Abstract
Osteoarthritis (OA) is a common joint disease characterized by chronic pain, and the perception of pain is closely associated with brain function and neuropeptide regulation. Rehmannia is common plant herb with anti-inflammatory and analgesic properties that is used to treat OA. However, it is unclear whether Rehmannia alleviates OA-related pain via regulation of neuropeptides and brain function. We examined the pain relief regulatory pathway in OA after treatment with Rehmannia by verifying the therapeutic effect of Rehmannia alcohol extract in vivo and vitro and exploring of the potential mechanism underlying the analgesic effect of Rahmanian using functional magnetic resonance imaging and measuring neuropeptide secretion. Our results showed that Rehmannia alcohol extract and the related active ingredient, Rehmannioside D, can delay cartilage degradation and alleviate inflammation in OA rats. The Rehmannia alcohol extract can also relieve OA pain, reduce the secretion of calcitonin gene-related peptide (CGRP) and substance P (SP), and reverse the pathological changes in the cerebral cortex and hippocampus. Our research results demonstrate that Rehmannia alleviates OA pain by protecting cartilage, preventing the stimulation of inflammatory factors on neuropeptide secretion, and influencing the relevant functional areas of the brain.
Keywords: Osteoarthritis, Rehmannia, Rehmannioside D, Neuropeptide, Pain
1. Introduction
Osteoarthritis (OA) is a chronic joint disease characterized primarily by chronic pain and involving all joint tissues, including degeneration of cartilage and meniscus, inflammation and fibrosis of synovium and infrapatellar fat pad, as well as remodeling of subchondral bone [1,2]. Due to the complexity of pain causing factors and the unclear mechanism of pain transmission, effective analgesia has become a key issue that troubles clinical treatment [3,4]. At present, chemical analgesics is one of the main treatments for osteoarthritis pain, but chemically synthesized painkillers will bring side effects currently such as gastrointestinal discomfort and risk of heart attack [5,6]. Therefore, it is of practical significance to develop new natural, safety and non-toxic analgesics.
The emergence of OA pain symptoms is the result of a combination of factors, often caused by mechanosensitive ion channels, inflammatory responses, and biomechanical imbalances [[7], [8], [9], [10]]. Recent studies have indicated a close association between neuropeptides and the perception and transmission of pain in OA [11,12]. In addition to the synovial membrane and infrapatellar fat pad, the subchondral bone contains a rich network of nerves and blood vessels, making it a major sensory organ for pain. Different neuropeptides are involved to varying degrees in pain and analgesic mechanisms, with SP and CGRP being the primary neuropeptides involved in pain perception [13]. Additionally, neuropeptides also interact with cytokines to modulate the transduction of noxious stimuli and regulate eventual pain perception as part of a complex chain of events [14,15]. Conversely, OA-related pain can be significantly alleviated by inhibiting the secretion of CGRP [16]. Therefore, the regulation of neuropeptide secretion may be a novel target for the development of new painkillers.
On the other hand, neuropeptide secretion can regulate the functional activities of the brain because pain perception is ultimately received by the brain [17,18]. Luo et al. findings demonstrated that CGRP might play a crucial role in nociceptive modulation in the ARC (arcuate nucleus of hypothalamus) during inflammatory pain, which was mediated by CGRP receptor in the ARC [19]. Additionally, Boll et al. found that the increase of blood-oxygen level dependent response in the caudate nucleus of the striatum could reduce the pain intensity level in patients with chronic low back pain [20]. Our previous studies found that the increased expression of pain-related neuropeptides CGRP and SP would lead to significant differences in signals in the hippocampus, bilateral orbitofrontal cortex, and frontal cortex [21].
Rehmannia glutinosa belongs to the perennial herb of Rehmannia in Scrophulariaceae which is mainly distributed in Henan and Shanxi provinces of China, and widely used in the treatment of osteoarthritis with excellent analgesic properties and lower side-effects [[22], [23], [24], [25], [26], [27]]. However, the specific analgesic pathway of Rehmannia glutinosa is still unclear. In the present study, it was hypothesized that Rehmannia may reduce pain symptoms by reducing neuropeptide secretion to regulate brain function changes. Firstly, we verified the therapeutic effect of Rehmannia on OA. Furthermore, the regulatory relationship between pain threshold, levels of pain-related neuropeptides CGRP and SP, and cortical - hippocampal regional homogeneity (ReHo) was further examined. This study can provide an experimental basis for Rehmannia to treat OA pain.
2. Materials and methods
2.1. Animals
Thirty 8-week-old male Sprague–Dawley (SD) rats aged weighing 280–290 g and ten 4-week-old male Sprague–Dawley rats aged weighing 90–95 g (Shanghai Slack Laboratory Animal Co., China) were reared in cages at the Experimental Animal Center of Fujian University of Traditional Chinese Medicine (Approval number: SYXK 2019–0007).
Thirty male SD rats were used for vivo experiment, randomly divided into sham (n = 10), OA model (n = 10), and Rehmannia group (n = 10). Meanwhile, chondrocytes were isolated from ten male normal SD rats. All animals were maintained at room temperature (21°C-22 °C) and had free access to food and sterile tap water under a 12 h/12 h light/dark cycle and treated as per the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Experiments were approved and supervised by the Animal Care and Use Committee of the Fujian University of TCM (approval number: 2,020,015).
2.2. High-performance liquid chromatography (HPLC)
Rehmannia glutinosa (Gaert.) was purchased from the Chinese pharmacy of the Third People's Hospital Affiliated to Fujian University of Traditional Chinese Medicine (220,314, HeNan, China). The quality was assessed using an HPLC fingerprint method using an Agilent 1200 HPLC system (Agilent, Santa Clara, CA, USA) and an Agilent 5 TC-C18 column (4.60 × 250.00 mm, 5 μm, Welch Materials, Inc., USA): 1 mL Rehmannia extract was added with methanol at a constant volume to 10 mL, and ultrasonic extraction (power 250 W, 50 kHz) was performed for 40 min. Centrifugation was performed at high speed (14000 rpm for 20 min). It was microfiltered and then refrigerated at 4 °C for future use. Standard samples were used to identify the components in Rehmannia. HPLC was performed with a 2504.6 mm Agilent 5 TC-C18 column. Mobile phase: “A" phase is acetonitrile (aladdin, Shanghai, China). Phase B is 0.1% phosphoric acid (Amresco,USA) aqueous solution, isometric elution method. The isometric elution process was 0–20 min, A: B = 17:83, the first 10 min wavelength was 230 nm, the last 10 min wavelength was 324 nm. Injection volume 10 μL, flow rate 1.0 mL/min, column temperature 35 °C.
