Abstract
Background:
Palliative care specialists are experts in conducting advance care planning (ACP) but are a limited resource. Oncology nurses often have special relationships with their patients and thus may be poised to provide primary palliative care. We sought to determine the impact of a nurse-led primary palliative care intervention on ACP uptake among patients with advanced cancer.
Methods:
We performed a secondary analysis of a cluster randomized controlled trial examining the impact of nurse-based primary palliative care. In the parent trial, patients with advanced cancer received either monthly primary palliative care visits with trained nurses within their cancer center or standard care. Nurses in the intervention arm received special training in ACP. ACP uptake was assessed at enrollment and 3 months later evaluating (1) whether an end-of-life conversation (EOLC) occurred with one’s oncologist, and (2) completion of an advance directive (AD). Multivariable logistic regression tested differences in ACP uptake by treatment arm adjusted for age, religious importance, education, time with current oncologist, and performance status.
Results:
Of 672 patients enrolled, 182/336 (54%) patients in the intervention arm and 196/336 (58%) in the standard care arm lacked an EOLC at baseline and completed the 3-month assessment. Of those, 82/182 (45.1%) patients in the intervention arm and 29/196 (14.8%) in the standard care arm reported having an EOLC at 3 months (adjusted odds ratio, 5.28; 95% CI, 3.10–8.97; P<.001). Similarly, 111/336 (33%) patients in the intervention arm and 105/336 (31%) in the standard care arm lacked an AD at baseline and completed the 3-month assessment. Of those, 48/111 (43.2%) patients in the intervention arm and 19/105 (18.1%) in the standard care arm completed an AD over the study period (adjusted odds ratio, 3.68; 95% CI, 1.89–7.16; P<.001).
Conclusions:
Nurse-led primary palliative care increased ACP uptake among patients with advanced cancer. Training oncology nurses embedded within community cancer centers to provide primary palliative care may help improve ACP access.
Background
As patients near the end of their lives, they are often unable to understand and engage in complex medical decision-making. Advance care planning (ACP), defined as “a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care,”1 is designed to allow patients to maintain a locus of control at the end of their lives.2,3 Although there has been recent controversy about the benefits of ACP,4 prior work has shown that engagement in ACP is associated with a higher likelihood of dying in a preferred location,5 superior hope at the end of life,6 less anxiety surrounding death for patients,7 and decreased decisional anxiety for caregivers.8 Thus, ACP remains an important aspect of care for patients with advanced cancer.4,9,10
Despite the acknowledged importance of ACP, data consistently show that ACP often occurs late in the course of a patient’s disease or not at all.11,12 Prognostic uncertainty, fear of “giving up hope,” lack of sufficient time, and inadequate training are among the principal reasons clinicians cite for deferring these important conversations with their patients.12–15 Although palliative care specialists have expertise in negotiating ACP, access to specialty palliative care is limited, particularly at community cancer centers that are geographically distant from larger academic facilities.16
The CONNECT study addressed this limitation by bringing oncology nurse–led primary palliative care to community cancer center clinics.17 Oncology nurses are uniquely suited to provide primary palliative care because they often have long-standing relationships with their patients and are the first to hear about patient preferences and concerns.18 Primary outcomes of this study were previously published.19 This analysis sought to determine whether the CONNECT intervention impacts ACP, measured as either a patient-reported conversation with the oncologist about end-of-life wishes or completion of an advance directive (AD). We hypothesized that patients randomized to the intervention would have increased uptake of ACP.
Methods
Overview
This is a secondary analysis of data from the Cluster Randomized Trial of a Primary Palliative Care Intervention (CONNECT), a trial that compared a primary palliative care intervention versus standard care in community cancer centers. The study was approved by the University of Pittsburgh Institutional Review Board (STUDY 19090204) and registered on ClinicalTrials.gov (NCT02712229). Previous reports detailed the study design and its primary outcomes.17,19,20 No prior analysis evaluated the impact of the intervention on ACP uptake.
Setting and Participants
The trial enrolled patients from July 2016 through October 2019 at 17 community clinics in the Hillman Cancer Center network in western Pennsylvania. Patients were eligible if they had advanced solid tumor malignancies, their oncologist responded “yes” to the surprise question (ie, the oncologist “would not be surprised” if the patient died within the next year),21 and had an ECOG performance status of 0–2. All enrolled participants provided informed consent.
