Abstract
Background
Antifungal prophylaxis can prevent invasive fungal diseases (IFDs) in high-risk, immunocompromised patients. This study assessed the real-world use of mold-active triazoles (MATs) for the prevention of IFDs.
Methods
This subgroup analysis of a multicenter, observational, prospective registry in the United States from March 2017 to April 2020 included patients who received MATs for prophylaxis (isavuconazole, posaconazole, and voriconazole) at study index/enrollment. The primary objective was to describe patient characteristics and patterns of MAT use. Exploratory assessments included the frequency of breakthrough IFDs and MAT-related adverse drug reactions (ADRs).
Results
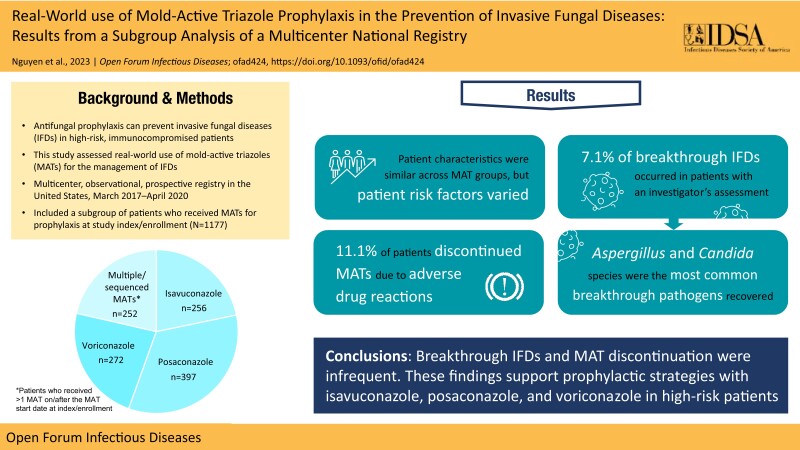
A total of 1177 patients (256 isavuconazole, 397 posaconazole, 272 voriconazole, and 252 multiple/sequenced MATs at/after index/enrollment) were included in the prophylaxis subgroup analysis. Patient characteristics were similar across MAT groups, but risk factors varied. Hematological malignancy predominated (76.5%) across all groups. Breakthrough IFDs occurred in 7.1% (73/1030) of patients with an investigator's assessment (5.0% [11/221] isavuconazole; 5.3% [20/374] posaconazole; 4.0% [9/226] voriconazole; and 15.8% [33/209] multiple/sequenced MATs). Aspergillus (29.5% [18/61]) and Candida (36.1% [22/61]) species were the most common breakthrough pathogens recovered. ADRs were reported in 14.1% of patients, and discontinuation of MATs due to ADRs was reported in 11.1% of patients (2.0% [5/245] isavuconazole; 8.2% [30/368] posaconazole; and 10.1% [27/267] voriconazole).
Conclusions
Breakthrough IFDs were uncommon in patients who received MATs for prophylaxis. Candida and Aspergillus species were the most commonly reported breakthrough pathogens. The discontinuation of MATs due to ADRs was infrequent. These findings support prophylactic strategies with isavuconazole, posaconazole, and voriconazole in high-risk patients.
Keywords: antifungal prophylaxis, isavuconazole, posaconazole, real-world, voriconazole
Graphical Abstract
Graphical Abstract.
Invasive fungal diseases (IFDs) are an important cause of morbidity and mortality in immunocompromised patients [1]. This has been largely driven by the widespread use of immunosuppressive and immune-modifying therapies and increases in solid organ and stem cell transplants [1–3]. Mortality rates from IFDs range from ∼40% to >80%, depending on the pathogen involved [4]. However, these estimates are likely an underrepresentation due to the lack of reliable diagnostic and surveillance methods [5].
Difficulties in establishing an early diagnosis and poor outcomes associated with IFDs have contributed to the adoption of antifungal prophylaxis in high-risk, immunocompromised patients, such as those with hematological malignancies [1, 6]. Prophylaxis with mold-active triazoles (MATs), including posaconazole, voriconazole, and isavuconazole, has been shown to be effective in patients at high risk for IFDs [7]. In patients considered to be high risk (eg, stem cell recipients with graft-vs-host disease [GVHD] or neutropenia), the Infectious Disease Society of America (IDSA) recommends posaconazole prophylaxis for invasive aspergillosis [8]. Voriconazole is also recommended as prophylaxis for invasive aspergillosis in high-risk patients, but clinical evidence to support improved survival is limited [8]. Isavuconazole is a broad-spectrum MAT, which is approved for the treatment of invasive aspergillosis and mucormycosis in adults [9]. It is recommended as an alternative treatment for invasive pulmonary aspergillosis by the IDSA [8], but in the absence of substantive clinical evidence, recommendations for isavuconazole to prevent IFDs are generally not yet available [10]. Other professional bodies provide general recommendations and do not specify any preferred prophylactic antifungal agent. For example, the International Society for Heart and Lung Transplantation recommends 4–6 months of universal prophylaxis or 3–4 months of preemptive therapy in heart and lung transplant recipients but does not state which agents should be used [11]. The choice of antifungal agent is frequently determined based on safety profiles, spectrum of activity, and pharmacologic variability among MATs [12, 13]. Real-world data from a registry-based study of patients receiving MATs for IFDs have been reported [14]; they are a useful tool to guide clinical practice and improve clinical outcomes. However, despite the challenges associated with preventive strategies in the management of IFDs [15], experience with MAT prophylaxis in the real-world setting is lacking. We aimed to generate clinical and healthcare-related utilization data relevant to the prophylaxis of IFDs with broad-spectrum agents in high-risk populations in order to characterize real-world choices and assess clinical outcomes outside a trial setting. Therefore, we analyzed the epidemiological, mycological, safety, and outcome data of a subgroup of patients who received MAT prophylaxis for IFDs in a registry-based observational study.
METHODS
Study Design and Population
The multicenter, observational, prospective registry study was conducted from March 2017 to April 2020, and patients were identified from 55 sites in the United States [14]. The study was approved by an institutional review board at each participating study site and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and any applicable laws and regulations. Patients who received a MAT (isavuconazole, posaconazole, or voriconazole) as antifungal prophylaxis at index/enrollment were included in this subgroup analysis. Patients were prospectively enrolled under 2 versions of the protocol: (1) after initiating a MAT and within 60 days of MAT initiation (“index”) or (2) while already receiving a MAT at the time of enrollment (“enrollment”). If patients received >1 MAT on or after the MAT start date at index/enrollment, they were assigned to the multiple/sequenced MATs group. Patients in the prophylaxis subgroup may have transitioned to treatment during the course of the study.