2.3. Rehmannia treatment of OA rat and cell models
As in previous research methods, the OA model was established in all the groups, except the sham group using modified Hulth surgery [28]. Detailed steps: After successful anesthesia with isoflurane (1.5–2%) (Rayward Life Technology Co., Ltd, China). The sham group performed a longitudinal incision of the medial epidermis on both sides of the rats knee joints and then sutured. The model group used a scalpel to cut the medial epidermis of the knee joint longitudinally, and bluntly separated the medial collateral ligament. And cut, then cut off the anterior cruciate ligament and excision of the medial meniscus, suture the inner and outer cortex after finishing, wipe the wound with iodophor, and put the rat back into the cage after waking up. After the operation, each rat was given an intramuscular injection of 200,000 U of penicillin (Macklin, Shanghai, China), once a day for 3 consecutive days to prevent infection. Two weeks after the surgery, 10 OA rats were randomly selected for treatment with 0.87 mg/mL (2 mL/kg, via intragastric administration. qd) alcohol extract of Rehmannia. Animals in the sham and OA groups also received intragastric administration of the same dose of 0.9% saline. After 12 weeks of intervention, the rats underwent pain behavior detection and MRI scanning. Blood was collected from the abdominal aorta for ELISA serum inflammatory factor detection. Then knee joint tissues were isolated and stored in liquid nitrogen for protein detection, and in 4% paraformaldehyde for morphological detection.
Ten rats were deeply anesthetized with isoflurane, rats were placed in a container containing 75% medical-grade alcohol and soaked for 5 min. Using ophthalmic scissors, the fur from the hind limbs of the mice was removed. The intact hind limbs were then dissected and transferred to a container with 75% medical-grade alcohol and moved to a laminar flow hood. Tissues were rinsed with PBS to remove residual alcohol. Using a disposable surgical blade, muscles, fascia, and fibula were sequentially dissected and removed from the tissues, obtaining intact tibia and femur. The cartilage was shaved using a disposable blade and added to a cell culture incubator with type II collagenase for cell lysis, performed in three separate 30 min steps. After removing the supernatant, the cells were resuspended in a culture medium and seeded at a density of 1 × 105 cells/mL in culture flasks. Differential adhesion method was used for purification, and the culture medium was replaced after 24 h. When the cells reached 90% confluence, passaging was performed. The cellular growth state was observed under a microscope, and cell passages with better growth were selected for subsequent experimental studies. Chondrocytes were firstly identified by type II collagen (Col II) immunocytochemistry (ab34712, AbCam, USA, 1:200), then exposed to 10 ng/mL lipopolysaccharide (LPS) (Sigma Aldrich, USA) for 8 h to establish the inflammation model (7,29). Identify the model and screen the best intervention conditions of drugs by ELISA kits (e.g., IL-6 and MMP-9) (ab234570, ab273243, AbCam, USA). Chondrocytes were divided into control (10%DMEM), model (10%DMEM + LPS 10 ng/mL), TAK-242 inhibitor (10%DMEM + TAK-242 10 μmol/mL + LPS 10 ng/mL), and RD (10%DMEM + TAK-242 10 μmol/mL + LPS 10 ng/mL + RD 5 μmoL/mL) groups and treated for 8 h.
2.4. Hematoxylin and eosin (HE) and safranin O-fast green staining
The joint tissues of the rats in each group were decalcified in EDTA-2Na (Xinyuhong, China) for 8 weeks, and then dehydrated and embedded in paraffin. The paraffin-embedded tissues were cut into 4 μm sections, immersed in xylene for 10 min for dewaxing, and then treated with graded ethanol (100%, 95%, 85%, 75%) for 5 min each. The HE and Safranin O-fast green kit (Beijing Solarbio Science & Technology Co., Ltd.) was used to dye according to the manufacturer's instructions. Finally, a quick rinse with distilled water, followed by dehydrated and sealed. The pathological changes of mouse cartilage were observed under a light microscope (DM4000 B; Leica Microsystems GmbH). Rat cartilage tissue slices were evaluated using an improved Wakitani scoring system, which has a 14-point scale and consists of the following five items: cell morphology, matrix or metachromatic staining, surface regularity, thickness of the cartilage, and integration of repaired tissue to the surrounding articular cartilage. The detailed scoring rules are shown in Table 1 (n = 3 rats in each group).
Table 1.
Modified Wakitani histological scoring system.
| Category | Item | Points |
|---|---|---|
| Cell morphology | ||
| Hyaline cartilage | 0 | |
| Mostly hyaline cartilage | 1 | |
| Mostly fibrocartilage | 2 | |
| Mostly noncartilage | 3 | |
| Noncartilage only | 4 | |
| Matrix staining (metachromasia)a | ||
| Normal (compared with host adjacent cartilage) | 0 | |
| Slightly reduced | 1 | |
| Markedly reduced | 2 | |
| No metachromatic stain | 3 | |
| Surface regularityb | ||
| Smooth (>3/4) | 0 | |
| Moderate (1/2 to 3/4) | 1 | |
| Irregular (1/4 to 1/2) | 2 | |
| Severely irregular (<1/4) | 3 | |
| Thickness of cartilagec | ||
| >2/3 | 0 | |
| 1/3 to 2/3 | 1 | |
| <1/3 | 2 | |
| Integration of donor with host adjacent cartilage | ||
| Both edges integrated | 0 | |
| One edge integrated | 1 | |
| Neither edge integrated | 2 | |
| Total maximum | 14 | |
Metachromasia matrixes including proteoglycan staining intensity by Safranin O and hematoxylin and eosin staining compared with host adjacent cartilage.
Total smooth area of the reparative cartilage compared with the entire area of the cartilage defect.
Average thickness of the reparative cartilage compared with that of the surrounding cartilage.