Randomization
Randomization was performed by clinic site rather than individual patient. This cluster randomized design was used to reduce the possibility of unintentional crossover.
CONNECT Intervention
Oncology infusion room nurses based at intervention clinics underwent an immersive 3-day training led by palliative care experts. This training included a focus on 4 key competencies: (1) addressing symptom needs, (2) engaging patients and caregivers in ACP, (3) providing emotional support to patients and caregivers, and (4) communicating and coordinating appropriate care.17 In the intervention arm, patients were invited to have at least monthly meetings with a CONNECT-trained nurse over the course of 3 months, usually either immediately preceding or following their otherwise scheduled oncology appointment. As part of this training, nurses built shared care plans with patients, which included an assessment of symptom burden and goals of care.17 At the first CONNECT visit, trained nurses would begin to engage in ACP with patients by assessing whether patients had a surrogate decision-maker. At subsequent visits, nurses delved further into ACP by assessing goals of care via questions such as, “What is important to you if you were to get sicker?” and encouraged communication with family and medical staff. Nurses would then take these plans to the patient’s oncologist, who would work with the patient and the CONNECT-trained nurse to address the patient’s needs. Patients were also offered a copy of the Pennsylvania Advance Health Care Directive and were encouraged to complete it with family. Although the CONNECT nurses discussed ADs and reviewed their role and function with the patient, nurses were not able to sign these legal documents. Full details of the intervention arm visits have been previously published.17,20
Standard Care
Patients in the standard care arm received standard oncology treatment from their oncologist and the in-clinic nurses, including any specialty supportive or palliative care services at the discretion of their oncologist. ACP was done at the discretion of the patient’s clinical team, but no additional support was provided to encourage ACP in this group.
Measures
All enrolled patients completed a full set of assessments at enrollment and at 3 months. Assessments were completed by either a blinded research assistant or a paper survey. Two validated questions from the ACP engagement survey were used to assess ACP uptake: (1) “Have you and any of your healthcare providers at the cancer center discussed any particular wishes you have about the care you would want to receive if you were dying?” (ie, end-of-life conversation [EOLC]); and (2) “Have you completed a living will or advance directive [AD]?”.22 These measures were chosen because they assess ACP outcomes from the patient perspective, which was the focus of this study.
Demographic and basic clinical information were collected at baseline, including age, sex, race, religious importance, education level, marital status, ability to manage on current income, and length of time receiving care from current oncologist (all self-reported). ECOG performance status was reported by oncologists, and patients completed the Edmonton Symptom Assessment System tool (scale, 0–100; higher scores indicate increased symptom burden).23
Statistical Analysis
This analysis included patients who reported not having a prior EOLC and/or AD at baseline, because our goal was to understand whether the intervention increased ACP uptake. Baseline characteristics of patients in the CONNECT intervention and standard care arms are presented using frequency and percentages for categorical variables and mean [SD] for continuous variables. Baseline characteristics are compared between treatment groups with chisquare tests for categorical variables and t tests for continuous variables.
Among patients who reported no previous EOLC at baseline and completed the 3-month assessment, we note the frequency and percentage of those who reported having an EOLC at the 3-month assessment. We used multivariable logistic regression to estimate the odds ratio for a new EOLC at the 3-month assessment in the CONNECT intervention group versus the standard care group when adjusting for variables known to be associated with ACP uptake (age, religious importance, education level, duration receiving care from current oncologist, and ECOG status).11,24–28 We followed an identical statistical approach for the AD analysis. All statistical analyses were performed using SAS 9:4 (SAS Institute Inc.).
