Abstract
Health inequities are differences in health that are ‘unjust’. Yet, despite competing ethical views about what counts as an ‘unjust difference in health’, theoretical insights from ethics have not been systematically integrated into epidemiological research. Using diabetes as an example, we explore the impact of adopting different ethical standards of health equity on population health outcomes. Specifically, we explore how the implementation of population-level weight-loss interventions using different ethical standards of equity impacts the intervention's implementation and resultant population health outcomes. We conducted a risk prediction modelling study using the nationally representative 2015-16 Canadian Community Health Survey (n = 75,044, 54% women). We used the Diabetes Population Risk Tool (DPoRT) to calculate individual-level 10-year diabetes risk. Hypothetical weight-loss interventions were modelled in individuals with overweight or obesity based on each ethical standard: 1) health sufficiency (reduce DPoRT risk below a high-risk threshold (16.5%); 2) health equality (equalize DPoRT risk to the low risk group (5%)); 3) social-health sufficiency (reduce DPoRT risk <16.5 in individuals with lower education); 4) social-health equality (equalize DPoRT risk to the level of individuals with high education). For each scenario, we calculated intervention impacts, diabetes cases prevented or delayed, and relative and absolute educational inequities in diabetes. Overall, we estimated that achieving health sufficiency (i.e., all individuals below the diabetes risk threshold) was more feasible than achieving health equality (i.e., diabetes risk equalized for all individuals), requiring smaller initial investments and fewer interventions; however, fewer diabetes cases were prevented or delayed. Further, targeting only diabetes inequalities related to education reduced the target population size and number of interventions required, but consequently resulted in even fewer diabetes cases prevented or delayed. Using diabetes as an example, we found that an explicit, ethically-informed definition of health equity is essential to guide population-level interventions that aim to reduce health inequities.
Keywords: Health equity, Social justice, Ethics, Social epidemiology, Risk prediction, Population health interventions
Highlights
-
•
Health inequities are differences in health that are ‘unjust’.
-
•
Ethical standards of health equity are not often integrated into epidemiology.
-
•
Modelling health sufficiency and health equality found disparate diabetes outcomes.
-
•
Ethically-informed population-level interventions are essential to reduce health inequities.
1. Introduction
The reduction of health inequities—a priority of public health and health systems worldwide—is predicated not simply on reducing differences in health, but reducing differences in health that are considered to be unjust (Braveman et al., 2011; Smith, 2015). Hence, efforts to reduce health inequities require at least two activities: (1) describing differences in health and the effect public health interventions have on those differences—the primary task of epidemiologists—and; (2) identifying the ethical standards that indicate which differences in health count as ‘unjust’ and what a ‘just’ state of population health resembles—the primary task of ethicists (NCCDH, 2020). Despite much ethical debate about which ethical standard ought to guide equity-focused population-level health interventions, these standards have not been systematically considered or integrated into epidemiological research and practice (Asada, 2005, 2007; Asada et al., 2014; Beauchamp, 1976; Norheim & Asada, 2009; Persad, 2019; Smith, 2023). Public health practitioners are therefore less likely to be aware that different ethical standards of health equity exist, which may in turn render them inattentive to population health outcomes that are, at least according to some account(s), considered unjust. As a result, existing population-level interventions often proceed with non-existent, implicit, and/or conflicting views about which differences in health are unjust and which distribution of health outcomes population-level interventions should aim to achieve as a matter of equity (Harper et al., 2010). This can lead to critical differences regarding which populations are targeted, which health inequities are prioritized, and how equity-related population-level interventions are evaluated.
Little empirical guidance is available to policymakers and practitioners on the selection and implications of adopting different standards when designing or implementing population-level interventions to reduce health inequities. To fill this knowledge gap, we use diabetes as a case study to estimate the extent to which distinct ethical standards of health equity differentially impact population health outcomes. Specifically, we explore how the implementation of population-level weight-loss interventions using just two ethical standards of health equity—‘health sufficiency’ and ‘health equality’—impact the interventions' target population, benefit and scope, and the potential for achieving its goal of reducing health inequities. In so doing, we illustrate the significant value of explicitly integrating ethical considerations in the design, implementation, and evaluation of population-level interventions that aim to reduce health inequities.
1.1. Theory: ethical standards of health equity
Ethicists have advanced many competing views about what justice means and requires for public health (Daniels, 2007; Powers & Faden, 2006; Ruger, 2010; Segall, 2009; Smith, 2023; Venkatapuram, 2011). Public health researchers and practitioners are generally familiar with at least one distinction between competing ethical standards of health equity, i.e., between ‘equality’ and ‘equity’ (Chang, 2002). According to this distinction, the ethical objective when pursuing health equity is not to treat people equally (e.g., by allocating resources equally to all or to achieve equal health outcomes), but rather to treat people in accordance with their unique needs. Yet, this ethical distinction represents just one important ethical insight regarding the different ways ‘health equity’ is or ought to be interpreted to inform population-level interventions in practice (NCCDH, 2020).
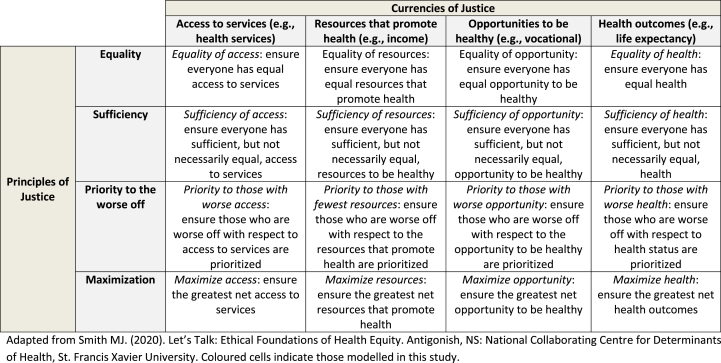
Several competing ethical standards of health equity exist and may serve as the basis for population-level interventions aiming to reduce health inequities. These standards are commonly defined across two dimensions of (distributive) justice (see Table 1) (NCCDH, 2020). The first dimension concerns the things we think people have an ethical claim to as a matter of justice—called the ‘currency’ of justice (e.g., resources like income; access to services like primary care; opportunities to be healthy like educational opportunities; actual health states themselves, like being free of diabetes or life expectancy). The second dimension concerns the pattern or basis upon which the currency of justice should be distributed among members of society—called ‘principles’ of justice (e.g., distributions that give people an equal amount of the currency; distributions that give people a sufficient amount of the currency; distributions that prioritize the worse off with respect to the currency; distributions that aim to maximize the currency). Multiple currencies and principles of justice mean that several permutations exist for the ethical standard(s) of health equity that could plausibly inform the design and implementation of population-level interventions. The choice of ethical standard has important implications for public health. For example, all inequalities should be reduced if one were to adopt an ‘equality’ standard, whereas inequalities above a ‘sufficient level of health’ would not need to be addressed, or would receive lower priority for being addressed, if one were to adopt a ‘sufficiency’ standard.
