Abstract
Gene therapies have potential to improve outcomes of severe diseases after only a single administration. Novel therapies are continually being developed using knowledge gained from prior successes, a concept known as scientific spillover. Gene therapy advancement requires extensive development at each stage: preclinical work to create and evaluate vehicles for delivery of the therapy, design of clinical development programs, and establishment of a large-scale manufacturing process. Pioneering gene therapies are generating spillover as investigators confront myriad issues specific to this treatment modality. These include frameworks for construct engineering, dose evaluation, patient selection, outcome assessment, and safety monitoring. Consequently, the benefits of these therapies extend beyond offering knowledge for treating any one disease to establishing new platforms and paradigms that will accelerate advancement of future gene therapies. This impact is even more profound in rare diseases, where developing therapies in isolation may not be possible. This review describes some instances of scientific spillover in healthcare, and specifically gene therapy, using delandistrogene moxeparvovec (SRP-9001), a gene therapy recently approved by the US Food and Drug Administration for the treatment of ambulatory pediatric patients aged 4–5 years with Duchenne muscular dystrophy with a confirmed mutation in the DMD gene, as a case study.
Keywords: delandistrogene moxeparvovec, Duchenne muscular dystrophy, gene therapy, rare disease, scientific spillover, SRP-9001
Graphical abstract

Rodino-Klapac and colleagues describe how knowledge gained from development and production of a gene therapy reaches beyond the therapy and its indication by helping accelerate further gene therapy advancements, a concept known as scientific spillover. An example of this is delandistrogene moxeparvovec, a novel gene therapy for Duchenne muscular dystrophy.
Introduction
Gene therapy offers a novel approach to treating monogenic diseases that, rather than only treating symptoms, targets the root cause of a disease by introducing a vector coding for a gene that compensates for a mutated or absent gene.1 The pathological consequences of a disease may be prevented or substantially delayed after only a single gene therapy treatment.2,3,4 Because diagnosis is made via genetic testing, appropriate use of and patient response to this treatment are anticipated to be high. The benefits may be even more enhanced if the gene therapy is administered before irreversible damage from the disease sets in.5 Many available and developing gene therapies are targeted at rare diseases with poor prognoses, making these therapies an exciting innovation that can offer hope to patients with diseases that have lacked effective treatment.
There are, however, significant challenges to successfully developing and manufacturing gene therapy. Fortunately, breakthroughs and further progress in scientific and medical research are frequently realized by building on lessons learned from prior developments (both successes and failures), a concept known as scientific spillover. The idea of spillover generally refers to the additional impact (or externalities) of an advancement or intervention that extends beyond the targeted recipient.6 An innovative therapy that may have benefited from earlier research could, in turn, produce knowledge that spurs advancement of other effective treatments.7,8 This is especially evident in the development of treatments for new disease states or for therapies with novel mechanisms of action, such as gene therapies.
Bringing innovative drugs and treatment modalities to the healthcare market requires significant resources and can be particularly challenging when targeting smaller subgroups, such as patients with rare diseases.9,10 Gene therapies live in the realm of personalized medicine, where a deeper understanding of disease processes has created treatments highly targeted to individuals with a certain genetic mutation. While the therapeutic potential of these treatments is pronounced, the cost of their manufacturing is burdensome, particularly in the rare disease setting, where that cost is not offset by selling in large volumes. Scientific spillover then becomes even more important for progress in gene therapies because the resources and expenditures involved in developing one of the first treatments in a disease area may have a broader perspective of value if it potentially lowers the development time and costs for subsequent therapies, both within that therapeutic area and for other indications.
Thus, the development of a novel gene therapy, especially for a rare disease, is expected to provide scientific spillover and corresponding value that extend beyond the patients who receive the gene therapy itself to society as a whole. This review will concentrate on the potential impact of scientific spillover in the development of gene therapies, focusing on delandistrogene moxeparvovec (SRP-9001), a gene therapy recently approved by the US Food and Drug Administration (FDA) for the treatment of ambulatory pediatric patients aged 4–5 years with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene11 as a case study.
Examples of scientific spillover in the biotechnology sector
To date, there are many examples of scientific spillover in the biotechnology sector, where development of new therapies has been greatly accelerated as a result of preexisting programs. For instance, monoclonal antibodies (mAbs) are currently one of the fastest growing therapeutic drug classes.12 Antibody-mediated immunotherapy has long been used as a tool to treat infectious diseases via passive transfer (i.e., from one individual to another) and has evolved into a technology that treats myriad conditions by targeting specific molecules using manufactured mAbs.13,14 The development of mAbs was made possible by a series of innovations, including hybridoma and recombinant DNA technologies, antibody isolation methods, and improvements in formulations and dosage forms.12 Further advancements have reduced mAb immunogenicity and increased their affinity for their antigens.15 In addition, optimization of cell culture processes and adoption of recombinant and separation technologies have increased mAb yields by several orders of magnitude, from <20 mg/L to up to 25 g/L.16 The first therapeutic mAb approved by the FDA in 1986 was OKT3, or muromonab-CD3, which is used to deplete T cells and thus prevent allograft rejection in renal transplant patients.17 Since then, over 100 mAbs have been approved to treat a range of diseases (e.g., cancer, infectious diseases, autoimmune diseases, and neurodegenerative disorders), with hundreds more in development.12,17
Another highly visible example of scientific spillover can be seen with mRNA vaccines. Both mRNA and liposomes (fatty membrane structures used to transport mRNA into cells) were first discovered in the 1960s.18 Basic research (both publicly and privately funded) propelled the development of RNA synthesis and methods for delivering mRNA into cells to induce gene expression. Based on experiences and insights gained from working with DNA, multiple innovations in mRNA technology ensued, including advancements in mRNA synthesis and modification as well as in lipid-based delivery systems (e.g., liposomes, lipid nanoparticles, scalable manufacturing methods).18 This led to the delivery in 1993 of the first mRNA vaccine (tested on mice) for influenza19 and has culminated in extraordinarily expedited vaccine development for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic.20,21 Advantages of this vaccine technology include its fast production and ability to quickly pivot to cover new strains, as is evidenced by the boosters specific to the Omicron variant of SARS-CoV-2.22,23 Furthermore, the lessons learned in initial studies of these vaccines informed best practices for safety monitoring, with the finding that the vast majority of adverse events (AEs) occur within days of receiving the vaccine.24 Therefore, just as scientific spillover contributed to the rapid advancements seen during the SARS-CoV-2 pandemic, similar contributions will continue to inform future vaccine development. Additionally, expedited development and manufacturing processes, as well as knowledge of how to better monitor patients and respond to AEs, are also applicable to gene therapy development.
