Abstract
Background
One of the major mediators of ischemic neuronal cell death is calcium. It has been found that elevated serum calcium is associated with a better prognosis in patients with ischemic stroke. This study highlights the association of serum calcium, albumin-corrected calcium, and ionic calcium with the size of acute ischemic stroke as well as severity outcome in terms of the National Institutes of Health Stroke Scale (NIHSS) score and Barthel Index.
Methods
This cross-sectional study was conducted on 85 cases of acute ischemic stroke (based on a computerized tomography scan of the brain) from September 2019 to October 2021. All included patients had undergone complete clinical history, systemic examination, as well as estimation of serum total calcium, albumin corrected calcium, and ionic calcium. NIHSS score and Barthel Index were used to access the severity of each subject.
Results
A significant positive correlation was seen between infarct size with NIHSS with a correlation coefficient of 0.35. A significant negative correlation was seen between infarct size with serum calcium, albumin-corrected calcium, and Barthel Index with a correlation coefficient of -0.483, -0.354, and -0.365 respectively. No correlation was seen between infarct size and ionic calcium with a correlation coefficient of 0.082.
Conclusion
It can be concluded that higher normal levels of serum calcium and albumin-corrected calcium are associated with a smaller-sized infarct and had less severity index among patients with acute ischemic stroke.
Keywords: albumin corrected calcium, ionic calcium, total calcium, barthel index, nihss, infarct size, stroke
Introduction
A stroke is a cerebrovascular event with signs and symptoms consistent with cerebral dysfunction. These develop rapidly over twenty-four hours and may result in chronic debility or mortality [1]. Occlusion in the cerebral blood flow due to a thrombus or embolus leads to ischemic stroke. Atheroma, cardioembolic phenomenon, and artery-to-artery embolism are some of the causes that lead to decreased blood flow [2]. Stroke presents in two regions: the inner ischemic core and the surrounding area of hypoperfusion, the penumbra [3]. Certain risk factors may be associated with stroke like diabetes mellitus, hypertension, hyperhomocysteinemia, raised levels of c-reactive protein, and uric acid [4].
The normal cerebral blood flow in humans is 45-50 ml/min/100 gm. When this rate decreases to 10ml/min/100gm depolarization of neurons occurs along with disturbances like rapid loss of intracellular potassium, reduction of adenosine triphosphate, and entry of calcium and sodium into the cells. Calcium influx occurs secondary to interruption in the O2-dependent generation of high-energy compounds. Calcium overload into the mitochondria causes a decrease in oxidative phosphorylation. There is the activation of membrane phospholipases and protein kinase which in turn causes the formation of free fatty acid [5]. Activation of the inflammatory cascade with mediators like prostaglandin, leukotrienes, and free radicals causes denaturation of intracellular proteins and enzymes. Thus, calcium homeostasis plays an important role in acute ischemic stroke [6,7].
Serum calcium is found as ionic calcium, protein-bound calcium, and in complex form. It binds to both albumin and globulin. This is about 40 % of the total calcium in the body. Calcium when present in complex form is absorbed by various tissues and is distributed throughout the body as calcium carbonate, calcium phosphate, and calcium oxalate [8]. Ionic calcium is free calcium that is utilized by the body for different functions and this account for 51% of total body calcium. Higher albumin-corrected calcium levels were directly associated with severity in terms of neurological outcomes like morbidity and mortality after acute ischemic stroke. Serum calcium also correlates with the size of cerebral infarction and clinical outcomes [9,10]. This study highlights the association of serum calcium, albumin-corrected calcium, and ionic calcium with the size of acute ischemic stroke as well as severity outcome in terms of the National Institutes of health stroke scale (NIHSS) Score and Barthel Index.
Materials and methods
This cross-sectional study was performed on 85 patients with acute ischemic stroke from September 2019 to October 2021 after institutional ethical committee approval.
