Summary
Bacillus Calmette-Guérin (BCG) confers heterologous immune protection against viral infections and has been proposed as vaccine against SARS-CoV-2 (SCV2). Here, we tested intravenous BCG vaccination against COVID-19 using the golden Syrian hamster model. BCG vaccination conferred a modest reduction on lung SCV2 viral load, bronchopneumonia scores, and weight loss, accompanied by a reversal of SCV2-mediated T cell lymphopenia, and reduced lung granulocytes. BCG uniquely recruited immunoglobulin-producing plasma cells to the lung suggesting accelerated local antibody production. BCG vaccination also recruited elevated levels of Th1, Th17, Treg, CTLs, and Tmem cells, with a transcriptional shift away from exhaustion markers and toward antigen presentation and repair. Similarly, BCG enhanced recruitment of alveolar macrophages and reduced key interstitial macrophage subsets, that show reduced IFN-associated gene expression. Our observations indicate that BCG vaccination protects against SCV2 immunopathology by promoting early lung immunoglobulin production and immunotolerizing transcriptional patterns among key myeloid and lymphoid populations.
Subject areas: Immune response, Virology, Model organism
Graphical abstract
Highlights
-
•
Single-cell transcriptomics of immune cells in BCG-vaccinated, SCV2-infected hamsters
-
•
BCG causes the reprogramming of myeloid cells, lymphocytes, and non-immune cells
-
•
BCG prevents SCV2-mediated lymphopenia and promotes antigen presentation
-
•
BCG vaccination promotes the expansion of plasma cells in SCV2-infected lungs
Immune response; Virology; Model organism
Introduction
COVID-19, the current global pandemic caused by the novel coronavirus SARS-CoV-2 (SCV2), has precipitated a severe health and economic crisis worldwide, dramatically affecting the lives of billions of people across all continents. Despite the widespread use of vaccines since December 2020, the incomplete efficacy of licensed vaccines and the development of novel SCV2 variants capable of breakthrough infections in vaccinated individuals poses a continuing public health threat.1,2 Further, there are indications that SCV2 and future variant coronaviruses may never be fully eliminated, and thus repetitive vaccination campaigns with updated vaccines may be necessary as occurs with influenza.3,4
SCV2 infection of ACE2 and TMPRSS2 expressing airway epithelial cells results in pyroptotic cell death and the subsequent release of infectious viral particles, damage-associated molecules (DAMPS), and inflammation inducing IL1-β.5,6 The release of DAMPs and viral particles and their sensing by epithelial cells and alveolar macrophages (AMs) results in a local exaggerated proinflammatory immune response primarily driven by cytokines (IL-6 and IFN-γ) and chemokines (CCL2 and CXCL10).7 This is followed by infiltration of monocytes, natural killer (NK) cells and lymphocytes from the peripheral blood to infected tissues causing even more pronounced inflammation eventually causing organ damage in individuals with severe disease.7 Longitudinal studies in infected individuals during the active and recovery phases of the disease have revealed that early host immune responses are dominated by cells of innate immune system, including neutrophils, monocytes, plasmacytoid dendritic cells (pDCs), and NK cells,8,9 while adaptive immune response are important for viral clearance and development of long term T and B cell memory responses.10
Because BCG has been shown to impart heterologous immunity and to protect against viral infections,11,12 a number of human clinical trials evaluating BCG for protection against COVID-19 were launched in early 2020. One such study now reports that indeed BCG re-vaccination reduces the risk of COVID-19 diagnoses by as much as 68% compared to unvaccinated controls,13 although others report little or no efficacy.14,15,16,17,18 BCG is also a well-known vaccine adjuvant and has been shown to boost immunogenicity of protein subunit, DNA, and viral vectored vaccines in pre-clinical models.19,20,21 Hence further studies of BCG as a COVID-19 vaccine either alone or in combination with the current arsenal of specific anti-COVID-19 vaccines may be warranted.
BCG has been shown to reprogram both myeloid cells and NK cells through processes collectively termed “trained immunity”. BCG is rapidly phagocytosed by macrophages and has been shown to elicit both epigenetic and metabolomic modifications that elevate their immune setpoint upon re-challenge with a heterologous antigens including viruses.22,23 Upon re-challenge with heterologous molecules, BCG-trained macrophages show elevated cytokine release and demonstrate reprogramming toward M1-like phenotypes. These initial events are associated with heterologous B and T-lymphocyte activation, elevated antibody titers24 and expansion of unconventional T cells such as innate lymphoid cells (ILCs) and mucosa-associated invariant T (MAIT) cells.25,26 BCG exposure also leads to expanded “trained” populations of hematopoietic stem cells (HSCs) and multipotent progenitors (MPPs) in the bone marrow that confer enhanced protection against subsequent pathogen challenges.27
Randomized control studies of BCG vaccination in humans have demonstrated improved control of live attenuated yellow fever virus,28 live-attenuated influenza A (H1N1),29 and human papilloma virus.30 Animal studies have similarly demonstrated a BCG benefit against at least eight viruses including two positive-sense, single-stranded RNA viruses (Cardiovirus A and Japanese encephalitis virus, neither of which is closely related to coronaviruses).22,31,32 Regarding animal studies of BCG for SCV2 protection, two studies now show that intravenous BCG does confer SCV2 protection in mice.33,34 In contrast, others have found that subcutaneous BCG does not protect in mouse models of SCV2 infection,33,35,36 and a study giving aerosolized BCG to non-human primates also failed to show protection against SCV2.37 A recent hamster study showed that both subcutaneous and intravenous BCG vaccination were ineffective in SCV2 disease prevention.36 These prior studies did not evaluate the comprehensive single cell immune landscape using robust, global immune profiling transcriptomic tools such as single-cell RNA sequencing.
In this study, we employed a non-lethal, self-resolving golden Syrian hamster model to evaluate the ability of intravenous BCG to protect against SCV2. We evaluated its impact on lung viral titers, tissue pathology using histopathology, immunohistochemistry (IHC) and PET/CT (positron emission tomography/computed tomography), and cell populations by flow cytometry and single-cell RNA sequencing (scRNA-seq). Our results reveal that BCG-vaccinated animals had significantly reduced viral loads and tissue pathology, reduced lung T cell lymphopenia, and a significant reprogramming of myeloid cell subsets. Moreover, we observed that during SCV2 infection BCG uniquely recruits immunoglobulin-producing plasma cells to the lung suggesting accelerated antiviral antibody production. BCG vaccination also resulted in elevated levels of several types of lung CD4+ T cells (Th1, Th17, Treg, CTLs, and Tmem), and these cells showed modified transcriptional programs toward antigen presentation and away from exhaustion. Similarly, BCG enhanced lung recruitment of AMs and reduced key interstitial macrophage subsets with both cells also showing reduced IFN-associated gene expression. Our results indicate that BCG vaccination has a tolerizing effect on SCV2-mediated lung inflammation and that it may accelerate antiviral antibody responses.
Results
BCG vaccination is associated with reduced viral load, a decrease in certain lung pathology parameters, alterations of lung immune cell infiltration, and prevention of weight loss in SCV2-infected hamsters
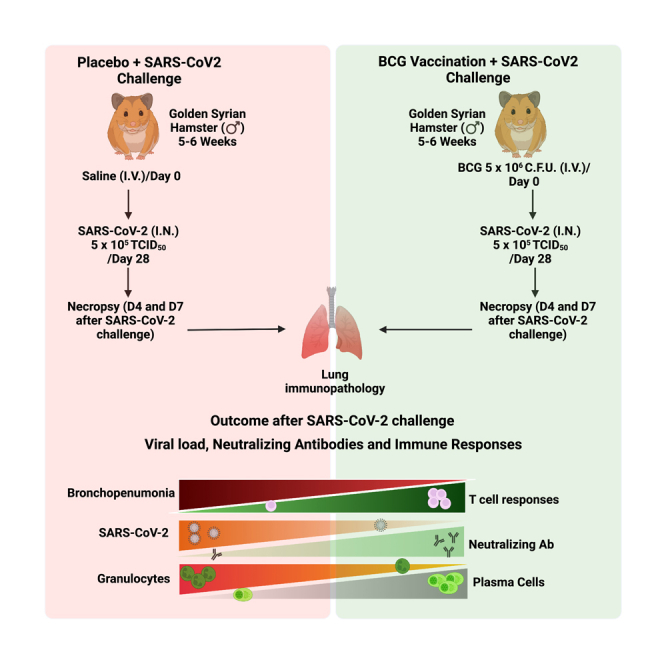
We set out to determine whether prior BCG vaccination alters the course of SCV2 infection in a golden Syrian hamster model (Figure 1A) which has previously been shown to exhibit a self-resolving, and non-lethal form of COVID-19. The experiment was performed once using a staggered design; the group size of vaccinated, challenged animals was 13 hamsters with 6 animals sacrificed at d4 and 7 animals at d7 post-SCV2 challenge; and for BCG-only treated hamsters the group size was 3 animals which were sacrificed on d35 post-BCG vaccination. We evaluated lung histopathologic changes at D4 and D7 post SCV2 challenge with and without prior BCG vaccination (Figures 1B–1C and S1A–S1F). While intra-alveolar inflammation (a common finding in viral pneumonitis) was unchanged by BCG vaccination (Figure 1C), we found that bronchopneumonia was significantly reduced by BCG-vaccination both at D4 and D7 (Figure 1D). In keeping with the bronchopneumonia findings, we also observed significantly reduced neutrophil infiltration in BCG vaccinated hamsters at D4 compared with unvaccinated animals (Figure 1E). In addition, BCG vaccination yielded a trend toward increased macrophage infiltration at D4 (Figure 1F). Regarding the well-described SCV2-mediated lymphopenia, BCG vaccination was able to partially correct infiltrating lung lymphocytes with BCG-vaccinated hamster lungs showing significantly higher levels of lung lymphocytes (CD3 positivity) both by traditional histology and IHC (Figures 1G and S2A), and this was true for perivascular lymphocytic infiltration as well (Figure S2B). We also noted atypical pneumocytes along the bronchial lining in unvaccinated hamsters (Figure S2C), and conversely, we observed granuloma formation, as expected, in BCG vaccinated hamsters (Figure S2C). The BCG-vaccinated hamsters which were not SARS-CoV-2 challenged showed normal lung pathology with the exception of small lung granulomas. Thus, prior BCG vaccination reduced SCV2-induced bronchopneumonia with its concomitant neutrophilic infiltration while simultaneously preventing lung lymphopenia and enhancing lung macrophage levels.
Figure 1.
BCG vaccination diminishes SARS-CoV-2 (SCV2) infection-induced lung inflammation, reduces viral load, and prevents weight loss in golden Syrian hamsters
(A) Experimental scheme showing hamster groups.
(B) High power micrograph of H&E-stained hamster lungs at day 4 (D4) and day 7 (D7) after SARS-CoV-2 infection with the arrow showing mild local inflammation in BCG + SCV2 lungs.
(C) Percent of high-power fields (HPF) showing intra-alveolar inflammation.
(D) Presence of bronchopneumonia in hamster lungs (0 absent, 1 present). Bronchopneumonia was defined as intrabronchial neutrophils with or without fibrin, red blood cells, or infiltration of the bronchial lining.
(E) Presence of neutrophil infiltration in hamster lungs (0 absent, 1 present).
(F) Presence of macrophage infiltration in hamster lungs (0 absent, 1 present).
(G) CD3+ lymphocyte positivity scores at D4 in hamster lungs after SARS-CoV-2 infection (0 absent, maximal score 18).
(H and I) Graph showing SARS-CoV-2 TCID50/mg of lung tissue and log10 viral load/100 ng of tissue in hamster lung at D4 and D7 post-infection in non-vaccinated and BCG-vaccinated hamsters. Briefly, infective viral particles (H) were quantified from a standard of infectious virus and expressed at TCID50 equivalents per mg lung tissue. The dotted lines indicate a lower limit of detection (LOD). Viral RNA levels (I) were determined in the lungs, normalized against human RNaseP, and were transformed to estimate viral RNA copies per 100 ng of total lung RNA. Data points represent the number of animals used per group.
(J) Mean change in hamster body weight at 0–6 days post-infection (DPI) with SARS-CoV-2. Statistical analysis was done using a two-sided Fisher exact test (D-E), Welch’s t-test (F), or two-tailed Student’s t-test (H–J) (significant when p < 0.05). Percent animals and 95% confidence intervals (CI) showing pathology were (D) bronchopneumonia in the BCG-SCV2 group at D4 0% (95% CI: 0, 45.9%), at D7 28.6% (95% CI: 36.7, 71.0%); (E) neutrophil infiltration in the BCG-SCV2 group at D4 50% (95% CI: 11.8, 88.2%); (f) macrophage infiltration in the BCG-SCV2 group 83.3% (95% CI: 35.9, 99.6%) and in the SCV2 only group at D4 60.0% (95% CI: 14.7, 94.7%). A description of CD3 positivity scoring is given in the STAR Methods section.
