Abstract
Background
Pharmacokinetic (PK) data underlying paediatric penicillin dosing remain limited, especially in critical care.
Objectives
The primary objective of the Neonatal and Paediatric Pharmacokinetics of Antimicrobials study (NAPPA) was to characterize PK profiles of commonly used penicillins using data obtained during routine care, to further understanding of PK variability and inform future evidence-based dosing.
Methods
NAPPA was a multicentre study of amoxicillin, co-amoxiclav, benzylpenicillin, flucloxacillin and piperacillin/tazobactam. Patients were recruited with informed consent. Antibiotic dosing followed standard of care. PK samples were obtained opportunistically or at optimal times, frozen and analysed using UPLC with tandem MS. Pharmacometric analysis was undertaken using NONMEM software (v7.3). Model-based simulations (n = 10 000) tested PTA with British National Formulary for Children (BNFC) and WHO dosing. The study had ethical approval.
Results
For the combined IV PK model, 963 PK samples from 370 participants were analysed simultaneously incorporating amoxicillin, benzylpenicillin, flucloxacillin and piperacillin data. BNFC high-dose regimen simulations gave these PTA results (median fT>MIC at breakpoints of specified pathogens): amoxicillin 100% (Streptococcus pneumoniae); benzylpenicillin 100% (Group B Streptococcus); flucloxacillin 48% (MSSA); and piperacillin 100% (Pseudomonas aeruginosa). Oral population PK models for flucloxacillin and amoxicillin enabled estimation of first-order absorption rate constants (1.16 h−1 and 1.3 h−1) and bioavailability terms (62.7% and 58.7%, respectively).
Conclusions
NAPPA represents, to our knowledge, the largest prospective combined paediatric penicillin PK study undertaken to date, and the first paediatric flucloxacillin oral PK model. The PTA results provide evidence supportive of BNFC high-dose IV regimens for amoxicillin, benzylpenicillin and piperacillin.
Introduction
Over 80 years since the first documented use of penicillin therapy in children,1 β-lactam antibiotics remain the most widely used class of medications in paediatrics.2 Furthermore, despite widespread antibiotic availability and the success of paediatric vaccination programmes, infectious diseases are still a leading cause of global childhood morbidity and mortality.3,4 The pharmacokinetic/pharmacodynamic (PKPD) data underlying paediatric β-lactam dosing regimens are limited, particularly in neonates,5 and standard dosing regimens in different formularies and countries vary enormously.6 Consequently, PKPD studies within these vulnerable populations, particularly those in ICU, are needed in order to support the development of updated evidence-based dosing regimens.7
The target PKPD index for penicillin therapy is the percentage of time within the dosing interval that the free (unbound) concentration of the penicillin antibiotic (as quantified in the blood) remains above the MIC, commonly abbreviated to %fT>MIC.7 The traditional PKPD target for penicillin therapy in humans aimed to achieve at least 40% fT>MIC; this target was originally obtained from in vitro and in vivo studies,8–10 where it was determined to be the best PKPD predictor of both bacterial killing and microbiological response.11 However, in recent years, this target has sometimes been increased to 70% fT>MIC (or up to 100% fT>MIC or 100% >4× MIC) in the context of critical illness.12–15 Given that many neonates receiving in-hospital antimicrobial therapy are critically ill or at risk of critical illness given their functional immunocompromise (relating to immune system immaturity), 70%–100% fT>MIC is sometimes advocated for neonates. These theoretical reasons for increasing the target %fT>MIC have led to extensive research into prolonged or continuous β-lactam infusions in critical care, the majority of which has focused on adult patients.16–18
Here we report the results from the Neonatal and Paediatric Pharmacokinetics of Antimicrobials study (NAPPA). NAPPA was a post-marketing prospective population pharmacokinetic study that aimed to use data acquired from sparse sampling strategies to develop a paediatric population pharmacokinetic model of five penicillins [amoxicillin, co-amoxiclav (amoxicillin/clavulanate), ampicillin, benzylpenicillin, flucloxacillin and piperacillin/tazobactam], which are collectively referred to as the ‘NAPPA penicillins’ in this manuscript. The objective was to characterize the PK profiles of these penicillins when used in hospital within routine clinical care, to further understanding of pharmacokinetic variability, and help inform future paediatric evidence-based dosing regimens.
Methods
Clinical study
Patients were recruited at nine NHS hospital sites from November 2013 until February 2016. Potentially eligible patients, identified by screening of participating ICUs and wards, were invited to enrol, and provided with study information. Written informed consent was obtained from each participant’s parent/guardian. Assent was sought from patients aged over 6 years. The inclusion criteria were: child aged under 16 years receiving one of the NAPPA penicillins (either IV or orally) and requiring IV access or blood test(s) as part of routine care. The exclusion criteria were: patient deemed unlikely to survive for 48 h after recruitment, pregnancy, known β-lactam or β-lactamase inhibitor (BLI) allergy/hypersensitivity, or care requiring renal replacement therapy, extracorporeal membrane oxygenation or cardiopulmonary bypass.
Clinical data were collected from participants’ medical records, including demographic information, weight, medical history, level of care [general, High Dependency Unit (HDU), Neonatal/Paediatric ICU], results of haematological, biochemical and microbiological investigations, antibiotic dosing regimen details and concomitant medications. Data were recorded on standardized case report forms. Central data processing used the REDCap electronic data capture tool.19
Study antibiotics were prescribed by the attending physician according to clinical need, at the usual dose, following local guidelines and the British National Formulary for Children (BNFC)20 (Table S1, available as Supplementary data at JAC Online) by the clinically appropriate route of administration. All participants had the usual blood test(s) required for routine care. In addition, at the time of other blood sampling where possible, an extra 0.5 mL aliquot of blood was obtained opportunistically. If intravascular access was in situ for clinical reasons, additional PK samples could also be taken at recommended times (Table S2), derived from the optimal design strategy, as previously described.21 Blood samples, from an indwelling vascular catheter, venepuncture or heel-prick, were collected into EDTA-containing tubes, centrifuged, and the plasma stored at −70°C to −80°C before transfer for retrospective, batched laboratory analysis. The maximum number of study-specific samples per enrolment was eight.
The London Dulwich Research Ethics Committee approved the protocol (reference 13/LO/0907). The study was registered with EudraCT (EudraCT 2013-002366-40) and ClinicalTrials.gov (NCT01975493).
Analyte quantification
Antibiotic concentrations were measured in the Analytical Services International Ltd laboratory using UPLC with tandem MS. The method development and validation details were previously published.22
PK analysis
Population PK modelling was undertaken using non-linear mixed-effects modelling software NONMEM® (version 7.3, ICON plc). A combined population-PK model was fitted simultaneously to the measured drug concentration–time data following IV administration for all study penicillins. The total (bound and unbound) concentration data were analysed to derive a base structural disposition model. The first-order conditional estimation method with interaction (FOCE-I) was utilized. First-order (linear) elimination kinetics were assumed. Standard compartmental linear models were tested (up to a maximum of two compartments). The M5 method was used to handle data below the limit of quantification (BLQ), setting BLQ data points to BLQ/2 for analysis.23 Interindividual variability was tested for all parameters (assuming a log-normal distribution, as all envisaged parameters were continuous). The residual variability model first tested was a combined proportional and additive residual error model. No other error models were tested as this gave a suitable fit. Since the data were sparse no interoccasion variability was included. R studio (version 1.1.414) with R software (version 3.3.3) was used for data management.
