Abstract
Objective
Men’s sexual health plays an important role in male fertility and childbearing, as it is associated with factors such as sexual desire, healthy spermatogenesis, and erectile function. In various cultures, medicinal plants have been utilized to address male sexual issues, including infertility and erectile dysfunction. Despite recent advancements in medical science for treating male impotence, some men opt for herbal supplements as an alternative, given that numerous herbs have the potential to enhance male sexual performance. The Apiaceae family is one of the oldest plant families used for medicinal purposes. Ferula, a genus within this family, comprises approximately 170 different species worldwide. Members of this genus possess numerous therapeutic properties due to the presence of various compounds. This article aims to explore the potential impacts of Ferula plants on the male reproductive system.
Methods
This review article was prepared by searching for terms including Ferula and “aphrodisiac,” Ferula and “spermatogenesis,” and Ferula and “male reproductive system.” Relevant information was gathered through electronic databases, including ISI Web of Knowledge, PubMed, and Google Scholar.
Results
The findings indicated that relatively comprehensive studies have been conducted in this area, revealing that certain Ferula species have been employed in folk medicine to boost fertility and libido. Recent research has corroborated these effects.
Conclusion
It is hoped that new aphrodisiac compounds with fewer side effects can be isolated from Ferula plants in the future.
Keywords: Aphrodisiacs, Ferula, Infertility, Spermatogenesis
Introduction
Erectile dysfunction (ED), loss of sexual interest, ejaculation disorders, and sperm abnormalities are key problems of the male reproductive system that play important roles in population growth and mental health [1]. Infertility in humans is characterized by the inability to conceive after 1 year of regular intercourse, affecting approximately 8% to 12% of couples worldwide. Male infertility accounts for roughly 40% to 50% of fertility failures [2]. Factors such as chemotherapy, antibiotics, radiation therapy, stress, pollution, poor eating habits, environmental factors, work, and lifestyle changes contribute to fertility issues and sperm abnormalities, which cause 30% to 40% of infertility cases [3]. Infertility treatments vary for several reasons and range from simple drug therapies to laboratory procedures and advanced surgery [4]. Primary testicular defects are a main cause of male infertility; these include abnormal sperm parameters such as number, morphology, and motility and account for 65% to 80% of cases [5]. In contrast, male sexual dysfunction refers to a collection of conditions, including ED and premature ejaculation (PE), which can ultimately result in male sexual dysfunction. ED is a globally prevalent disorder related to the male reproductive system that negatively impacts quality of life, particularly among older men [6]. This condition can be caused by factors such as androgen deficiency, atherosclerosis, diabetes, spinal cord injury, high cholesterol level, high blood pressure, prostate surgery, and psychological conditions such as depression [7]. Several medicinal compounds, known as phosphodiesterase type 5 inhibitors, are used to treat ED, including vardenafil, sildenafil, avanafil, and tadalafil (Figure 1) [8]. Separately, PE is defined as a lack of ejaculation control accompanied by distress, which can have physiological or psychological origins [9].
Figure 1.
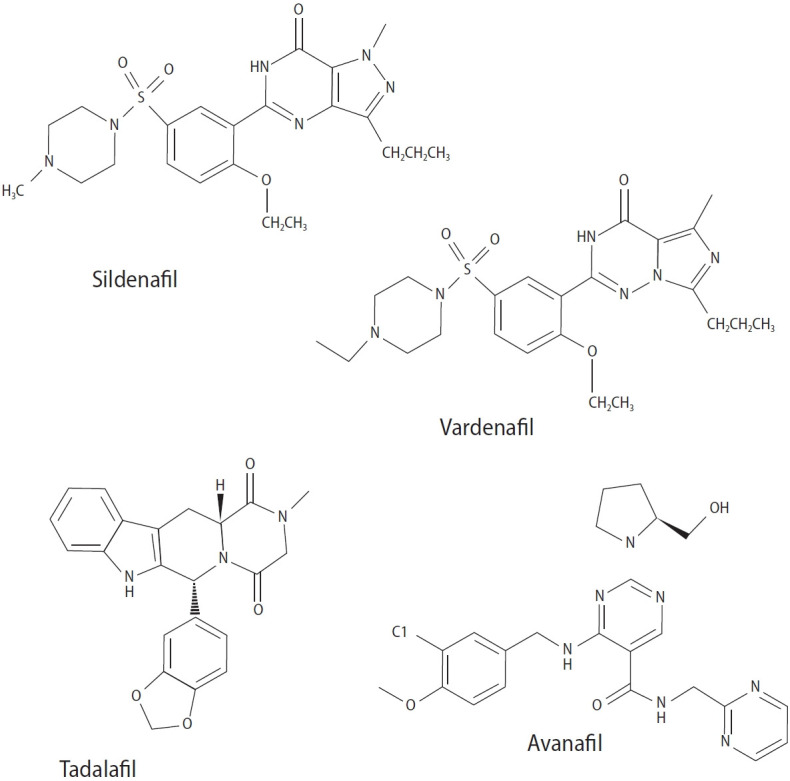
Chemical structures of common erectile dysfunction medications.
Generally, compounds used to increase sperm count or improve libido are associated with side effects. Complementary treatments and traditional medicine represent knowledge passed down through generations, based on local methods. Medicinal plants play an important role in the traditional medicine of various countries [10]. Apiaceae is a family of flowering plants that primarily grow in dry and temperate regions [11]. These plants typically have a pungent smell due to the presence of sulfide compounds and volatile essential oils [12]. The genus Ferula includes approximately 170 species that grow in areas ranging from Central Asia and the Mediterranean to North Africa [13]. These herbs have been reported in the scientific literature for their aphrodisiac and spermatogenic activity. Since ancient times, different species of Ferula have been used as aphrodisiacs in traditional and folk medicine across countries. In traditional Turkish medicine, several species of Ferula, such as the root and oleo gum resin of Ferula elaeochytris, Ferula communis, Ferula assa-foetida, and Ferula gummosa, have been used as aphrodisiacs to treat male sexual disorders. Ferula hermonis, also known as “zallouh” in the Middle East, is still used as a male aphrodisiac [14]. In Iranian traditional medicine, F. assa-foetida has also been used as an aphrodisiac [15]. In the Ayurvedic system of ancient India as well as in South American traditional medicine, such as in Brazil, asafoetida is considered an aphrodisiac [16]. In Nepal, asafoetida is used daily, primarily as an aphrodisiac for men [17]. Ibn Sina (Avicenna) and Al-Antaki also emphasized the aphrodisiac effects of F. assa-foetida [18]. Scientific investigations have demonstrated a number of medicinal properties of Ferula, including antinociceptive [19], antihemolytic and antioxidant [20], anticoagulant [21], anticonvulsant [22], relaxant [23], memory enhancement [24,25], antihyperglycemic [26], acetylcholinesterase inhibitory [27], antidepressant [28], antiulcer [15], antitumor [29], and anti-demyelination [30] properties. A phytochemical analysis of plants in this genus has shown that they contain various compounds, including terpenoids, coumarins, and many sulfide compounds (Figure 2) [31]. Due to the widespread use of these plants in the treatment and prevention of male sexual dysfunction, this review article is focused on scientific evidence of the effects of various compounds and extracts of these plants on increasing spermatogenesis and aphrodisiac properties.