Table 1.
Ethical Standards of Health Equity: Permutations of justice and their public health objectives and population targets.
Adapted from National Collaborating Centre for Determinants of Health. (2020). Let's Talk: Ethical Foundations of Health Equity. Antigonish, NS: National Collaborating Centre for Determinants of Health, St. Francis Xavier University. Coloured cells indicate those modelled in this study.
2. Material and methods
To empirically explore the real-world implications of adopting different ethical standards of health equity in practice, we have situated our inquiry in the context of diabetes prevention. Diabetes is associated with an increased risk of developing cardiovascular disease (The Emerging Risk Factors Collaboration, 2010) and all-cause mortality (Tancredi et al., 2015), and in 2020 was the 7th leading cause of mortality in Canada (Statistics Canada, 2023). In 2019/2020, approximately 9.2% of Canadians were living with diagnosed diabetes, increasing by 3.3% (201,000 new cases) each year from 2000 to 2018 (Public Health Agency of Canada, n.d.). There is strong evidence demonstrating associations between low socioeconomic position (SEP) and diabetes incidence (Agardh et al., 2011), prevalence (Brown et al., 2015), diabetes-related complications (Tatulashvili et al., 2020), and health care costs (Brown, 2004). The high burden and persistent social inequities in diabetes in Canada make it a high priority for intervention and an ideal health condition to explore the impacts of adopting different ethical standards of health equity. As such, we explore the impacts of adopting different ethical standards of health equity on the design and implementation of a population-level intervention using the following diabetes prevention case example.
2.1. Case example
Sarah is a public health practitioner tasked with designing a population-level weight-loss intervention as a means of reducing the incidence of, and inequities in, diabetes risk. Weight-loss interventions can reduce population-level diabetes risk (Hillmer et al., 2017) by targeting the strongest modifiable diabetes risk factor. Maintaining a healthy body weight, as part of adopting a healthy lifestyle, is a World Health Organization (WHO) ‘best buy’ intervention for the prevention and management of type 2 diabetes (World Health Organization, 2017; Zhang et al., 2020).
Sarah is aware there are at least two ways she might interpret ‘inequities in diabetes risk’, and hence, at least two competing ways she might design the intervention and evaluate its progress on reducing inequities: (1) the presence of some populations, but not others, falling above a threshold of what would be considered ‘acceptable’ risk, below which differences in diabetes risk are of no or less concern (i.e., what might be called the ‘sufficiency standard’) and; (2) the presence of differences in diabetes risk between populations (i.e., what might be called the ‘equality standard’). Moreover, Sarah is aware the health outcomes resulting from each approach might variably be considered ‘inequitable’ depending on whether shortfalls under the sufficiency standard or inequalities under the equality standard exist either (a) among any individual or segment of the population, such that her task is to ensure there are no differences or shortfalls in diabetes risk whatsoever, or (b) only as a result of unjust social conditions (e.g., if they co-vary with social variables like race, educational attainment, and so forth), such that her task is to ensure there are no differences or shortfalls that co-vary with these social conditions, but not that there are no differences or shortfalls whatsoever. She is aware that only one standard can inform the design of her intervention and that whichever she picks will have implications for how that intervention ought to be evaluated.
Sarah has her own intuitions about what equity should entail. However, she is not sure what adopting (1) vs. (2), understood in terms of (a) or (b), would look like if she designed the intervention accordingly. Perhaps her intuitions about what is equitable are laudable but are infeasible or have unfavourable implications in reality. She has designed population health interventions in the past for different diseases but is aware that some standards of equity may be appropriate and justified for some contexts and interventions and not others (e.g., equality may be an appropriate aim for some issues or interventions but not others). She believes that seeing some empirical data expressed in terms of how much diabetes risk would in fact be prevented by each scenario would be illuminating, as would seeing some data about how intensive the population-level weight-loss intervention would need to be to achieve ‘equity’ in each scenario. Sarah wonders whether a study that models the impacts of adopting these different standards of health equity could help her to design, implement, and evaluate her intervention.
2.2. Study design and participants
We conducted a risk prediction modelling study using data of the nationally representative 2015-16 Canadian Community Health Survey (CCHS). The CCHS is a cross-sectional survey conducted by Statistics Canada that collects self-reported information related to health status, health care utilization, and health determinants for the Canadian population (Statistics Canada, 2016). This survey used a multi-stage, cluster sampling approach to secure a sample of 103,766 Canadians aged 12 years and over. The overall sample is representative of 98% of the Canadian population, excluding full-time members of the Canadian Forces, institutionalized populations, and persons living on Indigenous reserves or in some remote communities (Statistics Canada, 2016). The data represent the most recent 2-year CCHS cycle accessible by our project team and is suitable for this case study.
We included respondents aged 20 years and older (n = 93,164). We excluded respondents who reported having diabetes or were pregnant (n = 9405), as required by the Diabetes Population Risk Tool (DPoRT – described below), had missing information on education or income (n = 3412) or other DPoRT model covariates or body mass index given that this measure was used for allocating interventions (n = 5303). The final sample consisted of 75,044 respondents (54% women). This study was reviewed and approved by the Western University Non-Medical Research Ethics Board (Project ID: 114328) and Public Health Ontario's Ethics Review Board (File number: 2019-049.01).
2.3. Outcome measure
We used the DPoRT version 2.0, a validated, population-based risk algorithm, to estimate average 10-year diabetes risk at baseline and model the number of diabetes cases prevented or delayed for each intervention scenario (described below).
DPoRT's development and validation are fully described elsewhere (Rosella et al., 2011, 2014). Briefly, DPoRT uses a Weibull survival distribution to predict an individual's risk of developing physician-diagnosed diabetes based on self-reported risk factor information with excellent model calibration (H-L Χ2 <20, p < 0.01) and discrimination (C-statistic = 0.77) (Manuel et al., 2013; Rosella et al., 2014). In women, age (<45 years; 45–64 years; ≥65 years) and body mass index (BMI) (<23 kg/m2; 23–24 kg/m2; 25–29 kg/m2; 30–34 kg/m2; ≥35 kg/m2; missing) specific categories were created to model age-BMI interactions. BMI was calculated with self-reported weight (kg) divided by height squared (m2). Further, self-reported hypertension, non-white ethnicity, immigrant, and attended post-secondary school were all dichotomized into present versus not. In men, DPoRT includes age (<45 years; ≥45 years) and BMI (<23 kg/m2; 23–24 kg/m2; 25–29 kg/m2; 30–34 kg/m2; ≥35 kg/m2) interactions, as well as dichotomous self-report hypertension, heart disease, non-white ethnicity, current smoker, attended post-secondary, and top income quintile. To calibrate CCHS data for use with DPoRT, mean-centred variables were used to calculate DPoRT risk.
