Abstract
Autologous vein is the optimal conduit for peripheral arterial bypass surgery, a standard recently highlighted by findings from the BEST-CLI trial. The Human Acellular Vessel is a novel biologic conduit produced using regenerative medicine technologies with structural and mechanical properties like a human blood vessel. Not yet approved by the United States Food and Drug Administration, the Human Acellular Vessel is being studied as an alternative bypass conduit in patients with peripheral arterial disease, vascular injury, and those in need of arteriovenous access for hemodialysis. This report describes and illustrates the technical aspects of intraoperative handling specific to the use of this new and innovative technology.
Keywords: Arterial reconstruction, Bioengineered vessel, Chronic limb-threatening ischemia, Critical limb-threatening ischemia, HAV, Human acellular vessel, Peripheral arterial disease, Regenerative medicine
Single-segment autologous saphenous vein is the optimal conduit for use in peripheral arterial bypass surgery, a standard recently underscored by findings from the BEST-CLI trial.1,2 For patients who do not have suitable vein to use as a bypass conduit, synthetic and biologic alternatives such as Dacron, polytetrafluoroethylene, and cryopreserved allografts are options. Unfortunately, these alternatives are not as good as autologous vein in terms of patency and durability, particularly for infrageniculate bypass targets. Additionally, synthetic conduits are not completely incorporated by the recipient and are prone to infection, which may obviate their use in the setting of tissue loss and gangrene.3 As shown in the BEST-CLI trial, the lack of a suitable bypass conduit places patients with critical limb ischemia at a high risk of amputation and reduced quality of life.4 Findings from BEST-CLI place a renewed emphasis on maximizing autologous conduit for bypass surgery and developing innovative technologies to improve the efficiency and effectiveness of limb salvage operations.
The Human Acellular Vessel (HAV), manufactured by Humacyte Inc, is a novel biologic produced in vitro by seeding human vascular smooth muscle cells onto a polyglycolic acid mesh scaffold within a bioreactor. After the growth process is complete, the HAV is decellularized to remove immunogenic potential, creating a biologically silent vessel with similar composition, biomechanics, and structure as native vascular tissue.5, 6, 7 Studies have demonstrated the propensity of the HAV to become populated by host cells and incorporated by surrounding soft tissue once it has been implanted, properties that portend resistance to infection and improved patency. This product requires only refrigeration; it does not need to be thawed and is thus immediately available “off the shelf.” Multi-center regulatory studies are ongoing, and data is being presented to the United States Food and Drug Administration (FDA) at this time.
In this report, we present instructive figures and videos that outline our approach to use of the HAV as a conduit for lower extremity arterial bypass. In the following cases, access to the HAV was attained through the FDA Expanded Access Program and an Investigational New Drug protocol approved and maintained at Mayo Clinic, Rochester, Minnesota.
Preoperative considerations
The HAV is an option for patients who require a lower extremity bypass and lack suitable autologous conduit and/or have infection or other anatomic patterns of disease obviating use of prosthetic material. Not yet approved by the FDA, at this time, the HAV is only available for use through an ongoing clinical trial or through the FDA Expanded Access Program.8, 9, 10 Although the vessel can be grown in various sizes or configurations, the HAV is currently available in a “one size fits most” 6-mm diameter by 40-cm length.7 The HAV is stored in a refrigerator at 2 °C to 8 °C and should not be frozen. The vessel can remain at room temperature for 48 hours once moved to a room-temperature environment. On the day of the operation, the outer carton of the HAV is dispensed from the pharmacy as a biologic to the surgical team in the operating theater. All other pre- and intraoperative procedures follow standard practice, and the operative sequence is generally the same as a conventional lower extremity arterial bypass. The following sections outline the steps specific to receipt, opening, preparation, and implantation of the HAV.