2.5. Detection of 23 inflammatory factors in rat serum by suspension chip
The contents of 23 inflammatory factors in serum of rats were detected by suspension chip. Perform the following experiments according to the instructions of the rat factor 23 multiplex assay kit (Bio-Plex Pro Rat Cytokine 23-Plex Assay, Bio-Rad, USA). The BioPlex® system needs to be warmed up for 30 min, and the rat serum to be tested was diluted 4 times and placed on ice. Next, a four-fold standard dilution series and blank were prepared and 20 × of the coupled beads were diluted to l × for use. Immediately after, add 50 μL of 1 × coupled beads into the 96 well plate, wash the plate twice, and then add 50 μL of standard solution, blank and diluted samples to each well, incubate for 1 h at room temperature with a shaker, and wash the plate for 3 times. Add 25 μL of 1 × detection antibodies to each well, incubate for 30 min in a shaker at room temperature, and wash the plate 3 times. Add 50 μL of 1 × SA-PE, incubate for 10 min in a shaker at room temperature, and wash the plate 3 times. Finally, add 125 μL assay buffer to each well to resuspend the coupled beads and shake them for 30 s at room temperature. Finally, the Bio-Plex 100, 200, and 3D systems were tested for sample factor content using low PMT, RP1 settings. Use the default instrument settings for the Bio-Plex®MAGPIX™ System.
2.6. Immunohistochemistry and immunofluorescence
The sections were dewaxed to water and then autoclaved to achieve antigen repair. UltraSensitive™ SP kits were used to measure protein levels in samples (KIT-9720, MXB biotechnologies, China). The endogenous enzyme was inactivated with 3% H2O2, then the sections were closed with 5% BSA (Beijing Solarbio Science & Technology Co., Ltd.). Col II primary antibody (ab34712, AbCam, USA, 1:200) was incubated overnight and biotin-labeled IgG polymer working solution (Beijing Solarbio Science & Technology Co., Ltd.) was added for 10 min incubation at room temperature. Then, the sections were stained with DAB (Jiangsu Kaiji Biology Co., Ltd, China) and hematoxylin and observed under an optical microscope. For immunofluorescence, the sections were deparaffinized to water and fixed in 4% paraformaldehyde for 30 min at room temperature. Then, the sections were sealed with goat serum and incubated with primary antibodies CGRP (14959 S, Cell Signaling Technology, USA, 1:400), SP (S8305, Sigma, USA, 1:5000), and NF-κB P65 (ab16502, AbCam, USA, 1:100), followed by conjugated with anti-rabbit IgG (4412 S, Cell Signaling Technology, USA, 1:500). Images were captured under a laser scanning confocal microscope (Olympus Corporation, Japan).
2.7. Q-PCR detection
Total RNA was extracted from chondrocytes using TRIzol (Invitrogen, USA). First, 1 μg of RNA was transcribed to cDNA using an RT kit (Thermo Fisher Scientific, Inc, USA), which was used for RT-PCR to determine mRNA expression with the SYBR Fluorescence Quantization Kit (Invitrogen) using a 7500 RT-qPCR System (Applied Biosystems, USA). The following thermocycling conditions were used for the PCR: Initial denaturation at 95 °C for 10 min; 40 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s; followed by melting curve analysis. The gene primers used are shown in (Table 2). Fold changes were calculated using the 2-ΔΔCt formula and expressed relative to expression in the sham group. GAPDH was used as the housekeeping gene.
Table 2.
Gene primer sequences.
| Gene | Sequences |
|---|---|
| TRL4 | forward 5′-CCGCTCTGGCAT CATCTTCA-3′ reverse 5′-CTCCCACTCGAGGTAGGTGT-3′; |
| Myd88 | forward 5′-CGGAGGAGATGGGTTTCGAG-3′ reverse 5′-CCAGGCATCCAAACT GC-3′; |
| NF-κB p65 | forward5′-AGAGAAGCACAGATACCACTAAGA-3′ reverse 5′-GTTCAGCCTCATAGAAGCCATC-3′; |
| IKK-β | forward 5′-CATCAAGCAATGCCGACAGG-3′ reverse 5′-CATTGGGTGCCAAGTTCTGC-3′; |
| GAPDH | forward 5′-ACGGCAAGTTCAACGGCACAG-3′ reverse 5′-GAAGACGCCAGTAGACTCCACGAC-3′. |
2.8. Western blotting
Total protein was extracted from chondrocytes/cartilage tissue using radio-immunoprecipitation assay lysis buffer containing 1 mM phenylmethanesulfonyl fluoride. Protein concentrations were measured using a bicinchoninic acid assay. Equal amounts of protein (20 μg/lane) were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with primary antibodies against TLR4, Myd88, IKK-β, β-actin, aggrecan, MMP-3, MMP-9 (ab13556, ab2064, ab124957, ab8227, ab36861, ab52915, ab76003, AbCam, USA, 1:1000, respectively), MMP-13 (ab39012, AbCam, USA, 1:5000), ADAMTS-4 (ab150370, AbCam, USA, 1:1000), ADAMTS-5 (A02802-1, Boster, China, 1:500), CGRP (14959 S, Cell Signaling Technology, USA, 1:1000), SP (S8305, Sigma, USA, 1:2000), NF-κB p65 (ab16502, AbCam, USA, 1:5000), and GAPDH (ab181602, AbCam, USA, 1:10,000) overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibody IgG (ab6721, AbCam, USA) at room temperature. Immune reactivity was visualized using an enhanced chemiluminescent reagent (Pierce; Thermo Fisher Scientific, Inc.). Band intensity was quantitatively analyzed by densitometric analysis on Image J software version 1.37 (National Institutes of Health, Bethesda, MD, USA).
2.9. MWT and TWL measurements
The measuring the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were assessed as follows. Briefly, a digital force gauge (YLS-3E, Yima Optoelec Co., Ltd., China) was used to measure the MWT with a continuously elevating force added to the hind paw until a withdrawal reflex response appeared. The measurement was repeated three times and the average value was considered as the MWT after removing the extreme values. For TWL measurement, animals underwent TWL measurements using a heat-pain stimulator (BME-410C, Institute of Biomedical Engineering, USA) with a 12-V/10-W halogen lamp, stimulation temperature of 45 °C-65 °C, time accuracy of 10 ms, and cutoff time of 20 s. Animals were placed in a glass box and stimulated. The time when the animal presented a withdrawal response was measured and the measurement repeated three times. The average value was considered as the TWL after removing the extreme values.
