Abstract
BACKGROUND
The number of frozen embryo transfers (FET) has increased dramatically over the past decade. Based on current evidence, there is no difference in pregnancy rates when natural cycle FET (NC-FET) is compared to artificial cycle FET (AC-FET) in subfertile women. However, NC-FET seems to be associated with lower risk of adverse obstetric and neonatal outcomes compared with AC-FET cycles. Currently, there is no consensus about whether NC-FET needs to be combined with luteal phase support (LPS) or not. The question of how to prepare the endometrium for FET has now gained even more importance and taken the dimension of safety into account as it should not simply be reduced to the basic question of effectiveness.
OBJECTIVE AND RATIONALE
The objective of this project was to determine whether NC-FET, with or without LPS, decreases the risk of adverse obstetric and neonatal outcomes compared with AC-FET.
SEARCH METHODS
A systematic review and meta-analysis was carried out. A literature search was performed using the following databases: CINAHL, EMBASE, and MEDLINE from inception to 10 October 2022. Observational studies, including cohort studies, and registries comparing obstetric and neonatal outcomes between singleton pregnancies after NC-FET and those after AC-FET were sought. Risk of bias was assessed using the ROBINS-I tool. The quality of evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation approach. We calculated pooled odds ratios (ORs), pooled risk differences (RDs), pooled adjusted ORs, and prevalence estimates with 95% CI using a random effect model, while heterogeneity was assessed by the I2.
OUTCOMES
The conducted search identified 2436 studies, 890 duplicates were removed and 1546 studies were screened. Thirty studies (NC-FET n = 56 445; AC-FET n = 57 231) were included, 19 of which used LPS in NC-FET. Birthweight was lower following NC-FET versus AC-FET (mean difference 26.35 g; 95% CI 11.61–41.08, I2 = 63%). Furthermore NC-FET compared to AC-FET resulted in a lower risk of large for gestational age (OR 0.88, 95% 0.83–0.94, I2 = 54%), macrosomia (OR 0.81; 95% CI 0.71–0.93, I2 = 68%), low birthweight (OR 0.81, 95% CI 0.77–0.85, I2 = 41%), early pregnancy loss (OR 0.73; 95% CI 0.61–0.86, I2 = 70%), preterm birth (OR 0.80; 95% CI 0.75–0.85, I2 = 20%), very preterm birth (OR 0.66, 95% CI 0.53–0.84, I2 = 0%), hypertensive disorders of pregnancy (OR 0.60, 95% CI 0.50–0.65, I2 = 61%), pre-eclampsia (OR 0.50; 95% CI 0.42–0.60, I2 = 44%), placenta previa (OR 0.84, 95% CI 0.73–0.97, I2 = 0%), and postpartum hemorrhage (OR 0.43; 95% CI 0.38–0.48, I2 = 53%). Stratified analyses on LPS use in NC-FET suggested that, compared to AC-FET, NC-FET with LPS decreased preterm birth risk, while NC-FET without LPS did not (OR 0.75, 95% CI 0.70–0.81). LPS use did not modify the other outcomes. Heterogeneity varied from low to high, while quality of the evidence was very low to moderate.
WIDER IMPLICATIONS
This study confirms that NC-FET decreases the risk of adverse obstetric and neonatal outcomes compared with AC-FET. We estimate that for each adverse outcome, use of NC-FET may prevent 4 to 22 cases per 1000 women. Consequently, NC-FET should be the preferred treatment in women with ovulatory cycles undergoing FET. Based on very low quality of evidence, the risk of preterm birth be decreased when LPS is used in NC-FET compared to AC-FET. However, because of many uncertainties—the major being the debate about efficacy of the use of LPS—future research is needed on efficacy and safety of LPS and no recommendation can be made about the use of LPS.
Keywords: frozen-thawed embryo transfer, artificial cycle, natural cycle, safety, hypertensive disorders of pregnancy, birthweight, luteal phase support
Graphical Abstract
Natural cycle FET decreases the risk of adverse obstetric and neonatal outcomes compared with artificial cycle FET. FET: frozen embryo transfer.
Introduction
It has been more than 30 years since the first successful frozen embryo transfer (FET) (Trounson and Mohr, 1983; Zeilmaker et al., 1984). Nowadays, FET is increasingly applied throughout the world and in 2015 FET accounted for about 40% of all IVF cycles in Europe (De Geyter et al., 2018; ESHRE, 2018; Pereira et al., 2019; European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) et al., 2021). The rise of FET is mainly due to improvement in laboratory techniques and use of the freeze-all strategy (Zaat et al., 2021a).
In FET one needs to synchronize the endometrium with the developmental stage of the embryo to facilitate implantation. The two most common ways to prepare the endometrium and to time the FET are artificial cycle FET (AC-FET) and natural cycle FET (NC-FET). In AC-FET exogenous estrogen is administered to develop the endometrium, subsequently progesterone is used to prepare the endometrium and time FET after optional downregulation using GnRH agonist. In NC-FET, there is a natural build-up of the endometrium while using detection of the LH surge to time the embryo transfer.
In spontaneous ovulatory cycles, the resulting corpus luteum (CL) is believed to effectively supply all that is necessary for embryo implantation. When hCG is used for ovulation triggering, its long half-life renders it also as a form of luteal phase support (LPS) (Casper and Yanushpolsky, 2016). A recent meta-analysis concluded—based on low-quality evidence with high heterogeneity in treatment protocols—that progesterone administration for LPS may be beneficial following NC-FET in terms of clinical pregnancy and live birth rates (Mizrachi et al., 2021). A Cochrane review that compared different types of endometrium preparation for FET found comparable effectiveness in terms of pregnancy chance (Glujovsky et al., 2020). Safety issues were not considered in the Cochrane review and no specific analysis was performed on NC-FET with or without LPS. Whether or not women should receive LPS following NC-FET is controversial but, nonetheless, NC-FET with LPS is often used in clinical practice (Weissman, 2020; Mizrachi et al., 2021).
The latest observational data assessing obstetrical and neonatal outcomes after FET suggests higher risk of early pregnancy loss, rates of hypertensive disorders of pregnancy (HDP), gestational diabetes (GDM), placental pathology, postpartum hemorrhage (PPH), higher birthweights, more babies being born as large for gestational age (LGA), and macrosomia in AC-FET cycles compared with NC-FET cycles (Hatoum et al., 2018; Saito et al., 2017; Ginstrom Ernstad et al., 2019; Saito et al., 2019; Wang et al., 2019; von Versen-Hoynck et al., 2019a,b; Wang et al., 2020a,b; Moreno-Sepulveda et al., 2021; Rosalik et al., 2021; Severino and Povoa, 2021; Zaat et al., 2021a; Busnelli et al., 2022; Vinsonneau et al., 2022). In these studies, no analysis was performed on the effectiveness of the use of LPS in NC-FET compared to NC-FET without using LPS. No meta-analyses have been performed on early pregnancy loss in AC-FET compared to NC-FET and in previous meta-analysis, there has not been a stratified analysis on women with or without polycystic ovary syndrome (PCOS). Given the increasing use of FET, it is important to evaluate the safety and effectiveness of all its specific elements. Knowledge on how to prepare the endometrium for FET should not only focus on of optimal pregnancy rates but also on the safest outcome for mother and baby.
With our systematic review, we aim to determine whether NC-FET with or without LPS decreases the risk of adverse obstetric and neonatal outcomes compared with AC-FET.
Methods
This systematic review and meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered at the International Prospective Register of Systematic Reviews [PROSPERO CRD42020163086].
Literature search and data extraction
A literature search was performed using the following databases: Pubmed, CINAHL, and EMBASE from inception to 10 October 2022. In addition, references of selected articles were examined to identify other relevant studies. Supplementary Data File S1 shows the complete search strategy. Following the search, we have used Covidence systematic review software where duplicates were removed (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). Two investigators (T.R.Z. and P.K.) independently reviewed titles, abstracts and full text articles, and selected the studies. Any disagreements were resolved by discussion with a third author (M.v.W.) until consensus was reached. Two authors (T.R.Z. and P.K.) independently assessed the quality of the included studies and the quality of the evidence (T.R.Z. and E.K.). Disagreement regarding type and quality of the study was resolved after discussion with the third author (M.v.W.).
Eligibility criteria
Three criteria for inclusion were determined, which all had to be fulfilled. First, the study design was a retrospective cohort study, post hoc analysis or follow-up studies of randomized controlled trials (RCTs), because it is not possible to accomplish conditions of prevalence in study designs other than cohort studies. Second, the studies should consist of a study population of women who conceived after NC-FET (including true NC-FET, defined as home-based monitoring of ovulation to time FET and modified NC-FET, defined as ultrasound monitoring and hCG trigger for ovulation to time FET) and a control group of women who conceived after AC-FET. Finally, data had to be available about the obstetric and/or neonatal outcomes. Exclusion criteria were other study designs, studies without control group or studies that did not contain data about obstetric and/or neonatal outcomes. In our research protocol we stated the exclusion criterion: studies including anovulatory women and women with polycystic ovary syndrome (PCOS). However, in the final review and meta-analysis, this exclusion criterion was rejected because of the small number of studies only including ovulatory women. We performed a subgroup analysis on studies that excluded women with PCOS.
Outcome measures
We chose birthweight as the main outcome and report this as: absolute birthweight (g); LGA (birthweight > 90th percentile); macrosomia (as defined by the authors of the included studies); low birthweight (LBW; birthweight < 2500 g); and small for gestational age (SGA; birthweight < p10).
Additional outcomes included:
-
Obstetric outcomes
Obstetric outcomes included: early pregnancy loss (defined as a miscarriage before 20 weeks of gestation expressed per woman with a registered pregnancy, although usually only first trimester pregnancy loss could be extracted); GDM (as defined by the authors of included studies); HDP (including pregnancy-induced hypertension, pre-eclampsia (PE) and hemolysis, elevated liver enzymes, and low platelets in the blood (HELLP syndrome); all as defined by the authors of included studies); PPH (as defined by the authors of included studies); placenta previa; preterm birth (PTB: delivery <37 weeks of gestation); and very preterm birth (very PTB; delivery <32 weeks of gestation).
-
Neonatal outcomes
Neonatal outcomes were congenital malformations and neonatal mortality.
