Abstract
Chronic wounds are among the major healthcare issues affecting millions of people worldwide with high rates of morbidity, losses of limbs and mortality. Microbial infection in wounds is a severe problem that can impede healing of chronic wounds. Accurate, timely and early detection of infections, and real time monitoring of various wound healing biomarkers related to infection can be significantly helpful in the treatment and care of chronic wounds. However, clinical methodologies of periodic assessment and care of wounds require physical visit to wound care clinics or hospitals and time-consuming frequent replacement of wound dressing patches, which also often adversely affect the healing process. Besides, frequent replacements of wound dressings are highly expensive, causing a huge amount of burden on the national health care systems. Smart bandages have emerged to provide in situ physiochemical surveillance in real time at the wound site. These bandages integrate smart sensors to detect the condition of wound infection based on various parameters, such as pH, temperature and oxygen level in the wound which reduces the frequency of changing the wound dressings and its associated complications. These devices can continually monitor the healing process, paving the way for tailored therapy and improved quality of patient's life. In this review, we present an overview of recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds in order to assess infection status. We have elaborated the recent progress in quantitative monitoring of several biomarkers important for assessing wounds infection status and its detection using smart biosensors. The review shows that real-time monitoring of wound status by quantifying specific biomarkers, such as pH, temperature and tissue oxygenation to significantly aid the treatment and care of chronic infected wounds.
Keywords: Biosensors, Smart dressings, Chronic wounds, pH
Graphical abstract
1. Introduction
Chronic wounds, that do not follow the standard physiological healing mechanism, have been estimated to impact 1–2% of the population in developed countries and 6.5 million patients in the US alone [1]. The management of chronic wounds patients costs over US$25 and US$4.6 billion in the US and UK, respectively [2]. The number of patients is projected to increase due to the worldwide estimated elderly population increase, i.e., Since 2009, the number of senior Americans has climbed by 14.4 million (or 36%), while the under-65 population has increased by 3% [2]. Consequently, treating chronic wounds will continue to be a pressing issue, requiring development of technologies to monitor wound status.
Conventionally, skin wounds are treated by applying a medical bandage that is designed to treat specific wounds and prevent pathogenic microorganisms from infecting the wound bed [[3], [4], [5], [6], [7], [8]]. In clinical practice, physicians assess the wound condition, visually, based on different factors such as the exudate amount and the wound color [9]. However, the assessment requires experienced practitioners to remove the wound dressing frequently, which interrupts the healing [9]. Therefore, engineering wound dressings with integrated sensors that can extract real-time information about the wound conditions and the healing status is crucial for physicians to limit the frequency of changing the wound dressing and reduce healthcare expenses [10].
Although current clinical wound dressings allow for active antimicrobial release to prevent infection and speed up the healing process, they are not designed for providing diagnostics information about the wound environment and the physiological conditions. Different colorimetric and electrochemical wearable sensors have been recently proposed for real-time monitoring of wound status [[11], [12], [13], [14], [15]]. These sensors detect changes in the wound biomarkers using visual or electrical detection tools and can be compatible with smart devices such as smart phones to alert the patient and the physician. The selection of suitable smart sensors depends on various parameters such as accuracy, precision, sensitivity, the limit of detection, integrability with current wound dressings, reusability, and cost [16]. Moreover, to lessen the environmental impact of these dressings, natural biodegradable materials have been recently used to replace the regular dressings to help with environmental sustainability [17]. A number of articles have been published on wearable technology for wound monitoring [11,18,19], smart materials used for wound dressings [17], and smart bandages based on different biomarkers [13]. However very little attention has been paid to highlight the characteristics of these sensors and the selection criteria according to the type of wound.
In this review, we present an overview of the recent advancements in various biosensors for monitoring chronic wound conditions. The normal wound healing and chronic wound healing processes have been discussed followed by a discussion on some of the most important types of biosensors for real time continual monitoring of wound conditions. The biosensors based on colorimetric and electrochemical detection methods, their fabrication processes, detection ranges and sensitivities have been discussed in the light of state-of-the-art technologies. The limitations of these currently available biosensors and the challenges in their applications in clinical settings have also been discussed. Finally, the directions of further research to overcome the limitations possessed by the currently available biosensors in monitoring the wound status have also been presented.
2. Basics of wound healing process
Normally Wounds follow a sequential wound healing process and typically heal without complications [[20], [21], [22], [23]]. However, due to improper wound management, aging, and health problems, some wounds become non-healing, known as chronic wounds, such as bedsores and diabetic foot ulcers [23].
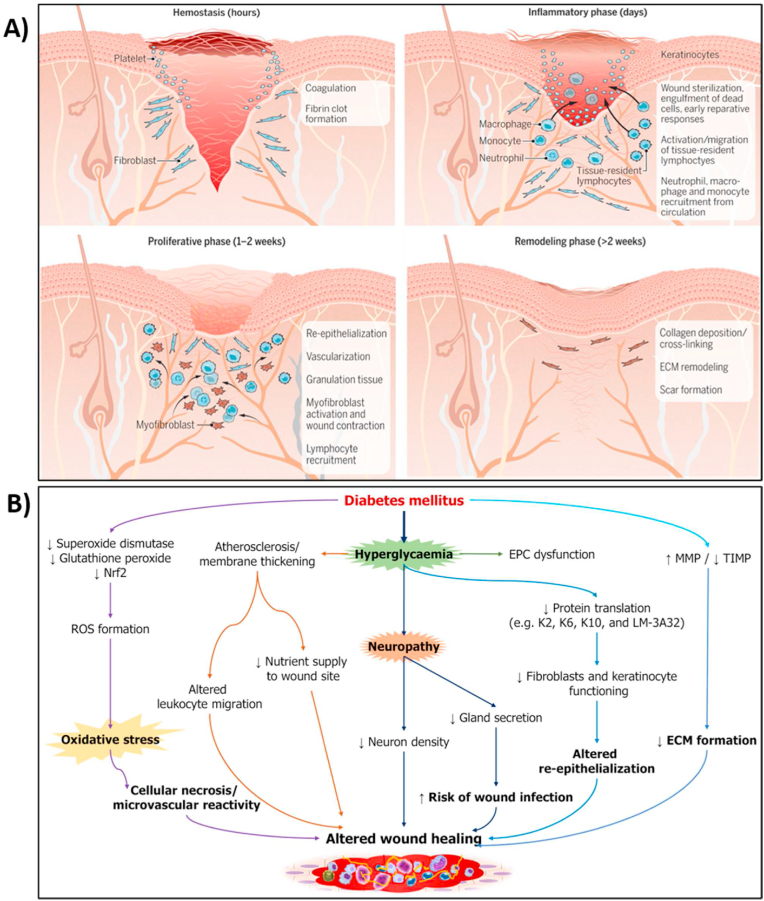
Naturally, the wounded tissue starts the repair process through a cascade of cellular signaling and behavioral events [20,23,24]. The healing process consists of four distinct stages (Fig. 1A): hemostasis, inflammation, proliferation, and remodeling. Immediately following a skin injury, hemostasis starts by constricting the blood vessels to prevent blood loss at the injury site. The blood coagulation forms a platelet-fibrin clot which prevents the wound from getting contaminated. Then, the blood vessels widen in a process called vasodilation to allow the release of growth factors, neutrophils, and macrographs to activate the inflammation process. The inflammatory responses help in cleaning the wound bed from pathogenic contaminants and tissue debris. Following the inflammatory phase, the proliferation phase occurs and creates granulation tissue using keratinocytes cells migration to cover the wound bed. New blood vessels are formed through angiogenesis for nutrients and oxygen supply to enhance proliferation. Finally, extensive layers of the extracellular matrix are remolded to close the wound.
Fig. 1.
Normal Wound healing vs Diabetic Wound healing (A) Wound healing starts with hemostasis to form a fibrin clot. Inflammation is then activated to sterilize the wound and remove debris and dead tissue. Then, through angiogenesis and cell migration, keratinocytes cells form granulation tissue. Finally, a robust extracellular matrix is remodeled for wound closure [25]. (B) Altered cellular factors and biochemical mediators involved in the development of diabetic wound [26].
Nrf2: Nuclear factor erythroid factor 2-related factor 2; ROS: Reactive oxygen species; EPC: Endothelial progenitor cell; MMP: Matrix metalloproteinase; TIMP: Tissue inhibitor of metalloproteinase; ECM: Extracellular matrix.
(Reproduced from Refs. [25,26] with permissions from Science and Baishideng Publishing Group).
However, the wound healing process in case of chronic wounds is not as identical as acute wound healing because the healing process may discontinue at any one or more phases [27]. In chronic wounds, the healing process is hindered, and the tissue fails to proliferate for wound closure due to various factors such as infection, inflammation, and poor tissue oxygenation and nutrition supply [28] (Fig. 1B). In chronic wounds such as diabetic wounds, increased oxygen intake brought on by hyperglycemia results in cellular hypoxia and promotes the generation of ROS. Through interactions with mitochondrial DNA and proteins, excessive ROS generation can disrupt endothelial cells. Additionally, diabetes patients' hypoxic states exacerbate their inflammatory processes [29]. Furthermore. Due to increasing levels of ROS, the hyperglycemic environment (high pH, high temperature) boosted the expression of ERK, EFGR and IL-8, which in turn resulted in excessive infiltration of neutrophil to the diabetic wound and ultimately to a protracted wound healing process [29].
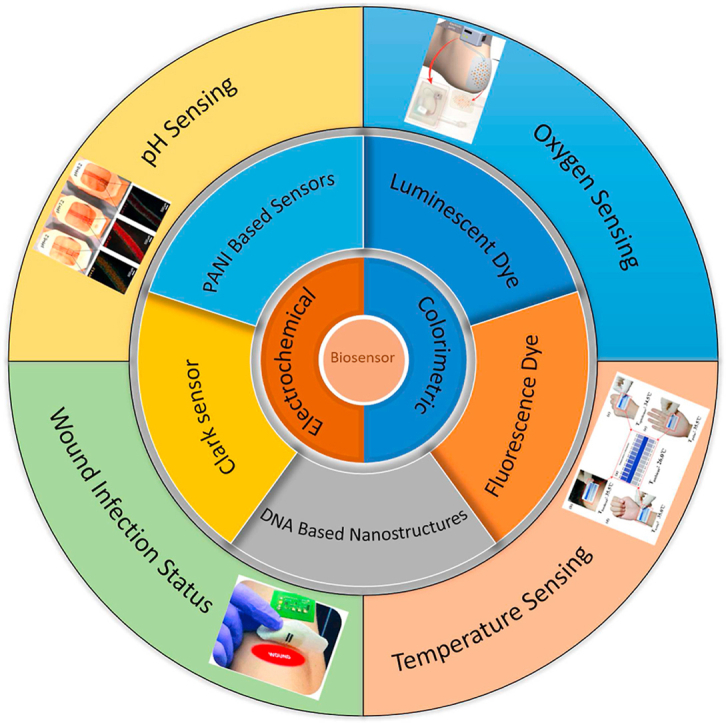
3. Biosensors for monitoring of wound
Different wound healing stages are accompanied by specific biomarkers, such as pH, temperature, bacterial infection, and tissue oxygenation, that provide information regarding the wound status [24]. Each of these biomarkers or a combination of them can be used for monitoring healing stages to allow for better wound management. Table 1 summarizes different types of sensors developed to monitor wound biomarkers.
Table 1.
Biosensors to monitor infection related biomarkers in wounds.
| Sensor | Type | Sensing Parameter | Advantage | Limitations |
|---|---|---|---|---|
| [30] | Colorimetric | pH | connected wirelessly to a smartphone for real time monitoring | Experiment on small sample size. |
| [31] | Colorimetric | pH | covalent bonding with cellulose beads limited the leaching of dye | There was no in vivo testing. |
| [32] | Colorimetric | pH | For long term stability, anti-inflammatory property. | Need further investigation on sensor's performance. |
| [33] | Colorimetric | pH | implementation in a clinical setting. | High deviation at pH of 7–8. |
| [34] | Electrochemical | pH | Good sensitivity | Large response time |
| [35] | Electrochemical | pH | considerably higher sensitivity and fast response time. | There was no in vivo testing. |
| [36] | Electrochemical | pH | attention to monitor the interstitial fluid through the skin | Low sensitivity |
| [37] | Colorimetric | Temperature | Both qualitative and quantitative analysis | Poor sensitivity |
| [38] | Colorimetric | Temperature | Flexible sensor | Less attention on performance |
| [39] | Colorimetric | Temperature | wide range of detection from 4 to 80 °C | no practical application was demonstrated |
| [40] | Electrochemical | Temperature | measuring the localized thermal conductivity of the skin around the wound and using it as a skin hydration sensor. | Low sensitivity |
| [41] | Electrochemical | Temperature | simple fabrication techniques | Fast degradation |
| [42] | Electrochemical | Temperature | excellent repeatability with high sensitivity. | Costly as it utilized gold electrode. |
| [43] | Colorimetric | Oxygen | two-dimensional mapping of the tissue oxygenation | Costly as it requires custom camera to read the data. |
| [44] | Colorimetric | Oxygen | Performance was assessed using in vivo wound models | Insufficient characterization |
| [45] | Colorimetric | Oxygen | flexible, paper-based, and biocompatible. | Requires expensive and complex optical systems |
| [46] | Electrochemical | Oxygen | implantable PDMS-based sensor, high sensitivity. | High response time |
| [47] | Electrochemical | Oxygen | Detection in flowing medium | Increase in flow rate can decrease sensor's sensitivity. |
| [48] | Electrochemical | Oxygen | can conform to any shape and enhances the sensor's mechanical stability under distortion | slow response time |
| [49] | Electrochemical | Oxygen | Can measure oxygen levels inside tissues. | a stable electrical signal was obtained after about 90s. |
3.1. pH sensors for assessing wound condition
The pH of the wound exudate is an important biomarker that can provide useful information regarding the infection and the healing status of wounds [13,18,19,50,51]. Generally, acute wounds display a more acidic environment with pH values between 4 and 6 to eliminate the growth of pathogenic microorganisms and promote cell proliferation and tissue remodeling through angiogenesis and epithelialization processes [18,51]. However, chronic wounds have an alkaline environment with pH of up to 10, making the wound environment suitable for growth of pathogenic bacteria. Therefore, monitoring the wound bed pH can help in detecting the presence of bacterial colonization and to initiate intervention [18,19]. Different types of pH sensors, colorimetric and electrochemical, have been prepared and integrated within bandages for healthcare applications, will be discussed below.