2.4. Modelling ethical standards of diabetes equity
We applied a percent weight-loss intervention in individuals that have overweight (BMI>25 kg/m2) or obesity (BMI>30 kg/m2) based on reported BMI to reduce diabetes risk. Individual behaviour modification targeting individuals that have overweight or obesity is a common clinical approach to reduce weight. This intervention efficacy is grounded with a weight loss of 2.32 kg (∼3%), identified in a meta-analysis of pragmatic lifestyle interventions (Dunkley et al., 2014), and is modelled such that one intervention is equal to 3% weight-loss and increased from 0% (baseline diabetes risk in the CCHS) to 30% weight-loss (10 years of intervention). The intervention was no longer applied to individuals whose BMI falls below 25 kg/m2 or until DPoRT risk is reduced to the target (i.e., diabetes risk inequities are eliminated), based on the distinct definition for each scenario. The upper intervention efficacy limits were set to explore the extent of the challenge in achieving each criterion, and at best likely require a suite of diabetes prevention interventions.
We modelled four counterfactual scenarios to examine the effectiveness of weight-loss interventions required to meet ‘sufficiency’ and ‘equality’ ethical standards of health equity. To estimate the impact of adopting a ‘health sufficiency’ standard, the intervention was targeted to individuals above the empirically-determined DPoRT high-risk threshold (≥16.5%), where equity was considered ‘achieved’ when diabetes risk for each individual in the population was reduced below 16.5% (Rosella et al., 2014), beyond which, based on the ethical standard, remaining inequalities were not considered ethically important to eliminate. To estimate the impact of adopting a ‘health equality’ standard, we considered health equity ‘achieved’ when each individual's diabetes risk was equalized across all populations to the average risk observed in the lowest diabetes risk group. Diabetes risk was categorized into low (<5% DPoRT risk), medium (5%-<16.5% DPoRT risk) and high (≥16.5% DPoRT risk) diabetes risk using previously defined risk groups (Rivera et al., 2015; Rosella et al., 2014). In this scenario, medium and high-risk groups received the weight-loss intervention.
Additionally, we modelled counterfactual scenarios to examine the ethical view that differences in diabetes risk are unjust only when they are the result of an injustice in social conditions (what we will refer to as ‘social inequities’). To do so, instead of estimating the achievement of ‘health sufficiency’ and ‘health equality’ across all populations, we considered equity to have been achieved when sufficiency or equality was achieved in populations for whom a shortfall from sufficiency or equality could be attributed to social conditions. For illustrative purposes, we operationalized social conditions using just one social condition known to impact health, i.e., educational attainment. This was measured using highest household educational attainment for participants aged 28 and over. Respondent educational attainment was used for participants under 28 years. Education was categorized as: less than high school, high school diploma, trades/certificate below Bachelor's degree, and, Bachelor's degree or above.
To estimate the impact of adopting an ethical standard of health sufficiency sensitive to social condition (which we label ‘social-health sufficiency’), we targeted respondents with an educational attainment below ‘Bachelor's degree or above’ who had a high baseline 10-year DPoRT risk (≥16.5%) and considered this ethical standard to be ‘achieved’ when each targeted individual's diabetes risk in the lower education groups was <16.5%, beyond which remaining inequalities across education were not considered ethically important to eliminate or prioritize. To estimate the impact of adopting an ethical standard of health equality sensitive to social condition (which we label ‘social-health equality’), we targeted respondents with an educational attainment below ‘Bachelor's degree or above’ and considered this ethical standard to be ‘achieved’ when each individual's diabetes risk was equalized to the average risk observed in the ‘Bachelor's degree or above’ group.
2.5. Statistical analysis
We applied DPoRT to the CCHS to calculate each respondents’ 10-year diabetes risk and incidence. Each CCHS respondent is assigned a survey weight, which corresponds to the number of persons in the entire population that is represented by a given survey respondent and therefore can be summed to provide a total count at the population level. Confidence intervals were calculated using bootstrap techniques to account for the complex survey design in variance estimation (Yeo et al., 1999).
We estimated average baseline DPoRT risk and 10-year predicted diabetes incidence across study characteristics. For each scenario, we estimated diabetes incidence by multiplying baseline DPoRT risk by the percent weight-loss intervention (intervention effectiveness) in the target populations, assuming 100% adherence (intervention coverage). For example, using DPoRT, we estimated 10-year diabetes risk and incidence in several scenarios, including a scenario where no weight-loss interventions are implemented, as well as scenarios where one or more interventions are implemented corresponding to a pre-specified percentage of weight-loss achieved across the population. Despite 100% adherence being unrealistic and no details being provided about the specific suite of weight-loss interventions that would result in such population-level outcomes, the value of DPoRT is that it is capable of informing policymakers about the degree of intervention effectiveness required to achieve certain objectives, measured in terms of 10-year diabetes risk and incidence.
For each percent weight-loss intervention effectiveness modelled, we assessed whether the ethical standard of health equity was achieved. For each counterfactual scenario, we estimated the number of diabetes cases prevented or delayed by comparing diabetes incidence to the baseline scenario. We also assessed relative and absolute social inequities in diabetes incidence at baseline and for each of the counterfactual scenarios.
3. Results
The 10-year diabetes risk and predicted diabetes incidence across baseline DPoRT characteristics are presented in Table 2. Our analysis included 75,044 participants (weighted n = 22,394,011) from the CCHS. Overall, without any intervention, we estimate 2.18 million new diabetes cases would occur by 2026, with higher diabetes incidence in men (1.25 million) than women (0.9 million). Overall, 10-year diabetes risk increased with age and with increasing BMI and was higher among those with hypertension and heart disease. Moreover, 10-year diabetes risk was higher among racialized Canadians and immigrants, with an inverse gradient observed across education and income categories.
Table 2.
