Abstract
Rapid and ongoing climate change increases global temperature, impacts feeding, and reproduction in insects. The olfaction plays an important underlying role in these behaviors in most insect species. Here, we investigated how changing temperatures affect odor detection and ensuing behavior in three drosophilid flies: Drosophila novamexicana, D. virilis and D. ezoana, species adapted to life in desert, global, and subarctic climates, respectively. Using a series of thermal preference assays, we confirmed that the three species indeed exhibit distinct temperature preferences. Next, using single sensillum recording technique, we classified olfactory sensory neurons (OSNs) present in basiconic sensilla on the antenna of the three species and thereby identified ligands for each OSN type. In a series of trap assays we proceeded to establish the behavioral valence of the best ligands and chose guaiacol, methyl salicylate and isopropyl benzoate as representatives of a repellent, attractant and neutral odor. Next, we assessed the behavioral valence of these three odors in all three species across a thermal range (10-35 °C), with flies reared at 18 °C and 25 °C. We found that both developmental and experimental temperatures affected the behavioral performance of the flies. Our study thus reveals temperature-dependent changes in odor-guided behavior in drosophilid flies.
Subject terms: Climate-change ecology, Behavioural ecology
Drosophila novamexicana, D. virilis, and D. ezoana are fly species that have respectively adapted to life in desert, global, or subarctic climates. These drosophilid species also exhibit distinct odor-guided behaviors in response to altered developmental or experimental temperatures.
Introduction
The olfactory system of insects underpins a large number of evolutionarily critical behaviors such as search for food, oviposition substrates, shelters, and mates, and avoidance of harmful organisms, including parasitoids, predators and pathogens1. Olfactory detection is carried out with an array of olfactory sensory neurons (OSNs) located on the antennae2 and maxillary palps3. In case of Drosophila melanogaster, the third antennal segment, the funiculus, is covered with a large number of hair-like structures, sensilla, which house dendrites of OSNs (Nava Gonzales et al., 2021). These OSNs express distinct olfactory receptors (ORs), which dimerize with the co-receptor Orco4,5. While OSNs housed in trichoid sensilla respond predominantly to pheromones6, and those associated with coeloconic sensilla mainly to amines and carboxylic acid7, general food and oviposition substrate odor detection mainly takes place in OSNs present in basiconic sensilla.
A consequence of human activities can be observed in terms of climate change and consistently increasing global temperatures, which in turn might affect the guided behavior of insects. Insects are ectotherms, meaning that their biology is closely related to environmental temperatures8. Any changes in the external temperature can thus affect the physiology of the olfactory sensory system leading to modulation of behavior triggered by the system9. For instance, at the behavioral level, the sensitivity of D. melanogaster to ethanol increases or decreases when flies are acclimatized to heat or cold, respectively10. Furthermore, electrophysiological recordings revealed changes in odor detection at the OSN level11. Another study showed that exposing D. melanogaster flies to 30 °C for 24 h altered the expression level of genes involved in odor detection, causing a reduction in avoidance of high ethanol concentrations12. Conclusions drawn from these studies were based on flies reared at 21 °C, acclimatized at 15 °C (cold treatment) or 30 °C (heat treatment) and subsequently tested at 24 °C using electrophysiology and behavoural studies. Similar studies conducted in other insect orders are based on insect acclimatization as well. Linn et al.13 reared the moths Grapholita molesta (Lepidoptera: Tortricidae) and Pectinophora gossypiella (Lepidoptera: Gelechiidae) at 25 °C or 26-27 °C, after which they were tested in a wind tunnel at 20 °C and 26 °C. These two temperatures induced differential specificity in male pheromone-directed responses. Processing of olfactory stimuli has also been shown to be temperature-dependent in other insects14.
The effect of developmental temperature and/or experimental temperature on odor detection and odor-dependent behavior in drosophilid species evolutionarily adapted to different climates has so far received little attention. In the present study, we used three closely related species belonging to the virilis group; Drosophila ezoana, D. novamexicana and D. virilis, which have adapted to live in subarctic, desert and global climates, respectively. Despite their different climatic preferences, all three species feed and breed on slime flux, saps and decaying bark of tree species belonging preferably to the Salicaceae and Betulaceae families15.
We first assessed the temperature preference of the three species when reared at 20, 23 and 25 °C. Next, we used a panel of 57 ecologically relevant odorants to characterize response profiles of distinct OSN classes and thereby identified active ligands for each OSN class. Further, we established the behavioral valence of key ligands identified for each OSN type using a two-choice trap assay. Lastly, we chose three odors that elicited attraction, aversion or neutrality, respectively, across the three species and tested whether behavioral responses would be affected by developmental and/or experimental temperatures.
Results
Temperature preference of Drosophila ezoana, D. novamexicana and D. virilis
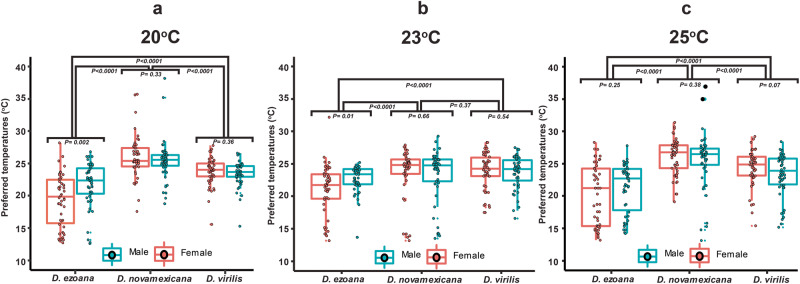
The temperature preference of the three species bred at 20, 23 or 25 °C temperature regimes was tested in mini thermal channels as described above. In initial control experiments, individual flies were released inside the channels in absence of a thermal gradient. After tracking their movements for 30 min, we observed that individuals of each species were distributed evenly inside the channels (Supplementary Fig. 1b). When the heat and cold generators were engaged, a linear temperature gradient from 10 to 40 °C was established inside each channel. The gradient was stable and remained unchanged even after 3 h (Supplementary Fig. 1c). When released inside the channels in presence of the thermal gradient, D. ezoana flies avoided the hot end of the gradient (Supplementary Fig. 2a); whereas D. novamexicana avoided the cold end (Supplementary Fig. 2b). D. virilis occupied an intermediate position in the gradient (Supplementary Fig. 2c). The temperature experienced by the three Drosophila species during their preimaginal development did not influence their thermal preference at the adult stage when tested within a species (Supplementary Fig. 3a-c, Supplementary Table 1). Males and females in general preferred the same temperature regime. Female D. ezoana did, however, show a significantly higher cold-loving propensity in comparison to the males of the same species. The three species investigated thus revealed clear temperature preferences matching their natural habitats (Fig. 1a–c).
Fig. 1. Temperature preference of the three Drosophila species.