3.1.1. Colorimetric detection of pH in wounds
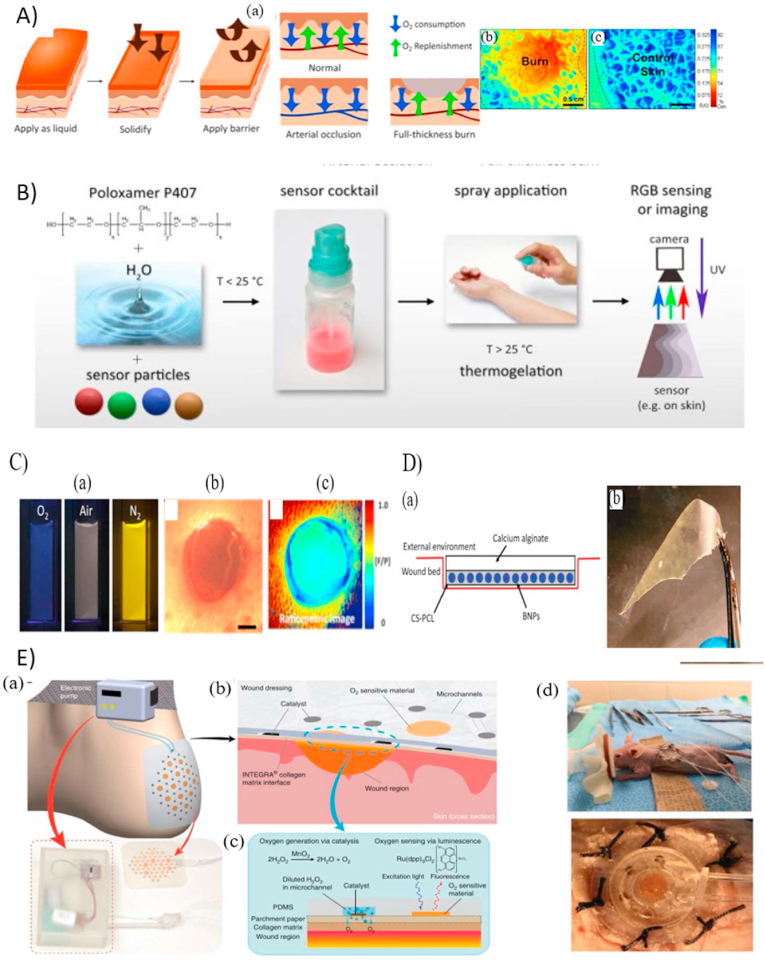
For colorimetric detection, pH-sensitive dyes are embedded within the sensor material to detect pH changes visually or to use image processing without the need for integrated electronics. The main pitfalls of these luminescence-based sensors are the possibility of the dye leaching out into the wound, the need for image processing to achieve high precision and sensitive detection, and range of detection of the dye. Tamayol et al. [30] exploited the microfluidics spinning technique (Fig. 2A) to fabricate pH-responsive hydrogel fibers for long-term monitoring of wound pH within the range of 6–8 and with a response time of 5 min. Mesoporous polyester beads encapsulated with brilliant yellow dye were incorporated into the hydrogel to protect the dye from leaching out into the wound while maintaining a biocompatible environment. The pH values could be estimated visually or through an integrated diode connected wirelessly to a smartphone for quantitative measurements. A smart wound dressing was developed by integrating the fibers into a medical tape to map the pH of the wound environment. In an experiment using this dressing, the retrieved pH values were 5.69, 6.26, 7.38, and 8.13, corresponding to the real pH of the pig skin samples (5.2, 6.2, 7.2, and 8.2, respectively). While the findings demonstrate a pretty close correlation in a pH range of 7–8.5, a more complete assessment, involving a bigger sample size and statistical analysis, would be advantageous to correctly assess the dependability and repeatability of the suggested technique.
Fig. 2.
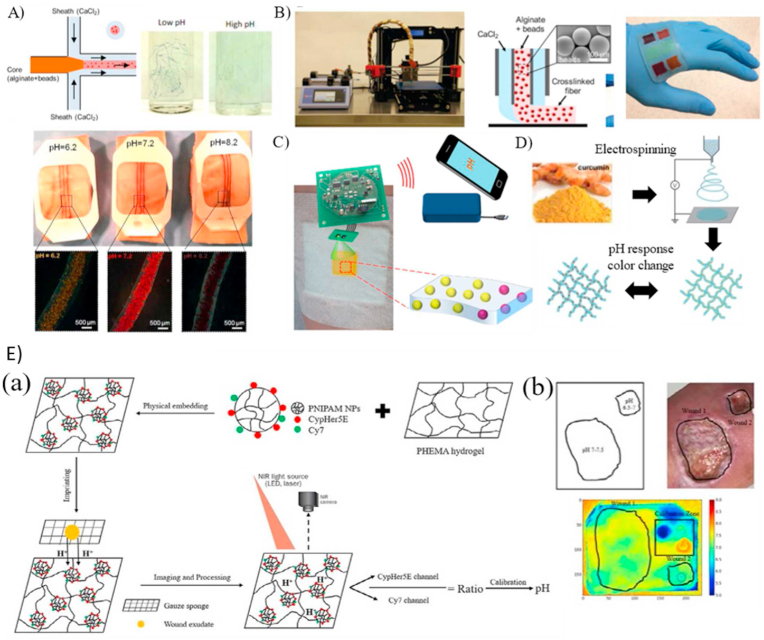
Wearable pH optical-based sensors. A) Production of alginate-based microfibers integrated with pH-responsive beads using microfluidics and coupled with a standard medical tape for pH monitoring [30]. (B) pH sensors fabricated with a 3D printer modified with a microfluidic head to deposit alginate-based films with pH-responsive beads.(C) Schematic of smart cellulose based wound dressings loaded with GJM534 dye and wirelessly connected to smartphone for real-time monitoring [52]. (D) Electrospinning for the development of fibers loaded with curcumin [32]. (E) The development process of a hydrogel-based pH sensing film and tested in-vivo with human volunteers in (b) [33].
(Reproduced from Refs. [30,32,33,52] with permissions from Wiley Online Library, Elsevier, Royal Society of Chemistry).
Liu et al. [31] synthesized a smart wound dressing based on the alginate/polyacrylamide (PAAm) hydrogel coupled with phenol red, a pH-responsive dye. To prevent the dye from leaching out, a methacrylate moiety was attached to the dye to form a covalent bond with the alginate-PAAm hydrogel during the radical polymerization reaction of PAAm. The developed colorimetric patches were tested against solutions of varying pH (5–9), corresponding to the pH range of infected wounds, and a visual color change from yellow to red was demonstrated. Kassal et al. [52] developed a wireless optical sensor for real-time detection of wound pH using pH indicator dye, 4-[4-(2-hydroxyethanesulfonyl)- phenylazo]-2,6-dimethoxyphenol (GJM-534), which transitions from yellow to purple between pH values of 4–12. A key limitation of the dye was its inability to covalently bond with commercial wound dressings due to the presence of reactive vinyl sulfonyl groups. Therefore, cellulose beads (Fig. 2C) were used as an intermediate mediator to covalently bond to the dye and be compatible with hydrogels such as Hydromed D4, a polyurethane-based hydrogel. The sensor characteristics were determined using artificial wound exudate solutions and a quantitative optical indicator that enhanced the sensors’ precision and accuracy. Next, wound bed simulations were performed for continuous monitoring using the same dye (GJM-534) and showed excellent spatial and temporal resolution. After an incubation time of 15 min, sensor exhibited a progressive color shift from yellow to dark orange as the pH increased from 7.3 to 10.0 [53]. Although, covalent bonding with cellulose beads limited the leaching of dye, long term stability study such as degradation study had not been done.
For long term stability, Pan et al. [32] utilized electrospinning to synthesize pH-responsive polycaprolactone (PCL) fibrous mats loaded with curcumin, a pH-sensitive dye, to measure the pH of wounds (Fig. 2D). Curcumin was not only used for pH detection, but also for its anti-inflammatory properties to help the wound healing process. The developed fibrous mat was tested using artificial wound exudate solutions and underwent a visible transition from yellow to red brown within pH values of 6–9. An exciting feature of the fibrous mats was their ability to be tailor-shaped into various irregular shapes (e.g., fibers, mats, cylinders) to be ergonomic. Moreover, integrating a portable detection method such as smartphones can be a convenient approach for physicians and patients. Due to slow degradation of PCL, this sensor can provide seamless performance over a long period of time.
Another interesting direction is integrating pH-dependent fluorophores within hydrogel patches for fluorometric wound monitoring. For instance, Jankowska et al. [54] proposed the concept of coupling 5(6)-carboxynaphthofluorescein, a fluorescent dye that changes color due to protonation or deprotonation, with the natural biocompatible polysaccharide hydrogel to develop a pH sensor that works in the range of 6–8. Due to the presence of a free carboxyl group at the fluorescent dye, a covalent bond with the hydrogel was formed to prevent the dye from leaching out. To mimic the wound environment, the sensitivity of the sensors was assessed against artificial wound exudate solutions with pH values between 6 and 8, and the sensors showed a linear correlation between the fluorescent intensity and pH values between 6 and 7.7. Chronic wounds are generally characterized by an increased infection rate. Therefore, Panzarasa et al. [55] coupled pyranine, a fluorescent dye, with Benzalkonium, a salt with antimicrobial properties, to form a material that can prevent bacterial growth while maintaining its pH sensing ability. This novel material might be an effective remedy for wound monitoring while enhancing the healing process.
To exploit colorimetric and fluorometric detection simultaneously, Yang et al. [56] utilized a microwave reactor system to obtain excitation-dependent orange-emissive carbon quantum dots (O-CDs) capable of exhibiting both colorimetric and fluorometric response to pH changes (5–9). The O-CDs were further immobilized on a typical medical cloth cotton to test the applicability for pH monitoring. The color change from red to yellow at pH values from 5 to 9, was visually verified, and it was attributed to the quinoidal formation due to the protonation of azo groups. Moreover, the O-CDs showed a distinct photoluminescence response to pH via the protonation and deprotonation of the azo surface groups for pH quantification. A wound dressing was developed and used as a dual-model pH visual indicator as well as precise pH quantification with excellent leachability, high biocompatibility, and high shelf-time. However, actual experimentation of the proposed O-CDs for real time-monitoring of chronic wounds were not tested.
Although earlier studies showed promising techniques for integrating various dyes for pH sensing, none of them demonstrated the implementation of the developed sensors in a clinical setting. Recently, Li et al. [33] developed easy-to-fabricate dual dye-labeled wound dressings by incorporating poly(N-isopropylacrylamide) nanoparticles covalently bonded with two fluorescence sensitive and insensitive pH dyes into 2-hydroxyethyl methacrylate hydrogel film (Fig. 2E). Using artificial wound exudates, the developed technique was characterized with a linear response in the pH range of 4–8.5 with a maximum deviation of 2.79% at pH 7.7. The film was immobilized on a freshly discarded wound gauze for clinical applications, and the pH distribution was determined using fluorescent imaging for six patients. Some samples showed low pH values corresponding to normal inflammatory responses and granulation tissue formation while others exhibited neutral pH environment indicating ongoing epithelization. Thus, the study showed the potential of correlating wound pH to the status of wound healing.
Pakolpakcıl et al. [57] utilized electrospinning to prepare a sodium alginate and poly (vinyl alcohol) hydrogel film loaded with purple cabbage anthocyanins for open wound pH monitoring. Anthocyanins were used due to their pH-sensitive behaviors and anti-inflammatory characteristics that are vital for wound healing. The developed film was placed on open wounds of rats for in-vivo experiments, and the pH was quantified at different time point. They showed color changes from purple to green in the pH range of 4–10 with a short response time; however, a fundamental limitation of the film was its inability to provide a visual color difference within 6–8 pH range, which is necessary for monitoring of wound pH. Therefore, there is a need to integrate image processing with the developed film for precise pH measurement.
Although the abovementioned biosensors exhibited the potential to detect pH in the range of 4–10 with minimal leaching out of dye into the wounds, response time and resolution of these sensors might be major obstacles in their implementation into clinical settings. However, these issues are resolvable with advanced image processing techniques, and machine learning and artificial intelligence-based image analysis methods. Thus, significant progress has been made recently in development of various colorimetric biosensors for monitoring of the real time pH of wounds. However, further advancements are required before its widespread clinical application.
3.1.2. Electrochemical detection of pH in wounds
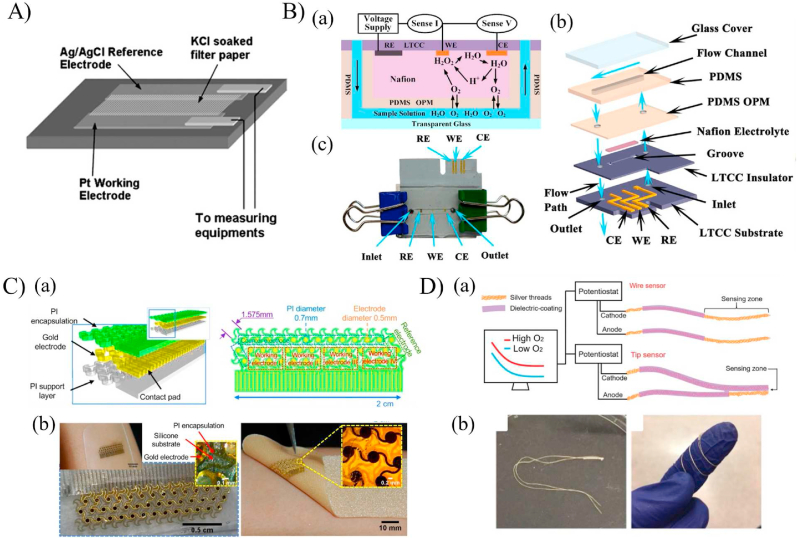
Several simple, low-cost, and compact wearable electrochemical sensors have been recently proposed for pH monitoring in wounds by integrating chemicals that have innate selectivity to pH [[11], [12], [13],18,19,50,58]. For instance, Guinovart et al. [34] incorporated the medical bandage with a potentiometric pH sensor (Fig. 3A) that uses the antibacterial and biocompatible pH-dependent conductive polymer polyaniline (PANI) as a working electrode. Owing to the high conductivity, stability, and ease of fabrication of PANI [58,59], it has been used for pH detection based on changes in resistance due to the increase or decrease in the mobility of ions passing through the working electrode. The reference electrode was fabricated from polyvinyl butyral polymer, a material with a well-known electrical potential in the pH range of interest [60]. Hence, the detection principle in a potentiometric sensor relies on the potential reduction generated between the working and reference electrodes due to the solution's pH. Upon characterizing the sensor with solutions with known-pH values between 4.35 and 8, the results showed a Nernstian sensitivity of −58 mV/pH with a response time <20 s [34]. Human serum solutions with varying pH values were mixed with polyethylene glycol to prepare a hydrogel over the working electrode to mimic the wound environment. The developed bandage demonstrated the ability to monitor the pH variations at a wound milieu within 100 min [34]. To enhance the sensors' response time, Yoon et al. [61] developed a thin and flexible pH sensor using PANI as the working electrode and Ag/AgCl as the reference electrode on a nanopillar backbone film (Fig. 3B). Integrating the nanopillar structure enhanced the sensor's mechanical durability and the response time to be less than 1s. The sensor exhibited a Nernstian sensitivity of (60.3 mV/pH) within a pH level from 2.38 to 11.61 in both standard and bent states. The practicality of the sensor for on-skin monitoring was mimicked by sensing the pH variation on the curved surfaces of oranges. A good agreement with commercial pH sensors was obtained [61].
Fig. 3.
