyw069. Epub 2016] [PMCID: PMC5841602.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anantharaman 2017
- Anantharaman D, Abedi-Ardekani B, Beachler DC, Gheit T, Olshan AF, Wisniewski K et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. International Journal of Cancer 2017;140(9):1968-75. [DOI: 10.1002/ijc.30608] [PMCID: PMC8969079] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ang 2010
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine 2010;363(1):24-35. [DOI: 10.1056/NEJMoa0912217. Epub 2010] [PMCID: PMC2943767] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bafeta 2012
- Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ 2012;344(1):e813. [DOI: 10.1136/bmj.e813] [PMCID: PMC3279328] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batsakis 1999
- Batsakis JG. Surgical excision margins: a pathologist's perspective. Advances in Anatomic Pathology 1999;6(3):140-8. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1991;50(4):1088-101. [PubMed] [Google Scholar]
Bouvard 2022
- Bouvard V, Nethan ST, Singh D, Warnakulasuriya S, Mehrotra R, Chaturvedi AK, et al. IARC Perspective on Oral Cancer Prevention. New England Journal of Medicine 2022;387(21):1999-2005. [DOI] [PubMed] [Google Scholar]
Bravi 2021
- Bravi F, Lee YA, Hashibe M, Boffetta P, Conway DI, Ferraroni M, et al. INHANCE Consortium investigators. Lessons learned from the INHANCE consortium: an overview of recent results on head and neck cancer. Oral Diseases 2021;27(1):73-93. [DOI: 10.1111/odi.13502] [PMCID: PMC7752834] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brennan 2017
- Brennan PA, Bradley KL, Brands M. Intensity-modulated radiotherapy in head and neck cancer – an update for oral and maxillofacial surgeons. British Journal of Oral and Maxillofacial Surgery 2017;55:770-4. [DOI] [PubMed] [Google Scholar]
Brizel 1998
- Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. New England Journal of Medicine 1998;338:1798-804. [DOI] [PubMed] [Google Scholar]
Brizel 2011
- Brizel DM, Lydiatt W, Colevas AD. Controversies in the locoregional management of head and neck cancer. Journal of National Comprehensive Cancer Network 2011;9(6):653-62. [DOI] [PubMed] [Google Scholar]
Bteich 2022
- Bteich YT, Hosri JE, Wehbi JA, Daou LR. Current landscape of clinical trials for HPV-positive head and neck squamous cell carcinoma (HNSCC). Ecancermedicalscience 2022;16(1):1447. [DOI: 10.3332/ecancer.2022.1447] [PCMID: PMC9666285] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carew 2003
- Carew JF, Singh B, Shah JP. Cervical lymph nodes. In: Shah JP, Johnson NW, Batsakis JG, editors(s). Oral Cancer. London: Martin Dunitz, 2003:215-49. [Google Scholar]
Chan 2015
- Chan KK, Glenny A-M, Weldon JC, Furness S, Worthington HV, Wakeford H. Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD010341. [DOI: 10.1002/14651858.CD010341.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaturvedi 2013
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of Clinical Oncology 2013;31(36):4550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conway 2015
- Conway DI, Brenner DR, McMahon AD, Macpherson LM, Agudo A, Ahrens W, et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. International Journal of Cancer 2015;136(5):1125-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creaney 2022
- Creaney G, McMahon AD, Ross AJ, Bhatti LA, Paterson C, Conway DI. Head and neck cancer in the UK: what was the stage before COVID-19? UK cancer registries analysis (2011-2018). British Dental Journal 2022;233(9):787-93. [DOI: 10.1038/s41415-022-5151-4] [PMCID: PMC9650177] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 1992
- Day GL, Blot WJ. Second primary tumours in patients with oral cancer. Cancer 1992;70(1):14-9. [DOI] [PubMed] [Google Scholar]
Deleyiannis 1997
