Abstract
Background
Chronic pain is a major health problem worldwide but the limited knowledge of its underlying pathophysiology impairs the opportunities for diagnostics and treatment. Biomarkers of chronic pain are greatly needed to understand the disease and develop new targets for interventions and drug treatments, and potentially introduce more precise diagnostic procedures. Much evidence points to a neuroimmune pathology for many chronic pain conditions and that important neuroimmune biomarkers exist in the cerebrospinal fluid (CSF) of patients with chronic pain. Systematic collection of CSF in large cohorts of chronic pain patients and healthy volunteers has proven difficult, however. We established the Danish Pain Research Biobank (DANPAIN-Biobank) with the aim of studying potential neuroimmune and glia-related biomarkers of chronic pain. In this paper, we describe the methods and the study population of the DANPAIN-Biobank.
Methods
In this cross-sectional study, we included (I) participants with high-impact (HI) chronic pain from a tertiary, interdisciplinary pain center; (II) participants with osteoarthritic pain scheduled for arthroplasty surgery of the hip or knee at a regional hospital; and (III) pain-free volunteers. All participants completed a questionnaire assessing pain, functional impairment, anxiety, depression, and insomnia before samples of blood and CSF were extracted. Quantitative sensory tests were performed on participants with HI chronic pain and pain-free volunteers, and postoperative outcome scores were available on participants with osteoarthritic pain.
Results
Of the 352 participants included, 201 had HI chronic pain (of which 71% had chronic widespread pain), 81 had chronic osteoarthritic pain, and 70 were pain-free volunteers. Samples were handled uniformly, and CSF samples were frozen within 30 minutes.
Conclusions
We describe the content of the DANPAIN-Biobank, which is unique in terms of the number of participants (including pain-free volunteers), extensive clinical data, and uniformity in sample handling. We believe it presents a promising new platform for the study of neuroimmune and glia-related biomarkers of chronic pain.
Keywords: Biomarkers, cerebrospinal fluid (CSF), chronic pain, neuroimmunology, microglia
Highlight box.
Key findings
• We present the Danish Pain Research Biobank (DANPAIN)-Biobank consisting of cerebrospinal fluid (CSF), blood and clinical data on 201 patients with high impact chronic pain, 81 patients with osteoarthritis pain and 70 healthy volunteers.
What is known and what is new?
• Neuroimmune mechanisms, including glia activation, associated with hyper excitability of dorsal horn neurons and chronic pain behavior is relatively well documented in animal models, but less so in human models.
• The DANPAIN-Biobank contributes as a resource for further research in neuroimmune biomarkers of chronic pain in humans.
What is the implication, and what should change now?
• We consider the DANPAIN-Biobank a unique resource for future research in biomarkers of chronic pain and potential neuroimmune mechanisms.
Introduction
Chronic pain is a major health problem worldwide (1), affecting more than 20% of the adult population (2). It causes disability and suffering for the individual (3) and is an immense societal burden (4). In the US alone, chronic pain is estimated to cost society $635 billion each year (5), and similar proportional costs are reported for other countries (6).
Chronic pain is a maladaptive process but we have limited understanding of the underlying pathophysiology (7). Diagnoses of chronic pain syndromes relies on subjective reports of pain in specific bodily areas, in the absence of biomarkers and concrete objective methods to discriminate between pain syndromes (8). Consequently, treatment options are limited (9,10). Biomarkers of chronic pain are greatly needed to better understand the pain processes, to introduce more precise diagnostic procedures, and potentially to develop new and effective treatment strategies.
Some important pathophysiological mechanisms in chronic pain have been uncovered, including amplification of the nociceptive signal in the central nervous system (CNS) and reduced pain inhibitory capacity leading to hypersensitivity to pain (11-13). Their importance is demonstrated in the characteristic changes in pain perception, such as reduced pain thresholds (i.e., hyperalgesia), increase in temporal summation of pain (TSP), and reduced conditioned pain modulation (CPM) (14-16). In addition, the finding of functional and structural changes in the CNS in several chronic pain syndromes has suggested a key role for neuroplastic changes that alter nociceptive function and central hypersensitivity to pain. This has led to the introduction of nociplastic pain as a third pain descriptor (7,17,18). A possible explanation for changed nociception is neuroinflammation due to glial cell activation (19). Glial cells (microglia, astrocytes and oligodendrocytes) are supportive cells of the CNS that have several roles, including immune functions (i.e., Immune regulation of CNS including production of cytokines, chemokines, removing apoptotic cells, etc.) (20,21). Particularly microglia and astrocytes have been associated with chronic pain (22,23), and when activated, they can release pro-inflammatory mediators that sensitize central nociceptive neurons, thus altering pain perception (24,25). Oligodendrocytes have also been involved in pain processes, however (26). In animal models, the association between activation of glial cells, hyper-excitability of dorsal horn neurons in the spinal cord, and chronic pain behavior is relatively well-documented (27). The theory is less established in humans, where systematic and high-quality collection of cerebrospinal fluid (CSF) in large cohorts of individuals with chronic pain and healthy volunteers has proven difficult. In humans, several studies have assessed inflammatory mediators in the CSF and demonstrated signs of central inflammation (28-34). Unfortunately, several limitations make it hard to draw any final conclusions from these studies. First, sample sizes are relatively small, limiting the statistical power. Second, control groups sometimes consist of participants with other diseases rather than healthy volunteers, which potentially can limit their value as a reference group. Finally, some of these studies have important variations in obtaining, handling, and storing samples, which may introduce important systematic bias and limitations to the results and conclusions.
The aim of the Danish Pain Research Biobank (DANPAIN-Biobank) was to systematically collect blood, CSF, and clinical data from a large cohort of patients with chronic pain and healthy volunteers in order to study potential neuroimmune and glia-related biomarkers of chronic pain. The data collection has now ended and biomarker analysis is ongoing. In this paper, we describe the methods and the study population of the DANPAIN-Biobank. We present this study in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5319/rc) (35).