Intraoperative handling
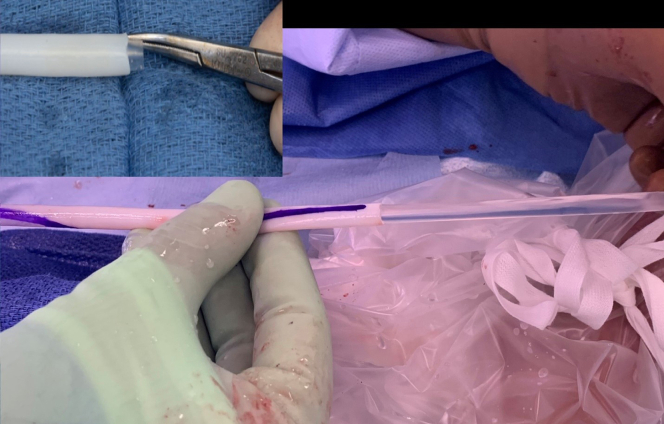
The sterile bioreactor bag containing the HAV is shipped within an outer non-sterile carton that can be discarded once the plastic package with the HAV is carefully placed onto the sterile operative field. Being mindful not to injure the vessel, the bioreactor bag is opened on the operating field with scissors, draining the sterile phosphate buffer solution within the container. The outer cover of the bioreactor bag is cut away and the HAV, which is secured to each end, is removed using scissors, leaving a several millimeters of the vessel on each end to be discarded with the rest of the sterile package (Supplementary Video 1, online only). Once the vessel is removed, a silastic mandril is gently removed from its inner lumen and discarded (Supplementary Video 2, online only; Fig 1). The video demonstrates the length of an HAV, illustrating the location and removal of the inner mandril, which is grasped with forceps or a mosquito hemostat and gently slid from the inner lumen. It is critical to ensure that this mandril has been removed from within the HAV and not inadvertently left in place and sutured when the vessel is implanted.
Fig 1.
Inner mandril removed from Human Acellular Vessel (HAV).
If two HAVs are required to obtain a required bypass length, the second vessel is prepared in a similar fashion, and the edges of the conduits spatulated and sewn end-to-end using standard anastomotic techniques and tools (Supplementary Video 3, online only). We perform this with minimal vessel handling and favor using fine (eg, 6-0 or 7-0) monofilament suture such as GORE-TEX or Prolene. As the HAV is new, we have chosen to use a slightly greater suture depth (ie, bigger “bites”) and smaller spacing than that for GSV, although experience to date suggests that the technical aspects of handling, clamping, and suturing are the same as vein or other commercially available conduits. Preclinical bench testing demonstrates excellent tensile strength of the HAV.
Following surgical exposure and control of the inflow and outflow target arteries, and prior to administering systemic heparin, a tunnel for the bypass is created using a sheathed device such as a Scanlon Vascular Tunneler (Supplementary Video 4, online only). Typically, the proximal anastomosis is performed next, following administration of intravenous heparin, using standard anastomotic techniques. Completing the proximal anastomosis prior to routing the vessel through the tunnel allows the conduit to be laid on top of the extremity to be closely inspected with pulsatile flow in its lumen (Fig 2). This sequence is necessary to visualize and assure hemostasis in the HAV-to-HAV anastomosis in cases where two vessels have been sewn together to attain bypass length.
Fig 2.
Confirming hemostasis at the Human Acellular Vessel (HAV)-to-HAV anastomosis.
Once the proximal anastomosis has been performed and the HAV(s) inspected with an arterial pulse, the top or “12:00 o’clock” position is marked prior to routing within the Scanlon Tunneler to assure proper orientation (ie, prevent twisting) as it is passed to the distal target vessel (Supplementary Video 5, online only). Supplementary Video 6 (online only) demonstrates the HAV being passed to the distal target: following placement of the tunneler, the end of the HAV is grasped, and gently passed or routed through the lumen of the tunneler from proximal to distal. As the vessel is pulled through, the proximal end is stabilized to prevent tension on the anastomosis or over-stretching of the HAV during routing and as the sheath of the tunneler is withdrawn. The distal anastomosis can next be performed using standard anatomic exposure and vascular control, opening, and suturing techniques. For tibial level bypass targets, leg exsanguination and use of tourniquet control has been used in several HAV bypass cases with good effect.
