Abstract
Gelada (Theropithecus gelada) is an Ethiopian endemic mammal whose range was previously believed to be limited to the Bale and Simien Mountain national parks. However, knowledge of the range of the species is still not satisfactory. This study was designed to investigate the distribution and population size of the gelada in selected highlands in Tigray. To achieve this, study areas were divided into census tracts identified for a direct field assessment using habitat-based counting approaches. Moreover, informal interviews were conducted parallel to the direct field assessment in different villages. Our large spatial scale survey, the first of its kind for the region, confirmed the presence of T. gelada in three mountain clusters in Tigray for the first time. A total of 223 individual geladas were recorded in Ganta Afeshum, Hawzen and Welkait escarpments. The highest number of individuals (35.7 ± 3.8) was recorded in Hawzen (with 6.99 individuals/km2), followed by Ganta Afeshum (with 4.3 individuals/km2). Agricultural expansion, settlement and a lack of community awareness are the key threats operating against the conservation of the gelada in its current range in Tigray. Further research on the overall ecology, feeding, and spatial distribution of the species should be projected.
Keywords: Conservation, Endemic mammals, Geographic range, Population ecology, Primates, Theropithecus
1. Introduction
Ethiopia is known for its diverse landscape and climate. The proprietorship of such a huge diversity in landscape and climate has bestowed it with a huge flora and fauna composition, making it to have the fifth largest floral composition in Tropical Africa [1]. According to Yalden et al. [2], Ethiopia has more than 284 species of mammals, out of which 11% of them are endemic. As a result of the country's possession of such great biodiversity in its natural ecosystem and biogeographically isolated highlands that support high species endemicity, it is internationally recognized as one of the most important conservation hotspots [3,4]. Landscape-wise, the country has a broad range of habitats, from an arid desert in the southeastern to an open grassy steppe and semi-arid savannas in the southwestern and northern parts of the country ([4]). It also has highland forests in the western and southern parts of the country and Afro-alpine moorlands clustered in the different parts of the country, which are strongly believed to have contributed to the country's rich biodiversity [[4], [5], [6]]. The country also has a wide range of elevation gradients, from 116 m below sea level in the Afar depression to a peak of 4620 m above sea level at Ras Dejen in the Semien Mountain [4]. This does contribute to the availability of a large diversity of ecological conditions, which are inhabited by different fauna adapted to the different elevation gradients. The highland regions, although possessing fewer species than lowland parts of the country, have a large number of endemic species of birds (n = 18), mammals (n = 55) and amphibians (n = 25) [2,[7], [8], [9], [10]]. What is more, the Ethiopian highlands are believed to be home to more than 31 endemic species of mammals such as the Ethiopian wolf (Canis simensis), walia ibex (Capra walie) and gelada (Theropithecus gelada) [2,5,7]. As one of the Ethiopian endemic mammals, the gelada has been previously reported from different highlands of Ethiopia: the Simien Mountains National Park [11], the Central highlands of Ethiopia, Debre-Libanos area [12], Arsi and Bale Mountains [[13], [14], [15]].
Previously, geladas were believed to be range limited to the well-known and relatively accessible regions of Bale and the Simien Mountain national park. There have been several empirical studies on the gelada population structure [13,[16], [17], [18]], ecology [[19], [20], [21]], phylogeography [22] and distribution [2,23] from different localities in Ethiopia. Nevertheless, despite the historical records and museum collections indicating the historical presence of the gelada in Tigray [2,23], Tigray has been removed from the list of gelada home ranges [24,25]. Some researchers have even speculated that the gelada population might have been locally extinct in Tigray [22]. However, recently, the presence of gelada in small pockets of Eastern Tigray has been confirmed [26,27]. This is self-evident that the gelada population's geographic range in Ethiopia is poorly known at the moment, and our knowledge of the distribution range of the species is still not satisfactory. Thus, the topic deserves further investigation.
There is a wealth of evidence that clearly shows the distribution and abundance of primates are determined by a complex set of historical, human and environmental factors [14,23]. But still, there is a major gap in our knowledge of the distribution of geladas and we need to search for further potential areas of the gelada range instead of limiting ourselves to research on the gelada population of localized regions in protected areas and some extensively studied areas in the southern part of the gelada range [12,13,20]. This on its own should have led to further exploration outside the protected areas and would enable and enhance our understanding of how the geladas interact with the ever-increasing human population and respond to frequent contact with humans as local farmers expand their cultivation and livestock grazing to steep hillsides once inhabited solely by wildlife [11].
Despite the scattered studies on population estimates of geladas from different localities [[14], [15], [16],28], there is little knowledge about the total number of geladas throughout Ethiopia [11]. Beehner et al. [11] gave an estimate for the gelada population in the wild to be 50,000–60,000 individuals. However, this estimate is difficult to corroborate because we still don't know the exact geographic range of the species. Even though the existence of the gelada population was witnessed by the local community living in the Eastern, Southern, South Eastern and Western parts of Tigray, no systematic research has been conducted on the distribution and population size of these species at a larger scale. The present study aimed to fill this gap and provide pertinent evidence on the population size, spatial distribution, and key threats of the endemic gelada in a large spatial scale inventory, the first of its kind in the highlands of Tigray National Regional State, northern Ethiopia. To this end, this research aimed to investigate the spatial distribution and population status of the gelada in the highlands of Tigray National Regional State. Inspired by the geladas' preferences for cliffs and highlands as their suitable habitat for living, we hypothesized that the highlands of Tigray are inhabited by geladas. We have presented here the first large-scale field survey of the gelada in Tigray National Regional State.