Assessment of heterogeneity
To ensure that pooling was valid, we assessed the similarity between the eligible studies in their design and clinical characteristics using the I2 statistic. An I2 >50% was labeled as marked heterogeneity (Higgins et al., 2003).
Quality and risk of bias assessment
The ROBINS-I bias tool, for assessing the quality of studies in meta-analyses was used to evaluate the included studies (Sterne et al., 2016). The quality of the included studies was evaluated according to the following variables: confounding, selection of participants, classification of intervention, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. The included studies were graded as low quality, high quality, or unknown. A risk of bias summary was created in Review Manager software (version 5.4; The Cochrane Collaboration, 2020). The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Atkins et al., 2004). Quality of evidence was downgraded by one level for serious concerns and by two levels for very serious concerns for risk of bias, inconsistency, indirectness, imprecision, and publication bias. The assessment of bias and grading of evidence was performed independently by two authors. Any disagreements were resolved by discussion or consultation with a third author.
Data analysis
Studies that met the inclusion criteria were selected for analysis. Extracted data from included studies were pooled using StataCorp (2015. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP). We calculated pooled odds ratios (ORs), pooled risk differences (RDs), pooled adjusted ORs, and prevalence estimates with 95% CI using a random effect model, while heterogeneity was assessed by the I2. We performed stratified analyses on LPS use. As sensitivity analysis, we stratified studies on completely excluding women with PCOS and studies that also included women with PCOS.
Results
Result of the searches
The conducted search identified 2436 studies, then 890 duplicates were removed and 1546 studies were screened based on the abstract. In total, 1509 studies were excluded based on abstract. The remaining 37 studies were considered eligible by at least one of the reviewers. Subsequently, we excluded seven studies by screening of full text. Thirty studies met the inclusion criteria (Nakashima et al., 2013; Lathi et al., 2015; Guan et al., 2016; Cerrillo et al., 2017; Saito et al., 2017; Ernstad et al., 2019; Jing et al., 2019; Saito et al., 2019; von Versen-Hoynck et al., 2019a,b; Bu et al., 2020; Levi Setti et al., 2020; Lin et al., 2020; Makhijani et al., 2020; Pan et al., 2020; Zong et al., 2020; Wang et al., 2020a,b; Aslih et al., 2021; Asserhoj et al., 2021; Hu et al., 2021; Li et al., 2021; Waschkies et al., 2021; Zaat et al., 2021b; Dallagiovanna et al., 2022; Gu et al., 2022; Li et al., 2022; Roelens et al., 2022; Xu et al., 2022; Yang et al., 2022; Zhou et al., 2022). Detailed information about the selection of studies for inclusion is shown in the PRISMA flow diagram in Fig. 1 Characteristics of the included studies are reported in Table 1.
Figure 1.
Prisma flow diagram of the systematic search.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Study period | Inclusion criteria* | Exclusion criteria | Embryo stage at transfer (%) | Luteal phase support NC-FET | Study groups (n) |
|---|---|---|---|---|---|---|---|---|
| Aslih et al. (2021) | Israel | RC | 2016–2018 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 10.6%; AC-FET 24.2%; P < 0.0001) | The use of donor oocytes, FET cancel due to endometrial polyps, premature progesterone elevation | Cleavage and blastocyst | Yes |
|
| Asserhoj et al. (2021) | Denmark |
|
2006–2014 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 2.9%; AC-FET 9.6%) | The use of donor oocytes or PGT |

|
No |
|
| Wang et al. (2020a,b) | China |
|
2014–2017 | All women with a singleton live birth >28 weeks after FET. Included women with anovulation and/or PCOS (NC-FET 2.4%; AC-FET 2.4%; P = 0.91). | The use of donor embryo’s, PGT, live-born singletons from twin deliveries with a stillbirth |

|
Yes |
|
| Bu et al. (2020) | China |
|
2010–2018 | All women with a singleton live birth after FET. Unclear whether women with anovulation and/or PCOS were included. | Uterus malformation, PGT, oocyte donation, a history of artificial multiple pregnancy reduction/vanish twin | N/A | N/A |
|
| Cerrillo et al. (2017) | Spain |
|
2011–2012 | Women with a singleton live birth after FET, age <40, regular cycles (26–35 days) and no more than 2 previous IVF cycles | The use of donor oocytes, PGT, irregular cycles, PCOS, endometriosis stage III/IV | N/A | Yes |
|
| Dallagiovanna et al. (2022) | Italy |
|
2014–2019 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 1.0%; AC-FET 26.0%). | Multiple pregnancies or risk factors for HDP | All frozen blastocysts transfer | No |
|
| Ernstad et al. (2019) | Sweden |
|
2005–2015 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 11.9%; AC-FET 34.0%). | N/A |

|
No |
|
| Gu et al. (2022) | China |
|
2016–2019 | All women with a singleton live birth after FET delivered after 28 weeks of gestation | Cycles with PGT, vanishing twins, and women with a history of preeclampsia, type 2 diabetes mellitus, prediabetes mellitus, hypertension, and PCOS | All frozen blastocysts transfer | Yes |
|
| Guan et al. (2016) | China |
|
2012–2013 | All women with a singleton live birth after FET, regular menstrual cycle | History of recurrent implantation failure or abortion | All vitrified-thawed Day 3 embryo’s, stage not reported | N/A |
|
| Hu et al. (2021) | China |
|
2013–2019 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 6.0%; AC-FET 29.0%). | The use of donor oocytes or donor sperm, PGT, twin deliveries or neonatal death. | All frozen blastocysts transfer | Yes |
|
| Jing et al. (2019) | China |
|
2013–2016 | Women with a singleton live birth after FET with at least one blastocyst or two cleavage-stage embryos in storage, regular ovulatory cycles, and at most two previous FET | Ovulation disorders, Asherman syndrome, PCOS and uterine malformation |

|
Yes |
|
| Lathi et al. (2015) | USA |
|
2007–2012 | All women with a singleton live birth after first attempt FET. I Unclear whether women with anovulation and/or PCOS were included | Cycles using embryos cryopreserved at the 2 PN stage or on Day 3 and the use of donor oocytes | All frozen blastocysts transfer | Yes |
|
| Levi Setti et al. (2020) | Italy |
|
2011–2017 | All women with a singleton live birth after single FET. Included women with anovulation and/or PCOS (tNC-FET 5.0%; mNC-FET 6.8%; AC-FET 23.9%; P < 0.0001) | Cycles using PGT or more than one embryo per transfer | All frozen blastocysts transfer | Yes |
|
| Li et al. (2021) | China |
|
2010–2017 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 1.3%; AC-FET 11.6%. | N/A |

|
No |
|
| Li et al. (2022) | China |
|
2018–2020 | All women with a singleton live birth after FET. | Women with uterine anatomic abnormalities, donor gametes, PGT, PCOS, chronic hypertension, diabetes, heart disease, foetal anomalies | 1599 blastocyst transfer, 594 cleavage stage transfer | Yes |
|
| Lin et al. (2020) | China |
|
2016–2017 | Women with a singleton live birth after FET who participated in the previously published RCT with regular cycles undergoing their first IVF cycle | N/A | All frozen blastocysts transfer | Yes |
|
| Makhijani et al. (2020) | USA |
|
2013–2018 | All women with a singleton live birth after FET, one cycle per participant. Included women with anovulation and/or PCOS (NC-FET 3.9%; AC-FET 37.3%). | The use of donor oocytes, cleavage stage embryos or slow freeze embryo’s. Multiple pregnancies | All frozen blastocysts transfer | Yes |
|
| Nakashima et al. (2013) | Japan | RRBC MC | 2007–2008 | All women with a singleton live birth after FET. Unclear whether women with anovulation and/or PCOS were included | The use of frozen-thawed oocytes, gamete intrafallopian transfer, oocyte intrauterine transfer, two-step embryo transfer cycles |
|
N/A |
|
| Pan et al. (2020) | China |
|
2015–2017 | Women with a singleton live birth after FET who participated in the previously published RCT, age >20 and ≤35 years, regular cycle (21–35 days), first IVF/ICSI cycle, >5 oocytes retrieved | Uterine anatomic abnormalities, one ovary removed, PCOS, PGT, recurrent miscarriages | All cleavage stage embryo’s | Yes |
|
| Roelens et al. (2022) | Belgium |
|
2010–2019 | All women with a singleton live birth after FET. Included women with anovulation and/or PCOS (NC-FET 6.5%; AC-FET 41.2%; P < 0.001). | Cycles with LPS, or FET after ovulation induction |

|
No |
|
| Saito et al. (2017) | Japan | RRBC MC | 2013 | All women with a singleton live birth >22 weeks after FET from autologous oocytes. Included women with anovulation and/or PCOS (percentages not stated) | Multiple pregnancies |

|
N/A |
|
| Saito et al. (2019) | Japan | RRBC MC | 2014 | All women with a singleton live birth after FET from autologous oocytes. Included women with anovulation and/or PCOS (percentages not stated) | FET cycles with ovarian stimulation |

|
Yes |
|
| von Versen-Hoynck et al. (2019a,b) | USA |
|
Not stated |
|
By administration of the Study Coordinator | N/A | Yes |
|
| Waschkies et al. (2021) | Germany |
|
1997–2019 | All women with a singleton live birth after FET from autologous oocytes. Included women with anovulation and/or PCOS (NC-FET 25.0%; AC-FET 20.2%; P = 0.39) | Multiple pregnancies | N/A | Yes |
|
| Xu et al. (2022) | China |
|
2016–2021 |
|
Cycles with PGT, women with chronic hypertension or diabetes mellitus or with congenital or secondary uterine abnormalities |

|
Yes |
|
| Yang et al. (2022) | China |
|
2014–2021 | Women with a singleton live birth after FET, age ≤40 years at the time IVF treatment | Cycles with oocyte donor, PGT or slow freeze. Women with presence of chromosomal abnormalities, history of uterine surgery, presence of intracavitary lesions | N/A | Yes |
|
| Zaat et al. (2021b) | Netherlands |
|
Women with a singleton live birth after FET who participated in the previously published RCT, age 18–40 years; first, second or third IVF, regular menstrual cycle | Multiple pregnancies |

|
No |
|
|
| Wang et al. (2020) | China |
|
2013–2018 | All women with a singleton live birth after FET | The use of frozen-thawed oocytes, age >40, BMI >35 kg/m2, PCOS, self-history or family history of PE, diagnosis of hypertension, diabetes, renal disease, or abnormal renal function, a history of failure to obtain clinical pregnancy after >3 times FET | All frozen blastocysts transfer | Yes |
|
| Zhou et al. (2022) | China |
|
2017–2020 | Women with a singleton live birth after autologous FET, maternal age ≤42 years; BMI <28, regular menstrual cycle (21–35 days). | Multiple pregnancies, congenital uterine malformations, intrauterine adhesions, PCOS. Women with chronic medical conditions that have been associated with adverse pregnancy outcomes |

|
Yes |
|
| Zong et al. (2020) | China |
|
2015–2018 | Women with a singleton live birth >28 weeks after FET, age 20–40 | Type II diabetes mellitus, preconceptional hypertension, PCOS, uterine malformation, intrauterine adhesion, the use of donor oocyte or PGT | All frozen blastocysts transfer | Yes |
|
The studies of Wang (2020a,b), Gu (2022), and Zong (2020) only included women with live birth after 28 weeks of gestation. N/A: not available; RRBC: retrospective register-based cohort; RC: retrospective cohort; PC: prospective cohort; MC: multiple center; SC: single center; FET: frozen embryo transfer; NC-FET: natural cycle FET (not specified whether true or modified); tNC-FET: true natural cycle FET; mNC-FET: modified natural cycle FET; sC: stimulated cycle FET; AC: artificial cycle FET; PGT: preimplantation genetic testing; PCOS: polycystic ovary syndrome.