We also evaluated the effect of BCG vaccination on lung viral load by measuring infectious viral particles (TCID50) and viral RNA in lung homogenates. We observed a modest but significant decrease in TCID50 in BCG vaccinated animals at D4 (Figure 1H), and a non-significant trend toward reduced viral mRNA at this same time point (Figure 1I). TCID50 and viral mRNA levels were significantly lower at D7 compared to D4 and levels BCG-vaccinated animals did not differ from the unvaccinated ones likely because of progressive disease resolution in both groups. Lastly, weight change has been found to be an important marker of hamster health during SARS-CoV-2 infection,38 and we found that BCG vaccinated hamsters stabilized the weight loss that began at the time of SARS-CoV-2 challenge, while unvaccinated hamsters lost weight throughout the 6-day monitoring period (Figure 1J).
BCG vaccinated hamsters show less SCV2-mediated lung T cell lymphopenia, higher levels of lung macrophages, and lower levels of lung granulocytes
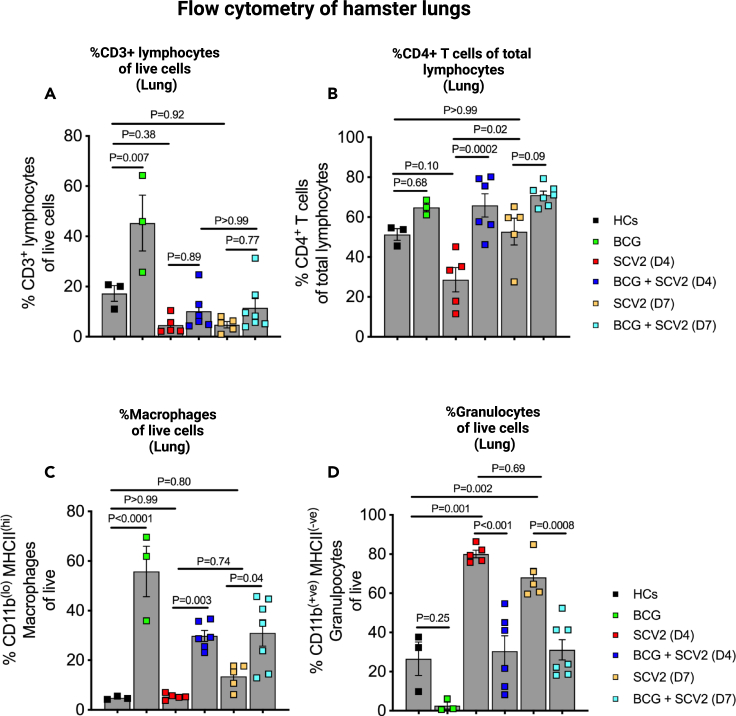
To assess overall cell population fluxes in hamster lungs, we performed multicolor flow cytometry. We found that lung T cell infiltration (CD3+ cells) was dramatically boosted by BCG vaccination in the absence of SCV2 (>40% of live cells) compared to age-matched healthy animals (18%) (Figure 2A). In unvaccinated animals challenged with SCV2, the total lung CD3+ population was reduced at D4 and D7 to 4–6%; while BCG vaccinated, SCV2 challenged animals showed a trend to have nearly twice as many CD3+ cells (10–12%) at these same time points (Figure 2A) supporting our histopathological observations. This same effect of BCG preventing T cell depletion in the lung was present to an even greater extent among lung CD4+ T cells (Figure 2B). We did not observe this same pattern in hamster spleens suggesting that the observed effects of BCG vaccination were limited to the site of active infection in the lungs (Figures S3A–S3B). Similarly, BCG vaccination in the absence of SCV2 infection prompted a pronounced macrophage recruitment to the lung (>50% of live cells) compared to healthy (unvaccinated) animals (5%) (Figure 2C). And while SCV2 infection led to a modest macrophage lung recruitment by D7 (12% of live cells), previously BCG vaccinated animals showed significantly elevated macrophage populations in the lungs (30% of live cells) on both D4 and D7 (Figure 2C). In contrast, for granulocytes, we found that SCV2-challenged, unvaccinated hamster lungs revealed dramatic infiltration by polymorphonuclear leukocytes (PMNs) on D4 (80% of live cells) and D7 (72%) (Figure 2D) in accordance with the profound bronchopneumonia observed (Figures 1B and 1D). Pre-vaccination with BCG had the effect of limiting the SCV2 mediated granulocytic infiltration to levels of 30% on D4 and 32% on D7 which were levels essentially equivalent to those in the lungs of vaccinated, unchallenged hamsters. A modest increase in macrophages at both D4 and D7 in the splenic compartment, not statistically significant, was observed in BCG-vaccinated, SCV2-infected animals compared to unvaccinated SCV2 infected animals (Figure S3C). Splenic granulocytes trended to be lower in BCG vaccinated animals compared to unvaccinated animals (Figure S3D), similar to the observations in the lungs.
Figure 2.
Differential abundance of lymphocytes, macrophages, and granulocytes in BCG-vaccinated golden Syrian hamster lungs after SARS-CoV-2 infection
(A) Differential percentages of a. CD3+ lymphocytes.
(B) CD4+ T cells within the lymphocyte compartment.
(C and D) Macrophages (CD11b(lo) MHCII(hi) of live cells) and (D) granulocytes (CD11b(+ve) MHCII(−ve) of live cells) in the lung of hamsters. Single cell preparations from lung tissues following necropsy were investigated for different lymphoid and myeloid cells using multicolor flow cytometry. The data are presented as mean values ±S.E.M. Statistical analyses were done using one-way ANOVA with the p values indicated.
Single cell transcriptional profiling in lung cells in BCG vaccinated and unvaccinated hamsters
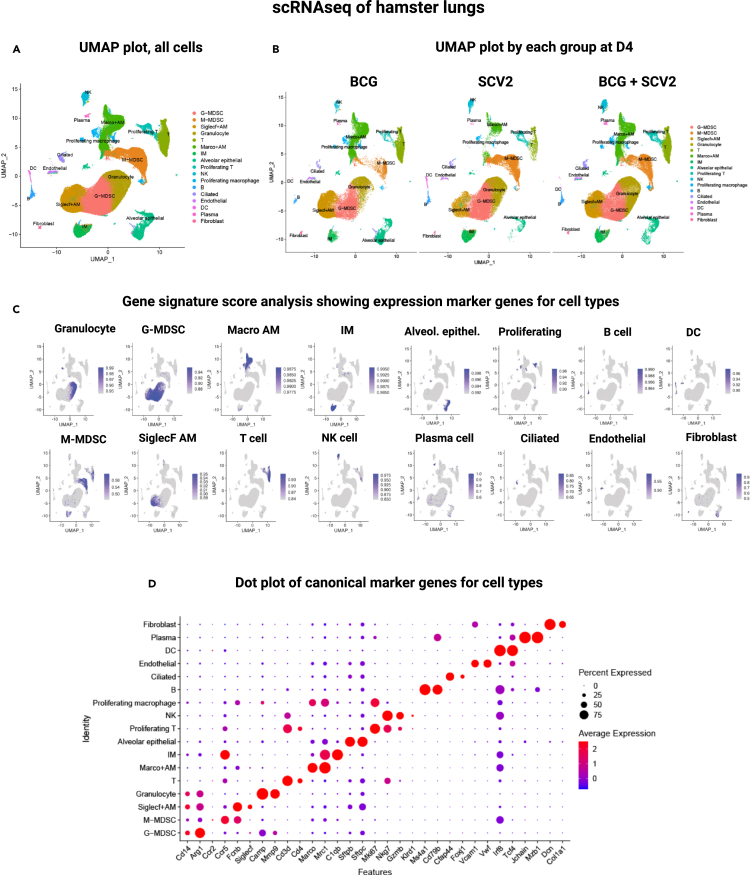
To obtain a higher resolution of pulmonary immune responses linked to BCG immunization during SCV2 infection, hamster lungs were examined using droplet-based single-cell RNA sequencing (10X Genomics) during the peak (day 4) and resolution (day 7) phases of COVID-19. BCG-vaccinated, unchallenged hamster lungs were also studied. A total of 13 hamsters were evaluated in the following three groups: BCG vaccination only (3 animals), SCV2 infection only (2 animals on D4; 2 on D7), and BCG vaccination with SCV2 challenge (3 animals on D4; 3 on D7). Sequencing was performed in a total of 194,536 cells and, after filtering out low-quality cells, red blood cells, and doublets, 112,928 cells were analyzed. (Table S1). The mean numbers of cells analyzed per animal in BCG vaccinated lungs, SCV2 infected lungs, and BCG-vaccinated + SCV2 infected lungs were 11,249, 8,643, and 7,434 respectively. By graph-based clustering of uniform manifold approximation and projection (UMAP), transcriptomes of 17 major cell types or subtypes were identified in hamster lungs (Table S2). These included lymphoid, myeloid, and non-immune cell types (Figures 3A–3C). These cell clusters were relatively homogenously distributed across all animal lungs at both timepoints and were based on the expression of well-defined canonical genes as shown in Figure 3D. The 5 most highly expressed genes in each cluster are shown in Figure S4.
Figure 3.
The cellular landscape of SARS-CoV-2 infected hamster lungs after BCG vaccination identified by scRNA-seq
(A) Uniform Manifold Approximation and Projection (UMAP) plot showing identification of 17 different major cell-type in hamster lungs integrated by all samples.
(B) UMAP plot showing lung cellular dynamics in hamster lungs in BCG vaccinated, SARS-CoV-2 (SCV2) infected, and BCG + SARS-CoV-2 (SCV2) infected animals at D4 after SCV2 infection.
(C) UCell score distribution in UMAP plot for cell type marker gene signatures (listed in Table S2) evaluated using UCell.
(D) Dot plots of log-normalized expression and fraction of cells expressing selected canonical marker genes for identification of 17 different cell types in hamster lungs across all samples.
BCG vaccination is associated with relatively higher Th1, Th17, Treg, CTLs, Tmem, and plasma cell populations during SCV2-infection
Next, we performed subset analyses to evaluate lung immune cells during SCV2 infection in vaccinated and unvaccinated hamsters. We were able to identify 10 distinct lymphocyte cell types (Figures 4A and S5A–S5B). Among the CD4+ T cells in hamster lungs, we were able to distinguish Th1, Th17, Treg (regulatory T cells) and Tmem (memory T cells) cells primarily based on Ifng, Itgb7, Foxp3, and Lef1 expression, respectively (Figures S5C and S6). CTLs (cytotoxic T cells) showed high Gzmk and Gzma but low Cd4 expression. Plasma cells were identified by their elevated expression of Jchain and Mzb1 (Figures S5C and S6). As may be seen in Figure 4A, both at D4 and D7 post-SCV2 challenge, hamsters that were BCG-vaccinated maintained high levels of Th1, Th17, Treg, CTLs, Tmem, and plasma cells in their lungs. With the exception of plasma cells, vaccination with BCG only (no SCV2 challenge) stimulated high levels of these cell types in the lungs, and these relatively high levels were maintained in hamsters challenged with SCV2. In contrast, a plasma cell response was not observed in BCG-vaccinated, unchallenged animals; rather elevated plasma cell numbers in the lungs were uniquely observed only in BCG-vaccinated, SCV2 infected animals.
Figure 4.
BCG vaccination strongly affects lung lymphoid dynamics and gene activation programs in different lymphoid subsets in SARS-CoV-2-infected hamster lungs
(A–E) (A) Bar graph showing the average proportion of different lymphocytic cells across groups. Violin plots showing gene signature scores for (B) immunoglobulin production on plasma cells and (C) exhaustion scores on Th1 cells across different groups. Horizontal lines represent mean values. All differences with p < 0.01 are indicated. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 (one-way ANOVA). Th1 lymphocyte-linked gene ontology enrichment analysis of biological processes showing gene sets that enriched in (D) SARS-CoV-2 (SCV2) versus BCG + SARS-CoV-2 (SCV2) and in (E) BCG + SARS-CoV-2 (SCV2) versus SARS-CoV-2 (SCV2) across both time-points. A darker color indicates a smaller p value.
