Abstract
Gap junctions (GJs) are aqueous channels that allow cells to communicate via physiological signals directly. The role of gap junctional connectivity in determining single-cell functions has long been recognized. However, GJs have another important role: the regulation of large-scale anatomical pattern. GJs are not only versatile computational elements that allow cells to control which small molecule signals they receive and emit, but also establish connectivity patterns within large groups of cells. By dynamically regulating the topology of bioelectric networks in vivo, GJs underlie the ability of many tissues to implement complex morphogenesis. Here, a review of recent data on patterning roles of GJs in growth of the zebrafish fin, the establishment of left-right patterning, the developmental dysregulation known as cancer, and the control of large-scale head-tail polarity, and head shape in planarian regeneration has been reported. A perspective in which GJs are not only molecular features functioning in single cells, but also enable global neural-like dynamics in non-neural somatic tissues has been proposed. This view suggests a rich program of future work which capitalizes on the rapid advances in the biophysics of GJs to exploit GJ-mediated global dynamics for applications in birth defects, regenerative medicine, and morphogenetic bioengineering.
Keywords: gap junctions, networks, bioelectric, patterning, morphogenesis
INTRODUCTION
Gap junctions (GJs), formed by connexin or innexins proteins, form aqueous channels directly connecting the internal cytoplasmic space of nearby cells for the transfer of ions and other small molecules (Phelan, 2005; Scemes et al., 2007). Along with ion channels, they are a key component by which most types of somatic cells organize into physiological networks (Fig. 1). The molecular biology and physiology of GJs, as well as their many roles in the nervous system, have recently been expertly reviewed (Sohl and Willecke, 2004; Steyn-Ross et al., 2007; Pereda et al., 2013; Baker and Macagno, 2014). Here, we focus not on the cellular-level signaling mechanics of GJs, but on their role in determining large-scale pattern formation. Tissue and organ patterning includes embryonic morphogenesis, regeneration of limbs and other organs in a range of model species, and continuous tumor suppression in adult metazoan organisms. Coordinating cell behaviors in vivo toward the creation and repair of complex structures depends on a tight coordination among cells. This coordination is implemented by a rich system of information-bearing signals that propagate throughout the body. Because GJs enable small molecule physiological signals (e.g., ion flow) to pass among cells, they serve as an important modality for cell–cell communication (Trosko, 2007). In this overview, we focus on intercellular channel functions of GJs, and do not discuss permeability-independent functions of connexin proteins (Dang et al., 2003, 2006; Vinken et al., 2012; Clasadonte and Haydon, 2014) or hemichannels.
Figure 1.
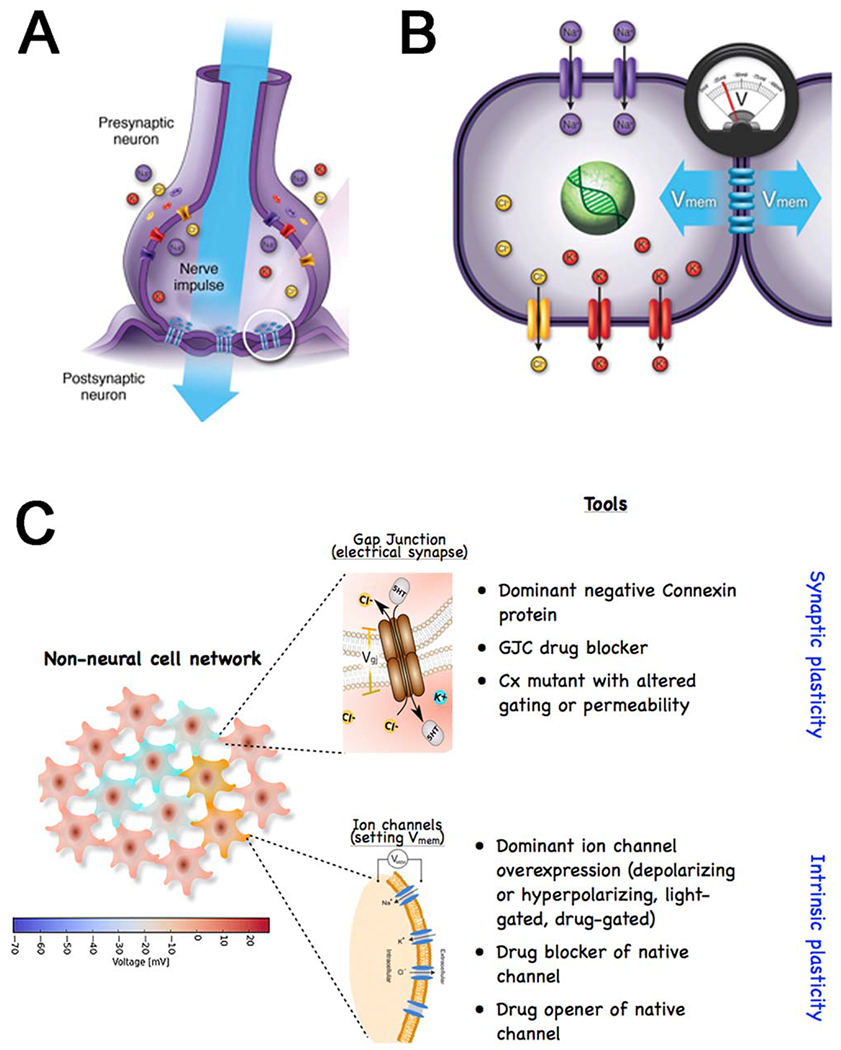
Gap junctions form bioelectric circuits in the brain and beyond. (A) Neurons are often coupled by gap junctions, which allows electrical activity to propagate and integrate across cells. (B) The same scheme, involving ion channels to set Vmem levels and gap junctions to communicate bioelectric state to neighboring cells, is present in most somatic tissues. (C) Non-neural cells assemble into GJ-coupled networks that have many of the properties of neural networks. Manipulating the function of somatic tissues during pattern formation, by modulating GJ activity, makes use of two basic approaches, using pharmacological or genetic techniques to target connectivity (GJ gating, akin to synaptic plasticity) or resting potential (ion channels, akin to intrinsic plasticity). Graphics courtesy of Alexis Pietak and Jeremy Guay.
