Abstract
Background
Imipenem/funobactam (formerly XNW4107) is a novel β-lactam/β-lactamase inhibitor with activity against MDR Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacterales strains. Using a neutropenic murine thigh infection model, we aimed to determine the pharmacokinetic/pharmacodynamic (PK/PD) index, relative to funobactam exposure, that correlated most closely with the in vivo efficacy of imipenem/funobactam combination and the magnitude of index required for efficacy against serine carbapenemase-producing clinical strains.
Methods
Dose-fractionation was conducted against three strains. Imipenem human-simulated regimen (HSR, 500 mg q6h 1 h infusion) efficacy in combination with escalating funobactam exposures against seven A. baumannii, four P. aeruginosa and four Klebsiella pneumoniae (imipenem/funobactam MICs 0.25–16 mg/L) was assessed as 24 h change in log10cfu/thigh.
Results
Increased funobactam fractionation enhanced efficacy, indicating time-dependent killing. Changes in log10cfu/thigh versus %fT > MIC were poorly predictive of efficacy; bactericidal activity was observed at %fT > MIC = 0%. Across different threshold plasma funobactam concentrations (CTs), %fT > CT(1 mg/L) had the highest correlation with efficacy. Normalizing the %fT > CT = 1 mg/L index to the respective isolate imipenem/funobactam MIC ([%fT > CT]/MIC) allowed integration of the isolate’s susceptibility, which further enhanced the correlation. Median (%fT > CT[1 mg/L])/MIC values associated with 1-log reductions were 9.82 and 9.90 for A. baumannii and P. aeruginosa, respectively. Median (%fT > CT[1 mg/L])/MIC associated with stasis was 55.73 for K. pneumoniae. Imipenem/funobactam 500/250 mg q6h 1 h infusion HSR produced >1-log kill against 6/7 A. baumannii, 4/4 P. aeruginosa and stasis against 4/4 K. pneumoniae.
Conclusions
Imipenem/funobactam showed potent in vivo efficacy against serine carbapenemase-producers. The novel PK/PD index (%fT > CT)/MIC appeared to best describe in vivo activity.
Introduction
Infections due to carbapenem-resistant Gram-negative bacteria are an urgent public health threat.1–3 Specifically, in 2017 the WHO published a list of bacteria for which new antibiotics are urgently needed and named carbapenem-resistant Enterobacterales (CRE), Pseudomonas aeruginosa and Acinetobacter baumannii (CRAB) among the most critical group.4 Although multiple novel agents have been recently introduced with activity against CRE and carbapenem-resistant P. aeruginosa,5 sparse therapeutic options exist for the treatment of CRAB infections.6 Additionally, current IDSA guidance recommends combination therapy for the treatment of moderate to severe CRAB infections.7 Among the limited armamentarium against CRAB, polymyxin and tetracycline derivatives have undesirable adverse effects and are often associated with poor clinical outcomes.8,9
Imipenem/funobactam (formerly XNW4107) is a novel β-lactam/β-lactamase inhibitor (BL/BLI) with in vitro activity against serine carbapenemase-producing A. baumannii, P. aeruginosa and Enterobacterales. Funobactam is a diazabicyclooctane BLI that confers protection against hydrolysis by Ambler Class A, C and D β-lactamases, including OXA-23 and OXA-24 found in A. baumannii.10 The investigational compound is advancing into Phase III clinical trials for complicated urinary tract infections and hospital acquired/ventilator-associated bacterial pneumonia (NCT05204368 and NCT05204563, respectively) as imipemen/cilastatin/funobactam 500/500/250 mg q6h. The objectives of this study were: (i) to determine and assess the magnitude of the pharmacokinetic/pharmacodynamic (PK/PD) index best predictive of efficacy when funobactam is co-administered with human-simulated plasma exposure of imipenem, and (ii) to assess the efficacy of a human-simulated regimen (HSR) of imipenem/funobactam 500/250 mg q6h against serine carbapenemase-producing A. baumannii, P. aeruginosa and Klebsiella pneumoniae in the neutropenic murine thigh infection model.
Methods
Antimicrobial test agents
For in vivo studies, commercially available imipenem-cilastatin vials (250/250 mg, Fresenius Kabi, Lake Zurich, IL, USA; lots 0001D95 and 0001E15) were utilized. Imipenem USP Reference-Standard was used for in vitro testing (Rockville, MD, USA; lot R038R0). Funobactam vials provided by WuXi Biologics Conjugation Co. Ltd (50 mg; Wuxi, Jiangsu, China; lots 20200907 and 20200504) were used for in vitro and in vivo studies. Placebo vials (lot 2216FFF1911010) provided by WuXi Biologics Conjugation Co. Ltd were diluted with 0.9% NaCl for dosing sham controls. Imipenem and funobactam were dissolved in 0.01 M phosphate buffer pH 7.2% and 0.9% NaCl, respectively, for in vitro studies.11,12 For in vivo testing, imipenem and funobactam were reconstituted and diluted with 0.9% NaCl to attain final concentrations that would deliver mean weight-based dosages.
Bacterial isolates and susceptibility testing
A total of 15 isolates (4 P. aeruginosa, 7 A. baumannii and 4 K. pneumoniae) were utilized (Table 1). Isolate selection for efficacy studies was based on phenotypic profiles, β-lactamases encoded and ability to establish infection in the model. Isolates were frozen in skim milk and stored at −80°C. Prior to experimentation, isolates were subcultured twice onto Trypticase Soy Agar with 5% sheep blood (Becton, Dickinson & Co., Sparks, MD, USA) and incubated for 18–24 h. Imipenem and imipenem/funobactam MICs (funobactam fixed at 8 mg/L) were determined by broth microdilution. P. aeruginosa ATCC 27853 and K. pneumoniae ATCC BAA 1705 were used as the quality controls for both imipenem11,12 and imipenem/funobactam.
Table 1.
MICs of imipenem and imipenem/funobactam combination at a fixed funobactam concentration of 8 mg/L against a selection of Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae isolates
| Modal MIC (mg/L) | |||||
|---|---|---|---|---|---|
| CAIRD isolate ID | Source | Alternate isolate ID | IPM | IPM/funobactam | β-Lactamase(s) encodeda |
| ACB 160 | CDC | 0277 | >64b | 16 | OXA-24, OXA-65, TEM-1B |
| ACB 179 | CDC | 0296 | 64b | 1 | ADC-25, OXA-23, OXA-223 |
| ACB 193 | CDC | 0310 | >64b | 8 | OXA-23, OXA-82 |
| ACB 194 | CDC | 0311 | 64b | 4 | ADC-25, OXA-23, OXA-82 |
| ACB 209 | CDC | 0101 | >64b | 8 | OXA-65, OXA-24 |
| ACB 246 | JMI | 990089 | 64 | 8 | ADC-33, OXA-23, OXA-82 |
| ACB 258 | JMI | 1043774 | 64 | 4 | ADC-222, OXA-23, OXA-95 |
| PSA 1844 | CDC | 0356 | >64b | 1 | KPC-2, PDC-42 |
| PSA 1862 | CDC | 0763 | 64b | 4 | GES-19, GES-20 |
| PSA 1866 | CDC | 0767 | 64b | 8 | GES-20 |
| PSA 1869 | CDC | 0770 | 16b | 2 | GES-19, GES-26 |
| KP 648 | CDC | 0113 | >64b | 1 | KPC-3 |
| KP 651 | CDC | 0120 | 16b | 0.5 | KPC-2 |
| KP 741 | CAIRD | N/A | >32 | 0.5 | SHV-11, CTX-M-55, OXA-48 |
| KP 827 | CDC | 0848 | >64b | 0.25 | OXA-48, CTX-M-15, OXA-1, SHV-11, TEM-1B |
ACB, A. baumannii; CAIRD, Center for Anti-Infective Research and Development; IPM, imipenem; KP, K. pneumoniae; N/A, not applicable; PSA, P. aeruginosa.
