Abstract
Background
Progressive motor impairment anatomically attributable to prominent, focally atrophic lateral column spinal cord lesions (“critical lesions”), can be seen in multiple sclerosis (MS); e.g., progressive hemiparetic MS.
Objective
To investigate whether similar spinal cord lesions are more frequent in longstanding MS patients with secondary progressive motor impairment (SPMS) vs those maintaining a relapsing-remitting course (RRMS).
Methods
We retrospectively identified Olmsted County (MN, USA) residents on December 31, 2011 with: 1) RRMS or SPMS for ≥25 years; and 2) available brain and spine MRI. A blinded neuroradiologist determined demyelinating lesion burden and presence of potential critical lesions (prominent focally atrophic spinal cord lateral column lesions).
Results
Thirty-two patients were included: RRMS, 18; SPMS, 14. Median (range) disease duration (34 [27–53] vs 39 [29–47] years) and relapse number (4 [1–10] vs 3 [1–15]) were similar. SPMS patients more commonly showed potential critical spinal cord lesions (8/18 [44%] vs 14/14 [100%]), higher spinal cord (median [range]: 4 [1–7] vs 7.5 [3–12]), and brain-infratentorial (median [range]: 1 [0–12] vs 2.5 [1–13]) lesion number; p<0.05. By multivariate analysis, only the presence of potential critical lesions independently associated with motor progression (p=0.02).
Conclusion
Critical spinal cord lesions may be important contributors to motor progression in MS.
Introduction
We previously reported MS patients with highly restricted CNS lesion burden (≤5 lesions),(1–3) or exclusively unilateral motor impairment,(4) in whom motor progression was anatomically attributable to single demyelinating lesions along critical corticospinal tract segments, typically in the spinal cord lateral columns (where a single demyelinating lesion has a higher likelihood of affecting the lateral corticospinal tract). These lesions, that we termed “critical lesions”, were designated as such to highlight their strong association with progressive motor impairment. On MRI, critical lesions are characterized by prominent size and severe focal atrophy.(4) In these prior studies we were able to show that motor progression was anatomically attributable to those lesions by analyzing patients with a limited CNS lesion burden or motor deficit restricted to a certain region (i.e., progressive unilateral hemiparesis).(4) However, in most MS patients the large number of CNS lesions and presence of bilateral motor impairment make it difficult to determine an anatomical association between individual lesions and motor progression.
While the majority of patients with relapsing-remitting MS (RRMS) develop secondary progressive disability (secondary progressive MS – SPMS) after many years of disease, and usually do so in the form of progressive motor impairment, a minority of RRMS patients do not develop a secondary progressive course.(5, 6) The reasons behind these different outcomes in patients with RRMS are unclear and the main drivers of secondary progression are still poorly understood. Direct comparisons between patients with RRMS and SPMS are often confounded by the differences in disease duration, typically shorter in patients with RRMS that have not yet developed secondary progressive disability. When undertaking a case-control study to compare patients that do develop motor progression (SPMS) to those that do not (and remain RRMS), it is crucial to study patients with longstanding disease to reduce the risk of including RRMS patients that would later convert to SPMS. Furthermore, when investigating the potential contributory role of severe cortical spinal tract lesions to motor progression, it is important to exclude patients with other types of secondary progressive disease (e.g., isolated secondary progressive ataxia). For this study, we hypothesized that spinal cord lesions with the aforementioned MRI characteristics of critical lesions (i.e., prominent, focally atrophic lesions in the spinal cord lateral columns) would be more common in longstanding MS patients who developed secondary progressive motor impairment than those maintaining a relapsing-remitting clinical course over a similar time-period.
Materials and Methods
The study was approved by the Mayo Clinic Institutional Review Board. All patients consented to the use of their medical records for research.
