Abstract
Substantial evidence suggests that periodontal disease increases the risk of developing and progressing extraoral manifestations such as diabetes, atherosclerosis, rheumatoid arthritis, and inflammatory bowel disease. The most probable causative mechanism behind this is the influx of bacteria and/or bacterial products (endotoxin) and inflammatory cytokines into the systemic circulation originating from inflamed periodontal tissues. However, recent studies have revealed that oral bacteria, especially periodontopathic bacteria, play a role in inducing dysbiosis of the gut microbiota resulting induction of gut dysbiosis-related pathology associated with systemic diseases. Conversely, the disruption of gut microbiota has been shown to have a negative impact on the pathogenesis of periodontal disease. Based on our study findings and the available literature, this review presents an overview of the relationship between periodontal disease and systemic health, highlighting the mouth-gut connection.
Keywords: Oral microbiota, Periodontopathic bacteria, Gut microbiota, Periodontitis, Systemic diseases
1. Introduction
The oral cavity harbors a microbiota with a diversity and abundance comparable to those found in the large intestine [1]. These microorganisms perform a crucial function in maintaining oral homeostasis by impeding the colonization of pathogenic bacteria and stimulating salivary antimicrobial components such as IgA and defensins that shape oral microbiota [2]. Nevertheless, when the bacterial community equilibrium is disturbed (i.e., dysbiosis) owing to risk factors like poor oral hygiene, unhealthy diet, smoking, and diabetes, the levels of periodontopathic bacteria such as Porphyromonas gingivalis elevate, leading to the onset of periodontitis [3]. Furthermore, in addition to oral afflictions, recent epidemiological investigations have demonstrated that periodontal disease heightens the likelihood of developing various extraoral ailments, such as diabetes, atherosclerotic diseases, autoimmune disorders, neurodegenerative disease, and malignant tumors [4].
The correlation between periodontal disease and these disorders is believed to be attributable to the dissemination of bacteria and/or bacterial products, along with inflammatory mediators, throughout the body, originating locally in periodontal disease [5]. However, these hypothetical mechanisms suffer from contradictory points, preventing comprehensive acceptance. For example, genes for periodontopathic bacteria have been reported to be detected in various tissues, including atherosclerotic lesions, but there are also reports of the detection of oral bacteria other than periodontopathic bacteria and enterobacteria [6]. Regarding inflammatory mediators, several studies have demonstrated elevated serum levels of high-sensitivity C-reactive protein (CRP) and interleukin (IL)− 6 [7]. Despite the elevated level of IL-6 in the gingival crevicular fluid of periodontitis lesion [8], there is no evidence that elevated systemic IL-6 is derived from inflamed periodontal lesions and the level is high enough for CRP induction in the liver. Nevertheless, it cannot be negated that there may be shared risk factors, such as susceptibility to disease and smoking, between periodontal disease and these disorders, and insufficient data are available to explicate the causal association [9].
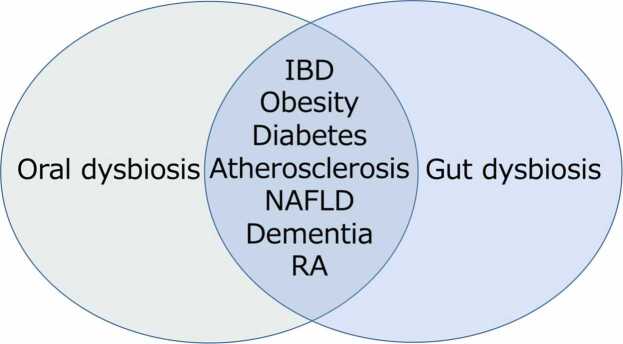
Recently, dysbiosis of the gut microbiota has emerged as a risk factor for various diseases, with many associated conditions overlapping with those linked to periodontal disease [10], [11] (Fig. 1). Our study has been the pioneer in demonstrating that oral bacteria instigate pathological alterations leading to various diseases by causing dysbiosis of the gut microbiota, based on the data of animal experiments [12]. In addition to the conventional concept of disease mechanism, this etiologic mechanism on the oral-gut-systemic axis can rationally explain the association between periodontal disease and systemic diseases. Furthermore, dysbiosis of the gut microbiota has been shown to influence the pathogenesis of periodontal disease. Therefore, it is expected that by integrating the conventional concept of disease mechanism with the causal mechanism founded on the oral-gut correlation, the association between periodontal disease and systemic diseases can be comprehended more rationally. This review will cover recent papers on the interaction between the oral and intestinal microbiota and the resulting systemic pathological changes, including periodontal tissue, in various mouse models, as well as the analysis of the intestinal microbiota in human periodontitis patients.
Fig. 1.
Effect of oral and gut dysbiosis on systemic diseases. Many of the systemic diseases affected by dysbiosis of the oral and gut microflora are similar.
2. Relationship between periodontal disease and systemic disease via the intestinal tract
Previously, it was assumed that oral bacteria were eradicated by gastric and bile acid and therefore did not reach the lower gastrointestinal tract as viable bacteria. Hence, little consideration was given to the influence of oral bacteria on the gut microbiota, except in instances of diseases such as cirrhosis and colorectal cancer [13], [14], [15]. However, recent studies have revealed that oral bacteria present in saliva can colonize the intestinal tract and become an integral component of the gut microbiota, even in the absence of specific conditions [16], [17], [18] though it remains controversial [19].
The saliva of individuals with severe periodontitis reportedly contains P. gingivalis in concentrations of 106 per milliliter. An average person produces 1–1.5 liters of saliva per day [20], those with severe periodontitis may ingest anywhere from 109 to 1010 P. gingivalis cells and 1012 to 1013 oral bacteria daily [21], [22], [23]. Moreover, P. gingivalis cultured in biofilm form exhibits remarkable resistance to acidic environments, as demonstrated by its high acid tolerance when exposed to simulated gastric fluid [24]. Consequently, the impact of periodontopathic bacteria in swallowed saliva on the intestinal environment has become a subject of intense interest as a possible mechanism connecting periodontal disease with systemic diseases.