β-lactamase genes reported as posted on the FDA-CDC Antimicrobial Resistance Isolate Bank website at the time of study execution.
MIC reported as posted on the FDA-CDC Antimicrobial Resistance Isolate Bank website at the time of study execution.
Animals
Specific-pathogen-free female ICR mice weighing 20 to 22 g were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) and allowed to acclimate for 48 h before study procedures. Mice were housed in groups of six at controlled room temperature in high-efficiency particulate air-filtered cages (Innovive, San Diego, CA, USA) with paper nesting material for enrichment. Food and water were provided ad libitum and a 12 h light/12 h dark cycle was maintained. Animals were utilized according to recommendations from the National Research Council of the National Academy of Sciences. The protocol was approved by the Institutional Animal Care and Use Committee of Hartford Hospital (Assurance #A3185-01).
Neutropenic murine thigh infection model
Mice were rendered neutropenic by injecting cyclophosphamide via intraperitoneal (IP) injections at doses of 150 mg/kg and 100 mg/kg 4 days and 1 day prior to inoculation, respectively. In order to reduce the renal clearance of the study drugs and generate human-simulated exposures, uranyl nitrate at 5 mg/kg was given by IP injection 3 days before inoculation. One thigh per mouse was intramuscularly inoculated with 0.1 mL of ∼107 cfu/mL bacterial suspension in normal saline (NS) 2 h prior to the initiation of antimicrobial or placebo therapy.
PK studies
Funobactam single-dose studies
Single-dose funobactam PK studies were carried out to determine murine PK parameters and assess if the co-administration of funobactam alters the disposition of imipenem. Escalating doses of funobactam (1, 10 and 20 mg/kg) were given subcutaneously with a previously established imipenem HSR13 to 108 infected mice (36 mice per funobactam dose). Groups of six were sacrificed at predefined timepoints. Blood was obtained via cardiac puncture and plasma was matched with an equal volume of stabilizing buffer (1:1 50% ethylene glycol/1 M pH 6.0 MES buffer) and stored at −80°C. Total imipenem and funobactam plasma concentrations were measured using a validated LC/MS-MS method by Keystone Bioanalytical, Inc. (North Wales, PA, USA). PK modeling and simulations were performed using Phoenix WinNonlin (Version 8.1, Pharsight Corp., Mountain View, CA, USA).
Ex vivo funobactam plasma protein-binding studies
Escalating doses of funobactam (1, 10 and 20 mg/kg) were administered subcutaneously in combination with the imipenem HSR to determine the funobactam plasma protein-binding using methods previously described.13 These doses covered the approximate exposure range seen in the dose-ranging and dose-fractionation studies. Triplicate plasma and ultrafiltrate samples were collected, treated with stabilizing buffer and stored at −80°C. Funobactam concentrations were determined by Keystone Bioanalytical, Inc.
Human-simulated exposure PK studies
PK studies were conducted to establish HSRs that approximated the target human exposures achieved after administration of imipenem/cilastatin 500/500 mg (imipenem HSR monotherapy) and imipenem/cilastatin/funobactam 500/500/250 mg (imipenem/funobactam HSR) q6h as 1 h infusions. To simulate the imipenem human profile in critical illness,14 previously reported murine PK parameters and percentage plasma protein-binding were utilized.13 The murine PK parameters generated during funobactam single doses, the estimated protein-binding percentage in mouse plasma, and the previously measured protein-binding percentage in humans (16.63%, unpublished data on file) were utilized to simulate a dosing regimen that approximated the target exposure in healthy volunteers.15
Following mathematical selection, confirmatory PK studies were undertaken following the same methodology as outlined above to establish that the murine HSRs approximated imipenem and funobactam target human exposures and confirm that the co-administration of funobactam did not alter imipenem exposures.
In vivo efficacy studies
All groups contained six mice. Controls were sacrificed just prior to antibiotic initiation (0 h controls) and 24 h later (24 h controls receiving placebo). Imipenem HSR monotherapy served as a negative treatment control. Treatments were continued for 24 h, then animals were euthanized by CO2 asphyxiation followed by cervical dislocation. Thighs were removed and individually homogenized in NS. Serial dilutions were plated on Trypticase soy agar with 5% sheep blood for cfu enumeration. Changes in log10cfu/thigh at 24 h, relative to 0 h controls, were evaluated.
Funobactam dose-fractionation
Funobactam total daily doses of 3.6 and 7.2 mg/kg in combination with the imipenem HSR were administered as once daily and fractionated as q12h and q6h against three isolates. For a single isolate (ACB 258), the total daily dose of 7.2 mg/kg was further fractionated (q4h) to maximize the %fT > threshold funobactam plasma concentration parameter (%fT > CT). Comparisons were made between the different regimens for bacterial densities after 24 h using one-way analysis of variance test followed by Tukey’s test where the P value was ≤0.05.
Funobactam dose-ranging and HSR studies
Fifteen isolates were tested against the imipenem HSR alone or in combination with 4–5 funobactam regimens [0.9, 1.8, 3.6, 7.2 (HSR of predicted clinical dose of 250 mg q6h) or 14.4 mg/kg, all administered q6h], representing escalating fractions of funobactam HSR. Additional PK studies were conducted to assess the funobactam exposures over the dose range for PK/PD analyses; funobactam 0.9 and 14.4 mg/kg (the lowest and highest doses utilized, respectively) were given with the finalized imipenem HSR of 500 mg q6h. Samples were obtained, processed and analysed, and exposures were estimated as described previously. A sigmoidal inhibitory maximum possible effect (Emax) model was fitted to the dose-ranging data using WinNonlin. The effective funobactam index required to achieve net stasis, 1-log and 2-log bacterial killing from the starting bacterial burden for each isolate as well as for the composite within each species was estimated and the goodness-of-fit for each relationship was characterized.
Results
In vitro susceptibility
Imipenem and imipenem/funobactam modal MICs for the 15 isolates are presented in Table 1. All isolates were resistant to imipenem per CLSI-established breakpoints.11 The MICs in combination with funobactam decreased for all isolates with a range of reduction of >4-fold to >256-fold.
PK studies
Total funobactam plasma concentrations versus time profiles achieved following single-dose administration of 1, 10 and 20 mg/kg given concomitantly with imipenem HSR are presented in Figure S1 (available as Supplementary data at JAC Online). Dose-proportionality was confirmed across the examined dose range (Figure S2). PK was best described using a one-compartment model. The best-fit average PK parameters were: apparent volume of distribution, 4.62 L/kg; first-order absorption rate constant, 7.21 h−1; and overall elimination rate constant, 0.41 h−1.