Patients
From a previously reported prevalent cohort (December 31, 2011) of 309 Olmsted County residents (Minnesota, USA; 85% Caucasian),(7) we identified those with: 1) diagnosis of RRMS or SPMS by 2017 revised McDonald’s MS criteria;(8) 2) ≥25 years of disease from MS clinical presentation; and 2) available brain and cervical (±thoracic) spinal cord MRI. SPMS patients without motor impairment (i.e., secondary progressive ataxia/cognitive impairment), or tumefactive demyelination were excluded (Figure 1). Demographics and clinical data were abstracted (E.S., M.B.), including number and type of clinical relapses (myelitis vs other), and prior disease-modifying treatments (stratified as highly effective [ocrelizumab, rituximab, natalizumab, alemtuzumab, mitoxantrone] vs low-moderately effective [interferon-β, glatiramer acetate, dimethyl fumarate, teriflunomide or fingolimod]).
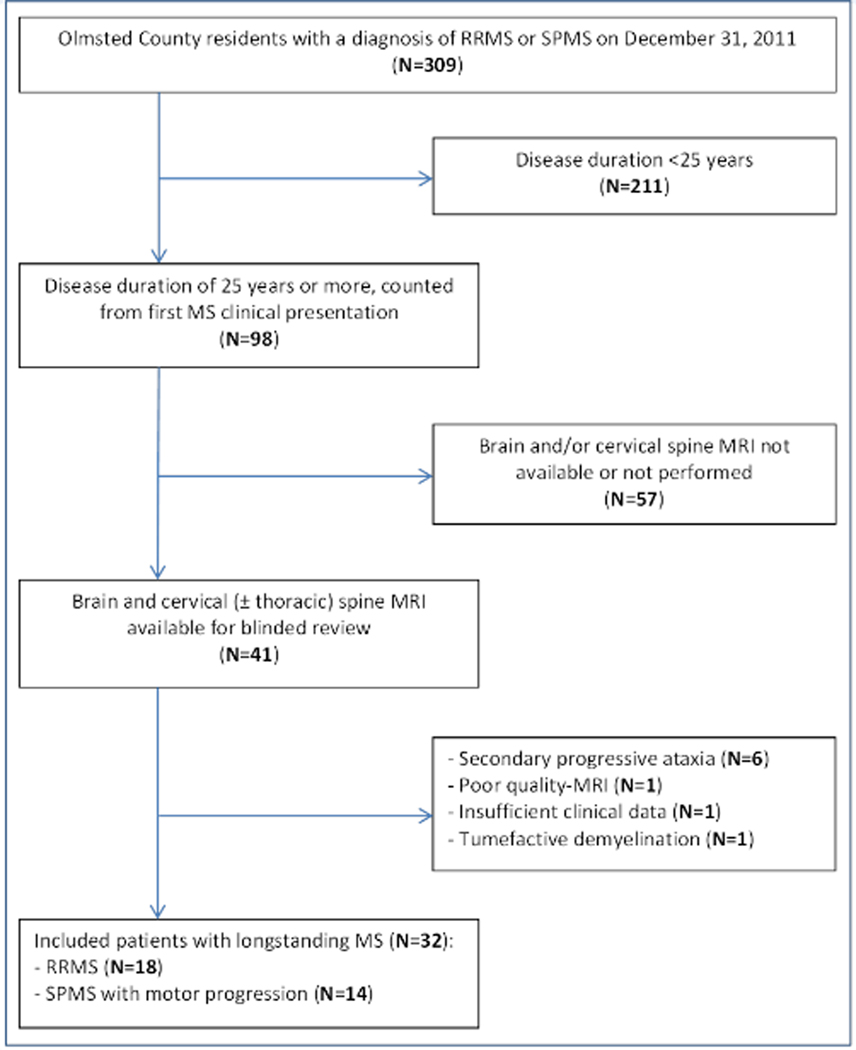
Figure 1 – Search strategy for identification of MS patients in Olmsted County.
The flow-chart illustrates the search strategy utilized to identify Olmsted County residents on December, 31 2011 with a diagnosis of RRMS or SPMS for ≥25 years.