Results
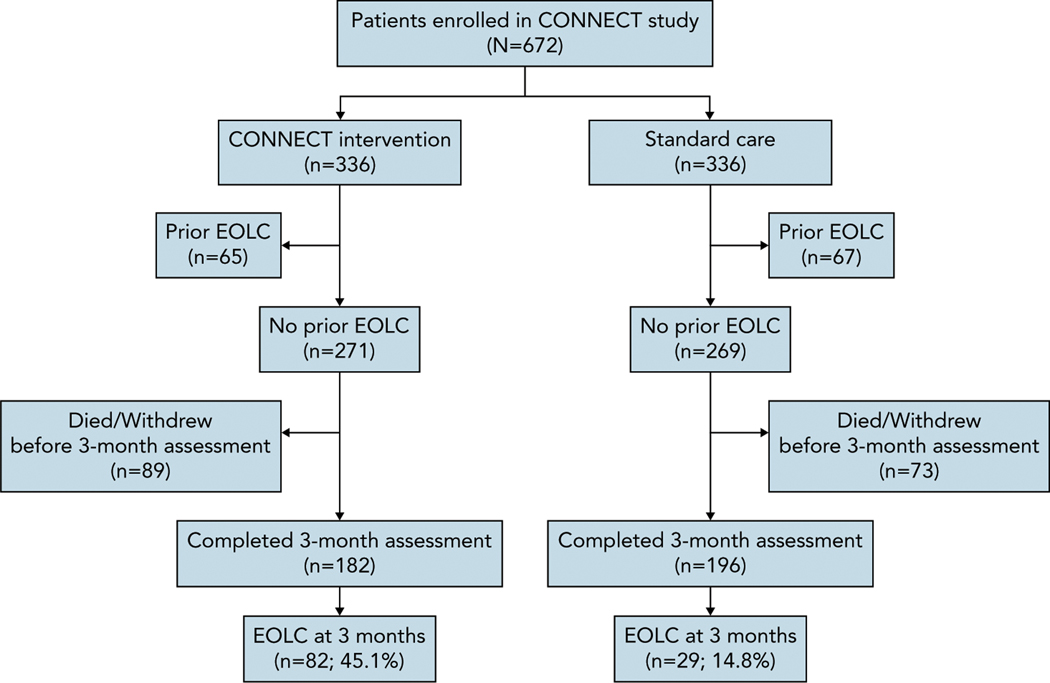
The CONNECT study enrolled 672 patients, of whom 378 did not report an EOLC at baseline and completed the 3-month assessment (Figure 1). Characteristics of study participants who did not report having a prior EOLC at baseline are shown in Table 1. Among these, 82/182 (45.1%) in the CONNECT intervention arm and 29/196 (14.8%) in the standard care arm reported having had an EOLC at 3 months (unadjusted odds ratio [OR], 4.72; P<.001). After adjustment, patients in the CONNECT arm remained significantly more likely than patients in the standard care (control) arm to report that they had engaged in an EOLC with their oncologist (adjusted OR, 5.28; P<.001) (Table 2).
Figure 1.
CONSORT diagram for EOLC.
Abbreviation: EOLC, end-of-life conversation.
Table 1.
Characteristics of Study Participants Who Did Not Report Having a Prior EOLC at Baseline
| All Patients Eligible for Analysis n (%) | CONNECT Intervention n (%) | Standard Care n (%) | P Value | |
|---|---|---|---|---|
| Patients, N | 378 | 182 | 196 | |
| Age, mean [SD], y | 69.1 [10.3] | 68.8 [10.1] | 69.4 [10.6] | .601 |
| Sex | .519 | |||
| Female | 210 (55.6) | 98 (53.8) | 112 (57.1) | |
| Male | 168 (44.4) | 84 (46.2) | 84 (42.9) | |
| Race | .983 | |||
| White | 360 (95.2) | 174 (95.6) | 186 (94.9) | |
| Black | 14 (3.7) | 6 (3.3) | 8 (4.1) | |
| Asian | 2 (0.5) | 1 (0.5) | 1 (0.5) | |
| Other | 2 (0.5) | 1 (0.5) | 1 (0.5) | |
| Religious importance | .867 | |||
| Not at all important | 10 (2.6) | 6 (3.3) | 4 (2.0) | |
| Not too important | 37 (9.8) | 20 (11.0) | 17 (8.7) | |
| Fairly important | 96 (25.4) | 46 (25.3) | 50 (25.5) | |
| Very important | 231 (61.1) | 108 (59.3) | 123 (62.8) | |
| Declined/No answer | 4 (1.1) | 2 (1.1) | 2 (1.0) | |
| Education level | .027 | |||
| Less than high school | 26 (6.9) | 15 (8.2) | 11 (5.6) | |
| High school diploma or GED | 160 (42.3) | 90 (49.5) | 70 (35.7) | |
| Some college/college degree | 158 (41.8) | 61 (33.5) | 97 (49.5) | |
| Graduate/Postgraduate degree | 30 (7.9) | 14 (7.7) | 16 (8.2) | |
| Declined/No answer | 4 (1.1) | 2 (1.1) | 2 (1.0) | |
| Current marital status | .431 | |||
| Never married | 25 (6.6) | 10 (5.5) | 15 (7.7) | |
| Married | 222 (58.7) | 104 (57.1) | 118 (60.2) | |