2.10. Resting-state fMRI scan and data preprocessing
Kendall's concordance coefficient of an individual voxel and its adjacent 26 voxels was calculated using previously reported parameter conditions and a brain map was constructed [21]. T2WI structural image was acquired by Bruker 9.4 T small animal MRI (Pharmascan, micro MRI, brucker medizenk, Germany) and 35 mm diameter surface orthogonal coil. T2 weighted imaging using rapid acquisition with relaxation enhancement, the parameters are as follows: repetition time = 2500 ms, echo time = 33 ms, field of view = 32 × 32mm, averages = 4, slices = 21, slice thickness = 1 mm, matrix = 256 × 256, bandwidth = 326,087 Hz, echo spacing = 10,000 ms, refocusing angle = 180°, excitation angle = 90°, echo train length = 8, k-zero = 3. fMRI scanning data were analyzed using SPM 12 software. A mask was defined as the brain areas that exhibited significant differences compared with ANOVA. One-way analysis of variance followed by Bonferroni post hoc analyses was performed among multiple groups for parametric data. P-values <0.001 were considered significant at the voxel level and in clusters exceeding 20 voxels.
2.11. ELISA analysis
ELISA kits were used to measure CGRP (1161, R&D Systems, USA) and SP (Trust Specialty Zeal Biological Trade Co., Ltd, USA) serum levels in samples of 100 μL. Microplate spectrophotometry (Omega Bio-Tek, Inc., Norcross, GA, USA) was performed at 405 nm.
2.12. Statistical analysis
Statistical analysis between two groups was analyzed by unpaired t-test. Multiple comparisons were performed using a one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. Data from at least three independent experiments were presented as the mean ± standard deviation. P-values <0.05 were considered statistically significant, ns., no significan. All statistical analyses were performed using SPSS (IBM) and GraphPad Prism. No outliers were excluded in this study.
3. Results
3.1. Rehmannia alcohol extract delays cartilage degeneration in OA
After using the modified Hulth method to suggest the OA rat model, analysis of the morphology of the cartilage demonstrated that the cartilage surface was rough, the hyaline cartilage layer was attenuated, chondrocytes were arranged disorderly, and there was reduced protein expression of Col-II in the OA model group compared with the sham and Rehmannia treatment groups, which received Rehmannia treatment for 12 weeks (Fig. 1A) (P<0.01). And there was no abnormal change in the body weight of Rehmannia group (Supplementary Fig. 1). We then examined the aggrecan content in the cartilage of the sham, OA model, and Rehmannia groups by Western blotting. The results showed that the protein expression of aggrecan was significantly increased in the Rehmannia group compared with the OA model group (Fig. 1B) (P<0.05). We also measured the expression of matrix metalloproteinases (MMPs), including MMP-3, MMP-9, MMP-13, ADAMTS-4, and ADAMTS-5, which destroy collagen and proteoglycan in cartilage tissue and exacerbate cartilage damage (30). The expression of these proteins was higher in the OA model group than the sham group. However, treatment with Rehmannia significantly reversed the increased expression of these proteins in the OA rat model (Fig. 1C–G) (P<0.05). These results indicate that Rehmannia attenuates cartilage degeneration.
Fig. 1.
Rehmannia delays OA degeneration. Representative images of H&E, safranin O-fast green staining, and Col-II immunohistochemistry (A). Original magnification × 100. Quantitative analysis of the relative protein expression of aggrecan (B), MMP-3 (C), MMP-9 (D), MMP-13 (E), ADAMTS-4 (F), and ADAMTS-5 (G) in cartilage. GAPDH was used as a loading control. Values indicate mean ± standard deviation (SD), where SD is shown as vertical bars. Data represent the mean of three independent experiments. *P < 0.05 and **P < 0.01 compared with the OA group.
Alcoholic extract and active components of Rehmannia inhibit the secretion of inflammatory factors and related mechanisms.
Analysis of the protein levels of 23 inflammatory factors showed that the expression of IL-1α, IL-1β, IL-5, IL-12, IL-17A, IL-18, M-CSF, RANTES, and TNF-α proinflammatory cytokines increased, whereas the expression of the anti-inflammatory cytokines, IL-13, was decreased in the OA model group compared with the sham group (P<0.05). Rehmannia treatment effectively reduced the expression of these proinflammatory factors and increased the expression of anti-inflammatory factors (Fig. 2A-J) (P<0.05). Meanwhile, the expression of inflammatory cytokines including IL-4 and IL-7 had no statistical significance compared with OA group (Supplementary Fig. 2). The TLR4/NF-κB pathway plays an important role in the regulation of inflammation [29]. Hence, we further examined the expression of the major proteins of TLR4/NF-κB pathway, including TLR4 and NF-κB p65. Protein expression of TLR4 and NF-κB p65 was significantly increased in the OA model group compared with the sham group (P<0.01). However, the upregulation of these proteins was significantly reversed by Rehmannia treatment for 12 weeks (Fig. 2K and L) (P<0.01).
Fig. 2.
Effect of Rehmannia on inflammatory factors. Quantitative analysis of the relative protein expression of IL-1α (A), IL-1β (B), IL-5 (C), IL-18 (D), M-CSF (E), IL-12 (F), IL-13 (G), IL-17A (H), RANTES (I), and TNF-α (J) in serum. Quantitative analysis of the relative cartilage tissue protein expression of TLR4 (K) and P–NF-κB p65/NF-κB p65 (L). GAPDH was used as a loading control. Values represent mean ± SD, where SD is shown as vertical bars. Data represent the mean of the three independent experiments. *P < 0.05 and **P < 0.01 compared with the OA group.
The major constituents of Rehmannia glutinosa including Catalpol, Rehmaionoside A, Rehmaionoside D, Acteoside, Oleanonic acid, P-hydroxybenzoic acid, Diosmetin, Luteolin, Apigenin, Total reducing sugars, and Glutamic acid et al. [30,31]. Next, we verified the concentration of Rehmannioside D (RD), which is the main active component of Rehmannia, in the alcohol extract of Rehmannia by HPLC [32] (Supplementary Fig. 3).