Weighted distribution of baseline characteristics for the study population, CCHS 2015-16.
| Characteristic | N | Percent | 10- year DPoRT risk | Predicted diabetes cases, 2025 |
|---|---|---|---|---|
| Overall | 22,394,011 | 100 | 9.7 | 2,177,738 |
| Health sufficiency diabetes risk* | ||||
| <16.5% | 18,397,958 | 82 | 6.2 | 1,136,574 |
| ≥16.5% | 3,996,053 | 18 | 26.1 | 1,041,164 |
| Health equality diabetes risk** | ||||
| Low | 9,302,101 | 41 | 2.5 | 232,428 |
| Medium | 9,095,857 | 41 | 9.9 | 904,145 |
| High | 3,996,053 | 18 | 26.1 | 1,041,164 |
| Gender | ||||
| Women | 11,297,217 | 50 | 8.2 | 926,141 |
| Men | 11,096,794 | 50 | 11.3 | 1,251,597 |
| Age | ||||
| <45 | 10,341,848 | 46 | 4.7 | 481,770 |
| 45-64 | 8,216,239 | 37 | 13.5 | 1,111,142 |
| ≥65 | 3,835,925 | 17 | 15.3 | 584,825 |
| BMI | ||||
| <23 kg/m2 | 6,264,712 | 28 | 3.3 | 207,289 |
| 23-24 kg/m2 | 4,175,076 | 19 | 5.9 | 248,038 |
| 25-29 kg/m2 | 7,740,505 | 35 | 10.6 | 820,648 |
| 30-34 kg/m2 | 2,989,928 | 13 | 19.1 | 571,636 |
| ≥35 kg/m2 | 1,223,791 | 5 | 27.0 | 330,127 |
| Hypertension | ||||
| No | 18,898,249 | 84 | 7.7 | 1,448,719 |
| Yes | 3,495,763 | 16 | 20.85 | 729,019 |
| Heart disease | ||||
| No | 21,529,635 | 96 | 9.3 | 2,003,320 |
| Yes | 840,483 | 4 | 20.4 | 171,482 |
| Ethnicity | ||||
| Non-white | 5,232,304 | 23 | 11.6 | 606,881 |
| White | 17,161,708 | 77 | 9.2 | 1,570,857 |
| Immigrant | ||||
| No | 16,575,814 | 74 | 9.2 | 1,526,799 |
| Yes | 5,808,398 | 26 | 11.2 | 650,053 |
| Current smoker | ||||
| No | 18,150,605 | 81 | 9.8 | 1,780,783 |
| Yes | 4,214,538 | 19 | 9.4 | 393,849 |
| Education | ||||
| Less than high school | 2,155,824 | 10 | 15.0 | 323,657 |
| High school grad | 4,365,443 | 19 | 12.3 | 534,605 |
| Trades/certificate below Bachelor's | 8,479,506 | 38 | 9.1 | 768,956 |
| Bachelor's degree or above | 7,393,239 | 33 | 7.5 | 550,521 |
| Income | ||||
| Quintile 1- low | 3,849,408 | 17 | 10.7 | 412,529 |
| Quintile 2 | 4,061,715 | 18 | 10.5 | 426,468 |
| Quintile 3 | 4,433,547 | 20 | 9.9 | 438,267 |
| Quintile 4 | 4,866,658 | 22 | 9.2 | 449,075 |
| Quintile 5 - high | 5,182,683 | 23 | 8.7 | 451,400 |
*In the health sufficiency diabetes risk scenarios, diabetes risk is dichotomized into above or below the empirically-determined DPoRT high-risk threshold (≥16.5%), where the equity goal is to reduce diabetes risk below that threshold in the entire population.
**In the health equality diabetes risk scenario, diabetes risk was categorized into low (<5% DPoRT risk), medium (5%-<16.5% DPoRT risk) and high (≥16.5% DPoRT risk) diabetes risk using previously defined risk groups (Rivera et al., 2015; Rosella et al., 2014) to enable weight loss interventions to be targeted to medium and high-risk groups.
Health sufficiency: equity is ‘achieved’ when diabetes risk in the entire population is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate. Health equality: equity is ‘achieved’ when average diabetes risk is equalized to that observed in the lowest diabetes risk group. Social-health sufficiency: equity is ‘achieved’ when diabetes risk co-varying with lower educational attainment is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate. Social-health equality: equity is ‘achieved’ when diabetes risk among those with lower educational attainment is equalized to that observed with higher educational attainment.
3.1. Target population
A description of each modelled scenario, including the definition of the ethical standard of health equity, target population, and operationalized intervention scenario, is summarized in Table 3. The size of the population that would need to be targeted for each scenario, which would inform the size of the initial public health investment that would be required to implement each scenario, varied from 3.84 million people (health sufficiency scenario) and 9.87 million people (health equality scenario), and from 3.04 million people (social-health sufficiency scenario) and 6.67 million people (social-health equality scenario).
Table 3.
Definitions of ethical standards of health equity and conceptualization for modelling each scenario.
| Ethical standard of health equity | Target population | Individuals targeted | Equity criteria |
|---|---|---|---|
| Health sufficiency | High diabetes risk (≥16.5% DPoRT risk) | 3.84 Million | DPoRT risk <16.5% |
| Health equality | Medium/high diabetes risk (≥5.0% DPoRT risk) | 9.87 Million | DPoRT risk <5.0% |
| Social-health sufficiency | 1) Below highest education level; 2) High diabetes risk (≥16.5% DPoRT risk) |
3.04 Million | DPoRT risk <16.5% in individuals with Education below ‘Bachelor's degree’ |
| Social-health equality | Below highest education level (≥6.0% DPoRT risk) | 6.67 Million | DPoRT risk <6.0% in individuals with Education below ‘Bachelor's degree’ |
Health sufficiency: equity is ‘achieved’ when diabetes risk in the entire population is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate.
Health equality: equity is ‘achieved’ when average diabetes risk is equalized to that observed in the lowest diabetes risk group.
Social-health sufficiency: equity is ‘achieved’ when diabetes risk co-varying with lower educational attainment is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate.
Social-health equality: equity is ‘achieved’ when diabetes risk among those with lower educational attainment is equalized to that observed with higher educational attainment.
3.2. Intervention benefit: diabetes inequities
Estimated 10-year diabetes risk, BMI, and the number of diabetes cases prevented or delayed at increasing efficacy of weight-loss interventions for the four modelled scenarios are presented in Table 4. In the ‘health sufficiency’ scenario, the equity criterion of reducing population-level diabetes risk below the DPoRT high-risk 16.5% cut off was achieved with an 18% mean population-level weight-loss, with an estimated 401,932 diabetes cases prevented or delayed and an average 10-year DPoRT risk of 15.8% in the intervention group. In the ‘health equality’ scenario, the post-intervention average 10-year DPoRT risk was 6.3% in the intervention group compared to 4.2% in the non-intervention group after modelling a 30% weight-loss, a large reduction that would require a suite of diabetes prevention efforts beyond weight-loss interventions to achieve (Zhang et al., 2020). Overall, we estimated that 1,029,645 diabetes cases would be prevented or delayed under this scenario, with the largest share from the high-risk group. While a large number of diabetes cases prevented or delayed were estimated, the inherent difficultly of achieving health equity in terms of equality of health outcomes is highlighted, with only 43% of the medium-risk and 2% of the high-risk intervention groups, respectively, able to reduce their diabetes risk to the average diabetes risk present in the no intervention scenario.
