Highlights
-
•
Fe(III)-assisted anammox exhibited superior properties treating saline wastewater.
-
•
Fe(III) promoted the synthesis of heme c and key enzymes of anammox species.
-
•
Candidatus Kuenenia could be enriched with Fe(III) addition under salt stress.
-
•
Fe(III) improve the energy metabolism of anammox species to resist high salinity.
Keywords: Anammox, Fe(III), Salinity, Microbial community, Candidatus Kuenenia
Abstract
Anammox process has attracted attention due to its excellent nitrogen removal properties in nitrogen-rich wastewater treatment. However, there were some obstacles for the application of anammox to treat high saline wastewater due to its sensitivity to salinity. In this study, Fe(III) addition strategy was developed to assist anammox to adapt high saline surroundings, with the defense mechanism involved in Fe(III)-assisted anammox emphasized. Nitrogen removal performance of anammox was deteriorated at 3.5% salinity, with the average total nitrogen removal rate of 0.85 kg/(m3·d) observed. The continuous addition of Fe(III) could significantly assist anammox to resist high salinity through facilitating the enrichment of anammox species. Candidatus Kuenenia was the main anammox species and outcompeted Candidatus Brocadia under high saline surrounding. The relative abundance of Candidatus Kuenenia increased with increased salinity and reached 41.04% under 3.5% salinity. The synthesis of key enzymes of anammox species were improved through Fe(III) addition and then facilitated the energy metabolism of anammox bacteria under 3.5% salinity. This study provides a new thought in Fe(III)-assisted anammox enhancement technologies and deepens the insight of anammox in high saline wastewater treatment.
Graphical abstract
1. Introduction
In anaerobic ammonium oxidation (anammox) process, with NO2−-N as electron acceptor, NH4+-N could be oxidized to N2 under anaerobic environment. Compared with traditional nitrification-denitrification process, anammox can save oxygen demand (nearly 60%) and organic carbon consumption (100%) (Wang et al., 2021a). Anammox has turned out to be a friendly and cost-saving alternative, showing broad prospect in nitrogen-rich wastewater treatment (Zhao et al., 2022).
High concentration of salinity was often encountered by industrial nitrogen-rich wastewaters, such as textile wastewater and coal chemical wastewater etc. (Lin et al., 2021). In biological process such as anammox, salinity was a serious inhibitor for microorganisms owing to the increased osmotic pressure, leading to cell dehydration and death (Pang et al., 2021). Fang et al. (2018) demonstrated that anammox activity and nitrogen removal would decrease under salinity higher than 3%, although anammox related species could adapt to high saline surroundings after long-term acclimatization. It was reported that 3.5% salinity could lead to a collapse of anammox performance even after a long period of stepwise acclimatization (Ya et al., 2021). The long acclimatization period of anammox species to the unfavorable factors such as salinity has become one of the main obstacles for the full-scale application of anammox in practical wastewater treatment. Thus, it is essential to find a more suitable alternative to relieve salt stress on anammox bacteria.
Iron, as a necessary component of iron-containing proteins, plays a vital role in the metabolism and growth of anammox species (Wang et al., 2021a; Erdim et al., 2019). For example, the iron-binding enzymes such as hydrazine synthase (HZS), hydrazine dehydrogenase (HDH) and nitrite reductase (Nir) are vital for anammox process (Peng et al., 2022). Heme c was involved in anaerobic conversion of NH4+-N to N2 in anammox process (van Niftrik et al., 2008). Although Fe(II) showed higher bioavailability for anammox species (Dai et al., 2022), soluble Fe(II) was often unstable and could be easily converted into Fe(III). Thus, Fe(III) was more suitable to supply anammox species for practical application. It was suggested that 5 mg/L Fe(III) significantly enhanced the nitrogen removal performance of anammox process (Zhang et al., 2021c). Furthermore, it was indicated that due to the decrease of Fe content inside anammox granule, the growth of anammox species was limited with the increased salinity (Jeong et al., 2022). Besides, iron could stimulate anammox species to secret extracellular polymeric substances (EPS) to resist adverse conditions (Dai et al., 2022). EPS is important for anammox bacteria to adapt to saline surroundings, which could act as a barrier between anammox bacteria and salinity conditions (Zhang et al., 2021b). Therefore, the additive supply of Fe(III) into anammox process is crucial under high saline conditions. Nevertheless, there was little information on the assistance of Fe(III) in anammox system to resist salinity. The defense mechanism of Fe(III)-assisted anammox toward salt stress deserved to be investigated.
Therefore, the effect of Fe(III) on anammox and defense mechanisms of Fe(III)-assisted anammox under salt stress were revealed in this study. The objectives of this work were as follows: (i) to investigate the effect of Fe(III) on anammox under salt stress; (ii) to analyze the variation of microbial community of anammox reactors under salinity exposure; (iii) to explore the defense mechanism of Fe(III)-assisted anammox in high-saline wastewater treatment. This work would support a new thought of the application of Fe(III)-assisted anammox treating nitrogen-rich saline wastewater.