Boxplot illustrating the preferred temperatures of males and females of a D. ezoana, b D. novamexicana and c D. virilis when reared at 20 °C (left), 23 °C (middle) and 25 °C (right). Boxplot whiskers indicate ± 1.5 interquartile range limits. Dots in each boxplot indicate individual temperature data points (n = 50 biologically independent samples). Significance (P < 0.05) was tested in an unpaired t-test (comparison between sexes of each species) and a one-way ANOVA followed by SNK multiple comparison post hoc tests (comparison across species).
Physiological characterization of olfactory sensory neurons in antennal sensilla basiconica of the three species under study
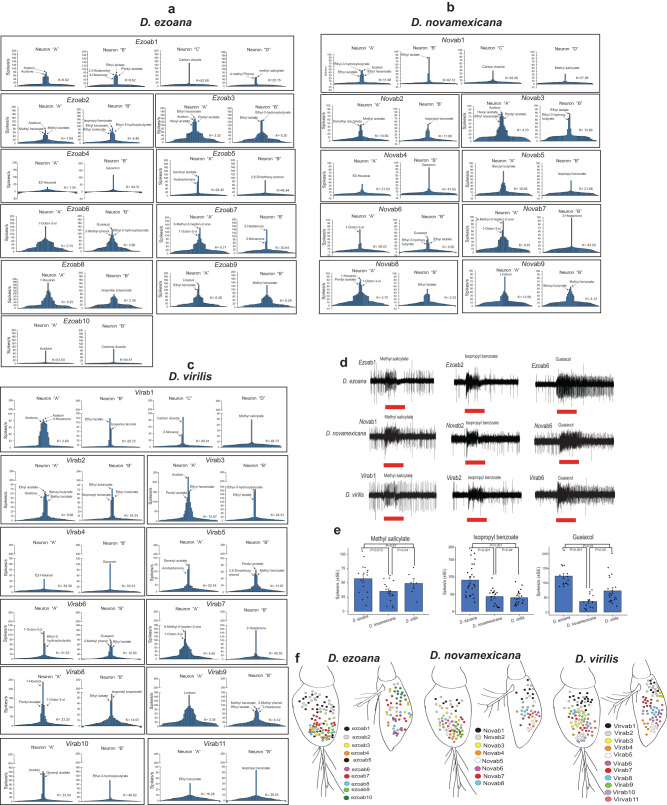
We used the well-characterized olfactory sensory neuron (OSN) types associated with basiconic sensilla in Drosophila melanogaster as a vantage point for our dissection of the same type of neurons in D. ezoana, D. novamexicana and D. virilis. In comparison to D. melanogaster, where 10 basiconic types have been physiologically classified, we identified 10, 9 and 11 types, respectively in D. ezoana (Supplementary Fig. 4), D. novamexicana (Supplementary Fig. 5), and D. virilis (Supplementary Fig. 6). When sensilla in these species contained an OSN collection with identical or almost identical response spectra as found in D. melanogaster, the sensillum types were given the same number as in D. melanogaster preceeded by a species identifier (Ezo, Nov, Vir) (Supplementary Tables 4-6). In a few cases, the key ligands of one OSN in a sensillum were identical to those of one D. melanogaster OSN, while the second OSN did not match the D. melanogaster pattern. Also, we observed that OSNs of D. novamexicana in general produced fewer action potentials as compared to D. ezoana and D. virilis (Fig. 2e). A spatial distribution map of all sensillum types identified from the three species is found in Fig. 2f.
Fig. 2. Odor coding in the three Drosophila species.
Tuning histograms of each olfactory sensory neuron present in basiconic sensillum types identified in (a) D. ezoana, (b) D. novamexicana and (c) D. virilis. x-axis represents the 57 compounds tested with the most active compound in the center; y-axis represents the number of action potentials (spikes) elicited. The kurtosis (k) value represents the ‘peakedness’ of each distribution curve. This value is high in narrowly tuned neurons, and low in broadly tuned neurons. d Representative single-sensillum recording (SSR) traces in D. ezoana, D. novamexicana and D. virilis, showing responses of ab1, ab2 and ab6 to methyl salicylate, isopropyl benzoate and guaiacol respectively. e Bar graphs showing the mean number of action potentials produced by OSNs housed in the ab1, ab2 and ab6 of D. ezoana, D. novamexicana and D. virilis when stimulated with their best ligands (ANOVA followed by the SNK posthoc tests). Error bars indicate standard error of the mean (SEM). f Spatial distribution of all identified sensillum types in D. ezoana, D. novamexicana and D. virilis.
As in D. melanogaster, three large basiconic (LB) types were identified in D. ezoana (Ezoab1, Ezoab2, Ezoab3), D. novamexicana (Novab1, Novab2, Novab3) and D. virilis (Virab1, Virab2, Virab3). In the three species, the Ezoab1, Novab1 and Virab1 sensillum type housed the typical four OSNs (Fig. 2d), the “A” neuron, characterized by large action potentials or spikes, responding strongly to acetoin and acetone, “B”, “C” and “D” neurons responding respectively to ethyl lactate, carbon dioxide and methyl salicylate. In D. ezoana, a similar LB sensillum (Ezoab10) was found. This sensillum type, however, contained only two responding neurons (A and B) strongly activated by acetone and carbon dioxide, respectively (Fig. 2a). This type was not found in D. novamexicana (Fig. 2b) or D. virilis (Fig. 2c). The Ezoab2, Novab2 and Virab2 sensillum types responded to similar stimulus spectra. Their “A” and “B” OSNs responded strongly to methyl acetate and isopropyl benzoate (Fig. 2d), respectively. In addition, in D. ezoana and D. virilis acetone stimulated the “A” neuron, while in D. novamexicana it elicited a very weak response. In this species, the “A” neuron responded strongly also to dimethyl disulfide. The Ezoab3, Novab3 and Virab3 sensillum types of all the species also exhibited similar response patterns. The “A” OSN responded strongly to ethyl hexanoate, acetoin and pentyl acetate, while the “B” OSN responded to ethyl lactate and ethyl-3-hydroxybutyrate.
Small basiconic (SB) sensillum types were present in all three Drosophila species. The Ezoab4, Novab4 and Virab4 sensillum types housed two neurons “A” and “B” tuned to E2-hexanal and geosmin, respectively. The Ezoab5 and Virab5 sensilla housed two OSNs: “A” that responded to geranyl acetate and acetophenone and “B” that responded to 2,6-dimethoxy-phenol. No similar sensillum type was found in D. novamexicana. Nonetheless, we found in this species a sensillum type that we called Novab5. It housed two neurons “A” and “B” responding respectively to benzyl butyrate and isopropyl benzoate. The Ezoab6, Novab6 and Virab6 sensillum types housed two neurons “A” and “B” (Fig. 2d) tuned to 1-octen-3-ol and guaiacol, respectively. The Ezoab7, Novab7 and Virab7 hosted two neurons “A” and “B” that mainly responded to 6-methyl-5-hepten-2-one and 2-heptanone respectively. The Ezoab8, Novab8 and Virab8 sensillum types responded strongly to 1-hexanol (A); while the B OSN responded to ethyl lactate and isopentyl propionate. The Ezoab9, Novab9 and Virab9 sensillum types hosted an “A” neuron responding to linalool and a “B” neuron responding to methyl benzoate and a variety of other odorants. Lastly, in D. virilis, we found two sensilla: Virab10 and Virab11 absent in D. ezoana and D. novamexicana. The Virab10 sensillum housed two neurons; “A” tuned to acetoin and geranyl acetate, “B” tuned to ethyl-3-hydroxybutyrate. The Virab11 sensillum type displayed an “A” neuron tuned to ethyl benzoate and a “B” neuron primarily responded to isopropyl benzoate.
