Abstract
Single-cell transcriptomics are powerful tools to define neuronal cell types based on co-expressed gene clusters. Limited RNA input in these technologies necessarily compromises transcriptome coverage and accuracy of differential expression analysis. We propose that bulk RNA-Seq of neuronal pools defined by spatial position offers an alternative strategy to overcome these technical limitations. We report a laser-capture microdissection (LCM)-Seq method which allows deep transcriptome profiling of fluorescently tagged neuron populations isolated with LCM from histological sections of transgenic mice. Mild formaldehyde fixation of ZsGreen marker protein, LCM sampling of ∼300 pooled neurons, followed by RNA isolation, library preparation and RNA-Seq with methods optimized for nanogram amounts of moderately degraded RNA enabled us to detect ∼15,000 different transcripts in fluorescently labeled cholinergic neuron populations. The LCM-Seq approach showed excellent accuracy in quantitative studies, allowing us to detect 2891 transcripts expressed differentially between the spatially defined and clinically relevant cholinergic neuron populations of the dorsal caudate-putamen and medial septum. In summary, the LCM-Seq method we report in this study is a versatile, sensitive, and accurate bulk sequencing approach to study the transcriptome profile and differential gene expression of fluorescently tagged neuronal populations isolated from transgenic mice with high spatial precision.
Keywords: spatial transcriptomics, laser-capture microdissection, basal forebrain, cholinergic, RNA-Seq, gene expression, formaldehyde, transcriptome profiling
Genome-wide quantification of mRNA transcripts is a powerful approach to characterize cellular states and molecular circuitries. High-throughput next-generation RNA-Seq increasingly replaces hybridization-based microarray technologies (1) given its much better dynamic range, higher reproducibility, and increased accuracy in differential expression analysis of both high- and low-abundance transcripts (2).
Spatially resolved transcriptomics includes a growing variety of RNA-Seq technologies, which preserve topographic information on the analyzed cells (3, 4, 5, 6, 7). Considering the extraordinary diversity of brain cell types, retaining the precise anatomical context is particularly important in neuroscience (8). Spatial transcriptomic methods are often complementary, with various inherent strengths and trade-offs in anatomical resolution, target coverage, and throughput. MERFISH (9) and other in situ single-cell transcriptomics (single-cell RNA-Seq [scRNA-Seq]), which use microscopic analysis of multiplexed FISH signals, provide unparalleled anatomical precision, with a necessary compromise in the detection of low-abundance transcripts. A large number of scRNA-Seq techniques use simultaneous ex situ sequencing of thousands of dispersed cells (10, 11, 12, 13). While this is an efficient approach to reveal neuronal heterogeneity and define cell types (14, 15), the limited amount of RNA in single cells (16) compromises detection sensitivity, accuracy, and precision and reduces the statistical power of differential gene expression analysis (10, 11, 12).
Bulk sequencing of neuronal pools can overcome several limitations of scRNA-Seq methods by increasing the amount of starting RNA (11, 17, 18). Use of laser-capture microdissection (LCM) (19) to collect a spatially defined cell population from frozen tissue sections, coupled to RNA-Seq (LCM-Seq) (17, 20, 21) is a powerful strategy to obtain a transcriptomic snapshot from anatomically similar cell populations. Accordingly, application of morphometric selection criteria for LCM has recently allowed us to carry out deep transcriptome profiling of spiny projection neurons and cholinergic interneurons (ChINs) from the human putamen (17). Adaptation of the LCM-Seq technology to neuronal populations expressing specific neurochemical phenotype markers remains technically challenging and unresolved.
To address an unmet need, here, we introduce a versatile LCM-Seq method for deep transcriptome analysis of spatially defined neuron populations expressing a fluorescent marker protein. Use of ZsGreen reporter protein fixed mildly with formaldehyde, LCM sampling of >300 pooled neurons, and RNA isolation, library preparation, and RNA-Seq methods optimized for nanogram amounts of moderately degraded RNA enabled us to detect ∼15,000 different transcripts in distinct cholinergic neuron populations of transgenic mice. Beyond this high sensitivity, the LCM-Seq method showed excellent accuracy and precision in quantitative studies, allowing us to identify 2891 genes expressed differentially between the spatially defined cholinergic neuron populations of the dorsal caudate-putamen (CPU) and the medial septum (MS), two clinically important cell types in various neurodegenerative disorders.
Results
ZsGreen fixed lightly with formaldehyde serves as an optimal fluorescent marker for LCM-based cell harvesting
Fluorescent proteins expressed in transgenic mice are widely used neuronal phenotype markers. Harvesting fluorescent neurons with LCM for downstream RNA applications, such as RT-qPCR, microarray, and RNA-Seq, requires stabilization of the fluorescent signals with the crosslinking fixative formaldehyde (18, 22, 23). As the first step toward an optimized LCM-Seq strategy, we compared the fluorescence stability and signal intensity of formaldehyde-fixed tdTomato and ZsGreen proteins expressed selectively in cholinergic systems. Both choline acetyltransferase (ChAT)-tdTomato and ChAT-ZsGreen mice contain the marker transgene in the ROSA 26 locus and use the CAG promoter and a loxP-flanked STOP cassette to drive fluorescent protein expression similarly in the two strains. In accordance with previous observations using GFP (18, 22, 23), the fluorescence of tdTomato vanished entirely from unfixed cryostat sections mounted and dried on microscope sides (Fig. 1A). To preserve signals, we perfused the vasculature of ChAT-tdTomato and ChAT-ZsGreen transgenic mice (N = 3/group) transcardially with either 0.5%, 2%, or 4% formaldehyde, followed by 20% sucrose. Fixed forebrain sections prepared with a cryostat were mounted on microscope slides and analyzed with fluorescence microscopy. We found that tdTomato fluorescence remained extremely weak using 0.5% formaldehyde, with very little improvement using 2% or 4% fixative. In contrast, all three formaldehyde concentrations preserved sufficiently the bright ZsGreen signal, allowing subsequent visualization and isolation of the labeled cholinergic neurons using LCM (Fig. 1B).
Figure 1.
LCM-Seq protocol developed for whole-transcriptome profiling of spatially resolved cholinergic neuron populations.A, loss of the tdTomato signal from the unfixed dorsal CPU section of a ChAT-tdTomato mouse. Endogenous fluorescence of ChINs entirely vanishes during tissue drying. B, effect of fixation on marker protein fluorescence. Microscopic analysis of sections obtained from the dorsal CPU of ChAT-tdTomato and ChAT-ZsGreen transgenic mice provides evidence for superior signal intensity of formaldehyde (FA)-fixed ZsGreen over tdTomato, irrespective of fixative concentration. C, bioanalyzer measurements on RNA isolated from 500 pooled ChINs. D, random primer-based RNA-Seq library preparation from 330 MS cholinergic neurons (MS330) and 300 dorsal CPU ChINs (CPU300) occupying equal section areas. Additional libraries from 30 ChINs (CPU30) are prepared to investigate how limited RNA input affects sequencing quality. E, illumina sequencing and bioinformatic analysis of two spatially distinguished cholinergic neuron populations. Quality control (QC), transcriptome profiling, and differential expression analysis are followed by neuroanatomical and functional confirmation of unique receptorial mechanisms revealed in the two distinct cell types. (Lower photomicrograph has been reused in Fig. 5A). The scale bars represent 25 μm (A and B, and high-power images in D), 100 μm (low-power images in D) and 10 μm (E). ChAT, choline acetyltransferase; ChIN, cholinergic interneuron; CPU, caudate-putamen; LCM, laser-capture microdissection.
