Abstract
Introduction
Low-attenuation non-calcified plaque (LAP) burden and vascular inflammation by pericoronary adipose tissue (PCAT) measured from coronary CT angiography (CCTA) have shown to be predictors of cardiovascular outcomes. We aimed to investigate the relationships of cardiometabolic risk factors including lipoprotein(a) and epicardial adipose tissue (EAT) with CCTA high-risk imaging biomarkers, LAP and vascular inflammation.
Methods
The patient population consisted of consecutive patients who underwent CCTA for stable chest pain and had a complete cardiometabolic panel including lipoprotein(a). Plaque, PCAT and EAT were measured from CT using semiautomated software. Elevated LAP burden and PCAT attenuation were defined as ≥4% and ≥70.5 HU, respectively. The primary clinical end-point was a composite of myocardial infarction, revascularization or cardiovascular death.
Results
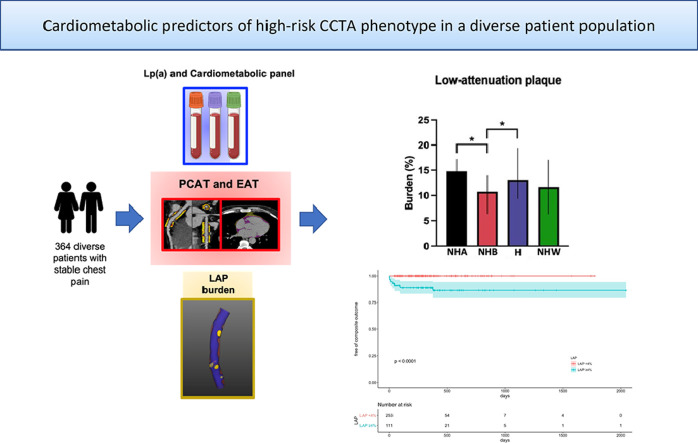
A total of 364 consecutive patients were included (median age 56 years, 64% female); the majority of patients were of Hispanic (60%), and the rest were of non-Hispanic Black (21%), non-Hispanic White (6%) and non-Hispanic Asian (4%) race/ethnicity. The prevalence of elevated LAP burden and PCAT attenuation was 31 and 18%, respectively, while only 8% had obstructive stenosis. There were significant differences in plaque characteristics among different racial/ethnic groups (p<0.001). Lipoprotein(a) correlated with LAP burden in Hispanic patients. Patients with elevated LAP were older, more likely to be have diabetes, hypertension, hyperlipidemia and smoke with higher CAC and EAT volume (all P<0.05). Patients with elevated LAP were more likely to develop the primary clinical outcome (p<0.001) but those with elevated PCAT were not (p=0.797).
Conclusion
The prevalence of LAP and PCAT attenuation were 31 and 18%, respectively. Lipoprotein(a) levels correlated with LAP burden in Hispanic patients. Age, male sex, hypertension and hyperlipidemia increased the odds of elevated LAP, which showed prognostic significance.
Keywords: High-risk plaque, CT coronary angiogram, Risk factors, PCAT, LAP, Epicardial adipose tissue
Graphical abstract
A total of 364 consecutive patients with stable chest pain and complete cardiometabolic panel who were referred for CCTA at Montefiore were included. We measured pericoronary adipose tissue, epicardial adipose tissue and quantitative plaque characteristics using semiautomated software (AutoPlaque and QFat, Cedars-Sinai Medical Center, Los Angeles, CA). There were significant differences in low-attenuation plaque (LAP) burden between racial/ethnic groups with an overall prevalence of elevated LAP (≥4%) of 31%. Patients with elevated LAP had higher incidence of the primary clinical outcome. NHA= non-Hispanic Asian, NHB= non-Hispanic Black, H= Hispanic, NHW= non-Hispanic White.
1. Introduction
A central focus of modern cardiology has been to identify vulnerable patients at increased risk for major adverse cardiovascular events to guide a patient-centric intensification of preventive therapies. Plaque burden is now recognized as the strongest predictor of future cardiovascular events and has led to a recent endorsement of coronary computed tomography angiography (CCTA) by major guidelines [1], [2], [3]. Recent advances in CCTA and post-processing software have allowed for a novel deep-learning driven characterization of plaque and quantitation of high-risk features (such as low-attenuation non-calcified plaque burden [LAP, as defined by <30 Hounsfield Units] and pericoronary adipose tissue [PCAT] attenuation) with an evolution from a qualitative visual assessment and tedious manual quantitation to a rapid semi-automated quantitative analysis [4]. Data from the Scottish Computed Tomography of the HEART (SCOT-HEART) trial has suggested that a high LAP burden and an elevated PCAT attenuation could be the strongest predictors of subsequent myocardial infarction (MI), superior to vessel stenosis or cardiovascular risk estimators [5]. Moreover, based on the Youden index of the ROC curves, the optimal cutoff of the RCA PCAT attenuation was defined as -70.5 HU and 4% for LAP burden for the primary endpoint of fatal or non-fatal MI. These findings represent a paradigm shift beyond stenosis into exploring a high-risk quantitative CCTA phenotype. Different studies have visually assessed the burden of plaque by CCTA in multiethnic U.S. populations, however significantly less data is available on quantitative plaque phenotyping as assessed by CCTA on non-White patients even though minority patients comprise an increasing proportion of the US population.
Despite the established association between cardiometabolic risk factors with atherosclerotic cardiovascular disease events (ASCVD), a significant proportion of patients do not develop atherosclerosis [6] or clinical ASCVD [7]. Furthermore, significant racial/ethnic differences have been observed with higher cardiovascular mortality in non-Hispanic Black people and poorer cardiometabolic risk profile in Hispanic and non-Hispanic Black people at each level of income and insurance [8]. Additionally, it has been suggested that the attributable risk of cardiometabolic variables including lipoprotein(a) [Lp(a)] and epicardial adipose tissue (EAT) may have an heterogenous racial/ethnic distribution [9,10]. Thus, there is a need for data on the prevalence of quantitative high-risk CCTA features and their cardiometabolic determinants in diverse patient cohorts.
The goals of our study were to 1) describe the prevalence of a quantitatively high-risk phenotype by CCTA [defined as elevated LAP burden (≥4%) and elevated RCA PCAT attenuation (≥-70.5 HU)] in a racial/ethnically diverse patient population and 2) characterize the association of cardiometabolic risk factors including Lp(a) and EAT with these imaging features. In addition, as an exploratory outcome, we also aimed to study the relationship between high-risk CCTA phenotype and clinical outcomes.
2. Methods
2.1. Population
We conducted a search from our CT registry and further retrospective post-hoc analysis of all patients with stable chest pain or angina equivalent undergoing CCTA between June 2016 and March 2022 at Montefiore Healthcare Network. Patients who had a complete cardiometabolic panel including hemoglobin A1c (HbA1c) and lipid panel up to 90 days before CCTA and Lp(a) at any point were included. As shown in Fig. 1, patients with acute chest pain, prior myocardial infarction (MI), percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were excluded. From the initially identified group consisting of 380 subjects undergoing CCTA with a complete cardiometabolic panel including Lp(a), we excluded 13 due to prior MI, PCI or CABG and 3 due to acute chest pain. A total of 364 subjects were included in the final study. Baseline demographic, clinical (as per ICD codes) and laboratory data were retrieved from our electronic medical records system using Clinical Looking Glass (CLG) software, a proprietary software used to abstract data from our system's records. Race and ethnicity were self-identified at the time of initial registration for care. We first identified self-reported Hispanics, and then only among non-Hispanics, we specified race. Being Hispanic is an ethnicity, but self-reported race among Hispanics is notoriously unreliable [11]. Thus, we categorized race/ethnicity as Hispanic, non-Hispanic Black (NHB), non-Hispanic White (NHW), non-Hispanic Asian (NHA), and declined/unknown as has been reported previously [12]. Coronary artery calcium score and coronary artery stenosis – reporting and data system (CAD-RADS) data were retrieved from imaging reports.
Fig. 1.
Flow-diagram of the included patients.
2.2. Laboratory measurements and risk calculator
Hematology, biochemistry, and lipid panels were determined according to standardized operating procedures in the hospital laboratory. Low-density-lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation [13]. Lipoprotein(a) was measured using an Immunoturbidimetric assay from Quest Diagnostics measured in molar concentration (nm/L). The 10-year risk of ASCVD was calculated using the pooled cohort equations risk calculator [14]. If multiple values were available, the closest one to the CCTA was used.
2.3. CT acquisition
Non-contrast, ECG-gated computed tomography CAC scans were performed before CCTA as per current SCCT guidelines [15]. Briefly, scans were performed at 120 kVp with mA varying per body habitus with 2.5 mm slice thickness. CCTA acquisition was performed after CAC scan using 64-slice Philips IQon and 64-slice GE VCT scanners as per SCCT guidelines using 80–100 mL of Isovue-370 contrast [15]. Metoprolol was administered for heart rate control and sublingual nitroglycerin given before image acquisition.