The sensillum classification and its corresponding nomenclature is unique to the individual species and should in general not be compared with the canonical D. melanogaster sensilla nomenclature and also not between the three species. However, we found some OSN types conserved across the three species that are comparable to D. melanogaster. For instance, the ab1-like class (diagnostic ligand: CO2), ab4-like (diagnostic ligand: geosmin) and ab6-like (diagnostic ligand: guaiacol). However, these classes also displayed changes in terms of the specificity of their co-innervating OSN types. For example, we observed that the A neuron in Dvirab1-like sensillum class responded strongest to acetoin as compared to ethyl acetate for the ab1A OSN type reported in D. melanogaster.
Behavioral valence of the best ligands identified and its modulation by temperature
Using the best ligands (eliciting the strongest response) identified for each class of OSN in the SSR recordings, we wanted to test their behavioral significance in the three species studied. Screening of the 20 best ligands in trap assays revealed that the ligand spectrum contained odors eliciting attraction, repulsion or neutral responses in D. ezoana (Fig. 3a), D. novamexicana (Fig. 3b) and D. virilis (Fig. 3c). Several compounds triggered different responses in the three species. For instance, 6-methyl-5-hepten-2-one and methyl benzoate elicited no response in D. ezoana, while in D. novamexicana and D. virilis they triggered repulsion. Acetoin and ethyl-3-hydroxybutyrate attracted D. virilis, while in D. ezoana and D. novamexicana, these compounds elicited no response. 1-hexanol attracted D. novamexicana, but in D. ezoana and D. novamexicana it triggered no response. Also, some compounds exhibited the same valence across the three species. Benzyl butyrate, linalool and methyl salicylate elicited attraction; guaiacol and hexyl acetate triggered aversion, while isopropyl benzoate and pentyl acetate did not induce any choice. No compounds elicited opposite responses.
Fig. 3. Behavioral response of the three Drosophila species to the key ligands.
Valence value of key ligands of olfactory sensory neurons in a D. ezoana, b D. novamexicana and c D. virilis. Box plots show the attraction index (based on a two-choice trap assay (odor vs solvent) with 1 depicting maximum attraction, 0 neutrality and -1 maximum repellency of the compound tested. In each boxplot, the ends of boxplot whiskers represent the minimum and maximum values of all the data and dots show individual data points (n = 10 replicates, 20 biologically independent samples per replicate). Boxplots filled with blue, red and white colors depict respectively a attraction, repulsion and neutrality. Based on distribution of the data, one sample t-test (normally distributed data: Shapiro test: P > 0.05) or one sample Wilcoxon (non-normally distributed data: Shapiro test: P < 0.05) statistical tests were used to test the significance of the attraction index. The n.s. symbol denotes a non-significant difference of the attraction index to the theoretical mean 0; while *, **, ***, **** indicate statistical significance of the attraction index to the theoretical mean 0, with P < 0.05, P < 0.01, P < 0.001, P < 0.0001, respectively.
We next asked whether the valence of these key ligands would change as a function of rearing and/or experimental temperatures. To address this, we conducted trap assays at 10, 15, 20, 25, 30, and 35 °C using flies reared at 18 and 25 °C. As representative attractant, repellent and neutral odorants, we used guaiacol (negative), methyl salicylate (positive) and isopropyl benzoate (neutral). We observed differences in the proportion of flies responding to a given cue at different temperatures (Fig. 4). In most of the cases, D. ezoana (Fig. 4a), D. novamexicana (Fig. 4b) and D. virilis (Fig. 4c) entered either into the control or treatment containers (the purple portion of the pie-chart) in trap assays conducted at 15, 20, 25 and 30 °C. In trap assays performed at 10 and 35 °C, however, we found a large proportion of flies outside the control and treatment containers (white portion of the pie-chart); suggesting that these two extreme temperatures considerably impaired the movement of the three species towards the trap containers. Based on this result we decided to exclude the extreme temperatures from further analysis.
Fig. 4. Effect of temperature on the proportion of responding vs non responding flies.
Pie charts showing the proportion of a D. ezoana, b D. novamexicana and c D. virilis individuals found in the control and treatment container (purple portion) in comparison to the proportion of individuals found outside the containers (white portion). Proportions compared using the Chi-square test. The symbols n.s indicate non-significant differences; *, **, ***, **** depicts P < 0.05, P < 0.01, P < 0.001 and P < 0.0001, respectively.
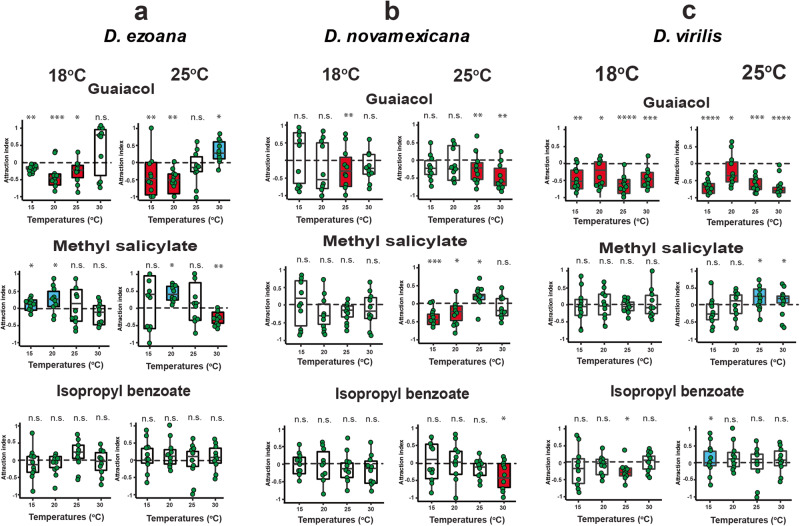
When proceeding with a more detailed analysis of the behavioral responses in the two-choice assay, we found that in D. ezoana (Fig. 5a) reared at 18 °C the repellency for guaiacol was significantly reduced (flies reacted neutrally) in trap assays conducted at 30 °C. Among individuals reared at 25 °C, the repellency disappeared in trap assays conducted at 25 and 30 °C. At 30 °C the flies even became attracted to guaiacol. High testing temperatures thus clearly affected the behavioral responses to guaiacol. For methyl salicylate we found conserved attraction in trap assays conducted at 15 and 20 °C with D. ezoana reared at 18 °C. However, when tested at 25 and 30 °C, the flies exhibited neutral behavior toward this compound. With individuals reared at 25 °C, we only observed attraction to methyl salicylate in trap assays performed at 20 °C, while at 15 and 25 °C these flies responded neutrally, and at 30 °C they were even significantly repelled by this compound. The positive response to methyl salicylate thus had a very narrow temperature range. The neutral response to isopropyl benzoate in D. ezoana remained across the different developmental and experimental temperatures.
