Abstract
BACKGROUND
Cardiovascular disease is a significant contributor to the disease burden in geriatric patients. Underlying systemic inflammation is thought to be the cause of age-related changes in the bone marrow and a major risk factor for atherosclerosis. The purpose of the study was to assess the accuracy of these hematological biomarkers in predicting 30-day mortality in older patients with acute coronary syndrome (ACS).
METHODS
This was a prospective observational study of 601 older adult patients (age > 60 years) with ACS who underwent percutaneous coronary intervention over two years (2017–2019). The relationship between baseline hematological parameters and mortality was assessed during the 30-day follow-up. Logistic regression analysis and receiver operating characteristic curve analysis were done to evaluate for diagnostic accuracy of various hematological parameters.
RESULTS
The mean age of presentation was 77 ± 17 years. The mean neutrophil-lymphocyte ratio (NLR) value was 5.07 ± 4.90 and the mean platelet-lymphocyte ratio (PLR) value was 108.65 ± 85.82. On univariate analysis, total leucocyte count [odds ratio (OR) = 0.85, P = 0.021], hematocrit (OR = 0.91, P = 0.018), NLR (OR = 1.10, P = 0.001) and PLR (OR = 1.05, P = 0.001) were associated with mortality. On receiver operating characteristic curve analysis, NLR predicted mortality with 68.1% and PLR with 65.7% accuracy. On multivariate analysis, NLR (OR = 1.096, 95% CI: 1.006–1.15, P = 0.035) was an independent predictor of 30-day mortality.
CONCLUSIONS
For the risk classification of all elderly ACS patients, we highly advise using NLR rather than the total white blood cell count.
With 30.3% of the total disease burden among those 60 years and older, cardiovascular illnesses most frequently brought on by atherosclerosis are the major contributors to disease burden in the older population.[1] Older patients frequently present with atypical symptoms of coronary artery disease (CAD) making diagnosis difficult and may partially increase morbidity and mortality.[2] The increasing occurrence of clonal hematopoiesis of indeterminate potential, driven by interleukin-6 signalling and/or inflammasome activation, is a process promoted by age-related changes in the bone marrow and is a key risk factor for atherosclerosis.[3] In a mouse model of induced myocardial infarction (MI), the sympathetic nervous system is activated. This causes the release of hematopoietic stem cells from bone marrow niches, which further stimulates atherosclerotic plaques and leads to atherogenesis.[4]
Hematological indices have recently attracted a lot of attention since they may offer independent information on pathophysiology and risk stratification.[5] The neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) is useful in predicting short- and long-term mortality in patients with ST-segment elevation MI (STEMI) and non-ST-segment elevation MI (NSTEMI), respectively.[6,7] Indirect inflammatory indicators of atherosclerosis, such as increased NLR and PLR, can predict major detrimental cardiovascular outcomes.[8,9]
The fundamental benefit of hematological indicators is their low cost, making them widely and conveniently accessible in routine clinical practice. Therefore, this study aimed to evaluate how well these hematological biomarkers predicted 30-day mortality in older patients with acute coronary syndrome (ACS).
METHODS
This was a single centre prospective observational study carried out on patients with ACS who were admitted to a 1200-bedded tertiary university teaching hospital in North India over two years (2017–2019) and underwent percutaneous coronary intervention (PCI). 601 consecutive patients were included in this study and were approved by the Institutional Ethics Committee. Informed written consent was obtained from the patients. Inclusion criteria included patients more than 60 years of age, presenting to emergency with ACS. Exclusion criteria included patients with stable ischemic heart disease on outpatient follow-up, and patients with underlying hematological diseases (myeloproliferative neoplasm, hematological malignancies, vitamin B12 deficiency, immune thrombocytopenia, drug-induced thrombocytopenia) were excluded. The study population was divided into two groups: the young-old group (60–70 years) and the old-old group (> 70 years). Risk factors for CAD and co-morbidities were documented at the time of catheterisation by completing a questionnaire. ACS was divided into STEMI, NSTEMI and unstable angina.
Laboratory Analysis
At baseline, venous blood samples were obtained within 30 min of admission to measure haematological indices (haemoglobin, total leucocyte count, differential count, platelets, NLR, mean corpuscular volume, mean corpuscular hemoglobin, and PLR). An automated Sysmex XN-1000™ hematology analyzer (Sysmex America) was used to measure haematological indices.