Included studies
Methodology of the included studies
Data from 30 studies (NC-FET n = 56 445; AC-FET n = 57 231) were included in the meta-analysis (Table 1). Five were retrospective register-based cohort studies (Nakashima et al., 2013; Saito et al., 2017; Ernstad et al., 2019; Saito et al., 2019; Asserhoj et al., 2021). Twenty-three were retrospective cohort studies (Lathi et al., 2015; Guan et al., 2016; Jing et al., 2019; Bu et al., 2020; Levi Setti et al., 2020; Lin et al., 2020; Makhijani et al., 2020; Pan et al., 2020; Zong et al., 2020; Wang et al., 2020a,b; Aslih et al., 2021; Hu et al., 2021; Li et al., 2021; Waschkies et al., 2021; Zaat et al., 2021b; Dallagiovanna et al., 2022; Gu et al., 2022; Li et al., 2022; Roelens et al., 2022; Xu et al., 2022; Yang et al., 2022; Zhou et al., 2022). Two were prospective cohort studies (Cerrillo et al., 2017; von Versen-Hoynck et al., 2019a,b). Twenty studies were single-center studies (Lathi et al., 2015; Guan et al., 2016; Cerrillo et al., 2017; Jing et al., 2019; von Versen-Hoynck et al., 2019a,b; Bu et al., 2020; Levi Setti et al., 2020; Makhijani et al., 2020; Zong et al., 2020; Wang et al., 2020a,b; Aslih et al., 2021; Hu et al., 2021; Li et al., 2021; Dallagiovanna et al., 2022; Li et al., 2022; Roelens et al., 2022; Xu et al., 2022; Yang et al., 2022; Zhou et al., 2022). The other 10 studies were multicenter studies (Nakashima et al., 2013; Saito et al., 2017; Ernstad et al., 2019; Saito et al., 2019; Lin et al., 2020; Pan et al., 2020; Asserhoj et al., 2021; Waschkies et al., 2021; Zaat et al., 2021b; Guet al., 2022). Across all studies, data were extracted from national registers or hospitals records (Table 1).
LPS protocols for NC-FET in the included studies
Twenty studies used LPS in NC-FET (Lathi et al., 2015; Cerrillo et al., 2017; Jing et al., 2019; Saito et al., 2019; von Versen-Hoynck et al., 2019a,b; Levi Setti et al., 2020; Lin et al., 2020; Makhijani et al., 2020; Pan et al., 2020; Zong et al., 2020; Wanget al., 2020a,b; Aslih et al., 2021; Hu et al., 2021; Waschkies et al., 2021; Gu et al., 2022; Li et al., 2022; Xu et al., 2022; Yang et al., 2022; Zhou et al., 2022), six studies did not use LPS (Ernstad et al., 2019; Asserhoj et al., 2021; Li et al., 2021; Zaat et al., 2021b; Dallagiovanna et al., 2022; Roelens et al., 2022), and for four studies this was unclear (Nakashima et al., 2013; Guan et al., 2016; Saito et al., 2017; Buet al., 2020). The protocols used for LPS in NC-FET varied widely between studies, as presented in Table 2. The use of LPS in case of pregnancy ranged from 5 to 12 weeks of gestation (Table 2).
Table 2.
Luteal phase support during NC-FET in the included studies.
| Study | Generic name, dose (brand name/manufacturer) | Administration route | Start of LPS use | Duration of LPS during gestation |
|---|---|---|---|---|
| Aslih (2021) |
|
|
Not reported | Until 10 weeks of gestation |
| Wang (2020a,b) | Dydrogesterone 10 mg (Duphastone® Abbott, Biologicals) | Not reported | Third day after hCG injection | Not reported |
| Cerrillo (2017) | Micronized progesterone 400 mg (Utrogestan® Seid, Barcelona, Spain) | Vaginal | Three or 5 days before FET | Until 5 weeks of gestation |
| Gu (2022) |
|
|
Day of ovulation | Until 10 weeks of gestation |
| Hu (2021) | Dydrogesterone 20 mg (Duphaston; Solvay Pharmaceuticals BV) | Oral | One day before FET | Not reported |
| Jing (2019) | Progesterone 600 mg (Duphaston, Abbott Biologicals B.V., The Netherlands) | Vaginal | Two days before FET | Not reported |
| Lathi (2015) | Progesterone 200 mg (not reported) | Vaginal |
|
Until 10–12 weeks of gestation |
| Levi Setti (2020) |
|
|
|
Not reported |
| Li (2022) |
|
|
When endometrial thickness reached 7 mm, serum E2 level peaked at 200 pg/ml, and the serum levels of P4 were <1.5 ng/ml | Until 10–12 weeks of gestation |
| Lin (2020) | Dydrogesterone 30 mg (not reported) | Oral | After day of ovulation | Until 10 weeks of gestation |
| Makhijani (2020) | Progesterone (Crinone, Merck, Kenilworth, NJ, USA; and Endometrin, Ferring Pharmaceuticals, Parsippany, NJ, USA) | Vaginal | Two days after LH-surge | Not reported |
| Pan (2020) | Dydrogesterone 20 mg (not reported) | Oral | Day of ovulation | Until 10 weeks of gestation |
| Saito et al. (2019) | Groups: progesterone alone; hCG; progesterone + hCG; estrogen + progesterone; estrogen + progesterone + hCG (not reported) | Not reported | Not reported | Not reported |
| von Versen-Hoynck (2019a,b) | Estradiol or progesterone (not reported) | Not reported | Not reported | Not reported |
| Waschkies (2021) | Progesterone 200–300 mg (not reported) | Vaginal | Day of hCG-injection | Until 10–12 weeks of gestation |
| Xu (2022) | Dydrogesterone 30 mg (Duphastone® Abbott, Biologicals) | Oral | Day of ovulation | Until 10 weeks of gestation |
| Yang (2022) |
|
|
After FET | Not reported |
| Wang (2020) | Progesterone 20–30 mg (not reported) | Not reported | After ovulation | Until 10 weeks of gestation |
| Zhou (2022) | Dydrogesterone 40 mg (Duphaston; Abbott, OLST, Netherlands) | Oral | On day of ovulation | Not stated |
| Zong (2020) | Dydrogesterone 30 mg (Duphaston, Abbott Biologicals B.V.) | Oral | On day of ovulation | Until 12 weeks of gestation |
FET: frozen embryo transfer; tNC-FET: true natural cycle FET; mNC-FET: modified natural cycle FET; E2: estradiol; P4 progesterone.
Outcomes reported in the included studies
Not all studies demonstrated all pre-defined outcomes, and included outcomes per study are presented in Table 3.
Table 3.
Outcomes reported per included study.
| Study | Birthweight | LGA | Macrosomia | SGA | LBW | EPL | GDM | HDP | PE | PPH | Placenta praevia | PTB | Very PTB | Cong malformations | Neonatal mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aslih (2021) | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Asserhoj (2021) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Wang (2020a,b) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Bu (2020) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | x | ✗ |
| Cerrillo (2017) | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | x | ✗ |
| Dallagiovanna (2022) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | x |
| Ernstad (2019) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gu (2022) | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Guan (2016) | ✓ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Hu (2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Jing (2019) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ |
| Lathi (2015) | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | x | ✗ |
| Levi Setti (2020) | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | x | ✗ |
| Li (2021) | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| Li (2022) | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Lin (2020) | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | x | ✗ |
| Makhijani (2020) | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Nakashima (2013) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | x | ✗ |
| Pan (2020) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ |
| Roelens (2022) | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ |
| Saito (2017) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Saito et al. (2019) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ |
| von Versen-Hoynck (2019a,b) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Waschkies (2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Xu (2022) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Yang (2022) | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Zaat (2021b) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Wang (2020) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Zhou (2022) | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ |
| Zong (2020) | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ |
LGA: large for gestational age; SGA: small for gestational age; LBW: low birthweight; EPL: early pregnancy loss; GDM: gestational diabetes mellitus; HDP: hypertensive disorders of pregnancy; PE: pre-eclampsia; PPH: postpartum hemorrhage; PTB: preterm birth; cong: congenital.