2.4. CCTA interpretation and plaque analysis
Coronary artery calcium scoring and CCTA were graded by cardiologists and radiologists with level 3 cardiac CT expertise. Coronary artery calcium scoring was scored as per the Agatston method [16] using TeraRecon iNtuition version 4.6.2.59 (TeraRecon, Durham, NC). Images from CCTA were reconstructed with TeraRecon and Philips Intellispace software and CAD-RADS graded as per SCCT guidelines [17].
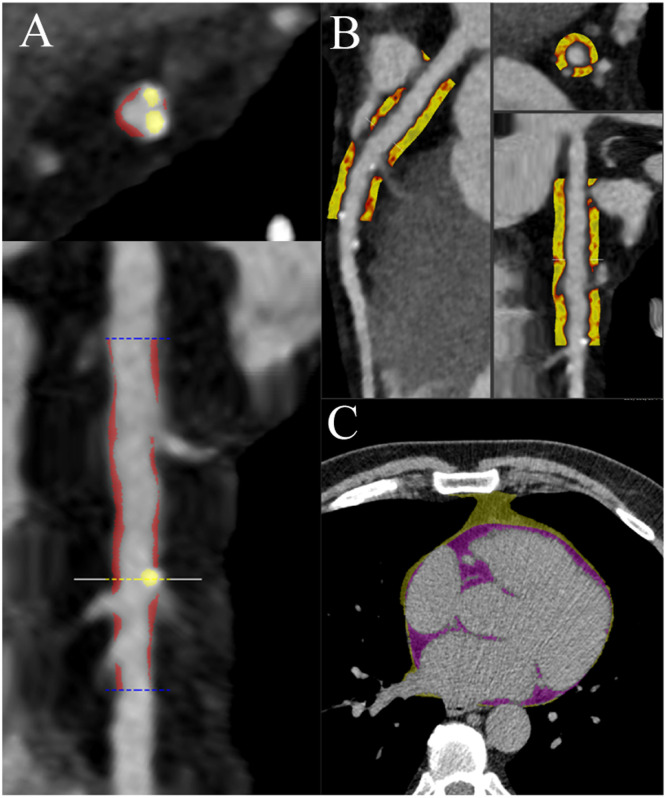
As shown on Fig. 2, plaque volumes and PCAT attenuation measurements were performed from CCTAs using a previously validated semiautomated software (AutoPlaque version 2.5, Cedars-Sinai Medical Center, Los Angeles, CA) in all patients with CAD-RADS >0, while PCAT was measured in all CCTAs. Semi-automatic measurements were performed by two readers (JA, SS) with level 3 certification equivalent reading experience, blinded from patient characteristics. Briefly, coronary artery centerlines were extracted in a semiautomated fashion for each major artery and any tributary of >2 mm with visually observed disease. A region of interest was placed in the aorta to define blood pool attenuation. All suitable vessel segments were manually identified and vessel wall and plaque constituents were automatically determined using scan-specific thresholds with manual adjustments made as required. Coronary atherosclerotic plaque volumes were measured for total plaque, calcific, non-calcific and low-attenuation plaque as a marker of necrotic core. For each patient, total plaque, non-calcified plaque (NCP), low-attenuation non-calcified plaque (LAP), calcified plaque (CP) burden (%) were assessed as described previously [18]. Plaque volumes (measured in mm3) for each plaque type were measured across all segments and summed to generate the total plaque volume on a per-patient level. Plaque burdens were calculated by dividing plaque volumes by the coronary vessel volume and multiplying by 100. Epicardial Adipose Tissue (EAT) volume was measured on the CAC dedicated scans for all patients using QFat software (JA and JD). PCAT analysis was performed by five experienced operators in proximal vessels of the coronary tree with diagnostic image quality and luminal diameter >2 mm. To standardize PCAT analysis, we focused on the proximal right coronary artery (RCA; PCAT-RCA 10–50 mm from ostium), left anterior descending coronary artery (LAD; PCAT-LAD 0–40 mm from left main stem bifurcation) and left circumflex (LCx; PCAT-LCx 0–40 mm from left main stem bifurcation). We considered PCAT attenuation (reported in Hounsfield units [HU]) as the average attenuation of all adipose tissue containing voxels (range -190 HU to -30 HU) within an outer radial distance from the vessel wall of 3 mm. If the vessel length was short (<40 mm) or of small diameter (<2 mm) or had a major motion artefact, it was excluded from analysis. We have previously demonstrated that this method has excellent intra-observer and inter-observer repeatability [19]. As previously described [20], QFAT uses convolutional deep learning for fully automated quantification of EAT volume. Accuracy and reproducibility of QFat have been previously validated [21]. High-risk CCTA features were defined as LAP ≥4% or RCA PCAT ≥-70.5 HU as previously reported [18].
Fig. 2.
Example of quantitative plaque burden, pericoronary adipose tissue and epicardial fat. Figure shows a patient example of plaque (A), pericoronary adipose tissue (PCAT, B) and epicardial adipose tissue (EAT, C) quantification.
2.5. Clinical outcomes
Incidence of invasive coronary angiography, percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), type 1 MI, cardiovascular and all cause death were assessed by chart review from researchers blinded from CCTA findings. Whenever available linked records from other institutions were reviewed. Myocardial infarction was defined as elevation of troponin with ECG changes and symptoms. Reason for revascularization was obtained from procedural reports. The primary clinical outcome was a composite of type 1 MI, revascularization and cardiovascular death. Data was censored on May 1st, 2022. Median follow-up time was 288 days [169.75, 403.00]. CLG system has previously shown to capture most events with events outside Montefiore occurring rarely (1.9%) [22]. In addition, events that occurred elsewhere and were reported to our institution were also included through our data mining software and manual chart review.
2.6. Statistical analysis
Normality of data was assessed with visual inspection of frequency histograms and probability-probability plots. Continuous data are presented as median (interquartile range) and compared with Wilcoxon test or Kruskal-Wallis test. Categorical data are presented as percentages and compared with chi-square test or Fisher's exact tests. Kaplan-Meier curves were created based on LAP ≥4% versus <4%, RCA PCAT ≥-70.5 HU versus <-70.5 and CAC ≥100 versus <100. Log-rank test was performed to examine the difference between groups.
The relationship between plaque characteristics and Lp(a) was estimated using a simple linear regression model. The model was fitted using least squares method. The assumptions of linear regression were examined.
Independent predictors for LAP≥4% and RCA PCAT ≥-70.5 HU on CCTA among patients were examined using multivariable logistic regression. The following variables were entered; age, male, non-Hispanic Black race, diabetes, hypertension, hyperlipidemia, smoking history, use of statin therapy, and Lp(a). A p-value <0.05 was considered statistically significant. All statistical calculations and analyses were performed using R 3.6.2 R Foundation for Statistical Computing (Vienna, Austria). This study was approved by the Albert Einstein College of Medicine institutional review board.
3. Results
3.1. Patients characteristics
A total of 364 consecutive patients were included in our study (age 56.00 [48.00, 63.00] years, 64% were female, 16% had diabetes, 47% hypertension, 40% hyperlipidemia and 31% reported current or previous smoking history) (Table 1, Central Illustration). Median body mass index (BMI) was 29.80 [26.27, 33.04]kg/m2. Majority of patients were of Hispanic race/ethnicity (60%), and the rest were of NHB (21%), NHW (6%), NHA (4%) and 9% unknown race/ethnicity. All patients were scanned in the setting of stable chest pain or angina equivalent. As shown on Supplementary Table 1 there was no difference in the 10-year ASCVD risk between the included and excluded patient cohorts without a full cardiometabolic panel (5.00 [2.00, 10.94]) vs 5.30 [1.98, 12.65]; p=0.331).
Table 1.