Statistical Analysis
For descriptive statistics, the continuous variable was presented as mean ± SD or medians (interquartile range) as appropriate. The categorical variables are described as counts (percentages). All data were assessed for normality. The Student’s t-test, Fisher’s exact probability test, and Mann-Whitney U test was used wherever applicable. Variables with P-value < 0.05 on univariate analysis were included in the multivariate logistic regression analysis. Receiver operating characteristic curve analysis was conducted to determine the prognostic accuracy of haematological markers in predicting short-term mortality. The backward stepwise likelihood ratio method was used to identify the independent predictors of 30-day mortality. All tests were two-tailed and P-value < 0.05 was considered statistically significant. Statistical analysis was done using SPSS 28.0 (SPSS Inc., IBM, Armonk, NY, USA). The significance level was set at P-value < 0.05.
RESULTS
The demographic, clinical risk factors and co-morbidities of the 601 study patients are described in Table 1. The mean age of presentation was 77 ± 17 years. The majority were males (n = 486, 80.9%) and the rest were females (n = 115, 19.1%). There were 272 patients (45.3%) belonged to rural areas and 329 patients (54.7%) lived in urban areas.
Table 1. Demography, risk factors and comorbidities.
| Variable | Young-old group (60–70 yrs) n = 467 |
Old-old group (> 70 yrs) n = 134 |
P-value |
| Data are presented as n (%). | |||
| Male | 381 (81.6%) | 105 (78.4%) | 0.403 |
| Hypertension | 355 (76.0%) | 114 (85.1%) | 0.026 |
| Diabetes mellitus | 153 (32.8%) | 45 (33.6%) | 0.859 |
| Smoker | 220 (47.1%) | 51 (38.1%) | 0.063 |
| Dyslipidemia | 29 (6.2%) | 3 (2.2%) | 0.071 |
| Family history of coronary artery disease | 44 (9.4%) | 12 (9.0%) | 0.87 |
| Chronic obstructive pulmonary disease | 31 (6.6%) | 15 (11.2%) | 0.080 |
| Previous myocardial infarction | 83 (17.7%) | 25 (18.7%) | 0.814 |
| Stroke | 27 (5.8%) | 8 (6.0%) | 0.935 |
| Chronic kidney disease | 16 (3.4%) | 5 (3.7%) | 0.865 |
The haematological indices in the study population are shown in Table 2. The mean NLR value was 5.07 ± 4.90 and the mean PLR value was 108.65 ± 85.82.
Table 2. Hematological indices of study participants.
| Indices | |
| *Refers to data are presented as means ± SD (95% CI). **Refers to data are presented as median (interquartile range). NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio. | |
| Hemoglobin, g/dL | 13.51 ± 1.76 (7.70–18.5)* |
| Total leucocyte count, mm3 | 8.54 ± 3.18 (1.06–21)* |
| Neutrophils, % | 70.36 ± 11.93 (13–98)* |
| Lymphocytes, % | 21.10 ± 9.98 (2–76)* |
| Platelet, × 103/mm3 | 166.38 ± 65.18 (55–422)* |
| Mean corpuscular volume, fL | 84.64 ± 5.83 (31–102)* |
| Mean corpuscular hemoglobin, pg | 30.05 ± 3.89 (18–88)* |
| NLR | 3.30 (2.17–6.15)** |
| PLR | 7.94 (5.18–13.31)** |
PCI was performed in all 601 study patients. Primary PCI was performed in 77 patients (11.8%). Forty-seven patients out of these presented to secondary care hospitals in remote areas and had initial thrombolysis. Elective PCI was performed in 524 patients (87.2%). In total, 565 patients had successful stent deployment and reperfusion and 36 patients were referred for coronary artery bypass grafting.
A total of 32 patients (5.3%) died post-PCI within 30 days. The thirty-day mortality was 2.7% in the young-old group and 14.1% in the old-old group (P < 0.0001), respectively. NLR, PLR, and hematocrit were associated with mortality and were statistically significant (Table 3).
Table 3. Haematological parameters related to 30-day mortality.
| Haematological parameters | Died (n = 32) | Survived (n = 569) | P-value |
| Data are presented as means ± SD or median (interquartile range). NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio. | |||
| Hemoglobin, g/dL | 13.23 ± 1.57 | 13.53 ± 1.78 | 0.374 |
| Total leucocyte count, mm3 | 7340 ± 2940 | 8740 ± 3290 | 0.217 |
| Platelet, × 103/mm3 | 163.94 ± 64.65 | 166.52 ± 65.27 | 0.831 |
| NLR | 6.76 (3.11–12.11) | 3.23 (2.16–5.84) | 0.019 |
| PLR | 13.32 (7.18–19.41) | 7.91 (5.12–12.85) | 0.034 |
| Hematocrit, % | 36.08 ± 5.81 | 38.50 ± 5.86 | 0.023 |
Higher mortality was seen in female patients (n = 11, 9.5%), as compared to male patients (n = 21, 4.32%) (P < 0.028). On univariate analysis, total leucocyte count [odds ratio (OR) = 0.85, P = 0.021], hematocrit (OR = 0.91, P = 0.018), NLR (OR = 1.10, P = 0.001) and PLR (OR = 1.05, P = 0.001) were associated with mortality (Table 4).