Neuroimaging
A blinded neuroradiologist (S.M.) reviewed the last available brain and spinal cord MRI to determine: 1) total CNS demyelinating lesion burden; 2) presence/absence of potential critical spinal cord lesions (defined as prominent lateral column T2-hyperintense lesions accompanied by severe focal atrophy appreciable on axial images); and 3) spinal cord cross-sectional area at C3 and C7 vertebral-body levels (manually assessed on representative T2-images; QREADS Clinical Image Viewer 5.10.8.). The presence of severe focal lesion atrophy that define a potential critical lesion was visually assessed by the neuroradiologist as a marked, unequivocal alteration of the physiologic rounded shape of a hemi-cord on axial T2-weighted images at the level and side of the spinal cord lesion (typically assuming a triangular shape; Figure 1, B2-B4). For identified potential critical lesions, the largest axial T2-lesion area (manually outlined on representative images) and length on sagittal T2-weighted images was assessed. In situations in which there was more than one spinal cord lateral column lesion on MRI with prominent size and severe atrophy in the same patient, the neuroradiologist was asked to identify and measure the most prominent lesion.
Assessment of demyelinating lesions (of any type) was performed on all available sequences including fluid attenuated inversion recovery (FLAIR), T2-weighted, and T1-weighted sequences pre- and post-gadolinium in the brain; while for the spinal cord T2-weighted sequences sometimes with complementary use of short tau inversion recovery (STIR) sequences, and T1-weighted sequences pre- and post-gadolinium were used. Regional lesion burden was stratified as follows: 1) overall brain lesion burden (more or less than 15 total lesions); 2) number of brain infratentorial lesions; and 3) number of cervical and, when available, thoracic spinal cord lesions. Only lesions that were felt by the neuroradiologist to be unequivocally demyelinating were recorded, similar to our prior studies.(2, 4) Examined MRIs were obtained at Mayo Clinic, Rochester, in all but one case.
Statistics
Continuous and categorical variables were reported as median (range) and number (percentages), and compared by using the Wilcoxon rank sum and Fisher’s exact tests, as appropriate. The simultaneous association between clinical/MRI variables found to be significantly different between the two groups and secondary progressive motor impairment was explored by multivariate logistic regression (JMP Pro 14.1.0). Odds ratios (OR) and 95% confidence intervals (CI) were reported; p-values <0.05 were considered statistically significant.
Results
Thirty-two patients were included: RRMS, 18; SPMS, 14. Their clinical and MRI characteristics are summarized (Table 1) and examples of different spinal cord lesions illustrated (Figure 2). Thoracic spinal cord MRI was available in 12/18 (67%) RRMS patients and 10/14 (71%) SPMS patients. No statistically significant difference was observed between the two groups in age at MS presentation, total number and type of clinical relapses, and treatment with disease modifying agents over years (Table 1).
Table 1 –
Comparison of clinical and MRI characteristics in RRMS and SPMS patients with longstanding disease; results are shown as number/total (%) or median (range).