When the HAV needs to be clamped, this is performed using standard, atraumatic instruments such as DeBakey or padded Fogarty hydragrip clamps with no more pressure than needed to occlude flow (ie, minimum number of “clicks” needed to occlude flow) (Fig 3). As with autologous vein, care must be taken to not damage, over stretch, or crush the walls of the HAV. For a short tunnel, (eg, passing through the interosseous membrane to an anterior tibial target), the HAV can be grasped with a vascular clamp and brought through the tunnel (Supplementary Video 7, online only). The HAV handles in a manner like other biologics such as saphenous vein or CryoVein as demonstrated in the videos.
Fig 3.
Human Acellular Vessel (HAV) clamped gently with a padded Fogarty hydrogrip.
Intraoperative assessment of technical adequacy and flow of the HAV bypass can be performed with continuous wave Doppler, duplex ultrasound, electromagnetic flow probes, or completion arteriography. Other post-procedure management steps follow protocols or surgeon preference with no specific accommodation necessary for the HAV. The HAV requires no special anticoagulation, and patients are given standard chemoprophylaxis for deep venous thrombosis and antiplatelet therapy (ie, aspirin and or clopidogrel) beginning on the first postoperative day. Intravenous heparin and/or oral anticoagulants can be used in select cases based on complexity of the bypass operation, surgeon preference, or as indicated for coexisting cardiac or other disease processes.
In our experience with more than 25 HAV bypasses for CLTI in 3 years, the HAV tolerates the spectrum of open and endovascular reintervention procedures, including open thrombectomy and catheter-directed pharmacomechanical thrombolysis. For open redo operations, the HAV has been observed to be densely incorporated into the surrounding tissue and even covered by newly formed vasa vasorum. As a tubular structure or vessel, the HAV is amenable to dissection, open embolectomy, and to a patch angioplasty (Fig 4). Although our experience is limited to a few cases, endovascular reintervention using catheter directed thrombolysis, angioplasty, and even placement of a covered stent has been well tolerated by the HAV.
Fig 4.
Reintervention on Human Acellular Vessel (HAV). A, Graft thrombosis; B, Patency restored with thrombolysis and pharmacomechanical (AngioJet) thrombectomy; C, Anastomotic stenosis D, treated with angioplasty with completion angiogram demonstrating patent bypass with good outflow (E). Patient presented with a recurrent outflow stenosis; this then was treated with repeat thrombolysis and redo patch angioplasty of the distal anastomosis; F, redo exposure demonstrating HAV (blue arrow); G, Bovine pericardial patch angioplasty (yellow arrow) of the HAV-PT distal anastomosis.
Recent publications, including a phase II study in patients with peripheral arterial disease in non-US centers by Gutowski et al,11 report 12-month primary and secondary patency rates were 63% and 84%, respectively. Currently, the HAV development program includes evaluation of the 6-mm diameter vessel in seven clinical trials conducted in coordination with the FDA: four in patients receiving hemodialysis, two in patients with peripheral arterial disease, and one in patients with vascular injury.5,11, 12, 13, 14
Conclusion
The HAV is a readily available (ie, “off the shelf”) biologic with the potential to expand open bypass options in patients with critical limb-threatening ischemia and absent or unsuitable autologous vein. The biologic and infection-resistant properties of the HAV may also provide an alternative in scenarios of contamination or infection where the use of prosthetic conduits is ill-advised or contraindicated. Understanding the composition of the HAV and the steps in its preparation and implantation will prepare surgeons as additional clinical data emerges and regulatory approval is considered.
Author Contributions
Conception and design: AF, IS, PA, FS, TR
Analysis and interpretation: AF, IS, PA, FS, TR
Data collection: AF, IS, PA, FS, TR
Writing the article: AF, IS, PA, FS, TR
Critical revision of the article: AF, IS, PA, FS, TR
Final approval of the article: AF, IS, PA, FS, TR
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: TR
Footnotes
Author conflict of interest: T.E.R. has served in a paid clinical advisory capacity to Humacyte.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at https://www.jvscit.org.
Appendix
Additional material for this article may be found online at https://www.jvscit.org.
Appendix (online only)
Removing Human Acellular Vessel (HAV) from bioreactor bag.
Removing inner mandril from Human Acellular Vessel (HAV).
Creation of a spatulated end-to-end anastomosis using two Human Acellular Vessel (HAV) grafts.