Table 4. Univariate analysis of parameters.
| Variables | Unadjusted OR (95% CI) | P-value |
| NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio. | ||
| Female | 2.34 (1.09–5.00) | 0.028 |
| Total leucocyte count, mm3 | 0.85 (0.74–0.97) | 0.021 |
| Hematocrit, % | 0.91 (0.85–0.98) | 0.018 |
| NLR | 1.10 (1.05–1.15) | 0.001 |
| PLR | 1.05 (1.02–1.07) | 0.001 |
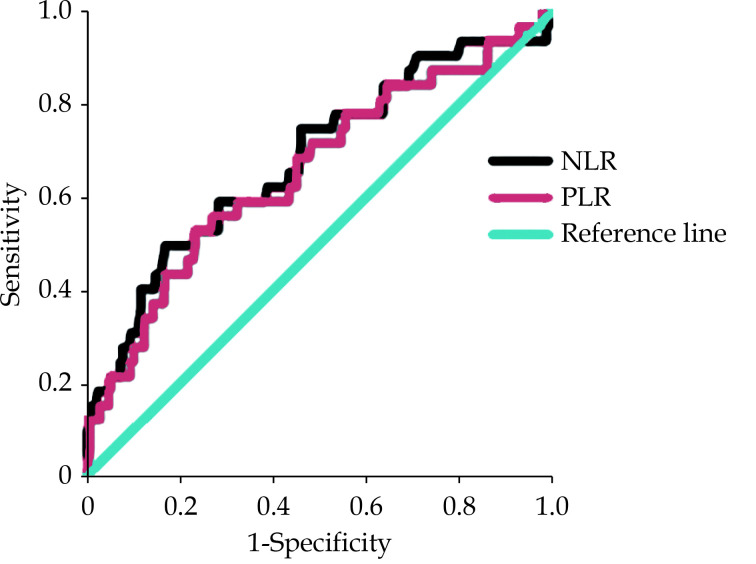
Figure 1 depicts the receiver operating characteristic curve analysis of NLR and PLR with the outcome as mortality. We found that NLR has a diagnostic accuracy of 68.1% for predicting mortality, whereas PLR has a slightly lower accuracy of 65.7%. A cut-off of 3.30 NLR (median NLR) was associated with a sensitivity of 75% and specificity of 52%. A cut-off of 7.94 PLR (median PLR) was associated with a sensitivity of 72% and specificity of 52%.
Figure 1.
ROC curve analysis of NLR and PLR as predictors of mortality.
The area under the ROC for NLR was 0.681 (P = 0.001) and the area under the ROC for PLR was 0.657 (P = 0.003). NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio; ROC: receiver operating characteristic.
Only NLR (OR = 1.096, 95% CI: 1.006–1.15, P = 0.035) was an independent predictor of 30-day mortality in elderly patients with ACS on multivariate analysis as depicted in Table 5.
Table 5. Multivariate logistic regression analysis.
| Variables | Adjusted OR (95% CI) | P-value |
| NLR: neutrophil-lymphocyte ratio; PLR: platelet-lymphocyte ratio. | ||
| Female | 2.024 (0.908–4.513) | 0.085 |
| Total leucocyte count, mm3 | 0.925 (0.786–1.089) | 0.352 |
| Hematocrit, % | 0.93 (0.852–1.195) | 0.096 |
| NLR | 1.096 (1.006–1.15) | 0.035 |
| PLR | 1.008 (0.958–1.061) | 0.755 |
DISCUSSION
Based on the results of this study, a higher NLR ratio was associated with higher 30-day mortality in elderly patients with ACS. The NLR of elderly patients with ACS who died was higher than that of those who survived. On multivariable analysis, this was also established as an independent predictor of 30-day mortality in elderly patients with ACS. Furthermore, on univariate analysis, the female sex, a lower hematocrit, and a lower total leucocyte count at admission were associated with an increased risk of mortality.