2. Results and Discussion
2.1. Nitrogen removal in Fe(III)-assisted anammox under salt stress
The nitrogen removal process of Fe(III)-assisted anammox reactors was divided to three periods according to salinity level (Text A in SI), which were low salinity (0-0.5%), moderate salinity (1%-2%) and high salinity (2.5%-3.5%), respectively. As depictured in Fig. 1a, in phase I, the mean value of total nitrogen removal efficiency (TNRE) of R0 (control reactor without Fe(III)) and R1 (reactor with Fe(III)) were 89.73 ± 2.28% and 89.12 ± 2.93%, respectively, which suggested that low salinity showed unsignificant effect on anammox process. Similar result was reported by Zhang et al. (2022), where 0.5% salinity showed slight impact on nitrogen removal in anammox. In phase II, the salinity was increased to 2% to further explore the influence of moderate salinity on Fe(III)-assisted anammox. TNRE of R0 and R1 were decreased to 84.56 ± 1.57% and 84.23 ± 2.02%, respectively, indicating that 2% salinity had slight influence on anammox. However, Li et al. (2018) reported that the nitrogen removal properties of anammox was significantly deteriorated at 2% salinity. In both R0 and R1, the relatively good performance at salinity of 2% could be attributed to the dense and compact structure of granular anammox sludge (Fig. S1), which could resist adverse conditions (Fang et al., 2018). Compared with the inoculated sludge, with increased salinity, the granular anammox sludge was divided to smaller granules due to high osmotic pressure. However, the anammox sludge was still granular to resist salt stress.
Fig. 1.
Nitrogen removal efficiency (a) and nitrogen removal rate (b) of R0 and R1 under salinity variation.
In phase III, with the increase of salinity from 2.5% to 3.5%, the nitrogen removal performance of R0 and R1 began to appear different. When the salinity increased to 3%, TNRE of R1 was well kept at 80.82 ± 3.59%, while TNRE of R0 was deteriorated significantly to 71.74 ± 7.15%. The discrepancy indicated that 3% salinity could inhibit the activity of anammox species. Jeong et al. (2020) also indicated that NRE was obviously decreased to 63.8% at 2.75% salinity in anammox system. Correspondingly, when the salinity was further increased to 3.5%, the average total nitrogen removal rate (TNRR) was 0.85 kg/(m3·d) in R1, which was higher than 0.77 kg/(m3·d) in the control R0 (Fig. 1b). The granular anammox sludge was taken at 3.5% salinity to analyze specific anammox activity (SAA). As shown in Fig. S2, SAA value of R1 was much higher than the control, probably due to the assistance of Fe(III). It was reported that Fe(III) addition significantly promoted SAA (Wang et al., 2022a). Overall, the nitrogen removal properties of R1 was always superior to R0, indicating that Fe(III) was quite helpful to alleviate salt stress for anammox. In addition, the value of ΔNO2−-N/ΔNH4+-N in R1 was lower than 1.32 (Fig. S3), which was also lower than the value of R0. It was reported that NH4+-N could be removed through Fe(III) reduction under anaerobic surroundings, namely Feammox (Yang et al., 2021). Fe(III) could combine with OH− to Fe(OH)3 under pH neutral condition and Fe(II) was not detected in effluent. Wang et al. (2023) also indicated that Fe(OH)3 could induce anammox bacteria to perform extracellular electron transfer to remove NH4+-N via Feammox pathway. Therefore, anammox might couple with Feammox to remove NH4+-N in this study.
2.2. Characteristics of granular anammox sludge under salt stress
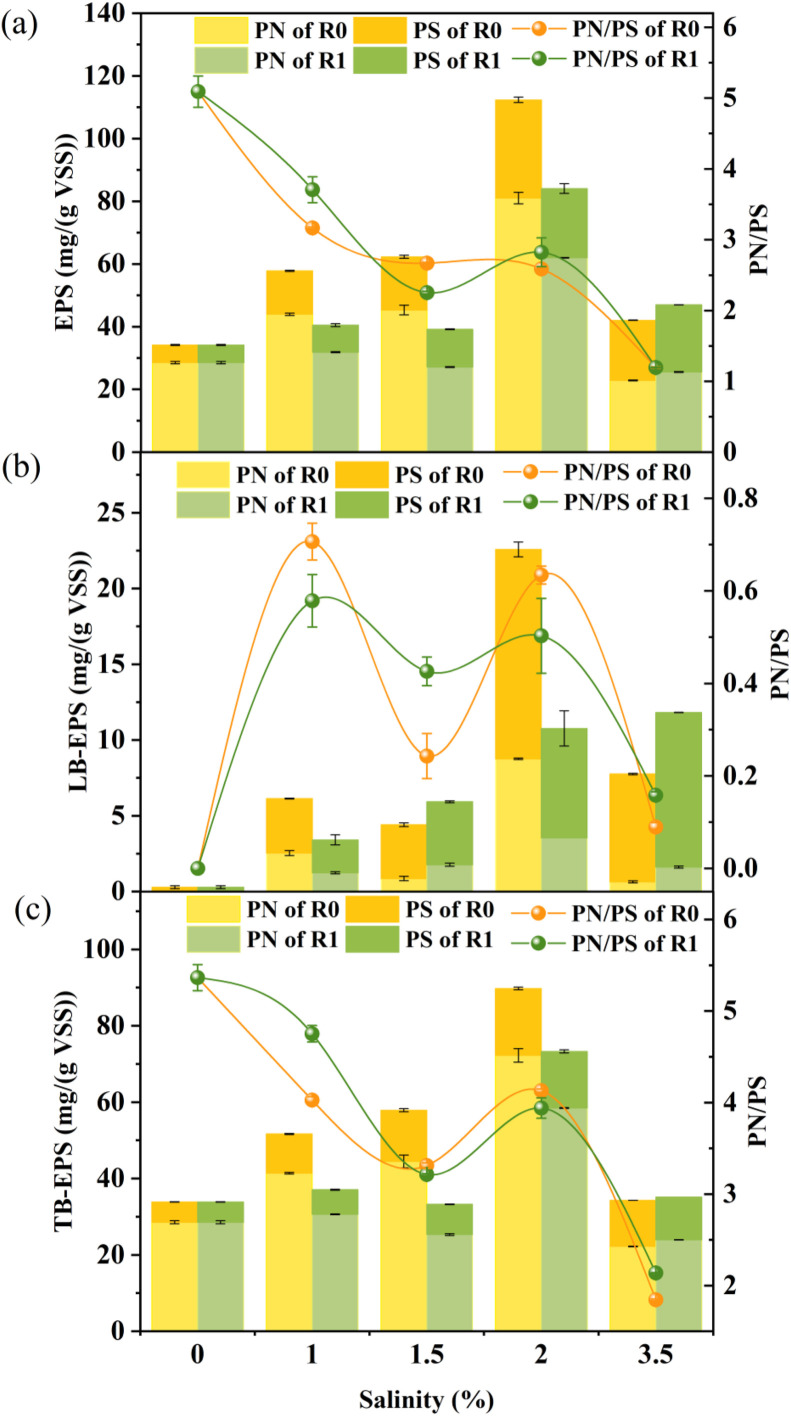