Table 4.
The impact of adopting sufficiency and equality ethical standards of health equity on prevention and inequalities in diabetes.
| N (weighted) |
Weight Loss (%) |
10-year DPoRT Risk |
BMI after intervention |
Achieved ethical standard (%) |
Number of diabetes cases averted |
|
|---|---|---|---|---|---|---|
| Health Sufficiency | Criteria met: 18% weight loss | |||||
| No intervention | 18,558,245 | 0 | 6.3 | 25 | ||
| Intervention | 3,835,766 | 12 | 15.8 | 28 | 71% | 401,932 |
| Health Equality | 30% weight loss | |||||
| No intervention | 12,524,240 | 0 | 4.2 | 23 | ||
| Intervention | 9,869,772 | 27 | 6.3 | 22 | 27% | 1,029,645 |
| Medium Risk | 6,034,006 | 25 | 3.9 | 21 | 43% | 404,365 |
| High Risk | 3,835,767 | 30 | 9.8 | 22 | 2% | 625,279 |
| Social-Health Sufficiency | Criteria met: 18% weight loss | |||||
| No intervention | 19,352,481 | 0 | 7.1 | 25 | ||
| Intervention | 3,041,531 | 12 | 16.0 | 28 | 69% | 322,143 |
| Less than high school | 702,008 | 13 | 17.1 | 28 | 64% | 73,924 |
| High school graduation | 1,067,227 | 12 | 16.0 | 28 | 69% | 117,445 |
| Trades/certificate below Bachelor's | 1,272,295 | 12 | 15.4 | 29 | 73% | 130,773 |
| Social-Health Equality | 30% weight loss | |||||
| No intervention | 15,588,658 | 0 | 6.0 | 24 | ||
| Intervention | 6,805,354 | 20 | 7.9 | 24 | 65% | 701,341 |
| Less than high school | 1,187,576 | 24 | 9.5 | 23 | 47% | 141,219 |
| High school graduation | 2,149,944 | 21 | 8.1 | 24 | 62% | 240,988 |
| Trades/certificate below Bachelor's | 3,467,835 | 18 | 7.2 | 25 | 74% | 319,136 |
Health sufficiency: equity is ‘achieved’ when diabetes risk in the entire population is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate.
Health equality: equity is ‘achieved’ when average diabetes risk is equalized to that observed in the lowest diabetes risk group.
Social-health sufficiency: equity is ‘achieved’ when diabetes risk co-varying with lower educational attainment is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate.
Social-health equality: equity is ‘achieved’ when diabetes risk among those with lower educational attainment is equalized to that observed with higher educational attainment.
In the ‘social-health sufficiency’ scenario, the equity criterion was achieved with a population-level weight-loss of 18%, with an estimated 322,143 diabetes cases prevented or delayed, with the largest share among individuals with trades/certificate below Bachelor's degree educational status due to higher sample size in this group. After the intervention, the 10-year DPoRT risk was 16.0% in the intervention group overall, but remained inversely patterned with educational attainment. In the ‘social-health equality’ scenario, the post-intervention average 10-year DPoRT risk was 7.9% in the intervention group compared to 6.0% in the non-intervention group. Overall, we estimated that 701,341 diabetes cases were prevented or delayed, with the largest share in the trades/certificate below Bachelor's degree educational status. More intervention was required in lower education groups to meet this equity criterion as diabetes risk was higher in these populations.
3.3. Intervention benefit: social inequities in diabetes
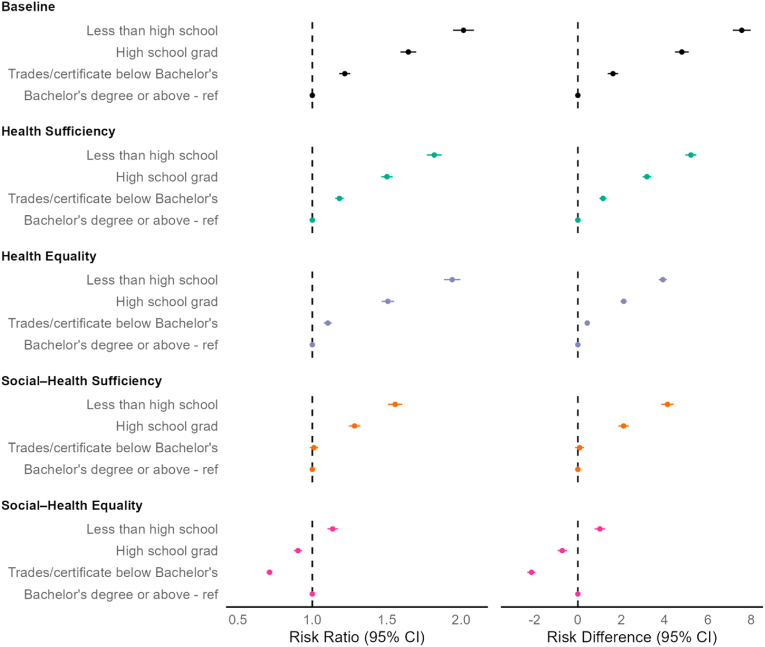
Relative and absolute educational inequalities in diabetes risk at baseline and after each intervention scenario are included in Fig. 1. At baseline, the 10-year diabetes risk among individuals with less than high school education was 2.02 (95%CI: 1.95, 2.09) times higher than individuals with Bachelor's degree or above, an absolute difference of 7.57% (95%CI: 7.15, 7.98) between these groups. Inverse associations on both the relative and absolute scales were observed across education where the diabetes risk decreased with higher educational attainment. Educational inequalities were reduced but remained in the ‘health sufficiency’ scenario (e.g., less than high school vs. Bachelor's or above: Relative Risk (RR) = 1.82, 95%CI: 1.77, 1.87; Risk difference (RD) = 1.82%, 95%CI: 1.77, 1.87). Relative educational inequalities in diabetes risk observed at baseline were largely unchanged in the ‘health equality’ scenario, with absolute differences remaining but more than halved in individuals in each education attainment group (e.g., less than high school vs. Bachelor's or above: RD = 3.92%, 95%CI: 3.73, 4.11). In the ‘social-health sufficiency’ scenario, relative and absolute educational inequalities in diabetes risk were reduced in the less than high school and high school graduation groups, and were eliminated in the trades/certificate below Bachelor's degree compared to Bachelor's degree or above group. In the ‘social-health equality’ scenario, educational inequalities in diabetes risk were largely eliminated, with only increased diabetes risk among individuals with less than high school education compared to individuals with Bachelor's degree or above (RR = 1.14, 95%CI: 1.10, 1.17; RD = 1.05%, 95%CI: 0.77, 1.26].
Fig. 1.
