Abstract
Starch is a vital component of wheat grain and flour, characterized by two distinct granule types: A-type starch (AS) with granules larger than 10 µm in diameter, and B-type starch (BS) with granules measuring no more than 10 µm in diameter. This review comprehensively evaluates the isolation, purification, and biosynthesis processes of these types of granules. In addition, a comparative analysis of the structure and properties of AS and BS is presented, encompassing chemical composition, molecular, crystalline and morphological structures, gelatinization, pasting and digestive properties. The variation in size distribution of granules leads to differences in physicochemical properties of starch, influencing the formation of polymeric proteins, secondary and micro-structures of gluten, chemical and physical interactions between gluten and starch, and water absorption and water status in dough system. Thus, starch size distribution affects the quality of dough and final products. In this review, we summarize the up-to-date knowledge of AS and BS, and propose the possible strategies to enhance wheat yield and quality through coordinated breeding efforts. This review serves as a valuable reference for future advancements in wheat breeding.
Keywords: Wheat quality, A- and B-type starch granules, Dough rheological properties, Flour-based food quality
1. Introduction
Common wheat (Triticum aestivum L.) is a major source of energy and proteins in the human diet and ranks among the most important cereal crops [1]. The area for wheat cultivation has exceeded 200 million hectares and its annual production has reached 700 million tons [2]. Compared to other crops (rice, maize, etc.), wheat is versatile: its unique and complex gluten properties allow for the production of a diverse range of products, including white bread, noodles, Chinese steamed bread, and chapatti [3], [4]. Gluten is a major determinant for end-use properties of wheat [5]. Notably, the high-molecular-weight glutenin subunits (HMW-GSs) take up only 12% of the total seed storage protein, but explain 45–70% of the variations in gluten, dough and end-use properties [6]. Previous reviews on composition and structure of gluten and its contribution to the quality of dough and final products have been widely published [7], [8], [9], [10]. In addition, gluten is the most common cause of food-related allergies and intolerances, which has been summarized in the previous reviews [11], [12].
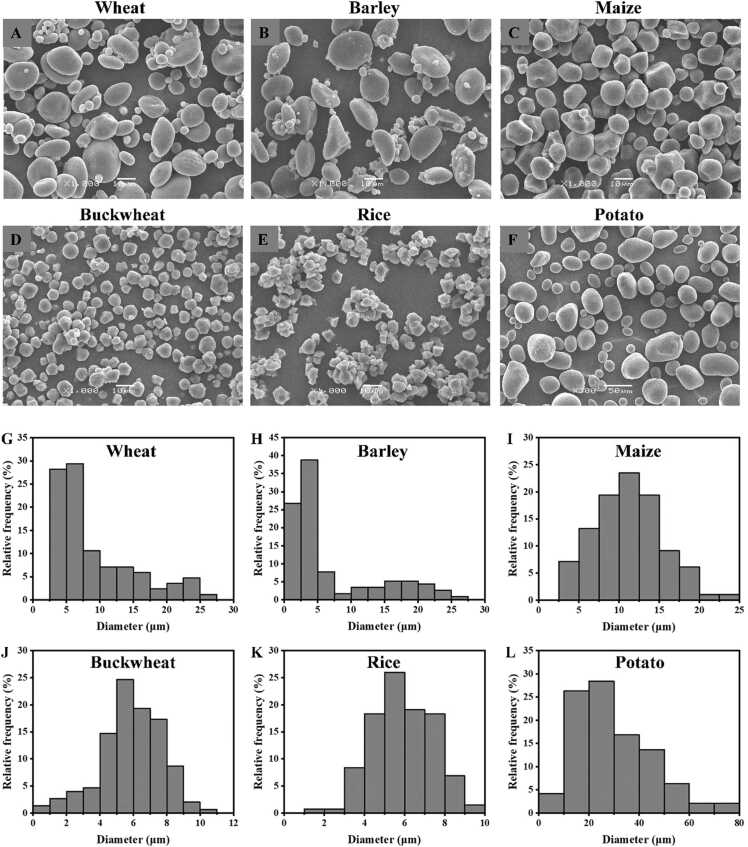
Starch is the primary component in wheat grain and flour, and it has a crucial role in determining the quality of flour-based food products, which has become the focus of research interest [13], [14]. Starch is synthesized as discrete granules with varying size ranges in specialized plant organelles called amyloplasts, and different crops produce starch granules that vary significantly in shape and size (Fig. 1). For instance, wheat and barley have disc-shaped, spherical or irregular starch granules (Fig. 1A and B), while maize and buckwheat have polygonal or spherical starch granules (Fig. 1C and D). Rice and potato starch granules exhibit irregular and lenticular shapes, respectively (Fig. 1E and F). Notably, potato starch granules are the largest, ranging from 2.7 to 70.7 µm, while rice starch granules are the smallest, measuring less than 10 µm. An interesting observation is that cereals in the tribe Triticeae, such as wheat and barley, demonstrate a bimodal size distribution (Fig. 1G and H), consisting of A-type starch granules (AS) and B-type starch granules (BS) [15], [16], [17]. In contrast, other crops such as maize, buckwheat, rice, and potato also have starch granules of various sizes, but they lack such specialized multiple size distributions (Fig. 1I, J, K, and L) [17]. Starch granules with different sizes show varied chemical compositions and molecular structures, leading to different physicochemical properties and thus various roles in food processing. Fully understanding the composition, structure, and function of different-sized wheat starch holds immense importance for wheat breeders and food producers.
Fig. 1.
The scanning electron microscopy images (A, B, C, D, E and F) of starch from six different botanical sources and their size distribution (G, H, I, J, K and L) analyzed by ImageJ software. A and G, wheat; B and H, barley; C and I, maize; D and J, buckwheat; E and K, rice; F and L, potato.
Over the past few decades, an extensive body of research on starch granules has been accumulated, continuously expanding over time. However, systematic summaries of data referring to the composition, structure and function of AS and BS are scarce. In this review, we compared the isolation techniques and biosynthesis of different-sized wheat starch granules, highlighted the differences in physicochemical properties between AS and BS, and explored their impacts on dough rheological properties and quality of the final products. Finally, we proposed potential strategies for coordinating improvements in the yield and quality of wheat.
2. Isolation and purification of wheat starch samples and their granule size distribution
In the laboratory, starch from many crops, including barley and highland barley, can be isolated using wet milling method which involves steeping, blending, screening, deproteinization and centrifugation [18]. Steeping with lye degrades or loosens the protein surrounding the starch granules, facilitating the separation of starch and protein [19]. In addition, the use of enzymes such as protease, cellulose, xylanase, lichenase, and glucanase can further improve the purity of starch [18]. In contrast, wheat flour behaves differently from barley and highland barley; when water is added in wheat flour and the mixtures are mixed mechanically, the glutenin and gliadin of wheat begin to interact with water molecules to form a specific three-dimensional network. Thus, the wheat starch samples are typically isolated from dough using physical methods, such as hand washing or stirring [20], [21]. Briefly, the wheat flour is moderately hydrated and mixed for 2 min to form the dough, which is then rested for 10 min at room temperature. The starch is then washed out of the dough using running water until no starch left in the gluten matrix [20]. Alternatively, the starch samples can be obtained by stirring the dough in water [21]. Thereafter, starch slurry is passed through a 120-mesh sieve or eight layers of gauze to remove gluten and other solid impurities. The starch is pelleted by centrifugation at 3000–4000g for 10–15 min, and the yellow-brown sludge fraction at the top layer is scraped off, while the remaining white starch in the bottom is washed with ethanol and dried in a freeze dryer or oven. Unlike starch separation from barley and highland barley flours, most of the impurities (gluten) in wheat flour are removed by filtration, resulting in reduced brown layer formation, and lower BS losses [22].
The size distribution of starch can be assessed using microscopic techniques (i.e. light microscopy and scanning electron microscopy (SEM)), sieving, electrical resistance method (Coulter counter), laser light scattering, and field flow fractionation, which have been previously reviewed [22]. Laser particle size analysis of wheat starch has shown a bimodal distribution consisting of AS and BS [23]. AS accounts for less than 10% of granules in number, while BS constitutes more than 90% [22]. However, the number distribution of AS and BS ranged from 43.96% to 64.56% and from 35.45% to 56.04%, respectively in a previous study [24], which may be attributed to variations in wheat varieties and methods of determination. Furthermore, small-sized BS granules aggregate more easily during drying, usually leading to an underestimation of BS number. To prevent clumping, it becomes necessary to periodically mash the starch during the drying process. By assuming that the starch granules are homogeneous with the same density, the weight distribution of wheat starch can be calculated. The results showed that AS accounts for 70–80% and BS less than 30% in weight [25], [26].
Sieving and sedimentation (gravity or centrifugation) are two commonly used techniques for granule size separation, as previously reviewed [17]. To separate potato starch into four fractions, three test sieves (270-mesh, 400-mesh, and 500-mesh) are employed, resulting in surface area-weighted average diameters (D [2], [3]) of 81.16 µm, 61.11 µm, 40.07 µm, and 35.23 µm, respectively [27]. However, complete separation of AS and BS from wheat flour cannot be achieved through a 10 µm sieve, because many small BS granules are adhered to the surface of large AS granules that they remained in the AS population [28]. Additionally, some AS granules pass through the nylon screen and remain in the filtrate along with BS [28]. According to Stokes’ Law, the large starch granules precipitate faster than the small ones in distilled water [29], and thus AS and BS can be individually fractionated from total starch by repeated suspension and sedimentation in distilled water [30], [31]. Briefly, wheat starch (50 g) is mixed with distilled water (500 g) and rested for 1 h. The upper 100 mL suspension is collected as the BS fraction. Afterwards, 100 mL distilled water is added and well mixed. This process is repeated about 10 times until the upper suspension is clear, and the final precipitate is collected as the AS fraction [30]. SEM observations confirm that AS and BS fractioned by this method are minimally contaminated, and the particle size analyses show that the purity of AS and BS fractions reaches up to 93.63% and 99.56%, respectively [30]. Centrifugation can improve the efficiency of sedimentation and shorten the time required. The purity of BS fraction separated by centrifugation in aqueous solutions of sucrose, maltose, and Percoll is 100%, while the purity of AS fraction is 91.98%, 89.56%, and 100% [28]. The slightly lower purity of AS fraction may be due to the adsorption of some BS granules onto the surface of AS granules in the process of precipitation or centrifugation, while Percoll can destroy this adsorption force, and thus higher purity of AS granules can be obtained. However, Percoll is not a cost-saving method, not allowing the wide application in food industry. Consequently, efficiently and rapidly separating AS and BS quickly and efficiently remains a challenge.
3. Biosynthesis of different-sized starch granules
The AS and BS granules are individually produced in two granule-initiation events that occur independently in time. The AS initiation takes place at 3–5 days after anthesis (DAA), and the final number is achieved about 4 days later, with each amyloplast containing one starch granule at this stage [15], [16]. Their volume increases over time with the development of grains, with a final diameter ranging from 10 to 45 µm, depending on the cultivar and environment [32]. BS granules, on the other hand, are initiated in amyloplast that contain only one AS granule at 11 DAA [33], 15 DAA [16], or 12–16 DAA [34]. The volume of BS granules increases gradually as the endosperm develops, but the diameter usually does not exceed 10 µm in mature grains. Usually, each endosperm plastid in mature wheat grain contains one AS and several BS granules.
The biosynthesis of starch requires the coordinated activities of several enzymes, including ADP-glucose pyrophosphorylase, granule bound starch synthase, soluble starch synthase, starch branching enzyme (SBE), and starch debranching enzyme. Previous reviews have extensively covered the biosynthesis of amylose (AM) and amylopectin (AP), as well as the functions of individual enzyme [13], [35], [36]. Two starch granule-bound proteins (SGP), SGP-140 and SGP-145, are variants of SBEIc, and their abundance in AS are much higher than in BS, indicating that the two polypeptides preferentially associated with the development of AS [37]. In addition, SGP140 or SGP145 was found in all crops containing AS and BS, including wheat, triticale, barley, and rye, yet they are not present in canary seed, rice, maize, or potato tubers. Hence, these two proteins are considered crucial factors in the formation of AS and BS [37]. Although most of the Triticeae tribe, including common wheat, barley, rye, wild wheat, and ‘goat grass’ species, have endosperm starch with a bimodal granule size distribution, a few wild wheat species (Aegilops) lack BS, such as Ae. peregrina, Ae. kotschyi, Ae. crassa, and Ae. Juvenalis [38]. In order to study the genetic basis of BS formation, a population lacking BS was generated by crossing the Ae. peregrina with a synthetic tetraploid (KU37, containing both AS and BS), and a major quantitative trait locus has been identified on the short arm of chromosome 4S, accounting for 44.4% of the phenotypic variation within the population [39]. Further studies have revealed that BGC1, an orthologue of the FLO6 in rice and barley, and PTST2 in Arabidopsis encoding PROTEIN TARGETING TO STARCH proteins, is involved in the initiation of BS, and plays a complex biological role in starch granule initiation [40], [41]. Specifically, BGC1 represses the initiation of AS at the early stage, but promotes the initiation of BS at middle stage of endosperm development. The influence of BGC1 on starch synthesis is dose dependent; the double-deletion mutant line (A- and D-genome deletion mutant) does not have BS, while the triple-deletion mutant line shows abnormal starch granules [40], [41]. Consistent with the studies in Arabidopsis that the STARCH SYNTHASE 4 (SS4) plays a central role in starch granule initiation and morphogenesis [42], [43], TaSS4 has been reported to associate with starch granule development in tetraploid wheat [44]. Morphologically, the wild-type and single mutants exhibit typical flatten AS granules and round BS granules, while the double-deletion mutant has irregular, polyhedral morphology, and few normal BS granules [44]. To summarize, in despite of the identification of essential genes like TaSS4 and TaBGC1 for starch granule initiation in wheat endosperm, our understanding of the complete pathway of different-sized starch granule biosynthesis and regulation is still limited.