| Widowed/Divorced/Separated | 127 (33.6) | 67 (36.8) | 60 (30.6) | |
| Declined/No answer | 4 (1.1) | 1 (0.5) | 3 (1.5) | |
| Ability to manage on current income | .732 | |||
| Cannot make ends meet | 22 (5.8) | 10 (5.5) | 12 (6.1) | |
| Just manage to get by | 133 (35.2) | 67 (36.8) | 66 (33.7) | |
| Have enough with a little extra | 142 (37.6) | 71 (39.0) | 71 (36.2) | |
| Money is not a problem | 62 (16.4) | 27 (14.8) | 35 (17.9) | |
| Declined/No answer | 19 (5.0) | 7 (3.8) | 12 (6.1) | |
| Duration receiving care from current oncologist | .351 | |||
| <1 mo | 21 (5.6) | 13 (7.1) | 8 (4.1) | |
| 1–6 mo | 113 (29.9) | 56 (30.8) | 57 (29.1) | |
| 6 mo–1 y | 69 (18.3) | 29 (15.9) | 40 (20.4) | |
| 1–2 y | 70 (18.5) | 39 (21.4) | 31 (15.8) | |
| 2–5 y | 64 (16.9) | 28 (15.4) | 36 (18.4) | |
| >5 y | 39 (10.3) | 16 (8.8) | 23 (11.7) | |
| Declined/No answer | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Cancer type | .937 | |||
| Genitourinarya | 46 (12.2) | 22 (12.1) | 24 (12.2) | |
| Brain | 2 (0.5) | 1 (0.5) | 1 (0.5) | |
| Breast/Gynecologicb | 71 (18.8) | 36 (19.8) | 35 (17.9) | |
| Gastrointestinalc | 74 (19.6) | 33 (18.1) | 41 (20.9) | |
| Hepatobiliaryd | 37 (9.8) | 15 (8.2) | 22 (11.2) | |
| Head and neck | 7 (1.9) | 4 (2.2) | 3 (1.5) | |
| Lung | 132 (34.9) | 68 (37.4) | 64 (32.7) | |
| Melanoma | 5 (1.3) | 2 (1.1) | 3 (1.5) | |
| Sarcoma | 3 (0.8) | 1 (0.5) | 2 (1.0) | |
| Other | 1 (0.3) | 0 (0.0) | 1 (0.5) | |
| ECOG performance status | <.001 | |||
| 0 | 104 (27.5) | 33 (18.1) | 71 (36.2) | |
| 1 | 222 (58.7) | 123 (67.6) | 99 (50.5) | |
| 2 | 52 (13.8) | 26 (14.3) | 26 (13.3) | |
| Symptom burden (ESAS), mean [SD] | 23.9 [15.7] | 24.7 [15.6] | 23.2 [15.8] | .374 |
Abbreviations: EOLC, end-of-life conversation; ESAS, Edmonton Symptom Assessment System.
Kidney, prostate, urethral.
Ovarian, endometrial.
Stomach, esophageal, colon, rectal.
Gallbladder, liver, pancreas.
Table 2.
Relationship Between CONNECT Intervention and New EOLC at 3 Months
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Unadjusted | 4.72 (2.89–7.71) | <.001 |
| Adjusteda | 5.28 (3.10–8.97) | <.001 |
Abbreviation: EOLC, end-of-life conversation.
Model adjusted for age, religious importance, education level, duration receiving care from current oncologist, and ECOG performance status.