Figure 2.
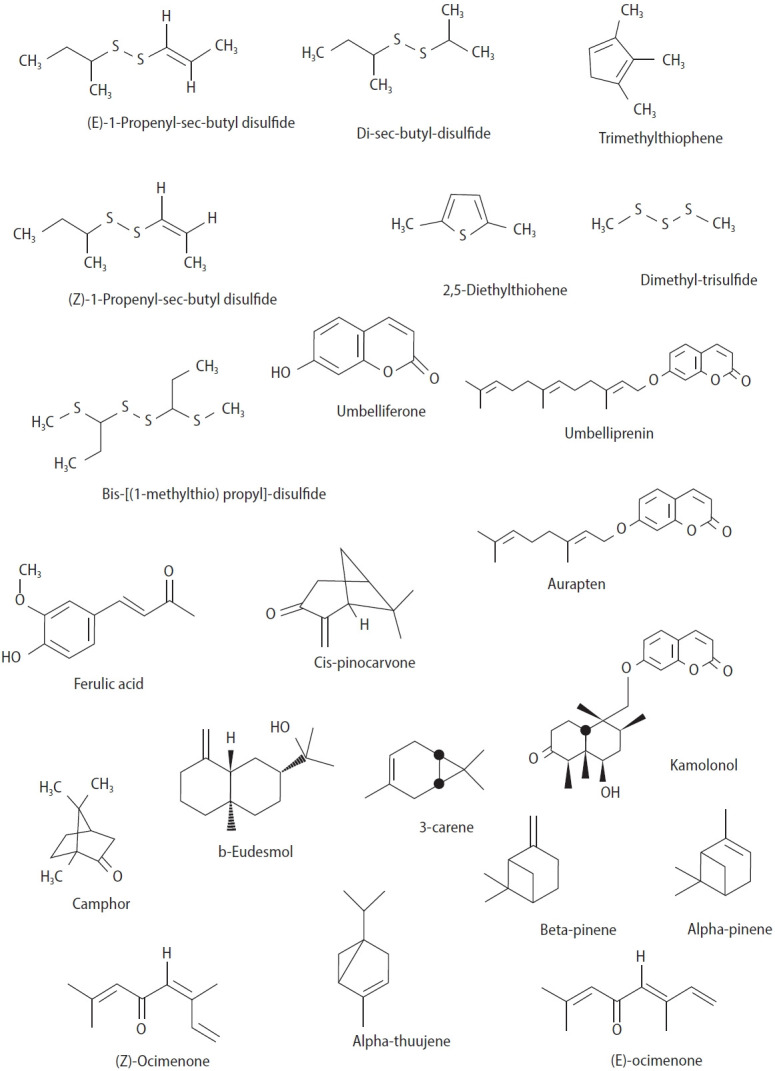
Chemical structures of notable compounds isolated from Ferula plants.
Methods
Articles were retrieved using various combinations of keywords, such as “male reproductive system,” “Ferula,” “aphrodisiac,” and “spermatogenesis,” from scientific databases such as PubMed, Google Scholar, and Science Direct. Studies published through the end of 2022 that focused on the male reproductive system and extracts or compounds isolated from the genus Ferula were summarized and included in this paper (Table 1). The primary limitations of this study were the inability to access the full texts of some articles and the lack of subscriptions to certain journals by our academic center.
Table 1.
Summary of the effects of the Ferula genus on sexual function and improvement of sperm parameters
| Species name | Extract/part used/ dose(s) | Animal | Study | Effects |
|---|---|---|---|---|
| Sperm parameters | Water extract/root/0.025 mL/100 g of Ferula hermonis | Rats | Hanafi et al. (2010) [33] | Sperm factors and testosterone levels improved. |
| Aqueous root extract/3 mg/kg of F. hermonis | Mice | Khleifat et al. (2001) [39] | Sperm motility was reduced, and sperm abnormalities increased. | |
| Root extract/100–200 mg of F. hermonis | Duck | Al-Salhie et al. (2019) [34] | Weight and volume of the testes and concentrations of LH, FSH, and testosterone increased in the 200 mg group. | |
| Alcoholic/roots (0.03 mL/50 mL) of F. hermonis | Holstein bulls | Abdulkareem et al. (2018) [35] | The percentages of sperm tail abnormalities decreased for all preservation periods (2, 30, and 60 days after freezing). | |
| 1% concentration extract of Ferula elaeochytris | Redfish | Inanan et al. (2021) [62] | Improved sperm parameters | |
| Aqueous extract of gum/50, 100, 200 mg/kg of Ferula assa-foetida | Rats | Bagheri et al. (2015) [54] | Increased sperm motility and improved sperm morphology | |
| 0.025/100 g methanol root extract of F. hermonis | Rats | Girgis et al. (2021) [37] | The sperm indices and testosterone level improved. | |
| Ethanolic root extract 0.2% percent of Ferula communis | Fish | Koca et al. (2021) [68] | The seminiferous lobules were larger, with considerably more sperm. | |
| 3 kg of F. assa-foetida seeds | Snowflake rams | O’g’li (2019) [51] | Sperm concentration increased by 20%, number of live sperm by 80%, and motility by 19.6%. | |
| Aqueous of gum extract/50–100 mg/kg of F. assa-foetida | Rats | Selyametovich et al. (2022) [52] | Improved quantitative and qualitative sperm indicators | |
| 50 mg/kg hydroalcoholic extract of seeds of F. assa-foetida | Rats | Manayi et al. (2017) [56] | Improved sperm count, morphology, and motility and increased testosterone and LH levels. | |
| Sexual function | Essential oil of seeds/0.05, 0.5–2 g/kg of F. hermonis | Rats | El-Thaher et al. (2001) [42] | Improved penile erection at 0.05, 0.5–2 g/kg |
| 30 and 60 mg/kg of F. hermonis | Rats | Zanoli et al. (2003) [43] | Increased the sexual motivation of normal animals with acute improvements in sluggish/impotent male rats. | |
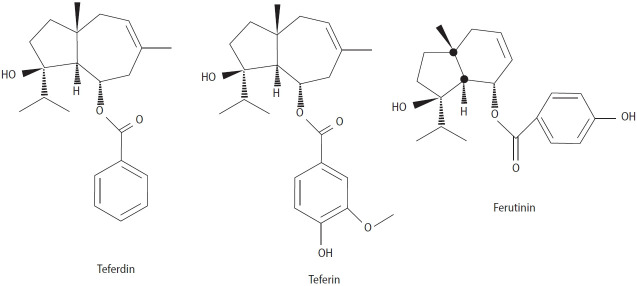
| Ferutinin, teferdin, and teferin in an acute (2.5 mg/kg) and sub-chronic (0.25 mg/kg) regimen of F. hermonis | Rats | Zanoli et al. (2005) [46] | Ferutinin and teferdin reduced mount and intromission latencies and shortened the ejaculation latency only in acute form. | |
| Seeds and 50% water-ethanol extracts of roots of F. assa-foetida | Rats and men | Kassis et al. (2009) [57] | Increased erection in rats and humans. Improved sperm count in people with untreatable azoospermia and incomplete azoospermia. | |
| Chloroform and deacetylkellerin, elaeochytrin-A and ferutinin/10 mg/kg of Ferula huber | Rats | Aydogan et al. (2020) [14] | Chloroform extract stimulated sexual behaviors, and ferutinin had the most effective aphrodisiac effect. | |
| Methanol root extract/40 and 60 mg/kg of F. elaeochytris | Rats | Eser et al. (2020) [61] | Significantly improved sexual parameters including ICP, testicular weight, and spermatogenesis. | |
| Root extract/40 and 20 mg/kg of F. elaeochytris | Older rats | Eser et al. (2020) [63] | Improved penile neuronal nitric oxide synthase, serum testosterone, and ICP/MAP. | |
| CHCl3 fraction (200 mg/kg) of Ferula drudeana and feselol, samarcandin, and 3′-O-acetyl samarcandin | Rats | Alqarni et al. (2020) [65] | Increased mount frequency and intromission frequency, ejaculation latency, and postejaculatory interval. Samarcandin showed a particularly strong aphrodisiac effect. | |
| 600 mg/kg/root extract (petroleum ether, ethyl acetate, methanol, and water) of F. hermonis | Rats | Hadidi et al. (2003) [36] | Petroleum ether and ethyl acetate extracts decreased MR, IR, and IL. Methanolic extract increased MR and water extract and decreased IL only. |
LH, luteinizing hormone; FSH, follicle-stimulating hormone; ICP, intracavernosal pressure; MAP, mean arterial pressure; MR, mount rate; IR, intromission rate; IL, intromission latency.
Ferula hermonis
