Abstract
One sex differences in the perception of emotion is that females, particularly those with high anxiety, often show heightened identification of fearful faces. To better understand the causal role of glucocorticoids in this sex difference, we examine these associations in people with HIV(PWH) where emotion perception is impaired and mental health disorders are frequent. In a double-blind, placebo-controlled, cross-over study, we used a single low-dose of hydrocortisone (10 mg; LDH) as a mechanistic probe of the effects of elevated glucocorticoids on negative emotion perception in 65 PWH (31 women). The primary outcome was accuracy in identifying emotional expressions on the Facial Emotion Perception Test (FEPT). Salivary cortisol, self-reported stress/anxiety, and childhood trauma were also assessed. LDH increased salivary cortisol levels versus placebo. The effect of LDH versus placebo on FEPT accuracy depended on the combined influence of facial expression and sex (P = 0.03). LDH influenced accuracy only for women (P = 0.03), specifically for fearful faces (Cohen’s d = 0.44, P = 0.04). Women’s enhanced threat detection varied with psychological burden (mood, anxiety, and post-traumatic stress symptoms), more pronounced among those with lower burden and trauma (P < 0.05). This result suggests a role of the HPA axis in sex differences for perception of fearful faces in women with HIV, potentially due to changes in glucocorticoid receptor availability/activity, or improved integration of signals from facial recognition and emotion processing regions. The blunting of this effect in men and in individuals with more severe trauma suggests that the mechanisms underlying threat detection differ by sex and trauma history and warrant further investigation.
Keywords: Glucocorticoids, HIV, Sex, Emotion
Introduction
Sex-specific differences in hypothalamic–pituitary–adrenal (HPA) axis functioning have previously been characterized (Ferrini et al. 1997; Goel et al. 2014; Oyola and Handa 2017) and may also underlie differences in processing of social stimuli such as emotional facial expressions. In men, both cortisol production and subsequent responses of the HPA axis are blunted compared to women (Panagiotakopoulos and Neigh 2014; Rohleder et al. 2003), who have also been shown to have higher cortisol responses in reaction to stimulation of glucocorticoid receptors by steroids like dexamethasone or corticotropin-releasing hormone (Rampp et al. 2018; Swaab and Bao 2020). Viewing and subconscious processing of negatively valenced emotional stimuli such as fearful or angry faces, or traumatic or stressful experiences, have also been shown to enhance this sex difference (Rohleder et al. 2001, 2003) and have been associated with the higher risk for brain changes associated with depression or anxiety disorder symptoms (Lok et al. 2012; Pooley et al. 2018).
Facial and emotional processing is a crucial factor in effective human social interactions. Accurate, rapid facial processing relies on functioning of the fusiform gyrus (expertise in recognizing nuances in human faces, familiarity/identification) (Nasr and Tootell 2012) and prefrontal regions (to integrate and prepare behavioral response) (Grill-Spector et al. 2004; Kitada et al. 2010). While “neutral” faces produce a fairly straightforward pattern of brain activation, faces with positive or negative valence involve more complex circuitry, to recognize and integrate an emotional reaction to the stimulus (e.g., fear, anger, happiness), mediated by activity in the hippocampus, frontal and limbic cortex, and the amygdala (Brooks et al. 2012; Szymkowicz et al. 2016).
Perception of “fearful” faces is particularly behaviorally relevant, in situations that pose a threat to an individual’s safety. Accurate perception of fearful faces has been shown to increase amygdala input relative to neutral faces (Vuilleumier et al. 2003), enhancing visual attention acuity with increased activity in the frontoparietal attention network and fusiform gyrus (Barbot and Carrasco 2018; Wang et al. 2017). However, there is considerable evidence that the way fearful faces are processed recruits different neurocircuitries in men compared to women, with men tending to have much higher neural activation to certain emotional faces (fear, anger) relative to women, as well as activating different cortical regions, such as unilateral activation in men versus bilaterally in women (Cahill et al. 2001; Im et al. 2018). Additionally, this circuitry is potentially heavily influenced by prior life experiences (trauma exposure), particularly in those with chronic disorders that impact CNS function, such as HIV (Clark et al. 2010, 2015). People with HIV (PWH) are at higher risk for stress or trauma exposure (Reif et al. 2011), subsequently higher risk for that stress and trauma to lead to a diagnosed mood disorder (Neigh et al. 2016), and potentially further altering the functioning of fear perception neural activity across the frontal and parietal lobes.
In PWH, facial emotion processing circuitry is altered in a number of ways. HIV has been shown to alter volume, activity, and connectivity in many of the same key regions mentioned above (Clark et al. 2010). Higher stress exposure and significant mood symptoms are also associated with chronic HIV, increasing the potential for stress-related dysfunction of emotional perception and processing circuitry (Cruess et al. 2001; Leserman 2003). Perception for fearful faces in particular has also been shown to be impaired in PWH relative to HIV-uninfected adults (Baldonero et al. 2013), likely related to impaired frontostriatal circuitry. Ultimately, these interrelated impairments alter how PWH perceive, process, and react to fearful facial expressions, which can potentially be associated with threat avoidance. In a population prone to stressful or threatening life experiences, as well as mood and anxiety disorders, these changes may also have deleterious effects on social connectedness, a key feature of coping for PWH.
