Abstract
Introduction Trigeminal schwannomas (TS) are rare skull base tumors that have been associated with significant neuropathic sequalae for patients. The authors aim to evaluate the clinical features, treatment outcomes, and neuropathic sequelae following endoscopic endonasal approach (EEA) for TS.
Methods The study involves a retrospective review of patients who underwent EEA for resection of TS at a single academic institution between 2004 and 2020. Radiographic and clinical data were recorded and analyzed.
Results A total of 16 patients were abstracted, with a mean age at the time of surgery of 44 years with a slight female (1.83:1) predominance. Primary preoperative symptomatology included facial pain/neuralgia ( n = 5, 31.3%), facial hypoesthesia ( n = 4, 25.0%), and headache ( n = 4, 25.0%). Following TS resection, patients were found to have facial hypoesthesia ( n = 11, 68.8%), neuropathic keratopathy ( n = 4, 25.0%), and mastication musculature atrophy ( n = 3, 18.8%). Patients with preoperative facial pain/neuralgia ( n = 5, 31.3%) were significantly more likely to try adjunctive pain therapies ( p = 0.018) as well as seek pain consultation ( p = 0.018). Patients with preoperative migraines ( n = 2, 12.5%) were significantly more likely to trial adjunctive pain therapies ( p = 0.025) and undergo evaluation with pain specialists ( p = 0.025). Finally, patients with preoperative pharmacologic agent utilization were significantly more likely to trial adjunctive pain therapies ( p = 0.036) and pursue pain consultation ( p = 0.036).
Conclusion Some degree of trigeminal dysfunction may be more common than previously reported following EEA for TS resection. Factors that appear to play a role in the development of trigeminal dysfunction include pre-existing pain syndromes such as facial pain/neuralgia or headache and preoperative medication utilization.
Keywords: endoscopic endonasal approach, trigeminal neuropathy, outcomes, trigeminal nerve, trigeminal schwannoma
Introduction
Schwannoma (neurilemoma) is a benign, slow-growing tumor of ectodermal origin derived from Schwann cells. Trigeminal schwannomas (TS) are a rare clinical entity, constituting approximately 0.36% of all intracranial tumors and 8% of intracranial schwannomas. 1 2 3 TS may arise from the trigeminal nerve root, the Gasserian ganglion, or one of the three peripheral branches; thus, the growth patterns of these tumors are extraordinarily complex. In 1955, Jefferson classified TS according to their principal location: tumors may be restricted to the middle cranial fossa (Type 1), posterior cranial fossa (Type 2), or extend across multiple compartments (Type 3). 4 Depending on the site of origin and growth pattern, these tumors have varied clinical manifestations (i.e., facial pain, headache, hypoesthesia, etc.) and unique radiographic imaging characteristics. Historically, surgery has been the preferred treatment for TS. Surgical outcomes are suboptimal, as they may not achieve gross-total or near-total resection. 1 5 6 Cushing and Eisenhardt were particularly pessimistic about TS, especially dumbbell-shaped tumors with both infratentorial and supratentorial components which they described as “sit[ting] like a saddle astride the anterior end of the petrous ridge.” Surgical approaches for TS, they believed, were ” [more] likely to hasten the inevitable than to prolong life or alleviate symptoms.” 7
Fortunately, significant advances in microsurgical techniques, endoscopic endonasal approaches (EEAs), and electrophysiological monitoring have allowed for notable improvements toward achieving the surgical goal of complete tumor resection with minimal morbidity. In recent years, reports of functional outcomes following EEA for TS resection are favorable with a low rate of surgical complications. 8 9 10 11 Despite these developments, limited information exists regarding the neuropathic sequelae associated with TS. In an effort to better understand long-term results, this work aims to explore trigeminal dysfunction following TS surgery.
Methods
Study Design and Subject Selection
A retrospective review was performed utilizing the University of Pittsburgh Medical Center Cranial Base Surgery Database to abstract all patients who underwent EEA for TS resection from 2004 to 2020. Clinical data collected include age, gender, tumor location, radiographic imaging, prior treatment, surgical approach, degree of resection, complication profile, radiation therapy, disease course, and follow-up period. Neuropathic parameters abstracted include perioperative symptomatology, pharmacologic agents, pain syndromes, adjunctive therapies, and specialist evaluations. This study was approved by the Institutional Review Board at the University of Pittsburgh Medical Center.
Surgical Technique
All TS resections were performed by the same surgical team comprised of an otolaryngologist and neurosurgeon utilizing an EEA. 8 In the supine position, the patient's head is secured in 3-pin Mayfield Head Holder and held in a neutral position with a slight neck turn to the right. The use of a triplanar imaging navigation system is employed for identification of key surgical landmarks and assessment of tumor resection margins. Throughout all operations, somatosensory evoked potentials and cranial nerve electromyography (V 3, mandibular division) were utilized to allow for safe microsurgical dissection of TS with functional preservation of nerve fibers and adjacent cranial nerves. In TS cases with Meckel's cave involvement, oculomotor nerve electromyography was also monitored.