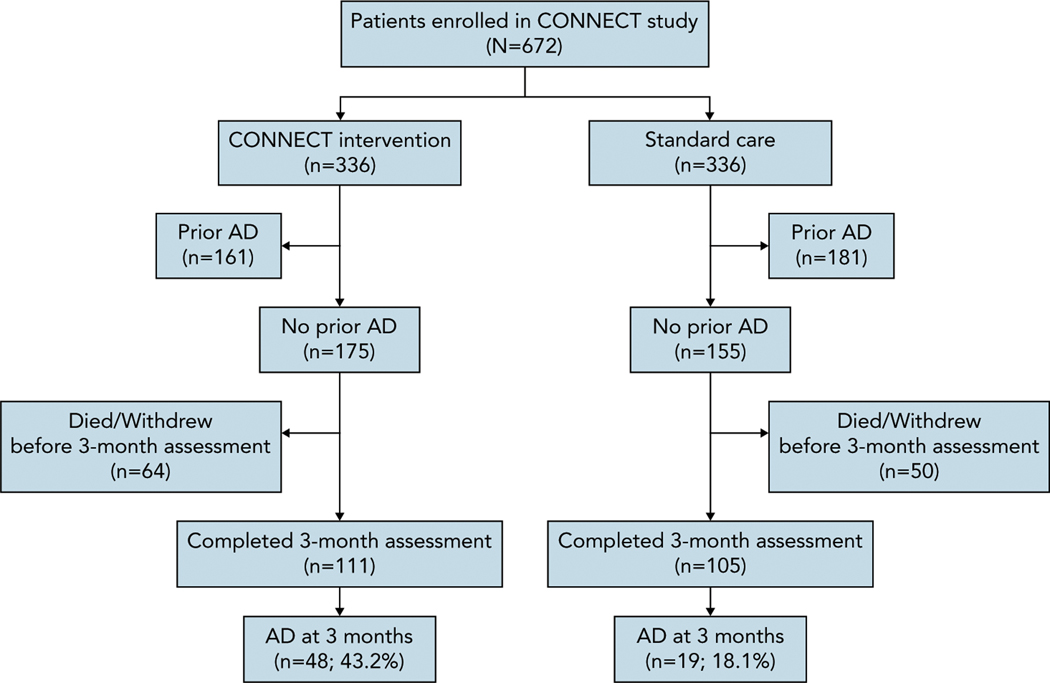
A total of 216 patients did not report an AD at baseline and completed the 3-month assessment (Figure 2). Characteristics of study participants who did not report having an AD at baseline are shown in Table 3. Among patients without an AD at baseline, 48/111 (43.2%) in the CONNECT arm and 19/105 (18.1%) in the standard care arm reported having an AD at the 3-month assessment (unadjusted OR, 3.45; P<.001). After adjustment, the odds of AD completion remained higher among patients in the CONNECT arm (adjusted OR, 3.68; P<.001) (Table 4).
Figure 2.
CONSORT diagram for completion of AD.
Abbreviation: AD, advance directive.
Table 3.
Characteristics of Study Participants Who Did Not Report Having an AD at Baseline
| All Patients Eligible for Analysis n (%) | CONNECT Intervention n (%) | Standard Care n (%) | P Value | |
|---|---|---|---|---|
| Patients, N | 216 | 111 | 105 | |
| Age, mean [SD], y | 66.0 [10.1] | 65.9 [9.4] | 66.1 [10.8] | .893 |
| Sex | .159 | |||
| Female | 119 (55.1) | 56 (50.5) | 63 (60.0) | |
| Male | 97 (44.9) | 55 (49.5) | 42 (40) | |
| Race | .986 | |||
| White | 195 (90.3) | 101 (91.0) | 94 (89.5) | |
| Black | 17 (7.9) | 8 (7.2) | 9 (8.6) | |
| Asian | 2 (0.9) | 1 (0.9) | 1 (1.0) | |
| Other | 2 (0.9) | 1 (0.9) | 1 (1.0) | |
| Religious importance | .461 | |||
| Not at all important | 7 (3.2) | 6 (5.4) | 1 (1.0) | |
| Not too important | 26 (12.0) | 12 (10.8) | 14 (13.3) | |
| Fairly important | 59 (27.3) | 30 (27.0) | 29 (27.6) | |
| Very important | 122 (56.5) | 62 (55.9) | 60 (57.1) | |
| Declined/No answer | 2 (0.9) | 1 (0.9) | 1 (1.0) | |
| Education level | .253 | |||
| Less than high school | 19 (8.8) | 12 (10.8) | 7 (6.7) | |
| High school diploma or GED | 99 (45.8) | 56 (50.5) | 43 (41.0) | |
| Some college/college degree | 86 (39.8) | 38 (34.2) | 48 (45.7) | |
| Graduate/Postgraduate degree | 9 (4.2) | 3 (2.7) | 6 (5.7) | |
| Declined/No answer | 3 (1.4) | 2 (1.8) | 1 (1.0) | |
| Current marital status | .183 | |||
| Never married | 18 (8.3) | 6 (5.4) | 12 (11.4) | |
| Married | 124 (57.4) | 66 (59.5) | 58 (55.2) | |
| Widowed/Divorced/Separated | 72 (33.3) | 39 (35.1) | 33 (31.4) | |
| Declined/No answer | 2 (0.9) | 0 (0.0) | 2 (1.9) | |
| Ability to manage on current income | .335 | |||
| Cannot make ends meet | 13 (6.0) | 6 (5.4) | 7 (6.7) | |
| Just manage to get by | 83 (38.4) | 44 (39.6) | 39 (37.1) | |