Higher exposure to stressful life experiences has been associated with dysregulated HPA axis function, though this effect is subject to sex differences. Uninfected healthy women tend to perform more accurately on facial emotion perception than men (Campbell et al. 2002), but not if those women endorse depression or PTSD symptoms (Wright et al. 2009). Among those with a history of stress or trauma exposure, sex-specific differences in amygdala connectivity and activity may be one of the underlying mechanisms responsible for alterations in cortisol availability and HPA function (Orozco and Ehlers 1998), increasing HPA activity and cortisol availability in men, while decreasing in women (Kajantie and Phillips 2006; Kirschbaum et al. 1999). Cortisol availability after a single dose of an exogenous corticosteroid is a useful biomarker to probe HPA axis functioning and subsequently integrity of facial processing circuitry in men and women living with HIV. To that end, we conducted a single-dose study of low-dose hydrocortisone (LDH) as a mechanistic probe of the effects of increased glucocorticoid availability on a facial emotion processing task in a cohort of men and women with HIV (MWH and WWH, respectively). We hypothesized that after LDH administration, men and WWH would display sex-specific differences in their ability to correctly identify positive, neutral, or negative facial emotions. Secondarily, we also expected that cortisol responsivity to LDH would be associated with change in facial emotion identification following LDH in PWH.
Methods
Participants
Participants included 65 PWH (31 WWH) (Rubin et al. 2018, 2017), recruited from HIV primary care clinics in the Chicago-land area via advertisements and websites. Participants included in this analysis were and were aged 18–45 with English as their first language and used effective antiretroviral therapy (ART) for at least 3 months. Exclusion criteria included the following: lifetime history of any psychotic disorder, neurological condition impacting cognition (e.g., loss of consciousness > 1 h), body mass index greater than 40, history of substance abuse/dependence in the past 6 months excluding alcohol/ nicotine, and inability to abstain from illicit substances 24 h prior to testing confirmed via urine toxicology screen.
Screening, baseline, and psychiatric assessment procedures
Participants provided written informed consent. As part of study Session 1, all participants completed a Diagnostic and Statistical Manual of Mental Disorders (SCID) IV interview, toxicology screen, vitals assessment, and questionnaires: Childhood Trauma Questionnaire (CTQ) (Bernstein and Fink 1998), Schedule of Life Events Checklist (SLE) (Bieliauskas et al. 1995), PTSD Checklist–Civilian version (PCL-C) (Ruggiero et al. 2003), Perceived Stress Scale (PSS-10) (Cohen et al. 1983), Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977), Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1991), and Medication Adherence Self-Report Inventory (MASRI) (Walsh et al. 2002).
Drug procedures
After screening measures at Session 1, qualifying participants returned for two subsequent visits (Sessions 2 and 3) nested in a randomized, double-blind, placebo-controlled, cross-over pharmacologic challenge study Sessions 2 and 3 occurred between 12:00 pm (± 30 min) and 6:00 pm (± 30 min) to account for diurnal variations in cortisol. Participants were randomized to receive either LDH or placebo (all pills encapsulated to obtain valid blinding) at Session 2 and then received the opposite treatment at Session 3. Parallel procedures were used at Sessions 2 and 3 and included a toxicology screen, pregnancy test (for women), blood draw, vitals assessment, completion of questionnaires (PCL-C, PSS-10, CES-D, PSQI, MASRI, State-Trait Anxiety Inventory [STAI]-collected concurrent with saliva collection), cognitive tests, and collection of saliva samples. The emotion processing assessment occurred 4 h post-pill administration. Session 2 occurred within 1 week of Session 1, and Session 3 occurred approximately 1 month after Session 2.
Emotion perception task
The Facial Emotion Perception Test (FEPT) (Langenecker et al. 2005) is a computerized task that assesses the ability to categorize facial expressions. The FEPT involves briefly showing participants pictures of faces with one of four target emotions (fear, anger, happiness, sadness) or a neutral expression or pictures of animals that fall into one of four categories (primate, dog, cat, bird). Each presentation of stimuli (faces and animals) began with an orienting cross in the center of the laptop that was presented for 500 ms. The cross was followed by the presentation of a stimulus for 300 ms, a visual mask for 100 ms, and then a response period of 2600 ms. Thus, each trial lasted for 3500 ms, and there was no inter-trial interval. The primary outcome measure was the accuracy of affect identification. Reaction time to correct trials and accuracy for categories were examined as secondary outcomes. The FEPT was only administered 4-h post-pill administration at Sessions 2 and 3. A final n of 31 WWH and 34 MWH completed this task (two female participants were excluded from analysis due to invalid data) as we added this task to study after a number of participants completed the study.
Cognitive assessments
A detailed description of the cognitive test battery that was administered 30 min and 4 h post-pill administration has been previously reported (Rubin et al. 2018, 2017) as they were the primary study endpoints. In brief, the battery administered 30 min post-pill administration at Sessions 2 and 3 included the Hopkins Verbal Learning Test (HVLT-R) (Benedict et al. 1998), Letter-Number Sequencing (LNS) task form the Wechsler Adults Intelligence Scale IV (Wechsler 2008), Trail Making Test (TMT) Part A and B (Reitan 1978), and a computerized Stroop Test (MacLeod 1991), and Line Orientation Task from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Benton et al. 1983). The battery administered 4 h post-pill administration at Sessions 2 and 3 included HVLT-R, LNS, and TMT. To minimize carryover effects, four alternate versions of the HVLT-R, LNS, and TMT were used. All forms were administered in a counterbalanced manner. For this analysis, we only focused on the tests that were administered 4 h post-pill administration (HVLT-R, LNS, TMT) as that is the same time that the FEPT was administered. These measures in part rely on prefrontal cortex function that is typically related to facial emotion processing.