F. hermonis Boiss is a perennial plant found in limited geographical areas, including Lebanon and Syria. Among the various Ferula species evaluated, this species has been most extensively studied for its aphrodisiac effects and capacity to improve sperm health. In different cultures and nationalities, this plant is used to increase libido. The plant’s root is commonly described as “Lebanese root” or “Lebanese viagra.” It has been used in folk medicine to reduce total body weight and plasma cholesterol levels, as well as to treat impotence and frostbite, skin infections, stomach disorders, fever, dysentery, and neurological disorders such as hysteria [32]. Thermal stress can adversely affect physiology in various ways, such as reducing fertility, activity, respiration rate, and food intake. A study examining the use of F. hermonis water root extract (0.025 mL/100 g for 8 weeks, orally) demonstrated that the extract can prevent the harmful effects of heat stress on sperm number, motility, morphology, and viability while significantly increasing testosterone level [33]. Examining the effects of 100 and 200 mg/kg of F. hermonis root extract in ducks revealed that the relative weight, length, width, and volume of the testicles, the diameter of the seminiferous tubules, and the thickness of the seminiferous epithelium increased. Additionally, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone levels significantly increased [34]. One study showed that F. hermonis alcoholic root extract (0.03 mL/50 mL) can reduce sperm abnormalities in Holstein bulls caused by freezing with different storage periods (2, 30, and 60 days after freezing) [35]. This study demonstrates that the plant extract has protective effects on sperm against freezing. A study on the effect of 600 mg/kg of oral F. hermonis root extracts (petroleum ether, ethyl acetate, methanol, and water) on the sexual behavior of male rats showed that the petroleum ether and ethyl acetate extracts significantly decreased mount rate, intromission rate, and intromission latency. The methanolic extract caused a significant increase in mount rate, while the water extract impacted only intromission latency [36]. The protective effects of F. hermonis are not limited to that study. Research has shown that F. hermonis not only increases testicular function and sexual desire but also can play a protective role against drugs that damage the testicles and the process of spermatogenesis. For example, Cycram is used in neoplastic treatment, but this medication also has destructive effects on testicular tissue. A related study showed that the simultaneous use of the methanol extract of F. hermonis root at a dose of 0.025/100 g improved sperm indices and testosterone levels, which apparently related to the reduction of plasma lipid peroxidation levels and the increase of antioxidant enzyme activity in the tested animals [37]. Furthermore, the simultaneous use of F. hermonis extract (3 mg/kg) and bee honey (1.5 mL/kg) for 10 consecutive days showed that this extract had a protective effect on the testis against gamma radiation (8 Gy). The weight of the testis and antioxidant indices improved, and the serum levels of testosterone, FSH, and LH were restored [38]. Contrary to these results, other studies have indicated that F. hermonis has no significant effect on the improvement of sperm or sexual behavior and may even cause damage and deterioration of sperm indicators. Administration of an aqueous extract of F. hermonis to male rats (3 mg/kg) for 6 weeks caused a decrease in the weight of the accessory sexual organs. Additionally, the number of epididymal sperm and their motility significantly decreased, and sperm abnormalities significantly increased [39]. In another study, oral administration of aqueous extract of F. hermonis root (6 mg/kg) to mice for 6 weeks significantly decreased testosterone levels and caused fertility impairment. Histopathological degenerative changes and a significant decrease in the expression of estrogen receptor b in the testes, epididymis, and seminal vesicles were also observed [40]. Consumption of 1.5, 3, and 6 mg/kg of the aqueous extract of F. hermonis root for 3 and 6 weeks was associated with decreased testosterone levels and severe histopathological damage in the testis [41]. This issue may be related to the duration of use, the type of extraction, and the concentration used. Studies have shown that long-term use of F. hermonis can also cause toxicity and testicular atrophy. El-Thaher et al. [42] showed that administration of the essential oil of F. hermonis seeds improved the penile erection index in a dose-dependent manner, but its use also caused toxicity after 28 days. According to some findings, F. hermonis can strongly impact sexual motivation only acutely, and it does not have a significant effect when consumed sub-chronically. Zanoli et al. [43] found that the injection of 30 and 60 mg/kg of F. hermonis extract increased the sexual motivation of normal animals and led to improvements among sluggish/impotent male rats, but long-term use did not have a significant effect. These results showed that F. hermonis can exert dual and opposite effects. Such impacts are related to the increase in testosterone levels, as the injection of 0.01 and 0.02 mL/100 g/body weight of 50% aqueous extract of F. hermonis has been shown to increase the testosterone level in male rats [44]. However, limited studies have been conducted on the effective compounds of this plant. Ferutinin, isolated from F. hermonis, is an oxygenated sesquiterpene that appears to act through the nitric oxide signaling pathway [45]. The injection of the three compounds of ferutinin, teferdin, and teferin (Figure 3) isolated from F. hermonis in sexually potent and sluggish/impotent animals with an acute (2.5 mg/kg) and sub-chronic (0.25 mg/kg per day for 10 days) regimen showed that ferutinin and teferdin can reduce mounting and intromission latencies and shorten ejaculation latency only in the acute regimen both compounds increased testosterone levels in rats, but only teferdin increased sex appetite and testosterone levels sub-chronically, while sub-chronic ferutinin had a negative effect and decreased testosterone levels with long-term use [46].