GJ-mediated intercellular signaling functions in parallel with the better-understood secreted gradients of extracellular signaling molecules (Ben-Zvi et al., 2011; Rogers and Schier, 2011; Hironaka and Morishita, 2012). GJ channels are restricted to small molecules (unlike extracellular signaling, which is often mediated by sizeable proteins). However, GJ signaling networks are extremely versatile because they allow cells to control coupling at the transcriptional, translational, and physiological levels (Solan and Lampe, 2014). GJs can be gated by a range of regulatory inputs (phosphorylation, voltage, pH, etc.) (Peracchia, 2004) and depending on their specific connexin composition, can exercise considerable selectivity over permeant molecules (Goldberg et al.,2004). Because of this, GJ-coupled cell networks can set up very rich patterns of connectivity in vivo—potentially, with far greater complexity than is possible for diffusing chemicals. Thus, it is no surprise that now classical (Chuang-Tseng et al., 1982; Weir and Lo, 1982; Warner, 1985; Guthrie and Gilula, 1989; Lo, 1996) and recent data have implicated gap junctional communication (GJC) in a range of patterning events in both vertebrate and invertebrate model systems (Sutor and Hagerty, 2005; Elias and Kriegstein, 2008; Ahir and Pratten, 2014; Irion et al., 2014; Merrifield and Laird, 2015).
GJs are a powerful mechanism for coordinating long-range signaling during pattern regulation, which is only beginning to be understood. Cells often upregulate gap junctional communication when they need to share ions with their neighbors (Aslanidi et al., 1991; Larre et al., 2006). Thus, GJC is a perfect conduit for information flow during development, which depends on the ability of cells and tissues to communicate. The converse, however, is also paramount—embryos contain independent compartments (Rela and Szczupak, 2004; Sutor and Hagerty, 2005) that must remain isolated for proper morphology to result. Mutations in GJ genes are now known to be involved in a wide spectrum of patterning defects (Table 1); however, the true impact of GJC will be realized not only through ever finer-resolution analysis of the protein dynamics in single cells, but also through an appreciation of their contribution to network dynamics. Here, we review several non-neural contexts in which GJ-mediated signals instruct growth and form. We also present a novel perspective on this field, proposing that GJs may underlie information processing during somatic remodeling that is very similar to the role of these electrical synapses (Bennett, 1997) in implementing plasticity and memory in the brain. This hypothesis sheds new light on emerging data in this exciting field and suggests a research program focused on understanding the global dynamics of GJ-mediated physiological networks.
Table 1.
Channelopathies of GJ Genes and the Resulting Patterning Defects
| Gap Junction Protein | Morphogenetic Role or LOF Phenotype | Species | Reference |
|---|---|---|---|
| Innexins | Gonad and germline morphogenesis | C. elegans | (Starich et al., 2014) |
| Innexin1,2 | Epithelial patterning, foregut development | Drosophila | (Bauer et al., 2002) |
| Innexin 2 | Eye size | Drosophila | (Richard and Hoch, 2015) |
| Innexins | Foregut, cuticle (epithelial) patterning | Drosophila | (Bauer et al., 2004) |
| Connexin 43 | Oculodentodigital dysplasia (ODDD), heart defects (outflow tract and conotruncal), left-right asymmetry randomization, Osteoblast differentiation problems, craniofacial defects, myogenesis | Human, mouse, chick | (Britz-Cunningham et al., 1995; Reaume et al., 1995; Ewart et al., 1997; Becker et al., 1999; Levin and Mercola, 1999; Lecanda et al., 2000; Araya et al., 2003; Pizzuti et al., 2004; Debeer et al., 2005; Civitelli, 2008) |
| Connexin 37 | Lymphatic system patterning | Mouse | (Kanady et al., 2011., 2015) |
| Connexin 45 | Cardiac defects (cushion patterning) | Mouse | (Kumai et al., 2000; Nishii et al., 2001) |
| Connexin 50, Connexin 46 | eye patterning (differentiation and proliferation, especially lens), | Mouse | (White, 2002) |
| Connexin 26 | Cochlear development | Mouse | (Chang et al., 2014) |
| Connexin 41.8 | Pigmentation pattern | Zebrafish | (Watanabe et al., 2006) |
| Connexin 43 | Fin size and pattern regulation Craniofrontonasal syndrome | Zebrafish Mouse |
(Iovine et al., 2005; Davy et al., 2006; Hoptak-Solga et al., 2008; Sims et al., 2009) |
| Innexin 4, Innexin 2 | Germline differentiation and spermatogenesis | Drosophila | (Smendziuk et al., 2015) |
| Pannexin 3 | Skeletal development | Mouse | (Oh et al., 2015) |
GJS IN CELLULAR REGULATION
The first patterning tasks required of any animal is embryogenesis—the remarkable self-assembly of a complex body from the descendants of a single egg cell. This process necessarily involves partitioning the embryo into diverse components with different fates and functions.