Extracellular polymeric substances (EPS), the macromolecules secreted from microorganisms, play a vital role in granular anammox sludge (Fang et al., 2018). As depicted in Fig. 2a, with the increase of salinity up to 2%, EPS content of R0 was increased, and EPS content of R0 was generally higher than R1, probably because Fe(III) addition weakened the unfavorable effects of salinity on anammox bacteria. It was found that EPS played an important role in osmotic pressure resistance and could prevent cells from salinity inhibition (Tang et al., 2021). However, EPS content of both reactors was decreased with the further increase of salinity to 3.0% and 3.5%. At salinity of 3.0% and 3.5%, EPS content of R1 was higher than that of R0, which could be attributed to the fact that Fe(III) could stimulate granular anammox sludge to secret EPS to resist the adverse effect in high saline environment. It was reported that polysaccharides (PS) were responsible for the negative charges, while proteins (PN) contained positively charges (Zhang et al., 2021a). With the increase of salinity, PS proportion in EPS was increased, as indicated by the decreased PN/PS ratios. The increase of PS proportion could be attributed to the significant increase of PS in loosely-bound EPS (LB-EPS) rather than tightly-bound EPS (TB-EPS) (Fig. 2b-c), which was beneficial for the enrich of negative charges on the anammox granules. Due to the negative surface charge of anammox species cells, Fe(III) ions could reduce electrostatic repulsion to promote the granulation of anammox species (Wang et al., 2022b). Fang et al. (2018) indicated that the increase in PS and decrease in PN could decrease the flocculating capacity of the sludge. It was reported that Fe(III) could reduce zeta potential through compressing the electric double layer and promote the granulation of anammox sludge (Dai et al., 2022). Besides, Dai et al. (2022) also indicated that Fe(III) could combine with OH− and stimulate the active flocculation groups, which were beneficial for sludge sedimentation. Therefore, Fe(III) could assist anammox granules to resist high salinity through promoting the granulation and EPS secretion.
Fig. 2.
Variation of EPS (a), loosely-bound EPS (b) and tightly-bound EPS (c) in R0 and R1 at various salinity levels.
The appearance of anammox granules were analyzed to reflect the effect of Fe(III) on anammox under salt stress. The color of anammox granules turned from typical red to orange under salt stress (Fig. S1). In order to confirm the role of Fe(III), the anammox sludge was further analyzed through X-ray photoelectron spectroscopy (XPS). As indicated in Fig. S4, the iron in anammox sludge of R1 was dominated by Fe(III), with two Fe2p peaks centered at binding energies of 712.1 eV and 725.7 eV, respectively (Yang et al., 2021), which indicated that Fe(III) was involved in the formation of network structure of anammox sludge. The size of inocula sludge was mainly distributed within diameter range of 1.4-2.8 mm, while the size of anammox granules was decreased with the increase of salinity (Fig. S5). However, it was also observed that the size of anammox granules in R1 was bigger than that in R0, which could be attributed to Fe(III) addition. Wang et al. (2022b) reported that Fe(III) was beneficial for EPS secretion, and iron-based particles could become “micro-nuclei” during granular sludge formation. Through the formation of iron hydroxides with high flocculation ability, granulation of anammox species could be induced (Wang et al., 2021b).