Fig. 5. Effect of temperature on odorant valence.
a Boxplot showing the temperature effect on aversion, attraction and neutral activity of guaiacol, methyl salicylate and isopropyl benzoate, respectively in D. ezoana reared at 18 °C (left) and 25 °C (right). b The same representation for D. novamexicana. c The same representation for D. virilis. The edges of the boxes are the first and third quartiles, thick lines mark the medians, and whiskers represent the data range. The dots in each box show individual data points (n = 10 replicates, 20 biologically independent samples per replicate). Box plots colored in red, blue and white depict aversion, attraction and no response, respectively. Significance of the attraction index to the theoretical mean 0 was tested using the one-sample t-test (normally distributed data: Shapiro test: P > 0.05) or one sample Wilcoxon test (non-normally distributed data: Shapiro test: P < 0.05).
In D. novamexicana (Fig. 5b), flies reared at 18 °C exhibited avoidance behavior towards guaiacol when tested at 25 °C. At 15, 20 and 30 °C they responded neutrally. With individuals reared at 25 °C, guaiacol was repellent at 25 and 30 °C. At 15 and 20 °C, the flies exhibited neutral behavior. The negative esponse to guaiacol was thus abolished at lower temperatures. D. novamexicana reared at 18 °C responded neutrally to methyl salicylate at all test temperatures. When reared at 25 °C, we noted attraction to this compound only when tested at 25 °C. These flies were instead repelled by methyl salicylate at 15 and 20 °C, while responding neutrally at 30 °C. As in D. ezoana, the positive response to methyl salicylate was extremely sensitive to the testing temperature. The neutral response to isopropyl benzoate did not change with test temperature when we used D. novamexicana reared at 18 °C. However, when reared at 25 °C, they showed repulsion at 30 °C testing temperature.
In D. virilis (Fig. 5c), flies reared at 18 and 25 °C avoided guaiacol under all different experimental temperatures. In the case of methyl salicylate, no attraction to this compound was observed with D. virilis developed at 18 °C. Instead, they exhibited neutral behavior across the different testing temperatures. When the flies were reared at 25 °C, they were attracted to methyl salicylate at 25 and 30 °C. At 15 and 20 °C, they did not respond to the compound. The neutral response to isopropyl benzoate persisted across most experimental temperatures. However, D. virilis flies developed at 18 °C showed aversion to this compound at 25 °C and flies reared at 25 °C were attracted at 15 °C. A comprehensive summary of all possible interactions between these factors is illustrated in Supplementary Table 7.
Discussion
We aimed to study how insects can cope with the global increase in overall temperature from an olfactory point of view. We studied odor-induced behavior in three Drosophila species representing arctic, desert and cosmopolitan distributions, namely Drososphila ezoana, D. novamexicana and D. virilis. After establishing differences in thermal preference range between the species, we used single sensillum recording technique to classify different basiconic sensillum types present on the antennae and identified best ligands for the corresponding olfactory sensory neurons (OSNs). In a series of trap assay experiments, we then established the behavior (attraction, aversion or no response) that these key ligands triggered in the three species. Finally, we tested both the effect of rearing and testing temperatures on the behavioral reactions by conducting trap assays at different testing temperatures using flies reared at 18 and 25 °C and odorants previously identified as an attractant, repellent or neutral in the three species.
As expected from their natural habitats, D. ezoana showed a significant preference towards cooler temperatures, D. novamexicana towards warmer, while D. virilis preferred an intermediate temperature range. These thermal preference patterns remained consistent across flies reared at 20, 23 and 25 °C, showing that despite different conditions of development, the thermal preference behavior stayed constant. Maclean et al.16 stated that fundamental species-specific ecological characteristics such as temperature tolerance do not eclipse during laboratory maintenance. D. ezoana, D. novamexicana and D. virilis have adapted to life in the arctic, desert and global climates, respectively. In comparison, Rajpurohit and Schmidt17 demonstrated that D. melanogaster from temperate areas exhibited a greater preference for cooler temperatures, while those from tropical habitats preferred higher temperatures. Similar patterns were found in other temperature-related parameters. D. ananassae flies living at high latitudes recovered faster from chill coma and more slowly to heat knockdown as compared to flies living at low latitudes18. Obviously, Drosophila species in general show clear physiological climatic limits and geographical variation of genes involved in temperature preference and adaptation19,20, that lead to a thermal preference matching the habitat they have evolved in.
As a base for the behavioral studies, we performed an extensive screening of antennal sensilla basiconica. The sampling (200 in each species) yielded 10, 9 and 11 basiconic sensillum types in D. ezoana, D. novamexicana and D. virilis, respectively. The recordings revealed both similarities and differences from the 10 basiconic types physiologically classified in D. melanogaster4,5,21,22, D. mojavensis23 and D. suzukii24. The differences observed likely stem from the fact that Drosophila flies colonize environments and display ecologies characterized by different types of sensory information that have shaped the molecular, physiological and anatomical organization of their sense of smell. Despite our extensive recordings, sampling effects might also play a role. Another factor is the fact that we did not investigate sensilla trichodea (typically involved in pheromone communication) and sensilla coeloconica (involved in detecting acids and amines).
In general, we observed both gain and loss of some sensillum types in the three Drosophila species studied here. In D. ezoana, we identified a large sensillum type (Ezoab10) containing two neurons “A” and “B” tuned to acetone and carbon dioxide, respectively, and thus seemingly lacking two of the OSNs present in the normal ab1 type. Across all three species, the Ezoab3, Novab3 and Virab3 sensilla showed some functional deviation from ab3 sensilla of D. melanogaster. In D. melanogaster, the “A” and “B” OSNs of this sensillum type respond mainly to ethyl hexanoate and 2-heptanone, respectively22,25. In our study, we found these two neurons in two distinct sensillum types. In the first sensillum type (Ezoab3, Novab3 and Virab3), the ethyl hexanoate-responding “A” neuron innervated the same sensillum lymph as a “B” neuron excited by ethyl lactate and ethyl-3-hydroxybutyrate. In the second sensillum type (Ezoab7, Novab7 and Virab7), the 2-heptanone-responding “B” neuron was housed in the same lymph as an “A” neuron responding mostly to 6-methyl-5-hepten-2-one and 1-octen-3-ol. In several Drosophila species, the ab3 sensilla (characterised by its larger amplitude “A” neuron) is known to exhibit within-species and interspecies variation in odorant responses. This sensillum type plays a major role in ecological adaptations to feeding26,27, and in egg-laying decisions28.