Allometric weight scaling was included a priori with fixed allometric exponents of 1.0 and 0.75 for volume and clearance terms, respectively. For clearance parameters, the allometric weight scaling was combined with a Hill model using postmenstrual age (PMA) in weeks to describe the maturation function (as derived by Rhodin et al.24 in 2009 given expected clearance is principally by glomerular filtration, particularly during the neonatal period and infancy):
| (1) |
where PMA50 is the PMA in weeks at which drug clearance reaches half the mature (adult) value. To further account for the changes in clearance during the early days of life, a covariate function based on postnatal age (PNA), as previously described,25 was also tested:
| (2) |
Serum creatinine (SeCr) was tested as a covariate on clearance, using a power model adjusted for the ratio to the mean age-adjusted creatinine, calculated using the Ceriotti formula:26
| (3) |
The output of this function reflects the expected fall in creatinine after birth (which is initially high as early measurements reflect maternal creatinine), followed by a steady rise with increasing age.
Further covariate analysis was not undertaken for the combined IV model as earlier covariate testing on the population PK data from the individual NAPPA penicillins had not identified any additional significant covariates (data not shown). Additional details are included in the Supplementary information.
Model evaluation and qualification procedures
Model selection was based on comparison of the objective function value (OFV) between competing models, in addition to the standard errors of parameter estimates, goodness-of-fit (GOF) plots and scientific plausibility. To aid model selection, simulation properties were tested with visual predictive checks (VPCs), generated using PsN software (version 4.7.0). A non-parametric bootstrap (with 1000 replicates) was used to assess the precision of final parameter estimates.
PTA evaluation
Model-based simulations, based on the final combined IV PK model, were used to evaluate the PTA with BNFC and WHO dosing regimens (Table 1) administered as an IV bolus.20,27 The simulated patient population (n = 10 000) was randomly generated using the study participants’ baseline demographics. The fraction of time spent above the MIC (fT>MIC) over the first 24 h of therapy was the primary PD endpoint. Fixed protein binding was assumed, based on literature values (Table 2).28–31 The EUCAST clinical breakpoints used are shown in Table 3.32,33
Table 1.
Dosing regimens used for the model-based simulations
| Penicillin | Age groups | BNFC high-dose regimen | BNFC low-dose regimen | WHO regimen |
|---|---|---|---|---|
| Amoxicillin | PNA < 7 days | 60 mg/kg q12h | 30 mg/kg q12h | 30 mg/kg q8h |
| PNA ≥ 7 days | 60 mg/kg (max 1 g) q8h | 30 mg/kg (max 500 mg) q8h | 30 mg/kg (max 500 mg) q8h | |
| Benzylpenicillin | PNA < 7 days | 50 mg/kg q12h | 25 mg/kg q8h | 30 mg/kg q8h |
| PNA 7–27 days | 50 mg/kg q8h | 25 mg/kg q8h | 30 mg/kg q8h | |
| PNA ≥ 28 days | 50 mg/kg (max 2.4 g) q4h | 25 mg/kg (max 2.4 g) q6h | N/A | |
| Flucloxacillin | PNA < 7 days | 100 mg/kg q12h | 50 mg/kg q12h | 50 mg/kg q12h |
| PNA 7–20 days | 100 mg/kg q8h | 50 mg/kg q8h | ||
| PNA ≥ 21–27 days | 100 mg/kg (max 2 g) q6h | 50 mg/kg (max 2 g) q6h | ||
| PNA ≥ 28 days | 25 mg/kg (max 2 g) q4h | |||
| Piperacillin | PNA < 28 days | 90 mg/kg q8h | N/A | 100 mg/kg q8h |
| PNA ≥ 28 days | 90 mg/kg (max. 4.5 g) q6h | N/A | 100 mg/kg (max 4.5 g) q8h |
Max, maximum. Expanded details of the BNFC dosing recommendations for different clinical indications are included in the Supplementary data.
Table 2.
Published protein binding percentages for the NAPPA penicillins
Table 3.
MIC cut-offs used for plots (mg/L)
| Antibiotic | MIC S cut-off | MIC R cut-off |
|---|---|---|
| Amoxicillin | 2 | 8 |
| Benzylpenicillin | 0.25 | 2 |
| Flucloxacillin | — | 2 |
| Piperacillin | 8 | 16 |
Analysis of oral amoxicillin and flucloxacillin PK data
The oral PK data for amoxicillin and flucloxacillin were analysed separately, together with the IV PK data from each drug, respectively; the parameters estimated from the combined IV PK model were fixed, and the absorption rate constant (ka) and bioavailability term, F, were then estimated.
Results
Across all the penicillins combined, 428 patients were enrolled in the NAPPA study. For the combined IV PK model incorporating amoxicillin (including participants receiving co-amoxiclav), benzylpenicillin, flucloxacillin and piperacillin PK data, a total of 963 evaluable PK samples from 370 participants were analysed simultaneously.
The baseline demographics of study participants and treatment indications are shown in Tables 4 and 5, respectively. Data points were excluded from model building if there were known protocol deviations, for example, if antibiotic dose administration details were not recorded (n = 3). Two piperacillin data points were excluded where the measured concentration were greater than 1000 mg/L, since sample contamination was suspected, as concentrations this high have not previously been reported. The ampicillin data were excluded as only three participants were enrolled.
Table 4.
Demographic characteristics of each study population for the combined IV model
| Demographic characteristics | Co-amoxiclav and amoxicillin (n = 174) | Benzylpenicillin (n = 64) | Flucloxacillin (n = 72) | Piperacillin (n = 70) |
|---|---|---|---|---|
| Weight (kg) | 3.76 (0.58–70) | 2.83 (0.57–64.3) | 3.1 (0.585–67) | 10.9 (0.6–85) |
| PNA (weeks) | 7.21 (0.1–815.9) | 0.3 (0.1–685.1) | 6.714 (0.143–818.6) | 90.4 (0.7–795) |
| Gestational age at birth (weeks) | 38.6 (22.86–41.86) | 37 (24–42.29) | 37 (23–41.43) | 40 (23–40) |
| PMA (weeks) | 41.9 (23.6–855.9) | 37.5 (24.1–725.1) | 41.6 (24.7–858.6) | 130 (24.7–835) |
| Female sex | 72 (41.4) | 28 (43.8) | 37 (51.4) | 32 (45.7) |
| ICU- or HDU-level care | 157 (90.2) | 56 (87.5) | 57 (79.2) | 63 (90) |
| Ventilation support or oxygen therapy | 121 (69.5) | 29 (45.3) | 39 (54.2) | 52 (74) |
| Renal impairment | 7 (4.0) | 2 (3.1) | 0 (0) | 2 (3) |
| Therapeutic hypothermia | 9 (5.1) | 2 (3.1) | 0 (0) | 0 (0) |
| Liver impairment | 2 (1.1) | 0 (0) | 1 (1.4) | 0 (0) |
| Baseline creatinine (μmol/L) | 33 (2–102) | 56 (14–105) | 37 (11–81) | 31 (6–95) |
| Urea (mmol/L) | 3.3 (0.8–12.4) | 3.1 (1.4–16.4) | 3.5 (0.6–17) | 3.4 (0.9–14.5) |
| Bilirubin (μmol/L) | 26 (2–250) | 118 (6–302) | 16.5 (2–255) | 6.5 (2–114) |
| AST (IU/L) | 37 (12–4153) | 37 (18–144) | 27.5 (11–166) | 25 (12–384) |
| ALT (IU/L) | 19 (3–2212) | 16 (5–191) | 17 (5–131) | 22 (5–342) |
| ALP (IU/L) | 359 (47–2010) | 249 (92–735) | 320 (23–1196) | 278 (2–1121) |
| Albumin (g/L) | 28 (17–47) | 30 (15–38) | 29 (16–47) | 27.5 (12–45) |
| Haematocrit | 0.34 (0.21–0.66) | 0.48 (0.25–0.74) | 0.35 (0.25–0.64) | 0.30 (0.21–0.49) |
| CRP (mg/L) | 18.5 (0.1–281) | 16.55 (0.2–172.3) | 6.85 (1–146.4) | 57 (0.4–388.7) |
| Median IV dose (mg/kg/dose) | 25.2 (9.7–112) | 25.4 (20.8–59.4) | 25.1 (12.4–100) | 80 (47–125) |
| Total sample number | 409 | 147 | 185 | 222 |
Continuous data are presented as median (range) and categorical data are presented with the number of subjects (% of total). ALP, alkaline phosphatase; CRP, C-reactive protein. Only those study participants contributing PK samples are included.