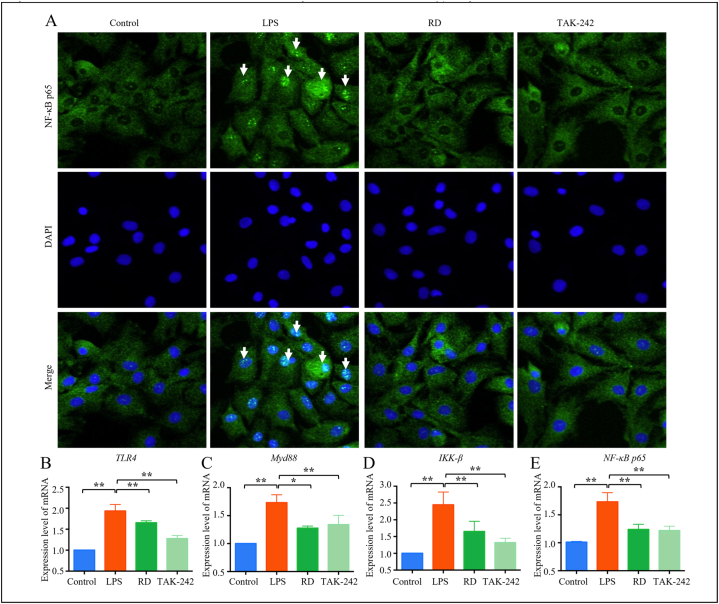
The chondrocytes were identified as brown by immunocytochemistry staining (Supplementary Fig. 4). When RD is present at a concentration of 5μmoL/mL, it promotes the proliferation of chondrocytes (Supplementary Fig. 5) (P<0.01). Moreover, LPS was used to simulate the activation of inflammatory signal pathway, and the best intervention condition of RD was screened by ELISA as 5μmoL/mL (Supplementary Fig. 6). 10 μmol/mL TAK-242 (a TLR4 pathway inhibitor) and 5 μmoL/mL RD were added to chondrocytes that were stimulated with 10 ng/mL LPS to examine the effect of RD on LPS-induced chondrocyte inflammation in vitro. LPS exposure significantly promoted translocation of NF-κB p65 from the cytoplasm to the nucleus (Fig. 3A). We then measured the protein expression levels of key biomarkers of the TLR4/NF-κB pathway in the control, LPS-induced cellular model (model), TAK-242, and RD-treatment groups. The expression of TLR4, MyD88, IKK-β, and NF-κB p65 mRNA significantly increased after LPS stimulation. Interestingly, TAK-242 and RD treatment could reverse the LPS-induced expression of these mRNA (Fig. 3B–E) (P<0.05).
Fig. 3.
RD inhibits the expression of inflammation-related genes and proteins. Representative images of NF-κB p65 nuclear translocation (A). Original magnification × 400. Quantitative analysis of the relative mRNA expression of TLR4 (B), MyD88 (C), IKK-β (D), and NF-κB p65 (E). GAPDH was used as a loading control. Values represent the mean ± standard deviation (SD), where SD is shown as vertical bars. Data represent the mean of the three independent experiments. *P < 0.05 and **P < 0.01 compared with the OA group.
3.2. Rehmannia alleviates OA-related pain via regulation of ReHo in the cortex–hippocampus
We investigated the effect of Rehmannia on pain threshold by MWT and TWL. The MWT and TWL decreased dramatically in the OA rat model group compared with the sham group; however, the thresholds in the Rehmannia treatment group were significantly higher than those in the model group (Fig. 4A and B) (P<0.01). We performed BOLD imaging of the whole areas using fMRI to elucidate the regulatory pathways involved in the response to Rehmannia treatment to examine OA-related pain relief. fMRI revealed that ReHo in the cortex was significantly reduced whereas ReHo in the hippocampus was dramatically increased in the Rehmannia treatment group compared with the model group (Fig. 4C, Table 3). We also examined the localization and quantification of CGRP and SP neuropeptides in the tibial plateau, which is related to OA-related pain sensitization. We found that expression of CGRP and SP was significantly enhanced in the subchondral bone in the model group. On the other hand, Rehmannia could downregulate neuropeptide expression (Fig. 4D and E). We also measured the pain-related neuromodulation proteins, CGRP and SP, in the serum and bone tissue. We found that secretion of CGRP and SP was decreased in the Rehmannia treatment group compared with the model group (Fig. 4F–I) (P<0.01). Thus, our results suggest that Rehmannia reduces OA-related pain via modulation of neuropeptide transmission and cortex and hippocampal signaling.
Fig. 4.
Rehmannia relieves OA pain by regulating neuropeptide-mediated changes in brain function. Effect of Rehmannia on MWT (A) and MWT (B). Changes in ReHo induced by Rehmannia treatment (C). Yellow regions represent brain areas with enhanced ReHo values and blue regions represent areas with reduced ReHo values. Changes in areas caused by OA and significantly reversed by Rehmannia are marked with white arrows. Quantitative analysis of the relative protein expression of CGRP (D, F, H) and SP (E, G, I) in serum and tibial plateau. Original magnification × 100. GAPDH was used as a loading control. Values represent the mean ± SD, where SD is shown as vertical bars. Data represent the mean of the three independent experiments. *P < 0.05 and **P < 0.01 compared with the OA group.
Table 3.
Brain areas with regional homogeneity changes induced by Rehmannia in OA rats.
| ROI | KE | MAX_T | X | Y | Z | |
|---|---|---|---|---|---|---|
| Rehmannia VS. OA | hippocampus_left | 21 | 4.206 | 3.8114 | 3.4374 | −5.8779 |
| hippocampus_right | 112 | 5.1803 | −5.0222 | 4.1566 | −4.9179 | |
| motor cortex_left | 2 | 3.3454 | 0.67826 | 0.24788 | −1.5579 | |
| motor cortex_right | 9 | 3.7196 | −1.851 | 0.018975 | −2.2779 | |
| striatum_right | 40 | 4.4923 | −2.5754 | 4.4371 | 1.8021 | |
| visual cortex_left | 55 | 4.3903 | 3.8312 | 0.79072 | −7.3179 | |
| visual cortex_right | 24 | 4.0691 | −1.8147 | 0.83738 | −4.9179 |
4. Discussion
This study evaluated the in vivo and in vitro therapeutic effects of Rehmannia and the relative active constituents using animal and cell models. In the in vivo experiments, ethanol extract of Rehmannia attenuated the inflammatory response and inhibited the degradation of cartilage matrix in the OA model. This effect was associated with the inhibition of key regulatory factors in the TLR4/NF-κB inflammatory pathway. Further validation was conducted through in vitro cell experiments. Subsequently, we investigated the relationship between Rehmannia, pain, brain functional changes, and neuropeptides by measuring pain thresholds, functional magnetic resonance imaging, and neuropeptide immunofluorescence. In the OA rat model, treatment with Rehmannia resulted in decreased pain thresholds, pathological changes in the cerebral cortex and hippocampus, and upregulation of relevant neuropeptides. These findings suggest that the pain-related mechanisms play a crucial role in the analgesic effects of Rehmannia. Therefore, our study indicates that Rehmannia may alleviate OA pain through cartilage protection and modulation of neuropeptide-mediated brain function, exerting a key role in OA-related pain sensitization. This may contribute to the development of Rehmannia as a novel therapeutic agent for OA.