Study population. Patient demographics, past medical history, CCTA findings and clinical outcomes.
| Overall | NHA | NHB | Hispanic | NHW | p | |
|---|---|---|---|---|---|---|
| n, % | 364 | 14 (3.8) | 76 (20.9) | 217 (59.6) | 21 (5.8) | |
| Age (years) | 56.00 [48.00, 63.00] | 49.00 [42.00, 53.25] | 57.00 [46.75, 62.00] | 57.00 [48.00, 64.00] | 63.00 [59.00, 66.00] | <0.001 |
| Male sex (n, %) | 133 (36.5) | 7 (50.0) | 19 (25.0) | 77 (35.5) | 9 (42.9) | 0.153 |
| BMI (kg/m2) | 29.80 [26.27, 33.04] | 24.85 [23.28, 27.79] | 31.00 [27.43, 34.13] | 29.95 [26.45, 33.07] | 27.98 [25.85, 31.70] | <0.001 |
| Diabetes mellitus (n, %) | 57 (16.2) | 2 (16.7) | 10 (13.3) | 36 (17.1) | 4 (20.0) | 0.856 |
| Hypertension (n, %) | 166 (47.3) | 3 (25.0) | 44 (58.7) | 94 (44.5) | 16 (80.0) | 0.002 |
| Hyperlipidemia (n, %) | 139 (39.6) | 6 (50.0) | 29 (38.7) | 84 (39.8) | 12 (60.0) | 0.300 |
| Smoking (n, %) | 0.238 | |||||
| Never | 250 (69.3) | 12 (85.7) | 57 (76.0) | 147 (68.1) | 12 (57.1) | |
| Past | 48 (13.3) | 0 (0.0) | 9 (12.0) | 29 (13.4) | 2 (9.5) | |
| Present | 63 (17.5) | 2 (14.3) | 9 (12.0) | 40 (18.5) | 7 (33.3) | |
| CAC (AU) | 0.00 [0.00, 32.00] | 0.00 [0.00, 11.25] | 0.00 [0.00, 10.00] | 0.00 [0.00, 32.00] | 56.00 [13.00, 229.00] | <0.001 |
| CAD-RADS (value) | 0.00 [0.00, 1.00] | 0.50 [0.00, 1.00] | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 2.00 [1.00, 2.00] | <0.001 |
| Total Cholesterol (mg/dL) | 183.00 [159.50, 216.00] | 207.00 [170.00, 225.00] | 175.00 [151.50, 211.00] | 183.00 [161.50, 212.50] | 193.00 [174.00, 213.00] | 0.34 |
| LDL-C (mg/dL) | 105.00 [82.00, 132.25] | 114.00 [98.00, 150.00] | 106.00 [80.00, 127.00] | 102.00 [82.00, 128.50] | 104.00 [92.00, 146.00] | 0.60 |
| HDL-C (mg/dL) | 49.00 [41.00, 59.00] | 44.00 [39.00, 52.00] | 53.00 [45.50, 63.50] | 49.00 [41.00, 57.00] | 54.00 [42.00, 63.00] | 0.039 |
| Creatinine (mg/dL) | 0.80 [0.72, 0.95] | 0.79 [0.72, 0.88] | 0.84 [0.77, 1.02] | 0.79 [0.70, 0.91] | 0.81 [0.67, 0.98] | 0.013 |
| HbA1c (%) | 5.60 [5.30, 6.10] | 5.40 [5.25, 6.20] | 5.60 [5.20, 6.10] | 5.60 [5.40, 6.10] | 5.50 [5.30, 6.20] | 0.779 |
| Lipoprotein(a) (nmol/L) | 64.50 [18.75, 150.25] | 22.50 [16.25, 62.00] | 120.50 [62.75, 201.00] | 52.00 [16.00, 122.00] | 31.00 [11.00, 158.00] | <0.001 |
| Statin use at baseline (%) | 128 (36.3) | 6 (46.2) | 29 (38.7) | 77 (36.5) | 8 (38.1) | 0.907 |
| SBP (mmHg) | 123.93 [115.46, 134.00] | 115.52 [111.09, 122.57] | 130.73 [122.82, 139.09] | 121.80 [115.06, 132.61] | 130.50 [119.07, 143.89] | <0.001 |
| DBP (mmHg) | 75.27 [70.39, 80.69] | 72.46 [69.23, 78.65] | 77.50 [73.23, 82.85] | 74.74 [69.97, 79.61] | 75.75 [70.32, 79.48] | 0.013 |
| NCP burden (%) | 0.00 [0.00, 38.88] | 17.80 [0.00, 48.16] | 0.00 [0.00, 30.10] | 0.00 [0.00, 38.45] | 43.78 [26.02, 49.99] | <0.001 |
| CP burden (%) | 0.00 [0.00, 1.51] | 0.07 [0.00, 2.29] | 0.00 [0.00, 0.15] | 0.00 [0.00, 1.60] | 1.16 [0.44, 4.60] | <0.001 |
| Total plaque burden (%) | 0.00 [0.00, 41.24] | 17.88 [0.00, 50.34] | 0.00 [0.00, 31.27] | 0.00 [0.00, 40.50] | 48.83 [26.29, 54.63] | <0.001 |
| LAP burden (%) | 0.00 [0.00, 5.13] | 2.71 [0.00, 6.39] | 0.00 [0.00, 2.78] | 0.00 [0.00, 5.17] | 5.38 [1.15, 8.11] | 0.002 |
| Total CP vol (mm3) | 0.00 [0.00, 9.43] | 0.36 [0.00, 9.31] | 0.00 [0.00, 2.32] | 0.00 [0.00, 10.49] | 13.39 [2.79, 77.94] | <0.001 |
| Total NCP vol (mm3) | 0.00 [0.00, 275.16] | 36.98 [0.00, 190.17] | 0.00 [0.00, 180.48] | 0.00 [0.00, 277.67] | 374.62 [164.88, 514.92] | <0.001 |
| Total LAP vol (mm3) | 0.00 [0.00, 38.56] | 10.58 [0.00, 30.84] | 0.00 [0.00, 15.05] | 0.00 [0.00, 38.14] | 51.31 [7.52, 75.92] | 0.002 |
| Total plaque vol (mm3) | 0.00 [0.00, 294.50] | 38.86 [0.00, 194.47] | 0.00 [0.00, 180.74] | 0.00 [0.00, 296.48] | 386.16 [175.10, 590.23] | <0.001 |
| EAT (mL) | 88.00 [63.00, 117.00] | 59.50 [50.50, 100.50] | 65.50 [53.00, 87.50] | 94.00 [71.50, 124.00] | 124.00 [97.00, 134.00] | <0.001 |
| LAD (HU) | -80.00 [-86.07, -74.88] | -84.83 [-89.83, -78.55] | -77.06 [-82.44, -72.53] | -80.99 [-86.61, -75.64] | -82.10 [-90.64, -77.11] | 0.008 |
| LCX (HU) | -77.44 [-82.87, -71.90] | -82.96 [-90.46, -78.03] | -75.44 [-80.44, -67.96] | -77.78 [-83.02, -72.72] | -78.98 [-83.16, -74.27] | 0.015 |
| RCA (HU) | -79.57 [-87.06, -72.50] | -83.62 [-88.10, -76.57] | -74.81 [-85.25, -68.94] | -80.53 [-87.12, -73.06] | -82.42 [-87.44, -76.80] | 0.071 |
| 10- year ASCVD risk (%) | 5.00 [2.00, 10.94] | 2.00 [1.20, 4.46] | 5.33 [2.52, 11.50] | 4.70 [1.67, 10.11] | 11.00 [6.58, 18.62] | 0.007 |
| LAP≥4% (n, %) | 111 (30.5) | 7 (50.0) | 13 (17.1) | 70 (32.3) | 11 (52.4) | 0.003 |
| RCA PCAT ≥-70.5HU (n, %) | 65 (17.9) | 3 (21.4) | 19 (25.0) | 33 (15.2) | 2 (9.5) | 0.18 |
| LAP≥4%+RCAPCAT≥-70.5HU (n,%) | 14 (3.8) | 2 (14.3) | 3 (3.9) | 5 (2.3) | 1 (4.8) | 0.11 |
| No stenosis (n,%) | 186 (51.1) | 7 (50.0) | 42 (55.3) | 110 (50.7) | 3 (14.3) | 0.003 |
| Non-obstructive stenosis (n,%) | 152 (41.8) | 5 (35.7) | 31 (40.8) | 92 (42.4) | 13 (61.9) | |
| Borderline stenosis (n,%) | 18 (4.9) | 1 (7.1) | 2 (2.6) | 10 (4.6) | 5 (23.8) | |
| Obstructive stenosis (n,%) | 8 (2.2) | 1 (7.1) | 1 (1.3) | 5 (2.3) | 0 (0.0) | |
| Invasive coronary angiogram (n,%) | 15 (4.1) | 0 (0) | 1 (1.3) | 10 (4.6) | 3 (14.3) | 0.058 |
| Revascularization(n,%) | 0.04 | |||||
| PCI (n,%) | 11 (3.0) | 0 (0.0) | 1 (1.4) | 7 (3.7) | 2 (12.5) | |
| CABG (n,%) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (4.8) | |
| MI (n,%) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (4.8) | 0.086 |
| CV death (n,%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Death (n,%) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0.913 |
BMI: Body mass index, CAC: coronary artery calcium, LDL-C: low-density lipoprotein cholesterol, HLD-C: high-density lipoprotein cholesterol, HbA1c: hemoglobin A1c, SBP: average systolic blood pressure, DBP: average diastolic blood pressure, NCP: non-calcified plaque, NHA= non-Hispanic Asian, NHB= non-Hispanic Black, NHW=non-Hispanic White, CP: calcified plaque, LAP: low-attenuation plaque, EAT: epicardial adipose tissue, ASCVD: atherosclerotic cardiovascular disease risk by pooled cohort equations.
Comparison of risk factors, laboratory values and measurements between different race/ethnicities are shown on Table 1. Briefly, NHW patients were older and had higher prevalence of hypertension when compared to Hispanic and NHA patients (p<0.001) but not when compared to NHB patients. Non-Hispanic Asian patients had the lowest BMI and NHB the highest.