Table 5.
Indications for antibiotic treatment for study populations of the combined IV model
| Indication for antibiotic therapy | Co-amoxiclav and amoxicillin (n = 174) | Benzylpenicillin (n = 64) | Flucloxacillin (n = 72) | Piperacillin (n = 70) |
|---|---|---|---|---|
| Suspected or proven sepsis (including bacteraemia), n (%) | 81 (46.6) | 49 (76.6) | 34 (47.2) | 18 (21.7) |
| Surgical prophylaxis, n (%) | 38 (21.8) | 0 (0) | 11 (15.3) | 4 (4.8) |
| LRTI (including CAP), n (%) | 18 (10.3) | 0 (0) | 5 (6.9) | 20 (24.1) |
| Intra-abdominal infection (including NEC), n (%) | 12 (6.9) | 0 (0) | 0 (0) | 4 (4.8) |
| Medical prophylaxis, n (%) | 9 (5.2) | 12 (18.8) | 1 (1.4) | 7 (8.4) |
| Meningitis, n (%) | 7 (4) | 1 (1.6) | 1 (1.4) | 0 (0) |
| Urinary tract infection, n (%) | 3 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Hospital-acquired pneumonia (HAP), n (%) | 2 (1.1) | 2 (3.1) | 0 (0) | 4 (4.8) |
| Ventilator-associated HAP, n (%) | 0 (0) | 1 (1.6) | 0 (0) | 4 (4.8) |
| Aspiration pneumonia, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (2.4) |
| Pharyngitis, n (%) | 1 (0.6) | 1 (1.6) | 0 (0) | 1 (1.2) |
| Skin or soft tissue infection, n (%) | 1 (0.6) | 3 (4.7) | 19 (26.4) | 0 (0) |
| Septic arthritis or osteomyelitis, n (%) | 1 (0.6) | 2 (3.1) | 4 (5.6) | 0 (0) |
| Febrile neutropenia or neutropenic sepsis, n (%) | 1 (0.6) | 0 (0) | 1 (1.4) | 6 (7.2) |
| Endocarditis, n (%) | 0 (0) | 1 (1.6) | 2 (2.8) | 0 (0) |
| Congenital pneumonia, n (%) | 0 (0) | 2 (3.1) | 0 (0) | 0 (0) |
| Other, n (%) | 15 (8.6) | 2 (3.1) | 2 (2.8) | 13 (15.7) |
LRTI, lower respiratory tract infection; CAP, community-acquired pneumonia, NEC, necrotizing enterocolitis. Note some patients had more than one indication for antibiotic therapy recorded, hence the total number of indications is greater than the total number of subjects. Note the total number of patients across all penicillin groups is higher than the overall total n = 370 because 10 participants contributed samples for two different penicillins.
The concentration was BLQ in 19 samples (representing 2% of the total). A further two BLQ data points obtained from a single participant on benzylpenicillin were excluded as no quantifiable samples were obtained from this participant (and their inclusion prevented model minimization).
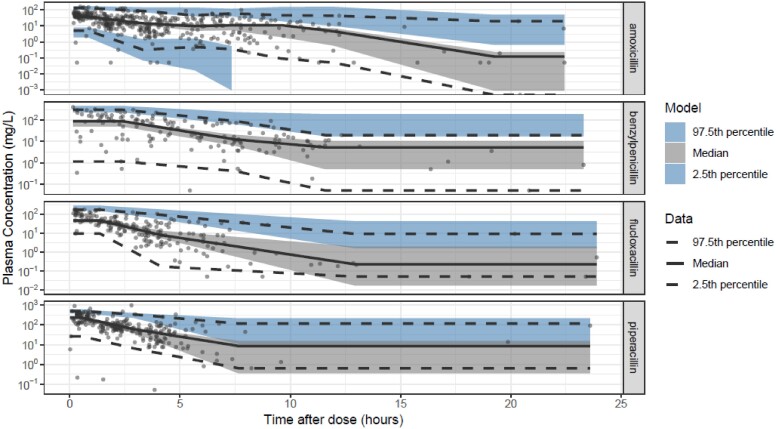
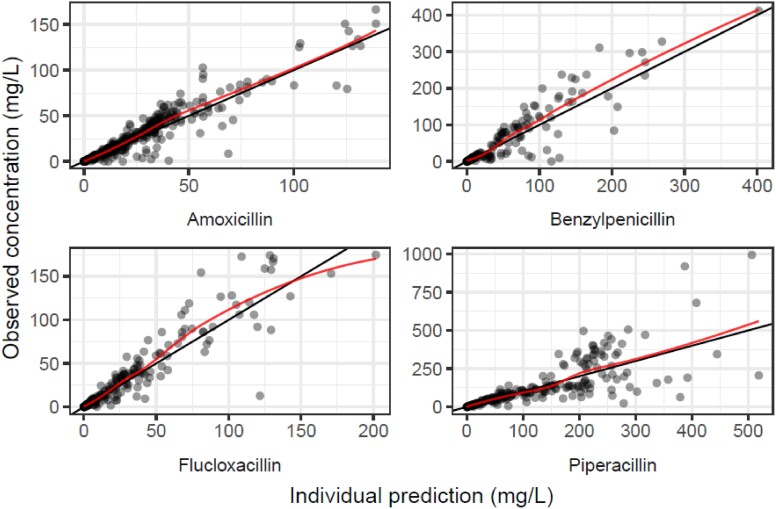
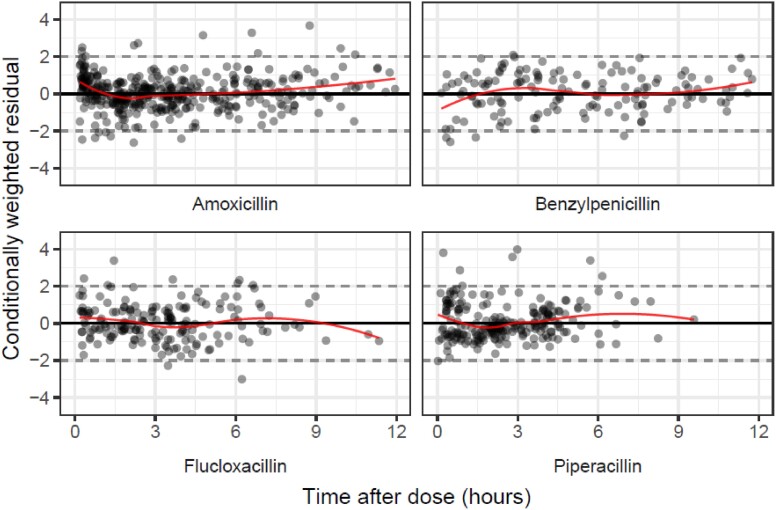
In the population PK analysis, inclusion of the PNA-driven covariate function (to reflect maturation of clearance after birth, independent of gestational age at birth) and the creatinine covariate function on clearance improved model fit over the base joint one-compartment model with a priori allometric weight scaling. The minimization and covariance step were successful. Table 6 summarizes the final parameter estimates, which were used for the PTA simulations. The GOF plots and VPCs are shown in Figures 1–4. Further GOF plots are included in the Supplementary data, together with the NONMEM code for the final model.
Table 6.