| Have enough with a little extra | 85 (39.4) | 47 (42.3) | 38 (36.2) | |
| Money is not a problem | 25 (11.6) | 8 (7.2) | 17 (16.2) | |
| Declined/No answer | 9 (4.2) | 5 (4.5) | 4 (3.8) | |
| Duration receiving care from current oncologist | .387 | |||
| <1 mo | 11 (5.1) | 7 (6.3) | 4 (3.8) | |
| 1–6 mo | 69 (31.9) | 35 (31.5) | 34 (32.4) | |
| 6 mo–1 y | 47 (21.8) | 19 (17.1) | 28 (26.7) | |
| 1–2 y | 38 (17.6) | 23 (20.7) | 15 (14.3) | |
| 2–5 y | 36 (16.7) | 17 (15.3) | 19 (18.1) | |
| >5 y | 14 (6.5) | 9 (8.1) | 5 (4.8) | |
| Declined/No answer | 1 (0.5) | 1 (0.9) | 0 (0.0) | |
| Cancer type | .923 | |||
| Genitourinarya | 21 (9.7) | 12 (10.8) | 9 (8.6) | |
| Brain | 2 (0.9) | 1 (0.9) | 1 (1.0) | |
| Breast/Gynecologicb | 36 (16.7) | 17 (15.3) | 19 (18.1) | |
| Gastrointestinalc | 46 (21.3) | 21 (18.9) | 25 (23.8) | |
| Hepatobiliaryd | 20 (9.3) | 10 (9.0) | 10 (9.5) | |
| Head and neck | 6 (2.8) | 4 (3.6) | 2 (1.9) | |
| Lung | 82 (38.0) | 45 (40.5) | 37 (35.2) | |
| Melanoma | 2 (0.9) | 1 (0.9) | 1 (1.0) | |
| Sarcoma | 1 (0.5) | 0 (0.0) | 1 (1.0) | |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ECOG performance status | .011 | |||
| 0 | 58 (26.9) | 21 (18.9) | 37 (35.2) | |
| 1 | 124 (57.4) | 74 (66.7) | 50 (47.6) | |
| 2 | 34 (15.7) | 16 (14.4) | 18 (17.1) | |
| Symptom burden (ESAS), mean [SD] | 25.5 [15.7] | 24.9 [15.6] | 26.2 [15.8] | .548 |
Abbreviations: AD, advance directive; ESAS, Edmonton Symptom Assessment System.
Kidney, prostate, urethral.
Ovarian, endometrial.
Stomach, esophageal, colon, rectal.
Gallbladder, liver, pancreas.
Table 4.
Relationship Between CONNECT Intervention and New AD at 3 Months
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Unadjusted | 3.45 (1.85–6.43) | <.001 |
| Adjusteda | 3.68 (1.89–7.16) | <.001 |
Abbreviation: AD, advance directive.
Model adjusted for age, religious importance, education, duration receiving care from current oncologist, and ECOG performance status.
Discussion
The primary goal of this analysis was to assess whether a nurse-led primary palliative care intervention improves ACP uptake in the forms of an EOLC with one’s oncologist and the completion of a living will or AD. We found that patients randomized to the intervention had significantly increased odds of engaging in EOLCs and completing an AD when compared with patients receiving standard oncology care.