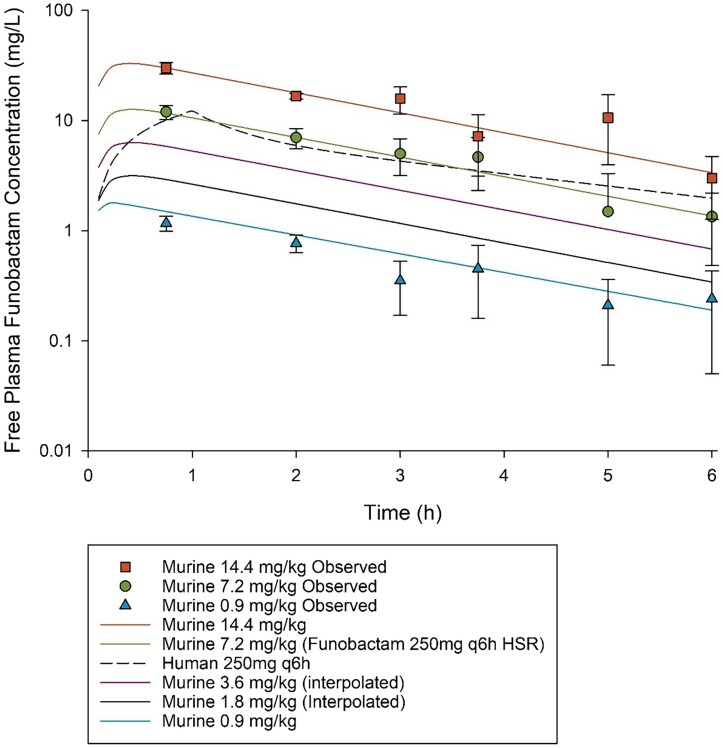
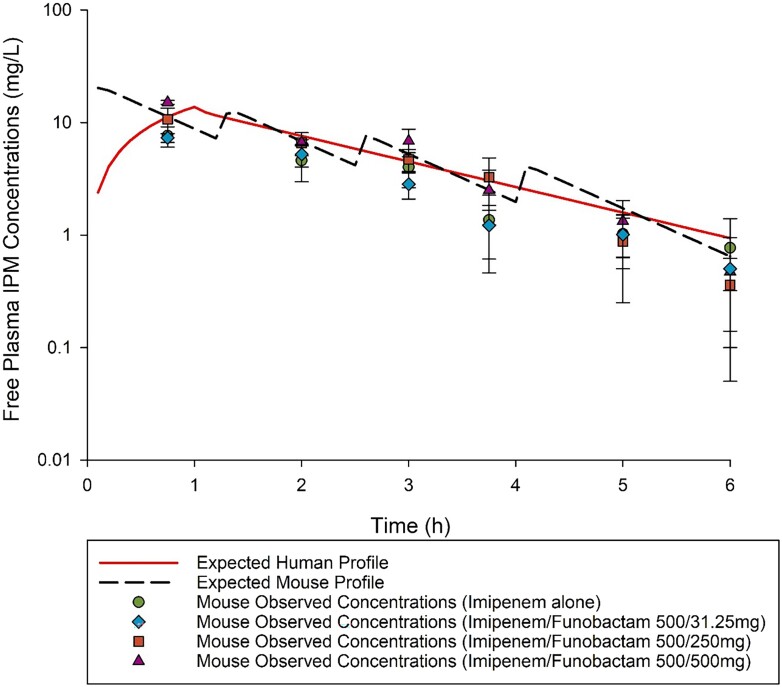
The PK exposures of the lowest and highest doses of funobactam used in dose-ranging, 0.9 and 14.4 mg/kg q6h, respectively, co-administered with the imipenem HSR of 500 mg q6h were comparable to those achieved in humans following clinical doses of 31.25 and 500 mg q6h, respectively. Interpolation was applied to estimate the exposures achieved with regimens utilized in dose-ranging between 0.9 mg/kg q6h to 7.2 mg/kg q6h (Figure 1). Funobactam exposures achieved with the escalating regimens administered in the dose-ranging studies are summarized in Table 2. Funobactam co-administration did not alter imipenem HSR exposure profile (Figure 2).
Figure 1.
Funobactam free plasma concentration–time profiles achieved with the regimens administered in dose-ranging studies. Data are presented as means ± SD.
Table 2.
Comparison of the funobactam pharmacokinetic exposures achieved with each regimen administered in the funobactam dose-ranging studies in the murine neutropenic thigh infection model
| Funobactam dose | %fT > MIC for MIC (mg/L): | fAUC0-24 (mg.h/L) | fC max (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ||||
| Murine dose q6h SC | Clinical-equivalent dose q6h (1 h infusion) | ||||||||||
| 0.9 mg/kg | 31.25 mg | 86% | 57% | 29% | 0% | 0% | 0% | 0% | 0% | 17.84a | 1.74a |
| 1.8 mg/kg | 62.5 mg | 100% | 86% | 57% | 29% | 0% | 0% | 0% | 0% | 35.68b | 3.48b |
| 3.6 mg/kg | 125 mg | 100% | 100% | 86% | 57% | 29% | 0% | 0% | 0% | 71.36b | 6.95b |
| 7.2 mg/kg | 250 mg | 100% | 100% | 100% | 86% | 57% | 29% | 0% | 0% | 142.71a | 13.90a |
| 14.4 mg/kg | 500 mg | 100% | 100% | 100% | 100% | 94% | 67% | 39% | 10% | 363.70a | 36.15a |
Values calculated from observed PK data.
Linear interpolation between 7.2 and 0.9 mg/kg.
Figure 2.
Imipenem (IPM) free plasma concentration–time profile after receiving imipenem human-simulated regimen alone and in combination with escalating doses of funobactam in a neutropenic thigh infection model compared with human receiving imipenem 500 mg q6h. Data are presented as means ± SD.
Ex vivo plasma protein-binding
The average ± SD protein-binding percentages of funobactam after single-dose administration of 1, 10 and 20 mg/kg in the presence of imipenem HSR were 3.11% ± 3.73%, 5.44% ± 3.23% and 2.03% ± 6.18%, respectively, with overall average of 3.53%. Thus, across the exposures tested, funobactam mouse plasma-bound fraction was low and not concentration-dependent.
Human-simulated exposure PK studies
The imipenem exposure attained following administration of 8 mg/kg at 0 h, 2.5 mg/kg at 1.25 h, 1.5 mg/kg at 2.5 h, and 0.9 mg/kg at 4 h repeated every 6 h, was similar to that expected in humans receiving 500 mg q6h 1 h infusion (Table 3). The imipenem/funobactam HSR (500/250 mg q6h 1 h infusion) reasonably approximated human exposures (Figures 1 and 2 and Table 3).
Table 3.