Saliva collection and cortisol analysis procedures
Participants were instructed to refrain from recreational drugs and alcohol for 1 day before study sessions, refrain from caffeine/physical exertion for 3 h before appointments, eat a light breakfast low in fat/protein, and refrain from smoking the day of appointments as a means to minimize the influence of external factors on cortisol levels. During Sessions 2 and 3, salivary samples were obtained at 10 time points. Baseline measures were taken 35 and 20 min before pill administration. Saliva was then measured 30, 60, 90, 180, 210, 240, 270, and 300 min after pill administration. Saliva was collected via straws into Nalgene tubes, stored at −80 degrees, and samples were batch shipped to Salimetrics and assayed for cortisol with an EIA kit (sensitivity < 0.007 μg/dL). Cortisol was determined by Salimetrics utilizing a commercially available 96-plate EIA kit specific for saliva samples. Sensitivity was 0.003 μg/dL; interassay coefficient of variation (CV) for high and low concentration samples was 5.1% and 6.3%, respectively; intraassay CV was 5.2%. Total cortisol (area under the curve from ground [AUCg] and increase [AUCi]) was calculated according to according to standard procedures (Pruessner et al. 2003). Outliers were winsorized (< 1% of the data). Participants also completed the State-Trait Anxiety Inventory: Short Form (Marteau and Bekker 1992) and a two-item Visual Analogue Scale measuring how “anxious” and “stressed” they felt, by placing a mark on a line broken into 10-mm segments (maximum rating = 80 mm) at 10 time points, concurrent with saliva sampling.
Statistical analysis
General linear models were conducted to examine sex differences in the effects of LDH versus placebo on cortisol levels and emotion perception performance. For cortisol, the grouping variable was Sex and the within-subject variables were Time (measured at 10 time points) and Treatment. For emotion perception, the grouping variable was Sex and the within-subject variables were Emotion (fear, angry, etc.) and Treatment. There were two interactions of particular interest. The first was the two-way Sex × Treatment interaction which tested the hypothesis that the effect of LDH relative to placebo differed for men and WWH. The second was the three-way Sex × Treatment × Emotion interaction which tested the idea that the effect of LDH relative to placebo depends on the combined influence of Sex and Emotion. All models adjusted for age, education (less than high school vs. not), depressive (CES-D) and PTSD symptoms (PCL-C), and a positive urine toxicology screen for marijuana. For emotion perception, treatment sequence was also included to minimize carry-over effects.
Pearson correlations were conducted to determine whether performance changes (LDH—placebo) were associated with cortisol responsivity to LDH, computed as AUCg and AUCi (Pruessner et al. 2003). Exploratory Pearson correlations were also conducted to determine whether performance changes (LDH—placebo) or performance during placebo or LDH were related to individual difference factors (trauma [CTQ], perceived stress [PSS-10], post-traumatic stress [PCL-C], depressive symptoms [CES-D], major life events [SLE]), HIV-related clinical characteristics (CD4 count, viral load), and cognitive performance (LNS, TMT, HVLT-R). All analyses were conducted in SAS (v.9.4 for Windows; SAS); significance was set at P < 0.05, and trends were set at P ≥ 0.05 and P < 0.10.
Results
Demographic, behavioral, and clinical results
Full details of the behavioral, demographic, and clinical characteristics of this study cohort are in Table 1. A total sample size of 65 participants (31 WWH) were included in this cohort and were well-matched across level of education, race, and employment, though the MWH were younger on average than WWH (P = 0.04). MWH endorsed considerably more current substance use than WWH including more alcoholic drinks per week (P < 0.001) and marijuana use (P < 0.001). Consistent with prior literature, the women in this sample had higher depressive symptoms (CES-D, P = 0.03), perceived stress (PSS-10, P = 0.008), and PTSD (PCL-C, P = 0.02) symptoms than MWH, though the prevalence of abuse and neglect were similar between groups. On average, WWH have been living with HIV longer than MWH (P = 0.04), though the majority of participants in both groups (83%) were virally suppressed with viral loads of ≤ 20 copies/ml.
Table 1.
Demographic and clinical characteristics of the sample at the enrollment visit, Session 1 by biological sex.
| Variables | Women with HIV (n = 31) | Men with HIV (n = 34) | p value |
|---|---|---|---|
| Socio-demographic factors | |||
| Age | 36.40 (7.40) | 31.85 (8.78) | 0.04* |
| Education | 0.21 | ||
| < High school | 11 (35) | 9 (26) | |
| High school graduate | 12 (39) | 9 (26) | |
| > High school | 8 (26) | 16 (47) | |
| Race/ethnicity | 0.40 | ||
| Black, not Hispanic | 29 (94) | 31 (91) | |
| White, not Hispanic | 1 (3) | 0 (0) | |
| Others | 1 (3) | 3 (9) | |
| Unemployed | 18 (58) | 24 (71) | 0.30 |
| Risky health behaviors | |||
| Currently smoking | 14 (45) | 23 (68) | 0.07 |
| Number of Alcohol drinks/week | 0.50 (0.74) | 1.31 (1.78) | < 0.001** |
| Current use | |||
| Marijuana | 7 (23) | 23 (68) | < 0.001** |