Data Collection
All enrolled patients received 1 or more MATs during the study; data were collected from patients receiving a MAT for primary and secondary prophylaxis at index/enrollment up to 90 days following completion of their MAT course or 1 year from their date of enrollment, whichever came first. If a patient switched MATs during the study, data were collected through 90 days following completion of the new agent. MAT exposure was based on the duration of the MAT from enrollment onwards. Patients who enrolled within 60 days of MAT initiation but then discontinued the MAT before enrollment in the study were counted as 0 days’ duration; this was reflected in the range for the median duration of MAT prophylaxis after index/enrollment. Retrospective antifungal therapy data up to 90 days before enrollment were also collected. Full details of how data were collected have been previously published [14].
Study Assessments
The primary objective of this report was to describe MAT prophylaxis patterns and patient characteristics. Previous antifungals were defined as MATs or non-MATs that were taken (but not necessarily started) within 90 days before MAT initiation at index/enrollment. Any use of non-MAT antifungals on or after study index/enrollment was recorded. Changes to the sequence of MAT utilization, including discontinuation after study index/enrollment, were captured. Exploratory objectives characterized patient outcomes, including the presence or absence of breakthrough IFDs (bIFDs), clinical factors associated with the risk of bIFDs, and mortality. Patients who initiated prophylaxis at index/enrollment and subsequently developed IFDs were determined to have bIFDs, which were classified at the end of the MAT course(s) as proven, probable, or possible bIFDs [16]. Breakthrough IFDs were documented at any time while the patient was receiving a MAT. Discontinuation due to lack of efficacy was reported for patients who received MAT prophylaxis, developed a bIFD, and then switched to a new MAT for treatment. Mortality was recorded at multiple time points following the start date of the MAT at index/enrollment, including end of MAT and end of MAT plus 90 days; total all-cause and fungal-specific mortality accounted for mortality across these time points. Fungal-specific mortality was determined by the investigator. Safety assessments included the frequency of serious and nonserious adverse drug reactions (ADRs; reported using MedDRA, version 20.0, preferred terms) suspected to be causally (possibly or probably) related to the MAT. ADRs were reported by submitting a form, which required a free-text description of the adverse event, onset and end dates, severity, and the outcome. A full list of study variables is included in the Supplementary Methods.
Data Analysis
The full analysis set (FAS) included patients who met study entry criteria. The safety analysis set (SAF) consisted of patients who had received ≥1 MAT either before or after study index/enrollment. Variables were summarized descriptively. No direct comparisons were made across MAT groups. Exploratory assessments of risk factors for the prophylactic response assessment were analyzed using logistic regression to provide the odds ratio (OR), 95% confidence interval of the OR, and a P value for each factor. The dependent variable was bIFD (yes vs no/not applicable). The independent variables included age, sex, race, body mass index, primary health insurance, underlying risk factors, bacterial infection, viral infection, and pathogens. A full list of independent variables is included in the Supplementary Methods. All data analyses were performed using SAS 9.4 or a higher version.
RESULTS
Patient Characteristics
A total of 2009 patients were enrolled and included in the SAF, and 1993 patients were included in the FAS. Of these, 1177 patients who received a MAT for prophylaxis (256 isavuconazole, 397 posaconazole, 272 voriconazole, and 252 multiple/sequenced MATs at index/enrollment) were included in this subgroup analysis (Supplementary Figure 1). The mean age (SD) of patients in the prophylaxis subgroup was 56.0 (16.2) years, and most patients were male (56.2%) and White (82.1%). Baseline characteristics were similar across MAT groups, with the exception of underlying risk factors (Table 1). Overall, hematological malignancy (76.5%) was the most frequent underlying condition in the prophylaxis subgroup analysis. Of those patients who received posaconazole for prophylaxis at index/enrollment, 89.9% (357/397) had hematological malignancy, compared with 80.2% (202/252) in the multiple/sequenced MATs group, 66.9% (182/272) in the voriconazole group, and 62.1% (159/256) in the isavuconazole group. Concomitant corticosteroid use was frequent (60.2%), with the numerically largest proportion of patients in the isavuconazole group (69.9%) compared with the multiple/sequenced MATs (59.5%), voriconazole (57.7%), and posaconazole (55.9%) groups. Neutropenia was present in 60.0% of patients who received MAT prophylaxis at index/enrollment; 76.8% of patients receiving posaconazole had neutropenia, compared with 61.9%, 48.4%, and 44.5% in the multiple/sequenced MATs, isavuconazole, and voriconazole groups, respectively.
Table 1.
Baseline Characteristics and Demographics in Patients who Received Mold-Active Triazole Prophylaxis at Index/Enrollment (Full Analysis Set)
| Isavuconazole (n = 256) | Posaconazole (n = 397) | Voriconazole (n = 272) | Multiple/Sequenced MATsa (n = 252) | Total (N = 1177) | |
|---|---|---|---|---|---|
| Male, No. (%) | 139 (54.3) | 219 (55.2) | 160 (58.8) | 144 (57.1) | 662 (56.2) |
| Age, y | |||||
| Mean ± SD | 58.0 (14.92) | 56.5 (15.66) | 52.5 (19.07) | 57.0 (14.48) | 56.0 (16.23) |
| Min | 17 | 4 | <1 | 4 | <1 |
| Median | 62.0 | 60.0 | 57.5 | 61.0 | 60.0 |
| Max | 92 | 97 | 86 | 80 | 97 |
| Age ≥18 y, No. (%) | 255 (99.6) | 392 (98.7) | 260 (95.6) | 248 (98.4) | 1155 (98.1) |
| Race, No. (%) | |||||
| White | 212 (82.8) | 335 (84.4) | 215 (79.0) | 204 (81.0) | 966 (82.1) |
| Black/African American | 21 (8.2) | 29 (7.3) | 28 (10.3) | 20 (7.9) | 98 (8.3) |
| Asian | 7 (2.7) | 8 (2.0) | 5 (1.8) | 7 (2.8) | 27 (2.3) |
| American Indian/Alaskan Native | 1 (0.4) | 1 (0.3) | 2 (0.7) | 1 (0.4) | 5 (0.4) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.3) | 1 (0.4) | 0 | 2 (0.2) |
| Other | 15 (5.9) | 23 (5.8) | 21 (7.7) | 20 (7.9) | 79 (6.7) |
| Hispanic ethnicity, No. (%) | 15 (5.9) | 23 (5.8) | 17 (6.3) | 15 (6.0) | 70 (6.0) |
| Underlying disease, No. (%) | |||||
| Hematological malignancy | 159 (62.1) | 357 (89.9) | 182 (66.9) | 202 (80.2) | 900 (76.5) |
| Receiving corticosteroids | 179 (69.9) | 222 (55.9) | 157 (57.7) | 150 (59.5) | 708 (60.2) |
| Neutropenia | 124 (48.4) | 305 (76.8) | 121 (44.5) | 156 (61.9) | 706 (60.0) |
| Receiving T-cell immunosuppressants | 115 (44.9) | 154 (38.8) | 129 (47.4) | 124 (49.2) | 522 (44.4) |
| HCT | 99 (38.7) | 131 (33.0) | 60 (22.1) | 103 (40.9) | 393 (33.4) |
| ICU | 69 (27.0) | 49 (12.3) | 73 (26.8) | 49 (19.4) | 240 (20.4) |
| Surgical (nontransplant) | 55 (21.5) | 68 (17.1) | 35 (12.9) | 32 (12.7) | 190 (16.1) |
| Solid organ transplant | 64 (25.0) | 11 (2.8) | 65 (23.9) | 32 (12.7) | 172 (14.6) |
| Solid tumor | 21 (8.2) | 34 (8.6) | 17 (6.3) | 17 (6.7) | 89 (7.6) |
| Trauma | 13 (5.1) | 9 (2.3) | 3 (1.1) | 2 (0.8) | 27 (2.3) |
| Iron overload | 5 (2.0) | 5 (1.3) | 6 (2.2) | 3 (1.2) | 19 (1.6) |
| Inherited immunodeficiency disorder | 3 (1.2) | 0 | 2 (0.7) | 3 (1.2) | 8 (0.7) |
| HIV/AIDS | 0 | 1 (0.3) | 2 (0.7) | 0 | 3 (0.3) |
| ≥1 previous MAT antifungal, No. (%)b | 137 (53.5) | 199 (50.1) | 81 (29.8) | 91 (36.1) | 508 (43.2) |
| ≥1 previous non-MAT antifungal, No. (%)c | 123 (48.0) | 190 (47.9) | 117 (43.0) | 139 (55.2) | 569 (48.3) |
Abbreviations: HCT, hematopoietic cell transplantation; HIV, human immunodeficiency virus; ICU, intensive care unit; max, maximum; min, minimum; MAT, mold-active triazole; SD, standard deviation.
