Introduction
Lichen planus (LP) is a chronic inflammatory skin disease that affects cutaneous and mucosal sites and is often recalcitrant to topical therapies. Erosive lichen planus (ELP) is a severe variant which is often less responsive to therapy. ELP tends to occur on the oral and anogenital mucosa and modified mucous membranes but can also involve other mucosal sites, such as the esophagus. ELP significantly affects quality of life (QOL) and if left untreated, can lead to chronic scarring.1 Erosive vulvovaginal LP (EVVLP) can lead to narrowing of the introitus and midline fusion, resulting in pain, itch, irritation, dyspareunia, dysuria, and recurrent urinary tract infections due to urinary retention and obstruction.
Topical immunosuppression with corticosteroids or calcineurin inhibitors are usually considered first-line for EVVLP, while resistant disease may be treated with intralesional, oral, or intramuscular steroids.2 Studies have estimated that up to 40% of women with EVVLP do not respond to first-line topical steroid therapy and require systemic agents.3 Steroid-sparing agents such as methotrexate, mycophenolate mofetil, hydroxychloroquine, and rituximab have been used with varying levels of efficacy.4 Thus, there is significant need for more efficacious treatments, such as molecularly-targeted therapies for EVVLP.
While the pathogenesis of ELP is not completely understood, cytokines in the Janus kinase – signal transducer and activator of transcription (JAK-STAT) signaling pathway are thought to play a role in disease development and persistence.5 IFN-gamma has been shown to activate the JAK-STAT pathway in keratinocytes.5 Previous reports have demonstrated efficacy of Janus kinase (JAK) inhibitors in lichen planopilaris, nail LP, and recently in 3 patients with oral ELP.6, 7, 8, 9 A recent review summarizes the cases of LP where 3 JAK inhibitors were used with success: tofacitinib, baricitinib, and upadacitinib.10 Here, we report a case series of 6 patients with a diagnosis of EVVLP who were treated with oral tofacitinib.
Case series
This was a retrospective case series of 6 patients with severe EVVLP who were seen in a vulvar clinic between 2017 and 2022 at one of 2 dermatology sites (Southeast Vulvar Clinic and University of California) and were treated with oral tofacitinib. This study was approved by the Institutional Review Board (IRB). Before initiation of tofacitinib, a discussion regarding the risks of the medication and its “off-label” use ensued between the patient and prescriber. All patients provided verbal informed consent prior to initiation of tofacitinib.
Six patients with a diagnosis of refractory ELP were treated with tofacitinib 5 mg twice a day. Mean follow-up time was 3 years (6 months-6 years). As there are no validated scoring systems for EVVLP, changes in EVVLP activity were assessed by clinical and symptomatic improvement. The following parameters were used – morphologic changes: erythema, erosions/ulcerations, white reticulation; symptomatologic changes – pain, pruritus, burning, dyspareunia (if patients were sexually active). Representative clinical images of improvement of EVVLP (Fig 1), oral ELP (Fig 2 and 3) and anal ELP (Fig 4) in selected patients were included.
Fig 1.
Clinical images of vulvovaginal erosive lichen planus (LP) at baseline (A), 3 months on tofacitinib 5 mg oral twice a day (B), and 6 months on tofacitinib (C).
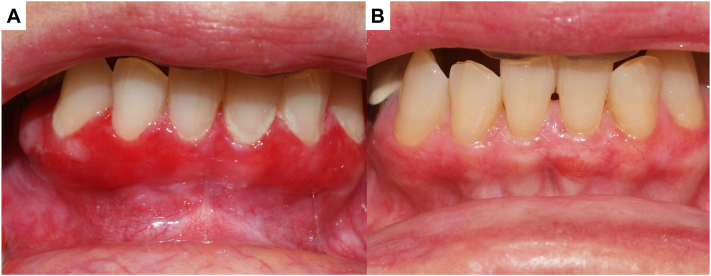
Fig 2.
Clinical images of oral erosive lichen planus (LP) at baseline (A), and 6 months on tofacitinib 5 mg oral twice a day (B).
Fig 3.
Clinical images of oral erosive lichen planus (LP) at baseline (A), and 6 months on tofacitinib 5 mg oral twice a day (B).