Scientific spillover in gene therapy for rare diseases
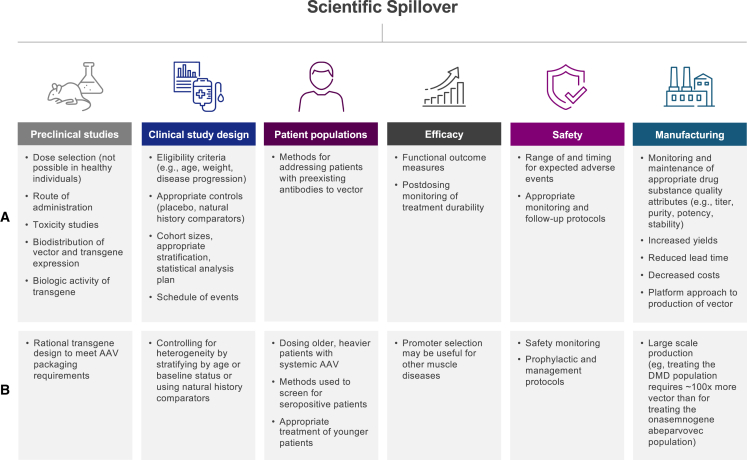
Because the approach to treating diseases with targeted gene therapies is novel, any knowledge derived from early attempts at repairing genetic errors by introducing a viral vector will provide important scientific spillover for subsequent attempts. The benefits reaped by spillover are even more profound when the disease is rare. For instance, Becker muscular dystrophy (BMD), a progressive neuromuscular disease (although with a slower trajectory, on average, than DMD) has an incidence of approximately 1 in 18,500 male births, while the incidence of DMD is approximately 1 in 3,500–5,000 male births worldwide.25,26 These diseases often lack robust natural history and outcome data and have a limited patient base from which to cull information, necessitating additional efforts to identify and develop relevant data. Thus, large portions of the evidence base (including optimal diagnostic testing and disease trajectory, preclinical and clinical trial designs, functional outcome measures, and even protocols for safety monitoring, site readiness, and patient education) must be created during the therapeutic development phase. This is not easy to do independently or in isolation with a scarcity of clinical data, so progress in developing gene therapies for these disease states is likely to rely on a certain amount of scientific spillover from prior development of similar therapies. Spillover may also aid in the development of treatments in other therapeutic classes, particularly for the same disease. Currently, approximately 5,000 gene therapy trials are either planned, ongoing, or completed (ClinicalTrials.gov, as of February 23, 2023), with more than 60 approved cell and gene therapies projected for 2030.27 This section will assess the opportunities for spillover of gene therapies across many facets of drug development (Figure 1A).
Figure 1.
Impact of scientific spillover on technology development
(A) Scientific spillover resulting from advances in gene therapy. (B) Potential scientific spillover resulting from development of delandistrogene moxeparvovec DMD gene therapy.
Preclinical studies
Preclinical studies that evaluate safety, efficacy, and appropriate dosage are particularly important because, unlike other therapies, gene therapies can be administered only once to humans due to the inhibitory immune response that follows, and they cannot be tested in healthy volunteers. Therefore, transduction, biodistribution, transgene expression, toxicology following gene transfer, and optimal dosing of the viral vectors must be assessed in stable cell lines and animal models, including mice and nonhuman primates.
Preclinical biodistribution studies are critical for determining the optimal route of administration for gene therapies to transduce a target tissue.28,29 For example, a recent preclinical study compared adeno-associated virus 9 (AAV9) biodistribution in the central and peripheral nervous systems following intracerebroventricular (i.c.v.), intracisternal magna (ICM), intrathecal-lumbar (IT-L), and intravenous (i.v.) delivery in nonhuman primates.30 Both IT-L and i.v. administration delivered 10- to 100-fold lower levels of AAV to the brain compared with i.c.v. and ICM administration.30 Furthermore, preclinical studies assessing AAV serotype tissue tropism (e.g., muscle vs. liver) and cell-type-specific expression directed by regulatory cassettes may also influence the design of subsequent investigational gene therapies.
Gene therapies also differ from many other therapeutic classes in that therapeutic dose ranges for drugs are often selected based on results from treating healthy volunteers. However, in gene therapy development, the use of healthy volunteers is not an option, as vector exposure can result in immunologic memory to the vector and/or transgene,31 thus precluding the future use of gene therapies for these individuals. Healthy volunteers are also excluded from gene therapy clinical trials due to the permanence of gene transfer and potentially nonbeneficial consequences of transgene expression in healthy individuals. Another issue in dose-escalation studies for gene therapy is that care must be taken to avoid doses that are too low, especially for those patients with serious, progressive, and fatal diseases.32,33,34,35,36 Gene therapy is unique in that a subtherapeutic low dose of the study drug could be considered harmful because the consequent immune barrier that develops could prevent the patient from receiving an effective dose in the future.37
Taken together, these factors place an unusually high burden on the preclinical stage of drug development for gene therapies and necessitate the establishment of new experimental models and methodologies. Fortunately, as the validity of these techniques is proven by subsequent clinical trials, they can also be used to accelerate the development of future therapies.
Clinical development
Gene therapy clinical trials may also generate scientific spillover that will be valuable for designing future trials, particularly for rare diseases for which data are often scarce. For example, the clinical trials of the first two gene therapies that were approved by the FDA—voretigene neparvovec-rzyl for the treatment of Leber congenital amaurosis and onasemnogene abeparvovec-xioi for the treatment of spinal muscular atrophy (SMA)—have included trials with fewer than 100 patients combined.38,39 These studies therefore required the development of protocols that could establish safety and efficacy using relatively small numbers of patients. Proper follow-up analysis for future clinical trials must also be modeled on earlier studies of this class of therapeutics. Since a one-time gene therapy differs from other therapeutic classes in that it cannot be discontinued, best practices for follow-up must be determined to assess its long-term safety, efficacy, and durability.
Patient populations
The composition of patient populations from previous gene therapy studies may be used to model subsequent studies in similar diseases, including eligibility criteria (e.g., age, weight, progression of disease) and anticipated exclusions to generate cohorts best suited for detecting treatment effects. Determining an appropriate treatment population requires a careful balance between safety and sensitivity, with the aim of including as many individuals as possible who will benefit. The age and weight of patients will influence what dosages are needed and potentially the immune responses to the gene therapy construct. These learnings will apply to future therapies as studies come to include both older and younger patients, as well as to establish effective dosing for heavier patients.