Figure 3.
Chemical structures of three oxygenated sesquiterpenes isolated from Ferula hermonis.
Ferula assa-foetida
F. assa-foetida is a native plant of Iran and Afghanistan that naturally grows in the hot and dry central regions of Iran. This plant is one of the most well-known species of Ferula and has been used to treat various diseases [47]. It is a perennial plant, approximately 2 m in length, with a strong and unpleasant odor. A sticky gum, called anghuzeh in Persian and “asafoetida” in scientific terms, is obtained from cutting its roots [30]. The main components of asafoetida generally include sulfide, glycosidic, and various sesquiterpene coumarin compounds [48]. In many countries, such as Iran, Afghanistan, Nepal, and Arabic countries, this plant is used as an aphrodisiac [49]. In Iranian traditional medicine, it has also been mentioned as an aphrodisiac, and pregnant women are advised to avoid consuming it due to the risk of miscarriage [50]. Feeding Karakul rams a diet of 6 kg of compound feed and 3 kg of F. assa-foetida seeds for 30 days resulted in a 27.3% increase in ejaculation capacity, a 20% increase in sperm concentration, an 80% increase in the number of live sperm, and a 19.6% increase in sperm motility. Additionally, sperm breathing increased significantly (114.3%) within 15 minutes [51]. Apparently, although asafoetida increases sperm count and improves its quality, it may cause a decrease in plasma testosterone concentration, especially at high doses. In one study, injecting 50 mg/kg of asafoetida aqueous extract resulted in a decreased plasma testosterone concentration in the treatment group, but sperm count and quality were increased significantly [52]. In separate research, administering 75, 150, and 300 mg of asafoetida extract to rats for 15 days led to a decrease in the thickness of the cell layers of the seminiferous tubules, and the number of Leydig and Sertoli cells, as well as blood testosterone concentration, decreased significantly compared to the control group [53]. Injecting asafoetida at doses of 25, 50, 100, and 200 mg/kg into male rats for 6 weeks showed that asafoetida can increase sperm motility and improve sperm morphology. However, at a high dose (200 mg/kg), Leydig cells became vacuolated, and testosterone levels decreased [54]. Although the positive effect of asafoetida on spermatogenesis has been determined in various studies, sufficient consideration should be given to the dose and duration of its use. These results suggest that additional studies are needed to clarify the toxicity of this plant. One study on this topic showed that long-term use with a dose of 200 mg/kg and above can increase liver enzymes and potentially cause liver damage [55]. In addition to the gum, the seeds of F. assa-foetida also appear to have aphrodisiac and spermatogenic effects. In rats, oral consumption of hydroalcoholic extract from the seeds of F. assa-foetida for 6 weeks at a dose of 50 mg/kg significantly improved fertility parameters, including sperm count, sperm morphology, and motility, and increased testosterone and LH levels in the treated group [56]. “Masculinity” tablets, prepared from seeds and 50% water-ethanol extracts from the roots of F. assa-foetida, were found to increase erection in rats and humans. This combination also led to a quantitative and qualitative improvement in sperm count for individuals with untreatable azoospermia and incomplete azoospermia after 2 months of treatment, by 17% and 60%, respectively. A significant improvement in libido and erectile function in men was also reported [57].
Ferula huber
Ferula huber is one of several species of the genus Ferula, native to Turkey and widely used in the traditional medicine of that country. Yusufoglu et al. [58] demonstrated that the methanol extract of F. huber has significant antioxidant and hypoglycemic activities. Like many other Ferula species, F. huber is utilized as an aphrodisiac in traditional Turkish medicine. The effects of aqueous and chloroform extracts from the root of the F. huber plant, as well as gummosin, mogoltavidin, deacetylkellerin, ferukrin acetate with kellerin, elaeochytrin-A, and ferutinin isolated from the chloroform extract, were tested on the aphrodisiac potential of rats. The results showed that only the chloroform extract and three compounds—deacetylkellerin, elaeochytrin-A, and ferutinin (Figure 4)—had a significant impact on stimulating sexual behaviors. Among these sesquiterpenoids, ferutinin was shown be the most effective aphrodisiac compound [14].