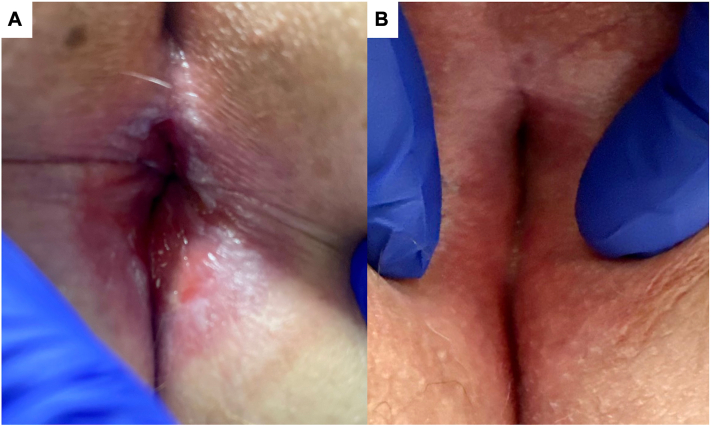
Fig 4.
Clinical images of anal erosive lichen planus (LP) at baseline (A), and 2 months on tofacitinib 5 mg oral twice a day (B).
The study population of 6 patients had a mean age of 61 years (35-76 years). All patients had both EVVLP and oral ELP. In addition, 1 had esophageal ELP, 1 had anal ELP, 2 had lichen planopilaris, and 2 had extragenital cutaneous LP. Except for 1 patient who had only undergone treatment with topical and systemic steroids prior to tofacitinib, all other patients were refractory to numerous treatments before initiation of tofacitinib (Table I). All 6 patients had marked improvement clinically and symptomatically in oral and vulvovaginal disease. One patient had clinical and symptomatic improvement in vulvovaginal disease as early as 6 weeks after starting tofacitinib. The patient with esophageal ELP and the patient with anal ELP also had improvement in esophageal and anal involvement, respectively, with tofacitinib.
Table I.
Summary of 6 patients with erosive vulvovaginal lichen planus (LP) treated with tofacitinib
| Patient | Age | Duration of disease prior to tofacitinib | Biopsy proven diagnosis | Prior systemic therapies | Prior topical therapies | Other skin/mucosal involvement | Duration of tofacitinib use | Adverse events |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | >15 y | Yes | Mycophenolate mofetil, prednisone, cyclosporine | High-potency topical steroids, tacrolimus | Anal, oral, scalp (lichen planopilaris) | 6 mo | ALT elevation |
| 2 | 58 | 6 y | Yes | Rituximab, prednisone, mycophenolate mofetil, hydroxychloroquine, methotrexate, cyclosporine, naltrexone, intravenous immunoglobulin | High-potency topical steroids, tacrolimus | Oral, scalp (lichen planopilaris) | 2 y | None |
| 3 | 71 | 10 y | Yes | Tacrolimus, mycophenolate mofetil, methotrexate, hydroxychloroquine, thalidomide, adalimumab, ustekinumab, naltrexone, apremilast | High-potency topical steroids, tacrolimus, pimecrolimus | Oral | 5 y | None |
| 4 | 76 | 20 y | Yes | Tacrolimus, mycophenolate mofetil, methotrexate, hydroxychloroquine, adalimumab, ustekinumab, naltrexone, etanercept, dapsone, ixekizumab | High-potency topical steroids, tacrolimus, intralesional steroids | Oral, esophageal | 3 y | GI bleeding while on tofacitinib. Resumed with no recurrence. |
| 5 | 55 | 3 y | Yes | Prednisone, cyclosporine, adalimumab | High-potency topical steroids, tacrolimus, intralesional steroids | Oral, extragenital cutaneous LP | 2 y | None |
| 6 | 35 | 2 mo | Yes | Prednisone | Topical high-potency steroids | Oral, extragenital cutaneous LP | 2 y | None |
Unfortunately, the patient with esophageal ELP experienced gastrointestinal (GI) bleeding while on the medication, though no source was identified. Her tofacitinib was discontinued per gastroenterology. However, tofacitinib was later resumed due to EVVLP severity, with clinical remission and no side effects or further gastrointestinal bleeding. In addition, the patient with anal involvement developed alanine aminotransferase (ALT) elevation, which is being followed but was deemed to not be of any clinical significance after evaluation by hepatology. One patient opted to self-discontinue tofacitinib as she had experienced complete resolution and she became concerned for risks of immunosuppression. However, within 2 months, she had recurrence of her disease and followed-up for flaring of vulvovaginal symptoms. After shared decision making, she opted to resume tofacitinib and has since resumed with improvement clinically and symptomatically.
All patients who continued oral tofacitinib remain clinically and symptomatically improved and are tolerating the medication without adverse effects. One patient has been lost to follow-up and 2 routinely require additional maintenance with intralesional triamcinolone injections for oral and vulvovaginal involvement.