Elevation of plasma cell abundance in BCG-vaccinated, SCV2 challenged hamsters
In order to estimate the numbers of lymphocytes in healthy control hamsters, we were able to compare our scRNA-seq cell abundance data with previously published data which included healthy hamster controls39(Figure S7A). Both Th1 and Treg lymphocytes were scarce in the lungs of healthy hamsters but were strongly recruited to the lung by BCG vaccination both in the presence and absence of SCV2 challenge (Figure S7B). Plasma cells were not seen abundantly in either healthy hamster lungs or unchallenged, BCG-vaccinated hamsters. Thus, an enhanced plasma cell response occurred only in the presence of prior BCG-vaccination followed by SCV2 challenge (Figure 4A). Moreover, an evaluation of gene signatures revealed elevated expression of immunoglobulin production by plasma cells in BCG-vaccinated and SCV2 challenged animals strongly suggesting that these plasma cells appear functionally active in antibody production (Figure 4B). To determine if the elevated plasma cell levels in the lung resulted in accelerated antibody production in the serum, we measured anti-spike receptor-binding domain (RBD) IgG, IgM, and neutralizing antibody (nAb) levels and found that no antibodies were detectable at d4 and that at d7 there were no significant differences between BCG-vaccinated and unvaccinated hamsters although there was a slight, non-statistically significant trend toward elevated IgM and nAb levels in BCG vaccinated animals (Figures S7C–S7E). Since serum antibody levels are largely driven by plasma cell production in lymph node germinal centers, it remains likely that the lung plasma cells we observed generate a local antibody response that is not detectable in plasma but it is also possible that the plasma cells observed do not produce antibody responses specific to SARS-CoV-2.
Lung CD4+ Th1 cells showed reduced exhaustion and mitochondrial stress gene signatures with BCG vaccination
By gene signature and gene ontology (GO) analysis, we found that Th1 cells in unvaccinated, SCV2-challenged showed evidence exhaustion markers (Figure 4C) and a high degree of mitochondrial respiratory gene expression and translation (Figure 4D), consistent with mitochondrial dysfunction in T cells that have been previously reported.40 In contrast, in Th1 cells from BCG-vaccinated, SCV2 challenged animals, pathways related to T cell activation, T cell immunity, and antigen processing were upregulated suggesting greater functional immunity (Figure 4E). As expected, these signatures were absent in hamsters which received BCG only.
Differentially expressed genes (DEGs) in lung CD4 Th1 cells
When we considered specific DEGs in Th1 cells in BCG-vaccinated, SCV2-challenged hamsters (Figures S8A and S8B), we found strong upregulation of genes associated with protection from injury. These include Cd74, a gene recently associated with antiviral responses and protection from lung injury,41,42 Cd70 a gene associated with T cell activation, Ltb (lymphotoxin-beta) a gene known to play a protective role in other viral infection, Gsdma (gasdermin A) which has been found to be deficient in autopsy studies of humans with severe COVID-19,43 and Ifng (IFN-γ) a prominent Th1 activator. Several of these same genes including Cd74, Ltb, and Gsdma were also strongly expressed in Th17 and Treg lung lymphocytes from BCG-vaccinated, SCV2-challenge hamsters (Figures S8C–S8D). We noted that the pro-inflammatory gene Gzmb (granzyme B) was strongly expressed in unvaccinated hamster Th1, Th17, and Treg cells but not in BCG-vaccinated animals (Figures S8B–S8D); high granzyme B levels have been reported in severe human COVID-19.44,45
In contrast, in Th1 cells from non-vaccinated, SCV2-challenged hamsters, we found DEGs that were associated with markers of exhaustion and ongoing viral infection (Figures S8A–S8B). Markers of exhaustion included Cd278 (Icos), a gene known to be up-regulated in SCV2,46 Ctla4, a well-known exhaustion marker which has also been found in human SCV2 convalescent plasma,47 and Tnfrsf18 (GITR). Genes associated with ongoing viral infection included Ifitm2 (IFN-induced transmembrane protein 2) which has recently been shown to be hijacked by SCV2 and to promote ongoing infection response,48 Bzw2 (Basic Leucine Zipper and W2 Domain-Containing Protein 2)49 which is a viral restriction factor shown to interact with the SCV2 M protein, and IFN-responsive regulatory proteins Mx1 (an IFN induced GTP-binding protein), Rgs10 (Regulator of G protein signaling-10), and Irf7 which is known to be involved in transcription activation of viral-inducible cellular genes and is a gene for which polymorphisms known to be associated with severe SCV2.50 We noted that DEG Saa3 encoding serum amyloid A3 protein which is an acute phase reactant marker of inflammation and infection was temporally down-regulated over time in BCG-vaccinated SCV2-challenged animals.51
Myeloid cell changes mediated by BCG vaccination: Enhanced AM abundance and diminished IM lung recruitment
Our scRNA-seq analysis of myeloid cells revealed 10 distinct myeloid cell subsets (Figures 5A and S9A–S9B). AMs are relatively immunotolerant cells that scavenge debris and play an important role in pathogen clearance.52 We identified two clusters of AMs, the first identified by the classic AM marker Siglecf (Siglecf+ AMs) and the second identified by the presence of the scavenger receptor Marco (Marco+ AMs). BCG vaccination has been shown to induce recruitment of Siglecf+ AMs to the lung in healthy human volunteers,53 and consistent with this, while we observed virtually no Siglecf+ AMs in healthy hamster lungs, this population was strongly induced by BCG vaccination (Figure S9C). In contrast, scavenging Marco+ AMs were present in high numbers in healthy hamster lungs, and BCG vaccination caused ∼2-fold increased in this population (Figure S9C). Unvaccinated, SCV-infected hamsters showed a high degree of depletion of both categories of AMs, but prior BCG vaccination served to prevent the loss of both AMs from the lung (Figures 5A and S9C).
Figure 5.
BCG vaccination curtails SCV2-mediated lung inflammation by modulating myeloid cell abundance and phenotypes
(A) Bar diagram showing the average proportion of different myeloid cells across groups.
(B) Dot plot showing the expression levels of a group of chemokines and cytokines in Isg15+ macrophages across different groups.
(C) Violin plots showing gene signature scores of hallmark type I IFN (Interferon-α) responses in 5 major myeloid cells in hamster lungs across different groups. All differences with p < 0.01 are indicated. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 (one-way ANOVA).
Interstitial macrophages (IM) are more inflammatory than AMs and are recruited from the periphery during lung infection.54,55 In addition to traditional IMs, we identified a second population which strongly expressed Isg15 (Isg15+ macrophages) indicating that they are highly responsive to IFN I and hence likely to be hyperinflammatory. Both IMs and Isg15+ macrophages were virtually absent in healthy hamster lungs (Figure S9C) and in unvaccinated hamsters, but both of these cell types were recruited to the lungs in high numbers following SCV2 challenge. Interestingly, prior BCG-vaccination significantly blunted this recruitment (Figures 5A and S9C). In keeping with the inflammatory nature of Isg15+ macrophages, we observed that SCV2 infection caused a strong upregulation of Ccr5, Ccl8, Ccl7, Ccl5, Ccl4, Ccl2 and Cxcl10 in IFN-I-responsive Isg15+ macrophages (Figure 5B). These inflammatory chemokines and chemokine receptors remained at basal expression levels in either BCG vaccinated animals or those challenged with SCV2 after vaccination. These observations support the notion that BCG vaccination leads to an elevated setpoint of scavenging AM in the lungs, but during SCV2 infection it prevents excess recruitment of pro-inflammatory Isg15+ macrophages and IMs which may be involved in SCV2-mediated, lung immunopathology.
Dendritic cells (DCs)
We observed an increase in dendritic cells following SCV2 infection at both time points. In contrast, levels of DCs remained essentially undetectable with BCG vaccination alone or BCG-vaccination in the presence of SCV2 challenge (Figure 5A). Patients with severe COVID-19 demonstrate reduced DC levels in blood possibly because of recruitment to infection sites.56 DCs, especially pDCs, are considered a chief source of IFN I, a cytokine which is found at unusually low levels during human COVID-19 suggesting that DCs have an exhaustion phenotype during SCV2 infection.57,58
Myeloid-derived suppressor cells (MDSCs)
Expansion of MDSCs have been described in humans with severe COVID-19, a phenomenon that correlates with lymphopenia and enhanced arginase activity, while milder forms of disease display a reduced MDSCs count.59,60 Increased recruitment of both G-MDSCs and M-MDSCs subtypes of myeloid cells are reported in COVID-19 patients and may contribute to acute respiratory distress syndrome (ARDS).61,62 BCG vaccination led to reductions of in both M-MDSCs and G-MDSCs among SCV2-challenged hamsters suggesting a protective effect (Figure 5A). The majority of G-MDSCs identified in our data showed marked elevation of Il1b and Arg1 both strong biomarkers of COVID-19 severity62(Figure S4).
Myeloid cell DEGs reveal divergent immune programs
Next, we evaluated DEGs in Siglecf+ AM, Marco+ AM, IMs, Isg15+ macrophages, and M-MDSCs. In unvaccinated animals infected with SCV2, there was high transcription of IFN-α (IFN-I) response genes (Figure 5C); these included Isg15, Irf7, and Mx1 as well as chemokine-encoding genes such as Ccl2, Ccl12, and Ccl4 and those associated with lymphocyte activation (Slamf9) all consistent with ongoing inflammatory responses (Figures S10A–S10B). However, in vaccinated animals challenged with SCV2, the predominant DEGs were shifted toward those involved in metabolic and repair processes such as Ubd (encoding ubiquitin), Ppa1 (encoding pyrophosphatase 1), Cdo (encoding cysteine dioxygenase) or genes for complement factor expression (C1qa, C1qc) as might be expected in a less inflammatory, repair-oriented environment (Figures S10A–S10B). Focused analysis of IFN-α and IFN-γ response gene signature scores revealed that for major myeloid cell subsets, BCG-vaccinated animals showed reduced expression of these interferons (Figure 5C), including for Isg15+ macrophages which had the highest levels of IFN-γ expression.
DEGs in Isg15+ macrophages
In unvaccinated, SCV2-challenged Isg15+ macrophages we observed robust upregulation of type I IFN-dependent ISGs (interferon-stimulated genes), (Bst2, Mxd1 and Ifitm2), ISGs known to be activated following viral genome sensing (Irf7, Fcgr4, Slamf9, and Irf9), antiviral restriction factors (Apobec1), and inflammatory mediators known to drive COVID-19 severity (Ccl2, Ccl4, Ccl7, Ccrl2, Cxcl10, and Ccl12) (Figure S10A). In contrast, Isg15+ macrophages among BCG-vaccinated, SCV2-challenged hamsters expressed high levels of oxidative phosphorylation genes including Atp5, Atp5c1, Atp5f1a and Atp5g3 as well as mitochondrial Chchd10 (a gene predicted to be involved in mitochondrial oxidative phosphorylation) suggesting upregulated oxidative phosphorylation, a characteristic hallmark of BCG training.63 Isg15+ macrophages among BCG-vaccinated animals showed increased expression of mitochondrial genes Suclg1 (succinyl-CoA ligase), and Sdhd (succinate dehydrogenase) suggested increased generation of succinate and fumarate respectively. Both metabolites play extensive roles in epigenetic reprogramming of macrophages, a characteristic also associated with macrophage training. Interestingly, increased expression of Aoah (encoding acyloxyacyl hydrolase), a gene usually upregulated on granulomas formed by mycobacteria64 which may promote resolution of inflammation was also observed in BCG-vaccinated Isg15+ macrophages. ISGs associated with immune clearance were also upregulated including Snx10 (phagosomal maturation), Gtgn2 (M1 polarization) and Itgal (T cell recruitment) were upregulated following BCG vaccination and BCG-vaccinated, SCV2-challenged hamsters (Figure S10A). Isg15+ macrophages from BCG-vaccinated, SCV2 challenged animals also showed up-regulation of genes associated with attenuation of inflammatory responses such as Socs3, Il18bp, Dusp1 and Fos as well as those which may counter tissue damage phenotype induced by inflammation such as Il1rn (regulator of IL-1α and IL-1β cytokines) and Timp1 (tissue inhibitor of MMP1) (Figure S10A). Certain genes in BCG-vaccinated, SCV2-challenged hamsters were showed low level expression at d4 but were strongly up- (Plaat3, C1qa, Mcoln2, C1qc, C1qb) or down-regulated (Ecm1, Dusp1, Nfkbia, Ccl3) at d7.
DEGs in Siglecf+ AMs
In unvaccinated, SCV2-infected animals Siglecf+ AMs highly expressed inflammatory genes (Irf7, Irf9, Apobec1, Mx2, Mx1, Isg15, Ifit2, Rasd2, Herc6, Gbp5, Gbp7, Ifi44, Ifi47, Dhx58, Ifitm2, Igitp, and Cnp) and genes involved in T cell recruitment and activation (Ccl5, Ccrl2, Clec5a, Ltb, Ccl4CL4, Marcs1, Tnfsf10, Cd48, Alxox5, C3ar1, Cd80, and Cxcr2). Genes characteristic of ER stress (Gadd45, Gadd45b, and Atf4) were also upregulated in Siglecf+ AMs of unvaccinated, SCV2-challenged animals (Figure S10B). In the Siglecf+ AMs of these same animals we also noted expression of genes are associated with signaling to plasmacytoid DCs and monocytic DCs (Unc93b1, Lsp1, Slamf9 and Batf) suggesting a program to keep these key sentinel cells in and activated state. In contrast, in Siglecf+ AMs from BCG-vaccinated, SCV2-challenged hamster lungs we found upregulation of genes associated extracellular microbial sensing linked to NF-κB signaling (Cox1, Cox2, Cox3, Tnfsf13b, Hsp90ab1, Apoe1, Gtgn2 and Ckap4P). In addition, genes involved in phagocytosis (Mrc1), antigen presentation (H2aa), and suppression of inflammation (Cdo1, Mertk, Mrc1) were also upregulated in Siglecf+ AMs from BCG-vaccinated, SCV2-challenged hamsters (Figure S10B).