At present, a major barrier to successful gene therapy is the possible presence of preexisting antibodies to vectors. People may coincidentally develop antibodies against viruses present in the environment, such as AAV, and these may cross-react with gene therapy vectors derived from these viruses. These preexisting antibodies may block transduction by the vector and/or stimulate an immune response that could lead to AEs. For these reasons, gene therapy clinical trials often exclude patients with certain levels of preexisting antibodies directed against the vector. There are several methods to test for anti-AAV antibodies, including neutralizing assays and total antibody binding assays,40 and methods for removing these antibodies prior to drug administration have been described.41 Many of these assays, however, are product-specific and have not been validated across multiple clinical trials, in essence, hindering spillover in this area. Yet, studies have established antibody levels that strike the best balance between maintaining safety and efficacy while excluding as few patients as possible,42,43 and investigations are underway to determine how to dose patients who have preexisting AAV antibodies. As research advances, it seems possible that this potential barrier to treatment may be overcome and the knowledge applied to development of future treatments.
Efficacy
Selection of functional outcome measures in gene therapy clinical studies sensitive enough to detect differences from baseline values or natural history controls, especially in heterogeneous patient populations, is critical for demonstrating therapeutic efficacy. When the disease being studied is rare, outcome measures are usually reported using small sample sizes, and when it is severe, clinical trials are of short duration. Earlier gene therapy studies may then help identify reliable and quantifiable functional outcome measures for individuals with these diseases, determine the time frames during which they should be assessed, and inform the interpretation of results. Longitudinal analyses will also help maximize statistical power in these limited studies.44 Further, outcome measures need to be sensitive enough to assess changes in as short a period of time as possible, particularly when studies of progressive diseases involve a placebo arm, as maintaining deteriorating patients on a placebo for extended periods raises ethical issues. In addition, methods of postdosing monitoring for the durability of efficacy findings may be applied based on experience from other studies.
Safety
Safety findings and management of treatment-related AEs (TRAEs) from initial gene therapy studies can inform investigators of the range of expected AEs (e.g., complement activation, hepatotoxicity) as well as the methods to manage or prevent such TRAEs from occurring in subsequent studies.45,46 For example, known safety events reported for initial AAV gene transfer differing among AAV serotypes (including hepatoxicity, thrombocytopenia, and thrombotic microangiopathy46,47,48) may inform the postdosing monitoring needs for subsequently developed AAV-based gene therapies. In addition, the importance of prednisolone prophylaxis in systemic AAV-based gene transfer has been highlighted by elevations in serum aminotransferase in one initial gene transfer study using an AAV9-based vector administered by i.v. injection.36 Best practices in prednisolone tapering in the absence or presence of persistent transaminase elevations can also be determined by collective analysis of previous studies.49
Safety analyses can also inform the design and delivery of future gene therapies. Current trials using AAV vectors are illuminating differences in safety profiles among different AAV vector serotypes, and this will inform the choice of optimal vector platforms for future therapies.
Manufacturing
Many steps in the manufacturing of AAV gene therapies can affect the stability and efficacy of the process, including plasmid development and production; cell expansion; plasmid(s) transfection; viral vector production, purification, and formulation; and analytical method development.50 It is anticipated that over time, advances in gene therapy manufacturing will lower costs and increase yield, thereby clearing the way for cheaper and more efficient manufacture of newer gene therapies. Thus far, efforts have focused on increasing vector yield and purity and decreasing lead time while minimizing empty capsids.51
Plasmid production is itself a multistep process, requiring Escherichia coli fermentation followed by amplification, harvesting, purification, and testing of plasmid.50 Unfortunately, lot-to-lot differences in plasmid yield and purity are still problematic51; however, the use of defined media and process controls can increase consistency and provide a spillover effect to improve the manufacturing of subsequent gene therapies.50
Regarding cell culture methods, researchers now use suspension cultures that allow for the scaling up of the process to achieve a desired cell density. Further, highly defined culture media that have lower levels of animal-based products (e.g., serum) can reduce product contamination.51 However, researchers have also discovered that such cultures are not always efficiently transfected, especially when co-transfection is required.50 Knowledge achieved from testing these processes is likely to guide the manufacture of future gene therapies.
The production of AAV originally required the use of an additional “helper” adenovirus (which lacked rep [replication] and cap [capsid] genes) that subsequently had to be removed from AAV preparations. Current methodologies have eliminated the need to use helper virus, thus streamlining AAV production. The “helper”-free triple-transfection method now in use co-transfects an adenovirus helper plasmid containing the E2a/b, E4, and VARNA genes with a cis plasmid, comprising the transgene cassette flanked by inverted terminal repeats, and a trans plasmid, which contains rep and cap gene components from helper adenovirus.50 Stable mammalian cell lines that constitutively express capsid cassettes and/or helper genes are also now in use. These cell lines not only increase yield for large-scale production capacity52,53 but also reduce potential sources of contamination. Further, the use of baculovirus expression vector systems is also being explored as another way to improve AAV production.54
Following transfection, AAV gene therapies must undergo purification to remove host cell material, plasmid DNA, and empty capsids.50 Recent advances in purification methods include microfiltration for cell fragment removal, affinity chromatography to remove protein contaminants, and transfer from centrifugation methods to anion-exchange chromatography to remove empty capsids.50 These improvements have enabled the transition from lab-scale production of vectors to processes and standardizations better suited to large-scale manufacturing that will aid in the production of commercial products targeting multiple diseases.
Case study of delandistrogene moxeparvovec, a gene therapy for DMD
The development of delandistrogene moxeparvovec, a gene therapy recently approved by the FDA for the treatment of ambulatory pediatric patients aged 4–5 years with DMD with a confirmed mutation in the DMD gene,11 can serve as an example of the potential for scientific spillover (Figure 1B). DMD is a rare, fatal neuromuscular disease caused by mutations in the X-linked DMD gene, which codes for a critical protein called dystrophin that protects muscle against contraction-induced injury.55 Loss of dystrophin protein culminates in the progressive loss of skeletal and cardiac muscle; patients generally experience loss of ambulation early in life and premature death from cardiac or respiratory failure by the third decade.56
Preclinical studies
Numerous therapeutic approaches have been attempted to restore muscle function in patients with DMD. DMD is a recessive monogenic disease and therefore especially well-suited for gene therapy, which is designed to compensate for underlying genetic mutation. Multiple preclinical studies have helped identify key components of the DMD gene, and these advances have run parallel with advancements in AAV technology (Figure 2).57,58,59,60,61,62,63,64 Dystrophin is a 427 kDa protein comprising more than 3,600 amino acids, and the 79 exons in the DMD gene exceed the carrying capacity of AAVs. However, genotype-phenotype analyses of patients with mild DMD or BMD, a milder form of dystrophinopathy, suggest that certain portions of the DMD gene could be deleted without major consequences for protein function.65,66 These findings from a natural history study were used to inform an extensive body of preclinical work to identify shortened dystrophin proteins that retain the protein’s significant functional elements but whose gene sequences are short enough to be engineered into an AAV.65 The SRP-9001 dystrophin transgene construct, therefore, retains key anchoring regions needed to produce a shortened, functioning version of the dystrophin protein.37
Figure 2.