Lipoprotein(a) was higher in NHB patients (120.50 nmol/L [62.75, 201.00]) when compared to NHW (31.00 nmol/L [11.00, 158.00], p=0.004), Hispanic (52.00 nmol/L [16.00, 122.00], p<0.001), and NHA patients (22.50 nmol/L [16.25, 62.00], p=0.002). There were no differences in HbA1c, total cholesterol, LDL-C, triglycerides (TGs) or statin therapy at baseline between race/ethnicities.
A total of 37 (10%) patients had CAC≥100 AU. Eight (2.2%) patients had obstructive coronary stenosis (as defined by CAD-RADS ≥4), 18 (4.9%) borderline (CAD-RADS 3), 152 (41.8%) non-obstructive coronary stenosis (CAD-RADS 1-2) and 186 (51.1%) had normal coronary anatomy.
Non-Hispanic White patients had higher CAC and CAD-RADS when compared to NHB and Hispanic patients (p<0.001).
3.2. Quantitative plaque
In total, 111 (30.5%) patients had elevated LAP. As shown in Table 1, NHW and NHA patients had higher plaque volume and burden (p<0.001). There were significant differences between race/ethnicity groups in the percentage with elevate LAP. Non-Hispanic White patients had a higher percentage of elevated LAP when compared to NHB patients (11 [52%] vs 13 [17%]; p=0.006). Seventy (32.3%) Hispanic and 7 (50%) NHA patients had elevated LAP. The majority (78%) of patients with elevated LAP had non-obstructive and 16% had borderline coronary stenosis. Nevertheless, there was a correlation between higher CAD-RADS grades and prevalence of elevated LAP (Table 4).
Table 4.
High-risk features and vessel stenosis. Table shows the percentage of patients with LAP ≥4% and PCAT ≥-70.5HU amongst different level of coronary artery stenosis as graded per CAD-RADS classification.
| CAD-RADS | 0 | 1 | 2 | 3 | 4 | 5 | p |
|---|---|---|---|---|---|---|---|
| N | 186 | 98 | 54 | 18 | 1 | 7 | |
| LAP≥4% (n,%) | 0 (0.0) | 53 (54.1) | 33 (61.1) | 17 (94.4) | 1 (100.0) | 7 (100.0) | <0.001 |
| PCAT RCA≥-70.5HU(n,%) | 42 (22.6) | 11 (11.2) | 8 (14.8) | 4 (22.2) | 0 (0.0) | 0 (0.0) | 0.152 |
LAP: low-attenuation plaque, PCAT: pericoronary adipose tissue, RCA: right coronary artery.
As seen in Table 2, patients with elevated LAP were older (59.00 [52.00, 64.50] vs 55.00 years [44.00, 61.00]; p<0.001), more likely to have diabetes (25.0 vs 12.3%; p=0.006), hypertension (69.4 vs 37.4%; p<0.001), hyperlipidemia (58.3 vs 31.1%; p<0.001) and smoke (24.3 vs 14.4%; p<0.05). There were no differences between HbA1c, total cholesterol, LDL-C, high-density lipoprotein cholesterol (HLD-C), TGs, Lp(a), systolic or diastolic average blood pressure.
Table 2.
Predictors of elevated low-attenuation plaque burden (LAP). Table shows the demographic, clinical, laboratory data and CCTA features of patients with LAP ≥4% versus <4%.
| LAP<4% | LAP≥4% | p | |
|---|---|---|---|
| n | 253 | 111 | |
| Age (years) | 55.00 [44.00, 61.00] | 59.00 [52.00, 64.50] | <0.001 |
| Race/ethnicity (n, %) | 0.003 | ||
| NHA | 7 (3.1) | 7 (6.9) | |
| NHB | 63 (27.8) | 13 (12.9) | |
| Hispanic | 147 (64.8) | 70 (69.3) | |
| NHW | 10 (4.4) | 11 (10.9) | |
| Male sex (n, %) | 81 (32.0) | 52 (46.8) | 0.01 |
| BMI (kg/m2) | 29.71 [26.22, 33.08] | 29.95 [26.42, 32.69] | 0.97 |
| Diabetes mellitus (n, %) | 30 (12.3) | 27 (25.0) | 0.005 |
| Hypertension (n, %) | 91 (37.4) | 75 (69.4) | <0.001 |
| Hyperlipidemia (n, %) | 76 (31.3) | 63 (58.3) | <0.001 |
| Smoking (n, %) | 0.023 | ||
| Never | 184 (73.6) | 66 (59.5) | |
| Past | 30 (12.0) | 18 (16.2) | |
| Present | 36 (14.4) | 27 (24.3) | |
| CAC (AU) | 0.00 [0.00, 0.00] | 40.00 [11.00, 83.00] | <0.001 |
| CAD-RADS (value) | 0.00 [0.00, 1.00] | 2.00 [1.00, 2.00] | <0.001 |
| Total Cholesterol (mg/dL) | 181.00 [160.00, 214.00] | 188.50 [157.25, 222.75] | 0.362 |
| LDL-C (mg/dL) | 104.00 [81.00, 128.50] | 109.00 [86.00, 140.00] | 0.278 |
| HDL-C (mg/dL) | 50.00 [41.00, 59.00] | 48.00 [41.25, 59.00] | 0.306 |
| Creatinine (mg/dL) | 0.80 [0.71, 0.94] | 0.81 [0.74, 0.97] | 0.26 |
| HbA1c (%) | 5.60 [5.30, 5.95] | 5.75 [5.38, 6.35] | 0.082 |
| Lipoprotein(a) (nmol/L) | 65.00 [19.00, 156.00] | 60.00 [18.00, 130.00] | 0.736 |
| Statin use at baseline (%) | 82 (32.9) | 46 (44.2) | 0.059 |
| SBP (mmHg) | 123.77 [115.04, 133.66] | 124.78 [117.25, 134.61] | 0.338 |
| DBP (mmHg) | 75.35 [70.87, 80.91] | 75.22 [70.18, 80.22] | 0.686 |
| NCP burden (%) | 0.00 [0.00, 0.00] | 43.77 [37.11, 50.88] | <0.001 |
| CP burden (%) | 0.00 [0.00, 0.00] | 1.61 [0.49, 3.65] | <0.001 |
| Total plaque burden (%) | 0.00 [0.00, 0.00] | 46.95 [39.14, 53.91] | <0.001 |
| Total CP vol (mm3) | 0.00 [0.00, 0.00] | 10.84 [3.68, 32.01] | <0.001 |
| Total NCP vol (mm3) | 0.00 [0.00, 0.00] | 366.26 [212.77, 627.19] | <0.001 |
| Total LAP vol (mm3) | 0.00 [0.00, 0.00] | 69.55 [35.30, 112.69] | <0.001 |
| Total plaque vol (mm3) | 0.00 [0.00, 0.00] | 378.88 [228.18, 656.93] | <0.001 |
| EAT (mL) | 79.00 [58.50, 108.50] | 105.00 [80.50, 135.50] | <0.001 |
| 10-year ASCVD risk (%) | 3.81 [1.40, 9.15] | 8.12 [4.00, 14.07] | <0.001 |
BMI: Body mass index, CAC: coronary artery calcium, LDL-C: low-density lipoprotein cholesterol, HLD-C: high-density lipoprotein cholesterol, HbA1c: hemoglobin A1c, SBP: average systolic blood pressure, DBP: average diastolic blood pressure, NCP: non-calcified plaque, NHA= non-Hispanic Asian, NHB= non-Hispanic Black, NHW=non-Hispanic White, CP: calcified plaque, LAP: low-attenuation plaque, EAT: epicardial adipose tissue, ASCVD: atherosclerotic cardiovascular disease risk by pooled cohort equations.
Patients with elevated LAP had higher median CAC (40 [11, 83] vs 0 [0, 0], p<0.001), higher median CAD-RADS (2 [1,2] vs 0 [0, 0]; p<0.001), and EAT volume (105 [80.5, 135.5] vs 79 mL [58.50, 108.5]; p<0.001), when compared to those with low LAP burden. Eleven patients had CAC=0 but CAD-RADS >0, of whom 4 (36.4%) had elevated LAP.
In a multivariable regression model for predictors of elevated LAP, age; OR: 1.03 [95%CI: 1.00, 1.05], male sex; OR: 2.05 [95%CI: 1.16 3.63], hypertension; OR:4.00 [95%CI: 2.15, 7.63] and hyperlipidemia; OR: 2.48 [95%CI: 1.33, 4.66] were associated with increased risk for elevated LAP whilst NHB race/ethnicity; OR: 0.35 [95%CI: 0.16, 0.72] decreased the risk.