Non-immune cells are modulated by BCG vaccination
Since we did not enrich for immune cells prior to scRNA-seq analysis, we were able to investigate non-immune cell responses. We were able to identify 5 types of non-immune cells (AT2, endothelial, ciliated, AT1, and fibroblasts) in hamster lungs. We identified both type I alveolar cells (AT1 cells), which are non-replicative, thin flat squamous cells that form the basic structure of alveoli, as well as Type II cells (AT2 cells), which are cuboidal, release pulmonary surfactant, and may differentiate into AT1 cells during lung injury.65 Ciliated cells from bronchial epithelium, fibroblasts, and endothelial cells were also present.
Consistent with ongoing inflammatory lung injury during SCV2 infection at D4 and D7, we found that AT1, AT2, ciliated, and fibroblast cells all appeared at significantly reduced frequencies in unvaccinated animals (Figure 6A). Interestingly, BCG vaccination prevented this depletion suggesting a global protective effect by BCG against viral lung injury. Since this reversal of lung damage was most profound for AT2 cells, we investigated the GO of these cells in BCG-vaccinated animals and found both an enhancement of genes involved in remodeling and response to injury as well as genes involved in immune responses (Figure 6B). We noted that antigen presentation via MHCII was enriched in AT2 cells, and recent studies indicate that antigen presentation may serve as a pan-epithelial feature playing a major role as a barrier tissue regulator of the location, plasticity and activity of resident memory T cells.66 In contrast, among non-vaccinated animals the gene groups most strongly expressed in AT2 cells were those playing a role in viral responses and host defenses (Figure 6C).
Figure 6.
BCG vaccination restores AT2 cells and causes extensive phenotypic changes in non-immune cells in SARS-CoV-2-infected hamster lungs
(A–D) (A) Bar graph showing the average proportion of different lung non-immune cells across groups. AT2 cell-linked gene ontology enrichment analysis of biological processes showing gene sets that enriched in (B) BCG + SARS-CoV-2 (SCV2) versus SARS-CoV-2 (SCV2), and in (C) SARS-CoV-2 (SCV2) versus BCG + SARS-CoV-2 (SCV2) across both time-points and D. heatmap depicting the average expression values of top differentially expressed genes (DEGs) across different groups in AT2 cells. Top DEGs were selected with adjusted p-value <0.05 and average fold change >0.7.
AT2 cells possess immunomodulatory functions and are considered the immune cells of the alveolar epithelium. Not surprisingly, when we evaluated DEGs in AT2 cells, we observed similar patterns to those seen in lymphoid and myeloid cells. In the absence of vaccination, prominent DEGs in AT2 cells were IFN-response genes (Mx1, Mx2, Irf7, Irf9, Ifit2, Ifitm2, Slamf9), antiviral response genes (Apobec1, Ifit2, and Ddx58) (Figure 6D). In contrast, in BCG-vaccinated hamsters, AT2 DEGs were involved in immune clearance pathways such as antigen presentation (H2-Ea, H2-Oa, H2Aa, Ctss [cathepsin S, lysosomal Ag processing]), suppression of inflammation (Ido1), lysosomal clearance (Ubd, lipA [lysosomal acid lipase]), and the complement pathway (C1qa, C1qb C1qc, Cfd). Indeed, epithelial cells are known sources of complement proteins,67 and we observed similar elevations in for C1qa, C1qc in Isg15+ macrophages (Figure S10A).
Quantification of lung pathology using PET/CT
To evaluate pulmonary lung disease, SCV2 infected animals were imaged using 18F-FDG PET/CT at the peak of lung disease (D4) after infection. The 18F-FDG PET/CT analysis of lung consolidations did not reveal significant differences across the groups at D4 (Figures S11A–S11C).
Discussion
In this study we evaluated the impact of intravenous BCG vaccination on the pathogenesis and immunology of SCV2 lung infection in the golden Syrian hamster model using viral quantification, histology, and flow cytometry supplemented by scRNA-seq analysis. The SCV2 virus levels, histology and flow results showed that BCG vaccination conferred a modest but statistically significant reduction in SCV2 replication at the peak of infection (D4), that it also reduced the development of bronchopneumonia both at D4 and D7, and that it prevented SCV2-associated weight loss. Concomitantly, BCG vaccination blunted T cell lymphopenia in the lungs and reduced granulocyte lung infiltration. BCG vaccination was also associated with a significant recruitment of macrophages to the lung.
Our study is the first to use single-cell transcriptional analysis in BCG-vaccinated, SCV2 infected animals. Our scRNAseq data revealed that BCG vaccination 4 weeks prior to SCV2 challenge was associated with significant shifts both in the populations of cell types present in the lungs and in the DEGs expressed by these cell types. In light of the short time interval between SCV2 challenge and the scRNA-seq analyses (4 or 7 days), the scRNA-seq changes observed are likely to reflect alterations in innate immune responses. Among lymphoid cells, we noted a unique lung recruitment of plasma cells in BCG vaccinated animals that was absent in SCV2-infected animals and also in BCG vaccinated animals that were not SCV2 infected. These plasma cells showed higher gene signature scores associated with elevated immunoglobulin production suggesting that they are involved in a BCG-mediated acceleration of antibody production against the SCV2. This expansion of plasma cells and humoral response may in part be an anamnestic reaction, since peptide epitopes from BCG proteins and SARS-CoV-2 NSP3 and NSP13 proteins have shown to share significant homology and this may allow for potential cross-reactive adaptive immunity.68,69 Interestingly, two recent randomized, controlled clinical trials suggest that BCG improves SARS-CoV-2 antibody responses: one showed that among patients who developed COVID-19 those with prior BCG develop higher levels of specific anti-SARS-CoV-2 antibodies,14 and the other found that prior BCG leads to improved anti-SARS-CoV-2 antibody responses after receipt of the COVISHIELD ChAdOx1nCoV-19 vaccine.70 Several categories of CD4 T cells (Th1, Th17, Treg, and Tmem cells) as well as CTLs were more abundant in the lungs of BCG vaccinated animals, and BCG-vaccination led to reduced exhaustion scores in the gene expression profiles of some of these T cells. Th1 and Tregs cells in the lung expressed high levels of type I IFN-associated genes in SCV2-infected animals as would be expected with viral infection; however, in these same cell types, among BCG-vaccinated, SCV2-infected lungs the predominant DEGs were shifted toward antigen presentation.
Among macrophages, scRNA-seq revealed that BCG vaccination alone recruits high levels AMs to the lungs, a cell type associated with low inflammatory potential and clearance of pathogens. Upon SCV2 infection, BCG-vaccinated animals retained these high levels of AMs, while they dropped to low levels in SCV2-challenged, unvaccinated hamsters. In contrast we identified several populations of interstitial macrophages that were considerably more abundant in lungs of unvaccinated animals than in those that were BCG-vaccinated. As IM are non-resident macrophages likely recruited from the periphery which are known to have high inflammatory capacity, it is possible that a salutary immunologic effect of BCG is prevention of excess pro-inflammatory IM recruitment. While the DEGs of both the AM and IM populations in unvaccinated animals showed high expression of IFN-associated and chemokine genes, BCG vaccinated lungs showed AM and IM that strongly expressed metabolic and repair genes. It is well known that mycobacteria such as Mtb and BCG elicit an antiviral Type 1 IFN response,71 and a recent preprint using scRNA-seq analysis indicates that in progressive TB lesions in mice this high Type I IFN response emanates from pDCs and certain IMs and is deleterious TB control.72 Our finding that prior BCG vaccination followed by SCV2 infection blunts the Type I IFN responsiveness of myeloid cells (Figure 5C) yet nevertheless leads to high level expression of antiviral Type I IFN genes such as Mx1, Mx2, Isg15, Ifitm2, and Irf7 in lymphoid cells (Figure S8A) suggests that prior BCG exposure may temper Type I IFN responses in a beneficial way.
This study shows that intravenous BCG has a modest beneficial effect against SCV2 infection in hamsters. We found a modest, statistically significant reduction of the SCV2 viral titer in BCG vaccinated animals at the peak of infection on day 4, and our results agree with two studies of IV BCG vaccination in the K-18 mouse model demonstrating protection against SCV2.33,34 In contrast another recent study of both intravenous and subcutaneous BCG in two species of hamsters (golden Syrian and Roborovski) found that neither route of vaccination protected either hamster species against SCV2 challenge.36 Our study differed from that of Kaufmann et al. in that we used a higher dose of intravenous BCG (5 × 106 as opposed to 1 × 106 CFU), a higher viral challenge dose (5x105 as opposed to 1x105 or 1.4 × 104 PFU), and our peak disease sacrifice time was one day later (day 4) than theirs (day 3). Four groups have evaluated non-intravenous administration of BCG to prevent SCV2 disease; these include three groups that studied subcutaneous administration in mice,33,35,36 and one group that evaluated aerosol BCG administration in non-human primates.37 All four of these studies failed to show a significant effect of BCG vaccination against SCV2 proliferation. It is important to note that the intravenous route of BCG administration is likely to have significant differences from the more clinically relevant subcutaneous or intradermal route, and this has been shown in non-human primates challenged with Mycobacterium tuberculosis.73 Also, despite being a well-defined animal model of COVID-19 with human-like pathology, one key limitation of using golden Syrian hamsters is the limited immunological resources such as well-characterized and validated antibodies (anti-CD8 Abs for example) and the relative paucity of immunologic data on hamster immune responses for correlation with our scRNA-seq data. Along these lines, it was surprising that no CD8+ T cells were observed across the hamster groups even though they have been well-described in human scRNA-seq studies of BAL samples in SCV2.74,75 Another limitation of our study is that live BCG organisms are very likely to have been present in hamster tissues given the short, 28-day interval between vaccination and challenge, and thus we cannot definitively attribute the efficacy of BCG to heterologous “trained” immune memory which has been demonstrated in humans as long as 90 days following intradermal BCG vaccination.28
While BCG vaccination is routinely used in most countries for TB prevention, the vaccine is generally given intradermally, and recent human studies showing protection by BCG against COVID-19 disease also used intradermal BCG.13 Like other animal studies using BCG in animal models of SCV2 (28) we used the intravenous route based on literature that IV BCG is far more potent against tuberculosis in non-human primates, and evidence that IV BCG reprograms bone marrow hematopoietic stem cells toward a more protective state against bacterial challenge.27 Nevertheless, SCV2 animal studies using subcutaneous BCG have shown significant immunologic benefits at the level of flow cytometry,33 and we anticipate that many of the scRNA-seq shifts which we observed in IV BCG vaccinated hamsters would be present following intradermal BCG albeit potentially at a lower level.
In summary, our study reveals that BCG vaccination reduces SCV2 replication and prevents bronchopneumonia in hamsters by mechanisms that involve enhanced numbers of lung AMs, blunting of SCV2-mediated T cell lymphopenia, and reduced lung granulocyte infiltration. BCG appears to accelerate the appearance of immunoglobulin-producing plasma cells in the lung suggesting accelerated antiviral antibody production. The fact that BCG elevates the abundance of lung Treg cells while shifting a number of cell types away from expression of IFN-associated genes, suggests that BCG has immunotolerizing activity. These observations indicate that BCG vaccination may play a beneficial role in either preventing COVID-19 or limiting its severity in humans and suggest that further studies of combining BCG with existing COVID-19 vaccines may offer synergistic protection.