Cartilage degeneration and upregulation of proinflammatory factors are characteristic symptoms of OA. Members of the MMPs and ADAMTS family are involved in the degeneration of OA [33]. MMPs are a family of proteases that are mainly involved in tissue remodeling and degeneration of the extracellular membrane (ECM), especially MMP-3, MMP-9, and MMP-13, and the denaturation of Col-II and aggrecan in the ECM and destruction of cartilage in OA [34,35].
ADAMTS-4 and ADAMTS-5 are the main proteoglycanases involved in cartilage matrix conversion [36,37]. In this study, we established an OA model using the modified Hulth method and found that the OA animal model showed articular cartilage degeneration, which could be reversed Rehmannia treatment. Rehmannia treatment also led to decreased expression of MMP-3, MMP-9, MMP-13, ADAMTS-4, and ADAMTS-5, and increased secretion of Col-II and aggrecan, which confirmed that Rehmannia effectively inhibited cartilage degeneration. We also found that Rehmannia could regulate the secretion of inflammatory factors, including IL-1α, IL-1β, IL-4, IL-5, IL-7, IL-12, IL-13, IL-17A, IL-18, M-CSF, RANTES, G-CSF, and TNF-α. These results confirmed the anti-inflammatory effects of Rehmannia.
TLR4 plays a crucial role in inflammation regulation. Inactive cytoskeletal Myd88 is released into the cytoplasm in response to TLR ligands. The Myd88-dependent pathway then activates NF-κB via degradation of phosphorylated IκB protein and NF-κB is released and translocated to the nucleus, resulting in the activation of proinflammatory mediators [38,39]. We explored the molecular mechanisms of the anti-inflammatory effects of Rehmannia in vivo and vitro experiments and showed that the RD down-regulated the expression of TLR4 gene, followed by decreased the expression of downstream genes MyD88 and IKK-β, and finally inhibited the activation of the TLR4/NF-κB pathway by suppressed the nuclear import of NF-κB p65. These results suggest that Rehmannia and its related active components can inhibit inflammation and alleviate cartilage degeneration in OA.
Pain is a common clinical symptom of OA [1,2]. We found that the thresholds of MWT and TWL were decreased significantly in OA model, whereas Rehmannia treatment significantly increased the pain tolerance threshold. Pain perception is a subjective and multidimensional experience that encompasses sensory-discriminative, affective-motivational, and cognitive-evaluative components. BOLD imaging changes in multiple brain regions may be involved after the onset of pain [40]. Studies have shown that stimulation of the motor cortex suppresses neuropathic pain compared with stimulation of the primary sensory cortex, which increases pain [41]. Acute and chronic pain are associated with changes to the hippocampus, which plays a significant role in emotional processing [42,43]. Therefore, we examined changes to the whole brain region using fMRI and found that Rehmannia regulated ReHo in the cerebral cortex and hippocampus in the OA model group. We also examined the expression of pain-related neuropeptides, including CGRP and SP, and found that these neuropeptides were highly expressed in the subchondral bone area of OA model, which could be significantly reversed by Rehmannia treatment. We then measured CGRP and SP protein levels in serum and bone tissue and found consistent results. Therefore, neuropeptides may be important participants in the transmission of information between bone and brain, which may explain the potential attenuation of OA-related pain after treatment with Rehmannia.
In conclusion, this study used in vivo and in vitro experiments to confirm the effect of Rehmannia on OA: Rehmannia can alleviate the pain phenotype by inhibiting the secretion of pro-inflammatory factors (and its active ingredient can inhibit the TLR4/NFKB inflammatory pathway), promoting the secretion of anti-inflammatory factors, inhibiting the degradation of cartilage matrix, and promoting the production of proteoglycans. The studies also demonstrated that neuropeptide secretion in subchondral bone was significantly altered with ReHo in cerebral cortex and hippocampus.
However, there are limitations in this study. In the future studies, we will continue to explore the mechanism of the active ingredients of Rehmannia on OA-related pain in vivo rather than in vitro. In vitro experiments, it's unable to further explore the effect of active components of Rehmannia on pain due to the inability to detect pain indicators in vitro cell experiments. Therefore, we will continue to explore relevant studies in vivo experiments. Furthermore, osteoarthritis pain is the result of the complex interplay of multiple factors. In future studies, we will also focus on investigating the involvement of other tissues in pain perception and transmission.
5. Conclusions
Our study suggests that Rehmannia alleviates OA pain by protecting cartilage, preventing the stimulation of inflammatory factors on neuropeptide secretion, and influencing the relevant functional areas of the brain. Therefore, Rehmannia is considered as a novel analgesic medication for treating OA.
Author contribution statement
Yanfeng Huang, Qing Lin, Xue Tan: Performed the experiments; Analyzed and interpreted the data; Contributed analysis tools and data; Wrote the paper. Liangliang Jia, Hui Li, Zaishi Zhu, Changlong Fu, Lili Wang, Linlong Liu, Min Mao, Zhouping Yi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Xihai Li: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed analysis tools and data; Wrote the paper. Dezun Ma: Conceived and designed the experiments; Performed the experiments, Analyzed and interpreted the data; Contributed analysis tools and data; Wrote the paper.
Data availability statement
Data associated with this study has been deposited at the jianguoyun [https://www.jianguoyun.com/p/DbrTFsIQxr6jCxjn3u4EIAA].
Ethics approval and consent to participate
Animal use procedures were approved by the animal welfare ethics committee of Fujian University of TCM (approval no. 2020015). In addition, all animal protocols conform to the Guide for the Care and Use of Laboratory Animals (8th edition) published by the US National Institute of Health (37).
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82074461); the Science and Technology Programs Pilot Project of Fujian Province (Grant No. 2021Y0032); Scientific Research Foundation for the Highlevel Talents Fujian University of Traditional Chinese Medicine (X2021007-talents).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19322.
Contributor Information
Dezun Ma, Email: 2021025@fjtcm.edu.cn.