Figure 4.
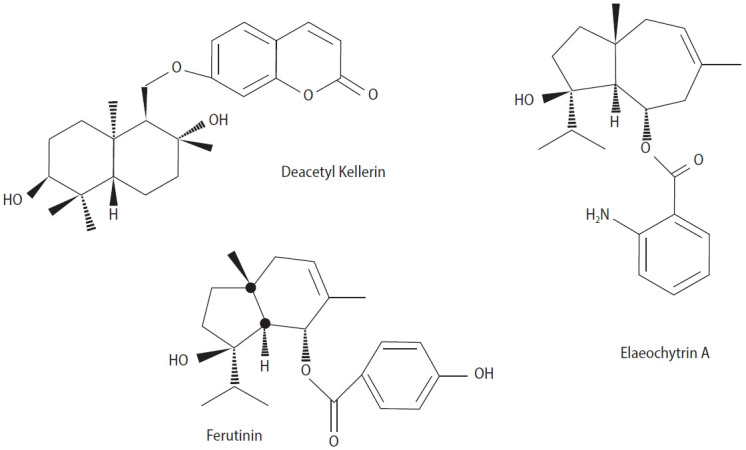
Chemical structures of three components isolated from Ferula huber.
Ferula orientalis
Ferula orientalis, known as heliz in Turkey, is used as an antiseptic, sedative, antispasmodic, laxative, digestive, expectorant, diuretic, aphrodisiac, and stimulant [59]. The application of root extract from this plant has been shown to induce 98% relaxation in corpus cavernosum strips in response to acetylcholine (1 mM), electrical field stimulation (10 Hz), and sodium nitroprusside (0.1 μM), thereby enhancing erectile function in diabetic animals [60].
Ferula elaeochytris
F. elaeochytris, a perennial herb native to Turkey and known as çakşır in Anatolia, is widely used for its aphrodisiac, antioxidant, anti-inflammatory, and anti-diabetic properties. ED is an important complication of diabetes, leading to impotence in men with the condition. One study demonstrated that the methanol root extract of F. elaeochytris, at concentrations of 40 and 60 mg/kg, improved sexual parameters such as intracavernosal pressure (ICP) and testicular weight after 8 weeks, while also reducing blood sugar levels. Histopathological results indicated that extract consumption significantly increased spermatogenesis, the ICP/mean arterial pressure (MAP) ratio, and testis weight in diabetic animals [61]. Another study found that using a 1% concentration of F. elaeochytri extract improved sperm parameters in adult male redfish (Carassius auratus) after 60 days, whereas a 0.5% dose decreased sperm parameters and increased oxidative stress [62]. Thus, it is crucial to consider the appropriate concentration of medicinal plants before use. ED is an age-related side effect that can diminish quality of life among older individuals. Consequently, discovering new compounds to alleviate this disorder can greatly enhance the lives of the elderly. A study involving the oral administration of F. elaeochytris extract at concentrations of 40 and 20 mg/kg in aged rats revealed that the extract could positively impact changes in smooth muscle cells and collagen fibrils, tumor necrosis factor-α level, penile neuronal nitric oxide synthase expression, serum testosterone level, neurogenic and endothelial-dependent relaxation of the corpus cavernosum, the ICP/MAP ratio, total antioxidant status, and total oxidant status in corpus cavernosal tissue. The extract also increased serum testosterone levels, decreased tumor necrosis factor-α levels, and balanced oxidative and antioxidant parameters [63].
Ferula drudeana
Ferula drudeana is an indigenous plant found in the central regions of Turkey, traditionally used as an aphrodisiac. The primary components of the fruit’s essential oil are shyobunone (44.2%) and 6-epishyobunone (12.6%), and it has been established that various parts of the plant, such as the root and fruit, possess antibacterial properties [64]. Additionally, another study has demonstrated anti-diabetic and antioxidant effects [58]. A CHCl3 solution fraction (200 mg/kg body weight) and three compounds isolated from this plant, namely feselol, samarcandin, and 3′-O-acetyl samarcandin (Figure 5), were found to significantly increase mount frequency, intromission frequency, ejaculation latency, and postejaculatory interval in male rats. The mount and intromission latencies were notably reduced, while ejaculation latency was extended. Both feselol and samarcandin contributed to the decrease in postejaculatory interval, with samarcandin exhibiting a more potent aphrodisiac effect than feselol [65].
Figure 5.
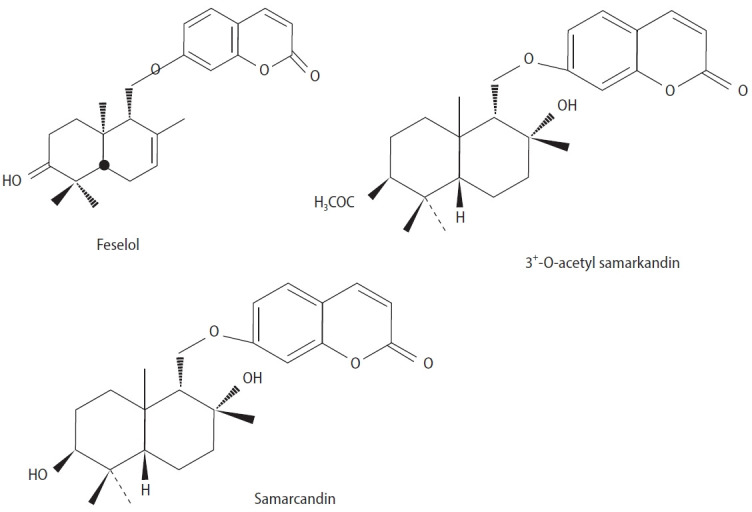
Chemical structures of three compounds isolated from Ferula drudeana.