| Number of times used/week | 4.78 (2.77) | 2.11 (1.89) | 0.02* |
| Urine toxicology screen positive for marijuana | 8 (25) | 19 (56) | 0.01* |
| Cocaine | 2 (6) | 1 (3) | 0.50 |
| Methadone | 1 (3) | 0 (0) | 0.29 |
| Methamphetamines | 0 (0) | 1 (3) | 0.35 |
| Ever dependent/abuse alcohol | 2 (6) | 3 (9) | 0.72 |
| Ever dependent/abuse substances | 12 (39) | 8 (24) | 0.20 |
| Psychological profile | |||
| Major depression† in lifetime but not past year | 15 (48) | 14 (41) | 0.56 |
| Depressive symptoms (CES-D) (range: 0–60) | 16.06 (9.61) | 10.91 (6.77) | 0.03* |
| Perceived stress (PSS-10) (range: 0–40) | 22.16 (5.99) | 18.47 (4.79) | 0.008* |
| PTSD symptoms (PCL-C) (range: 17–85) | 35.29 (15.18) | 25.41 (8.04) | 0.02* |
| Childhood Trauma (CTQ) | |||
| Emotional abuse (range: 5–25) | 11.58 (5.99) | 9.34 (4.66) | 0.12 |
| Physical abuse (range: 5–25) | 10.87 (6.56) | 8.94 (4.73) | 0.35 |
| Sexual abuse (range: 5–25) | 9.19 (7.11) | 9.44 (6.28) | 0.42 |
| Emotional neglect (range: 5–25) | 12.52 (4.81) | 11.26 (5.29) | 0.30 |
| Physical neglect (range: 5–25) | 9.97 (4.53) | 8.79 (4.02) | 0.26 |
| Negative Life events (SLE) (range: 0–54) | 7.42 (4.82) | 6.88 (4.60) | 0.62 |
| Reported exposure to interpersonal violence | 10 (32) | 11 (32) | 0.99 |
| Reported exposure to sexual abuse | 11 (35) | 8 (24) | 0.29 |
| Clinical characteristics | |||
| Body mass index | 28.48 (5.54) | 23.74 (4.66) | < 0.001** |
| Years living with HIV | 12.08 (6.27) | 8.62 (6.59) | 0.04* |
| Medication adherence (MASRI) missing ≥ 1 dose: | |||
| 3 days summated before visit | 9 (29) | 8 (24) | 0.62 |
| 2 weeks before visit | 11 (35) | 11 (32) | 0.79 |
| Last month ± | 14 (45) | 13 (38) | 0.57 |
| CD4 Count (cells/μl) | 0.18 | ||
| > 500 | 15 (48) | 22 (65) | |
| ≥ 200 and ≤ 500 | 13 (42) | 7 (21) | |
| < 200 | 3 (10) | 5 (15) | |
| Viral Load (HIV RNA (cp/ml)) | 0.53 | ||
| Undetectable | 12 (39) | 16 (47) | |
| Lowest detectable limit (20 cp/ml) | 8 (26) | 8 (24) | |
| < 10,000 | 8 (26) | 6 (18) | |
| ≥ 10,000 | 3 (10) | 4 (11) |
Note. Current use = use in the last month. CES-D Center for Epidemiologic Studies Depression Scale; PSS-10 Perceived Stress Scale; PCL-C PTSD Checklist—Civilian version; CTQ Childhood Trauma Questionnaire; SLE Schedule of Negative Life Events Checklist; PSQI Pittsburgh Sleep Quality Index; MASRI Medication Adherence Self-Report Inventory. †based on the Structural Clinical Interview of Mental Health Disorders (SCID); ± visual analogue scale for proportion of doses taken in the last month; based on information extracted from the PTSD module on the SCID. Groups were compared on continuous variables using Wilcoxon two-sample tests and on categorical variables using chi-square tests
Cortisol results
After administration of LDH, but not placebo, salivary cortisol levels increased sharply in both MWH and WWH from the time point 20 min before pill administration to 30 min after pill administration (P’s < 0.001), without differences between the sexes (P = 0.79). Thereafter, for 90 min, salivary cortisol levels remained increased for both MWH and WWH (P’s > 0.70) with no significant differences between the sexes (P’s > 0.46 comparing 30 to 60 and 30 to 90 min after pill administration). Salivary levels returned to levels comparable to baseline (levels 20 min before pill administration) for MWH and WWH 240 min after pill administration at which point the FEPT was administered (P’s > 0.10). There was no difference between the sexes in salivary levels at this time point (P = 0.06).
Effects of LDH on facial emotion perception task
Overall, during the active LDH treatment, 52% of the WWH showed at least a 10% improvement in recognition accuracy for fearful faces, and 39% of the WWH participants showed at least a 20% improvement in recognition accuracy for fearful faces on the FEPT. Although the Sex × Treatment interaction was not significant on the FEPT, (F(1, 56) = 0.51, P = 0.48), the three-way interaction between Emotion × Treatment × Sex—was significant (F(4, 224) = 2.62, P = 0.03). This indicated that the magnitude of change in performance (accuracy) on the FEPT after LDH relative to placebo depended on the combined influence of Emotion and Sex (Fig. 1). Restricting a follow-up analysis to PWH of each sex, the Emotion × Treatment interaction was significant for WWH (F(4, 224) = 2.75, P = 0.03), but not MWH (F(4, 224) = 0.17, P = 0.95). Among WWH, the effect of LDH relative to the placebo treatment improved accuracy on task performance for fearful faces (F(1, 224) = 4.48, P = 0.04, Cohen’s d = 0.44), but not for angry (d = 0.04), sad (d = 0.38), happy (d = 0.32), or neutral (d = 0.23) faces (P’s > 0.11). Among MWH, the effect of LDH relative to the placebo treatment was small for all faces: neutral (d = 0.14), sad (d = 0.13), fearful (d = 0.07), angry (d = 0.07), and happy (d = 0.04). The Sex × Treatment interaction on task performance for categorizing animals trials was not significant (F(1, 58) = 3.47, P = 0.07; WWH d = 0.34; MWH d = 0.18). There were no significant effects on reaction time for identifying faces correctly on the FEPT: main effect of treatment, (F(1,224) = 0.10, P = 0.75); Sex × Treatment interaction (F(1,224) = 0.01, P = 0.93); and Emotion × Treatment × Sex interaction (F(4,224) = 0.27, P = 0.90).
Fig. 1.