Endogenous Expression and GJC-Dependent Domains
Embryos of frog, fruit fly, fish, and nematodes have long been known to possess compartments—discrete regions of GJ-coupled cells with distinct selectivity of permeable signals (Lo and Gilula, 1979; Weir and Lo, 1982, 1984; Blennerhassett and Caveney, 1984; Pitts et al., 1988; Bozhkova, 1998). For example, the wing imaginal disk in Drosophila is subdivided into a number of communication compartments during differentiation (Weir and Lo, 1984). Such developmental domains are cell groups with distinct physiological properties, which thus could underlie the diversification and patterning of different embryonic regions. Early mollusk development includes a number of specific GJC patterns that functionally determine cell determination (de Laat et al., 1980). Chick limbs exhibit a gradient of GJC along the AP axis (Coelho and Kosher, 1991). The highest GJC was observed in cells adjacent to the zone of polarizing activity, while no GJC was present at the opposite end of the limb bud. Mesenchymal tissues in the middle of the limb had an intermediate level of dye coupling and it has been hypothesized that polarizing region cells communicate to anterior mesenchyme cells via GJs (Allen et al., 1990).
The potential complexity of GJ-based regionalization is augmented by the fact that GJs composed of different connexin subunits provide a degree of functional compensation (Vink et al., 2004), but also confer the ability to sense different signals upon cells that express them (Elfgang et al., 1995). For example, homomeric Cx32 channels are permeable to both cAMP and cGMP, whereas heteromeric Cx32/Cx26 channels retain permeability to cAMP but prevent transfer of cGMP (Bevans et al., 1998). Indeed, substitution studies in tissues such as lens showed that distinct connexins play different roles in development, forming signaling conduits that are not interchangeable (White, 2002). In addition to molecular selectivity, GJs also enable rectification and unidirectional transfer (Robinson et al., 1993; Bruzzone and Giaume, 1999; Zhang et al., 2003; Fan et al., 2005; Palacios-Prado et al., 2014), enabling embryogenesis to take advantage of a very rich and dynamic set of signaling paths. For example, unidirectional junctions are thought to form between Cx32 and Cx43 (Robinson et al., 1993; Xin and Bloomfield, 1997), potentially allowing an embryo to establish one-way signaling paths.
GJC and Stem Cells
Pattern formation is the result of a tight spatiotemporal regulation of cell proliferation, differentiation, migration, and programmed cell death. Recent data have shown that GJ-mediated signaling is a powerful regulator of these properties, especially in stem cells (Oviedo and Levin, 2007; Wong et al., 2008). GJs are involved in regulating multipotency (Dyce et al., 2014), differentiation (Bani-Yaghoub et al., 1999; Zhang et al., 2002; Araya et al., 2003; Gu et al., 2003b; Hirschi et al., 2003; Araya et al., 2005; Li et al., 2015), self-renewal (Hitomi et al., 2015), proliferation (Paraguassu-Braga et al., 2003; Pearson et al., 2005; Starich et al., 2014), and motility (Huang et al., 1998a; Xu et al., 2001; Zahler et al., 2003; Marins et al., 2009; Kotini and Mayor, 2015). Neural stem cells exhibit a unique signature based on GJC and ion transporters, and GJC based on Cx43 and Cx45 is essential for their survival and proliferation (Cai et al., 2004). For example, the GJ protein Zero Population Growth (zpg) is required for germ cell differentiation in Drosophila ovary. In the absence of ZPG, the stem cell daughter destined to differentiate instead dies (Gilboa et al., 2003). Germ line stem cells differentiate upon losing contact with their niche, which likely involves GJC; as an example, it has been proposed that GJ-mediated cAMP signaling between blastomeres and somatic cells results in changes in somatic cell gene expression (Burnside and Collas, 2002). Many instances of GJC-dependent regulation is bidirectional, with several cell types exchanging instructive information, such as occurs in the communication between stem cells and their neighbors in spermatogenesis during germline differentiation (Smendziuk et al., 2015).
Pigmentation Control Networks
One of the exciting recent developments is the elegant merging of genetic evidence for GJC roles with a mathematical analysis of the resulting pattern formation that has taken place in the context of zebrafish pigment patterning. In 1953 Alan Turing wrote an article entitled “The chemical basis of morphogenesis.” In it, Turing describes a mathematical analysis of reaction-diffusion systems that could account for the formation of stable periodic patterns in organisms. His system consisted of two substances with differing diffusion rates. One of those unknown compounds was an activator that could enhance its own formation and that of the other unknown compound, the inhibitor. The inhibitor not only was capable of inhibiting the formation of the activator, but it also possessed the faster diffusion rate of the two (Turing, 1953). The model predicted self-organization of spatial patterns, which would change as the size of the organism changed, but the chemical identity of the morphogens was unknown (Schiffmann, 2005). Confirmation came in 1995 when Kondo and Asai found that pigmentation patterns on angelfish changed as the fish grew, and showed a traveling wave that behaved similar to the computer simulations of Turing’s reaction-diffusion system (Kondo and Asai, 1995). In order to elucidate the molecules that played the role of the activator and inhibitor, research began to focus on zebrafish pigmentation patterns due to the increasing amount of molecular tools available for experimentation in that model organism (Asai et al., 1999; Kondo, 2002; Iwashita et al., 2006; Watanabe et al., 2006; Yamaguchi et al., 2007; Watanabe and Kondo, 2014).
It was found that the zebrafish leopard mutation, which confers spots instead of the typical stripes, gives rise to distinct pigmentation changes that suggested a reaction-diffusion wave as the mechanism controlling pigment pattern (Asai et al., 1999). Interestingly, it was then discovered that the leopard mutation is in connexin41.8 (Watanabe et al., 2006), suggesting that in this case of pigment system morphogenesis, the relevant signaling molecules may be moving through GJs. The zebrafish mutant, jaguar, also showed changes in pigmentation pattern. This mutant had irregular spacing of its stripes that was caused by a mutation in the gene for the inward rectifying potassium channel 7.1 (Kir7.1) (Iwashita et al., 2006). Since Kir7.1 is responsible for maintaining transmembrane voltage potential and is required in the black pigment cells (melanophores), experiments were done to determine how membrane potential may be involved in pigment patterning changes. These experiments found that melanophore membrane potential was more depolarized in the jaguar mutants and that this interfered with the transient depolarization signal conferred by contact with xanothopores, the yellow pigment cells. Loss of this transient signal resulted in melanophores that stayed in close contact with xanothopores, rather than moving away from each other as was the case in the wildtype (Inaba et al., 2012).