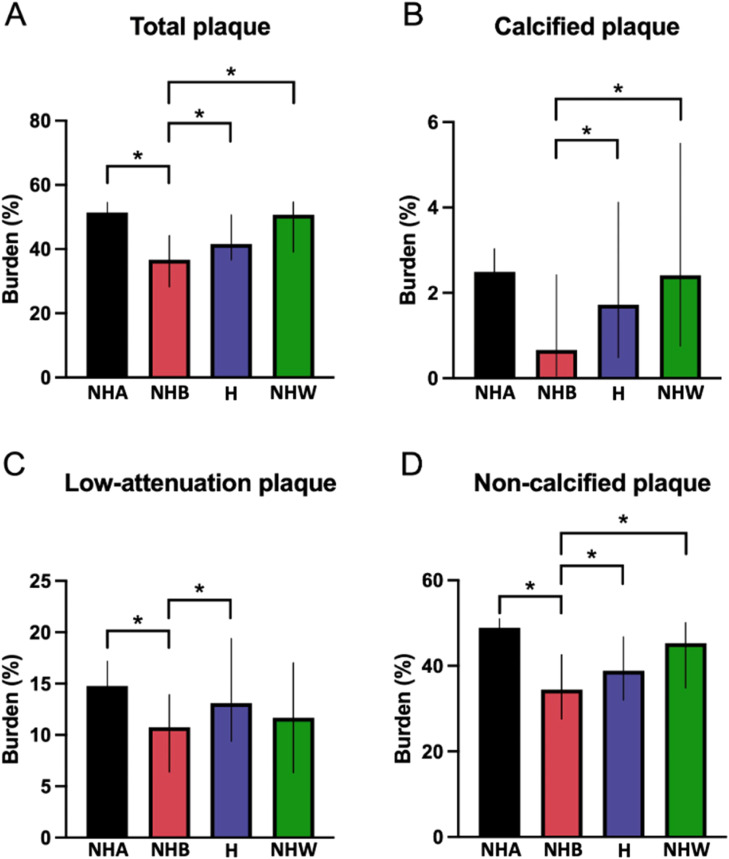
When only patients with plaque were compared, NHB patients had a lower burden of total, calcified, non-calcified and LAP (Fig. 3).
Fig. 3.
Differences in plaque burden between ethnicities in patients with plaque. Graph bars show the median and interquartile range for total (A), calcified (B), non-calcified (C) and low-attenuation (D) plaque burden. *=p<0.05.
Hispanic (n=107), NHB= non-Hispanic Black (n=34), NHW= non-Hispanic White (n=18), NHA=non-Hispanic Asian (n=7).
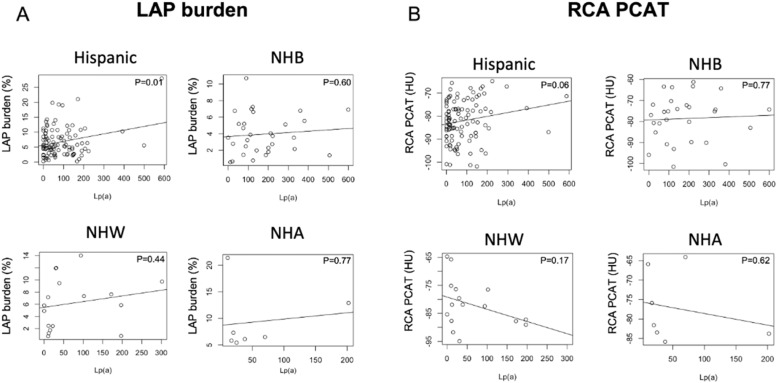
When Lp(a) and plaque characteristics were analyzed by race/ethnicity (Fig. 4), Lp(a) showed a correlation with LAP burden in Hispanic patients but not in NHA, NHB or NHW patients.
Fig. 4.
Relationship between lipoprotein (a) levels and low-attenuation plaque burden (LAP) and right coronary artery pericoronary adipose tissue attenuation (RCA PCAT). Graphs show correlation between lipoprotein(a) levels (nm/L) and the LAP burden (A) and RCA PCAT attenuation (B) among different ethnicities in patients with plaque.
Hispanic (n=107), NHB= non-Hispanic Black (n=34), NHW= non-Hispanic White (n=18), NHA=non-Hispanic Asian (n=7).
3.3. Pericoronary adipose tissue and epicardial adipose tissue
A total of 65 (17.9%) patients had elevated RCA PCAT attenuation, and of those, 23 had CAD-RADS>0. As shown in Table 1, there were no differences in RCA PCAT attenuation between race/ethnicities.
When patients with plaque were analyzed (Table 3), patients with elevated RCA PCAT attenuation showed a trend toward higher levels of Lp(a) (116.00 [43.00, 202.5] vs 56.00 nmol/L [17.50, 142.50]; p=0.065). Patients with elevated RCA PCAT attenuation had higher total plaque burden (46.61 [43.40, 55.05] vs 40.50% [32.21, 49.13]; p=0.025) and calcified plaque volume (16.09 [9.18, 66.24] vs 9.53 mm3 [2.00, 31.51]; P=0.038). There were no differences in ASCVD risk or traditional risk factors between the groups. A comparison of all patients (with and without plaque) with PCAT measured can be seen in Supplementary Table 2.
Table 3.
Predictors of elevated pericoronary adipose tissue (PCAT) attenuation in patients with coronary plaque. Table shows the demographic, clinical, laboratory data and CCTA features of patients with RCA PCAT ≥-70.5 versus <70.5 HU.
| PCAT<-70.5HU | PCAT≥-70.5HU | p | |
|---|---|---|---|
| n | 155 | 23 | |
| Age (years) | 60.00 [55.00, 66.00] | 60.00 [55.50, 65.00] | 0.776 |
| Race (%) | 0.313 | ||
| NHA | 5 (3.4) | 2 (10.0) | |
| NHB | 28 (19.2) | 6 (30.0) | |
| Hispanic | 97 (66.4) | 10 (50.0) | |
| NHW | 16 (11.0) | 2 (10.0) | |
| Male sex (%) | 71 (45.8) | 5 (21.7) | 0.051 |
| BMI (kg/m2) | 29.43 [26.58, 32.56] | 31.08 [26.19, 34.85] | 0.287 |
| Diabetes mellitus (%) | 38 (24.8) | 4 (18.2) | 0.677 |
| Hypertension (%) | 108 (70.6) | 16 (72.7) | 1.00 |
| Hyperlipidemia (%) | 92 (60.1) | 13 (59.1) | 1.00 |
| Smoking (%) | 0.825 |
||
| Never | 90 (58.4) | 14 (60.9) | |
| Past | 28 (18.2) | 3 (13.0) | |
| Present | 36 (23.4) | 6 (26.1) | |
| CAC (AU) | 32.00 [6.00, 80.50] | 57.00 [18.00, 163.50] | 0.106 |
| CAD-RADS (value) | 1.00 [1.00, 2.00] | 2.00 [1.00, 2.00] | 0.512 |
| Total Cholesterol (mg/dL) | 183.50 [157.00, 211.50] | 191.00 [173.50, 227.00] | 0.255 |
| LDL-C (mg/dL) | 103.00 [79.00, 130.50] | 108.00 [94.50, 143.50] | 0.309 |
| HDL-C (mg/dL) | 48.50 [41.00, 60.00] | 48.00 [42.50, 59.00] | 0.994 |
| Creatinine (mg/dL) | 0.83 [0.74, 0.98] | 0.78 [0.73, 0.84] | 0.104 |
| HbA1c (%) | 5.80 [5.40, 6.20] | 5.50 [5.30, 5.90] | 0.229 |
| Lipoprotein (a) (nmol/L) | 56.00 [17.50, 142.50] | 116.00 [43.00, 202.50] | 0.065 |
| Statin use at baseline (%) | 64 (43.8) | 12 (57.1) | 0.362 |
| SBP (mmHg) | 127.92 [117.08, 135.80] | 121.42 [117.75, 129.71] | 0.195 |
| DBP (mmHg) | 75.44 [70.33, 80.92] | 72.00 [70.09, 76.20] | 0.075 |
| NCP burden (%) | 38.92 [30.57, 46.49] | 43.45 [38.33, 50.54] | 0.057 |
| CP burden (%) | 1.52 [0.22, 3.73] | 2.35 [1.03, 3.31] | 0.102 |
| Total plaque burden (%) | 40.50 [32.21, 49.13] | 46.61 [43.40, 55.06] | 0.025 |
| LAP burden (%) | 5.41 [2.73, 8.40] | 5.34 [3.67, 7.91] | 0.935 |
| Total CP vol (mm3) | 9.53 [2.00, 31.51] | 16.09 [9.18, 66.24] | 0.038 |
| Total NCP vol (mm3) | 280.70 [149.87, 521.70] | 498.63 [170.76, 857.22] | 0.07 |
| Total LAP vol (mm3) | 40.78 [17.60, 85.68] | 52.28 [18.03, 119.22] | 0.49 |
| Total plaque vol (mm3) | 296.75 [159.02, 559.87] | 545.02 [179.12, 947.00] | 0.064 |
| EAT (mL) | 103.00 [76.50, 132.00] | 81.00 [70.00, 106.00] | 0.04 |
| 10- year ASCVD risk (%) | 10.00 [5.32, 18.45] | 7.00 [4.94, 10.41] | 0.212 |
BMI: Body mass index, CAC: coronary artery calcium, LDL-C: low-density lipoprotein cholesterol, HLD-C: high-density lipoprotein cholesterol, HbA1c: hemoglobin A1c, SBP: average systolic blood pressure, DBP: average diastolic blood pressure, NCP: non-calcified plaque, NHA= non-Hispanic Asian, NHB= non-Hispanic Black, NHW=non-Hispanic White, CP: calcified plaque, LAP: low-attenuation plaque, EAT: epicardial adipose tissue, ASCVD: atherosclerotic cardiovascular disease risk by pooled cohort equations.