Methods
Setting and ethical considerations
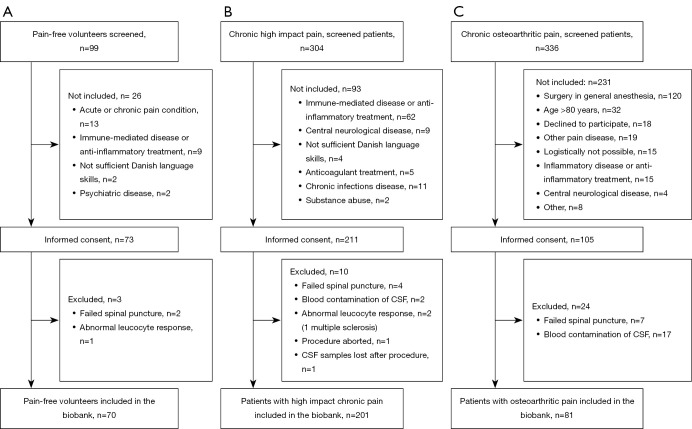
Between April 2017 and June 2019, collection of blood, CSF, measures of pain sensitivity, and clinical data on pain-free volunteers (pain-free group) and patients with chronic high-impact pain (HI-pain group) was performed at the Pain Center of Southern Denmark, Odense University Hospital, Denmark, which is a tertiary interdisciplinary pain center. The chronic HI-pain group was defined as individuals with persistent pain and substantial restriction of life activities lasting 6 months or more and classified as such, based on clinical evaluation. Collection of blood, CSF, and clinical data on patients scheduled for arthroplasty surgery of the hip or knee due to painful osteoarthritis (OA-group) was performed at the Department of Orthopedic Surgery of the regional Lillebaelt Hospital, Vejle, Denmark from January to June 2018 (Figure 1).
Figure 1.
Inclusion of participants. (A) Pain-free volunteers; (B) chronic high impact pain; (C) chronic osteoarthritic pain. CSF, cerebrospinal fluid.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Regional Ethics Committee (Nos. S-20160173, S-20180003) and was reported to the Danish Data Protection Agency (No. 17/3391). Written informed consent was obtained from all participants. The risk for the participants undergoing lumbar puncture was considered minimal due to the precautions taken as outlined below and because it was part of standard perioperative care for the OA-group.
Study flow
All participants completed an online questionnaire within 24 hours of blood and CSF collection. To mimic the impact of possible confounders (36), participants in the pain-free and HI-pain groups fasted for both food and liquids for at least two hours before sampling. Participants in the OA-group needed to keep a 6-hour fast for food and dairy products and 2-hour fast for non-dairy liquids before surgery (and sampling). Participants were instructed to keep their diet, sleeping pattern, and activity levels stable “as usual” for two days before sampling.
Samples were collected between 7.30 am and 2.30 pm with sample collection time spread out during this interval for all three groups. Time for sample collection, flow time of CSF during sample collection, and time from sampling to freezing were noted (Table S1). After the lumbar puncture, participants rested in a supine or lateral position for at least 30 minutes to avoid post-dural-puncture headache and were recommended to avoid heavy physical activity for the rest of the day. Participants were contacted after a week to be interviewed about complications and were encouraged to ring in the case of unpleasant symptoms so they could receive treatment.
Quantitative sensory tests on participants in the pain-free and HI-pain groups were scheduled within one week (before or after) of the sample collection but never on the same day.
Participants
All participants were between 18 to 80 years of age and with sufficient skills in speaking, reading, and writing Danish. Participants with high impact chronic pain were identified by the US National Pain Strategy definition of chronic pain with substantial restriction in work, social, and self-care activities for at least 6 months (37,38). These participants were recruited from the Pain Center of Southern Denmark, Odense University Hospital, and from individuals who contacted the Pain Center after coverage of the study by patient associations and social media and who were eligible for treatment in the Pain Center due to high-impact chronic pain. Pain-free participants were individuals with no acute or chronic pain conditions and were recruited via local advertisements. Participants with OA were recruited from patients scheduled for arthroplasty surgery of the hip or knee in spinal anesthesia at the Department of Orthopedic Surgery, Lillebaelt Hospital, Vejle on the indication painful OA.
Exclusion criteria were immunologic or inflammatory disease, suspected or manifest conditions with increased intracranial pressure, common cold or any other acute or chronic infectious disease, pregnancy, malignant disease, ongoing immunotherapy or chemotherapy, steroid treatment, non-steroid anti-inflammatory drug within three days of biologic sample collection, anticoagulant therapy or disease involving the coagulation system, acute pain during the previous week, dementia or a similar condition, central neurological disease, or abuse of alcohol or other psychoactive drugs. Additionally, patients were not included in the OA-group if they had arthroplasty surgery or any other operation of a similar size within the previous three months, had dysfunction or complications to existing hip or knee arthroplasty, or any other chronic pain than hip or knee pain. Participants in the pain-free group were excluded if they had any pain during the previous week.
Participants with chronic pain received no compensation for participating in the study as payment of patients under active treatment is prohibited by Danish law. The pain-free participants received 2,000 DKK (taxable, approximately 270 euro) as compensation for inconvenience.
Sample collection
Prior to lumbar puncture, 24 mL of blood was collected via peripheral vein puncture. Of this, 12 mL of blood for serum was collected in two 6 mL plain tubes and left untouched for 30 minutes before centrifugation. Blood for plasma was collected in two 4 mL ethylenediaminetetraacetic acid (EDTA) tubes and centrifuged immediately. All blood samples for serum and plasma, were centrifuged at 2,000 G for 10 minutes at 4 ℃, aliquoted on ice blocks, frozen on dry ice, and stored in a freezer at −80 ℃. Serum and plasma were stored in 0.5 mL Sarstedt polypropylene tubes. The remaining blood sample was collected in an EDTA tube for full blood, then distributed into four RNAse-free tubes of 1.25 mL. Buffy coat from each of the two plasma-EDTA tubes was aliquoted into two RNAse-free tubes of 700 µL.
CSF was collected via lumbar puncture at the L3–L5 spinal level using a sterile technique and lidocaine 10 mg/mL for local analgesia. All samples from participants in the HI-pain and pain-free groups were collected by the same anesthesiologist (MRBE) with a 25 or 27 G pencil point needle (0.4–0.5 mm non-traumatic point). The procedure was aborted if spinal puncture was not achieved within three attempts, if there was more than minor discomfort from the needle insertion, or if the participant withdrew consent. Samples from participants in the OA-group were collected by the anesthetist in charge of the anesthesia, using department procedures (39,40), and were immediately handed to a project laboratory technician. After reaching the intrathecal space, the first 10 drops of CSF were unused to minimize blood contamination. In case of visible blood contamination, the CSF was left dripping until it became clear. Following this, 1 mL CSF was collected for immediate routine analysis of erythrocytes, leucocytes, protein, and glucose, and then 6 mL was collected in a plain tube stored in ice water and transferred immediately to the laboratory. Here they were centrifuged at 2,000 G for 10 minutes at 4 ℃, aliquoted on ice blocks into 0.5 mL polypropylene Sarstedt tubes, and frozen on dry ice or stored directly in a freezer at −80 ℃.
We registered sample collection times, the time interval from sample collection until freezing, and outflow time for CSF (Table S1).