Detailed clinical history and examination were done and investigations specific to the study like complete hemogram, renal function test, liver function test, lipid profile, serum calcium, ionic calcium, and computed tomography (CT) of the brain were performed. Patients with acute ischemic stroke aged >18 years who were diagnosed within the previous 72 hours by examination and confirmed by a CT scan were included in the study. Patients with hemorrhagic stroke, subarachnoid hemorrhage, cerebral venous sinus thrombosis, those presenting with ischemic stroke after 72 hours of onset, and those with renal or hepatic failure (both of which may affect albumin level and thus alter the results) were excluded from the study. The flow chart of the study is shown in Figure 1.
Figure 1. Flow chart of the study.
Hemogram was obtained from a peripheral venous sample collected in an ethylenediamine tetraacetic acid (EDTA) bulb on admission and was run using an automated machine- UnicelDxH 800 Coulter Cellular Analysis System (Beckman Coulter), Danaher Corporation Company, Brea, California, United States.
Ionic calcium was estimated using an automated blood gas analyzer. Albumin-corrected calcium level was calculated as the sum of serum total calcium level and 0.8 times the difference between the normal population albumin level (4 mg/dl) and the patient’s albumin level. Albumin-corrected calcium level = serum total calcium level + 0.8 × (4 − patient's albumin level).
CT imaging was performed to calculate the size of the infarct, and the largest slice in the affected area was selected. A ruler tool was used to measure the longest axis in the lesion which was labeled as the x [A] axis. Another line was drawn perpendicular to it at the widest dimension and was labeled as the y [B] axis. The third axis was calculated by multiplying the number of slices with slice thickness and was the z [C] axis. The x, y, and z axis were calculated in mm. The infarct size was then calculated by ABC/2 which has been deemed the most accurate for calculating the size of the ischemic stroke [8]. The infarct size as used in the study was in cubic mm.
Outcome measures
The primary outcome of the study was to study serum calcium, ionic calcium, and albumin-corrected calcium in relation to the size of the ischemic stroke. The secondary outcome was to study the severity in terms of NIHSS score and Barthel Index about the size of ischemic stroke.
Statistical analysis
Statistical analysis was done using the Spearman rank correlation coefficient. The final analysis was done with the use of Statistical Package for Social Sciences (SPSS) software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp). For statistical significance, a p-value of less than 0.05 was considered statistically significant.
Results
Among 85 patients, the mean age of study subjects was 58.6 ± 13.8 years. 8.24% patients had brainstem infarct, 3.53% had cerebellar infarct, 11.76% had an infarct in the corona radiata, 12.94% had an infarct in ganglio-capsular region, 5.88% had an infarct in the internal capsule, 3.53% had infarct in thalamus and 54.12% had cortical infarcts. The mean value of serum calcium (mg/dl) was 8.96 ± 0.91, albumin-corrected calcium was 9.17 ± 0.92 and ionic calcium was 4.35 ± 0.53. Other baseline characteristics are shown in Table 1.
Table 1. Baseline characteristics and baseline profile of the study subjects.