A highly cited trial conducted among patients with non–small cell lung cancer showed that early specialty palliative care improved end-of-life outcomes, including ACP uptake.29 A 2014 systematic review indicated significant disparities in end-of-life outcomes and ACP based on access to palliative care.30 Although recent clinical practice guidelines advocate palliative care involvement for all patients with advanced malignancies,31 specialty palliative care resources remain limited in community settings.32 Based on the intervention providing palliative care led by oncology nurses, our findings represent a promising approach for meeting an unmet need across community oncology centers and a novel way to address the current NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Palliative Care recommending that ACP is facilitated for all patients with advanced malignancies.33
At baseline, <20% of patients reported an EOLC with their provider and fewer than half had an AD. These findings are consistent with decades of research showing substantial room for improvement in these domains of ACP.5,13,15,34 Previous work has shown that although oncologists feel that ACP is important, they are often reluctant to engage in it with their patients, citing insufficient time and a concern about taking away patient hope as barriers.35–37 The clear and structured role of CONNECT nurses may have helped incorporate ACP within oncology practices, bridging a gap between patients and oncologists. In a previously published in-depth interview study, CONNECT nurses reported that delivering primary palliative care was beneficial to their own careers and clinically meaningful but also required additional time and emotional investment.38 Future nurse-led interventions to increase patient engagement in ACP must ensure that nurses are adequately supported to fulfill these roles.
This analysis has limitations. First, the primary outcome of the parent study was quality of life; as such, the study was not designed to assess for differences in ACP. Second, sociodemographic and cultural factors play an important role in end-of-life care. The study population was drawn from a limited geographic area in suburban and rural western Pennsylvania and >90% of participants were White, possibly limiting generalizability to a more diverse population. Third, all participants agreed to participate in a palliative care study; a priori, this may select for patients more willing or open to engage in ACP.
Conclusions
Our findings show that a nurse-led primary palliative care intervention improves ACP uptake, assessed as an EOLC with one’s oncologist or completion of an AD, among patients with advanced cancer. Nurse-led primary palliative care is a promising approach to improve ACP among patients with advanced cancer, particularly for those without access to specialty palliative care.
Acknowledgments
The following individuals contributed to the writing, reviewing, or editing of the manuscript before publication: Shane Belin, Carolyn Impagliazzo, and Julian Hall.
Funding:
This trial was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA197103. This project used resources provided through the Clinical Protocol and Data Management and the Protocol Review and Monitoring System, which are supported in part by award P30CA047904. Dr. White reports additional support by the National Institutes of Health under grant number K24 HL148314. Dr. Schenker is supported by K24AG070285.
Footnotes
Disclosures: The authors have not received any financial consideration from any person or organization to support the preparation, results, analysis, or discussion of this article.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funders had any role in the conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript or decision to submit the paper for publication.
References
- 1.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017;53:821–832.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown AJ, Sun CC, Urbauer D, et al. Targeting those with decreased meaning and peace: a supportive care opportunity. Support Care Cancer 2015;23:2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa T, Fukui S, Okamoto Y. Advance care planning and home death in patients with advanced cancer: a structured interview analysis. Int J alliat Nurs 2018;24:418–426. [DOI] [PubMed] [Google Scholar]
- 4.Morrison RS, Meier DE, Arnold RM. What’s wrong with advance care planning? JAMA 2021;326:1575–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orlovic M, Callender T, Riley J, et al. Impact of advance care planning on dying in hospital: evidence from urgent care records. PLoS One 2020;15:e0242914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MG, Althouse AD, Arnold RM, et al. Hope and advance care planning in advanced cancer: is there a relationship? Cancer 2022;128:1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AJ, Shen MJ, Ramondetta LM, et al. Does death anxiety affect end-of-life care discussions? Int J Gynecol Cancer 2014;24:1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiarchiaro J, Buddadhumaruk P, Arnold RM, et al. Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc 2015;12:1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang ST, Chen JS, Wen FH, et al. Advance care planning improves psychological symptoms but not quality of life and preferred end-of-life care of patients with cancer. J Natl Compr Canc Netw 2019;17:311–320. [DOI] [PubMed] [Google Scholar]