Clinical data and pain outcomes
Questionnaire data were collected online via a clinical pain registry (PainData) (41) and included demographic data on biological sex, age, height, weight, smoking and alcohol habits, education, and work situation. Furthermore, participants were asked to estimate their general physical activity into either primarily sedentary purposes, physical activity at least 4 hours per week, active sports/heavy work or competitive sports and to rate the experience of general symptoms of fatigue, waking unrefreshed, reduced cognition into: no problems, mild problems, moderate problems or severe problems. Finally participants were asked whether they had experienced headache or abdominal pain during the last 6 months.
Clinical outcome questionnaires
Functional impairment was assessed using a modified version of the functional domain on the Symptom Impact Questionnaire (SIQR) (42,43). The SIQR consists of nine questions about normal daily activities. The difficulty of each activity is scored on a 0–10 Likert scale, where 0 is “no difficulty” and 10 is “very difficult”. The total score is the sum of all nine scores, giving a total score of range 0–90, where 90 expresses the highest level of functional impairment. Depressive symptoms were assessed using the Major Depression Inventory (MDI) (44,45), in which 10 items ask about various depressive symptoms using a 0–5 Likert scale where 0 reflects absence of the symptom and 5 reflects constant presence of the symptom. The scores of the items are summed to a total score of 0–50, where a higher score expresses a higher load of depressive symptoms. Symptoms of anxiety were assessed using the Generalized Anxiety Disorder Assessment (GAD7) (46,47), which consists of seven items about anxiety symptoms that are, scored on a 0–3 Likert scale, where 0 is “not experienced at all” and 3 is “experienced nearly every day”. The item scores are summed to a total score of 0–21, where a higher score represents more severe anxiety symptoms. Finally, insomnia was assessed using the Insomnia Severity Index (ISI) (48,49), in which seven items on the patient’s subjective perception of sleep as well as satisfaction and distress about the sleep are scored on a 0–4 Likert scale where 0 is “no problem/distress” and 4 is “severe problem/distress”. The items scores are summed to a total score of 0–28, where a higher score represents more severe insomnia.
Pain outcome questionnaires
Participants with chronic pain also noted the approximate date of pain onset and the pain pattern (number of days per week with pain and variations in pain during the day). Pain distribution during the previous week was reported on the online body chart and based on this, participants were classified as having widespread pain (located axially, above and below the waist, and on both sides of the body) back pain or other pain. Pain intensity (worst, least, and average pain) and pain during physical activity during the last week were scored on a numerical rating scale (NRS) ranging from 0–10, where 0 was “no pain” and 10 was “worst imaginable pain”. Interference of pain in daily life was assessed using the Pain Disability index (PDI) (50), in which pain interference with daily activities is scored on a 0–10 points Likert scale, where 0 is “no disability” and 10 is “worst disability”. In this study, only the five voluntary activity items were collected as previous psychometric analyses had indicated that the obligatory activation subscale (self-care activities and life-support activities) has low internal reliability in this population (51). We thus had a total score of 0–50 expressing the highest level of pain interference.
Postoperative outcome questionnaires
In the OA group, postoperative outcome data at 3 and 12 months as well as baseline scores, were available on the Oxford Hip Score (OHS) (52) and the Oxford Knee Score (OKS) (53), which assess the patient’s pain and functional status in the hip or knee whith12 items scored on a 0–4 Likert scale (0= worst, 4= best). The item scores are summed to a composite score of 0–48, with higher score representing better hop or knee function. The University of California, Los Angeles (UCLA) activity score (54,55) was used to monitor changes in activity levels before and after arthroplasty. This is a 10-point scale that evaluates patient activity based on 10 descriptive activity levels ranging from wholly inactive (level 1) to regular participation in impact sports such as jogging or tennis (level 10). Finally, self-perceived general health was assessed using the 0–100 visual analog scale (VAS) included in the EQ-5D instrument (EQ VAS), where a higher score represents a better self-perceived health (56).
Quantitative sensory testing (QST)
Two operators with extensive experience and supervised training in the QST-protocol (16,57), performed QST on participants in the pain-free and HI-pain groups. During this session, the tests were administrated in the order they are presented below.
Sensitivity to heat stimulus
Heat detection threshold (HDT) and heat pain threshold (HPT) were assessed over the most painful area and the thenar eminence of the left hand using a MSA Thermotester (Somedic AB, Hörby, Sweden). In pain-free participants the same area on the lower back was used as substitute of the most painful area. HDT were defined as the first subtle change of temperature and HPT as when the heat from the thermode was experienced as pain. Thresholds were obtained with ramped stimuli (1 C/s) that were terminated when the participant pushed a button. Baseline temperature was 32 ℃, and cut-off temperature was 50 ℃. The final thresholds were calculated as the mean of three consecutive trials (58).
Sensitivity to pinprick
Pain intensity ratings to a single pinprick over the most painful area and the thenar eminence of the left hand were assessed using a custom-made weighted pinprick stimulator (flat contact area of 0.2 mm diameter) exerting a force of 512 mN (MRC systems, Heidelberg, Germany). To assess TSP, pain intensity ratings after a single pinprick was compared to ratings after a train of 10 stimuli of the same force (repeated at a 1/s rate and given within an area of 1 cm2) over the most painful area and the thenar eminence of the left hand. Pain intensity ratings were provided on 0–10 NRS, where 0 was “no pain” and 10 was “worst imaginable pain”. TSP was calculated as the ratio between NRS-scores after the train of 10 stimuli and the single pinprick, with positive values indicating an increase in NRS-scores during the repeated stimulation (58).
Sensitivity to pressure
A computer-controlled cuff pressure algometer (CPAR, NociTech, Denmark) was used to assess deep tissue pressure pain sensitivity. Two 13 cm wide silicone tourniquet cuffs (VBM, Sulz, Germany) with equal-sized proximal and distal chambers were wrapped around the right and left lower legs 8 cm from the tibial tuberosity. The cuff pressure was increased by the computer at a rate of 1 kPa/s, and the maximal pressure was 96.5 kPa at one leg at time (left first, followed by the right leg).
Participants used an electronic 10 cm VAS to rate the intensity of the pressure-induced pain in a continuous fashion where 0 was “no pain” and 10 was “maximal, intolerable pain”. The electronic VAS was sampled at 10 Hz and equipped with a stop button. Participants were informed that they could stop the pressure at any time by pushing the stop button, thus indicating that they were not willing to tolerate more pressure. If the VAS reached a score of 10, i.e., intolerable pain, the pressure would be released from the cuff automatically. The cuff pressure pain threshold (cPPT) was defined as the pressure value when the participant rated the sensation of pain as 1 cm on the VAS. The cuff pressure pain tolerance (cPTT) was defined as the pressure at termination by the stop button or when the VAS rating was 10. The test was always performed on the left leg first and then the right leg.