ChAT-ZsGreen neurons collected with LCM provide ∼4 pg/cell total RNA with a RIN value of ∼5.5
To minimize fixation-related RNA damages (24), we optimized further the LCM-Seq protocol using ChAT-ZsGreen brains fixed lightly with 0.5% formaldehyde. Twelve-micrometer-thick coronal sections of three mice were mounted on polyethylene naphthalate (PEN) membrane slides. Fluorescent ChINs were microdissected individually and collected from the dorsal CPU with LCM (Fig. 1C). To estimate the amount and quality of total RNA recovered from LCM-isolated neurons, we prepared RNA samples (N = 3) from 500 pooled neurons with the Arcturus Paradise PLUS FFPE RNA Isolation Kit (Thermo Fisher Scientific). RNA yield we determined with Bioanalyzer analysis was 1.9 ± 0.5 ng (∼3.8 ± 1 pg/neuron), and RNA integrity number (RIN) used as a sample quality measure was 5.5 ± 0.4 (mean ± SEM) (Fig. 1C).
Preparation of RNA-Seq libraries from LCM-isolated neurons combines rRNA removal and random primer-based reverse transcription
Orthodox RNA-Seq library preparation protocols rely on the reverse transcription of mRNA with oligo(dT) primers, which becomes inefficient using low-quality and low-quantity input RNA (25). Available commercial kits optimized for low amounts and fragmented RNA require inventive hybridization–based removal of rRNA or complementary DNA (cDNA) sequences with highly efficient random primer-based reverse transcription of RNA transcripts (25, 26). From a variety of approaches available (25), we chose to prepare cholinergic cDNA libraries with the TruSeq Stranded Total RNA Library Preparation Gold kit (Illumina), which is compatible with sequencing of formaldehyde-fixed material (17, 18, 27) (Fig. 1D). To exceed safely the cDNA sample requirements of Illumina sequencing, we increased the number of amplification cycles from 15 to 16, while preparing libraries from 330 LCM-isolated basal forebrain cholinergic cells of the MS (MS330; N = 4) and 300 microdissected ChINs of the dorsal CPU (CPU300; N = 4). Choosing these cell numbers ensured that MS and dorsal CPU neurons occupied identical section areas (CPU300: 66,476 ± 1796 μm2; MS330: 67,666 ± 1569 μm2). PEN membranes of the same total area with no tissue were also microdissected and used as a negative control to assess possible nucleic acid contamination in the cDNA library. To also address the impact of limited RNA input on sequencing quality, we generated and studied additional libraries (CPU30; N = 4) from 30 microdissected dorsal CPU ChINs, using 19 PCR cycles for fragment enrichment (Fig. 1D). The amount of cDNA in the 12 amplified cholinergic samples varied between 278 to 5933 pg/μl. Following RNA isolation and library preparation from empty PEN membranes, no cDNA was observed on the DNA electropherograms, confirming that libraries to be sequenced are derived from the microdissected cells (Fig. S1). Twenty microliters of a 1 nM library mix representing proportionally the indexed cholinergic cDNA samples was subjected to single-end sequencing with the Illumina NextSeq500/550 High Output kit (v2.5; 75 Cycles) and an Illumina NextSeq500 instrument (Fig. 1E).
LCM-Seq studies of 300 to 330 microdissected neurons allow the identification of ∼15,000 different transcripts in two spatially defined cholinergic neuron populations
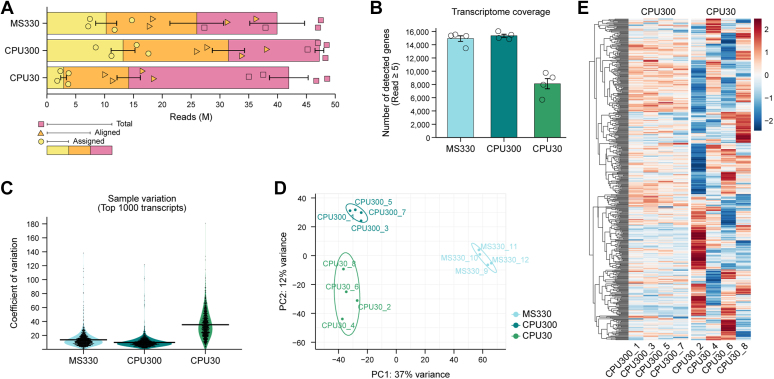
Sequencing of the twelve RNA-Seq libraries generated 516.5 M raw reads (27.3 M–48.6 M per sample). After trimming with Trimmomatic and adapter removal with Cutadapt, mapping to the mouse reference genome (Ensembl; release 107) with STAR (v 2.7.9a; https://github.com/alexdobin/STAR) resulted in 26 ± 4.7 M and 31.5 ± 2.8 M aligned reads in the MS330 and CPU300 samples (Fig. 2A), respectively, from which 10.3 ± 1.8 M MS330 reads and 13.2 ± 2.1 M CPU300 reads were assigned to unique genes (Fig. 2A), and quantified with featureCounts (subread v 2.0.2). For gene body coverage and read distribution between exons, introns, and intergenic regions, see Fig. S2. LCM-Seq studies of the 300 to 330 pooled cholinergic neurons allowed us to detect 14,926 ± 422 different transcripts in MS330 and 15,353 ± 231 transcripts in CPU300 samples (cut-off: five reads/sample; Fig. 2B). All transcripts identified in MS and dorsal CPU cholinergic neurons and selected functional categories, such as ion channels, transporters, transcription factors, and receptors (defined in the Kyoto Encyclopedia of Genes and Genomes [KEGG] BRITE database) are reported in Table S1.
Figure 2.
Quality assessment of LCM-Seq results.A, number of total reads, reads alignable to the mouse reference genome (Ensembl; release 107) and reads assigned to unique genes. While total reads are distributed evenly among the cosequenced 12 samples, there is a large drop in both aligned and assigned reads using RNA harvested from 30 (CPU30 samples), instead of 300 to 330 pooled cholinergic neurons (CPU300 and MS330 samples; N = 4 for each). B, effect of RNA input on library complexity. The 2.9 to 13.2 M assigned reads allow the identification of ∼15,000 different transcripts (raw reads ≥ 5) in the MS330 and CPU300 transcriptomes. For full listing and categorization of these transcripts, see Table S1. The number of genes detected with the same cut-off is compromised substantially in the CPU30 (N = 4) transcriptomes (∼8100 transcripts). C, CV for the most highly expressed 1000 transcripts in each library. Violin plot illustrates enhanced sample variability in the CPU30 (mean CV: 35.4) versus the MS330 (mean CV: 13.7) and the CPU300 (mean CV: 9.83) transcriptomes. D, principal component plot. Cell type–dependent differences (MS versus dorsal CPU neurons) are best reflected in clear separation of the MS330 from both the CPU300 and the CPU30 transcriptomes in PC1. Difference between CPU30 and CPU300 in PC2 reveals a library amplification bias due to low amounts of starting RNA in the former. Note also the reduced clustering of the CPU30 transcriptomes. E, heat map of the top 1000 transcripts identified in the CPU300 (N = 4) and CPU30 (N = 4) transcriptomes. Enhanced CPU30 sample variability indicates largely reduced detection accuracy and precision. CPU, caudate-putamen; LCM, laser-capture microdissection; MS, medial septum.
Limited RNA input compromises sequencing quality, library complexity, and detection accuracy
While bulk sequencing of 300 to 330 microdissected neurons ensured excellent transcriptome coverage, we found that several qualitative parameters of sequencing were compromised when the number of pooled neurons was reduced to 30. Accordingly, the number of reads aligned to the mouse genome (Fig. 2A), assigned to unique genes (Fig. 2A), and mapped to exonic sequences (Fig. S2B) were dropped in the CPU30 transcriptomes. The number of different transcripts (cut-off: raw reads ≥5) decreased from 15,353 ± 231 in the CPU300 to 8118.25 ± 768 in the CPU30 transcriptomes (Fig. 2B), indicating reduced library complexity. Limited RNA input was also harmful for detection accuracy. The coefficient of variation increased from 9.83 (mean of the most abundant 1000 transcripts) in the CPU300 to 35.4 in the CPU30 transcriptomes (Fig. 2C). Further, CPU30 transcriptomes exhibited impaired clustering in the principal component analysis plot and differed from the CPU300 transcriptomes in PC2 (Fig. 2D). Along with an increased color variability of heat maps (Fig. 2E), these observations indicated that the low-RNA input introduced amplification biases and unwanted variations in the CPU30 libraries.
