Abstract
Interrelationships between periodontal infection and systemic conditions such as cardiovascular disease, adverse pregnancy outcomes, and head-and-neck cancer have become increasingly appreciated in recent years. Periodontitis is associated with cardiovascular disease (CVD) and, experimentally, with measures of atherosclerosis and endothelial dysfunction. Periodontal therapy may reduce atherosclerotic changes and improve endothe-lial function. Preliminary findings suggest a role for the genetic locus ANRIL in the pathobiology of both CVD and periodontitis. Periodontal pathogens induce anticardiolipin in periodontitis patients by molecular mimicry of the serum protein ß-2 glyco-proteinI. These antibodies have biological and pathological activities consistent with those reported for other infection-induced antiphospholipid antibodies. Anticardiolipin may explain some of the observed associations between periodontitis and systemic conditions such as CVD and adverse pregnancy outcomes. The oral commensal Fusobacterium nucleatum (Fn) becomes pathogenic on migration to extra-oral sites. Fn infection of the fetal-placental unit has been linked to pregnancy complications, including preterm birth, stillbirth, and earlyonset neonatal sepsis. Reagents aimed at inhibiting or resolving inflammatory responses may be used to treat or prevent pregnancy complications due to bacterial infection. Chronic periodontitis may be independently associated with head-and-neck squamous cell carcinoma (HNSCC) through direct toxic effects of bacteria and their products, and/or through indirect effects of inflammation. Additionally, chronic periodontitis may facilitate the acquisition and persistence of oral HPV infection, a recently emerged risk factor for HNSCC.
Keywords: periodontitis, cardiovascular diseases, molecular mimicry, anticardiolipin antibodies, pregnancy complications, head-and-neck neoplasms
Introduction
The role of periodontal disease in the development and/or progression of systemic diseases and its potential contribution as a risk factor for such conditions have been the subject of much interest and investigation in recent years. Particular attention has been paid to the relationship between periodontitis and cardiovascular disease (CVD), given the high prevalence and the social and economic impact of cardiovascular events. There are also accumulating data supporting a possible relationship between maternal periodontal disease and adverse pregnancy outcomes.
Periodontal pathogens have long been implicated in the induction of systemic diseases, and ongoing research is revealing some of the direct and indirect mechanisms by which they are thought to induce or contribute to systemic disease processes.
In this article, evidence linking periodontal disease to systemic diseases, such as CVD and pregnancy complications, is described, and potential underlying pathogenic mechanisms are explored. The possible influence of periodontitis on the development of head-and-neck squamous cell carcinoma is also discussed.
Periodontitis and Cardiovascular Disease: Systemic, Genetic, and Mechanistic Connections
Cardiovascular diseases (CVD) are caused mostly by atherosclerosis. Atherosclerosis typically leads to the highly prevalent myocardial infarction (MI) or the less prevalent cerebrovascular accident (stroke). Atherogenesis results in endothelial dysfunction, arterial stiffness, reduction of blood vessel lumen size, high blood pressure, and, ultimately, ischemic events. Preliminary findings implicate the possible involvement of the genetic locus ANRIL in the pathobiology of both CVD and periodontitis.
Periodontitis Is Associated with CVD
A strong association between periodontitis and CVD is supported by evidence from numerous cross-sectional and longitudinal studies. In 2009, the editors of the American Journal of Cardiology and the Journal of Periodontology wrote a joint consensus report and concluded that the association between periodontitis and cardiovascular disease really exists (Friedewald et al., 2009). Evidence for how periodontitis could play an etio-logical role in CVD has been recently reviewed (Schenkein and Loos, 2013).
Measurement of Surrogate Markers of Atherosclerosis Associated with Periodontitis
The association between periodontitis and atherosclerosis has been evaluated by several indirect measures of atherosclerosis.
Periodontitis and Intima Media Thickness (IMT)
The IMT of the larger arteries that are relatively superficially positioned (e.g., carotids, brachial arteries) can be measured non-invasively by ultrasound. An increase in IMT has been shown to predict cardiovascular events, and the IMT measurements of the carotid arteries are often used as surrogate clinical markers for the extent of atherosclerosis (Greenland et al., 2010). The IMT of the carotid arteries in relation to periodontitis has been assessed in 19 studies, 11 of which found a higher IMT in periodontitis patients. After statistical adjustments for potential confounding factors, the reported association between peri-odontitis and increased IMT remained significant in nine studies.
Periodontitis and Flow-mediated Dilatation (FMD)
Flow-mediated dilatation (FMD) is a non-invasive atherosclerotic parameter evaluating endothelial function; it measures the % dilatation of the brachial artery in response to pharmacological and physiological stimuli (Anderson, 2006). Six case-control studies evaluated FMD in relation to periodontitis. Nearly all studies reported a significant endothelial dysfunction in peri-odontitis patients. However, a relatively large variation in endo-thelial function can be detected (2.35-12.4% reduced endothelium-dependent dilation [EDD] in individuals with peri-odontitis compared with control individuals).
Periodontitis and Pulse-wave Velocity (PWV)
Recently, arterial stiffness (AS), as assessed by pulse-wave velocity (PWV), has been examined to determine its relation to CVD. PWV is a reproducible non-invasive measure of large artery stiffness and has emerged as a novel biomarker for CVD (Graham et al., 2007). AS represents a measure of whole arterial function, determined by both endothelial function and that of the intima, media, and adventitia of arteries. PWV has been established as a clinical parameter for the prediction of cardiovascular mortality and morbidity, independent of traditional cardiovascular risk factors (Vlachopoulos et al., 2010).