Xihai Li, Email: lixihaifz@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chatterjee I., Baumgartner L., Cho M. Detection of brain regions responsible for chronic pain in osteoarthritis: an fMRI-based neuroimaging study using deep learning. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1195923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi Y., Uchida K., Fukushima K., Inoue G., Takaso M. Mechanisms of peripheral and central sensitization in osteoarthritis pain. Cureus. 2023;15(2) doi: 10.7759/cureus.35331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoyama M., Iijima H., Kubota K., Kanemura N. Exploring the modification factors of exercise therapy on biomechanical load in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin. Rheumatol. 2023;42(7):1737–1752. doi: 10.1007/s10067-023-06553-4. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S.P., Vase L., Hooten W.M. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 5.Turk D., Boeri M., Abraham L., Atkinson J., Bushmakin A.G., Cappelleri J.C., Hauber B., Klein K., Russo L., Viktrup L., et al. Patient preferences for osteoarthritis pain and chronic low back pain treatments in the United States: a discrete-choice experiment. Osteoarthritis Cartilage. 2020;28(9):1202–1213. doi: 10.1016/j.joca.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Abdel Shaheed C., Awal W., Zhang G., Gilbert S.E., Gallacher D., McLachlan A., Day R.O., Ferreira G.E., Jones C.M., Ahedi H., et al. Efficacy, safety, and dose-dependence of the analgesic effects of opioid therapy for people with osteoarthritis: systematic review and meta-analysis. Med. J. Aust. 2022;216(6):305–311. doi: 10.5694/mja2.51392. [DOI] [PubMed] [Google Scholar]
- 7.Gao W., Hasan H., Anderson D.E., Lee W. The role of mechanically-activated ion channels Piezo1, Piezo 2, and TRPV4 in chondrocyte mechanotransduction and mechano-therapeutics for osteoarthritis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.885224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmi A., Stocco E., Boscolo-Berto R., Contran M., Belluzzi E., Favero M., Ramonda R., Porzionato A., Ruggieri P., De Caro R., et al. Infrapatellar fat pad-synovial membrane anatomo-fuctional unit: microscopic basis for piezo1/2 mechanosensors involvement in osteoarthritis pain. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.886604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H., Huang T., Lu W.W., Tong L., Chen D. Osteoarthritis pain. Int. J. Mol. Sci. 2022;23(9):4642. doi: 10.3390/ijms23094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coaccioli S., Sarzi-Puttini P., Zis P., Rinonapoli G., Varrassi G. Osteoarthritis: new insight on its pathophysiology. J. Clin. Med. 2022;11(20):6013. doi: 10.3390/jcm11206013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki R., Honda Y., Oga S., Fukushima T., Tanaka N., Kajiwara Y., Nakagawa K., Takahashi A., Sakamoto Y., Morita H., et al. Effect of exercise and/or educational interventions on physical activity and pain in patients with hip/knee osteoarthritis: a systematic review with meta-analysis. PLoS One. 2022;17(11) doi: 10.1371/journal.pone.0275591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber A.E., Jalali O., Limfat S., Shkhyan R., Van Der Horst R., Lee S., Lin Y., Li L., Mayer E.N., Wang L., et al. Modulation of hedgehog signaling by kappa opioids to attenuate osteoarthritis. Arthritis Rheumatol. 2020;72(8):1278–1288. doi: 10.1002/art.41250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu P., Liu Y., Xie J., Li J. Spatiotemporally controlled calcitonin delivery: long-term and targeted therapy of skeletal diseases. J. Contr. Release. 2021;338:486–504. doi: 10.1016/j.jconrel.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 14.Duarte F.C.K., Hurtig M., Clark A., Brown S., Simpson J., Srbely J. Experimentally induced spine osteoarthritis in rats leads to neurogenic inflammation within neurosegmentally linked myotomes. Exp. Gerontol. 2021;149 doi: 10.1016/j.exger.2021.111311. [DOI] [PubMed] [Google Scholar]
- 15.Gatenholm B., Brittberg M. Neuropeptides: important regulators of joint homeostasis. Knee Surg. Sports Traumatol. Arthrosc. 2019;27(3):942–949. doi: 10.1007/s00167-018-5074-4. [DOI] [PubMed] [Google Scholar]
- 16.Rees T.A., Hendrikse E.R., Hay D.L., Walker C.S., Beyond C.G.R.P. The calcitonin peptide family as targets for migraine and pain. Br. J. Pharmacol. 2022;179(3):381–399. doi: 10.1111/bph.15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schramm S., Börner C., Reichert M., Baum T., Zimmer C., Heinen F., Bonfert M.V., Sollmann N. Functional magnetic resonance imaging in migraine: a systematic review. Cephalalgia. 2023;43(2) doi: 10.1177/03331024221128278. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J., Zeng F., Cheng S., Dong X., Jiang N., Zhang X., Tang C., He W., Chen Y., Sun N., et al. Modulation effects of different treatments on periaqueductal gray resting state functional connectivity in knee osteoarthritis knee pain patients. CNS Neurosci. Ther. 2023;29(7):1965–1980. doi: 10.1111/cns.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo L., Qi W., Zhang Y., Wang J., Guo L., Wang M., Wang H.B., Yu L.C. Calcitonin gene-related peptide and its receptor plays important role in nociceptive regulation in the arcuate nucleus of hypothalamus of rats with inflammatory pain. Behav. Brain Res. 2023;443 doi: 10.1016/j.bbr.2023.114351. [DOI] [PubMed] [Google Scholar]
- 20.Boll S., Ueltzhoeffer K., Roth C., Bertsch K., Desch S., Nees F., Grinevich V., Herpertz S.C. Pain-modulating effects of oxytocin in patients with chronic low back pain. Neuropharmacology. 2020;171 doi: 10.1016/j.neuropharm.2020.108105. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Xu Y., Li H., Jia L., Wang J., Liang S., Cai A., Tan X., Wang L., Wang X., et al. Verification of pain-related neuromodulation mechanisms of icariin in knee osteoarthritis. Biomed Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112259. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Li H., He X., Huang Y., Wang S., Wang L., Fu C., Ye H., Li X., Asakawa T. Identification of the key role of NF-κB signaling pathway in the treatment of osteoarthritis with bushen zhuangjin decoction, a verification based on network pharmacology approach. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.637273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G., Zhang J., Chen W., Chen S., Huang Y., Lin R., Huang M., Li Z., Zheng L., Li X. Tougu Xiaotong capsule exerts a therapeutic effect on knee osteoarthritis by regulating subchondral bone remodeling. Mol. Med. Rep. 2019;19(3):1858–1866. doi: 10.3892/mmr.2018.9778. [DOI] [PubMed] [Google Scholar]