4. Physicochemical properties of different-sized starch granules
Starch primarily comprises carbohydrates as its major components, along with minor components like proteins and lipids. Starch granules possess a complex structure, mainly consisting of six levels: individual chains formed by glucose monomers with α-(1→4) glycosidic linkages, with these linear chains being linked together by α-(1→6) glycosidic linkages as branches; fully branched individual starch molecules, including AM and AP; the semi-crystalline structure (7–11 nm) formed by crystalline lamellae (5–8 nm) and amorphous lamellae (2–3 nm); the semi-crystalline and amorphous growth rings; the starch granules; and the arrangement of starch granules in the cell and cell locations within the entire endosperm/tuber. These varying structures of starch result in its different gelatinization, pasting and digestive properties. In this section, a comparison is made between the structure and properties of wheat starch granules with different sizes to gain a deeper understanding of the relationship between starch structure and its property.
4.1. Chemical composition
AS and BS granules exhibit significantly different chemical compositions (Table 1). As mentioned earlier, obtaining pure starch from wheat flour is difficult due to the presence of other constituents such as proteins and lipids. The ‘purified’ AS fraction contains higher proportion of starch (95.99–99.43%) compared to the BS fraction (89.02–98.89%) [30], [31], [45], [46]. Additionally, the AS fraction has a higher AM content ranging from 22.88% to 37.08% while the BS fraction contains 19.06–32.15% [31], [45], [46], [47]. In general, AP branches build up the crystalline lamellae, while AM is present in amorphous lamellae or intersperses in AP crystallites [48]. Therefore, the AM-to-AP ratio is a key factor affecting the crystalline structure of starch. Moreover, starch granules tend to be broken into smaller particles by mechanical forces (such as shear, impingement, collision, and friction) during milling [49]. The damaged starch content has been reported as 1.28% and 16.2 UCD for AS, and 4.35% and 23.2 UCD for BS, which may be attributed to some fragmented damaged starch with smaller size mixed with the BS sample during precipitation or centrifugation [30], [46]. Another possible explanation could be that small starch granules tend to bind more closely to other components (such as proteins) in wheat grain, leading to higher levels of damaged starch during milling [49]. The higher content of damaged starch decreases the relative crystallinity, viscosity, and average molecular weight of starch, but increases the solubility, thereby affecting the dough rheological and end-use properties [49].
Table 1.
Chemical composition of A-type (AS) and B-type (BS) starch granule samples.
| Sample | Starch (%) | Amylose (%) | Damaged starch | Protein (%) | Lipid (%) | Phosphorus (ppm) | Ash (%) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Six soft wheat varieties | AS | 98.27–99.29 (+) | 22.88–25.49 (+) | nd | 0.21–0.25 (-) | 0.28–0.30 (-) | nd | 0.23–0.29 (-) | [31] |
| BS | 94.99–98.89 (+) | 19.06–21.53 (+) | nd | 0.28–0.36 (-) | 0.29–0.32 (-) | nd | 0.26–0.34 (-) | ||
| Six hard wheat varieties | AS | 95.99–99.27 (+) | 25.11–28.15 (+) | nd | 0.22–0.24 (-) | 0.28–0.31 (-) | nd | 0.25–0.33 (-) | [31] |
| BS | 95.89–97.01 (+) | 21.56–24.36 (+) | nd | 0.42–0.47 (-) | 0.30–0.32 (-) | nd | 0.40–0.47 (-) | ||
| Commercial wheat starch | AS | 98.07 (+) | nd | 1.28% (-) | 0.68 (-) | nd | nd | nd | [30] |
| BS | 97.17 (+) | nd | 4.35% (-) | 0.81 (-) | nd | nd | nd | ||
| Xinong 9718 (hard red winter wheat) | AS | 99.43 (+) | 25.26 (+) | nd | 0.16 (-) | nd | nd | nd | [45] |
| BS | 97.85 (+) | 19.19 (+) | nd | 0.23 (-) | nd | nd | nd | ||
| Shannong 138 (soft red winter wheat) | AS | 98.12 (+) | 25.30 (+) | nd | 0.18 (-) | nd | nd | nd | [45] |
| BS | 97.29 (+) | 21.58 (+) | nd | 0.28 (-) | nd | nd | nd | ||
| AK58 | AS | 96.02 (+) | 37.08 (+) | 16.2 UCD (-) | 1.02 (-) | nd | nd | nd | [46] |
| BS | 89.02 (+) | 32.15 (+) | 23.2 UCD (-) | 3.02 (-) | nd | nd | nd | ||
| Commercial wheat starch | AS | nd | 28.9 (+) | nd | 0.54 (-) | 0.25 (-) | nd | nd | [47] |
| BS | nd | 25.0 (+) | nd | 0.86 (-) | 0.45 (-) | nd | nd | ||
| IDO630 (Waxy) | AS | nd | nd | nd | nd | nd | 90.5 (-) | nd | [50] |
| BS | nd | nd | nd | nd | nd | 240.0 (-) | nd | ||
| Jubilee (Commercial wheat starch) | AS | nd | nd | nd | nd | nd | 470.0 (-) | nd | [50] |
| BS | nd | nd | nd | nd | nd | 585.0 (-) | nd | ||
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS; “nd” represents no data.
The content ranges of protein, lipid, phosphorus, and ash in AS samples (i.e. 0.16–1.02%, 0.25–0.31%, 90.5–470.0 ppm, and 0.23–0.33%) are lower than those in BS samples (i.e. 0.23–3.02%, 0.29–0.45%, 240.0–585.0 ppm, and 0.26–0.47%) [30], [31], [45], [46], [47], [50]. The protein in starch sample comprises starch granule-associated proteins, mainly enzymes involved in starch biosynthesis, and storage proteins adsorbed to the surface of starch granules after starch extraction [51]. The higher protein content in BS may be related to its higher enzyme content. Another explanation is that BS has much higher specific surface area (2.3–2.8 m2/g) compared to AS (0.8–0.9 m2/g) [50], causing BS to absorb more storage proteins during the starch separation process [31]. Lipids in starch granules vary in their compositions and types depending on their locations. Lipids in the surface of starch granules mainly comprise triacylglycerides, free fatty acids, digalactosyldigylceride, monogalactosyldigylceride, phosphatidylcholine and lysophosphatidylcholine, while the lipids in the internal part of starch granules consisted of monoacyl lipids, free fatty acids, and lysophospholipids [14]. The content of phospholipid, which accounts for 86–94% of total starch lipids, is negatively correlated with mean starch granule diameter, indicating that the smaller granules have higher lipid and phosphorus content [52]. However, further studies are needed to investigate the component differences in protein and lipid of different-sized starch granules.
4.2. Molecular structure
The fine structures of starch molecules, including fine structures of AM and AP, are crucial in determining hierarchical structures and functionalities. The chain-length distribution (CLD) is the fundamental to starch granules and their starch physicochemical properties. CLD analysis is commonly conducted using size exclusion chromatography (SEC), fluorophore-assisted capillary electrophoresis (FACE) and high-performance anion exchange chromatography (HPAEC) [53]. SEC can characterize the full range of starch CLD, including both AP and AM branches, but it cannot determine the accurate CLD of AP due to column adsorption and shear degradation. On the other hand, FACE and HPAEC provide more accurate results of starch CLD, but they are limited to measuring specific ranges (degree of polymerization (DP)< 180 and < 70, respectively) of starch chain [54]. Thus, a combination of SEC and FACE (or HPACE) can complementarily characterize the starch CLD in full details: SEC for AM molecules and FACE (or HPACE) for AP [55]. Based on the DP, the chain length of AP can be divided into four categories, namely A chains (6 ≤DP≤12), B1 chains (13 ≤DP≤24), B2 chains (25 ≤DP≤36), and B3 chains (DP≥37). It is generally accepted that most wheat varieties have AP with more B2 chains but fewer A chains in AS compared to the BS (Table 2) [56], [57], [58]. Recent study has also measured short chains (DP<6), showing that AS from both normal and waxy wheat contains more short chains, while BS contains more A chains, B2 chains, and B3 chains (Table 2) [59]. The CLD of AP is an essential factor influencing the structure of starch granules. In the case of AS, an AP molecule with more B2 chains has a cylindrical shape and tends to pack in a parallel pattern and develop into a disk-shaped granule [57]. Conversely, an AP molecule with more short chains i.e. A and B1 chains, and fewer B2 chains, has a conical shape and is more easily embedded into a spherical BS granule [57]. However, there is still limited understanding of the differences in the molecular structure of AM between AS and BS, as well as their contribution to the structure of starch granules.
Table 2.
The amylopectin branch chain length distribution of A-type (AS) and B-type (BS) starch granule samples.
| Sample | DP< 6 (%) | 6 ≤DP≤ 12 (%) | 13 ≤DP≤ 24 (%) | 25 ≤DP≤ 36 (%) | DP≥ 37 (%) | Reference | |
|---|---|---|---|---|---|---|---|
| Hard wheat | AS | nd | 32.5 (-) | 49.4 (+) | 13.7 (+) | 4.4 (+) | [56] |
| BS | nd | 35.3 (-) | 48.1 (+) | 12.6 (+) | 4.1 (+) | ||
| Soft wheat | AS | nd | 31.8 (-) | 49.4 (+) | 14.5 (+) | 4.4 (+) | [56] |
| BS | nd | 34.0 (-) | 48.6 (+) | 13.2 (+) | 4.1 (+) | ||
| Wesley | AS | nd | 21.8 (-) | 43.3 (-) | 15.1 (+) | 19.7 (+) | [57] |
| BS | nd | 25.2 (-) | 46.8 (-) | 13.5 (+) | 14.2 (+) | ||
| Xinong 979 (normal wheat) | AS | 16.92 (+) | 34.97 (-) | 22.42 (-) | 12.98 (+) | 12.71 (-) | [59] |
| BS | 6.95 (+) | 39.07 (-) | 29.10 (-) | 10.84 (+) | 14.04 (-) | ||
| Nongda3471 (waxy wheat) | AS | 22.43 (+) | 33.06 (-) | 23.40 (-) | 11.88 (-) | 9.23 (-) | [59] |
| BS | 9.21 (+) | 35.31 (-) | 29.28 (-) | 13.52 (-) | 12.68 (-) | ||
| Sunco | AS | nd | 39.9 (-) | 48.3 (+) | 8.8 (+) | 3 (+) | [58] |
| BS | nd | 43.3 (-) | 46 (+) | 7.9 (+) | 2.8 (+) | ||
| Sunsoft | AS | nd | 40.4 (-) | 49.4 (+) | 8.5 (+) | 1.7 (-) | [58] |
| BS | nd | 43.1 (-) | 46.6 (+) | 7.5 (+) | 2.8 (-) | ||
| SM1118 | AS | nd | 40.6 (-) | 47.9 (+) | 9.2 (+) | 2.3 (+) | [58] |
| BS | nd | 42.7 (-) | 47.1 (+) | 8.5 (+) | 1.8 (+) | ||
| SM1028 | AS | nd | 41.3 (-) | 48.6 (+) | 8.6 (+) | 1.5 (-) | [58] |
| BS | nd | 43.3 (-) | 47.2 (+) | 7.8 (+) | 1.6 (-) | ||
| Waxy wheat | AS | nd | 38.0 (-) | 50.2 (-) | 9.8 (+) | 2 (+) | [58] |
| BS | nd | 39.3 (-) | 50.3 (-) | 8.6 (+) | 1.9 (+) | ||
DP: degree of polymerization.
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS; “nd” represents no data.
