Abstract
Chronic uveitis comprises heterogeneous clinical entities characterized by sustained and recurrent intraocular inflammation that is believed to be driven by autoimmune responses. The management of chronic uveitis is challenging with the limited availability of efficacious treatments, and the underlying mechanisms mediating disease chronicity remain poorly understood as the majority of experimental data are derived from the acute phase of the disease (the first 2–3 weeks post-induction). Herein, we investigated the key cellular mechanisms underlying chronic intraocular inflammation using our recently established murine model of chronic autoimmune uveitis. We demonstrate unique long-lived CD44hiIL-7R+IL-15R+CD4+ memory T cells in both retina and secondary lymphoid organs after 3 months post-induction of autoimmune uveitis. These memory T cells functionally exhibit antigen-specific proliferation and activation in response to retinal peptide stimulation in vitro. Critically, these effector-memory T cells are capable of effectively trafficking to the retina and accumulating in the local tissues secreting both IL-17 and IFN-γ upon adoptively transferred, leading to retinal structural and functional damage. Thus, our data reveal the critical uveitogenic functions of memory CD4+ T cells in sustaining chronic intraocular inflammation, suggesting that memory T cells can be a novel and promising therapeutic target for treating chronic uveitis in future translational studies.
Keywords: chronic autoimmune uveitis, immunological memory, retinal inflammation, T cells
1. Introduction
Chronic uveitis is a sight-threatening intraocular inflammatory condition of the uveal tract of the eye, and the outcomes are inferior when adjacent structures, such as the retina, become inflamed. It represents one of the major causes leading to severe visual impairment globally, with exceptionally high prevalence in young or middle age individuals, generating a considerable socioeconomic burden close to that of diabetic retinopathy.1–5 Encompassing a variety of heterogeneous clinical entities, chronic uveitis can present either as a stand-alone ocular condition or as a component of systemic disorders, and is generally driven by the presumed autoimmune responses.6 The current first-line mainstay of treatment for chronic uveitis is corticosteroids and other broad immunosuppressants, which cannot cure the disease but only limit the intraocular inflammation, and further have common and serious side effects associated with their long-term or systemic use.7,8 Thus, developing novel corticosteroid-sparing therapies with a better benefit/risk ratio is the primary goal of current research, which requires a better understanding of the basic mechanisms of chronic uveitis. This will help identify critical disease mediators that can be specifically targeted without suppressing other immune components germane to host self-surveillance and defense against pathogens.
Experimental animal models are used to generate essential pre-clinical data to develop an understanding of disease pathogenesis and thus play a critical role in providing foundations for prioritizing the development of new, targeted treatments. Accumulating knowledge gained through the popular experimental autoimmune uveitis (EAU) models that primarily focus on the acute disease peak (2–3 weeks post-immunization) strongly implicates the dominant role of T helper-17 (Th17) cells in the induction of acute inflammation of uvea and retina.6,9,10 However, few studies have investigated into the chronic stage of disease, which presents a lower-grade but prolonged intraocular inflammation up to 2–3 months.11,12 The resolution of acute inflammation in EAU has been attributed to the generation of antiuveitic inducible regulatory T cells (Tregs) after 2–3 weeks,11,13 but this regulatory immunity does not restore the eye to pre-disease status. Thus, we have an incomplete understanding of the immunopathogenic mechanisms that perpetuate inflammation in chronic uveitis. This is clinically relevant because acute uveitis often resolves after a short course of topical corticosteroids, and it is chronic uveitis that is treatment-resistant and harbors a higher risk of sight-threatening complications.14 We have recently established a robust murine model of chronic autoimmune uveitis (CAU) in wild-type mice modified from the interphotoreceptor retinoid-binding protein (IRBP)-based EAU protocol.15 This active immunization approach is superior to passive transfer16 or transgenic approach17 in better mimicking the natural disease process of human chronic uveitis. Our CAU model at more than 3 months post-induction exhibits clinical features consistent with noninfectious, blinding uveitis observed in humans, characterized by chronic chorioretinitis with damage to the outer blood-retina barrier, retinal degeneration, photoreceptor destruction, and impaired retinal function.15
In contrast to a previous study demonstrating that IRBP-responsive CD4+ T cells primarily reside in the bone marrow in mice with chronic uveitis,18 we have demonstrated increased Th17 cells in the retinal tissues, draining lymph nodes (LN) and spleen, but not in the bone marrow or peripheral blood in our CAU model.15 In the present study, we have further characterized specific memory phenotypes of those retinal infiltrating CD4+ T cells in chronic uveitis for the first time, and examined their antigen-specific functions in vitro and uveitogenic capabilities in vivo.
2. Materials and Methods
2.1. Animals
Wild-type (WT) C57BL/6J mice and B6.129S7-Rag1tm1Mom/J (B6.Rag1−/−) mice (The Jackson Laboratory, Bar Harbor, ME) at 8 – 10 weeks of age were used for this study. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Experimental chronic autoimmune uveitis
Chronic uveitis was induced in WT female C57BL/6J mice using our CAU protocol.15 In brief, mice were immunized with 150μg human interphotoreceptor retinoid-binding protein (IRBP) peptide (residues 161–180, Cat #: AS-60183, AnaSpec, Fremont, CA) and 300μg human IRBP peptide (residues 1–20, Cat #: AS-62297, AnaSpec) emulsified in 0.2ml CFA (containing 2.5 mg/ml Mycobacterium tuberculosis strain H37RA, Difco) (Cat #: F5881, Sigma, St Louis, MO), along with injection of 0.2μg Bordetella pertussis toxin (Cat #: P7208, Sigma). Animals at 12 weeks or later post-immunization were used as the chronic model. The immunization was performed via subcutaneous injection of both flanks (50μl each side) as well as the base of the tail (100μl).
2.3. Digital fundus imaging and scoring
A Micron III (Phoenix, Pleasanton, CA) retinal imaging system was used for taking fundus photographs weekly post-immunization. Mice were anesthetized using ketamine (NDC #: 17033-100-10, Dechra Veterinary Products) and xylazine (NDC #: 59399-110-20, Akorn) (100 mg/kg+20 mg/kg, respectively), and pupils were dilated using 0.5% tropicamide (NDC #: 24208-585-64, Bausch & Lomb). Eyes were kept moist by application of ocular lubricant (Genteal® gel, Alcon, Fort Worth, TX), and were examined for optic disc inflammation, retinal vessel changes, retinal infiltrates, and structural damage. Fundus images were taken and scored by a masked grader using a valid and reproducible grading system that provides a more detailed and refined approach by assessing the aforementioned clinical parameters on a scale of 0–4 for each of them with sum of them reported as the summary score of clinical disease, as previously described.12,15,19–21
2.4. Retinal imaging by spectral domain optical coherence tomography
After anesthesia and pupil dilation as detailed above, mice were restrained in a mounting tube that was fixed on a six-axis platform. Genteal® gel was applied to both eyes to prevent the drying of the cornea. A spectral domain optical coherence tomography (OCT) system (Bioptigen, version 1.4.0, Durham, NC) was used for in vivo non-contact imaging of eyes. B scan was obtained with images centered on the optic nerve head. Average thickness and heat map of the total retina and each individual layers were determined and generated automatically by the machine algorithms. The hyperreflective foci (HRF) is defined as discrete and well-circumscribed dots or roundish lesions within retinal layers with reflectivity comparable to the retinal nerve fiber layer or RPE layer,22,23 and counted by a masked grader.
2.5. Electroretinography
Following overnight dark adaptation, mice were prepared for electroretinography (ERG) recording under dim red light. After anesthesia and pupil dilation as detailed above, one drop of 0.9% sterile saline was applied to the cornea to prevent dehydration and to allow electrical contact with the recording electrode (gold wire loop). A 25-gauge platinum needle, inserted subcutaneously in the forehead, served as reference electrode, while a needle inserted subcutaneously near the tail served as the ground electrode. A series of flash intensities were produced by an Espion Ganzfeld (Diagnosys, Lowell, MA) to test both scotopic (dark-adapted) and photopic (light-adapted) responses. The major ERG components (a-wave and b-wave) were measured using the Espion software (version 6, Diagnosys).17,18 The a-wave amplitude was measured from the baseline to the trough of the a-wave, and the b-wave amplitude was measured from the trough of the a-wave to the peak of the b-wave.