2. Materials and methods
2.1. Description of the study animal and area
The gelada (Theropitecus gelada, Rüppell 1835) is the only living species of the genus Theropitecus, which is endemic to the Ethiopian grassland plateaus [7]. An essential distinguishing field mark of geladas is their characteristic red area of skin on the chest. This is also believed to play a role in communication among geladas [29]; as a sign of sexual maturity in females [30] and as a status indicator for alfa males [29]. Furthermore, adult males have a long, heavy cape of hair on their backs. The gelada's shorter jaws, longer face, snub snout and bulging cheek pouches distinguish it from other baboons in Ethiopia. Additionally, they have very long canines [[31], [32], [33]]. Despite the absence of clear morphological distinction between geladas from different localities and museum species [2,7,23], two subspecies of gelada in the northern and central highlands of Ethiopia: T. gelada gelada and T. gelada obscurus and a third one, T. gelada arsi, from the southern highlands of Ethiopia have been suggested based on molecular work [22,34]. Tigray, which is the focus of this study, is one of the nine administrative national regional states in the Federal Democratic Republic of Ethiopia (FDRE). The region has highlands reaching peaks of more than 3960 m above sea level (mount Tsibet, in the southern zone of Tigray National Regional State). Following Fishpool and Evans [35], the entire region can be classified into three major biomes namely: Sudan-Guinea Savanna biomes (which encompass the entire southwestern part of the region); Afrotropical Highland biomes (which expand from the southern part of the region all along the central to the northern part of the region); and Somali-Masai biomes (which are limited to the eastern escarpment and a small section in the northwestern part of the region bordering Eritrea).
This study was carried out in different mountain clusters (escarpments) of Tigray National Regional State (called TNRS hereafter). Included in these clusters are: (1) an Eastern escarpment, which refers to the highlands in the Eastern zone of TNRS and the study is confined to the mountains of Ganta Afeshum and Hawzen districts (Fig. 1). This study area, located at a distance of 135 km from Mekelle, the capital city of Tigray, and is home to stunning mountains and more than 20 rock-hewn churches [36]; (2) the Western escarpment, which refers to mountains in the Western Zone of TNRS. The study in this escarpment is delimited to the mountain chains of Welkait district (Fig. 1). Welkait is located at a distance of 660 km from Mekelle, the capital city of Tigray National Regional State, and at a distance of 119 km from Humera, the capital city of the Western Zone of TNRS.
Fig. 1.
Digital elevation map of Tigray National Regional State, with the study sites indicated by a black star and the Eastern escarpment and the Western escarpment indicated by a Pentagon.
2.2. Methods
A preliminary survey was conducted, in 2017, through informal interviews with local residents in the different escarpments in Tigray National Regional State (TNRS). Respondents were provided with photo plates of four different baboons: the hamadryas baboon (Papio hamadryas), yellow baboon (Papio cynocephalus), olive baboon (Papio anubis) and gelada (Theropithecus gelada) and asked if they had seen the animal in the photo. Informants who claimed to have seen the gelada (Theropithecus gelada) and correctly identified it from the photos provided to them were further asked about (1) the number of geladas they saw in their locality; (2) how they themselves and/or someone in their locality interact with the geladas; and (3) if they could identify any perceived threats to the geladas in their localities. Then, using this information from interviewees, two main escarpments were selected for field observation that was carried out in 2017 and 2018. The two escarpments selected for a detailed field observation were the Eastern escarpment and the Western escarpment of TNRS (Fig. 1). The study areas/escarpments were divided into counting blocks (census tracts) identified by an initial preliminary survey in the respective escarpments. ‘Counting blocks’ in this study's context refers to small areas with natural and artificial boundaries that can easily be identified on the map as well as on the ground (see supplementary material, Fig. S1). Thus, our field survey design follows a habitat-based counting approach where the distance and expanse of the consecutive counting blocks studied vary depending on the natural boundaries and the topography of the area. The census was conducted from a suitable vantage point and/or while moving along counting blocks from the bottom of a cliff at around 2400 m above sea level (in Hawezen district), 2600 m above sea level in Ganta Afeshum and starting at 1797 m above sea level in Welkait district. Any sign of the species left (like fecal droppings and footprints; see supplementary material Fig. S2) was recorded to identify potential distribution ranges of the baboons in the escarpments investigated. Any individual observed as we move along the census track was recorded along with its age structure (i.e., juvenile, adult female or adult male). Total counting of the gelada population was carried out through direct observation and silent detection from a fitting vantage point or while moving along the established counting blocks. Thus, the total count, based on direct observation, is used to determine the population size of geladas in the study area using the ground survey techniques of Southwood and Henderson [37]. Then, based on the total count, we estimated population density using the density formula:
All block counts within an escarpment were conducted during the morning hours (7:00–11:00 a.m.) to avoid double counting. Each count was carried out with the help of the local guide (‘someone with a good knowledge of the geography of the escarpment/s) in the study areas. Binoculars were used for counting geladas at a distance. When a gelada is encountered, the following data were recorded: the activity engaged in (i.e., feeding, moving, resting and socializing) and the habitat type (cliff vs. flat top) where the groups are spotted. Then the collected data is analyzed using descriptive statistics and presented using tables and percentage representations.