Only D. novamexicana possessed a Novab5 sensillum housing an “A” neuron responding to benzyl butyrate and a “B” neuron excited by isopropyl benzoate. Out of the 200 basiconic sensilla recorded from in the D. ezoana and D. virilis funiculus, no sister types of this sensillum were identified. D. novamexicana did not display a sensillum type equivalent to Ezoab5 and Virab5. In D. ezoana and D. novamexicana, on the other hand, we could not find the sister type of the Virab11 sensillum. Several studies (reviewed in Anholt29) have shown that the ecological adaptation of drosophilid flies to specific environments is accompanied by rapid evolutionary changes in their olfactory system. Absence of specific sensillum types has e.g. been noticed in D. sechellia, where Stensmyr et al.30 observed the loss of the ab2 sensillum type paralleled by an over-representation of the ab3 type. This change has been hypothesized to be driven by adaptation to the food source of D. sechellia, the noni fruit. The loss of specific sensillum types in D. ezoana and D. novamexicana could also be the result of adaptations to their lifestyle, which is restricted to arctic and desert regions, respectively. Still, all three species under study here find their food in tree sap from a restricted spectrum of tree species, which would provide similar selection pressures in shaping the sensillum arsenal.
A broad behavioral two-choice experiment revealed the preferences of the three species when presented with 20 key ligands from the SSR experiments at a fixed testing temperature of 23 °C. Some of the tested compounds elicited differential behavioral responses in the species. For instance, acetoin and ethyl-3-hydroxybutyrate elicited clear attraction in D. virilis but no response in D. ezoana and D. novamexicana. Methyl benzoate and 6-methyl-5-hepten-2-one attracted significantly fewer flies than the control in D. novamexicana and virilis, while it elicited a neutral response in D. ezoana. Such differences have also been observed among other fruitfly species. Dweck et al.31 found that 4-ethylguaiacol and methyleugenol derived from yeast fermentation induce strong attraction in D. melanogaster but elicit no response in D. suzukii. Similarly, the avoidance behaviour triggered by CO2 in D. melanogaster is not conserved in D. suzukii32. The behavioral differences observed between the present three species might represent a taxon-specific adaptation to their respective environment. Such ecological adaptations are often accompanied by functional changes in some olfactory circuits33,34, leading to situations where some odors elicit strong physiological responses but weak behavioral responses or vice versa35.
In contrast, we found that guaiacol, methyl salicylate and isopropyl benzoate elicited conserved avoidance, attraction and neutral response, respectively, in D. ezoana, D. novamexicana and D. virilis. This suggests that the olfactory circuits responsible for the detection of these compounds are possibly conserved in the three species. Insects possess several olfactory circuits that are highly conserved, allowing them to accomplish complex behaviors, such as courtship, feeding, oviposition avoidance of enemies or toxic microorganisms. An interesting example to such a circuit is the geosmin-dedicated olfactory circuitry. This odor, emitted by molds, activates a specific, narrowly tuned olfactory receptor expressed in the ab4B neuron (Or56a) in the majority of drosophilid species (including the three studied here). In D. melanogaster specifically, the stimulation of the ab4B OSNs by geosmin triggers activity in a single glomerulus (DA2) and inhibits feeding, attraction and oviposition36. However, if guaiacol (conserved repellent in our study) acts as antifeedant, attraction inhibitor, or oviposition deterrent like geosmin does to D. melanogaster still remains to be elucidated. Nonetheless, in another Diptera system, guaiacol (one of the waterbuck body odors) was found among the main odors composing the formulation (Waterbuck Repellent Blend (WRB)) that repels the tsetse fly species Glossina fuscipes fuscipes37. Further, methyl salicylate, identified as an attractant for the three species tested here, is an herbivore-induced plant volatile (HIPV) known to attract herbivore predators and parasitoids38. In D. melanogaster, this odor exhibits a neutral valence when tested in a trap assay39. At the same time, yellow traps baited with methyl salicylate significantly enhances catches of the dance fly, Rhamphomyia gibba (Diptera, Empididae)40.
The temperature-modulated behavioral response assays revealed that, when tested at the extreme temperatures of 10 and 35 °C, a large proportion of flies in all three species remained passive outside the traps, not making a choice to enter, indicating that these two extreme temperatures significantly impacted the ability to orientate and move. Recently, Ito and Awasaki41 also showed that the locomotor activity in 11 Drosophila flies (including D. virilis) matched the temperatures they frequently encounter in their habitats. As the species tested in our study were less likely to be active at 10 °C or 35 °C in their environment and showed both impaired activity in the choice assay at these temperatures (less than 50% made a choice in the two-choice bioassay), and a clear avoidance of the same in our thermal preference assay, we decided to remove these data from further consideration.
In the remaining data, where flies reared at 18 and 25 °C were tested for their preference at 15, 20, 25 and 30 C, we observed some temperature-dependent effects. Below we discuss these for each of the compounds tested.
We noticed that the compound guaiacol, which is associated with a generally negative behavioral effect, exhibited changes in response based on temperature. In the cold- and warm-loving species the negative response was abolished or even reversed when in suboptimal temperature environments. In D. ezoana, repulsion disappeared at higher temperatures and was even reversed to attraction in flies reared at 25 °C. In D. novamexicana, a species adapted to warmer climates, we observed the opposite temperature response, with lower testing temperatures abolishing the negative response to guaiacol. In the temperate D. virilis, the response remained largely unaffected over the temperatures tested. Taken together, these results indicate that the ambient testing temperature had a clear impact on the behavior towards guaiacol, while the developmental temperature had little effect on this behavior. At less permissive temperatures the behavior vanished or was even reversed. Failing to respond to signals of danger might significantly reduce the survival of these flies, as this behavior often serves to escape pathogens, parasitoids and predators. For instance, Hangartner et al.42 found that D. melanogaster exposed to extreme heat had a lower survival rate when faced with predation. These authors postulated that extreme temperatures affect the flies’ physiological performance and impair their ability to detect and escape predators.
The attraction behavior of all three Drosophila species to methyl salicylate was notably affected by development under suboptimal temperatures. Attraction also appeared only in a very narrow temperature window suggesting that exposing the preimaginal or adult stages of flies to uncommon temperatures can modulate their odor-guided attraction. Several studies outside the field of olfaction have pointed in a similar direction. For example, it was found that at low temperatures, the feeding preference for yeast in D. melanogaster was strongly reduced43. In D. suzikii, the oviposition activity on blueberries decreased continuously above 28 °C and below 15 °C44. Our results also show the importance of the temperature in the environment where the behavior is performed. All three species displayed attraction to methyl salicylate in a 5-10-degree window, which seems lower in the cold-loving species as compared to the temperate and warm-living ones.
Very little impact of rearing and testing temperatures was observed when testing isopropyl benzoate that elicited no choice at 23 °C. In D. ezoana it remained neutral through all tested temperatures and under both rearing regimes. In the other two species, a few tests showed weak attraction or repulsion but did not reveal any clear patterns.
In summary, our study showed that drosophilid flies choose locations with a temperature matching their natural habitats. We also found that temperature modulation can affect odor-guided behavior in three drosophilid species of different geographical and climatic origins. By recording olfactory responses, we established relevant stimuli of differing behavioral importance and further demonstrated that the innate thermal preference of the three drosophilids to some extent also dictates their odor-mediated behaviors to ecologically relevant odors. The innate valence of such compounds can thus change at suboptimal temperatures outside of the innate thermal range. The mechanisms underpinning the changes observed might reside at different levels, from sensory detection to muscle action. In the future, it will be worth investigating the cellular and molecular mechanisms underlying the plastic character of olfactory-mediated behavior of drosophilid flies when exposed to different temperatures.