Limitations of the study
A key limitation of our study is the limited resources and reagents available for hamster-related immunological investigations and the relative paucity of data on immune responses in hamsters for correlation with the scRNA-seq data. Another limitation of the study is that although we show that BCG uniquely recruited immunoglobulin-producing plasma cells to the lung, we have not determined if this was a specific antibody response to SCV2 antigens or BCG or both, or merely a recruitment of non-specific plasma cells that enhanced local antibody response. Yet another limitation of our study is that live BCG is known to persist 3–9 months post-vaccination and is very likely to have been present in hamster tissues. Therefore, given the short, 28-day interval between vaccination and SCV2 challenge, we cannot definitively attribute the efficacy of BCG against SCV2 to heterologous “trained” immune memory.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-CD3 | Bio-Rad | MCA1477PB, CD3-12; RRID: AB_10843429 |
| Anti-CD4 | BioLegend | 100451, GK1.5; RRID: AB_2564591 |
| Anti-CD11b | Novus | NB110-89474, PECY7Poly; RRID: AB_1216361 |
| Anti-RT1D | BioLegend | 110211, 14-4-4S; RRID: AB_493214 |
| Anti-CD3 | Thermo-Fisher | RM-9107-S1; RRID: AB_149924 |
| HRP-conjugated secondary IgG | Abcam | ab6892; RRID: AB_955427 |
| HRP-conjugated secondary IgG | Brookwood BioMed | sab3003M |
| Bacterial and virus strains | ||
| Bacillus Calmette-Guérin (BCG)-Tice or OncoTICE | Merck | TICE® BCG |
| SARS-CoV-2 virus | CDC | Wuhan-1/2020 |
| WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) | BEI Resources | NR-52281 |
| Chemicals, peptides and recombinant proteins | ||
| SARS-CoV-2 N gene | Integrated DNA Technologies | 10006625 |
| Spike receptor-binding domain (S-RBD) | BEI Resources, NIAID | NR-52309 |
| 7H9 Middlebrook Medium | Fisher Scientific | B271310 |
| OADC | Fisher Scientific | B11886 |
| Glycerol | Sigma | G55116 |
| Tween-80 | Fisher Scientific | BP338 |
| Minimum Essential Media (MEM) | Thermo Fisher | 11095080 |
| Fetal Bovine Serum (FBS) | Thermo Fisher | 16140071 |
| ACK lysis buffer | Thermo Fisher | A1049201 |
| IC fixation buffer | eBiosciences | 00-8222-49 |
| RBC lysis solution | Miltenyi Biotec | 130-094-183 |
| Mouse lung dissociation kit | Miltenyi Biotec | 130-095-927 |
| Mouse spleen dissociation kit | Miltenyi Biotec | 130-095-926 |
| TRizol | Invitrogen | 15596026 |
| RNeasy kit | Qiagen | 74004 |
| TaqMan Fast Advanced Master Mix | Applied Biosystems | 4444556 |
| 18F-FDG | SOFIE | F-18- fludeoxyglucose |
| Critical commercial assays | ||
| Zombie Aqua™ Fixable Viability Kit | Biolegend | 423101 |
| Chromium Next GEM Single Cell 3ʹ Reagent Kits v3.1 (Dual Index) | 10X Genomics | CG000315 |
| UltraView DAB detection kit | Roche | 05269806001 |
| CellTiter-Glo® Luminescent Cell Viability Assay Kit | Promega | G9241 |
| 2019-nCoV CDC Research Use Only (RUO) kit | Integrated DNA Technologies | 10006625 |
| Deposited data | ||
| scRNA seq data | NCBI | GSE229135 |
| Experimental models: Cell lines | ||
| Vero C1008 [Vero 76, clone E6] Cells: For vial growth, and vital stock preparation | American Type Culture Collection (ATCC) | ATCC CRL-1586 |
| VeroE6-TMPRSS2 Cells: For determination of virus neutralization assay | Japanese Collection of Research Bioresources Cell Bank | JCRB1819 |
| Experimental models: Organisms and strains | ||
| Male golden Syrian hamsters (Mesocrietus auratus), Strain HsdHan®: AURA | Envigo | HsdHan®:AURA |
| Software and algorithms | ||
| FACSDiva | BD | www.bdbiosciences.com |
| FlowJo (v10) | FlowJo | TreeStar |
| Cell Ranger v4.0.0 | 10X Genomics | www.10xgenomics.com |
| Seurat v4.0.4 | (Hao et al., 2021) | github.com/satijalab/seurat |
| UCell | (Andreatta et al., 2021) | github.com/carmonalab/UCell |
| Metascape | (Zhou et al., 2019) | https://metascape.org |
| GraphPad Prism | Dotmatics, Prism - GraphPad | https://www.graphpad.com |
| Other | ||
| Animal bedding | Envigo | Teklad 7099 TEK-Fresh |
| Animal feed | Envigo | Teklad 2018 SX |
| GentleMACS Octo Dissociator with heaters | Mitenyi Biotec | 130-096-427 |
Resource availability
Lead contact
Additional information and requests for resources and reagents used in this study should be directed to and will be fulfilled by the lead contact, William R. Bishai (wbishai1@jhmi.edu).
Materials availability
There are restrictions on the availability of the live viruses SARS-CoV-2/Wuhan-1/2020 virus (U.S. Center for Disease Control and Prevention) and WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) (BEI Resources) due to shipping restrictions of BSL-3 pathogens.
Vero C1008 [Vero 76, clone E6] cells can be obtained from ATCC (ATCC CRL-1586), while VeroE6-TMPRSS2 cells could be obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB1819).
Mycobacterium bovis (M. bovis) Bacillus Calmette-Guérin (BCG)-Tice or OncoTICE is commercially available from Merck (TICE® BCG). Also, BCG-Tice (used in this study) can be available upon request and written formal MTA documents.
Experimental models and study participant details
This study does not involve any patients or healthy control participants.
Ethics
All experiments with infectious SCV2 were carried out in Institutional Biosafety Committee-approved BSL3 and ABSL3 facilities at The Johns Hopkins University School of Medicine using recommended positive-pressure air respirators and protective equipment. Experimental procedures involving live animals were carried out in agreement with the protocol (#HA20M310) approved by the Institutional Animal Care and Use Committee (IACUC) at The Johns Hopkins University School of Medicine.
Sources of BCG vaccine strain and SARS-CoV-2
Mycobacterium bovis (M. bovis) Bacillus Calmette-Guérin (BCG)-Tice (Onco-Tice©) was purchased from Merck (TICE® BCG). SARS-CoV-2/Wuhan-1/2020 virus (U.S. Center for Disease Control and Prevention) was provided by Dr. Andrew Pekosz. WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) was obtained from BEI resources (NR-52281).
Sources of vero cells
Vero C1008 [Vero 76, clone E6, Vero E6] (ATCC CRL-1586) cells were used for viral growth and for viral growth and determination of virus stock titers. VeroE6-TMPRSS2 cells were used for the determination of virus neutralization assays and were obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB1819).
Sources of antibodies
The antibodies used in this study were purchased from different vendors as follows:
Flow cytometry antibodies: Anti-CD3 (MCA1477PB, CD3-12): Bio Rad; Anti-CD4 (100451, GK1.5): BioLegend; Anti-CD11b (NB110-89474PECY7, Poly): Novus; Anti-RT1D (110211, 14-4-4S): BioLegend.
Other antibodies used in the study: Anti-CD3 (RM-9107-S1): Thermo Fisher Scientific; Horseradish peroxidase (HRP)-conjugated secondary IgG (ab6892): Abcam; Horseradish peroxidase (HRP)-conjugated secondary IgG (sab3003M): Brookwood Biomedical.
Sources of reagents and kits used in single cell preparation and single cell RNA sequencing
We used Mouse lung dissociation kit (130-095-927) and Mouse spleen dissociation kit (130-095-926) that were purchased from Miltenyi Biotec and are commercially available. Chromium Next GEM Single Cell 3ʹ Reagent Kits v3.1 (Dual Index) (CG000315) used for library preparation was purchased from 10X Genomics and is available commercially.
Experimental models
Culture and propagation of Mycobacterium bovis
We used Mycobacterium bovis (M. bovis) Bacillus Calmette-Guérin (BCG)-Tice (Onco-Tice©, Merck) for immunization experiments. The lyophilized bacterial stock was resuspended in 1ml of 7H9 Middlebrook liquid medium (Cat. B271310, Fisher Scientific) supplemented with (OADC) (Cat. B11886, Fisher Scientific), 0.5% glycerol (Cat. G55116, Sigma) and 0.05% Tween-80 (Cat. BP338, Fisher Scientific). The culture was streaked on 7H11 plate supplemented with oleic-albumin-dextrose-catalase (OADC) and single colonies were picked and propagated in 7H9 Middlebrook liquid medium for preparation of seed-stock. Individual seed-stock vial was randomly picked from frozen stock and was subsequently propagated in 7H9 medium before immunization.
Culture conditions and propagation of vero cells and SARS-CoV-2 strains
All cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Vero C1008 [Vero 76, clone E6, Vero E6] (ATCC CRL-1586) cells were used for viral growth and determination of virus stock titers. Vero E6 cells were grown in MEM with 10% fetal bovine serum (FBS), L-glutamine, and penicillin-streptomycin at 37°C with 5% CO2. VeroE6-TMPRSS2 cells were used for the determination of virus neutralization assays. These cells were grown in complete media (CM) consisting of DMEM containing 10% FBS (Gibco, Thermo Fisher Scientific), 1 mM glutamine (Invitrogen, Thermo Fisher Scientific), 1 mM sodium pyruvate (Invitrogen, Thermo Fisher Scientific), 100 U/mL penicillin (Invitrogen, Thermo Fisher Scientific), and 100 μg/mL streptomycin (Invitrogen, Thermo Fisher Scientific). Cells were incubated at 37°C in a humidified incubator with 5% CO2. The viral stocks of SARS-CoV-2/Wuhan-1/2020 virus was stored at -80°C and titers were determined by tissue culture infectious dose 50 (TCID50) assay. WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) was obtained from BEI resources (NR-52281).
Golden Syrian hamster model, BCG vaccination, SARS-CoV-2 challenge, and experimental design
In vivo experiments involving BCG vaccination and SCV2 infection were carried out using male golden Syrian hamsters (Mesocricetus auratus). Male golden Syrian hamsters (age 5 to 6 weeks) were purchased from Envigo (Haslett, MI). Animals were housed individually under standard housing conditions (68 to 76°F, 30 to 70% relative humidity, 12-h light/12-h dark cycle) in cages with proper bedding (Teklad 7099 TEK-Fresh, Envigo, Indianapolis, IN) in the Animal Biosafety Level 3 (ABSL3) facility at Koch Cancer Research Building 2 (CRB2) at School of Medicine at The Johns Hopkins University. Animals were given ad libitum reverse osmosis (RO) water and feed (2018 SX Teklad, Envigo, Madison, WI). After 7 days of acclimatization animals were intravenously vaccinated through the lateral vein of the penis using live 5 x 106 C.F.U. of BCG-Tice in a total volume of 100 ul saline under ketamine (60 to 80 mg/kg) and xylazine (4 to 5 mg/kg) anesthesia administered intraperitoneally. This dose of BCG was selected in light of the known toxicity of BCG to hamsters at doses higher than 1x107 CFU IV76 and also in light of the commonly used dose of 1x106 CFU of BCG for mice.33,36 Control animals received an equivalent volume of saline. Animals were challenged using 5 x 105 TCID50 of SCV2/Wuhan-1/2020 virus in 100 μl of DMEM (50 μl/naris) through the intranasal route under ketamine (60 to 80 mg/kg) and xylazine (4 to 5 mg/kg) anesthesia administered intraperitoneally. Control animals received an equivalent amount of DMEM. Animals were randomly assigned to be euthanized by isoflurane overdose at end points (day 4 and day 7) following SCV2 infection.
Experimental design: Groups of hamsters were intravenously (I.V.) administered saline or were vaccinated (I.V.) with 5x106 CFU of BCG-Tice. 30 days after vaccination animals were challenged with 5x105 TCID50 units of SARS-CoV-2 (SCV2) (Wuhan-1/2020) by the intranasal route. At D4 and D7 post-challenge animals were sacrificed for analysis. 3 animals (BCG only) were not infected with SCV2 and were sacrificed 30 days post-vaccination to serve as controls (Figure 1A).
Method details
Flow cytometry analysis
For cellular immune profiling cell surface staining was performed on single cells from hamster lung and spleen tissues at the experimental end point. Briefly, tissues were harvested and stored in sterile PBS in individual tubes before single cell preparations. Lung was extensively perfused using sterile PBS. We used mouse lung (Miltenyi Biotec; 130-095-927) and spleen dissociation kits (Miltenyi Biotec; 130-095-926) for preparation of single cells as per manufacturer’s instruction using a gentleMACS™ Octo Dissociator with heaters (Miltenyi Biotec; 130-096-427). Cells were passed through a 70 μm filter and washed twice using ice-cold PBS followed by RBC lysis using ACK lysis buffer (Thermo Fisher Scientific: A1049201) at room temperature for 5 minutes. The cell viability was determined using Trypan blue dye staining and determine total live cells per lobe. For surface staining a total of 5 million cells per animal lung were used in this study. Briefly, cells were washed again using ice-cold PBS and stained using Zombie Aqua™ Fixable Viability Kit (Biolegend; 423101) for 20 min at room temperature. Cells were subsequently washed and resuspended in FACS buffer (1% BSA, 2mM EDTA in PBS) and incubated for 30 minutes at 4 °C in block buffer consisting of PBS and 2% FBS, 2% normal rat serum (Sigma Aldrich) and 2% normal mouse serum (Sigma Aldrich) prior to surface staining. Cells were again washed and stained with conjugated primary antibodies as per manufacturer’s protocol. Following antibodies were used for cell surface staining: anti-CD3 (Bio-Rad, #MCA1477PB, CD3-12), anti-CD4 (BioLegend, #100451, GK1.5), anti-CD11b (Novus, #NB110-89474PECY7, Poly), and anti-RT1D (BioLegend, #110211, 14-4-4S). Cells were subsequently fixed using IC fixation buffer (eBiosciences™; 00-8222-49) for 60 minutes at 4°C. Cells were washed three times using FACS buffer and acquired using BD LSRII with FACSDiva Software. Data analyses was carried out using FlowJo (v10) (TreesStar).