For daily activity, a fixed-point counting method was applied in which the observer remained at one point and recorded the groups. To calculate the proportion of time the study animals spent engaged in each activity, we divided the number of records for each activity category by the total number of activity records. We used the behavioral records of the group to calculate the activity budget for each day. The grand mean proportions of the total 5250 scans (150 scans*35 study days/visits in the study area) provided the overall activity budgets for the entire study period.
2.2.1. Ethics committee approval
The study complies with all regulations for ethical clearance. This study does not involve the use of human subjects (human experimentation) but involves direct field observation of vertebrates (geladas). Permission to carry out field observation was obtained from Mekelle University, College of Natural and Computational Sciences, along with project registrations for VPRS/25/2012 and CRPO/CNCS/PhD/MU-NMBU/001/2011. Besides, the people interviewed were asked for their consent to participate in the interview.
3. Results
3.1. Spatial distribution and population size of geladas in Tigray
Our large-scale inventory, the first of its kind for the region, has revealed that the endemic gelada (Theropithecus gelada) is fairly abundant in different highlands of Tigray from the eastern highlands to the western highlands in Welkait district. In the eastern highlands, geladas were recorded in two clusters and seven counting blocks namely: Ganta-Afeshum (n = 4 counting blocks) and Hawzen district (n = 3 counting blocks), while in the western highlands, they were recorded in four counting blocks (Table 1 and supplementary information, Fig. S1 and see Fig. 1). Furthermore, the informants’ survey revealed the presence of geladas, an additional range-distribution, in the central highlands (Temebien-Nae-der area highland clusters) and mountain ranges in the southern highlands of Tigray National Regional State (Supplementary Information Table S1). The spatial distribution of the geladas among the mountain clusters is different in both population size and the area occupied in terms of size (Table 1). In the eastern escarpment, in both Ganta Afeshum and Hawzen districts, a total of 172 individual geladas were recorded. The highest number of individuals (35.7 ± 3.8) was recorded from three localities in Hawzen district, mainly composed of a few adult males (n = 19) followed by a high number of females (n = 55). While the number of juveniles in Ganta Afeshum district (n = 39) was higher than the number of juveniles in Hawzen district. Overall, 41.9% of the total population in the eastern escarpment was mainly juveniles (Table 1). The density (ind./km2) was also higher in Hawzen (6.99 ind./km2) than in Ganta Afeshum (4.3 ind./km2) in the eastern escarpment.
Table 1.
Spatial distribution, population size, and structure of the endemic gelada in selected mountains of Tigray.
| Zone | Districts | Kebelle | Counting Blocks | Age-sex |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Adult male | Adult female | Juveniles | Total | % | ||||
| Eastern | G.Afeshum | Adi Asefah | Adi Asefah -01 | 15.3 | 1 | 2 | 10 | 13 | 0.08 |
| Eastern | G.Afeshum | Adi Asefah | Adi Asefah -02 | 3 | 5 | 17 | 25 | 0.15 | |
| Eastern | G.Afeshum | Zeban Erar | Zeban Erar | 4 | 2 | 7 | 13 | 0.08 | |
| Eastern | G.Afeshum | Erar Senefti | Erar Sn'efti | 1 | 8 | 5 | 14 | 0.08 | |
| Eastern | Hawzen | Debre-Abay | Degu'at-01 | 15.5 | 6 | 25 | 9 | 40 | 0.23 |
| Eastern | Hawzen | Debre-Abay | Degu'at-02 | 8 | 14 | 12 | 34 | 0.20 | |
| Eastern | Hawzen | Dega'mba | Mai-da-Gobo | 5 | 16 | 12 | 33 | 0.19 | |
| Subtotal Total | 30.8 | 28 | 72 | 72 | 172 | ||||
| Western | Welkait | Adi Gaba | Mai Gebo-01 | 15.33 | 4 | 2 | 24 | 30 | 0.59 |
| Western | Welkait | Adi Gaba | Mai Gebo-02 | 9.33 | 3 | 3 | 9 | 15 | 0.29 |
| Western | Welkait | Bil'amba | Medacha-01 | 10.84 | 1 | 2 | 2 | 5 | 0.10 |
| Western | Welkait | Wef-argf | Qakha-01 | 5.00 | 1 | 0 | 0 | 1 | 0.02 |
| Subtotal Total | 40.5 | 9 | 7 | 35 | 51 | ||||
| Grand Total | 223 | ||||||||
G.Afeshum = Ganta Afeshum; Area = refers to the area of the counting block we scanned and is estimated based on GPS readings of the study sites. Age-sex: Adult male doesn't refer to alpha males but includes all adult males irrespective of their social status.
The western escarpment, in Welkait district, was found to be another home for the endemic gelada in the western zoogeographic corner of TNRS. In this escarpment, a total of 51 individual geladas were encountered in the open cliffs of the Bil’Amba and Adi-Geba mountain chains (Table 1). Out of the 51 geladas recorded from four counting blocks in this escarpment, nine (17.6%) were adult males, seven (13.7%) were adult females, and the majority (68.6%) of the population in the western escarpment were juveniles (Table 1). The mean number of individuals in the counting blocks was 12.8 ± 10.3 (±SD) (Table 1). In this escarpment, the highest gelada population, among the counting blocks, was recorded in Mai-Gebo-01. However, in the fourth locality surveyed, Qakha, only a single adult male was detected during our field campaign. The density of geladas (1.3 ind./km2) for this escarpment is lower than the eastern escarpment (Table 1).