NAPPA combined IV model: final parameter estimates and bootstrap results
| Drug: | Amoxicillin | Benzylpenicillin | Flucloxacillin | Piperacillin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Point estimate (RSE) | S (%) | BS (95% CI) | Point estimate (RSE) | S (%) | BS (95% CI) | Point estimate (RSE) | S (%) | BS (95% CI) | Point estimate (RSE) | S (%) | BS (95% CI) |
| TVCL | 16.4 (7.1) | — | 16.5 (14.0–18.8) | 7.17 (13) | — | 7.25 (5.29–9.61) | 14.6 (8.4) | — | 14.7 (12.5–17.5) | 8.59 (7.9) | — | 8.57 (7.44–10.1) |
| TVV | 46.2 (4.8) | — | 46.2 (42.4–51.0) | 11.8 (10.5) | — | 11.8 (9.86–14.6) | 23.3 (8.5) | — | 23.7 (20.3–28.3) | 18.6 (8.7) | — | 18.6 (15.4–22.1) |
| Var IIV (1) | 0.167 (26.4) | 39.2 | 0.152 (0.085–0.278) | 0.249 (25.6) | 60.6 | 0.238 (0.119–0.373) | 0.237 (19.6) | 58.8 | 0.225 (0.136–0.321) | 0.225 (32.2) | 59 | 0.220 (0.084–0.374) |
| Var IIV (2) | 0.045 (57.3) | 70.3 | 0.043 (0.005–0.099) | — | — | — | 0.024 (137.4) | 85.6 | 0.033 (0.0005–0.178) | — | — | — |
| Var prop RE | 0.135 (16.4) | 16.6 | 0.134 (0.092–0.184) | 0.241 (15.5) | 12.3 | 0.234 (0.168–0.315) | 0.179 (15.7) | 13.5 | 0.170 (0.123–0.229) | 0.212 (15.4) | 8.8 | 0.209 (0.153–0.280) |
| Var add RE | — | — | — | 0.012 (163.1) | 12.3 | 0.011 (0.001–0.836) | — | — | — | — | — | — |
| Parameter | Point estimate (RSE, %) | BS (95% CI) | ||||||||||
| T50 | 42.6 (9.1) | 43.5 (35.6–50.7) | ||||||||||
| Hill | 2.68 (6.3) | 2.80 (2.16–3.34) | ||||||||||
| M | 0.516 (13.1) | 0.486 (0.340–0.659) | ||||||||||
| N | 0.020 (50.5) | 0.022 (0.011–0.176) | ||||||||||
| Creat on CL | −0.302 (27.7) | −0.301 (−0.471 to −0.136) | ||||||||||
S shrinkage (%); BS, bootstrap; TVCL, typical value of clearance in L/h/70 kg; TVV, typical value of volume in L/70 kg; T50, maturation half time (PMA in weeks); Hill, Hill coefficient; M, fraction of CL on day of birth; N, rate of maturation post birth; Creat on CL, creatinine covariate on clearance; Var, variance; IIV (1), variance of IIV on CL; IIV (2), variance of IIV on V; prop RE, proportional component of the residual error; add RE, additive component of the residual error. All bootstrap results are presented as median (2.5th–97.5th percentiles).
Figure 1.
GOF plot for combined IV model, stratified by drug: observed concentrations plotted against population predictions for amoxicillin, benzylpenicillin, flucloxacillin and piperacillin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 4.
VPC of combined IV model, showing observed concentrations (dots) in mg/L and prediction intervals (shaded areas) against time after dose in hours. The solid black line is the 50th percentile and the dashed lines are the 2.5th and 97.5th percentiles of the observed data, respectively. Each shaded area is a non-parametric 95% CI for the corresponding predicted concentrations. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 2.
GOF plots for combined IV model, stratified by drug: observed concentrations plotted against individual predictions for amoxicillin, benzylpenicillin, flucloxacillin and piperacillin. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
GOF plots for combined IV model, stratified by drug: conditionally weighted residual errors plotted against time after dose (for 0–12 h following the IV dose) for amoxicillin, benzylpenicillin, flucloxacillin and piperacillin. Additional plot available in the Supplementary data. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Model-based simulations
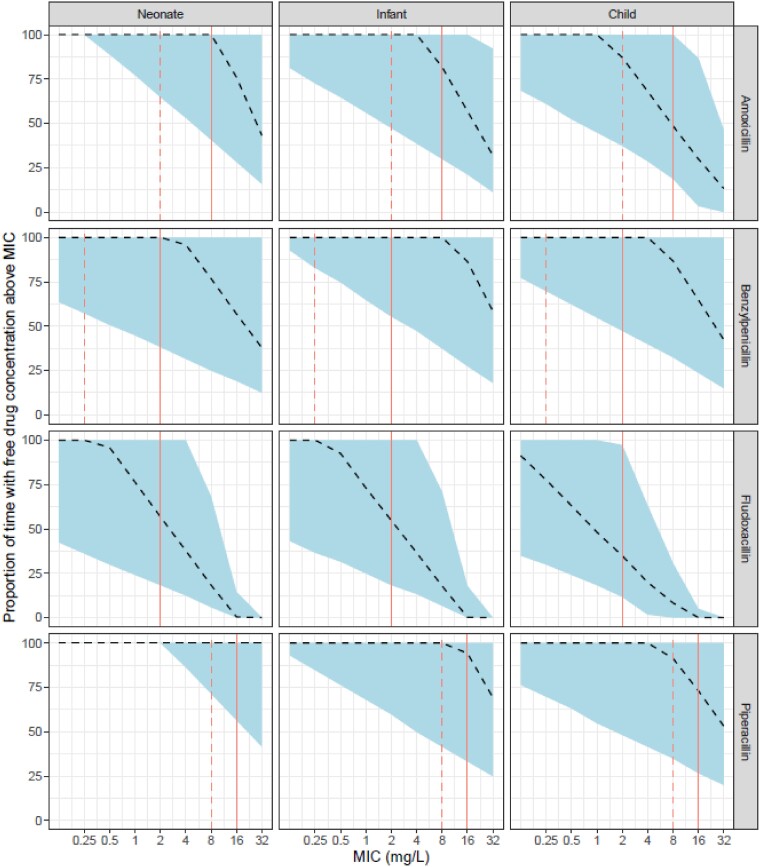
Figure 5 shows the results of the model-based simulations for each NAPPA penicillin, which demonstrates the %fT>MIC after the first 24 h of therapy using BNFC high-dose regimens (Table 1). Further simulation results, including those using WHO dosing recommendations, are included for comparison in Figures S1–S6 and Tables S4–S6.27
Figure 5.
Results of the model-based simulations testing the PTA for the BNFC high-dose regimens for all NAPPA penicillins. The panels each show the proportion of time above a range of MICs over the first 24 h of therapy for simulated patients (n = 10 000). The dashed line represents the simulated median and the shaded area represents 95% of simulated patients’ fT>MIC. The solid vertical line indicates the EUCAST breakpoint MIC R (resistant). The dashed vertical line indicates the EUCAST MIC S (susceptible). Further simulation results are included in the Supplementary data. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Analysis of PK data from participants on oral amoxicillin and flucloxacillin
Sparse oral PK data were available from participants receiving oral flucloxacillin (n = 9) and amoxicillin (including co-amoxiclav) (n = 7). The demographics and indications are summarized in Tables S7 and S8. Table 7 shows the final parameter estimates. The GOF plots are shown in Figures S7–S14. The NONMEM code for these models is also included at the end of the Supplementary data.
Table 7.