Further research by Kondo et al. showed that interactions between the two main pigment cells, xanothophores and melanophores, also involved Delta/Notch signals. These signals were activated in the cells by long processes that connect the different chromatophores. The xanothophore processes is short and presents the Delta ligand that binds to the Notch receptor on the membrane of the melanophore and promotes its survival. If the melanophore is near a xanothophore then it can bind to the Delta ligand and survive. This relationship would satisfy the requirement for an activator with a short range. In addition, the melanophores have a long process that reach out to xanothophores, but after a certain distance they can no longer reach, resulting in long-range inactivation (Hamada et al., 2014; Watanabe and Kondo, 2014). However, it is possible that Delta/Notch works in conjunction with a molecule that still depends on diffusion through GJs (Hamada et al., 2014). Indeed, recent work on mutated Connexins 41.8 and 39.4 show that heterotypic GJs do play a part in zebrafish pigmentation patterning. These experiments also show that a third class of chromatophore called an iridophore is involved in patterning on the trunk of the animal but not on the tail, and that this relationship is possibly mediated by molecules that are diffused by GJs (Bullara and Decker, 2015; Irion et al., 2014; Kondo, 2002; Takagi and Kaneko, 2005). Together, these studies form an elegant body of work on the relationship of voltage and gap junctional signaling in pattern regulation.
The theoretical discussion as to the identity of these diffusible molecules has suggested the GJC-permeable molecules cAMP and ATP may be the relevant Turing couple. Whereby cAMP is the short range activator and ATP a long range inhibitor (Schiffmann, 1991). However, the small molecule serotonin may also play a role in the reaction-diffusion model of zebrafish coloring pattern formation, as it does in other examples of GJC-regulated patterning (Blackiston et al., 2015; Fukumoto et al., 2005b; Gairhe et al., 2012). Recently it was found that the zebrafish leopard mutation was correlated with behavioral abnormalities that pointed to lowered levels of serotonin transport and that treatments with serotonergic drugs resulted in altered pigmentation patterns (Maximino et al., 2013; Stewart et al., 2013).
GJC and Nerve Sprouting Control
In addition to development, GJs are also important for physiological maintenance (Maeda and Tsukihara, 2011), regeneration (Umino and Saito, 2002; Hoptak-Solga et al., 2008), and remodeling. A key cell type involved in these processes is the neuron. Axonal processes must interact with surrounding tissues and make decisions about direction and magnitude of sprouting, in order to correctly pattern and maintain innervation to target organs. The signaling underlying this, and the involvement of GJs, was recently revealed by using a transplantation assay in which eye primordial from a fluorescently labeled donor embryo were transplanted onto the flank of a host (Vandenberg et al., 2014). Normally, the ectopic eye produced one major nerve bundle, which extended to the host’s spinal cord. However, when the host cells were depolarized, the eye instead generated a huge overproliferation of nerve which spread throughout the tissue. Testing a number of transduction mechanisms for this effect on nerve growth, the authors showed that inhibiting GJC in the host abolished the ability of depolarization to cause hyperinnervation from the implanted organ. The authors showed that the effect only applied to ectopic innervation (not the host’s native nerve pattern), and that serotonergic signaling was both required for the hyperinnervation and could rescue the blockade of GJC. A model was advanced in which ectopic nerve, searching for guidance cues, was making proliferation and extension decisions based on serotonin molecules arriving via GJs. As will be seen in the discussion of left-right patterning below, the movement of serotonin via GJs, under an electrophoretic force, is a conserved theme among a number of patterning systems.
GJs in Cancer
Another example in which GJs play an important role is the occasional defection of cells away from the normal anatomical plan toward carcinogenesis (Omori et al., 1998; Mesnil et al., 2005; Trosko, 2005) and subsequent metastasis (Defamie et al., 2014). In a sense, cancer is the derangement of developmental patterning—a disease of geometry (Rubin, 1985; Maffini et al., 2005; Soto and Sonnenschein, 2011; Tarin, 2012; Chernet and Levin, 2013a). Cancer cells abandon correct morphogenesis in favor of unrestrained growth reminiscent of unicellular organisms prior to the appearance of multicellularity and to the GJ-mediated coupling of cells to other cells within a larger somatic context (Loch-Caruso and Trosko, 1985). Reduction of GJ-dependent communication with other somatic tissues is an important early step in the process in which cells begin to treat the rest of the body as an “external environment” within which they must survive by any means necessary.
Studies have long noticed that lowered GJC was associated with induction of tumorigenesis (Potter, 1980; Yamasaki et al., 1984; Loch-Caruso and Trosko, 1985); normal tissue generally possesses a much higher degree of GJC than tumor tissue, and a loss of GJC accompanies early steps in neoplastic transformation (Pitts et al., 1988). A neoplastic phenotype can be induced in cell culture by ectopic closing of GJs using pharmacological agents or dominant-negative constructs (Omori and Yamasaki, 1998). Most interestingly, neoplastic characteristics can be suppressed by ectopic induction of GJC in tumor tissue (Mehta et al., 1991; Rose et al., 1993; Hellmann et al., 1999). Connexin32-deficient mice have a 25-fold increased incidence of spontaneous liver tumors (Temme et al., 1997), and Connexin32 is also an anti-invasive agent in renal cell carcinoma (Yano et al., 2006). Thus, gap junctional isolation is known to be a tumor-promoting agent (Loewenstein and Kanno, 1966; Loewenstein, 1969, 1979, 1980; Rose et al., 1993; Yamasaki et al., 1995; Leithe et al., 2006; Mesnil et al., 2005). This is consistent with a view of GJs as mediating morphogenetic cues that could be keeping cells under differentiation and growth limitation consistent with adult morphostasis. It should be noted, however, that a few studies have indicated the opposite (Stoletov et al., 2013), for example the finding that Connexins 26 and 43 mediate metastasis in melanoma and breast cancer (Stoletov et al., 2013), and a role for GJs in promoting metastasis by transfer of cGAMP (Chen et al., 2016).