Outcomes
Main outcomes
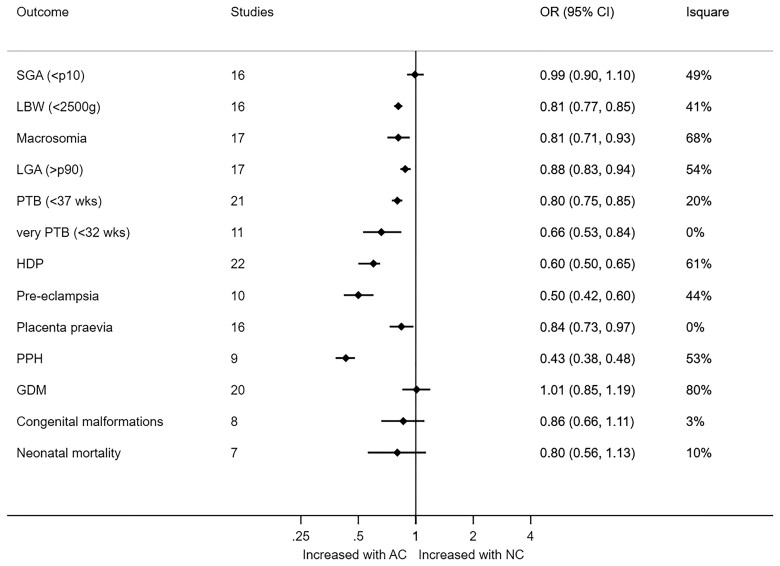
Birthweight was lower following NC-FET versus AC-FET (MD 26.35 g; 95% CI 11.61–41.08, I2 = 63%). NC-FET compared to AC-FET resulted in a lower risk of LGA (OR 0.88, 95% 0.83–0.94, I2 = 54%; RD −0.016, 95% CI −0.024 to −0.008), macrosomia (OR 0.81; 95% CI 0.71–0.93, I2 = 68%; RD −0.007, 95% CI −0.012 to −0.002), and LBW (OR 0.81, 95% CI 0.77–0.85, I2 = 41%; RD −0.012, 95% CI −0.018 to −0.005) (Table 4, Fig. 2, Supplementary Figs S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17, S18, S19, S20, S21, S22, S23, S24, S25, S26, S27, and S28). Prevalence estimates for these main outcomes following NC-FET and AC-FET are depicted in Supplementary Table S1.
Table 4.
The summary of findings for NC-FET versus AC-FET with the grading of the evidence.
| Outcomes | Mean difference (95% CI) | Number of studies | GRADE | ||
|---|---|---|---|---|---|
| Birthweight | 26.35 (11.61–41.08) | 23 studies | MODERATE | ||
|
| |||||
| Pooled odds ratio (95% CI) | Pooled adjusted odds ratio (95% CI) | Absolute risk difference (95% CI) | |||
|
| |||||
| LGA | 0.88 (0.83–0.94) | 0.87 (0.80–0.93) | −0.016 (−0.024 to −0.008) | 17 studies | MODERATE |
| Macrosomia | 0.81 (0.71–0.93) | 0.77 (0.69–0.86) | −0.007 (−0.012 to −0.002) | 17 studies | LOW |
| SGA | 0.99 (0.90–1.10) | 0.97 (0.88–1.06) | −0.001 (−0.006 to −0.004) | 16 studies | LOW |
| LBW | 0.79 (0.72–0.87) | 0.77 (0.66–0.89) | −0.012 (−0.018 to −0.005) | 15 studies | MODERATE |
| Early pregnancy loss | 0.73 (0.61–0.86) | NA | −0.040 (−0.060 to −0.030) | 10 studies | LOW |
| GDM | 1.01 (0.85–1.19) | 1.02 (0.92–1.14) | 0.000 (−0.010 to 0.010) | 20 studies | VERY LOW |
| HDP | 0.60 (0.50–0.65) | 0.52 (0.47–0.58) | −0.022 (−0.031 to −0.020) | 20 studies | MODERATE |
| PE | 0.50 (0.42–0.60) | 0.43 (0.37–0.51) | −0.036 (−0.053 to −0.019) | 10 studies | MODERATE |
| PPH | 0.43 (0.38–0.48) | 0.44 (0.36–0.47) | −0.052 (−0.096 to −0.009) | 9 studies | VERY LOW |
| Placenta previa | 0.84 (0.73–0.97) | 0.85 (0.66–1.10) | −0.002 (−0.004 to 0.001) | 16 studies | MODERATE |
| PTB | 0.80 (0.75–0.85) | 0.79 (0.75–0.85) | −0.015 (−0.020 to −0.010) | 21 studies | MODERATE |
| Very PTB | 0.66 (0.53–0.84) | 0.56 (0.40–0.78) | −0.004 (−0.007 to −0.001) | 11 studies | MODERATE |
| Cong malformations | 0.86 (0.66–1.11) | 0.99 (0.75–1.30) | 0.000 (−0.010 to 0.010) | 8 studies | VERY LOW |
| Neonatal mortality | 0.80 (0.56–1.13) | NA | −0.000 (−0.000 to 0.000) | 7 studies | VERY LOW |
| Stratified analysis LPS PTB | 0.75 (0.70–0.81) | NA | NA | 14 studies | VERY LOW |
LGA: large for gestational age; SGA: small for gestational age; LBW: low birthweight; GDM: gestational diabetes mellitus; HDP: hypertensive disorders of pregnancy; PE: pre-eclampsia; PPH: postpartum hemorrhage; PTB: preterm birth; LPS: luteal phase support; NC: natural cycle; AC: artificial cycle; FET: frozen embryo transfer; NA: not available.
Figure 2.
Difference in birthweight for NC-FET versus AC-FET, stratified by luteal phase support use. NC: natural cycle; AC: artificial cycle; LPS: luteal phase support.
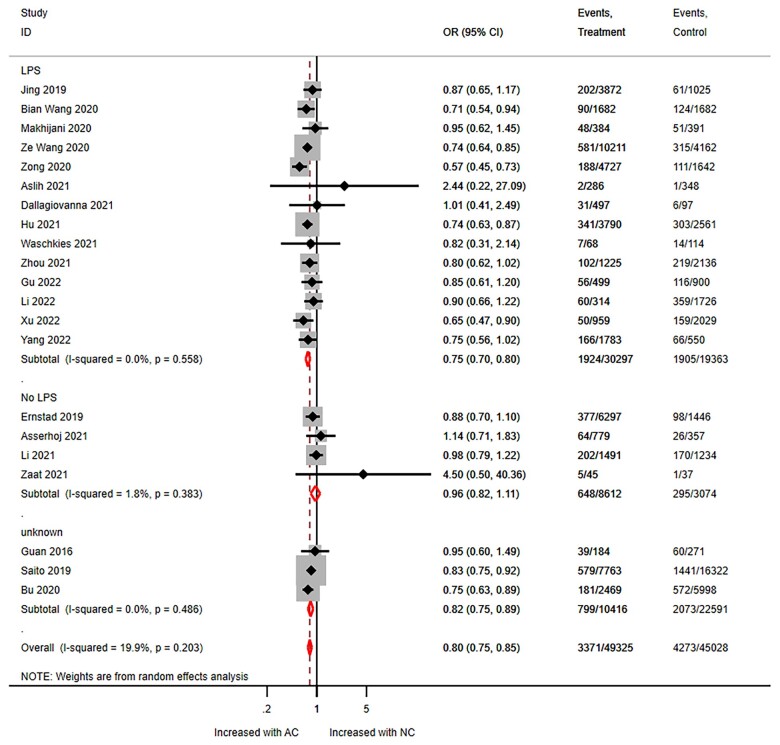
Stratified analyses on LPS use in NC-FET suggested that compared to AC-FET, NC-FET with LPS decreased PTB risk, while NC-FET without LPS did not (OR 0.75, 95% CI 0.70–0.81).
Pooled adjusted OR resulted in similar estimates (Table 4).
Additional outcomes: obstetric outcomes
NC-FET compared to AC-FET resulted in a lower risk of early pregnancy loss (OR 0.73; 95% CI 0.61–0.86, I2 = 71%; RD −0.04, 95% CI −0.06 to −0.03). NC-FET compared to AC-FET resulted in a lower risk of PTB (OR 0.80; 95% CI 0.75–0.85, I2 = 20%; RD −0.015, 95% CI −0.020 to −0.010) and very PTB (OR 0.66, 95% CI 0.53–0.84, I2 = 0%; RD −0.004, 95% CI −0.007 to −0.001), HDP (OR 0.60, 95% CI 0.50–0.65, I2 = 61%; RD −0.022, 95% CI −0.031 to −0.020), PE (OR 0.50; 95% CI 0.42–0.60, I2 = 44%; RD −0.036, 95% CI −0.053 to −0.019), placenta previa (OR 0.84, 95% CI 0.73–0.97, I2 = 0%; RD −0.002, 95% CI −0.004 to 0.001), and PPH (OR 0.43; 95% CI 0.38–0.48, I2 = 53%; RD −0.052, 95% CI −0.963 to −0.009).
Pooled adjusted OR resulted in similar estimates. The risk of GDM did not differ between NC-FET and AC-FET (OR 1.01, 95% CI 0.85–1.19, I2 = 80%; RD 0.000, 95% −0.010 to 0.010) (Table 4, Fig. 3, Supplementary Figs S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, and S12 and S15, S16, S17, S18, S19, S20, S21, S22, S23, S24, S25, and S26).
Figure 3.
Summary of all averaged secondary outcomes for NC-FET versus AC-FET, expressed as odds ratio with 95% CI. NC: natural cycle; AC: artificial cycle; OR: odds ratio.
Prevalence estimates for these outcomes following NC-FET and AC-FET are depicted in Supplementary Table S1.
Additional outcomes: neonatal outcomes
The risk of congenital malformations (OR 0.86; 95% CI 0.66–1.11, I2 = 3%; RD −0.000, 95% CI −0.010 to 0.010) and neonatal mortality (OR 0.80; 95% CI 0.56–1.13, I2 = 10%; RD −0.000, 95% CI −0.010 to 0.0010) did not differ between NC-FET compared to AC-FET (Table 4, Supplementary Table S1, Supplementary Figs S12 and S13 and S27 and S28).
Stratified analysis on studies with or without inclusion of women with or without PCOS
We performed subgroup analyses on studies that excluded or included women with PCOS (Table 1), for all outcomes. No statistically significant differences were observed, and the direction of effect was the same, but there were some differences in size of the effect. Compared to studies including women with PCOS, we found that in studies excluding women with PCOS there may be an increased risk of LGA (OR 0.84, 95% CI 0.76–0.92 for studies excluding PCOS versus OR 0.92, 95% CI 0.84–1.01 for studies included women with PCOS) and early pregnancy loss (OR 0.65, 95% CI 0.52–0.81 for studies excluding PCOS versus OR 0.88, 95% CI 0.63–1.23 for studies including women with PCOS) after AC-FET.
In studies excluding women with PCOS, there may be a lower risk of macrosomia (OR 0.91, 95% CI 0.79–1.05 for studies excluding PCOS versus OR 0.76, 95% CI 0.61–0.95 for studies including women with PCOS) after AC-FET, compared to studies including women with PCOS (Supplementary Figs S1, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, and S14).