Parameter estimates for separate oral flucloxacillin and amoxicillin PK models
| Parameter | Parameter estimates | |
|---|---|---|
| Flucloxacillin model | Amoxicillin model | |
| TVCL | 14.6 (fixed) | 16.4 (fixed) |
| TVV | 23.3 (fixed) | 46.2 (fixed) |
| T50 | 42.6 (fixed) | 42.6 (fixed) |
| Hill | 2.68 (fixed) | 2.68 (fixed) |
| M | 0.516 (fixed) | 0.516 (fixed) |
| N | 0.0202 (fixed) | 0.0202 (fixed) |
| Creat on CL | −0.302 (fixed) | −0.302 (fixed) |
| TVKA | 1.16 (RSE 28.4%) | 1.3 (RSE 52.4%) |
| F1 | 0.627 (RSE 8.1%) | 0.587(RSE 15.3%) |
| Var IIV (1) | 0.237 (fixed) (s: 8.1%) | 0.167 (fixed) (s: 11.8%) |
| Var IIV (2) | 0.024 (fixed) (s: 67.7%) | 0.045 (fixed) (s: 57.3%) |
| Var prop RE | 0.196 (RSE 14.5%) (s: 12.7%) | 0.141(RSE 14.2%)(s: 16.3%) |
TVCL, typical value of clearance in L/h/70 kg; TVV, typical value of volume in L/70 kg; TVQ, typical value of intercompartmental clearance in L/h/70 kg; TVV2, typical value of peripheral volume in L/70 kg; T50, maturation half time (PMA in weeks); Hill, Hill coefficient; M, fraction of CL on day of birth; N, rate of maturation post birth; Creat on CL, creatinine covariate on clearance; TVKA, typical value for the first-order absorption rate constant in h−1; F1, bioavailability term; Var, variance; Var IIV (1), variance of IIV on CL; Var IIV (2), variance of IIV on V; s, shrinkage; prop RE, proportional residual error.
Discussion
The NAPPA study represents, to our knowledge, the largest prospective combined penicillin PK study undertaken in children and neonates to date. The method demonstrated that recruitment to a paediatric clinical PK study is feasible within routine NHS care. Simultaneous modelling of the PK data from amoxicillin, benzylpenicillin, flucloxacillin and piperacillin together enabled use of a shared maturation function, which was thus informed by the combined data from these four different penicillins including neonatal, paediatric, and adolescent participants. This approach can help us to better understand that maturational component of developmental PK, capturing the physiological and pharmacological changes that evolve from birth to adulthood. However, it is important to note that joint PK modelling approaches combining data from different drugs should only be used for drugs that share similar clearance mechanisms.
Interpreting paediatric population PK parameter estimates necessitates comparison with the pre-existing literature, and the summaries of product characteristics (SPCs). Tables S9–S12 summarize comparisons of our results with previously published paediatric population PK studies. We adopted a standardized approach to scaling clearance, employing a standardized base model parametrization using allometric weight0.75 and a sigmoidal maturation function, in order to facilitate model comparison and future extrapolation.34,35 However, few previous studies adopted this method, and the parameterization of previous paediatric models varied significantly, making direct comparison difficult.5
Nevertheless, it remains necessary to explore potential explanations for differences between our results and previously published PK parameter estimates. We focused on comparing the NAPPA study estimates of clearance and volume of distribution (Vd) with those reported in the ABDose study, presented in Table S13.36 The ABDose study recruited ICU patients of all ages. The majority of NAPPA participants were receiving ICU/HDU care, and the methods and standard operating procedures were consistent between the studies.
The estimate of clearance was very similar between NAPPA and ABDose for amoxicillin, whereas the NAPPA results were lower for piperacillin, and—much more markedly so—for benzylpenicillin. In contrast, the NAPPA estimate of flucloxacillin clearance was almost 2-fold higher. Depending on the study population for each drug under comparison, distinct factors are likely responsible for these different results. For example, the NAPPA benzylpenicillin study population was principally neonates (median PNA 2 days), when a lower clearance is expected in relation to the participants’ age and renal maturation stage. For flucloxacillin, the majority of ABDose participants were critically ill adults [52/59 PK samples (88%)], in whom a combination of age-related decline in renal function, and acute kidney injury (AKI) will have contributed to the lower clearance. Where one study population has an apparently increased clearance estimate, another probable contributing factor in ICU settings is augmented renal clearance (ARC).37,38 These differences demonstrate that, even with the use of standardized scaling, the study population clinical context and demographics will contribute to different PK parameter estimates for clearance. Similarly, the SPC-reported clearance values for amoxicillin and piperacillin were higher than parameter estimates from both studies, reflecting their estimation from studies in healthy adult volunteers.
With respect to the apparent Vd values, the estimate was higher for ABDose for benzylpenicillin, similar for piperacillin, and higher for NAPPA for flucloxacillin and—much more strikingly so (61% higher)—for amoxicillin. Where elevated Vd estimates are identified, this is also likely secondary to several factors, including (in neonates) an increased fraction of unbound drug and relatively increased total body water,39,40 and hypoalbuminaemia within the critically ill patient population.41,42Vd can also increase during sepsis, and secondary to pathophysiological changes (increased capillary permeability, interstitial oedema and altered protein binding), and iatrogenic factors.43
PTA and MIC selection
The PTA simulations (Figure 5) for BNFC high-dose regimens demonstrate mixed performances for these four penicillins. Combining the results for all age groups, and focusing specifically on the EUCAST non-species-related PKPD breakpoints (Table 3), the benzylpenicillin results are reassuring, with a median of 100% fT>MIC in the first 24 h. The BNFC low-dose regimen PTAs (Figure S2) are supportive of the NICE treatment guidelines for early-onset neonatal sepsis. Similarly, the piperacillin results are reassuring, with a median of 100% fT>MIC in the first 24 h. For amoxicillin, the median across all age groups was 79% fT>MIC. The worst PTA results were for flucloxacillin, with a median of 48% fT>MIC across all age groups. This is largely a consequence of the assumption of 95% protein binding, discussed further below.
For PTA evaluation, MIC selection poses various challenges. Firstly, antibiotics prescribed for children are usually started empirically, in the absence of microbiological evidence.44 A broad-spectrum therapeutic beginning is followed by de-escalation to narrow therapy agents when possible.45 There are, however, some clinical contexts where selection of a lower MIC may be acceptable. For example, the use of the non-species-related breakpoint for benzylpenicillin will negatively influence the PTA interpretation, which improves if focusing instead on the MIC of 0.12 mg/L for Group B Streptococcus, which is most relevant for neonates. Consideration of different MICs should be determined by the clinical scenario. For example, the amoxicillin breakpoint for Streptococcus pneumoniae, a leading cause of sepsis in children, is 1.0 mg/L, whereas we have specified the median PTA above based on the EUCAST non-species-related PKPD breakpoints of 8.0 mg/L. For detailed consideration of the suitability of specific pathogen–penicillin combinations, the Supplementary data includes a tabular version of the PTA results for all simulated age groups combined. For example, when considering amoxicillin for the treatment of resistant S. pneumoniae, this shows a median PTA of 100% fT>MIC of 1 mg/L, with 95% patients achieving >63% fT>MIC.