Convergence of AAV and shortened dystrophin technologies, leading to systemic administration of DMD gene therapies in patients87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103
This type of transgene engineering may also be applied to other monogenic diseases. In fact, many of the estimated 5,000–8,000 monogenic diseases identified to date67 similarly require construct optimization, as the genes exceed the packaging capacity of AAV. Current ongoing studies in which the gene is too large to fit into an AAV vector include dysferlinopathies (dysferlin), cystic fibrosis (CFTR), Usher syndrome 1B (Myosin VIIA), Stargardt disease (ABCA4), hemophilia A (Factor VIII),68 Wilson disease (ATP7B),69 and GSDIII (Agl),70 as well as CEP290-associated Leber congenital amaurosis.71
Clinical development
Patient populations
As mentioned, referral to previous studies is useful when selecting the optimal patient population for initial clinical trials of a new therapy. In the delandistrogene moxeparvovec program, the DMD patient population has been expanded from those aged 4–7 years in initial studies to older (≥8 to <18 years [ClinicalTrials.gov: NCT04626674]) as well as younger (3 months to 3 years [ClinicalTrials.gov: NCT03375164] and ≥3 to <4 years [ClinicalTrials.gov: NCT04626674]) patients. These new studies will examine efficacy in these patient populations and determine the impact of treatment on different age groups. Lessons learned from these patient populations will also inform research regarding the safety and monitoring needs of future therapies.72
Initial studies are also critical when assessing the percentage of the patient population with preexisting immunity to AAV and how to successfully treat these patients. In one study, removing AAV-binding antibodies in seropositive nonhuman primates by plasmapheresis prior to isolated limb perfusion increased transduction levels of rAAVrh74.micro-dys.FLAG to those observed in seronegative animals.42 A follow-on study with i.v. delivery in nonhuman primates confirmed the utility of plasmapheresis (L.R.-K., unpublished data). Circumventing preexisting immunity to the vector holds promise for patients who previously were ineligible for treatment because of the level of AAV-binding antibodies detected in their serum. This is a prime example of the benefits of scientific spillover: knowledge of how to overcome this obstacle in nonhuman primates was gained in investigations using the vector that had been developed for delandistrogene moxeparvovec. These findings may be extended to clinical applications so that this knowledge will continue to be useful for the development of gene therapies in these and other disease states.
DMD is a systemic disease affecting the muscles, and gene therapies currently under development for DMD use i.v. administration based on the patient’s body weight. With a median age at diagnosis of 4.6 years,73 the average patient with DMD receiving gene therapy will be substantially heavier than a patient treated with onasemnogene abeparvovec, a gene therapy indicated for patients aged less than 2 years with SMA.49 Another gene therapy approved in the US, voretigene neparvovec, is directly administered via subretinal injection.74 Therefore, patients receiving gene therapy for DMD will require substantially more viral vectors compared with the low viral loads of both onasemnogene abeparvovec and voretigene neparvovec. Thus, safety monitoring of patients with DMD who receive greater viral loads may result in critical scientific spillover that will inform the dosing of older and heavier individuals in current and future gene therapies.
Efficacy and safety
Delandistrogene moxeparvovec for patients with DMD is currently the most advanced clinical program of systemic gene therapy for the disease, and advances made during its development have already and will continue to inform the development of additional therapies for DMD and other diseases. For example, trials of other treatments for muscular dystrophies have looked to studies of delandistrogene moxeparvovec for consistent outcome measures of muscle function to help identify therapeutics that successfully inhibit disease progression or permit muscle functional recovery. Similarly, delandistrogene moxeparvovec has the potential to establish the precedent for other gene therapies seeking the use of biomarker-based endpoints, as expression of the therapeutic gene itself (SRP-9001) is being used in clinical trials to demonstrate that restoring the functionality of the gene of interest is effective in altering the disease trajectory (ClinicalTrials.gov: NCT04626674, NCT03769116, NCT03375164, and NCT05096221). This could greatly speed the development and approval of future gene therapies for slowly progressing or highly heterogeneous diseases, where detection of clinical effects would require long trials or challenging cohort sizes. Further, extensive preclinical and clinical analyses of vectors and promoters for gene therapies are crucial, and the extent of transgene expression that occurs via different routes of administration must be understood. Future studies of vectors and promoters can also draw on knowledge obtained from studies of the SRP-9001 dystrophin transgene construct. In fact, the vector used in delandistrogene moxeparvovec, AAVrh74, is being used in several programs for limb-girdle muscular dystrophies (LGMDs). The delandistrogene moxeparvovec MHCK7 promoter is also being evaluated for use in other new gene therapies. The MHCK7 promoter, comprising an α-myosin heavy chain (α-MHC) enhancer and a muscle creatine kinase (MCK) enhancer/promoter region, induces high transgene expression in skeletal and cardiac muscle.75 It is therefore being used in the development of therapies for certain LGMD subtypes (including LGMD2B [dysferlinopathy], LGMD2C, LGMD2D, LGMD2E, and LGMDR25)76,77,78,79 and may be useful for other muscular dystrophies. The promoter has also been shown to enable muscle-specific CRISPR-mediated gene editing in cultured cells and in mice.80,81 As more is learned about the MHCK7 promoter, its therapeutic potential will likely be further elucidated.