Stratified analysis on the use of LPS in NC-FET
Stratified analyses on LPS use in NC-FET suggested that, compared to AC-FET, NC-FET with LPS decreased PTB risk, while NC-FET without LPS did not (OR 0.75, 95% CI 0.70–0.81 with LPS versus OR 0.96, 95% CI 0.82–1.11 without LPS). LPS use did not modify the other outcomes (Table 4, Fig. 4, Supplementary Figs S15, S16, S17, S18, S19, S20, S21, S22, S23, S24, S25, S26, S27, and S28).
Figure 4.
Preterm birth for NC-FET versus AC-FET, stratified by luteal phase support use. NC: natural cycle; AC: artificial cycle; LPS: luteal phase support.
Subgroup analysis on true NC-FET versus modified NC-FET
In total, three studies reported on birthweight of babies born after true NC-FET versus modified NC-FET. Birthweight did not differ between true NC-FET when compared with modified NC-FET (MD 44.90 g; 95% CI −186.8–96.9), I2 = 70%). The significance of the use of LPS in this groups remains to be studied (Fig. 5). Owing to a lack of data, the comparison between true NC-FET versus modified NC-FET could not be pooled for other outcomes.
Figure 5.
Subgroup analysis on birthweight for true NC-FET versus modified NC-FET. LPS: luteal phase support; NC: natural cycle; FET: frozen embryo transfer; mNC: modified natural cycle; tNC: true natural cycle.
Quality and risk of bias assessment
Two of the included studies in the meta-analysis were ranked as having a high risk of bias on the domains of confounding bias, selection bias and reporting bias. One study was ranked as low risk of bias. The other 27 included studies were ranked as moderate risk of bias (Supplementary Fig. S29). The GRADE tool was used for grading the quality of evidence. The quality of evidence ranged from very low to moderate (Table 4).
Discussion
Principal findings
This systematic review and meta-analysis shows an increase in normal range birthweight and a decrease in LGA, macrosomia, LBW, early pregnancy loss, PTB, very PTB, HDP, PE, placenta previa, and PPH in NC-FET compared to AC-FET. Therefore, the risk of adverse obstetric and neonatal outcomes is lower in NC-FET compared with AC-FET.
The use of LPS in NC-FET decreases PTB risk when NC-FET with or without LPS is compared to AC-FET.
The quality of evidence was very low to moderate mainly because this is a review based on observational studies and because of the substantial inter-study heterogeneity obtained, which was assumed to be caused by the variation between study populations.
We estimate that for each adverse outcome the use of NC-FET may prevent 4 to 22 cases per 1000 women with a singleton live birth.
Study strengths
The large sample size of 113 676 live births is a major strength of this study. This is a comprehensive and updated systematic review, which includes analyses of pregnancies following NC-FET and AC-FET. As LPS might have an impact on obstetric and perinatal outcomes, we provided separated analyses of pregnancies resulting from NC-FET with or without LPS. The present systematic review and meta-analysis was carried out in accordance with the PRISMA statement, ensuring high methodological quality. Moreover, the risk of bias of the included studies was assessed using the ROBINS-I tool. The validity of our results is notably improved owing to these factors.
Study limitations
The majority of published articles in this review comprised observational studies. There is a great variety in the included studies in terms of study populations, timeline of the study, development stage of the embryos transferred with FET, freezing protocols, the use of pre-implantation genetic testing, and numbers of embryos transferred. Protocols for LPS can hardly be compared between studies because of the major variety in medication used, starting day of LPS and continuation of LPS in case of gestation (Table 2). Adjustment for relevant confounders was not possible in our main analysis owing to lack of individual patient data. We did perform an adjusted analysis by pooling adjusted ORs of the included studies, resulting in no differences in outcomes (Table 4). It should be noted however that confounders, such as vanishing twins, could not be analyzed and may have influenced the outcomes. In addition, unpublished data as full-text articles and in languages other than English were excluded from the meta-analysis. Clear definitions for some of the outcomes were not reported in all publications. Definitions of GDM, HDP, PE, PPH congenital anomalies, and perinatal mortality were inconsistent across the included studies.
Discrepancies with research protocol
In total, 17 of the included studies also included women with irregular cycles, anovulation and/or PCOS (Table 1). In our research protocol, we stated to exclude women with PCOS/anovulation and revised this during the execution of the study.
This deviation deserves attention because including these women may be associated with a higher risk of perinatal complications, such as HDP, PE, GDM, and PTB, possibly distorting the outcomes of the analyses (Palomba and La Sala, 2016). Therefore, we performed a subgroup analysis on studies that excluded women with PCOS. Although this did not affect our general findings, the results suggest that in studies excluding women with PCOS the RD between NC-FET and AC-FET was larger for LGA and early pregnancy loss and smaller for macrosomia, compared to studies that also included women with PCOS. For the other outcomes, including HDP, PE, GDM, and PTB, no differences were found.
Furthermore, we aimed to include only singleton deliveries in our meta-analysis. Unfortunately, in five studies (Saito et al., 2019; Levi Setti et al., 2020; Pan et al., 2020; Li et al., 2022; Roelens et al., 2022), data were not presented separately for singleton and multiple deliveries. We contacted the authors of these studies for separate data on singleton births. One author responded but was not able to provide data in the short term. The other authors did not respond to our request. The number of multiple deliveries in these studies was comparable between study groups and therefore not likely to have influenced the results of our meta-analysis.
Comparison with other studies
The comparison of our findings with those of three recently published meta-analyses (Moreno-Sepulveda et al., 2021; Rosalik et al., 2021; Busnelli et al., 2022) exploring the same topic are reported in Table 5. Busnelli et al. (2022) included 19 studies in the meta-analysis, Moreno-Sepulveda et al. (2021) included 13 studies and Rosalik et al. (2021) included 15 studies, where we included 30 studies. The findings of a decreased risk in LGA and macrosomia in NC-FET compared to AC-FET were found in all four studies (Table 5). The decrease in birthweight after NC-FET compared to AC-FET was also found in the study of Rosalik et al. (2021) but not reported in the other two studies (Table 5). Our findings concerning a decrease in the risk of HDP, PE, PPH and PTB after NC-FET compared to AC-FET were also reported by two studies (Moreno-Sepulveda et al., 2021; Busnelli et al., 2022) (Table 5). We found a higher risk of early pregnancy loss after AC-FET compared to NC-FET, and none of the other meta-analyses reported on this outcome (Table 5). We found no difference in SGA between groups and this was also reported by the studies of Busnelli et al. (2022) and Moreno-Sepulveda et al. (2021) (Table 5). Our finding of a decrease in LBW after NC-FET compared to AC-FET was in line with the result of Moreno-Sepulveda et al.; however, Busnelli et al., did not find a difference in LBW between groups (Table 5). The decrease in risks of placenta previa and very PTB after NC-FET compared to AC-FET was also reported by Busnelli et al. (2022) (Table 5). No difference was found in congenital malformations between babies born following NC-FET and those following AC-FET in any meta-analysis (Table 5). No difference in neonatal mortality was found based on our results and the results of Moreno-Sepulveda et al. (2021) (Table 5).
Table 5.
Comparison of results with those of three previous meta-analyses.
| Outcomes | Present analysis |
(Busnelli et al., 2022) |
(Moreno-Sepulveda et al., 2021) |
(Rosalik et al., 2021) |
||||
|---|---|---|---|---|---|---|---|---|
| NC-FET | AC-FET | NC-FET | AC-FET | NC-FET | AC-FET | NC-FET | AC-FET | |
| Birthweight | ↓ | ↑ | Not reported | Not reported | ↓ | ↑ | ||
| LGA | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ |
| Macrosomia | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ |
| SGA | = | = | = | = | = | = | Not reported | |
| LBW | ↓ | ↑ | = | = | ↓ | ↑ | Not reported | |
| Early pregnancy loss | ↓ | ↑ | Not reported | Not reported | Not reported | |||
| GDM | = | = | = | = | = | = | Not reported | |
| HDP | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | Not reported | |
| PE | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | Not reported | |
| PPH | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | Not reported | |
| Placenta previa | ↓ | ↑ | ↓ | ↑ | = | = | Not reported | |
| PTB | ↓ | ↑ | ↓ | ↑ | ↓ | ↑ | Not reported | |
| Very PTB | ↓ | ↑ | ↓ | ↑ | Not reported | Not reported | ||
| Cong malformations | = | = | = | = | = | = | Not reported | |
| Neonatal mortality | = | = | Not reported | = | = | Not reported | ||
| Stratification PCOS | ↑ LGA, EPS, very PTB in AC-FET | Not reported | Not reported | Not reported | ||||
| Stratification LPS | ↓ PTB and placenta previa | Not reported | Not reported | Not reported | ||||
=: no difference; ↑: increased risk; ↓: reduced risk. LGA: large for gestational age; SGA: small for gestational age; LBW: low birthweight; GDM: gestational diabetes mellitus; HDP: hypertensive disorders of pregnancy; PE: pre-eclampsia; PPH: postpartum hemorrhage; PTB: preterm birth; EPS: early pregnancy loss.
None of the other meta-analysis reported on the use of LPS in NC-FET.
Discrepancies among different meta-analyses could be explained by the different criteria used to assess study eligibility and by the varying amount of data covering different periods of time.
Interpretation of the results
Birthweight, large for gestational age, macrosomia, small for gestational age, and low birthweight
For a while now it has been known that babies born from FET have a higher mean birthweight and are more likely to be LGA compared with babies born from fresh embryo transfer (Maheshwari et al., 2018). The biological explanation of this phenomenon is still unknown. It has been hypothesized that epigenetic disturbances during the early embryonic stages, occurring as a result of the freezing and warming procedures, might affect the development of fetal and placental tissues. This may result in asynchrony between the embryo and endometrium and cause disturbance in fetal growth, resulting in an increase in birthweight after FET compared to fresh embryo transfer (Pinborg et al., 2016).
Historically, FET cycles have been scheduled using AC-FET and therefore this type of endometrial preparation has already previously been suggested as a possible confounder for increased birthweight, LGA and macrosomia after FET (Ginstrom Ernstad et al., 2019). Based on our meta-analysis and the meta-analysis of Rosalik et al. (2021), we now can conclude that birthweight and the risk of LGA and macrosomia are indeed increased in AC-FET compared to NC-FET.