2.6. Flow cytometry analysis and reagents
Retinal tissues, lymph nodes (LN), and spleen of mice were collected, and single-cell suspensions were prepared using a 70-μm cell strainer (BD Biosciences, Franklin Lakes, NJ). The following antibodies (Abs) were used for flow cytometry analysis (the same fluorescence-conjugated Abs were not used together): Brilliant Violet 421-conjugated anti-CD4 (clone RM4–5, Cat #: 100544, BioLegend, San Diego, CA), PE-conjugated anti-CD3 (clone 17A2, Cat #: 100205, BioLegend), FITC-conjugated anti-IFN-γ (clone XMG1.2, Cat #: 505806, BioLegend), PerCP/Cy5.5-conjugated anti-CD44 (clone IM7, Cat #: 103032, BioLegend), PE-conjugated anti-IL-7R (clone A7R34, Cat #: 135009, BioLegend), Alexa Fluor 700-conjugated anti-IL-15R (clone 888220, Cat #: FAB5511N-100UG, R&D Systems, Minneapolis, MN), and APC-conjugated or PE-Cy7-conjugated anti-IL-17A (clone eBio17B7, Cat #: 17-7177-81 or 25-7177-82, ThermoFisher Scientific, Waltham, MA). For intracellular IL-17A and IFN-γ staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin (Cat #: P8139 and I0634, Sigma-Aldrich) for 6 hours at 37°C and 5% CO2 in the presence of GolgiStop™ (4 μl per 6 mL cell culture, Cat#: 554724, BD Biosciences) to inhibit cytokine secretion. Stained cells were examined with an LSR II flow cytometer (BD Biosciences), and the results were analyzed using FlowJo software (version 10, Tree Star, Ashland, OR).
2.7. Cell sorting and culture
Retina and secondary lymphoid organs (SLO, including draining LN and spleens) from CAU mice (week 12 – 16 post-induction) were harvested and pooled, and CD4+ T cells were isolated via negative selection with a magnetic CD4+ T cell isolation kit (Cat #: 130-104-454, Miltenyi Biotec Inc.). The isolated cells were >98% CD4+ as confirmed by flow cytometry, and they were stained with FITC- or PerCP/Cy5.5-anti-CD44 (clone IM7, Cat #: 103006 or 103032, BioLegend) antibody, and sorted for CD44hi memory and CD44−~lo control subpopulations using a MoFlo® FACS sorter (Dako Cytomation) or a BD FACSAria™ III sorter (BD Biosciences). The sorted cells in equal live numbers (1×105) were cultured for 5 days and stained with 7-AAD (Cat #: 00-6993-50, ThermoFisher), and the viable cells were determined as 7-AAD unstained populations by flow cytometric analysis.
2.8. T cell proliferation assay
The sorted memory and control T cells were labeled with 5μM CellTrace™ CFSE (Cat #: C34570, Invitrogen) according to the manufacturer’s protocol, and stimulated with 20 μg/ml IRBP1–20 and 1 μg/ml CD28 Ab (clone 37.51, Cat #: 102112, BioLegend) for 3 days at 37 °C. No IRBP-stimulated memory T cells served as the unstimulated control. Cell division index was determined by flow cytometric analysis.
2.9. Antigen-specific activation assay
The sorted memory and control T cells were stimulated with 20 μg/ml IRBP1–20 and 1 μg/ml anti-CD28 Ab for 18 hours at 37 °C, and the Golgi-stop was added to the culture medium for the last 4 hours to stabilize CD154 intracellularly, as cell surface expression of CD154 is transient during the course of T cell activation and could not be directly assayed.24 No IRBP-stimulated memory T cells served as the unstimulated control. The stimulation protocol also allowed adequate time (the first 14 hours) for the cells to secret cytokines to the media. After the culture supernatant was collected for ELISA, the cells were washed and stained with the 1 μl Fixable Viability Dye eFluor 660 (Cat #: 65–0864, Invitrogen), followed by intracellular staining with PE-conjugated anti-CD154 (clone MR1, Cat #: 12-1541-82, Invitrogen). The expression of CD154 by the T cells was determined by flow cytotmetric analysis as a marker for antigen-specific activation.24,25
2.10. T cell adoptive transfer
The memory and control T cells were sorted from CAU mice as described above. 1×105 sorted cells were immediately injected intravenously into naive syngeneic B6.Rag1−/− mice (the Jackson Laboratory, Bar Harbor, ME).
2.11. ELISA
The cell culture supernatant was assayed for the levels of IL-17 and IFN-γ using commercial ELISA kits (Cat #: BMS6001 and BMS606, eBioscience), according to the manufacturer’s protocol.
2.12. Statistical analyses
For the comparison of multiple groups, the statistical significance of endpoints was evaluated by one-way ANOVA followed by Bonferroni’s multiple comparisons post hoc test. For the comparison of two groups, the unpaired two-tailed Student’s t test was used. Data are summarized as mean ± SEM. All statistical analyses were performed with Prism software (version 9.3.1; GraphPad Software), and differences were considered significant at p < 0.05.
3. Results
3.1. Retinal infiltrating T cells in chronic autoimmune uveitis exhibit distinct memory phenotypes
A robust chronic uveoretinitis was induced in mice using our recently established CAU model,15 which exhibited optic disc inflammation, retinal vessel cuffing, and multiple small retinal solitary lesions by 16 weeks post-induction (Fig. 1A). Our previous work has shown increased Th17 response in retina and the secondary lymphoid organs (SLO) of CAU mice including the cervical eye-draining lymph nodes (ELN), the inguinal lymph nodes (ILN) draining injected antigens, and spleen, but not in the peripheral blood or bone marrow.15 We thus further examined the phenotypes of CD4+ T cells in these relevant tissues. Flow cytometric analysis of the retina showed prominent CD4+ T cell infiltration in chronic uveitis, in contrast to few T cells in the healthy retina, consistent with previous reports.15,26 Furthermore, these retinal infiltrating CD4+ T cells were primarily CD44+/hi cells, which co-expressed IL-7R and IL-15R (Fig. 1B). High levels of CD44, IL-7R, and IL-15R are recognized as both phenotypic and functional markers for memory T cells.15,27–30 In addition, both CD44hi and CD44−~lo CD4+ T cell populations were present in the relevant SLO of CAU mice (ELN, ILN, and spleen), and CD44hiCD4+ T cells similarly exhibited co-expressions of IL-7R and IL-15R, in contrast to their CD44−~loCD4+ counterpart (Fig. 1C), suggesting that uveitis-associated memory CD4+ T cells also reside in these lymphoid tissues in chronic uveitis.
Figure 1. Chronic uveoretinitis is characterized by memory-phenotype CD4+ T cells infiltrated in the retina and present in secondary lymphoid organs (SLO).