Placement of a sheathed tunneler and creation of the proximal anastomosis.
Marking the top 12:00 o’clock position.
Passing the Human Acellular Vessel (HAV) through the sheathed tunneler after confirming hemostasis at the HAV-to-HAV anastomosis.
Passing the Human Acellular Vessel (HAV) through a shorter tunnel without a sheathed tunneler.
References
- 1.Almasri J., Adusumalli J., Asi N., Lakis S., Alsawas M., Prokop L.J., et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. 2018;68:624–633. doi: 10.1016/j.jvs.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 2.Farber A., Menard M.T., Conte M.S., Kaufman J.A., Powell R.J., Choudhry N.K., et al. Surgery or endovascular therapy for chronic limb-threatening ischemia. N Engl J Med. 2022;387:2305–2316. doi: 10.1056/NEJMoa2207899. [DOI] [PubMed] [Google Scholar]
- 3.Ambler G.K., Twine C.P. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2018;2:CD001487. doi: 10.1002/14651858.CD001487.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siracuse J.J., Rowe V.L., Menard M.T., Rosenfield K., Conte M.S., Powell R., et al. Relationship between WIfI (wound, ischemia, foot infection) stage and quality of life at revascularization in the BEST-CLI (best endovascular versus best surgical therapy in patients with chronic limb threatening ischemia) trial. J Vasc Surg. 2023;77:1099–1106.e4. doi: 10.1016/j.jvs.2022.11.050. [DOI] [PubMed] [Google Scholar]
- 5.Morrison J.J., McMahon J., DuBose J.J., Scalea T.M., Lawson J.H., Rasmussen T.E. Clinical implementation of the Humacyte human acellular vessel: implications for military and civilian trauma care. J Trauma Acute Care Surg. 2019;87(Suppl 1):S44–S47. doi: 10.1097/TA.0000000000002350. [DOI] [PubMed] [Google Scholar]
- 6.Dahl S.L.M., Koh J., Prabhakar V., Niklason L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. [PubMed] [Google Scholar]
- 7.Lauria A.L., Sen I., Rasmussen T.E. The human acellular vessel for vascular reconstruction or bypass: a novel biologic conduit for vascular bypass and repair. JAMA Surg. 2022;157:731–732. doi: 10.1001/jamasurg.2022.1214. [DOI] [PubMed] [Google Scholar]
- 8.A military-civilian perspective on real-world evidence to support regulatory decision making. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.raps.org/RAPS/media/news-images/Feature%20PDF%20Files/20-7_Rasmussen_RWE.pdf Available at:
- 9.Expanded Access to Experimental Biologics. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/expanded-access-experimental-biologics Available at:
- 10.Lauria A.L., Kersey A.J., Propper B.W., Twerdahl E.H., Patel J.A., Clouse W.D., et al. Preliminary experience with the human acellular vessel: a descriptive case series detailing early use of a bioengineered blood vessel for arterial repair. Ann Vasc Surg. 2022;87:100–112. doi: 10.1016/j.avsg.2022.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Gutowski P., Gage S.M., Guziewicz M., Ilzecki M., Kazimierczak A., Kirkton R.D., et al. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056. [DOI] [PubMed] [Google Scholar]
- 12.Kakisis J.D., Liapis C.D., Breuer C., Sumpio B.E. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Kirkton R.D., Santiago-Maysonet M., Lawson J.H., Tente W.E., Dahl S.L.M., Niklason L.E., et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11:eaau6934. doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson J.H., Glickman M.H., Ilzecki M., Jakimowicz T., Jaroszynski A., Peden E.K., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Removing Human Acellular Vessel (HAV) from bioreactor bag.
Removing inner mandril from Human Acellular Vessel (HAV).
Creation of a spatulated end-to-end anastomosis using two Human Acellular Vessel (HAV) grafts.
Placement of a sheathed tunneler and creation of the proximal anastomosis.
Marking the top 12:00 o’clock position.
Passing the Human Acellular Vessel (HAV) through the sheathed tunneler after confirming hemostasis at the HAV-to-HAV anastomosis.
Passing the Human Acellular Vessel (HAV) through a shorter tunnel without a sheathed tunneler.