Surgical exposure was then performed in the coronal plane (level III-IV procedure) as defined by Snyderman and colleagues, 12 which chiefly includes a transpterygoid approach 13 ± anterior maxillotomy 14 to provide an adequate corridor for tumor extirpation. The transpterygoid approach provides access to the anteromedial part of Meckel's cave, anterior wall of the cavernous sinus, and paraclival internal carotid artery. An anterior maxillotomy (Caldwell-Luc procedure) provides additional access to the anterior, lateral, and posterior portions of Meckel's cave and middle cranial fossa. Microsurgical TS tumor resection was performed using a Kartush Dissector (The Magstim Company Limited, Spring Gardens, Whitland, Carmarthenshire, United Kingdom) to preserve motor fibers of the trigeminal nerve and identify adjacent cranial nerves. In cases with Meckel's cave involvement, oculomotor nerve electromyography was also monitored. Standard nasoseptal flap harvest was performed for some patients, particularly in cases where an intradural dissection was anticipated; this technique was performed as described by Hadad and Bassagasteguy. 15 Following reconstruction, resorbable nasal packing (Gelfoam and Surgicel) followed by nonresorbable nasal packing (Merocel) is placed to gently buttress the surgical site and minimize the risk of construct migration during the immediate postoperative period.
Statistical Analyses
Mann-Whitney U test was used to compare continuous variables such as tumor size between independent groups. Fisher's Exact test was used to compare all other categorical variables. p -Values were all two-tailed and significance was set at p < 0.05 level. Statistical analysis was conducted on SPSS Version 27 (IBM Corporation Armonk, New York, United States).
Results
Clinical Characteristics
A total of 16 patients were included in this analysis, with a mean age at the time of surgery of 44 years (range, 19–88 years) with a slight female (1.83:1) predominance. Thirteen patients underwent single-stage EEA and three patients required multistage surgery in the form of repeat EEA ( n = 1, 6.3%) or retromastoid craniotomy (RMC) ( n = 2, 12.5%). Four patients reported a prior history of craniotomy; patients 5, 6, 7, and 11 underwent surgery 40, 10, 7, and 1 years prior, respectively. Two patients had undergone previous radiation therapy; patient 6 received Gamma Knife Radiosurgery (GKRS) 8 and 10 years prior and patient 5 received external beam radiation therapy >10 years prior to their initial surgery for TS ( Table 1 ). Preoperative radiographic imaging obtained included both computed tomography (CT) and magnetic resonance imaging (MRI) for 87.5% of patients and solely CT imaging in 12.5% of patients. Predominant tumor location across the course of the trigeminal nerve included Meckel's Cave ( n = 6, 37.5%),V 2 ( n = 2, 12.5%), and V 3 ( n = 8, 50.0%), with a median tumor area of 1,100 mm 2 (95% CI 783–1,620 mm 2 ) ( Table 1 ). Radiographically, six cases (patients 4, 7, 9, 10, 11, 15) had TS extension into the posterior cranial fossa. Neither tumor location nor size was significantly associated with any of the indexed categorical pain variables.
Table 1. Series characteristics of endoscopic endonasal approach for trigeminal schwannoma resection.