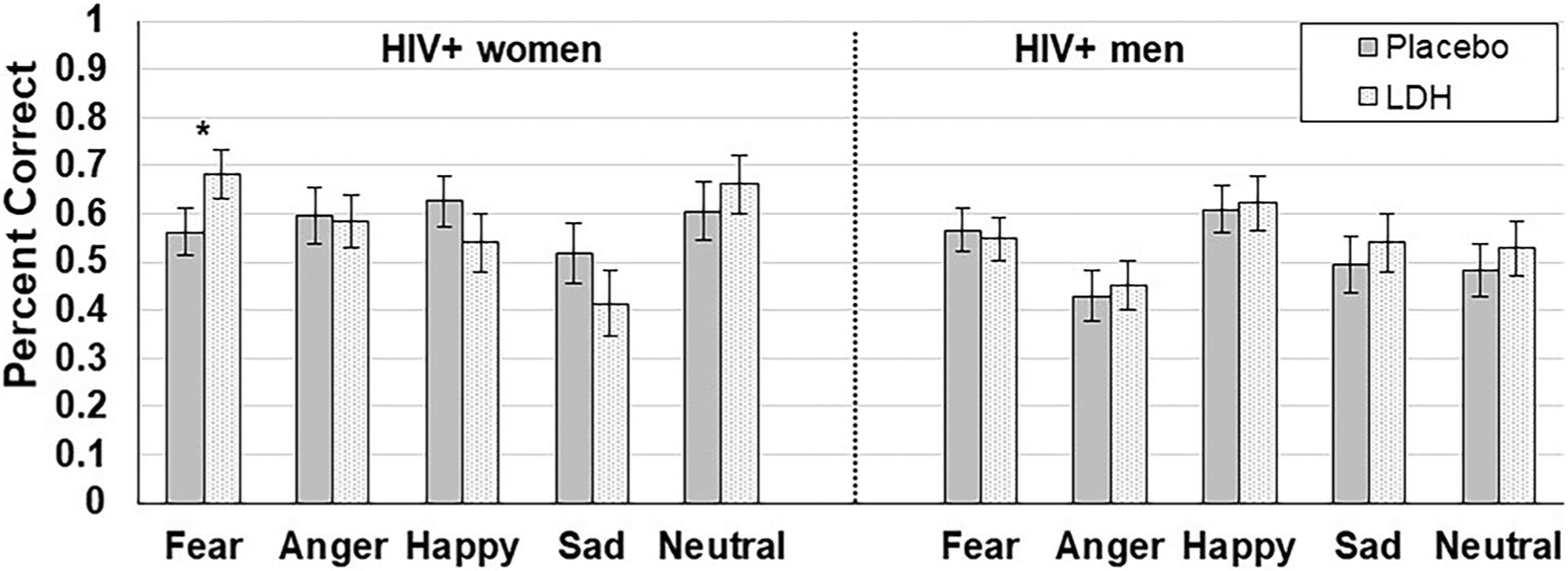
Sex differences in the effects of low-dose hydrocortisone (LDH) versus placebo on cognition in people with HIV. Note: LDH = low-dose hydrocortisone. The Emotion × Treatment (placebo vs LDH) × Sex interaction was significant at p < 0.05 (*)
Correlations were conducted to understand whether cortisol responsivity to LDH, psychological burden (mood, anxiety, and PTSD symptoms), cognitive function, or HIV-related clinical factors was associated with the degree of change in identifying fearful faces on the FEPT, with LDH relative to placebo in WWH. These women were more likely to perform more accurately identifying fearful faces with LDH relative to placebo if women reported less overall childhood trauma (CTQ; r = −0.40, P = 0.02), childhood physical abuse (CTQ; r = −0.49, P = 0.005), or childhood physical neglect (CTQ; r = −0.43, P = 0.01), trend on childhood emotional abuse (CTQ; r = −0.35, P = 0.05) and anxiety (STAI; r = −0.32, P = 0.08) (Fig. 2A–D). Better accuracy identifying fearful faces with LDH relative to placebo was associated with slower performance on TMT Part A (r = −0.42, P = 0.02) and Part B (r = −0.39, P = 0.02) and better performance on the working memory condition of the LNS (r = 0.40, P = 0.02) and trend on the attention condition of LNS (r = 0.31, P = 0.09) (Fig. 2E–H). Salivary cortisol responsivity to LDH (AUCg and AUCi) was not associated with improvement in fear identification on the FEPT (r = 0.02, P = 0.93 and r = −0.01, P = 0.96, respectively), and there were no significant associations between performance identifying fearful faces or with any other HIV-related clinical characteristics after LDH administration, relative to placebo.
Fig. 2.

In women with HIV (LDH – placebo), correlations between childhood trauma (A-D), cognition (E-H), and change inidentifying fearful faces with low-dose hydrocortisone relative to placebo in women with HIV. Note: LDH: low-dosehydrocortisone, CTQ: Childhood Trauma Questionnaire, LNS: WAIS-IV Letter-Number Sequencing Task
While there were no significant correlations between individual difference factors and performance accuracy on fearful faces during placebo (P’s > 0.09), there were a number of factors identified during LDH administration. During the active LDH treatment, accuracy for fearful faces was higher when WWH reported less overall childhood trauma (CTQ; r = −0.58, P = 0.001), childhood physical abuse (CTQ; r = −0.58, P = 0.001), childhood physical neglect (CTQ; r = −0.54, P = 0.002), childhood sexual abuse (CTQ; r = −0.51, P = 0.003), childhood emotional abuse (CTQ; r = −0.45, P = 0.01), anxiety (STAI; r = −0.44, P = 0.01), depressive symptoms (CES-D; r = −0.39, P = 0.03), and post-traumatic stress symptoms (PCL-C; r = −0.36, P = 0.04), relative to the placebo treatment (Fig. 3).
Fig. 3.

In women with HIV, correlations between childhood trauma (A-D), mental health questionnaires (E-H), and identifying fearful faces during low-dose hydrocortisone. Note: LDH: low-dose hydrocortisone, CTQ: Childhood Trauma Questionnaire, STAI: State-Trait Anxiety Index, CES-D: Center for Epidemiological Studies—DepressionScale, PCL-C: PTSD Checklist—Civilian
Discussion
In this study, we showed that in WWH, a single dose of LDH improved accuracy of identifying fearful facial expressions. This improvement in performance (higher % accurate) was most significantly influenced by the combined effect of biological sex and the emotional facial expression being presented. We also found that in this subset of WWH, experience of childhood abuse, neglect, or overall childhood trauma was negatively correlated with this effect of LDH on accuracy for fearful faces. Potentially, LDH influences HPA axis function in WWH and in particular those who experienced low, but not high levels of childhood adversity, such that they are better able to identify and subsequently react to fearful faces, which signals a potential imminent threat. This LDH effect was not observed for MWH, and LDH also did not improve accuracy for any other facial emotion (anger, happiness, etc.).