Three cross-sectional studies determined PWV in systemically diseased individuals with and without periodontitis. No significant associations between periodontitis and PWV were found after adjustment for potential confounders. The prevalence of severe periodontitis was significantly higher in individuals with a PWV ≥ 14 m/sec, compared with those with a lower PWV. However, after adjustment for potential confounding factors, no significant difference remained. Therefore, to date, no clear association has been observed between periodontitis and PWV.
Periodontal Intervention and Arterial Function
Interestingly, non-surgical periodontal treatment has been shown to improve arterial function and/or reduce severity of atherosclerosis (Tables 1 and 2). Two studies noted no difference in IMT between baseline and 3 mos after periodontal therapy (Seinost et al., 2005; Piconi et al., 2009). However, a longer follow-up of 6 and 12 mos revealed a significant reduction in IMT compared with baseline (Piconi et al., 2009). Thus, it is likely that the regeneration rate in the intima-medial layer is relatively slow and is clinically detectable only 6 mos after intervention.
Table 1.
Studies Evaluating the Effect of Non-surgical Periodontal Therapy on the Cardiovascular and/or Endothelial Function System in Individuals with Periodontitis: Duration, Population, and Periodontal Measures (relevant studies identified by literature review)
| Study Duration | Study Population | Periodontal Definition | |
|---|---|---|---|
| Mercanoglu et al., 2004 | 6 wks | 28; systemically healthy | CAL, PPD, GI, PI |
| Seinost et al., 2005 | 3 mos | 30; systemically healthy | CAL, PPD, GI, PI |
| Blum et al., 2007 | 3 mos | 13; systemically healthy | CAL, PPD, BOP |
| Elter et al., 2006 | 1 mo | 22; systemically healthy | CAL, PPD |
| Li et al., 2011 | 3 mos | 25; systemically healthy | CAL and PPD (interproximal), BOP, PI |
| Tonetti et al., 2007 | 6 mos | 59; systemically healthy | PPD |
| Higashi et al., 2008 | 6 mos | 32; systemically healthy | Self-reported questionnaire |
| Higashi et al., 2008 | 6 mos | 26; individuals with hypertension | Self-reported questionnaire |
| Higashi et al., 2009 | 6 mos | 48; individuals with CVD | Self-reported questionnaire |
| Piconi et al., 2009 | 12 mos | 35; systemically healthy | PSR |
CVD, cardiovascular disease; CAL, clinical attachment level; PPD, probing pocket depth; GI, gingival index; PI, plaque index; BOP, bleeding upon probing; PSR, Periodontal Screening and Recording.
Table 2.
Studies Evaluating the Effect of Non-surgical Periodontal Therapy on the Cardiovascular and/or Endothelial Function System in Individuals with Periodontitis: Changes in the Vessel Wall as Measured by Intima Media Thickness (IMT) and Endothelial Function as Measured by Flow-mediated Dilatation (FMD) or Endothelium-dependent Digital Pulse Amplitude Testing (EndoPAT) (relevant studies identified by literature review)
| Reference | Atherosclerotic Measure | Study Outcome |
Conclusion /Significance | |
|---|---|---|---|---|
| Baseline | Final | |||
| Mercanoglu et al., 2004 | FMD | Diameter: 3.9 ± 0.4 mm; | Diameter: 3.8 ± 0.5 mm; | NS |
| EDD: 8.4 ± 4.0% | EDD: 177 ± 57% | p < .0001 | ||
| EID: 13.3 ± 6.3% | EID: 24.9 ± 7.3% | p < .0001 | ||
| Seinost et al., 2005 | FMD | Diameter: 3.9 ± 0.4 mm; | Diameter: 3.9 ± 0.4 mm; | NS |
| EDD: 6.1 ± 4.4% | EDD: 8.7 ± 57% | p = .003 | ||
| EID: 13.3 ± 6.3% | EID: 13.3 ± 6.3% | NS | ||
| IMT | m-IMT (A. brachialis): | m-IMT (A. brachialis): | NS | |
| 0.27 ± 0.07 mm | 0.27 ± 0.06 mm | |||
| Blum et al., 2007 | FMD | EDD: 4.12 ± 3.96% | EDD: 11.12 ± 7.22 | p = .0007 |
| EID: 20.97 ± 10.7% | EID: 17.94 ± 6.23 | NS | ||
| Elter et al., 2006 | FMD | EDD: 8.6 ± 4.7% | EDD: 10.2 ± 3.9% | p = .034 |
| EID: 19.8 ± 4.7% | EID: 21.3 ± 8.0% | NS | ||
| Li et al., 2011 | Endo-PAT | 2.41 ± 0.71 | 2.22 ± 0.62 | NS; Adj. calculations for the treatment effect, p = .03 |
| Tonetti et al., 2007 | FMD | # | Diameter changes: 0.06 ± 0.15 mm | NS |
| EDD changes: 1.48 ± 0.8% | p < .001 | |||
| EID changes: −0.07 ± 2.44% | NS | |||
| Higashi et al., 2008 | FMD | Diameter: 5.2 ± 1.3 mL/min | Diameter: 5.4 ± 1.4 mL/min | NS |
| EDD: # | EDD: # | p < .001 | ||
| EID: # | EID: # | NS | ||
| Higashi et al., 2008 | FMD | EDD: # | EDD: # | p < .001 |
| EID: # | EID: # | NS | ||
| Higashi et al., 2009 | FMD | EDD: # | EDD: # | p < .001 |
| EID: # | EID: # | NS | ||
| Piconi et al., 2009 | IMT | Bifurcation: | Bifurcation: | |
| 0.55 ± 0.03 mm | 0.45 ± 0.04 mm | p = .01 | ||
| 1-cm: 0.49 ± 0.02 | 1-cm: 0.37 ± 0.03 mm | p < .001 | ||
| 2-cm: 0.5 ± 0.02 | 2-cm: 0.39 ± 0.03 mm | p = .001 | ||
Indicates not recorded.