2.3. Microbial community analysis
2.3.1. Correlations analysis of microbial communities
The anammox sludge samples were taken at 0, 1%, 2% and 3.5% salinity to explore the dynamics of microbial community under salt stress. As shown in Table S1, as compared with inocula sludge, the low Shannon and high Simpson indexes observed in R0 and R1 indicated the low diversity of the community under salinity stress. It was reported that harsh environment such as high salinity would lead to simple community structure (Li et al., 2020). Compared with R0, Shannon index of R1 was relatively low, while Simpson index of R1 was relatively high, indicating the low community diversity in R1, probably due to the enrichment of certain species at the presence of Fe(III). Principal component analysis (PCA) was depicted in Fig. 3a, with the increase of salinity, the microbial community in R0 and R1 presented clear separation with the inocula sludge. Under 3.5% salinity, the microbial community in R1 was rather different from R0, implying the divergence of microbial structure under the action of Fe(III). Redundancy analysis (RDA) was used to elucidate the influence of Fe(III) and salinity on variation of microbial structure (Fig. 3b). It could be found that Candidatus Kuenenia was positive with Fe(III), demonstrating that Fe(III) played an important role in anammox reactor under salt stress. Candidatus Brocadia and Denitratisoma were susceptible to high salinity, as indicated by the negative correlation with salinity.
Fig. 3.
Principal component analysis (PCA) of microbial community (a) and redundancy analysis (RDA) of functional microorganisms (b).
2.3.2. Functionalized microbial community in Fe(III)-assisted anammox system
The anammox sludge samples were taken at 0, 1%, 2% and 3.5% salinity to explore the dynamics of microbial community. As shown in Fig. 4a, at the phylum level, the main phyla were Planctomycetes, Proteobacteria, Chloroflexi and Bacteroidetes. Compared with R0, with the increase of salinity in R1, the relative abundance of Planctomycetes increased to 44.04%. Anammox species was reported to be a phylogenetically deep-branching group in the phylum Planctomycetes (Wang et al., 2022a). The relative abundance of Proteobacteria, which was often found in anammox system (Zhang et al., 2021c), decreased under salt stress. Chloroflexi were important symbiotic microbes with anammox bacteria through providing scaffolding for granular anammox sludge (Wang et al., 2020). Compared with R0, the relative abundance of Chloroflexi in R1 decreased at 3.5% salinity, indicating that Fe(III) could facilitate sludge granulation instead of Chloroflexi under salt stress. Bacteroidetes were inhibited by salinity with the decrease in the relative abundance.
Fig. 4.
Dynamics of microbial community at phylum level (a), genus level (b), main anammox bacteria (c) and relative abundance of N-related and Fe-related genes predicted by PICRUST 2 (d) under salinity variation.
As depicted in Fig. 4b, at the genus level, Candidatus Kuenenia, Chloroflexi bacterium OLB13 and Denitratisoma were dominant species. In the inoculation sludge, Candidatus Kuenenia and Candidatus Brocadia were main anammox bacteria. The relative abundance of anammox bacteria varied with increased salinity (Fig. 4c). Initially, Candidatus Kuenenia out-competed Candidatus Brocadia with the salinity increased to 1%, which was consistent with previous studies. It was indicated that Candidatus Kuenenia had a more higher salinity tolerance than Candidatus Brocadia (Lin et al., 2021). When the salinity increased to 3.5%, the content of Candidatus Kuenenia obviously decreased to 15.32% in R0, while increased to 41.04% in R1. Through long-term salinity acclimatization, the inherit adaption of the anammox process might be one reason for the enrichment of Candidatus Kuenenia. However, the content of Candidatus Kuenenia in R1 was much higher than that of R0 under 3.5% salinity. The results demonstrated that Fe(III) played a vital role in the enrichment of anammox bacteria under high saline conditions. Several studies had indicated that Fe(III) addition could stimulate the growth of anammox bacteria and promote the enrichment of anammox bacteria (Wang et al., 2021a; Yin et al., 2021). In addition, it was reported that Fe(III) addition could enhance the removal of NH4+ in anammox system via Feammox pathway (Wang et al., 2023). Candidatus Kuenenia might participate in the Feammox process. As a result, Candidatus Kuenenia could survive at high saline (3.5% salinity) environment through Fe(III) assistance.
Denitratisoma was inhibited by high salinity, which was decreased from 17.11% to 2.12% and 0.97% in R0 and R1 under 3.5% salinity, respectively. Denitratisoma is a denitrifying bacterium, which was often detected in anammox process (Liu et al., 2021). Nitrosomonas, as the known genus of ammonia oxidizing bacteria, could cooperate with anammox bacteria to remove NH4+-N (Guo et al., 2020). Its relative abundance decreased at 1% salinity, because Nitrosomonas was reported to only adapt to a low salinity environment (Li et al., 2018). Chloroflexi bacterium OLB13 could strengthen the structure of granules in the form of filamentous biomass (Xu et al., 2021). The relative abundance of Chloroflexi bacterium OLB13 of R1 was lower than R0 under 3.5% salinity, probably due to that Fe(III) addition accelerated the formation of anammox sludge structure. Under salinity exposure, compared with other microbes, Candidatus Kuenenia exhibited better resistance to salt stress by Fe(III) addition, which was the dominant microbe to remove nitrogen compounds under saline conditions.
2.3.3. Microbial function prediction based on KEGG database
The abundance of predicted functional genes related to nitrogen and iron metabolism were also analyzed. As depicted in Fig. 4d, the gene coding for hydroxylamine dehydrogenase (hao) was varied with increased salinity. Compared with R1, the genes of R0 that coding for nitrite transporter (nirC), nitrate reductase (narG, narZ), nitrite oxidoreductase (nxrA), and nitrite reductase (nirB) were down-regulated at 3.5% salinity. These results showed that Fe(III) improved the abundance of genes associated with nitrogen metabolism under high saline surroundings. The abundance of ferrous iron transport protein B (feoB) and iron complex outermembrane recepter protein (TC.FEV.OM) in R0 was always lower than that of R1. The genes coding for TC.FEV.OM were down-regulated with increased salinity. TC.FEV.OM is the receptor enzyme for the iron complex in the outer membrane of anammox bacteria (Wang et al., 2023). These results indicated that Fe(III) addition could change the iron metabolism inside anammox under high salt stress.