Genes implicated in Alzheimer’s disease and Parkinson’s disease are highly enriched in cholinergic neurons
Cholinergic systems are susceptible to degeneration in Alzheimer’s disease (AD) and Parkinson’s disease (PD). Analysis of Human Disease Ontology databases revealed an interesting enrichment of AD and PD genes in the MS330 and CPU300 transcriptomes. Half of the genes listed in AD (N = 156) and PD (N = 133) databases were present (mean reads > 5) and ∼27 to 35% were highly expressed (>50 CPM) in the MS330 and CPU300 transcriptomes (Fig. S3).
Human and murine striatal ChINs exhibit conserved receptor profiles
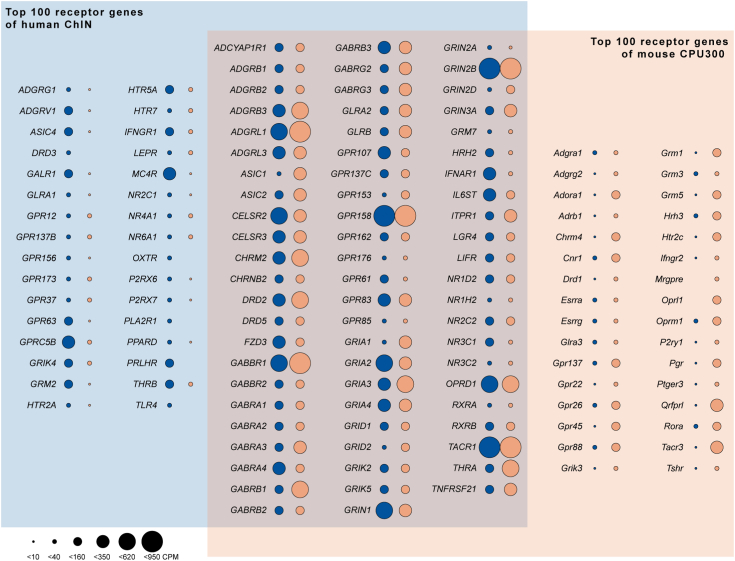
We have reported recently the detailed gene expression profile of LCM-isolated human ChINs identified by size in cresyl violet–stained putamen sections (17). This allowed us to address similarities/differences between the functionally analogous striatal neurons of the two species. The most abundant 100 receptor transcripts (identified based on the KEGG BRITE database), including the Drd2 and Drd5 dopaminergic receptor isoforms, were expressed at strikingly similar levels in humans and mice (Fig. 3). This finding revealed evolutionarily conserved regulatory mechanisms acting in striatal ChINs. The few exceptions showing species-specific expression included Drd3 in human and the opioid receptor Oprl in murine ChINs (Fig. 3 and Table S2).
Figure 3.
Receptor expression landscape of striatal ChINs from the human putamen and the mouse dorsal CPU. ChINs of the human putamen (blue field) (17) and the murine dorsal CPU (orange field) exhibit striking similarities in receptor expression profiles. Venn diagram illustrates the most abundant 100 receptors (KEGG BRITE database) from each species. Dark blue (human) and dark orange (mouse) dot areas change in proportion to transcript abundances (counts per million [CPM] units). Items in the overlapping area occur among the top 100 ChIN receptors in both species. Note obvious species similarities in all three fields and the few receptors detected only in human or murine ChINs. See also Table S2. ChIN, cholinergic interneuron; CPU, caudate-putamen; KEGG, Kyoto Encyclopedia of Genes and Genomes.
The MS330 and CPU300 transcriptomes contain 2891 differentially expressed transcripts
Both the MS330 and the CPU300 transcriptomes contained cholinergic as well as GABAergic neuronal phenotype markers (Fig. 4A). This finding indicated simultaneous use of acetylcholine and GABA for neurotransmission at least by subsets of cholinergic neurons, confirming earlier reports by others (28, 29). Differential expression analysis of the two transcriptomes using the DESeq2 R-package with RUVSeq normalization revealed 2891 transcripts with significantly different levels (p.adj. < 0.05) between MS and dorsal CPU neurons (Fig. 4B and Table S3). Homogenous heat maps of these transcripts (Fig. 4C) indicated excellent accuracy and precision of the LCM-Seq approach for studies of differential gene expression.
Figure 4.
Differential gene expression analysis of cell type–specific regulatory mechanisms acting in MS and dorsal CPU cholinergic neurons.A, expression of known cholinergic and GABAergic phenotype markers in each cell type. B, differentially expressed transcripts illustrated in a volcano plot. Genes with |log2 fold change (FC)|>12 and -log10 p.adj. >150 are tagged with gene symbols. C, heat map of 2891 transcripts expressed at significantly different levels in the MS330 and CPU300 transcriptomes (p.adj. < 0.05). D, Tukey’s box plots with examples of differentially expressed ion channels, transporters, transcription factors, and receptors. For full listing, see Table S3. Abundant expression of Drd2 only in dorsal CPU and Trhr only in MS cholinergic neurons forecast differential responsiveness of the two cell types to D2 agonists and to TRH, respectively. CPU, caudate-putamen; MS, medial septum; TRH, thyrotropin-releasing hormone.
MS and dorsal CPU cholinergic neurons have unique ion channel, transporter, transcription factor, and receptor profiles
Cholinergic projection neurons of the MS and the diagonal band of Broca give rise to the septohippocampal pathway. This connection plays a critical role in hippocampal theta rhythms and memory encoding, among other functions (30, 31). Cholinergic neurons of the dorsal CPU, in turn, contribute as local interneurons to the regulation of cortico-striato-thalamocortical neural pathways and are involved in motor control, learning, language, reward, cognitive functioning, and addiction (32). Different regulatory functions of MS and dorsal CPU neurons were reflected in 108 ion channels, 111 transporters, 140 transcription factors, and 81 receptors among the 2891 transcripts we found to be expressed differentially (p.adj. < 0.05) between the MS330 and CPU300 transcriptomes (Fig. 4D and Table S3). Using Synaptic Gene Ontology pathway analysis, we identified hundreds of differentially expressed transcripts related to various aspects of synaptic neurotransmission (Fig. S4 and Table S3).
Cell type–specific receptor profiles are in line with the distinct functional properties of MS and dorsal CPU cholinergic neurons
Differential expression analysis revealed selective presence of the Drd1, Drd2, and Drd5 dopamine receptor isoforms in dorsal CPU cholinergic neurons (Fig. 4D), suggesting that dopamine plays a key role in the afferent control of this cell group. Indeed, the immunofluorescent detection of the dopaminergic marker enzyme tyrosine hydroxylase (TH) revealed a dense dopaminergic fiber network in the dorsal CPU. In contrast, we could only label scattered TH fibers in tissue sections of the MS (Fig. 5A). We used whole-cell current-clamp studies of ChAT-ZsGreen neurons to address the differential responsiveness of the two cholinergic cell types to a D2 receptor agonist. Corroborating distinct Drd2 expression profiles and TH innervation patterns, a single bolus of the D2 agonist sumanirole (30 μM) decreased the rate of cholinergic neuron firing in the dorsal CPU to 25.7 ± 5.8% (mean ± SEM) of the control. Wash-out started to occur ∼4 to 6 min later. Sumanirole had no effect on the mean firing frequency of MS cholinergic neurons (108.6 ± 7.5% of the control frequency) (Fig. 5, B and C and Table S4), in accord with the extremely low Drd2 expression in this cell type (Fig. 4D). Among the differentially expressed receptors, Trhr encoding the Type-1 thyrotropin-releasing hormone (TRH) receptor showed the opposite distribution pattern, with an overwhelming expression in the MS330 transcriptome (Fig. 4D). TRH responsiveness of this cell type was supported further by the high density of neuronal fibers immunoreactive to proTRH in the MS (Fig. 5D). Interestingly, a subset (8.4 ± 2.1%) of MS cholinergic neurons showed immunoreactivity to proTRH (Fig. S5, A and B). Indeed, Trh mRNA expression could be verified in the MS330 transcriptome (Fig. S5C and Table S1). Identification of endogenous TRH and Trhr in a subset of MS cholinergic neurons raised the intriguing possibility of an autocrine regulatory mechanism acting in these cells. Cholinergic neurons in the dorsal CPU, unlike in the MS, were not surrounded by TRH immunoreactive fibers (Fig. 5D). Together with the low level of Trhr expression in this cell type, this suggested that TRH does not play an important role in the afferent regulation of ChINs.