| Parameter | Percentage/ Mean |
| Age | |
| Mean Age ± SD | 58.6 ± 13.8 |
| 18-30 | 3.53% |
| 31-60 | 48.24% |
| >60 | 48.24% |
| Gender | |
| Female | 35.29% |
| Male | 64.71% |
| Common clinical presentations | |
| Right-sided Hemiparesis | 36.5% |
| Left-sided Hemiparesis | 33% |
| Abnormal Body movements | 2.4% |
| Drowsy/ loss of consciousness | 5.8% |
| Giddiness and ataxia | 1.2% |
| Sudden loss of vision/ Diminution of vision | 2.4% |
| Altered sensorium | 5.8% |
| Headache | 3.5% |
| Speech disorders and deviation of angle of mouth | 8.2% |
| No weakness | 1.2% |
| Total | 100.00% |
| Mean NIHSS and Barthel Index on admission | |
| NIHSS | 12.07 ± 4.7 |
| Barthel Index | 74.41 ± 17.24 |
| Co-morbidities | |
| Diabetes mellitus | 20.00% |
| Hypertension | 56.47% |
| Others Ischemic heart disease Rheumatic heart disease Chronic obstructive pulmonary disease Old case of pulmonary tuberculosis) | 9.42% |
| More than 1 co-morbidity | 18.82% |
| Area involved | |
| Lobar | 54.12% |
| Combined lesion | 34.12% |
| Frontal region | 7.06% |
| Occipital | 2.35% |
| Parietal | 10.59% |
| Brainstem | 8.24% |
| Cerebellum | 3.53% |
| Corona radiata | 11.76% |
| Gangliocapsular region | 12.94% |
| Internal capsule | 5.88% |
| Thalamus | 3.53% |
| Total | 100.00% |
| Lipid profile | |
| Total cholesterol(mg/dL) | 170.71 ± 44.09 |
| LDL (mg/dL) | 111.66 ± 39.01 |
| HDL (mg/dL) | 38.04 ± 13.02 |
| Triglycerides(mg/dL) | 126.48 ± 57.74 |
| Risk Factors: | |
| Smoking | 40.00% |
| Alcohol | 34.12% |
| Serum calcium, albumin-corrected calcium, ionic calcium | |
| Serum calcium(mg/dL) | 8.96 ± 0.91 |
| Albumin corrected calcium(mg/dL) | 9.17 ± 0.92 |
| Ionic calcium(mg/dL) | 4.35 ± 0.53 |
A significant positive correlation was seen between NIHSS with infarct size (mm³) with a correlation coefficient of 0.35. A significant negative correlation was seen between NIHSS with serum calcium and albumin-corrected calcium with a correlation coefficient of -0.713, and -0.556 respectively. No correlation was seen between NIHSS with ionic calcium with a correlation coefficient of 0.053. A significant positive correlation was seen between the Barthel Index with serum calcium, albumin corrected calcium with a correlation coefficient of 0.779, and 0.566 respectively. A significant negative correlation was seen between the Barthel Index with infarct size with a correlation coefficient of -0.365. No correlation was seen between the Barthel Index with ionic calcium with a correlation coefficient of 0.002 as shown in Figures 2, 3, 4, 5 demonstrating a correlation with the NIHSS score, and Figures 6, 7, 8, 9 demonstrating a correlation with Barthel Index.
Figure 2. Correlation of the volume of infarct with NIHSS score.
Figure 3. Correlation of serum calcium with NIHSS score.
Figure 4. Correlation of albumin-corrected calcium with NIHSS score.
Figure 5. Correlation of ionic calcium with NIHSS score.
Figure 6. Correlation of volume of infarct with Barthel Index.
Figure 7. Correlation of serum calcium with Barthel Index.
Figure 8. Correlation of albumin-corrected calcium with Barthel Index.
Figure 9. Correlation of ionic calcium with Barthel Index.
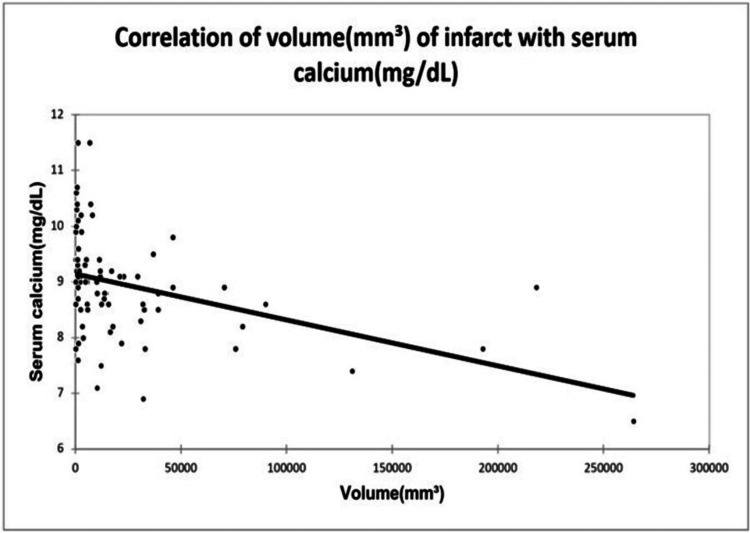
A significant positive correlation was seen between infarct size with NIHSS with a correlation coefficient of 0.35. A significant negative correlation was seen between infarct size with serum calcium, albumin-corrected calcium, and Barthel Index with a correlation coefficient of -0.483, -0.354, and -0.365 respectively. No correlation was seen between infarct size and ionic calcium with a correlation coefficient of 0.082 as shown in Figures 10, 11, 12.