| Patient | Gender | Age | Prior treatment | Radiographic imaging | Tumor location | Surgical approach | Intraoperative CSF leak | Reconstruction | Complications | GTR | NTR | STR | Surveillance imaging (# Scans) | Recurrence | Persistent disease | GKRS | Revision surgery | Follow-up (mo) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Radiation | Modality | Size (mm) | Transpterygoid | Maxillotomy | Vessel embolization/ligation | CT | MRI | |||||||||||||||
| 1 | F | 24 | N | N | CT | 37 × 30 | R V 2 | Y | N | Y (IMAX) | N | Tisseel | None | Y | N | N | 0 | 3 | N | N | N | N | 15 |
| 2 | F | 34 | N | N | CT | 88 × 49 | L V 3 | Y | N | Y (IMAX) | N | None | Left VI Palsy | N | N | Y | 1 | 10 | N/A | Y | Y | N | 137 |
| 3 | F | 46 | N | N | CT/MR | 20 × 19 | L MC | Y | N | N | N | NSF | None | Y | N | N | 0 | 4 | N | N | N | N | 63 |
| 4 | F | 19 | N | N | CT/MR | 30 × 28 | R MC | Y | N | N | Y | Fat + NSF | None | N | Y | N | 5 | 15 | N | Y | Y | Y (RMC) | 124 |
| 5 | M | 57 | Y | Y | CT/MR | 64 × 49 | L V 3 | Y | N | N | N | Fat + NSF | None | N | Y | N | 1 | 1 | N/A | Y | N | N | 1 |
| 6 | M | 25 | Y (RMC) | Y | CT/MR | 38 × 31 | L MC | Y | N | N | Y | NSF | Left VI Palsy | N | N | Y | 0 | 7 | N/A | Y | N | N | 115 |
| 7 | M | 50 | Y (RMC) | N | CT/MR | 45 × 42 | R V 3 | Y | N | N | Y | NSF | None* | N | N | Y | 2 | 10 | N/A | Y | Y | Y (RMC) | 66 |
| 8 | F | 24 | N | N | CT/MR | 60 × 20 | L V 3 | Y | Y | N | Y | NSF | None | Y | N | N | 5 | 12 | Y | N/A | Y | Y (EEA) | 115 |
| 9 | F | 20 | N | N | CT/MR | 24 × 21 | L MC | Y | N | N | Y | NSF | None | Y | N | N | 2 | 3 | N | N | N | N | 38 |
| 10 | F | 88 | N | N | MR | 49 × 38 | L V 3 | Y | N | N | N | Tisseel | None | N | Y | N | 1 | 1 | N | N | N | N | 1 |
| 11 | F | 37 | Y (RMC) | N | CT/MR | 19 × 16 | L MC | Y | N | N | Y | NSF | None | Y | N | N | 1 | 7 | N | N | N | N | 78 |
| 12 | F | 41 | N | N | CT/MR | 29 × 27 | R V 3 | Y | N | N | N | Tisseel | None | Y | N | N | 1 | 6 | N | N | N | N | 95 |
| 13 ⸸ | F | 61 | N | N | CT/MR | 43 × 32 | L V 3 | Y | Y | N | N | Tisseel | None | Y | N | N | 1 | 5 | N | N | N | N | 36 |
| 14 | F | 61 | N | N | CT/MR | 20 × 15 | L MC | Y | N | N | N | Fat + NSF | L ICA Injury | N | Y | N | 2 | 7 | N/A | Y | Y | N | 48 |
| 15 | M | 54 | N | N | CT/MR | 39 × 28 | L V 3 | Y | N | N | N | Fat + NSF | None | N | N | Y | 2 | 3 | N | N | N | N | 14 |
| 16 | M | 55 | N | N | CT/MR | 54 × 30 | L V 2 | Y | Y | Y (IMAX) | N | NSF | None | Y | N | N | 0 | 3 | N | N | N | N | 10 |
Stage I transcervical excision for parapharyngeal tumor extension.
Surgical Characteristics
All patients underwent a transpterygoid approach as described by Fortes and colleagues 13 to provide an adequate corridor to allow for TS resection. Three patients also required an anterior maxillotomy 14 as well as internal maxillary artery embolization/ligation given tumor extension into the parapharyngeal and infratemporal fossae. An intraoperative cerebrospinal fluid leak was encountered in 37.5% of cases ( n = 6), which were uniformly repaired with abdominal fat followed by nasoseptal flap reconstruction with no instances of postoperative cerebrospinal fluid leak. Multistage resection was required in three cases (patients 4, 7, and 8: two [RMC] planned a month later and the other [EEA] 3 years later ).
With respect to degree of resection, eight patients (50.0%) underwent gross total resection, four patients (25.0%) underwent near-total (>90%) resection, and four patients (25.0%) underwent subtotal resection. Complications were uncommon in this series, with two cases of transient abducens nerve palsy (12.5%) and one case of an internal carotid artery injury (6.3%) that was repaired endoscopically with a non-occlusive aneurysm clip without postoperative neurological deficit ( Table 2 ). The latter was followed for 5 years with yearly MR angiograms without pseudoaneurysm formation or carotid artery disease.
Table 2. Neuropathic parameters for trigeminal schwannoma patients.