Figure 5.
Anatomical and functional support for differential responsiveness of MS and dorsal CPU cholinergic cell types to a D2 agonist and to TRH.A, confocal microscopic analysis of ChAT-ZsGreen cholinergic neurons (green) and dopaminergic fibers immunoreactive to tyrosine hydroxylase (TH; magenta). While dopaminergic axons are scarce in the MS, they form a dense network around ChAT-ZsGreen neurons of the dorsal CPU, suggesting strong dopaminergic control of the latter cell type (high-power insets; Upper photographic inset has been reused from Fig. 1E). B and C, results of whole-cell patch clamp recordings from MS and dorsal CPU cholinergic neurons treated with a single bolus of the selective D2 receptor agonist sumanirole (30 μM). While representative traces (B) and statistical analyses (C) reveal no change in the mean firing frequency of MS cholinergic neurons, sumanirole causes robust inhibition of dorsal CPU cholinergic neurons. These observations are in line with high expression of Drd2 only in the latter cell group. D, simultaneous detection of ChAT-ZsGreen cells (green) and TRH immunoreactive axons (magenta). TRH axons are present in high numbers and surround cholinergic neurons in the MS, whereas they occur rarely in sections of the dorsal CPU (high-power insets). E and F, electrophysiological responses of MS and dorsal CPU cholinergic neurons to TRH in representative traces (E) and in statistics (F). A single bolus of TRH causes robust excitation of cholinergic neurons in the MS, without affecting the mean firing rate of ChINs in the dorsal CPU. This observation is in accord with the selective RNA-Seq detection of Trhr in the former cell group. ∗p < 0.05 versus ctrl with Student’s t test. Vertical arrows in (B) and (E) show application of sumanirole or TRH, respectively, whereas the horizontal arrows indicate wash-out of the drug effects. For statistics, see Table S4. The scale bars represent 250 μm in (A) and (D) and 7.5 μm in all high-power insets. ChAT, choline acetyltransferase; CPU, caudate-putamen; MS, medial septum; TRH, thyrotropin-releasing hormone.
Electrophysiological studies established that a single bolus of TRH (10 μM) increased the firing rate of MS cholinergic neurons to 230.7 ± 47.2% of the control rate. Wash-out of this excitatory effect became obvious in ∼5 to 7 min. The mean firing rate of dorsal CPU cholinergic neurons, Trhr expression, remained unaffected by TRH (91.7 ± 7.1% of the control rate) (Fig. 5, E and F and Table S4). Altogether, these immunohistochemical and electrophysiological observations provided functional support for different regulatory mechanisms acting in the two spatially defined cholinergic cell types.
Discussion
In this study we are reporting a versatile LCM-Seq method developed for deep transcriptome profiling of fluorescently-tagged neuron populations isolated with LCM from histological sections of transgenic mice. Key findings are that, (i) light formaldehyde fixation preserves the bright fluorescence of expressed ZsGreen, but not tdTomato, during tissue preparation; (ii) ChAT-ZsGreen neurons isolated with LCM from 12-μm-thick sections of the dorsal CPU provide ∼4 pg/cell total RNA with only minimally compromised integrity (RIN: ∼5.5); (iii) bulk RNA-Seq of RiboZero libraries prepared from 300 to 330 LCM-isolated cholinergic neurons (∼1.2 ng total RNA) can reliably identify ∼15,000 different transcripts with sufficient accuracy and precision to allow differential expression analysis of the two spatially defined cholinergic cell types of the MS and the dorsal CPU; and finally, (iv) both detection sensitivity and accuracy of RNA-Seq become compromised using only 30 neurons to prepare the libraries.
This versatile LCM-Seq method has unique advantages over various scRNA-Seq technologies (10, 11, 12, 13) due to the use of ng, instead of pg (16) amounts of RNA, which can substantially improve detection sensitivity, accuracy, and precision (11). Conversely, we have shown formally that the number of the identified transcripts drops from ∼15,000 to ∼8,100, the CV for the top 1000 transcripts increases and PCR amplification of the cDNA libraries introduces biases to the LCM-Seq results if RNA samples are prepared from 30, instead of 300 to 330 pooled neuronal cells. We also have to point out that a general compromise of LCM-Seq is the loss of information about cellular heterogeneity within the cosequenced cell pool. Murine ChINs also exhibit variable anatomical, functional, and developmental characteristics (28) and the GABAergic phenotype characterizes the Lhx6-positive population making up ∼53% of ChINs (29).
Fluorescence-activated cell sorting (FACS) of dispersed living neurons (33) offers an alternative protocol to LCM for cell enrichment. One major advantage of LCM over FACS is the superior anatomical precision of sample collection. In addition, the LCM-Seq approach provides a functional snapshot on the transcriptome, whereas the lengthy FACS protocol may allow unwanted transcriptomic changes to occur. Stabilizing the transcriptome with our approach will be critically important in future studies of differential gene expression. Accordingly, the LCM-Seq method has recently allowed us to identify ∼2300 significant changes in the kisspeptin neuron transcriptome of gonadectomized female mice in response to estrogen treatment (18).
Alcohol-based precipitating (molecular) fixatives were unable to preserve the signals of expressed fluorescent proteins in our pilot studies, and the use of formaldehyde in the LCM-Seq protocol was inevitable. Although this crosslinking fixative was reported to reduce RNA quality and recovery (34) and to interfere with the RT-PCR amplification of RNA extracts (24), the amount (∼1.2 ng) and integrity (RIN∼5.5) of RNA we were able to isolate from ∼300 microdissected neurons satisfied the requirements of library preparation protocols optimized for low-quality/low-quantity RNA samples (25, 26). The TruSeq Stranded Total RNA Library Preparation Gold kit we chose here relies on the RiboZero technology to remove rRNA, followed by reverse transcription of RNA with random primers and cDNA fragment enrichment with PCR. This procedure requires >100 ng RNA for optimal results according to the manufacturer (Illumina). However, it was tested and reported to perform very well using 5 to 10 ng and well using 1–2 ng degraded RNA (26). Our decision to pool ∼300 neurons for the LCM-Seq method relies on practical considerations: (i) ∼300 pooled dorsal CPU neurons were enough to provide >1 ng total RNA, the minimal amount tested successfully by other investigators (26); (ii) collecting thousands of neurons within a workday with LCM would be technically infeasible; (iii) preparation of libraries from 30 neurons greatly reduced detection sensitivity and precision; (iv) genetically tagged neuron populations with a strictly defined topography often consist of a few hundred microdissectable cells only (18, 23).