Multiple/sequenced MATs describes patients receiving >1 MAT for prophylaxis throughout the study since index/enrollment.
Safety analysis set population; antifungal therapy taken (but not necessarily started) 90 days before MAT initiation at index/enrollment.
Any antifungal other than isavuconazole, posaconazole, or voriconazole.
Breakthrough Infection Characteristics
A total of 1030 patients who received a MAT for prophylaxis at index/enrollment had a prophylactic response assessment, and of these, bIFDs occurred in 7.1% (73/1030) of patients (Table 2). Breakthrough IFDs were reported most frequently (15.8% [33/209]) in patients in the multiple/sequenced MATs group. Rates of bIFDs were similar across the single MAT groups (5.0% [11/221] isavuconazole; 5.3% [20/374] posaconazole; and 4.0% [9/226] voriconazole). Aspergillus (29.5% [18/61]) and Candida (36.1% [22/61]) species were the most common breakthrough pathogens recovered from patients with microbiology data. Aspergillus was the most common pathogen in the isavuconazole (40% [4/10]) and multiple/sequenced MATs (37.9% [11/29]) groups, and Candida was the most common in the posaconazole (55.6% [10/18]) group. Aspergillus fumigatus and Candida glabrata were the most frequently recovered pathogens among patients receiving isavuconazole (30.0% [3/10] for each). Among patients receiving posaconazole, Candida glabrata was the most common pathogen (27.8% [5/18]). Fusarium species and Mucorales (Mucor, Rhizomucor, and Rhizopus species) were also recovered from 5 (8.2%) and 3 (4.9%) patients, respectively. Breakthrough IFDs were most frequently reported in the respiratory samples of patients who received isavuconazole (63.6% [7/11]) vs infection sites for the other MAT groups.
Table 2.
Infection Characteristics in Patients who Received Mold-Active Triazole Prophylaxis at Index/Enrollment and Had a Breakthrough Infection (Full Analysis Set)
| Isavuconazole (n = 256) | Posaconazole (n = 397) | Voriconazole (n = 272) | Multiple/Sequenced MATsa (n = 252) | Total (N = 1177) | |
|---|---|---|---|---|---|
| Prophylaxis patients with assessment, No.b | 221 | 374 | 226 | 209 | 1030 |
| Breakthrough IFD | 11 (5.0) | 20 (5.3) | 9 (4.0) | 33 (15.8) | 73 (7.1) |
| Not applicable,c No. | 35 | 22 | 46 | 42 | 145 |
| Missing, No. | 0 | 1 | 0 | 1 | 2 |
| Highest level of diagnosis during study, No. | 9 | 16 | 3 | 30 | 58 |
| Proven | 7 (77.8) | 10 (62.5) | 3 (100.0) | 15 (50.0) | 35 (60.3) |
| Probable | 2 (22.2) | 5 (31.3) | 0 | 9 (30.0) | 16 (27.6) |
| Possible | 0 | 1 (6.3) | 0 | 6 (20.0) | 7 (12.1) |
| Missing, No. | 2 | 4 | 6 | 3 | 15 |
| Pathogen recovered,d No. | 10 | 18 | 4 | 29 | 61 |
| Aspergillus spp. | 4 (40.0) | 3 (16.7) | 0 | 11 (37.9) | 18 (29.5) |
| fumigatus | 3 (30.0) | 1 (5.6) | 0 | 3 (10.3) | 7 (11.5) |
| niger | 1 (10.0) | 1 (5.6) | 0 | 2 (6.9) | 4 (6.6) |
| Not specified | 1 (10.0) | 1 (5.6) | 0 | 5 (17.2) | 7 (11.5) |
| Missing, No. | 0 | 0 | 0 | 2 | 2 |
| Candida spp. | 3 (30.0) | 10 (55.6) | 1 (25.0) | 8 (27.6) | 22 (36.1) |
| albicans | 0 | 3 (16.7) | 0 | 1 (3.4) | 4 (6.6) |
| glabrata | 3 (30.0) | 5 (27.8) | 1 (25.0) | 1 (3.4) | 10 (16.4) |
| krusei | 0 | 1 (5.6) | 0 | 1 (3.4) | 2 (3.3) |
| parapsilosis | 1 (10.0) | 1 (5.6) | 0 | 0 | 2 (3.3) |
| tropicalis | 0 | 1 (5.6) | 0 | 2 (6.9) | 3 (4.9) |
| Not specified | 0 | 1 (5.6) | 0 | 2 (6.9) | 3 (4.9) |
| Missing, No. | 0 | 0 | 0 | 2 | 2 |
| Fusarium spp. | 0 | 2 (11.1) | 1 (25.0) | 2 (6.9) | 5 (8.2) |
| Mucoralese.e | 0 | 0 | 0 | 3 (10.3) | 3 (4.9) |
| Penicillium spp. | 1 (10.0) | 1 (5.6) | 0 | 0 | 2 (3.3) |
| Coccidioides spp. | 1 (10.0) | 0 | 0 | 0 | 1 (1.6) |
| Histoplasma spp. | 0 | 0 | 1 (25.0) | 0 | 1 (1.6) |
| Otherf | 1 (10.0) | 2 (11.1) | 1 (25.0) | 5 (17.2) | 9 (14.8) |
| Infection site, No. | 11 | 18 | 4 | 32 | 65 |
| Abdominal cavity | 1 (9.1) | 0 | 0 | 0 | 1 (1.5) |
| Chest | 0 | 1 (5.6) | 0 | 6 (18.8) | 7 (10.8) |
| Lung | 7 (63.6) | 6 (33.3) | 0 | 11 (34.4) | 24 (36.9) |
| Maxillary sinus | 0 | 1 (5.6) | 0 | 1 (3.1) | 2 (3.1) |
| Oropharynx | 0 | 3 (16.7) | 0 | 3 (9.4) | 6 (9.2) |
| Otherg | 4 (36.4) | 8 (44.4) | 4 (100.0) | 13 (40.6) | 29 (44.6) |
| Skin | 0 | 0 | 1 (25.0) | 1 (3.1) | 2 (3.1) |
| Missing, No. | 0 | 2 | 5 | 1 | 8 |
Data are reported as No. (%), except where indicated. No. represents the number of applicable patients who received the prophylactic assessment. FAS included patients meeting study entry criteria for the relevant enrollment protocol. Prophylaxis groups were not randomized, and prophylactic assessment results were not adjusted; it is possible that any differences between prophylaxis groups could be due to other confounders rather than effects of MAT prophylaxis. Patients in the prophylaxis subgroup may have transitioned to treatment during the course of the study. Patients who initiated prophylaxis at index/enrollment and subsequently developed IFDs were determined to have bIFDs, which were classified at the end of the MAT course(s) as proven, probable, or possible bIFDs [16].