3.2. Habitat use and activity patterns of gelada
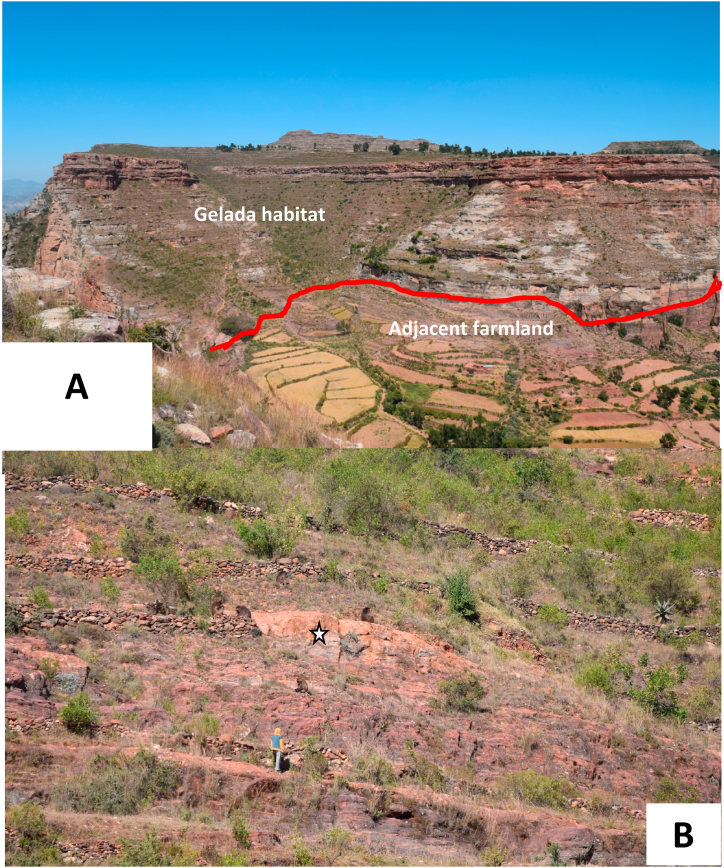
In the two escarpments, we observed two patterns of habitat use. In the eastern escarpment, geladas use the very steep, middle part of the cliff for nighttime spending while they move down to 2400 m above sea level for feeding (in Hawzen district) and move up to 2700 m above sea level to the flat plains of mountain chains (Fig. 2A and supplementary information, Fig. S3). Despite the presence of potential food sources in the area, we have not observed geladas moving down below the line at 2000 m above sea level in Hawzen district (Fig. 2A). The only potential human-gelada conflict was between the lines 2000–2400 and 2600–2700 (the flat top part of the escarpment, see Fig. S4 in the supplementary information for the topography of the escarpment). According to local informants, there were ten gelada killed in one season as a result of crop raiding at 2600–2700 m above sea level. Similarly, geladas were spotted on the cliff faces and top mountain plains of Ganta-Afeshum districts above 2000 m above sea level (Fig. 1). The species usually spends the night on the open cliff faces and takes off to the flat surface during the early morning hours in both mountain clusters of the eastern escarpments.
Fig. 2.
The 2000 m above sea level line and potential source of human-gelada conflict due to the presence of adjacent farmlands (A) and a boy chasing away five geladas in Mai-da-Gobo counting block, Hawzen district (B, cf. star mark).
Meanwhile, in the western escarpment, the cliff faces are prosperous in resting physical lands fitted with the behavioral aspects of the gelada. In comparison to the eastern escarpment, the landscape occupied by geladas in the western escarpment is too steep, and it all forms tapering chains of mountains. They are physically separated by massive gorges, making access by domestic animals and other agricultural activities to the top part very difficult. Besides, the present range of the species is less disturbed and free from grazing by domestic animals because of its difficult access to domestic animals, unlike habitats in Hawzen district of the eastern escarpment where access to the home range of geladas is possible both from the line 2000 m above sea level and again from the line 2700 m above sea level-the flat plains of the mountain. Such kinds of ecological consistency can sustain a high abundance of biota. However, the flat plains on top of the cliff in Hawzen district, at Maida-Gobo counting block, above 2600 m above sea level, are a potential source of human-gelada conflict (Fig. 2B). Here, more than three gelada were killed by traps while raiding crops in this area (personal obs.). Moreover, the spatial distribution and habitat use of the gelada in the Hawzen area start at 2400 m above sea level and reach about 2700–2800 m on the top flat part of the cliff. Whereas the gelada habitats used in the Western escarpment start at a lower elevation (1798 m above sea level), and the cliffs have tapering tops unlike the flat plains on top in Hawzen counting blocks (supplementary information, Fig. S4).
The activity pattern of individuals along their respective counting blocks, recorded during 150 scans conducted on 35 study days, is presented in Fig. 3. In these scans, the geladas spent 49.1% (SD ± 5.3%) of their time feeding (2578 of the total scans), 28.3% (SD ± 4.7%) moving, and 22.6% (1187 of the total scans) of their time engaging in resting and some social activities (i.e., grooming, playing and sexual activities, see supplementary information in Fig. S3).
Fig. 3.