Single cell RNA sequencing
Sample and preparation for scRNAseq
For single-cell RNA seq (scRNA-seq) of lung tissue derived single cells, whole right lung superior lobe was isolated from each animal. For single cell preparation, a mouse lung dissociation kit (Miltenyi Biotec) was used. Following single cell preparation, cell suspensions were applied to a MACS Smart Strainer (70 μm) and washed twice with 10 ml of DMEM. Cells were pelleted by spinning at 300 x g for a total of 7 minutes at 4°C. Cells were resuspended in 1ml DMEM containing 200 μl DNase (1μg/μl) for 5 minutes at room temperature. Cells were washed by adding 10 ml sterile PBS containing 0.5% BSA and pelleted at 4°C. The RBC lysis was carried out using 1X RBC lysis solution (Miltenyi Biotec; 130-094-183) in a total volume of 1ml for 10 minutes at 4°C. Cells were resuspended in 10 ml chilled DPBS containing 0.5% BSA and pelleted subsequently. Cells were filtered using 35 μm Falcon cell strainer and transferred to a 2 ml lo-binding tube in a total volume of 1ml DPBS containing 0.04% BSA. Cells were subsequently counted using trypan blue dye exclusion assay to determine cell viability and total cell number.
Single-cell RNA sequencing and data pre-processing
Cells and gel beads were partitioned using the 10X Genomics Chromium platform aiming for recovery of 10,000 cells per sample using the Chromium Next GEM Single Cell 3ʹ Reagent Kits v3.1 (Dual Index) (CG000315). After RNA capturing, cDNA synthesis and library construction, the 3’DGE libraries were sequenced on NovaSeq 6000 instrument to achieve a target sequencing depth of ∼ 50,000 reads per cell.
Cell Ranger v4.0.0 was used to demultiplex the FASTQ reads and align them to the hamster transcriptome. The resulting gene expression matrix were then loaded into Seurat (v4.0.4)77,78 in R (v4.0.3). ND1, ND2, ND4, ND5, ND6 were used to determine the percentage of mitochondrial genes. The quality of cells was assessed based on the number of genes detected per cell and the proportion of mitochondrial gene counts. Low-quality cells were filtered out if the number of detected genes were below 500 or above 5,000. Cells were filtered out if the proportion of mitochondrial gene counts was higher than 10%. In addition, genes that were expressed in less than 5 cells were excluded.
Data integration and cell type identification
To compare cell types and proportions across different groups, we applied the integration methods using the SCTransform workflow, which can be used to assemble multiple distinct datasets into an integrated one and eliminate potential batch effect.77 Briefly, top 3000 highly variable features were identified and used to find anchors between each dataset with FindIntegrationAnchors function of Seurat. Then these anchors were used to integrate different datasets together with IntegrateData function to create a batch-corrected data assay for downstream analysis.
Principal component analysis (PCA) was performed on the integrated data for dimensionality reduction and top 30 significant PCs were used for cluster analysis. After using FindNeighbors and FindClusters functions, we performed nonlinear dimensional reduction with the RunUMAP function to obtain a two-dimensional representation of the cells. Cells with similar transcriptome were clustered together. The FindAllMarkers function in Seurat was used to find marker genes of each cluster for cell type identification. Clusters were then annotated based on the expression of canonical cell marker genes described in previous study39 and a single cell sequencing database PanglaoDB (https://panglaodb.se/).79 Basically, Arg1, Ccr2 and Ccr5 for MDSCs , Camp and Mmp9 for granulocyte, Siglecf, Marco and Mrc1 for alveolar macrophage (AM), C1qb for interstitial macrophages (IMs), Cd3d and Cd4 for T cell, Sftpb and Sftpc for alveolar epithelial cell, Nkg7 and Klrd1 for NK cell, Mki67 for proliferating cell, Ms4a1 and Cd79b for B cell, Cfap44 and Foxj1 for ciliated cell, Vcam1 and Vwf for endothelial cell, Irf8 and Tcf4 for dendritic cells (DCs), Jchain and Mzb1 for plasma cell, Dcn and Col1a1 for fibroblast. Clusters expressing two or more canonical marker genes characteristic of different cell types, were classified as doublets and excluded from further analysis. Lymphoid, myeloid, and non-immune cell subpopulations were further sub-setted and extracted, and separately analyzed by dimensionality reduction with PCA and UMAP.
To compare the abundance of each cell types across different samples, cell type proportion was calculated as the number of cells within each cell cluster divided by the total number of cells of that sample.
Differentially expresses genes (DEGs) and pathway analysis
The FindMarker function in Seurat package was performed to identify differentially expressed genes between BCG-WT + SCV2 and SCV2 groups in a particular cell cluster. Benjamini–Hochberg method was used to adjust p-values for multiple tests. Genes expressed in at least 25% of cells in a cluster with adjusted P values < 0.05 were considered as differentially expressed genes. The gene filtering parameters used to generate each heatmap from differential gene expression analysis were described in each figure legend. GO term enrichment analysis of the DEGs was performed using Metascape (www.metascape.org).80
UCell analysis
UCell81 is an R package, based on the Mann-Whitney U statistic, for calculating gene signatures in single-cell datasets . We used UCell score to evaluate the degree to which individual cells expressed a certain gene set. HALLMARK_INTERFERON_ALPHA_RESPONSE from MsigDB, Immunoglobulin production involved in immunoglobulin-mediated immune response (GO:0002381) and 6 well-defined exhaustion associated genes (Lag3, Tigit, Pdcd1, Ctla4, Havcr2 and Tox)82 were used as gene signature input to calculate UCell scores for each individual cell. UCell score violin plots were grouped by different samples and cell types.
Publicly available healthy control data
We additionally processed scRNA-seq data on hamster lung tissues by Nouailles et al.39 Three datasets of lung samples from healthy hamsters (GSM4946629, GSM4946630, GSM4946631) were downloaded from the GEO database under accession code GSE162208. We processed the data using the similar strategy described above to filter low quality cells. Then MapQuery function in Seurat (v4.0.4) was performed to identify cell types using our own data reference. In brief, we transferred cell type labels from our reference data. Second, we integrated reference with query by correcting the low-dimensional embeddings. Finally, we projected the query data onto the UMAP structure of the reference, which allowed us to compare the abundance of the same cell type between datasets.
Histochemistry and immunohistochemistry
Formalin-fixed paraffin embedded lung sections were stained with hematoxylin-eosin (H&E) for analysis. Lung tissue was scored using a panel of specific lung inflammation parameters according to criteria previous published on animal models of acute pneumonia.83 All examinations were performed by a board-certified pulmonary pathologist. Immunohistochemistry was performed on FFPE lung tissue sections for CD3 using an automated Ventana Discovery (Roche, Basel, Switzerland) autostainer. Heat induced antigen retrieval was achieved using ETDA pH9 solution (CC1, Roche). Primary antibody for CD3 (1:200 dilution; Cat# RM-9107-S1, Thermo Fisher Scientific) was incubated for 30m at 4°C and detection was achieved using UltraView DAB detection kit (Roche). Protein expression was scored by a board-certified pulmonary pathologist blinded to cohort status or treatment group. Intra-alveolar inflammation was defined as the presence (score of 1) or absence (score of 0) of intra-alveolar neutrophils with or without fibrin, red blood cells, or infiltration of alveolar spaces. Bronchopneumonia was defined as the presence (score of 1) or absence (score of 0) of intrabronchial neutrophils with or without fibrin, red blood cells, or infiltration of the bronchial lining. CD3 membranous staining was evaluated separately in perivascular or peribronchial inflammation, bronchial epithelium, alveolar wall as well as pneumocytes and in areas of pneumonia (when present) or alveolar spaces (in absence of pneumonia). CD3 staining was scored as 1+ (scattered single cells), 2+ (cells in clusters or sheets with at least 2 layers) or 3+ (several clusters or sheets). Scores were then quantified using a point system (focal 1+= 0.5, 1+=1, focal 2+=1.5, 2+=2, 3+=3) and total points were analyzed between treatment groups. CD3 scoring was performed for perivascular, peribronchial, bronchial epithelium, alveolar wall, pneumocytes, and alveolar spaces. Total CD3 expression was calculated by combining all point scores for all 6 criteria such that the maximal score was 18. Statistical analysis was performed between groups at each timepoint (day 4 post infection and day 7 post infection) using Welch’s t-test.
SCV2 viral quantification
Quantification of SARS-CoV-2 infectious viral load in lung tissues
Following perfusion with phosphate-buffered saline (PBS), lung sections were collected, weighed and snap frozen on dry ice. Ice-cold virus titer buffer (Dulbecco’s Modified Eagle Medium, 2% fetal bovine serum, 2 mM L-glutamine, 200 U/mL penicillin and 200 μg/mL streptomycin, 100 μg/mL gentamicin, 0.5 μg/mL amphotericin B) was added to the frozen lung samples at a 10% weight to volume ratio. The tissue was homogenized with ceramic 1.4 mm beads for 2 cycles of 20 seconds at a speed setting of 5000 rpm using a Precelley’s Evolution homogenizer. Samples were centrifuged at 10,000 xg for 1 minute at 4°C to pellet the beads and tissue debris. In brief, lung homogenates were serially diluted in seven-point half-log dilutions and incubated on Vero C1008 cells in six technical replicates. Each sample was measured in triplicate. Plates were scored for CPE five days post infection using the CellTiter-Glo® Luminescent Cell Viability Assay per the manufacture’s protocol (Promega). TCID50 was calculated using the Reed and Muench method.84
RNA extraction and RT-qPCR
Total RNA from hamster lung homogenates were extracted using TRIzol Reagent (Invitrogen) followed by the RNeasy kit (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized from total RNA using qScript cDNA SuperMix containing random hexamers and oligo-dT primers (Quanta Biosciences) following the manufacturer’s protocol. Real-time PCR was performed in triplicate using TaqMan Fast Advanced Master Mix (Applied Biosystems) on a StepOnePlus Real Time PCR system (Applied Biosystems). SARS-CoV-2 RNA was detected using premixed forward (5′-TTACAAACATTGGCCGCAAA-3′) and reverse (5′-GCGCGACATTCCGAAGAA-3′) primers and probe (5′-FAM-ACAATTTGCCCCCAGCGCTTCAG-BHQ1-3′) designed by the CDC as part of the 2019-nCoV CDC Research Use Only (RUO) kit (Integrated DNA Technologies, Catalog #10006713) to amplify a region of the SARS-CoV-2 (SCV2) nucleocapsid (N) gene. PCR conditions were as follows: 50°C for 2 min, 95°C for 2 min, followed by 45 cycles of 95°C for 3 s and 55°C for 30 s. Serially diluted (10-fold) plasmid containing the complete SARS-CoV-2 N gene (Integrated DNA Technologies, Catalog #10006625) was measured to generate a standard curve for quantification of viral RNA copies. The limit of detection for the assay was 1 x 102 RNA copies. Viral copies were normalized to the human RNase P (RP) gene using premixed forward (5′-AGATTTGGACCTGCGAGCG-3′) and reverse (5′-GAGCGGCTGTCTCCACAAGT-3′) primers and probe (5′-FAM-TTCTGACCTGAAGGCTCTGCGCG-BHQ-1-3′) included in the same 2019-nCoV CDC RUO kit.
Antibody ELISAs
The hamster antibody ELISA protocol was described previously85 with modifications. ELISA plates (96-well plates; Immunol 4HBX, Thermo Fisher Scientific) were coated with spike receptor-binding domain (S-RBD) in 1x phosphate-buffered saline (PBS) and incubated at 4°C overnight. Coated plates were washed three times with wash buffer (1x PBS10.1% Tween 20), blocked with 3% nonfat milk solution in wash buffer, and incubated at room temperature for 1 h. After incubation, blocking buffer was discarded, 2-fold serially diluted plasma (starting at a 1:100 dilution) was added, and plates were incubated at room temperature for 2 h. After washing the plates 3 times, horseradish peroxidase (HRP)-conjugated secondary IgG (1:10,000; Abcam, MA, USA) or IgM (1:250; Brookwood Biomedical, AL, USA) antibodies were added. After addition of secondary IgG antibody, plates were incubated at room temperature for 1 h, while for IgM antibody, plates were incubated at 4°C overnight. Sample and antibody dilutions were done in 1% nonfat milk solution in wash buffer. Following washing, reactions were developed by adding 100ml/well of Sigmafast OPD (o-phenylenediamine dihydrochloride) (MilliporeSigma) solution for 10 min and stopped by adding 3 M hydrochloric acid (HCl) solution; plates were read at a 490-nm wavelength using an ELISA plate reader (BioTek Instruments). The area under the curve for each sample was determined by plotting normalized optical density values against sample dilution using a cutoff value that is three times the absorbance of the first dilution of mock (uninfected) animal samples.