| Patient | Preoperative | Postoperative | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptomatology | Pharmacologic agents | Pain syndrome | Symptomatology | Pharmacologic Agents | Adjunctive therapies | Pain syndrome | Consultations | ||||||||||||
| Diplopia | Facial Pain | Headache | Hypoesthesia | Analgesics | Antidepressants | Neuromodulators | Hypoesthesia | Neuropathic keratopathy | Mastication musculature atrophy | Analgesics | Antidepressants | Neuromodulators | Migraines | Trigeminal neuropathy | Neurology | Pain | |||
| 1 | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 2 | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N |
| 3 | Y | N | N | N | N | N | N | N | Y | Y | N | N | N | N | N | N | N | N | N |
| 4 | N | N | Y | N | N | N | N | N | Y | N | Y | Y (Excedrin, Naproxen, Flurbiprofen, Ibuprofen, Indomethacin, Tylenol) | Y (Nortriptyline, Duloxetine) | Y (Gabapentin) | N | Y | Y | Y | N |
| 5 | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | |
| 6 | N | Y | N | N | N | N | N | N | Y | Y | N | N | N | N | N | N | N | N | N |
| 7 | N | N | N | Y | N | N | N | N | Y | Y | N | N | N | N | N | Y | N | N | N |
| 8 | N | Y | N | N | Y (Hydrocodone) | N | N | N | Y | N | Y | Y (Ibuprofen, Percocet, Tramadol) | Y (Fluoxetine, Sertraline, Nortriptyline) | Y (Carbamazepine, Gabapentin, Rizatriptan, Sumatriptan) | Y (Chiropractic Manipulation, Acupuncture) | Y | Y | Y | Y |
| 9 | N | N | N | Y | Y (Tylenol) | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 10 | N | Y | Y | N | Y (Tylenol #3) | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 11 | N | Y | N | N | Y (Ibuprofen) | N | Y (Gabapentin) | Y (Migraines) | Y | Y | Y | N | N | Y (Carbamazepine, Gabapentin, Pregabalin) | Y (C1-C2 Nerve Blocks) | Y | Y | Y | Y |
| 12 | N | Y | Y | N | N | Y (Sertraline) | Y (Gabapentin, Topiramate, Almotriptan) | Y (Migraines) | Y | N | N | N | N | Y (Carbamazepine, Gabapentin, Topiramate) | Y (Botulinum Toxin Injections) | Y | Y | Y | Y |
| 13 | N | N | N | Y | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N |
| 14 | Y | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 15 | N | N | Y | N | N | N | Y (Gabapentin) | N | N | N | N | N | N | N | N | N | N | N | N |
| 16 | N | N | N | Y | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | N |
Neuropathic Parameters
Primary preoperative symptomatology included facial neuralgia/pain ( n = 5, 31.3%), facial hypoesthesia ( n = 4, 25.0%), headache ( n = 4, 25.0%), and diplopia ( n = 2, 12.5%). With regards to preoperative trigeminal motor function, only one patient (6.3%) was found to have a V 3 motor deficit. Two patients carried a preoperative diagnosis of migraines ( n = 2, 12.5%) and preoperative pharmacologic agents included analgesics ( n = 4, 25.0%), antidepressants ( n = 1, 6.3%), and neuromodulators ( n = 3, 18.8%). Following TS resection, patients were found to have facial hypoesthesia ( n = 11, 68.8%), neuropathic keratopathy ( n = 4, 25.0%), and atrophy of mastication musculature ( n = 3, 18.8%). All cases of neuropathic keratopathy were closely followed by ophthalmology without the need for surgical intervention and patients with preoperative diplopia ( n = 2, 12.5%) had resolution of their visual symptoms. New trigeminal motor dysfunction following TS resection occurred in two patients. During the study period, the number of patients formally diagnosed by a neurologist or pain specialist postoperatively with trigeminal neuropathy ( n = 4, 25.0%) and migraines ( n = 5, 31.3%) considerably increased following TS resection. Absolute improvement in trigeminal nerve function, defined as either sensory and/or motor recovery following TS resection, was observed in 40% of patients.
Preoperative pharmacologic agent utilization ( n = 5, 31.3%) was significantly associated with trialing adjunctive pain therapies ( p = 0.036) and seeking intervention with pain specialists ( p = 0.036) postoperatively. Similarly, patients with preoperative facial pain/neuralgia ( n = 5, 31.3%) were also significantly more likely to postoperatively trial adjunctive pain therapies ( p = 0.018) as well as seek consultation with pain specialists ( p = 0.018). Finally, patients with a preoperative diagnosis of migraines ( n = 2, 12.5%) were significantly more likely to trial adjunctive pain therapies ( p = 0.025) and seek evaluation with pain specialists ( p = 0.025) ( Table 3 ).
Table 3. Effect of preoperative clinical factors of trigeminal schwannoma on postoperative management.
| Postoperative management | Preoperative pharmacologic agents | Preoperative facial pain | Preoperative migraines |
|---|---|---|---|
| Adjunctive therapies | p = 0.036 a | p = 0.018 a | p = 0.025 a |
| Prolonged pharmacologic agent utilization | p = 0.300 | p = 0.245 | p = 0.083 |
| Postoperative pain syndrome | p = 0.300 | p = 0.245 | p = 0.083 |
| Neurology consultation | p = 0.118 | p = 0.063 | p = 0.050 |
| Pain consultation | p = 0.036 a | p = 0.018 a | p = 0.025 a |
Fisher's Exact Test: significance p <0.05.