2.4. Effects of Fe(III) on nitrogen metabolism of anammox species under 3.5% salinity
To further reveal the influence of Fe(III) on metabolism of anammox species, the contents of key functional enzymes (Nir, HZS, HDH) and heme c were analyzed at 3.5% salinity. In anammox metabolism process, NO2− was initially catalyzed to NO by Nir, and then NO combined with NH4+ to form N2H4 through HZS, and finally N2H4 was catalyzed to N2 by HDH (Kartal et al., 2011). As depicted in Fig. 5, the contents of Nir, HZS and HDH of R1 was higher than that of R0, which indicated that Fe(III) obviously regulated functional enzymes synthesis of anammox bacteria under 3.5% salinity. It was reported that Fe addition could promote the activities of Fe-involved enzymes (Wang et al., 2022a). Dai et al. (2022) also indicated that moderately increasing Fe concentration of influent could enhance the activity of HDH. As a result, Fe(III) promote the functional enzymes synthesis of anammox bacteria to alleviate salt stress.
Fig. 5.
The content of Nir and HZS (a), HDH and Heme c (b) of anammox bacteria metabolism process under 3.5% salinity (p < 0.05).
In R1, Heme c content was higher than that of the control (Fig. 5b), which indicated that Fe(III) could stimulate the production of Heme c of anammox bacteria under 3.5% salinity. Zhang et al. (2021c) reported that Heme c content was significantly increased at 10 mg/L Fe(III) addition. Jeong et al. (2022) indicated that the content of Fe in granular anammox sludge decreased with increased salinity and thus limited the growth of anammox species. Furthermore, Heme c is correlated positively with the relative abundance of anammox bacteria (Kang et al., 2020). Therefore, Fe(III) addition could increase Heme c content, which would be helpful for Candidatus Kuenenia and then promote its enrichment to resist salt stress.
2.5. Defense mechanism of Fe(III)-assisted anammox under high-salt stress
The defense mechanism of Fe(III)-assisted anammox under high salt stress was depicted in Fig. 6. Firstly, with the addition of Fe(III), the relative abundance of Candidatus Kuenenia increased with salinity, which was higher than the control under 3.5% salinity. Yin et al. (2019) also reported that Fe(III) addition significantly accelerated the growth of marine anammox species in high saline wastewater treatment. Secondly, Fe(III) strengthened the resistance of granular anammox sludge through stimulating EPS secretion and sludge granulation under high-salt stress. It was indicated that Fe could stimulate anammox sludge to secrete EPS and then improve the resistance of anammox species to inhibitors (Dai et al., 2022). Besides, Fe(III) could combine with OH− to Fe(OH)3 under pH neutral condition, and Fe(OH)3 promoted anammox granulation by acting as “micro-nuclei” (Wang et al., 2022b). The granular anammox sludge could resist salt stress through its dense and compact structure with Fe(III) addition. Thirdly, Fe(III) addition facilitated the synthesis of key enzymes and Heme c and thus alleviated the influence of salt stress on anammox bacteria. It was found that the activity of key enzymes in anammox was severely inhibited under saline surroundings (Zhang et al., 2021b). Several studies had reported that Fe could promote heme c synthesis and increase the activity of key enzymes of anammox (Feng et al., 2023; Wang et al., 2022a; Wang et al., 2023).
Fig. 6.
Defense mechanism of Fe(III)-assisted anammox under high-salt stress.
3. Conclusions
This work investigated the defense mechanism of Fe(III)-assisted anammox under salt stress. The nitrogen removal efficiency and specific anammox activity was enhanced due to the addition of Fe(III) under as high as 3.5% salinity. Compared with the control group, Fe(III) could stimulate the EPS secretion of granular anammox sludge, and facilitate the synthesis of Nir, HZS, HDH and Heme c to relieve salt stress. Fe(III) addition significantly increased the relative abundance of Candidatus Kuenenia under 3.5% salinity exposure. Overall, this work would give a new sight of anammox species on resisting salt stress through Fe(III) assistance.
4. Materials and methods
4.1. Experiment setup and reactor operation
Two identical upflow anaerobic sludge blanket (UASB) reactors were conducted with a working volume of 3 L. 5 mg/L Fe(III) was continuously added to influent in R1, while R0 was operated without Fe(III) as the control reactor. In particular, the background iron of influent was supplied for the normal growth of anammox. The only difference between R0 and R1 was the exogenous Fe(III) addition. Thus, the background value of iron was negligible. FeCl3 was applied to provide Fe(III). Reasons for choosing 5 mg/L Fe(III) in this study were shown in the supplementary information (Text B in SI). The operation temperature was kept at 35 ± 1 ℃ through a circulating water bath. The hydraulic retention time was set at 12 h. The entire experiment period was divided into three phases based on the variation of salinity (Table S2).