IMT, intima-media thickness; FMD, flow-mediated dilatation; EndoPAT, endothelium-dependent digital pulse amplitude testing; EDD, endothelium-dependent dilatation; EID, endothelium-independent dilatation; NS, not significant; a, arteria; Adj, statistically adjusted for potential confounding factors.
Six studies utilizing FMD to measure endothelial function suggested a positive effect of periodontal therapy on FMD at 4 to 28 post-operative wks (see Tables 1 and 2). Tonetti et al. (2007) observed a significant improvement of the FMD at 6 mos, but not at 2 mos, after therapy; moreover, the FMD at 6 mos was significantly better than the FMD in those in the control group with periodontitis who obtained only community dental care.
A Common Genetic Risk Factor for CVD and Periodontitis?
Complex diseases like CVD, type 2 diabetes, and Crohn's disease may have similar and overlapping common causative genetic variants (Sivakumaran et al., 2011); this is termed ‘plei-otropy of complex diseases’. The ANRIL locus is the best-replicated coronary heart disease (CHD)-associated risk locus to date (Schunkert et al., 2011). A highly increased risk for aggressive periodontitis (AgP) and limited evidence for increased risk with chronic periodontitis (CP) were observed with genetic variants in the ANRIL locus (Table 3) (Schaefer et al., 2009, 2011). We speculate that there are likely to be common patho-physiologic pathways for both CVD and periodontitis.
Table 3.
Results from Genotyping of Independent Case-Control Population for Various Single-nucleotide Polymorphisms (SNP) in the ANRIL Locus (Schaefer et al., 2009, 2011)
| Type of Periodontitis | Ethnicity | N Patients | N Controls | SNP p value | Odds Ratio |
|---|---|---|---|---|---|
| G-AgP | German | 151 | 736 | 6.9 × 10−4 | 1.99 |
| L-AgP | German | 137 | 368 | 2.6 × 10−2 | 1.72 |
| G and L-AgP | Dutch | 164 | 421 | 7.0 × 10−3 | 2.53 |
| G and L-AgP | German | 301 | 962 | 4.0 × 10−4 | 1.48 |
| CP | Dutch | 154 | 421 | 4.0 × 10−3 | 0.57 |
| CP | German | 740 | 962 | 2.5 × 10−2 | 0.66 |
Abbreviations: N, number; SNP, single-nucleotide polymorphism; G-AgP, generalized aggressive periodontitis; L-AgP, localized aggressive peri-odontitis; C P, chronic periodontitis.
Antiphospholipids and Molecular Mimicry: a Link Between Periodontitis and Systemic Disease?
It is well-known that micro-organisms can produce pathology due to the phenomenon known as “molecular mimicry”. Many microbes bear molecular structures of sufficient similarity to human tissue components so as to induce an immune response that is cross-reactive with human tissue. A group of autoantibodies termed “antiphospholipids” is related to pathology present in the Antiphospholipid Syndrome (APS) (Mehdi et al., 2010). Patients who develop APS have greatly increased risk of thrombosis, fetal loss, and possibly early atherosclerosis. Analysis of data linking periodontitis to stroke, myocardial infarction, adverse pregnancy outcomes, and atherosclerosis prompted interest in these antibodies. In particular, anticardiolipin (anti-Cl) appeared to be of potential relevance to periodontitis, for two reasons. First, it was shown that monoclonal antibodies raised against the serum protein beta-2 glycoprotein I (β2GPI), which contains the target antigen for pathogenic anti-Cl (the peptide sequence TLRVYK), could induce APS-like pathology in mice (Bakimer et al., 1992); and second, it was demonstrated that a variety of microbial pathogens could induce anti-Cl-like antibodies because they contained antigenic epitopes similar to TLRVYK in β2GPI (Blank et al., 2002). These antibodies also produced APS-like symptoms in animal models.
Utilizing a commercially available ELISA kit, investigators examined serum samples from periodontally characterized patients for anti-Cl (Schenkein et al., 2003). The proportion of chronic periodontitis (CP) and generalized aggressive periodontitis (GAP) patients testing positive for anti-Cl was significantly higher than that in periodontally healthy individuals (16-19% vs. 6.8%, p = .0033). It was hypothesized that the presence of these antibodies could be due to molecular mimicry of the TLRVYK peptide sequence of β2GPI by periodontal bacteria. Examination of the Swiss-prot database revealed a peptide sequence in the arg-gingipain (RGP) of P. gingivalis with homology to TLRVYK. When anti-Cl positive sera were absorbed from periodontitis patients with various strains of P. gingivalis, it was noted that all strains other than an RGP-defective mutant could remove most of the anti-Cl antibody from serum (Schenkein, 2005). Other investigators have found sequence homologies, and mutual cross-reactivity, between peptide sequences in both Aggregatibacter actinomycetemcomitans and Treponema denti-cola and β2GPI as well (Wang et al., 2008; Chen et al., 2009). Thus, elevated levels of anti-Cl in sera from periodontitis patients could very well be induced by periodontal pathogenic micro-organisms.