4.3. Crystalline structure
Starches exhibit different crystalline structures depending on their types: A-type starches have an orthorhombic crystalline structure; B-type starches have a hexagonal crystalline structure, and C-type starches display a combination of both orthorhombic and hexagonal crystalline structures [60]. The starches type and relative crystallinity are usually determined by X-ray diffraction (XRD). The A-type starches, found in wheat, maize, and rice starch, exhibit diffraction peaks at around 15°, 17°, 18°, and 23°. The B-type starches, found in high AM maize starch and tuber starches such as potato starch, show diffraction peaks at around 5.6°, 17.2°, 22.2°, and 24°. The C-type starches, derived from pea and cassava, display diffraction peaks at around 5.6°, 15°, 17°, and 23° [61], [62]. AS and BS samples show similar XRD patterns, indicating that both of them contain orthorhombic crystalline structures [63]. In most wheat varieties, the relative crystallinity of AS (26.78–34.90%) is higher than that of BS (25.03–30.98%) (Table 3) [31], [45], [59], [63]. The AP chains are arranged in order and make it possible for starch to form a crystalline structure. The shorter chains may not contribute to the formation of crystalline structure, causing defects in the crystalline structure [48]. Based on the knowledge that AP chains with 12 ≤DP≤ 24 allow the formation of more stable crystalline structures [48], [64], it is speculated that the higher relative crystallinity of AS may be related to its higher proportion of B2 chains. However, the relationship between the CLD of AP and relative crystallinity requires further investigation. On the contrary, another study showed that the BS showed higher relative crystallinity compared to AS, which may be attributed to variations in the sample preparation and techniques used for measurement and calculation [57].
Table 3.
Crystalline structure of A-type (AS) and B-type (BS) starch granule samples.
| Sample | Relative crystallinity (%) | q (nm−1) | d (nm) | α | Dm | Ds | Reference | |
|---|---|---|---|---|---|---|---|---|
| WAN50 (soft wheat variety) | AS | 26.78 (+) | nd | nd | nd | nd | nd | [31] |
| BS | 25.03 (+) | nd | nd | nd | nd | nd | ||
| ZHENG366 (hard wheat variety) | AS | 27.65 (+) | nd | nd | nd | nd | nd | [31] |
| BS | 25.98 (+) | nd | nd | nd | nd | nd | ||
| Xinong 9718 (hard red winter wheat) | AS | 34.47 (+) | nd | nd | nd | nd | nd | [45] |
| BS | 31.05 (+) | nd | nd | nd | nd | nd | ||
| Shannong 138 (soft red winter wheat) | AS | 34.82 (+) | nd | nd | nd | nd | nd | [45] |
| BS | 30.98 (+) | nd | nd | nd | nd | nd | ||
| Wesley | AS | 32.40 (-) | nd | nd | nd | nd | nd | [57] |
| BS | 35.50 (-) | nd | nd | nd | nd | nd | ||
| Commercial wheat starch | AS | 31.95 (+) | 0.5956 (-) | 10.54 (+) | 2.81 (-) | 2.81 | na | [63] |
| BS | 29.38 (+) | 0.6265 (-) | 10.02 (+) | 3.99 (-) | na | 2.01 | ||
| Xinong 979 (normal wheat) | AS | 33.10 (+) | 0.679 (-) | 9.25 (+) | 2.80 (-) | 2.80 | na | [59] |
| BS | 27.23 (+) | 0.693 (-) | 9.06 (+) | 3.91 (-) | na | 2.09 | ||
| Nongda3471 (waxy wheat) | AS | 34.90 (+) | 0.617 (-) | 10.18 (+) | 3.01 (-) | 3.01 | na | [59] |
| BS | 30.80 (+) | 0.684 (-) | 9.18 (+) | 3.63 (-) | na | 2.37 | ||
q: the scattering vector; d: thickness; α: the power-law exponent; Dm: mass fractal structure; Ds: surface fractal structure.
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS; “nd” represents no data.
“na” represents not applicable.
Small angle X-ray scattering (SAXS) is a useful technique for analyzing the nano-structural and fractal properties of starch. The exact q value of the scattering peak at round 0.6 nm−1 is usually used to calculate the average repeat distance (d=2π/q) of semi-crystalline lamellae in starch [65]. AS shows lower q values (0.5956–0.679 nm−1) compared to BS (0.6265–0.693 nm−1), but higher average repeat distance (9.25–10.54 nm) than BS does (9.06–10.02 nm) (Table 3). These results suggest that the average repeat distance of the semi-crystalline lamellae in BS is smaller, indicating a denser layered structure [59], [63]. The fractal structure of scattering objects can be characterized by the fractal dimension D, and the fractal system for starches is classified as mass dimension (Dm) and surface dimension (Ds). The scattering law relates scattering intensity to scattering vector q: I(q)∝q−α, where I is the SAXS intensity and the exponent α refers to the slope of lnI-lnq in SAXS graph. For starch sample, if α is between 1 and 3, the sample is mass fractal with fractal dimension Dm= α. If α is between 3 and 4, the sample is surface fractal with fractal dimension of Ds = 6-α [66]. α ranges from 2.80 to 3.01 for AS and from 3.63 to 3.99 for BS (Table 3). These findings indicate that AS has a mass fractal structure with regular arrangement, whereas BS exhibits a surface fractal structure with a compact and smooth surface [59], [63].
4.4. Morphological structure
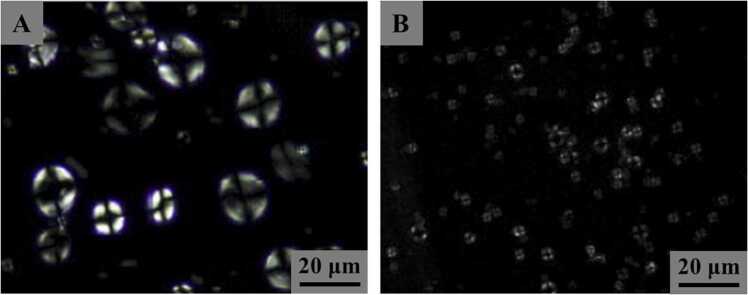
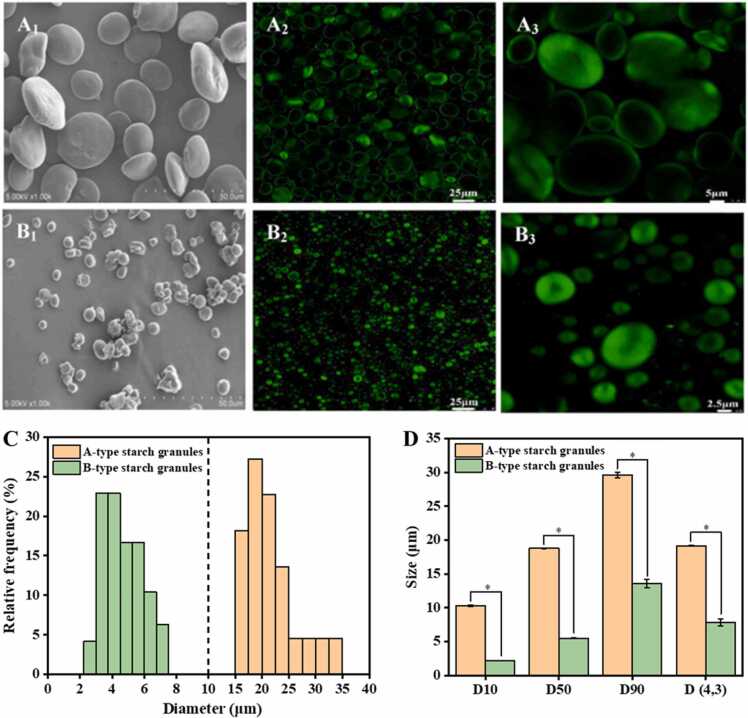
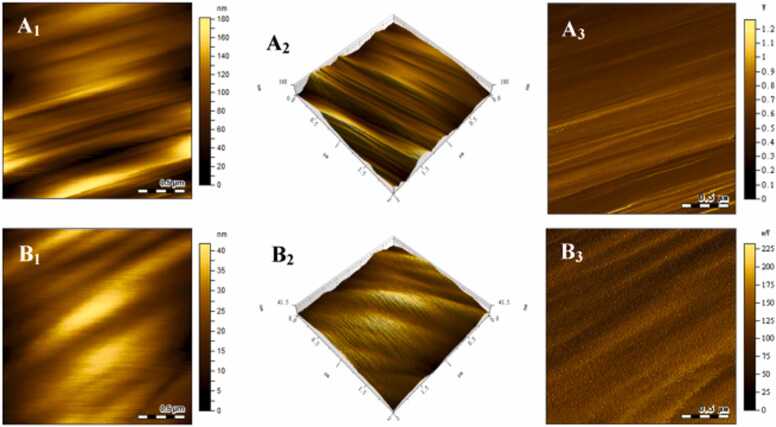
Various advanced microscopic techniques, including polarized light microscopy, SEM, confocal laser scanning microscopy (CLSM), and atomic force microscopy (AFM), have been applied to observe the morphologies of AS and BS [64]. The crystalline region of starch granules exhibits an orderly arrangement of starch molecules, whereas the amorphous region shows a more random arrangement. This leads to an anisotropic phenomenon, visible as a polarization cross (Maltese cross), when polarized light passes through starch granules. AS displays larger and clearer birefringence compared to BS (Fig. 2) [31], [45], [56], [63]. The clearer birefringence of AS is attributed to its higher crystallinity, as the intensity of birefringence depends on the dimensions of the granules and their relative crystallinity and micro-crystalline orientation [67]. SEM can provide useful information on starch size and morphology. AS granules are generally disc-shaped or lenticular, while BS granules are spherical or irregular (Fig. 3A1 and B1). The diameters of AS and BS granules range from 15.7 µm to 33.1 µm and 2.3–7.1 µm, respectively (Fig. 3C). However, the diameters of AS and BS granules vary among different studies. Some other studies report diameters of AS granules to be 10–40 µm [45], 10–35 µm [57], or 12–24 µm [68]. On the other hand, the diameter of BS granules is reported to be 2–5 µm [45], 2 µm [57], or 4–6 µm [68]. As determined by laser particle size analyzer, the D10, D50, D90 and D (4,3) of AS are significantly higher than those of BS (Fig. 3D). However, it is worth noting that these parameters of AS or BS can vary considerably in different studies [45], [59]. The variations in the size of AS or BS among the studies can be attributed to variations in wheat genotypes and extraction methods. For example, the size range of BS granules can be reduced by extending the rest time or increasing the centrifugal force during the extraction process. CLSM has been used to analyze the internal structure of starch granules, including the distribution of AM and AP, hilum, growth rings, pores, and channels [69], [70], [71], [72]. APTS (8-amino-1,3,6-pyrenetrisulfonic acid) is used to specifically label the reducing ends of starch molecules, and the captured fluorescence signals can reveal the position of the reducing ends. AM, being a smaller molecule than AP, exhibits a higher molar ratio of reducing ends per anhydrous glucose residue, resulting in a greater labeling of amylase per unit weight [69]. The fluorescence intensity does not show significant differences between AS and BS (Fig. 3A2, A3, B2 and B3), which may be attributed to the small difference in AM content between AS and BS [59]. Previous study demonstrated that AS exhibits clearer hilum and growth rings compared with BS [70]. However, in another study, the growth rings of both types of starch can be easily distinguished [72]. The discrepancies in results may be due to differences in experimental materials or testing techniques. Therefore, the identification of starch growth rings depends on experimental protocols and experimenter's expertise. Channels within starch granules have been demonstrated to be filled with proteins, when stained with CBQCA (3-(4-carboxybenzoyl) quinoline-2-carboxaldehyde) and methanolic merbromin [71]. In addition, the AS possesses large channels in equatorial groove region and finer channels in other regions of the granule, while BS contains predominantly larger, less-defined, and void-like channels [71]. These channels within wheat starch granules play a role in facilitating the flow of enzymes or other chemical reagents into the granule matrix, which is crucial for the digestion or modification of starch and requires further investigation. When characterizing the starch surface structures at the nanoscale using AFM, AS shows a ravine-like, uneven surface morphology, while the surface of BS appears relatively smooth (Fig. 4) [59]. The differences in morphologies of AS and BS may be attributed to variations in starch biosynthesis, the ratio of AM to AP, and the molecular structure of starch [14], [59].
Fig. 2.
Polarized light microscopic images of the A-type (A) and B-type (B) starch granules of wheat starch. Data are derived from the previous study [63].
Fig. 3.
The micro-structure and size distribution of A- and B-type starch granules. Fig. A1 and B1 show the scanning electron microscopy images of A- and B-type starch granules, respectively. Fig. A2, B2, A3 and B3 show the confocal laser scanning microscope images of A- and B-type starch granules, respectively. Fig. C and D show the size distribution of A- and B-type starch granules analyzed by ImageJ software and laser diffraction analyzer, respectively. Data are derived from the previous study [59].