The mechanisms underlying the impact of dysbiotic gut microbiota on various diseases include 1) disruption of gut barrier function, leading to endotoxemia, systemic inflammation, and other consequences [25], [26]; 2) effects on gut immunity, including an increase in T helper 1 (Th1) and Th17 responses, as well as a decrease in Treg responses [27]; and 3) changes in bacterial metabolites, such as a decrease in short-chain fatty acids and an increase in secondary bile acids, branched-chain amino acids, and aromatic amino acids [10], [28]. Swallowing large amounts of oral bacteria that have become dysbiotic due to periodontitis may result in an imbalance in gut bacteria, leading to alterations in bacterial metabolites, impaired gut barrier function, and immune dysregulation.
3. Verification of causal mechanisms via gut microbiota in a mouse model
We were the pioneering researchers to document that oral administration of P. gingivalis induces dysbiosis of the gut microbiota in mice [12]. In addition to alterations in the microbial composition, we observed decreased expression of tight junction protein genes in intestinal tissues, which play a critical role in maintaining the barrier function, as well as increased expression of inflammatory cytokine genes and elevated levels of endotoxin in the bloodstream (endotoxemia). Our investigation also revealed that both adipose and liver tissues exhibited macrophage infiltration and fat accumulation, respectively, and increased expression of inflammatory cytokine genes in both tissues. Furthermore, glucose tolerance and insulin tolerance tests revealed mild glucose intolerance, indicative of obesity, non-alcoholic fatty liver disease (NAFLD), and early-stage type 2 diabetes. Later, Actinobacillus actinomycetemcomitans, another periodontopathic bacterium, was reported to induce gut dysbiosis [29].
In addition to disruption of gut barrier function, P. gingivalis stimulates the gene expressions of interferon-gamma, the cytokine derived from Th1 cells in the colon [12], [30], and IL-17 produced by Th17 in mesenteric lymph nodes [24]. Regarding the effect of other oral bacteria on the gut immune system, Klebsiella isolated from the human oral cavity was found to colonize the mouse intestinal tract and induce Th1 cells, leading to intestinal inflammation [31].
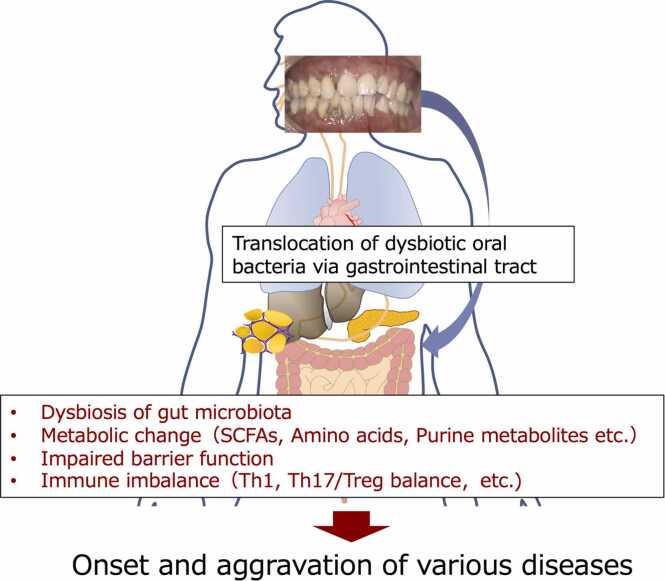
Through predictive metagenomic analysis of the gut microbiota, we identified a preponderance of genes involved in the synthesis of aromatic amino acids such as phenylalanine, tyrosine, and tryptophan and observed a significant rise in the blood levels of these amino acids [32]. These findings are relevant to the link between periodontitis and cardiometabolic diseases via oral-gut connection because of the previous reports that elevated levels of branched-chain and aromatic amino acids in the bloodstream are a potential predictive marker for the development of diabetes [33], [34] and cardiovascular disease [35]. These alterations align with our study's outcomes, suggesting that dysbiosis of the gut microbiota is the mechanism that links it to various diseases, thus implicating gut bacteria in the association between periodontitis and systemic diseases (Fig. 2).
Fig. 2.
Effect of oral dysbiosis on gut ecosystem. Dysbiotic oral microbiota is considered to affect gut microbiota resulting in 1) disruption of gut barrier function, leading to endotoxemia, systemic inflammation, and other consequences; 2) effects on gut immunity, including an increase in Th1 and Th17 responses, as well as a decrease in Treg responses; and 3) changes in bacterial metabolites, such as a decrease in short-chain fatty acids and an increase in secondary bile acids, branched-chain amino acids, and aromatic amino acids. Th, T helper. SCFAs, Short-chain fatty acids.
4. Adverse effects of periodontal disease or periodontopathic bacteria on disease models via the gut microbiota
The systemic effects of periodontitis via the gut microbiota have been analyzed using various models.
4.1. P. gingivalis-administered model
It has been reported that oral administration of P. gingivalis induces the deterioration of pathological conditions in various disease models through changes in the gut microbiota as follows:
4.1.1. Atherosclerosis
Recent evidence suggested that trimethylamine oxide, which is produced in the liver through the involvement of intestinal bacteria, promotes atherosclerosis and induces cardiovascular disease, mainly by foam cell formation [36], [37]. P. gingivalis infection abrogated the beneficial effect of dietary intervention on atherosclerotic plaque formation by altering the gut microbiota [38]. Subsequently, it became evident that P. gingivalis was involved in the aggravation of atherosclerotic lesions in ApoE gene-deficient mice through this process [39].
4.1.2. Rheumatoid arthritis
IL-17 and its producer, Th17, are the key factors for rheumatoid arthritis [40]. In a collagen-induced arthritis model, the immune system of the gut shifted towards Th17 dominance by P. gingivalis, resulting in an elevation in blood IL-17 levels, which led to a deterioration of the disease state, correlating with changes in the gut microflora [24]. In this study, P. gingivalis did not elevate the anti-citrullinated protein antibody, reported to be an etiological agent of rheumatoid arthritis associated with periodontitis [41]. In arthritis-prone SKG mice, P. gingivalis oral administration also affected gut microbiota dysbiosis and joint destruction via increased citrullinated protein generation [42].
4.1.3. Diabetes
Glucose intolerance associated with gut dysbiosis has been reported in mice that received oral administration of P. gingivalis [12], [43], [44]. In obese mice mimicking type 2 diabetes (T2D), P. gingivalis treatment increased fasting blood glucose levels and induced changes in the intestinal microbiota, an increase in metabolites related to gluconeogenesis in the liver, and a decrease in metabolites involved in glycogen storage and energy production, which were attributed to impaired glucose tolerance [45].