The ability of P. gingivalis and A. actinomycetemcomitans to induce anti-Cl was subsequently assessed in rabbits and mice. It was found that immunization of rabbits with P. gingivalis induced anti-Cl. Furthermore, affinity purification of anti-P. gingivalis antisera with either P. gingivalis or A. actinomy-cetemcomitans resulted in an approximately 40-fold purification of anti-Cl regardless of which bacterium was used. Some strains of A. actinomycetemcomitans were also observed to induce elevated anti-Cl in mice. Thus, it is clear that some periodontal pathogens can induce anti-Cl.
Effects of Anti-Cl on Human Endothelial Cell Activation
We have taken two approaches to assessing the biological activities of human anti-Cl in periodontitis patients. First, associations between elevated levels of anti-Cl and soluble markers of endothelial cell activation ICAM-1, VCAM-1, and E-selectin in serum were studied (Schenkein et al., 2007). In a study of 290 periodontally characterized patients, it was observed that sera with elevated anti-Cl also contained elevated soluble markers of endothelial cell activation. This was especially apparent in patients with GAP and was observed whether or not the patients were current smokers. The individuals with elevated anti-Cl also demonstrated significantly elevated serum levels of C-reactive protein. Second, IgG was purified from sera of 53 individuals who were periodontally healthy or who were diagnosed with CP or GAP. These IgG preparations were added to cultures of human umbilical vein endothelial cells (HUVEC), and production of monocyte chemotactic protein-1 (MCP-1) was assessed. There was a strong correlation between anti-Cl titer and MCP-1 production (p < .0001, r2 = .48), with higher MCP-1 production when titers of anti-Cl were elevated, regardless of clinical diagnoses. Furthermore, reduction of the anti-Cl content of the IgG preparations resulted in significantly decreased production of MCP-1 by patient IgG (Schenkein et al., 2013b). Thus, it appears that anti-Cl is biologically active, affecting endothelial cell function.
Effects of Anti-Cl on Pregnancy Outcomes in a Mouse Model
Experiments have also been undertaken to examine the effect of anti-Cl induced by P. gingivalis on pregnancy outcomes in a mouse model. This involved preparation of a series of mouse antisera to antigens that included β2GPI, P. gingivalis strain W83, and P. gingivalis strain HF 18 (an RGP-defective mutant of P. gingivalis strain W83). The results showed that anti-Cl titers were increased in sera from mice immunized with peri-odontal pathogens, other than the P. gingivalis strain (HF18) lacking the arg-gingipain protease (Table 4). Anti-Cl was then removed from these preparations by immuno-absorption on affinity columns containing cardiolipin complexed with β2GPI. Antibodies to the P. gingivalis strains were passively administered to mated mice at day 0 of pregnancy, and fetuses were harvested and evaluated at day 15. It was observed that anti-P. gingivalis induced fetal resorption at rates equivalent to that induced by anti-β2GPI, and that reduction of anti-Cl content of the antibody preparations resulted in a proportional reduction in fetal resorption. Furthermore, the RGP-defective mutant of P. gingivalis (strain HF18) failed to induce fetal loss, consistent with the presence of the cross-reactive epitope being present on the arg-gingipain protease (Schenkein et al., 2013a).
Table 4.
Relative Anti-cardiolipin Titers in Mouse Sera following Immunization
| Antigen | IgG Anti-Cl Titer ± SEM a |
|---|---|
| β2GPI | 2,438 ± 514 |
| P. gingivalis W83 | 2,228 ± 283 |
| P. gingivalis HF18 b | 228 ± 68 |
| A. actinomycetemcomitans DB03A c | 3,176 ± 93 |
| A. actinomycetemcomitans DO45D c | 1,313 ± 59 |
| Alum alone | 290 ± 183 |
Titer was calculated as the inverse of the dilution of antibody for which OD = 1.0 in ELISA assay. Titers represent mean value from 5 antibody preparations
P. gingivalis HF18 is an arg-gingipain defective mutant of P. gingivalis W83.
Both strains of A. actinomycetemcomitans are clinical isolates from individuals with aggressive periodontitis.
Periodontitis and Adverse Pregnancy Outcomes
Fusobacterium nucleatum: a Commensal Turned Pathogen
Increasing evidence suggests that oral bacteria can enter the systemic circulation under certain circumstances. Members of the oral microbiome can migrate away from the mouth, causing infections and inflammation at extra-oral sites (Han and Wang, 2013). Fusobacterium nucleatum, a Gram-negative common oral anaerobe, is one such “mobile” micro-organism. As an opportunistic oral commensal, F. nucleatum exists in both peri-odontally healthy and diseased sites. It is a heterogeneous species, with 5 established subspecies (subsp): subsp animalis, subsp fusiforme, subsp nucleatum, subsp polymorphum, and subsp vincentii. All 5 subspecies are present in the oral cavity. Outside the oral cavity, Fn becomes a “bona fide” pathogen, having been isolated from infections and abscesses from a wide range of organs and tissues, including liver, spleen, lung, kidney, blood, brain, and obstetrical and intestinal sites (Han and Wang, 2013). This section focuses on F. nucleatum infection in the intra-uterine cavity and its role in adverse pregnancy outcomes.