Virus neutralization assays
SARS-CoV-2 neutralization assays were performed essentially as described.86 WA-1 (SARS-CoV-2/USA-WA1/2020 EPI_ISL_404895) was obtained from BEI Resources. Infectious virus titers were determined by 50% tissue culture infectious dose as previously described.87 After making twofold dilutions of plasma (1:20 to 1:2560), infectious virus was added at a final concentration of 1 x 103 TCID50/mL to the serial dilutions and incubated for 1h at room temperature, then transferred to a 96-well plate of VeroE6-TMPRSS2 cells in sextuplet, and incubated until cytopathic effect was observed in the controls and the highest sera dilutions. The cells were fixed, stained, and the nAb titer was calculated as the highest serum dilution that eliminated the cytopathic effect in 50% of the wells (NT50). The area under the curve (AUC) was calculated with Graphpad Prism using a lower limit of detection of 1.7.
CT and PET imaging
Four days post infection, live SCV2-infected male hamsters (n= 5 SCV2 and n= 6 BCG + SCV2) underwent chest CT using the nanoScan positron emission tomography (PET)/CT (Mediso USA, MA, USA) small animal imager. Prior to PET imaging, hamsters were administered ∼10.22 MBq of 18F-FDG (SOFIE, Sterling VA, USA) via the surgically implanted center venous catheter. Given that SCV2 is designated as a BSL-3 pathogen, live SCV2-infected animals were imaged inside transparent and sealed biocontainment cells developed in-house, compliant with BSL-3 containment and capable of delivering air-anesthetic mixture to sustain live animals during imaging.85,88 A 15-min PET acquisition and subsequent CT were performed using the nanoScan PET/CT (Mediso, Arlington, VA). CT images were visualized and analyzed using the VivoQuant 2020 lung segmentation tool (Invicro, MA, USA).85 Briefly, an entire lung volume (LV) was created, and volumes of interests (VOIs) were shaped around the pulmonary lesions using global thresholding for Hounsfield Units (HU) ≥ 0, and disease severity (CT score) was quantified as the percentage of diseased lung in each animal. The investigators were blinded to the group assignments. The data are represented as CT score [(pulmonary lesions volume/whole lung volume) × 100]. The investigators analyzing the CT were blinded to the group assignments. VivoQuant™ 2020 (Invicro, Boston, MA, USA) was used for visualization and quantification. Scatter and attenuation corrections were applied to the PET data and multiple VOIs were manually drawn per animal using the CT as a reference.
Quantification and statistical analysis
Histopathological scoring, CT scoring and scRNASeq analsysis were performed in a blinded fashion. Data were graphed and analyzed using Graphpad Prism 9.0. Statistical analyses were performed using either a two-sided Fisher exact test, or Welch's t-test, or two-tailed Student's t-test or one-way ANOVA. Data are presented as mean values ± S.E.M. Specific statistical tests used, significance, number of animals used and other details are indicated in individual figure legends.
Acknowledgments
The generous support of grant #2167 from Emergent Ventures at the Mercatus Center, George Mason University is gratefully acknowledged. This study was also supported by NIH grants U54CA260492 (providing support to S.Y. and R.W.), T32AI007417-26 (providing support to P.S.C.) and NIH contract N7593021C00045 to the Johns Hopkins Center of Excellence in Influenza Research and Response (to A.P.). We thank the members of the Sidney Kimmel Comprehensive Cancer Center’s Experimental and Computational Genomics Core, supported by NIH Grant P30CA006973, for their support of the next generation sequencing experiments. The authors also thank Ada Tam and Lee Blosser for technical assistance.
Author contributions
A.K.S., R.W., W.R.B., S.Y., and T.J.B co-led the study through conceptualization, design, oversight, and interpretation of results. W.R.B. obtained the funding for the study. A.K.S. designed, conducted, and interpreted the results of the experiments. A.K.S., R.W., K.A.L., M.P., C.K.B., P.U., M.G., G.S., S.D., O.K., P.B.I., A.A.O., M.B., J.H., A.G., K.J.P., P.S.C., M.L., A.P., and S.L.K. conducted experiments and/or provided key expert advice. S.K.J., and T.J.B. assisted in the design of the experiments and provided key expert advice. A.K.S. and W.R.B wrote the manuscript. A.K.S., R.W., K.A.L., M.P., C.K.B., G.S., A.A.O., K.J.P., A.P., S.L.K, S.K.J., T.J.B., S.Y., and W.R.B. revised and edited the manuscript. A.K.S., R.W., and W.R.B. designed and produced figures for this manuscript.
Declaration of interests
The authors have declared that no conflict of interest exists.
Published: August 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107733.
Supplemental information
Data and code availability
-
•
The single cell sequencing data is publicly available at NCBI through the GEO Series accession number GSE229135 provides access to all the data on GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL28997). The publicly available datasets that we used in this study are available from GEO GSE162208 (Nouailles et al., 2021).
-
•
This study does not report any original code.
-
•
Any additional information related to the data on flow cytometry, IHC, neutralization assay, viral quantification, and antibody ELISA can be obtained from the lead contact (wbishai1@jhmi.edu) upon request. Any additional information required to reanalyze the data reported in this study is available from the lead contact (wbishai1@jhmi.edu).
References
- 1.Brinkley-Rubinstein L., Peterson M., Martin R., Chan P., Berk J. Breakthrough SARS-CoV-2 Infections in Prison after Vaccination. N. Engl. J. Med. 2021;385:1051–1052. doi: 10.1056/NEJMc2108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., COVID-19 Genomics UK COG-UK Consortium. Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., Huppert A. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Kent S.J., Triccas J.A., Khoury D.S., Davenport M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet. Microbe. 2022;3:e52–e61. doi: 10.1016/s2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L., et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y., Carpp L.N., Miller H.E.R., Zager M., Newell E.W., Gottardo R. Single-cell immunology of SARS-CoV-2 infection. Nat. Biotechnol. 2022;40:30–41. doi: 10.1038/s41587-021-01131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat. Rev. Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Domínguez-Andrés J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183:315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea M.G., van der Meer J.W., van Crevel R. BCG vaccination in health care providers and the protection against COVID-19. J. Clin. Invest. 2021;131 doi: 10.1172/jci145545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsilika M., Taks E., Dolianitis K., Kotsaki A., Leventogiannis K., Damoulari C., Kostoula M., Paneta M., Adamis G., Papanikolaou I., et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moorlag S.J.C.F.M., Taks E., Ten Doesschate T., van der Vaart T.W., Janssen A.B., Müller L., Ostermann P., Dijkstra H., Lemmers H., Simonetti E., et al. Efficacy of BCG Vaccination Against Respiratory Tract Infections in Older Adults During the Coronavirus Disease 2019 Pandemic. Clin. Infect. Dis. 2022;75:e938–e946. doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czajka H., Zapolnik P., Krzych Ł., Kmiecik W., Stopyra L., Nowakowska A., Jackowska T., Darmochwał-Kolarz D., Szymański H., Radziewicz-Winnicki I., Mazur A. A Multi-Center, Randomised, Double-Blind, Placebo-Controlled Phase III Clinical Trial Evaluating the Impact of BCG Re-vaccination on the Incidence and Severity of SARS-CoV-2 Infections Among Symptomatic Healthcare Professionals during the COVID-19 Pandemic in Poland-First Results. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Anjos L.R.B., da Costa A.C., Cardoso A.d.R.O., Guimarães R.A., Rodrigues R.L., Ribeiro K.M., Borges K.C.M., Carvalho A.C.d.O., Dias C.I.S., Rezende A.d.O., et al. Efficacy and Safety of BCG Revaccination With M. bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.841868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ten Doesschate T., van der Vaart T.W., Debisarun P.A., Taks E., Moorlag S.J.C.F.M., Paternotte N., Boersma W.G., Kuiper V.P., Roukens A.H.E., Rijnders B.J.A., et al. Bacillus Calmette-Guérin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 2022;28:1278–1285. doi: 10.1016/j.cmi.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton C.M., van Wijk R.C., Mockeliunas L., Simonsson U.S.H., McHarry K., van den Hoogen G., Muller C., von Delft A., van der Westhuizen H.M., van Crevel R., et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48 doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J., Tian M., Fan X., Yu Q., Jing Y., Wang W., Li L., Zhou Z. Mycobacterium tuberculosis multistage antigens confer comprehensive protection against pre- and post-exposure infections by driving Th1-type T cell immunity. Oncotarget. 2016;7:63804–63815. doi: 10.18632/oncotarget.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman M.J., Fernández C. Neonatal vaccination with Mycobacterium bovis BCG: potential effects as a priming agent shown in a heterologous prime-boost immunization protocol. Vaccine. 2009;27:4038–4046. doi: 10.1016/j.vaccine.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y., Cai M., Ma J., Teng X., Tian M., Bassuoney E.B.M.B., Fan X. Heterologous Boost Following Mycobacterium bovis BCG Reduces the Late Persistent, Rather Than the Early Stage of Intranasal Tuberculosis Challenge Infection. Front. Immunol. 2018;9:2439. doi: 10.3389/fimmu.2018.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Singh A.K., Netea M.G., Bishai W.R. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J. Clin. Invest. 2021;131 doi: 10.1172/jci148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A.B., Jacobs C., van Loenhout J., Xavier R.J., Aaby P., van der Meer J.W.M., et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua W.J., Truscott S.M., Eickhoff C.S., Blazevic A., Hoft D.F., Hansen T.H. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect. Immun. 2012;80:3256–3267. doi: 10.1128/iai.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steigler P., Daniels N.J., McCulloch T.R., Ryder B.M., Sandford S.K., Kirman J.R. BCG vaccination drives accumulation and effector function of innate lymphoid cells in murine lungs. Immunol. Cell Biol. 2018;96:379–389. doi: 10.1111/imcb.12007. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann E., Sanz J., Dunn J.L., Khan N., Mendonça L.E., Pacis A., Tzelepis F., Pernet E., Dumaine A., Grenier J.C., et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against. Tuberculosis. Cell. 2018;172:176–190.e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R., Rimmelzwaan G.F., Pickkers P., Netea M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 30.Salem A., Nofal A., Hosny D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr. Dermatol. 2013;30:60–63. doi: 10.1111/j.1525-1470.2012.01848.x. [DOI] [PubMed] [Google Scholar]
- 31.Lodmell D.L., Ewalt L.C. Enhanced resistance against encephalomyocarditis virus infection in mice, induced by a nonviable Mycobacterium tuberculosis oil-droplet vaccine. Infect. Immun. 1978;19:225–230. doi: 10.1128/iai.19.1.225-230.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulkarni S., Mukherjee S., Pandey A., Dahake R., Padmanabhan U., Chowdhary A.S. Bacillus Calmette-Guérin Confers Neuroprotection in a Murine Model of Japanese Encephalitis. Neuroimmunomodulation. 2016;23:278–286. doi: 10.1159/000452171. [DOI] [PubMed] [Google Scholar]