4.1.4. NAFLD
Mouse models of diet-induced NAFLD have clearly shown the aggravation of the pathology concomitant alteration of gut microbiota by P. gingivalis [46], [47]. Although the changes in bacterial composition within the gut were variable between studies with less pronounced in the latter study, the metagenomic analysis revealed that the genes of amino acid metabolism were enriched. Furthermore, in addition to an increase in the levels of endotoxin in the blood, there was a surge in the expression of genes related to endoplasmic reticulum stress, oxidative stress, and tumorigenesis in the liver. Changes in the expression of genes related to the circadian rhythm were also noted, as were variations in blood metabolite profiles [47]. These findings demonstrate that the blood metabolite profiles undergo alterations.
4.1.5. Neurodegenerative disease
In a Parkinson's disease model, mice that have mutations in the leucine-rich repeat kinase 2 gene, administration of P. gingivalis demonstrated a decrease in dopaminergic neurons and an increase in activated microglia in the substantia nigra. These changes are associated with changes in the intestinal microbiota, a concomitant decrease in the intestinal barrier function and inflammation of the intestinal tract, an increase in IL-17 in the blood, and increased expression of IL-17 receptors in the brain [48].
Functional impairment of the glymphatic system is suggested to be associated with neurodegenerative diseases such as Alzheimer’s disease [49]. In addition, P. gingivalis administration to ordinary mice induced cognitive impairment as evidenced by a slowed rate of spatial learning and gut dysbiosis. The proportions of lymphocytes in the periphery and myeloid cells infiltrating the brain were increased in P. gingivalis-administered animals. In addition, the solute clearance efficiency of the glymphatic system decreased. Concomitantly, neurons in the hippocampus and cortex regions were reduced, whereas microglia, astrocytes, and apoptotic cells were increased in the P. gingivalis-administered mice [50].
These findings indicate that P. gingivalis may play an important role in neurodegeneration via gut dysbiosis.
4.1.6. Inflammatory bowel disease
It has been reported that IL-10-deficient mice, a model of inflammatory bowel disease (IBD), show exacerbation of intestinal inflammation when treated with P. gingivalis and that transplantation of fecal microbiota from P. gingivalis-treated mice can reproduce the pathological state of the disease. It was shown that the background of this phenomenon is a decrease in the intestinal barrier function caused by a certain type of T cells induced by changes in the intestinal bacteria and the consequent induction of inflammation [51].
4.2. Ligature-induced periodontitis model
The ligature-induced periodontitis is also a frequently used animal model for the investigation of periodontal pathology and periodontitis-systemic disease associations. Studies using this model to analyze the effects on inflammatory bowel disease have shown that dysbiosis of gut microbiota is induced with the colonization of oral bacteria (Klebsiella) in the intestinal tract and that homing of orally sensitized Klebsiella-specific Th17 cells to the intestinal tract exacerbates inflammation [52]. Alterations in the gut microbiota due to the ligation-induced model for periodontitis have also been associated with the development of diabetes [53], cognitive dysfunction [54], and hepatic steatosis [55] through elevation of systemic inflammation, and promotion of multiple sclerosis-like symptoms through ectopic colonization of oral pathobionts and expansion of Th17 cells [56].
These findings underscore the potential significance of the ligation-induced periodontitis model in comprehending the intricate association between oral and systemic health.
4.3. Human salivary microbiome-administered models
Oral administration of periodontopathic bacteria and ligature-induced periodontitis are commonly used animal models, but they do not necessarily reflect actual clinical conditions. To establish a more authentic model, the impact of orally administered salivary bacteria from patients with periodontitis and healthy controls on the gut microbiota and related pathological conditions in mice have been examined.
It has been reported that human salivary bacteria administered to germ-free mice colonize the intestinal tract [57]; however, the composition of gut microbiota differs significantly between saliva from healthy volunteers and those with periodontitis [58]. Moreover, mice treated with patient saliva bacteria displayed inflammatory changes in the liver and adipose tissue, which were absent in mice treated with healthy saliva [58] and exacerbated NAFLD in high-fat diet-induced obese mice [59].
Furthermore, experimental studies using IBD [60] and Alzheimer's disease model mice [61] with commensal flora have demonstrated that the condition of mice treated with saliva from patients with periodontitis was worse than that of mice treated with saliva from normal patients.
5. Effect of oral microbiome on gut microbiome in patients with periodontitis
Studies on the effects of oral bacteria, periodontopathic bacteria, or periodontal disease on the gut microbiota in humans have just begun.
It has been reported that the alpha diversity of the gut microbiota is decreased in patients with chronic periodontitis [62] and that dysbiosis of the gut microbiota is observed in patients with severe chronic [63] and invasive periodontitis [64].
A study of the impact of periodontitis-induced gut dysbiosis on systemic health in older adult patients with T2D and those with both T2D and periodontitis found that 25 of the 34 taxa that characterized differences between the two were correlated with diabetes duration and inflammatory cytokines in the peripheral blood. Thus, the authors concluded that gut dysbiosis driven by periodontitis may contribute to systemic inflammation and metabolic dysfunction during the progression of T2D [65].
For the association between IBD and periodontitis, the gut microbiome was reported to be significantly more similar to the oral microbiome in patients with ulcerative colitis and Crohn’s disease (CD) compared with healthy patients, suggesting that ectopic gut colonization by oral bacteria is increased in patients with IBD. The study also demonstrated that early periodontitis may associate with worse clinical symptoms in some patients with CD [66]. In relation to this, it is reported that intestinal colonization of pathogenic oral bacteria Haemophilus parainfluenzae in periodontitis is associated with intestinal inflammation in patients with CD [67].
Although clinical significance was not investigated, in addition to a close relationship between the oral microbiota and pregnant periodontitis, significant changes occurred in both the oral and gut microbiome when periodontitis was coupled with gestational diabetes [68].
6. Does the intestinal environment affect the pathogenesis of periodontal disease?
The dysbiosis of the gut microbiota disrupts the gut barrier function, alters the immune system, and modifies the profile of bacterial metabolites. Consequently, it is hypothesized to cause mild systemic inflammation, impaired glucose, and lipid metabolism, and enhance the likelihood of various systemic diseases by changing blood metabolites. Considering that the host's general condition influences the pathogenesis of periodontal disease [69], it is conceivable that the dysbiosis of the gut microbiota may adversely impact the development of periodontal disease. In support of this idea, IBD is a risk factor for periodontal disease [70].