Surveillance
The mean follow-up period was 59.8 months (range, 1–137 months), with an average of two CT and six MRI surveillance scans performed during this timeframe ( Fig. 1 ). Specifically, four patients (25.0%) underwent GKRS and one patient (6.3%) underwent RMC for persistent disease. Postoperative GKRS was associated with revision surgery ( p = 0.025) as it was utilized to treat persistent or recurrent disease. With regards to recurrent disease, one patient required repeat EEA + GKRS (6.3%) that was detected on radiographic imaging 23 months after the index EEA. Finally, it appears that larger tumors trended toward persistent or recurrent disease ( p = 0.055, 1,200 mm 2 95% CI 840–4312 mm 2 vs. 1092 mm 2 95% CI 504–1620 mm 2 ) but did not correlate with the above pain variables.
Fig. 1.
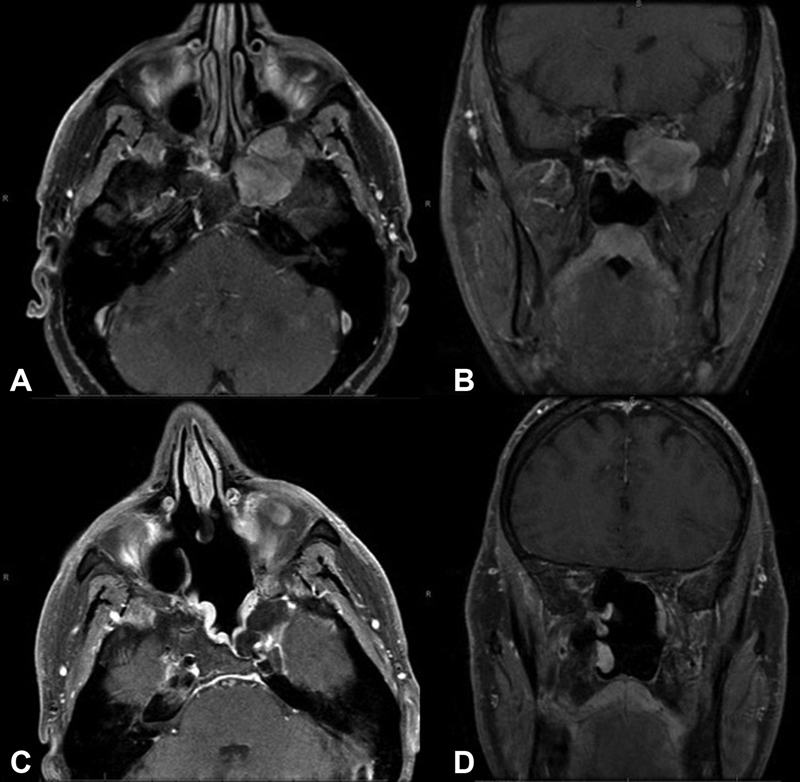
Radiographic MRI Imaging. Preoperative MRI axial (A) and coronal (B) T1-weighted sequences with contrast of a left trigeminal schwannoma. Postoperative MRI axial (C) and coronal (D) images T1-weighted sequences with contrast demonstrate gross-total resection and expected enhancement of nasoseptal flap reconstruction.
Discussion
“It is possible of course that a method may someday be evolved whereby a Gasserian neurinoma…even after it has crossed the ridge, may be safely approached and removed. Should this come to pass, it will be another conquest for neurosurgery.” 7 In the modern era, it appears the surgical management of TS is steadily approaching Dr. Cushing's prediction. Despite its low incidence, it is apparent that, with rare exceptions, TS are benign tumors for which complete surgical resection can offer patients a durable cure. 16 In this series, 75% of patients were able to undergo a gross-total or near-total resection, with only one patient requiring repeat EEA + GKRS for recurrent disease and four patients requiring GKRS for persistent disease (25%) during the follow-up period. Not surprisingly, GKRS utilization was associated with revision surgery ( p = 0.025). This is comparable to prior descriptions utilizing EEA for TS, which includes gross-total and near-total resection data by Park 17 ( n = 19, 75%) and Raza 9 ( n = 3, 75%). However, it is important to note the small number of cases reported by Raza and Shin as well as the fact that a transorbital approach was exclusively employed for 12 subjects in the Park study, which skews gross-total and near-total outcomes from a purely EEA perspective. This report details the surgical experience of 16 EEA cases for TS over 17 years which includes some hybrid approaches utilized for complex cases. In comparison, the GKRS experience at UPMC for TS has been previously described with 50 patients from 1989 to 2017, 17 of which underwent prior surgery. 18
EEA for TS offers unique advantages compared with open lateral cranial base approaches as it obviates the need for temporal lobe exposure or retraction, avoids unnecessary manipulation of adjacent cranial nerves, and provides a direct corridor for tumor ablation via the transpterygoid approach ± anterior maxillotomy. This is increasingly evident when the pattern of iatrogenic cranial nerve dysfunction for lateral skull base approaches is examined; Samii 2 (8.3%, facial nerve), Al-Mefty 19 (8.0%, abducens nerve and 3.7%, trochlear nerve), Goel 16 (4.1%, abducens nerve, 2.7%, trochlear nerve, facial nerve, 2.7%, and vestibulocochlear nerve, 2.7%), and Wanibuchi 20 (oculomotor nerve, 1.9%) describe a variety of cranial nerve deficits following TS resection with a major complication rate ranging from 1.4 to 33% and a mortality rate of 2.7%. 4 Complications following EEA for TS were uncommon in this cohort; two patients (12.5%) developed a transient abducens nerve palsy and one patient (6.3%) had an internal carotid artery injury which was addressed with an endoscopic aneurysm clip without neurological sequala. This is analogous to findings reported by Park 17 ( n = 1, middle cerebral artery vasospasm) and Raza 9 ( n = 1, abducens nerve palsy). Although EEA does not replace conventional skull base approaches, it does provide another option in the management of this complex pathology, especially V 3 and some V 2 tumors.