A hemogram is available to all patients who visit the emergency department of any hospital. A routine hemogram contains many parameters that have been studied to assess factors in the prediction of mortality in ACS, such as NLR and white blood cell (WBC).[10,11] Most of these studies had a small number of elderly patients. The current study sought to investigate these haematological parameters only in this age group (age > 60 years). In addition, we determined the NLR cut-off value in a larger patient cohort. In our cohort, a higher NLR was associated with a higher likelihood of predicting mortality. NLR can be calculated simply by dividing the neutrophil count by the lymphocyte count in a differential WBC sample. It is one of the most well-studied haematological biomarkers in ACS, providing prognostic and diagnostic information. The combination of neutrophil and lymphocyte parameters outperforms each parameter separately in terms of prognosis. Tamhane, et al.[12] described the admission NLR as a predictor of in-hospital and 6-month mortality in patients undergoing PCI. NLR is useful in predicting short- and long-term mortality in NSTEMI patients with an NLR of more than 4.7.[13]
According to Sahin, et al.,[14] NLR is also related to the severity of CAD in STEMI patients. The NLR of the high SYNTAX score group was higher than that of the low SYNTAX score group (P < 0.001). NLR was found to be a predictor of all-cause mortality and cardiovascular events in patients undergoing angiography or cardiac revascularization in one of the meta-analyses.[15] In patients with acute inferior STEMI and stent thrombosis, NLR is also an independent predictor of right ventricular dysfunction.[16,17]
The link between atherosclerosis and inflammation is not new and has been extensively researched.[18,19] Atherosclerosis is primarily caused by inflammation, which also plays a significant role in plaque rupture, the development of an acute coronary event, and the size of the infarct and subsequent fibrosis.[20,21]
Due to their effect on the instability of atherosclerotic plaques, leukocytes play an important role in the pathophysiology of ACS. Leukocytes permeate endothelial cells and become activated when they reach the tunica intima, causing the formation of microvascularity and, as a result, making plaques more prone to rupture.[22] Many studies have linked leukocytosis to an increased risk of cardiovascular disease. Chia, et al.[23] discovered that elevated leucocytes and neutrophils are predictors of adverse cardiac events in 363 STEMI patients in a prospective study.
Polymorphonuclear cell accumulation in the coronary culprit lesion site thrombus is an independent predictor of mortality in patients with ACS, according to Distelmaier, et al.[24] At the site of the culprit lesion, polymorphonuclear cell release neutrophil extracellular traps. Neutrophil extracellular traps are prothrombotic and pro-inflammatory fibres that can entrap leucocytes and spread thrombosis.[24] Lymphocytes, particularly B cells and T helpers, as components of the adaptive immune system, on the other hand, can mute and limit inflammation. Lower lymphocyte counts were linked to atherosclerosis progression and poor outcomes in ACS patients.[25,26]
Platelets are important in the pathophysiology of ACS. Platelets, when combined with fibrin, form a coronary thrombus. According to the CADILLAC study, platelet levels (which do not affect the effectiveness of percutaneous interventions) are significantly correlated with the incidence of restenosis and stent thrombosis.[27,28] There was no statistically significant difference in platelet levels between patients who died and/or survived in our study. The PLR is an important metric for describing the systemic inflammatory response. PLR has been identified as a risk factor for all-cause mortality following NSTEMI. In patients with ACS, PLR at admission is significantly related to the severity of coronary atherosclerosis.[29,7] In addition, a higher PLR is linked to recurrent MI, heart failure, ischemic stroke, and all-cause mortality in patients with STEMI.[30] In our study, PLR between survivors and those who died was not significant in multivariate analysis.
On multivariate analysis, NLR and PLR were found to be independent predictors of the Global Registry of Acute Coronary Events (GRACE) score in STEMI patients.[31] On multivariate analysis, our study found that only NLR was an independent predictor of short-term mortality (30 days) in elderly patients.
LIMITATIONS
Our study had a few disadvantages, including being a single center study, having a limited sample size for the disease’s prevalence in the region, having a preponderance of male patients, and focusing solely on interventional care.