Fig. 4.
The surface morphology (1), three-dimensional morphology (2) and amplitude diagram (3) of A-type starch granules (A) and B-type starch granules (B) observed by atomic force microscopy. Data are derived from the previous study [59].
4.5. Gelatinization and pasting properties
Gelatinization is the process in which starch is heated above a critical temperature with enough water to cause an irreversible phase transition. In this context, gelatinization begins with the glass transition stage of the amorphous region, leading to AM molecule leaching and subsequent disassociation of AP crystallites, causing crystalline structure loss [73]. Differential scanning calorimetry is used to characterize the gelatinization properties of starch, including onset temperature (To), peak temperature (Tp), conclusion temperature (Tc), and enthalpy of gelatinization (ΔH). Among these parameters, gelatinization temperatures are indicators of the thermal stability of crystallites. It is generally observed that in most wheat varieties, AS exhibits lower To, Tp, and Tc compared to BS (Table 4) [28], [45]. However, this observation cannot be generalized to all crop varieties. For example, in three varieties AS shows higher To than BS does, and in one variety, AS shows higher Tp and Tc than BS does (Table 4) [28], [63]. The conflicting reports may be attributed to imprecise measurements and variations in the genotype of wheat varieties [28]. In addition, these discrepancies could arise from separation efficiency as well as the retention of extremely small granules [17]. ΔH reflects the amount of energy required for the dissociation of starch crystalline structure [63]. The ΔH of AS (6.78–12.2 J/g) is higher than that of BS (4.46–10.0 J/g) for all tested wheat varieties (Table 4) [28], [45], [63]. More heat needed when the structure of the AS is disrupted, which is attributed to its higher relative crystallinity. Furthermore, the chemical composition of starch is also considered to be an important factor affecting ΔH. AS shows higher starch content, resulting in more heat needed during the gelatinization process. Moreover, the higher ΔH of AS can be attributed to its lower damaged starch content, which has been suggested to negatively correlate with the ΔH [49].
Table 4.
Gelatinization properties of A-type (AS) and B-type (BS) starch granule samples.
| Sample | Starch: water ratio (w:w) | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) | Reference | |
|---|---|---|---|---|---|---|---|
| Xinong 9718 (hard red winter wheat) | AS | 1:4 | 56.52 (-) | 60.91 (-) | 66.10 (-) | 6.86 (+) | [45] |
| BS | 1:4 | 58.73 (-) | 62.41 (-) | 68.84 (-) | 4.46 (+) | ||
| Shannong 138 (soft red winter wheat) | AS | 1:4 | 56.60 (-) | 61.05 (-) | 66.48 (-) | 6.78 (+) | [45] |
| BS | 1:4 | 58.03 (-) | 62.75 (-) | 67.34 (-) | 5.87 (+) | ||
| CDC Teal | AS | 1:3 | 56.2 (+) | 62.2 (-) | 69.4 (-) | 11.2 (+) | [28] |
| BS | 1:3 | 55.4 (+) | 63.4 (-) | 71.6 (-) | 9.2 (+) | ||
| McKenzie | AS | 1:3 | 52.2 (-) | 60.2 (-) | 70.3 (-) | 10.2 (+) | [28] |
| BS | 1:3 | 53.8 (-) | 61.3 (-) | 70.8 (-) | 8.0 (+) | ||
| AC Karma | AS | 1:3 | 53.0 (-) | 60.7 (-) | 70.8 (-) | 12.2 (+) | [28] |
| BS | 1:3 | 54.4 (-) | 62.4 (-) | 73.2 (-) | 9.0 (+) | ||
| AC Crystal | AS | 1:3 | 54.2 (-) | 61.3 (-) | 71.0 (-) | 10.4 (+) | [28] |
| BS | 1:3 | 55.0 (-) | 62.3 (-) | 72.6 (-) | 8.3 (+) | ||
| Fielder | AS | 1:3 | 52.8 (-) | 60.6 (-) | 68.0 (-) | 11.4 (+) | [28] |
| BS | 1:3 | 53.4 (-) | 61.8 (-) | 68.4 (-) | 10.0 (+) | ||
| Plenty | AS | 1:3 | 52.8 (+) | 58.0 (-) | 63.8 (-) | 10.0 (+) | [28] |
| BS | 1:3 | 52.2 (+) | 59.6 (-) | 69.6 (-) | 8.8 (+) | ||
| Commercial wheat starch | AS | nd | 65.48 (+) | 70.74 (+) | 78.01 (+) | 10.27 (+) | [63] |
| BS | nd | 60.89 (+) | 65.98 (+) | 73.38 (+) | 8.22 (+) | ||
To: onset temperature; Tp: peak temperature; Tc: conclusion temperature; ΔH: enthalpy of gelatinization
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS.
“nd” represents no data.
Rapid viscosity analyzer is used to characterize the pasting properties of starch, including peak viscosity (PV), trough viscosity (TV), breakdown (BD), final viscosity (FV), setback (SB), and pasting temperature (PT). PV represents the maximum viscosity before the onset of cooling process. BD, the difference between PV and TV, indicates the extent of granule disintegration. As the cooled-cooked paste stabilizes during cooling, FV gradually increases. SB, the difference between FV and PV, reflects the stability of hot paste and the retrogradation tendency of starch [45]. PT is the temperature at which the viscosity begins to increase during heating [45]. Generally, AS exhibits higher PV, TV, and FV (2269–4748, 1619–2448, and 2704–3634 cP, respectively), but lower PT (54.4–78.75 °C) than BS (540–3385, 240–1347, 842–2985 cP, and 61.3–94.18 °C) (Table 5). These variations can be explained by differences in the chemical compositions and molecular structures of AS and BS. Specifically, protein and lipids in starch pastes may suppress granule swelling and maintain the integrity of starch granules, resulting in lower viscosity [31], [57]. Additionally, damaged starch with reduced particle size and broken granular surface could enhance the permeability of water into granules, thus decreasing viscosity [49]. AS samples contain less protein, lipid, and damaged starch, resulting in higher PV, TV, and FV, but lower PT. Furthermore, A chains negatively correlate with FV, while B1 chains positively correlate with FV, which is attributed to longer AP chains interacting with AM molecules during retrogradation [74]. The higher FV of AS may be related to its lower content of A chains and higher content of B1 chains. The relative magnitude of BD and SB between AS and BS varies due to variations in material genotypes or experimental protocols (Table 5). The method of starch isolation also affects viscosity parameters. The increase in AS size resulting from the isolation procedure may increase starch viscosity, while the retention of extremely small granules may reduce the viscosity of BS. These indicate that the viscosity parameters of starch are affected by both intrinsic (starch granule size, chemical compositions, and molecular structures) and extrinsic (experimental protocols and isolation method) factors, with chemical compositions and molecular structures being the dominant factors for viscosity differences between AS and BS. Further investigations are needed to understand the contribution of AM molecular structure of AS and BS to starch viscosity.
Table 5.
Pasting properties of A-type (AS) and B-type (BS) starch granule samples.
| Sample | Starch: water ratio (w:w) | PV (cP) | TV (cP) | BD (cP) | FV (cP) | SB (cP) | PT (°C) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Six soft wheat varieties | AS | nd | 2269–3132 (+) | 1619–2448 (+) | 519–648 (∼) | 2704–3634 (+) | 1021–1186 (∼) | 56.7–69.1 (∼) | [31] |
| BS | nd | 1060–1821 (+) | 526–1094 (+) | 503–915 (∼) | 1276–2175 (+) | 750–1253 (∼) | 61.3–81.3 (∼) | ||
| Six hard wheat varieties | AS | nd | 2127–2779 (+) | 1628–2138 (+) | 399–685 (+) | 2858–3079 (+) | 693–1031 (+) | 57.2–66.7 (-) | [31] |
| BS | nd | 540–892 (+) | 240–451 (+) | 259–441 (+) | 842–1232 (+) | 602–835 (+) | 67.4–85.9 (-) | ||
| Commercial wheat starch | AS | 3:25 | 2883.5 (+) | 2421.5 (+) | 462.0 (+) | 3527.5 (+) | 1106.0 (+) | 78.75 (-) | [30] |
| BS | 3:25 | 1648.5 (+) | 1347.0 (+) | 301.0 (+) | 1737.5 (+) | 390.5 (+) | 94.18(-) | ||
| Xinong 9718 (hard red winter wheat) | AS | 2:25 | 3981 (+) | 1530 (+) | 1297 (-) | 3291 (+) | 1761 (-) | 69.3 (-) | [45] |
| BS | 2:25 | 2302 (+) | 1005 (+) | 2451 (-) | 2985 (+) | 1890 (-) | 75.1(-) | ||
| Shannong 138 (soft red winter wheat) | AS | 2:25 | 4748 (+) | 1504 (+) | 3244 (+) | 3206 (+) | 1522 (+) | 54.4 (-) | [45] |
| BS | 2:25 | 3385 (+) | 803 (+) | 2582 (+) | 2008 (+) | 1205 (+) | 67.1 (-) | ||
PV: peak viscosity; TV: trough viscosity; BD: breakdown; FV: final viscosity; SB: setback; PT: pasting temperature.
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS; “∼” in parentheses represents the range for AS and BS partially overlapped.
“nd” represents no data.
4.6. Digestive properties
Starch can be categorized into three groups based on its resistance to enzymatic hydrolysis: rapidly digestible starch (RDS, digested within 20 min), slowly digestible starch (SDS, digested between 20 and 120 min), and resistant starch (RS, digested beyond 120 min). RDS is rapidly and completely hydrolyzed in the small intestine, causing a rapid increase in blood glucose levels, and long-term consumption of high RDS content may lead to type 2 diabetes [14]. However, RDS is essential for individuals who suffer from maltrition due to a lack of energy [49]. SDS is digested slowly and completely in small intestine and helps to stabilize blood glucose levels, making it the most desirable group [14]. RS, consumed as dietary fiber, has a hypoglycemic effect and improves intestinal transit [75].
The digestive properties of AS and BS remain uncertain due to conflicting results from different studies (Table 6) [70], [76], [77], [78]. Starch digestibility is influenced by factors such as granule size, chemical composition, crystalline structure, and molecular structure [79]. Starch granules with a larger specific surface area and more enzyme action sites tend to be hydrolyzed faster [80]. Additionally, damaged starch, with more cell disintegration and a larger contact area for enzymatic catalysis [49], exhibits increased starch digestibility. In general, starch digestibility is negatively correlated with AM content [53], and the crystalline regions of starch granules would resist amylase hydrolysis [81]. The higher digestibility of BS can be attributed to its larger specific surface area, more enzyme action sites, and higher damaged starch content, along with its lower AM content and crystallinity. However, some studies have shown that AS may have greater digestibility (Table 6), potentially due to its higher starch content, the specific fine structure of AM, AM lipid complex, and phenolic substances, which require further investigation. The conflicting digestive properties observed between AS and BS may be attributed to differences in the sources of experimental materials or variations in the digestive enzymes and their activities.
Table 6.
Digestibility of A-type (AS) and B-type (BS) starch granule samples.
| Sample | RDS (%) | SDS (%) | RS (%) | Reference | |
|---|---|---|---|---|---|
| Yangmai No. 9 | AS | 40.42 (+) | 30.93 (-) | 28.65 (-) | [70] |
| BS | 29.34 (+) | 38.18 (-) | 32.48 (-) | ||
| Nuo Mai 2 | AS | 30.95 (+) | 55.03 (-) | 14.02 (+) | [78] |
| BS | 25.03 (+) | 61.57 (-) | 13.40 (+) | ||
| Commercial wheat starch | AS | 30.49 (-) | 13.54 (+) | 56.10 (+) | [77] |
| BS | 45.24 (-) | 12.58 (+) | 42.18 (+) | ||
| Commercial wheat starch (uncooked) | AS | 24.72 (-) | 25.86 (+) | 49.42 (-) | [76] |
| BS | 25.96 (-) | 20.07 (+) | 53.97 (-) | ||
| Commercial wheat starch (cooked) | AS | 35.33 (-) | 50.19 (+) | 14.58 (-) | [76] |
| BS | 35.45 (-) | 50.02 (+) | 14.64 (-) | ||
RDS: rapidly digestible starch; SDS: slowly digestible starch; RS: resistant starch.
“+ ” in parentheses represents the value of AS is higher than that of BS; “-” in parentheses represents the value of BS is higher than that of AS.