Discussion
There are currently no Food and Drug Administration (FDA)-approved therapies for LP and off-label therapeutic algorithms have been proposed, without a clear standard of care treatment for EVVLP. Because of the detrimental effects on QOL and potential for irreversible scarring as well as possible increased risk of squamous cell carcinoma, EVVLP should be treated early and adequately to mitigate inflammation and long-term effects of chronic inflammation.1,2 Unfortunately, potent topical agents alone are often not sufficient for disease control of EVVLP.3 Systemic steroid-sparing options are important additional agents to prevent chronic systemic steroid use for recurrent flares in severe cases of EVVLP.
While this case series suggests that JAK inhibitors may be an effective treatment for refractory EVVLP, a detailed conversation should ensue between provider and patient when any oral JAK inhibitor is being considered for this diagnosis, including its off-label use and risks as well as cautions. It is important to consider the warnings with oral tofacitinib which include the increased risk of serious infections, major adverse cardiac events (MACEs), a higher rate of all-cause mortality, malignancies, and thrombosis.11, 12, 13 Dermatologists should use caution when prescribing JAK inhibitors, while also considering disease severity and the patient’s quality of life (QOL).
In summary, we report 6 patients with EVVLP who had clinical and symptomatic response with treatment with tofacitinib 5 mg twice daily. Five patients had severe EVVLP complicated by various stages of scarring and most patients had treatment-refractory disease. Limitations of our study include the retrospective nature, small number of patients, and lack of standardized outcome measures to assess disease severity. The results of this case series study suggest that JAK inhibition with tofacitinib may be effective in the treatment of refractory EVVLP. Future larger scale studies and randomized controlled trials with standardized outcomes are necessary to evaluate efficacy of oral tofacitinib for the treatment of EVVLP.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
Patient consent: All patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
IRB approval status: IRB was approved by the University of California, Irvine.
References
- 1.Ranum A., Pearson D.R. The impact of genital lichen sclerosus and lichen planus on quality of life: a review. Int J Womens Dermatol. 2022;8(3) doi: 10.1097/JW9.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauskar M.M., Marathe K., Venkatesan A., Schlosser B.J., Edwards L. Vulvar diseases: conditions in adults and children. J Am Acad Dermatol. 2020;82(6):1287–1298. doi: 10.1016/j.jaad.2019.10.077. [DOI] [PubMed] [Google Scholar]
- 3.Kherlopian A., Fischer G. Identifying predictors of systemic immunosuppressive treatment of vulvovaginal lichen planus: a retrospective cohort study of 122 women. Australas J Dermatol. 2022;63(3):335–343. doi: 10.1111/ajd.13851. [DOI] [PubMed] [Google Scholar]
- 4.Dunaway S., Tyler K., Kaffenberger J. Update on treatments for erosive vulvovaginal lichen planus. Int J Dermatol. 2020;59(3):297–302. doi: 10.1111/ijd.14692. [DOI] [PubMed] [Google Scholar]
- 5.Shao S., Tsoi L.C., Sarkar M.K., et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11(511) doi: 10.1126/scitranslmed.aav7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iorizzo M., Haneke E. Tofacitinib as treatment for nail lichen planus associated with alopecia universalis. JAMA Dermatology. 2021;157(3):352–353. doi: 10.1001/jamadermatol.2020.4555. [DOI] [PubMed] [Google Scholar]
- 7.Moussa A., Bhoyrul B., Asfour L., Kazmi A., Eisman S., Sinclair R.D. Treatment of lichen planopilaris with baricitinib: a retrospective study. J Am Acad Dermatol. 2022;87(3):663–666. doi: 10.1016/j.jaad.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Yang C.C., Khanna T., Sallee B., Christiano A.M., Bordone L.A. Tofacitinib for the treatment of lichen planopilaris: a case series. Dermatol Ther. 2018;31(6) doi: 10.1111/dth.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damsky W., Wang A., Olamiju B., Peterson D., Galan A., King B. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145(6):1708–1710.e2. doi: 10.1016/j.jaci.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Motamed-Sanaye A., Khazaee Y.F., Shokrgozar M., Alishahi M., Ahramiyanpour N., Amani M. JAK inhibitors in lichen planus: a review of pathogenesis and treatments. J Dermatolog Treat. 2022;33(8):3098–3103. doi: 10.1080/09546634.2022.2116926. [DOI] [PubMed] [Google Scholar]
- 11.Verden A., Dimbil M., Kyle R., Overstreet B., Hoffman K.B. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 2018;41(4):357–361. doi: 10.1007/s40264-017-0622-2. [DOI] [PubMed] [Google Scholar]
- 12.Mease P., Charles-Schoeman C., Cohen S., et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 2020;79(11):1400–1413. doi: 10.1136/annrheumdis-2019-216761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoisnard L., Lebrun-Vignes B., Maury S., et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-10777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]