This study is the first to critically evaluate trigeminal dysfunction following EEA for TS. In general, the clinical features of TS vary according to the site of origin as well as the direction and extent of growth. 21 The degree of trigeminal nerve dysfunction for patients presenting with TS has been cited at approximately 70 to 95%. 22 In this series, primary preoperative symptomatology includes facial pain/neuralgia ( n = 5, 31.3%), facial hypoesthesia ( n = 4, 25.0%), headache ( n = 4, 25.0%), and diplopia ( n = 2, 12.5%). This is in line with prior reports of preoperative facial pain for TS, which varies from 10 to 45%. 21 In this analysis, patients with preoperative facial pain/neuralgia ( n = 5, 31.3%) were also significantly more likely to trial adjunctive pain therapies ( p = 0.018) as well as seek consultation with pain specialists ( p = 0.018) postoperatively. These findings highlight the fact that complete pain resolution following TS resection may not be durable in long term, despite short-term data supporting facial pain improvement (73–100%) postoperatively. 22
Following TS resection, patients were found to have facial hypoesthesia ( n = 11, 68.8%), neuropathic keratopathy ( n = 4, 25.0%), and atrophy of mastication musculature ( n = 3, 18.8%). All cases of neuropathic keratopathy were closely followed by ophthalmology without the need for surgical intervention and patients with preoperative diplopia ( n = 2, 12.5%) had resolution of their visual symptoms following surgery. The rate of neuropathic keratopathy appears to be also similar to what has been described by Raza 9 ( n = 1, 25%), and Shin 8 ( n = 3, 27.3%). Previous studies have also demonstrated that trigeminal sensory disturbance improved in only 19 to 44% of patients and preoperative trigeminal sensation deteriorated in 20 to 70% of cases. 22 For this group, a net change of 48.8% ( n = 7) was appreciated between preoperative and postoperative facial hypoesthesia. Despite these findings, it still compares favorably to open cranial base approaches for TS, where 70% of patients had worsened trigeminal sensory deficits and 56% of patients had worsened trigeminal motor deficits. 23 In summary, it appears that trigeminal sensory function will rarely if ever fully recover if present preoperatively and atrophy of mastication musculature remains permanent due to V 3 denervation. 16
Preoperative pharmacologic agent utilization ( n = 5, 31.3%) was significantly associated with trialing adjunctive pain therapies ( p = 0.036) and seeking intervention with pain specialists ( p = 0.036). In an analysis of 38 patients with benign skull base tumors undergoing GKRS ( n = 5, TS), 18 patients (58%) were taking neuromodulators specifically for their facial pain (carbamazepine [ n = 13], gabapentin [ n = 4], lamotrigine [ n = 1], and pregabalin [ n = 1]). 24 In this study, preoperative pharmacologic agents taken by TS patients include analgesics ( n = 4, 25.0%), antidepressants ( n = 1, 6.3%), and neuromodulators ( n = 3, 18.8%). Interestingly, only 25% of patients who underwent GKRS maintained durable relief of tumor-related trigeminal pain without the need for pharmacologic agents. 24 This is the first surgical cohort to shed light on pharmacologic agent utilization in TS; further analysis is required to shed light on the exact role it may play in the control of perioperative symptomatology.