The exogenous oestrogen and progesterone that is used in AC-FET may affect the endometrium and subsequent placental development. Furthermore, in early pregnancy, progesteron has been described to induce decidualization of endometrial stromal cells, regulate extravillous trophobast (EVT) invasion, and vascular remodeling (Beltrame et al., 2018). However, aberrant progesterone and oestradiol levels in early pregnancy after AC-FET may lead to abnormal invasion of the EVT, impaired spiral artery remodeling and dysfunction of the trophoblast cells (Schatz et al., 2016; Labarrere et al., 2017; Beltrame et al., 2018). This non-physiological increase in steroids during AC-FET in early pregnancy has been linked to PE, abnormal placentation, stillbirth, fetal growth restriction (FGR), and many cases of PTB (Schatz et al., 2016; Labarrere et al., 2017; Beltrame et al., 2018). It is hypothesized that HDP is an etiologically heterogeneous disorder that occurs in at least two subsets, one involving placental dysfunction and FGR, and another with normal or enhanced placental function (Rasmussen and Irgens, 2003; Pinborg et al., 2014). Notably, our results showed that SGA was not different between NC-FET and AC-FET but LBW was increased during AC-FET compared to NC-FET. We hypothesize that AC-FET may more often result in the latter subset of HDP, both clinical and subclinical, causing enhanced placental function owing to very early alterations in implantation and placental development. This hypothesis may explain the increase in birthweight, LGA and macrosomia after AC-FET. However, the majority of the included studies did use progesterone supplementation for LPS in NC-FET, which makes it difficult to tease out the role of progesterone, leaving mainly exogenous estrogen and/or the lack of CL as possible explanations. This needs to be further explored to determine the biological plausibility underlying these adverse outcomes.
Hypertensive disorders of pregnancy and abnormal placentation
As reported in our results and in the other recent meta-analysis, the risk of HDP, PE, and abnormal placentation (placenta previa) is decreased in NC-FET compared to AC-FET. The hypothesised rationale of the biological plausibility is discussed in the previous paragraph. Another possible, or perhaps combined, explanation is the absence of the CL during AC-FET. During AC-FET, estrogen substitution causes suppression of a dominant follicle and therefore no ovulation and CL will appear. Already in 1991, a cohort study showed that relaxin, a vasoactive hormone produced by the CL, was not detected in serum of anovulatory women who conceived with oocyte donation in an artificially prepared endometrium throughout pregnancy (Johnson et al., 1991). In 2019, a group from Stanford-university/University-of-Florida performed a similar study up to 12 weeks pregnancy in ovulatory women who conceived with AC-FET and could not detect relaxin (von Versen-Hoynck et al., 2019a,b). Blood pressure, endothelial function, and the number of circulating endothelial progenitor cells were also affected. The findings of this study support that conception following an artificially prepared endometrium (hence, the lack of CL) has a negative influence on maternal vascular health in early pregnancy compared with pregnancies conceived following NC-FET or natural conception (von Versen-Hoynck et al., 2019a,b). In 2020, a prospective study of two periconception cohorts showed that, during the first trimester, pregnancies conceived in the absence of a CL are characterized by lower circulating renin and prorenin concentrations compared with those conceived naturally (Wiegel et al., 2020). The absence of vasoactive factors produced by the CL in AC-FET led to deficient circulatory adaptations during early gestation and probably led to increased risks of abnormal placentation and HDP (Conrad, 2011; Conrad and Baker, 2013; Conrad et al., 2019; 2019; von Versen-Hoynck et al., 2019a,b; Conrad et al., 2020; Singh et al., 2020; Pereira et al., 2021).
Abnormal placenta invasion in its turn is associated with an increased risk of PTB (Morgan, 2016) and may also be the reason for an increased risk of PPH (Busnelli et al., 2022).
Luteal phase support
Based on our exploratory stratified analysis, the use of LPS in NC-FET decreases the risk of PTB and when NC-FET, with or without LPS, is compared to AC-FET (very low quality of evidence). The other outcomes were not influenced by the use of LPS. We need to take into account that the decrease in risk of PTB may be a spurious finding, given the limited number of included studies and the observational nature of these studies. Also, it should be noted that the protocols used for LPS in our meta-analysis vary widely and are barely comparable, which leads to a high level of clinical heterogeneity. Half of the studies on LPS did not report the duration of LPS in case of pregnancy. It was not clear from the included studies if PTB had a spontaneous onset or was induced because of pregnancy complications.
To determine a biological rationale for our findings on LPS we searched the literature. For prevention of spontaneous PTB in high-risk women, daily vaginal progesterone or weekly 17-hydroxyprogesterone caproate is recommended from 16 weeks of gestation to 34 weeks of gestation by the guidelines of the National Institute for health and Care Excellence, the American College for Obstetricians and Gynecologists and the International Federation of Gynaecology and Obstetrics ((NICE) NIfHaCE, 2022; American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics, 2021; Shennan et al., 2021). The use of progesterone supplementation limited to the first trimester as a preventative measure for spontaneous PTB has never been studied to our knowledge. Two RCTs included women with recurrent miscarriage and compared the use of vaginal micronized progesterone supplementation to placebo vaginal capsules until 12 and 16–17 completed weeks of gestation, respectively. Progesterone lowered the risk of another miscarriage. The risk of PTB was not reported in this trial but no difference in live birth before 34 weeks of gestation was found (Coomarasamy et al., 2016, 2020).
A recently published systematic review assessed the long-term effect of prenatal progesterone treatment on child development, behavior, and health. All included studies compared progesterone to placebo in second and/or third trimester for the prevention of PTB. The authors concluded that based on the latest evidence there is no effect of prenatal progesterone on child development. Outcomes after first trimester progesterone alone remain unclear (Simons et al., 2021).
The use of LPS following NC-FET may be beneficial in terms of pregnancy rates, based on a low level of evidence (Mizrachi et al., 2021). In clinical practice, NC-FET with LPS is often applied (Weissman, 2020; Mizrachi et al., 2021). Currently, a multicenter RCT is comparing the efficacy of NC-FET with or without LPS in China (trial registration number: ChiCTR2200057498). In terms of safety, LPS after NC-FET has not been assessed thus far. The explanation for the decrease in risk of PTB after NC-FET with LPS remains unclear. Perhaps the use of LPS somehow reduces any alterations in adaption of the cardiovascular system and placental invasion during early pregnancy. Future research on the efficacy and safety of LPS use is of great importance as it remains a major gap in knowledge in the field of ART. Owing to these uncertainties, we cannot make any recommendation about the use of LPS. Future research should focus on the efficacy of LPS in NC-FET and follow-up studies need to investigate any safety issues concerning its use.
To trigger or not to trigger ovulation
The efficacy of triggering ovulation compared to monitoring natural ovulation has been investigated in the Antarctica-2 RCT, which is currently in its follow-up phase (Zaat et al., 2021c).
When hCG is used for ovulation triggering, its long half-life renders it also as a form of LPS (Casper and Yanushpolsky, 2016). This form of LPS has not been proven to be beneficial following NC-FET in terms of clinical pregnancy and live birth rates, based on low quality of evidence (Mizrachi et al., 2021). In terms of safety, we performed a sub-analysis on true NC-FET (natural ovulation) compared to modified NC-FET (triggering ovulation) and did not find any differences in birthweight between groups. For other outcomes this sub-analysis could not be performed owing to lack of data. This question remains to be studied in future research.
Future implications
Implications for clinical practice
The association between the endometrium preparation method for FET and obstetric and neonatal complications merits further attention and awareness in clinical practice in order to optimize the health of both mothers and children after FET. It has been more than 30 years since the first baby was conceived after FET. From the start, FET was performed in an artificially prepared endometrium because its first application was in fresh oocyte donation cycles. A solution for women without oocytes was to apply an ‘artificial cycle’ to grow the endometrium, in which the natural hormones, as produced in ovulatory women by the CL, are partly substituted with estrogen pills and progesterone vaginal capsules. AC-FET has been a very reliable, effective and predictable protocol for the fertility laboratory and therefore is still popular for FET in ovulatory women. However, the time has come to re-evaluate the use of AC-FET. Many studies, including our meta-analysis, report on increased risks for mother and child in AC-FET compared to NC-FET. The advantage of AC-FET nowadays is the easy alignment of the time point of thawing and transferring embryos with organizational necessities of the IVF laboratory, the treating doctors and the patient, which does not outweigh the disadvantages in terms of adverse obstetric and neonatal outcomes (Zaat et al., 2022). These data on safety outcomes suggest that NC-FET is preferred over AC-FET in ovulatory women.
NC-FET in combination with LPS might be considered, as LPS use may be beneficial in terms of pregnancy rates, did not result in worse safety outcomes and might result in a lower PTB risk. Our recommendations do not concern anovulatory women.
Implications for research
Now that so many studies on safety are available, an individual patient data meta-analysis that includes the original databases of these studies would be welcome. Such an analysis allows to study whether there are differences in safety profile in specific subgroups while adjusting for confounders. This could also provide us with compound adverse outcomes in terms of pregnancy complications.
Concerning implications for future research, the development and use of an extended core outcome set for obstetric and neonatal outcomes in fertility care is needed (Duffy et al., 2017, 2018, 2021). In our meta-analysis, we captured several outcomes, such as early pregnancy loss, HDP, and GDM, based on individual study definition, which introduces heterogeneity. A standardized set of outcomes across studies would facilitate evidence synthesis in meta-analyses and systematic reviews. Furthermore, data on the actual growth during pregnancy of babies born after FET should be collected and analyzed. Previous research demonstrates that crown-rump-length (CRL) is increased in babies after FET and leads to higher birthweight (Zaat et al., 2021b). It would be of great interest to investigate the association between CRL and actual birthweight in a large study in order to look at the risk of FGR after FET. Even babies born with a normal range birthweight could suffer from FGR, which can lead to increased risk of perinatal mortality and morbidity (De Reu et al., 2010; Audette and Kingdom, 2018). FGR is commonly defined as a condition in which the fetus does not reach its intrinsic growth potential (Marijnen et al., 2022). Placental insufficiency, resulting from a variety of placental lesions, is the common underlying pathophysiologic mechanism (Burton and Jauniaux, 2018).