When the relationship between Lp(a) and PCAT was analyzed per race/ethnicity, Hispanic patients showed a trend for a correlation between higher PCAT attenuation in the RCA and higher Lp(a) values (p=0.06) but this was not seen in NHA, NHB or NHW patients.
As seen in Table 1, median EAT volume was 88.00 mL [63.00, 117.00]. Non-Hispanic White patients had higher EAT (124.00 mL [97.00, 134.00]) when compared to NHA (59.50 mL [50.50, 100.50]; p<0.002), NHB (65.50 mL [53.00, 87.50]; P<0.001) and Hispanic patients (94.00 mL [71.50, 124.00]; p=0.016).
3.4. Clinical outcomes
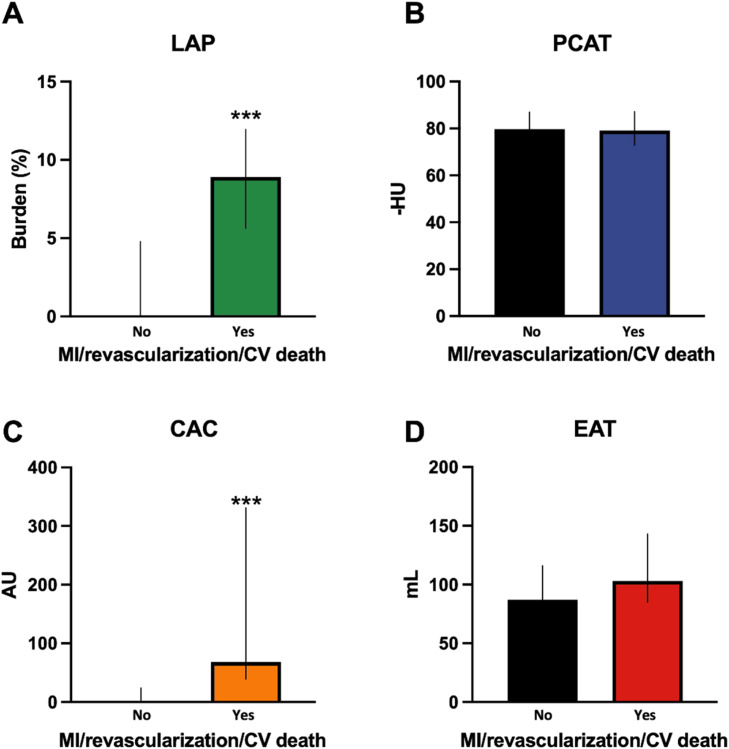
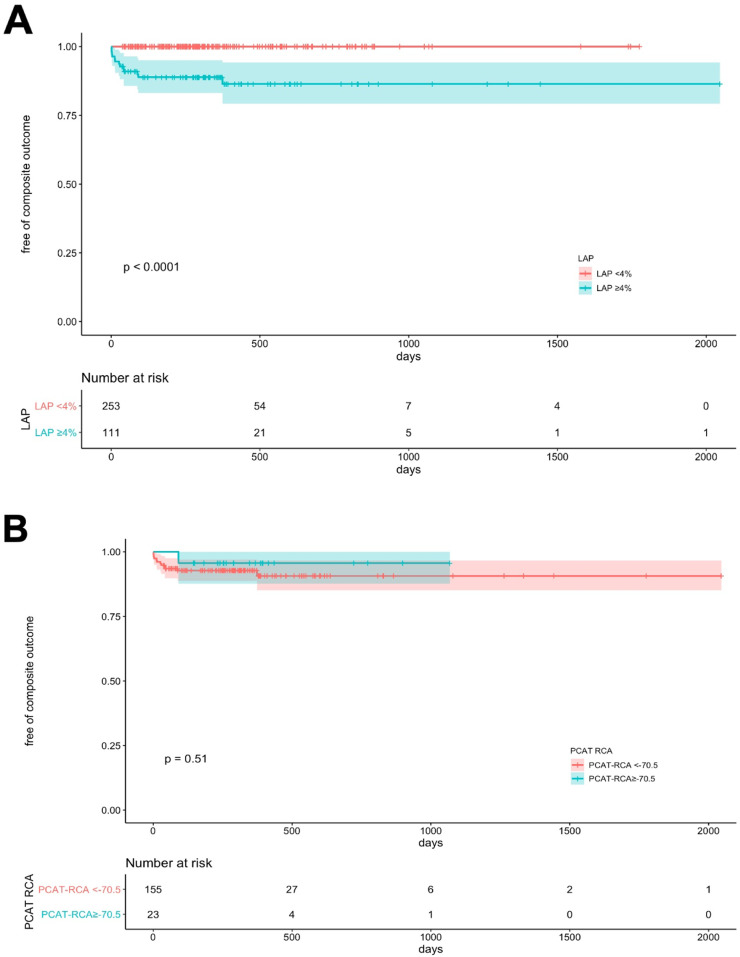
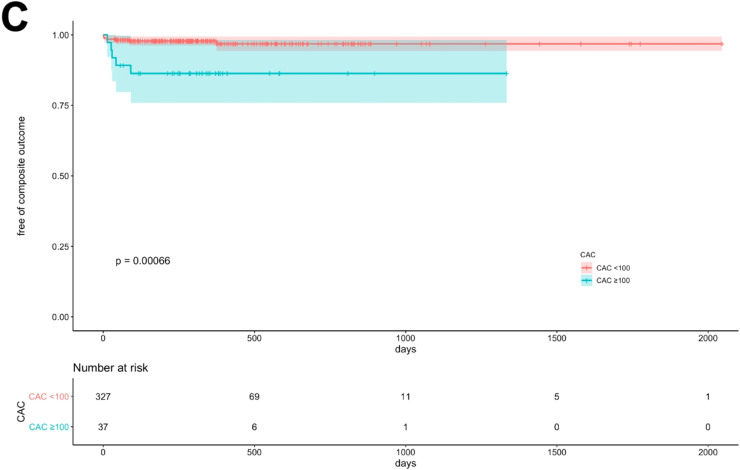
As seen in Table 1, there were a total of fifteen (4.1%) invasive coronary angiograms, eleven (3.0%) percutaneous coronary interventions (PCI), two (0.6%) coronary artery bypass graft surgeries (CABG), two MIs (0.6%) and one (0.3%) non-cardiac death during the follow-up period. Seven revascularizations were performed in the setting of unstable angina, two in non-ST elevation MI (NSTEMI), two due to high-risk/ulcerated plaque features by optimal coherence tomography, one based on high-risk stenosis by invasive coronary findings and one for unknown reason was performed at an outside hospital. Death was non-cardiac in origin due to COVID-19 pneumonia. Hispanic and NHW patients had higher revascularizations than NHB and NHA (p=0.04). As shown on Fig. 5, patients with occurrence of the primary clinical outcome had greater LAP burden (A, p<0.001) and CAC (C, p<0.001) but no significant differences in RCA PCAT attenuation (B) or EAT volume (D). Patients with elevated LAP burden (Fig. 6A) or CAC>100 (Fig. 6C) had a higher accumulative incidence of the primary clinical outcome (p<0.001). Moreover, as shown in Fig. 6B, there was no significant difference in clinical events for patients that had elevated RCA PCAT attenuation when compared to those without it. All events occurred in patients with elevated LAP burden.
Fig. 5.
CCTA predictors of composite of myocardial infarction, revascularization and cardiovascular death. Graph bars show the LAP burden (A), PCAT attenuation (B), CAC (C) and EAT volume (D) between patients that had the composite clinical outcome of MI, revascularization and cardiovascular death and those who did not.
LAP: low-attenuation plaque, AU: Agatston units, CV death: cardiovascular death, EAT: epicardial adipose tissue, HU: Hounsfield units, MI: myocardial infarction, mL: milliliters, PCAT: pericoronary adipose tissue. ***= p<0.001.
Fig. 6.
Unadjusted cumulative risk for MI, revascularization or cardiovascular death. Kaplan-Meier curves show cumulative risk for revascularization, MI and cardiovascular death according to LAP burden(A), RCA PCAT attenuation (B) and CAC (C).
CAC: coronary artery calcium score, LAP: Low-attenuation plaque burden, MI: Myocardial infarction, PCAT: pericoronary adipose tissue attenuation, RCA: right coronary artery. Log-rank p<0.001.