5. Effect of starch morphology on dough physicochemical and rheological properties
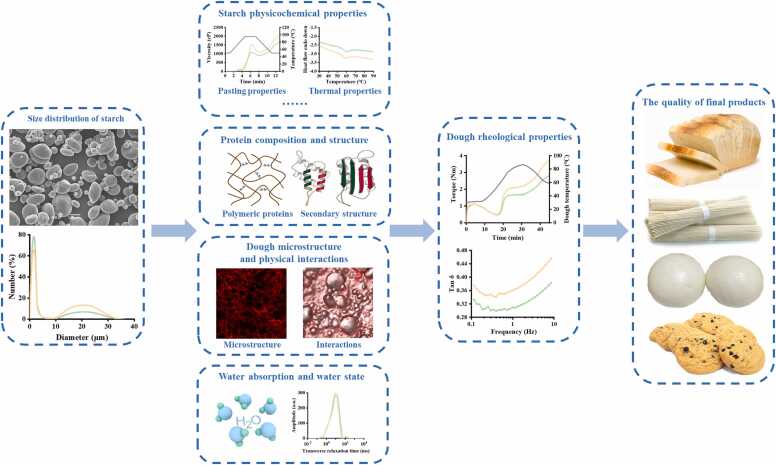
Starch is a major component of dough, a crucial factor influencing the structure and properties of wheat dough. The presence of starch affects the gluten network, involving the polymeric proteins, chemical interactions, secondary structures of gluten, micro-structure, physical interactions between gluten and starch, and water absorption and water state, consequently impacting the rheological properties of dough (Fig. 5). This section compares the effects of different-sized wheat starch on dough properties to further understand the relationship between starch structure and its functionalities.
Fig. 5.
Schematic diagram of starch size distribution affecting dough rheological properties and final product quality.
5.1. Starch physicochemical properties
The physicochemical properties of starch have a significant influence on the rheological properties of dough. In native wheat flour, the FV and ΔH is negatively correlated with dough development time (DDT) and stability time (ST) [82], [83]. However, the presence of AM can enhance dough stability and deformation resistance [82], [84]. Starch with low relatively crystallinity is less likely to form hydrogen bonds with water, which allows more hydrogen bonds to interact with gluten, thereby improving gluten-starch interactions and dough stability [82]. However, another study reported that dough with high relative crystallinity of starch shows greater stability [85]. This inconsistency may be attributed to the genotypic variations in the experimental materials. In general, AS shows higher AM content, FV, ΔH, and relative crystallinity, compared to BS (Table 1, Table 3, Table 4, Table 5). However, further investigations are required to understand the relationship between these parameters and the quality of doughs with varying AS/BS (the ratio of AS to BS by weight). Previous studies have indicated differences in the CLD of AP between AS and BS [56], [57], [58], [59], whereas their specific effect on dough properties needs to be further studied.
5.2. Polymeric proteins
When flour is mixed with water, glutenin and gliadin become hydrated and start to interact to form a specific three-dimensional network. At the molecular scale, glutenin forms glutenin polymers through disulfide bonds, while gliadin typically attaches to glutenin through non-covalent interactions such as ionic bonds, hydrophobic interactions, and hydrogen bonds [86]. In study of reconstituted model doughs with different AS/BS, the glutenin macropolymer (GMP) and relative content of γ-gliadin generally decrease as the AS/BS ratio increases, indicating that BS can promote glutenin polymerization and strengthen the cross-linking of γ-gliadin with other polypeptide chains [46]. Based on their solubility in 0.5% SDS solution, the polymeric protein can be divided into SDS-unextractable polymeric proteins (UPP) and SDS-extractable polymeric proteins (EPP) [87]. When the content of different-sized starch is increased at the same percentage level, the BS reconstituted flours show higher UPP% but lower EPP%, indicating that BS promotes the polymerization of UPP with higher molecular weight [30]. In addition, the content of high molecular weight proteins (Mw=91,000–688,000 Da) and low molecular weight proteins (Mw<91,000 Da) significantly decreases as the AS/BS ratio increases [88], which is consistent with the finding that BS promotes the formation of GMP and UPP. This observation can be explained by previous finding that BS with smaller size facilitates polymerization of gluten [30].
5.3. Chemical interactions
The disulfide bonds reflect the cross-linkages between protein molecules and can be oxidized from free sulfhydryl [89]. The free sulfhydryl content increases gradually with the increase of AS/BS ratio [46]. When AS or BS is added to native wheat flours, the content of free sulfhydryl and disulfide bonds decreases gradually due to the dilution of gluten [30]. BS reconstituted flours show a greater decrease in free sulfhydryl content and a smaller decrease in disulfide bonds than AS reconstituted flours, indicating that BS can facilitate the formation of disulfide bonds through the oxidation of sulfhydryl groups, compared to AS [30]. This is mainly due to the presence of large-sized AS granules, which impede the formation of UPP and interfere with the continuity of gluten.
In addition to disulfide bonds, the formation and stability of dough system also depend on non-covalent bonds, including ionic bonds, hydrogen bonds, and hydrophobic interactions [90]. In gluten, ionic bonds form through free side chain groups in amino acids residues. As the AS/BS ratio increases, ionic bonds decreased gradually, possibly due to large-sized AS granules packing into gluten network as nonionic polymers, impeding ionic interactions between proteins [91]. Moreover, the amount of hydrogen bonds is gradually decreased, as AS/BS ratio increases from 5:5–10:0 [46]. Correspondingly, the BS reconstituted doughs show higher hydrogen bond content than AS reconstituted doughs do [30]. Given that hydrogen bonds can be formed between hydroxyl groups on starch surface and hydrophilic amino acids in gluten, BS exhibits higher damaged starch content than AS does, resulting in more hydrogen bonds formed with gluten [90]. On the other hand, the AS reconstituted dough contains more hydrophobic interactions compared to the BS reconstituted dough, which can be attributed to the smaller specific surface area of AS, where fewer hydrophobic interactions occur in the protein-starch interface [46]. However, the effects of different-sized wheat starch on specific chemical groups in the dough system have not been determined.
5.4. Secondary structures of gluten
Secondary structures of gluten can be characterized by the contents of β-sheets, α-helices, β-turns and random coils, which are typically analyzed using FTIR spectroscopy [87]. The Amide I band (1600–1700 cm−1) in the FTIR spectrum is particularly sensitive for studying gluten's secondary structures, as it is associated with the C O stretching vibration [92]. In starch-gluten model doughs with varying AS/BS ratios, β-sheets and β-turns are the dominant secondary structures of gluten, as observed in noodle dough [46], [88]. Specifically, the β-sheet content increases gradually with the starch granule AS/BS ratio increasing from 5:5–10:0 in starch-gluten model dough, attributed to the formation of hydrogen bonds between HMW-GSs or between amino acid side chains and free hydroxyl groups in starch [46]. However, in noodle dough, the content of β-sheets gradually increases as BS content increases [88], which may be attributed to the differences in the hydration levels of the wheat doughs, as hydration level significantly influences the secondary structures of gluten [89]. Specifically, full hydration is achieved (optimum water absorption) in the starch-gluten model dough, while the noodle dough is in low hydration state. Moreover, in noodle dough, the content of β-turns decreases gradually as BS content increases [88], likely due to the replacement of β-turns by β-sheets [93]. The secondary structures of gluten, particularly the presence and proportion of β-sheets and β-turns, significantly impact the rheological properties of dough. Since starch size distribution affects these secondary structures, it can consequently influence the quality of dough [94].
5.5. Micro-structure of dough
Various microscopy techniques, including optical microscopy, SEM, and CLSM, can be used to observe the micro-structure of dough [30], [88], [95]. Dough with a high AS content exhibits more cracks and voids between the starch granules and gluten network, indicating a loose dough structure [30], [46], [88]. In contrast, lower AS content or higher BS content leads to a more uniform and compact structure with fewer cracks and voids [30], [46], [88]. Similarly, in gluten-free bread dough, the continuous phase (liquid phase) formed by starch granules surrounding gas bubbles becomes more consistent with the increase of BS content [95]. These phenomena can be explained by the differences in size distribution of AS and BS: (1) small-sized BS can be homogeneously and tightly packed into the gluten network, while large-sized AS granules are more likely to be exposed or disengaged from the gluten network [30], [46], [88], [95]; (2) BS granules can fill the voids and cracks between AS granules and gluten network, thus increasing the continuity of dough structure [96]. A recent study showed that the addition of AS decreases protein junctions, indicating that AS hinders the formation of protein cross-linkages, while BS addition has the opposite effect [30]. Lacunarity, which reflects the void distribution in the gluten network, is a measure of dough strength, where a lower value indicates stronger dough [97]. This parameter can be determined by analyzing images of dough micro-structure observed by CLSM with AngioTool64 software [98], [99]. Compared with AS reconstituted dough, BS reconstituted dough shows lower lacunarity, supporting the finding that dough systems with higher BS content show fewer voids [30]. Taken together, these results demonstrate that BS can strengthen dough micro-structure, resulting in improved dough strength and stability.
5.6. Physical interactions between gluten and starch
Dough formation and stability are influenced by not only chemical but also physical interactions. The micro-structure of dough shows that the gluten network embedded with AS granules has many cracks and voids, while BS can be tightly packed into the network. Therefore, the concept of gluten-starch physical interaction has been proposed to indicate the strength of physical connections between starch granules and gluten networks [23]. The ratio of BS to AS by number (B/A) is used to characterize the size distribution of starch granules, and lacunarity is used to represent the uniformity of voids in gluten networks. Three parameters, i.e. B/lacunarity, A/lacunarity, and B/A/lacunarity, have been used to quantitatively characterize the physical interactions between gluten and starch at the micro-structure level [23]. The filling degree, represented by B/A/lacunarity, has been found to be positively related to dough stability time (r = 0.71, P < 0.01), indicating that gluten-starch physical interactions strongly affect the strength and stability of dough [24]. Higher B/A/lacunarity reflects that starch granules have greater ability to fill into the voids in the gluten network, where starch and gluten interact with each other closely, thus improving the dough strength. This understanding provides a better way to interpret and predict the stability of dough. The addition of BS to native flour increases B/A/lacunarity, enhances gluten-starch physical interactions, and thus improves the mixing properties of dough. Even during sequential thermo-mechanical treatment, dough with higher BS content shows a more stable network structure due to stronger gluten-starch physical interactions [100]. These results indicate that the dough with strengthened gluten-starch physical interactions exhibits greater stability during mixing and thermo-mechanical processes.
5.7. Water absorption and water state
The interactions among dough components, including gluten-gluten and gluten-starch interactions, can be influenced by water absorption and water state, which in turn affect the quality and end-use of the dough. The water absorption of flour can be measured by farinograph or Mixolab [30], [97]. Due to its smaller volume, larger specific surface area, and higher content of damaged starch, BS exhibits stronger water absorption ability compared to AS [68]. Doughs reconstituted with BS also demonstrate higher water absorption than AS reconstituted doughs [30]. Similarly, in the model dough, water absorption increases with the increased proportion of BS [101]. Moreover, BS may compete with gluten for water, resulting in insufficient hydration of gluten, and increases dough rigidity [46].
The water state of dough can be analyzed using low field nuclear magnetic resonance. Three distinct water populations i.e. bound water, weakly bound water, and free water can be analyzed in dough samples by transverse relaxation (T2), and the relaxation time points of the peak positions are recorded as T21, T22, and T23, respectively [102]. A21, A22, and A23 represent the relative peak areas, indicating the relative content of the three types of water [102]. In a recent study, it was found that the AS/BS ratio has no significant effect on T21, T22, A21, or A22; however, T23 shows an upward trend, while A23 exhibits an opposite trend with the increase in BS content [88]. In a previous study, only two distinct water populations were observed, and the addition of BS at a proportion of 5% increases bound water content but decreases free water content [30]. These results indicate that increasing the content of BS can reduce the moisture fluidity of dough, which is responsible for its uniform and compact structure.
5.8. Dough rheological properties
The rheological properties of dough are crucial factors for determining the end-use of dough and quality of final products, and can be measured using a rheometer, farinograph, Mixolab, and texture analyzer [30], [88], [97]. The storage modulus (G′) and loss modulus (G′′) reflect the elasticity and viscosity of the dough, respectively. The loss tangent (tan δ) is the ratio of G′′ to G′, reflecting the relative magnitude of viscosity and elasticity. In various dough systems such as gluten-free bread dough, noodle dough, and reconstituted flour dough, an increase in the BS content has been observed to elevate both G′ and G′′, while decrease tan δ [30], [88], [95]. This indicates that BS can enhance the dough's elasticity. Moreover, the addition of BS at a proportion of 5% increases DDT, ST, hardness, and springiness of dough, indicating an improvement in the strength and stability of dough [30]. Creep and recovery tests are commonly used to evaluate dough stability and resistance to deformation. In the creep phase, the dough sheets with higher BS content show lower maximum creep compliance (Jmax), indicating stronger resistance to deformation. In the recovery phase, the relative elastic part of Jmax increases with the addition of BS, while the relative viscous part of Jmax decreases, indicating that increasing the content of BS can enhance the elasticity of dough [88]. The improvements in dough properties are due to the small size ranges of BS granules, which facilitate close packing into the gluten network, enhancing gluten-starch physical interactions, and leading to a more stable network structure. Additionally, BS can promote the polymerization of gluten protein and reduce the fluidity of water in dough. In contrast, AS granules are more likely to be exposed from the gluten network, resulting in reduced filling degree. Furthermore, a high proportion of AS decreases the covalent (disulfide bonds) and non-covalent interactions (ionic bonds and hydrogen bonds) of dough [30], [46]. Different contributions of AS and BS to the dough rheological properties need to be further investigated, and their different effects on the end-use of dough and the quality of final products need to be further clarified.