Headaches ( n = 4, 25.0%) appear to be a common preoperative symptom in TS patients with rates ranging from 16 to 74% 2 25 ; reduction of headaches was noted in two patients following TS resection in this analysis. Naturally, patients with a preoperative diagnosis of migraines ( n = 2) continued to have headaches following TS resection. However, the number of patients with postoperative migraines ( n = 5, 31.3%) increased following TS resection. Exploring this result further, it appears patients with a preoperative diagnosis of migraines ( n = 2, 12.5%) were significantly more likely to trial adjunctive pain therapies ( p = 0.025) and seek evaluation with pain specialists ( p = 0.025). These findings emphasize the importance of assessing relevant comorbidities when considering surgical intervention for TS and counseling patients on their particular long-term course. Table 2 demonstrates that a preoperative pain syndrome results in a postoperative pain syndrome, which was managed with neuromodulators and adjunctive therapies. Of the remaining 14 patients without a preoperative pain syndrome, three patients developed a postoperative pain syndrome. These cases were similarly treated with pharmacologic as well as adjunctive therapies. In addition, 40% of patients had improvement in trigeminal function. By comparison, the GKRS experience at UPMC demonstrates the rate of improvement for pain syndrome following treatment at roughly 44%. 18 Concerning degree of resection, 75% of patients achieved either a gross total (50%) or near-total resection (25%) via EEA. EEA is one of many tools that should be considered in the multimodality treatment paradigm of TS. Certainly, additional research is warranted to establish direct causality for both pain and the potential for nerve injury with resection must be considered.
Limitations
This study is limited by its small sample size secondary to disease process rarity and retrospective design. This restricts the ability to trend key parameters in trigeminal dysfunction, particularly quality of life and patient-reported outcome measures which would capture alterations in pain, sensation, and motor function over time. Given the retrospective nature of this analysis, documentation of perioperative trigeminal nerve function as neuralgia, neuropathy, etc. was inconsistently characterized. It is clear that to better understand the intricate complexities of the trigeminal nerve and its dysfunction, future studies will require prospective classification of these key details with established trigeminal nerve parameters. In an attempt to address this deficiency, previously described preoperative trigeminal nerve nomenclature by Niranjan and colleagues 18 was employed in this comprehensive analysis of surgical outcomes. Postoperative classification remains even more difficult to categorize given acute pain versus chronic pain as well as multimodality treatments that could potentially be employed to address clinical symptoms. For this reason, long-term pain syndromes as defined by neurologists and chronic pain specialists were specifically captured during the surveillance period to standardize outcomes. In addition, approach selection biases toward V 2 and V 3 schwannomas may impact outcomes for certain complications such as corneal keratopathy. However, the data collected in this clinical work represents decades of experience with TS at a single institution, with the largest endoscopic cohort for TS to date in the literature with a robust follow-up period and delineation of long-term trigeminal dysfunction. Further prospective studies are essential in understanding the natural history of TS as well as developing a standardized grading system to accurately assess trigeminal neuropathy in the extended perioperative period.
Conclusion
This series demonstrates long-term neuropathic sequelae associated with EEA for TS resection in which 75% of patients underwent gross total or near-total resection. Factors that appear to play a role in the development of trigeminal dysfunction include preoperative facial pain/neuralgia, medication utilization, and preexisting pain or headache syndromes. Additional prospective studies are necessary to further elucidate the natural history of trigeminal neuropathy as well as develop a standardized grading system to accurately assess trigeminal dysfunction.
Conflict of Interest Dr. Snyderman is a consultant for SPIWay, LLC; Dr. Gardner is a consultant for Peter Lazic US, Inc. and SPIWay, LLC.
Note
This manuscript was presented as a virtual on-demand presentation at the North American Skull Base Society Annual Meeting on February 13, 2021.