Comparison of the activity budgets of geladas in mountain clusters of Tigray National Regional State.
3.3. Threats to geladas in the mountains of Tigray National Regional State
Three major threats operating against geladas are identified based on interviews and personal observations in the two escarpments, which differ in their degree of threat to geladas. Agricultural expansion, the proximity of settlements to the natural range of gelada (with less than 1.5 km of settlements in Maida Gobo) and the misperception of the adjoining local communities owing to crop losses. In addition to this, the absence of delineated in-situ conservation areas (recognized by 10% of respondents in the interview) is threatening the wild status of the species and resulting in intense human-wildlife conflict in the eastern escarpments. As a result, 13 individuals were reported killed during the study period.
In Welkait district, agricultural and settlement expansion into the home range of the adjacent localities were identified as key threat factors operating against the conservation practices of the species. Coupled with this, the absence of delineated in-situ conservation areas is notably disturbing the wild behavioral state of the species. This might lead to a difference in the distribution of the species across habitat variability, with prominent conservation implications. However, the top section of the cliff in the western escarpment is not accessible, thus providing a safe refuge for the species. Nonetheless, geladas move down to lower elevations (up to 1797 m above sea level) and raid crops. Yet, we have not received any reports of gelada kills in this escarpment.
4. Discussion
Here, we reported the results of the first-ever large-scale spatial survey for the gelada outside its perceived home range, in the northernmost part of Ethiopia. Based on field observation for the first time, we confirm that gelada occur, but not exclusively, on three mountain clusters, with more potential areas in Tigray National Regional State. The three clusters we surveyed during our study have different densities of gelada, with the highest in the Hawzen cluster, followed by the Ganta Afeshum and Welkait clusters. Contrary to claims that geladas of Tigray might have been locally extinct because of draught and prolonged war in the region [22], we have recorded the gelada in larger areas of Tigray. Our result also showed that the gelada range extends to the western escarpment in addition to the eastern escarpments reported earlier [26]. This indicates that the distribution range of the species has not been properly documented so far and is not as range-limited as it was proclaimed to be [24,25]. Understanding the distribution pattern of geladas is important for a population estimate and the conservation of the species that need to be prioritized. We believe a lot of work remains to be done in terms of mapping potential habitats and investigating the distribution patterns of the geladas. Within the previously known location of the gelada, about 1000 individuals of T. gelada arsi and about 4300 individuals of T. gelada gelada are estimated [11,13]. Some studies have suggested that the gelada population could be less than 25,000 individuals throughout Ethiopia [11]. This clearly indicates that population size does vary from region to region and is profoundly undetermined. However, scientific studies indicate that an accurate population size estimation is critical to establishing conservation and management policies. The new record of the gelada population from TNRS mountain clusters is a new opportunity for further study, but it is still essential to determine the exact population size to understand whether the gelada population in Ethiopia is stable or in decline status.
In this study, we reported the highest relative abundance of juvenile population size compared to a study in Gosh-Meda area, central Ethiopia [17] and Debre-Libanos [28]. This implies that there is a promising population structure despite the underlying anthropogenic threats in these mountain clusters. Previous studies indicated that variation in population size of the species is associated with food availability, nutritive demand and protection from predation [21,[38], [39], [40]]. Thus, the difference in population size observed in the three mountain clusters might be associated with the difference in the degree of human disturbance, the availability of food, and the suitability of the habitats [31,32,41]. The finding of the current study is consistent with a study done by Abie and Bekele [12], which noted that the distribution of geladas across habitats depends on the availability of food and distance from a human settlement in Debre-Libanos, northwest Shewa zone, Ethiopia [12].
We have detected different patterns of habitat use in the two escarpments surveyed: with the geladas in the Western escarpment occupying bare rocky cliffs and moving to lower elevations up to 1797 m above sea level. This is evidenced by the low population density of the geladas in this escarpment. This pattern is in line with the conclusion from other studies that a reduction in habitat quality pushes geladas to marginal areas, mainly to bare rocky cliffs (Iwamoto and Dunbar, 1983). This leads to a reduction in the population density of geladas [31,32,42]. Similar to reports of other studies, we noticed that the species usually spends the night at the open cliff faces and takes off to the flat surface during the early morning hours. This might be associated with the avoidance of the risk of predation by potential nocturnal predators such as hyenas and leopards [21]. Previous reports indicated that leopards, foxes, hyenas and even a very large bird of prey such as Lammergeyer (Gypaetus barbatus) are implicated among several potential and actual predators [19,21,40]. Despite the absence of a distribution study of these predators in the study areas, anecdotally, we suppose that there could be a difference in the potential and actual predators in the different areas due to the topographic differences of the escarpments. We believe further research should be conducted in this area.
Activity pattern studies (animal daily time budget) help us to understand how animals maximize their energy earning and spending from time to time (diurnal activity) and season-to-season (temporal variation). Despite the differences in the methods used in the study of time budgets broad comparisons could be made. In the majority of studies, feeding was a prominent activity pattern [13,30,39]. Geladas of Ganta Afeshum and Hawzen districts spend more time foraging and moving than resting and socializing. Overall, our study suggests that geladas in the TNRS mountain chains spend more time foraging and less time on social activities. This is in line with the findings of other studies where they reported that geladas spend less time on social activities such as grooming, playing, sexual activities and aggression compared to foraging activities [23,30]. We noticed that geladas in the western escarpment move more in terms of area covered compared to their eastern escarpment relatives, but this could possibly not be because of a lack of resources but because of free-range areas from threats. However, studies suggest that geladas move more if they do not get enough food resources [20]. So, to fulfill their energy requirements, they have to spend more time feeding and moving. In animals, the time spent on different activities is an indication of balancing the energy budget. A gelada that can easily obtain food spends more time resting and grooming than feeding and moving [33]. This perhaps might be related to a reduced availability of preferred foods [13,30,43]. However, to make such a bold conclusion, there needs to be an extensive habitat suitability study and a comprehensive survey of the diet of the geladas in the different mountain clusters of TNRS, which are non-existent.