Besides the epidemiological findings, previous studies have suggested a link between gut microbiota and periodontal disease pathogenesis. In an experiment in which probiotics (Bacillus subtilis) were administered to a ligature-induced periodontitis rat model in drinking water, the periodontitis group treated with probiotics showed greater microvilli height and crypt depth in the jejunal epithelium than that in the periodontitis group without probiotics, i.e., less injury to the intestinal tissue and less alveolar bone resorption. [71]. These findings imply that probiotics can suppress injury to intestinal tissue and weaken alveolar bone resorption in the probiotic-treated and periodontitis groups. Moreover, the functional recovery of the intestinal tract has an inhibitory effect on periodontal tissue destruction.
In the periodontitis model where P. gingivalis was administered, adding fish [rich in n-3 polyunsaturated fatty acids such as α-linoleic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which are useful in preventing lifestyle-related diseases] and corn oil (rich in n-6 polyunsaturated fatty acids containing high linoleic acid and lacking EPA or DHA) to the diet was found to result in lower bone resorption in the fish oil-fed group [72]. A metabolite of dietary n-3 polyunsaturated fatty acids (10-oxo-cis-12-cis-15-octadecadienoic acid) by gut microbiota is reported to exert anti-inflammatory properties through a peroxisome proliferator-activated receptor-gamma-dependent pathway [73]. Another metabolite (10-hydroxy-cis-12-octadecenoic acid) was shown to suppress alveolar bone resorption via protection of gingival epithelial barrier disruption in experimental periodontitis in mouse induced by ligature placement and P. gingivalis administration [74]. In addition, metformin, a known diabetic drug, demonstrated suppression of gingival inflammation and alveolar bone resorption by drinking water administration in a rat model of ligature-induced periodontitis [75]. Metformin has been implicated in the promotion and maintenance of a healthy gut microbiome and reduces many age-related degenerative pathologies though the precise mechanisms remain to be elucidated [76]. These results suggest that the gut microbiota can indirectly influence the pathogenesis of the periodontal disease.
7. Gut bacteria linking obesity and periodontal disease risk
Recently, a more direct association between gut microbiota and periodontal disease has been observed. Our study demonstrated that changes in gut microbiota and its metabolism contribute to the heightened risk of periodontitis in individuals with obesity [77]. To investigate this, we conducted an experiment utilizing both normal and high-fat diets. In this study, we collected fecal material from mice fed both diets and transferred them to mice with suppressed gut bacteria through antibiotics, followed by periodontitis induced by silk thread ligation (known as fecal microbiota transplantation or FMT). Our findings revealed that the FMT group of obese mice fed a high-fat diet had significantly more severe alveolar bone destruction than that in the FMT group fed a normal diet. Serum metabolome analysis indicated that uric acid was associated with periodontal tissue destruction. To verify this, experimental periodontitis was induced in mice with artificially induced hyperuricemia by intraperitoneal injection of uric acid rather than FMT, resulting in more severe periodontitis. Allopurinol, an inhibitor of uric acid synthesis administered during FMT, prevented the severity of periodontitis, indicating the involvement of uric acid. The ratio of Enterococcus, Akkermansia, and Turicibacter increased in high-fat-fed mice, whereas Lactobacillus and Prevotella decreased, but these bacterial groups maintained their characteristic flora composition after FMT. Metabolomic analysis of fecal samples from obese mice showed an increase in purine nucleosides such as inosine and guanosine, as well as uric acid, suggesting that Akkermansia and Turicibacter have a purine metabolic pathway and may be associated with elevated uric acid levels. On the other hand, Lactobacillus, which degrades purine nucleosides to purine bases, decreased, suggesting that an increase in purine nucleosides, which are easily absorbed in the intestinal tract, contributes to the rise in blood uric acid levels. Although uric acid has an antioxidant effect at low concentrations, high concentrations enhance IL-1β production via activation of the NLRP inflammasome [78] and are associated with periodontal tissue destruction [79]. Thus, our study suggests that uric acid may contribute to periodontal tissue destruction.
8. Influence of the gut microbiota on periodontal disease via bone metabolism
Similar to patients with metabolic and inflammatory diseases, gut dysbiosis has been reported in patients with osteoporosis [80], [81]. In the initial investigation into the impact of gut microbiota on bone metabolism, higher bone mineral densities and lower osteoclast counts were reported in germ-free (GF) mice compared to wild-type mice [82]. Similar findings have been observed in the alveolar bone of GF mice compared to SPF mice [83].
On the other hand, conflicting results have been reported, and the reasons for these conflicting results are thought to be due to the duration of intestinal bacterial colonization, sex differences, and the genetic background of the mice [84].
In addition to the effects of bacterial metabolites and bacteria-derived components, gut microbiota are involved in bone metabolism by influencing the immune and endocrine systems.
Short-chain fatty acids are the metabolites of enterobacteria that can stimulate osteoblast proliferation by increasing insulin-like growth factor-1. Conversely, butyrate inhibits preosteoclast differentiation into osteoclasts by suppressing histone deacetylases. Additionally, butyrate promotes the differentiation and proliferation of regulatory T cells that possess anti-inflammatory properties, producing the cytokine transforming growth factor-beta, which promotes osteoblast differentiation and proliferation and induces osteoclast apoptosis via the cell surface molecule CTLA-4 [85].
From an osteoimmunological perspective, the relationship between the intestinal microbiota and bone metabolism involves two pathways. Firstly, bone resorption is inhibited by the activation of butyrate-mediated Tregs or bone formation is promoted through the activation of osteoblasts, as mentioned earlier. Secondly, there is a decrease in bone mass due to the promotion of osteoclast formation through the activation of Th17. Notably, specific gut bacteria, known as segmented filamentous bacteria (SFB), can differentiate and induce Th17, leading to inflammation and exacerbation of autoimmune arthritis [86].
Gut microbiota regulates estrogen levels in the body specifically through the production of enzymes that act on steroid hormone metabolic pathways [87]. Estrogen deficiency results in decreased expression of tight junction proteins in the intestinal epithelium, which induces endotoxemia and increases systemic inflammation. Parathyroid hormone is involved in blood calcium concentration homeostasis by releasing bone minerals from bone tissue. Persistent elevation of parathyroid hormone (PTH) levels can lead to osteoporosis, while the transient and repetitive action of PTH (intermittent action) markedly stimulates bone formation [88]. In a recent study, it was revealed that the gut microbiota is indispensable for PTH-induced bone formation and augmentation of bone mass via the butylate-Treg-Wnt signaling pathway [89]. Consequently, the gut microbiota profoundly influences bone homeostasis through the regulation of the endocrine pathway.