The fact that some connexins are pro-, and others anti-oncogenic, or even can exert both types of effects in different contexts (El-Saghir et al., 2011; Sin et al., 2012), is less puzzling if GJs are considered not as typical proteins that induce or suppress a particular phenotype, but rather as conduits for cell–cell communication. The outcome is expected to be a function of what signals arrive via the GJs (Mendoza-Naranjo et al., 2007; Lim et al., 2011), and are thus hard to predict from knowledge of the connexin type, because it’s partially dependent on the neighboring cells, and physiological parameters that dictate the open/closed state of the GJs and what permeant molecule might be traversing it. This has implications for therapies targeting GJs in cancer and other diseases (Kandouz and Batist, 2010; Grek et al., 2014; Cogliati et al., 2016), because it shifts the focus onto the signaling molecules and their transfer profiles, in addition to targeting connexin gene products themselves.
In Xenopus, cells expressing KRAS mutations make tumor structures; the incidence of tumors can be significantly reduced, despite the strong presence of the oncogenic mutation, by hyperpolarizing cells in vivo by misexpression of hyperpolarizing ion channels (Chernet and Levin, 2013b). Remarkably, however, this also works if cells at the opposite end of the animal are targeted (Chernet and Levin, 2014). How can such long-range communication of physiological state occur? Functional experiments implicated the movement of serotonin and butyrate in bioelectric control of tumorigenesis (Blackiston et al., 2011; Lobikin et al., 2012; Chernet and Levin, 2014), and a recent study looked at the role of GJs in this process. Chernet et al. showed that loss of GJC taking place within nascent tumor sites, within remote host tissues, or between the host and the prospective tumor region significantly lowered the incidence of tumorigenesis, with the most pronounced suppression taking place when GJC inhibition occurred far from the oncogene-expressing cells (Chernet et al., 2015). In contrast, overexpression of wild-type Connexin26 increased tumor incidence. The authors presented a mechanistic model, based on an oscillating signal that propagates through GJs and controls proliferation, that quantitatively explained these puzzling data (and made an unexpected, and subsequently validated, prediction about the role of the left–right axis in this process).
From Single Cell Properties to Multiscale Pattern
Active GJ communication allows cells to make sophisticated decisions comparing relative levels of specific compounds between themselves and their neighbors (Moreno, 2008), and to integrate information across anatomical distances. Thus, they underlie the transmission of physiological patterning signals (Levin and Mercola, 1998b; Levin and Mercola, 1999; Warner, 1999; Levin and Mercola, 2000; Lecanda et al., 2000; Pizard et al., 2005; Levin, 2007; Jin et al., 2008; Schiffmann, 2008; Dobrowolski et al., 2009; Oviedo et al., 2010). Cells utilize GJs to communicate directly with their neighbors, or with more distant cells via tunneling nanotubes—extended cell processes with GJs at their end (Wang et al., 2010; Wittig et al., 2012; Antanaviciute et al., 2014; Sherer, 2013; Rimkute et al., 2016).
Some of the earliest data implicating GJs in development came from studies with functional antibodies. Introduction of antibodies raised to specific portions of connexin proteins in mouse embryos resulted in developmental defects (Becker et al., 1995). In Xenopus, microinjection of antibodies has been reported to disrupt axial patterning (Warner et al., 1984); the treated embryos contained differentiated mesodermal derivatives such as notochord and muscle tissue and Warner et al. argued that GJC has a role in pattern formation per se, rather than in the induction of specific cell types.
In invertebrate systems, Caenorhabditis elegans and Drosophila have been paramount in revealing GJC roles via genetic approaches. Numerous defects were observed in a C. elegans innexin-3 mutant, including rupture of hypodermis, failure of elongation, and failure of pharynx to attach to anterior (Starich et al., 2003). Likewise, epithelial organization and polarity of the embryonic epidermis is dependent on heteromerization of innexins 2 and 3 in Drosophila (Bauer et al., 2004; Lehmann et al., 2006). Establishment of the proventriculus requires Innexin-2, which is a target of Wingless signaling (Bauer et al., 2001, 2002). This work is particularly interesting because it links GJC to a canonical signaling pathway. Wnt signaling is likewise upstream of GJC patterns in vertebrates (Olson et al., 1991; Olson and Moon, 1992; van der Heyden et al., 1998; Ai et al., 2000), suggesting possible conservation of signaling modules involving GJC. Interestingly, overexpression of Cx43 provides a partial recovery of the formation of the cerebellum in Wnt-1 knock-out mice (Melloy et al., 2005), strengthening the link to the Wnt pathway and suggesting that establishing ectopic domains of cell:cell communication may be a useful modality to control certain patterning defects. Gene targeting studies of connexins in mice have provided evidence that GJs are important for cardiac septation (Kirchhoff et al., 2000) and morphogenesis of the outflow tract (Gu et al., 2003a) and endocardial cushions (Kumai et al., 2000). Many of these effects appear mediated by effects on the behavior of neural crest (Ewart et al., 1997; Lo et al., 1997; Huang et al., 1998a,b; Sullivan et al., 1998; Lo et al., 1999; Waldo et al., 1999; Waller et al., 2000; Xu et al., 2001; Li et al., 2002).