Perception of fearful stimuli is a necessary behavioral signal, for appropriate psychosocial response to environmental threats. Fearful stimuli, in particular, faces, is known to strongly activate the amygdala (Mattavelli et al. 2014) the dorsolateral prefrontal cortex, and fusiform gyrus (Wang et al. 2017) relative to other emotions. Former studies have shown that accurate identification for fearful faces is prioritized over other expressions (Stein et al. 2009), even when competing with distracting stimuli (Stein et al. 2010). Stressful life experiences, particularly those severe enough to trigger symptoms of PTSD, alter this ability, with prior evidence showing that individuals with previous stress exposure are more likely to pay less attention to fearful face stimuli (Stienen and de Gelder 2011) and are more easily distracted by task irrelevant stimuli (Milders et al. 2006) performing less accurately. Stressful life experiences have also been shown to negatively affect cortisol availability (Petrowski et al. 2012), and although the mechanisms underlying the current results are unknown, the stress-related dysregulation has far-reaching consequences on neural and behavioral responses to stressful stimuli.
Cortisol availability is associated with multiple neurobiological processes relevant to perception of fear signals, learning, and coping, as a result of functional modulation of neural circuitry between the medial prefrontal cortex (PFC), limbic cortex, and particularly the amygdala, where glucocorticoid receptors are densely distributed (Fusar-Poli et al. 2009; Ma et al. 2017). A previous study found that low-dose cortisol administration improved PTSD symptoms in a cohort of seronegative adults (Aerni et al. 2004), though it was a small pilot study of less than five patients. However, glucocorticoid stimulation has also been shown to have opposite effects in women, compared to men, particularly in regard to speed and accuracy identifying of threatening/fearful stimuli (Merz et al. 2010). This has been attributed to sex-specific differences in connectivity between the amygdala and the hippocampus and frontal cortices (Kogler et al. 2016; Roca et al. 2005). Together, these neurobiological differences may be involved in enhancing vigilance and perception to potential threats, such as a fearful face, when cortisol availability is increased, though one would expect that the efficacy of such a treatment to be sex-dependent as well.
Focusing on PWH, prior studies have shown that impairment in facial emotion processing is associated with smaller amygdala and anterior cingulate volumes in PWH (Baldonero et al. 2013; Clark et al. 2015). WWH are at a higher risk of experiencing significant traumatic or stressful life events than MWH (Katz and Nevid 2005; Spies et al. 2017). Interestingly in our results, however, the WWH who endorsed high levels of childhood traumatic life experiences did not experience any improvement in fearful face accuracy after LDH treatment. In these individuals, the combination of their HIV and trauma history exerting a combined negative effect on CNS and HPA functioning may instead serve to blunt emotional processing capacity regardless of emotion presented, and a one-time LDH treatment is not sufficient to rescue that ability. Our results could also indicate that in this population of HIV-infected persons, the curve representing the relationship between trauma severity and HPA axis function/GC responsivity takes an inverted “U” shape, and as a consequence performance is negatively impacted except in those near the top of the curve. It is not fully understood the mechanism at work that results in this behavioral difference, whether it is a reorganization of the circuitry between the amygdala and frontal/parietal lobes, a decrease in glucocorticoid receptor availability, or some other as yet unknown mechanism. The effect of LDH also had no effect on accuracy for any other facial emotion (i.e. angry, happy, neutral, etc.), suggesting this is not an overall effect on emotion processing in general. Conversely, our results may instead suggest that LDH treatment is potentially impairing perception for these other emotions, while fearful perception is unaffected, through a mechanism not yet understood.
When examining the correlations between “fear” perception and cognitive and behavioral factors in WWH, we found that poorer performance following LDH administration was associated with higher severity of childhood trauma and poorer working memory and attention performance. This study is one of few studies examining the combined effects of HIV, sex, and glucocorticoid stimulation on cognitive performance. Our results reported here seem to indicate that LDH treatment produces enhanced vigilance in particular for fearful facial expressions in these women, to effectively “prime” the neurocircuitry necessary for a subsequent response, but this response may become maladaptive in those with much higher levels of trauma exposure.
Limitations
The main limitation for this study was the relatively small sample, the relationship between the degree of childhood trauma and response to LDH, which could and should be better examined in a larger cohort. This study was powered to address cognitive endpoints (Rubin et al. 2018, 2017), and the FEPT was added after study initiation. A sample size of 41 MWH and 41 WWH was determined using PASS 14 software (2015, NCSS, LLC) and was based on a mixed design with one between-subjects factor (Sex) and one within-subjects factor (Treatment) and a Geisser-Greenhouse Corrected F-test with 5% significance level. We had 80% power (d = 0.43, a = 0.05, two-tailed) to test the main effect of Treatment and the Sex × Treatment interaction. Though our analysis controlled for substance use and depressive symptoms, a large proportion of this cohort reports regular marijuana use and elevated depressive symptoms, which have been previously shown to alter HPA axis functionality (Cservenka et al. 2018; Knorr et al. 2010; Vreeburg et al. 2009), and could potentially have impacted our results. Exploring sex-differences in the acute effects of LDH would have been an interesting approach, though that was not possible with the design of this study, where all participants began testing 4 h post-LDH administration. It is important to recognize that the effects of LDH on outcomes may differ in the acute versus longer-term timeframe, with immediate effects perhaps reflecting alterations in glucocorticoid receptors and longer-term effects reflecting alterations in the HPA axis such as negative feedback inhibition. Although an uninfected control cohort would have been an interesting comparison group to better determine the degree to which HIV status plays a role in these disparate neurobiological effects between MWH and WWH, the goal of this study was to understand HPA axis function in a particularly vulnerable group, who are also at significantly higher risk of neurological, endocrine, and emotional changes, due to the unique combination of pharmacological, behavioral, and social factors relevant to PWH, but less so to the uninfected population. This study also did not evaluate the interaction of other steroid hormones such as estradiol. Estradiol for example can effect HPA axis function and fear processing in a sex-dependent manner.