Figure 10. Correlation of volume of infarct with serum calcium.
Figure 11. Correlation of volume of infarct with albumin-corrected calcium.
Figure 12. Correlation of volume of infarct with ionic calcium.
Discussion
In our study, we found that higher normal levels of serum calcium and albumin-corrected calcium were associated with a smaller-sized infarct and better prognosis in terms of NIHSS and Barthel Index among patients with acute ischemic stroke. Ionic calcium did not correlate with the size of the infarct or the prognosis of the patients.
Ischemic stroke occurs following critical stenosis in the cerebral circulation leading to a decrease in blood flow and ischemia. Hypoxia leads to disturbance in cellular homeostasis and movement of calcium from extracellular space into the cells. This leads to enzyme cascade activation and lipid peroxidation further increasing the influx of calcium into the cells. Ovbiagele et al. conducted a study in 2008 on 826 patients on patients with acute ischemic stroke and found that increased levels of serum Ca predict higher independence three months following ischemic stroke [10]. They also found that very early serum calcium appears not to have any prognostic significance, whereas in our study higher normal levels of serum calcium and albumin-corrected calcium were associated with a smaller-sized infarct and better prognosis in terms of NIHSS and Barthel Index. Buck et al. in 2007 found that the infarct size was comparatively smaller in patients with higher levels of serum calcium on admission [6]. Appel et al. performed a study in 2011 on 784 and found that serum calcium was a marker of mortality among patients with ischemic stroke [11]. Albumin-corrected calcium was the only factor to be associated with long-term mortality. Suryawan et al. in 2017 found that patients with poor outcomes had lower levels of serum-adjusted calcium [12,13,14]. A retrospective study done by Chung et al. in 2014 found that there was a significant association between serum calcium and albumin-corrected calcium as linear variables [15,16]. There was no significant association of calcium with NIHSS and Barthel Index as noted by Sivasubramaniyam et al. in 2017. Ishfaq et al. in their study of 138 patients revealed that low levels of serum calcium may be related to more severe clinical findings at the stroke onset [14,17]. A study by Borah et al. revealed that total calcium, albumin-corrected calcium, and ionized calcium had a statistically significant negative correlation with infarct size [5,18].
Strength of the study
Our study correlated biochemical serum calcium, albumin-corrected calcium, and ionic calcium along with radiological parameters. Functional and clinical indicators such as The National Institute of Health Stroke Scale and Barthel Index were included in this study.
Limitations
Potential confounding factors like socioeconomic status, dietary pattern, etc. were not adjusted. The study was done on a small sample size and the statistical association needs a larger sample size for further validation.