To assess temporal summation of cuff pressure pain (cTSP) , 10 repeated cuff pressure stimulations (2 s duration and 1 s interval between stimuli) were delivered to the leg with an intensity equivalent to the cPTT recorded during the previous assessment. Using the electronic VAS, participants rated their pressure pain intensity continuously during the sequential stimulation. They were instructed not to return the VAS to zero between stimulations. In the period between stimuli, a constant non-painful pressure of 5 kPa was retained to prevent the cuff from moving. The VAS score immediately after each stimulus was extracted. cTSP scores were calculated for the main statistical analysis as the ratio between the mean pain intensity rating of the 8th to 10th stimuli compared to the mean pain intensity of the 1st to 4th stimuli. cTSP scores above 1 reflect increased pain ratings during repeated stimulations, while a score below 1 reflects a decrease in pain ratings.
Pain inhibitory capacity
Pain inhibitory capacity was assessed using the CPM paradigm. The conditioning stimulus was delivered by the tourniquet cuff wrapped around the right lower leg (conditioning stimulus cuff). Within 1 second, the cuff was inflated to the pressure equal to VAS =5 during the assessment of cPTT, and the pressure was kept constant throughout the CPM protocol (maximum of 100 seconds). Pressure intensity equal to VAS =5 was chosen to ensure that the conditioning cuff was above cPPT and thus would be perceived as moderately painful, as recommended (59). Five seconds after inflation of the conditioning stimulus cuff, the cuff on the left leg (test stimulus cuff) was inflated at a rate of 1 kPa/s, and the cPPT and cPTT were reassessed as described above. Patients were informed that the conditioning stimulus cuff would be moderately painful and that they should focus their attention on the test stimulus cuff on the left leg. The CPM effect is defined as the difference in the cPPT or cPTT recorded during conditioning and at baseline assessments, with positive values indicating hypoalgesia.
Statistical analysis and sample size
We made an a priori power analysis based on a previous pilot study (unpublished data). We considered a difference of 30% in possible biomarkers between participants with and without pain relevant and expected that participants with and without pain could be recruited at a 4:1.4 ratio, and we chose α=0.01 and β=0.80. Based on this, and depending on the method for calculation, we would need between 16 and 183 participants in the pain group to detect differences in interleukin (IL)-8, prostaglandin E2 (PGE2), IL-1β, or IL-6 respectively and between 6 and 64 participants in the pain-free group. Thus, as a conservative approach, we planned to include 200 participants in the HI-pain group and 70 in the pain-free group. For logistic reasons, we planned to include 100 participants in the OA-group and succeeded to include 81.
Continuous variables with normal distribution are presented as mean ± standard deviation (SD), alternatively as median and interquartile range (IQR). Categorical variables are expressed in absolute figures and proportion of the total.
Results
From April 2017 until June 2019, 389 persons consented to participate (Figure 1A-1C), of whom 352 were finally included in the DANPAIN-Biobank (pain-free: n=70; HI-pain: n=201, OA-group: n=81). Spinal puncture failed in 13 (3%), and 19 participants (5%) were excluded due to blood contamination of CSF (erythrocyte count >300×106/L). In 15 participants, routine leucocyte count was elevated above the cutoff level of 5×106/L, and they were referred to the neurological department for further investigation. Of these, one participant was diagnosed with multiple sclerosis and was excluded from the biobank, while 12 participants had only marginally elevated leucocyte count {[6–8]×106/L} and no signs of neurological disease and were therefore included in the biobank. Two participants were excluded due to abnormally high leucocyte counts (12×106/L and 20×106/L) but displayed no other signs of neurological disease.
Complications
No serious complications were reported from the participants (Table S2). Six participants suffered post-dural-puncture headache (1.5% of lumbar punctures, one from the pain-free group and five from the HI-pain group): one participant reported the headache as mild and two participants as moderate the first two days with mild headache the following three-four days. Three participants reported severe headache; one of these received an epidural blood patch after which the headache disappeared, while the other two participants reported the headache reduced slowly during the first week after puncture. Twenty-four participants experienced back pain for 1–7 days after lumbar puncture, and 38 participants experienced some soreness at the puncture site after the local anesthetic wore off. Eight participants experienced unspecific worsening of well-known, existing headache with no postural element the first few days after the procedure. In three participants, the existing headache was migraine and the procedure provoked a migraine attack as recognized by the participants. One participant experienced worsening of well-known sciatic pain for a few days and one participant experienced dizziness in the week after the procedure.
Socio-demographics
Sociodemographic factors are presented in Table 1. Mean age and BMI was significantly higher in both pain groups compared to the pain-free group while physical activity was significantly was significantly lower. Participants in the HI-pain group smoked less and drank less alcohol than the participants in the pain-free group while participants in the OA-group had a slightly higher alcohol intake than participants in the pain-free group. Overall, smoking and alcohol consumption were relatively limited in all three groups.
Table 1. Sociodemographic data of participants in the DANPAIN-Biobank.
| Variables | Pain-free (n=70) | High impact chronic pain | Osteoarthritis pain | |||
|---|---|---|---|---|---|---|
| Number (n=201) | P value | Number (n=81) | P value | |||
| Age (years), mean ± SD [range] | 37.4±16.2 [18–77] | 45.4±11.5 [20–75] | <0.001 | 67.1±8.9 [44–79] | <0.001 | |
| Sex, female, n (%) | 43 (61.4) | 172 (85.6) | <0.001 | 46 (56.8) | 0.56 | |
| BMI (kg/m2), mean ± SD [range] | 25.7±4.6 [18–42] | 28.3±5.5 [17–48] | 0.001 | 28.4±5.7 [19–55] | 0.002 | |
| Civil status, cohabiting n (%) | 40 (57.1) | 129 (64.2) | 0.21 | 58 (71.6) | 0.05 | |
| Smoking, n (%) | 0.013 | 0.70 | ||||
| No | 50 (71.4) | 103 (51.2) | 56 (69.1) | |||
| Yes | 8 (11.4) | 44 (21.9) | 13 (16.0) | |||
| Previous smoking | 11 (15.7) | 50 (24.9) | 11 (13.6) | |||
| Alcohol consumption (units of alcohol per week), n (%) | 0.019 | 0.013 | ||||
| 0 | 30 (42.9) | 116 (57.7) | 15 (18.5) | |||
| 1–7 | 32 (45.7) | 74 (36.8) | 52 (64.2) | |||
| 8–14 | 5 (7.1) | 8 (4.0) | 7 (8.6) | |||
| ≥15 | 2 (2.9) | 0 | 5 (6.2) | |||
| Highest education, n (%) | <0.001 | <0.001 | ||||
| Primary school education | 5 (7.1) | 18 (9.0) | 19 (23.5) | |||
| Upper secondary education | 19 (27.1) | 19 (9.5) | 4 (4.9) | |||
| Vocational education/training | 6 (8.6) | 60 (29.9) | 22 (27.2) | |||
| Short cycle education | 5 (7.1) | 29 (14.4) | 11 (13.6) | |||
| Vocational bachelor education | 24 (34.3) | 53 (26.4) | 15 (18.5) | |||
| Master’s program | 10 (14.3) | 12 (6.0) | 3 (3.7) | |||
| Others | 1 (1.4) | 6 (3.0) | 5 (6.2) | |||
| Work situation, n (%) | <0.001 | <0.001 | ||||
| Normal working hours | 34 (48.6) | 28 (13.9) | 20 (24.7) | |||
| Reduced working hours | 0 | 41 (20.3) | 1 (1.2) | |||
| Sick leave (partly or fulltime) | 1 (1.4) | 44 (21.9) | 4 (4.9) | |||
| Pension | 5 (7.1) | 44 (21.9) | 45 (55.6) | |||
| Studying | 24 (34.3) | 13 (6.5) | 0 | |||
| Unemployed | 0 | 10 (5.0) | 2 (2.5) | |||
| Other | 6 (8.6) | 17 (8.5) | 8 (9.9) | |||
| Physical activity, n (%) | <0.001 | <0.001 | ||||
| Primarily sedentary pursuits | 10 (14.3) | 65 (32.3) | 16 (19.8) | |||
| Physical activity at least 4 hours per week | 27 (38.6) | 102 (50.7) | 48 (59.3) | |||
| Active sports activities/heavy work | 30 (42.9) | 21 (10.4) | 13 (16.0) | |||
| Competitive sports | 2 (2.9) | 1 (0.5) | 0 | |||
In categories where the number of participants is not equal to the total number of participants in the group, data are missing. P values represents differences compared to the pain-free group. DANPAIN, the Danish Pain Research Biobank; SD, standard deviation; BMI, body mass index.