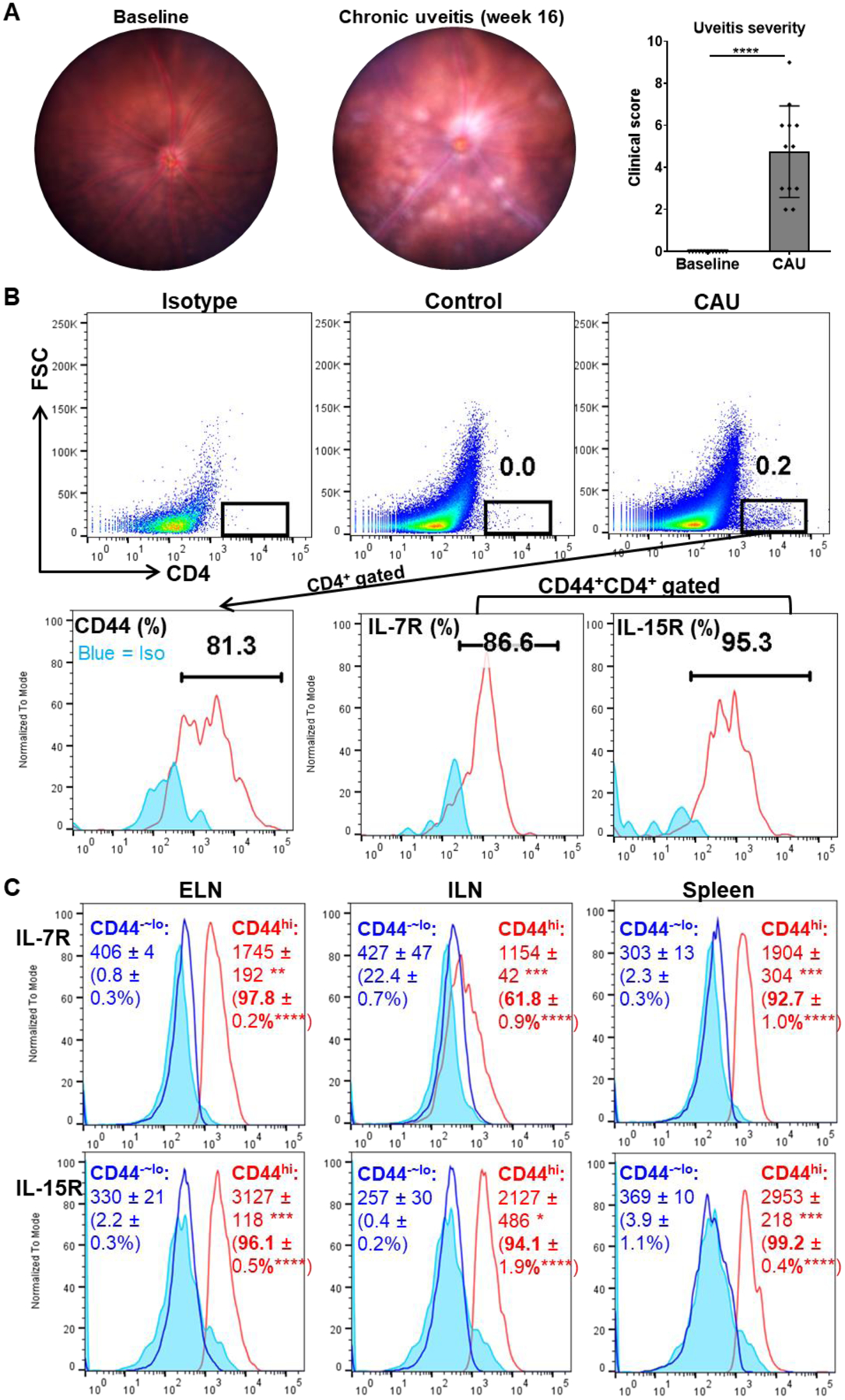
(A) Representative digital fundus images from one same animal before disease induction (baseline) and 16 weeks post-induction (CAU). Chronic uveitis is characterized by optic disc inflammation with masked vessels, blood vessels cuffing, and multiple retinal solitary, small lesions, and disease severity is scored and summarized in the bar graph. (B) Pooled retinal tissues of each group (n = 4) from one representative experiment were analyzed by flow cytometry. CAU exhibits considerable CD4+ T cell infiltration in retinal tissues, and they are phenotyped as dominant CD44+/hi cells which are also primarily L-7R+IL-15R+ cells. Numbers in the upper panel indicate percentages among total retinal cells and in the lower panel indicate percentages among parent CD4+ (for CD44) or CD44+CD4+ (for IL-7R and IL-15R) populations. (C) The cervical eye-draining lymph nodes (ELN), the inguinal lymph nodes (ILN), and spleen in CAU mice were analyzed for IL-7R and IL-15R expressions by CD44hiCD4+ memory T cells and CD44−~loCD4+ cells. Numbers in histograms indicate mean ± SEM of MFI and percentage (in the brackets) of indicated molecules (n = 4 per group). Data shown are one representative out of two performed. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
3.2. Memory-phenotype T cells from chronic uveitis exhibit better in vitro survival and higher expansion upon secondary stimulation
We next determined whether these memory-phenotype T cells in chronic uveitis act like functional memory T cells with long-lived, self-renewing properties. As shown in Fig. 1, CD44hiCD4+ cells are primarily CD44hiIL-7R+IL15R+CD4+, we were thus able to sort CD44hiCD4+ cells as memory T cells, and those CD44−~loCD4+ cells from the same CAU mice (week 12 – 16 post-induction) were used as control cells to determine the function of the specific memory subset in our studies (Fig. 2A). Given the similar phenotypes of memory T cells in retina and SLO (Fig. 1) and limited numbers of cells that can be isolated from retina alone, the sorted memory T cells from relevant tissues were pooled together (~40% from retina, ~20% from LN, and ~40% from spleen) for subsequent experiments. Equal number of the live cells were cultured in vitro, and after 5 days nearly all CD44−~lo control T cells died while over 20% of the CD44hi memory T cells were still viable (Fig. 2B). In addition, we re-stimulated the T cells with the peptide antigen that was used to immunize the source mice and performed the CFSE-dilution assay. We observed significantly more cell divisions of CD44hi memory T cells than the control cells (Fig. 2C).
Figure 2. Chronic uveitis-derived memory T cells exhibit better survival and increased proliferative capacity.
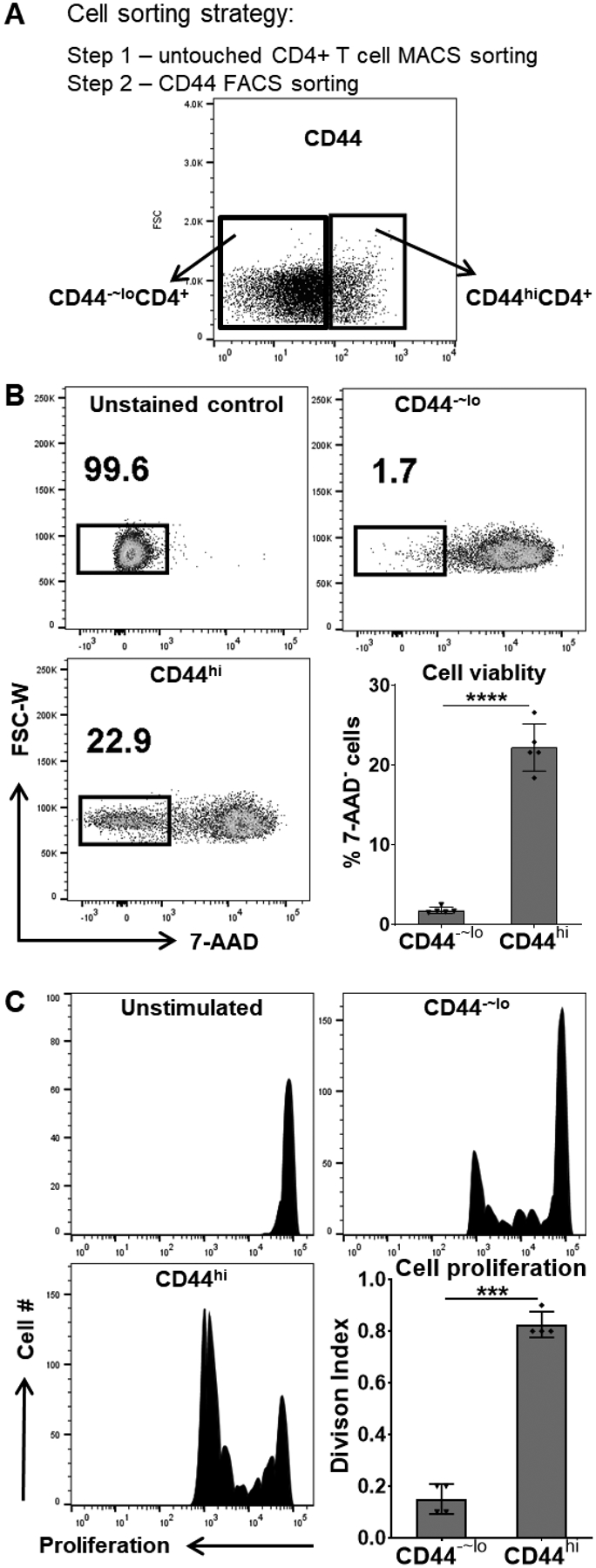
(A) The CD44hiCD4+ memory T cells and the CD44−~loCD4+ control T cells were sorted from mice with CAU. (B) An equal number of live, sorted cells were in vitro cultured for 5 days and stained with 7-AAD, and the viable cells were determined as 7-AAD unstained populations by flow cytometric analysis. (C) The sorted cells were also in vitro stimulated with the uveitogenic antigen (IRBP) for 3 days. Cell proliferation was detected by CFSE dilution, and T cell Division Index (the average number of cell divisions that a cell in the original population has undergone) was determined by flow cytometric analysis. No antigen-stimulated CD44hiCD4+ cell cultures served as the unstimulated control. Data in bar graphs represent mean ± SEM from one experiment out of two performed. ***, p < 0.001; ****, p < 0.0001.
3.3. Memory-phenotype T cells from chronic uveitis are autoantigen-specific responders
As antigen-experienced memory T cells are characterized by their antigen specificity and robust recall response due to a lower activation threshold, we subsequently assessed whether these memory T cells in CAU are uveitis-associated or belong to a non-specific memory pool in the host. The CAU-derived memory T cells or control T cells were in vitro re-stimulated with the retinal antigen (a uveitogenic peptide used in CAU induction) for 18 hours, and antigen-specific activation of T cells were examined by flow cytometric analysis of CD154 expression24,25 and quantification of effector cytokines in the culture supernatants. More than one-third of the memory T cells showed up-regulation of CD154, while control T cells barely showed any expression of CD154 upon stimulation (Fig. 3A). Further, the re-stimulated memory T cells produced significantly higher amounts of both IL-17 and IFN-γ (Fig. 3B), the two principal cytokines implicated in the pathogenesis of autoimmune uveitis.20,31
Figure 3. Chronic uveitis-derived memory T cells show antigen-specific activation in response to in vitro re-stimulation.