4.2. Inoculation and synthetic wastewater
The sludge derived from a mature granular anammox reactor was inoculated into both R1 and R0 at initial mixed liquid suspended solids (MLSS) of 3600 mg/L. The synthetic wastewater was used as the influent, which was consisted of KH2PO4 (0.029 g/L), MgSO4 (0.3 g/L), CaCl2·H2O (0.136 g/L), KHCO3 (1.2 g/L) and trace element solutions (1 ml/L) (Yin et al., 2019). Thereinto, ferrous iron (1 mg/L) was added as trace element to keep the normal metabolic activity of anammox bacteria. Influent NH4+-N and NO2−-N concentrations were controlled at 230.0 mg/L and 303.6 mg/L, respectively, at theoretical molar ratio of 1.32. The effect of salinity on anammox was studied with salt concentrations varying from 0 to 3.5% (calculated by NaCl). Influent pH was controlled at 7.5 ± 0.2 through adding 2 mol/L HCl. N2 was aerated to the synthetic wastewater to expel dissolved oxygen.
4.3. Analytical methods
Samples were collected and filtered through 0.22 μm filters. The concentrations of NH4+-N, NO2−-N and NO3−-N were measured according to the standard methods (APHA, 2005). Anammox sludge was taken from R1 and R0 to analyze SAA at the salinity of 0, 10, 20, 35 g/L, respectively. SAA was calculated through the removal rate of NH4+-N and NO2−-N concentrations from batch tests. EPS, including LB-EPS and TB-EPS, were extracted through heat-extraction method (Gao et al., 2021). The contents of PN and PS were measured by Lowry method and phenol-sulfuric acid method, respectively (DuBois et al., 1956; Lowry et al., 1951). XPS (ESCALAB 250Xi, hv=1486.6 eV) was employed to determine the element composition and chemical state. The binding energy was calibrated with C 1s at 284.8 eV. XPS peak was applied to fit the XPS spectra peaks. The enzyme contents of Nir, HZS, HDH related to nitrogen metabolism process were detected by double antibody sandwich enzyme-linked immunoassay kit (Jingkang Biological Technology Co.,Ltd, Shanghai, China), as well as the content of Heme c. The procedures of enzyme contents were measured according to Xue et al. (2023). Statistical analysis was performed using SPSS 26 statistical software with a confidence interval of 95%. Independent sample T test was used to measure the contents of key enzymes between R1 and R0. Differences were considered statistically significant when p < 0.05.
4.4. Microbial community analysis
The anammox sludge was taken from R1 and R0 to analyze microbial community variation at the influent salinity of 0, 10, 20 and 35 g/L. Total bacterial DNA were extracted from the four samples using the FastDNA Spin Kit for soil. The V3-V4 region of bacterial 16S rRNA gene was amplified with the primers 338F (5′ -ACCTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The sequence was performed on the Illumina Miseq platform (Majorbio Biotechnology Co., Ltd, Shanghai, China). PCA and RDA were performed to analyze the correlation of microbial community and environment paraments. PICRUST2 was applied to predict the functional genes of microbial community. The functional genes related to nitrogen and iron were obtained from KEGG database (Hou et al., 2022).
Author contributions
J. Shen and X. Jiang: conceived and designed the experiments. S. Yin and Y. Wang: carried out the experiment. S. Yin and X. Jiang: co-wrote the paper. Y. Yang and D. Chen: provided constructive suggestions for the manuscript revision. J. Shen: provided constructive suggestions for results and discussion. All authors participated in the discussion.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is financed by National Key Research and Development Program of China (No. 2021YFA1201704), National Natural Science Foundation of China (No. 52170084) and Natural Science Foundation of Jiangsu Province (BK20211574).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2023.100188.
Contributor Information
Xinbai Jiang, Email: xinbai_jiang@njust.edu.cn.
Jinyou Shen, Email: shenjinyou@mail.njust.edu.cn.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- APHA . 21th ed. APHA; Washington, DC, USA: 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Dai B., Yang Y.F., Wang Z.B., Wang J.M., Yang L., Cai X., Wang Z.Y., Xia S.Q. Enhancement and mechanisms of iron-assisted anammox process. Sci. Total Environ. 2022;858(Pt 3) doi: 10.1016/j.scitotenv.2022.159931. [DOI] [PubMed] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry. 1956;28(3):350–356. [Google Scholar]
- Erdim E., Yucesoy Ozkan Z., Kurt H., Alpaslan Kocamemi B. Overcoming challenges in mainstream Anammox applications: Utilization of nanoscale zero valent iron (nZVI) Sci. Total Environ. 2019;651(Pt 2):3023–3033. doi: 10.1016/j.scitotenv.2018.09.140. [DOI] [PubMed] [Google Scholar]