6. Effect of different-sized starch granules on quality of final products
The physicochemical properties of starch, particularly its size distribution, are key factors that influence the quality of final products, including bread, noodles, and Chinese steamed bread. Recent studies have shown increasing interest in this area. For example, bread with an AS/BS ratio of 3:1 exhibits the highest specific volume [95], in agreement with the previous findings that breads with 25–35% [103] or 30% [104] BS content (by weight) exhibit higher volumes. However, these results are inconsistent with the findings that the dough with high BS content shows higher G′, G′′, DDT, and ST, where other factors may influence bread volume, such as water absorption, gas volume during fermentation, and changes in starch properties during baking [95]. When the AS/BS ratio is 1:1, raw noodles exhibit the highest binding strength and least immobilized water, whereas cooked noodles show the greatest hardness, chewiness, and resilience, but lowest cooking loss and water absorption [105]. Furthermore, adding 20% (by weight) BS to native flour increased the density of wet and dried noodles, along with cooking yield, and decreased cooking loss [106]. These effects can be attributed to the formation of a stronger gluten network during kneading and sheeting with higher BS content [106]. For raw white noodles, increasing the BS content improves the color, viscoelasticity, and smoothness of raw white noodles, and those noodles made from flours reconstituted with 30–40% BS exhibit moderate firmness [101]. The total score of Chinese steamed bread can be improved by increasing BS content, and the Chinese steamed bread made from the reconstituted flours with 30% BS content exhibits the best crumb structure and highest total score [101]. It is essential to consider that the quality criteria of flour for different food products such as bread, noodles, and Chinese steamed bread vary significantly. Accordingly, the optimal ratio of AS to BS is significantly different for the various end-use. Moreover, the optimal AS/BS ratio for making same food product differed among multiple studies, which may be attributed to the variations in content and strength of flour glutens resulting from different genetic backgrounds or production procedures [107]. Taken together, adjusting the size distribution of starches from different flour backgrounds can improve the quality of bread, noodles, and Chinese steamed bread. Further studies are required to investigate the optimal range of different-sized starch granules in the flour or dough systems with varying gluten strength specific to various food applications.
7. Possible strategies for coordinately improving yield and quality of wheat
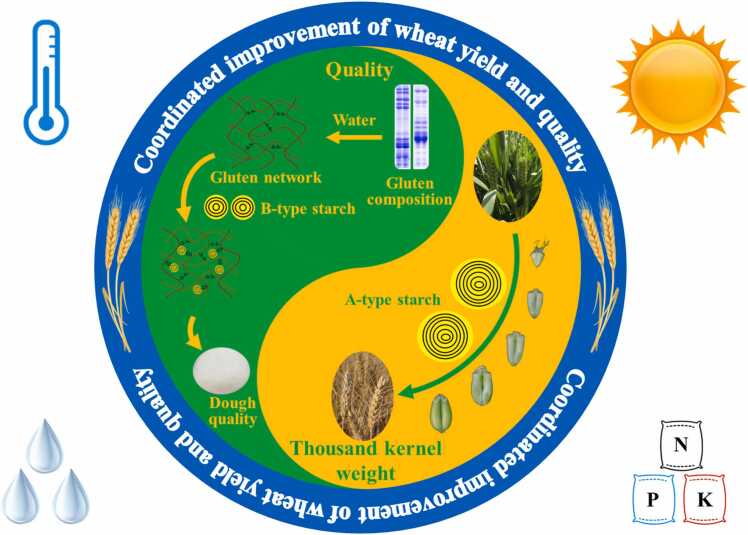
Though wheat processing quality is crucial, it cannot come at the expense of yield in wheat breeding. The balance between quality and yield in wheat is essentially a trade-off between chemical compositions, such as starch and protein. Therefore, coordinated improvements in wheat yield and quality can be achieved by optimizing the content and compositions of protein and starch through breeding and cultivation techniques (Fig. 6). Firstly, the content and compositions of protein are the key factors determining the dough rheological properties and processing quality. However, reversing the negative correlation between protein content and yield is challenging [108], limiting strategies for improving processing properties by increasing protein content. HMW-GSs, such as Dx5, Dy10, and Bx7OE, play an essential role in governing the strength and stability of dough, making them valuable targets in wheat breeding [11]. On the other hand, gliadin, albumin, and globulin have little or negative impact on the processing properties of wheat [6]. The molecular biology techniques, such as gene editing, provide opportunities to reduce these proteins and thus improve the quality of dough. For example, the knockout mutants of γ-gliadin γ1–1D and γ2–1B have shown higher SDS-sedimentation volume, gluten index, and longer dough stability time compared to their wild type [109]. Secondly, as mentioned earlier, the size distribution of starch is a key factor affecting the dough rheological properties and the quality of flour-based food. Recent study has shown that adding 5% BS to native flour reduces protein content while optimizing dough micro-structure and enhancing gluten-starch interactions, thereby improving dough strength and stability [30]. This suggests that the dough quality can be improved by optimizing the size distribution of starch, which can avoid the yield loss caused by increasing protein content. In addition, wheat yield is influenced by factors such as thousand-kernel weight (TKW), grain number per spike, and spike number per unit area, with TKW being a primary target trait for improving grain yield in modern wheat breeding [110]. Among grain filling substances, starch is the most abundant compound in the endosperm, and therefore has the greatest effect on grain weight and yield [14]. The larger size and greater weight of AS may contribute to the improvement of grain weight. Achieving a balance between AS and BS content in wheat grains may help the breeders to break the trade-off between yield and quality of wheat. Lastly, environmental factors, such as light, temperature, water and fertilizer also affect wheat yield and quality. For example, compared with the white light regimen provided by fluorescent lamps, the RedFR regimen consisting of red light and far-red light has been shown to lead to the higher grain yield, Zeleny sedimentation, and ratio of HMW-GS to LMW-GS [111]. Integrated crop and soil management strategies, including improved newer cultivars, timely sowing date, appropriate plant density and fertilizer rates, and adequate soil nutrients supply, can be applied in the complex and changeable wheat production environments to improve yield and protein content synchronously [112]. These two cases indicate the potential to improve the yield and quality of wheat by adopting appropriate cultivation measures. Further improvement in wheat yield and quality can be achieved when the factors of grain components are fully taken into consideration.
Fig. 6.
Schematic diagram of coordinated improvement of wheat yield and quality.
8. Conclusions and prospects
Considerable progress has been made in understanding of the biosynthesis, composition, structure, and function of different-sized wheat starch granules. The AS and BS granules are produced in two separate granule-initiation events and they can be isolated using sedimentation or centrifugation. The variations in physicochemical properties between AS and BS are mainly attributed to their CLD of AP. Specifically, AS exhibits lower content of A chains, but higher content of B chains, leading to higher relative crystallinity, ΔH and FV. Moreover, the distinct contributions of AS and BS to dough rheological properties are primarily influenced by their sizes. The small-sized BS granules can be closely packed into gluten network, enhancing gluten-starch physical interactions, promoting the polymerization of gluten proteins, reducing the fluidity of water in dough, and thereby improving the elasticity, strength, resistance to deformation, and stability of dough. On the contrary, AS granules are more likely to disengage from gluten network, resulting in weakened covalent and non-covalent interactions, and adversely affecting dough rheological properties. Additionally, adjusting the size distribution of starch offers an avenue for improving the quality of wheat varieties and flour-based food products.
Although extensive research has been carried out on AS and BS, numerous opportunities remain for further exploration and utilization of wheat starches with various sizes. Potential areas for future research include: (1) characterization of the genetic basis for the initiation and development of starch granules, and the genetic manipulation of starch size distribution, (2) investigation of the AM chain length distribution, and its correlations with other starch properties, including morphology, gelatinization, pasting, and digestive properties, (3) elucidation of the mechanisms by which AS and BS affecting the quality of final products, including bread, noodles, biscuits and Chinese steamed bread, along with exploration of methods for producing high-quality end products, (4) characterization of the effects of starch size distribution on wheat yield and development of the new wheat varieties with optimized AS and BS ratio to achieve high yield and quality. Extensive and in-depth studies on different-sized starches can address people's growing demand for nutritious and healthy food while advancing food industry. Understanding the contribution of starch size distribution to wheat quality can assist breeders to respond to market demands by breeding new wheat varieties. With continued research, we can pave the way for innovative improvements in wheat processing and enhance the overall quality of food products.
CRediT authorship contribution statement
Lei Guo: Investigation, Literature analysis, Writing – original draft, Writing – review & editing. Heng Chen: Investigation, Literature analysis, Writing – original draft. Yizhi Zhang: Investigation, Literature analysis. Shuai Yan: Investigation, Literature analysis. Xueyan Chen: Funding acquisition, Project administration, Writing – review & editing. Xin Gao: Funding acquisition, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Mr. Yun Jiang for his assistance in language corrections. We would like to thank Prof Jianjun Liu and Prof Haosheng Li at Shandong Academy of Agricultural Sciences for their helpful comments that improved this article. This work was supported by the Taishan Scholars Program (tsqnz20221161), Taishan Industrial Experts Programme (LJNY202006), Key Research and Development Program of Shandong Province (Major Science and Technology Innovation Project) (2021LZGC013 and 2021LZGC025), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2022E01).
Contributor Information
Xueyan Chen, Email: chenxueyan@shandong.cn.
Xin Gao, Email: bestgaoxin@nwsuaf.edu.cn, bestgaoxin@126.com.