References
- 1.Lesoin F, Rousseaux M, Villette Let al. Neurinomas of the trigeminal nerve Acta Neurochir (Wien) 198682(3-4):118–122. [DOI] [PubMed] [Google Scholar]
- 2.Samii M, Migliori M M, Tatagiba M, Babu R. Surgical treatment of trigeminal schwannomas. J Neurosurg. 1995;82(05):711–718. doi: 10.3171/jns.1995.82.5.0711. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Kawase T. Trigeminal neurinomas extending into multiple fossae: surgical methods and review of the literature. J Neurosurg. 1999;91(02):202–211. doi: 10.3171/jns.1999.91.2.0202. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson G. The trigeminal neurinomas with some remarks on malignant invasion of the gasserian ganglion. Clin Neurosurg. 1953;1:11–54. doi: 10.1093/neurosurgery/1.cn_suppl_1.11. [DOI] [PubMed] [Google Scholar]
- 5.Arseni C, Dumitrescu L, Constantinescu A. Neurinomas of the trigeminal nerve. Surg Neurol. 1975;4(06):497–503. [PubMed] [Google Scholar]
- 6.Schisano G, Olivecrona H. Neurinomas of the Gasserian ganglion and trigeminal root. J Neurosurg. 1960;17:306–322. doi: 10.3171/jns.1960.17.2.0306. [DOI] [PubMed] [Google Scholar]
- 7.Cushing H EL. Springfield, Ill: Charles C Thomas; 1938. Meningiomas: Their Classification, Regional Behavior, Life History, and Surgical End Results. [Google Scholar]
- 8.Shin S S, Gardner P A, Stefko S T, Madhok R, Fernandez-Miranda J C, Snyderman C H.Endoscopic endonasal approach for nonvestibular schwannomas Neurosurgery 201169051046–1057., discussion 1057 [DOI] [PubMed] [Google Scholar]
- 9.Raza S M, Donaldson A M, Mehta A, Tsiouris A J, Anand V K, Schwartz T H. Surgical management of trigeminal schwannomas: defining the role for endoscopic endonasal approaches. Neurosurg Focus. 2014;37(04):E17. doi: 10.3171/2014.7.FOCUS14341. [DOI] [PubMed] [Google Scholar]
- 10.Karligkiotis A, Turri-Zanoni M, Sica E et al. Role of endoscopic surgery in the management of sinonasal and skull base schwannomas. Head Neck. 2016;38 01:E2074–E2082. doi: 10.1002/hed.24383. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Chen J, Chen T et al. Neuroendoscopic resection of trigeminal schwannoma in the pterygopalatine/infratemporal fossa via the transnasal perpendicular plate palatine bone or transnasal maxillary sinus approach. World Neurosurg. 2018;120:e1011–e1016. doi: 10.1016/j.wneu.2018.08.216. [DOI] [PubMed] [Google Scholar]
- 12.Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello D M. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117(04):699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]
- 13.Fortes F S, Sennes L U, Carrau R L et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118(01):44–49. doi: 10.1097/MLG.0b013e318155a492. [DOI] [PubMed] [Google Scholar]
- 14.Truong H Q, Sun X, Celtikci E et al. Endoscopic anterior transmaxillary “transalisphenoid” approach to Meckel's cave and the middle cranial fossa: an anatomical study and clinical application. J Neurosurg. 2018;130(01):227–237. doi: 10.3171/2017.8.JNS171308. [DOI] [PubMed] [Google Scholar]
- 15.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 16.Goel A, Muzumdar D, Raman C.Trigeminal neuroma: analysis of surgical experience with 73 cases Neurosurgery 20035204783–790., discussion 790 [DOI] [PubMed] [Google Scholar]
- 17.Park H H, Hong S D, Kim Y H et al. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: a retrospective multicenter analysis (KOSEN-005) J Neurosurg. 2020;133(02):467–476. doi: 10.3171/2019.3.JNS19492. [DOI] [PubMed] [Google Scholar]
- 18.Niranjan A, Raju S S, Kano H, Flickinger J C, Lunsford L D. Clinical and imaging response to trigeminal schwannoma radiosurgery: a retrospective analysis of a 28-year experience. J Neurol Surg B Skull Base. 2021;82(05):491–499. doi: 10.1055/s-0040-1714110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded Meckel cave and outcomes of cranial nerve function. J Neurosurg. 2002;96(03):453–463. doi: 10.3171/jns.2002.96.3.0453. [DOI] [PubMed] [Google Scholar]
- 20.Wanibuchi M, Fukushima T, Zomordi A R, Nonaka Y, Friedman A H.Trigeminal schwannomas: skull base approaches and operative results in 105 patientsNeurosurgery 2012;70(suppl operative 1):132–143, discussion 143–144 [DOI] [PubMed]
- 21.Pollack I F, Sekhar L N, Jannetta P J, Janecka I P. Neurilemomas of the trigeminal nerve. J Neurosurg. 1989;70(05):737–745. doi: 10.3171/jns.1989.70.5.0737. [DOI] [PubMed] [Google Scholar]
- 22.MacNally S P, Rutherford S A, Ramsden R T, Evans D G, King A T. Trigeminal schwannomas. Br J Neurosurg. 2008;22(06):729–738. doi: 10.1080/02688690802272172. [DOI] [PubMed] [Google Scholar]
- 23.Taha J M, Tew J M, Jr, van Loveren H R, Keller J T, el-Kalliny M. Comparison of conventional and skull base surgical approaches for the excision of trigeminal neurinomas. J Neurosurg. 1995;82(05):719–725. doi: 10.3171/jns.1995.82.5.0719. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Pollock B E, Stafford S L, Link M J.Stereotactic radiosurgery for trigeminal pain secondary to benign skull base tumors World Neurosurg 201380(3-4):371–377. [DOI] [PubMed] [Google Scholar]
- 25.Guthikonda B, Theodosopoulos P V, van Loveren H, Tew J M, Jr, Pensak M L. Evolution in the assessment and management of trigeminal schwannoma. Laryngoscope. 2008;118(02):195–203. doi: 10.1097/MLG.0b013e3181596091. [DOI] [PubMed] [Google Scholar]