Our recent observation implies that the species spend the night at the complex open cliffs, start feeding grass, and socialize at the top plain areas during the early sunrise (supplementary information, Fig. S4E). Our result here is consistent with studies from other parts of Ethiopia that noted gelada prefers open cliffy habitats to avoid human conflict and other predators [17,40]. Besides, the surveyed habitats are highly compatible with the previously known ecological requirements of the gelada range areas [7,32,39].
4.1. Threats to gelada
A study by Abie and Bekele [12] indicated that major threats to geladas are habitat destruction, livestock grazing and the expansion of agricultural activities. This is in line with the major threats identified in our study. In the face of unprecedented population growth and expansion of agricultural lands to even previously unsuitable and inaccessible areas to meet the demands of local communities for food, there is potential for conflict between geladas and humans in its current range. The anthropogenic killing of 13 geladas reported in this study evidences this.
We have reports of crop raid effects and anthropogenic kills of geladas in the eastern escarpment. This is in line with other studies that reported crop raids as a potential source of human-gelada conflict during the harvesting season [13,43]. The absence of anthropogenic kills of geladas in the western escarpment, despite the identification of crop raids as a common source of conflict, might indicate that people are more tolerant of gelada crop raids in this escarpment. The local communities living in gelada range areas are connected with traditional ecological knowledge on the distribution and feeding ecology of the species. This can mark efforts to enhance participatory gelada conservation by integrating useful local community knowledge into the existing management frameworks. Unlike other regions where male geladas are being killed for ceremonial headdresses, such activity is not reported in Tigray. However, the species has been reported as one of the zootherauptic resources for several ailments, including bruised skin and chloasma ([44], unpublished project report). Furthermore, grazing land competition with livestock has also been reported elsewhere [43]. Our recent survey reported that, the local communities living adjacent to the gelada home range area can easily differentiate the gelada from other baboons, based on morphological features of the species, when provided with photo plates. This shows the long-lasting community knowledge base associated with the species, which has also been reported elsewhere [18].
The agricultural encroachment of members of the adjoining local communities into the present range of the species led to a significant decrease in the habitat used by geladas. Owing to the habitat overlap of the species with the adjoining local communities, there is a potential for continued intense human-gelada conflict, threatening the conservation practices of the species. Additionally, due to their specialized diet, geladas are severely affected by soil erosion, drought, and possibly even global warming [20,38,39,45].
The species has not been classified as threatened or vulnerable for a long time, despite research indicating otherwise (Dunbar 1998). Recently, the northern gelada has been classified as vulnerable, while the other subspecies of geladas are of least concern under IUCN conservation designation status [22]. Given the taxonomic uniqueness, restricted habitat range, and small population size of geladas, as well as the anthropogenic threats they face, they need particular conservation policies and deserve detailed landscape population genetic studies and the development and implementation of empirically based conservation strategies to improve their chances of long-term survival.
5. Conclusions and a way forward
Studies on the spatial distribution of geladas have been restricted to the previously known protected areas in Ethiopia. Apart from historical records and small scale studies from eastern Tigray [26], no comprehensive report of the gelada in Tigray is made available to the body of scientific literature. To the best of our knowledge, geladas have not been reported from Tigray National Regional State in such a large country wide study. Our large-scale spatial survey confirmed the presence of the gelada in different mountain clusters of Tigray National Regional State for the first time in the region. Hence, this is the first report of its kind in such a large-scale spatial survey reporting the spatial distribution, population size, and potential threat to the gelada. Furthermore, our result indicated that the species is found at the open cliff faces and plains of the surveyed mountain chains of Tigray. Unlike previous studies, which set its elevation limit at 2400 m above sea level [2], we have recorded the species in mountain chains starting at 1797 m above sea level (in Blamabmichael, in the Eastern escarpments) reaching 2800 m above sea level in Hawzen mountain chains. The present study reported a total of 223 individuals of geladas with a promising reproductively active population. The mountain chains of the gelada range areas are important tourism hubs in the eastern escarpments of the Tigray National Regional State [36]. Hence, it is a good prospect to extend the alternative eco-tourism destinations to the existing tourism packages in the region. We have identified anthropogenic threat factors underlying the conservation status of the geladas, such as agricultural expansion, expansion of settlements adjoining the geladas’ habitats, and a lack of local community awareness towards the species, as key conservation challenges that have to be addressed in the future.
The gelada distribution is poorly known in Tigray National Regional State. Due to this, there is a knowledge gap in its full range of distribution and population ecology across the region. Thus, further intensive research on the conservation of the gelada should be carried out. Coupled with this, the local communities living adjoining the gelada's distribution range should be given awareness creation training. The currently spotted gelada distribution range should be delineated with the consent of the local community and district administrators. This will enhance the sustainability of conservation practices. The regional government should give due emphasis to the conservation and development of the sites where the gelada resides, for eco-tourism accessibility. Most importantly, the regional tourism bureau should work to boost its ecotourism potential to the larger tourism community and the global market demands.