Comparison of the pharmacokinetic profiles and %fT > MIC values achieved with imipenem and funobactam at each MIC in humans versus mice receiving human-simulated regimens
| %fT > MIC for a MIC (mg/L) of: | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | Species | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | fAUC0-24 (mg·h/L) | fC max (mg/L) |
| IPM 500 mg q6h | Humana | 100% | 98% | 76% | 53% | 25% | 0% | 0% | 128.0 | 14.4 |
| Mouse | 100% | 92% | 78% | 55% | 28% | 6% | 0% | 146.8 | 20.9 | |
| Funobactam 250 mg q6h |
Humanb | 100% | 100% | 99% | 65% | 25% | 0% | 0% | 142.7 | 14.1 |
| Mouse | 100% | 100% | 86% | 57% | 29% | 0% | 0% | 142.7 | 13.9 | |
IPM, imipenem.
Human exposures estimated based on previously published population PK parameters in critically ill patients.14
Human exposures estimated from Phase I PK study in healthy volunteers (NCT04482569).15
In vivo efficacy studies
Funobactam dose-fractionation
Funobactam exposures achieved with examined dosing regimens are available in Table S1. At 0 h, bacterial burden was 5.69 ± 0.57 log10cfu/thigh across all three species. Adequate growth was observed in controls and imipenem HSR monotherapy groups: increase in burden of 3.51 ± 0.46 and 3.13 ± 0.37 log10cfu/thigh, respectively. Statistical differences in the 24 h change in log10cfu/thigh were identified among some fractionations of the same total daily dose against ACB 258 and KP 827 (P < 0.05) (Figures S3–S5). The dosing frequency appeared to consistently impact the in vivo activity of funobactam, favouring the more fractionated administration. Importantly, statistical and visual trends suggested that the PD index best correlated with efficacy of funobactam was likely %fT > CT, as opposed to %fT > MIC, as bactericidal activity was observed with regimens that achieved %fT > MIC = 0% (Table S1).
Funobactam dose-ranging and HSR studies
A. baumannii.
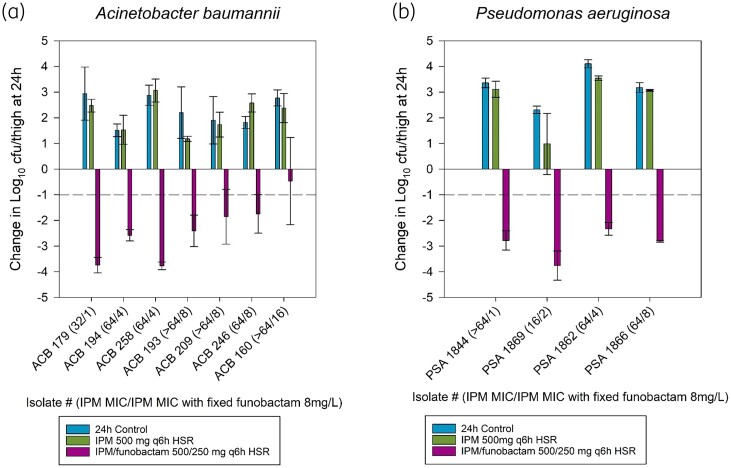
At 0 h, bacterial burden was 5.97 ± 0.18 log10cfu/thigh, and increased over 24 h by 2.32 ± 0.84 log10cfu/thigh in untreated controls. Imipenem in vitro resistance was confirmed in vivo; bacterial burden of imipenem HSR monotherapy groups increased by 2.16 ± 0.74 log10cfu/thigh. All A. baumannii isolates reached 1-log kill upon administration of imipenem/funobactam 500/250 mg HSR, except for ACB 160, which had the highest imipenem/funobactam MIC of 16 mg/L, and five of seven isolates achieved ∼2-log kill (Figure 3a).
Figure 3.
Comparative efficacy of the human-simulated exposure of imipenem (500 mg q6h) alone and in combination with human-simulated exposure of funobactam (250 mg q6h) against (a) seven Acinetobacter baumannii isolates and (b) four Pseudomonas aeruginosa isolates. Data are means ± SDs. ACB, Acinetobacter baumannii; HSR, human-simulated regimen; IPM, imipenem; PSA, Pseudomonas aeruginosa.
P. aeruginosa.
0 h bacterial burden was 5.64 ± 0.41 log10cfu/thigh, and increased at 24 h by 3.33 ± 0.62 and 2.64 ± 1.18 log10cfu/thigh in sham controls and imipenem HSR monotherapy groups, respectively. All four P. aeruginosa isolates reached 2-log kill with imipenem/funobactam 500/250 mg HSR (Figure 3b).
K. pneumoniae.
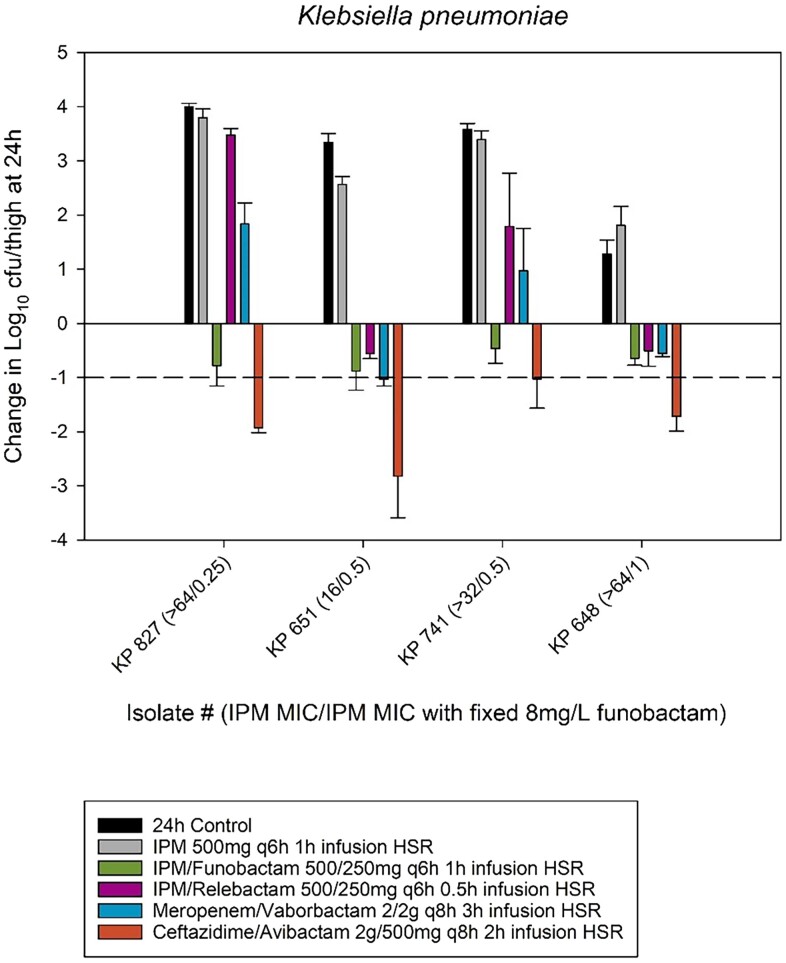
0 h bacterial burden was 5.92 ± 0.14 log10cfu/thigh and increased over 24 h by 3.15 ± 1.00 and 2.83 ± 0.81 log10cfu/thigh in untreated controls and imipenem HSR monotherapy groups, respectively. All strains achieved stasis with the imipenem/funobactam 500/250 mg HSR; however, none reached 1-log kill (Figure 4). The figure also includes comparative efficacy of plasma HSRs of commercially available BL/BLIs (imipenem/relebactam, ceftazidime/avibactam and meropenem/vaborbactam) in a similar model during prior experiments.16
Figure 4.