Initial clinical data from studies of delandistrogene moxeparvovec may also inform subsequent safety protocols. Clinical analyses can help minimize off-target effects derived from transgene expression in nontarget tissues that may have potential safety implications. For instance, in three clinical studies of 84 young patients with DMD treated with delandistrogene moxeparvovec, common safety findings included vomiting and increased levels of transaminases (clinical data cutoff date: April 2022; L.R.-K., unpublished data). Both prophylactic and management protocols from these studies were used to inform subsequent studies of SRP-9003 for patients with LGMD. Furthermore, prophylactic steroid regimens, which were initially implemented to minimize and manage liver toxicity following AAV-mediated gene transfer,36 are now universally implemented.31 In addition, a recent serious AE (SAE) of myositis was reported in a patient treated with delandistrogene moxeparvovec. The incident was specific to the participant’s mutation and was resolved with treatment.45 However, a pattern of similar SAEs has emerged in multiple gene therapy programs for DMD, and all patients with this SAE had deletions in specific regions of the DMD gene.82 As a result, future trials may exclude patients with certain at-risk genotypes. These discoveries have also increased understanding of immune reactions to transgene-expressed proteins, as SAEs in patients with DMD were thought to arise from T cell-mediated reactions to the protein in a cross-reactive immunologic material (CRIM)-negative setting.82 Determining the etiology of specific AEs is currently an area of active research to inform best practices, as evidenced by a recent pooled safety analysis of DMD gene therapies conducted by a number of manufacturers,82 which, in addition to setting a precedent for cross-company collaboration to address important issues of wide concern, may affect gene therapy programs beyond DMD.
Manufacturing
Advances in the large-scale production of delandistrogene moxeparvovec are also likely to result in scientific spillover for subsequent gene therapies. As mentioned, because dosing is based on the patient’s body weight, the quantity of AAV needed per patient is larger for patients with DMD. Specifically, treating one patient with an average weight of 51 kg with delandistrogene moxeparvovec requires 22,610 times the vector needed to treat a patient with voretigene neparvovec-rzyl in both eyes83 and 7 times the vector needed to treat a patient with onasemnogene abeparvovec.84 In addition, not only does each patient with DMD require more AAV, but the size of the patient population is larger than that of the populations dosed with voretigene neparvovec-rzyl or onasemnogene abeparvovec. RPE65-associated inherited retinal dystrophy affects 1,000–2,000 patients in the US,38 and SMA affects 1 in 10,000 live births.85 The manufacturing burden of producing a therapeutic agent for patients with DMD will therefore be substantially greater than for the other diseases. Treating the full prevalent DMD population with delandistrogene moxeparvovec requires about 150,000 times the vector needed to treat the eligible voretigene neparvovec-rzyl population in both eyes and about 100 times the vector needed to treat the prevalent SMA type 1 population with onasemnogene abeparvovec.
To produce enough delandistrogene moxeparvovec to treat the population of patients with DMD, the sponsor company has invested in improving the manufacturing process in collaboration with external partners. Faced with the substantially increased demand compared with currently approved gene therapies, experience gained from the manufacture of delandistrogene moxeparvovec may have spillover benefits by increasing efficiency (i.e., greater yields and lower cycle time). This strategy may potentially decrease the costs of gene therapy manufacturing, which are significantly higher compared with other biologics.86
As the delandistrogene moxeparvovec development program continues to mature, there are likely to be additional aspects of scientific spillover that will benefit other areas of drug development, such as diagnostic testing, regulatory oversight, site readiness, and patient and caregiver education/advocacy. Future research should give thought as to whether and how these aspects could be quantified over time.
Conclusions
Gene therapy is an exciting new area of medical research and drug development that targets the root cause of a disease (i.e., at the genetic level) and holds the promise to meaningfully improve the lives of many patients. Scientific spillover from initial gene therapy development such as that for delandistrogene moxeparvovec can greatly affect each stage of development for subsequent gene therapies, expediting their design and manufacture while decreasing time, waste, and therefore cost. This case study with delandistrogene moxeparvovec suggests that effects may be especially profound for rare diseases such as DMD and other muscular dystrophies. Ultimately, scientific spillover provides value for the development of subsequent treatments, including for those targeted to treat rare diseases with high unmet needs; these potential benefits to society should be considered when evaluating the value of novel therapies.
Acknowledgments
Medical writing and editorial support were provided by Marjet Heitzer, PhD, of 360 Medical Writing and Elizabeth Smith, MS, of Symbiotix, LLC, and funded by Sarepta Therapeutics, Inc.
Author contributions
Conceptualization, D.A., A.C.K., L.E.S., K.L.G., and L.R.-K.; visualization, D.A., D.D., A.C.K., L.E.S., K.L.G., and L.R.-K.; writing – original draft, D.A., A.C.K., L.E.S., and K.L.G.; writing – review & editing, D.A., D.D., A.C.K., L.E.S., K.L.G., and L.R.-K.
Declaration of interests
All authors are employees of Sarepta Therapeutics, Inc., and may hold stock/options in the company. L.R.-K. is the coinventor of the AAVrh74 microdystrophin technology and is eligible to receive financial consideration as a result.
References
- 1.National Institutes of Health What is gene therapy? 2022. https://medlineplus.gov/genetics/understanding/therapy/genetherapy/
- 2.Buchlis G., Podsakoff G.M., Radu A., Hawk S.M., Flake A.W., Mingozzi F., High K.A. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119:3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendell J.R., Al-Zaidy S.A., Lehman K.J., McColly M., Lowes L.P., Alfano L.N., Reash N.F., Iammarino M.A., Church K.R., Kleyn A., et al. Five-year extension results of the phase 1 START trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78:834–841. doi: 10.1001/jamaneurol.2021.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ruiten H.J.A., Straub V., Bushby K., Guglieri M. Improving recognition of Duchenne muscular dystrophy: a retrospective case note review. Arch. Dis. Child. 2014;99:1074–1077. doi: 10.1136/archdischild-2014-306366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir K.J., Keim-Malpass J. Analyzing the concept of spillover effects for expanded inclusion in health economics research. Methodology. 2020;9:755–766. doi: 10.2217/cer-2020-0051. [DOI] [PubMed] [Google Scholar]