The impact of adopting sufficiency and equality ethical standards of health equity on educational inequities in diabetes risk
Health sufficiency: equity is ‘achieved’ when diabetes risk in the entire population is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate. Health equality: equity is ‘achieved’ when average diabetes risk is equalized to that observed in the lowest diabetes risk group. Social-health sufficiency: equity is ‘achieved’ when diabetes risk co-varying with lower educational attainment is reduced below a threshold (i.e., 16.5%), beyond which remaining inequalities are not considered ethically important to eliminate. Social-health equality: equity is ‘achieved’ when diabetes risk among those with lower educational attainment is equalized to that observed with higher educational attainment.
3.4. Intervention scope
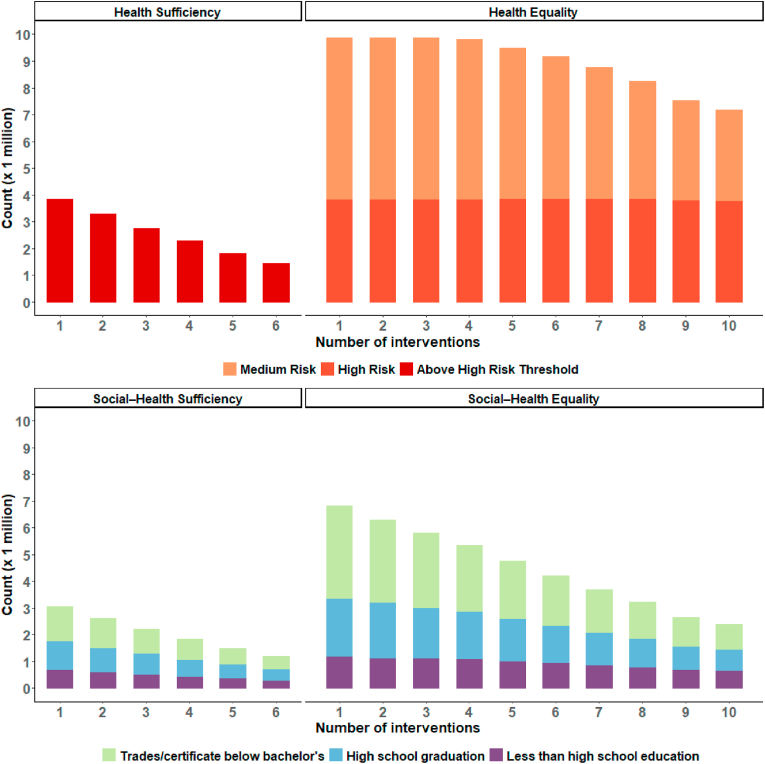
The number of interventions implemented under each intervention scenario is presented in Fig. 2. We estimated that the number of interventions required in each scenario varied substantially, which tells us something about the relative amount of resources that would likely be necessary to implement each intervention scenario in practice. For example, we estimated 34.0 million interventions over 6 years would be required to achieve equity in the ‘health sufficiency’ scenario, compared to 89.8 million over 10 years in the ‘health equality’ scenario. Similarly, we estimated the ‘social-health sufficiency’ scenario would require 12.4 million interventions over 6 years to achieve its standard of equity, compared to 45.2 million interventions over 10 years in the ‘social-health equality’ scenario.
Fig. 2.
The number of interventions received under each scenario adopting sufficiency and equality ethical standards of health equity
The number of interventions received under each intervention scenario, assuming that 1 intervention is equal to 3% weight loss each year from baseline.
4. Discussion
This study estimated the impacts of adopting different ethical standards of health equity on diabetes incidence and inequities in a population-representative sample of Canadians. We estimated that for ethical standards of equity that locate injustice in both health outcomes themselves as well as health outcomes that are the result of unjust social conditions, achieving ‘health sufficiency’ was more feasible than achieving ‘health equality’, requiring smaller initial investments and fewer interventions overall. However, as the sufficiency scenarios aim to reduce diabetes risk below a high-risk threshold, fewer diabetes cases were ultimately prevented or delayed compared to the equality scenarios. This highlights where a value judgment (i.e., a judgment about what is important or of worth, the assumption of which can be the basis for ethical action) about trade-offs is required, i.e., between investing more resources to achieve more equal health outcomes versus investing fewer resources to bring population-level diabetes risk below a high-risk threshold but with fewer absolute number of diabetes cases prevented. Estimating these scenarios may help to inform such value judgments. For instance, one may intuitively value the achievement of more equal health outcomes but have a strong reason to reject this as a suitable aim given what these data reveal about what would be required to achieve it in practice. Alternatively, such data may tell us which standard would result in the most favorable balance between competing objectives.
Further, targeting only diabetes inequalities related to education (rather than diabetes inequalities irrespective of their cause) reduced the size of the target populations and number of interventions required, but consequently resulted in preventing or delaying fewer cases of diabetes compared to health sufficiency and health equality scenarios. However, social-health sufficiency and social-health equality scenarios eliminated more relative and absolute educational inequalities in diabetes compared to the baseline scenario. While small educational inequalities in diabetes remained in the social-health equality scenario, inequities were effectively eliminated by intentionally aiming to reduce diabetes risk in the lower education groups to that observed in the Bachelor's degree or above group. Overall, we found very disparate effects of the ethical standards of health equity on population and educational inequalities in diabetes, including the target population, intervention benefit and remaining differences (inequalities or inequities), intervention scope, and the timeframe in which health equity is considered ‘achieved’.
These findings illustrate the importance of modelling approaches to empirically inform the design, implementation, and evaluation of population-level interventions, like in the case example of Sarah discussed earlier. While Sarah may have a priori convictions about what health equity requires for the design, implementation, and evaluation of a diabetes-related weight-loss intervention, these findings can help test the strength of her convictions by estimating how they would ‘cash out’ if implemented in reality, whether alternative views about what health equity requires would produce similar or more preferable population-level health outcomes, and whether the varying views that her colleagues might have about what health equity requires would make a real difference if used to design, implement, and evaluate her intervention. And because it is unlikely to be the case that there is just one ‘correct’ ethical standard that ought to be applied across all population health interventions, such findings may help to discern which standard is most appropriate and justified for the specific intervention under consideration. Ultimately, while this approach may do little to identify some unwavering ethical ‘truth’ about what is or is not equitable, it can help ensure population health interventions are explicitly justified in ethical terms while being grounded in the ‘realities’ of public health policy and practice. It affirms the practical nature of ethics as an inquiry directed at ‘what to do’ rather than a mere theoretical inquiry directed towards ‘what we ought to think’ (Finlay, 2007).