Comparative efficacy of the human-simulated exposure of imipenem (500 mg q6h) alone and in combination with human-simulated exposure of funobactam (250 mg q6h) against four Klebsiella pneumoniae isolates. The comparative efficacy of human-simulated exposures of three commercially available β-lactam/β-lactamase inhibitors (imipenem/relebactam, ceftazidime/avibactam and meropenem/vaborbactam) against these isolates from previous unrelated experiments are also included. Data are means ± SDs. Imipenem/relebactam, meropenem/vaborbactam, ceftazidime/avibactam MICs for: KP 827 (>32, >64, 1 mg/L), KP 651 (1, ≤ 0.06, 1 mg/L), KP 741 (>32, 64, 1 mg/L) and KP 648 (4, 1, 2 mg/L). HSR, human-simulated regimen; IPM, imipenem; KP, Klebsiella pneumoniae.
PK/PD analyses
Conventional PK/PD indices: fCmax/MIC, fAUC/MIC and %fT > MIC
The relationships between funobactam fCmax/MIC, fAUC/MIC and %fT > MIC (based on the imipenem/funobactam combination MIC) and change in bacterial burden seen in dose-ranging studies were explored for each isolate (Table 4). fCmax/MIC and fAUC/MIC were both strong predictors of activity, whereas %fT > MIC was a poor predictor, as activity was observed at 0%fT > MIC against multiple isolates. The estimated funobactam %fT > MIC values for different efficacy endpoints were also equivalent to 0%, which disagreed with dose-ranging results demonstrating reasonable funobactam dose–response against the majority of isolates. Given that neither fCmax/MIC nor %fT > MIC was a better predictor of the exposure–response relationship, fAUC/MIC defaulted to the best predictive index of the conventional indices. However, and importantly, funobactam dose-fractionation studies provided evidence that imipenem/funobactam displayed time-dependent bactericidal activity in vivo. Whereas %fT > MIC was poorly predictive, several additional approaches were adopted to analyse alternative time-dependent funobactam exposure–response relationships.
Table 4.
Comparison of the funobactam stasis, 1-log kill, and 2-log kill exposure targets for the three traditional pharmacodynamic indices and goodness-of-fit (R2) across all tested isolates in the presence of imipenem human-simulated plasma exposures in dose-ranging studies utilizing the murine neutropenic thigh infection model
| Funobactam required to achieve: | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %fT > MIC | fAUC0-24/MIC | fC max/MIC | |||||||||||
| Isolate (MIC, mg/L) | Enzyme(s) | Stasis | 1-Log reduction | 2-Log reduction | R 2 | Stasis | 1-Log reduction | 2-Log reduction | R 2 | Stasis | 1-Log reduction | 2-Log reduction | R 2 |
| Acinetobacter baumannii | |||||||||||||
| ACB 179 (1) | ADC-25, OXA-23, OXA-223 | 12.60 | 14.05 | 15.81 | 0.99 | 8.65 | 9.51 | 10.54 | 0.99 | 0.91 | 0.99 | 1.09 | 0.99 |
| ACB 194 (4) | ADC-25, OXA-23, OXA-82 | 0.00 | 1.03 | 12.64 | 0.24 | 2.77 | 3.75 | 5.84 | 0.79 | 0.25 | 0.35 | 0.58 | 0.79 |
| ACB 258 (4) | ADC-222, OXA-23, OXA-95 | 3.71 | 7.75 | 15.20 | 0.87 | 8.75 | 11.89 | 16.41 | 0.91 | 0.85 | 1.16 | 1.60 | 0.91 |
| ACB 193 (8) | OXA-23,OXA-82 | 0.02 | 0.97 | 2.13 | 0.21 | 0.47 | 0.93 | 2.77 | 0.93 | 0.05 | 0.09 | 0.27 | 0.93 |
| ACB 209 (8) | OXA-65,OXA-24 | 1.60 | 7.88 | 32.73 | 0.66 | 6.24 | 11.71 | 24.07 | 0.79 | 0.62 | 1.14 | 2.36 | 0.79 |
| ACB 246 (8) | ADC-33, OXA-23, OXA-82 | 0.00 | 0.00 | N/A | 0.27 | 5.24 | 5.93 | N/A | 0.81 | 0.51 | 0.58 | N/A | 0.81 |
| ACB 160 (16) | OXA-24, OXA-65, TEM-1B | 0.19 | 1.33 | N/A | 0.38 | 6.49 | 13.28 | N/A | 0.75 | 0.63 | 1.30 | N/A | 0.75 |
| Mean | 2.59 | 4.71 | 15.70 | 0.52 | 5.51 | 8.14 | 11.92 | 0.85 | 0.54 | 0.80 | 1.18 | 0.85 | |
| Median | 0.19 | 1.33 | 15.20 | 0.38 | 6.24 | 9.51 | 10.54 | 0.81 | 0.62 | 0.99 | 1.09 | 0.81 | |
| Composite | 0.31 | 4.14 | 20.04 | 0.54 | 4.38 | 8.15 | 16.22 | 0.73 | 0.43 | 0.79 | 1.58 | 0.73 | |
| Pseudomonas aeruginosa | |||||||||||||
| PSA 1844 (1) | KPC-2, PDC-42 | 31.55 | 37.16 | 46.16 | 0.88 | 19.42 | 22.91 | 28.62 | 0.88 | 1.89 | 2.24 | 2.79 | 0.88 |
| PSA 1869 (2) | GES-19, GES-26 | 0.00 | 1.08 | 5.37 | 0.74 | 2.74 | 6.49 | 12.78 | 0.89 | 0.27 | 0.64 | 1.24 | 0.89 |
| PSA 1862 (4) | GES-19, GES-20 | 0.00 | 0.00 | 0.00 | 0.25 | 3.72 | 4.08 | 4.89 | 0.97 | 0.36 | 0. 39 | 0.47 | 0.97 |
| PSA 1866 (8) | GES-20 | 0.00 | 0.00 | 0.00 | 0.53 | 4.54 | 5.63 | 7.68 | 0.97 | 0.45 | 0.55 | 0.75 | 0.97 |
| Mean | 7.89 | 9.56 | 12.88 | 0.60 | 7.60 | 9.78 | 13.49 | 0.93 | 0.74 | 0.95 | 1.31 | 0.93 | |
| Median | 0.00 | 0.54 | 2.69 | 0.64 | 4.13 | 6.06 | 10.23 | 0.93 | 0.40 | 0.59 | 1.00 | 0.93 | |
| Composite | 10.55 | 21.84 | 38.38 | 0.49 | 4.03 | 6.48 | 12.10 | 0.77 | 0.39 | 0.60 | 1.09 | 0.77 | |
| Klebsiella pneumoniae | |||||||||||||
| KP 827 (0.25) | OXA-48, CTX-M-15, OXA-1, SHV-11, TEM-1B | 93.86 | N/A | N/A | 0.89 | 82.76 | N/A | N/A | 0.89 | 8.08 | N/A | N/A | 0.89 |
| KP 651 (0.5) | KPC-2, CDC-120 | 17.38 | N/A | N/A | 0.93 | 19.90 | N/A | N/A | 0.95 | 1.94 | N/A | N/A | 0.95 |
| KP 741 (0.5) | SHV-11, CTX-M-55, OXA-48 | 66.55 | N/A | N/A | 0.98 | 49.88 | N/A | N/A | 0.98 | 4.91 | N/A | N/A | 0.98 |
| KP 648 (1) | KPC-3, CDC-113 | 13.20 | N/A | N/A | 0.96 | 8.26 | N/A | N/A | 0.96 | 0.84 | N/A | N/A | 0.96 |
| Mean | 47.75 | N/A | N/A | 0.94 | 40.20 | N/A | N/A | 0.95 | 3.94 | N/A | N/A | 0.95 | |
| Median | 41.96 | N/A | N/A | 0.94 | 34.89 | N/A | N/A | 0.96 | 3.42 | N/A | N/A | 0.96 | |
| Composite | 28.81 | N/A | N/A | 0.80 | 21.50 | N/A | N/A | 0.81 | 1.53 | N/A | N/A | 0.81 | |
N/A, target not achieved based on the curve of best-fit.