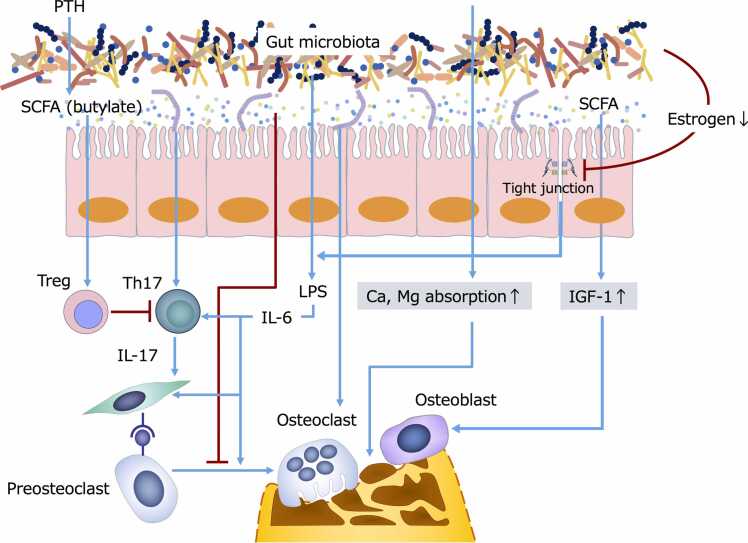
The effect of gut microbiota on bone metabolism is illustrated in Fig. 3.
Fig. 3.
Effect of gut microbiota and metabolites on bone metabolism. The gut ecosystem can affect bone homeostasis via (a) modulating gut barrier integrity; (b) enhancing the absorption of Ca2+ and Mg2+; (c) regulating the inflammatory response by balancing Th17 and Treg cells differentiation; and (e) directly promoting the activation of osteoblasts while suppressing the differentiation of preosteoclasts. Blue line: Stimulation or activation. Red line: Suppression or infibition. IGF-1, Insulin-like growth factor-1. LPS, Lipopolysaccharide. PTH, Parathyroid hormone. SCFAs, Short-chain fatty acids. Th, T helper. Treg, T-regulatory.
The specific effects of gut bacteria on alveolar bone resorption have also been reported. Osteoporosis represents a risk factor for periodontal disease. In a periodontitis model of ovariectomized rats treated with ligation induction plus P. gingivalis, probiotic administration was observed to inhibit alveolar bone resorption. This effect might be attributed to the increase in butyrate-producing bacteria and butyrate in the gut flora. The accompanied decrease in Th17 in the bone marrow and increase in Treg and IL-17 in the blood, as well as the increased Th17 inducibility in the bone marrow, supported this finding [90].
In a comparative study between germ-free and SFB gnotobiotic mice with Th17-inducing ability, an increase in IL-17 gene expression and production in the gingival tissue and an increase in alveolar bone resorption were reported compared with control sterile mice. In SFB mice, dendritic cells and activated Th1 and Th17 in the jaw bone marrow were increased, indicating that IL-17 and tumor necrosis factor produced by these cells might contribute to the generation of pro-osteoclast activating factors by osteoblasts [91].
Furthermore, dysbiosis of the gut microbiota and inflammation of the intestinal tract were observed in ApoE-deficient mice with ligation-induced periodontitis. The transfer of gut bacteria through co-housing caused periodontal and intestinal tissues in recipient mice to resemble those of the ligation-induced periodontitis mice model, indicating the involvement of gut bacteria in the pathogenesis of ligation-induced periodontitis [92].
9. Impact of the intereaction between oral and gut microbiota on the pathogenesis of periodontal disease through the immune system
A recent report indicated that orally administered P. gingivalis is taken up through Peyer's patches and specifically activates Th17 under the influence of gut bacteria and that the activated Th17 homing to periodontal tissues is involved in alveolar bone destruction [93]. The results also suggest that changes in the gut microbiota are involved in the responsiveness of periodontal tissues to oral bacteria.
10. Conclusion
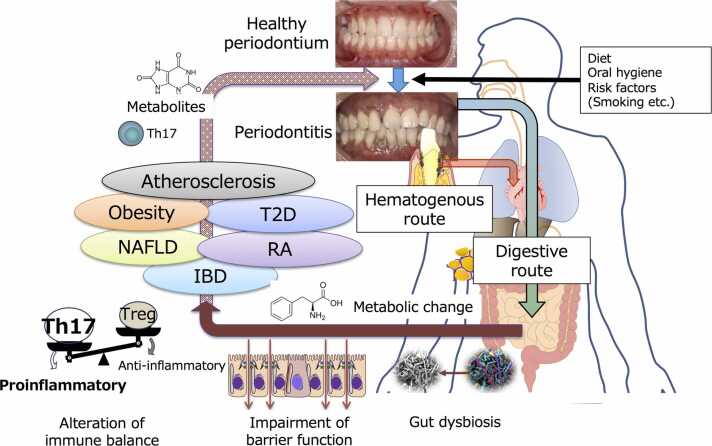
To date, the pathogenesis of systemic diseases resulting from periodontal disease has been attributed to the action of bacterial components and inflammatory mediators from lesions (hematogenous dissemination). However, a novel mechanism (gastrointestinal transmission) has recently garnered attention due to experimental findings in mice. This mechanism involves periodontopathic bacteria, ingested orally, that alter the gut microbiota and could plausibly explain the causative relationship between periodontal disease and systemic diseases that has yet to be entirely elucidated by conventional hypotheses (Fig. 4). Conversely, it has become apparent that dysbiosis of the gut microbiota influences both periodontitis and metabolic diseases. The oral and gut microbiota are thought to interact and impact our health status, but the intricacies of this mechanism are still being unraveled. Future studies should focus on determining the bacterial and host factors responsible for the oral bacteria establishment in the intestinal tract, identifying gut bacteria that vary with oral bacteria and their pathogenicity, assessing changes in metabolites and their consequences, evaluating effects on the immune system, and establishing their connection with periodontitis in actual patients.
Fig. 4.