| RRMS (n=18) | SPMS (n=14) | P | |
|---|---|---|---|
| Demographics | |||
| Female gender | 15/18 (83%) | 12/14 (86%) | 1.0 |
| Caucasian | 18/18 (100%) | 15/15 (100%) | 1.0 |
| Clinical | |||
| Age at MS onset | 25 (12–43) | 23 (14–51) | 0.83 |
| Age at last follow-up | 60 (46–76) | 65 (54–84) | 0.13 |
| Total number of relapses | 4 (1–10) | 3 (1–15) | 0.69 |
| Total number of myelitis relapses | 2 (0–9) | 2 (0–10) | 0.59 |
| DMT ever | 10/18 (56%) | 6/14 (43%) | 0.72 |
| Highly effective DMT | 2/18 (11%) | 1/14 (7%) | 1.0 |
| EDSS last follow-up | 2 (1–4) | 6 (4–8) | <0.001 |
| Follow-up duration, years | 34 (27–53) | 39 (29–47) | 0.22 |
| Brain MRI | |||
| Brain lesions (>15 lesions) | 10/18 (56%) | 10/14 (71%) | 0.47 |
| Infratentorial lesion number | 1 (0–12) | 2.5 (1–13) | 0.02 |
| Onset to last brain MRI, years | 32 (26–50) | 37 (29–44) | 0.34 |
| Spinal cord MRI | |||
| Spinal cord lesion number | 4 (1–7) | 7.5 (3–12) | 0.002 |
| Cervical spinal cord | 2.5 (0–6) | 4 (2–8) | 0.02 |
| Thoracic spinal cord | 1 (0–4) | 4 (0–6) | 0.01 |
| Frequency of any CST lesion (≥1) | 17/18 (94%) | 14/14 (100%) | 1.0 |
| Cervical spinal cord | 14/18 (78%) | 13/14 (93%) | 0.35 |
| Thoracic spinal cord | 6/12 (50%) | 7/10 (70%) | 0.41 |
| Frequency of “potential critical lesions” (≥1) | 8/18 (44%) | 14/14 (100%) | 0.001 |
| Cervical spinal cord | 5/18 (28%) | 12/14 (86%) | 0.002 |
| Thoracic spinal cord | 3/12 (25%) | 3/10 (30%) | 1.0 |
| Lesion cross-sectional area, mm2 | 15 (8–18.1) | 13.1 (4.2–23.4) | 0.9 |
| Lesion sagittal length, mm2 | 10.9 (7–22.3) | 13.5 (7.4–22.1) | 0.45 |
| C3 cross-sectional area, mm2 | 74.2 (59.5–99.2) | 71.2 (49.4–91.8) | 0.3 |
| C7 cross-sectional area, mm2 | 62.7 (51–82.5) | 60.3 (41.3–79.5) | 0.47 |
| Onset to last cervical MRI, years | 31 (21–49) | 36 (27–44) | 0.30 |
| Onset to last thoracic MRI, years | 30 (24–40) | 38 (20–44) | 0.06 |
Abbreviations: CST = corticospinal tracts; DMT = disease-modifying treatment; EDSS = expanded disability status scale score; RRMS = relapsing remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis*
Figure 2 – Representative MRI examples of brain lesions and spinal cord lateral column lesions in patients with RRMS (left column) and SPMS (right column).
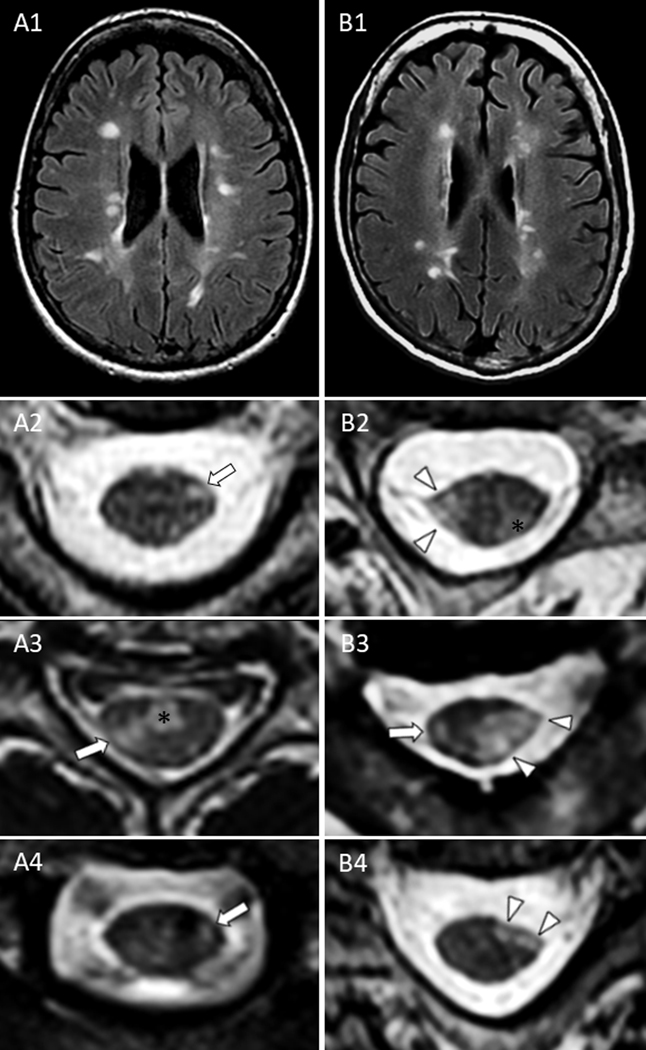
Despite a similar brain lesion burden on axial fluid attenuated inversion recovery (A1-B1), cervical spine lateral column lesions in patients with RRMS were typically characterized by smaller foci of T2-hyperintensity (less likely to involve the lateral corticospinal tracts) without atrophy on axial images (A2-A4, arrows) that differed from the large, markedly atrophic lesions of SPMS patients (B2-B4, arrowheads). The asterisks (*) indicate additional ventral (A3) and dorso-lateral (B2) prominent demyelinating lesions similarly noted in both groups, but located outside the corticospinal tracts and therefore unlikely to contribute to motor progression.
Comparison of MRI characteristics