Alternative time-dependent PK/PD indices
%fT > CT
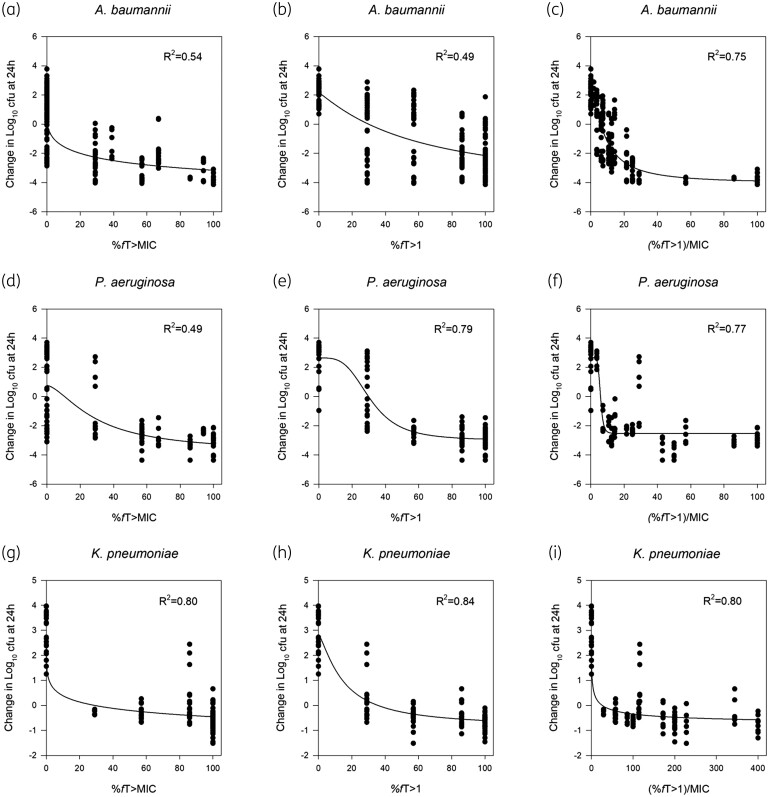
The %fT > CT (at funobactam CT ranging from 0.25 to 8 mg/L) were explored. For individual isolates, %fT > CT(1 mg/L) was the best predictive threshold concentration as assessed by R2 values, but was poorly predictive on the cumulative species level, particularly among the A. baumannii strains (Figure 5). Notably, estimated targets varied considerably within species and appeared to be higher for isolates with higher MICs compared with those with lower MICs (i.e. more resistant isolates required longer periods of time above the concentration threshold) (Table S2).
Figure 5.
Comparison of the 24 h change in cfu/thigh versus funobactam exposure using different time-dependent PK/PD indices in serine carbapenemase-producing Acinetobacter baumannii (n = 7) (a–c), Pseudomonas aeruginosa (n = 4) (d–f) and Klebsiella pneumoniae (n = 4) (g–i) isolates after receiving escalating doses of funobactam in combination with an imipenem human-simulated regimen of 500 mg q6h in the neutropenic murine thigh infection model.
(%fT > CT)/MIC.
Given that efficacy studies collectively showed that imipenem/funobactam bactericidal activity varied by the combination MIC, with isolates of lower MIC values showing enhanced killing at lower funobactam exposures, and the fact that %fT > MIC as well as %fT > CT offered poor predictions at the cumulative species level, a novel analysis was employed that normalizes the %fT > CT (for CT with the highest correlation to the efficacy data) to imipenem/funobactam combination MIC. In other words, the magnitude of time during which the inhibitor free plasma concentration remains above a threshold concentration required for in vivo killing would vary based on the combination MIC. Thus, whereas %fT > CT discounts the in vitro potency of the compound against the specific isolate, the novel index (%fT > CT)/MIC allowed for a better comparison of PK/PD targets across isolates of the same species and was strongly predictive on both an individual isolate and cumulative species basis.
Across the different threshold plasma funobactam concentrations assessed, individual isolate analyses involving %fT > CT(1 mg/L) had the highest correlation with efficacy. Normalizing the %fT > CT(1 mg/L) index to imipenem/funobactam MIC [(%fT > CT[1 mg/L])/MIC] allowed the integration of the isolate’s susceptibility, which further enhanced the correlation and best described the bacterial killing (Figure 5). Funobactam PK/PD target thresholds for in vivo efficacy endpoints are summarized in Table 5.
Table 5.