Protein binding
An important factor contributing to flucloxacillin PK variability is its variable protein binding, which is influenced by many dynamic variables, including body temperature, pH and hypoalbuminaemia. In our study, total flucloxacillin concentrations were measured in plasma, and the simulations assumed protein binding of 95%.30 However, as a highly protein-bound drug, small absolute differences in protein binding can result in significant relative differences in the pharmacologically active unbound concentration.46 Marked protein binding variability has been reported in neonates [34.3%–89.7% (mean 74.5%)]47 and adults.48–50 A recent study in 33 adult ICU patients found that the unbound fraction of flucloxacillin ranged from 7.0% to 71.7%.48 Previously, Wong et al.49 identified significant differences between measured and predicted concentrations of unbound flucloxacillin in critically ill adults, and identified a non-linear correlation. Variable, concentration-dependent protein binding of flucloxacillin has also been reported in non-critically ill adult patients,51 while another study demonstrated poor, biased performance of model-based predictions of unbound concentrations.50 For our interpretation, we have assumed what might be viewed as a worst-case scenario with 95% protein binding (as per the SPC) and it is possible that particularly neonatal participants may have markedly reduced protein binding. This would lead to marked improvements in the PTA curves. Given the literature evidencing extensive interindividual variability in protein binding, this highlights the importance of exploring the feasibility and cost-effectiveness of measuring unbound concentrations in future flucloxacillin PKPD studies. Standardization of protein binding research methods will help to elucidate the clinical and laboratory determinants of variability,52 since methodological differences also impact on the microbiology and PD.53
PKPD variability and individualized dosing
The significant variability in β-lactam PKPD, particularly in critically ill patients, as also demonstrated in this study, has previously been presented as evidence supporting β-lactam therapeutic drug monitoring (TDM).54,55 Some experts also recommend this approach in paediatrics.55 However, it remains essential to clarify clinical utility, cost-effectiveness and feasibility in children. A correlation between PTA and clinical outcomes has been identified in some adult research, such as the DALI study,15 reinforcing interest in β-lactam TDM in adult ICU patients,56,57 which is now formally advocated.58 Demonstrating an equivalent correlation in children will prove difficult because of small sample sizes in paediatric/neonatal studies. It can be argued that it is scientifically acceptable to assume that this correlation should also be clinically relevant in children, given the shared underlying microbiological and pharmacological principles of antimicrobial therapy. The prevalence of ARC in paediatric ICU gives added justification to pursue this further.37
PK of oral flucloxacillin
The results in Table 7 represent, to our knowledge, the first published paediatric oral flucloxacillin population PK model. Our estimate for F of 62.7% was similar to the 54.4% ± 18.8% (in seven elderly patients) reported by Gath et al.,59 and to an earlier study reporting 55.4%±9.2% and 48.%±15.5% (for 24 adults given a 250 and 500 mg capsule, respectively).60 In contrast, Herngren et al.61 estimated F for nine neonates to be 31.6% (range 21.3% to 45.0%). These studies did not publish the estimated ka values for flucloxacillin so we could not compare our estimate. However, Gardiner et al.62 estimated ka for the free concentrations of flucloxacillin to be 0.74 h−1 (95% CI 0.15–3.8) and 3.6 h−1 (95% CI 0.71–18.2), in the fed and fasting states, respectively, in 12 adult volunteers, and both these 95% CIs overlap with our group estimate of 1.16 h−1 (relative standard error, RSE 28.4%). Furthermore, although fasting was associated with increased drug exposure, the PTAs were largely equivalent, suggesting food is unlikely to have a clinically significant effect on flucloxacillin efficacy.62 Similar studies in children could help to ascertain whether current fasting recommendations can be relaxed.
PK of oral amoxicillin
Our estimate of F for amoxicillin was 58.7% (RSE 15.3%), slightly lower than the 70% reported in the SPC.28 As oral amoxicillin therapy is potentially of interest for the treatment of suspected neonatal sepsis in resource-limited settings,63 further neonatal oral PK studies will be valuable. In addition, the PediCAP PK substudy results,64 investigating amoxicillin and co-amoxiclav PK in pneumonia within a randomized controlled trial, will be of great interest.
Study limitations
Total antibiotic concentrations were measured in plasma; however, measurement of unbound concentrations is recommended for future studies where feasible.65 It was not possible to measure antibiotic concentrations at other sites (e.g. in CSF), which could be investigated in studies focusing on specific clinical infection syndromes. For the oral PK analysis, the sample sizes were small; larger studies could improve parameter estimate precision.
The study included two β-lactam/BLI combinations but BLI concentrations were not measured. Amoxicillin and co-amoxiclav PK data were merged for the final analysis, since amoxicillin PK is independent of the presence of clavulanic acid.66 Future paediatric BLI PKPD studies are warranted.67,68
Conclusions
We have reported, to our knowledge, the largest prospective combined paediatric penicillin PK study undertaken to date, and the first paediatric oral flucloxacillin PK model. The PTA results demonstrated reassuring performance of BNFC dosing regimens for amoxicillin, benzylpenicillin and piperacillin but the flucloxacillin PTA was suboptimal. Further research is needed to investigate the clinical relevance of protein binding variability, particularly for flucloxacillin, and the potential clinical utility of β-lactam TDM in children.
Supplementary Material
Acknowledgements
The population pharmacokinetics of the individual penicillins in the NAPPA study were previously analysed separately and these results were presented within C.I.S.B.’s PhD thesis (ISNI: 0000 0005 0665 2680); an adapted summary of these previous results is included in the Supplementary data for information. The final results described in this paper were presented at the PKUK Conference in November 2022.
We and the consortium members are grateful to all the NAPPA study participants and their families. NAPPA was an adopted study on the UK NIHR Clinical Research Network Portfolio; the consortium extends thanks to the NIHR Children and Young People Specialty network, particularly to the healthcare and clinical research staff involved in study delivery at all nine participating NHS hospitals: St George’s Hospital, St George’s University Hospitals NHS Foundation Trust, London; Liverpool Women’s Hospital Neonatal Unit, Liverpool Women’s NHS Foundation Trust, Liverpool; Alder Hey Children’s Hospital, Alder Hey Children’s NHS Foundation Trust, Liverpool; John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, Oxford; Great Ormond Street Hospital for Children NHS Foundation Trust, London; Queen Alexandra Hospital, Portsmouth Hospitals University NHS Trust, Portsmouth; University Hospital Coventry, University Hospitals Coventry and Warwickshire NHS Trust; Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust, Truro; and Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust.
Contributor Information
Charlotte I S Barker, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Paediatric Infectious Diseases Department, St George’s University Hospitals NHS Foundation Trust, London, UK; Infection, Immunity and Inflammation Research & Teaching Department, UCL Great Ormond Street Institute of Child Health, University College London, London, UK; Department of Medical & Molecular Genetics, King’s College London, London, UK.
Karin Kipper, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Analytical Services International, St George’s, University of London, London, UK; Analytical Chemistry Department, Epilepsy Society, Chesham Lane, Chalfont St Peter, Buckinghamshire, UK; Institute of Chemistry, University of Tartu, Tartu, Estonia.
Dagan O Lonsdale, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Paediatric Infectious Diseases Department, St George’s University Hospitals NHS Foundation Trust, London, UK.
Kirstie Wright, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK.
Georgina Thompson, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK.
Min Kim, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Infection, Immunity and Inflammation Research & Teaching Department, UCL Great Ormond Street Institute of Child Health, University College London, London, UK.
Mark A Turner, Department of Women’s and Children’s Health, University of Liverpool, Liverpool Health Partners, Liverpool, UK.
Atholl Johnston, Analytical Services International, St George’s, University of London, London, UK; Clinical Pharmacology, William Harvey Research Institute, Queen Mary University of London, London, UK.
Mike Sharland, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Paediatric Infectious Diseases Department, St George’s University Hospitals NHS Foundation Trust, London, UK.
Joseph F Standing, Centre for Neonatal and Paediatric Infection, Level 2 Jenner Wing, Institute for Infection and Immunity, St George’s, University of London SW17 0RE, London, UK; Infection, Immunity and Inflammation Research & Teaching Department, UCL Great Ormond Street Institute of Child Health, University College London, London, UK; Pharmacy Department, Great Ormond Street Hospital for Children, NHS Foundation Trust, London, UK.
NAPPA study consortium members
Tatiana Múnera Huertas, Hana Tabusa, Isabelle Hubbard, Suzie Wright, Jennifer Stuart, Amelia Floresca, Andrew Collinson, Andrew Riordan, Barbara Bromage, Bronagh Howell, Christopher Knight, Debs Heard, Emma Harper, Gita Modgil, Jane Lovatt, Jennie Godwin, Joanne Windrow, Karen Harvey, Kerri McGowan, Kirsty O’Brien, Louise Newbury, Louise Willis, Mark Anthony, Michele Voysey, Michelle Pople, Nasreen Aziz, Nicola Howell, Nicola Thorne, Nigel Klein, Patrick McGowan, Piper Griffin, Prakash Satodia, Rebecca Beckley, Rooba Kauppayamootoo, Sara Davis, Sarah Siner, Sharon Northey, Shaun Larkin, Shelly Segal, Sheula Barlow, Susan Dale, Tamsyn Crane, Tim Scorrer and Zoe Young.