- Deleyiannis FW, Weymuller EA, Coltrera MD. Quality of life of disease-free survivors of advanced (stage III or IV) oropharyngeal cancer. Head and Neck 1997;19(6):466-73. [DOI] [PubMed] [Google Scholar]
Dias 2001
- Dias FL, Kligerman J, Matos De Sa G, Arcuri RA, Freitas EQ, Farias T, et al. Elective neck dissection versus observation in stage I squamous carcinomas of the tongue and floor of the mouth. Otolaryngology, Head and Neck Surgery 2001;125:23-9. [DOI] [PubMed] [Google Scholar]
Duprez 2017
- Duprez F, Berwouts D, De Neve W, Bonte K, Boterberg T, Deron P, et al. Distant metastases in head and neck cancer. Head & Neck 2017;39(9):1733-43. [DOI] [PubMed] [Google Scholar]
Ebrahimi 2012
- Ebrahimi A, Ashford BG, Clark JR. Improved survival with elective neck dissection in thick early-stage oral squamous cell carcinoma. Head & Neck 2012;34(5):709-16. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elrefaey 2014
- Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngologica Italica 2014;34(5):299-309. [PMCID: PMC4299160] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Ewing 1952
- Ewing MR, Martin H. Disability following radical neck dissection. An assessment based on the postoperative evaluation of 100 patients. Cancer 1952;5:873-83. [DOI] [PubMed] [Google Scholar]
Fasunla 2011
- Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncology 2011;47(5):320-4. [DOI] [PubMed] [Google Scholar]
Ferlay 2020
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. International Agency for Research on Cancer, Lyon, France 2020.
Gillison 2000
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of National Cancer Institute 2000;92:709-20. [DOI] [PubMed] [Google Scholar]
Gillison 2015
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. Journal of Clinical Oncology 2015;33(29):3235-42. [DOI: 10.1200/JCO.2015.61.6995. Epub 2015 Sep 8] [PMCID: PMC4979086] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glenny 2010
- Glenny AM, Furness S, Worthington HV, Conway DI, Oliver R, Clarkson JE, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: radiotherapy. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD006387. [DOI: 10.1002/14651858.CD006387.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gormley 2022
- Gormley M, Creaney G, Schache A, Ingarfield K, Conway DI. Reviewing the epidemiology of head and neck cancer: definitions, trends and risk factors. British Dental Journal 2022;233(9):780-6. [DOI: 10.1038/s41415-022-5166-x] [PMCID: PMC9652141] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddadin 2000
- Haddadin KJ, Soutar DS, Webster MH, Robertson AG, Oliver RJ, MacDonald DG. Natural history and patterns of recurrence of tongue tumours. British Journal of Plastic Surgery 2000;53(4):279-85. [DOI] [PubMed] [Google Scholar]
Helliwell 2016
- Helliwell TR, Giles TE. Pathological aspects of the assessment of head and neck cancers: United Kingdom National Multidisciplinary Guidelines. Journal of Laryngology & Otology 2016;130(S2):S59-S65. [DOI: 10.1017/S0022215116000451] [PCMID: PMC4873923] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Wiley, 2011. Available from handbook.cochrane.org. [Google Scholar]
Higgins 2022
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. Wiley, 2022. [Google Scholar]
Holden 2018
- Holden AM, Sharma D, Schilling C, Gnanasegaran G, Odell EW, Sassoon I, et al. Biopsy of the sentinel lymph node in oral squamous cell carcinoma: analysis of error in 100 consecutive cases. British Journal of Oral and Maxillofacial Surgery 2018;56(7):615-20. [DOI: 10.1016/j.bjoms.2018.06.019] [DOI] [PubMed] [Google Scholar]
Hughes 1993
- Hughes CJ, Gallo O, Spiro RH, Shah JP. Management of occult neck metastases in oral cavity squamous carcinoma. American Journal of Surgery 1993;166(4):380-3. [DOI] [PubMed] [Google Scholar]
Hughes 2012
- Hughes C, Homer J, Bradley P, Nutting C, Ness A, Persson M, et al. An evaluation of current services available for people diagnosed with head and neck cancer in the UK (2009-2010). Clinical Oncology 2012;24(10):187-9. [DOI] [PubMed] [Google Scholar]