Comparison of the funobactam (%fT > CT)/MIC (based on the MICs of imipenem in combination at a fixed funobactam concentration of 8 mg/L, and a CT of 1 mg/L) stasis, 1-log kill and 2-log kill pharmacodynamic targets and goodness-of-fit (R2) across all tested isolates in the presence of imipenem HSR in dose-ranging studies utilizing the murine neutropenic thigh infection model
| Isolate (MIC, mg/L) | Enzyme(s) | Funobactam (%fT > CT[1 mg/L])/MIC required to achieve: | |||
|---|---|---|---|---|---|
| Stasis | 1-Log reduction | 2-Log reduction | R 2 | ||
| Acinetobacter baumannii | |||||
| ACB 179 (1) | ADC-25, OXA-23, OXA-223 | 12.60 | 14.05 | 15.81 | 0.99 |
| ACB 194 (4) | ADC-25, OXA-23, OXA-82 | 4.52 | 6.09 | 9.31 | 0.79 |
| ACB 258 (4) | ADC-222, OXA-23, OXA-95 | 15.06 | 17.45 | 19.92 | 0.90 |
| ACB 193 (8) | OXA-23,OXA-82 | 0.62 | 1.41 | 4.62 | 0.93 |
| ACB 209 (8) | OXA-65,OXA-24 | 9.46 | 11.00 | 12.47 | 0.76 |
| ACB 246 (8) | ADC-33, OXA-23, OXA-82 | 7.11 | 9.82 | N/A | 0.75 |
| ACB 160 (16) | OXA-24, OXA-65, TEM-1B | 6.14 | 6.38 | N/A | 0.65 |
| Mean | 7.93 | 9.46 | 12.42 | 0.82 | |
| Median | 7.11 | 9.82 | 12.47 | 0.79 | |
| Composite | 5.84 | 9.17 | 14.48 | 0.75 | |
| Pseudomonas aeruginosa | |||||
| PSA 1844 (1) | KPC-2, PDC-42 | 31.55 | 37.16 | 46.16 | 0.88 |
| PSA 1869 (2) | GES-19, GES-26 | 5.54 | 11.51 | 19.40 | 0.88 |
| PSA 1862 (4) | GES-19, GES-20 | 5.95 | 6.56 | 7.91 | 0.97 |
| PSA 1866 (8) | GES-20 | 6.98 | 8.29 | 10.10 | 0.97 |
| Mean | 12.50 | 15.88 | 20.89 | 0.93 | |
| Median | 6.46 | 9.90 | 14.75 | 0.92 | |
| Composite | 5.81 | 6.55 | 7.90 | 0.77 | |
| Klebsiella pneumoniae | |||||
| KP 827 (0.25) | OXA-48, CTX-M-15, OXA-1, SHV-11, TEM-1B | 134.52 | N/A | N/A | 0.89 |
| KP 651 (0.5) | KPC-2, CDC-120 | 33.85 | N/A | N/A | 0.95 |
| KP 741 (0.5) | SHV-11, CTX-M-55, OXA-48 | 77.60 | N/A | N/A | 0.98 |
| KP 648 (1) | KPC-3, CDC-113 | 13.20 | N/A | N/A | 0.96 |
| Mean | 64.80 | N/A | N/A | 0.95 | |
| Median | 55.73 | N/A | N/A | 0.96 | |
| Composite | 26.07 | N/A | N/A | 0.80 | |
N/A, target not achieved based on the curve of best-fit.
The responses observed for each strain evaluated per each PK/PD index as detailed above are presented in Figures S6–S35.
Discussion
The isolates utilized in the current investigation are representative of imipenem/funobactam MIC distribution from recent surveillance of 261 clinical imipenem non-susceptible isolates from China, where imipenem/funobactam MIC90 values for A. baumannii (n = 106), P. aeruginosa (n = 101) and K. pneumoniae (n = 54) were 8, 32 and 2 mg/L, respectively.10 Through investigating the PD of funobactam in combination with imipenem HSR in the neutropenic thigh infection model, it was clear that funobactam co-administration markedly enhanced the in vivo efficacy of the latter against A. baumannii, P. aeruginosa and K. pneumoniae isolates harbouring various serine carbapenemases. Dose-fractionation revealed time-dependent killing and a novel PK/PD index of (%fT > CT[1 mg/L])/MIC best described the change in log10cfu/thigh against clinical imipenem-non-susceptible isolates harbouring an array of serine BLs.
Traditional PK/PD indices of fAUC/MIC, fCmax/MIC and %fT > MIC have been used to predict antimicrobial activity and guide optimal dosing strategies for decades.17 In the case of BLIs, which often have little-to-no intrinsic antimicrobial activity, these classic indices may be insufficient to characterize activity when co-administered in a combination.18 It is well understood that there is high degree of co-linearity between these three conventional indices. Thus, well-designed dose-fractionation studies are required to deparameterize and pull apart the indices to appreciate a meaningful effect of one over the others, suggesting that it is more difficult to conclude that an index other than fAUC/MIC is best predictive of activity. Indeed, an additional fractionation of every 4 h was necessary against the A. baumannii isolate to emphatically demonstrate time-dependent activity. Importantly, and often overlooked, fractionating the BLI ought to be done in the presence of humanized, or at least clinically relevant, fixed BL backbone exposures. Although funobactam fAUC/MIC was a strong predictor of change in cfu/thigh for this dataset, it could not be ignored that overall, dosing frequency appeared to consistently impact in vivo activity of funobactam across all three species, favouring the more fractionated administration. However, across numerous isolates and species, activity was observed at 0% fT > MIC, thus making this traditional index a poor descriptor. Alternatively, %fT > CT was a more accepted model of describing time-dependent antibacterial killing and is the currently welcomed index of the non-β-lactam BLI avibactam, when co-administered with ceftazidime.19 Upon investigating %fT > CT at thresholds from 0.25 to 8 mg/L in doubling dilutions, a free concentration of 1 mg/L funobactam with imipenem HSR was best predictive of change in log10cfu/thigh on an individual isolate basis. Importantly, the estimated %fT > CT targets varied considerably within species, with notable discrepancies by MIC values—they appeared to be greater for isolates with higher MICs compared with those with lower MICs. Previous investigations of another BL/BLI, ceftolozane/tazobactam, similarly found that in four BL-producing Escherichia coli strains, tazobactam %fT > CT satisfactorily described the change in log10cfu for individual isolates (R2 range = 0.90–0.99), but did not co-model well.20 However, a translational relationship that did allow for the co-modelling (R2 = 0.90) was assigning an isolate-specific CT equal to the product of 0.5 and the MIC. Indeed, an inherent limitation of %fT > CT is that, by definition, it does not consider the susceptibility (i.e. MIC) of the isolates. However, the application of this approach to our dataset yielded stasis or net bacterial killing at 0% fT > CT(0.5*MIC) in multiple isolates (data not shown). Other novel PK/PD indices have been introduced recently—notably AUC:MIC * 1/τ.21 Reportedly, this index has utility for compounds with especially short half-lives in animals to gain better certainty around human dose predictions.22 Funobactam did not display a short half-life in our model with uranyl nitrate and therefore this PK/PD index was not investigated.
In this study, we explored an alternative approach using MIC as a correction factor applied to the best predictive %fT > CT of 1 mg/L, producing the novel PK/PD index of (%fT > CT[1mg/L])/MIC. Although we attempted to include isolates with sufficient diversity so that the finding from our study would better forecast for the target isolate populations, whether CT of 1 mg/L will provide good correlation for strains and species beyond those assessed in our investigation cannot be ascertained. Accounting for the in vitro potency parameter, i.e. the MIC of the organism, yielded comparable goodness-of-fit for individual isolates, but markedly improved the predictive ability of the cumulative analyses. This MIC correction factor makes intuitive sense and suggests that isolates with higher MICs require more time above the threshold concentration than those with lower MICs to achieve similar efficacy endpoints.
The magnitude of killing achieved with imipenem/funobactam 500/250 mg q6h HSR was profound against the non-fermenters: 24 h change in log10cfu/thigh ranged from −0.46 ± 1.69 to −3.77 ± 0.15, and −2.33 ± 0.25 to −3.76 ± 0.57 among A. baumannii and P. aeruginosa isolates, respectively. In preclinical models, stasis has been linked to clinical efficacy for lower inoculum infection such as intra-abdominal or those involving the urinary tract, whereas a 1-log reduction has correlated similarly for higher inoculum infections including pneumonia, endocarditis or bacteraemia.23 Going further, a 2-log reduction may be beneficial for optimized time-to-response or prevention of resistance development.23 The bacterial killing against the four K. pneumoniae strains was lesser (−0.47 ± 0.28 to −0.88 ± 0.35), despite lower imipenem/funobactam MICs. However, these K. pneumoniae isolates were selected in part due to available comparative data with commercial BL/BLIs. For the KPC-producing K. pneumoniae isolates, imipenem/funobactam 500/250 mg q6h HSR achieved cfu reductions comparable to those seen previously with HSRs of imipenem/relebactam and meropenem/vaborbactam in the same model.16 The ceftazidime/avibactam HSR on the other hand provided greater cfu reductions compared with all the examined regimens. Imipenem/funobactam and ceftazidime/avibactam HSRs both led to net bacterial reduction against the OXA-48-harbouring K. pneumoniae strains, whereas growth was observed on imipenem/relebactam and meropenem/vaborbactam HSRs as expected due to lack of inhibition of enzyme-mediated hydrolysis with these latter compounds.16 Although the enzyme-mediated resistance mechanisms have been well characterized for these isolates, there may be additional underlying mechanisms of resistance at play, such as porin loss or alterations in efflux pump expression. Further experimentation with additional K. pneumoniae isolates and other Enterobacterales spp. is warranted.