4. Discussion
The main finding of our study is the high prevalence of elevated LAP burden and PCAT attenuation (31 and 18%, respectively) among patients with stable chest pain or angina equivalent undergoing CCTA, while only 8% had obstructive stenosis. All patients with revascularization/MI or cardiovascular death (mostly driven by unstable angina or NSTEMI) had elevated LAP burden. On the opposite, elevated PCAT attenuation was not associated with the primary clinical outcome. Secondly, we found that age, male sex, hypertension and hyperlipidemia, were associated with increased risk for elevated LAP burden whilst NHB race/ethnicity decreased the risk. Thirdly, we identified an association between EAT volume and elevated LAP burden and between elevated RCA PCAT attenuation and total plaque burden but no association between lipid parameters, Lp(a), HbA1c and high-risk CCTA phenotype in the overall cohort. When the relationship between Lp(a) and high-risk phenotype was analyzed by race/ethnicity, we found a correlation between Lp(a) and LAP burden and a trend between Lp(a) levels and elevated PCAT attenuation in Hispanic patients.
This is the first study comparing the prevalence of quantitative high-risk CCTA phenotype characteristics in a diverse patient cohort and analyzing their associated cardiometabolic predictors and clinical outcomes. While the science behind quantitative plaque analysis has made significant progress in the last decade, showing plaque to be superior to stenosis severity assessment in the ability to predict MACE, very little data has been produced on diverse patient cohorts. A previous study in asymptomatic patients with a diverse patient population (47% Hispanic, 43% NHW and 3.5% NHB) found that 13% had CAC≥100, while 6% had stenosis ≥50% and 2% ≥70% [23]. Even that our patients were symptomatic, the prevalence of CAC≥100 and obstructive stenosis were similar with 10% having CAC≥100 and 7 and 2% coronary stenosis ≥50% and 70%, respectively. Previous studies have reported a lower prevalence of CAC in asymptomatic Black when compared to White patients [24]. Another study by Zhang et al. in patients with stable chest pain from a nested observational cohort study within the PROMISE trial, found that Black patients had a lower prevalence of CAC>0 (45.1 vs 63.2%); p<0.001), coronary stenosis ≥50% (8.7 vs 14.6%; p=0.001), and high-risk plaque using qualitative plaque analysis (37.6% vs 52.4%; p<0.001) [25]. Our study found similar results using quantitative analysis with NHB patients having higher Lp(a), but lower percentage of elevated LAP burden and clinical events.
Increased PCAT attenuation measured from CCTA is associated with biopsy-proven coronary inflammation [26] and with plaque progression [27], and can predict MACE [28,29] and mortality [30]. Our results did not demonstrate any differences in high-risk PCAT between ethnicities. Goeller et al. had similar findings in an Australian patient population without differences in PCAT between South Asian, East Asian and European-origin White patients [31].
Lipoprotein(a) is predominantly genetically determined with significant variation among different ethnicities [32]. A recent study, in a Scottish White population from a post-hoc analysis of the DIAMOND (Dual Antiplatelet Therapy to Inhibit Coronary Atherosclerosis and Myocardial Injury in Patients With Necrotic High-Risk Coronary Plaque Disease) trial, found that Lp(a) was associated with progression of an adverse plaque phenotype in a cohort of patients with advanced stable CAD who were already using guideline-directed preventative therapies [33]. Another recent study found an association between Lp(a), total plaque volume and fat attenuation index in CCTAs from a Chinese cohort [34]. We found a correlation between LAP burden and Lp(a) levels and a trend between higher Lp(a) and high-risk PCAT in Hispanic patients. Our results were not observed in other race/ethnic subgroups. These findings could be due to the predominantly Hispanic population from our sample and the smaller size of the other race/ethnicity subgroups and are therefore not conclusive suggesting the need for larger studies. Levels of Lp(a) are 70-90% genetically determined, and Black individuals from African descent are known to have higher Lp(a) primarily due to medium apo(a) alleles [35]. Lipoprotein(a) levels are known to be associated with higher risk of ASCVD [36] since Lp(a) promotes inflammation and atherosclerosis by multiple mechanisms [37]. Similar to other lipoproteins, Lp(a) is susceptible to oxidative modifications leading to the formation of pro-inflammatory and pro-atherogenic oxidized phospholipids [38]. In particular, Lp(a) is associated with accelerated progression of LAP [33], and PCAT has been suggested as a storage site and possible supply for oxidized LDL [39], however its association with Lp(a) needs further research. Moreover, in our CT registry, Lp(a) was measured only in a small percentage of patients, reinforcing the need for further education among clinicians on Lp(a) as an important risk factor.
Additionally, we demonstrated that NHW patients had higher EAT volume and an association between elevated LAP burden and EAT volume. The higher level of EAT volume in NHW versus NHB patients has been previously described in patients with diabetes [40]. Its association with CAC seems to be maintained in different ethnicities in the MESA study [41]. Furthermore, EAT is known to be associated with high-risk plaque features [42]. Its association with quantitative high-risk plaque features and PCAT in different races/ethnicities has not been previously explored.
Lastly, our findings are in alignment with prior reports on the importance of high coronary LAP burden in predicting coronary clinical outcomes as all our patients with mostly unstable angina and NSTEMI driven revascularization had elevated LAP burden. We did not find elevated PCAT (as compared to non-elevated PCAT) attenuation to be a predictor of clinical outcomes. Our findings on PCAT are consistent with a recent article by Chaterjee et al [43] and may be related to different stages of CAD in these patient populations.
4.1. Limitations
Our study has several limitations. First, given the sample size and study design, our patients were not propensity matched for cardiovascular risk factors, limiting the ability to individualize the impact of risk factors or race/ethnicity on the high-risk CCTA phenotype and clinical outcomes despite efforts to adjust for confounders. Further studies are needed with larger cohorts including propensity-matching for cardiovascular risk factors. Additionally, as only patients referred for CCTA with complete metabolic panel were included, referral bias cannot be excluded. Second, this study was limited to those with a full cardiometabolic panel including Lp(a), rather than all comers undergoing CCTA for stable chest pain. However, when patients with Lp(a) measured were compared to those that did not, there were no differences in 10-year ASCVD risk by PCE, baseline statin therapy, age, sex or smoking and only minimal differences in ethnicity, diabetes, hyperlipidemia and creatinine (Supplementary Table 1). Third, we are not able to determine if the lipid panels were performed in a fasting or non-fasting state. Fourth, we had a small number of clinical events with most of them being revascularizations and a short follow-up. Nonetheless, most of these revascularizations were for unstable coronary artery disease (unstable angina or NSTEMI). Further larger studies with longer follow-up including racial/ethnically diverse patients are needed. Lastly, we only found a significant correlation between Lp(a) and LAP burden in Hispanic patients, this is most likely due to the higher representation of Hispanic patients in our cohort. Larger studies including diverse race/ethnicity populations are needed.
5. Conclusions
In our racial/ethnically diverse cohort with high number of Hispanic and NHB patients presenting with stable chest pain or angina equivalent and referred for CCTA at a single healthcare network, elevated LAP burden and PCAT attenuation had high prevalence despite low-number of patients with obstructive stenosis. Age, male sex, hypertension and hyperlipidemia, were associated with increased and NHB race/ethnicity decreased odds of elevated LAP burden. Hispanic patients showed a correlation between Lp(a) and higher LAP burden.
Funding and disclosures
S.V. has received research support from the Department of Veterans Affairs, NIH, Tahir and Jooma Family and Honorarium from American College of Cardiology (Associate Editor for Innovations, acc.org). L.S. has received consulting honorarium from Amgen, BMS and Philips; and Grant support from Amgen. P.J.S, D.S.B. and D.D. have received software royalties from Cedars-Sinai Medical Center. D.D. was supported by grants from National Heart, Lung and Blood institute (1R01HL133616 and 1R01HL148787-01A1). Other authors declare no conflict.
Declaration of Competing Interest
S.V. has received research support from the Department of Veterans Affairs, NIH, Tahir and Jooma Family and Honorarium from American College of Cardiology (Associate Editor for Innovations, acc.org). L.S. has received consulting honorarium from Amgen, BMS and Philips; and Grant support from Amgen. P.J.S, D.S.B. and D.D. have received software royalties from Cedars-Sinai Medical Center. D.D. was supported by grants from National Heart, Lung and Blood institute (1R01HL133616 and 1R01HL148787-01A1). Other authors declare no conflict.
Acknowledgments
Authors would like to thank Matthew Miller for his assistance with the manuscript figures.
Contributor Information
Damini Dey, Email: Damini.dey@cshs.org.
Leandro Slipczuk, Email: lslipczukb@montefiore.org.