Pain and clinical outcomes
Pain outcomes are presented in Table 2 and general clinical outcomes in Table 3. NRS scores between the two pain groups did not differ, but participants in the HI-pain group had significantly longer pain duration and widespread pain index and rated themselves worse on both scales for functional impairment [PDI and Revised Fibromyalgia Impact Questionnaire (FIQR)], anxiety, depression and insomnia. In the OA-group, participants generally reported better postoperative outcome scores (Table 4) than at baseline. In the quantitative sensory tests (Table 5), participants in the HI-pain group reported higher sensitivity to pain stimuli for most parameters.
Table 2. Pain outcomes of participants in the DANPAIN-Biobank.
| Variables | Pain-free (n=70) | High impact chronic pain (n=201) | Osteoarthritis pain (n=81) | P |
|---|---|---|---|---|
| Pain duration, years, median (25th–75th percentiles) | 0 | 12.0 (6.5–21.8) | 2.0 (1.0–4.0) | <0.001 |
| Peak pain intensity last week, NRS 0–10, mean (SD) | 0 | 7.2 (1.8) | 6.8 (2.1) | 0.10 |
| Average pain intensity last week, NRS 0–10, mean (SD) | 0 | 5.7 (1.9) | 5.5 (2.2) | 0.56 |
| Least pain intensity last week, NRS 0–10, mean (SD) | 0 | 3.9 (2.1) | 3.6 (2.6) | 0.30 |
| Pain intensity at physical activity last week, NRS 0–10 mean (SD) | 0 | 6.3 (2.3) | 6.4 (2.2) | 0.77 |
| Widespread pain index, pain sites, 0–19, mean (SD) | 0 | 11.5 (5.1) | 2.0 (1.3) | <0.001 |
| Pain diagnoses, n (%) | ||||
| Wide spread pain | 0 | 143 (71%) | 0 | |
| Back pain | 0 | 24 (12%) | 0 | |
| Osteoarthritic pain | 0 | 0 | 81 (100%) | |
| Other | 0 | 34 (17%) | 0 | |
| Pain disability (PDI 5 items: 0–50, higher is worse), mean (SD) | 0 | 37.1 (11.6) | 26.1 (13.6) | <0.001 |
| PDI family/home responsibilities, 0–10 mean (SD) | 0 | 6.4 (2.2) | 4.8 (2.9) | <0.001 |
| PDI recreation 0–10, mean (SD) | 0 | 7.3 (2.2) | 6.9 (2.8) | 0.2 |
| PDI social activity 0–10, mean (SD) | 0 | 6.3 (2.5) | 4.1 (2.9) | <0.001 |
| PDI occupation 0–10, mean (SD) | 0 | 7.1 (2.5) | 4.4 (3.4) | <0.001 |
| PDI sexual behavior 0–10, mean (SD) | 0 | 5.9 (3.0) | 3.8 (3.4) | <0.001 |
| Analgesic treatment, n (%) | ||||
| No analgesic treatment | 70 (100%) | 22 (11%) | 12 (15%) | |
| Paracetamol | 0 | 130 (65%) | 62 (77%) | |
| NSAID | 0 | 52 (26%) | 21 (26%) | |
| Opioid | 0 | 67 (33%) | 10 (12%) | |
| Antidepressants†, pain indication | 0 | 63 (31%) | 1 (1%) | |
| Antiepileptics, pain indication | 0 | 38 (19%) | 2 (2%) | |
| Cannabinoids | 0 | 11 (5%) | 0 | |
| Low dose naltrexone | 0 | 11 (4%) | 1 (1%) | |
| Local treatments‡ | 0 | 7 (3%) | 0 |
†, tricyclic antidepressants, duloxetine, venlafaxine; ‡, transcutaneous electric nerve stimulation =2, Qutenza =1, Versatis =1, Botox =1, peripheral nerve field stimulation =1, spinal cord stimulation =1. P values represents differences between the two pain groups. DANPAIN, the Danish Pain Research Biobank; SD, standard deviation; NRS, Numerical Rating Scale; PDI, Pain Disability Index; NSAID, non-steroidal anti-inflammatory drugs.
Table 3. Clinical outcomes of participants in the DANPAIN-Biobank.
| Variables | Pain-free (n=70) | High impact chronic pain | Osteoarthritis pain | ||||
|---|---|---|---|---|---|---|---|
| n=201 | P* | n=81 | P* | P** | |||
| Functional impairment (SIQR; 0–90, higher is worse), mean (SD) | 1.3 (2.6) | 42.6 (18.3) | <0.001 | 28.1 (17.9) | <0.001 | <0.001 | |
| Depression (MDI; 0–50, higher is worse), mean (SD) | 4.7 (5.1) | 19.2 (9.6) | <0.001 | 7.5 (6.0) | 0.002 | <0.001 | |
| Anxiety (GAD–7; 0–21, higher is worse), mean (SD) | 1.8 (2.6) | 6.0 (4.8) | <0.001 | 2.0 (2.8) | 0.64 | <0.001 | |
| Insomnia (ISI; 0–28, higher is worse), mean (SD) | 3.4 (3.6) | 13.6 (6.8) | <0.001 | 7.6 (5.7) | <0.001 | <0.001 | |
*, P values represent differences compared to the pain-free group; **, P values represent differences compared to the high impact chronic pain group. DANPAIN, the Danish Pain Research Biobank; SIQR, Symptom Impact Questionnaire; SD, standard deviation; MDI, Major Depression Inventory; GAD-7, Generalized Anxiety Disorder Assessment; ISI, Insomnia Severity Index.