Abbreviations: bIFD, breakthrough invasive fungal infection; FAS, full analysis set; IFD, invasive fungal infection; MAT, mold-active triazole; spp., multiple species.
Multiple/sequenced MATs describe patients receiving >1 MAT throughout the study since index/enrollment.
Only patients receiving prophylaxis at index/enrollment were included in the prophylactic response assessment.
Patients deemed not applicable by the investigator, including those who transitioned to treatment for a non-bIFD at the time of assessment by an investigator.
Pathogen subgroups were determined by considering all recorded fungal infections, including those that started before the start date of the MAT at index/enrollment. More than 1 pathogen species could be recovered from a single patient.
Mucorales includes the genera Mucor, Rhizomucor, and Rhizopus.
“Other” pathogens include (but are not limited to) Zygomycetes (most frequently reported) and Simplicillium subtropicum.
“Other” sites include (but are not limited to) blood (most frequently reported), sputum, bladder, eye, esophagus, abdomen, urine, and sinus.
Patients who received MAT prophylaxis at index/enrollment with concomitant bacterial or viral infections were at significantly greater risk of bIFDs than those who did not acquire a concomitant infection (bacterial infection: OR, 2.67; 95% CI, 1.59–4.50; P = .0002; or viral infection: OR, 1.99; 95% CI, 1.20–3.30; P = .0075). Further results for the risk factor analysis are reported in Supplementary Table 1.
Antifungal Prophylaxis
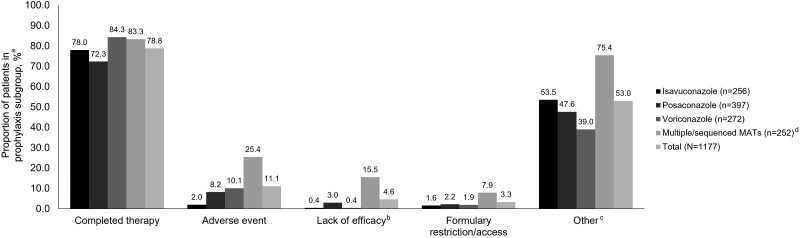
The majority (95.4% [1123/1177]) of patients received primary vs secondary MAT prophylaxis at index/enrollment. Most (78.8% [892/1132]) patients remained on their MAT agent for the study duration (Figure 1); discontinuation data were missing for 45 patients. Among the single MAT groups, 84.3% (225/267) in the voriconazole group, 78.0% (191/245) in the isavuconazole group, and 72.3% (266/368) in the posaconazole group remained on their MAT agent throughout the study.
Figure 1.
Reasons for the discontinuation of mold-active triazoles in the prophylaxis subgroup (safety analysis set). Patients could have been counted in multiple categories but were only counted once for each category. aPercentages are derived from the number of patients without missing data in each category; isavuconazole n = 245, posaconazole n = 368, voriconazole n = 267, multiple/sequenced MATs n = 252, and total n = 1132. bLack of efficacy was reported for a patient who received MAT prophylaxis, developed a bIFD, and then switched to a new MAT for treatment. c“Other” reasons for discontinuation of MATs included but were not limited to hospital visits, hospital admission, discharge, lack of intravenous formulation, and inpatient and outpatient switching. dMultiple/sequenced MATs described patients receiving >1 MAT for prophylaxis throughout the study since index/enrollment. Abbreviations: bIFD, breakthrough invasive fungal infection; MAT, mold-active triazole.
In patients who received MAT prophylaxis at index/enrollment, the majority (78.6% [925/1177]) received a single MAT agent (rather than multiple/sequenced MATs), with posaconazole being the most frequently administered agent (33.7% [397/1177]) (Supplementary Table 2). Voriconazole and isavuconazole were administered to 23.1% (272/1177) and 21.8% (256/1177) of patients, respectively, and multiple/sequenced MATs to 21.4% (252/1177) of patients. The most frequent first- and second-line sequences of MATs in patients who received prophylaxis at index/enrollment were posaconazole and voriconazole (3.4% [40/1177]), respectively. The most frequent first-, second-, and third-line sequences were posaconazole, voriconazole, and posaconazole (1.9% [22/1177]), respectively. Almost all (97.5% [1148/1177]) patients received oral MAT prophylaxis at index/enrollment (94.5% [242/256] isavuconazole; 99.5% [393/397] posaconazole; 96.0% [261/272] voriconazole; and 99.2% [250/252] multiple/sequenced MATs). Twenty percent (235/1177) of patients received intravenous MAT prophylaxis (21.5% [55/256] isavuconazole; 5.3% [21/397] posaconazole; 16.2% [44/272] voriconazole; and 45.6% [115/252] multiple/sequenced MATs). Non-MAT antifungals started on or after index/enrollment were received by 45.2% (532/1177) of patients (Supplementary Table 3). Fluconazole (20.5% [241/1177]) and micafungin (18.9% [223/1177]) were the most commonly used non-MAT antifungal agents.