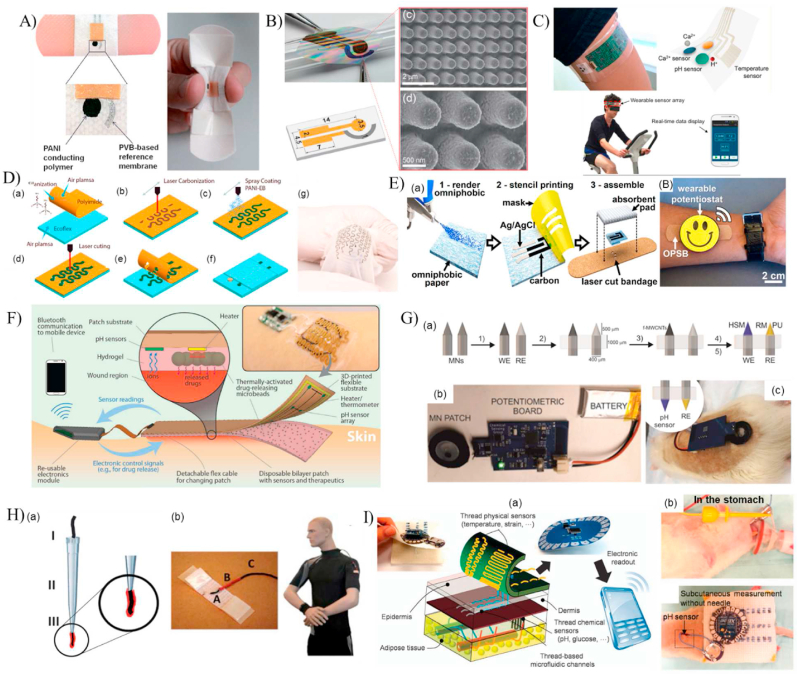
Electrochemical based pH sensors. (A) A pH-sensitive bandage fabricated from PANI and Ag/AgCl electrodes [34]. (B) A PANI coated nanopillar array for enhancing the sensor's sensitivity [61]. (C) An integrated wearable pH sensor patterned on a flexible PET substrate and used for real-time monitoring of sweat pH during human activities [62]. (D) A high stretchable serpentine like pH sensor fabricated using laser carbonization of PANI [63]. (E)The fabrication process of the omniphobic paper-based smart bandages (OPSB) [64]. (F)An automated smart bandage for pH sensing and antibiotic drug release [65]. (G) In vivo transdermal pH sensing with microneedles electrodes [36]. (H) Details of the fabrication procedure of the cotton yarn-based band-aid [66]. (I) A toolkit of thread-based chemical and physical sensors, microfluidic channels, and interconnects for the realization of a thread-based diagnostic device [67].
(Reproduced from Refs. [34,36,[61], [62], [63], [64], [65], [66], [67]] with permissions from Wiley online library, Elsevier, ACS publications, pubs.rsc.org and Nature).
A wearable electrochemical sensor based on PANI was developed by Nyein et al. [62] to enable real-time and continuous monitoring of the pH, ionized calcium, and temperature of biofluids (Fig. 3C). The PANI electrode was fabricated through cyclic voltammetry onto a gold electrode. A linear correlation between the measured electrical potentials and the corresponding pH values of 3–8 was obtained with an average Nernstian sensitivity of 62.7 mV/pH with a slight drift of performance over a prolonged sensing period of 1 h. The sensor was used to perform ex-situ analysis of body fluids such as sweat, urine, and tear and on-body evaluation of sweat pH changes during physical activities. This sensor showed incremental improvement when compared to previously developed PANI based sensors. However, its drift in performance over time can be a major drawback while clinical implementation.
Rahimi et al. [63] synthesized a highly stretchable potentiometric wearable pH sensor for point-of-care applications using direct laser carbonization. The sensing serpentine-like element was synthesized by CO2 laser pyrolization and cutting of a polyimide (PI) sheet followed by permeation with PANI as the pH-sensitive electrodes (Fig. 3D). The fabricated working electrode was attached to a stretchable Ecoflex substrate to conform easily to the human skin. Then, the device characterization was conducted using standard pH solutions, varying from 4 to 10 and potentiometric measurements. The change in electrical potential was inversely proportional to the pH value with a sensitivity of 53 mV/pH and an average response time of 58s. The sensor's biocompatibility was tested using the in-vitro live/dead assay of cultured fibroblasts cells. To verify the applicability of the sensor for wound pH sensing, a microfluidic platform was designed to imitate the dermal wound bed. The developed platform consisted of an agarose pad, where the sensor was placed, bonded to a PET film, and enclosed with a PDMS layer embedded with microfluidic channels where the test fluids were flowing. Real time detection of the agarose pad was determined when fluids of pH values in the physiological range (5–8) were flowing in the microfluidic channels, demonstrating the potential for wound pH monitoring. However, the sensor was not transparent, hindering the ability to monitor the wound healing progress, and was not a standalone platform, requiring the integration of expensive and bulky instruments. Therefore, the same group developed another flexible pH sensor that was transparent and coupled with near-field communication for wireless monitoring [68]. The transparency was achieved by using a PET substrate for laser scrubbing of an ITO film as the conductive electrode [69]. The reference Ag/AgCl and the PANI working electrodes were fabricated on the ITO conductive electrodes by screen printing and electro-polymerization, respectively. Upon characterizing the sensor, it displayed a linear Nernstian sensitivity of −55 mV/pH with a response time of 28s within the physiologically relevant pH range of 4–10. Again, a microfluidic platform with an in-vitro infected wound model was used to examine the function of the sensor to detect the pH variations in a chronic wound. The stretchable sensors demonstrated a notable decrease in sensitivity compared to previously developed sensors. Moreover, the response time experienced a significant increase, posing limitations for their clinical applications where real-time monitoring is of utmost importance.
Chronic wounds frequently need long-term monitoring, perhaps for months or even years. Cost-effective methods enable continuous monitoring while not dramatically increasing the financial load on patients. In this context, Pal et al. [64] developed a low-cost omniphobic paper-based smart bandage (OPSB) for continuous and wireless monitoring of pH of open wounds (Fig. 3E). The pH sensing element consisted of a pair of Ag/AgCl electrode separated by a layer of the pH-sensitive Ag/PANI composition and printed on an omniphobic paper. The sensor demonstrated a linear response within the clinically relevant pH range of 5.5–8.5 with a sensitivity of 0.72 MΩ/pH. In another work, Mostafalu et al. [65] developed a dual-function wireless and wearable wound dressing that is capable of in-situ detection of pH and temperature of infected wounds and the on-demand delivery of antibiotics drugs (Fig. 3F). The pH sensor consisted of four carbon/PANI based electrode and one Ag/AgCl reference electrode, printed on a transparent PET substrate. The on-demand drug-release system was developed by embedding stimuli-responsive microcapsules containing antibiotics (cefazolin) within a permeable alginate hydrogel layer. The entire system, consisting of a pH sensor, a thermo-responsive layer, and the wireless electronics chip, was incorporated within a transparent medical tape for wound monitoring. The potential measurements of the pH sensor showed a linear response with a sensitivity of 50 mV/pH within pH of 4–10. The antibiotics release was achieved by incorporating a heating coil to trigger the thermally responsive microcapsule. An in-vitro scratch assay using keratinocytes was used to mimic the cell migration rate of an infected and cefazolin-treated wounds, indicating faster growth rate in the presence of cefazolin. Unlike other sensors, it provided wound therapeutics along with wound monitoring, making it a good candidate in clinical applications.
The conventional potentiometric technique has been widely exploited in the literature for pH sensing [58]. However, other configurations have been developed for pH detection such as the ion-sensitive field-effect transistor (ISEFT) and microneedle sensors [58]. ISEFT technique depends on measuring the current flowing through the transistor due to the potential difference between the gate and reference electrodes caused by the change in ion-concentrations of the electrolyte. DU et al. [35] developed a sensor gauze for pH sensing of chronic wounds using the ISEFT concept. The gauze was designed to directly cover the wound and measure the pH value of the wound exudate with a high sensitivity of 100 mV/pH and a fast response time of 1 s within the pH values of 3–10. This technique exhibits considerably higher sensitivity compared to the potentiometric technique. The enhanced sensitivity allows it to detect even the slightest changes in wound pH, providing a distinct advantage for monitoring chronic wounds in clinical settings.
Previous studies exploited the wound exudate or body fluids to monitor the pH changes, giving less attention to monitor the interstitial fluid through the skin [[70], [71], [72], [73]]. Thus, García-Guzmán et al. [36] used a microneedle-based technique for developing an on-body in-vivo transdermal pH sensor that was capable of continuous monitoring of subcutaneous pH changes using different in-vivo models (Fig. 3G). Sharp stainless-steel microneedles were modified with carbon nanotubes and Ag/AgCl coatings to fabricate the working and reference electrodes, respectively. Then, the developed microneedles were attached to a silicone substrate and connected to a standard potentiometric detection chip for acquiring the changes in the electrical potential with pH. The sensor was characterized using an artificial interstitial fluid with varying pH values from 5 to 8.5. A linear Nernstian response was observed with a sensitivity of 54.4 mV/pH and a fast response time of less than 5s. The sensor's applicability for transdermal pH monitoring was verified using ex-vivo and in-vivo experiments with chicken and rat models.
Although the above sensors hold great potential in the development of smart and wearable sensors for monitoring a patient's health status, they have been limited to biofluids, open wounds, transdermal and subcutaneous pH monitoring, giving less attention to the biofluids of closed wounds or organs to the sensing element with non-invasive techniques. Thread-based diagnostic devices have been recently exploited for healthcare monitoring due to their flexibility, small dimensions, and ease of fabrication, making them excellent candidates for synthesizing wearable sensors. For instance, Guinovart et al. [66] synthesized a pH sensor using commercial cotton yarns by coating them with a conductive ink and a potassium ion-selective membrane (Fig. 3H). The sensor displayed a linear response with a sensitivity of 59 mV/pH and a response time of less than 60s. Recently, Smith et al. [74] further enhanced the sensor by incorporating a method for coating the cotton yarns with multiwalled carbon nanotubes and PANI to make them more sensitive and flexible. Mostafalu et al. [67] exploited the flexibility of nanomaterial-infused conductive threads to develop a thread-based diagnostic device (TDD) that can deliver the bodily fluids to the sensing element for pH, temperature, glucose, and strain sensing (Fig. 3I). For the delivery of fluids, microfluidic-like interconnections were constructed by embedding hydrophilic cotton threads onto a hydrophobic fabric, acting as microchannels due to their wicking property. As the sensor relies on the concept of potentiometric measurements, conductive threads were obtained by coating the thread with different conductive inks based on the targeted biomarker. For instance, pH sensing was achieved by coating the threads with carbon/PANI and Ag/AgCl for the working and reference electrodes, respectively. An in-vitro chicken skin model was used to mimic the subcutaneous measurements and characterize the sensor. A sensitivity of 59.63 mV/pH and a response time of <30s were reported. The TDD system was integrated with an electronic chip and used for in-vivo wireless measurement of pH, both subcutaneously and in the stomach of a rat model. Subcutaneous and gastric pH values were obtained by passing the threads through an implanted surgical needle or using oral gavage, respectively. Next, threads functionalized with PANI and Ag/AgCl were sewn into a commercial wound bandage and used for wirelessly mapping the pH values at different areas on the bandage [75]. Although the threads' sensitivity did not change significantly when incorporated within the bandage, the response time increased considerably (∼2min). To enhance the response time further, Lyu et al. [76] increased the surface area of the sensing threads by changing the sewing technique from a standard 2D line stitching to a knot-like 3D structure, archiving a response time of 37.30s.
Although the developed devices possessed a good sensitivity (>50 mV/pH) with relatively short response time, difficulty in miniaturizing the potentiostats might be an obstacle yet to be overcome. However, fabrication techniques such as lithography, bioprinting and laser cutting might hold the potential of enabling manufacturing in nanometer scale for monitoring the status of wounds of any size and shape.
3.2. Temperature sensors for assessing wound condition
Temperature is the second important, commonly monitored biomarker for wound healing. Natural skins exhibit temperature fluctuations from 31.1 to 36.5 °C. Acute wounds exhibit a slight increase in temperature around 37.8 °C during the inflammatory phase of the healing process due to the vascular expansion. However, a prolonged inflammatory phase and wound infection at the wound milieu are associated with a significant increase in the wound temperature close to 39–40 °C, indicating the transition from an acute to a chronic wound. Moreover, some wounds like local ischemia are associated with a temperature decrease at the wound bed. Therefore, temperature monitoring can provide valuable diagnostic information on wound status during inflammation and infection. The commonly used technique for temperature monitoring is based on infrared imaging because of the infrared electromagnetic waves emitted percutaneously. However, infrared imaging is done on an open wound, requiring frequent removal of the wound dressing, and affecting the healing progress. Therefore, several wearable sensors, optical and electrochemical, have been developed to monitor temperature changes.
3.2.1. Colorimetric detection of temperature in wounds
Optical detection relies on the concept of lizard skin that changes its color with the ambient temperature to adapt to its environment. This can be achieved in sensors by using thermochromic materials that exhibit color changes with temperature to monitor the skin temperature instantly. When the temperature changes, protonation or deprotonation of the materials causes the corresponding visible color. For instance, Kim et al. [77] developed a skin-like flexible pressure sensor to detect temperature changes by integrating a layer of polydimethylsiloxane (PDMS) with leuco dyes. The temperature-sensitive PDMS layer was sandwiched between two AgNW spray-coated PDMS layers that work as electrodes for pressure sensing (Fig. 4A). The temperature sensor was provided as a potential application, but it was not characterized. Similarly, Charaya et al. [37] fabricated an array of electrochemical pressure and colorimetric temperature sensors. The sensor, shown in Fig. 4B, consists of a dielectric PDMS layer containing thermochromic liquid crystal for temperature detection, sandwiched between two transparent, flexible, and ion-conductive polyampholyte hydrogel layers for capacitive pressure sensing. Temperature monitoring was achieved qualitatively using color changes and quantitively by calibrating the sensor through image-processing of images taken by a digital camera. The calibration curve showed an uncertainty of 0.1–0.4 °C between 26 and 40 °C. Although the developed device has several potential applications as electronic skin, it has not been exploited as a sensor for wound temperature monitoring. Its poor sensitivity of 0.4 °C at temperatures near 39–40 °C might hinder its applicability for wound monitoring.
Fig. 4.
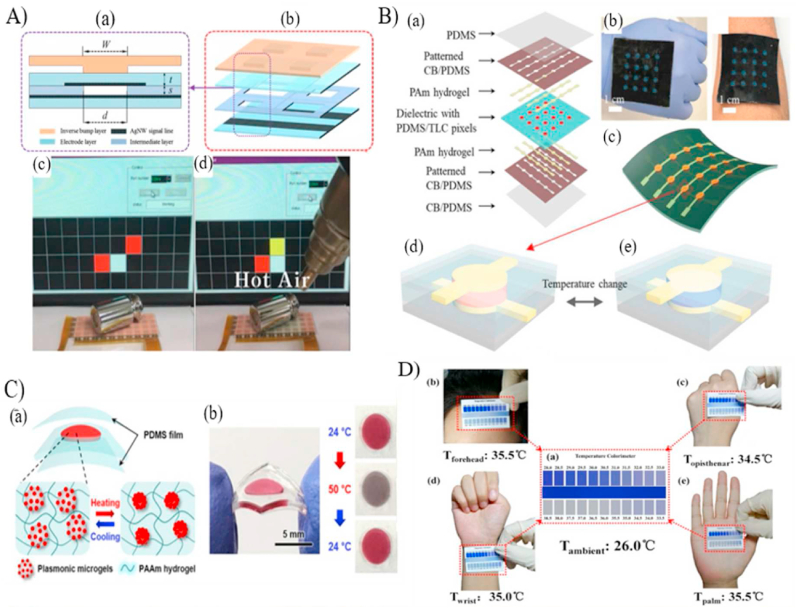
Smart optical temperature sensors. (A) Schematic of the coupled pressure and temperature step sensor with skin like color. (a) A cross section of a single cell. (b) A four-cell unit. (c–d) Pressure sensing and change in the sensors' color at high temperatures [77]. (B) Combined thermochromic and pressure sensor. (a) Layers of the fabricated sensor with the fully assembly pictures in (b) and (c). (d) Illustration of the temperature sensing functionality [37]. (C) A plasmonic thermos responsive microgel based sensor. (a) Schematic illustration of the microgel film encapsulated between to PDMS layers in the hot and cold environments. (b) The fabricated temperature sensor changes color at different temperatures [78]. (D) Portable temperature sensor showing temperature readings at different body parts [38].