- 33.Hilligan K.L., Namasivayam S., Clancy C.S., O'Mard D., Oland S.D., Robertson S.J., Baker P.J., Castro E., Garza N.L., Lafont B.A.P., et al. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022;219 doi: 10.1084/jem.20211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B.Z., Shuai H., Gong H.R., Hu J.C., Yan B., Yuen T.T.T., Hu Y.F., Yoon C., Wang X.L., Hou Y., et al. Bacillus Calmette-Guérin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight. 2022;7 doi: 10.1172/jci.insight.157393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Counoupas C., Johansen M.D., Stella A.O., Nguyen D.H., Ferguson A.L., Aggarwal A., Bhattacharyya N.D., Grey A., Hutchings O., Patel K., et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines. 2021;6:143. doi: 10.1038/s41541-021-00406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann E., Khan N., Tran K.A., Ulndreaj A., Pernet E., Fontes G., Lupien A., Desmeules P., McIntosh F., Abow A., et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White A.D., Sibley L., Sarfas C., Morrison A.L., Bewley K., Churchward C., Fotheringham S., Gkolfinos K., Gooch K., Handley A., et al. Influence of Aerosol Delivered BCG Vaccination on Immunological and Disease Parameters Following SARS-CoV-2 Challenge in Rhesus Macaques. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.801799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sia S.F., Yan L.M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., Poon L.L.M., Nicholls J.M., et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nouailles G., Wyler E., Pennitz P., Postmus D., Vladimirova D., Kazmierski J., Pott F., Dietert K., Muelleder M., Farztdinov V., et al. Temporal omics analysis in Syrian hamsters unravel cellular effector responses to moderate COVID-19. Nat. Commun. 2021;12:4869. doi: 10.1038/s41467-021-25030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson E.A., Cascino K., Ordonez A.A., Zhou W., Vaghasia A., Hamacher-Brady A., Brady N.R., Sun I.H., Wang R., Rosenberg A.Z., et al. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majdoul S., Compton A.A. Lessons in self-defence: inhibition of virus entry by intrinsic immunity. Nat. Rev. Immunol. 2022;22:339–352. doi: 10.1038/s41577-021-00626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farr L., Ghosh S., Moonah S. Role of MIF Cytokine/CD74 Receptor Pathway in Protecting Against Injury and Promoting Repair. Front. Immunol. 2020;11:1273. doi: 10.3389/fimmu.2020.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartmann C., Miggiolaro A.F.R.D.S., Motta J.D.S., Baena Carstens L., Busatta Vaz De Paula C., Fagundes Grobe S., Hermann de Souza Nunes L., Lenci Marques G., Libby P., Zytynski Moura L., et al. The Pathogenesis of COVID-19 Myocardial Injury: An Immunohistochemical Study of Postmortem Biopsies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.748417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenarruzabeitia O., Astarloa-Pando G., Terrén I., Orrantia A., Pérez-Garay R., Seijas-Betolaza I., Nieto-Arana J., Imaz-Ayo N., Pérez-Fernández S., Arana-Arri E., Borrego F. T Cell Activation, Highly Armed Cytotoxic Cells and a Shift in Monocytes CD300 Receptors Expression Is Characteristic of Patients With Severe COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.655934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin J., Law R., Korosec C.S., Zhou C., Koh W.H., Ghaemi M.S., Samaan P., Ooi H.K., Matveev V., Yue F., et al. Longitudinal Assessment of SARS-CoV-2-Specific T Cell Cytokine-Producing Responses for 1 Year Reveals Persistence of Multicytokine Proliferative Responses, with Greater Immunity Associated with Disease Severity. J. Virol. 2022;96 doi: 10.1128/jvi.00509-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomić S., Đokić J., Stevanović D., Ilić N., Gruden-Movsesijan A., Dinić M., Radojević D., Bekić M., Mitrović N., Tomašević R., et al. Reduced Expression of Autophagy Markers and Expansion of Myeloid-Derived Suppressor Cells Correlate With Poor T Cell Response in Severe COVID-19 Patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.614599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou H., Zhang Y., Tang G., Luo Y., Liu W., Cheng C., Jiang Y., Xiong Z., Wu S., Sun Z., et al. Immunologic memory to SARS-CoV-2 in convalescent COVID-19 patients at 1 year postinfection. J. Allergy Clin. Immunol. 2021;148:1481–1492.e2. doi: 10.1016/j.jaci.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prelli Bozzo C., Nchioua R., Volcic M., Koepke L., Krüger J., Schütz D., Heller S., Stürzel C.M., Kmiec D., Conzelmann C., et al. IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro. Nat. Commun. 2021;12:4584. doi: 10.1038/s41467-021-24817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y., Zhang G., Vong C.T., Ye R.D. Serum amyloid A3 confers protection against acute lung injury in Pseudomonas aeruginosa-infected mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318:L314–L322. doi: 10.1152/ajplung.00309.2019. [DOI] [PubMed] [Google Scholar]
- 52.Allard B., Panariti A., Martin J.G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018;9:1777. doi: 10.3389/fimmu.2018.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koeken V.A.C.M., van der Pasch E.S., Leijte G.P., Mourits V.P., de Bree L.C.J., Moorlag S.J.C.F.M., Budnick I., Idh N., Lerm M., Kox M., et al. The effect of BCG vaccination on alveolar macrophages obtained from induced sputum from healthy volunteers. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dewhurst J.A., Lea S., Hardaker E., Dungwa J.V., Ravi A.K., Singh D. Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci. Rep. 2017;7:7143. doi: 10.1038/s41598-017-07101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Upadhyay A.A., Hoang T.N., Pino M., Boddapati A.K., Viox E.G., Lee M.Y.H., Corry J., Strongin Z., Cowan D.A., Beagle E.N., et al. TREM2+ and interstitial macrophages orchestrate airway inflammation in SARS-CoV-2 infection in rhesus macaques. bioRxiv. 2021 doi: 10.1101/2021.10.05.463212. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez-Gómez A., Vitallé J., Gasca-Capote C., Gutierrez-Valencia A., Trujillo-Rodriguez M., Serna-Gallego A., Muñoz-Muela E., Jiménez-Leon M.d.L.R., Rafii-El-Idrissi Benhnia M., Rivas-Jeremias I., et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell. Mol. Immunol. 2021;18:2128–2139. doi: 10.1038/s41423-021-00728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T., Sigal N., Feng Y., Bristow L., Tak-Yin Tsang O., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reizine F., Lesouhaitier M., Gregoire M., Pinceaux K., Gacouin A., Maamar A., Painvin B., Camus C., Le Tulzo Y., Tattevin P., et al. SARS-CoV-2-Induced ARDS Associates with MDSC Expansion, Lymphocyte Dysfunction, and Arginine Shortage. J. Clin. Immunol. 2021;41:515–525. doi: 10.1007/s10875-020-00920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agrati C., Sacchi A., Bordoni V., Cimini E., Notari S., Grassi G., Casetti R., Tartaglia E., Lalle E., D'Abramo A., et al. Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19) Cell Death Differ. 2020;27:3196–3207. doi: 10.1038/s41418-020-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacchi A., Grassi G., Bordoni V., Lorenzini P., Cimini E., Casetti R., Tartaglia E., Marchioni L., Petrosillo N., Palmieri F., et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020;11:921. doi: 10.1038/s41419-020-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takano T., Matsumura T., Adachi Y., Terahara K., Moriyama S., Onodera T., Nishiyama A., Kawana-Tachikawa A., Miki S., Hosoya-Nakayama K., et al. Myeloid cell dynamics correlating with clinical outcomes of severe COVID-19 in Japan. Int. Immunol. 2021;33:241–247. doi: 10.1093/intimm/dxab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arts R.J.W., Carvalho A., La Rocca C., Palma C., Rodrigues F., Silvestre R., Kleinnijenhuis J., Lachmandas E., Gonçalves L.G., Belinha A., et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou B., Jiang W., Han H., Li J., Mao W., Tang Z., Yang Q., Qian G., Qian J., Zeng W., et al. Acyloxyacyl hydrolase promotes the resolution of lipopolysaccharide-induced acute lung injury. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olajuyin A.M., Zhang X., Ji H.L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Dis. 2019;5:63. doi: 10.1038/s41420-019-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shenoy A.T., Lyon De Ana C., Arafa E.I., Salwig I., Barker K.A., Korkmaz F.T., Ramanujan A., Etesami N.S., Soucy A.M., Martin I.M.C., et al. Antigen presentation by lung epithelial cells directs CD4(+) T(RM) cell function and regulates barrier immunity. Nat. Commun. 2021;12:5834. doi: 10.1038/s41467-021-26045-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaudhary N., Jayaraman A., Reinhardt C., Campbell J.D., Bosmann M. A single-cell lung atlas of complement genes identifies the mesothelium and epithelium as prominent sources of extrahepatic complement proteins. Mucosal Immunol. 2022;15:927–939. doi: 10.1038/s41385-022-00534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urbán S., Paragi G., Burián K., McLean G.R., Virok D.P. Identification of similar epitopes between severe acute respiratory syndrome coronavirus-2 and Bacillus Calmette-Guérin: potential for cross-reactive adaptive immunity. Clin. Transl. Immunology. 2020;9:e1227. doi: 10.1002/cti2.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eggenhuizen P.J., Ng B.H., Chang J., Fell A.L., Cheong R.M.Y., Wong W.Y., Gan P.Y., Holdsworth S.R., Ooi J.D. BCG Vaccine Derived Peptides Induce SARS-CoV-2 T Cell Cross-Reactivity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.692729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rakshit S., Adiga V., Ahmed A., Parthiban C., Chetan Kumar N., Dwarkanath P., Shivalingaiah S., Rao S., D'Souza G., Dias M., et al. Evidence for the heterologous benefits of prior BCG vaccination on COVISHIELD™ vaccine-induced immune responses in SARS-CoV-2 seronegative young Indian adults. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.985938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berry M.P.R., Graham C.M., McNab F.W., Xu Z., Bloch S.A.A., Oni T., Wilkinson K.A., Banchereau R., Skinner J., Wilkinson R.J., et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kotov D.I., Lee O.V., Langner C., Guillen J.V., Peters J.M., Moon A., Burd E.M., Witt K.C., Stetson D.B., Jaye D.L., et al. Cellular Sources and Targets of Type I Interferons that Drive Susceptibility to Tuberculosis. bioRxiv. 2022 doi: 10.1101/2022.10.06.511233. Preprint at. [DOI] [Google Scholar]
- 73.Darrah P.A., Zeppa J.J., Maiello P., Hackney J.A., Wadsworth M.H., 2nd, Hughes T.K., Pokkali S., Swanson P.A., 2nd, Grant N.L., Rodgers M.A., et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren X., Wen W., Fan X., Hou W., Su B., Cai P., Li J., Liu Y., Tang F., Zhang F., et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895–1913.e19. doi: 10.1016/j.cell.2021.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu G., Qi F., Li H., Yang Q., Wang H., Wang X., Liu X., Zhao J., Liao X., Liu Y., et al. The differential immune responses to COVID-19 in peripheral and lung revealed by single-cell RNA sequencing. Cell Discov. 2020;6:73. doi: 10.1038/s41421-020-00225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zwilling B.S., Davis G.W. Effect of systemic BCG infection in syrian golden hamsters. Infect. Immun. 1976;14:271–276. doi: 10.1128/iai.14.1.271-276.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franzén O., Gan L.M., Björkegren J.L.M. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database. 2019 doi: 10.1093/database/baz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andreatta M., Carmona S.J. UCell: Robust and scalable single-cell gene signature scoring. Comput. Struct. Biotechnol. J. 2021;19:3796–3798. doi: 10.1016/j.csbj.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J.Y., Wang X.M., Xing X., Xu Z., Zhang C., Song J.W., Fan X., Xia P., Fu J.L., Wang S.Y., et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020;21:1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 83.Dietert K., Gutbier B., Wienhold S.M., Reppe K., Jiang X., Yao L., Chaput C., Naujoks J., Brack M., Kupke A., et al. Spectrum of pathogen- and model-specific histopathologies in mouse models of acute pneumonia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindenbach B.D. Measuring HCV infectivity produced in cell culture and in vitro. Methods Mol. Biol. 2009;510:329–336. doi: 10.1007/978-1-59745-394-3_24. [DOI] [PubMed] [Google Scholar]
- 85.Dhakal S., Ruiz-Bedoya C.A., Zhou R., Creisher P.S., Villano J.S., Littlefield K., Ruelas Castillo J., Marinho P., Jedlicka A.E., Ordonez A.A., et al. Sex Differences in Lung Imaging and SARS-CoV-2 Antibody Responses in a COVID-19 Golden Syrian Hamster Model. mBio. 2021;12 doi: 10.1128/mBio.00974-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M., Beck E.J., Laeyendecker O., Eby Y., Tobian A.A.R., Caturegli P., Wouters C., Chiklis G.R., Block W., McKie R.O., et al. Convalescent plasma with a high level of virus-specific antibody effectively neutralizes SARS-CoV-2 variants of concern. Blood Adv. 2022;6:3678–3683. doi: 10.1182/bloodadvances.2022007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klein S.L., Pekosz A., Park H.S., Ursin R.L., Shapiro J.R., Benner S.E., Littlefield K., Kumar S., Naik H.M., Betenbaugh M.J., et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Invest. 2020;130:6141–6150. doi: 10.1172/jci142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruiz-Bedoya C.A., Mota F., Ordonez A.A., Foss C.A., Singh A.K., Praharaj M., Mahmud F.J., Ghayoor A., Flavahan K., De Jesus P., et al. (124)I-Iodo-DPA-713 Positron Emission Tomography in a Hamster Model of SARS-CoV-2 Infection. Mol. Imag. Biol. 2022;24:135–143. doi: 10.1007/s11307-021-01638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The single cell sequencing data is publicly available at NCBI through the GEO Series accession number GSE229135 provides access to all the data on GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL28997). The publicly available datasets that we used in this study are available from GEO GSE162208 (Nouailles et al., 2021).
-
•
This study does not report any original code.
-
•
Any additional information related to the data on flow cytometry, IHC, neutralization assay, viral quantification, and antibody ELISA can be obtained from the lead contact (wbishai1@jhmi.edu) upon request. Any additional information required to reanalyze the data reported in this study is available from the lead contact (wbishai1@jhmi.edu).