The median duration (range) of MAT prophylaxis after index/enrollment was 66.0 (0–903) days and was numerically longer for patients who received isavuconazole (76.5 [0–864] days) and multiple/sequenced MATs (100.5 [0–887] days) than for those who received posaconazole (46.0 [0–903] days) or voriconazole (46.5 [0–584] days). Overall, therapeutic drug monitoring (TDM) was measured in 34.6% (407/1176) of patients who received MAT prophylaxis at index/enrollment (7.8% [20/256] isavuconazole; 37.9% [150/397] posaconazole; and 43.4% [118/272] voriconazole). Median TDM levels (range) were reported for the isavuconazole (3.5 [0.4–9.4] mg/mL), posaconazole (1.4 [0.1–6.5] mg/mL), and voriconazole (2.0 [0.1–17.8] mg/mL) groups.
Safety
Adverse Drug Reactions
Overall, ADRs were reported in 14.1% of patients who received a MAT for prophylaxis at index/enrollment (Table 3). The rate of ADRs was numerically lower in the isavuconazole group (3.1%), followed by posaconazole (12.1%) and voriconazole (13.2%). ADRs were numerically more frequent (29.4%) among patients receiving multiple/sequenced MATs than in the single MAT groups. Rates of ADRs were analyzed by the MAT suspected to have caused an ADR in the multiple/sequenced MATs group and were numerically lower in those who received isavuconazole (4.8% [12/252]) than in the posaconazole (12.7% [32/252]) and voriconazole (17.9% [45/252]) groups (Supplementary Table 4).
Table 3.
Adverse Drug Reactions Occurring in ≥1% of Patients in Any Group who Received Any Mold-Active Triazole for Prophylaxis at Index/Enrollment (Safety Analysis Set)
| Adverse Drug Reaction | Isavuconazole (n = 256) | Posaconazole (n = 397) | Voriconazole (n = 272) | Multiple/Sequenced MATsa (n = 252) | Total (N = 1177) |
|---|---|---|---|---|---|
| Overall | 8 (3.1) | 48 (12.1) | 36 (13.2) | 74 (29.4) | 166 (14.1) |
| Liver toxicityb | 4 (1.6) | 31 (7.8) | 25 (9.2) | 48 (19.0) | 108 (9.2) |
| Hepatic enzyme increasedc | 1 (0.4) | 15 (3.8) | 9 (3.3) | 21 (8.3) | 46 (3.9) |
| Aspartate aminotransferase increased | 0 | 0 | 1 (0.4) | 4 (1.6) | 5 (0.4) |
| Alanine aminotransferase increased | 0 | 0 | 1 (0.4) | 4 (1.6) | 5 (0.4) |
| Bilirubin increasedd | 2 (0.8) | 7 (1.8) | 9 (3.3) | 11 (4.4) | 29 (2.5) |
| Liver function test increasede | 0 | 9 (2.3) | 7 (2.6) | 13 (5.2) | 29 (2.5) |
| Blood alkaline phosphatase increased | 1 (0.4) | 0 | 0 | 3 (1.2) | 4 (0.3) |
| Hallucinationf | 0 | 0 | 1 (0.4) | 21 (8.3) | 22 (1.9) |
| Nausea | 1 (0.4) | 9 (2.3) | 1 (0.4) | 9 (3.6) | 20 (1.7) |
| Electrocardiogram QT prolonged | 0 | 4 (1.0) | 1 (0.4) | 7 (2.8) | 12 (1.0) |
| Vomiting | 1 (0.4) | 1 (0.3) | 0 | 4 (1.6) | 6 (0.5) |
| Photosensitivity reaction | 1 (0.4) | 0 | 3 (1.1) | 0 | 4 (0.3) |
| Vision blurred | 0 | 0 | 1 (0.4) | 3 (1.2) | 4 (0.3) |
| Acute kidney injury | 0 | 0 | 3 (1.1) | 0 | 3 (0.3) |
Data are reported as No. (%). Only ADRs that started on or after the start date of the index MAT or the MAT at enrollment are included. ADRs are grouped using MedDRA, version 20.0, preferred terms.
Abbreviations: ADR, adverse drug reaction; ICG, indocyanine green; LFT, liver function test; MAT, mold-active triazole; MedDRA, Medical Dictionary for Regulatory Activities.
Multiple/sequenced MATs describe patients receiving >1 MAT for prophylaxis throughout the study since index/enrollment.
“Liver toxicity” summarizes ADRs related to abnormal liver function or liver injury: “hepatic enzyme increased,” bilirubin increased,” “liver function test increased,” and “blood alkaline phosphatase increased.” Only ADRs occurring in ≥1% of patients in any MAT group are included.
Preferred terms under “hepatic enzyme increased” are mutually exclusive and include “hepatic enzyme increased,” “transaminases increased,” “alanine transaminase increased,” and “aspartate transaminase increased.”
Preferred terms under “bilirubin increased” are mutually exclusive and include “blood bilirubin increased” and “hyperbilirubinemia.”
Preferred terms under “liver function test increased” are mutually exclusive and could include “ICG increased,” “LFTs raised,” “liver function test increased,” “liver function tests raised,” “raised LFTs,” and “raised liver function tests.”
Preferred terms under “hallucination” are mutually exclusive and include “hallucination” and “hallucination, visual.”
Discontinuation of MAT due to ADRs was reported in 11.1% (126/1177) of patients; patients in the voriconazole and posaconazole groups had numerically higher rates of MAT discontinuation (10.1% [27/267] and 8.2% [30/368], respectively) than those in the isavuconazole group (2.0% [5/245]) (Figure 1). A total of 25.4% (64/252) of patients in the multiple/sequenced MATs group discontinued MAT due to an ADR. Adverse events leading to MAT discontinuation can be found in Supplementary Table 5. MedDRA system organ class preferred terms relating to liver function and injury (including the terms liver function test increased, hyperbilirubinemia, blood bilirubin increased, hepatic enzyme increased, transaminases increased, alkaline phosphatase increased, alanine transaminase increased, and aspartate transaminase increased) were the most common ADRs (9.2%) (Table 3). In patients who received multiple/sequenced MATs, elevated markers of liver function and injury were reported at a frequency of 19.0%. Among the single MAT groups, 9.2%, 7.8%, and 1.6% of patients receiving voriconazole, posaconazole, and isavuconazole, respectively, reported ADRs related to abnormal liver function or liver injury.