(Reproduced from Refs. [37,38,77,78] with permissions from ACS Publications, Wiley online library, Nature and Elsevier). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To develop smart portable temperature monitoring patches, Choe et al. [78] developed a wearable and stretchable colorimetric patch for temperature monitoring through sandwiching a thermo-responsive raspberry-shaped plasmonic microgel-based poly-acrylamide (PAAM) hydrogel film between two PDMS layers (Fig. 4C). The thermo-responsive microgel-hydrogel layer was fabricated by coating plasmonic gold nanoparticles with the thermo-responsive poly(N-isopropyl acrylamide) hydrogel and incorporating them into PAAM hydrogel. Efficient plasmon-coupling between the gold nanoparticles and the thermo-responsive material showed a remarkable and fast (1 s) shift in colors as a function of temperature, exploiting the concept of localized surface plasmon resonance. Furthermore, integrating the developed hydrogel within PDMS substrates enhanced its stretchability without affecting color detection. The patch showed an accuracy of 0.2 °C in the range of 29–40 °C, suggesting the applicability for wound temperature monitoring.
He et al. [38] synthesized a flexible, sensitive, and reversible facile wearable temperature sensor by incorporating the reversible thermochromic micro/nano encapsulated phase change materials (TC-M/NPCMs) within a polyvinyl alcohol polyurethane membrane. The micro/nanocapsules are temperature-sensitive materials as they encapsulate temperature-sensitive luminescent or leuco dyes. A portable temperature sensing pad (Fig. 4D) was fabricated by integrating the thermochromic membrane to visually determine the body surface temperature up to 38.5 °C by comparing the color of the pad with standard colors, like the well-established pH sensing pads. However, the developed membrane was characterized in terms of its tensile properties, chemical structures, morphology, and thermal properties to the content of micro/nanocapsules, paying less attention to determining its sensitivity, accuracy, precision, and response time for temperature sensing. Therefore, its suitability for human health monitoring applications requires further investigation.
Previous studies have established various colorimetric sensors based on temperature-responsive materials such as hydrogels and microcapsules with leuco dyes [37,38,[77], [78], [79]]. Another exciting material, but not well studied is DNA-based nanostructures, they are highly temperature-sensitive and have a fast response time. This method works by exploiting the associated temperature-dependent hydrogen bonding between the DNA strands. For instance, the DNA goes through a melting process at high temperatures due to broken hydrogen bonds, and the melted DNA works as a temperature sensor. Lee et al. [39] exploited the concept of DNA melting of the guanine (G)-rich DNAzyme (Dz) and peptide nucleic acid (PNA) and developed a polyethylene glycol-functionalized graphene oxide (PEG-GO) colorimetric sensor. The working theory of this sensor relies on the conformation of G-Dz into a G-quadruplex structure in the presence of a catalyst at high temperatures, causing a visual change in the sensor color [39,80]. The developed sensor has a wide range of detection from 4 to 80 °C that could be tuned by adjusting the melting temperature of the DNA. The developed system has been integrated with a smartphone to enhance precision and accuracy. However, no practical application was demonstrated, and the sensor's characteristics was not explicitly investigated.
To sum up, colorimetric temperature detection techniques can be used to rapidly monitor the skin temperature with the help of thermochromic materials that demonstrate changes in color depending on temperature change. Unlike colorimetric pH sensors, these sensors have considerably better resolution (around 0.2 °C) and shorter response time (1–50 s). However, both resolution and response time can be further improved using advance image processing techniques.
3.2.2. Electrochemical detection of temperature in wounds
Several electrochemical wearable temperature sensors have been developed and used in biomedical applications due to their skin-like mechanical stability, temperature resistance, and low power consumption. For instance, Kim et al. [81] exploited standard photolithography techniques to develop thin, flexible sutures with integrated on-skin heating and temperature monitoring. Th sensor (1 mm × 30.6 mm) was fabricated by integrating four silicone nanomembranes or platinum resistor-based temperature sensors and four gold micro-heaters on a polyester fabric strip coated with PDMS. The sensor had a wide range of detection i.e. 0–120 °C, with a resolution of ∼ 0.2 °C and a sensitivity of ∼ 2.7 Ω/°C. Integrating a thin layer of PDMS coating allowed for increasing the flexibility of the device not only to be used for suturing, but also for use as on-skin temperature sensing through wrapping it around a fingertip. For in-vivo testing, the sensor was used to suture a wound on a mouse model, and local temperature monitoring was achieved through the four sensing points to be 25.1, 26.8, 26.1, and 25.9 °C. This new type of biocompatible sutures is the first of its kind to enable surgical applications with real-time temperature monitoring while providing local heating to promote the wound healing process [81].
Next, the same group as above built two skin like flexible dual-functional temperature sensors/heaters for providing a real-time and continuous thermal characterization of skin [82]. The first sensor was fabricated using standard microlithographic techniques to incorporate serpentine-shaped gold traces, which exhibit a relative change of resistance due to temperature changes. The second sensor adopted the concept of Si nanomembranes diodes that cause changes in voltage due to temperature changes. Both sensors were integrated on wound dressing or attached directly to the skin and tested under various mechanical deformations, like twisting and stretching, to determine that changes in strain will not alter the temperature measurements. An exceptionally fast response time of 5 ms was reported with millikelvin precision. Measurements of a sensor attached to the palm of subjects exposed to mental and physical stimuli were compared to results obtained from a high-precision infrared camera. The remarkable level of agreement between both techniques gives credence to the potential applications of the sensors in biomedical applications and health care monitoring [82].
In 2014, Hattori et al. [40] developed an epidermal temperature sensor that conforms to the skin and maps the temperature distribution in the wound area. The developed sensor, consisted of a serpentine-like sensing element made from copper sandwiched between two polyimide layers and silicon layers. The electrical resistance increased linearly with the temperature increase in the range of 23–50 °C with a sensitivity of ∼ 0.075 Ω/°C. Clinical studies on patients with granulated wounds showed higher temperatures close the wound area at the middle of the wound healing process, indicating the inflammatory phase [40]. However, granulated but sutured wounds had a prolonged temperature increase, reflecting a more intense inflammation. Another exciting advantage of the sensor is measuring the localized thermal conductivity of the skin around the wound and using it as a skin hydration sensor. The results obtained through human subjects illustrated the impact this technology can have for monitoring wounds. However, the resolution of this sensor was not investigated. Resolution plays a vital role in detecting even the slightest changes in wound temperature, which is crucial for early detection of chronicity in wounds. In addition, sensitivity of this sensor is significantly low when compared with other available sensors.
Similarly, Trung et al. [41] demonstrated simple fabrication techniques to synthesize a transparent and stretchable temperature sensor based on the resistance change of graphene oxide nanosheets. Due to the flexibility of the sensor, it was used to measure the temperature changes of human skin during drinking activities with a sensitivity of ∼1.34% resistance change per °C, and a resolution of 0.2 °C. In another study, Lu et al. [83] presented a novel implantable, bioresorbable, wireless, and battery-free resonance-based temperature sensor that facilitate monitoring the temperature in the intracranial space without the need for extracting it post-implantation. The sensor was designed to dissolve within 4–6 days in the body naturally. The sensing concept of the resonance-based sensor relied on the use of polyethylene glycol (PEG), which holds a strong temperature-dependent dielectric constant, in between two parallel capacitors made from magnesium foils and poly-l-lactic acid. Then, the sensing element was sandwiched between two biodegradable poly-lactic-co-glycolic acid layers and a layer of natural wax. The sensor exhibited a steady sensitivity of 2% change in frequency/°C, a precision of <0.05 °C, and an accuracy of nearly 0.5 °C prior to the time of degradation at day 6. Wireless monitoring was verified through in-vivo implantation of the sensor in the subcutaneous pocket of the dorsal region of a rat's skull. The obtained temperature values were compared to the temperatures acquired in parallel from an epidermal thermocouple. Interestingly, the results were on-par with the implantable wired-based sensors, showing the applicability of the sensor for monitoring infections that occurred at internal wounds with high accuracy and precision. However, fast degradation rate and its associated issues such as drop in performance might hinder its clinical application.
Diabetic foot ulcers are a common complication of a severe wound in patients with diabetes, leading to severe disability and, in some cases, limb amputations. Therefore, continuous monitoring of a foot ulcer status even when patients are not hospitalized is inevitable. Thus, Salvo et al. [42] developed, an electrochemical-based wearable temperature and pH sensors for monitoring diabetic foot ulcers. The temperature sensor consisted of two gold electrodes fabricated on a Kapton substrate; one coated with a temperature-sensitive material and the other uncoated. The temperature-sensitive material, thermoplastic elastomer mixed with multi-walled carbon nanotubes, changed the electrical resistance linearly with temperatures from 25 to 50 °C. The sensor's sensitivity of ∼85 Ω/°C and a relatively slow response time of 30 s were reported; although, the sensor was not tested either in-vivo or in clinical trials, its excellent repeatability (standard deviation of 0.1% maximum after seven repeated observations) with high sensitivity hold the potential for clinical translation.
Some studies reported the development of temperature sensors, but have not shown applications to wound monitoring [[84], [85], [86]]. For instance, Ota et al. [85] reported the development of an innovative wearable sensor to monitor the core body temperature through the tympanic membrane on the ear based on an integrated infrared sensor. This smart sensor was 3D printed to be entirely user-customized and help in preliminary medical screening but it was not capable of wound status monitoring. Wearable sensors require suitable attachment with the skin and less noise to signal ratio to obtain trustable information. Therefore, Yamatomo et al. [86] focused on enhancing the sensor's attachment and investigated the effect of device thickness on the temperature readings. To do so, a gel-less adhesive conductive layer was developed, and the temperature sensing material (multiwall carbon nanotubes mixed with PDMS and polyethyleneimine) was fabricated on different thicknesses (25, 50, and 100 μm) of polyethylene terephthalate (PET). The results obtained through hot plate-based experiments revealed that a thicker PET film would cause a significant temperature difference of 1.8 °C than the actual skin temperature, depending on the ambient temperature. Thus, it was recommended to use thin layers for wearable temperature sensors to reach a sensitivity of ∼0.85%/°C. In another study, Oh et al. [84] fabricated a resistor-type temperature sensor on a bioinspired octopus sucker-like adhesive layer for improving its adhesion to the skin. The sensing element composed of carbon nanotubes, a conductive polymer (poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)) (PEDOT:PSS), and a thermoresposnive hydrogel was fabricated on a PDMS film. The sensor exhibited a thermal sensitivity of 2.6%/°C with a response time of 30s within the dynamic range of 25–40 °C.
The abovementioned sensors provide high precision and sensitivity temperature and thermal conductivity sensing for health monitoring. However, for improving human health, developing wearable electronics that can sense the wound status and respond to the wound through the delivery of targeted drugs is inevitable [12,13,87]. Hence, Honda et al. [88] developed for the first time a low-cost wearable human-interactive bandage integrated with temperature and capacitive touch sensors and a microfluidic-based drug delivery pump. To achieve a low-cost fabrication, large-scale lithography-free processes like shadow mask printing were used to print the sensors on a flexible Kapton substrate. The developed device consisted of three stacked layers: the middle thin Kapton layer with the temperature and wireless coil sandwiched between a bottom Kapton layer of an array of touch sensors and a PDMS top layer for incorporating the drug delivery channel. A carbon nanotube (CNT) paste was mixed with a conductive (PEDOT: PSS) to synthesize the resistive temperature sensor. The sensor was characterized by measuring the resistance change at temperatures from 22 to 48 °C and showed a relatively high sensitivity of ∼0.61% change in resistance/°C and a slow response time of ∼18s. As a proof of concept of the ‘sensor's functionality, the developed integrated bandage was used to detect an increase in an adult ‘male's arm temperature during lunch and exercise. The slow response time of the above might hinder its feasibility for wound status monitoring. Therefore, very recently, Zhou et al. [89] improved the response time (<3s) of PEDOT: PSS-based temperature sensor through the fabrication of micro/nano-sized PEDOT: PSS wires doped with graphene oxide (GO). The response speed was inversely proportional to the surface-to-volume ratio of the wires and not affected by the GO ratio.
Gong et al. [90] prepared a dual function temperature sensor with a thermally triggered drug release capability for anti-infection therapy. The developed sensor consisted of a thermo-responsive polymer nanomesh loaded with moxifloxacin hydrochloride (MOX), an anti-inflammatory agent, and a conductive coil pattern for temperature sensing and triggering the release of MOX on-demand. An elevated temperature at the wound site can indicate the start of infection or inflammation and trigger the release of MOX autonomously. The results showed that the electrical resistance is linearly correlated to the temperature change with a high sensitivity of 0.23% change in resistance/°C in the detection range between 30 and 70 °C. In-vitro bacteria colonization test was used to assess the efficacy of controlling the released MOX concentration with time by controlling the heating temperature. Over 6 h and cycles of heating and cooling to control the release rate, complete bacterial growth inhibition was achieved, showing the great potential for monitoring the wound status and enhancing the healing cascade. Recently, instead of a thermally triggered release approach, Pang et al. [91] developed a flexible wearable sensor for real-time wound temperature monitoring, early infection alerting (>40 °C), and on-demand infection treatment. The detector consisted of a PDMS layer integrated with a temperature sensor and ultraviolet light-emitting diodes to stimulate a UV-responsive antibacterial hydrogel to release antibiotics. To verify the sensor's functionality, it was used to monitor the temperature change of a pig with a full-thickness-infected wound. The obtained results demonstrated an increase in the wound temperature at the time of bacteria inoculation and a decrease in temperature after UV irradiation. The same group further enhanced the system by integrating a biocompatible chitosan dermal equivalent pro-healing layer for skin regeneration [92]. These wound dressings with real-time detection and on-demand treatment hold great potential for wound therapy and health care monitoring [11].
Wang et al. [93] synthesized a self-healable multifunctional wearable sensor (electronic tattoo) for temperature, strain and humidity monitoring based on the integration of graphene with silk fibroin and Ca2+ ions. The simple fabrication process enabled direct printing of the sensor onto human skin like a temporary tattoo. The addition of graphene [94] facilitated the use of the sensor's resistance change for temperature sensing due to the hopping of electrons at the interface of graphene structures. The resistance was found linearly changing with the temperature from 20 to 50 °C with a sensitivity of 2.1%/°C. To demonstrate the practicability of the sensor to monitor temperature, the resistance changes of an electronic tattoo attached to a human hand were recorded upon exposing the hand to cold and hot conditions. Although the developed electronic tattoo showed not only applications in temperature sensing, but also in humidity, strain, and electrocardiogram monitoring with a self-healing ability, its sensitivity is in the lower range compared to other temperature based wearable sensors [13,19]. Hence, further focus on improving its sensitivity is needed.