Kaanders 2022
- Kaanders JHAM, den Bosch S, Kleijnen J. Comparison of patients with head and neck cancer in randomized clinical trials and clinical practice: a systematic review. JAMA Otolaryngology—Head & Neck Surgery 2022;148(7):670-6. [DOI: 10.1001/jamaoto.2022.0890] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kowalski 2007
- Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngologica Italica 2007;27(3):113-7. [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Lo Nigro 2017
- Lo Nigro C, Denaro N, Merlotti A, Merlano M. Head and neck cancer: improving outcomes with a multidisciplinary approach. Cancer Management and Research 2017;18(9):363-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Louie 2015
- Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncology 2015;51(4):341-8. [DOI] [PubMed] [Google Scholar]
McGurk 2005
- McGurk M, Vhan C, Jones J, O'Regan E, Sherriff M. Delay in diagnosis and its effect on outcome in head and neck cancer. British Journal of Oral and Maxillofacial Surgery 2005;43(4):281-4. [DOI] [PubMed] [Google Scholar]
Mehanna 2016
- Mehanna H, Franklin N, Compton N, Robinson M, Powell N, Biswas-Baldwin N, et al. Geographic variation in human papillomavirus-related oropharyngeal cancer: data from 4 multinational randomized trials. Head and Neck 2016;38(1):E1863-69. [DOI: 10.1002/hed.24336.] [PMCID: PMC4869674] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Cancer Institute 2022
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. seer.cancer.gov/statfacts/html/oralcav.html (accessed February 2023) October 2022.
NICE 2018
- National Institute for Health and Care Excellence. Cancer of the upper aerodigestive tract: assessment and management in people aged 16 and over. www.nice.org.uk/guidance/ng36 (accessed prior to 6 December 2018). [www.nice.org.uk/guidance/ng36] [PubMed]
O Leary 2022
- O Leary B, Young A, Nutting C. Recent advances in the oncological management of head and neck cancer and implications for oral toxicity. British Dental Journal 2022;233(9):737-43. [DOI: 10.1038/s41415-022-5195-5. Epub 2022 Nov 11] [PMID: ] [DOI] [PubMed] [Google Scholar]
Paleri 2016
- Paleri V, Urbano TG, Mehanna H, Repanos C, Lancaster J, Roques T, et al. Management of neck metastases in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. Journal of Laryngology and Otology 2016;130(Suppl S2):S161-9. [DOI: 10.1017/S002221511600058X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analysis of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Parmar 2021
- Parmar A, Macluskey M, Mc Goldrick N, Conway DI, Glenny A-M, Clarkson JE, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD006386. [DOI: 10.1002/14651858.CD006386.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Partridge 2000
- Partridge M, Li SR, Pateromichelakis S, Francis R, Phillips E, Huang XH, et al. Detection of minimal residual cancer to investigate why oral tumors recur despite seemingly adequate treatment. Clinical Cancer Research 2000;6(7):2718-25. [PubMed] [Google Scholar]
Purkayastha 2016
- Purkayastha M, McMahon AD, Gibson J, Conway DI. Trends of oral cavity, oropharyngeal and laryngeal cancer incidence in Scotland (1975-2012) – a socioeconomic perspective. Oral Oncology 2016;61:70-5. [DOI] [PubMed] [Google Scholar]
Robbins 2002
- Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Archives of Otolaryngology - Head & Neck Surgery 2002;128(7):751-8. [DOI] [PubMed] [Google Scholar]
Schache 2016
- Schache AG, Powell NG, Cuschieri KS, Robinson M, Leary S, Mehanna H, et al. HPV-related oropharynx cancer in the United Kingdom: an evolution in the understanding of disease etiology. Cancer Research 2016;76(22):6598-606. [DOI: 10.1158/0008-5472.CAN-16-0633.] [PMCID: PMC9158514] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schilling 2015
- Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. European Journal of Cancer 2015;51:2777-84. [DOI] [PubMed] [Google Scholar]
Schilling 2017
- Schilling C, Shaw R, Schache A, McMahon J, Chegini S, Kerawala C, et al. Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? British Journal of Oral and Maxillofacial Surgery 2017;55:757-62. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Available from guidelinedevelopment.org/handbook. The GRADE Working Group, 2003. [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. Wiley, 2022. [Google Scholar]
Shah 1990
- Shah JP. Cervical lymph node metastases – diagnostic, therapeutic, and prognostic implications. Oncology (Williston Park, N.Y.) 1990;4(10):61-9. [PubMed] [Google Scholar]
Simard 2014
- Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncology 2014;50(5):387-403. [DOI] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Studer 2007
- Studer G, Zwahlen RA, Graetz KW, Davis BJ, Glanzmann C. IMRT in oral cavity cancer. Radiation Oncology 2007;2(16):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sung 2021
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer Journal for Clinicians 2021;17(3):209-49. [DOI: 10.3322/caac.21660] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sutton 2003
- Sutton DN, Brown JS, Rogers SN, Vaughan ED, Woolgar JA. The prognostic implications of the surgical margin in oral squamous cell carcinoma. International Journal of Oral and Maxillofacial Surgery 2003;32(1):30-4. [DOI] [PubMed] [Google Scholar]
Umeda 2010
- Umeda M, Shigeta T, Takahashi H, Oguni A, Katakoa T, Minamikawa T, et al. Shoulder mobility after spinal accessory nerve-sparing modified radical neck dissection in oral cancer patients. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2010;109:820-4. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2009
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncology 2009;45(4-5):309-16. [DOI] [PubMed] [Google Scholar]
WHO 1990
- World Health Organization. International Classification of Diseases for Oncology (ICD-O). 2nd edition. Geneva: World Health Organization, 1990. [Google Scholar]
WHO 2022
- WHO. Global oral health status report—towards universal health coverage for oral health by 2030. www.who.int/team/noncommunicable-diseases/global-status-report-on-oral-health-2022 (date accessed: February 2023) 18 November 2022.
Winn 2015
- Winn DM, Lee YC, Hashibe M, Boffetta P. The INHANCE consortium: toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Diseases 2015;21(6):685-93. [DOI] [PubMed] [Google Scholar]
Woolgar 2003
- Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncology 2003;39(2):130-7. [DOI] [PubMed] [Google Scholar]
Yang 2017
- Yang Y, Zhoug J, Wu H. Diagnostic value of sentinel node biopsy for cT1/T2N0 tongue squamous cell carcinoma: a meta-analysis. European Archives of Oto-Rhino-Laryngology 2017;274:3843-52. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bessell 2011
- Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD006205. [DOI: 10.1002/14651858.CD006205.pub3] [DOI] [PubMed] [Google Scholar]
Bulsara 2018
- Bulsara VM, Worthington HV, Glenny AM, Clarkson JE, Conway DI, Macluskey M. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD006205. [DOI: 10.1002/14651858.CD006205.pub4] [PMICD: PMC6517307] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2006
- Oliver RJ, Clarkson JE, Conway D, Glenny AM, Macluskey M, Pavitt S, Sloan P, The CSROC Expert PA, Worthington HV. Interventions for the treatment of oral cancer: surgical treatment. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006205. [DOI: 10.1002/14651858.CD006205] [DOI] [PubMed] [Google Scholar]
Oliver 2007
- Oliver R, Clarkson JE, Conway D, Glenny AM, Macluskey M, Pavitt S, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006205. [DOI: 10.1002/14651858.CD006205.pub2] [DOI] [PubMed] [Google Scholar]