Mortality
Among patients in the prophylaxis subgroup with bIFDs, the rate of total all-cause mortality was 43.8% (32/73), including 45.5% (5/11) in the isavuconazole group, 35.0% (7/20) in the posaconazole group, 33.3% (3/9) in the voriconazole group, and 51.5% (17/33) in the multiple/sequenced MATs group (Table 4). Total fungal-specific mortality was reported in 6.8% (5/73) of patients with bIFDs (9.1% [1/11] isavuconazole; 10.0% [2/20] posaconazole; and 6.1% [2/33] multiple/sequenced MATs). No fungal-specific deaths were reported in the voriconazole group.
Table 4.
All-Cause and Fungal-Specific Mortality in Patients who Received Mold-Active Triazole Prophylaxis at Index/Enrollment and Had Invasive Fungal Disease or a Breakthrough Infection (Full Analysis Set)
| Isavuconazole (n = 256) | Posaconazole (n = 397) | Voriconazole (n = 272) | Multiple/Sequenced MATsa (n = 252) | Total (N = 1177) | |
|---|---|---|---|---|---|
| Patients with an IFD, No.b | 248 | 385 | 263 | 244 | 1140 |
| Total all-cause mortalityc | 69 (27.8) | 96 (24.9) | 44 (16.7) | 94 (38.5) | 303 (26.6) |
| All-cause mortality at end of MATd,e | 27 (10.8) | 29 (7.4) | 18 (6.8) | 28 (11.4) | 102 (8.9) |
| Total fungal-specific mortalityc | 2 (0.8) | 2 (0.5) | 3 (1.1) | 8 (3.3) | 15 (1.3) |
| Fungal-specific mortality at end of MATd,e | 2 (0.8) | 1 (0.3) | 2 (0.8) | 3 (1.2) | 8 (0.7) |
| Patients with a breakthrough IFD, No.b | 11 | 20 | 9 | 33 | 73 |
| Total all-cause mortalityc | 5 (45.5) | 7 (35.0) | 3 (33.3) | 17 (51.5) | 32 (43.8) |
| All-cause mortality at end of MATd | 3 (27.3) | 2 (10.0) | 0 | 3 (9.1) | 8 (11.0) |
| Total fungal-specific mortalityc | 1 (9.1) | 2 (10.0) | 0 | 2 (6.1) | 5 (6.8) |
| Fungal-specific mortality at end of MATd | 1 (9.1) | 1 (5.0) | 0 | 1 (3.0) | 3 (4.1) |
Data are reported as No. (%). Patients in the prophylaxis subgroup may have transitioned to treatment during the course of the study.
Abbreviations: IFD, invasive fungal disease; MAT, mold-active triazole.
Multiple/sequenced MATs describe patients receiving >1 MAT throughout the study since index/enrollment.
No. is the number of patients still in the study at the given time point (excluding patients who discontinued or were lost to follow-up).
Total mortality counted all cases of death, including those that occurred after the end of MAT.
Mortality at the end of MAT included cases of death before the end of MAT.
Proportions of patients who died at the end of MAT were based on the total number of patients with an IFD at the end of MAT: isavuconazole n = 250, posaconazole n = 390, voriconazole n = 266, multiple/sequenced MATs n = 245, total n = 1151. If a patient received >1 MAT, they were categorized into the multiple/sequenced MATs group, and the mortality at the end of MAT was for the last MAT received.
DISCUSSION
Antifungal prophylaxis with MATs has emerged as an important standard of care based on data from prospective studies with posaconazole [17, 18], voriconazole [19–21], and isavuconazole [22]. This subgroup analysis expands on a previous report of this registry [14] by providing previously unpublished and important real-world data from a database of >1000 patients who received MATs for the prevention of IFDs. We analyzed patient demographics, choice of MAT agent, ADRs, and bIFD characteristics in a subgroup of patients who received MAT prophylaxis. Overall, the prophylaxis subgroup was representative of the broader at-risk patient population, with patients experiencing low and nearly equivalent rates of bIFDs across all 3 individual MAT groups. The duration of isavuconazole prophylaxis was numerically longer than the duration of posaconazole and voriconazole prophylaxis, and the incidence of ADRs and discontinuation rate due to ADRs were numerically lower in the isavuconazole group. These findings are consistent with a previous report from this registry, in which the duration of exposure to isavuconazole for the treatment and prophylaxis of IFDs was numerically longer and ADRs were proportionally less common compared with the other MATs [14]. This prophylaxis subgroup analysis also showed some real-world alignment with published recommendations on MAT prophylaxis [8]. The results reported herein contribute to a body of evidence that has been limited since the introduction of isavuconazole.
Most patients (78.8%) who received MATs for prophylaxis at index/enrollment completed their MAT course. Posaconazole was administered at a numerically higher rate (33.7%) than voriconazole or isavuconazole. It is possible that isavuconazole was less frequently administered as prophylaxis at index/enrollment due to the relatively brief time since its approval when this study was conducted. Thus, fewer published randomized controlled trials demonstrating the prophylactic efficacy of isavuconazole may have deterred physicians from its use in this setting.
Variation was observed in underlying risk factors across the patients who received MAT prophylaxis at index/enrollment. However, hematological malignancy and neutropenia were among the most common underlying diseases in this patient population, which is consistent with other reports [23]. Other patient populations, such as those in the intensive care unit or undergoing nontransplant surgery, also received MAT prophylaxis, suggesting recognition of nonclassical risk factors as potential predisposing factors for invasive aspergillosis.
There is some alignment of this real-word study with the IDSA recommendations for MAT prophylaxis of patients at high risk for invasive aspergillosis [8]. The IDSA recommends prophylaxis with posaconazole or voriconazole during prolonged neutropenia, or posaconazole for allogeneic HCT recipients with GVHD [8]. In the present study, the most frequently administered prophylactic MAT agent at index/enrollment was posaconazole, and this was a cohort for which hematological malignancy and neutropenia were common risk factors. In solid organ transplant recipients, the IDSA and American Society of Transplantation Infectious Diseases Community of Practice (AST-IDCOP) recommend antifungal prophylactic strategies (either universal or targeted) in lung, heart, or liver transplant recipients, including MATs such as voriconazole, posaconazole, and itraconazole [8, 13]. In the present analysis, most solid organ transplant patients who were not in the multiple/sequenced MATs group received voriconazole (23.9%) or isavuconazole (25.0%) for prophylaxis at index/enrollment; only 2.8% received posaconazole.