- 7.Garrison L.P., Jr., Kamal-Bahl S., Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20:213–216. doi: 10.1016/j.jval.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Lakdawalla D.N., Doshi J.A., Garrison L.P., Jr., Phelps C.E., Basu A., Danzon P.M. Defining elements of value in health care-a health economics approach: an ISPOR Special Task Force report [3] Value Health. 2018;21:131–139. doi: 10.1016/j.jval.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen J., Kefalas P. Reimbursement of licensed cell and gene therapies across the major European healthcare markets. J. Mark. Access Health Policy. 2015;3 doi: 10.3402/jmahp.v3.29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horgan D., de Braud F., Jonsson B., Vallone S., Jagielska B., Koeva J., Geanta M. The three-way pendulum of healthcare innovation. Biomed. Hub. 2017;2:22–25. doi: 10.1159/000479489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elevidys (delandistrogene moxeparvovec) prescribing information. 2023. https://www.elevidys.com/PI
- 12.Lu R.M., Hwang Y.C., Liu I.J., Lee C.C., Tsai H.Z., Li H.J., Wu H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham B.S., Ambrosino D.M. History of passive antibody administration for prevention and treatment of infectious diseases. Curr. Opin. HIV AIDS. 2015;10:129–134. doi: 10.1097/COH.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenzel A., Schirrmann T., Hust M. Phage display-derived human antibodies in clinical development and therapy. mAbs. 2016;8:1177–1194. doi: 10.1080/19420862.2016.1212149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronemeyer P., Ditz R., Strube J. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering. 2014;1:188–212. doi: 10.3390/bioengineering1040188. [DOI] [PubMed] [Google Scholar]
- 17.Mullard A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021;20:491–495. doi: 10.1038/d41573-021-00079-7. [DOI] [PubMed] [Google Scholar]
- 18.Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–324. doi: 10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F., Krishnan S., Lenzen G., Magné R., Gomard E., Guillet J.G., Lévy J.P., Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993;23:1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 20.FDA approves first COVID-19 vaccine. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
- 21.FDA Coronavirus (COVID-19) update: FDA takes key action by approving second COVID-19 vaccine. 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine
- 22.Moderna Moderna announces first participant dosed in phase 2 study of Omicron-specific booster candidate and publication of data on booster durability against Omicron variant. 2022. https://www.accesswire.com/685660/Moderna-Announces-First-Participant-Dosed-in-Phase-2-Study-of-Omicron-Specific-Booster-Candidate-and-Publication-of-Data-on-Booster-Durability-Against-Omicron-Variant
- 23.Pfizer Pfizer and BioNTech initiate study to evaluate Omicron-based COVID-19 vaccine in adults 18 to 55 years of age. 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based
- 24.Possible side effects after getting a COVID-19 vaccine. 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html
- 25.Emery A.E. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul. Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 26.Crisafulli S., Sultana J., Fontana A., Salvo F., Messina S., Trifirò G. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020;15:141. doi: 10.1186/s13023-020-01430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MIT Research Brief Pipeline Analysis. 2020. https://newdigs.tuftsmedicalcenter.org/updated-projection-of-us-durable-cell-and-gene-therapies-product-indication-approvals-based-on-december-2019-development-pipeline/
- 28.Gray S.J., Nagabhushan Kalburgi S., McCown T.J., Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liguore W.A., Domire J.S., Button D., Wang Y., Dufour B.D., Srinivasan S., McBride J.L. AAV-PHP.B administration results in a differential pattern of CNS biodistribution in non-human primates compared with mice. Mol. Ther. 2019;27:2018–2037. doi: 10.1016/j.ymthe.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neurogene Neurogene’s AAV biodistribution study shows route of administration essential component in optimizing gene therapy treatment for neurological disease. 2021. https://www.neurogene.com/press-releases/neurogenes-aav-biodistribution-study-shows-route-of-administration-essential-component-in-optimizing-gene-therapy-treatment-for-neurological-disease/
- 31.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cehajic-Kapetanovic J., Xue K., Martinez-Fernandez de la Camara C., Nanda A., Davies A., Wood L.J., Salvetti A.P., Fischer M.D., Aylward J.W., Barnard A.R., et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuer W.J., Schiffman J.C., Davis J.L., Porciatti V., Gonzalez P., Koilkonda R.D., Yuan H., Lalwani A., Lam B.L., Guy J. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire A.M., High K.A., Auricchio A., Wright J.F., Pierce E.A., Testa F., Mingozzi F., Bennicelli J.L., Ying G.S., Rossi S., et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 36.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 37.Asher D.R., Thapa K., Dharia S.D., Khan N., Potter R.A., Rodino-Klapac L.R., Mendell J.R. Clinical development on the frontier: gene therapy for duchenne muscular dystrophy. Expet Opin. Biol. Ther. 2020;20:263–274. doi: 10.1080/14712598.2020.1725469. [DOI] [PubMed] [Google Scholar]
- 38.FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss
- 39.FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy, a rare disease and leading genetic cause of infant mortality. 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease
- 40.Day J.W., Finkel R.S., Mercuri E., Swoboda K.J., Menier M., van Olden R., Tauscher-Wisniewski S., Mendell J.R. Adeno-associated virus serotype 9 antibodies in patients screened for treatment with onasemnogene abeparvovec. Mol. Ther. Methods Clin. Dev. 2021;21:76–82. doi: 10.1016/j.omtm.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue L., Clements-Egan A., Amaravadi L., Birchler M., Gorovits B., Liang M., Myler H., Purushothama S., Manning M.S., Sung C. Recommendations for the assessment and management of pre-existing drug-reactive antibodies during biotherapeutic development. AAPS J. 2017;19:1576–1586. doi: 10.1208/s12248-017-0153-x. [DOI] [PubMed] [Google Scholar]