Lately, Wu et al. [95] reported the development of a new set of temperature sensors with built-in microheaters for self-calibration without the need for bulky equipment or thermal cameras to externally calibrate the sensors. The fabricated device consisted of a layer of serpentine-like platinum microheaters attached to a liquid crystal polymer substrate and to another layer of 3D reduced graphene oxide as the temperature sensing element. The integrated microheaters were used for producing a localized heating to obtain different temperatures for calibrating the sensor. Although the sensor has not been exploited for health monitoring applications, it exhibited a thermal sensitivity of 2.04%/°C within a wide range of detection from 26 to 101 °C and, most importantly, a resolution of 0.2 °C that make it applicable for wound monitoring and biomedical applications.
Briefly, electrochemical detection of temperature is based on the material's change in resistance with respect to temperature. Although most of the developed sensors exhibited sensitivity within the physiological range of temperature (20–50), resolution of around 0.2 °C might hinder their applicability in wound monitoring. However, some sensors exploited polyethylene glycol (PEG) or copper as a sensing element to improve the resolution. Sensors with PEG as a sensing element showed resolution of <0.05 °C making them a good candidate in monitoring wounds temperature.
3.3. Oxygen sensors for assessing wound condition
Wound oxygenation is determinantal for the progression of the natural wound healing cascade [21,96]. Providing the wound bed with sufficient oxygen enhances cell proliferation and angiogenesis and works as an antibacterial agent [13,45,96,97]. In all biological processes, cells utilize oxygen to generate energy through synthesizing high-energy adenosine triphosphate (ATP) in a process called aerobic respiration [98]. ATP is considered as the energy source for many biological processes inside the cells, like wound healing [98]. Moreover, oxygen initiates a respiratory burst process to help cells produce reactive oxygen species (ROS). ROS are not only responsible for protecting the wound from infection, but also play a role in the release of growth factors which are vital in stimulating the healing process. Impaired angiogenesis and vascular complications are commonly associated with chronic wounds due to hypoxia, inadequate oxygen supply at the wound site, making the wound vulnerable to infection [1,96,99,100].
Chronic wounds are characterized by a reduced oxygen partial pressure (pO2) of 30 mmHg compared to acute wounds of 60 mmHg [28]. A plethora of oxygen sensors have been introduced to provide a comprehensive mapping of the wound oxygen level using electrochemical measurements or colorimetric detection.
3.3.1. Colorimetric detection of oxygen in wounds
Colorimetric detection of tissue oxygenation depends on quenching the phosphorescence of a molecular fluorescent dye by collision with oxygen molecules, causing energy transfer from the donor, phosphor molecules, to the acceptor, oxygen molecules [1,101,102]. Consequently, an oxygen-dependent phosphorescence quenching is occurred and is inversely proportional to the concentration of the dissolved oxygen in the sample. Several oxygen-sensing phosphors have been introduced to develop sensing films for continuous monitoring of surface tissue oxygenation in real-time [103]. In the review paper by Wang et al. [103], a comprehensive collection of the optical methods for sensing oxygen was provided up to 2014, giving an insight into the potential applications of these techniques in monitoring wounds. In addition, a list of fluorescent and luminescent probes for oxygen sensing was provided, along with their quench ability. Therefore, we review sensors for oxygen sensing using colorimetric detection since 2015.
Li et al. [43] exploited the metalloporphyrin, an oxygen-sensing phosphor, to develop a bandage that was painted on the skin for real-time two-dimensional mapping of the tissue oxygenation of burns and skin grafts. A polymeric transparent liquid bandage incorporated with the sensing molecules was painted and dried on the skin, forming the sensing film. Another transparent wound dressing was used to seal the sensing layer from being altered due to the room air. A custom camera accompanied the developed platform for capturing the phosphorescence emission and correlating it with the level of oxygen using a ratio metric or a lifetime-based approach. The sensor had a high sensitivity of 0.0625 intensity change and 0.018 ms-1 per mmHg of pO2 for ratiometric and lifetime measurements, respectively, within the range of detection between 0 and 160 mmHg pO2. The sensing-bandage was validated using both the in-vivo rat model for tissue ischemia and in-vivo and ex-vivo porcine burn models. Next, Wang et al. [104] developed a water-spray biomaterial capable of sensing pH, oxygen, and temperature changes. The final sensing layer was obtained by dispersing microparticles integrated with luminescent probes and inert reference dyes in thermogelation polymer matrix of Pluronic F127 (Fig. 5B). Different luminescent probes were used based on the targeted biomarker. For instance, the red-emitting oxygen probe Pt(II) meso-tetrakis(pentafluorophenyl)-porphyrin (PtTFPP), fluorescein, and an europium(III) chelate complex tris (benzoylacetonato) mono(phenanthroline) europium(III) were used for oxygen, pH, and temperature sensing, respectively. The sensing film was sprayed in the liquid form at low temperatures (20 °C), and, at elevated temperatures (>25 °C), a uniform solid gel layer was formed, providing high conformability with uneven surfaces such as wounds. The imaging was conducted using a commercial digital camera. The oxygen sensor showed a range of detection between 0 and 100% saturation with a linear response between 0 and 50%. The sensitivity was estimated as 0.11 intensity ratio per 1% O2. Although the oxygen sensor has not been used for real applications, a similar sprayable pH sensor has been utilized for in-vivo pH monitoring of chronic wounds [105].
Fig. 5.
(A) Application of the oxygen sensing bandage using full thickness wound model showing the ability for colorimetric detection [43]. (B) A water-spray biomaterial capable of sensing pH, oxygen, and temperature changes [104]. (C) The development of boron dye derivatives for oxygen sensing validated using a murine wound model [106]. (D) An oxygen-sensing wound bandage developed through incorporating the iodide-derivative boron dye into oxygen-permeable chitosan polycaprolactone [44]. (E) Design of the integrated oxygen sensing and delivery patch (a–c) with in-vivo testing (d) [45].
(Reproduced from Refs. [[43], [44], [45],104,106] with permissions from Nature, OPTICA, Elsevier, ACS publications and NCBI).
Boron dyes are another category of dual emission oxygen luminescent dyes. Difluoroboron β-diketonate poly (lactic acid) (BF2bdkPLA) are a new group of boron dyes with high photostability and distinctive fluorescent and oxygen room-temperature phosphorescence peaks. DeRosa et al. [106] optimized the oxygen sensitivity of the BF2bdkPLA materials by developing a series of dye derivatives through changing a single dye molecule (Hydrogen (H), Bromide (Br), Iodide (I)) using solvent-free ring-opening polymerization technique (Fig. 5C). The developed materials in integration with portable digital cameras was used for oxygen monitoring using lifetime measurements and ratiometric imaging. To determine the optical characteristics and oxygen sensing ranges of each dye, the dye derivatives were fabricated as nanoparticles (NP) and each dye showed a distinctive dynamic range with higher sensitivity. For instance, the hydrogen, bromide, and iodide derivatives indicated a linear response to oxygen levels of ∼0−0.3% (anoxia), 0−1% (hypoxia), and 0−100% (normoxia), respectively. The iodide derivative covers the physiological range 0–21%; thus, was used for in-vivo wound oxygenation monitoring of a murine wound model (Fig. 5C). Similarly, Tavakol et al. [44] incorporated the iodide-derivative boron dye into oxygen-permeable chitosan polycaprolactone to form an oxygen-sensing wound bandage (Fig. 5D). Another oxygen-impermeable calcium alginate layer was bonded to the sensing later for isolating the wound milieu from ambient air, resulting in a more sensitive and accurate oxygen monitoring. In-vivo murine and porcine wound models were used to assess the oxygen-sensing performance of the boron-dye film. While the study demonstrated the monitoring of wound oxygen levels using an in vivo model, further characterization is required to ensure the sensitivity, resolution, and accuracy of the sensor. This additional evaluation is necessary to determine its efficacy before considering its transfer into clinical settings.
The abovementioned sensors allow for detection of tissue oxygenation within wound beds, paying less attention to adjusting the oxygen level of the wound bed through controlled delivery of oxygen to prevent hypoxia. Currently, oxygen delivery is achieved via hyperbaric oxygen therapy [[107], [108], [109]] or topical oxygen therapy [110] using several commercial systems such as OXYGENESYS, OxyBand, and EPIFLO. However, both techniques require expensive equipment and repetitive treatments while suffering from the ability to selectively target the wounded region. Therefore, Ochoa et al. [45] proposed for the first time the use of flexible, paper-based, and biocompatible wound dressing (E) for non-invasive oxygen delivery and sensing in a wounded area. Oxygen generation was achieved through incorporating a network of microfluidic channels for the delivery of hydrogen peroxide to the catalyst location. The catalyst manganese dioxide (kMnO4) and the phosphorescent oxygen sensing dye (Ru(dpp)3Cl2) were printed on a parchment paper using an inkjet printer then boned to the microfluidic layer. Hydrogen peroxide was delivered to the catalyst location and decomposed to generate oxygen at the desired location. In-vitro bilateral full-thickness wounds covered using the developed wound showed higher inflammatory responses indicated by an increased number of neutrophils throughout the healing process. Integrating inkjet printing for developing a wearable wound dressing with oxygen sensing and delivery capabilities enables the development of non-invasive, customized, and wound-specific therapies.
Although using porphyrins or boron molecules for oxygen sensing is beneficial and provides sensitive and fast monitoring techniques, their optical characteristics require expensive and complex optical systems for excitation and detection. Therefore, a new group of metalloporphyrin has been designed for biomedical visualization of tissue oxygenation under ambient light [111]. The new material was incorporated in a liquid bandage formulation and utilized to map the oxygen consumption of an ex-vivo procaine burn model. These new materials allow for the detection of tissue oxygenation with the naked eye using standard lighting and without the need for incorporating expensive optical stimulation and image acquisition and processing systems [111].
Taken all together, colorimetric pH sensors exploit the energy transfer from a phosphor molecule to an oxygen molecule. Porphyrins and Boron dyes have been widely exploited in oxygen sensing because of their high photostability and distinctive fluorescent and oxygen room-temperature phosphorescence peaks. However, iodide derivative of Boron dyes covers the physiological range 0–21%; thus, was used for in-vivo wound oxygenation monitoring of a murine wound model. Additionally, in parallel to oxygen sensing systems, non-invasive oxygen delivery to the wounded site has also been explored by researchers.
3.3.2. Electrochemical detection of oxygen in wounds
Although optical based sensors are non-invasive and capable of obtaining a two-dimensional mapping of the wound area, they are easily affected by environmental light and require the integration of light emitting diodes and cameras, making them less sensitive [13,43,103,106]. Therefore, electrochemical devices have been widely utilized in the detection of oxygen due to their high sensitivity, low cost, portability, and ease of fabrication using traditional microfabrication techniques [[47], [48], [49],[112], [113], [114]].
| (1) |
| (2) |
The conventional commonly used amperometric Clark sensor developed by Clark consists of two electrodes: a working electrode from platinum or gold, and an Ag/AgCl reference electrode, and a permselective membrane that separates the device KCL solution form the external solution [115]. The sensor measures the dissolved oxygen in an aqueous solution through applying a potential to the electrodes to induce a chemical reaction, resulting in the reduction of oxygen (Equations (1), (2))), and changing the passing electric current which is proportional to the oxygen level [115]. Different electrode materials have been proposed to enhance the electrochemical reduction of oxygen, hence enhancing the sensor's sensitivity [103,115]. For instance, Hutton et al. [116] developed a new electrode material through functionalizing polycrystalline boron-doped diamond (pBDD) with Pt deposition for the amperometric detection of oxygen. pBDD material was chosen because of its wide potential in aqueous solutions, high temperature stability, low background noise, and corrosion resistance [117]. A linear correlation between the dissolved oxygen concentration and the current was obtained at percentages of oxygen to nitrogen of 0–100%.
Koley et al. [46] developed an implantable PDMS-based oxygen and pressure sensor for measurements in blood vessels. The sensor, shown in Fig. 6A, consisted of three layers mounted on a glass substrate: an oxygen permeable PDMS thin membrane, a filter paper soaked with the electrolyte KCl, and a glass substrate coated with Pt and Ag/AgCl as the working and counter electrodes, respectively. Oxygen sensing was achieved based on measuring the changes in current flowing between the working and counter electrodes that are in contact with the KCl soaked filter and separated from the test sample using the thin-PDMS membrane. The sensor demonstrated a high sensitivity of 2.75 μA/% O2 in the range of 10–30% and a low sensitivity of 0.83 μA/% O2 in the range of 60–90%. The sensor was used to measure the oxygen content in sensitive liquids where no contamination was allowed by encasing in PDMS. However, the PDMS membrane significantly altered the response time of the sensor from 40s to a few minutes. In another study, Wang et al. [118] proposed the use of a solid-state proton conductive matrix (PCM) as a solid electrolyte layer instead of the commonly used KCl liquid electrolyte, making the Clark type sensor more suitable for biomedical implantation. Integrating a solid electrolyte layer allowed for standard micro-machining techniques for the development of a miniaturized (1.1mm × 7.3 mm) oxygen sensor [118]. The sensor was fabricated on a Si substrate through the deposition of silicon dioxide layer followed by forming the titanium and platinum electrodes through electron beam evaporation. The sensor was intended for post-operative tissue monitoring where the oxygen level varied between 0 and 21% atm. For the intended range of detection, the output current was linearly proportional to the oxygen concentration with a sensitivity of 4.7 ± 1.8 nA/% O2 and a resolution of 2.6% O2. The potential use of the device for low-level oxygen monitoring such as the case of ischemia was verified through obtaining a significant difference in the output current when comparing a normal solution to an oxygen-depleted solution.
Fig. 6.
Oxygen electrochemical detection techniques. (A) Schematic diagram of the implantable oxygen sensor (B) low temperature co-fired ceramic sensor for measuring oxygen in a flowing environment [46]. (a) Conceptual design of the microchannels with the reaction mechanism happening at the electrodes. (b) The assembly and fabrication process of the sensor with an assembled version in (c) [47]. (C) A skin like oxygen sensor based on gold electrodes patterned in a horseshoe structure to enhance the mechanical properties and integrated onto a medical bandage [48]. (D) Thread-based electrochemical oxygen sensor for area and local measurements, showing the flexibility of the sensor wrapped around human finger [49].
(Reproduced from Refs. [[46], [47], [48], [49]], with permissions from Royal Society of Chemistry and Elsevier).