F. nucleatum and Pregnancy Complications
A series of clinical studies has linked F. nucleatum to a plethora of pregnancy complications, including preterm birth, stillbirth, and early-onset neonatal sepsis. By 16S rRNA gene-based PCR, F. nucleatum was found to be a highly prevalent species in amniotic fluid associated with preterm birth, while no microbial DNA was detected in normal pregnancies (Han et al., 2009). A term stillbirth case in which F. nucleatum was isolated as a pure culture from the infant's lung and stomach was reported (Han et al., 2010). An F. nucleatum clone identical to that from the stillborn infant was detected in the subgingival plaque of the mother, who had pregnancy-associated gingivitis with frequent gingival bleeding during gestation. It is possible that F. nuclea-tum translocated hematogenously from the mother's oral cavity to her uterus as a result of frequent dental bacteremia. Recently, it was reported that Fn was often concurrently detected in paired amniotic fluid and cord blood associated with early-onset neonatal sepsis (Wang et al., 2013). From these studies and others, it was discovered that intra-uterine Fn infection is dominated by subsp animalis, followed by subsp polymorphum (Han and Wang, 2013).
Mechanism of Fn in Intra-uterine Infection
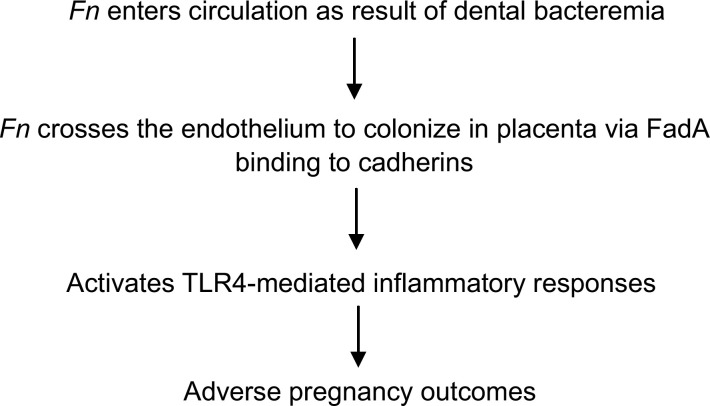
The mechanism of F. nucleatum in intra-uterine infection has been investigated in a pregnant mouse model. It has been shown that hematogenous injection of F. nucleatum (to mimic dental bacteremia) induces preterm and term fetal death in a dose-dependent manner, while injection of E. coli does not cause fetal loss (Han et al., 2004). The pattern of infection resembles cho-rioamnionitis in humans (Han et al., 2004, 2010). F. nucleatum colonize and proliferate specifically in the fetal-placental unit after crossing the endothelium (Han et al., 2004). F. nucleatum colonization is dependent on its unique FadA adhesin (Ikegami et al., 2009), a previously identified surface protein (Han et al., 2005; Xu et al., 2007; Nithianantham et al., 2009; Han, 2011; Temoin et al., 2012). FadA exists in two forms: the intact pre-FadA, consisting of 129 amino acids; and the secreted mature FadA (mFadA), consisting of 111 amino acids (Han et al., 2005). Both forms are required for the formation of the active complex, FadAc, for binding and invasion of host cells (Xu et al., 2007; Temoin et al., 2012). FadA binding to VE-cadherin on the endothelial cells causes translocation of the latter to intra-cellular compartments and loosens the cell-cell junction, allowing bacteria in the vicinity, such as E. coli, to percolate through the endothelium (Fardini et al., 2011). F. nucleatum crosses the endothelium either by direct invasion into the endothelial cells or through permeabilized cell junctions, and both mechanisms may be utilized for its hematogenous dissemination from the oral cavity.
Following colonization in the fetal-placental unit, F. nuclea-tum induces TLR4-mediated inflammatory responses (Liu et al., 2007). In TLR4-/- mice or in wild-type mice treated with a TLR4 antagonist, F. nucleatum colonizes the placenta without inducing inflammation, leading to significantly improved fetal survival (Liu et al., 2007). These observations suggest that reagents aimed at inhibiting or resolving inflammatory responses may be used to treat or prevent pregnancy complications due to bacterial infection. A model of oral Fn inducing adverse pregnancy outcomes is illustrated in Fig. 1.
Figure 1.
A model of oral Fn in induction of adverse pregnancy outcomes.
Periodontitis and Head-and-Neck Cancer
The epidemiology of head-and-neck squamous cell carcinoma (HNSCC) has changed in an unpredictable way over the last 3 decades. A steady increase in the incidence of oropharyngeal cancers has been observed in many parts of the world since the early 1970s, in spite of advances in prevention and treatment (Gillison et al., 2012). A better understanding of HNSCC etiology, interactions among risk factors, and new approaches to prevention and treatment are necessary to change the course of this disease.
Chronic Periodontitis and Head-and-Neck Cancer
Clinical, epidemiologic, and animal studies have established a strong association between chronic inflammation and cancer of several organs (Kipanyula et al., 2013). In the oral cavity, peri-odontitis is a chronic inflammatory disease associated with Gram-negative anaerobic dental plaque bacteria that promote the continuous release of bacteria and inflammatory cytokines into saliva (Scannapieco et al., 2007). The prevalence of peri-odontitis in the general population is estimated to be 47% (Eke et al., 2012).