Methods
Flies stocks and husbandry
We obtained Drosophila ezoana (stock number: E-15701) from the fly stocks of Kyorin university, Japan (https://shigen.nig.ac.jp/fly/kyorin/index_ja.html), while D. novamexicana (stock number: 15010-1031.08) and D. virilis (stock number: 15010-1051.00) were obtained from the National Drosophila Species Stock Center of Cornell University (https://www.drosophilaspecies.com/). We reared D. ezoana at 20 °C, 16 h Light: 8 h Dark, D. virilis at 23 °C 12 h Light: 12 h Dark, and D. novamexicana at 25 °C 12 h Light: 12 h Dark. All flies were fed on autoclaved cornmeal- yeast-sucrose-agar food. D. ezoana and D. novamexicana flies used in our bioassays were kept for two generations after arrival from the above-mentioned stock center. D. virilis was kept in our laboratory for more than 50 generations.
Temperature preference assay
An apparatus with thermally conductive material that can be heated in one end and cooled at the other offers the most suitable way of measuring thermal preference in animals45. Based on this, we designed a thermal gradient choice bioassay based on a design developed by Lynch et al.46. The apparatus (Supplementary Fig. 1a, right) was composed of 10 aluminium channels measuring each 24 × 1 × 0.8 cm and separated from each other by 0.5 cm (Supplementary Fig. 1a, bottom) and two heat and cool Quick-Ohm generators (https://www.quick-ohm.de/) to allow the establishment of a thermal gradient (going from 10 to 40 °C) along each aluminium channel. On top of the aluminium channels, a sheet of plexiglass was placed to maintain flies in the channels whilst enabling continuous observations. The plexiglass was drilled in the middle with 10 holes of 5 mm in diameter (covered with a screw) to ease the introduction of individual flies into each channel. To avoid phototaxis effects during the experiment, we kept the apparatus in darkness using a darkened cage, and infrared light and camera (Supplementary Fig. 1a, right and left).
The movements of the three species were first observed without turning on the heat and cold generators. To do this, we used 50 males and 50 females (4-to-6 days old) of each species and gently introduced them individually (using a mouth aspirator) inside each channel (Supplementary Fig. 1A, bottom). Their movements along the channels were recorded for 30 min using a camera placed on top of the bioassay. At the end of each recording, the channels were cleaned with ethanol before introducing the next set of 10 individuals. Using the recorded videos, the movement of the flies was tracked by determining their position in the channels every two minutes. To check for the consistency of the thermal gradient in the assay, we recorded (using a data logger thermometer equipped with two probes (https://www.tcdirect.de/)) the temperature of 12 fixed positions in each channel 1, 2 and 3 h after turning on the heat and cold generators.
After these control experiments, the heat and cold generators were again turned on. After 10 min a thermal gradient, spanning from 10 to 40 °C was established along the aluminium channels. Individual flies were then introduced into each channel. Fifty males and 50 females of each fly species were tested and their movements were videotaped for 30 min. We used the temperature of the region of the channel where an individual fly spent most of its time as a proxy for its preferred temperature46. To determine possible effects of developmental temperature, D. ezoana, D. novamexicana and D. virilis individuals used in this experiment were reared at three different temperatures; 20, 23 and 25 °C.
Electrophysiology
Single sensillum recordings were performed following the protocol described by Olsson and Hansson47. Briefly, adult flies of each species were restrained in a 100 µl plastic pipette tip with the wide end closed with dental wax and the narrow end cut to allow only the head with antennae to protrude. The preparation was fixed on dental wax placed on a microscope slide with the ventral side of the fly facing upward. The funiculus of the antenna was fixed with a sharpened glass capillary (placed between the second and third antennal segment) held with dental wax onto a cover slide, which in turn was held in place by dental wax on the microscope slide. Afterwards, the preparation was placed under a light microscope (BX51WI, Olympus, Tokyo, Japan) equipped with a ×50 magnification objective (LMPLFLN 50X, Olympus) and 4x eyepieces. The preparation was continuously flushed by clean air through a plastic tube of 0.4 cm diameter delivering a 1.5 l min−1 flux of charcoal-filtered and humified air. The tube ended approximately 6 cm m from the preparation.
Stimulus cartridges were prepared by placing a circular filter paper (1.2 cm diameter) in the large opening end of a Pasteur pipette and pipetting 10 µl of odorant solution onto the paper before closing the pipette with a 1 ml plastic pipette closed at its small opening with wax to prevent evaporation. The antenna of the fly was stimulated by a 500 ms air pulse (0.6 l min−1) through the stimulation cartridge into the permanent air flux (1.5 l min−1).
To record action potentials electrochemically sharpened tungsten electrodes were used. The electrodes were sharpened using saturated potassium nitrite (KNO2) solution. The reference electrode was inserted into the eye of the fly with the aid of a manually controlled micromanipulator. With the aid of a motor-controlled DC-3K micromanipulator (Märzhäuser, Wetzlar, Germany) equipped with a PM-10 piezo translator (Märzhäuser), the recording electrode was inserted at the base of a sensillum. The electrical signal was amplified using a USB-IDAC connection to a computer (Syntech, The Netherlands). The frequency of action potentials during a 1-second pre- and post-stimulation period was established using Auto Spike software (Syntech, version 3.7). Approximately 200 basiconic sensilla from the funiculus of each of the three Drosophila species were recorded from, using the D. melanogaster sensilla distribution map as a reference Lin and Potter48. 2,6-dimethoxy-phenol, 3,4,5-trimethoxyphenol, oleamide, acetovanillone, salicylaldehyde and hexadecanamide were diluted in dimethyl sulfoxide (DMSO) at 10−4 concentration (1:10000 volume:volume), while all remaining odorants were diluted in mineral oil at 10−4 concentration. All synthetic odorants used (Supplementary Table 2) were of the highest purity available from Sigma (www.sigmaaldrich.com) and Bedoukian (www.bedoukian.com). The entire odorant panel was tested on maximally 3 sensilla per fly. Our screening was performed exclusively from basiconic sensillum types, thus excluding sensilla trichodea and sensilla coeloconica as investigations in other drosophilid species have shown that food odors are primarily detected by OSNs located in basiconic sensilla.