(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The abovementioned sensors have been fabricated to monitor dissolved oxygen concentration in static environment such as a solution of cells for obtaining the cellular oxygen consumption rate, paying less attention to monitoring oxygen levels in a flowing medium. Therefore, Luo et al. [47] developed a microfluidic-based Clark type oxygen sensor using low temperature co-fired ceramic (LTCC) material for the real-time sensing of dissolved oxygen concentration from 0 to 8.1 mg/l in a flowing medium (Fig. 6B). The LTCC material was used as a substrate due to the ability to both integrate the electrodes as well as fabricate microfluidic channels using scalable manufacturing techniques. The developed sensor composed of multiple layers sandwiched together (Fig. 6B). The bottom LTCC layer was patterned with three electrodes: gold working and counter electrodes and an Ag/AgCl reference electrode. Then, the patterned layer was attached to another LTCC layer to work as an insulator, the Nafion solid-state electrolyte, an oxygen permeable PDMS thin membrane to separate the test sample from the electrolyte while allows for oxygen diffusion, and a PDMS-based microchannel sealed with a glass cover slip. Similar to the typical Clark type sensor, the sensing is based on the changes in current due to the electrochemical reduction of the diffused oxygen through the PDMS membrane to the electrolyte layer. The proposed sensor sensitivity decreased from 36.25 ± 0.31 to 60.42 ± 0.25 nA/mg/l with increasing the flow rate from 0 to 1 ml/min, respectively. Integrating the LTCC material increased the sensor lifetime as well as enhanced the response time to 10.9 s, making it suitable for biological applications.
Research in electrochemical detection has enabled the development of several oxygen biosensors that are suitable for human health care monitoring, but in general, are not flexible and cannot be fully integrated with the human body [47,118]. Therefore, Ashley et al. [48] exploited the concept of epidermal electronics to develop a skin-like wearable biosensor for real-time oxygen and lactate monitoring. The oxygen sensor relied on an innovative horseshoe-like structure with repeated circular gold working electrodes encased in a flexible polyimide layer or commercially available wound dressing (Fig. 6C). As such, the sensor can conform to any shape and enhances the sensor's mechanical stability under distortion (twisting, pinching, and stretching). Two oxygen-permeable membranes, nafion and PDMS with varying concentrations, were used to coat the gold electrodes for improving the oxygen selectivity, hence enhancing the electrochemical responses. Although the developed oxygen sensor achieved a high sensitivity of 21.6 nA/[O2] %, the slow response time of 69s and non-medical related dynamic range of 58.5–178 [O2] % hindered the use of the sensor for wound monitoring where the oxygen concentration varies between 0 and 27.7 [O2] %. Therefore, further studies are needed to integrate the skin-like mechanical properties into a sensor that could potentially be used for wound status monitoring.
Thread-based sensors have several advantages: high stability and durability, large surface area, flexibility, and inexpensive fabrication processes, making them suitable for developing point-of-care biosensors [[119], [120], [121], [122]]. Another vital advantage of utilizing thread-based sesnors in oxygen sensing is the ability to place them inside the tissue for deep-tissue measurements [67]. For instance, Xia et al. [49] fabricated a flexible silver-coated thread-based electrochemical oxygen sensor capable of quantifying local dissolved oxygen levels using point measurements or the average oxygen levels over a surface area using wire measurements (Fig. 6D). Local point measurements were achieved by adopting a novel clean-room free fabrication technique to confine the sensing element at the tip of the thread. Upon characterizing the sensor, a stable electrical signal was obtained within 90s with a linear response when exposing the sensor to dissolved oxygen concentrations of 0–55.47 mg L−1, covering the physiological oxygen levels between 0 and 12.42 mg L−1. The limit of detection of local and surface measurements was obtained to be 1.68 ppm and 0.95 ppm, respectively. The sensors showed good repeatability and high stability during mechanical deformation and at different pH levels with high sensitivity of 1.94 ± 0.35 nA mg−1 L−1 and 867.8 ± 87 nA mg−1 L−1 for local and surface measurements, respectively. Deep tissue measurements were mimicked using a hydrogel-based tissue model, showing the sensor's capability in measuring oxygen levels inside tissues.
In closing, electrochemical devices have been widely utilized in the detection of oxygen due to their high sensitivity, low cost, portability, and ease of fabrication using traditional microfabrication techniques. Although most of the sensors have been fabricated to monitor dissolved oxygen concentration in static environment, real time oxygen sensing in a flowing medium have also been explored. Most of the developed sensors are not flexible and cannot be fully integrated with the human body. Thus, textile like fabrics has been exploited in measuring oxygen levels inside tissues due to its flexibility, high stability and durability.
4. Challenges and future perspectives
Various colorimetric and electrochemical sensors have been designed to monitor the wound environment continuously. The main challenge with biosensors is their reliance on a precise volume of wound fluid for optimal functioning. However, the collection of wound fluid directly at the site is a complex task. As a result, only a limited number of devices are suitable for in vivo environments. To overcome this, advanced technologies such as microneedle arrays, microfluidic channels, and innovative materials must be utilized. These advancements enable the controlled and efficient collection of wound fluid, facilitating the development of biosensors that can effectively operate in real-world scenarios.
Additionally, researchers currently face the challenge of ensuring cost-effectiveness and ease of use for these sensors. However, the application of fabrication techniques such as lithography, bioprinting, and laser cutting has the potential to overcome these hurdles by enabling large-scale manufacturing with customizable structures. By utilizing these methods, the production process can be streamlined, leading to more affordable sensor manufacturing. Additionally, the ability to customize the sensor structures allows for tailoring them to specific applications, enhancing usability and optimizing their performance. These fabrication techniques offer promising solutions for improving the cost-effectiveness and user-friendliness of sensors.
Furthermore, Colorimetric sensors, particularly, face a significant limitation in terms of accuracy. For example, a temperature sensor based on PDMS (polydimethylsiloxane) exhibited an accuracy of 0.2 °C within the range of 29–40 °C, which hampers its effectiveness when compared to electrochemical sensors. However, the incorporation of advanced image processing techniques and artificial intelligence-driven image analysis holds promise in improving accuracy and precision levels [123]. By leveraging these technologies, it becomes possible to achieve an accuracy level of 0.001 °C or even surpass it, thereby enhancing the reliability of colorimetric sensors.
The current focus of research is primarily on the detection of individual biomarkers using biosensors. However, relying on a single type of biosensor often limits our ability to gain a comprehensive understanding of wound infection status. Therefore, it becomes necessary to combine different sensing modalities. By integrating multiple sensors into a single system, it becomes possible to analyze multiple biomarkers simultaneously [124]. Achieving this combination requires consistent sampling sources and a unified method for interpreting outputs. This approach aims to minimize the size of the sensing system, enhance biocompatibility, and provide users with a more comprehensive understanding. Ultimately, multifunctional sensors can reduce errors, improve data accuracy, and expand the overall capabilities of biosensors.
In addition, while current research trends predominantly focus on biomarker detection, there is a growing need for collaborative efforts to incorporate therapeutic compounds into in situ wound monitoring systems [125]. By combining the detection of biomarkers with the administration of therapeutic agents, the overall effectiveness of these devices can be significantly enhanced. This approach emphasizes not only the diagnosis of wound conditions but also the active promotion of wound healing. By integrating both aspects, researchers can develop more comprehensive and efficient systems that have the potential to improve patient outcomes and accelerate the healing process.
Despite the significant focus on technological advancements and evaluations in laboratory settings or animal models, it is imperative to thoroughly assess the validity and usability of wearable bioelectronic devices on human subjects, adhering to ethical guidelines and regulations. Extensive testing and scrutiny are essential to ensure the safety, efficacy, and ethical compliance of these devices when applied to real-world scenarios involving human beings.
5. Conclusions
Microbial infections in wounds pose a significant challenge to the healing of chronic wounds. The precise and timely detection of infections through real-time monitoring of diverse biomarkers associated with wound healing, can play a crucial role in the effective treatment and care of chronic wounds. By enabling early detection and continuous monitoring, healthcare providers can take proactive measures to address infections promptly and optimize the healing process. In this context, various colorimetric and electrochemical biosensors have been proposed for real-time monitoring of wound infection status by quantifying specific biomarkers, such as pH, temperature and tissue oxygenation.
This review provides a comprehensive overview of recent scientific advancements in the field of monitoring wound infection status using biosensors. The article delves into various scientific methodologies employed for measuring key parameters like pH, temperature, and oxygen levels in wounds, utilizing both colorimetric and electrochemical sensors. Furthermore, the review emphasizes the innovative strategies devised by researchers to address specific limitations associated with biosensors, such as the issue of dye leaching in colorimetric detection, as well as challenges related to temporal stability and spatial resolution. Through precise descriptions and relevant illustrations, this article offers valuable insights into the progress made in biosensor-based wound infection monitoring.
The review also highlights significant limitations of current biosensors in monitoring wound status, including their reliance on precise volume collection, cost-effectiveness, performance level, and single biomarker focus. These limitations hinder the clinical translation of proposed sensors. However, the integration of modern technologies such as microneedle arrays, artificial intelligence, and advanced fabrication techniques holds the potential to enhance sensor performance and cost-effectiveness. By overcoming these challenges, biosensors could be effectively translated into clinical settings to improve wound monitoring and patient care.
Statement of significance
Chronic wounds are among the major healthcare issues affecting millions of people worldwide with high rates of morbidity, losses of limbs and mortality. Accurate, timely and early detection of infections, and real time monitoring of various wound healing biomarkers can be significantly helpful in the treatment and care of chronic wounds. Thus, numerous smart bandages have emerged to provide in situ physiochemical surveillance in real time at the wound site. These bandages integrate smart sensors to detect the condition of wound infection based on various parameters, such as pH, temperature and oxygen level in the wound which reduces the frequency of changing the wound dressings and its associated complications.
In biosensors category, one latest review article similar to this topic named “Wearable Bioelectronics for Chronic Wound Management” was published in December 2021. However, the article provided a brief idea to measure individual parameters via biosensors. This article mainly focused on the mechanism of detecting those parameters rather than providing detailed overview about the latest innovation regarding monitoring of wound status in real time. Similarly, a review article named “Real-Time Monitoring of Wound States via Rationally Engineered Biosensors” was published in December 2022. Though this paper included sensors with different working mechanism/principles, it included very few research paper of each type. Thus, there was a room to expand the knowledge on this field by providing detailed information of the working principles of different types of biosensors with detailed overview of recent explorations.
Declaration of competing interest
As the lead author of this article, I can confirm that neither I nor any of my co-authors have any financial or personal relationships that could be perceived as a potential conflict of interest. We have no affiliations or financial involvement with any organization or entity that has a direct or indirect interest in the subject matter of this article.
We believe that it is important to disclose any potential conflicts of interest in scientific publications to maintain the integrity and transparency of the research process. As such, we confirm that we have no conflicts of interest to declare and that the research presented in this article has been conducted in an unbiased and objective manner.
Acknowledgement
The authors, A Hasan would like to acknowledge the grant NPRP12S-310-190-276, funded by Qatar National Research Foundation (QNRF) a part of Qatar Foundation. The authors, AA, PR, KY would like to acknowledge the financial support of the European Space Agency through the MAP program (WHISKY), and Lassonde Innovation Fund. All statements made herein are sole responsibilities of the authors.
Contributor Information
Anwarul Hasan, Email: hasan.anwarul.mit@gmail.com.
Alidad Amirfazli, Email: alidad2@yorku.ca.
Data availability
No data was used for the research described in the article.