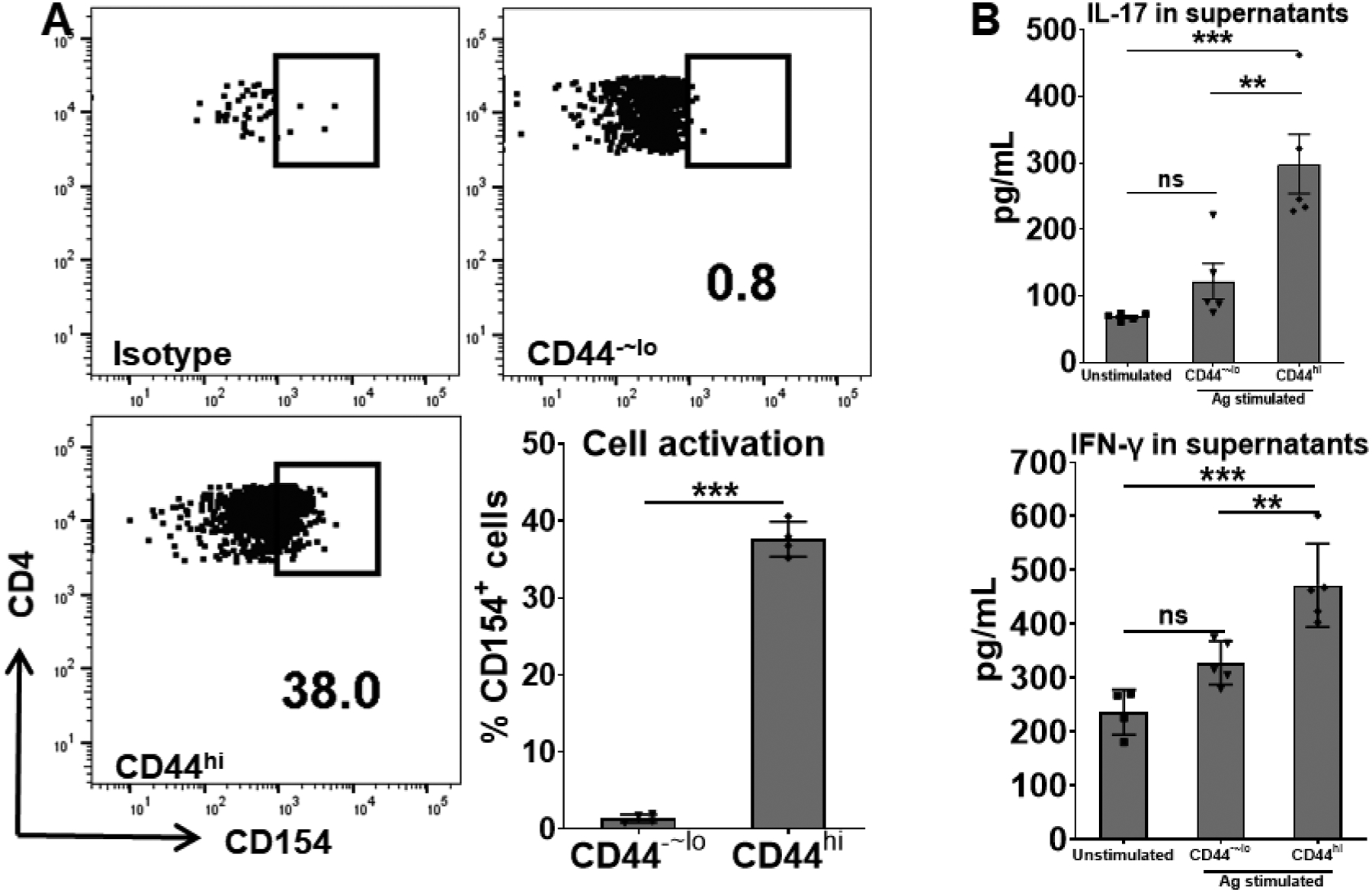
The sorted memory (CD44hi) and control (CD44−~lo) cells from CAU mice were in vitro stimulated with the uveitogenic antigen (IRBP) for 18 hours. Antigen-responding T cells were detected by CD154 expression (A) and pathogenic cytokines production (B). No antigen-stimulated CD44hiCD4+ cell cultures served as the unstimulated control. Data in bar graphs represent mean ± SEM from one experiment out of two performed. **, p < 0.01; ***, p < 0.001; ns, not significant.
3.4. Chronic uveitis-derived memory T cells induce retinal damage in vivo
To determine whether the memory T cells generated in chronic uveitis are indeed pathogenic, we next adoptively transferred the memory T cells freshly isolated from CAU mice into naive Rag1−/− mice which do not have their own T cells, thus permitting us to evaluate the functions of transferred cells specifically. Mice receiving the memory T cell progressively developed uveitis-associated clinical disease evidenced by the fundoscopic observations of retinal blood vessel cuffing and multiple small retinal lesions, and these abnormalities emerged at day 7 post-transfer, and persisted until the end of our observation at day 21 (Fig. 4A). The disease severity induced by adoptively transferred memory T cells is milder than that induced by active antigen immunization (Fig. 1A). Similarly, adoptive transfer of total CD4+ T cells (without differentiating specific subsets) from acute uveitis (EAU) (with additional in vitro activation before transfer) also induced milder disease in recipients than that observed in actively immunized EAU model.10 In contrast, those receiving CD44−~lo non-memory CD4+ T cells did not develop any retinal abnormalities on fundoscopy. The retina was further evaluated by spectral domain OCT, which showed engorged retinal blood vessels along with hyperreflective vitreous opacities as well as multiple hyperreflective foci (HRF, an indicator of inflammation)22 in the retina, including within the inner plexiform layer (IPL) and outer plexiform layer (OPL) in memory T cell recipients, but not in the control T cell recipients (Fig. 4B). We also measured the retinal thickness in the recipients and found a significant increase in the memory T cell recipients but not in the control recipients by week 2 post-transfer, and the increased thickness primarily involved the IPL, outer nuclear layer (ONL), and retinal pigment epithelium (RPE) layers (Supplemental Fig. 1A–D), suggesting an acute inflammatory process compromising both the inner and outer blood-retina barriers (BRB). The retina thickness in the memory T cell recipients returned to normal by week 3 (Supplemental Fig. 1E). Moreover, we performed full-field ERG to evaluate the retinal function. Both dark-adapted responses (evaluating scotopic vision mediated by rod cells) and light-adapted responses (assessing photopic vision mediated by cone cells) in the memory T cell recipients showed an electronegative or electronegative-like pattern that is selectively decreased amplitudes of b-waves without significant changes of a-waves. In contrast, the control recipients did not show any significant changes in amplitudes from the baseline (Fig. 5 and Supplemental Fig. 2).
Figure 4. Chronic uveitis-derived memory T cells specifically induce uveitis.