Behavioral assay
The single sensillum recordings allowed us to identify key odorants that elicited strong responses in each of the basiconic OSN types of the three Drosophila species. With this information as a base we turned to a dual choice bioassay to establish the valence (i.e. attraction, aversion, or no response) that the key odorants triggered in the three species. Trap assay experiments were performed in a climate chamber (23 °C, 70% humidity, 12 h Light: 12 h Dark). Transparent plastic boxes of 500 ml (with 30 ventilation holes in the lid) held treatment and control traps made from small, transparent plastic vials (25 ml) with a paper cone (cut at the apex (4 mm)), inserted into the opening of the vial and fixed with transparent tape. Treatment and control traps were loaded with a lid of an Eppendorf tube containing either 2 µl of the odorant to be tested (diluted in 200 µl mineral oil at 10−2 concentration) or 200 µl of solvent (mineral oil). For each species, we transferred 20 flies (10 males and 10 females, 4–6 days old, starved for 24 h before the experiment) inside the large box. We counted the number of flies that had entered the traps and those that remained outside after 24 h. With this data, an attraction index (AI) could be calculated as:
| 1 |
where O is the number of flies that entered the trap containing the odorant and C is the number of flies that entered the trap containing the solvent. The AI ranged from -1 (maximum avoidance) to 1 (maximum attraction). Zero thus denoted no choice. For each species, 20 odorants were tested and for each odorant the trap assay was replicated 10 times.
Among the 20 odorants tested in the trap assay, we identified some that triggered attraction, some aversion, while some were neutral across all the three species. Next, we aimed to see whether the developmental and/or the experimental temperatures could modulate these behaviors. For this, the three species were reared at 18 and 25 °C, after which the emerged adults (4–6 days old) were tested in trap assays as described above, with the particularity that all the experiments were conducted inside a thermo-controlled Percival incubator (www.percival-scientific.com). The experiments were run at 10, 15, 20, 25, 30 and 35 °C, all with 70% humidity and 12 h Light:12 h Dark. As stimuli, methyl salicylate (positive), guaiacol (negative) and isopropyl benzoate (neutral) were used. The compounds were diluted in mineral oil 10−2 concentration. No significant difference in release rate across the different temperatures and times was observed (Supplementary Figs. 7 and 8).
Statistics and reproducibility
All statistical analyses were performed in R software version 4.0.349 (R Core Team, 2020) and all graphs were assembled in Adobe Illustrator CC 2017 (version 21.0). The temperature preference data were normally distributed (Shapiro-Wilk test: P > 0.05) and their variances were homogeneous (Barlett test: P > 0.05), therefore we ran an unpaired t-test to see whether males and females of the same species significantly preferred different temperatures. The three-way analysis of variance was computed followed by Student-Neuman-Keuls (SNK) post hoc multiple comparisons tests using the R software package called ‘Agricolae’50 to analyze the combined effect of developmental temperature, species and sex on the temperature preference across the three Drosophila species. With the SSR data recorded from each species, we employed the heatmap () function embedded in the R software to generate the heatmaps illustrating the neuronal responses of each OSN type when individually stimulated by the 57 odorant panels. We used the one-sample t-test (normally distributed data: Shapiro test: P > 0.05) or one-sample Wilcoxon (non-normally distributed data: Shapiro test: P < 0.05) statistical tests to compare the attraction indices (calculated from the trap assay data) with the theoretical mean zero (0). We ran a two-way analysis of variance to see the effect of the developmental and experimental temperature on the attraction. Statistical results were considered significant when P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Silke Trautheim for helping with the ordering of the three Drosophilid species used in this study. We thank also Dr. Daniel Veit for conceiving the thermal gradient apparatus used to test the temperature preference in the three drosophilid flies. This research was supported through funding by the Max Planck Society and specifically through funding to the Max Planck Center “Next Generation Insect Chemical Ecology.”
Author contributions
S.B.S.S, M.K, and B.S.H. designed the research plan. S.B.B.S performed all the experiments, analysed the data and prepared the figures. VPM performed initial SSR screening of D. virilis antenna. S.B.S.S, VPM, M.K, and B.S.H. discussed the results and wrote the manuscript.
Peer review
Peer review information
Communications Biology thanks Zainulabeuddin Syed and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Luke R. Grinham and George Inglis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data supporting the conclusions of this article are included within the article. Source data underlying main figures are provided in Supplementary Data 1. All other data collected during this study were archived in the FigShare data repository (10.6084/m9.figshare.23923296).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-023-05280-5.
References
- 1.Hansson BS, Stensmyr MC. Evolution of Insect Olfaction. Neuron. 2011;72:698–711. doi: 10.1016/j.neuron.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Shanbhag, S. R. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect. Morphol. Embryol.21 (1999).
- 3.de Bruyne M, Clyne PJ, Carlson JR. Odor Coding in a Model Olfactory Organ: The Drosophila Maxillary Palp. J. Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto A, Alenius M, Dickson BJ. Molecular, Anatomical, and Functional Organization of the Drosophila Olfactory System. Curr. Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Hallem EA, Carlson JR. Coding of Odors by a Receptor Repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 6.Khallaf MA, et al. Large-scale characterization of sex pheromone communication systems in Drosophila. Nat. Commun. 2021;12:4165. doi: 10.1038/s41467-021-24395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao CA. Chemosensory Coding by Neurons in the Coeloconic Sensilla of the Drosophila Antenna. J. Neurol. 2005;25:8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colinet H, Sinclair BJ, Vernon P, Renault D. Insects in Fluctuating Thermal Environments. Annu. Rev. Entomol. 2015;60:123–140. doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- 9.Knaden M, et al. Human Impacts on Insect Chemical Communication in the Anthropocene. Front. Ecol. Evol. 2022;10:791345. doi: 10.3389/fevo.2022.791345. [DOI] [Google Scholar]
- 10.Riveron J, Boto T, Alcorta E. The effect of environmental temperature on olfactory perception in Drosophila melanogaster. J. Insect Physiol. 2009;55:943–951. doi: 10.1016/j.jinsphys.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Riveron J, Alcorta E. Environmental temperature modulates olfactory reception in Drosophila melanogaster. J. Insect Physiol. 2011;57:1631–1642. doi: 10.1016/j.jinsphys.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Riveron J, Boto T, Alcorta E. Transcriptional basis of the acclimation to high environmental temperature at the olfactory receptor organs of Drosophila melanogaster. BMC Genomics. 2013;14:259. doi: 10.1186/1471-2164-14-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linn CE, Campbell MG, Roelofs WL. Temperature modulation of behavioural thresholds controlling male moth sex pheromone response specificity. Physiol. Entomol. 1988;13:59–67. doi: 10.1111/j.1365-3032.1988.tb00909.x. [DOI] [Google Scholar]
- 14.Zeiner R, Tichy H. Integration of temperature and olfactory information in cockroach antennal lobe glomeruli. J. Comp. Physiol. A. 2000;186:717–727. doi: 10.1007/s003590000125. [DOI] [PubMed] [Google Scholar]
- 15.Throckmorton, L. H. in The Genetics and Biology of Drosophila. Vol. 3b, (eds. M. Ashburner et al.), pp. 227–296 (Academic Press, London) (1982).