All patients had spinal cord lesions. Potential critical lesions (severe focally atrophic lateral column spinal cord lesions) were more common in SPMS patients (14/14 [100%]) compared to RRMS patients (8/18 [44%]); p=0.001. This difference remained statistically significant if one imputed that all RRMS patients with missing thoracic spinal cord MRI had one or more potential critical lesion in the thoracic spinal cord (p=0.02). In four patients >1 potential critical lesions were identified (SPMS, 2; RRMS, 2). SPMS patients also had a higher number of lesions in the cervical spinal cord (p=0.02), and infratentorial lesions on brain MRI (p=0.02); Table 1. On multivariate analysis controlling for the number of brain infratentorial lesions, number of cervical spinal cord lesions , and presence/absence of potential critical lesions in the cervical spinal cord, only the presence of potential critical lesions remained independently associated with motor progression (unadjusted OR for presence vs absence of potential cervical critical lesions = 15.6 [95% CI, 2.5–96]; adjusted OR = 8.5 [95% CI, 1.2–59.8]; p=0.02).
Location of lesions
In all SPMS patients the most prominent potential critical lesion was identified in the cervical spinal cord, except for two patients (one with paraparesis and one with lower limb monoparesis) in whom the potential critical lesion was identified in the thoracic spinal cord. In five patients with SPMS, the progressive motor impairment was unilateral (hemiparesis, 2; lower-limb monoparesis, 3) and always affected the side of the identified potential critical lesion. All patients with bilateral motor progression had bilateral spinal cord lateral column demyelinating lesions of variable prominence and atrophy.
Discussion
In this population-based case-control study of patients with longstanding MS, those with secondary progressive motor impairment were more likely to have severe focally atrophic corticospinal tract lesions in the spinal cord than those maintaining a RRMS course despite similar disease duration, and number of clinical relapses. The presence of such severely atrophic corticospinal tract lesions in the spinal cord was the only variable independently associated with secondary progressive motor impairment.
Numerous studies have now shown the importance of spinal cord involvement in MS,(9–11) and that motor progression in some MS patients can be anatomically attributed to severe lesions located in eloquent corticospinal tract regions, which we previously termed critical lesions.(1–4) In this study we assessed the presence of spinal cord lateral column lesions with similar MRI characteristics of critical lesions (prominent size, severe focal atrophy) in patients with longstanding MS and showed that they are over-represented in those with secondary progressive motor impairment versus those that have maintained a relapsing-remitting course. These findings suggest that patients with RRMS who fail to develop corticospinal tract lesions in the spinal cord, or those in whom corticospinal tract lesions are smaller and do not develop severe atrophy over time, might be less likely to develop secondary progressive motor impairment.