References
- 1.de Sousa T., Ribeiro M., Sabenca C., Igrejas G. The 10,000-year success story of wheat! Foods. 2021;10:2124. doi: 10.3390/foods10092124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazard B., Trafford K., Lovegrove A., Griffiths S., Uauy C., et al. Strategies to improve wheat for human health. Nat Food. 2020;1(8):475–480. doi: 10.1038/s43016-020-0134-6. [DOI] [PubMed] [Google Scholar]
- 3.Shewry P.R., Hey S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015;4(3):178–202. doi: 10.1002/fes3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kundu M., Khatkar B.S., Gulia N. Assessment of chapatti quality of wheat varieties based on physicochemical, rheological and sensory traits. Food Chem. 2017;226:95–101. doi: 10.1016/j.foodchem.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Goesaert H., Brijs K., Veraverbeke W.S., Courtin C.M., Gebruers K., et al. Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol. 2005;16:12–30. [Google Scholar]
- 6.Wang D., Zhang K., Dong L., Dong Z., Li Y., et al. Molecular genetic and genomic analysis of wheat milling and end-use traits in China: Progress and perspectives. Crop J. 2018;6(1):68–81. [Google Scholar]
- 7.Delcour J.A., Joye I.J., Pareyt B., Wilderjans E., Brijs K., et al. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu Rev Food Sci Technol. 2012;3:469–492. doi: 10.1146/annurev-food-022811-101303. [DOI] [PubMed] [Google Scholar]
- 8.Ortolan F., Steel C.J. Protein characteristics that affect the quality of vital wheat gluten to be used in baking: a review. Compr Rev Food Sci Food Saf. 2017;16(3):369–381. doi: 10.1111/1541-4337.12259. [DOI] [PubMed] [Google Scholar]
- 9.Veraverbeke W.S., Delcour J.A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit Rev Food Sci Nutr. 2002;42(3):179–208. doi: 10.1080/10408690290825510. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M., Jia R., Ma M., Yang T., Sun Q., et al. Versatile wheat gluten: functional properties and application in the food-related industry. Crit Rev Food Sci Nutr. 2022:1–17. doi: 10.1080/10408398.2022.2078785. [DOI] [PubMed] [Google Scholar]
- 11.Rasheed A., Xia X., Yan Y., Appels R., Mahmood T., et al. Wheat seed storage proteins: advances in molecular genetics, diversity and breeding applications. J Cereal Sci. 2014;60(1):11–24. [Google Scholar]
- 12.Morita E., Kunie K., Matsuo H. Food-dependent exercise-induced anaphylaxis. J Dermatol Sci. 2007;47(2):109–117. doi: 10.1016/j.jdermsci.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R., Mukherjee S., Ayele B.T. Molecular aspects of sucrose transport and its metabolism to starch during seed development in wheat: a comprehensive review. Biotechnol Adv. 2018;36(4):954–967. doi: 10.1016/j.biotechadv.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Shevkani K., Singh N., Bajaj R., Kaur A. Wheat starch production, structure, functionality and applications-a review. Int J Food Sci Technol. 2017;52(1):38–58. [Google Scholar]
- 15.Wei C., Zhang J., Chen Y., Zhou W., Xu B., et al. Physicochemical properties and development of wheat large and small starch granules during endosperm development. Acta Physiol Plant. 2010;32(5):905–916. [Google Scholar]
- 16.Yin Y.-a, Qi J.-c, Li W.-h, Cao L.-p, Wang Z.-b. Formation and developmental characteristics of A- and B-type starch granules in wheat endosperm. J Integr Agric. 2012;11(1):73–81. [Google Scholar]
- 17.Li M., Daygon V.D., Solah V., Dhital S. Starch granule size: does it matter? Crit Rev Food Sci Nutr. 2023;63(19):3683–3703. doi: 10.1080/10408398.2021.1992607. [DOI] [PubMed] [Google Scholar]
- 18.Punia S. Barley starch: Structure, properties and in vitro digestibility - a review. Int J Biol Macromol. 2020;155:868–875. doi: 10.1016/j.ijbiomac.2019.11.219. [DOI] [PubMed] [Google Scholar]
- 19.Xie J., Hong Y., Gu Z., Cheng L., Li Z., et al. Highland barley starch: structures, properties, and applications. Foods. 2023;12:387. doi: 10.3390/foods12020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song L., Zhao L., Liu Z., Li L., Zheng J., et al. Effects of exogenous starch on the structural-thermal properties of gluten in wheat with HMW-GS variations at Glu-D1 locus. Food Res Int. 2020;130 doi: 10.1016/j.foodres.2019.108950. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Yue Q., Liu C., Zheng X., Hong J., et al. Comparative study of rheology and steamed bread quality of wheat dough and gluten: starch doughs. J Food Process Preserv. 2021;45(2) [Google Scholar]
- 22.Lindeboom N., Chang P.R., Tyler R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch/Stärke. 2004;56(34):89–99. [Google Scholar]
- 23.Gao X., Tong J., Guo L., Yu L., Li S., et al. Influence of gluten and starch granules interactions on dough mixing properties in wheat (Triticum aestivum L.) Food Hydrocoll. 2020;106 [Google Scholar]
- 24.Yu L., Guo L., Liu Y., Ma Y., Zhu J., et al. Novel parameters characterizing size distribution of A and B starch granules in the gluten network: effects on dough stability in bread wheat. Carbohydr Polym. 2021;257 doi: 10.1016/j.carbpol.2021.117623. [DOI] [PubMed] [Google Scholar]
- 25.Eliasson A.C., Karlsson R. Gelatinization properties of different size classes of wheat starch granules measured with differential scanning calorimetry. Starch/Stärke. 1983;35:130–133. [Google Scholar]
- 26.Soulaka A.B., Morrison W.R. The amylose and lipid contents, dimensions, and gelatinisation characteristics of some wheat starches and their A- and B-granule fractions. J Sci Food Agric. 1985;36:709–718. [Google Scholar]
- 27.Zhou T., Zhang L., Liu Q., Liu W., Hu H. Rheological behaviors and physicochemical changes of doughs reconstituted from potato starch with different sizes and gluten. Food Res Int. 2021;145 doi: 10.1016/j.foodres.2021.110397. [DOI] [PubMed] [Google Scholar]
- 28.Peng M., Gao M., Abdel-Aal E.-S.M., Hucl P., Chibbar R.N. Separation and characterization of A- and B-type starch granules in wheat endosperm. Cereal Chem. 1999;76(3):375–379. [Google Scholar]
- 29.Chen L., Ma R., Zhang Z., Huang M., Cai C., et al. Comprehensive investigation and comparison of surface microstructure of fractionated potato starches. Food Hydrocoll. 2019;89:11–19. [Google Scholar]
- 30.Guo L., Wang Q., Chen H., Wu D., Dai C., et al. Moderate addition of B-type starch granules improves the rheological properties of wheat dough. Food Res Int. 2022;160 doi: 10.1016/j.foodres.2022.111748. [DOI] [PubMed] [Google Scholar]
- 31.Shang J., Li L., Zhao B., Liu M., Zheng X. Comparative studies on physicochemical properties of total, A- and B-type starch from soft and hard wheat varieties. Int J Biol Macromol. 2020;154:714–723. doi: 10.1016/j.ijbiomac.2020.03.150. [DOI] [PubMed] [Google Scholar]
- 32.Briarty L.G., Hughes C.E., Evers A.D. The developing endosperm of wheat-A stereological analysis. Ann Bot. 1979;44(6):641–658. [Google Scholar]
- 33.Langeveld S.M.J., van Wijk R., Stuurman N., Kijne J.W., de Pater S. B‐type granule containing protrusions and interconnections between amyloplasts in developing wheat endosperm revealed by transmission electron microscopy and GFP expression. J Exp Bot. 2000;51(349):1357–1361. [PubMed] [Google Scholar]
- 34.Parker M.L. The relationship between A-type and B-type starch granules in the developing endosperm of wheat. J Cereal Sci. 1985;3(4):271–278. [Google Scholar]
- 35.Jeon J.-S., Ryoo N., Hahn T.-R., Walia H., Nakamura Y. Starch biosynthesis in cereal endosperm. Plant Physiol Bioch. 2010;48(6):383–392. doi: 10.1016/j.plaphy.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Bahaji A., Li J., Sánchez-López Á.M., Baroja-Fernández E., Muñoz F.J., et al. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol Adv. 2014;32(1):87–106. doi: 10.1016/j.biotechadv.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Peng M., Gao M., Båga M., Hucl P., Chibbar R.N. Starch-branching enzymes preferentially associated with A-type starch granules in wheat endosperm. Plant Physiol. 2000;124:265–272. doi: 10.1104/pp.124.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoddard F.L., Sarker R. Characterization of starch in Aegilops species. Cereal Chem. 2000;77(4):445–447. [Google Scholar]
- 39.Howard T., Rejab N.A., Griffiths S., Leigh F., Leverington-Waite M., et al. Identification of a major QTL controlling the content of B-type starch granules in Aegilops. J Exp Bot. 2011;62(6):2217–2228. doi: 10.1093/jxb/erq423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chia T., Adamski N.M., Saccomanno B., Greenland A., Nash A., et al. Transfer of a starch phenotype from wild wheat to bread wheat by deletion of a locus controlling B-type starch granule content. J Exp Bot. 2017;68(20):5497–5509. doi: 10.1093/jxb/erx349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chia T., Chirico M., King R., Ramirez-Gonzalez R., Saccomanno B., et al. A carbohydrate-binding protein, B-GRANULE CONTENT 1, influences starch granule size distribution in a dose-dependent manner in polyploid wheat. J Exp Bot. 2020;71(1):105–115. doi: 10.1093/jxb/erz405. [DOI] [PubMed] [Google Scholar]
- 42.Lu K.J., Pfister B., Jenny C., Eicke S., Zeeman S.C. Distinct functions of STARCH SYNTHASE 4 domains in starch granule formation. Plant Physiol. 2018;176(1):566–581. doi: 10.1104/pp.17.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malinova I., Alseekh S., Feil R., Fernie A.R., Baumann O., et al. Starch synthase 4 and plastidal phosphorylase differentially affect starch granule number and morphology. Plant Physiol. 2017;174(1):73–85. doi: 10.1104/pp.16.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawkins E., Chen J., Watson-Lazowski A., Ahn-Jarvis J., Barclay J.E., et al. STARCH SYNTHASE 4 is required for normal starch granule initiation in amyloplasts of wheat endosperm. New Phytol. 2021;230(6):2371–2386. doi: 10.1111/nph.17342. [DOI] [PubMed] [Google Scholar]
- 45.Li W., Shan Y., Xiao X., Luo Q., Zheng J., et al. Physicochemical properties of A- and B-starch granules isolated from hard red and soft red winter wheat. J Agric Food Chem. 2013;61(26):6477–6484. doi: 10.1021/jf400943h. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Liu C., Zheng X., Hong J., Bian K., et al. Interaction between A-type/B-type starch granules and gluten in dough during mixing. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129870. [DOI] [PubMed] [Google Scholar]
- 47.Tao H., Wang P., Wu F., Jin Z., Xu X. Particle size distribution of wheat starch granules in relation to baking properties of frozen dough. Carbohydr Polym. 2016;137:147–153. doi: 10.1016/j.carbpol.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert R.G., Witt T., Hasjim J. What is being learned about starch properties from multiple-level characterization. Cereal Chem. 2013;90(4):312–325. [Google Scholar]
- 49.Wang Q., Li L., Zheng X. A review of milling damaged starch: Generation, measurement, functionality and its effect on starch-based food systems. Food Chem. 2020;315 doi: 10.1016/j.foodchem.2020.126267. [DOI] [PubMed] [Google Scholar]
- 50.Kim H.-S., Huber K.C. Physicochemical properties and amylopectin fine structures of A- and B-type granules of waxy and normal soft wheat starch. J Cereal Sci. 2010;51(3):256–264. [Google Scholar]
- 51.Li W., Wu G., Luo Q., Jiang H., Zheng J., et al. Effects of removal of surface proteins on physicochemical and structural properties of A- and B-starch isolated from normal and waxy wheat. J Food Sci Technol. 2016;53(6):2673–2685. doi: 10.1007/s13197-016-2239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raeker M.Ö., Gaines C.S., Finney P.L., Donelson T. Granule size distribution and chemical composition of starches from 12 soft wheat cultivars. Cereal Chem. 1998;75(5):721–728. [Google Scholar]
- 53.Chi C., Li X., Huang S., Chen L., Zhang Y., et al. Basic principles in starch multi-scale structuration to mitigate digestibility: A review. Trends Food Sci Technol. 2021;109:154–168. [Google Scholar]
- 54.Li C., Hu Y., Huang T., Gong B., Yu W.W. A combined action of amylose and amylopectin fine molecular structures in determining the starch pasting and retrogradation property. Int J Biol Macromol. 2020;164:2717–2725. doi: 10.1016/j.ijbiomac.2020.08.123. [DOI] [PubMed] [Google Scholar]
- 55.Li C., Wu A., Yu W., Hu Y., Li E., et al. Parameterizing starch chain-length distributions for structure-property relations. Carbohydr Polym. 2020;241 doi: 10.1016/j.carbpol.2020.116390. [DOI] [PubMed] [Google Scholar]
- 56.Liu Q., Gu Z., Donner E., Tetlow I., Emes M. Investigation of digestibility in vitro and physicochemical properties of A- and B-type starch from soft and hard wheat flour. Cereal Chem. 2007;84(1):15–21. [Google Scholar]
- 57.Ao Z., Jane J.-l. Characterization and modeling of the A- and B-granule starches of wheat, triticale, and barley. Carbohydr Polym. 2007;67:46–55. [Google Scholar]