Ferula communis
F. communis is a Mediterranean umbelliferous plant with a rich history of medicinal and ecologically interesting uses. It naturally grows in the barren lands of the eastern Mediterranean mountainous regions of Turkey [66]. Some individuals consume the ground root of F. communis mixed with honey to boost libido. Goat herders and indigenous peoples assert that this plant has a sexually enhancing effect on both animals and humans. Additionally, F. communis is traditionally used as an anti-hysteria treatment and for treating dysentery. Research on F. communis has revealed that this plant contains two main types of phytochemicals: sesquiterpene daucane esters and prenylated coumarins [67]. A study involving the administration of a food supplement containing 0.2% F. communis extract for 90 days in fish demonstrated that the seminiferous lobules in the treatment group were larger and contained considerably more sperm, while no degenerative changes were observed. These findings suggest that this extract improves reproductive performance and may be effective in increasing ovarian and testicular capacity [68].
Conclusion
Findings from studies on the aphrodisiac and spermatogenic effects of the Ferula genus indicate that these plants hold promise for further research. It is well-known that these plants have been utilized as aphrodisiacs in the Middle East since ancient times. The recent results largely support the claims made by traditional medicine in this area. Among the plants in this genus, F. hermonis stands out as particularly noteworthy, and it appears that ferutinin, one of the isolated compounds, has a more potent effect on the male reproductive system than other compounds. These discoveries provide valuable insights for researchers to build upon in future studies. Such studies should focus on isolating additional compounds, determining the toxicity of these isolated compounds, and ultimately conducting human and clinical trials.
Acknowledgments
We are very grateful to the staff of the Yazd Neuroendocrinology Research Center. We also very thankful from Dr. Fatemeh Zare Mehrjardi for facilitating the project.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SMB, NA. Data curation: AS, JAG, MY, NA. Formal analysis: MY. Funding acquisition: AS. Methodology: SMB. Project administration: SMB. Visualization: AS. Writing-original draft: SMB, AS. Writing-review & editing: MY.
References
- 1.Monga M, Bernie J, Rajasekaran M. Male infertility and erectile dysfunction in spinal cord injury: a review. Arch Phys Med Rehabil. 1999;80:1331–9. doi: 10.1016/s0003-9993(99)90039-4. [DOI] [PubMed] [Google Scholar]
- 2.Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med. 2020;13:29–41. doi: 10.2147/IJGM.S241099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25:S35–40. doi: 10.12968/bjon.2016.25.18.S35. [DOI] [PubMed] [Google Scholar]
- 4.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM Guideline Part I. J Urol. 2021;205:36–43. doi: 10.1097/JU.0000000000001521. [DOI] [PubMed] [Google Scholar]
- 5.Leslie SW, Soon-Sutton TL, Khan MA. Male infertility. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 6.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 7.Ludwig W, Phillips M. Organic causes of erectile dysfunction in men under 40. Urol Int. 2014;92:1–6. doi: 10.1159/000354931. [DOI] [PubMed] [Google Scholar]
- 8.Agrahari N, Lakshameesha C, Roy S, Awadhesh NC. Regulatory insight for aphrodisiac drugs. J Drug Des Res. 2021;8:1077. [Google Scholar]
- 9.Rew KT. Men’s health: male sexual dysfunction. FP Essent. 2021;503:28–33. [PubMed] [Google Scholar]
- 10.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amiri MS, Joharchi MR. Ethnobotanical knowledge of Apiaceae family in Iran: a review. Avicenna J Phytomed. 2016;6:621–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41:683–93. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Moran J, van Rijswijk B, Traicevski V, Kitajima EW, Mackenzie AM, Gibbs AJ. Potyviruses, novel and known, in cultivated and wild species of the family Apiaceae in Australia. Arch Virol. 2002;147:1855–67. doi: 10.1007/s00705-002-0865-8. [DOI] [PubMed] [Google Scholar]
- 14.Aydogan F, Baykan S, Soliman GA, Yusufoglu H, Bedir E. Evaluation of the potential aphrodisiac activity of sesquiterpenoids from roots of Ferula huber-morathii Pesmen in male rats. J Ethnopharmacol. 2020;257:112868. doi: 10.1016/j.jep.2020.112868. [DOI] [PubMed] [Google Scholar]
- 15.Bagheri SM, Yadegari M, Zare-Mohazabiye F, Momeni-Asl H, Mirjalili A, Anvari M, et al. Effect of Ferula assa foetida oleo-gum-resin on gastric ulcer in indomethacin-ulcerated rats. J Curr Res Sci Med. 2018;4:42–6. [Google Scholar]
- 16.Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin): a review. J Ethnopharmacol. 2011;134:1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 17.Eigner D, Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 18.Bagheri SM, Yadegari M, Behpur M, Javidmehr D. Antilithiatic and hepatoprotective effects of Ferula assa-foetida oleo-gum-resin on ethylene glycol-induced lithiasis in rats. Urol Sci. 2018;29:180–5. [Google Scholar]