- 42.Chicoine L.G., Montgomery C.L., Bremer W.G., Shontz K.M., Griffin D.A., Heller K.N., Lewis S., Malik V., Grose W.E., Shilling C.J., et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 2014;22:338–347. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendell J.R., Sahenk Z., Lehman K., Nease C., Lowes L.P., Miller N.F., Iammarino M.A., Alfano L.N., Nicholl A., Al-Zaidy S., et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy; a nonrandomized controlled trial. JAMA Neurol. 2020;77:1122–1131. doi: 10.1001/jamaneurol.2020.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghasemi M., Emerson C.P., Jr., Hayward L.J. Outcome measures in facioscapulohumeral muscular dystrophy clinical trials. Cells. 2022;11:687. doi: 10.3390/cells11040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaidman C.M., Proud C.M., McDonald C.M., Lehman K.J., Goedeker N.L., Mason S., Murphy A.P., Guridi M., Wang S., Reid C., et al. Delandistrogene moxeparvovec gene therapy in ambulatory patients (aged ≥4 to <8 years) with Duchenne muscular dystrophy: 1-year interim results from Study SRP-9001-103 (ENDEAVOR) Ann. Neuro. 2021 doi: 10.1002/ana.26755. [DOI] [PubMed] [Google Scholar]
- 46.Day J.W., Mendell J.R., Mercuri E., Finkel R.S., Strauss K.A., Kleyn A., Tauscher-Wisniewski S., Tukov F.F., Reyna S.P., Chand D.H. Clinical trial and postmarketing safety of onasemnogene abeparvovec therapy. Drug Saf. 2021;44:1109–1119. doi: 10.1007/s40264-021-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chand D., Mohr F., McMillan H., Tukov F.F., Montgomery K., Kleyn A., Sun R., Tauscher-Wisniewski S., Kaufmann P., Kullak-Ublick G. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 2021;74:560–566. doi: 10.1016/j.jhep.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Chand D.H., Zaidman C., Arya K., Millner R., Farrar M.A., Mackie F.E., Goedeker N.L., Dharnidharka V.R., Dandamudi R., Reyna S.P. Thrombotic microangiopathy following onasemnogene abeparvovec for spinal muscular atrophy: a case series. J. Pediatr. 2021;231:265–268. doi: 10.1016/j.jpeds.2020.11.054. [DOI] [PubMed] [Google Scholar]
- 49.Zolgensma . Novartis Gene Therapies, Inc.; 2021. Prescribing Information.https://www.fda.gov/media/126109/download [Google Scholar]
- 50.Srivastava A., Mallela K.M.G., Deorkar N., Brophy G. Manufacturing challenges and rational formulation development for AAV viral vectors. J. Pharmaceut. Sci. 2021;110:2609–2624. doi: 10.1016/j.xphs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 51.Carbonell B.R., Mukherjee A., Dordick J., Roberts C.J. A technology roadmap for today’s gene therapy manufacturing challenges. Cell Gene. 2019:1–7. [Google Scholar]
- 52.Gao G.P., Qu G., Faust L.Z., Engdahl R.K., Xiao W., Hughes J.V., Zoltick P.W., Wilson J.M. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum. Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 53.Liu X.L., Clark K.R., Johnson P.R. Production of recombinant adeno-associated virus vectors using a packaging cell line and a hybrid recombinant adenovirus. Gene Ther. 1999;6:293–299. doi: 10.1038/sj.gt.3300807. [DOI] [PubMed] [Google Scholar]
- 54.Felberbaum R.S. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015;10:702–714. doi: 10.1002/biot.201400438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blake D.J., Weir A., Newey S.E., Davies K.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 56.Darras B.T., Urion D.K., Ghosh P.S. In: GeneReviews®. Adam M.P., Everman D.B., Mirzaa G.M., Pagon R.A., Wallace S.E., editors. University of Washington, Seattle; 2000. Dystrophinopathies. [Google Scholar]
- 57.Harper S.Q., Crawford R.W., DelloRusso C., Chamberlain J.S. Spectrin-like repeats from dystrophin and alpha-actinin-2 are not functionally interchangeable. Hum. Mol. Genet. 2002;11:1807–1815. doi: 10.1093/hmg/11.16.1807. [DOI] [PubMed] [Google Scholar]
- 58.Jung D., Yang B., Meyer J., Chamberlain J.S., Campbell K.P. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J. Biol. Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- 59.Koenig M., Kunkel L.M. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J. Biol. Chem. 1990;265:4560–4566. [PubMed] [Google Scholar]
- 60.Legardinier S., Hubert J.F., Le Bihan O., Tascon C., Rocher C., Raguénès-Nicol C., Bondon A., Hardy S., Le Rumeur E. Sub-domains of the dystrophin rod domain display contrasting lipid-binding and stability properties. Biochim. Biophys. Acta. 2008;1784:672–682. doi: 10.1016/j.bbapap.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Nelson D.M., Lindsay A., Judge L.M., Duan D., Chamberlain J.S., Lowe D.A., Ervasti J.M. Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins. Hum. Mol. Genet. 2018;27:2090–2100. doi: 10.1093/hmg/ddy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Norwood F.L., Sutherland-Smith A.J., Keep N.H., Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure. 2000;8:481–491. doi: 10.1016/s0969-2126(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki A., Yoshida M., Yamamoto H., Ozawa E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 1992;308:154–160. doi: 10.1016/0014-5793(92)81265-n. [DOI] [PubMed] [Google Scholar]
- 64.Zhao J., Kodippili K., Yue Y., Hakim C.H., Wasala L., Pan X., Zhang K., Yang N.N., Duan D., Lai Y. Dystrophin contains multiple independent membrane-binding domains. Hum. Mol. Genet. 2016;25:3647–3653. doi: 10.1093/hmg/ddw210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.England S.B., Nicholson L.V., Johnson M.A., Forrest S.M., Love D.R., Zubrzycka-Gaarn E.E., Bulman D.E., Harris J.B., Davies K.E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 66.Wells D.J., Wells K.E., Asante E.A., Turner G., Sunada Y., Campbell K.P., Walsh F.S., Dickson G. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum. Mol. Genet. 1995;4:1245–1250. doi: 10.1093/hmg/4.8.1245. [DOI] [PubMed] [Google Scholar]
- 67.Prakash V., Moore M., Yáñez-Muñoz R.J. Current progress in therapeutic gene editing for monogenic diseases. Mol. Ther. 2016;24:465–474. doi: 10.1038/mt.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chamberlain K., Riyad J.M., Weber T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum. Gene Ther. Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murillo O., Moreno D., Gazquez C., Barberia M., Cenzano I., Navarro I., Uriarte I., Sebastian V., Arruebo M., Ferrer V., et al. Liver expression of a miniATP7B gene results in long-term restoration of copper homeostasis in a Wilson disease model in mice. Hepatology. 2019;70:108–126. doi: 10.1002/hep.30535. [DOI] [PubMed] [Google Scholar]
- 70.Vidal P., Pagliarani S., Colella P., Costa Verdera H., Jauze L., Gjorgjieva M., Puzzo F., Marmier S., Collaud F., Simon Sola M., et al. Rescue of GSDiii phenotype with gene transfer requires liver- and muscle-targeted GDE expression. Mol. Ther. 2018;26:890–901. doi: 10.1016/j.ymthe.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W., Li L., Su Q., Gao G., Khanna H. Gene therapy using a minicep290 fragment delays photoreceptor degeneration in a mouse model of Leber congenital amaurosis. Hum. Gene Ther. 2018;29:42–50. doi: 10.1089/hum.2017.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waldrop M.A., Karingada C., Storey M.A., Powers B., Iammarino M.A., Miller N.F., Alfano L.N., Noritz G., Rossman I., Ginsberg M., et al. Gene therapy for spinal muscular atrophy: safety and early outcomes. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0729. [DOI] [PubMed] [Google Scholar]