Table 4. Postoperative outcomes of participants undergoing hip and knee arthroplasty because of painful osteoarthritis.
| Variables | Osteoarthritis pain (n=81), mean (SD) |
|---|---|
| Oxford | |
| Oxford hip/knee score, preoperatively; 0–48, lower is worse | 23.1 (6.8) |
| Oxford hip/knee score, 3 months postoperatively; 0–48, lower is worse | 37.7 (8.8) |
| Oxford hip/knee score, 12 months postoperatively; 0–48, lower is worse | 41.4 (7.7) |
| UCLA | |
| UCLA activity score, 3 months postoperatively; 1–10, lower is worse | 5.9 (1.7) |
| UCLA activity score, 12 months postoperatively; 1–10, lower is worse | 6.5 (1.9) |
| EQ VAS | |
| EQ VAS, preoperatively; 0–100, lower is worse | 62.0 (22.1) |
| EQ5DVAS, 3 months postoperatively; 0–100, lower is worse | 80.1 (19.7) |
| EQ5DVAS, 12 months postoperatively; 0–100, lower is worse | 83.1 (16.8) |
SD, standard deviation; UCLA, University of California, Los Angeles; VAS, visual analogue scale.
Table 5. Quantitative sensory testing outcome in participants with high impact chronic pain and pain-free volunteers.
| Variables | Pain-free (n=70), mean (SD) | High impact chronic pain (n=201), mean (SD) |
|---|---|---|
| Experimental pain, ℃ | ||
| HDT hand | 34.6 (1.3) | 34.3 (1.0) |
| HDT pain area | 35.6 (1.3) | 35.8 (2.1) |
| HPT hand | 44.1 (3.5) | 41.7 (4.3) |
| HPT pain area | 42.7 (3.3) | 40.9 (4.0) |
| Pinprick, NRS | ||
| Pinprick hand | 1.8 (1.6) | 2.7 (2.5) |
| Pinprick pain area | 1.5 (1.4) | 2.5 (2.3) |
| cPPT, kPa | ||
| cPPT right | 27.0 (11.6) | 19.1 (11.9) |
| cPPT left | 25.8 (12.8) | 18.8 (11.0) |
| cPTT right | 59.0 (22.4) | 39.7 (21.5) |
| cPTT left | 59.6 (22.3) | 43.2 (23.5) |
| Pain modulation | ||
| TSP pinprick hand, NRS | 2.4 (1.7) | 3.4 (2.1) |
| TSP pinprick back, NRS | 2.2 (1.7) | 3.9 (2.3) |
| TSP ratio, cuff | 2.6 (4.6) | 5.6 (11.2) |
| CPM-response, kPa | 8.2 (11.6) | 2.4 (8.1) |
SD, standard deviation; HDT, heat detection threshold; HPT, heat pain threshold; NRS, numerical rating scale; cPPT, cuff pressure pain threshold; cPTT, cuff pressure pain tolerance; TSP, temporal summation of pain; CPM, conditioned pain modulation.
Sample handling
Samples in the three groups were handled similarly (Table S1), and CSF samples were frozen within 30 minutes after sample collection. There were no clinically important differences in extraction time or in time from sample collection to freezing. However, plasma samples were on average frozen 40 minutes later for participants in the OA-group and puncture time was slightly earlier for samples from the OA-group, because it was dependent of the operation. The CSF composition is presented in Table 6.
Table 6. CSF analysis of participants in the DANPAIN-Biobank.
| Variables | Pain-free (n=70) | High impact chronic pain (n=201) | Osteoarthritis pain (n=81) |
|---|---|---|---|
| Erythrocyte count, 106/L | <300 | <300 | <300 |
| Leucocyte count, 106/L, mean (SD) | 0.2 (1.0) | 0.3 (1.4) | 0.3 (1.6) |
| Total protein, g/L, mean (SD) | 0.4 (0.1) | 0.4 (0.2) | 0.5 (0.2) |
| Glucose, mmol/L, mean (SD) | 3.4 (0.7) | 3.4 (0.5) | 3.7 (0.6) |
CSF, cerebrospinal fluid; DANPAIN, the Danish Pain Research Biobank; SD, standard deviation.
Discussion
We present here the DANPAIN-Biobank that comprises blood samples, CSF samples, QST, and extensive clinical data for the purpose of detecting neuroimmune and glia-related biomarkers of chronic pain. We believe the biobank to be unique in terms of the number of participants (especially pain-free volunteers) and in the extensive clinical data and QST that can be used to phenotype participants, which is important for biomarker research.
The DANPAIN-Biobank includes both participants with complex and often widespread high impact pain syndromes, who experience lifelong symptoms as well as participants with more localized osteoarthritic pain in hip or knee, where the pain can often be relieved or at least markedly reduced by arthroplasty. This presents the possibility to test potential biomarkers in a variety of different pain syndromes. Interestingly, the pain scores in the two groups were similar but the impairment of physical function and psychological health scores, were significantly different in the two groups, indicating that pain disability is not solely determined by pain intensity. We believe that the inclusion of 70 pain-free volunteers who were included for the biobank alone and not via other contacts to the health care system provides advantages compared with several other CSF biomarker studies testing potential biomarkers in a control group that resembles the background population. The comprehensive data on different pain outcomes, including clinical outcomes such as mental health, functional capacity, accompanying symptoms and pain sensitivity, enable investigation into possible clinical implications due to associations between potential biomarker candidates and a variety of clinical outcomes.
Another strength of the DANPAIN-Biobank is the rigidity of sample handling, with very high uniformity in sample handling, the instant and stable cooling of CSF samples until freezing, and the short interval from sample collection to freezing (60).
Complication rates for the participants were lower than in other studies, especially considering the relatively low age of the participants (61,62). The blood contamination rate was also considerably lower than the 14–20% previously reported and was possibly due to the special considerations and precautions taken for the spinal puncture procedure. The CSF composition in terms of cell count, protein, and glucose was comparable to that in other studies (63,64).