It has been observed that chronic periodontitis was associated with increased risks of potentially malignant disorders (Tezal et al., 2005) and HNSCC (Tezal et al., 2007, 2009a). The strength of the association was greatest in the oral cavity, followed by the oropharynx and larynx. In addition, a history of periodontitis predicted poorly differentiated tumor status in patients with cancer of the oral cavity. An unexpected finding was that the association between periodontitis and HNSCC was weaker in current smokers compared with former and never-smokers. Supporting these results, other studies have also reported that the associations of oral health variables with head-and-neck, esophageal, upper gastrointestinal, and pancreatic cancers were weaker in smokers compared with non-smokers (Abnet et al., 2005; Michaud et al., 2007; Hiraki et al., 2008). These observations, seemingly paradoxical, are consistent with the biological effects of tobacco smoke, which causes acute vasoconstriction and inhibits angiogenesis, proliferation, and production of inflammatory mediators (Laan et al., 2004). However, these potent suppressive effects of smoking are reversible within a few hours of cessation. It was shown that smoking one cigarette every 2 hours inhibited the LPS-induced production of inflammatory cytokines in bronchial epithelial cells (Laan et al., 2004). Clinical signs of gingival inflammation also increase after smoking cessation (Nair et al., 2003). It is thus possible that while multiple toxic components can initiate carcinogenesis, other components in tobacco may delay clinical manifestations. Therefore, treatment of sources of inflammation in the oral cavity before smoking cessation should be an important component of both smoking cessation and cancer management.
Chronic Periodontitis-HPV Synergy in Head-and-Neck Cancers
Oral human papillomavirus (HPV) infection has emerged as an etiological factor for a subset of HNSCC. A vaccine is available for cervical HPV infection, which is recommended for females aged 9 to 26 years and males aged 9 to 21 years (Giuliano et al., 2011). However, oral HPV has been found in 4 to 87% of new-borns, and in 52% of children aged 3 to 11 years, suggesting transmission early in life (Martinelli et al., 2012). Therefore, a large percentage of the general population does not benefit from the vaccine. Conversely, HPV is commonly transmitted, and most infections are cleared by the immune system without resulting in pathology. Persistence of HPV infection is the strongest risk factor in the development of cancer (Gillison et al., 2012). Thus, the identification of factors influencing not only the acquisition but also the persistence of HPV infection would lead to more effective prevention and treatment strategies, also benefiting those who are already infected.
HPV has a specific tropism for basal cells of squamous epithelium. The infection begins when the virus gains access through breaks in the mucosa, and the replication of the virus depends on the proliferation of basal cells (Stubenrauch and Laimins, 1999). Mucosal ulcerations and the proliferative state caused by chronic inflammation may facilitate HPV acquisition and persistence within the oral mucosa. The chronically inflamed epithelium is characterized by rete-ridge formation, increasing the surface area exposed. In addition, the candidate HPV receptor, cell-surface heparan sulphate expressed during wound healing, is found extensively in the inflamed epithelium (Hormia et al., 2005). The results of recent studies have suggested that a history of chronic periodontitis is associated with tumor HPV status in patients with HNSCC. The strength of this association was greater among patients with oropharyn-geal compared with those with oral cavity and laryngeal cancers (Tezal et al., 2009b, 2012).
Summary
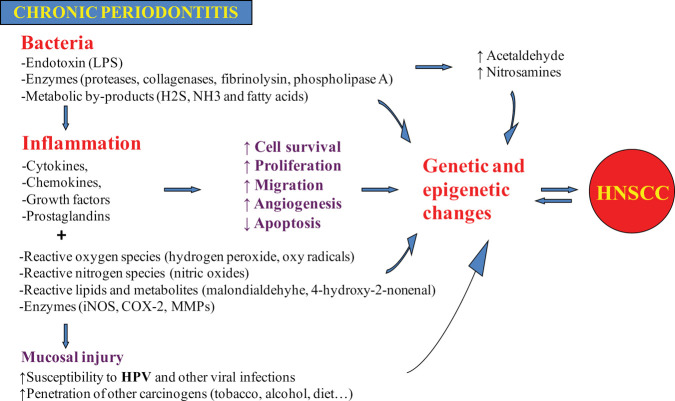
A model for the role of chronic peri-odontitis in HNSCC etiology is summarized in Fig. 2. Briefly, chronic periodontitis may be associated with HNSCC by direct toxic effects of bacteria and their products, and/or by indirect effect through inflammation. In addition to its independent effect on carcinogen-esis, chronic periodontitis may also facilitate the acquisition and persistence of oral HPV infection.
Figure 2.
A model for the role of chronic periodontitis in head-and-neck cancer.
Conclusions
The association between periodontitis and CVD is no longer disputed. Several possible biological mechanisms, including common genetic variants that may explain the link between CVD and periodontitis, have been reviewed. Indeed, it is likely that there are multiple mechanisms underlying these links, with inflammatory, infectious, immune, and genetic components. For example, anti-cardiolipin antibodies are induced by periodontal pathogens in periodontitis patients by molecular mimicry of β2GPI, and these antibodies have biological and pathological activities consistent with those previously reported for other infection-induced antiphospholipid antibodies. These results suggest the hypothesis that anti-Cl may explain some of the observed associations between periodontal infections and systemic conditions such as cardiovascular diseases and adverse pregnancy outcomes. Strategies to inhibit microbial invasion and translocation or to resolve inflammatory responses may be used to treat or prevent pregnancy complications due to bacterial infection.