Author contributions statement
1 - Conceived and designed the experiments
2 - Performed the experiments
3 - Analyzed and interpreted the data
4 - Contributed reagents, materials, analysis tools or data
5 - Wrote the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to extend our heartfelt thanks to the College of Natural and Computational Sciences, Mekelle University (MU) and the NORAD (MU-NMBU) project office of Mekelle University for their logistical support of the fieldwork. The fieldwork of THH was supported through the Tigray-Afar Eco-region project (VPRS/25/2012) and NORAD funds for small scale research projects (CRPO/CNCS/PhD/MU-NMBU/001/2011). Our thanks also go to Mr. Weldegebriel Hadera (from the MU-NMBU project office) and Mr. Fitsum Redae (from Mekelle Biodiversity Center) for their support during the fieldwork.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19346.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Brenan J.P. Some aspects of the phytogeography of tropical Africa. Ann. Mo. Bot. Gard. 1978;65(2):437–478. [Google Scholar]
- 2.Yalden D., et al. Catalogue of the mammals of Ethiopia and Eritrea. 7. Revised checklist, zoogeography and conservation. Trop. Zool. 1996;9(1):73–164. [Google Scholar]
- 3.Vavilov N.I. vol. 72. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 1951. p. 482. (The Origin, Variation, Immunity and Breeding of Cultivated Plants). [Google Scholar]
- 4.Ethiopia's Fifth National Report to the Convention on Biological Diversity. Ethiopian Biodiversity Institute; Addis Ababa: 2014. p. 86. EBI, Government of the Federal Democratic Republic of Ethiopia. [Google Scholar]
- 5.Yalden D. The extent of high ground in Ethiopia compared to the rest of Africa. Sinet. 1983;6(1):35–39. [Google Scholar]
- 6.Hillman J.C. Ethiopia: compendium of wildlife conservation information. Ethiopian Wildlife Conservation Organisation; Addis Ababa, Ethiopia: 1993. p. 230. [Google Scholar]
- 7.Yalden D., Largen M. The endemic mammals of Ethiopia. Mamm Rev. 1992;22(3‐4):115–150. [Google Scholar]
- 8.Lavrenchenko L.A., Bekele A. Diversity and conservation of Ethiopian mammals: what have we learned in 30 years? Ethiop. J. Biol. Sci. 2017;16(1):1–20. [Google Scholar]
- 9.Urban E.K., Brown L. Addis Ababa, Ethiopia. Dept. of Biology, Haile Sellassie I University; 1971. A checklist of the birds of Ethiopia. [Google Scholar]
- 10.Largen M.J. Catalogue of the amphibians of Ethiopia, including a key for their identification. Trop. Zool. 2001;14(2):307–402. [Google Scholar]
- 11.Beehner J., et al. Population estimate for geladas (Theropithecus gelada) living in and around the simien mountains national park, Ethiopia. Sinet. 2007;30(2):149–154. [Google Scholar]
- 12.Abie K., Bekele A. Threats to gelada baboon (Theropithecus gelada) around Debre Libanos, northwest Shewa zone, Ethiopia. International Journal of Biodiversity. 2016;2016:1–7. [Google Scholar]
- 13.Abu K., et al. Diet and activity patterns of Arsi geladas in low-elevation disturbed habitat south of the Rift Valley at Indetu, Ethiopia. Primates. 2018;59(2):153–161. doi: 10.1007/s10329-017-0640-9. [DOI] [PubMed] [Google Scholar]
- 14.Mori A., et al. Sociological and demographic characteristics of a recently found Arsi gelada population in Ethiopia. Primates. 1999;40(2):365–381. [Google Scholar]
- 15.Mori A., Belay G. The distribution of baboon species and a new population of gelada baboons along the Wabi-Shebeli river, Ethiopia. Primates. 1990;31:495–508. [Google Scholar]
- 16.Asfafaw H., Subramanian C. Population size and structure of gelada baboon (Theropithecus gelada, Ruppel, 1835) in simien mountains national park, Ethiopia. Global Journal of Biological and Agricultural Health Sciences. 2013;2:102–106. [Google Scholar]
- 17.Goshme B., Yihune M. Population structure and habitat use of gelada baboon (Theropithecus gelada) in Wof-Washa Forest (Gosh-Meda Area), Central Ethiopia. Journal of Ecology and Environment. 2018;42(35):1–6. [Google Scholar]
- 18.Kifle Z., Belay G., Bekele A. Population size, group composition and behavioral ecology of geladas (Theropithecus gelada) and human-gelada conflict in Wonchit Valley, Ethiopia. Pakistan J. Biol. Sci. 2013;16:1248–1259. doi: 10.3923/pjbs.2013.1248.1259. [DOI] [PubMed] [Google Scholar]
- 19.Ohsawa H. In: Ecological and Sociological Studies of Gelada Baboons. Kawai M., editor. Kodansha; Tokyo: 1979. The local gelada population and environment of the Gich area; pp. 3–45. Karger. [Google Scholar]
- 20.Jarvey J.C., et al. Graminivory and fallback foods: annual diet profile of geladas (Theropithecus gelada) living in the Simien Mountains National Park, Ethiopia. Int. J. Primatol. 2018;39(1):105–126. [Google Scholar]
- 21.Iwamoto T., et al. Anti–predator behavior of gelada baboons. Primates. 1996;37:389–397. [Google Scholar]
- 22.Zinner D., et al. Phylogeography, mitochondrial DNA diversity, and demographic history of geladas (Theropithecus gelada) PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0202303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gippoliti S. Theropithecus gelada distribution and variations related to taxonomy: history, challenges and implications for conservation. Primates. 2010;51(4):291–297. doi: 10.1007/s10329-010-0202-x. [DOI] [PubMed] [Google Scholar]
- 24.Hill, W.C.O., Primates comparative Anatomy and Taxonomy VIII. 1970, Edinburgh, UK: Edinburgh University Press..