4.1. Benefits of our approach
While a paucity of research exists in this area, previous attempts have variably been made to explicitly integrate and examine competing ethical views about health equity in quantitative population health research (Bak, 2022; Fleurbaey & Schokkaert, 2009; Harper et al., 2010). Moreover, some philosophers and ethicists have hypothesized, usually via thought experiments, what the real-world outcomes and differences might be of adopting different ethical positions on health equity and justice (Eyal, 2018; Temkin, 1996). However, to date, no epidemiologists or ethicists have sought to model the impacts of adopting different ethical standards of health equity on population health outcomes.
The approach described in this study represents an opportunity to more explicitly bridge the gap between ethics and epidemiology to generate unique policy-relevant evidence for public health decision-makers, and specifically those tasked with ‘reducing health inequities’. We demonstrate the importance of intentionally identifying which ethical standard of health equity should underlie population-level interventions and using that standard to evaluate whether such interventions are successful in achieving their aims. Our approach allows for the impacts of the choice of ethical standard to be estimated, not just theoretically, but empirically, by comparing four modelled scenarios. Risk prediction and simulation modelling approaches are flexible tools to estimate the benefits of population-level interventions under multiple scenarios (Manuel & Rosella, 2010; Smith et al., 2014). However, these tools are not morally neutral, and thus we have attempted to externalize the ethical considerations by defining distinct intervention scenarios to enable direct manipulation of these factors while examining both effectiveness and equity outcomes.
4.2. Towards implementation: reflections
This study represents a first-of-its-kind attempt to explicitly integrate ethical standards of health equity in epidemiological modelling. Countless opportunities exist to extend this work and its value to decision-makers, including by utilizing different risk prediction and simulation models, applying such tools to different interventions and conditions, and by modelling additional ethical standards of health equity. The selection of model inputs and desired outcomes can be improved with procedural justice, achieved in part through deliberation with other stakeholders (Bak, 2022).
4.3. Limitations
Our approach is not without limitations. First, we understand that deeply held views about health equity may not be amenable to change when presented with empirical findings about the implications of those views in practice. However, we suspect that while this may be the case for some who have systematically engaged with the philosophical literature, those practicing in public health do not commonly have this experience or familiarity, and so may benefit from understanding the implications of different ethical standards of health equity on population health outcomes. And even for those with deeply held convictions about what health equity requires in practice, evidence generated by approaches like ours may still work to test the strength of those convictions or illuminate the extent to which philosophical distinctions concerning the desiderata of health equity actually make a difference for population health outcomes. Further, our risk prediction approach does not incorporate dynamic transitions between health or intervention states over time. We also focus on a simplified reality, modelling a single intervention, which does not represent the complexity of individual and population-level interventions concurrently existing in the real world. Further, we considered the ethical standards of health equity ‘achieved’ when all individuals met the defined criteria (which may be contrasted with an approach where population averages, but not all individuals, meet the defined criteria). This is only one potential operationalization that could be considered, which, again, illustrates the importance of explicitly considering different ethical standards and their operationalization for work in the area of health equity. We also modelled only a few existing ethical standards of health equity. Our study focused on empirically assessing the impact of distinct ethical standards of health equity on health outcomes in order to demonstrate the value of such an approach, rather than estimating reductions in diabetes risk per se (which would be necessary if actually attempting to design a diabetes intervention strategy).
Future work is required to extend our findings to understand more complex interactions and examine a more complete range of ethical standards of health equity. Although it was beyond the focus of this work, it would be important to model the resources required and cost-effectiveness of different population-level interventions that adopt different ethical standards of health equity.
4.4. Strengths
Our study is among the first to empirically evaluate the intervention benefits of adopting different ethical standards of health equity, a noted gap between ethics and epidemiology. For our initial exploration, we used population-representative data in Canada and prioritized a transparent modelling approach a priori that includes an existing, well-validated model with demonstrated utility and success in advising policymakers (Rosella et al., 2011, 2014). Further, we provided an example of how different ethical standards of health equity can be incorporated into the design and implementation of population-level interventions so as to produce the most ethically desirable population health outcomes.
5. Conclusion
Using the case study of diabetes, we found that an explicit, ethically-informed definition of health equity is essential to guide population-level interventions aiming to reduce health and social inequities. Modelling different ethical standards of health equity reveals disparate impacts on the target population, intervention benefit, remaining health and social inequalities in diabetes, and the intervention scope. Our findings reinforce that public health research and practice must consider the ethical underpinnings of work in health equity if it is to take health equity seriously.
Funding
This research was supported by a Western University Medical and Health Science Review Board Seed Research Grant (ID# 46074).
Author statement
BTS and MJS contributed to the conception and design of this work and co-led this work's supervision. BTS, LCR and MJS acquired funding for this work. CW conducted the formal analysis. BTS, CW and MJS contributed to the writing of the original draft. BTS, CW, LCR and MJS contributed to this study's methodology; the interpretation of data and results; to review and editing of this work. All authors read and approved the final manuscript.