Conclusion
This systematic review and meta-analysis has shown that singletons born from NC-FET might have a lower birthweight and a lower risk of early pregnancy loss, LGA, macrosomia, SGA, LBW, HDP, PE, PPH, and PTB compared to singletons born from AC-FET, based on low to moderate quality of evidence.
In combination with comparable effectiveness of the two approaches, the interpretation is that NC-FET is the preferred treatment in women undergoing FET when the risks of obstetrical complications and potential neonatal complications are considered.
Based on the analysis of current evidence on the effectiveness and safety outcomes reported in this review, NC-FET should be the preferred treatment in women with ovulatory cycles undergoing FET. The difference between NC-FET and AC-FET may be partly related to the use of LPS in NC-FET for the outcome of PTB. This finding warrants further research on the efficacy of LPS before applying LPS in all NC-FET cycles since no head-to-head studies are currently available.
Supplementary Material
Acknowledgements
We thank Charlotte Leseman for her participation in the screening process of the included studies. We thank Luca den Tenter for the first draft of the search and bachelor thesis on this subject.
Contributor Information
T R Zaat, Department of Obstetrics and Gynecology, Amsterdam UMC Location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
E B Kostova, Department of Obstetrics and Gynecology, Amsterdam UMC Location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
P Korsen, University Medical Center Groningen, Groningen, The Netherlands.
M G Showell, Department of Obstetrics and Gynaecology, University of Auckland, Auckland, New Zealand.
F Mol, Department of Obstetrics and Gynecology, Amsterdam UMC Location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
M van Wely, Department of Obstetrics and Gynecology, Amsterdam UMC Location University of Amsterdam, Amsterdam, The Netherlands; Amsterdam Reproduction and Development Research Institute, Amsterdam, The Netherlands.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
T.R.Z.: involved in study design, data collection, data analysis, and manuscript preparation. E.K.: involved in quality assessments and manuscript preparation. P.K.: involved in data collection, quality assessment, and manuscript preparation. M.G.S.: involved data collection and manuscript preparation. F.M.: involved in study design, data collection, supervision, and manuscript preparation. M.v.W.: involved in study design, data analysis, supervision, and manuscript preparation. All authors contributed to the final version of the manuscript.
Funding
No external funding was sought in preparing this article.
Conflict of interest
T.R.Z. is supported by a grant of the Netherlands Organisation for Health Research and Development (ZonMw 843002807) in order to perform the The Antarctica-2 RCT (Trial NL6414 (NTR6590)).
All reported competing interests are outside the submitted work. No other relationships or activities that could appear to have influenced the submitted work. The remaining authors have no conflicts of interest to declare.
References
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Prediction and prevention of spontaneous preterm birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol 2021;138:e65–e90. [DOI] [PubMed] [Google Scholar]
- Aslih N, Dorzia D, Atzmon Y, Estrada D, Ellenbogen A, Bilgory A, Shalom-Paz E.. Ovulatory-based FET cycles may achieve higher pregnancy rates in the general population and among anovulatory women. J Clin Med 2021;10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asserhoj LL, Spangmose AL, Aaris Henningsen AK, Clausen TD, Ziebe S, Jensen RB, Pinborg A.. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril 2021;115:947–956. [DOI] [PubMed] [Google Scholar]
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O'Connell D, Oxman AD, Phillips B. et al. ; GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette MC, Kingdom JC.. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med 2018;23:119–125. [DOI] [PubMed] [Google Scholar]
- Beltrame JS, Sordelli MS, Canumil VA, Alonso CAI, Perez Martinez S, Ribeiro ML.. Steroid hormones induce in vitro human first trimester trophoblast tubulogenesis by the lysophosphatidic acid pathway. Mol Cell Endocrinol 2018;478:126–132. [DOI] [PubMed] [Google Scholar]
- Bu Z, Zhang J, Hu L, Sun Y.. Preterm birth in assisted reproductive technology: an analysis of more than 20,000 singleton newborns. Front Endocrinol (Lausanne) 2020;11:558819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E.. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018;218:S745–S761. [DOI] [PubMed] [Google Scholar]
- Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE.. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod 2022;37:1619–1641. [DOI] [PubMed] [Google Scholar]
- Casper RF, Yanushpolsky EH.. Optimal endometrial preparation for frozen embryo transfer cycles: window of implantation and progesterone support. Fertil Steril 2016;105:867–872. [DOI] [PubMed] [Google Scholar]
- Cerrillo M, Herrero L, Guillen A, Mayoral M, Garcia-Velasco JA.. Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J 2017;8:e0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Baker VL.. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 2013;304:R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Graham GM, Chi YY, Zhai X, Li M, Williams RS, Rhoton-Vlasak A, Segal MS, Wood CE, Keller-Wood M.. Potential influence of the corpus luteum on circulating reproductive and volume regulatory hormones, angiogenic and immunoregulatory factors in pregnant women. Am J Physiol Endocrinol Metab 2019;317:E677–E685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Lingis M, Sautina L, Li S, Chi YY, Qiu Y, Li M, Williams RS, Rhoton-Vlasak A, Segal MS.. Maternal endothelial function, circulating endothelial cells, and endothelial progenitor cells in pregnancies conceived with or without in vitro fertilization. Am J Physiol Regul Integr Comp Physiol 2020;318:R1091–R1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Petersen JW, Chi YY, Zhai X, Li M, Chiu KH, Liu J, Lingis MD, Williams RS, Rhoton-Vlasak A. et al. Maternal cardiovascular dysregulation during early pregnancy after in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019;74:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Semin Nephrol 2011;31:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A, Harb HM, Devall AJ, Cheed V, Roberts TE, Goranitis I, Ogwulu CB, Williams HM, Gallos ID, Eapen A. et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technol Assess 2020;24:1–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, Gupta P, Dawood F, Koot YE, Atik RB. et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages—a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess 2016;20:1–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. 2021.
- Dallagiovanna C, Cappellari M, D'Ambrosi F, Reschini M, Kordas K, Li Piani L, Filippi F, Somigliana E.. Endometrial preparation does not affect the risk of hypertensive disorders of pregnancy in low-risk women undergoing frozen embryo transfer. Gynecol Endocrinol 2022;38:238–242. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V; European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;33:1586–1601.30032255 [Google Scholar]
- De Reu PA, Oosterbaan HP, Smits LJ, Nijhuis JG.. Avoidable mortality in small-for-gestational-age children in the Netherlands. J Perinat Med 2010;38:311–318. [DOI] [PubMed] [Google Scholar]
- Duffy JM, Rolph R, Gale C, Hirsch M, Khan KS, Ziebland S, McManus RJ, Pre‐Eclampsia I, van‘t Hooft J, Brown M; International Collaboration to Harmonise Outcomes in Pre-eclampsia (iHOPE). Core outcome sets in women's and newborn health: a systematic review. BJOG 2017;124:1481–1489. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, AlAhwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, Farquharson RG, Franik S, Giudice LC, Khalaf Y. et al. ; Core Outcome Measure for Infertility Trials (COMMIT) initiative. Developing a core outcome set for future infertility research: an international consensus development study. Fertil Steril 2021;115:191–200. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, Bhattacharya S, Curtis C, Evers JLH, Farquharson RG, Franik S, Khalaf Y, Legro RS, Lensen S, Mol BW. et al. ; COMMIT: Core Outcomes Measures for Infertility Trials. A protocol developing, disseminating and implementing a core outcome set for infertility. Hum Reprod Open 2018;2018:hoy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstad EG, Wennerholm U-B, Khatibi A, Petzold M, Bergh C.. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126.e1–126.e18. [DOI] [PubMed] [Google Scholar]
- ESHRE. ART Fact Sheet. 2018. https://www.eshre.eu/-/media/sitecore-files/Press-room/ESHREARTFactSheetv73.pdf (14 July 2022, date last accessed).