One major contribution of GJs to large-scale patterning is by sculpting signaling via developmental bioelectricity—spatial gradients of distinct resting potential across tissues which instructs pattern regulation in embryogenesis and regeneration (Bates, 2015; Levin and Stevenson, 2012; Levin, 2012a,a,b). GJs both determine cellular voltage (by allowing current from nearby cells) and in turn are regulated by resting potential (Peracchia, 2004). They, thus, implement physiological feedback loops with complex behavior, akin and parallel to the bidirectional coupling between genes and the transcription factors they encode. These regulatory layers function in parallel (Fig. 2), each supporting unique dynamics that instruct pattern regulation and physiology. The brain and non-neural tissues both exploit GJ networks for processing information; indeed, there are remarkable parallels between the way bioelectric signaling, gene regulatory networks, and signals from the environment integrate within and outside the CNS, to generate instructive cues (Fig. 3). We next discuss a number of specific recent examples.
Figure 2.
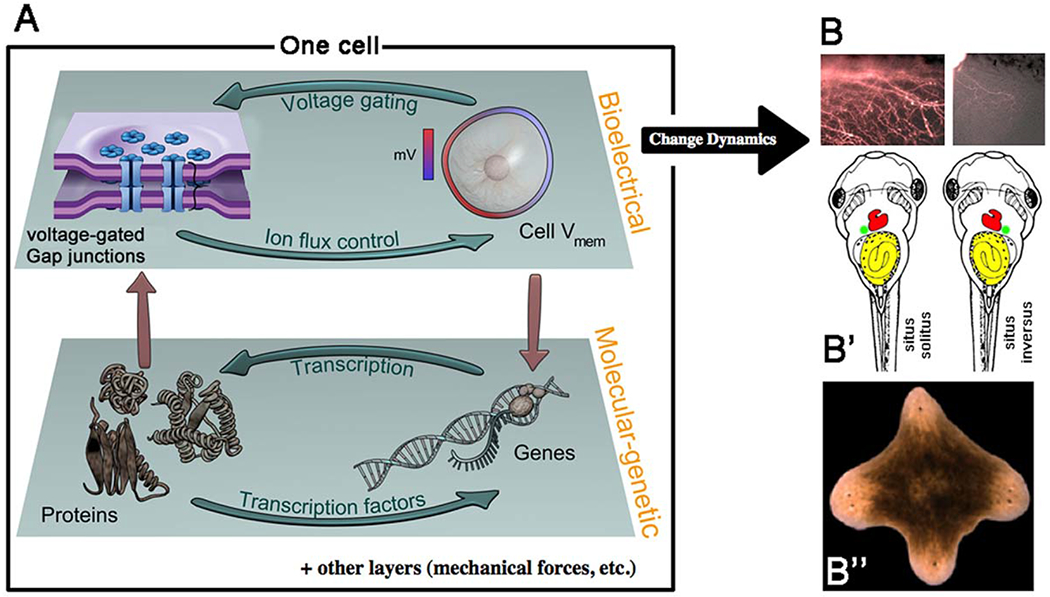
Information in physiological circuits instructs pattern formation. Gap junctions are key regulators of bioelectric cell state. (A) Like gene regulatory networks, which contain numerous feedback loops among gene loci, gap junctions, and ion channels both regulate and are regulated by resting potential. This establishes an autonomous layer of physiological dynamics that is coupled to transcriptional cascades, but has its own unique information and functions. Modulating bioelectric dynamics by induced changes of GJC results in large-scale alterations of pattern formation, including hyperinnervation (B), left-right organ inversions (B′), and multiple head formation in regenerating planaria (B″). Graphics courtesy of Alexis Pietak and Jeremy Guay.
Figure 3.
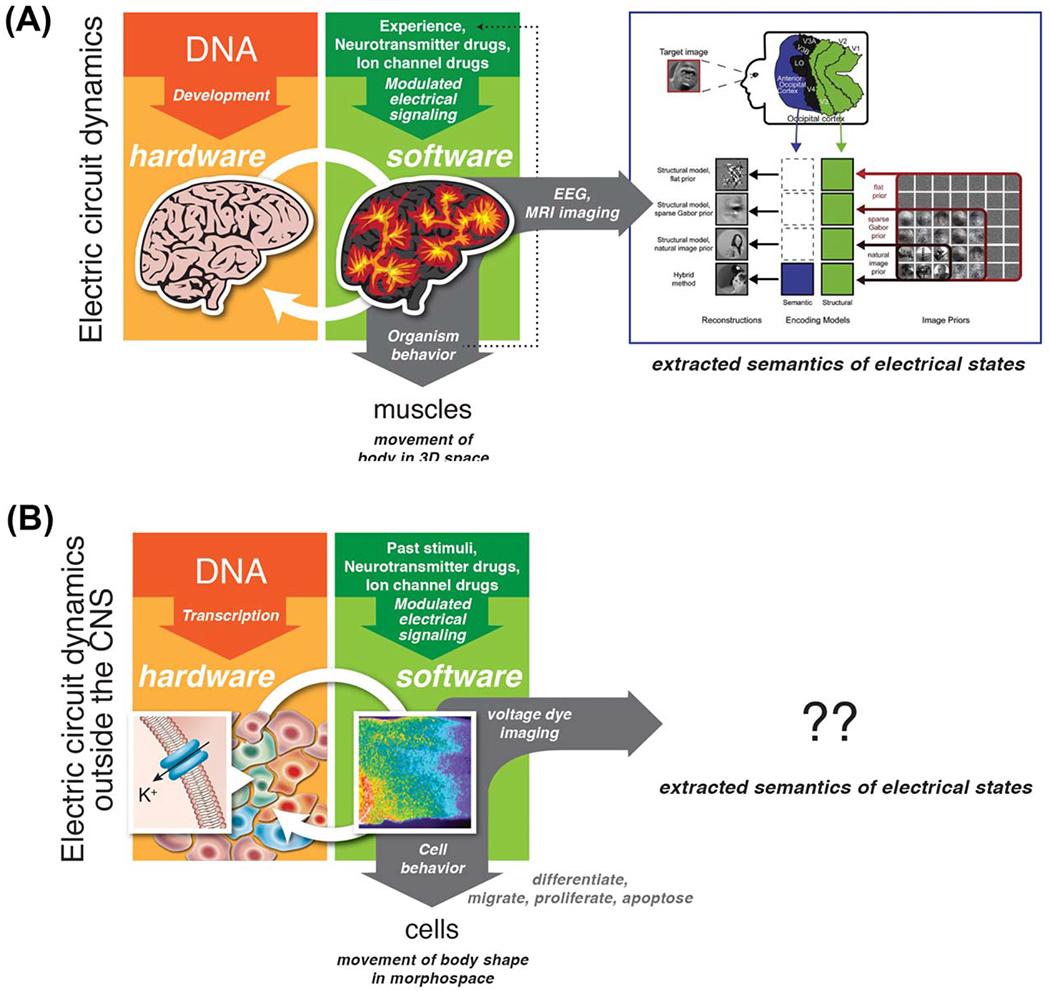
Parallelism between brain and body. (A) In the brain, DNA sets the structure of the central nervous system—the hardware. Electrical circuit dynamics process information and store memories, resulting in experience-dependent (and self-organizing) patterns that control muscles resulting in the movement of the animal in three-dimensional space. Experiences (external sensory stimuli) and reagents targeting GJs, ion channels, and neurotransmitters alter the electric dynamics. Computational pipelines are beginning to be developed and applied to dynamics observed via EEG and MRI imaging, to extract the semantics—the memories represented by these bioelectric states. (B) In somatic tissues of developing or regenerating organisms, DNA sets the complement of connexins, ion channels, and neurotransmitter machinery in all cells—the hardware. Bioelectric dynamics (slow patterns of resting potential within tissues) process information and establish prepatterns for gene expression and morphogenesis, resulting in chemical signal-dependent (and self-organizing) patterns that control cell functions like migration and differentiation, resulting in anatomical changes that move the body through morphospace. Stimuli (chemical and other signals) and reagents targeting GJs, ion channels, and neurotransmitters alter the electric dynamics. Computational pipelines need to be developed and applied to these dynamics as imaged with voltage-sensitive dyes and GJ tracers, to extract the semantics—the instructive anatomical patterns represented by these bioelectric states. Graphics by Jeremy Guay of Peregrine Creative and Alexis Pietak. Neural decoding panel in (A) is reproduced with permission from Naselaris et al., 2009. Voltage pattern panel in (B right) is courtesy of Douglas J. Blackiston.
GAP JUNCTIONAL COMMUNICATION IN GROWTH AND PATTERNING OF THE ZEBRAFISH FIN
Zebrafish fin patterning is an excellent model of joint formation due to the ability of the fin to regenerate once amputated, and the continuation of fin growth in adult animals. The caudal fin is made up of 16–18 fin rays, which are made up of multiple bone segments separated by multiple joints (Sims et al., 2009). GJs have been shown to regulate the morphology of bone segments and joints in the zebrafish fins. The fin length mutant short fin (sof) was found to be caused by mutations in the expression of Connexin43 (Cx43) protein (Iovine et al., 2005). Analysis showed that reduced GJC caused by lower expression of Cx43 or missense alleles reduces the total number of dividing cells that form the fin ray segments, resulting in shorter segments. However, Cx43 could also be allowing the diffusion of signaling molecules from proximal regions to distal regions acting as a biological ruler that could inform the formation of new segments that extend the length of the fin (Hoptak-Solga et al., 2007). The new bone segments in the fin are dependent on new joint formation that occurs in a proximal to distal manner. Cx43 was found to take part in joint formation by first determining the location of the future joint in the mesenchymal compartment. Later, the Cx43 proteins polarize themselves toward the medial surface of the newly differentiating cells that will form the new joint. Lowered levels of Cx43 resulted in premature joint formation and small fins, while increased expression resulted in joint failure and large fins (Hoptak-Solga et al., 2007).
Recently, microarray investigation into the mechanism by which Cx43 exerts its effect on fin size found that expression of the gene encoding the signaling molecule Semaphorin3d (Sema3d) was perturbed in Cx43 mutants. Semaphorins are involved in the patterning of a variety of tissues and organs. The semaphorin receptors Neruopilin2a (Nrp2a) and PlexinA3 (PlxnA3), were found to be involved in the changes in cell proliferation and joint forming phenotypes associated with Cx43 expression in fin formation, respectively (Ton and Iovine, 2012). Cx43 involvement in suppressing joint formation was also found to influence the timing of expression of the homeobox gene that encodes the transcription factor Even Skipped 1 (Evx1). Overexpression of Cx43 resulted in loss of evx-1 and decreased expression resulted in evx-1 expression that was more distal than wildtype and led to the premature expression of the genes involved in joint formation (Ton and Iovine, 2013).