In this study, we used the new LCM-Seq method to characterize the transcriptome landscape of two clinically important cholinergic neuron populations in the MS and the dorsal CPU, respectively. Information we are reporting on ∼15,000 expressed genes in each cell type contribute to our understanding of the molecular regulation of the two distinct neuronal systems in normal and disease conditions. Combined results of RNA-Seq, immunofluorescence, and electrophysiological studies supported the critical involvement of dopamine and specifically, the most abundant dopaminergic receptor, D2 in the inhibitory control of striatal ChINs. Future LCM-Seq studies of ChINs will be able to address the molecular consequences of losing this dopaminergic regulation in animal models of PD.
Important functions associated with cholinergic neurons of the MS and other basal forebrain regions include cortical activation, affect, attention, sensory coding, motivation, and memory, and the demise of this system has been strongly implicated in the pathophysiology of AD (31). Basal forebrain cholinergic neurons consist of heterogeneous subpopulations which differ in cortical, amygdala, and hippocampal projections as well as in sources and neurochemistry of their synaptic afferentation (31). LCM-Seq studies of subpopulations will require the identification and selective transgenic expression of marker genes for distinct cell types. In this study, we carried out transcriptome profiling of ChAT-ZsGreen neurons from the MS. Out of ∼15,000 expressed transcripts, we selected Trhr for anatomical and electrophysiological studies and provided evidence for a strong excitatory regulation of MS cholinergic neurons by TRH. The unexpected finding of TRH cosynthesis in a subset of cholinergic neurons indicates that some of the receptor ligand may originate from endogenous sources. TRH agonists emerged as promising therapeutics to restore cholinergic functions in various neurodegenerative disorders (35, 36). The well-established analeptic effects of TRH were attributed, at least partly, to TRH activation of the septo-hippocampal cholinergic pathway over 40 years ago (37). Our study now provides solid molecular, anatomical, and functional support to the role of TRH signaling in the excitatory regulation of the septo-hippocampal cholinergic pathway.
In conclusion, this versatile LCM-Seq method allowed deep transcriptome profiling of fluorescently tagged neuron populations isolated with LCM from histological sections of transgenic mice. Bulk sequencing of neuronal pools defined by identical spatial position showed excellent sensitivity as well as accuracy and precision in quantitative studies on the clinically relevant cholinergic neuron populations isolated from the MS and the dorsal CPU regions.
Experimental procedures
Animals
Genetically modified postnatal day 20–30 and young adult (postnatal day 60–90) male mice (N = 32) were housed under standard conditions (lights on between 0600 and 1800 h, temperature 22 °C ± 1 deg. C, chow and water ad libitum) in the animal facility of the Institute of Experimental Medicine. ChAT-tdTomato and ChAT-ZsGreen transgenic mice were generated by crossing male ChAT-IRES-Cre knock-in mice (Jackson Laboratory, Bar Harbor, ME; RRID: IMSR_JAX:006410) with female B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Jackson Laboratory, IMSR_JAX:007914) and Ai6(RCL-ZsGreen) (Jackson Laboratory, IMSR_JAX:007906) fluorescent indicator strains, respectively. The resulting two lines showed fluorescent labeling restricted to known cholinergic systems, including basal forebrain cholinergic neurons of the MS-diagonal band of Broca complex and large ChINs of the dorsal CPU.
Microscopic assessment of fluorescent protein signals in formaldehyde-fixed sections
ChAT-tdTomato (N = 3) and ChAT-ZsGreen (N = 3) mice heterozygous for both the Cre and the indicator gene alleles were anesthetized with a cocktail of ketamine (25 mg/kg), xylavet (5 mg/kg), and pipolphen (2.5 mg/kg) in saline and perfused transcardially with 80 ml of ice-cold 0.5%, 2%, or 4% formaldehyde (prepared freshly from paraformaldehyde) in 0.1 M PBS (0.1 M; pH 7.4), followed by 50 ml of ice-cold 20% sucrose solution in PBS. The brains were removed, snap-frozen in powdered dry ice, and stored at −80 °C until sectioned at 12 μm in the coronal plane with a Leica CM1860 UV cryostat (Leica Biosystems). Sections corresponding to Atlas plates 20 to 26 of Paxinos (Bregma +1.34 to + 0.62 mm) (38) were thaw-mounted on glass slides. Fluorescent images of dorsal CPU cholinergic neurons were captured with a Zeiss AxioImager M1 microscope (Carl Zeiss) using a 40× objective lens, filter sets for fluorescein isothiocyanate or rhodamine, an AxioCam MRc5 digital camera (Carl Zeiss), and the AxioVision Se64 Rel.4.9.1 software (https://www.micro-shop.zeiss.com/en/de/softwarefinder/software-categories/zen-core/). The digital photomicrographs were prepared using identical manual exposure settings for all ChAT-tdTomato and ChAT-ZsGreen specimens fixed with varying formaldehyde concentrations. Editing with the Adobe Photoshop CS5 software (Adobe System Inc; https://www.adobe.com/products/photoshop.html) was applied to the composite table to maintain technical variations between samples. To address the possibility that unfixed tdTomato maintains its fluorescence during tissue preparation for LCM, dorsal CPU sections were prepared and studied from the unfixed brain of a ChAT-tdTomato mouse.
Transcriptomic studies
Section preparation for LCM
For all experiments, reagents were of molecular biology grade. Buffers were pretreated overnight with diethylpyrocarbonate (Merck; 1 ml/l) and autoclaved or prepared using diethylpyrocarbonate-treated and autoclaved water as diluent. The working area was cleaned with RNaseZAP (Merck KGaA). To minimize possible adverse effects of fixation on RNA integrity and recovery, 0.5% formaldehyde in 0.1 M PBS (80 ml) was chosen for transcardiac perfusion of ChAT-ZsGreen mice, followed by ice-cold 20% sucrose in PBS (50 ml). The brains were snap-frozen on pulverized dry ice and stored at −80 °C until sectioned with a cryostat. Twelve-micrometer-thick sections containing the MS and the dorsal CPU (Atlas plates 20–26 of Paxinos; Bregma +1.34 to +0.62 mm) (38) were thaw-mounted onto PEN membrane glass slides (Membrane Slide 1 PEN, Carl Zeiss), air-dried in the cryostat chamber, and preprocessed for LCM as reported elsewhere (18, 22, 23). In brief, the slides were immersed sequentially in 50% EtOH (20 s), n-butanol:EtOH (25:1; 90 s) and xylene substitute:n-butanol (25:1; 60 s). Then, they were stored at −80 °C in slide mailers with silica gel desiccants or processed immediately for LCM.
Laser capture microdissection
ChAT-ZsGreen neurons were microdissected individually and pressure-catapulted into 0.5 ml tube caps (Adhesive Cap 200, Carl Zeiss) with a single laser pulse using a 40× objective lens and the PALM Microbeam system and Robo Software (Carl Zeiss; https://www.zeiss.com/microscopy/en/products/light-microscopes/laser-microdissection/palm-microbeam.html). Neurons pooled from the MS or the dorsal CPU were stored in the LCM tube caps at −80 °C until RNA extraction. Functionally heterogeneous cholinergic cell groups occur in several compartments in the CPU (39). ChINs included in our transcriptomic analysis belong to the dorsal portion of the anterior striatum. Neurons in the shell and the core of the ventral striatum and in the dorsomedial and dorsolateral areas of the posterior striatum were not studied.
RNA extraction
RNA samples were prepared with the Arcturus Paradise PLUS FFPE RNA Isolation Kit (Applied Biosystems).
RNA analytics
RNA yield and RIN were determined with the Agilent 2100 Bioanalyzer system using the Eukaryotic Total RNA Pico Chips and the 2100 Expert software (Agilent; https://www.agilent.com/en/product/automated-electrophoresis/bioanalyzer-systems/bioanalyzer-software/2100-expert-software-228259#productdetails). To obtain enough RNA for this test, each sample (N = 3) included 500 cholinergic neurons isolated from the dorsal CPU. The calculated RNA yield/neuron and RIN values were provided as mean ± SEM of the three samples.