In this study, we found all SPMS patients had at least one severe focally atrophic lesion along the spinal cord lateral columns, similar to our prior findings that all MS patients with unilateral motor progression had critical corticospinal tract lesions anatomically corresponding to their motor deficit.(4) Corticospinal tracts lesions were also detected in the majority (89%) of RRMS patients but many were smaller and/or not accompanied by focal atrophy (Figure 2). SPMS patients also had a higher number of spinal cord and infratentorial brain lesions, although these associations were no longer significant after accounting for the presence of severe focally atrophic lesions along the spinal cord lateral columns. These findings confirm the importance of accounting for both brain and spinal cord MRI variables in assessing potential associations with disability in MS, as previously shown.(10) A recent 30-year observational study of patients with MS found the baseline presence of brain infratentorial lesions to be the strongest predictor of secondary progressive MS, although the concomitant effect of spinal cord MRI variables was not assessed.(12)
In contrast to many prior studies showing a strong association between cervical spinal cord atrophy and SPMS,(13–15) the difference in cervical spinal cord atrophy in our population-based cohorts did not reach statistical significance and the presence of focally atrophic lateral column lesions had a stronger association with SPMS. The retrospective design and analysis of MRIs obtained over two decades required a visual-based assessment for severe focal atrophy by an experienced neuroradiologist, rather than specialized quantitative imaging techniques to measure atrophy.(16) Our results are consistent with prior findings showing greater spinal cord lesion volume in MS (equivalent to the prominent size that characterize critical lesions) better correlates with disability than spinal cord lesion number or smaller cross-sectional area.(17) Furthermore, the motor progression of progressive solitary sclerosis and some MS patients with ≤5 lesions in which a single corticospinal tract lesion anatomically explains motor progression suggests lesion location is a more important contributor to motor progression than total spinal cord lesion number or global atrophy.(1, 2)
The number of clinical relapses, including myelitis, was similar in the two groups despite the higher spinal cord lesion burden in SPMS, indicating accumulation of asymptomatic spinal cord lesions in some patients. It is notable that one patient in the SPMS group developed motor progression without prior overt myelitis attacks, suggesting critical lesions may be initially asymptomatic, and become symptomatic over time in conjunction with progressive lesion atrophy. This resembles the occurrence of progressive disability in patients with radiologically isolated syndrome transitioning to primary progressive MS, or patients with progressive solitary sclerosis or unilateral motor progression and a progressive course from onset.(1, 4, 18) This is also consistent with the recently described “silent progression” observed in some MS patients after years of disease despite no clinical/MRI evidence of disease activity.(19)
Why seemingly severe focally atrophic lesions along spinal cord lateral columns are also found in patients without motor progression (44% of RRMS patients in this study) is unclear. It is possible that these patients may develop motor progression attributable to that lesion with a longer follow-up duration, that they would need more than one lesion with similar characteristics to develop motor progression (e.g., some patients with unilateral motor progression in our prior study had two tandem critical lesions anatomically explanatory for their motor impairment),(4) or that they have a greater functional neural reserve that does not allow morphologic changes to become clinically overt.(20) Intra-lesional factors as well as patient-specific factors underlying progression development warrant further study; the presence of chronic inflammatory changes that are undetectable with conventional MRI scans might explain the different evolution of certain lesions over time (e.g., progressive atrophy vs stabilization).(21)
Our study is limited by its retrospective nature, lack of standardized MRI parameters (i.e., the degree of prominent size and atrophy was variable and often dependent on the size of the other corticospinal tract lesions present), and the small sample size due to the relative rarity of population-based patients with longstanding disease. However, the selective inclusion of these patients allowed us to highlight major differences between the two groups despite the limited statistical power, that could have been less evident in patients with shorter disease-duration (i.e., less likely to have already developed SPMS despite the presence of critical lesions). The long follow-up of included patients is also consistent with the relatively low frequency of patients ever treated with disease-modifying agents (mostly low-moderately effective) during the study period (i.e., patients were required to have MS symptoms for 25 years or longer on December 2011) in which many drugs were being approved for MS treatment, and the specific effect of highly effective MS drugs on critical lesions development/evolution still has to be determined. Brain atrophy, always considered an important contributor for MS progression,(22, 23) was not analyzed in this study but it would be unlikely to explain asymmetric/unilateral face-sparing motor progression (five in this cohort). Despite the blinded assessment of MRI images, the single neuroradiologist could have been more prone to call a high proportion of potential critical spinal cord lesions as expected from the study design. However, most of the potential critical lesions were identified in patients with SPMS while the majority of RRMS patients did not have a single potential critical lesion identifiable, which is consistent with our study hypothesis. Further studies will clarify the frequency of potential critical lesions in RRMS patients with short disease-history that we excluded due to the selected study design focusing on patients with longstanding disease. Lastly, MS patients excluded due to lacking brain and/or cervical spine MRI potentially may differ from the analyzed cohort regarding disease severity and MRI features, but the median EDSS is typical of that of unselected patients with SPMS and RRMS.