Associations between periodontal disease and adverse pregnancy outcomes have also been noted. The bacterium F. nuclea-tum has been implicated as a common cause of intra-uterine infection. F. nucleatum uses a unique protein to bind to VE-cadherin on endothelial cells and to invade them. These interactions may facilitate translocation of the cadherins to intracellular compartments, thus loosening the cell-cell junction and allowing bacteria in the vicinity to pass through the endo-thelium (Fardini et al., 2011).
Evidence of an association between chronic periodontitis and HNSCC has practical implications for prevention, early diagnosis, and treatment. Chronic periodontitis may represent a clinical high-risk profile for both oral HPV infection and HNSCC. Periodontal treatment, as an adjunct to conventional oncologic management, may improve the prognosis of HNSCC.
Future research will yield a greater understanding of the relationship between periodontal infection and systemic disease and may enable improved management and prevention strategies to be developed that will benefit both oral and general health.
Acknowledgements
YWH acknowledges RO1 grants DE014924 and DE023332 from the National Institute of Dental and Craniofacial Research (NIDCR); BGL was funded in part by a grant from the University of Amsterdam for the focal point Oral Infections and Inflammation; HAS received support from NIDCR grant RO1DE018125 and grant P60MD002256 from the National Institute on Minority Health and Health Disparities; MT received grants 1R03CA119262 from the National Cancer Institute and T32-DE07034 from the NIDCR. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. (2005). Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int J Epidemiol 34: 467–474. [DOI] [PubMed] [Google Scholar]
- Anderson TJ. (2006). Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol 22(Suppl B): 72B–80B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakimer R, Fishman P, Blank M, Sredni B, Djaldetti M, Shoenfeld Y. (1992). Induction of primary antiphospholipid syndrome in mice by immunization with a human monoclonal anticardiolipin antibody (H-3). J Clin Invest 89: 1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. (2002). Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest 109: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Front E, Peleg A. (2007). Periodontal care may improve systemic inflammation. Clin Invest Med 30: E114–E117. [DOI] [PubMed] [Google Scholar]
- Chen YW, Nagasawa T, Wara-Aswapati N, Ushida Y, Wang D, Takeuchi Y, et al. (2009). Association between periodontitis and anti-cardiolipin antibodies in Buerger disease. J Clin Periodontol 36: 830–835. [DOI] [PubMed] [Google Scholar]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. (2012). Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83: 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elter JR, Hinderliter AL, Offenbacher S, Beck JD, Caughey M, Brodala N, et al. (2006). The effects of periodontal therapy on vascular endothelial function: a pilot trial. Am Heart J 151: 47. [DOI] [PubMed] [Google Scholar]
- Fardini Y, Wang X, Temoin S, Nithianantham S, Lee D, Shoham M, et al. (2011). Fusobacterium nucleatum adhesin FadA binds vascular endo-thelial cadherin and alters endothelial integrity. Mol Microbiol 82: 1468–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. (2009). The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol 104: 59–68. [DOI] [PubMed] [Google Scholar]
- Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. (2012). Prevalence of oral HPV infection in the United States, 2009-2010. J Am Med Assoc 307: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C, et al. (2011). Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 364: 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. (2007). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 14(Suppl 2): S1–S113. [DOI] [PubMed] [Google Scholar]
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. (2010). 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 122: e584–e636. [DOI] [PubMed] [Google Scholar]
- Han YW. (2011). Fusobacterium nucleatum interaction with host cells. In: Oral microbial communities: genomic inquiry and interspecies communication. Kolenbrander P, editor. Washington, DC: ASM Press, pp. 221–232. [Google Scholar]
- Han YW, Wang X. (2013). The mobile oral microbiome: oral bacteria in extra-oral infection and inflammation. J Dent Res 92: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. (2004). Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 72: 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, et al. (2005). Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol 187: 5330–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol 47: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, et al. (2010). Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol 115(2 Pt 2): 442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, et al. (2008). Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 51: 446–453. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Goto C, Hidaka T, Soga J, Nakamura S, Fujii Y, et al. (2009). Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis 206: 604–610. [DOI] [PubMed] [Google Scholar]
- Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. (2008). Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev 17: 1222–1227. [DOI] [PubMed] [Google Scholar]
- Hormia M, Willberg J, Ruokonen H, Syrjänen S. (2005). Marginal periodon-tium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol 76: 358–363. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Chung P, Han YW. (2009). Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun 77: 3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipanyula MJ, Seke Etet PF, Vecchio L, Farahna M, Nukenine EN, Nwabo Kamdje AH. (2013). Signaling pathways bridging microbial-triggered inflammation and cancer. Cell Signal 25: 403–416. [DOI] [PubMed] [Google Scholar]
- Laan M, Bozinovski S, Anderson GP. (2004). Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator Protein-1 in bronchial epithelial cells. J Immunol 173: 4164–4170. [DOI] [PubMed] [Google Scholar]