References
- 1.Mahnke A., Meier R.J., Schatz V., Hofmann J., Castiglione K., Schleicher U., Wolfbeis O.S., Bogdan C., Jantsch J. Hypoxia in leishmania major skin lesions impairs the NO-dependent leishmanicidal activity of macrophages. J. Invest. Dermatol. 2014;134:2339–2346. doi: 10.1038/jid.2014.121. [DOI] [PubMed] [Google Scholar]
- 2.Sen C.K. Human wounds and its burden: an updated compendium of estimates. Adv. Wound Care. 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R., Afreen A., Tariq M., Zahid A.A., Masoud M.S., Ahmed M., Ali I., Akram Z., Hasan A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021;16 doi: 10.1088/1748-605X/abc28b. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R., Tariq M., Ali I., Asghar R., Noorunnisa Khanam P., Augustine R., Hasan A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018;120:385–393. doi: 10.1016/j.ijbiomac.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 5.Augustine R., Hasan A., Dalvi Y.B., Rehman S.R.U., Varghese R., Unni R.N., Yalcin H.C., Alfkey R., Thomas S., Al Moustafa A.E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111519. [DOI] [PubMed] [Google Scholar]
- 6.Rezvani Ghomi E., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Ramakrishna S. Wound dressings: current advances and future directions. J. Appl. Polym. Sci. 2019;136 doi: 10.1002/app.47738. [DOI] [Google Scholar]
- 7.Zahid A.A., Augustine R., Dalvi Y.B., Reshma K., Ahmed R., Raza ur Rehman S., Marei H.E., Alfkey R., Hasan A. Development of nitric oxide releasing visible light crosslinked gelatin methacrylate hydrogel for rapid closure of diabetic wounds. Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111747. [DOI] [PubMed] [Google Scholar]
- 8.Zehra M., Zubairi W., Hasan A., Butt H., Ramzan A., Azam M., Mehmood A., Falahati M., Anwar Chaudhry A., Ur Rehman I., Yar M. Oxygen generating polymeric nano fibers that stimulate angiogenesis and show efficient wound healing in a diabetic wound model. Int. J. Nanomed. 2020;15:3511–3522. doi: 10.2147/IJN.S248911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Guo M., He B., Gao B. Intelligent patches for wound management: in situ sensing and treatment. Anal. Chem. 2021 doi: 10.1021/acs.analchem.0c04956. [DOI] [PubMed] [Google Scholar]
- 10.Tang N., Zheng Y., Cui D., Haick H. Multifunctional dressing for wound diagnosis and rehabilitation. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202101292. [DOI] [PubMed] [Google Scholar]
- 11.Brown M.S., Ashley B., Koh A. Wearable technology for chronic wound monitoring: current dressings, advancements, and future prospects. Front. Bioeng. Biotechnol. 2018;6:1–21. doi: 10.3389/fbioe.2018.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargaville T.R., Farrugia B.L., Broadbent J.A., Pace S., Upton Z., Voelcker N.H. Sensors and imaging for wound healing: a review. Biosens. Bioelectron. 2013;41:30–42. doi: 10.1016/j.bios.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Derakhshandeh H., Kashaf S.S., Aghabaglou F., Ghanavati I.O., Tamayol A. Smart bandages: the future of wound care. Trends Biotechnol. 2018;36:1259–1274. doi: 10.1016/j.tibtech.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francesko A., Petkova P., Tzanov T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2019;25:5782–5797. doi: 10.2174/0929867324666170920161246. [DOI] [PubMed] [Google Scholar]
- 15.McColl D., MacDougall M., Watret L., Connolly P. 2009. Monitoring Moisture without Disturbing the Wound Dressing. (Wounds UK) [Google Scholar]
- 16.Ates H.C., Brunauer A., von Stetten F., Urban G.A., Güder F., Merkoçi A., Früh S.M., Dincer C. Integrated devices for non-invasive diagnostics. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202010388. [DOI] [Google Scholar]
- 17.Suarato G., Bertorelli R., Athanassiou A. Borrowing from nature: biopolymers and biocomposites as smart wound care materials. Front. Bioeng. Biotechnol. 2018;6:1–11. doi: 10.3389/fbioe.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin M., Guo H., Dai Z., Yan X., Ning X. Advances in flexible and wearable pH sensors for wound healing monitoring. J. Semiconduct. 2019 doi: 10.1088/1674-4926/40/11/111607. [DOI] [Google Scholar]
- 19.Tang N., Zheng Y., Haick H., Jiang X., Zhou C., Jin H., Jin K., Wu W. Wearable sensors and systems for wound healing-related ph and temperature detection. Micromachines. 2021 doi: 10.3390/mi12040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez A.C.D.O., Andrade Z.D.A., Costa T.F., Medrado A.R.A.P. Wound healing - a literature review. An. Bras. Dermatol. 2016 doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindley L.E., Stojadinovic O., Pastar I., Tomic-Canic M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016;138:18S–28S. doi: 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorg H., Tilkorn D.J., Hager S., Hauser J., Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. 2017;58:81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson H.N., Hardman M.J. Wound healing: cellular mechanisms and pathological outcomes: cellular Mechanisms of Wound Repair. Open Biol. 2020;10 doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yussof S.J.M., Omar E., Pai D.R., Sood S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012;45:220–228. doi: 10.4103/0970-0358.101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boothby I.C., Cohen J.N., Rosenblum M.D. Regulatory T cells in skin injury: at the crossroads of tolerance and tissue repair. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.aaz9631. eaaz9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty R., Borah P., Dutta P.P., Sen S. Evolving spectrum of diabetic wound: mechanistic insights and therapeutic targets. World J. Diabetes. 2022;13:696–716. doi: 10.4239/wjd.v13.i9.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijayakumar V., Samal S.K., Mohanty S., Nayak S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019;122:137–148. doi: 10.1016/j.ijbiomac.2018.10.120. [DOI] [PubMed] [Google Scholar]
- 28.Frykberg R.G., Banks J. Challenges in the treatment of chronic wounds. Adv. Wound Care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fui L.W., Lok M.P.W., Govindasamy V., Yong T.K., Lek T.K., Das A.K. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J. Tissue Eng. Regen. Med. 2019;13:2218–2233. doi: 10.1002/term.2966. [DOI] [PubMed] [Google Scholar]
- 30.Tamayol A., Akbari M., Zilberman Y., Comotto M., Lesha E., Serex L., Bagherifard S., Chen Y., Fu G., Ameri S.K., Ruan W., Miller E.L., Dokmeci M.R., Sonkusale S., Khademhosseini A. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv. Healthcare Mater. 2016;5:711–719. doi: 10.1002/adhm.201500553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Li X., Nagao M., Elias A.L., Narain R., Chung H.J. A pH-Indicating colorimetric tough hydrogel patch towards applications in a substrate for smart wound dressings. Polymers (Basel) 2017;9:558. doi: 10.3390/polym9110558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan N., Qin J., Feng P., Li Z., Song B. Color-changing smart fibrous materials for naked eye real-time monitoring of wound pH. J. Mater. Chem. B. 2019;7:2626–2633. doi: 10.1039/c9tb00195f. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Vu H., Senkowsky J., Hu W., Tang L. A near-infrared fluorescent pH sensing film for wound milieu pH monitoring. Exp. Dermatol. 2020;29:107–111. doi: 10.1111/exd.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guinovart T., Valdés-Ramírez G., Windmiller J.R., Andrade F.J., Wang J. Bandage-based wearable potentiometric sensor for monitoring wound pH. Electroanalysis. 2014;26:1345–1353. doi: 10.1002/elan.201300558. [DOI] [Google Scholar]
- 35.Du Y.C., Ciou W.S. A Sensor gauze with multi-channel moisture and ph monitoring for chronic wound care. IEEE Access. 2019;7:29185–29192. doi: 10.1109/ACCESS.2019.2901061. [DOI] [Google Scholar]
- 36.García-Guzmán J.J., Pérez-Ràfols C., Cuartero M., Crespo G.A. Toward in vivo transdermal pH sensing with a validated microneedle membrane electrode. ACS Sens. 2021;6:1129–1137. doi: 10.1021/acssensors.0c02397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charaya H., La T.G., Rieger J., Chung H.J. Thermochromic and piezocapacitive flexible sensor array by combining composite elastomer dielectrics and transparent ionic hydrogel electrodes. Adv. Mater. Technol. 2019;4 doi: 10.1002/admt.201900327. [DOI] [Google Scholar]
- 38.He Y., Li W., Han N., Wang J., Zhang X. Facile flexible reversible thermochromic membranes based on micro/nanoencapsulated phase change materials for wearable temperature sensor. Appl. Energy. 2019;247:615–629. doi: 10.1016/j.apenergy.2019.04.077. [DOI] [Google Scholar]
- 39.Lee J., Kim W.K. PEGylated graphene oxide-based colorimetric sensor for recording temperature. J. Ind. Eng. Chem. 2021;94:457–464. doi: 10.1016/j.jiec.2020.11.021. [DOI] [Google Scholar]
- 40.Hattori Y., Falgout L., Lee W., Jung S.Y., Poon E., Lee J.W., Na I., Geisler A., Sadhwani D., Zhang Y., Su Y., Wang X., Liu Z., Xia J., Cheng H., Webb R.C., Bonifas A.P., Won P., Jeong J.W., Jang K.I., Song Y.M., Nardone B., Nodzenski M., Fan J.A., Huang Y., West D.P., Paller A.S., Alam M., Yeo W.H., Rogers J.A. Multifunctional skin-like electronics for quantitative, clinical monitoring of cutaneous wound healing. Adv. Healthcare Mater. 2014;3:1597–1607. doi: 10.1002/adhm.201400073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trung T.Q., Ramasundaram S., Hwang B.U., Lee N.E. An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 2016;28:502–509. doi: 10.1002/adma.201504441. [DOI] [PubMed] [Google Scholar]
- 42.Salvo P., Calisi N., Melai B., Dini V., Paoletti C., Lomonaco T., Pucci A., Di Francesco F., Piaggesi A., Romanelli M. Temperature-and pH-sensitive wearable materials for monitoring foot ulcers. Int. J. Nanomed. 2017;12:949–954. doi: 10.2147/IJN.S121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Z., Roussakis E., Koolen P.G.L., Ibrahim A.M.S., Kim K., Rose L.F., Wu J., Nichols A.J., Baek Y., Birngruber R., Apiou-Sbirlea G., Matyal R., Huang T., Chan R., Lin S.J., Evans C.L. Non-invasive transdermal two-dimensional mapping of cutaneous oxygenation with a rapid-drying liquid bandage. Biomed. Opt Express. 2014;5:3748. doi: 10.1364/boe.5.003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavakol D.N., Schwager S.C., Jeffries L.A., Bruce A., Corliss B.A., Derosa C.A., Fraser C.L., Peirce S.M., Cottler P.S. Oxygen-sensing biomaterial construct for clinical monitoring of wound healing. Adv. Skin Wound Care. 2020;33:428–436. doi: 10.1097/01.ASW.0000666912.86854.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochoa M., Rahimi R., Zhou J., Jiang H., Yoon C.K., Maddipatla D., Narakathu B.B., Jain V., Oscai M.M., Morken T.J., Oliveira R.H., Campana G.L., Cummings O.W., Zieger M.A., Sood R., Atashbar M.Z., Ziaie B. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020;6 doi: 10.1038/s41378-020-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koley G., Liu J., Nomani M.W., Yim M., Wen X., Hsia T.Y. Miniaturized implantable pressure and oxygen sensors based on polydimethylsiloxane thin films. Mater. Sci. Eng. C. 2009;29:685–690. doi: 10.1016/j.msec.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo J., Dziubla T., Eitel R. A low temperature co-fired ceramic based microfluidic Clark-type oxygen sensor for real-time oxygen sensing. Sensor. Actuator. B Chem. 2017;240:392–397. doi: 10.1016/j.snb.2016.08.180. [DOI] [Google Scholar]
- 48.Ashley B.K., Brown M.S., Park Y., Kuan S., Koh A. Skin-inspired, open mesh electrochemical sensors for lactate and oxygen monitoring. Biosens. Bioelectron. 2019;132:343–351. doi: 10.1016/j.bios.2019.02.041. [DOI] [PubMed] [Google Scholar]
- 49.Xia J., Sonkusale S. Flexible thread-based electrochemical sensors for oxygen monitoring. Analyst. 2021;146:2983–2990. doi: 10.1039/d0an02400g. [DOI] [PubMed] [Google Scholar]
- 50.Khan M.A., Ansari U., Ali M.N. Real-time wound management through integrated pH sensors: a review. Sens. Rev. 2015;35:183–189. doi: 10.1108/SR-08-2014-689. [DOI] [Google Scholar]
- 51.Schneider L.A., Korber A., Grabbe S., Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 2007 doi: 10.1007/s00403-006-0713-x. [DOI] [PubMed] [Google Scholar]
- 52.Kassal P., Zubak M., Scheipl G., Mohr G.J., Steinberg M.D., Murković Steinberg I. Smart bandage with wireless connectivity for optical monitoring of pH. Sensor. Actuator. B Chem. 2017;246:455–460. doi: 10.1016/j.snb.2017.02.095. [DOI] [Google Scholar]
- 53.Nischwitz S.P., Bernardelli de Mattos I., Hofmann E., Groeber-Becker F., Funk M., Mohr G.J., Branski L.K., Mautner S.I., Kamolz L.P. Continuous pH monitoring in wounds using a composite indicator dressing — a feasibility study. Burns. 2019;45:1336–1341. doi: 10.1016/j.burns.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Jankowska D.A., Bannwarth M.B., Schulenburg C., Faccio G., Maniura-Weber K., Rossi R.M., Scherer L., Richter M., Boesel L.F. Simultaneous detection of pH value and glucose concentrations for wound monitoring applications. Biosens. Bioelectron. 2017;87:312–319. doi: 10.1016/j.bios.2016.08.072. [DOI] [PubMed] [Google Scholar]
- 55.Panzarasa G., Osypova A., Toncelli C., Buhmann M.T., Rottmar M., Ren Q., Maniura-Weber K., Rossi R.M., Boesel L.F. The pyranine-benzalkonium ion pair: a promising fluorescent system for the ratiometric detection of wound pH. Sensor. Actuator. B Chem. 2017;249:156–160. doi: 10.1016/j.snb.2017.04.045. [DOI] [Google Scholar]
- 56.Yang P., Zhu Z., Zhang T., Zhang W., Chen W., Cao Y., Chen M., Zhou X. Orange-emissive carbon quantum dots: toward application in wound pH monitoring based on colorimetric and fluorescent changing. Small. 2019;15 doi: 10.1002/smll.201902823. [DOI] [PubMed] [Google Scholar]
- 57.Pakolpakçıl A., Osman B., Göktalay G., Özer E.T., Şahan Y., Becerir B., Karaca E. Design and in vivo evaluation of alginate-based pH-sensing electrospun wound dressing containing anthocyanins. J. Polym. Res. 2021;28:3. doi: 10.1007/s10965-020-02400-1. [DOI] [Google Scholar]
- 58.Ghoneim M.T., Nguyen A., Dereje N., Huang J., Moore G.C., Murzynowski P.J., Dagdeviren C. Recent progress in electrochemical pH-sensing materials and configurations for biomedical applications. Chem. Rev. 2019;119:5248–5297. doi: 10.1021/acs.chemrev.8b00655. [DOI] [PubMed] [Google Scholar]
- 59.Lu Y., Aimetti A.A., Langer R., Gu Z. Bioresponsive materials. Nat. Rev. Mater. 2016;2 doi: 10.1038/natrevmats.2016.75. [DOI] [Google Scholar]
- 60.Guinovart T., Crespo G.A., Rius F.X., Andrade F.J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta. 2014;821:72–80. doi: 10.1016/j.aca.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 61.Yoon J.H., Hong S.B., Yun S.O., Lee S.J., Lee T.J., Lee K.G., Choi B.G. High performance flexible pH sensor based on polyaniline nanopillar array electrode. J. Colloid Interface Sci. 2017;490:53–58. doi: 10.1016/j.jcis.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 62.Nyein H.Y.Y., Gao W., Shahpar Z., Emaminejad S., Challa S., Chen K., Fahad H.M., Tai L.C., Ota H., Davis R.W., Javey A. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano. 2016;10:7216–7224. doi: 10.1021/acsnano.6b04005. [DOI] [PubMed] [Google Scholar]
- 63.Rahimi R., Ochoa M., Tamayol A., Khalili S., Khademhosseini A., Ziaie B. Highly stretchable potentiometric pH sensor fabricated via laser carbonization and machining of Carbon−Polyaniline composite. ACS Appl. Mater. Interfaces. 2017;9:9015–9023. doi: 10.1021/acsami.6b16228. [DOI] [PubMed] [Google Scholar]