- 19.Bagheri SM, Dashti-R MH, Morshedi A. Antinociceptive effect of Ferula assa-foetida oleo-gum-resin in mice. Res Pharm Sci. 2014;9:207–12. [PMC free article] [PubMed] [Google Scholar]
- 20.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Dehpour AA. Antioxidant activity of hydroalcholic extract of Ferula gummosa Boiss roots. Eur Rev Med Pharmacol Sci. 2011;15:658–64. [PubMed] [Google Scholar]
- 21.Lamnaouer D. Anticoagulant activity of coumarins from Ferula communis L. Therapie. 1999;54:747–51. [PubMed] [Google Scholar]
- 22.Bagheri SM, Rezvani ME, Vahidi AR, Esmaili M. Anticonvulsant effect of Ferula assa-foetida oleo gum resin on chemical and amygdala-kindled rats. N Am J Med Sci. 2014;6:408–12. doi: 10.4103/1947-2714.139296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagheri S, Hejazian Sh, Dashti-R M. The relaxant effect of seed’s essential oil and oleo-gum-resin of Ferula assa-foetida on isolated rat’s ileum. Ann Med Health Sci Res. 2014;4:238–41. doi: 10.4103/2141-9248.129050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijayalakshmi, Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S. Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44:82–7. doi: 10.4103/0253-7613.91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagheri SM, Dashti-R MH. Influence of asafoetida on prevention and treatment of memory impairment induced by d-galactose and NaNO2 in mice. Am J Alzheimers Dis Other Demen. 2015;30:607–12. doi: 10.1177/1533317515576388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iranshahi M, Alizadeh M. Antihyperglycemic effect of Asafoetida (Ferula assafoetida Oleo-Gum-Resin) in streptozotocin-induced diabetic rats. World Appl Sci J. 2012;17:157–62. [Google Scholar]
- 27.Deveci E, Tel-Cayan G, Duru ME. Phenolic profile, antioxidant, anticholinesterase, and anti-tyrosinase activities of the various extracts of Ferula elaeochytris and Sideritis stricta. Int J Food Prop. 2018;21:771–83. [Google Scholar]
- 28.Kumar TB, Reddy VJ, Rushendran R, Mamatha T, Roja J, Roopavani T. Antidepressant activity of ethanolic extract of oleo gum resins of Ferula asafoetida Linn. J Pre-Clin Clin Res. 2017;11:50–60. [Google Scholar]
- 29.Bagheri SM, Abdian-Asl A, Moghadam MT, Yadegari M, Mirjalili A, Zare-Mohazabieh F, et al. Antitumor effect of Ferula assa foetida oleo gum resin against breast cancer induced by 4T1 cells in BALB/c mice. J Ayurveda Integr Med. 2017;8:152–8. doi: 10.1016/j.jaim.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagheri SM, Maghsoudi MJ, Yadegari M. Preventive effect of Ferula asafoetida oleo gum resin on histopathology in cuprizone-induced demyelination mice. Int J Prev Med. 2020;11:179. doi: 10.4103/ijpvm.IJPVM_108_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayed-Ahmad B, Talou T, Saad Z, Hijazi A, Merah O. The Apiaceae: ethnomedicinal family as source for industrial uses. Ind Crops Prod. 2017;109:661–71. [Google Scholar]
- 32.Sattar Z, Iranshahi M. Phytochemistry and pharmacology of Ferula hermonis Boiss: a review. Drug Res (Stuttg) 2017;67:437–46. doi: 10.1055/s-0043-109100. [DOI] [PubMed] [Google Scholar]
- 33.Hanafi EM, Raouf AA, Kassem SS, Abdel-Kader MM, Elkadrawy HH. A novel herbal remedy to alleviate drawbacks of heat stress in rats with special references to some reproductive and molecular alterations. Glob J Biotechnol Biochem. 2010;5:145–52. [Google Scholar]
- 34.Al-Salhie KC, Al-Hummod SK. Ferula hermonis roots extract on testicular biometry and reproductive hormones in local ducks. Indian Vet J. 2019;96:14–7. [Google Scholar]
- 35.Abdulkareem TA, Khalil RI, Salman AH. Effect of adding Ferula hermonis Boiss roots and some antioxidants to Tris extender on post-cryopreserved sperm abnormalities percentage of Holstein bulls. Al-Anbar J Vet Sci. 2018;11:70–81. [Google Scholar]
- 36.Hadidi KA, Aburjai T, Battah AK. A comparative study of Ferula hermonis root extracts and sildenafil on copulatory behaviour of male rats. Fitoterapia. 2003;74:242–6. doi: 10.1016/s0367-326x(03)00032-7. [DOI] [PubMed] [Google Scholar]
- 37.Girgis SM, ElRaouf AA, Abdou HS. Protective effect of Ferula hermonis root extract against cycraminduced DNA, biochemical and testicular damage in rats. Jordan J Biol Sci. 2021;14:105–10. [Google Scholar]
- 38.Osman NN. Antioxidant effects of Ferula hermonis and bee honey on γ-radiation-induced oxidative testicular damage in rats. J Radiat Res Appl Sci. 2011;4(4A):1201–19. [Google Scholar]
- 39.Khleifat K, Homady MH, Tarawneh KA, Shakhanbeh J. Effect of Ferula hormonis extract on social aggression, fertility and some physiological parameters in prepubertal male mice. Endocr J. 2001;48:473–82. doi: 10.1507/endocrj.48.473. [DOI] [PubMed] [Google Scholar]
- 40.Ayuob NN, Al-Harbi MS, Abdulhadi SS. Is the chronic use of Ferula harmonis to enhance mice erectile function effective and safe?: a histopathological study. Syst Biol Reprod Med. 2014;60:282–92. doi: 10.3109/19396368.2014.923059. [DOI] [PubMed] [Google Scholar]
- 41. Al-Harbi MS. The effect of Ferula hermonis roots extract on some physiological, biochemical and behavioral aspects in laboratory mice [dissertation]. King Abdulaziz University Faculty of Sciences; 2010.