RNA-Seq library preparation
Sequencing libraries were prepared with the TruSeq Stranded Total RNA Library Preparation Gold kit (Illumina) from 300 cholinergic neurons microdissected and pooled from the dorsal CPU (CPU300; N = 4) and, to achieve similar microdissected section areas, from 330 cholinergic neurons of the MS (MS330; N = 4). Additional libraries were generated from 30 dorsal CPU cholinergic neurons (CPU30; N = 4). The 15 PCR cycles recommended by the manufacturer for DNA fragment enrichment was increased to 16 using 300 to 330 neurons (18) and to 19 using 30 cells. As a negative control, empty PEN membrane covering the same total area as neurons of the CPU300 and MS330 samples was microdissected and used for RNA isolation, cDNA preparation, and Bioanalyzer analysis. A 1 nM library mix (20 μl) containing the twelve indexed cholinergic neuron samples proportionally was sequenced with an Illumina NextSeq500 instrument using the NextSeq500/550 High Output v2.5 kit (75 cycles).
Bioinformatics
Trimmomatic 0.38 (settings: LEADING:3, TRAILING:3, SLIDINGWINDOW:4:15, MINLEN:36) (40) and Cutadapt (41) were used to remove low quality and adapter sequences, respectively. Remaining reads were mapped to the Ensembl mm107 mouse reference genome using STAR (v 2.7.9a) (42). Read assignment to genes, read summarization, and gene level quantification were performed by featureCounts (Subread v 2.0.2) (43). The CPM read values were calculated with the edgeR R-package (44; https://bioconductor.org/packages/release/bioc/html/edgeR.html). The CV of CPM values for each of the four CPU300, CPU30, and MS330 transcriptomes were determined for the most abundant 1000 transcripts and illustrated in violin plot. Transcriptome coverage was defined as the number of different transcripts (cut-off: reads ≥ 5) in each sample and presented as the mean ± SEM of four transcriptomes for each library type. RSeQC analysis (45) was used to analyze read distribution over gene body and the distribution of reads between exons, introns, and the genomic space between annotated genes.
Species similarities in the receptor profile of striatal ChINs from the mouse dorsal CPU and the human putamen
Transcripts with the highest mean CPMs were identified in the CPU300 transcriptomes as well as in the analogous ChIN database of the human putamen, we reported recently (17). The top 100 receptors (KEGG BRITE database) (46) in each species, and the corresponding receptors in the other one, were illustrated with dot plots embedded in a Venn diagram.
Functional classification of MS330 and CPU300 transcripts
Transcripts present at CPM >1 in the CPU300 and MS330 transcriptomes were arranged by mean CPMs and ranked according to their presence in four, three, two, or only one of the four samples. Selected functional categories (ion channels, transporters, transcription factors, and receptors) were defined using the KEGG BRITE database.
Identification of neurodegenerative disease genes in cholinergic neurons
Identification of AD and PD genes in the two spatially defined cholinergic neuron populations was based on the Human Disease Ontology database (47).
Differential expression analysis of cholinergic neuron populations
Differential expression analysis of the dorsal CPU and MS cholinergic cell types was performed via comparing the MS330 and CPU300 transcriptomes with the DESeq2 R-package (48), following correction for unwanted variations with RUVSeq (49). Differences in mRNA expression levels were quantified by log2 fold changes, using approximate posterior estimation for generalized linear model coefficients (Apeglm method) (50). To take multiple testing into account, p values were corrected by the method of Benjamini and Hochberg (51). Volcano plot and heat map, made with the EnhancedVolcano and Pheatmap program packages, respectively, were used to illustrate all transcripts that were expressed differentially in the spatially defined CPU300 and MS330 samples. Top ten transcripts in the ion channels, transporters, transcription factors, and receptors categories were illustrated in box plots. Synaptic gene ontology (52) analysis was used to classify synapse-related genes.
Studying the effects of limited RNA input of sequencing quality
Quality of sequencing in three types of transcriptomes (MS330, CPU300, and CPU30), each including four independent samples, was determined from several technical parameters. These included total reads, aligned reads, and assigned reads. Library homogeneity was assessed from the mean CV of the top 1000 most abundant transcripts and from the results of principal component analysis.
In addition, homogeneity of heat maps was assessed for the top 1000 dorsal CPU transcripts (highest mean CPMs in the eight dorsal CPU transcriptomes). Transcriptome coverage was defined as the number of different transcripts in each library.
Immunofluorescence experiments
Perfusion fixation and section preparation
To provide morphological support for the roles of dopamine and TRH in the regulation MS and dorsal CPU cholinergic neurons, respectively, ChAT-ZsGreen mice (N = 5) were anesthetized and sacrificed by transcardiac perfusion with 40 ml 4% formaldehyde in 0.1 M PBS (pH 7.4). The brains were removed, postfixed for 1 h, infiltrated with 20% sucrose overnight, and then, snap-frozen on dry ice.
Section preparation
Twenty-micrometer-thick coronal sections containing the MS and the dorsal CPU were prepared with a Leica SM 2000R freezing microtome (Leica Microsystems) and stored at −20 °C in 24-well tissue culture plates containing antifreeze solution (30% ethylene glycol, 25% glycerol, 0.05 M phosphate buffer [pH 7.4]).
Fluorescent visualization of cholinergic neurons and their afferents
Floating sections were pretreated with 1% H2O2 and 0.5% Triton X-100 and then, processed for the immunofluorescent detection of either the dopaminergic marker enzyme TH or proTRH. Cholinergic cells were recognized based on their ZsGreen fluorescence. Dopaminergic neuronal elements were detected with chicken anti-TH antibodies (AB_10013440, Aves Laboratories; 1:1000; 48 h; 4 °C) (53) and TRH neurons with rabbit proTRH antibodies (PA5-57331, Invitrogen; 1:1000; 48 h; 4 °C) (54). Primary antibodies were reacted with Cy3-conjugated secondary antibodies (Jackson ImmunoResearch; 1:500; 2 h; room temperature). The labeled sections were mounted on microscope slides, coverslipped with the aqueous mounting medium Mowiol, and analyzed with confocal microscopy.
Confocal microscopy
Fluorescent signals were studied with a Zeiss LSM780 confocal microscope. High-resolution images were captured using 20 × /0.8 NA and 63 × /1.4 NA objective lenses, a 0.6 to 1× optical zoom and the Zen software (Carl Zeiss; https://www.micro-shop.zeiss.com/en/de/softwarefinder/software-categories/zen-core/). ZsGreen was detected with the 488 nm and Cy3 with the 561 nm laser line. Emission filters were 493 to 556 nm for ZsGreen and 570 to 624 nm for Cy3. Emission crosstalk between the fluorophores was prevented using “smart setup” function. To illustrate the results, confocal Z-stacks (Z-steps: 0.85–1 μm, pixel dwell time: 0.79–1.58 μs, resolution: 1024 × 1024 pixels, pinhole size: set at 1 Airy unit) were merged using maximum intensity Z-projection (ImageJ; https://imagej.nih.gov/ij/index.html). The final figures were adjusted in Adobe Photoshop using the magenta-green color combination and saved as tagged image file format (TIF) files.
Slice electrophysiology
Brain slice preparation
Brain slices were prepared as described earlier (55) with slight modifications. Briefly, 20- to 30-day old male ChAT-Cre/ZsGreen mice (N = 13) were decapitated in deep isoflurane anesthesia. The brain was immersed in ice-cold low-Na cutting solution bubbled with carbogen (mixture of 95% O2 and 5% CO2). The cutting solution contained the following (in mM): saccharose 205, KCl 2.5, NaHCO3 26, MgCl2 5, NaH2PO4 1.25, CaCl2 1, and glucose 10. Brain blocks including the MS and the dorsal CPU were dissected. Two hundred twenty–micrometer-thick coronal slices were prepared with a VT-1000S vibratome (Leica Microsystems) and transferred into oxygenated artificial cerebrospinal fluid (aCSF; 33 °C) containing (in mM) the following: NaCl 130, KCl 3.5, NaHCO3 26, MgSO4 1.2, NaH2PO4 1.25, CaCl2 2.5, and glucose 10. The solution was then allowed to equilibrate to room temperature for 1 h.