Conclusions
LDH administration was sufficient to improve accuracy of identifying fearful faces in WWH, but not MWH, suggesting a sex difference for the influence of glucocorticoids on perception of fearful/threatening facial expressions. This pattern of LDH-associated improved fear/threat detection appears to associated with adverse childhood experiences (as determined by the CTQ) and is more profound in WWH who report experiencing moderate, rather than severe childhood trauma experiences. This effect may be an adaptive response to moderate trauma exposure, allowing these individuals to more readily identify and subsequently respond to social fear cues. Our study shows that while HIV, trauma history, and glucocorticoid availability each play a role in perception of fearful or threatening stimuli, biological sex is an additional influential factor. Larger treatment studies are needed to verify sex specific effects of LDH on fear perception and the subsets of women, including those living and aging with HIV, who may benefit most.
Acknowledgements
We would like to thank Bruni Hirsch, Alana Aziz-Bradley, Jacob Ellis, Sheila D’Sa, Shannon Dowty, Lauren Drogos, Lacey Wisslead, Aleksa Anderson, and Preet Dhillon for their assistance with this study. We would also like to thank all of our participants, for without you this work would not be possible.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers Award Numbers K01MH098798 (Rubin), R21MH099978 (Rubin), R01MH113512 (Rubin), and U54 AG062334. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. This project was also supported in part by a University of Illinois at Chicago Campus Review Board (CRB) Grant (Rubin) and a Chicago Developmental Center for AIDS Research pilot grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest The authors declare no competing interests.
References
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ (2004) Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry 161:1488–1490 [DOI] [PubMed] [Google Scholar]
- Baldonero E, Ciccarelli N, Fabbiani M, Colafigli M, Improta E, D’Avino A, Mondi A, Cauda R, Di Giambenedetto S, Silveri MC (2013) Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychology 1:3–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Carrasco M (2018) Emotion and anxiety potentiate the way attention alters visual appearance. Sci Rep 8:5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J (1998) Hopkins Verbal Learning Test - Revised: normative data and analysis of inter-form and test-retest relability. Clin Neuropsychol 12(1):43–55 [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O (1983) Judgment of line orientation. Oxford University Press, Inc. [Google Scholar]
- Bernstein D, Fink L (1998) Childhood trauma questionnaire: a retrospective self-report. The Psychological Coorporation: San Antonio, TX [Google Scholar]
- Bieliauskas L, Counte M, Glandon G (1995) Inventorying stressing life events as related to health change in the elderly. Stress Med 11(1):93–103 [Google Scholar]
- Brooks SJ, Savov V, Allzen E, Benedict C, Fredriksson R, Schioth HB (2012) Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage 59:2962–2973 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ (1991) Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 14:331–338 [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT (2001) Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 75:1–9 [DOI] [PubMed] [Google Scholar]
- Campbell R, Elgar K, Kuntsi J, Akers R, Terstegge J, Coleman M, Skuse D (2002) The classification of “fear” from faces is associated with face recognition skill in women. Neuropsychologia 40:575–584 [DOI] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT (2010) Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc: JINS 16:1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Walker KA, Cohen RA, Devlin KN, Folkers AM, Pina MJ, Tashima KT (2015) Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia 70:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24:385–396 [PubMed] [Google Scholar]
- Cruess DG, Leserman J, Petitto JM, Golden RN, Szuba MP, Morrison MF, Evans DL (2001) Psychosocial-immune relationships in HIV disease. Semin Clin Neuropsychiatry 6:241–251 [DOI] [PubMed] [Google Scholar]
- Cservenka A, Lahanas S, Dotson-Bossert J (2018) Marijuana use and hypothalamic-pituitary-adrenal axis functioning in humans. Front Psychiatry 9:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini MG, Grillo CA, Piroli G, de Kloet ER, De Nicola AF (1997) Sex difference in glucocorticoid regulation of vasopressin mRNA in the paraventricular hypothalamic nucleus. Cell Mol Neurobiol 17:671–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P (2009) Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci: JPN 34:418–432 [PMC free article] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V (2014) Sex differences in the HPA axis. Compr Physiol 4:1121–1155 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N (2004) The fusiform face area subserves face perception, not generic within-category identification. NatNeurosci 7:555–562 [DOI] [PubMed] [Google Scholar]
- Im HY, Adams RB Jr, Cushing CA, Boshyan J, Ward N, Kveraga K (2018) Sex-related differences in behavioral and amygdalar responses to compound facial threat cues. Hum Brain Mapp 39:2725–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI (2006) The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31:151–178 [DOI] [PubMed] [Google Scholar]
- Katz S, Nevid JS (2005) Risk factors associated with posttraumatic stress disorder symptomatology in HIV-infected women. AIDS Patient Care STDS 19:110–120 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–162 [DOI] [PubMed] [Google Scholar]
- Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ (2010) Brain networks involved in haptic and visual identification of facial expressions of emotion: an fMRI study. Neuroimage 49:1677–1689 [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J (2010) Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology 35:1275–1286 [DOI] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Seidel E-M, Boubela R, Kalcher K, Moser E, Habel U, Gur RC, Eickhoff SB, Derntl B (2016) Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 134:410–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S (2005) Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol 27:320–333 [DOI] [PubMed] [Google Scholar]
- Leserman J (2003) The effects of stressful life events, coping, and cortisol on HIV infection. CNS Spectr 8:25–30 [DOI] [PubMed] [Google Scholar]