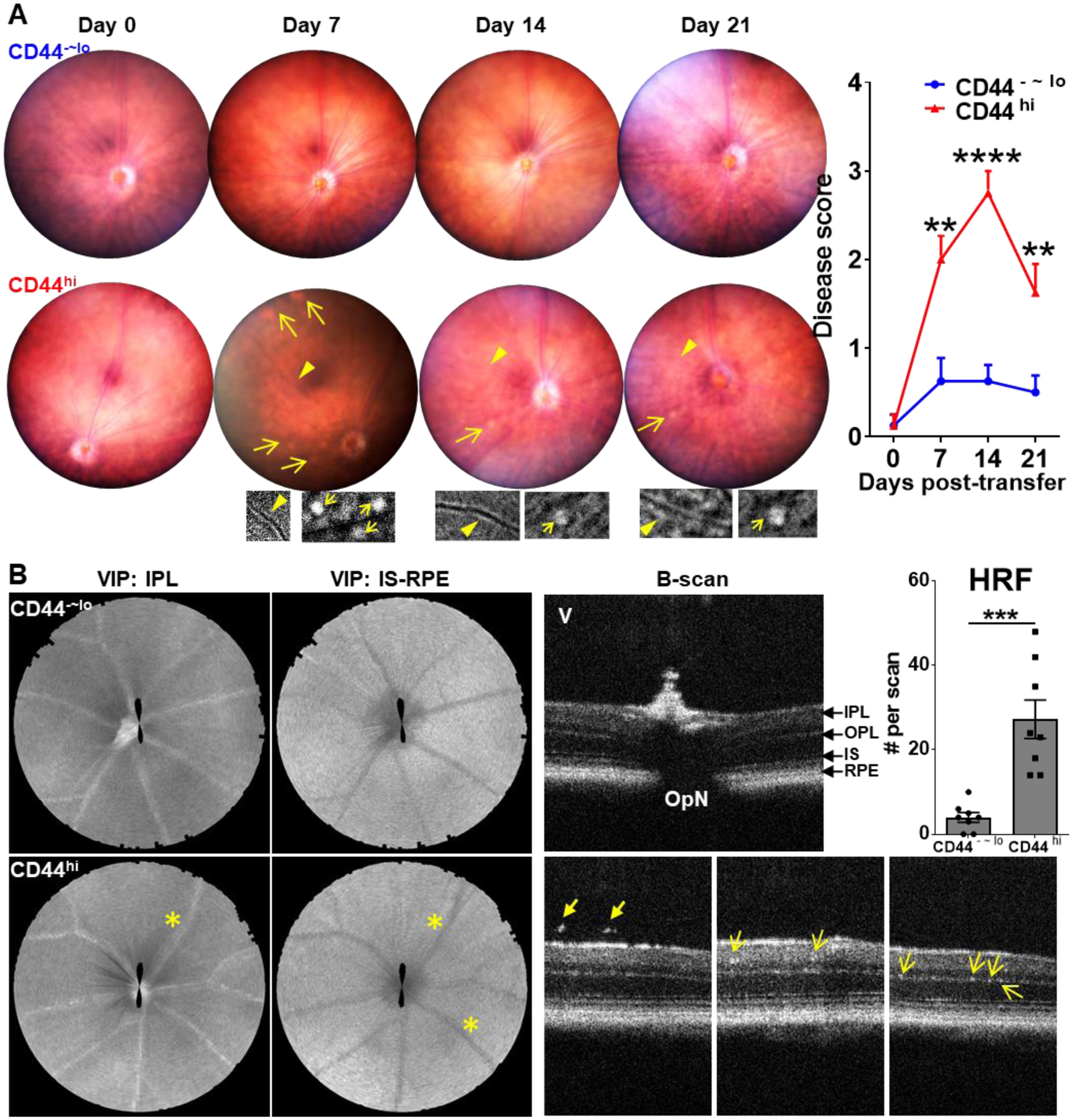
The freshly sorted memory (CD44hi) and control (CD44−~lo) T cells from CAU mice were adoptively transferred (AT) to naive Rag1−/− mice, and the recipients were evaluated by weekly fundoscopic and OCT examinations for 21 days. (A) Representative weekly digital fundus images from one same animal receiving memory T cells exhibit retinal blood vessel cuffing (arrows) and multiple retinal small lesions (arrowheads). Inserts show the enlarged changes in the corresponding areas in a grayscale. Disease scores during the observation period are summarized as mean ± SEM (n = 8 per group) from one experiment out of two performed. (B) OCT in segmental VIP views shows engorged blood vessels at IPL and IS-RPE layers (asterisk), and B-scan shows the hyperreflective vitreous opacities (thick arrow) and multiple hyperreflective foci (HRF, indicated by thin arrow) in the retina at day 14. Data in summary bar graphs represent mean ± SEM from one experiment out of two performed. IPL, inner plexiform layer; IS, inner segment; OPL, outer plexiform layer; OpN, optic nerve; RPE: retinal pigment epithelium; V, vitreous; VIP, volume intensity projection. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Figure 5. Memory T cell recipients show compromised retinal function evaluated by full-field ERG.
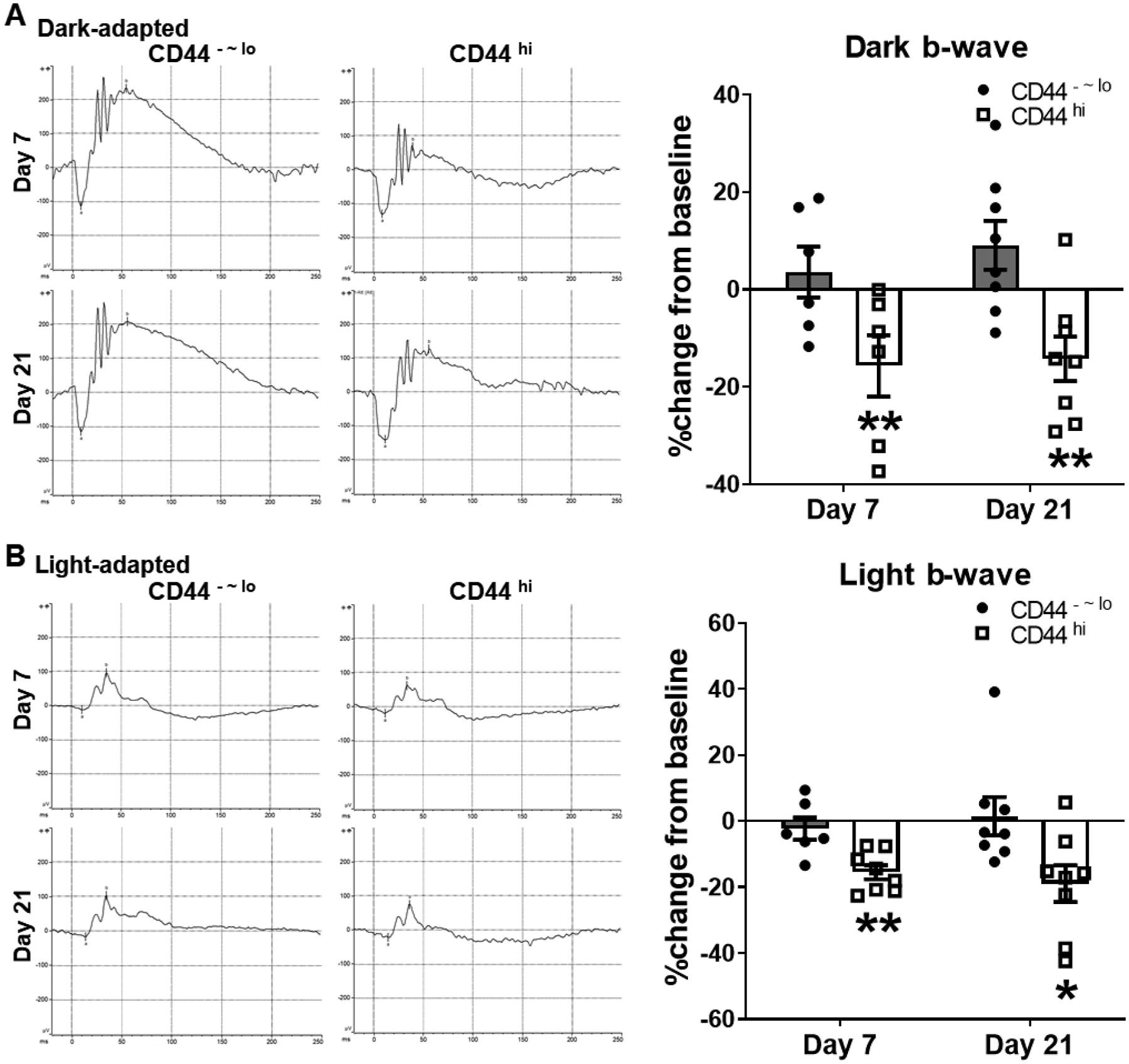
Representative ERG responses to light stimuli at 24.1 and 25.6 cd s/m2 for scotopic dark- (A) and photopic light-adapted (B) eyes are shown on the left panels. The b-wave amplitude changes from baseline are depicted in bar charts (mean ± SEM) on the right panels. Data are summarized from one experiment out of two performed. *, p < 0.05; **, p < 0.01.
3.5. Adoptively transferred memory T cells are capable of migrating to the retina and draining lymph nodes, and giving rise to IFN-γ-secreting subsets
We next examined the trafficking and localization of the transferred T cells in the recipients. Significant T cell infiltration in the retina was found in the memory T cell recipients but not in the control recipients, and the infiltrated T cells produced both IL-17 and IFN-γ, evidenced by a prominent population of IL-17/IFN-γ-double-positive Th17/1 cells (Fig. 6A). In the eye-draining lymph nodes, similar numbers of transferred T cells were recovered from both groups of recipients; however, only those from memory T cell recipients comprised of major IL-17-single positive and IL-17/IFN-γ-double positive subsets (Fig. 6B and Supplemental Fig. 3). In the distal inguinal lymph nodes, a considerable amount of transferred T cells were similarly recovered in both groups of recipients with more cells in the control recipients; and those recovered T cells did not express either IL-17 or IFN-γ in either group (Supplemental Fig. 4).