- Li X, Tse HF, Yiu KH, Li LS, Jin L. (2011). Effect of periodontal treatment on circulating CD34(+) cells and peripheral vascular endothelial function: a randomized controlled trial. J Clin Periodontol 38: 148–156. [DOI] [PubMed] [Google Scholar]
- Liu H, Redline RW, Han YW. (2007). Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol 179: 2501–2508. [DOI] [PubMed] [Google Scholar]
- Martinelli M, Zappa A, Bianchi S, Frati E, Colzani D, Amendola A, et al. (2012). Human papillomavirus (HPV) infection and genotype frequency in the oral mucosa of newborns in Milan, Italy. Clin Microbiol Infect 18: E197–E199. [DOI] [PubMed] [Google Scholar]
- Mehdi AA, Uthman I, Khamashta M. (2010). Antiphospholipid syndrome: pathogenesis and a window of treatment opportunities in the future. Eur J Clin Invest 40: 451–464. [DOI] [PubMed] [Google Scholar]
- Mercanoglu F, Oflaz H, Oz O, Gokbuget AY, Genchellac H, Sezer M, et al. (2004). Endothelial dysfunction in patients with chronic periodontitis and its improvement after initial periodontal therapy. J Periodontol 75: 1694–1700. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. (2007). A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst 99: 171–175. [DOI] [PubMed] [Google Scholar]
- Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. (2003). Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol 30: 435–437. [DOI] [PubMed] [Google Scholar]
- Nithianantham S, Xu M, Yamada M, Ikegami A, Shoham M, Han YW. (2009). Crystal structure of FadA adhesin from Fusobacterium nuclea-tum reveals a novel oligomerization motif, the leucine chain. J Biol Chem 284: 3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconi S, Trabattoni D, Luraghi C, Perilli E, Borelli M, Pacei M, et al. (2009). Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J 23: 1196–1204. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. (2007). Salivary biomarkers associated with alveolar bone loss. Ann NY Acad Sci 1098: 496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Richter G, Dommisch H, Reinartz M, Nothnagel M, Noack B, et al. (2011). CDKN2BAS is associated with periodontitis in different European populations and is activated by bacterial infection. J Med Genet 48: 38–47. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, et al. (2009). Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet 5: e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA. (2005). The host response in periodontitis: anti-phospholipid autoantibodies as a link between plaque bacteria and extraoral disease. Oral Biosci Med 2: 221–225. [Google Scholar]
- Schenkein HA, Loos BG. (2013). Inflammatory mechanisms linking peri-odontal diseases to cardiovascular diseases. J Clin Periodontol 40(14 Suppl): 51S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Berry CR, Burmeister JA, Brooks CN, Barbour SE, Best AM, et al. (2003). Anti-cardiolipin antibodies in sera from patients with periodontitis. J Dent Res 82: 919–922. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Best AM, Brooks CN, Burmeister JA, Arrowood JA, Kontos MC, et al. (2007). Anti-cardiolipin and increased serum adhesion molecule levels in patients with aggressive periodontitis. J Periodontol 78: 459–466. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Bradley JL, Purkall DB. (2013a). Anticardiolipin in Porphyromonas gingivalis antisera causes fetal loss in mice. J Dent Res 92:814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein HA, Sabatini R, Koertge TE, Brooks CN, Purkall DB. (2013b). Anti-cardiolipin from periodontitis patients induces MCP-1 production by human umbilical vein endothelial cells. J Clin Periodontol 40:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, et al. (2011). Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 43: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinost G, Wimmer G, Skerget M, Thaller E, Brodmann M, Gasser R, et al. (2005). Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 149: 1050–1054. [DOI] [PubMed] [Google Scholar]
- Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, et al. (2011). Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet 89: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Laimins LA. (1999). Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol 9: 379–386. [DOI] [PubMed] [Google Scholar]
- Temoin S, Wu KL, Wu V, Shoham M, Han YW. (2012). Signal peptide of FadA adhesin from Fusobacterium nucleatum plays a novel structural role by modulating the filament's length and width. FEBS Lett 586: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezal M, Grossi SG, Genco RJ. (2005). Is periodontitis associated with oral neoplasms? J Periodontol 76:406–410. [DOI] [PubMed] [Google Scholar]
- Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. (2007). Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg 133: 450–454. [DOI] [PubMed] [Google Scholar]
- Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, et al. (2009). Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 18: 2406–2412. [DOI] [PubMed] [Google Scholar]
- Tezal M, Sullivan MA, Stoler D, Hyland A, Melendy T, Smaldino PJ, et al. (2009). Chronic periodontitis-human papillomavirus synergy in base of tongue cancers. Arch Otolaryngol Head Neck Surg 135: 391–396. [DOI] [PubMed] [Google Scholar]
- Tezal M, Scannapieco FA, Wactawski-Wende J, Hyland A, Marshall JR, Rigual NR, et al. (2012). Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg 138: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. (2007). Treatment of periodontitis and endothelial function. N Engl J Med 356: 911–920. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. (2010). Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327. [DOI] [PubMed] [Google Scholar]
- Wang D, Nagasawa T, Chen Y, Ushida Y, Kobayashi H, Takeuchi Y, et al. (2008). Molecular mimicry of Aggregatibacter actinomycetemcomitans with beta2 glycoprotein I. Oral Microbiol Immunol 23: 401–405. [DOI] [PubMed] [Google Scholar]
- Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. (2013). Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS ONE 8: e56131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. (2007). FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem 282: 25000–25009. [DOI] [PubMed] [Google Scholar]