To conclude, in this rare population-based cohort of patients with longstanding MS we found the presence of critical spinal cord lesions to be the main discriminant factor between those who developed secondary progressive motor impairment and those who maintained a relapsing-remitting course. Preventing the development of these lesions might be crucial to prevent disability in MS.
Acknowledgements
The authors would like to acknowledge the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology that provided support through a translational clinical fellowship grant assigned to E.S. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Ethical approval
The study was approved by the Institutional Review Board of the Mayo Clinic.
Informed consent
All patients consented to the use of their medical records for research.
Declaration of Conflicting Interests
B.M.K. is funded by Biogen, and receives publishing royalties for Common Pitfalls in Multiple Sclerosis and CNS Demyelinating Diseases. He is an Editorial Board member of Multiple Sclerosis and Related Disorders. B.G.W. receives royalties from RSR Ltd, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen GbR for a patent of NMO-IgG as a diagnostic test for NMO and related disorders. He serves as a member of an adjudication committee for clinical trials in NMO being conducted by VielaBio and Alexion pharmaceutical companies. He is a consultant for Chugai Pharma and Mitsubishi Tanabe regarding potential clinical trials for NMO. S.J.P. reports grants, personal fees and non-financial support from Alexion Pharmaceuticals, Inc.; grants from Grifols, Autoimmune Encephalitis Alliance; grants, personal fees, non-financial support and other from MedImmune, Inc.; Dr. Pittock has a patent # 9,891,219 (Application#12–573942) “Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an indivuidual that is Aquaporin-4 (AQP4)-IgG Autoantibody positive”. O.H.K. received speaker honoraria (paid to Mayo Clinic) from Novartis and Biogen; performed a grant review for The National Multiple Sclerosis Society; and received research support from Biogen, the Multiple Sclerosis Society, the Mayo Foundation, and the Hilton Foundation. E.P.F. receives research support as a site principal investigator in a randomized placebo-controlled clinical trial of Inebilizumab (A CD19 inhibitor) in neuromyelitis optica spectrum disorders funded by MedImmune/Viela Bio. E.S., S.M., and M.B. declare that there is no conflict of interest.