Funding
The research leading to these results received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 261060 (Global Research in Paediatrics—GRiP Network of Excellence). This study was also supported by the National Institute for Health Research (NIHR) Clinical Research Network (UKCRN ID 14960). C.I.S.B. was funded by the GRiP Network, with support from the Penta Foundation, and by the NIHR (ACF-2016-18-016 and ACF-2019-17-004). Support at the institutional level was received from the NIHR Biomedical Research Centres based at Great Ormond Street Hospital (C.I.S.B. and J.F.S.) and at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London (C.I.S.B.). J.F.S. received funding from UK Medical Research Council Fellowships (grants G1002305 and M008665). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Transparency declarations
All authors declared no competing interests for this work.
Author contributions
C.I.S.B., K.K., M.A.T., A.J., M.S. and J.F.S. were involved in the conception and design of the study. This group, together with K.W., G.T. and M.K., also contributed to study delivery and data acquisition. K.K. analysed the plasma samples (quantifying the antibiotic concentrations). C.I.S.B. coordinated study delivery, led the PK data analysis and interpretation, and drafted the manuscript, with support from D.O.L., M.A.T., A.J., M.S. and J.F.S.. D.O.L. undertook the model-based simulations, with support from C.I.S.B., M.S. and J.F.S. All authors critically reviewed the manuscript and approved the final version.
Supplementary data
Figures S1 to S18, Tables S1 to S13, Supplementary information and Supplementary code are available as Supplementary data at JAC Online.
References
- 1. Fairbrother RW, Daber KS. Oral penicillin. Lancet 1954; 266: 858–60. 10.1016/S0140-6736(54)91421-X [DOI] [PubMed] [Google Scholar]
- 2. Versporten A, Bielicki J, Drapier Net al. The worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 2016; 71: 1106–17. 10.1093/jac/dkv418 [DOI] [PubMed] [Google Scholar]
- 3. United Nations Inter-Agency Group for Child Mortality Estimation (UN IGME) . Levels and trends in child mortality: UN IGME Report 2022.https://data.unicef.org/resources/levels-and-trends-in-child-mortality/
- 4. Perin J, Mulick A, Yeung Det al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health 2022; 6: 106–15. 10.1016/S2352-4642(21)00311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuchs A, Li G, van den Anker JNet al. Optimising β -lactam dosing in neonates: a review of pharmacokinetics, drug exposure and pathogens. Curr Pharm Des 2017; 23: 5805–38. 10.2174/1381612823666170925162143 [DOI] [PubMed] [Google Scholar]
- 6. Gastine S, Hsia Y, Clements Met al. Variation in target attainment of beta-lactam antibiotic dosing between international pediatric formularies. Clin Pharmacol Ther 2021; 109: 958–70. 10.1002/cpt.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barker CI, Standing JF, Turner MAet al. Antibiotic dosing in children in Europe: can we grade the evidence from pharmacokinetic/pharmacodynamic studies—and when is enough data enough? Curr Opin Infect Dis 2012; 25: 235–42. 10.1097/QCO.0b013e328353105c [DOI] [PubMed] [Google Scholar]
- 8. Eagle H, Fleischman R, Levy M. “Continuous” vs. “discontinuous” therapy with penicillin; the effect of the interval between injections on therapeutic efficacy. N Engl J Med 1953; 248: 481–8. 10.1056/NEJM195303192481201 [DOI] [PubMed] [Google Scholar]
- 9. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–10; quiz 1-2. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 10. Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol 2004; 2: 289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 11. Lodise TP, Lomaestro BM, Drusano GLet al. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on β-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006; 26: 1320–32. 10.1592/phco.26.9.1320 [DOI] [PubMed] [Google Scholar]
- 12. Ja R, Norris R, Paterson DLet al. Therapeutic drug monitoring of antimicrobials. Br J Clin Pharmacol 2012; 73: 27–36. 10.1111/j.1365-2125.2011.04080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007; 44: 357–63. 10.1086/510590 [DOI] [PubMed] [Google Scholar]
- 14. Roberts JA, Lipman J, Blot Set al. Better outcomes through continuous infusion of time-dependent antibiotics to critically ill patients? Curr Opin Crit Care 2008; 14: 390–6. 10.1097/MCC.0b013e3283021b3a [DOI] [PubMed] [Google Scholar]
- 15. Roberts JA, Paul SK, Akova Met al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58: 1072–83. 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
- 16. Roberts JA, Abdul-Aziz MH, Davis JSet al. Continuous versus intermittent β-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 2016; 194: 681–91. 10.1164/rccm.201601-0024OC [DOI] [PubMed] [Google Scholar]
- 17. Rizk NA, Kanafani ZA, Tabaja HZet al. Extended infusion of beta-lactam antibiotics: optimizing therapy in critically-ill patients in the era of antimicrobial resistance. Expert Rev Anti Infect Ther 2017; 15: 645–52. 10.1080/14787210.2017.1348894 [DOI] [PubMed] [Google Scholar]
- 18. Masich AM, Heavner MS, Gonzales JPet al. Pharmacokinetic/pharmacodynamic considerations of beta-lactam antibiotics in adult critically ill patients. Curr Infect Dis Rep 2018; 20: 9. 10.1007/s11908-018-0613-1 [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke Ret al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paediatric Formulary Committee . British National Formulary for Children (BNFC).https://bnfc.nice.org.uk/
- 21. Barker CI, Sharland M, Turner MAet al. Synthesising pragmatic and optimal design: NAPPA - a paediatric penicillin population pharmacokinetic study. PAGE 23, Alicante, Spain, June 2014. Abstract 3235. https://www.page-meeting.org/default.asp?abstract=3235
- 22. Kipper K, Barker CIS, Standing JFet al. Development of a novel multi-penicillin assay and assessment of the impact of analyte degradation: lessons for scavenged sampling in antimicrobial pharmacokinetic study design. Antimicrob Agents Chemother 2017; 62: e01540-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 24. Rhodin MM, Anderson BJ, Peters AMet al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009; 24: 67–76. 10.1007/s00467-008-0997-5 [DOI] [PubMed] [Google Scholar]
- 25. Kane Z, Gastine S, Obiero Cet al. IV and oral fosfomycin pharmacokinetics in neonates with suspected clinical sepsis. J Antimicrob Chemother 2021; 76: 1855–64. 10.1093/jac/dkab083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ceriotti F, Boyd JC, Klein Get al. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 2008; 54: 559–66. 10.1373/clinchem.2007.099648 [DOI] [PubMed] [Google Scholar]
- 27. WHO . The WHO Essential Medicines List Antibiotic Book: improving antibiotic AWaReness.2021. https://www.who.int/publications/m/item/the-who-essential-medicines-list-antibiotic-book-improving-antibiotic-awareness
- 28. Electronic Medicines Compendium . Amoxicillin 500 mg Capsules. Summary of Product Characteristics. 2017. https://www.medicines.org.uk/emc/product/526/smpc
- 29. Electronic Medicines Compendium . Benzylpenicillin sodium 600 mg Powder for Injection. Summary of Product Characteristics.2016.https://www.medicines.org.uk/emc/product/3828/smpc
- 30. Electronic Medicines Compendium . Flucloxacillin 1 g Powder for Solution for Injection or Infusion. Summary of Product Characteristics.2018. https://www.medicines.org.uk/emc/product/2239/smpc
- 31. Electronic Medicines Compendium . Tazocin 4 g / 0.5 g Powder for Solution for Infusion. Summary of Product Characteristics. 2021. https://www.medicines.org.uk/emc/product/1267/smpc
- 32. EUCAST . Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0, valid from 1 January 2022.https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf
- 33. Dien Bard J, Hindler JA, Gold HSet al. Rationale for eliminating Staphylococcus breakpoints for β-lactam agents other than penicillin, oxacillin or cefoxitin, and ceftaroline. Clin Infect Dis 2014; 58: 1287–96. 10.1093/cid/ciu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Germovsek E, Barker CI, Sharland Met al. Scaling clearance in paediatric pharmacokinetics: all models are wrong, which are useful? Br J Clin Pharmacol 2017; 83: 777–90. 10.1111/bcp.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Germovsek E, Barker CIS, Sharland Met al. Pharmacokinetic-pharmacodynamic modeling in pediatric drug development, and the importance of standardized scaling of clearance. Clin Pharmacokinet 2019; 58: 39–52. 10.1007/s40262-018-0659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lonsdale DO, Kipper K, Baker EHet al. β-Lactam antimicrobial pharmacokinetics and target attainment in critically ill patients aged 1 day to 90 years: the ABDose study. J Antimicrob Chemother 2020; 75: 3625–34. 10.1093/jac/dkaa363 [DOI] [PubMed] [Google Scholar]
- 37. Rhoney DH, Metzger SA, Nelson NR. Scoping review of augmented renal clearance in critically ill pediatric patients. Pharmacotherapy 2021; 41: 851–63. 10.1002/phar.2617 [DOI] [PubMed] [Google Scholar]
- 38. Dhont E, Van Der Heggen T, De Jaeger Aet al. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 2020; 35: 25–39. 10.1007/s00467-018-4120-2 [DOI] [PubMed] [Google Scholar]
- 39. Kearns GL, Abdel-Rahman SM, Alander SWet al. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349: 1157–67. 10.1056/NEJMra035092 [DOI] [PubMed] [Google Scholar]
- 40. Ehrnebo M, Agurell S, Jalling Bet al. Age differences in drug binding by plasma proteins: studies on human foetuses, neonates and adults. Eur J Clin Pharmacol 1971; 3: 189–93. 10.1007/BF00565004 [DOI] [PubMed] [Google Scholar]
- 41. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet 2013; 52: 1–8. 10.1007/s40262-012-0018-5 [DOI] [PubMed] [Google Scholar]
- 42. Ulldemolins M, Rello J. The relevance of drug volume of distribution in antibiotic dosing. Curr Pharm Biotechnol 2011; 12: 1996–2001. 10.2174/138920111798808365 [DOI] [PubMed] [Google Scholar]
- 43. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77: 3–11. 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 44. Bielicki JA, Cromwell DA, Sharland M. Fifteen-minute consultation: the complexities of empirical antibiotic selection for serious bacterial infections—a practical approach. Arch Dis Child Educ Pract Ed 2017; 102: 117–23. 10.1136/archdischild-2016-310527 [DOI] [PubMed] [Google Scholar]
- 45. Ashiru-Oredope D, Sharland M, Charani Eet al. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: Start Smart—Then Focus. J Antimicrob Chemother 2012; 67Suppl 1: i51–63. 10.1093/jac/dks202 [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez D, Schmidt S, Derendorf H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev 2013; 26: 274–88. 10.1128/CMR.00092-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pullen J, Stolk LM, Degraeuwe PLet al. Protein binding of flucloxacillin in neonates. Ther Drug Monit 2007; 29: 279–83. 10.1097/FTD.0b013e318063e30f [DOI] [PubMed] [Google Scholar]
- 48. Wallenburg E, Ter Heine R, de Lange DWet al. High unbound flucloxacillin fraction in critically ill patients. J Antimicrob Chemother 2021; 76: 3220–8. 10.1093/jac/dkab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong G, Briscoe S, Adnan Set al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 2013; 57: 6165–70. 10.1128/AAC.00951-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chin PKL, Drennan PG, Gardiner SJet al. Total flucloxacillin plasma concentrations poorly reflect unbound concentrations in hospitalized patients with Staphylococcus aureus bacteraemia. Br J Clin Pharmacol 2018; 84: 2311–6. 10.1111/bcp.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilkes S, van Berlo I, Ten Oever Jet al. Population pharmacokinetic modelling of total and unbound flucloxacillin in non-critically ill patients to devise a rational continuous dosing regimen. Int J Antimicrob Agents 2019; 53: 310–7. 10.1016/j.ijantimicag.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 52. Dalhoff A. Seventy-five years of research on protein binding. Antimicrob Agents Chemother 2018; 62: e01663-17. 10.1128/AAC.01663-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dalhoff A, Schubert S. The impact of protein binding on antibacterial activities of antibiotics is more than predicted by considering its numerical value alone: impact of preparative and incubation methods on different pharmacodynamic endpoints of β-lactams, macrolides, or fluoroquinolones against Gram-positive and Gram-negative bacteria-Part I. J Clin Infect Dis Pract 2016; 4: 110. 10.4172/2476-213X.1000110 [DOI] [Google Scholar]
- 54. Roberts JA, Abdul-Aziz MH, Lipman Jet al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14: 498–509. 10.1016/S1473-3099(14)70036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tu Q, Cotta M, Raman Set al. Individualized precision dosing approaches to optimize antimicrobial therapy in pediatric populations. Expert Rev Clin Pharmacol 2021; 14: 1383–99. 10.1080/17512433.2021.1961578 [DOI] [PubMed] [Google Scholar]
- 56. Fratoni AJ, Nicolau DP, Kuti JL. A guide to therapeutic drug monitoring of beta-lactam antibiotics. Pharmacotherapy 2021; 41: 220–33. 10.1002/phar.2505 [DOI] [PubMed] [Google Scholar]
- 57. Guilhaumou R, Benaboud S, Bennis Yet al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—guidelines from the French society of pharmacology and therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit Care 2019; 23: 104. 10.1186/s13054-019-2378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdul-Aziz MH, Alffenaar J-WC, Bassetti Met al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 2020; 46: 1127–53. 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gath J, Charles B, Sampson Jet al. Pharmacokinetics and bioavailability of flucloxacillin in elderly hospitalized patients. J Clin Pharmacol 1995; 35: 31–6. 10.1002/j.1552-4604.1995.tb04742.x [DOI] [PubMed] [Google Scholar]
- 60. GSK Clinical Studies Register . Bioavailability of Flucloxacillin Capsules (250 and 500 mg). GSK Study ID: 103811. NCT00358371. EudraCT number 2004-004493-81.https://www.gsk-studyregister.com/en/trial-details/?id=103811
- 61. Herngren L, Ehrnebo M, Broberger U. Pharmacokinetics of free and total flucloxacillin in newborn infants. Eur J Clin Pharmacol 1987; 32: 403–9. 10.1007/BF00543977 [DOI] [PubMed] [Google Scholar]
- 62. Gardiner SJ, Drennan PG, Begg Ret al. In healthy volunteers, taking flucloxacillin with food does not compromise effective plasma concentrations in most circumstances. PLoS One 2018; 13: e0199370. 10.1371/journal.pone.0199370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mir F, Pearce RE, Baig-Ansari Net al. Serum amoxicillin levels in young infants (0–59 days) with sepsis treated with oral amoxicillin. Arch Dis Child 2020; 105: 1208–14. 10.1136/archdischild-2019-317342 [DOI] [PubMed] [Google Scholar]
- 64. MRC Clinical Trials Unit . PediCAP. https://mrcctu.ucl.ac.uk/studies/all-studies/p/pedicap/.
- 65. Wong G, Briscoe S, McWhinney Bet al. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother 2018; 73: 3087–94. 10.1093/jac/dky314 [DOI] [PubMed] [Google Scholar]
- 66. Adam D, de Visser I, Koeppe P. Pharmacokinetics of amoxicillin and clavulanic acid administered alone and in combination. Antimicrob Agents Chemother 1982; 22: 353–7. 10.1128/AAC.22.3.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Monogue ML, Nicolau DP. Pharmacokinetics-pharmacodynamics of β-lactamase inhibitors: are we missing the target? Expert Rev Anti Infect Ther 2019; 17: 571–82. 10.1080/14787210.2019.1647781 [DOI] [PubMed] [Google Scholar]
- 68. Keij FM, Tramper-Stranders GA, Koch BCPet al. Pharmacokinetics of clavulanic acid in the pediatric population: a systematic literature review. Clin Pharmacokinet 2022; 61: 637–53. 10.1007/s40262-022-01116-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.