- 16.Maclean HJ, Kristensen TN, Sørensen JG, Overgaard J. Laboratory maintenance does not alter ecological and physiological patterns among species: a Drosophila case study. J. Evol. Biol. 2018;31:530–542. doi: 10.1111/jeb.13241. [DOI] [PubMed] [Google Scholar]
- 17.Rajpurohit S, Schmidt PS. Measuring thermal behavior in smaller insects: A case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly. 2016;10:149–161. doi: 10.1080/19336934.2016.1194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisodia S, Singh BN. Resistance to environmental stress in Drosophila ananassae: latitudinal variation and adaptation among populations. J. Evol. Biol. 2010;23:1979–1988. doi: 10.1111/j.1420-9101.2010.02061.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergland AO, Behrman EL, O’Brien KR, Schmidt PS, Petrov DA. Genomic Evidence of Rapid and Stable Adaptive Oscillations over Seasonal Time Scales in Drosophila. PLoS Genet. 2014;10:e1004775. doi: 10.1371/journal.pgen.1004775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabian DK, et al. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol. Ecol. 2012;21:4748–4769. doi: 10.1111/j.1365-294X.2012.05731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmore T, Ignell R, Carlson JR, Smith DP. Targeted Mutation of a Drosophila Odor Receptor Defines Receptor Requirement in a Novel Class of Sensillum. J. Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruyne M, Foster K, Carlson JR. Odor Coding in the Drosophila Antenna. Neuron. 2001;30:537–552. doi: 10.1016/S0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 23.Crowley-Gall A, et al. Population differences in olfaction accompany host shift in Drosophila mojavensis. Proc. R. Soc. B. 2016;283:20161562. doi: 10.1098/rspb.2016.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keesey IW, et al. Functional olfactory evolution in Drosophila suzukii and the subgenus Sophophora. iScience. 2022;25:104212. doi: 10.1016/j.isci.2022.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez, F., Witzgall, P. & Walker, W. B. Protocol for Heterologous Expression of Insect Odourant Receptors in Drosophila. Front. Ecol. Evol. 4, (2016).
- 26.Dekker T, Ibba I, Siju KP, Stensmyr MC, Hansson BS. Olfactory Shifts Parallel Superspecialism for Toxic Fruit in Drosophila melanogaster Sibling, D. sechellia. Curr. Biol. 2006;16:101–109. doi: 10.1016/j.cub.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 27.Keesey IW, Knaden M, Hansson BS. Olfactory Specialization in Drosophila suzukii Supports an Ecological Shift in Host Preference from Rotten to Fresh Fruit. J. Chem. Ecol. 2015;41:121–128. doi: 10.1007/s10886-015-0544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linz J, et al. Host plant-driven sensory specialization in Drosophila erecta. Proc. R. Soc. B. 2013;280:20130626. doi: 10.1098/rspb.2013.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anholt, R. R. H. Chemosensation and Evolution of Drosophila Host Plant Selection. 13. [DOI] [PMC free article] [PubMed]
- 30.Stensmyr MC, Dekker T, Hansson BS. Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc. R. Soc. Lond. B. 2003;270:2333–2340. doi: 10.1098/rspb.2003.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweck HKM, Ebrahim SAM, Farhan A, Hansson BS, Stensmyr MC. Olfactory Proxy Detection of Dietary Antioxidants in Drosophila. Curr. Biol. 2015;25:455–466. doi: 10.1016/j.cub.2014.11.062. [DOI] [PubMed] [Google Scholar]
- 32.Krause Pham C, Ray A. Conservation of Olfactory Avoidance in Drosophila Species and Identification of Repellents for Drosophila suzukii. Sci. Rep. 2015;5:11527. doi: 10.1038/srep11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bruyne M, Smart R, Zammit E, Warr CG. Functional and molecular evolution of olfactory neurons and receptors for aliphatic esters across the Drosophila genus. J. Comp. Physiol. A. 2010;196:97–109. doi: 10.1007/s00359-009-0496-6. [DOI] [PubMed] [Google Scholar]
- 34.Robertson HM, Kent LB. Evolution of the Gene Lineage Encoding the Carbon Dioxide Receptor in Insects. J. Insect Sc. 2009;9:1–14. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew, D. et al. Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc. Natl. Acad. Sci. USA. 110, (2013). [DOI] [PMC free article] [PubMed]
- 36.Stensmyr MC, et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 37.Saini RK, et al. Protecting cows in small holder farms in East Africa from tsetse flies by mimicking the odor profile of a non-host bovid. PLoS Negl. Trop. Dis. 2017;11:e0005977. doi: 10.1371/journal.pntd.0005977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 39.Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. Spatial Representation of Odorant Valence in an Insect Brain. Cell Rep. 2012;1:392–399. doi: 10.1016/j.celrep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Shamshev IV, Selitskaya OG. Methyl salicylate as an attractant for the dance fly Rhamphomyia gibba (Fallén) (Diptera, Empididae) Entmol. Rev. 2016;96:1003–1007. doi: 10.1134/S0013873816080054. [DOI] [Google Scholar]
- 41.Ito F, Awasaki T. Comparative analysis of temperature preference behavior and effects of temperature on daily behavior in 11 Drosophila species. Sci. Rep. 2022;12:12692. doi: 10.1038/s41598-022-16897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hangartner S, Dworkin I, DeNieu M, Hoffmann AA. Does increased heat resistance result in higher susceptibility to predation? A test using Drosophila melanogaster selection and hardening. J. Evol. Biol. 2017;30:1153–1164. doi: 10.1111/jeb.13084. [DOI] [PubMed] [Google Scholar]
- 43.Brankatschk, M. A Temperature-Dependent Switch in Feeding Preference Improves Drosophila Development and Survival in the Cold. 22. [DOI] [PubMed]
- 44.Zerulla FN, Augel C, Zebitz CPW. (2017) Oviposition activity of Drosophila suzukii as mediated by ambient and fruit temperature. PLoS ONE. 2017;12:e0187682. doi: 10.1371/journal.pone.0187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casterlin ME, Reynolds WW. Behavioral response of the New England dog whelk, Nassarius trivittatus, to a temperature gradient. Hydrobiologia. 1980;69:79–81. doi: 10.1007/BF00016539. [DOI] [Google Scholar]
- 46.Lynch KE, White TE, Kemp DJ. The effect of captive breeding upon adult thermal preference in the Queensland fruit fly (Bactrocera tryoni) J. Therm. Biol. 2018;78:290–297. doi: 10.1016/j.jtherbio.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Olsson, S. B. & Hansson, B. S. Electroantennogram and Single Sensillum Recording in Insect Antennae. in Pheromone Signaling (ed. Touhara, K.). 1068 157–177 (Humana Press, 2013). [DOI] [PubMed]
- 48.Lin C-C, Potter CJ. Re-Classification of Drosophila melanogaster Trichoid and Intermediate Sensilla Using Fluorescence-Guided Single Sensillum Recording. PLoS ONE. 2015;10:e0139675. doi: 10.1371/journal.pone.0139675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team. R: A Language and Environment for Statistical Computing_. Vienna, Austria: R Foundation for Statistical Computing; 2023. _. [Google Scholar]
- 50.de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research_. R package version 1.3-6. https://CRAN.R-project.org/package=agricolae. (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Data supporting the conclusions of this article are included within the article. Source data underlying main figures are provided in Supplementary Data 1. All other data collected during this study were archived in the FigShare data repository (10.6084/m9.figshare.23923296).