Possible mechanism connecting periodontitis and systemic diseases. Endotoxemia derived from localized periodontitis is a potential causal mechanism linking periodontitis and systemic disease. In addition, large amounts of swallowed dysbiotic oral microbiota can affect the intestinal environment, resulting in impaired barrier function, altered metabolites, and immune system imbalance, increasing the risk of various systemic diseases. On the other hand, changes in the gut microbiota can also negatively affect the pathogenesis of periodontitis by disrupting metabolism and increasing systemic inflammatory conditions. IBD, Inflammatory bowel disease. NAFLD, Non-alcoholic fatty liver disease. T2D, Type 2 diabetes. Th, T helper. Treg, T-regulatory.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant number JP23H03085).
Acknowledgments
The author would like to thank Editage (www.editage.com) for English language editing.
Conflict of interests
None.
Scientific field of dental science
Periodontology.
References
- 1.Segata N., Haake S.K., Mannon P., Lemon K.P., Waldron L., Gevers D., et al. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13(6):R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koren N., Zubeidat K., Saba Y., Horev Y., Barel O., Wilharm A., et al. Maturation of the neonatal oral mucosa involves unique epithelium-microbiota interactions. Cell Host Microbe. 2021;29(2):197–209. doi: 10.1016/j.chom.2020.12.006. e5. [DOI] [PubMed] [Google Scholar]
- 3.Scannapieco F.A., Dongari-Bagtzoglou A. Dysbiosis revisited: Understanding the role of the oral microbiome in the pathogenesis of gingivitis and periodontitis: A critical assessment. J Periodo. 2021;92(8):1071–1078. doi: 10.1002/JPER.21-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajishengallis G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000. 2022;89(1):9–18. doi: 10.1111/prd.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki K. In: The Human Microbiota and Chronic Disease: Dysbiosis as Cause of Human Pathology. Nibali L., Henderson B., editors. John Wiley & Sons, Inc.; Oxford: 2016. New paradigm in the relationship between periodontal disease and systemic diseases: effects of oral bacteria on the gut microbiota and metabolism; pp. 243–261. (irst ed.) (irst ed.) [Google Scholar]
- 6.Armingohar Z., Jorgensen J.J., Kristoffersen A.K., Abesha-Belay E., Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. 2014:6. doi: 10.3402/jom.v6.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart P.B., Bolger A.F., Papapanou P.N., Osinbowale O., Trevisan M., Levison M.E., et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 8.Stadler A.F., Angst P.D., Arce R.M., Gomes S.C., Oppermann R.V., Susin C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: a meta-analysis. J Clin Periodo. 2016;43(9):727–745. doi: 10.1111/jcpe.12557. [DOI] [PubMed] [Google Scholar]
- 9.Cullinan M.P., Seymour G.J. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 2013;62(1):271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 11.Brusca S.B., Abramson S.B., Scher J.U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheuma. 2014;26(1):101–107. doi: 10.1097/BOR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arimatsu K., Yamada H., Miyazawa H., Minagawa T., Nakajima M., Ryder M.I., et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. doi: 10.1038/srep04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin N., Yang F., Li A., Prifti E., Chen Y., Shao L., et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 14.Warren R.L., Freeman D.J., Pleasance S., Watson P., Moore R.A., Cochrane K., et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1(1):16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellarin M., Warren R.L., Freeman J.D., Dreolini L., Krzywinski M., Strauss J., et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt T.S., Hayward M.R., Coelho L.P., Li S.S., Costea P.I., Voigt A.Y., et al. Extensive transmission of microbes along the gastrointestinal tract. Elife. 2019:8. doi: 10.7554/eLife.42693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwauchi M., Horigome A., Ishikawa K., Mikuni A., Nakano M., Xiao J.Z., et al. Relationship between oral and gut microbiota in elderly people. Immun Inflamm Dis. 2019;7(3):229–236. doi: 10.1002/iid3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kageyama S., Takeshita T., Asakawa M., Shibata Y., Takeuchi K., Yamanaka W., et al. Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashidi A., Ebadi M., Weisdorf D.J., Costalonga M., Staley C. No evidence for colonization of oral bacteria in the distal gut in healthy adults. Proc Natl Acad Sci USA. 2021;118(42) doi: 10.1073/pnas.2114152118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 21.von Troil-Linden B., Torkko H., Alaluusua S., Jousimies-Somer H., Asikainen S. Salivary levels of suspected periodontal pathogens in relation to periodontal status and treatment. J Dent Res. 1995;74(11):1789–1795. doi: 10.1177/00220345950740111201. [DOI] [PubMed] [Google Scholar]
- 22.Saygun I., Nizam N., Keskiner I., Bal V., Kubar A., Acikel C., et al. Salivary infectious agents and periodontal disease status. J Periodontal Res. 2011;46(2):235–239. doi: 10.1111/j.1600-0765.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- 23.Boutaga K., Savelkoul P.H., Winkel E.G., van Winkelhoff A.J. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J Periodo. 2007;78(1):79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 24.Sato K., Takahashi N., Kato T., Matsuda Y., Yokoji M., Yamada M., et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci Rep. 2017;7(1):6955. doi: 10.1038/s41598-017-07196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 26.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 27.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 29.Komazaki R., Katagiri S., Takahashi H., Maekawa S., Shiba T., Takeuchi Y., et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci Rep. 2017;7(1):13950. doi: 10.1038/s41598-017-14260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Huang W., Dai K., Liu N., Wang J., Lu X., et al. Inflammatory response of gut, spleen, and liver in mice induced by orally administered Porphyromonas gingivalis. J Oral Microbiol. 2022;14(1) doi: 10.1080/20002297.2022.2088936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atarashi K., Suda W., Luo C., Kawaguchi T., Motoo I., Narushima S., et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358(6361):359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato T., Yamazaki K., Nakajima M., Date Y., Kikuchi J., Hase K., et al. Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere. 2018;3(5) doi: 10.1128/mSphere.00460-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A., et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson M., Lewis G.D., Ericson U., Orho-Melander M., Hedblad B., Engstrom G., et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer C.D., Simas A.M., He X., Ingalls R.R., Weinberg E.O., Genco C.A. Distinct roles for dietary lipids and Porphyromonas gingivalis infection on atherosclerosis progression and the gut microbiota. Anaerobe. 2017;45:19–30. doi: 10.1016/j.anaerobe.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L., Huang L., Zhou X., Zhao D., Wang Y., Min H., et al. Experimental periodontitis deteriorated atherosclerosis associated with trimethylamine N-oxide metabolism in mice. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.820535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Berg W.B., Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheuma. 2009;5(10):549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 41.Quirke A.M., Lugli E.B., Wegner N., Hamilton B.C., Charles P., Chowdhury M., et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis. 2014;73(1):263–269. doi: 10.1136/annrheumdis-2012-202726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamamoto Y., Ouhara K., Munenaga S., Shoji M., Ozawa T., Hisatsune J., et al. Effect of Porphyromonas gingivalis infection on gut dysbiosis and resultant arthritis exacerbation in mouse model. Arthritis Res Ther. 2020;22(1):249. doi: 10.1186/s13075-020-02348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe K., Katagiri S., Takahashi H., Sasaki N., Maekawa S., Komazaki R., et al. Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J. 2021;35(2) doi: 10.1096/fj.202001158R. [DOI] [PubMed] [Google Scholar]
- 44.Dong Z., Lv W., Zhang C., Chen S. Correlation analysis of gut microbiota and serum metabolome with Porphyromonas gingivalis-induced metabolic disorders. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.858902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kashiwagi Y., Aburaya S., Sugiyama N., Narukawa Y., Sakamoto Y., Takahashi M., et al. Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci Rep. 2021;11(1):18398. doi: 10.1038/s41598-021-97868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simas A.M., Kramer C.D., Genco C.A. Diet-induced non-alcoholic fatty liver disease and associated gut dysbiosis are exacerbated by oral infection. Front Oral Health. 2021;2 doi: 10.3389/froh.2021.784448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki K., Kato T., Tsuboi Y., Miyauchi E., Suda W., Sato K., et al. Oral pathobiont-induced changes in gut microbiota aggravate the pathology of nonalcoholic fatty liver disease in mice. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.766170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng Y.K., Wu Q.L., Peng Y.W., Liang F.Y., You H.J., Feng Y.W., et al. Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J Neuroinflamm. 2020;17(1):347. doi: 10.1186/s12974-020-02027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeves B.C., Karimy J.K., Kundishora A.J., Mestre H., Cerci H.M., Matouk C., et al. Glymphatic system impairment in alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. 2020;26(3):285–295. doi: 10.1016/j.molmed.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chi L., Cheng X., Lin L., Yang T., Sun J., Feng Y., et al. Porphyromonas gingivalis-Induced Cognitive Impairment Is Associated With Gut Dysbiosis, Neuroinflammation, and Glymphatic Dysfunction. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.755925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohn J., Li L., Zhang L., Settem R.P., Honma K., Sharma A., et al. Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4(+) T cells. Mol Oral Microbiol. 2021 doi: 10.1111/omi.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitamoto S., Nagao-Kitamoto H., Jiao Y., Gillilland M.G., 3rd, Hayashi A., Imai J., et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182(2):447–462. doi: 10.1016/j.cell.2020.05.048. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Bao J., Chang Y., Wang M., Chen B., Yan F. Gut microbiota may mediate the influence of periodontitis on prediabetes. J Dent Res. 2021;100(12):1387–1396. doi: 10.1177/00220345211009449. [DOI] [PubMed] [Google Scholar]
- 54.Hu Y., Zhang X., Zhang J., Xia X., Li H., Qiu C., et al. Activated STAT3 signaling pathway by ligature-induced periodontitis could contribute to neuroinflammation and cognitive impairment in rats. J Neuroinflamm. 2021;18(1):80. doi: 10.1186/s12974-021-02071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing T., Liu Y., Cheng H., Bai M., Chen J., Ji H., et al. Ligature induced periodontitis in rats causes gut dysbiosis leading to hepatic injury through SCD1/AMPK signalling pathway. Life Sci. 2022;288 doi: 10.1016/j.lfs.2021.120162. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L.J., Lin W.Z., Liu T., Chen B.Y., Meng X.Q., Li Y.L., et al. Oral pathobionts promote MS-like symptoms in mice. J Dent Res. 2023;102(2):217–226. doi: 10.1177/00220345221128202. [DOI] [PubMed] [Google Scholar]
- 57.Li B., Ge Y., Cheng L., Zeng B., Yu J., Peng X., et al. Oral bacteria colonize and compete with gut microbiota in gnotobiotic mice. Int J Oral Sci. 2019;11(1):10. doi: 10.1038/s41368-018-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamazaki K., Miyauchi E., Kato T., Sato K., Suda W., Tsuzuno T., et al. Dysbiotic human oral microbiota alters systemic metabolism via modulation of gut microbiota in germ-free mice. J Oral Microbiol. 2022;14(1) doi: 10.1080/20002297.2022.2110194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M., Li L., Qian J., Wang N., Bao J., Lu J., et al. Periodontitis salivary microbiota exacerbates nonalcoholic fatty liver disease in high-fat diet-induced obese mice. iScience. 2023;26(4) doi: 10.1016/j.isci.2023.106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian J., Lu J., Huang Y., Wang M., Chen B., Bao J., et al. Periodontitis salivary microbiota worsens colitis. J Dent Res. 2022;101(5):559–568. doi: 10.1177/00220345211049781. [DOI] [PubMed] [Google Scholar]
- 61.Lu J., Zhang S., Huang Y., Qian J., Tan B., Qian X., et al. Periodontitis-related salivary microbiota aggravates Alzheimer's disease via gut-brain axis crosstalk. Gut Microbes. 2022;14(1) doi: 10.1080/19490976.2022.2126272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lourenco T.G.B., de Oliveira A.M., Tsute Chen G., Colombo A.P.V. Oral-gut bacterial profiles discriminate between periodontal health and diseases. J Periodontal Res. 2022;57(6):1227–1237. doi: 10.1111/jre.13059. [DOI] [PubMed] [Google Scholar]