- 73.Soim A., Wallace B., Whitehead N., Smith M.G., Mann J.R., Thomas S., Ciafaloni E., Muscular Dystrophy Surveillance, Tracking, and Research Network MD STARnet Health profile of preterm males with Duchenne muscular dystrophy. J. Child Neurol. 2021;36:1095–1102. doi: 10.1177/08830738211047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spark Therapeutics, Inc Luxturna. Prescribing information. 2017. https://www.fda.gov/media/109906/download
- 75.Salva M.Z., Himeda C.L., Tai P.W., Nishiuchi E., Gregorevic P., Allen J.M., Finn E.E., Nguyen Q.G., Blankinship M.J., Meuse L., et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 76.rAAVrh74.MHCK7.DYSF.DV for treatment of dysferlinopathies. ClinicalTrials.gov identifier: NCT02710500. 2021. https://clinicaltrials.gov/ct2/show/NCT02710500?term=02710500&draw=2&rank=1
- 77.Griffin D.A., Pozsgai E.R., Heller K.N., Potter R.A., Peterson E.L., Rodino-Klapac L.R. Preclinical systemic delivery of adeno-associated α-sarcoglycan gene transfer for limb-girdle muscular dystrophy. Hum. Gene Ther. 2021;32:390–404. doi: 10.1089/hum.2019.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pozsgai E.R., Griffin D.A., Heller K.N., Mendell J.R., Rodino-Klapac L.R. Systemic AAV-mediated beta-sarcoglycan delivery targeting cardiac and skeletal muscle ameliorates histological and functional deficits in LGMD2E mice. Mol. Ther. 2017;25:855–869. doi: 10.1016/j.ymthe.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H., Wang P., Hsu E., Pinckard K.M., Stanford K.I., Han R. Systemic AAV9.BVES delivery ameliorates muscular dystrophy in a mouse model of LGMDR25. Mol. Ther. 2022 doi: 10.1016/j.ymthe.2022.1011.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu L., Zhao L., Gao Y., Xu J., Han R. Empower multiplex cell and tissue-specific CRISPR-mediated gene manipulation with self-cleaving ribozymes and tRNA. Nucleic Acids Res. 2017;45:e28. doi: 10.1093/nar/gkw1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu L., Park K.H., Zhao L., Xu J., El Refaey M., Gao Y., Zhu H., Ma J., Han R. CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol. Ther. 2016;24:564–569. doi: 10.1038/mt.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonnemann C.G., Belluscio B.A., Braun S., Morris C., Singh T., Muntoni F. A collaborative analysis by clinical trial sponsors and academic experts of anti-transgene SAEs in studies of gene therapy for DMD. Mol. Ther. 2022;30:4. [Google Scholar]
- 83.Spark Therapeutics, Inc. FDA approves Spark Therapeutics’ LUXTURNA™ (voretigene neparvovec-rzyl), a one-time gene therapy for patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy. 2017. https://sparktx.com/press_releases/fda-approves-spark-therapeutics-luxturna-voretigene-neparvovec-rzyl-a-one-time-gene-therapy-for-patients-with-confirmed-biallelic-rpe65-mutation-associated-retinal-dystrophy/
- 84.O'Shea T. Clinical Insights: Zolgensma for Spinal Muscular Atrophy. 2019. https://www.pharmacytimes.com/view/clinical-insights-zolgensma-for-spinal-muscular-atrophy
- 85.Verhaart I.E.C., Robertson A., Wilson I.J., Aartsma-Rus A., Cameron S., Jones C.C., Cook S.F., Lochmüller H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J. Rare Dis. 2017;12:124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris E. Breaking down pricing of cell & gene therapies. 2019. https://www.cellandgene.com/doc/breaking-down-pricing-of-cell-gene-therapies-0001#:∼:text=Cost%20of%20goods%2Fmanufacturing%20alone,to%20provide%20access%20to%20patients
- 87.Atchison R.W., Casto B.C., Hammon W.M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 88.Friedmann T., Roblin R. Gene therapy for human genetic disease? Science. 1972;175:949–955. doi: 10.1126/science.175.4025.949. [DOI] [PubMed] [Google Scholar]
- 89.Tratschin J.D., West M.H., Sandbank T., Carter B.J. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol. Cell Biol. 1984;4:2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermonat P.L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Food and Drug Administration Draft of FDA's Points to Consider in human somatic cell therapy and gene therapy. Hum. Gene Ther. 1991;2:251–256. doi: 10.1089/hum.1991.2.3-251. [DOI] [PubMed] [Google Scholar]
- 92.Fisher K.J., Jooss K., Alston J., Yang Y., Haecker S.E., High K., Pathak R., Raper S.E., Wilson J.M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 93.Flotte T., Carter B., Conrad C., Guggino W., Reynolds T., Rosenstein B., Taylor G., Walden S., Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 94.Duchenne, G.B.A. (1861). 2 Edition. In: De l'electrisation localisee et de son application a la pathologie et a la therapeutique, 2, (Paris), pp. 353-356.
- 95.Bulfield G., Siller W.G., Wight P.A., Moore K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monaco A.P., Neve R.L., Colletti-Feener C., Bertelson C.J., Kurnit D.M., Kunkel L.M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 97.Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 99.Yuasa K., Ishii A., Miyagoe Y., Takeda S. [Introduction of rod-deleted dystrophin cDNA, delta DysM3, into mdx skeletal muscle using adenovirus vector] Nihon Rinsho. 1997;55:3148–3153. [PubMed] [Google Scholar]
- 100.Rodino-Klapac L.R., Janssen P.M.L., Montgomery C.L., Coley B.D., Chicoine L.G., Clark K.R., Mendell J.R. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J. Transl. Med. 2007;5:45–55. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duncan F.J., Naughton B.J., Zaraspe K., Murrey D.A., Meadows A.S., Clark K.R., Newsom D.E., White P., Fu H., McCarty D.M. Broad functional correction of molecular impairments by systemic delivery of scAAVrh74-hSGSH gene delivery in MPS IIIA Mice. Mol. Ther. 2015;23:638–647. doi: 10.1038/mt.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Potter R.A., Griffin D.A., Heller K.N., Peterson E.L., Clark E.K., Mendell J.R., Rodino-Klapac L.R. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum. Gene Ther. 2021;32:375–389. doi: 10.1089/hum.2019.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendell J.R., Shieh P.B., McDonald C.M., Sahenk Z., Lehman K.J., Lowes L.P., Reash N.F., Iammarino M.A., Alfano L.N., Sabo B., et al. Expression of SRP-9001 dystrophin and stabilization of motor function up to 2 years post-treatment with delandistrogene moxeparvovec gene therapy in individuals with Duchenne muscular dystrophy. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1167762. [DOI] [PMC free article] [PubMed] [Google Scholar]