Breakthrough IFDs were uncommon in patients receiving MAT prophylaxis at index/enrollment in the present study and were reported in ∼7% of patients. Similar incidences of bIFDs were reported among the isavuconazole (5.0%), posaconazole (5.3%), and voriconazole (4.0%) groups. Breakthrough IFDs were most frequently reported in the respiratory samples of patients who received isavuconazole compared with other MATs and other infection sites. Aspergillus species were most often recovered in patients who received isavuconazole for prophylaxis at index/enrollment, while Candida species were most often recovered in the posaconazole group. In a phase 2 trial, in treatment-naïve adults with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) undergoing induction chemotherapy and receiving isavuconazole, the incidence of probable or proven bIFDs was 6% [22]. A slightly higher rate of bIFDs (8.3%) was reported in a retrospective review among adult hematological malignancy patients and HCT recipients who were receiving isavuconazole prophylaxis for >7 days; Aspergillus fumigatus was most often recovered in these patients, followed by Mucorales and Fusarium species [24]. In adult patients with newly diagnosed AML or high-risk MDS undergoing induction chemotherapy and receiving MAT prophylaxis for >5 days, proven or probable bIFDs were observed in 4% of patients overall, with bIFD incidences of 3% for posaconazole, 5% for voriconazole, and 6% for isavuconazole [7]. In a single-center retrospective study among lung transplant recipients, the incidence of bIFDs was ∼3% for transplant recipients receiving either isavuconazole or voriconazole prophylaxis; Candida glabrata and Aspergillus fumigatus were the most commonly recovered yeast and mold pathogens, respectively [25]. The rates of bIFDs reported in the present study were within the ranges reported in other studies in various populations for voriconazole (3%–5%) and isavuconazole (3%–8%), but were slightly higher than that reported in the posaconazole study (3%) [7, 22, 24, 25]. Comparisons across studies may be confounded by differences in definitions of bIFDs, heterogeneity among patient populations, and study design. Also, therapeutic drug monitoring data would have been informative for this study, but correlation with bIFDs was not feasible.
In the present study, numerically fewer patients discontinued voriconazole (15.7%) than isavuconazole (22.0%) or posaconazole (27.7%). In contrast, a retrospective study of lung transplant recipients reported that fewer patients discontinued isavuconazole (30%) than voriconazole (41%) prophylaxis [25]. The majority of patients in the present study discontinued isavuconazole due to reasons other than adverse events, which may have included hospital visits, hospital admission, discharge, or inpatient and outpatient switching. It is possible that this pattern is reflective of the lack of coverage by payers due to the more recent approval of isavuconazole. However, it is important to note that a patient receiving multiple MATs (or initiating the same MAT after discontinuation of the previous episode) could have a different reason for discontinuation of each distinct MAT (or each episode).
MAT discontinuation due to ADRs occurred at a numerically lower rate in patients receiving isavuconazole than in those receiving posaconazole or voriconazole prophylaxis at index/enrollment. Elevated markers of abnormal liver function and/or hepatic injury were the most common (9.2%) ADRs overall, with numerically lower rates in the isavuconazole group (1.6%) than the posaconazole (7.8%) and voriconazole (9.2%) groups. While reasons for discontinuation due to specific adverse events were not captured, this trend is consistent with other reports, in which the discontinuation of MATs for prophylaxis due to hepatotoxicity was generally lower for patients receiving isavuconazole (5%–6%) compared with those receiving voriconazole (18%–23%) [22, 25, 26]. In a study among adult patients with AML undergoing induction chemotherapy, the discontinuation rate of MAT prophylaxis, most often due to hepatotoxicity, was similar among the MATs: 13% for isavuconazole and posaconazole and 15% for voriconazole [7]. In the present study, a numerically larger proportion of patients who received posaconazole for prophylaxis at index/enrollment experienced nausea and vomiting than did patients receiving either isavuconazole or voriconazole. The association between gastrointestinal disorders and posaconazole prophylaxis has been corroborated in clinical trial settings [17, 27].
The strengths of this subgroup analysis include a prospective and observational design and subsequent characterization of the frequency of bIFDs relative to MAT prophylaxis at index/enrollment. Moreover, the real-world nature of this analysis allowed us to broadly assess safety, choice of MAT agent, and clinical outcomes of MAT prophylaxis. This subgroup analysis was limited by several factors, some of which were derived from the primary study and have been discussed in depth previously [14]. In general, there is a possibility of selection bias due to favoring high-performing clinical trial sites with sufficiently large patient populations using MATs. Our study was also conducted in a single country (the United States), meaning our results may not be generalizable to the global population. Many patients received non-MAT antifungal agents or other drugs with similar therapeutic indications, but outcomes were not stratified by concomitant drug use, which may limit their interpretation. Also, patients in the prophylaxis subgroup may have transitioned from prophylaxis for bIFDs to treatment and/or empiric treatment during the study period, but as prophylaxis and treatment subgroups were defined based on the MAT at index/enrollment, transitions from the prophylaxis to the treatment subgroup were not differentiated in this analysis. The MAT was decided by the physician or by institutional protocol at the time of index/enrollment; however, this was an inherited limitation due to the nature of real-world studies. In this study, we demonstrated that the 3 MATs were comparable in prophylactic antifungal activity, while isavuconazole appeared to be associated with numerically fewer reported adverse events than the other MATs. However, data for the highest level of diagnosis for bIFDs were missing for 15 of 73 patients with a bIFD.
We acknowledge that because there was no direct comparison between MAT groups we cannot evaluate or reach any conclusions regarding the noninferiority or superiority of these MATs. We are also cautious to note that these observations would warrant a randomized controlled trial to definitively address the comparative safety of voriconazole, posaconazole, and isavuconazole. Furthermore, patients received non-MAT antifungals during this study, but the impact of these agents on study outcomes was not assessed. Also, we did not conduct a formal analysis to evaluate confounding by indication (ie, higher- vs lower-risk patients), but there were differences between the MAT groups in terms of baseline characteristics, which provides some assessment of the patient population that received each MAT. This was a real-world study; thus the frequency and type of laboratory tests were based on the standard of care at each institution taking part in the study. However, the majority of patients had hematological malignancies, hematopoietic stem cell transplant, or solid organ transplant, and therefore basic laboratory tests were regularly performed for monitoring by standard protocols. There may have also been workup bias toward more monitoring for voriconazole due to its reported hepatoxicity. Finally, ADRs were reported using free text, rather than predefined terms, which may have introduced inconsistencies in the reporting.
In conclusion, the clinical characteristics and risk factors of patients in this real-world study were representative of the wider population of patients at risk for IFDs. The incidence of bIFDs was low across MAT groups. Candida and Aspergillus species were the most common pathogens involved in bIFDs, and potentially more resistant mold pathogens (eg, Mucorales and Fusarium species) were less common. A small proportion of patients who received MAT prophylaxis at index/enrollment discontinued due to ADRs. Taken together, these findings support prophylactic strategies with isavuconazole, posaconazole, and voriconazole to mitigate risk of IFDs.