- 64.Pal A., Goswami D., Cuellar H.E., Castro B., Kuang S., Martinez R.V. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 2018;117:696–705. doi: 10.1016/j.bios.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 65.Mostafalu P., Tamayol A., Rahimi R., Ochoa M., Khalilpour A., Kiaee G., Yazdi I.K., Bagherifard S., Dokmeci M.R., Ziaie B., Sonkusale S.R., Khademhosseini A. Smart bandage for monitoring and treatment of chronic wounds. Small. 2018;14 doi: 10.1002/smll.201703509. [DOI] [PubMed] [Google Scholar]
- 66.Guinovart T., Parrilla M., Crespo G.A., Rius F.X., Andrade F.J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst. 2013;138:5208–5215. doi: 10.1039/c3an00710c. [DOI] [PubMed] [Google Scholar]
- 67.Mostafalu P., Akbari M., Alberti K.A., Xu Q., Khademhosseini A., Sonkusale S.R. A toolkit of thread-based microfluidics, sensors, and electronics for 3D tissue embedding for medical diagnostics. Microsyst. Nanoeng. 2016;2:1–10. doi: 10.1038/micronano.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahimi R., Brener U., Ochoa M., Ziaie B. Flexible and transparent pH monitoring system with NFC communication for wound monitoring applications. Proc. - IEEE Int. Conf. Micro Electro Mech. Syst. (MEMS) 2017:125–128. doi: 10.1109/MEMSYS.2017.7863356. [DOI] [Google Scholar]
- 69.Rahimi R., Brener U., Chittiboyina S., Soleimani T., Detwiler D.A., Lelièvre S.A., Ziaie B. Laser-enabled fabrication of flexible and transparent pH sensor with near-field communication for in-situ monitoring of wound infection. Sensor. Actuator. B Chem. 2018;267:198–207. doi: 10.1016/j.snb.2018.04.004. [DOI] [Google Scholar]
- 70.Antohe I., Jinga L.I., Antohe V.A., Socol G. Sensitive ph monitoring using a polyaniline-functionalized fiber optic—surface plasmon resonance detector. Sensors. 2021;21 doi: 10.3390/s21124218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manjakkal L., Szwagierczak D., Dahiya R. Metal oxides based electrochemical pH sensors: current progress and future perspectives. Prog. Mater. Sci. 2020;109 doi: 10.1016/j.pmatsci.2019.100635. [DOI] [Google Scholar]
- 72.Nakata S., Shiomi M., Fujita Y., Arie T., Akita S., Takei K. A wearable pH sensor with high sensitivity based on a flexible charge-coupled device. Nat. Electron. 2018;1:596–603. doi: 10.1038/s41928-018-0162-5. [DOI] [Google Scholar]
- 73.Sridhar V., Takahata K. A hydrogel-based passive wireless sensor using a flex-circuit inductive transducer. Sensors Actuators, A Phys. 2009;155:58–65. doi: 10.1016/j.sna.2009.08.010. [DOI] [Google Scholar]
- 74.Smith R.E., Totti S., Velliou E., Campagnolo P., Hingley-Wilson S.M., Ward N.I., Varcoe J.R., Crean C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sensor. Actuator. B Chem. 2019;287:338–345. doi: 10.1016/j.snb.2019.01.088. [DOI] [Google Scholar]
- 75.Punjiya M., Rezaei H., Zeeshan M.A., Sonkusale S. TRANSDUCERS 2017 - 19th Int. Conf. Solid-State Sensors. 2017. A flexible pH sensing smart bandage with wireless CMOS readout for chronic wound monitoring. Actuators Microsystems 1700–1702. [DOI] [Google Scholar]
- 76.Lyu B., Punjiya M., Matharu Z., Sonkusale S. An improved pH mapping bandage with thread-based sensors for chronic wound monitoring. Proc. - IEEE Int. Symp. Circuits Syst. 2018-May. 2018:1–4. doi: 10.1109/ISCAS.2018.8351878. [DOI] [Google Scholar]
- 77.Kim S., Oh S., Jung Y., Moon H., Lim H. Customizable, flexible pressure, and temperature step sensors with human skinlike color. ACS Omega. 2018;3:1110–1116. doi: 10.1021/acsomega.7b01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choe A., Yeom J., Shanker R., Kim M.P., Kang S., Ko H. Stretchable and wearable colorimetric patches based on thermoresponsive plasmonic microgels embedded in a hydrogel film. NPG Asia Mater. 2018;10:912–922. doi: 10.1038/s41427-018-0086-6. [DOI] [Google Scholar]
- 79.Chen Y., Xi Y., Ke Y., Li W., Long Y., Li J., Wang L.N., Zhang X. A skin-like stretchable colorimetric temperature sensor. Sci. China Mater. 2018;61:969–976. doi: 10.1007/s40843-018-9266-8. [DOI] [Google Scholar]
- 80.Lee J., Kim Y., kwan, Lee S., Yoon S., Kim W. keun. Graphene oxide-based NET strategy for enhanced colorimetric sensing of miRNA. Sensor. Actuator. B Chem. 2019;282:861–867. doi: 10.1016/j.snb.2018.11.149. [DOI] [Google Scholar]
- 81.Kim D.H., Wang S., Keum H., Ghaffari R., Kim Y.S., Tao H., Panilaitis B., Li M., Kang Z., Omenetto F., Huang Y., Rogers J.A. Thin, flexible sensors and actuators as “instrumented” surgical sutures for targeted wound monitoring and therapy. Small. 2012;8:3263–3268. doi: 10.1002/smll.201200933. [DOI] [PubMed] [Google Scholar]
- 82.Webb R.C., Bonifas A.P., Behnaz A., Zhang Y., Yu K.J., Cheng H., Shi M., Bian Z., Liu Z., Kim Y.S., Yeo W.H., Park J.S., Song J., Li Y., Huang Y., Gorbach A.M., Rogers J.A. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013;12:938–944. doi: 10.1038/nmat3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu D., Yan Y., Avila R., Kandela I., Stepien I., Seo M.H., Bai W., Yang Q., Li C., Haney C.R., Waters E.A., MacEwan M.R., Huang Y., Ray W.Z., Rogers J.A. Bioresorbable, wireless, passive sensors as temporary implants for monitoring regional body temperature. Adv. Healthcare Mater. 2020;9 doi: 10.1002/adhm.202000942. [DOI] [PubMed] [Google Scholar]
- 84.Oh J.H., Hong S.Y., Park H., Jin S.W., Jeong Y.R., Oh S.Y., Yun J., Lee H., Kim J.W., Ha J.S. Fabrication of high-sensitivity skin-attachable temperature sensors with bioinspired microstructured adhesive. ACS Appl. Mater. Interfaces. 2018;10:7263–7270. doi: 10.1021/acsami.7b17727. [DOI] [PubMed] [Google Scholar]
- 85.Ota H., Chao M., Gao Y., Wu E., Tai L.C., Chen K., Matsuoka Y., Iwai K., Fahad H.M., Gao W., Nyein H.Y.Y., Lin L., Javey A. 3D printed “earable” smart devices for real-time detection of core body temperature. ACS Sens. 2017;2:990–997. doi: 10.1021/acssensors.7b00247. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto Y., Yamamoto D., Takada M., Naito H., Arie T., Akita S., Takei K. Efficient skin temperature sensor and stable gel-less sticky ECG sensor for a wearable flexible healthcare patch. Adv. Healthcare Mater. 2017;6 doi: 10.1002/adhm.201700495. [DOI] [PubMed] [Google Scholar]
- 87.Alven S., Nqoro X., Aderibigbe B.A. Polymer-based materials loaded with curcumin for wound healing applications. Polymers (Basel) 2020;12:1–25. doi: 10.3390/polym12102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Honda W., Harada S., Arie T., Akita S., Takei K. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv. Funct. Mater. 2014;24:3299–3304. doi: 10.1002/adfm.201303874. [DOI] [Google Scholar]
- 89.Zhou C., Tang N., Zhang X., Fang Y., Jiang Y., Zhang H., Duan X. Simultaneously optimize the response speed and sensitivity of low dimension conductive polymers for epidermal temperature sensing applications. Front. Chem. 2020;8:194. doi: 10.3389/fchem.2020.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gong M., Wan P., Ma D., Zhong M., Liao M., Ye J., Shi R., Zhang L. Flexible breathable nanomesh electronic devices for on-demand therapy. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201902127. [DOI] [Google Scholar]
- 91.Pang Q., Lou D., Li S., Wang G., Qiao B., Dong S., Ma L., Gao C., Wu Z. Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds. Adv. Sci. 2020;7 doi: 10.1002/advs.201902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lou D., Pang Q., Pei X., Dong S., Li S., Tan W., qiang, Ma L. Flexible wound healing system for pro-regeneration, temperature monitoring and infection early warning. Biosens. Bioelectron. 2020;162 doi: 10.1016/j.bios.2020.112275. [DOI] [PubMed] [Google Scholar]
- 93.Wang Q., Ling S., Liang X., Wang H., Lu H., Zhang Y. Self-healable multifunctional electronic tattoos based on silk and graphene. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201808695. [DOI] [Google Scholar]
- 94.Sun Y., Yang L., Xia K., Liu H., Han D., Zhang Y., Zhang J. Snowing” graphene using microwave ovens. Adv. Mater. 2018;30 doi: 10.1002/adma.201803189. [DOI] [PubMed] [Google Scholar]
- 95.Wu J., Huang W., Liang Y., Wu Z., Zhong B., Zhou Z., Ye J., Tao K., Zhou Y., Xie X. Self-calibrated, sensitive, and flexible temperature sensor based on 3D chemically modified graphene hydrogel. Adv. Electron. Mater. 2021;7 doi: 10.1002/aelm.202001084. [DOI] [Google Scholar]
- 96.Sen C.K. Wound Repair Regen. 2009. Wound healing essentials: let there be oxygen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bishop A. Role of oxygen in wound healing. J. Wound Care. 2008;17:399–402. doi: 10.12968/jowc.2008.17.9.30937. [DOI] [PubMed] [Google Scholar]
- 98.Dunn J., Grider M.H. StatPearls; 2020. Physiology, Adenosine Triphosphate (ATP) [PubMed] [Google Scholar]
- 99.Sano H., Ichioka S., Sekiya N. Influence of oxygen on wound healing dynamics: assessment in a novel wound mouse model under a variable oxygen environment. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Younis I. Role of oxygen in wound healing. J. Wound Care. 2020;29:S4–S10. doi: 10.12968/jowc.2020.29.Sup5b.S4. [DOI] [PubMed] [Google Scholar]
- 101.Hofmann J., Meier R.J., Mahnke A., Schatz V., Brackmann F., Trollmann R., Bogdan C., Liebsch G., Wang X.D., Wolfbeis O.S., Jantsch J. Ratiometric luminescence 2D in vivo imaging and monitoring of mouse skin oxygenation. Methods Appl. Fluoresc. 2013;1 doi: 10.1088/2050-6120/1/4/045002. [DOI] [PubMed] [Google Scholar]
- 102.Lin H., Shen Y., Chen D., Lin L., Wilson B.C., Li B., Xie S. Feasibility Study on quantitative measurements of singlet oxygen generation using singlet oxygen sensor green. J. Fluoresc. 2013;23:41–47. doi: 10.1007/s10895-012-1114-5. [DOI] [PubMed] [Google Scholar]
- 103.Wang X.D., Wolfbeis O.S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 2014 doi: 10.1039/c4cs00039k. [DOI] [PubMed] [Google Scholar]
- 104.Wang X.D., Meier R.J., Schmittlein C., Schreml S., Schäferling M., Wolfbeis O.S. A water-sprayable, thermogelating and biocompatible polymer host for use in fluorescent chemical sensing and imaging of oxygen, pH values and temperature. Sensor. Actuator. B Chem. 2015;221:37–44. doi: 10.1016/j.snb.2015.05.082. [DOI] [Google Scholar]
- 105.Schreml S., Meier R.J., Weiß K.T., Cattani J., Flittner D., Gehmert S., Wolfbeis O.S., Landthaler M., Babilas P. A sprayable luminescent pH sensor and its use for wound imaging in vivo. Exp. Dermatol. 2012 doi: 10.1111/exd.12042. [DOI] [PubMed] [Google Scholar]
- 106.DeRosa C.A., Seaman S.A., Mathew A.S., Gorick C.M., Fan Z., Demas J.N., Peirce S.M., Fraser C.L. Oxygen sensing difluoroboron β-diketonate polylactide materials with tunable dynamic ranges for wound imaging. ACS Sens. 2016;1:1366–1373. doi: 10.1021/acssensors.6b00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordillo G.M., Schlanger R., Wallace W.A., Bergdall V., Bartlett R., Sen C.K. Protocols for topical and systemic oxygen treatments in wound healing. Methods Enzymol. 2004;381:575–585. doi: 10.1016/S0076-6879(04)81037-1. [DOI] [PubMed] [Google Scholar]
- 108.Howard M.A., Asmis R., Evans K.K., Mustoe T.A. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21:503–511. doi: 10.1111/wrr.12069. [DOI] [PubMed] [Google Scholar]
- 109.Kranke P., Bennett M.H., Martyn-St James M., Schnabel A., Debus S.E. Hyperbaric oxygen therapy for treating chronic wounds. Diving Hyperb. Med. 2012;42:237. doi: 10.1002/14651858.CD004123.PUB3. [DOI] [Google Scholar]
- 110.Dissemond J., Kröger K., Storck M., Risse A., Engels P. Topical oxygen wound therapies for chronic wounds: a review. J. Wound Care. 2015;24:53–63. doi: 10.12968/jowc.2015.24.2.53. [DOI] [PubMed] [Google Scholar]
- 111.Roussakis E., Li Z., Nowell N.H., Nichols A.J., Evans C.L. Bright, “clickable” porphyrins for the visualization of oxygenation under ambient light. Angew. Chem. Int. Ed. 2015;54:14728–14731. doi: 10.1002/anie.201506847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim Jeonghyun, Salvatore G.A., Araki H., Chiarelli A.M., Xie Z., Banks A., Sheng X., Liu Y., Lee J.W., Jang K.I., Heo S.Y., Cho K., Luo H., Zimmerman B., Kim Joonhee, Yan L., Feng X., Xu S., Fabiani M., Gratton G., Huang Y., Paik U., Rogers J.A. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mostafalu P., Lenk W., Dokmeci M.R., Ziaie B., Khademhosseini A., Sonkusale S.R. Wireless flexible smart bandage for continuous monitoring of wound oxygenation. IEEE Trans. Biomed. Circuits Syst. 2015;9:670–677. doi: 10.1109/TBCAS.2015.2488582. [DOI] [PubMed] [Google Scholar]
- 114.Salvo P., Dini V., Di Francesco F., Romanelli M. The role of biomedical sensors in wound healing. Wound Med. 2015;8:15–18. doi: 10.1016/j.wndm.2015.03.007. [DOI] [Google Scholar]
- 115.Van Der Weerd B., Bierl R., Matysik F.M. Trends in electrochemical sensing of blood gases. Bioanal. Rev. 2017;6:263–280. doi: 10.1007/11663_2016_1. [DOI] [Google Scholar]
- 116.Hutton L., Newton M.E., Unwin P.R., Macpherson J.V. Amperometric oxygen sensor based on a platinum nanoparticle-modified polycrystalline boron doped diamond disk electrode. Anal. Chem. 2009;81:1023–1032. doi: 10.1021/ac8020906. [DOI] [PubMed] [Google Scholar]
- 117.Compton R.G., Foord J.S., Marken F. Electroanalysis at diamond-like and doped-diamond electrodes. Electroanalysis. 2003;15:1349–1363. doi: 10.1002/elan.200302830. [DOI] [Google Scholar]
- 118.Wang P., Liu Y., Abruña H.D., Spector J.A., Olbricht W.L. Micromachined dissolved oxygen sensor based on solid polymer electrolyte. Sensor. Actuator. B Chem. 2011;153:145–151. doi: 10.1016/j.snb.2010.09.075. [DOI] [Google Scholar]
- 119.Acar G., Ozturk O., Golparvar A.J., Elboshra T.A., Böhringer K., Kaya Yapici M. Wearable and flexible textile electrodes for biopotential signal monitoring: a review. Electron. 2019;8:479. doi: 10.3390/electronics8050479. [DOI] [Google Scholar]
- 120.Hatamie A., Angizi S., Kumar S., Pandey C.M., Simchi A., Willander M., Malhotra B.D. Review—textile based chemical and physical sensors for healthcare monitoring. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab6827. [DOI] [Google Scholar]
- 121.Mostafalu P., Tamayol A., Rahimi R., Ochoa M., Khalilpour A., Kiaee G., Yazdi I.K., Bagherifard S., Dokmeci M.R., Ziaie B., Sonkusale S.R., Khademhosseini A. Smart bandage for monitoring and treatment of chronic wounds. Small. 2018;14 doi: 10.1002/smll.201703509. [DOI] [PubMed] [Google Scholar]
- 122.Terse-Thakoor T., Punjiya M., Matharu Z., Lyu B., Ahmad M., Giles G.E., Owyeung R., Alaimo F., Shojaei Baghini M., Brunyé T.T., Sonkusale S. Thread-based multiplexed sensor patch for real-time sweat monitoring. npj Flex. Electron. 2020;4:1–10. doi: 10.1038/s41528-020-00081-w. [DOI] [Google Scholar]
- 123.Zheng X.T., Yang Z., Sutarlie L., Thangaveloo M., Yu Y., Salleh N.A.B.M., Chin J.S., Xiong Z., Becker D.L., Loh X.J., Tee B.C.K., Su X. Battery-free and AI-enabled multiplexed sensor patches for wound monitoring. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adg6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shirzaei Sani E., Xu C., Wang C., Song Y., Min J., Tu J., Solomon S.A., Li J., Banks J.L., Armstrong D.G., Gao W. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pang Q., Yang F., Jiang Z., Wu K., Hou R., Zhu Y. Smart wound dressing for advanced wound management: real-time monitoring and on-demand treatment. Mater. Des. 2023;229 doi: 10.1016/j.matdes.2023.111917. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.