Whole-cell patch clamp experiments
Recordings were carried out in oxygenated aCSF at 33 °C using Axopatch-200B patch-clamp amplifier, Digidata-1322A data acquisition system, and pClamp 10.4 software (Molecular Devices Co; https://support.moleculardevices.com/s/article/Axon-pCLAMP-10-Electrophysiology-Data-Acquisition-Analysis-Software-Download-Page). Neurons were visualized with a BX51WI IR-DIC microscope (Olympus Co). ChAT-ZsGreen neurons showing green fluorescence were identified by a brief illumination (CoolLED, pE-100) at 470 nm using an epifluorescent filter set. The patch electrodes (outer diameter= 1.5 mm, thin wall; WPI) were pulled with a Flaming-Brown P-97 puller (Sutter Instrument Co). Electrode resistance was 2 to 3 MΩ. The intracellular pipette solution contained (in mM) the following: K-gluconate 130, KCl 10, NaCl 10, Hepes 10, MgCl2 0.1, EGTA 1, Mg-ATP 4, Na-GTP 0.3, and pH = 7.2 to 7.3 with KOH. Osmolarity was adjusted to 300 mOsm with D-sorbitol. Pipette offset potential, series resistance (Rs), and capacitance were compensated before recording. Only cells with low-holding current (<≈50 pA) and stable baseline were used. Input resistance (Rin), Rs, and membrane capacitance (Cm) were also measured before each recording by using 5 mV hyperpolarizing pulses. To ensure consistent recording qualities, only cells with Rs <20 MΩ, Rin >300MΩ, and Cm >10 pF were accepted.
Spontaneous firing activity of ChAT-ZsGreen neurons were recorded in whole-cell current clamp mode. Measurements started with control recording (1 min). Then a single bolus of the dopamine D2 receptor agonist sumanirole (30 μM, Tocris No.2773) (56) or TRH (10 μM, Thermo Fisher Scientific[Bachem] No.4002023.0025) (57) was pipetted into the aCSF-filled measurement chamber, and the recording continued for another 7 min. Each neuron served as its own control when treatment effects were evaluated.
Statistical analysis
Recordings were stored and analyzed off-line. Event detection was performed using the Clampfit module of the pClamp 10.4 software (Molecular Devices Co). Mean firing rates were calculated from the number of action potentials over the control and treatment periods, respectively. All the recordings were selfcontrolled in each neuron and the effects were expressed as relative percentages of the control periods. Treatment group data were expressed as mean ± SEM. Statistical significance was determined with two-tailed Student’s t tests using the Prism 3.0 software (GraphPad Inc; https://www.graphpad.com/features). Differences were considered significant at p <0.05.
Data availability
RNA Seq files generated in the current study are available in BioProject with the accession number PRJNA901862.
Code availability
Scripts are available at https://github.com/goczbalazs/PRJNA901862.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Animal experiments were carried out in accordance with the Institutional Ethical Codex, Hungarian Act of Animal Care and Experimentation (1998, XXVIII, section 243/1998) and the European Union guidelines (directive 2010/63/EU) and approved by the Animal Welfare Committee of the Institute of Experimental Medicine. All efforts were made to minimize potential pain or suffering and to reduce the number of animals used.
Author contributions
É. R., B. G., K. S., M. S., S. T., I. F., S. P., M. P., N. S., and E. H. conceptualization; É. R., B. G., K. S., M. S., S. T., I. F., S. P., M. P., N. S., and E. H. methodology; É. R., B. G., K. S., M. S., S. T., I. F., and E. H. visualization; É. R., B. G., K. S., M. S., S. T., I. F., and E. H. investigation; É. R., B. G., K. S., and E. H. writing–reviewing and editing; K. S. and E. H. supervision; K. S. and E. H. funding acquisition.
Funding and additional information
This work was supported by grants to Project no. RRF-2.3.1-21-2022-00011, titled National Laboratory of Translational Neuroscience implemented with the support provided by the Recovery and Resilience Facility of the European Union within the framework of Programme Széchenyi Plan Plus, the National Research, Development and Innovation Office (K128317 and 138137 to E. H. and PD134837 to K. S.) and the Eötvös Loránd Research Network (SA-104/2021) of Hungary. We thank Ethan Tyler, Lex Kravitz, and Emmett Thompson for open access science art at SciDraw (accessible at doi.org/10.5281/zenodo.3925901, doi.org/10.5281/zenodo.3925971).
Reviewed by members of the JBC Editorial Board. Edited by Brian Strahl
Contributor Information
Éva Rumpler, Email: rumpler.eva@koki.hu.
Balázs Göcz, Email: gocz.balazs@koki.hu.
Erik Hrabovszky, Email: hrabovszky.erik@koki.hu.
Supporting information
References
- 1.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Gong B., Bushel P.R., Thierry-Mieg J., Thierry-Mieg D., Xu J., et al. The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 2014;32:926–932. doi: 10.1038/nbt.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marx V. Method of the year: spatially resolved transcriptomics. Nat. Methods. 2021;18:9–14. doi: 10.1038/s41592-020-01033-y. [DOI] [PubMed] [Google Scholar]
- 4.Moses L., Pachter L. Museum of spatial transcriptomics. Nat. Methods. 2022;19:534–546. doi: 10.1038/s41592-022-01409-2. [DOI] [PubMed] [Google Scholar]
- 5.Risso D., Perraudeau F., Gribkova S., Dudoit S., Vert J.P. A general and flexible method for signal extraction from single-cell RNA-seq data. Nat. Commun. 2018;9:284. doi: 10.1038/s41467-017-02554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnoli J.W., Ziegenhain C., Janjic A., Wange L.E., Vieth B., Parekh S., et al. Sensitive and powerful single-cell RNA sequencing using mcSCRB-seq. Nat. Commun. 2018;9:2937. doi: 10.1038/s41467-018-05347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y., Zhou X. Spatially informed cell-type deconvolution for spatial transcriptomics. Nat. Biotechnol. 2022;40:1349–1359. doi: 10.1038/s41587-022-01273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Close J.L., Long B.R., Zeng H. Spatially resolved transcriptomics in neuroscience. Nat. Methods. 2021;18:23–25. doi: 10.1038/s41592-020-01040-z. [DOI] [PubMed] [Google Scholar]
- 9.Chen K.H., Boettiger A.N., Moffitt J.R., Wang S., Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science. 2015;348 doi: 10.1126/science.aaa6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieth B., Parekh S., Ziegenhain C., Enard W., Hellmann I. A systematic evaluation of single cell RNA-seq analysis pipelines. Nat. Commun. 2019;10:4667. doi: 10.1038/s41467-019-12266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegenhain C., Vieth B., Parekh S., Reinius B., Guillaumet-Adkins A., Smets M., et al. Comparative analysis of single-cell RNA sequencing methods. Mol. Cell. 2017;65:631–643.e4. doi: 10.1016/j.molcel.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Svensson V., Natarajan K.N., Ly L.H., Miragaia R.J., Labalette C., Macaulay I.C., et al. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods. 2017;14:381–387. doi: 10.1038/nmeth.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macosko E.Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R., Wu X., Jiang L., Zhang Y. Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 2017;18:3227–3241. doi: 10.1016/j.celrep.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D., et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017;20:484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boon W.C., Petkovic-Duran K., Zhu Y., Manasseh R., Horne M.K., Aumann T.D. Increasing cDNA yields from single-cell quantities of mRNA in standard laboratory reverse transcriptase reactions using acoustic microstreaming. J. Vis. Exp. 2011 doi: 10.3791/3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skrapits K., Sarvari M., Farkas I., Gocz B., Takacs S., Rumpler E., et al. The cryptic gonadotropin-releasing hormone neuronal system of human basal ganglia. Elife. 2021;10 doi: 10.7554/eLife.67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gocz B., Rumpler E., Sarvari M., Skrapits K., Takacs S., Farkas I., et al. Transcriptome profiling of kisspeptin neurons from the mouse arcuate nucleus reveals new mechanisms in estrogenic control of fertility. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2113749119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmert-Buck M.R., Bonner R.F., Smith P.D., Chuaqui R.F., Zhuang Z., Goldstein S.R., et al. Laser capture microdissection. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe A.E., Hoeppner D.J., Saito T., Blanpain L., Ukaigwe J., Burke E.E., et al. Profiling gene expression in the human dentate gyrus granule cell layer reveals insights into schizophrenia and its genetic risk. Nat. Neurosci. 2020;23:510–519. doi: 10.1038/s41593-020-0604-z. [DOI] [PubMed] [Google Scholar]