References
- 1.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74 doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visseren F.L.J., MacH F., Smulders Y.M., Carballo D., Koskinas K.C., Bäck M., et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42 doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 3.B M.J., Y R., B G., C J.J., D R.C., F A.R., et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA) Eur Heart J. 2018;39 doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams M.C., Earls J.P., Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2021 doi: 10.1016/j.jcct.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Tzolos E., Williams M.C., McElhinney P., Lin A., Grodecki K., Flores Tomasino G., et al. Pericoronary adipose tissue attenuation, low-attenuation plaque burden, and 5-year risk of myocardial infarction. JACC Cardiovasc Imaging. 2022;15:1078–1088. doi: 10.1016/j.jcmg.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin S.S., Blaha M.J., Blankstein R., Agatston A., Rivera J.J., Virani S.S., et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease. Circulation. 2014;129:77–86. doi: 10.1161/CIRCULATIONAHA.113.003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.al Rifai M., Blaha M.J., Nambi V., Shea S.J.C., Michos E.D., Blumenthal R.S., et al. Determinants of incident atherosclerotic cardiovascular disease events among those with absent coronary artery calcium: multi-ethnic study of atherosclerosis. Circulation. 2022;145:259–267. doi: 10.1161/CIRCULATIONAHA.121.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javed Z., Maqsood M.H., Amin Z., Nasir K. Race and ethnicity and cardiometabolic risk profile: disparities across income and health insurance in a national sample of US adults. J Public Health Manag Pract. 2022;28 doi: 10.1097/PHH.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 9.Steffen B.T., Thanassoulis G., Duprez D., Stein J.H., Karger A.B., Tattersall M.C., et al. Race-based differences in lipoprotein(a)-associated risk of carotid atherosclerosis: The multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39 doi: 10.1161/ATVBAHA.118.312267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams D.B., Narayan O., Munnur R.K., Cameron J.D., Wong D.T.L., Talman A.H., et al. Ethnic differences in coronary plaque and epicardial fat volume quantified using computed tomography. Int J Cardiov Imaging. 2017;33 doi: 10.1007/s10554-016-0982-1. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez C.J., Allison M., Daviglus M.L., Isasi C.R., Keller C., Leira E.C., et al. Status of cardiovascular disease and stroke in hispanics/latinos in the united states: A science advisory from the american heart association. Circulation. 2014;130 doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasir K., Cainzos-Achirica M., Valero-Elizondo J., Ali S.S., Havistin R., Lakshman S., et al. Coronary atherosclerosis in an asymptomatic U.S. population. JACC Cardiovasc Imaging. 2022 doi: 10.1016/j.jcmg.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald W.T., Levy R.I., Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18 doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 14.Goff D.C., Lloyd-Jones D.M., Bennett G., Coady S., D'Agostino R.B., Gibbons R., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbara S., Blanke P., Maroules C.D., Cheezum M., Choi A.D., Han B.K., et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the north american society for cardiovascular imaging (NASCI) J Cardiovasc Comput Tomogr. 2016;10 doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15 doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 17.Cury R.C., Abbara S., Achenbach S., Agatston A., Berman D.S., Budoff M.J., et al. Coronary artery disease - reporting and data system (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging. 2016;9 doi: 10.1016/j.jcmg.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Williams M.C., Kwiecinski J., Doris M., McElhinney P., D'Souza M.S., Cadet S., et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (scottish computed tomography of the HEART) Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzolos E., McElhinney P., Williams M.C., Cadet S., Dweck M.R., Berman D.S., et al. Repeatability of quantitative pericoronary adipose tissue attenuation and coronary plaque burden from coronary CT angiography. J Cardiovasc Comput Tomogr. 2021;15:81–84. doi: 10.1016/j.jcct.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otaki Y., Rajani R., Cheng V.Y., Gransar H., Nakanishi R., Shmilovich H., et al. The relationship between epicardial fat volume and incident coronary artery calcium. J Cardiovasc Comput Tomogr. 2011 doi: 10.1016/j.jcct.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato R., Shmilovich H., Tamarappoo B.K., Cheng V.Y., Slomka P.J., Berman D.S., et al. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr. 2011 doi: 10.1016/j.jcct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southern W.N., Bellin E.Y., Arnsten JH. Longer lengths of stay and higher risk of mortality among inpatients of physicians with more years in practice. Am J Med. 2011;124:868–874. doi: 10.1016/j.amjmed.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasir K., Cainzos-Achirica M., Valero-Elizondo J., Ali S.S., Havistin R., Lakshman S., et al. Coronary atherosclerosis in an asymptomatic u.s. population: miami heart study at baptist health South Florida. JACC Cardiovasc Imaging. 2022;15:1604–1618. doi: 10.1016/j.jcmg.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Doherty T.M., Tang W., Detrano RC. Racial differences in the significance of coronary calcium in asymptomatic black and white subjects with coronary risk factors. J Am Coll Cardiol. 1999;34 doi: 10.1016/S0735-1097(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Olalere D., Mayrhofer T., Bittner D.O., Emami H., Meyersohn N.M., et al. Differences in cardiovascular risk, coronary artery disease, and cardiac events between black and white individuals enrolled in the PROMISE trial. JAMA Cardiol. 2022;7 doi: 10.1001/jamacardio.2021.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonopoulos A.S., Sanna F., Sabharwal N., Thomas S., Oikonomou E.K., Herdman L., et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 27.Yerramasu A., Dey D., Venuraju S., Anand D.V., Atwal S., Corder R., et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Yuvaraj J., Lin A., Nerlekar N., Munnur R.K., Cameron J.D., Dey D., et al. Pericoronary adipose tissue attenuation is associated with high-risk plaque and subsequent acute coronary syndrome in patients with stable coronary artery disease. Cells. 2021;10 doi: 10.3390/cells10051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzolos E., Williams M.C., McElhinney P., Lin A., Grodecki K., Guadalupe F.T., et al. Pericoronary adipose tissue attenuation, low-attenuation plaque burden and 5-year risk of myocardial infarction. Eur Heart J. 2021;42 doi: 10.1093/eurheartj/ehab724.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikonomou E.K., Marwan M., Desai M.Y., Mancio J., Alashi A., Hutt Centeno E., et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392 doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goeller M., Rahman Ihdayhid A., Cadet S., Lin A., Adams D., Thakur U., et al. Pericoronary adipose tissue and quantitative global non-calcified plaque characteristics from CT angiography do not differ in matched South Asian, East Asian and European-origin Caucasian patients with stable chest pain. Eur J Radiol. 2020;125 doi: 10.1016/j.ejrad.2020.108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W., Cao J., Steffen B.T., Post W.S., Stein J.H., Tattersall M.C., et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: The multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35 doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser Y., Daghem M., Tzolos E., Meah M.N., Doris M.K., Moss A.J., et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79 doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai N., Chen Z., Zhou F., Zhou Y., Hu N., Duan S., et al. Association of lipoprotein (a) with coronary-computed tomography angiography–assessed high-risk coronary disease attributes and cardiovascular outcomes. Circ Cardiovasc Imaging. 2022;15 doi: 10.1161/CIRCIMAGING.122.014611. [DOI] [PubMed] [Google Scholar]
- 35.Enkhmaa B., Anuurad E., Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57 doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A.P., Wang M., Pirruccello J.P., Ellinor P.T., Ng K., Kathiresan S., et al. Lp(a) (lipoprotein [a]) concentrations and incident atherosclerotic cardiovascular disease new insights from a large national biobank. Arterioscler Thromb Vasc Biol. 2021;41 doi: 10.1161/ATVBAHA.120.315291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes-Soffer G., Ginsberg H.N., Berglund L., Duell P.B., Heffron S.P., Kamstrup P.R., et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42 doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibundgut G., Scipione C., Yin H., Schneider M., Boffa M.B., Green S., et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54 doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida Y., Uchida Y., Shimoyama E., Hiruta N., Kishimoto T., Watanabe S. Human pericoronary adipose tissue as storage and possible supply site for oxidized low-density lipoprotein and high-density lipoprotein in coronary artery. J Cardiol. 2017;69 doi: 10.1016/j.jjcc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Divers J., Wagenknecht L.E., Bowden D.W., Carr J.J., Hightower R.C., Register T.C., et al. Ethnic differences in the relationship between pericardial adipose tissue and coronary artery calcified plaque: African-American-diabetes heart study. J Clin Endocrinol Metab. 2010;95 doi: 10.1210/jc.2010-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding J., Kritchevsky S.B., Hsu F.C., Harris T.B., Burke G.L., Detrano R.C., et al. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2008;88 doi: 10.1093/ajcn/88.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nerlekar N., Brown A.J., Muthalaly R.G., Talman A., Hettige T., Cameron J.D., et al. Association of epicardial adipose tissue and high-risk plaque characteristics: A systematic review and meta-analysis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee D., Shou B.L., Matheson M.B., Ostovaneh M.R., Rochitte C., Chen M.Y., et al. Perivascular fat attenuation for predicting adverse cardiac events in stable patients undergoing invasive coronary angiography. J Cardiovasc Comput Tomogr. 2022 doi: 10.1016/j.jcct.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]