There are several limitations to the DANPAIN-Biobank. First, the basic demographic variables, especially age, varied significantly among the three groups. As the distribution of these variables in all groups was rather broad, however, it should be possible to correct for this variation statistically in the search for potential biomarkers. Second, CSF and blood were collected at several time points during the day. As biochemical changes in several CNS diseases are known to fluctuate (65), this could both be an advantage and a disadvantage. For those biomarkers where circadian rhythms in the CSF is unclarified, it could bring new knowledge to the importance of the timing of sample collection. For biomarkers with circadian fluctuations, it could introduce potential bias, but it should be possible to correct for this potential bias statistically, since the material contains samples from all time points during the day in all three groups. Finally, both the self-report questionnaires and the quantitative sensory tests are based on subjective response and recollection, so the results could be biased. However, the clinical and pain outcome data are comparable to other studies on patients with high-impact chronic pain (16,41).
Conclusions
We describe here the content of the DANPAIN-Biobank, the methodology by which biological samples and clinical data are collected, the clinical characteristics of the participants and the initial biochemical analyses of the material. The high volume of participants, including pain-free volunteers, and the extensive clinical data and uniformity in sample handling present a promising platform for exploring neuroimmune and glia-related biomarkers of chronic pain. We remain open to collaborations that can facilitate the overall objective of the biobank.
Supplementary
The article’s supplementary files as
Acknowledgments
We acknowledge late Niels Henrik Heegaard for participating in the conception, design and methodology of the biobank project, before he passed away much too early and Claire Gudex (BRIDGE, Brain Research-Inter Disciplinary Guided Excellence, Department of Clinical Research, University of Southern Denmark, J. B. Winsløws Vej 19, 3, Odense DK-5000, Denmark) for copy-editing the manuscript.
Funding: The work was supported by: The Danish Rheumatism Association (Nos. R155-A4866-B1363, R175-A6088-B1363); Aase og Ejnar Danielsens Foundation; Karen S Jensens Foundation; Oberstinde Kirsten Jensa la Cours legat; Professor, Overlæge Sophus H. Johansens Foundation of August 23, 1981; Foundation of the Danish Association for Anaesthesia and Intensive Care; Fonden til Lægevidenskabens Fremme; the Development Foundation, Lillebaelt Hospital; OUH Fund for Free Research; Research fund of the Region of Southern Denmark; and Læge Sofus Carl Emils og Hustru Olga Doris Friis grant. All grants were received by MRBE. None of the funders participated in any aspect of design, data collection, analyses, interpretation of data, or writing of the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Regional Ethics Committee (Nos. S-20160173, S-20180003) and was reported to the Danish Data Protection Agency (No. 17/3391). Written informed consent was obtained from all participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5319/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5319/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5319/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5319/coif). CV received travel expenses from Stryker. The other authors have no conflicts of interest to declare.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2.Sjøgren P, Ekholm O, Peuckmann V, et al. Epidemiology of chronic pain in Denmark: an update. Eur J Pain 2009;13:287-92. 10.1016/j.ejpain.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 3.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 2014;95:986-995.e1. 10.1016/j.apmr.2013.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew J, Singh SB, Garis S, et al. Backing up the stories: The psychological and social costs of chronic low-back pain. Int J Spine Surg 2013;7:e29-38. 10.1016/j.ijsp.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gereau RW, 4th, Sluka KA, Maixner W, et al. A pain research agenda for the 21st century. J Pain 2014;15:1203-14. 10.1016/j.jpain.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flachs E EL, Koch M, Ryd J, Juel K. The disease burden in Denmark. Copenhagen: Statens Institut for Folkesundhed, Syddansk Universitet 2015. Available online: https://www.sst.dk/da/sygdom-og-behandling/~/media/00C6825B11BD46F9B064536C6E7DFBA0.ashx
- 7.Fitzcharles MA, Cohen SP, Clauw DJ, et al. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397:2098-110. 10.1016/S0140-6736(21)00392-5 [DOI] [PubMed] [Google Scholar]
- 8.Finnerup NB, Kuner R, Jensen TS. Neuropathic Pain: From Mechanisms to Treatment. Physiol Rev 2021;101:259-301. 10.1152/physrev.00045.2019 [DOI] [PubMed] [Google Scholar]
- 9.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021;397:2082-97. 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 10.The Lancet. Rethinking chronic pain. Lancet 2021;397:2023. 10.1016/S0140-6736(21)01194-6 [DOI] [PubMed] [Google Scholar]
- 11.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926. 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2-S15. 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendt-Nielsen L, Skou ST, Nielsen TA, et al. Altered Central Sensitization and Pain Modulation in the CNS in Chronic Joint Pain. Curr Osteoporos Rep 2015;13:225-34. 10.1007/s11914-015-0276-x [DOI] [PubMed] [Google Scholar]
- 14.Petersen KK, Arendt-Nielsen L, Finocchietti S, et al. Age Interactions on Pain Sensitization in Patients With Severe Knee Osteoarthritis and Controls. Clin J Pain 2017;33:1081-7. 10.1097/AJP.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 15.Petersen KK, Siebuhr AS, Graven-Nielsen T, et al. Sensitization and Serological Biomarkers in Knee Osteoarthritis Patients With Different Degrees of Synovitis. Clin J Pain 2016;32:841-8. 10.1097/AJP.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 16.Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain 2016;157:1480-8. 10.1097/j.pain.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 17.Kosek E, Cohen M, Baron R, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016;157:1382-6. 10.1097/j.pain.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 18.IASP. IASP Terminology. Available online: https://www.iasp-pain.org/resources/terminology/
- 19.Albrecht DS, Forsberg A, Sandström A, et al. Brain glial activation in fibromyalgia - A multi-site positron emission tomography investigation. Brain Behav Immun 2019;75:72-83. 10.1016/j.bbi.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peferoen L, Kipp M, van der Valk P, et al. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014;141:302-13. 10.1111/imm.12163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Benveniste EN. Immune function of astrocytes. Glia 2001;36:180-90. 10.1002/glia.1107 [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Zhang YQ, Qadri YJ, et al. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018;100:1292-311. 10.1016/j.neuron.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci 2019;20:667-85. 10.1038/s41583-019-0218-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013;154 Suppl 1:S10-28. 10.1016/j.pain.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji RR, Nackley A, Huh Y, et al. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018;129:343-66. 10.1097/ALN.0000000000002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malta I, Moraes T, Rodrigues G, et al. The role of oligodendrocytes in chronic pain: cellular and molecular mechanisms. J Physiol Pharmacol 2019. doi: . 10.26402/jpp.2019.5.02 [DOI] [PubMed] [Google Scholar]