- Fang F., Yang M.M., Wang H., Yan P., Chen Y.P., Guo J.S. Effect of high salinity in wastewater on surface properties of anammox granular sludge. Chemosphere. 2018;210:366–375. doi: 10.1016/j.chemosphere.2018.07.038. [DOI] [PubMed] [Google Scholar]
- Feng F., Liu Z.G., Tang X., Wu X., Qu C.Y., How S.W., Wu D., Xiao R.Y., Tang C.J., Lin Z., Chai L.Y., Chen G.H. Dosing with pyrite significantly increases anammox performance: Its role in the electron transfer enhancement and the functions of the Fe-N-S cycle. Water Res. 2023;229 doi: 10.1016/j.watres.2022.119393. [DOI] [PubMed] [Google Scholar]
- Gao Y.Y, Li J., Dong H.Y., Qiang Z.M. Nitrogen removal mechanism of marine anammox bacteria treating nitrogen-laden saline wastewater in response to ultraviolet (UV) irradiation: High UV tolerance and microbial community shift. Bioresour. Technol. 2021;320(Pt A) doi: 10.1016/j.biortech.2020.124325. [DOI] [PubMed] [Google Scholar]
- Guo Y., Sugano T., Song Y., Xie C.L., Chen Y.J., Xue Y., Li Y.Y. The performance of freshwater one-stage partial nitritation/anammox process with the increase of salinity up to 3.0% Bioresour. Technol. 2020;311 doi: 10.1016/j.biortech.2020.123489. [DOI] [PubMed] [Google Scholar]
- Hou C., Jiang X.B., Chen D., Zhang X.Y., Liu X.D., Mu Y., Shen J.Y. Ag-TiO2/biofilm/nitrate interface enhanced visible light-assisted biodegradation of tetracycline: The key role of nitrate as the electron accepter. Water Res. 2022;215 doi: 10.1016/j.watres.2022.118212. [DOI] [PubMed] [Google Scholar]
- Jeong D., Kim W., Lim H., Bae H. Shift in bacterial community structure in response to salinity in a continuous anaerobic ammonium oxidation (anammox) reactor. Int. Biodeterior. Biodegradation. 2020;147 [Google Scholar]
- Jeong S., Kim J., Direstiyani L.C., Kim Y., Yu J., Lee T. Long-term adaptation of two anammox granules with different ratios of Candidatus Brocadia and Candidatus Jettenia under increasing salinity and their application to treat saline wastewater. Sci. Total Environ. 2022 doi: 10.1016/j.scitotenv.2022.160494. [DOI] [PubMed] [Google Scholar]
- Kang D., Li Y.Y., Xu D.D., Li W.J., Li W., Ding A.Q., Wang R., Zheng P. Deciphering correlation between chromaticity and activity of anammox sludge. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116184. [DOI] [PubMed] [Google Scholar]
- Kartal B., Maalcke W.J., de Almeida N.M., Cirpus I., Gloerich J., Geerts W., Op den Camp H.J.M., Harhangi H.R., Janssen-Megens E.M., Francoijs K.-J., Stunnenberg H.G., Keltjens J.T., Jetten M.S.M., Strous M. Molecular mechanism of anaerobic ammonium oxidation. Nature. 2011;479(7371):127–130. doi: 10.1038/nature10453. [DOI] [PubMed] [Google Scholar]
- Li X., Yuan Y., Yuan Y., Bi Z., Liu X., Huang Y., Liu H.W., Chen C.J., Xu S.S. Effects of salinity on the denitrification efficiency and community structure of a combined partial nitritation- anaerobic ammonium oxidation process. Bioresour. Technol. 2018;249:550–556. doi: 10.1016/j.biortech.2017.10.037. [DOI] [PubMed] [Google Scholar]
- Li Y.Z., Chen Z., Peng Y.Y., Zheng K.M., Ye C.S., Wan K., Zhang S.H. Changes in aerobic fermentation and microbial community structure in food waste derived from different dietary regimes. Bioresour. Technol. 2020;317 doi: 10.1016/j.biortech.2020.123948. [DOI] [PubMed] [Google Scholar]
- Lin L.M., Pratt S., Li Z.H., Ye L. Adaptation and evolution of freshwater Anammox communities treating saline/brackish wastewater. Water Res. 2021;207 doi: 10.1016/j.watres.2021.117815. [DOI] [PubMed] [Google Scholar]
- Liu Y.X., Liu W., Li Y.Y., Liu J.Y. Layered inoculation of anaerobic digestion and anammox granular sludges for fast start-up of an anammox reactor. Bioresour. Technol. 2021;339 doi: 10.1016/j.biortech.2021.125573. [DOI] [PubMed] [Google Scholar]
- Lowry O., Robert N.R., Leiner K.Y., Wu M.L., Farr A. Protein measurement with the FOLIN phenol reagent. J. Biol. Chem. 1951;207 [PubMed] [Google Scholar]
- Pang J.X., Li J., Chen R., Lu H. Synergistic biological removal of nitrogen and sulfide from saline mariculture wastewater by halophilic consortia. Chem. Eng. J. 2021;423 [Google Scholar]
- Peng M.W., Qi J., Yan P., Guan Y., Liu Y.Y., Sun Z.H., Zhang L.J., Weng X., Shen Y., Fang F., Guo J.S., Chen Y.P. Insight into the structure and metabolic function of iron-rich nanoparticles in anammox bacteria. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150879. [DOI] [PubMed] [Google Scholar]
- Tang W.C., Wu M.J., Lou W., Yang C.P. Role of extracellular polymeric substances and enhanced performance for biological removal of carbonaceous organic matters and ammonia from wastewater with high salinity and low nutrient concentrations. Bioresour. Technol. 2021;326 doi: 10.1016/j.biortech.2021.124764. [DOI] [PubMed] [Google Scholar]