- 21.Nichterwitz S., Nijssen J., Storvall H., Schweingruber C., Comley L.H., Allodi I., et al. LCM-seq reveals unique transcriptional adaptation mechanisms of resistant neurons and identifies protective pathways in spinal muscular atrophy. Genome Res. 2020;30:1083–1096. doi: 10.1101/gr.265017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khodosevich K., Inta D., Seeburg P.H., Monyer H. Gene expression analysis of in vivo fluorescent cells. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vastagh C., Csillag V., Solymosi N., Farkas I., Liposits Z. Gonadal cycle-dependent expression of genes encoding peptide-, growth factor-, and orphan G-protein-coupled receptors in gonadotropin- releasing hormone neurons of mice. Front. Mol. Neurosci. 2020;13 doi: 10.3389/fnmol.2020.594119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groelz D., Sobin L., Branton P., Compton C., Wyrich R., Rainen L. Non-formalin fixative versus formalin-fixed tissue: a comparison of histology and RNA quality. Exp. Mol. Pathol. 2013;94:188–194. doi: 10.1016/j.yexmp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Adiconis X., Borges-Rivera D., Satija R., DeLuca D.S., Busby M.A., Berlin A.M., et al. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat. Methods. 2013;10:623–629. doi: 10.1038/nmeth.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuierer S., Carbone W., Knehr J., Petitjean V., Fernandez A., Sultan M., et al. A comprehensive assessment of RNA-seq protocols for degraded and low-quantity samples. BMC Genomics. 2017;18:442. doi: 10.1186/s12864-017-3827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gocz B., Takacs S., Skrapits K., Rumpler E., Solymosi N., Poliska S., et al. Estrogen differentially regulates transcriptional landscapes of preoptic and arcuate kisspeptin neuron populations. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.960769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed N.Y., Knowles R., Dehorter N. New Insights Into Cholinergic Neuron Diversity. Front. Mol. Neurosci. 2019;12:204. doi: 10.3389/fnmol.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozovaya N., Eftekhari S., Cloarec R., Gouty-Colomer L.A., Dufour A., Riffault B., et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat. Commun. 2018;9:1422. doi: 10.1038/s41467-018-03802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M.G., Chrobak J.J., Sik A., Wiley R.G., Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 31.Zaborszky L., Gombkoto P., Varsanyi P., Gielow M.R., Poe G., Role L.W., et al. Specific basal forebrain-cortical cholinergic circuits coordinate cognitive operations. J. Neurosci. 2018;38:9446–9458. doi: 10.1523/JNEUROSCI.1676-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fazl A., Fleisher J. Anatomy, physiology, and clinical syndromes of the basal ganglia: a brief review. Semin. Pediatr. Neurol. 2018;25:2–9. doi: 10.1016/j.spen.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkash J., Messina A., Langlet F., Cimino I., Loyens A., Mazur D., et al. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat. Commun. 2015;6:6385. doi: 10.1038/ncomms7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cieslik M., Chugh R., Wu Y.M., Wu M., Brennan C., Lonigro R., et al. The use of exome capture RNA-seq for highly degraded RNA with application to clinical cancer sequencing. Genome Res. 2015;25:1372–1381. doi: 10.1101/gr.189621.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarbrough G.G., Pomara N. The therapeutic potential of thyrotropin releasing hormone (TRH) in Alzheimer's disease (AD) Prog. Neuropsychopharmacol. Biol. Psychiatry. 1985;9:285–289. doi: 10.1016/0278-5846(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 36.Khomane K.S., Meena C.L., Jain R., Bansal A.K. Novel thyrotropin-releasing hormone analogs: a patent review. Expert Opin. Ther. Pat. 2011;21:1673–1691. doi: 10.1517/13543776.2011.623127. [DOI] [PubMed] [Google Scholar]
- 37.Kalivas P.W., Simasko S.M., Horita A. Effect of septohippocampal lesions on thyrotropin-releasing hormone antagonism of pentobarbital narcosis. Brain Res. 1981;222:253–265. doi: 10.1016/0006-8993(81)91031-3. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G., Franklin K.B.J. The Mouse Brain in Stereotaxic Coordinates. 2nd Edition. Academic Press; San Diego: 2001. [Google Scholar]
- 39.Matamales M., Gotz J., Bertran-Gonzalez J. Quantitative imaging of cholinergic interneurons reveals a distinctive spatial organization and a functional gradient across the mouse striatum. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011;17:10–12. [Google Scholar]
- 42.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 44.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Wang S., Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 46.Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schriml L.M., Mitraka E., Munro J., Tauber B., Schor M., Nickle L., et al. Human disease ontology 2018 update: classification, content and workflow expansion. Nucleic Acids Res. 2019;47:D955–D962. doi: 10.1093/nar/gky1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Risso D., Ngai J., Speed T.P., Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu A., Ibrahim J.G., Love M.I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35:2084–2092. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 52.Koopmans F., van Nierop P., Andres-Alonso M., Byrnes A., Cijsouw T., Coba M.P., et al. SynGO: an evidence-based, expert-curated knowledge base for the synapse. Neuron. 2019;103:217–234.e4. doi: 10.1016/j.neuron.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bardoczi Z., Wilheim T., Skrapits K., Hrabovszky E., Racz G., Matolcsy A., et al. GnRH neurons provide direct input to hypothalamic tyrosine hydroxylase immunoreactive neurons which is maintained during Lactation. Front. Endocrinol. (Lausanne) 2018;9:685. doi: 10.3389/fendo.2018.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos A.M.P., Teixeira P.D.S., Wasinski F., Klein M.O., Bittencourt J.C., Metzger M., et al. Differences between rats and mice in the leptin action on the paraventricular nucleus of the hypothalamus: implications for the regulation of the hypothalamic-pituitary-thyroid axis. J. Neuroendocrinol. 2020;32 doi: 10.1111/jne.12895. [DOI] [PubMed] [Google Scholar]
- 55.Farkas I., Kallo I., Deli L., Vida B., Hrabovszky E., Fekete C., et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Real J.I., Simoes A.P., Cunha R.A., Ferreira S.G., Rial D. Adenosine A2A receptors modulate the dopamine D2 receptor-mediated inhibition of synaptic transmission in the mouse prefrontal cortex. Eur. J. Neurosci. 2018;47:1127–1134. doi: 10.1111/ejn.13912. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Molina V., Patino J., Vargas Y., Sanchez-Jaramillo E., Joseph-Bravo P., Charli J.L. TRH regulates action potential shape in cerebral cortex pyramidal neurons. Brain Res. 2014;1571:1–11. doi: 10.1016/j.brainres.2014.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA Seq files generated in the current study are available in BioProject with the accession number PRJNA901862.
Code availability
Scripts are available at https://github.com/goczbalazs/PRJNA901862.