- 27.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533-48. 10.1038/nrd4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backonja MM, Coe CL, Muller DA, et al. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol 2008;195:157-63. 10.1016/j.jneuroim.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Kadetoff D, Lampa J, Westman M, et al. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 2012;242:33-8. 10.1016/j.jneuroim.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 30.Bäckryd E, Tanum L, Lind AL, et al. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J Pain Res 2017;10:515-25. 10.2147/JPR.S128508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bäckryd E, Lind AL, Thulin M, et al. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: a cross-sectional study of 2 cohorts of patients compared with healthy controls. Pain 2017;158:2487-95. 10.1097/j.pain.0000000000001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundborg C, Hahn-Zoric M, Biber B, et al. Glial cell line-derived neurotrophic factor is increased in cerebrospinal fluid but decreased in blood during long-term pain. J Neuroimmunol 2010;220:108-13. 10.1016/j.jneuroim.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 33.Kosek E, Altawil R, Kadetoff D, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain--interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J Neuroimmunol 2015;280:49-55. 10.1016/j.jneuroim.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosek E, Finn A, Ultenius C, et al. Differences in neuroimmune signalling between male and female patients suffering from knee osteoarthritis. J Neuroimmunol 2018;321:49-60. 10.1016/j.jneuroim.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou X, Fragala MS, McElhaney JE, et al. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care 2010;13:541-7. 10.1097/MCO.0b013e32833cf3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You DS, Ziadni MS, Hettie G, et al. Comparing Perceived Pain Impact Between Younger and Older Adults With High Impact Chronic Pain: A Cross-Sectional Qualitative and Quantitative Survey. Front Pain Res (Lausanne) 2022;3:850713. 10.3389/fpain.2022.850713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Committee. IPRC. A comprehensive population healthlevel strategy for pain: National pain strategy. Washington, DC. Available online: https://www.iprcc.nih.gov/sites/default/files/documents/NationalPainStrategy_508C.pdf
- 39.Internal guideline for alloplastic knee surgery, Vejle Hospital. Available online: https://infonet.regionsyddanmark.dk/?DokID=4457
- 40.Internal guideline for hip alloplastic surgery, Vejle Hospital. Available online: https://infonet.regionsyddanmark.dk/?DokID=4421
- 41.Vaegter HB, Christoffersen LO, Enggaard TP, et al. Socio-Demographics, Pain Characteristics, Quality of Life and Treatment Values Before and After Specialized Interdisciplinary Pain Treatment: Results from the Danish Clinical Pain Registry (PainData). J Pain Res 2021;14:1215-30. 10.2147/JPR.S306504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett RM, Friend R, Jones KD, et al. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther 2009;11:R120. 10.1186/ar2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friend R, Bennett RM. Distinguishing fibromyalgia from rheumatoid arthritis and systemic lupus in clinical questionnaires: an analysis of the revised Fibromyalgia Impact Questionnaire (FIQR) and its variant, the Symptom Impact Questionnaire (SIQR), along with pain locations. Arthritis Res Ther 2011;13:R58. 10.1186/ar3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bech P, Rasmussen NA, Olsen LR, et al. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord 2001;66:159-64. 10.1016/S0165-0327(00)00309-8 [DOI] [PubMed] [Google Scholar]
- 45.Olsen LR, Jensen DV, Noerholm V, et al. The internal and external validity of the Major Depression Inventory in measuring severity of depressive states. Psychol Med 2003;33:351-6. 10.1017/S0033291702006724 [DOI] [PubMed] [Google Scholar]
- 46.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 47.Seo JG, Park SP. Validation of the Generalized Anxiety Disorder-7 (GAD-7) and GAD-2 in patients with migraine. J Headache Pain 2015;16:97. 10.1186/s10194-015-0583-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297-307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 49.Dieperink KB, Elnegaard CM, Winther B, et al. Preliminary validation of the insomnia severity index in Danish outpatients with a medical condition. J Patient Rep Outcomes 2020;4:18. 10.1186/s41687-020-0182-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills 1984;59:974. 10.2466/pms.1984.59.3.974 [DOI] [PubMed] [Google Scholar]
- 51.Soer R, Köke AJ, Vroomen PC, et al. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine (Phila Pa 1976) 2013;38:E562-8. 10.1097/BRS.0b013e31828af21f [DOI] [PubMed] [Google Scholar]
- 52.Dawson J, Fitzpatrick R, Murray D, et al. Comparison of measures to assess outcomes in total hip replacement surgery. Qual Health Care 1996;5:81-8. 10.1136/qshc.5.2.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson J, Fitzpatrick R, Murray D, et al. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63-9. 10.1302/0301-620X.80B1.0800063 [DOI] [PubMed] [Google Scholar]
- 54.Amstutz HC, Thomas BJ, Jinnah R, et al. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am 1984;66:228-41. 10.2106/00004623-198466020-00010 [DOI] [PubMed] [Google Scholar]
- 55.Mørup-Petersen A, Skou ST, Holm CE, et al. Measurement properties of UCLA Activity Scale for hip and knee arthroplasty patients and translation and cultural adaptation into Danish. Acta Orthop 2021;92:681-8. 10.1080/17453674.2021.1977533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 57.Plinsinga ML, Vuvan V, Maclachlan L, et al. Pain-related cognitions and emotional distress are not associated with conditioned pain modulation: an explorative analysis of 1142 participants with acute, subacute, and chronic pain. Pain 2023;164:1593-9. 10.1097/j.pain.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 58.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231-43. 10.1016/j.pain.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 59.Wilder-Smith OH, Schreyer T, Scheffer GJ, et al. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119-28. 10.3109/15360281003706069 [DOI] [PubMed] [Google Scholar]
- 60.Teunissen CE, Tumani H, Engelborghs S, et al. Biobanking of CSF: international standardization to optimize biomarker development. Clin Biochem 2014;47:288-92. 10.1016/j.clinbiochem.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 61.Peskind E, Nordberg A, Darreh-Shori T, et al. Safety of lumbar puncture procedures in patients with Alzheimer's disease. Curr Alzheimer Res 2009;6:290-2. 10.2174/156720509788486509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zorrilla-Vaca A, Mathur V, Wu CL, et al. The Impact of Spinal Needle Selection on Postdural Puncture Headache: A Meta-Analysis and Metaregression of Randomized Studies. Reg Anesth Pain Med 2018;43:502-8. 10.1097/AAP.0000000000000775 [DOI] [PubMed] [Google Scholar]
- 63.Tigchelaar C, Atmosoerodjo SD, van Faassen M, et al. The Anaesthetic Biobank of Cerebrospinal fluid: a unique repository for neuroscientific biomarker research. Ann Transl Med 2021;9:455. 10.21037/atm-20-4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petzold A, Sharpe LT, Keir G. Spectrophotometry for cerebrospinal fluid pigment analysis. Neurocrit Care 2006;4:153-62. 10.1385/NCC:4:2:153 [DOI] [PubMed] [Google Scholar]
- 65.Bateman RJ, Wen G, Morris JC, et al. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology 2007;68:666-9. 10.1212/01.wnl.0000256043.50901.e3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as