Financial disclosure statement
None to declare.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank members of the Modelling Ethical Standards of Health Equity (MESHE) Study team members (Dr. A.M. Viens, Dr. Arjumand Siddiqi; Dr. Douglas MacKay; Dr. Katherine Saylor, Dr. Nicholas King, Dr. Sam Harper, Dr. Yukikio Asada) for their insightful comments on earlier versions of this work. We also thank Sze Hang Fu for assistance with data visualization and the anonymous reviewers for their valuable suggestions which helped improve the clarity of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2023.101481.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The authors do not have permission to share the data. Code is available through accessing a public repository found here: https://github.com/BtsmithPHO/
References
- Agardh E., Allebeck P., Hallqvist J., Moradi T., Sidorchuk A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. International Journal of Epidemiology. 2011;40:804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]
- Asada Y. A framework for measuring health inequity. Journal of Epidemiology & Community Health. 2005;59:700–705. doi: 10.1136/jech.2004.031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada Y. University of Toronto Press; Toronto: 2007. Health inequality: Morality and measurement. [Google Scholar]
- Asada Y., Hurley J., Norheim O.F., Johri M. A three-stage approach to measuring health inequalities and inequities. International Journal for Equity in Health. 2014;13:98. doi: 10.1186/s12939-014-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M.A. Computing fairness: Ethics of modeling and simulation in public health. SIMULATION. 2022;98:103–111. doi: 10.1177/0037549720932656. [DOI] [Google Scholar]
- Beauchamp D.E. Public health as social justice. Inquiry. 1976;13:3–14. [PubMed] [Google Scholar]
- Braveman P.A., Kumanyika S., Fielding J., Laveist T., Borrell L.N., Manderscheid R., Troutman A. Health disparities and health equity: The issue is justice. American Journal of Public Health. 2011;101(Suppl 1):S149–S155. doi: 10.2105/AJPH.2010.300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.F. Socioeconomic position and health among persons with diabetes mellitus: A conceptual framework and review of the literature. Epidemiologic Reviews. 2004;26:63–77. doi: 10.1093/epirev/mxh002. [DOI] [PubMed] [Google Scholar]
- Brown K., Nevitte A., Szeto B., Nandi A. Growing social inequality in the prevalence of type 2 diabetes in Canada, 2004–2012. Canadian Journal of Public Health. 2015;106:e132–e139. doi: 10.17269/CJPH.106.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.-C. The meaning and goals of equity in health. Journal of Epidemiology & Community Health. 2002;56:488–491. doi: 10.1136/jech.56.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels N. 1st ed. Cambridge University Press; 2007. Just health: Meeting health needs fairly. [DOI] [Google Scholar]
- Dunkley A.J., Bodicoat D.H., Greaves C.J., Russell C., Yates T., Davies M.J., Khunti K. Diabetes prevention in the real world: Effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- Eyal N. Inequality in political philosophy and in epidemiology: A remarriage: Inequality in political philosophy and in epidemiology. Journal of Applied Philosophy. 2018;35:149–167. doi: 10.1111/japp.12150. [DOI] [Google Scholar]
- Finlay S. Four faces of moral realism. Philosophy Compass. 2007;2:820–849. doi: 10.1111/j.1747-9991.2007.00100.x. [DOI] [Google Scholar]
- Fleurbaey M., Schokkaert E. Unfair inequalities in health and health care. Journal of Health Economics. 2009;28:73–90. doi: 10.1016/j.jhealeco.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Harper S., King N.B., Meersman S.C., Reichman M.E., Breen N., Lynch J. Implicit value judgments in the measurement of health inequalities. The Milbank Quarterly. 2010;88:4–29. doi: 10.1111/j.1468-0009.2010.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmer M., Sandoval G.A., Elliott J.A., Jain M., Barker T., Prisniak A., Astley S., Rosella L. Diabetes risk reduction in primary care: Evaluation of the Ontario primary care diabetes prevention program. Canadian Journal of Public Health. 2017;108:e176–e184. doi: 10.17269/CJPH.108.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel D.G., Rosella L.C. Commentary: Assessing population (baseline) risk is a cornerstone of population health planning—looking forward to address new challenges. International Journal of Epidemiology. 2010;39:380–382. doi: 10.1093/ije/dyp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel D.G., Rosella L.C., Tuna M., Bennett C., Stukel T.A. Effectiveness of community-wide and individual high-risk strategies to prevent diabetes: A modelling study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0052963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCDH . National Collaborating Centre for Determinants of Health, St. Francis Xavier University; Antigonish, NS: 2020. Let's Talk - ethical foundations of health equity. [Google Scholar]
- Norheim O., Asada Y. The ideal of equal health revisited: Definitions and measures of inequity in health should be better integrated with theories of distributive justice. International Journal for Equity in Health. 2009;8:40. doi: 10.1186/1475-9276-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad G. In: The oxford handbook of public health ethics. Oxford university press. Mastroianni A.C., Kahn J.P., Kass N.E., editors. United States of America; New York, NY: 2019. Justice and public health. [Google Scholar]
- Powers M., Faden R.R. Oxford University Press; Oxford ; New York: 2006. Social justice: The moral foundations of public health and health policy, issues in biomedical ethics. [Google Scholar]
- Public Health Agency of Canada, n.d. Publiv Health Infobase.Canadian chronic disease surveillance system (CCDSS).
- Rivera L.A., Lebenbaum M., Rosella L.C. The influence of socioeconomic status on future risk for developing type 2 diabetes in the Canadian population between 2011 and 2022: Differential associations by sex. International Journal for Equity in Health. 2015;14:101. doi: 10.1186/s12939-015-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosella L.C., Lebenbaum M., Li Y., Wang J., Manuel D.G. Risk distribution and its influence on the population targets for diabetes prevention. Preventive Medicine. 2014;58:17–21. doi: 10.1016/j.ypmed.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Rosella L.C., Manuel D.G., Burchill C., Stukel T.A., PHIAT-DM team A population-based risk algorithm for the development of diabetes: Development and validation of the diabetes population risk tool (DPoRT) Journal of Epidemiology & Community Health. 2011;65:613–620. doi: 10.1136/jech.2009.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruger J.P. Oxford University Press; Oxford ; New York: 2010. Health and social justice. [Google Scholar]
- Segall S. Princeton University Press; Princeton: 2009. Health, luck, and justice. [Google Scholar]
- Smith M.J. Health equity in public health: Clarifying our commitment. Public Health Ethics. 2015;8:173–184. doi: 10.1093/phe/phu042. [DOI] [Google Scholar]
- Smith M.J. In: The routledge handbook of philosophy of public health, routledge handbooks in applied ethics. Routledge. Venkatapuram S., Broadbent A., editors. Taylor & Francis Group; London ; New York: 2023. Social justice and public health. [Google Scholar]
- Smith B.T., Smith P.M., Harper S., Manuel D.G., Mustard C.A. Reducing social inequalities in health: The role of simulation modelling in chronic disease epidemiology to evaluate the impact of population health interventions. Journal of Epidemiology & Community Health. 2014;68:384–389. doi: 10.1136/jech-2013-202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada . 2016. Canadian community health survey 2015-16. [Google Scholar]
- Statistics Canada . 2023. Leading causes of death, total population. by age group. [DOI] [Google Scholar]
- Tancredi M., Rosengren A., Svensson A.-M., Kosiborod M., Pivodic A., Gudbjörnsdottir S., Wedel H., Clements M., Dahlqvist S., Lind M. Excess mortality among persons with type 2 diabetes. New England Journal of Medicine. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- Tatulashvili S., Fagherazzi G., Dow C., Cohen R., Fosse S., Bihan H. Socioeconomic inequalities and type 2 diabetes complications: A systematic review. Diabetes & Metabolism. 2020;46:89–99. doi: 10.1016/j.diabet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Temkin L.S. Oxford ethics series. Paperback Edition. Oxford Univ. Press; New York, NY: 1996. Inequality, 1. [Google Scholar]
- The Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatapuram S. Polity; Cambridge, UK ; Malden, MA: 2011. Health justice: An argument from the capabilities approach. [Google Scholar]
- World Health Organization . 2017. ‘Best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. [Google Scholar]
- Yeo D., Mantel H., Liu T.-P. Bootstrap variance estimation for the national population health survey. American Statistical Association. Proceedings of the Survey Research Methods Section. 1999:778–783. [Google Scholar]
- Zhang Y., Pan X.-F., Chen J., Xia L., Cao A., Zhang Y., Wang J., Li H., Yang K., Guo K., He M., Pan A. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63:21–33. doi: 10.1007/s00125-019-04985-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share the data. Code is available through accessing a public repository found here: https://github.com/BtsmithPHO/