References
- 1.Keegan BM, Kaufmann TJ, Weinshenker BG, Kantarci OH, Schmalstieg WF, Paz Soldan MM, et al. Progressive solitary sclerosis: Gradual motor impairment from a single CNS demyelinating lesion. Neurology. 2016;87(16):1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keegan BM, Kaufmann TJ, Weinshenker BG, Kantarci OH, Schmalstieg WF, Paz Soldan MM, et al. Progressive motor impairment from a critically located lesion in highly restricted CNS-demyelinating disease. Mult Scler. 2018;24(11):1445–52. [DOI] [PubMed] [Google Scholar]
- 3.Schmalstieg WF, Keegan BM, Weinshenker BG. Solitary sclerosis: progressive myelopathy from solitary demyelinating lesion. Neurology. 2012;78(8):540–4. [DOI] [PubMed] [Google Scholar]
- 4.Sechi E, Keegan BM, Kaufmann TJ, Kantarci OH, Weinshenker BG, Flanagan EP. Unilateral motor progression in MS: Association with a critical corticospinal tract lesion. Neurology. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson EJ, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler. 2013;19(2):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol. 1994;36 Suppl:S6–11. [DOI] [PubMed] [Google Scholar]
- 7.Asnafi SM PP; Sechi E; Pittock SJ; Weinshenker BG; Palace J; Messina S; Flanagan EP The Frequency of Longitudinally Extensive Transverse Myelitis in MS; A Population-Based Study. Mult Scler Relat Disord. 2019. [DOI] [PubMed] [Google Scholar]
- 8.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. [DOI] [PubMed] [Google Scholar]
- 9.Eden D, Gros C, Badji A, Dupont SM, De Leener B, Maranzano J, et al. Spatial distribution of multiple sclerosis lesions in the cervical spinal cord. Brain. 2019;142(3):633–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearney H, Altmann DR, Samson RS, Yiannakas MC, Wheeler-Kingshott CA, Ciccarelli O, et al. Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology. 2015;84(4):367–73. [DOI] [PubMed] [Google Scholar]
- 11.Rocca MA, Valsasina P, Meani A, Gobbi C, Zecca C, Rovira A, et al. Clinically relevant cranio-caudal patterns of cervical cord atrophy evolution in MS. Neurology. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Chung KK, Altmann D, Barkhof F, Miszkiel K, Brex PA, O’Riordan J, et al. A 30-Year Clinical and Magnetic Resonance Imaging Observational Study of Multiple Sclerosis and Clinically Isolated Syndromes. Ann Neurol. 2020;87(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukas C, Knol DL, Sombekke MH, Bellenberg B, Hahn HK, Popescu V, et al. Cervical spinal cord volume loss is related to clinical disability progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(4):410–8. [DOI] [PubMed] [Google Scholar]
- 14.Rocca MA, Horsfield MA, Sala S, Copetti M, Valsasina P, Mesaros S, et al. A multicenter assessment of cervical cord atrophy among MS clinical phenotypes. Neurology. 2011;76(24):2096–102. [DOI] [PubMed] [Google Scholar]
- 15.Zeydan B, Gu X, Atkinson EJ, Keegan BM, Weinshenker BG, Tillema JM, et al. Cervical spinal cord atrophy: An early marker of progressive MS onset. Neurol Neuroimmunol Neuroinflamm. 2018;5(2):e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moccia M, Prados F, Filippi M, Rocca MA, Valsasina P, Brownlee WJ, et al. Longitudinal spinal cord atrophy in multiple sclerosis using the generalized boundary shift integral. Ann Neurol. 2019;86(5):704–13. [DOI] [PubMed] [Google Scholar]
- 17.Pravata E, Valsasina P, Gobbi C, Zecca C, Riccitelli GC, Filippi M, et al. Influence of CNS T2-focal lesions on cervical cord atrophy and disability in multiple sclerosis. Mult Scler. 2019:1352458519865989. [DOI] [PubMed] [Google Scholar]
- 18.Kantarci OH, Lebrun C, Siva A, Keegan MB, Azevedo CJ, Inglese M, et al. Primary Progressive Multiple Sclerosis Evolving From Radiologically Isolated Syndrome. Ann Neurol. 2016;79(2):288–94. [DOI] [PubMed] [Google Scholar]
- 19.University of California SFMSET, Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger SC, Cook K, De Nino S, Fletcher M. The topographical model of multiple sclerosis: A dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–76. [DOI] [PubMed] [Google Scholar]
- 23.Fadda G, Brown RA, Magliozzi R, Aubert-Broche B, O’Mahony J, Shinohara RT, et al. A surface-in gradient of thalamic damage evolves in pediatric multiple sclerosis. Ann Neurol. 2019;85(3):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]