- 42.El-Thaher TS, Matalka KZ, Taha HA, Badwan AA. Ferula harmonis ‘zallouh’ and enhancing erectile function in rats: efficacy and toxicity study. Int J Impot Res. 2001;13:247–51. doi: 10.1038/sj.ijir.3900706. [DOI] [PubMed] [Google Scholar]
- 43.Zanoli P, Benelli A, Rivasi M, Baraldi C, Vezzalini F, Baraldi M. Opposite effect of acute and subchronic treatments with Ferula hermonis on copulatory behavior of male rats. Int J Impot Res. 2003;15:450–5. doi: 10.1038/sj.ijir.3901051. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Kader MM, Kassem SS, Danial EN, El-Raouf AA, Hanafi EM. Evaluation of Ferula hermonis root extract as a growt promoter. Int J Acad Res. 2011;3:140–7. [Google Scholar]
- 45.Colman-Saizarbitoria T, Boutros P, Amesty A, Bahsas A, Mathison Y, Garrido Mdel R, et al. Ferutinin stimulates nitric oxide synthase activity in median eminence of the rat. J Ethnopharmacol. 2006;106:327–32. doi: 10.1016/j.jep.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 46.Zanoli P, Rivasi M, Zavatti M, Brusiani F, Vezzalini F, Baraldi M. Activity of single components of Ferula hermonis on male rat sexual behavior. Int J Impot Res. 2005;17:513–8. doi: 10.1038/sj.ijir.3901346. [DOI] [PubMed] [Google Scholar]
- 47.Bagheri S, Javidmehr D, Ghaffari M, Ghoderti-Shatori E. Chemical compositions and antiproliferative effect of essential oil of asafoetida on MCF7 human breast cancer cell line and female wistar rats. Cancer Transl Med. 2020;6:34. [Google Scholar]
- 48.Bafghi AF, Bagheri SM, Hejazian SH. Antileishmanial activity of Ferula assa-foetida oleo gum resin against Leishmania major: an in vitro study. J Ayurveda Integr Med. 2014;5:223–6. doi: 10.4103/0975-9476.146567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagheri SM, Mohamadsadeghi H, Hejazian ES. Antinociceptive effect of seed’s essential oil of Ferula assa-foetida in mice. Int J Clin Exp Physiol. 2017;4:34–7. [Google Scholar]
- 50.Shieh A, Bagheri SM, Yadegari M, Javidmehr D, Farhadi Z. Therapeutic effect of Ferula assa-foetida oleo-gum resin in rats with letrozole-induced polycystic ovary syndrome. Clin Exp Reprod Med. 2022;49:239–47. doi: 10.5653/cerm.2022.05449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’g’li E. Influence of herbaceous grain on Ferula assafoet on the quantitative and qualitative characteristics of snowflake rams sperm. Asian J Multidimens Res. 2019;8:6–10. [Google Scholar]
- 52.Selyametovich SR, Nabievich NA, Khakimovich TS. Study of Asphervon gum effect on diuresis, spermatogenesis and its effect on testosterone level in rat male blood. Indian J Forensic Med Toxicol. 2022;16:1056–63. [Google Scholar]
- 53.Ayoubi AR, Valizadeh R, Arshami J, Mousavi Z. The effect of water-alcholic extracted gum of Ferula asafoetida on body and testes weight, testosterone and spermatogenesis in adult male wistar rat. Iran J Anim Sci Res. 2014;6:173–80. [Google Scholar]
- 54.Bagheri SM, Yadegari M, Porentezari M, Mirjalili A, Hasanpor A, Dashti RM, et al. Effect of Ferula assa-foetida oleo gum resin on spermatic parameters and testicular histopathology in male wistar rats. J Ayurveda Integr Med. 2015;6:175–80. doi: 10.4103/0975-9476.146552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bagheri SM, Yadegari M, Mirjalily A, Rezvani ME. Evaluation of toxicity effects of asafetida on biochemical, hematological, and histological parameters in male Wistar rats. Toxicol Int. 2015;22:61–5. doi: 10.4103/0971-6580.172258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manayi A, Aliakbari F, Hadjiakhondi A. Effects of Ferula assa-foetida extract on spermatogenesis of rats. Res J Pharmacogn. 2017;4(Supplement):97. [Google Scholar]
- 57.Kassis E, Fulder S, Khalil K, Hadieh B, Nahhas F, Saad B, et al. Efficacy and safety assessments of Ferula assa-foetida L., traditionally used in Greco-Arab herbal medicine for enhancing male fertility, libido and erectile function. Open Complement Med J. 2009;1:102–9. [Google Scholar]
- 58.Yusufoglu HS, Soliman GA, Abdel-Rahman RF, Abdel-Kader MS, Genaie MA, Bedir E, et al. Antioxidant and antihyperglycemic effects of Ferula drudeana and Ferula huber-morathii in experimental diabetic rats. Int J Pharmacol. 2015;11:738–48. [Google Scholar]
- 59.Kiziltas H, Goren AC, Bingol Z, Alwasel SH, Gulcin I. Anticholinergic, antidiabetic and antioxidant activities of Ferula orientalis L. determination of its polyphenol contents by LC-HRMS. Rec Nat Prod. 2021;15:513–28. [Google Scholar]
- 60.Karakaya S, Yilmaz Oral D, Gur S, Kilic CS. Effect of aerial part and root extracts from Ferula orientalis L. growing in Turkey on erectile dysfunction in streptozotocin-induced diabetic rats. Biol Divers Conserv. 2019;12:1–6. [Google Scholar]
- 61.Eser N, Buyuknacar HS, Cimentepe OO, Gocmen C, Ucar Y, Erdogan S, et al. The effect of Ferula elaeochytris root extract on erectile dysfunction in streptozotocin-induced diabetic rat. Int J Impot Res. 2020;32:186–94. doi: 10.1038/s41443-019-0137-8. [DOI] [PubMed] [Google Scholar]
- 62.Inanan BE, Acar U, Inanan T. Effects of dietary Ferula elaeochytris root powder concentrations on haematology, serum biochemical parameters, spermatozoa parameters, and oxidative status in tissues of males goldfish (Carassius auratus) Aquaculture. 2021;544:737087. [Google Scholar]
- 63.Eser N, Yoldas A, Yigin A, Yumusak N, Bozkurt AS, Kokbas U, et al. The protective effect of Ferula elaeochytris on age-related erectile dysfunction. J Ethnopharmacol. 2020;258:112921. doi: 10.1016/j.jep.2020.112921. [DOI] [PubMed] [Google Scholar]
- 64.Tosun F, Goger F, Iscan G, Kurkcuoglu M, Kuran FK, Miski M. Biological activities of the fruit essential oil, fruit, and root extracts of Ferula drudeana Korovin, the putative Anatolian ecotype of the Silphion plant. Plants (Basel) 2023;12:830. doi: 10.3390/plants12040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alqarni MH, Soliman GA, Salkini MA, Alam P, Yusufoglu HS, Baykan S, et al. The potential aphrodisiac effect of Ferula drudeana Korovin extracts and isolated sesquiterpene coumarins in male rats. Pharmacogn Mag. 2020;16:404–9. [Google Scholar]
- 66.Seki Y, Sarikanat M, Sever K, Durmuskahya C. Extraction and properties of Ferula communis (chakshir) fibers as novel reinforcement for composites materials. Compos B Eng. 2013;44:517–23. [Google Scholar]
- 67.Akaberi M, Iranshahy M, Iranshahi M. Review of the traditional uses, phytochemistry, pharmacology and toxicology of giant fennel (Ferula communis L. subsp. communis) Iran J Basic Med Sci. 2015;18:1050–62. [PMC free article] [PubMed] [Google Scholar]
- 68.Koca SB, Ozdogan HB, Ozmen O, Biyikli M, Yigit NO. Effects of Tribulus terrestris and Ferula communis extracts on growth and gonad histology of red zebra cichlid Maylandia estherae (Konings, 1995) Indian J Fish. 2021;68:157–63. [Google Scholar]