- 24.Lu M.K., Chang C.C., Chao C.H., Hsu Y.C. Structural changes, and anti-inflammatory, anti-cancer potential of polysaccharides from multiple processing of Rehmannia glutinosa. Int. J. Biol. Macromol. 2022;206:621–632. doi: 10.1016/j.ijbiomac.2022.02.112. [DOI] [PubMed] [Google Scholar]
- 25.Jhun J.Y., Na H.S., Shin J.W., Jung K.A., Seo H.B., Ryu J.Y., Choi J.W., Moon S.J., Park H.J., Oh S.W., et al. Notoginseng radix and rehmanniae radix preparata extract combination (YH23537) reduces pain and cartilage degeneration in rats with monosodium iodoacetate-induced osteoarthritis. J. Med. Food. 2018;21(8):745–754. doi: 10.1089/jmf.2017.4041. [DOI] [PubMed] [Google Scholar]
- 26.Ren H., Li K., Min Y., Qiu B., Huang X., Luo J., Qi L., Kang M., Xia P., Qiao H., et al. Rehmannia glutinosa polysaccharides: optimization of the decolorization process and antioxidant and anti-inflammatory effects in LPS-stimulated porcine intestinal epithelial cells. Antioxidants. 2023;12(4):914. doi: 10.3390/antiox12040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Yue Y., Zhang Q., Liang L., Li C., Chen Y., Li W., Peng M., Yang M., Zhao M., et al. Structural characterization and anti-inflammatory effects of an arabinan isolated from Rehmannia glutinosa Libosch. Carbohydr. Polym. 2023;303 doi: 10.1016/j.carbpol.2022.120441. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Chen N., Zhang T., Lin J., Huang Y., Wu G. Rongjin Niantong Fang ameliorates cartilage degeneration by regulating the SDF-1/CXCR4-p38MAPK signalling pathway. Pharm. Biol. 2022;60(1):2253–2265. doi: 10.1080/13880209.2022.2143533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Xu L., Wang S., Wang L., Wang X., Xu H., Li X., Ye H. Confirmation of inhibitingTLR4/MyD88/NF-κB signalling pathway by duhuo jisheng decoction on osteoarthritis: a network pharmacology approach-integrated experimental study. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.784822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Ma S., Su H., Cheng J. Isoliquiritigenin inhibits IL-1β-induced production of matrix metalloproteinase in articular chondrocytes. Mol Ther Methods Clin Dev. 2018;9:153–159. doi: 10.1016/j.omtm.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bian Z., Zhang R., Zhang X., Zhang J., Xu L., Zhu L., Ma Y., Liu Y. Extraction, structure and bioactivities of polysaccharides from Rehmannia glutinosa: a review. J. Ethnopharmacol. 2023;305 doi: 10.1016/j.jep.2022.116132. [DOI] [PubMed] [Google Scholar]
- 32.Yang H., Zhai B., Wang M., Fan Y., Wang J., Cheng J., Zou J., Zhang X., Shi Y., Guo D., et al. The influence of rhein on the absorption of rehmaionoside D: in vivo, in situ, in vitro, and in silico studies. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114650. [DOI] [PubMed] [Google Scholar]
- 33.Di Francesco M., Fragassi A., Pannuzzo M., Ferreira M., Brahmachari S., Decuzzi P. Management of osteoarthritis: from drug molecules to nano/micromedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(3) doi: 10.1002/wnan.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai K., Fujimaki H., Yamada S., Nejima S., Matsubara J., Inaba Y. Changes of synovial fluid biomarker levels after opening wedge high tibial osteotomy in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2021;29(7):1020–1028. doi: 10.1016/j.joca.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Ł Pulik, Łęgosz P., Motyl G. Matrix metalloproteinases in rheumatoid arthritis and osteoarthritis: a state of the art review. Reumatologia. 2023;61(3):191–201. doi: 10.5114/reum/168503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanakis I., Liu K., Poulet B., Javaheri B., van 't Hof R.J., Pitsillides A.A., Bou-Gharios G. Targeted inhibition of aggrecanases prevents articular cartilage degradation and augments bone mass in the STR/ort mouse model of spontaneous osteoarthritis. Arthritis Rheumatol. 2019;71(4):571–582. doi: 10.1002/art.40765. [DOI] [PubMed] [Google Scholar]
- 37.Núñez-Carro C., Blanco-Blanco M., Montoya T., Villagrán-Andrade K.M., Hermida-Gómez T., Blanco F.J., de Andrés M.C. Histone extraction from human articular cartilage for the study of epigenetic regulation in osteoarthritis. Int. J. Mol. Sci. 2022;23(6):3355. doi: 10.3390/ijms23063355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemmati S., Sadeghi M.A., Mohammad Jafari R., Yousefi-Manesh H., Dehpour A.R. The antidepressant effects of GM-CSF are mediated by the reduction of TLR4/NF-ĸB-induced Ido expression. J. Neuroinflammation. 2019;16(1):117. doi: 10.1186/s12974-019-1509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang S.H., Mori D., Kobayashi H., Mori Y., Nakamoto H., Okada K., Taniguchi Y., Sugita S., Yano F., Chung U.I., et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat. Commun. 2019;10(1):1442. doi: 10.1038/s41467-019-09491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi Y., Hou X., Zhong J., Hu L. Test-retest reliability of laser evoked pain perception and fMRI BOLD responses. Sci. Rep. 2021;11(1):1322. doi: 10.1038/s41598-020-79196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavrov I., Latypov T., Mukhametova E., Lundstrom B., Sandroni P., Lee K., Klassen B., Stead M. Pre-motor versus motor cerebral cortex neuromodulation for chronic neuropathic pain. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-91872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunz M., Chen J.I., Rainville P. Keeping an eye on pain expression in primary somatosensory cortex. Neuroimage. 2020;217 doi: 10.1016/j.neuroimage.2020.116885. [DOI] [PubMed] [Google Scholar]
- 43.Guerreiro S.R., Guimarães M.R., Silva J.M., Dioli C., Vamvaka-Iakovou A., Sousa R., Gomes P., Megalokonomou A., Campos-Marques C., Cunha A.M., et al. Chronic pain causes Tau-mediated hippocampal pathology and memory deficits. Mol. Psychiatr. 2022;27(11):4385–4393. doi: 10.1038/s41380-022-01707-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited at the jianguoyun [https://www.jianguoyun.com/p/DbrTFsIQxr6jCxjn3u4EIAA].