- 10.Zwakman M, van Delden JJ, Caswell G, et al. Content analysis of advance directives completed by patients with advanced cancer as part of an advance care planning intervention: insights gained from the ACTION trial. Support Care Cancer 2020;28:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu D, Yen YF, Hu HY, et al. Factors associated with advance directives completion among patients with advance care planning communication in Taipei, Taiwan. PLoS One 2018;13:e0197552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vleminck A, Houttekier D, Pardon K, et al. Barriers and facilitators for general practitioners to engage in advance care planning: a systematic review. Scand J Prim Health Care 2013;31:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulmer T, Escobedo M, Berman A, et al. Physicians’ views on advance care planning and end-of-life care conversations. J Am Geriatr Soc 2018;66:1201–1205. [DOI] [PubMed] [Google Scholar]
- 14.Prod’homme C, Jacquemin D, Touzet L, et al. Barriers to end-of-life discussions among hematologists: a qualitative study. Palliat Med 2018;32:1021–1029. [DOI] [PubMed] [Google Scholar]
- 15.Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One 2015;10:e0116629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley P. Barriers to access to palliative care. Palliat Care 2017;10: 1178224216688887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker CL, Arnold RM, Park SY, et al. A cluster randomized trial of a primary palliative care intervention (CONNECT) for patients with advanced cancer: protocol and key design considerations. Contemp Clin Trials 2017;54:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R, Epstein AS. Advance care planning and end-of-life decision making for patients with cancer. Semin Oncol Nurs 2018;34:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenker Y, Althouse AD, Rosenzweig M, et al. Effect of an oncology nurse-led primary palliative care intervention on patients with advanced cancer: the CONNECT cluster randomized clinical trial. JAMA Intern Med 2021;181:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins-Welty GA, Mueser L, Mitchell C, et al. Interventionist training and intervention fidelity monitoring and maintenance for CONNECT, a nurse-led primary palliative care in oncology trial. Contemp Clin Trials Commun 2018;10:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss AH, Lunney JR, Culp S, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med 2010;13:837–840. [DOI] [PubMed] [Google Scholar]
- 22.Sudore RL, Heyland DK, Barnes DE, et al. Measuring advance care planning: optimizing the advance care planning engagement survey. J Pain Symptom Manage 2017;53:669–681.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6–9. [PubMed] [Google Scholar]
- 24.Cooney TM, Shapiro A, Tate CE. End-of-life care planning: the importance of older adults’ marital status and gender. J Palliat Med 2019;22:902–907. [DOI] [PubMed] [Google Scholar]
- 25.Kubi B, Istl AC, Lee KT, et al. Advance care planning in cancer: patient preferences for personnel and timing. JCO Oncol Pract 2020;16:e875–883. [DOI] [PubMed] [Google Scholar]
- 26.Huang IA, Neuhaus JM, Chiong W. Racial and ethnic differences in advance directive possession: role of demographic factors, religious affiliation, and personal health values in a national survey of older adults. J Palliat Med 2016;19:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruff H, Jacobs RJ, Fernandez MI, et al. Factors associated with favorable attitudes toward end-of-life planning. Am J Hosp Palliat Care 2011;28:176–182. [DOI] [PubMed] [Google Scholar]
- 28.Hirschman KB, Abbott KM, Hanlon AL, et al. What factors are associated with having an advance directive among older adults who are new to long term care services? J Am Med Dir Assoc 2012;13:82.e7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742. [DOI] [PubMed] [Google Scholar]
- 30.Bazargan M, Bazargan-Hejazi S. Disparities in palliative and hospice care and completion of advance care planning and directives among non-Hispanic Blacks: a scoping review of recent literature. Am J Hosp Palliat Care 2021;38:688–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112. [DOI] [PubMed] [Google Scholar]
- 32.Schenker Y, Arnold R. Toward palliative care for all patients with advanced cancer. JAMA Oncol 2017;3:1459–1460. [DOI] [PubMed] [Google Scholar]
- 33.Dans M, Smith T, Back A, et al. NCCN Guidelines Insights: palliative care, version 2.2017. J Natl Compr Canc Netw 2017;15:989–997. [DOI] [PubMed] [Google Scholar]
- 34.Brown AJ, Shen MJ, Urbauer D, et al. Room for improvement: an examination of advance care planning documentation among gynecologic oncology patients. Gynecol Oncol 2016;142:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elit L, Charles C, Gafni A, et al. Walking a tightrope: oncologists’ perspective on providing information to women with recurrent ovarian cancer (ROC) during the medical encounter. Support Care Cancer 2012;20:2327–2333. [DOI] [PubMed] [Google Scholar]
- 36.Waller A, Turon H, Bryant J, et al. Medical oncology outpatients’ preferences and experiences with advanced care planning: a cross-sectional study. BMC Cancer 2019;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson VA, Mack J, Matsuyama R, et al. A qualitative study of oncologists’ approaches to end-of-life care. J Palliat Med 2008;11:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feldenzer K, Rosenzweig M, Soodalter JA, et al. Nurses’ perspectives on the personal and professional impact of providing nurse-led primary palliative care in outpatient oncology settings. Int J Palliat Nurs 2019;25:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]