- 25.Dunbar R. In: Prince Rainier HSH III. Bourne G., editor. Academic; New York, USA: 1977. The gelada baboons: status and conservation. Primate conservation; pp. 363–383. [Google Scholar]
- 26.Girmay T., Tesfay H. New geographical range of geladas (Theropithecus gelada) in Tigray region, northern Ethiopia. Afr. J. Ecol. 2020;59:277–280. [Google Scholar]
- 27.Girmay T., Dati D. Population size, group and age structure of geladas (Theropithecus gelada) in escarpments of Eastern Tigray, Ethiopia: implication for conservation. Journal of Ecology and Environment. 2020;44(1):1–7. [Google Scholar]
- 28.Abie K., Bekele A. Population estimate, group size and age structure of the gelada baboon (Theropithecus gelada) around Debre-Libanos, northwest Shewa zone, Ethiopia. Glob. J. Sci. Front. Res. (GJSFR) 2017;17(1):27–33. [Google Scholar]
- 29.Bergman T.J., Ho L., Beehner J.C. Chest color and social status in male geladas (Theropithecus gelada) Int. J. Primatol. 2009;30(6):791–806. [Google Scholar]
- 30.Filipčík R., et al. The daily pattern of main activities in the gelada baboon (Theropithecus gelada) Acta Univ. Agric. Silvic. Mendelianae Brunensis. 2014;62(5):891–896. [Google Scholar]
- 31.Dunbar R. Structure of gelada baboon reproductive units: IV. Integration at group level. Z. Tierpsychol. 1983;63(4):265–282. [Google Scholar]
- 32.Dunbar R. Impact of global warming on the distribution and survival of the gelada baboon: a modelling approach. Global Change Biol. 1998;4(3):293–304. [Google Scholar]
- 33.Dunbar R.I. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;22(6):469–493. [Google Scholar]
- 34.Shotake T., et al. Genetic diversity within and among gelada (Theropithecus gelada) populations based on mitochondrial DNA analysis. Anthropol. Sci. 2016;124:157–167. [Google Scholar]
- 35.Fishpool L.D., Evans M.I. BirdLife International; Cambridge, UK: 2001. Important Bird Areas in Africa and Associated Islands: Priority Sites for Conservation. [Google Scholar]
- 36.Batistoni, M., A Comprehensive Guide to the Rock Hewn Churches of Tigray. 2015, Addis Ababa, Ethiopia: Arada Books..
- 37.Southwood T.R.E., Henderson P.A. John Wiley & Sons, Inc; New Jersey, USA: 2009. Ecological Methods. [Google Scholar]
- 38.Roberts S.C., Dunbar R.I. Climatic influences on the behavioural ecology of Chanter's mountain reedbuck in Kenya. Afr. J. Ecol. 1991;29(4):316–329. [Google Scholar]
- 39.Fashing P.J., et al. Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 2014;155:1–16. doi: 10.1002/ajpa.22559. [DOI] [PubMed] [Google Scholar]
- 40.Dunbar R.I., Dunbar E.P. Ecological relations and niche separation between sympatric terrestrial primates in Ethiopia. Folia primatologica; international journal of primatology. 1974;21(1):36–60. doi: 10.1159/000155595. [DOI] [PubMed] [Google Scholar]
- 41.Crook J.H. Gelada baboon herd structure and movement: a comparative report. Symp. Zool. Soc. Lond. 1972;18:237–258. [Google Scholar]
- 42.Iwamoto T., Dunbar R.I.M. Thermoregulation, habitat quality and behavioural ecology of gelada baboons. J. Anim. Ecol. 1983;52:357–366. [Google Scholar]
- 43.Mesele Y., Afework B., Zelealem T. Human–gelada baboon conflict in and around the simien mounains national park, Ethiopia. Journal of African Ecology. 2009;47:276–282. [Google Scholar]
- 44.Haileselasie T.H. Report to the Office of Vice President for Research and Community Services. Mekelle University; Mekelle, Tigray: 2022. Assessment of biotic natural resources of Tigray and Afar eco-region: traditional zootherapeutics studies in Tigray national regional state, northern Ethiopia; p. 140. (VPRCS/RB/25/2012) Mekelle University. [Google Scholar]
- 45.Frost S.R., Jablonski N.G., Haile-Selassie Y. Early pliocene Cercopithecidae from Woranso-mille (central Afar, Ethiopia) and the origins of the Theropithecus oswaldi lineage. J. Hum. Evol. 2014;76:39–53. doi: 10.1016/j.jhevol.2014.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.