Supplementary Material
Acknowledgments
This study was initiated and supported by Astellas Pharma Global Development, Inc. Medical writing support was provided by Sadie van Dyne, PhD, and Anne-Marie Edwards, MChem of Lumanity, funded by Astellas Pharma, Inc.
Author contributions. Conception: L.O.Z. Study design and conduct: L.O.Z. Data acquisition: L.O.Z., M.H.N., J.B., B.D.A., M.H.M., P.G.P., G.R.T., T.J.W. Analysis and interpretation: L.O.Z., M.H.N., J.B., B.D.A., J.J., Y.S., G.R.T., T.J.W. Writing: L.O.Z., M.H.N., J.B., B.D.A., M.H.M., P.G.P., J.J., G.R.T., T.J.W.
Data sharing. Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Patient consent. Please see primary study: Ostrosky-Zeichner et al. Infect Dis Ther 2022 [14].
Prior presentation. These data were presented in part at the 10th Trends in Medical Mycology (TIMM) bi-annual meeting of the European Confederation of Medical Mycology (ECMM), October 8–11, 2021, Aberdeen, Scotland, UK.
Financial support. This work was supported and initiated by Astellas Pharma Global Development, Inc. Medical writing support was funded by Astellas Pharma, Inc.
Contributor Information
M Hong Nguyen, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Luis Ostrosky-Zeichner, McGovern Medical School, Houston, Texas, USA.
Peter G Pappas, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Thomas J Walsh, Weill Cornell Medicine, Cornell University, New York, New York, USA; Institute for Innovative Therapeutics and Diagnostics, Richmond, Virginia, USA.
Joseph Bubalo, Oregon Health and Science University Hospital and Clinics, Portland, Oregon, USA.
Barbara D Alexander, Duke University, Durham, North Carolina, USA.
Marisa H Miceli, University of Michigan, Ann Arbor, Michigan, USA.
Jeanette Jiang, Astellas Pharma Global Development, Inc., Northbrook, Illinois, USA.
Yi Song, Astellas Pharma Global Development, Inc., Northbrook, Illinois, USA.
George R Thompson, III, UC Davis Health, Sacramento, California, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Jenks JD, Mehta SR, Hoenigl M. Broad spectrum triazoles for invasive mould infections in adults: which drug and when? Med Mycol 2019; 57(Suppl 2):S168–78. [DOI] [PubMed] [Google Scholar]
- 2. Floros L, Pagliuca A, Taie AA, et al. . The cost-effectiveness of isavuconazole compared to the standard of care in the treatment of patients with invasive fungal infection prior to differential pathogen diagnosis in the United Kingdom. J Med Econ 2020; 23:86–97. [DOI] [PubMed] [Google Scholar]
- 3. Parslow BY, Thornton CR. Continuing shifts in epidemiology and antifungal susceptibility highlight the need for improved disease management of invasive candidiasis. Microorganisms 2022; 10:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Formanek PE, Dilling DF. Advances in the diagnosis and management of invasive fungal disease. Chest 2019; 156:834–42. [DOI] [PubMed] [Google Scholar]
- 5. Firacative C. Invasive fungal disease in humans: are we aware of the real impact? Mem Inst Oswaldo Cruz 2020; 115:e200430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamdy RF, Zaoutis TE, Seo SK. Antifungal stewardship considerations for adults and pediatrics. Virulence 2017; 8:658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rausch CR, DiPippo AJ, Jiang Y, et al. . Comparison of mold active triazoles as primary antifungal prophylaxis in patients with newly diagnosed acute myeloid leukemia in the era of molecularly targeted therapies. Clin Infect Dis 2022; 75:1503–10. [DOI] [PubMed] [Google Scholar]
- 8. Patterson TF, Thompson GR III, Denning DW, et al. . Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. CRESEMBA (Isavuconazonium Sulfate) [Prescribing Information]. Astellas Pharma US; 2022.
- 10. Maertens JA, Girmenia C, Brüggemann RJ, et al. . European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 2018; 73:3221–30. [DOI] [PubMed] [Google Scholar]
- 11. Husain S, Sole A, Alexander BD, et al. . The 2015 International Society for Heart and Lung Transplantation guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: executive summary. J Heart Lung Transplant 2016; 35:261–82. [DOI] [PubMed] [Google Scholar]
- 12. Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. Overview of treatment options for invasive fungal infections. Med Mycol 2011; 49:561–80. [DOI] [PubMed] [Google Scholar]
- 13. Husain S, Camargo JF. Invasive aspergillosis in solid-organ transplant recipients: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant 2019; 33:e13544. [DOI] [PubMed] [Google Scholar]
- 14. Ostrosky-Zeichner L, Nguyen MH, Bubalo J, et al. . Multicenter registry of patients receiving systemic mold-active triazoles for the management of invasive fungal infections. Infect Dis Ther 2022; 11:1609–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teh BW, Yeoh DK, Haeusler GM, et al. . Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2021. Intern Med J 2021; 51(Suppl 7):67–88. [DOI] [PubMed] [Google Scholar]
- 16. De Pauw B, Walsh TJ, Donnelly JP, et al. . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ullmann AJ, Lipton JH, Vesole DH, et al. . Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356:335–47. [DOI] [PubMed] [Google Scholar]
- 18. Cornely OA, Maertens J, Winston DJ, et al. . Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–59. [DOI] [PubMed] [Google Scholar]
- 19. Wingard JR, Carter SL, Walsh TJ, et al. . Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116:5111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marks DI, Pagliuca A, Kibbler CC, et al. . Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol 2011; 155:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandhaniya S, Swaroop C, Thulkar S, et al. . Oral voriconazole versus intravenous low dose amphotericin B for primary antifungal prophylaxis in pediatric acute leukemia induction: a prospective, randomized, clinical study. J Pediatr Hematol Oncol 2011; 33:e333–41. [DOI] [PubMed] [Google Scholar]
- 22. Bose P, McCue D, Wurster S, et al. . Isavuconazole as primary antifungal prophylaxis in patients with acute myeloid leukemia or myelodysplastic syndrome: an open-label, prospective, phase 2 study. Clin Infect Dis 2021; 72:1755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rüping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs 2008; 68:1941–62. [DOI] [PubMed] [Google Scholar]
- 24. Fontana L, Perlin DS, Zhao Y, et al. . Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic cell transplant recipients. Clin Infect Dis 2020; 70:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samanta P, Clancy CJ, Marini RV, et al. . Isavuconazole is as effective as and better tolerated than voriconazole for antifungal prophylaxis in lung transplant recipients. Clin Infect Dis 2021; 73:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogler Y, Stern A, Su Y, et al. . Efficacy and safety of isavuconazole compared with voriconazole as primary antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. Med Mycol 2021; 59:970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornely OA, Duarte RF, Haider S, et al. . Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 2016; 71:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.