CONCLUSIONS
Inflammatory processes are crucial in the onset of atherosclerosis, destabilisation of atherosclerotic plaques, and formation of clots on the plaque surface. In elderly individuals with ACS, NLR and PLR both predict 30-day mortality. For the risk classification of all elderly ACS patients, we highly advise using NLR rather than the total WBC count. It serves as a key instrument for intra-hospital clinical surveillance. However, more research is needed on these hematological indicators.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.Prince MJ, Wu F, Guo Y, et al The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 2.Jung YJ, Yoon JL, Kim HS, et al Atypical clinical presentation of geriatric syndrome in elderly patients with pneumonia or coronary artery disease. Ann Geriatr Med Res. 2017;21:158–163. doi: 10.4235/agmr.2017.21.4.158. [DOI] [Google Scholar]
- 3.Tyrrell DJ, Goldstein DR Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18:58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta P, Courties G, Wei Y, et al Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budzianowski J, Pieszko K, Burchardt P, et al The role of hematological indices in patients with acute coronary syndrome. Dis Markers. 2017;2017:3041565. doi: 10.1155/2017/3041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Liu Q, Tang Y Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta-analysis. Sci Rep. 2017;7:40426. doi: 10.1038/srep40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtul A, Murat SN, Yarlioglues M, et al Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2014;114:972–978. doi: 10.1016/j.amjcard.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Avci BŞ, Avci A, Dönmez Y, et al The effectiveness of neutrophil-lymphocyte ratio in predicting in-hospital mortality in non-ST-elevation myocardial infarction. Emerg Med Int. 2020;2020:8718304. doi: 10.1155/2020/8718304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado GP, Araujo GN, Carpes CK, et al Comparison of neutrophil-to-lymphocyte ratio and mean platelet volume in the prediction of adverse events after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Atherosclerosis. 2018;274:212–217. doi: 10.1016/j.atherosclerosis.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Bajari R, Tak S Predictive prognostic value of neutrophil-lymphocytes ratio in acute coronary syndrome. Indian Heart J. 2017;69:S46–S50. doi: 10.1016/j.ihj.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat T, Teli S, Rijal J, et al Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 12.Tamhane UU, Aneja S, Montgomery D, et al Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Azab B, Zaher M, Weiserbs KF, et al Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 14.Sahin DY, Elbasan Z, Gür M, et al Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology. 2013;64:423–429. doi: 10.1177/0003319712453305. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Zhang G, Jiang X, et al Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis. 2014;234:206–213. doi: 10.1016/j.atherosclerosis.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Yaylak B, Ede H, Baysal E, et al Neutrophil/lymphocyte ratio is associated with right ventricular dysfunction in patients with acute inferior ST-segment elevation myocardial infarction. Cardiol J. 2016;23:100–106. doi: 10.5603/CJ.a2015.0061. [DOI] [PubMed] [Google Scholar]
- 17.Ayça B, Akın F, Celik O, et al Neutrophil to lymphocyte ratio is related to stent thrombosis and high mortality in patients with acute myocardial infarction. Angiology. 2015;66:545–552. doi: 10.1177/0003319714542997. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Okamoto Y, Rocha VZ, et al Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.CJ-09-0706. [DOI] [PubMed] [Google Scholar]
- 19.Wolf D, Ley K Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 21.Palasubramaniam J, Wang X, Peter K Myocardial infarction: from atherosclerosis to thrombosis. Arterioscler Thromb Vasc Biol. 2019;39:e176–e185. doi: 10.1161/ATVBAHA.119.312578. [DOI] [PubMed] [Google Scholar]
- 22.Madjid M, Awan I, Willerson JT, et al Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Chia S, Nagurney JT, Brown DF, et al Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi: 10.1016/j.amjcard.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 24.Distelmaier K, Winter MP, Dragschitz F, et al Prognostic value of culprit site neutrophils in acute coronary syndrome. Eur J Clin Invest. 2014;44:257–265. doi: 10.1111/eci.12228. [DOI] [PubMed] [Google Scholar]
- 25.Major AS, Fazio S, Linton MF B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.ATV.0000039169.47943.EE. [DOI] [PubMed] [Google Scholar]
- 26.Zouridakis EG, Garcia-Moll X, Kaski JC Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86:449–451. doi: 10.1016/S0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- 27.Falk E, Nakano M, Bentzon JF, et al Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 28.Nikolsky E, Grines CL, Cox DA, et al Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 29.Azab B, Shah N, Akerman M, et al Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012;34:326–334. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun XP, Li J, Zhu WW, et al Impact of platelet-to-lymphocyte ratio on clinical outcomes in patients with ST-segment elevation myocardial infarction. Angiology. 2017;68:346–353. doi: 10.1177/0003319716657258. [DOI] [PubMed] [Google Scholar]
- 31.Acet H, Ertaş F, Akıl MA, et al Relationship between hematologic indices and Global Registry of Acute Coronary Events risk score in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2016;22:60–68. doi: 10.1177/1076029614533145. [DOI] [PubMed] [Google Scholar]