Figure 6. Adoptively transferred memory T cells specifically migrate to the retina and its draining lymph nodes by producing both IL-17 and IFN-γ.
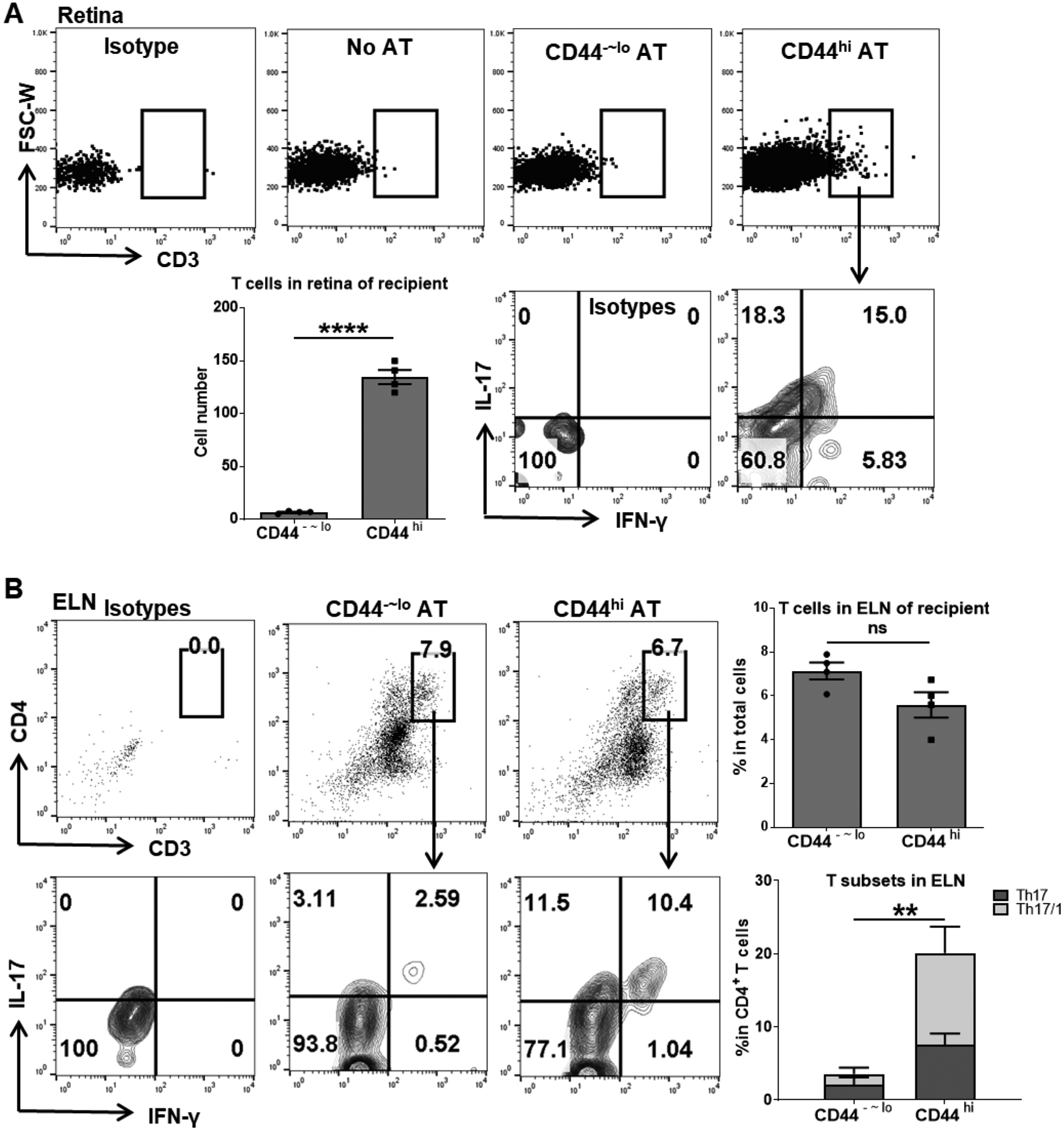
At day 14 post-transfer, the retina (A) and eye-draining lymph nodes (ELN) (B) of recipients were analyzed for T cell infiltration and their cytokines production by flow cytometry. Bar charts summarize the total T cells or Th17 and Th17/1 subsets as mean ± SEM (n = 4 per group) from one representative experiment out of two performed. The absolute numbers of T cells infiltrated in the retinal tissue were extrapolated from the total numbers of live cells isolated from each eye. AT, adoptive transfer. **, p < 0.01; ****, p < 0.0001; ns, not significant.
4. Discussion
Chronic uveitis is one of the major blinding conditions globally, particularly in developed countries. However, few studies have investigated the precise pathogenesis mediating the chronicity of this condition, and thus there is a lack of effective treatment for chronic uveitis. Herein, using a robust murine model of chronic uveitis we have recently established, we demonstrate distinct memory-phenotype T cells infiltrating the retina during the chronic course. These memory T cells exert retinal antigen-specific effector functions that cause damage to retinal tissues impairing visional functions.
Many studies have demonstrated the pivotal roles of T cells, particularly Th17 subsets, in the induction/acute phase of uveitis using the classic EAU models by focusing on the first 2–3 weeks when the disease reaches peak severity.9,10,26 Blockade of the Th17 pathway immunity has been shown to reduce acute uveitis through neutralization of various Th17-associated cytokines, such as IL-17 and IL-6.9,26,32 Interestingly, the natural process in EAU presents with spontaneous resolution of acute inflammation after 2–3 weeks, along with the decline of effector Th17 cells9,33 and emergence of anti-inflammatory inducible Tregs.11,13 However, with time, the retina and choroid do not return to normal immune homeostasis, but exhibit persistent, low-grade intraocular inflammation,11,12,34 for which the underlying mechanisms are incompletely understood. Herein, we demonstrate that the principal retinal infiltrating T cells in chronic uveitis are memory T cells, defined by their high expression of “memory markers”, including CD44 – an adhesion receptor critically promoting cell survival,35 as well as IL-7R and IL-15R – both critically promoting memory CD4+ T cell survival.30,36,37 In contrast to short-lived effector T cells in acute uveitis that rapidly decline probably through undergoing the well-known process of activation-induced cell death,38 these memory-phenotype T cells persist for a long term in the chronic uveitis. They are resistant to apoptosis while highly proliferative upon antigen re-stimulation in vitro, suggesting that the superior surviving ability of these memory T cells may enable them to maintain the disease chronicity in CAU.
In addition to the retina, memory CD4+ T cells are present in the draining LN and spleen in mice with chronic uveitis,15 and in the peripheral blood in human patients with chronic uveitis,9,39 indicating that these memory cells recirculate through the blood to non-lymphoid tissues, and lymphatics to lymph nodes. This is a characterized trafficking pattern of the “effector-memory” subset, which is in contrast to the “central-memory” subset recirculating through the blood and lymphoid tissues, or the “tissue-resident memory” subset parked in non-lymphoid tissues without recirculating.40 Functional analysis of the memory T cells from CAU shows robust reactivation of these cells upon re-stimulation with the retinal antigen, as well as rapid secretion of effector cytokines IL-17 and IFN-γ, consistent with the biological behaviors of effector-memory T cells.41 Our results also demonstrate these memory T cells with antigen-specificity and a lower activation threshold, evidenced by their ability to mount a robust recall response in the presence of the same antigen IRBP without additional T cell stimulators. Further, although memory T cells in CAU primarily produce IL-17 but not IFN-γ,15 upon re-stimulation, they start to produce IFN-γ, a recently recognized functional plasticity of Th17 cells that is critical in promoting autoimmune inflammation.42–44 In line with our findings, the peripheral blood mononuclear cells (PBMCs) collected from patients with chronic uveitis have been shown robust IL-17 and IFN-γ production upon IRBP stimulation specifically, which is significantly higher than the response of PMBCs from healthy controls.45 It is worth noting that we analyzed the pooled memory T cells from the retina and SLO due to limited cell numbers that can be isolated from the retina alone for subsequent experiments. Our previous study has demonstrated almost 10 times higher IL-17-producing memory T cells in the retina than in SLO in CAU,15 suggesting that retinal memory T cells are more functionally active than memory T cells in SLO.