In conclusion, the therapeutic armamentarium against CRAB is limited and represents a serious unmet medical need on the global scale.6 Imipenem/cilastatin/funobactam stands to bolster the sparse options available for treatment due to funobactam’s broad inhibition of serine carbapenemases, including OXA-23 and OXA-24 enzymes, providing an advantage over commercially available BL/BLIs. Imipenem/funobactam combination (500/250 mg q6h as 1 h infusion) showed potent in vivo efficacy against serine carbapenemase-producing A. baumannii and P. aeruginosa, and bacterial reduction comparable to commercially available BL/BLIs against K. pneumoniae isolates, in the neutropenic thigh infection model. These data support the consideration of this combination and the dosage for the treatment of serious infections due to these organisms in Phase III trials.
Supplementary Material
Acknowledgements
This work was presented in part at IDWeek 2022 (Washington D.C.) as Poster #1722.
We acknowledge the team from the Center for Anti-Infective Research and Development for their vital assistance in the conduct of this study.
Contributor Information
Andrew J Fratoni, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Angela V Berry, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Xiao Liu, Department of Research and Development, Evopoint Biosciences Co., Ltd, Beijing, China.
Xi Chen, Department of Research and Development, Evopoint Biosciences Co., Ltd, Beijing, China.
Yuchuan Wu, Department of Research and Development, Evopoint Biosciences Co., Ltd, Beijing, China.
David P Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA; Division of Infectious Diseases, Hartford Hospital, Hartford, CT, USA.
Kamilia Abdelraouf, Center for Anti-Infective Research and Development, Hartford Hospital, 80 Seymour Street, Hartford, CT 06102, USA.
Funding
This project was sponsored by Evopoint Biosciences Co., Ltd (Beijing, China).
Transparency declarations
X.L., X.C. and Y.W. are employed by Evopoint Biosciences Co. K.A. has received research funding from the study sponsor. A.J.F., A.V.B. and D.P.N. have none to declare.
Supplementary data
Figures S1 to S35 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Cai B, Echols R, Magee Get al. . Prevalence of carbapenem-resistant gram-negative infections in the United States predominated by Acinetobacter baumannii and Pseudomonas aeruginosa. Open Forum Infect Dis 2017; 4: ofx176. 10.1093/ofid/ofx176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215: S28–36. 10.1093/infdis/jiw282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cerceo E, Deitelzweig SB, Sherman BMet al. . Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist 2016; 22: 412–31. 10.1089/mdr.2015.0220 [DOI] [PubMed] [Google Scholar]
- 4. Tacconelli E, Carrara E, Savoldi Aet al. . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 5. van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation beta-lactam/beta-lactamase inhibitor combinations. Clin Infect Dis 2016; 63: 234–41. 10.1093/cid/ciw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdul-Mutakabbir JC, Griffith NC, Shields RKet al. . Contemporary perspective on the treatment of Acinetobacter baumannii infections: insights from the Society of Infectious Diseases pharmacists. Infect Dis Ther 2021; 10: 2177–202. 10.1007/s40121-021-00541-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamma PD, Aitken SL, Bonomo RAet al. . Infectious Diseases Society of America guidance on the treatment of AmpC beta-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74: 2089–114. 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 8. Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis 2010; 51: 79–84. 10.1086/653120 [DOI] [PubMed] [Google Scholar]
- 9. Stein GE, Craig WA. Tigecycline: a critical analysis. Clin Infect Dis 2006; 43: 518–24. 10.1086/505494 [DOI] [PubMed] [Google Scholar]
- 10. Li Y, Yan M, Xue Fet al. . In vitro and in vivo activities of a novel beta-lactamase inhibitor combination imipenem/XNW4107 against recent clinical gram-negative bacilli from China. J Glob Antimicrob Resist 2022; 31: 1–9. 10.1016/j.jgar.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 11. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022. [Google Scholar]
- 12. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018. [Google Scholar]
- 13. Reyes S, Abdelraouf K, Nicolau DP. In vivo activity of human-simulated regimens of imipenem alone and in combination with relebactam against Pseudomonas aeruginosa in the murine thigh infection model. J Antimicrob Chemother 2020; 75: 2197–205. 10.1093/jac/dkaa145 [DOI] [PubMed] [Google Scholar]
- 14. Sakka SG, Glauner AK, Bulitta JBet al. . Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 2007; 51: 3304–10. 10.1128/AAC.01318-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Study to Evaluate Safety, Tolerability, and Pharmacokinetics of XNW4107 Alone or in Combination With Imipenem/Cilastatin. ClinicalTrials.gov identifier: NCT04482569. https://clinicaltrials.gov/search?id=NCT04482569
- 16. Asempa TE, Kois AK, Gill CMet al. . Phenotypes, genotypes and breakpoints: an assessment of beta-lactam/beta-lactamase inhibitor combinations against OXA-48. J Antimicrob Chemother 2023; 78: 636–45. 10.1093/jac/dkac425 [DOI] [PubMed] [Google Scholar]
- 17. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–10. quiz 1–2. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 18. Bhagunde P, Chang KT, Hirsch EBet al. . Novel modeling framework to guide design of optimal dosing strategies for beta-lactamase inhibitors. Antimicrob Agents Chemother 2012; 56: 2237–40. 10.1128/AAC.06113-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berkhout J, Melchers MJ, van Mil ACet al. . Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 2016; 60: 368–75. 10.1128/AAC.01269-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanscoy B, Mendes RE, McCauley Jet al. . Pharmacological basis of beta-lactamase inhibitor therapeutics: tazobactam in combination with ceftolozane. Antimicrob Agents Chemother 2013; 57: 5924–30. 10.1128/AAC.00656-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McEntee L, Johnson A, Farrington Net al. . Pharmacodynamics of tebipenem: new options for oral treatment of multidrug-resistant gram-negative infections. Antimicrob Agents Chemother 2019; 63: e00603-19. 10.1128/AAC.00603-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lakota EA, Bader J, Bhavnani SMet al. . Traditional PK-PD indices for efficacy - can we do better? Open Forum Infect Dis 2017; 4Suppl 1: S298. 10.1093/ofid/ofx163.685 [DOI] [Google Scholar]
- 23. Trang M, Dudley MN, Bhavnani SM. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr Opin Pharmacol 2017; 36: 107–13. 10.1016/j.coph.2017.09.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.