- European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), Wyns D, Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA. et al. ART in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open 2021;2021:hoab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginstrom Ernstad E, Spangmose AL, Opdahl S, Henningsen AA, Romundstad LB, Tiitinen A, Gissler M, Wennerholm UB, Pinborg A, Bergh C. et al. Perinatal and maternal outcome after vitrification of blastocysts: a Nordic study in singletons from the CoNARTaS group. Hum Reprod 2019;34:2282–2289. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A.. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2020;10:CD006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Wu Y, Tan M, Hu R, Chen Y, Li X, Lin B, Duan Y, Zhou C, Li P. et al. Programmed frozen embryo transfer cycle increased risk of hypertensive disorders of pregnancy—a multicenter cohort study in ovulatory women. Am J Obstet Gynecol MFM 2022;5:100752. [DOI] [PubMed] [Google Scholar]
- Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, Sun L, Wang X, Zhang Z.. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med 2016;62:335–342. [DOI] [PubMed] [Google Scholar]
- Hatoum I, Bellon L, Ouazana M, Swierkowski N, Bouba S, Fathallah K, Paillusson B, Bailly M, Boitrelle F, Alter L. et al. Disparities in reproductive outcomes according to the endometrial preparation protocol in frozen embryo transfer: The risk of early pregnancy loss in frozen embryo transfer cycles. J Assist Reprod Genet 2018;35:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KL, Zhang D, Li R.. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril 2021;115:1487–1494. [DOI] [PubMed] [Google Scholar]
- Jing S, Li XF, Zhang S, Gong F, Lu G, Lin G.. Increased pregnancy complications following frozen-thawed embryo transfer during an artificial cycle. J Assist Reprod Genet 2019;36:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Abdalla H, Allman AC, Wren ME, Kirkland A, Lightman SL.. Relaxin levels in ovum donation pregnancies. Fertil Steril 1991;56:59–61. [PubMed] [Google Scholar]
- Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, Haas DM, Kassab GS, Romero R.. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol 2017;216:287.e1–287.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathi RB, Chi YY, Liu J, Saravanabavanandhan B, Hegde A, Baker VL.. Frozen blastocyst embryo transfer using a supplemented natural cycle protocol has a similar live birth rate compared to a programmed cycle protocol. J Assist Reprod Genet 2015;32:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi Setti PE, Cirillo F, De Cesare R, Morenghi E, Canevisio V, Ronchetti C, Baggiani A, Smeraldi A, Albani E, Patrizio P.. Seven years of vitrified blastocyst transfers: comparison of 3 preparation protocols at a single ART center. Front Endocrinol (Lausanne) 2020;11:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, He YC, Xu JJ, Wang Y, Liu H, Duan CC, Shi CY, Chen L, Wang J, Sheng JZ. et al. Perinatal outcomes of neonates born from different endometrial preparation protocols after frozen embryo transfer: a retrospective cohort study. BMC Pregnancy Childbirth 2021;21:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xie Q, Luan T, Su Y, Zhang J, Zhang J, Zhao C, Ling X.. Maternal and child-health outcomes in different endometrial preparation methods for frozen-thawed embryo transfer: a retrospective study. Hum Fertil (Camb) 2022;1–12. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhao J, Hao G, Tan J, Pan Y, Wang Z, Jiang Q, Xu N, Shi Y.. Maternal and neonatal complications after natural vs. hormone replacement therapy cycle regimen for frozen single blastocyst transfer. Front Med (Lausanne) 2020;7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- Makhijani R, Bartels C, Godiwala P, Bartolucci A, Nulsen J, Grow D, Benadiva C, Engmann L.. Maternal and perinatal outcomes in programmed versus natural vitrified-warmed blastocyst transfer cycles. Reprod Biomed Online 2020;41:300–308. [DOI] [PubMed] [Google Scholar]
- Marijnen MC, Damhuis SE, Smies M, Gordijn SJ, Ganzevoort W.. Practice variation in diagnosis, monitoring and management of fetal growth restriction in the Netherlands. Eur J Obstet Gynecol Reprod Biol 2022;276:191–198. [DOI] [PubMed] [Google Scholar]
- Mizrachi Y, Horowitz E, Ganer Herman H, Farhi J, Raziel A, Weissman A.. Should women receive luteal support following natural cycle frozen embryo transfer? A systematic review and meta-analysis. Hum Reprod Update 2021;27:643–650. [DOI] [PubMed] [Google Scholar]
- Moreno-Sepulveda J, Espinos JJ, Checa MA.. Lower risk of adverse perinatal outcomes in natural versus artificial frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Reprod Biomed Online 2021;42:1131–1145. [DOI] [PubMed] [Google Scholar]
- Morgan TK. Role of the placenta in preterm birth: a review. Am J Perinatol 2016;33:258–266. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Araki R, Tani H, Ishihara O, Kuwahara A, Irahara M, Yoshimura Y, Kuramoto T, Saito H, Nakaza A. et al. Implications of assisted reproductive technologies on term singleton birth weight: an analysis of 25,777 children in the national assisted reproduction registry of Japan. Fertil Steril 2013;99:450–455. [DOI] [PubMed] [Google Scholar]
- NICE (National Institute for Health and Care Excellence). Preterm Labour and Birth [NG25]. https://www.nice.org.uk/guidance/ng25/resources/preterm-labour-and-birth-pdf-1837333576645 (12 July 2022, date last accessed).
- Palomba S, La Sala GB.. Pregnancy complications in women with polycystic ovary syndrome: importance of diagnostic criteria or of phenotypic features? Hum Reprod 2016;31:223–224. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li B, Wang Z, Wang Y, Gong X, Zhou W, Shi Y.. Hormone replacement versus natural cycle protocols of endometrial preparation for frozen embryo transfer. Front Endocrinol (Lausanne) 2020;11:546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MM, Mainigi M, Strauss JF.. Secretory products of the corpus luteum and preeclampsia. Hum Reprod Update 2021;27:651–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira N, Petrini AC, Hancock KL, Rosenwaks Z.. Fresh or frozen embryo transfer in in vitro fertilization: an update. Clin Obstet Gynecol 2019;62:293–299. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN.. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod 2014;29:618–627. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Romundstad LB, Wennerholm UB, Soderstrom-Anttila V, Bergh C, Aittomaki K.. Epigenetics and assisted reproductive technologies. Acta Obstet Gynecol Scand 2016;95:10–15. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Irgens LM.. Fetal growth and body proportion in preeclampsia. Obstet Gynecol 2003;101:575–583. [DOI] [PubMed] [Google Scholar]
- Roelens C, Racca A, Mackens S, Van Landuyt L, Buelinckx L, Gucciardo L, Tournaye H, De Vos M, Blockeel C.. Artificially prepared vitrified-warmed embryo transfer cycles are associated with an increased risk of pre-eclampsia. Reprod Biomed Online 2022;44:915–922. [DOI] [PubMed] [Google Scholar]
- Rosalik K, Carson S, Pilgrim J, Luizzi J, Levy G, Heitmann R, Pier B.. Effects of different frozen embryo transfer regimens on abnormalities of fetal weight: a systematic review and meta-analysis. Hum Reprod Update 2021;28:1–14. [DOI] [PubMed] [Google Scholar]
- Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, Fukami M, Miyasaka N, Ishihara O, Irahara M. et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod 2019;34:1567–1575. [DOI] [PubMed] [Google Scholar]
- Saito K, Miyado K, Yamatoya K, Kuwahara A, Inoue E, Miyado M, Fukami M, Ishikawa T, Saito T, Kubota T. et al. Increased incidence of post-term delivery and Cesarean section after frozen-thawed embryo transfer during a hormone replacement cycle. J Assist Reprod Genet 2017;34:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz F, Guzeloglu-Kayisli O, Arlier S, Kayisli UA, Lockwood CJ.. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum Reprod Update 2016;22:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino AI, Povoa AM.. Frozen embryo transfer and preeclampsia risk. J Gynecol Obstet Hum Reprod 2021;50:102167. [DOI] [PubMed] [Google Scholar]
- Shennan A, Suff N, Leigh Simpson J, Jacobsson B, Mol BW, Grobman WA; FIGO Working Group for Preterm Birth. FIGO good practice recommendations on progestogens for prevention of preterm delivery. Int J Gynaecol Obstet 2021;155:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons NE, Leeuw M, Van't Hooft J, Limpens J, Roseboom TJ, Oudijk MA, Pajkrt E, Finken M, Painter RC.. The long-term effect of prenatal progesterone treatment on child development, behaviour and health: a systematic review. BJOG 2021;128:964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Reschke L, Segars J, Baker VL.. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril 2020;113:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A, Mohr L.. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983;305:707–709. [DOI] [PubMed] [Google Scholar]
- Vinsonneau L, Labrosse J, Porcu-Buisson G, Chevalier N, Galey J, Ahdad N, Ayel JP, Rongieres C, Bouet PE, Mathieu d'Argent E. et al. Impact of endometrial preparation on early pregnancy loss and live birth rate after frozen embryo transfer: a large multicenter cohort study (14 421 frozen cycles). Hum Reprod Open 2022;2022:hoac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen-Hoynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, Winn VD.. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension 2019a;73:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR. et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019b;73:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Murugappan G, Kort J, Westphal L.. Hormone replacement versus natural frozen embryo transfer for euploid embryos. Arch Gynecol Obstet 2019;300:1053–1060. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang J, Zhu Q, Yang X, Wang Y.. Effects of different cycle regimens for frozen embryo transfer on perinatal outcomes of singletons. Hum Reprod 2020a;35:1612–1622. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhu Q, Wang Y.. Pregnancy outcomes after different cycle regimens for frozen-thawed embryo transfer: a retrospective study using propensity score matching. Front Med (Lausanne) 2020b;7:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu H, Song H, Li X, Jiang J, Sheng Y, Shi Y.. Increased risk of pre-eclampsia after frozen-thawed embryo transfer in programming cycles. Front Med (Lausanne) 2020;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschkies F, Kroning L, Schill T, Chandra A, Schippert C, Topfer D, Ziert Y, von Versen HF.. Pregnancy outcomes after frozen-thawed embryo transfer in the absence of a corpus luteum. Front Med (Lausanne) 2021;8:727753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. Frozen-Thawed Embryo Transfer. 2020. https://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryo-transfer.html (December 2020, date last accessed).
- Wiegel RE, Fares DA, Danser AHJ, Laven JSE, Willemsen SP, Steegers EAP, Steegers-Theunissen RPM.. The impact of the mode of conception on periconceptional maternal cardiovascular adaptation to pregnancy: the renin-angiotensin-aldosterone-system. Reprod Sci 2020;27:332A. [Google Scholar]
- Xu J, Zhou H, Zhou T, Guo Y, Liang S, Jia Y, Li K, Teng X.. The impact of different endometrial preparation protocols on obstetric and neonatal complications in frozen-thawed embryo transfer: a retrospective cohort study of 3,458 singleton deliveries. Reprod Biol Endocrinol 2022;20:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bai X, Han Y, Zou Z, Fan Y, Wang X, Luo H, Zhang Y.. Adverse obstetric and perinatal outcomes in 2333 singleton pregnancies conceived after different endometrial preparation protocols: a retrospective study in China. BMC Pregnancy Childbirth 2022;22:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S.. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2021a;2:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat TR, Brink AJ, de Bruin JP, Goddijn M, Broekmans FJM, Cohlen BJ, Macklon NS, van Wely M, Groenewoud ER, Mol F. et al. Increased obstetric and neonatal risks in artificial cycles for frozen embryo transfers? Reprod Biomed Online 2021b;42:919–929. [DOI] [PubMed] [Google Scholar]
- Zaat TR, de Bruin JP, Goddijn M, van Baal M, Benneheij EB, Brandes EM, Broekmans F, Cantineau AEP, Cohlen B, van Disseldorp J. et al. Is home-based monitoring of ovulation to time frozen embryo transfer a cost-effective alternative for hospital-based monitoring of ovulation? Study protocol of the multicentre, non-inferiority Antarctica-2 randomised controlled trial. Hum Reprod Open 2021c;2021:hoab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat TR, de Bruin JP, Mol F, van Wely M.. Facilitators and barriers for home-based monitoring to time frozen embryo transfers in IVF among women and healthcare providers. Hum Reprod Open 2022;2022:hoac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilmaker GH, Alberda AT, Van Gent I, Rijkmans CM, Drogendijk AC.. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril 1984;42:293–296. [DOI] [PubMed] [Google Scholar]
- Zhou R, Zhang X, Huang L, Wang S, Li L, Dong M, Zhu X, Liu F.. The impact of different cycle regimens on birthweight of singletons in frozen-thawed embryo transfer cycles of ovulatory women. Fertil Steril 2022;117:573–582. [DOI] [PubMed] [Google Scholar]
- Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y.. Increased risk of maternal and neonatal complications in hormone replacement therapy cycles in frozen embryo transfer. Reprod Biol Endocrinol 2020;18:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.