Total CD4+ T cells from acute EAU have been widely shown to adoptively induce disease after being re-stimulated with IRBP with or without polarization (to Th1 or Th17 effectors) before being transferred to normal recipients.10,26,46,47 Our study directly demonstrates the uveitogenicity of the specific memory CD44hiCD4+ T cell subset in CAU through adoptive transfer of freshly isolated cells without any ex vivo manipulations, distinct from a previous study transferring all bone marrow cells (without differentiating specific populations) isolated from chronic uveitis mice with additional in vitro re-stimulation before transfer.18 Further, we used syngeneic Rag1−/− mice as recipients to track the injected T cells for their migration and function. Interestingly, unlike the induction of CAU through active immunization, passive transfer of memory T cells induces inflammatory retinal damage in normal recipients without the need for antigen immunization or Bordetella pertussis toxin injection. Instead, the transferred memory T cells can specifically migrate to and accumulate in the retina and its draining lymph nodes, indicating that the uveitogenic memory T cells harbor the capabilities of homing to the target tissues and breaking down the BRB. The possible mechanisms may include their expressions of specific adhesion molecules (such as integrins), chemokine receptors, and inflammatory cytokines mediating their capture and sustained arrest in retinal vessels, subsequently inducing local vascular endothelial permeability and their extravasation from blood vessels to the retina.48–54 This is supported by our findings that the T cells recovered from the retina and draining lymph nodes of the recipients actively produce IL-17 and IFN-γ consisting of a prominent IL-17/IFN-γ-double-positive Th17/1 population, while those sequestrated in the distal lymph nodes do not produce either effector cytokines. In addition, comparable numbers of the transferred T cells are recovered from the draining lymph nodes and distal lymph nodes of the control T cell recipients. However, those cells barely produce IL-17 or IFN-γ, suggesting that the in vivo non-pathogenicity of the control T cells is unlikely attributed to their inferior survival ability as observed in the in vitro culturing setting, but primarily due to their functional distinction from the memory T cells. Our clinical findings show engorged retinal blood vessels with cuffing and increased retinal thickness by 2 weeks post-transfer of memory T cells, indicating acute inflammatory edema induced by the pathogenic cells. A recent study has shown the presence of Th17/1 in acute uveitis and their resistance to corticosteroid treatment.20 Further analysis reveals the inner plexiform layer, outer nuclear layer, and retinal pigment epithelium layer all involved, suggesting the passage of memory T cells into the retina across both inner (from microvascular venules) and outer (from choroid plexus) BRB. However, the precise process and specific mediators involved in the multi-step trafficking of memory T cells across the BRB remain to be determined.
A unique OCT finding in the memory T cell recipients is the multiple hyperreflective foci (HRF) within the retina. HRF has received much attention recently as a clinical biomarker for intraocular inflammation in retinitis pigmentosa,55 diabetic retinopathy,23 and age-related macular degeneration.22 The possible histopathological correlates of HRF vary in different diseases. In patients with chronic uveitis, HRF has been presumed to represent intraretinal exudates, infiltrating lymphocytes, or clumping of photoreceptors/intraretinal RPE cells, depending on its structural features and topographic location.56–58 The observed HRF in our study probably represents retinal infiltrating T cells or inflammatory exudates. The association of HRF in pre-clinical uveitis models with disease severity and treatment response deserves further studies. Along with the structural changes by memory T cells, the visual functional assessment on the retina shows a significant “electronegative” pattern on the full-field ERG (shown as the selective b-wave reduction) as early as 1 week post-transfer and sustained until the end of follow-up (3 weeks), suggesting that retinal dysfunction takes precedence over significant structural changes that can be visually detected by fundoscopy. The electronegative ERG indicates the abnormality occurring at the photoreceptor to bipolar synapse that can either be in the photoreceptor terminal or the bipolar dendrite,59 an area where multiple HRF are observed on OCT. The electronegative ERG has also been reported in patients with chronic uveitis and may resolve with treatment.60
Immunological memory is well known for its protection against infection and solid tumors. Our present study, along with previous,28,61–63 has demonstrated that CD4+ T cell-mediated immunological memory can be detrimental in causing autoimmune disorders and chronic inflammation. This has important therapeutic implications, particularly in chronic uveitis: the past failure of targeting IL-17 to prevent disease relapse in human chronic uveitis64 may be due to its ineffectiveness in eliminating pathogenic memory T cells.30 Development of novel immunomodulatory strategy targeting critical factors governing memory T cell survival and function might thus be promising to achieve sustained clinical improvement, with a hope to cure this debilitating disease.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants EY031781 (Y.C.) and P30EY003790 (Massachusetts Eye and Ear), the Harvard Medical School Shore Faculty Development Award (Y.C.), and the Massachusetts Lions Eye Research Awards (Y.C.). Dr. Nai-Wen Fan would like to thank the Yin Shu-Tien Foundation for its support through the Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program (No. 106-V-A-002).
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- 1.Nussenblatt RB: The natural history of uveitis. Int Ophthalmol, Int Ophthalmol, 1990, 14:303–308. [DOI] [PubMed] [Google Scholar]
- 2.Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, Uchida A: Incidence and prevalence of uveitis: Results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol, JAMA Ophthalmol, 2013, 131:1405–1412. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein H: The reported demography and causes of blindness throughout the world. Adv Ophthalmol, Adv Ophthalmol, 1980, 40:1–99. [PubMed] [Google Scholar]
- 4.LUGOSSY G: Epidemiology of uveitis. Szemeszet, Arch Ophthalmol, 1962, 99:197–204. [PubMed] [Google Scholar]
- 5.Suttorp-Schulten MSA, Rothova A: The possible impact of uveitis in blindness: A literature survey. Br. J. Ophthalmol 1996, pp. 844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi RR: Understanding autoimmune uveitis through animal models: The friedenwald lecture. Investig. Ophthalmol. Vis. Sci 2011, pp. 1873–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabs DA, Rosenbaum JT, Foster CS, Holland GN, Jaffe GJ, Louie JS, Nussenblatt RB, Stiehm ER, Tessler H, Van Gelder RN, Whitcup SM, Yocum D: Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am J Ophthalmol 2000, 130:492–513. [DOI] [PubMed] [Google Scholar]
- 8.Suhler EB, Thorne JE, Mittal M, Betts KA, Tari S, Camez A, Bao Y, Joshi A: Corticosteroid-Related Adverse Events Systematically Increase with Corticosteroid Dose in Noninfectious Intermediate, Posterior, or Panuveitis: Post Hoc Analyses from the VISUAL-1 and VISUAL-2 Trials. Ophthalmology 2017, 124:1799–1807. [DOI] [PubMed] [Google Scholar]
- 9.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE: TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med, Nat Med, 2007, 13:711–718. [DOI] [PubMed] [Google Scholar]
- 10.Eskandarpour M, Alexander R, Adamson P, Calder VL: Pharmacological Inhibition of Bromodomain Proteins Suppresses Retinal Inflammatory Disease and Downregulates Retinal Th17 Cells. J Immunol, J Immunol, 2017, 198:1093–1103. [DOI] [PubMed] [Google Scholar]
- 11.Lee DJ, Taylor AW: Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J Leukoc Biol 2015, 97:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers CE, Calder VL, Greenwood J, Eskandarpour M: Experimental Autoimmune Uveitis: An Intraocular Inflammatory Mouse Model. J Vis Exp 2022, 2022. [DOI] [PubMed] [Google Scholar]
- 13.Muhammad F, Wang D, McDonald T, Walsh M, Drenen K, Montieth A, Foster CS, Lee DJ: TIGIT+ A2Ar-Dependent anti-uveitic Treg cells are a novel subset of Tregs associated with resolution of autoimmune uveitis. J Autoimmun 2020, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durrani OM, Meads CA, Murray PI: Uveitis: A potentially blinding disease. Ophthalmologica. 2004, pp. 223–236. [DOI] [PubMed] [Google Scholar]
- 15.Fan NW, Li J, Mittal SK, Foulsham W, Elbasiony E, Huckfeldt RM, Chauhan SK, Chen Y: Characterization of Clinical and Immune Responses in an Experimental Chronic Autoimmune Uveitis Model. Am J Pathol 2021, 191:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D: Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res 2006, 82:323–331. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Qian H, Horai R, Chan CC, Falick Y, Caspi RR: Comparative Analysis of Induced vs. Spontaneous Models of Autoimmune Uveitis Targeting the Interphotoreceptor Retinoid Binding Protein. PLoS One 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H-M, Yu C-R, Lee Y, Chan C-C, Maminishkis A, Egwuagu CE: Autoreactive Memory CD4 + T Lymphocytes That Mediate Chronic Uveitis Reside in the Bone Marrow through STAT3-Dependent Mechanisms. J Immunol, J Immunol, 2011, 187:3338–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Koch P, Chen M, Lau A, Reid DM, Forrester JV.: A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res, Exp Eye Res, 2008, 87:319–326. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Eskandarpour M, Gondrand A, Zhang X, Gu R, Galatowicz G, Lightman SL, Calder VL: Functionally distinct IFN-γ+IL-17A+ Th cells in experimental autoimmune uveitis: T-cell heterogeneity, migration, and steroid response. Eur J Immunol 2020, 50:1941–1951. [DOI] [PubMed] [Google Scholar]