- 58.Salman H., Blazek J., Lopez-Rubio A., Gilbert E.P., Hanley T., et al. Structure–function relationships in A and B granules from wheat starches of similar amylose content. Carbohydr Polym. 2009;75(3):420–427. [Google Scholar]
- 59.Sun X., Sun Z., Saleh A.S.M., Zhao K., Ge X., et al. Understanding the granule, growth ring, blocklets, crystalline and molecular structure of normal and waxy wheat A- and B- starch granules. Food Hydrocoll. 2021;121 [Google Scholar]
- 60.Rodriguez-Garcia M.E., Hernandez-Landaverde M.A., Delgado J.M., Ramirez-Gutierrez C.F., Ramirez-Cardona M., et al. Crystalline structures of the main components of starch. Curr Opin Food Sci. 2021;37:107–111. [Google Scholar]
- 61.Sun Y., Wang M., Ma S., Wang H. Physicochemical characterization of rice, potato, and pea starches, each with different crystalline pattern, when incorporated with Konjac glucomannan. Food Hydrocoll. 2020;101 [Google Scholar]
- 62.Ouyang Q., Wang X., Xiao Y., Luo F., Lin Q., et al. Structural changes of A-, B- and C-type starches of corn, potato and pea as influenced by sonication temperature and their relationships with digestibility. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129858. [DOI] [PubMed] [Google Scholar]
- 63.Zhang B., Li X., Liu J., Xie F., Chen L. Supramolecular structure of A- and B-type granules of wheat starch. Food Hydrocoll. 2013;31(1):68–73. [Google Scholar]
- 64.Huang J., Wang Z., Fan L., Ma S. A review of wheat starch analyses: methods, techniques, structure and function. Int J Biol Macromol. 2022;203:130–142. doi: 10.1016/j.ijbiomac.2022.01.149. [DOI] [PubMed] [Google Scholar]
- 65.Blazek J., Gilbert E.P. Effect of enzymatic hydrolysis on native starch granule structure. Biomacromolecules. 2010;11:3275–3289. doi: 10.1021/bm101124t. [DOI] [PubMed] [Google Scholar]
- 66.Li Q., Wu Q.Y., Jiang W., Qian J.Y., Zhang L., et al. Effect of pulsed electric field on structural properties and digestibility of starches with different crystalline type in solid state. Carbohydr Polym. 2019;207:362–370. doi: 10.1016/j.carbpol.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Obadi M., Qi Y., Xu B. Highland barley starch (Qingke): structures, properties, modifications, and applications. Int J Biol Macromol. 2021;185:725–738. doi: 10.1016/j.ijbiomac.2021.06.204. [DOI] [PubMed] [Google Scholar]
- 68.Chiotelli E., Le Meste M. Effect of small and large wheat starch granules on thermomechanical behavior of starch. Cereal Chem. 2002;79(2):286–293. [Google Scholar]
- 69.Glaring M.A., Koch C.B., Blennow A. Genotype-specific spatial distribution of starch molecules in the starch granule: a combined CLSM and SEM approach. Biomacromolecules. 2006;7:2310–2320. doi: 10.1021/bm060216e. [DOI] [PubMed] [Google Scholar]
- 70.Zhang B., Zhao K., Su C., Gong B., Ge X., et al. Comparing the multi-scale structure, physicochemical properties and digestibility of wheat A- and B-starch with repeated versus continuous heat-moisture treatment. Int J Biol Macromol. 2020;163:519–528. doi: 10.1016/j.ijbiomac.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Kim H.-S., Huber K.C. Channels within soft wheat starch A- and B-type granules. J Cereal Sci. 2008;48(1):159–172. [Google Scholar]
- 72.Naguleswaran S., Li J., Vasanthan T., Bressler D., Hoover R. Amylolysis of large and small granules of native triticale, wheat and corn starches using a mixture of α-amylase and glucoamylase. Carbohydr Polym. 2012;88(3):864–874. [Google Scholar]
- 73.Wani A.A., Singh P., Shah M.A., Schweiggert-Weisz U., Gul K., et al. Rice starch diversity: effects on structural, morphological, thermal, and physicochemical properties-a review. Compr Rev Food Sci F. 2012;11(5):417–436. [Google Scholar]
- 74.Ral J.-P., Cavanagh C.R., Larroque O., Regina A., Morell M.K. Structural and molecular basis of starch viscosity in hexaploid wheat. J Agric Food Chem. 2008;56:4188–4197. doi: 10.1021/jf800124f. [DOI] [PubMed] [Google Scholar]
- 75.Arp C.G., Correa M.J., Ferrero C. Resistant starches: a smart alternative for the development of functional bread and other starch-based foods. Food Hydrocoll. 2021;121 [Google Scholar]
- 76.Li M.N., Xie Y., Chen H.Q., Zhang B. Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A- and B-type starch granules. Food Chem. 2019;272:523–529. doi: 10.1016/j.foodchem.2018.08.079. [DOI] [PubMed] [Google Scholar]
- 77.Han N., Fan J.-L., Chen N., Chen H.-Q. Effect of ball milling treatment on the structural, physicochemical and digestive properties of wheat starch, A- and B-type starch granules. J Cereal Sci. 2022;104 [Google Scholar]
- 78.Zhang K., Zhao D., Guo D., Tong X., Zhang Y., et al. Physicochemical and digestive properties of A- and B-type granules isolated from wheat starch as affected by microwave-ultrasound and toughening treatment. Int J Biol Macromol. 2021;183:481–489. doi: 10.1016/j.ijbiomac.2021.04.180. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z., Zhang Y., Wu Y., Ouyang J. Factors influencing the starch digestibility of starchy foods: a review. Food Chem. 2023;406 doi: 10.1016/j.foodchem.2022.135009. [DOI] [PubMed] [Google Scholar]
- 80.Hong J., An D., Zeng X.-A., Han Z., Zheng X., et al. Behaviors of large A-type and small B-type wheat starch granules esterified by conventional and pulsed electric fields assisted methods. Int J Biol Macromol. 2020;155:516–523. doi: 10.1016/j.ijbiomac.2020.03.184. [DOI] [PubMed] [Google Scholar]
- 81.Tester R.F., Qi X., Karkalas J. Hydrolysis of native starches with amylases. Anim Feed Sci Technol. 2006;130(1–2):39–54. [Google Scholar]
- 82.Cao X., Tong J., Ding M., Wang K., Wang L., et al. Physicochemical properties of starch in relation to rheological properties of wheat dough (Triticum aestivum L.) Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.125000. [DOI] [PubMed] [Google Scholar]
- 83.Kaur A., Shevkani K., Katyal M., Singh N., Ahlawat A.K., et al. Physicochemical and rheological properties of starch and flour from different durum wheat varieties and their relationships with noodle quality. J Food Sci Technol. 2016;53(4):2127–2138. doi: 10.1007/s13197-016-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCann T.H., Homer S.H., Øiseth S.K., Day L., Newberry M., et al. High amylose wheat starch increases the resistance to deformation of wheat flour dough. J Cereal Sci. 2018;79:440–448. [Google Scholar]
- 85.Li H., Ma Y., Pan Y., Yu L., Tian R., et al. Starch other than gluten may make a dominant contribution to wheat dough mixing properties: a case study on two near-isogenic lines. LWT. 2021;152 [Google Scholar]
- 86.Hu X., Cheng L., Hong Y., Li Z., Li C., et al. An extensive review: How starch and gluten impact dough machinability and resultant bread qualities. Crit Rev Food Sci Nutr. 2023;63(13):1930–1941. doi: 10.1080/10408398.2021.1969535. [DOI] [PubMed] [Google Scholar]
- 87.Liu T., Gao X., Li L., Du D., Cheng X., et al. Effects of HMW-GS at Glu-B1 locus on the polymerization of glutenin during grain development and on the secondary and micro-structures of gluten in wheat (Triticum aestivum L.) J Cereal Sci. 2016;72:101–107. [Google Scholar]
- 88.Shang J., Zhao B., Liu C., Li L., Hong J., et al. Impact of wheat starch granule size on viscoelastic behaviors of noodle dough sheet and the underlying mechanism. Food Hydrocoll. 2023;134 [Google Scholar]
- 89.Jia R., Zhang M., Yang T., Ma M., Sun Q., et al. Evolution of the morphological, structural, and molecular properties of gluten protein in dough with different hydration levels during mixing. Food Chem X. 2022;15 doi: 10.1016/j.fochx.2022.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M., Liu C., Hong J., Zheng X., Lu Y., et al. Influence of wheat starch on rheological, structural and physico‐chemical properties gluten–starch dough during mixing. Int J Food Sci Technol. 2021;57(4):2069–2079. [Google Scholar]
- 91.Rosell C.M., Foegeding A. Interaction of hydroxypropylmethylcellulose with gluten proteins: Small deformation properties during thermal treatment. Food Hydrocoll. 2007;21(7):1092–1100. [Google Scholar]
- 92.Wang P., Chen H., Mohanad B., Xu L., Ning Y., et al. Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014;39:187–194. [Google Scholar]
- 93.Georget D.M.R., Belton P.S. Effects of temperature and water content on the secondary structure of wheat gluten studied by FTIR spectroscopy. Biomacromolecules. 2006;7:469–475. doi: 10.1021/bm050667j. [DOI] [PubMed] [Google Scholar]
- 94.Gao X., Liu T., Yu J., Li L., Feng Y., et al. Influence of high-molecular-weight glutenin subunit composition at Glu-B1 locus on secondary and micro structures of gluten in wheat (Triticum aestivum L.) Food Chem. 2016;197:1184–1190. doi: 10.1016/j.foodchem.2015.11.085. [DOI] [PubMed] [Google Scholar]
- 95.Roman L., Cal Edl, Gomez M., Martinez M.M. Specific ratio of A-to B-type wheat starch granules improves the quality of gluten-free breads: optimizing dough viscosity and pickering stabilization. Food Hydrocoll. 2018;82:510–518. [Google Scholar]
- 96.Martínez M.M., Gómez M. Rheological and microstructural evolution of the most common gluten-free flours and starches during bread fermentation and baking. J Food Eng. 2017;197:78–86. [Google Scholar]
- 97.Guo L., Yu L., Tong J., Zhao Y., Yang Y., et al. Addition of Aegilops geniculata 1Ug chromosome improves the dough rheological properties by changing the composition and micro-structure of gluten. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129850. [DOI] [PubMed] [Google Scholar]
- 98.Bernklau I., Lucas L., Jekle M., Becker T. Protein network analysis-A new approach for quantifying wheat dough microstructure. Food Res Int. 2016;89:812–819. doi: 10.1016/j.foodres.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Gao X., Liu T., Ding M., Wang J., Li C., et al. Effects of HMW-GS Ax1 or Dx2 absence on the glutenin polymerization and gluten micro structure of wheat (Triticum aestivum L.) Food Chem. 2018;240:626–633. doi: 10.1016/j.foodchem.2017.07.165. [DOI] [PubMed] [Google Scholar]
- 100.Yu L., Ma Y., Zhao Y., Rehman A.U., Guo L., et al. Interaction of B-type starch with gluten skeleton improves wheat dough mixing properties by stabilizing gluten micro-structure. Food Chem. 2022;371 doi: 10.1016/j.foodchem.2021.131390. [DOI] [PubMed] [Google Scholar]
- 101.Guo Q., He Z., Xia X., Qu Y., Zhang Y. Effects of wheat starch granule size distribution on qualities of Chinese steamed bread and raw white noodles. Cereal Chem. 2014;91(6):623–630. [Google Scholar]
- 102.Wang R., Li M., Chen S., Hui Y., Tang A., et al. Effects of flour dynamic viscosity on the quality properties of buckwheat noodles. Carbohydr Polym. 2019;207:815–823. doi: 10.1016/j.carbpol.2018.09.048. [DOI] [PubMed] [Google Scholar]
- 103.Soulaka A.B., Morrison W.R. The bread baking quality of six wheat starches differing in composition and physical properties. J Sci Food Agric. 1985;36(8):719–727. [Google Scholar]
- 104.Park S.-H., Chung O.K., Seib P.A. Effects of varying weight ratios of large and small wheat starch granules on experimental straight-dough bread. Cereal Chem. 2005;82(2):166–172. [Google Scholar]
- 105.Yan H.-L., Lu Q.-Y. Effect of A- and B-granules of wheat starch on Chinese noodle quality. J Cereal Sci. 2020;91 [Google Scholar]
- 106.Hong J., An D., Wang M., Liu C., Buckow R., et al. Wheat noodles enriched with A‐type and/or B‐type wheat starch: physical, thermal and textural properties of dough sheet and noodle samples from different noodle‐making process. Int J Food Sci Technol. 2021;56(6):3111–3122. [Google Scholar]
- 107.Park S.-H., Wilson J.D., Seabourn B.W. Starch granule size distribution of hard red winter and hard red spring wheat: its effects on mixing and breadmaking quality. J Cereal Sci. 2009;49(1):98–105. [Google Scholar]
- 108.Mosleth E.F., Wan Y., Lysenko A., Chope G.A., Penson S.P., et al. A novel approach to identify genes that determine grain protein deviation in cereals. Plant Biotechnol J. 2015;13(5):625–635. doi: 10.1111/pbi.12285. [DOI] [PubMed] [Google Scholar]
- 109.Liu D., Yang H., Zhang Z., Chen Q., Guo W., et al. An elite γ‐gliadin allele improves end‐use quality in wheat. New Phytol. 2023;239(1):87–101. doi: 10.1111/nph.18722. [DOI] [PubMed] [Google Scholar]
- 110.Ma X., Ouyang X., Liu D., Zhang A. The 218th amino acid change of Ser to Ala in TaAGPS-7A increases enzyme activity and grain weight in bread wheat. Crop J. 2023;11(1):140–147. [Google Scholar]
- 111.Monostori I., Heilmann M., Kocsy G., Rakszegi M., Ahres M., et al. LED lighting - modification of growth, metabolism, yield and flour composition in wheat by spectral quality and Intensity. Front Plant Sci. 2018;9:605. doi: 10.3389/fpls.2018.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li C., Yang J., Li Z., Wang X., Guo Z., et al. Integrating crop and soil nutrient management for higher wheat grain yield and protein concentration in dryland areas. Eur J Agron. 2023;147 [Google Scholar]