- van Niftrik L., Geerts W.J.C., van Donselaar E.G., Humbel B.M., Yakushevska A., Verkleij A.J., Jetten M.S.M., Strous M. Combined structural and chemical analysis of the anammoxosome: A membrane-bounded intracytoplasmic compartment in anammox bacteria. J. Struct. Biol. 2008;161(3):401–410. doi: 10.1016/j.jsb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Wang H., Fan Y.F., Zhou M.D., Wang W.G., Li X., Wang Y.Y. Function of Fe(III)-minerals in the enhancement of anammox performance exploiting integrated network and metagenomics analyses. Water Res. 2022;210 doi: 10.1016/j.watres.2021.117998. [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Peng L., Mao N.J., Geng J.J., Ren H.Q., Xu K. Effects of Fe3+ on microbial communities shifts, functional genes expression and nitrogen transformation during the start-up of Anammox process. Bioresour. Technol. 2021;320(Pt A) doi: 10.1016/j.biortech.2020.124326. [DOI] [PubMed] [Google Scholar]
- Wang P.C., He Y., Ding J.Q., Wang W.H., Sheng H., Wei Z., Huang M.S., Zhang H.Q. Feasibility of iron scraps for enhancing nitrification of domestic wastewater at low temperatures. Environ. Sci. Pollut. Res. 2021;28(21):26819–26827. doi: 10.1007/s11356-021-12607-4. [DOI] [PubMed] [Google Scholar]
- Wang P.C., Lu B., Liu X.J., Chai X.L. Accelerating the granulation of anammox sludge in wastewater treatment with the drive of “micro-nuclei”: A review. Sci. Total Environ. 2022 doi: 10.1016/j.scitotenv.2022.160238. [DOI] [PubMed] [Google Scholar]
- Wang W.G., Wang J.J., Wang H., Ma J., Wu M., Wang Y.Y. Anammox Granule Enlargement by Heterogenous Granule Self-assembly. Water Res. 2020;187 doi: 10.1016/j.watres.2020.116454. [DOI] [PubMed] [Google Scholar]
- Wang Z.X., Wang X.P., Sun Y., Yu Q., Zhao Z.Q., Zhang Y.B. Fe(OH)3 induced the Anammox system to perform extracellular electron transfer for enhancement of NH4+ removal. Chem. Eng. J. 2023;423:460. 141768. [Google Scholar]
- Xu D.D., Ying S.Y., Wang Y.H., Zheng H.Y., Zhang M., Li W.J., Chen W.D., Pan C., Kang D., Zheng P. A novel SAD process: Match of anammox and denitrification. Water Res. 2021;193 doi: 10.1016/j.watres.2021.116874. [DOI] [PubMed] [Google Scholar]
- Xue Y.T., Liu X.Y., Dang Y., Shi T.J., Sun D.Z. Enhancement of nitrogen removal in coupling Anammox and DAMO via Fe-modified granular activated carbon. J Environ Manage. 2023;340 doi: 10.1016/j.jenvman.2023.118001. [DOI] [PubMed] [Google Scholar]
- Ya T., Du S., Li Z.Y., Liu S.D., Zhu M.H., Liu X.J., Jing Z.B., Hai R.T., Wang X.H. Successional Dynamics of Molecular Ecological Network of Anammox Microbial Communities under Elevated Salinity. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116540. [DOI] [PubMed] [Google Scholar]
- Yang Y.F., Xiao C.C., Yu Q., Zhao Z.Q., Zhang Y.B. Using Fe(II)/Fe(III) as catalyst to drive a novel anammox process with no need of anammox bacteria. Water Res. 2021;189 doi: 10.1016/j.watres.2020.116626. [DOI] [PubMed] [Google Scholar]
- Yin S.Y., Li J., Dong H.Y., Qiang Z.M. Enhanced nitrogen removal through marine anammox bacteria (MAB) treating nitrogen-rich saline wastewater with Fe(III) addition: Nitrogen shock loading and community structure. Bioresour. Technol. 2019;287 doi: 10.1016/j.biortech.2019.121405. [DOI] [PubMed] [Google Scholar]
- Yin S.Y., Li J., Dong H.Y., Qiang Z.M. Unraveling the nitrogen removal properties and microbial characterization of “Candidatus Scalindua”-dominated consortia treating seawater-based wastewater. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147470. [DOI] [PubMed] [Google Scholar]
- Zhang A.Y., Wang S., Yang M.M., Li H.X., Wang H., Fang F., Guo J.S. Influence of NaCl salinity on the aggregation performance of anammox granules. J. Water Process. Eng. 2021;39 [Google Scholar]
- Zhang Q., Fu J.J., Wu Q.Y., Chen J.Y., Fan N.S., Huang B.C., Jin R.C. Build the expressway for the salt-tolerant anammox process: Acclimation strategy tells the story. J. Clean. Prod. 2021;278 [Google Scholar]
- Zhang S.Q., Zhang L.Q., Yao H.N., Rong H.W., Li S.G. Responses of anammox process to elevated Fe(III) stress: Reactor performance, microbial community and functional genes. J. Hazard Mater. 2021;414 doi: 10.1016/j.jhazmat.2021.125051. [DOI] [PubMed] [Google Scholar]
- Zhang X.J., Zhang H., Ma B.B., Song Y.L., Wang L., Wang Q., Ma Y.P. Can anammox process be adopted for treating wastewater with high salinity exposure risk? Chemosphere. 2022;293 doi: 10.1016/j.chemosphere.2022.133660. [DOI] [PubMed] [Google Scholar]
- Zhao K.X., Wang Y., Gao L.J., Chai B.H., Zhuang P.Y., Kou X.M. Anammox in a biofilter reactor to treat wastewater of high strength nitrogen. J. Water Process. Eng. 2022;49 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.