Conclusions
It can be concluded that higher levels of serum calcium and albumin-corrected calcium are associated with a smaller-sized infarct and better prognosis among patients with acute ischemic stroke. Serum calcium, albumin-corrected calcium, and ionic calcium are often overlooked or not assessed in patients with acute ischemic stroke. Thus, efforts must be made to create awareness with regard to the use of serum calcium in assessing the size of ischemic stroke and its prognostic significance. A multi-centric study with a larger number of patients should be done in order to further assess the role of serum calcium, albumin-corrected calcium, and ionic calcium in acute ischemic stroke.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Ethical Committee (DMIMS) issued approval DMIMS (DU)/IEC/Aug-2019/8222
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Incidence & prevalence of stroke in India: a systematic review. Kamalakannan S, Gudlavalleti AS, Gudlavalleti VS, Goenka S, Kuper H. Indian J Med Res. 2017;146:175–185. doi: 10.4103/ijmr.IJMR_516_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Sacco RL, Kasner SE, Broderick JP, et al. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Saver JL, Altman H. Stroke. 2012;43:1537–1541. doi: 10.1161/STROKEAHA.111.636928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annual rhythmic and non-rhythmic biological variation of magnesium and ionized calcium concentrations. Valero-Politi J, Ginard-Salvá M, González-Alba JM. Clin Chem Lab Med. 2001;39:45–49. doi: 10.1515/CCLM.2001.011. [DOI] [PubMed] [Google Scholar]
- 5.Association of serum calcium levels with infarct size in acute ischemic stroke: observations from Northeast India. Borah M, Dhar S, Gogoi DM, Ruram AA. J Neurosci Rural Pract. 2016;7:0–5. doi: 10.4103/0976-3147.196461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low serum calcium levels contribute to larger hematoma volume in acute intracerebral hemorrhage. Inoue Y, Miyashita F, Toyoda K, Minematsu K. https://doi.org/10.1161/STROKEAHA.113.001187. Stroke. 2013;44:2004–2006. doi: 10.1161/STROKEAHA.113.001187. [DOI] [PubMed] [Google Scholar]
- 7.Correlation of serum calcium levels with severity and functional outcome in acute ischemic stroke patients. Gupta A, Dubey U, Kumar A, Singh S. Int J Res Med Sci. 2015;3:3698–3702. [Google Scholar]
- 8.ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinico-radiological correlation between serum calcium and acute ischemic stroke. Kasundra GM, Sood I, Bhushan B, Bohra GK, Supriya PS. Int J Adv Med Health Res. 2014;1:69–74. [Google Scholar]
- 10.Serum calcium as prognosticator in ischemic stroke. Ovbiagele B, Starkman S, Teal P, et al. Stroke. 2008;39:2231–2236. doi: 10.1161/STROKEAHA.107.513499. [DOI] [PubMed] [Google Scholar]
- 11.Serum calcium levels and long-term mortality in patients with acute stroke. Appel SA, Molshatzki N, Schwammenthal Y, Merzeliak O, Toashi M, Sela BA, Tanne D. Cerebrovasc Dis. 2011;31:93–99. doi: 10.1159/000321335. [DOI] [PubMed] [Google Scholar]
- 12.Low adjusted serum calcium level as a predictor of poor outcome in patients with acute ischemic stroke. Suryawan A, Nuartha AA, EkoPurwata T, Samatra DP, Widyadharma IP. IJSR. 2017;6:638–641. [Google Scholar]
- 13.Correlation of serum calcium with severity of acute ischaemic stroke. Ishfaq M, Ullah F, Akbar S, Rahim F, Afridi AK. https://doi.org/10.47391/JPMA.04-593. J Pak Med Assoc. 2017;67:20–23. [PubMed] [Google Scholar]
- 14.A study on the role of serum calcium, albumin, and uric acid as predictors of neurological severity and short term outcome in acute ischemic stroke. Sivasubramaniyam S, Umesha HB, Ramesh B. Int J Med Health Res. 2017;3:102–105. [Google Scholar]
- 15.Elevated calcium after acute ischemic stroke: association with a poor short-term outcome and long-term mortality. Chung JW, Ryu WS, Kim BJ, Yoon BW. J Stroke. 2015;17:54–59. doi: 10.5853/jos.2015.17.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serum uric acid as a biomarker inpredicting outcome in patients of acute ischemic stroke: a cross-sectionalstudy at limited resources rural setup. Khanna S, Kumar S, Acharya S. Int J Nutr Pharmacol Neurol Dis. 2023;13:68–73. [Google Scholar]
- 17.A study protocol for estimating the association of de ritis ratio (AST/ALT) with outcomes in patients of acute ischemic stroke. Nimkar S, Varma A, Acharya SS. J Pharm Res Int. 2021;33:186–193. [Google Scholar]
- 18.Development and validation of a daily monitoring stroke scale: a three-month follow up study. Aradhey P, Kumar S, Acharya S. Journal of Pharmaceutical Research International. 33:175–180. [Google Scholar]