- Lok A, Mocking RJ, Ruhe HG, Visser I, Koeter MW, Assies J, Bockting CL, Olff M, Schene AH (2012) Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology 37:892–902 [DOI] [PubMed] [Google Scholar]
- Ma ST, Abelson JL, Okada G, Taylor SF, Liberzon I (2017) Neural circuitry of emotion regulation: effects of appraisal, attention, and cortisol administration. Cogn Affect Behav Neurosci 17:437–451 [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991) Half a century of research on the stroop effect: an integrative review. Psychol Bull 109:163–203 [DOI] [PubMed] [Google Scholar]
- Marteau TM, Bekker H (1992) The development of a six-item short-form of the state scale of the Spielberger state-trait anxiety inventory (STAI). Br J Clin Psychol 31(Pt 3):301–306 [DOI] [PubMed] [Google Scholar]
- Mattavelli G, Sormaz M, Flack T, Asghar AUR, Fan S, Frey J, Manssuer L, Usten D, Young AW, Andrews TJ (2014) Neural responses to facial expressions support the role of the amygdala in processing threat. Soc Cogn Affect Neurosci 9:1684–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT (2010) Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35:33–46 [DOI] [PubMed] [Google Scholar]
- Milders M, Sahraie A, Logan S, Donnellon N (2006) Awareness of faces is modulated by their emotional meaning. Emotion 6:10–17 [DOI] [PubMed] [Google Scholar]
- Nasr S, Tootell RBH (2012) Role of fusiform and anterior temporal cortical areas in facial recognition. Neuroimage 63:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Rhodes ST, Valdez A, Jovanovic T (2016) PTSD co-morbid with HIV: Separate but equal, or two parts of a whole? Neurobiol Dis 92:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL (1998) Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry 44:281–289 [DOI] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ (2017) Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress (amsterdam, Netherlands) 20:476–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN (2014) Development of the HPA axis: where and when do sex differences manifest? Front Neuroendocrinol 35:285–302 [DOI] [PubMed] [Google Scholar]
- Petrowski K, Wintermann GB, Siepmann M (2012) Cortisol response to repeated psychosocial stress. Appl Psychophysiol Biofeedback 37:103–107 [DOI] [PubMed] [Google Scholar]
- Pooley AE, Benjamin RC, Sreedhar S, Eagle AL, Robison AJ, Mazei-Robison MS, Breedlove SM, Jordan CL (2018) Sex differences in the traumatic stress response: the role of adult gonadal hormones. Biol Sex Differ 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–931 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977) The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- Rampp C, Eichelkraut A, Best J, Czamara D, Rex-Haffner M, Uhr M, Binder EB, Menke A (2018) Sex-related differential response to dexamethasone in endocrine and immune measures in depressed in-patients and healthy controls. J Psychiatr Res 98:107–115 [DOI] [PubMed] [Google Scholar]
- Reif S, Mugavero M, Raper J, Thielman N, Leserman J, Whetten K, Pence BW (2011) Highly stressed: stressful and traumatic experiences among individuals with HIV/AIDS in the Deep South. AIDS Care 23:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R (1978) Manual for administration of neuropsychological test batteries for adults and children. Neuropsychology Laboratories Inc, Tuscon, AZ [Google Scholar]
- Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR (2005) Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab 90:4224–4231 [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C (2001) Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med 63:966–972 [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Kirschbaum C (2003) Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress 6:207–222 [DOI] [PubMed] [Google Scholar]
- Rubin LH, Phan KL, Keating SM, Maki PM (2018) A single low-dose of hydrocortisone enhances cognitive functioning in HIV-infected women. AIDS 32:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Phan KL, Keating SM, Weber KM, Maki PM (2017) Brief report: low-dose hydrocortisone has acute enhancing effects on verbal learning in HIV-infected men. J Acquir Immune Defic Syndr 75:e65–e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE (2003) Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress 16:495–502 [DOI] [PubMed] [Google Scholar]
- Spies G, Fennema-Notestine C, Cherner M, Seedat S (2017) Changes in cognitive function in women with HIV infection and early life stress. AIDS Care 29:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein T, Peelen MV, Funk J, Seidl KN (2010) The fearful-face advantage is modulated by task demands: evidence from the attentional blink. Emotion 10:136–140 [DOI] [PubMed] [Google Scholar]
- Stein T, Zwickel J, Ritter J, Kitzmantel M, Schneider WX (2009) The effect of fearful faces on the attentional blink is task dependent. Psychon Bull Rev 16:104–109 [DOI] [PubMed] [Google Scholar]
- Stienen BMC, de Gelder B (2011) Fear modulates visual awareness similarly for facial and bodily expressions. Front Hum Neurosci 5:132–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Bao A-M (2020) Chapter 21 - Sex differences in stress-related disorders: major depressive disorder, bipolar disorder, and posttraumatic stress disorder. In: Handb Clin Neurol. Lanzenberger R, Kranz GS, Savic I, (eds). Elsevier, pp 335–358 [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, Persson J, Lin T, Fischer H, Ebner NC (2016) Hippocampal brain volume is associated with faster facial emotion identification in older adults: preliminary results. Front Aging Neurosci 8:203–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW (2009) Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ (2003) Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 6:624–631 [DOI] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, Gazzard BG (2002) Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 16:269–277 [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Zhao L, Huang H, Yao X, Sang N, Hou X, Mao Y, Bi T, Qiu J (2017) The structural and functional correlates of the efficiency in fearful face detection. Neuropsychologia 100:1–9 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008) Wechsler adult intelligence scale. 4. Pearson: San Antonio, TX [Google Scholar]
- Wright SL, Langenecker SA, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, Own LS, Akil H, Young EA, Zubieta JK (2009) Gender-specific disruptions in emotion processing in younger adults with depression. Depress Anxiety 26:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]