- 63.Kawamoto D., Borges R., Ribeiro R.A., de Souza R.F., Amado P.P.P., Saraiva L., et al. Oral dysbiosis in severe forms of periodontitis is associated with gut dysbiosis and correlated with salivary inflammatory mediators: a preliminary study. Front Oral Health. 2021;2 doi: 10.3389/froh.2021.722495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amado P.P.P., Kawamoto D., Albuquerque-Souza E., Franco D.C., Saraiva L., Casarin R.C.V., et al. Oral and fecal microbiome in molar-incisor pattern periodontitis. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.583761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Lu H., Wu H., Huang S., Chen L., Gui Q., et al. Periodontitis in elderly patients with type 2 diabetes mellitus: impact on gut microbiota and systemic inflammation. Aging. 2020;12(24):25956–25980. doi: 10.18632/aging.202174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imai J., Ichikawa H., Kitamoto S., Golob J.L., Kaneko M., Nagata J., et al. A potential pathogenic association between periodontal disease and Crohn's disease. JCI Insight. 2021;6(23) doi: 10.1172/jci.insight.148543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sohn J., Li L., Zhang L., Genco R.J., Falkner K.L., Tettelin H., et al. Periodontal disease is associated with increased gut colonization of pathogenic Haemophilusparainfluenzae in patients with Crohn's disease. Cell Rep. 2023;42(2) doi: 10.1016/j.celrep.2023.112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X., Wang P., Ma L., Guo R., Zhang Y., Wang P., et al. Differences in the oral and intestinal microbiotas in pregnant women varying in periodontitis and gestational diabetes mellitus conditions. J Oral Microbiol. 2021;13(1) doi: 10.1080/20002297.2021.1883382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jepsen S., Caton J.G., Albandar J.M., Bissada N.F., Bouchard P., Cortellini P., et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodo. 2018;89(Suppl 1):S237–S248. doi: 10.1002/JPER.17-0733. [DOI] [PubMed] [Google Scholar]
- 70.Chandan J.S., Thomas T. The impact of inflammatory bowel disease on oral health. Br Dent J. 2017;222(7):549–553. doi: 10.1038/sj.bdj.2017.318. [DOI] [PubMed] [Google Scholar]
- 71.Messora M.R., Oliveira L.F., Foureaux R.C., Taba M., Jr., Zangeronimo M.G., Furlaneto F.A., et al. Probiotic therapy reduces periodontal tissue destruction and improves the intestinal morphology in rats with ligature-induced periodontitis. J Periodo. 2013;84(12):1818–1826. doi: 10.1902/jop.2013.120644. [DOI] [PubMed] [Google Scholar]
- 72.Kesavalu L., Bakthavatchalu V., Rahman M.M., Su J., Raghu B., Dawson D., et al. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22(4):232–239. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 73.Nagatake T., Kishino S., Urano E., Murakami H., Kitamura N., Konishi K., et al. Intestinal microbe-dependent omega3 lipid metabolite alphaKetoA prevents inflammatory diseases in mice and cynomolgus macaques. Mucosal Immunol. 2022;15(2):289–300. doi: 10.1038/s41385-021-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada M., Takahashi N., Matsuda Y., Sato K., Yokoji M., Sulijaya B., et al. A bacterial metabolite ameliorates periodontal pathogen-induced gingival epithelial barrier disruption via GPR40 signaling. Sci Rep. 2018;8(1):9008. doi: 10.1038/s41598-018-27408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.AAd Araújo, AdSBF Pereira, CACXd Medeiros, GAdC Brito, RFdC Leitão, LdS Araújo, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLOS One. 2017;12(8) doi: 10.1371/journal.pone.0183506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Induri S.N.R., Kansara P., Thomas S.C., Xu F., Saxena D., Li X. The gut microbiome, metformin, and aging. Annu Rev Pharm Toxicol. 2022;62:85–108. doi: 10.1146/annurev-pharmtox-051920-093829. [DOI] [PubMed] [Google Scholar]
- 77.Sato K., Yamazaki K., Kato T., Nakanishi Y., Tsuzuno T., Yokoji-Takeuchi M., et al. Obesity-related gut microbiota aggravates alveolar bone destruction in experimental periodontitis through elevation of uric acid. mBio. 2021;12(3) doi: 10.1128/mBio.00771-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rock K.L., Kataoka H., Lai J.J. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheuma. 2013;9(1):13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banu S., Jabir N.R., Mohan R., Manjunath N.C., Kamal M.A., Kumar K.R., et al. Correlation of Toll-like receptor 4, interleukin-18, transaminases, and uric acid in patients with chronic periodontitis and healthy adults. J Periodo. 2015;86(3):431–439. doi: 10.1902/jop.2014.140414. [DOI] [PubMed] [Google Scholar]
- 80.Li C., Huang Q., Yang R., Dai Y., Zeng Y., Tao L., et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30(5):1003–1013. doi: 10.1007/s00198-019-04855-5. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Wang Y., Gao W., Wang B., Zhao H., Zeng Y., et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. 2017;5 doi: 10.7717/peerj.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sjogren K., Engdahl C., Henning P., Lerner U.H., Tremaroli V., Lagerquist M.K., et al. The gut microbiota regulates bone mass in mice. J Bone Min Res. 2012;27(6):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novince C.M., Whittow C.R., Aartun J.D., Hathaway J.D., Poulides N., Chavez M.B., et al. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep. 2017;7(1):5747. doi: 10.1038/s41598-017-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 85.Lu L., Chen X., Liu Y., Yu X. Gut microbiota and bone metabolism. FASEB J. 2021;35(7) doi: 10.1096/fj.202100451R. [DOI] [PubMed] [Google Scholar]
- 86.Wu H.J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flores R., Shi J., Fuhrman B., Xu X., Veenstra T.D., Gail M.H., et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silva B.C., Bilezikian J.P. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharm. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J.Y., Yu M., Pal S., Tyagi A.M., Dar H., Adams J., et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. 2020;130(4):1767–1781. doi: 10.1172/JCI133473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jia L., Tu Y., Jia X., Du Q., Zheng X., Yuan Q., et al. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021;54(7) doi: 10.1111/cpr.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hathaway-Schrader J.D., Carson M.D., Gerasco J.E., Warner A.J., Swanson B.A., Aguirre J.I., et al. Commensal gut bacterium critically regulates alveolar bone homeostasis. Lab Invest. 2022;102(4):363–375. doi: 10.1038/s41374-021-00697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L., Wang M., Bao J., Wang N., Huang Y., He S., et al. Periodontitis may impair the homeostasis of systemic bone through regulation of gut microbiota in ApoE(-/-) mice. J Clin Periodo. 2022;49(12):1304–1319. doi: 10.1111/jcpe.13708. [DOI] [PubMed] [Google Scholar]
- 93.Nagao J.I., Kishikawa S., Tanaka H., Toyonaga K., Narita Y., Negoro-Yasumatsu K., et al. Pathobiont-responsive Th17 cells in gut-mouth axis provoke inflammatory oral disease and are modulated by intestinal microbiome. Cell Rep. 2022;40(10) doi: 10.1016/j.celrep.2022.111314. [DOI] [PubMed] [Google Scholar]