- 21.Bradley LJ, Ward A, Hsue MCY, Liu J, Copland DA, Dick AD, Nicholson LB: Quantitative Assessment of Experimental Ocular Inflammatory Disease. Front. Immunol 2021,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fragiotta S, Abdolrahimzadeh S, Dolz-Marco R, Sakurada Y, Gal-Or O, Scuderi G: Significance of Hyperreflective Foci as an Optical Coherence Tomography Biomarker in Retinal Diseases: Characterization and Clinical Implications. Milani P, editor. J Ophthalmol [Internet] 2021, 2021:6096017. Available from: https://www.hindawi.com/journals/joph/2021/6096017/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Jang H, Choi YA, Kim HC, Chung H: Association between soluble cd14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Investig Ophthalmol Vis Sci 2018, 59:715–721. [DOI] [PubMed] [Google Scholar]
- 24.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A: Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 2005, 11:1118–1124. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhoff D, Frentsch M, Leclerk P, Bumann D, Rausch S, Hartmann S, Thiel A, Scheffold A: Identification and isolation of murine antigen-reactive T cells according to CD154 expression. Eur J Immunol 2007, 37:2370–2377. [DOI] [PubMed] [Google Scholar]
- 26.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR: Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med 2008, 205:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama T, Yamashita M: Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin Immunol, Academic Press, 2009, 21:78–83. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Chauhan SK, Soo Lee H, Saban DR, Dana R: Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol 2014, 7:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seddon B, Tomlinson P, Zamoyska R: Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 2003, 4:680–686. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Chauhan SK, Tan X, Dana R: Interleukin-7 and −15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun 2017, 77:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horai R, Caspi RR: Cytokines in autoimmune uveitis. J Interf Cytokine Res 2011, 31:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, Kobayashi T, Yoshida H, Yoshimura A: Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology 2009, 48:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Wan F, Song J, Tang K, Zheng F, Guo J, Guo D, Bi H: Imbalance Between Th17 Cells and Regulatory T Cells During Monophasic Experimental Autoimmune Uveitis. Inflammation 2016, 39:113–122. [DOI] [PubMed] [Google Scholar]
- 34.Kerr EC, Raveney BJE, Copland DA, Dick AD, Nicholson LB: Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun 2008, 31:354–361. [DOI] [PubMed] [Google Scholar]
- 35.Baaten BJG, Li CR, Bradley LM: Multifaceted regulation of T cells by CD44. Commun Integr Biol 2010, 3:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM: Interleukin 7 Regulates the Survival and Generation of Memory CD4 Cells. J Exp Med 2003, 198:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadzadeh M, Hussain SF, Farber DL: Heterogeneity of the Memory CD4 T Cell Response: Persisting Effectors and Resting Memory T Cells. J Immunol 2001, 166:926–935. [DOI] [PubMed] [Google Scholar]
- 38.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y: IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A 2000, 97:11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, Huang X, Kijlstra A: Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Investig Ophthalmol Vis Sci 2008, 49:3058–3064. [DOI] [PubMed] [Google Scholar]
- 40.Jameson SC, Masopust D: Understanding Subset Diversity in T Cell Memory. Immunity. 2018, pp. 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F, Geginat J, Lanzavecchia A: Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol, Annu Rev Immunol, 2004, 22:745–763. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R: IFN-γ–Expressing Th17 Cells Are Required for Development of Severe Ocular Surface Autoimmunity. J Immunol 2017, 199:1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B: Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 2011, 12:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT: Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 2015, 112:7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeuchi M, Usui Y, Okunuki Y, Zhang L, Ma J, Yamakawa N, Hattori T, Kezuka T, Sakai J ichi, Goto H: Immune responses to interphotoreceptor retinoid-binding protein and s-antigen in behçet’s patients with uveitis. Investig Ophthalmol Vis Sci 2010, 51:3067–3075. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal RK, Silver PB, Caspi RR: Rodent models of experimental autoimmune uveitis. Methods Mol Biol, Methods Mol Biol, 2012, 900:443–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chong WP, Mattapallil MJ, Raychaudhuri K, Bing SJ, Wu S, Zhong Y, Wang WW, Chen Z, Silver PB, Jittayasothorn Y, Chan CC, Chen J, Horai R, Caspi RR: The Cytokine IL-17A Limits Th17 Pathogenicity via a Negative Feedback Loop Driven by Autocrine Induction of IL-24. Immunity 2020, 53:384–397.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitcup SM, Chan CC, Li Q, Nussenblatt RB: Expression of Cell Adhesion Molecules in Posterior Uveitis. Arch Ophthalmol 1992, 110:662–666. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Forrester JV., Liversidge J, Crane IJ: Leukocyte trafficking in experimental autoimmune uveitis: Breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Investig Ophthalmol Vis Sci 2003, 44:226–234. [DOI] [PubMed] [Google Scholar]
- 50.Chen YH, Eskandarpour M, Zhang X, Galatowicz G, Greenwood J, Lightman S, Calder V: Small-molecule antagonist of VLA-4 (GW559090) attenuated neuro-inflammation by targeting Th17 cell trafficking across the blood-retinal barrier in experimental autoimmune uveitis. J Neuroinflammation 2021, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu P, Pollard J, Hunt N, Chan-Ling T: Microvascular and cellular responses in the retina of rats with acute experimental allergic encephalomyelitis (EAE). Brain Pathol 1998, 8:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu P, Pollard JD, Chan-Ling T: Breakdown of the blood-retinal barrier induced by activated T cells of nonneural specificity. Am J Pathol 2000,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchetti L, Engelhardt B: Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol 2020, 2:H1–H18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engelhardt B, Ransohoff RM: The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005, pp. 485–495. [DOI] [PubMed] [Google Scholar]
- 55.Nagasaka Y, Ito Y, Ueno S, Terasaki H: Number of Hyperreflective Foci in the Outer Retina Correlates with Inflammation and Photoreceptor Degeneration in Retinitis Pigmentosa. Ophthalmol Retin 2018, 2:726–734. [DOI] [PubMed] [Google Scholar]
- 56.Keane PA, Allie M, Turner SJ, Southworth HS, Sadda SR, Murray PI, Denniston AK: Characterization of birdshot chorioretinopathy using extramacular enhanced depth optical coherence tomography. JAMA Ophthalmol 2013, 131:341–350. [DOI] [PubMed] [Google Scholar]
- 57.Agrawal R, Arora R, Keane PA, Agarwal A, Pavesio C: Morphometric features on enhanced depth imaging optical coherence tomography scans in idiopathic posterior uveitis or panuveitis. Int. Ophthalmol 2018, pp. 993–1002. [DOI] [PubMed] [Google Scholar]
- 58.Gobuty M, Adhi M, Read SP, Duker JS: Visual response and anatomical changes on sequential spectral-domain optical coherence tomography in birdshot chorioretinopathy treated with local corticosteroid therapy. Int J Retin Vitr 2016, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang X, Mahroo OA: Negative electroretinograms: genetic and acquired causes, diagnostic approaches and physiological insights. Eye. 2021, pp. 2419–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holder GE, Robson AG, Pavesio C, Graham EM: Electrophysiological characterisation and monitoring in the management of birdshot chorioretinopathy. Br J Ophthalmol 2005, 89:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W: Human TH17 cells are long-lived effector memory cells. Sci Transl Med, NIH Public Access, 2011, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP: Th17 Cells Are Long Lived and Retain a Stem Cell-like Molecular Signature. Immunity 2011, 35:972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, Porth K, Boniface K, Mattson J, Basham B, Anderton SM, McClanahan TK, Sadekova S, Cua DJ, McGeachy MJ: Autoimmune Memory T Helper 17 Cell Function and Expansion Are Dependent on Interleukin-23. Cell Rep 2013, 3:1378–1388. [DOI] [PubMed] [Google Scholar]
- 64.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, Androudi S: Secukinumab in the treatment of noninfectious uveitis: Results of three randomized, controlled clinical trials. Ophthalmology 2013, 120:777–787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