The other gene identified in the microarray investigation into downstream effects of changes in Cx43 expression, was hyaluronan and “proteoglycan link protein 1a” (hapln1a). Hapln1a is a protein that links the carbohydrate polymer, hyaluronic acid (HA) with proteoglycans in the extracellular matrix (ECM). This link stabilizes HA creating the right ECM environment that works in parallel with the Sema3d pathway for correct fin formation (Govindan and Iovine, 2014). Further studies on Cx43 expression in zebrafish and how it affects fin formation have found that the gene called “establishment of cohesion1 homolog 2” (esco2) is responsible for regulating Cx43 levels. ESCO2 is an acetyltransferase that modifies cohesion proteins that are responsible for joining sister chromatids during mitosis. Mutations in esco2 cause disruptions in mitosis that increase cell apoptosis and decrease cell proliferation. Together, these data reveal elements both upstream and downstream of connexin function. However, the perturbation in mitosis caused by the mutant form of esco2 in humans could not completely explain the phenotype seen in the resulting syndrome. This syndrome, called Robert’s syndrome is characterized by craniofacial abnormalities and limb malformation that could be a more severe form of oculodentodigital dysplasia (OCDD) that arises from mutations in the human Cx43 (Banerji et al., 2016).
GJS AND LEFT-RIGHT PATTERNING
Establishing Vertebrate Laterality
All vertebrates, and many invertebrates, exhibit a fundamental asymmetry of the bilateral bodyplan, with some internal organs (the heart, viscera, and brain) consistently biased to one side of the midline (Speder et al., 2007; Vandenberg and Levin, 2009). Early discoveries of left- and right-specific transcriptional signaling cascades had suggested that each side develops independently (Levin, 1998; Ramsdell and Yost, 1998). However, surgical experiments in the chick showed that in the absence of the right side, the left-sided (and not directly manipulated) tissue became confused, sometimes failing to turn on left-sided markers such as Sonic hedgehog and Nodal (Levin and Mercola, 1999). Seeking a mechanistic understanding of why distant tissue is required for laterality decisions, gap junctional communication was tested as a mechanism to coordinate sidedness decisions across the whole early embryo in frog and chick models (Levin and Mercola, 1998b). The data showed that either universal blockade of GJs with dominant negative connexins, or universal expression of constitutively-open connexins, randomized both the expression of laterality marker genes and the sidedness of the internal organs. Targeted misexpression experiments in the frog showed that normal asymmetry requires open GJC across the embryos’ dorsal side, while lack of GJC is required across the ventral midline. These endogenous differences in GJC had been shown to be set up by Wnt pathway signaling as part of dorso-ventral axis determination (Olson et al., 1991; Olson and Moon, 1992).
A model was proposed, in which signals traversed the blastoderm in a chiral (left to right) manner, propagating through GJs and accumulating on one side of a midline zone of junctional isolation (Levin and Nascone, 1997; Levin and Mercola, 1998a). This scheme was suggested by the molecular functional data, although LR asymmetric GJ transfer was actually present in earlier data examining connectivity among frog blastomeres (Turin and Warner, 1980; Guthrie, 1984; Guthrie et al., 1988; Nagajski et al., 1989). Subsequent work identified the neurotransmitter serotonin (Fukumoto et al., 2005a,b; Adams et al., 2006; Vandenberg et al., 2012) as one left-right morphogen that moves through GJs (long before the nervous system appears) to control downstream gene expression via HDAC1-dependent chromatin modification (Carneiro et al., 2011) and several proton pumps and potassium channels that provide the electrophoretic force for unidirectional accumulation of charged morphogens (Levin et al., 2002; Adams et al., 2006; Aw et al., 2008; Morokuma et al., 2008; Aw et al., 2010). These mechanisms and the role of GJs in left-right axial patterning appear to be implemented very similarly in chick and frog, despite quite different early embryonic architectures. In chick, the relevant connexin appears to be Cx43, which is also important for laterality in zebrafish, where it is required for morphogenesis of the Kupffer’s Vesicle (Hatler et al., 2009). Together, these data have also led to quantitative models of the distribution of serotonin through long-range GJ paths, and a study of the main system-level behaviors of this electrophoretic system (Esser et al., 2006; Zhang and Levin, 2009). A number of open questions remain, including possible roles of unidirectional transfer (by distinct connexins expressed in neighboring cells).
Innexin-Based GJs also Pattern the Nematode LR Axis
Interestingly, despite a completely different bodyplan and long evolutionary distance, GJs are also involved in LR patterning in the nematode C. elegans. An elegant set of studies (Chuang et al., 2007) focused on neural lateralization showed that establishing left-right asymmetry in C. elegans olfactory neurons involves Ca++ signaling, tight junctions, and communication through GJs. The central nervous system of C. elegans contains two bilaterally symmetrical odor sensory neurons (AWC). During late embryogenesis only one of these neurons will express the str-2 G-protein coupled olfactory receptor. The determination of which neuron is going to express str-2 (AWCON) is stochastic, and the remaining neuron acquires the AWCOFF phenotype. Chuang et al. identified nsy-5, a gene encoding a member of the innexin/pannexin GJ family of proteins as nsy-5 mutants are unable to sense odorants normally processed by AWCON neurons. Reduction of nsy-5 function resulted in both neurons having an AWCOFF phenotype. NSY-5 can form functional hemichannels and provide electrical coupling between cells. Genetic approaches showed that nsy-5 has specific site of actions within different neuronal cell bodies. Strikingly, nsy-5 can act autonomously to induce the future AWCON neuron based on a feedback mechanism between both AWC neurons. Subsequent work showed that calcium levels in non-AWC sensory neurons, determined the AWCON bias, demonstrating that a network of neurons communicate with AWC via signaling dependent on nsy-5 to determine asymmetric expression of the str-2 gene. It was also found that the modulation and propagation of the calcium signals was not mediated by serotonin or inositol triphosphate (IP3) (Schumacher et al., 2012). It is important to note that NSY-5 mediated connectivity also works in parallel with the NSY-4 claudin, which is related to the gamma transmembrane protein subunit of voltage-activated calcium channels and genetically down-regulates calcium channels which inhibits the calcium signaling pathway in the AWCON cell (VanHoven et al., 2006; Chuang et al., 2007). Inhibition of the calcium signaling pathway, calcium-calmodulin-dependent kinase II (CaMKII)-MAP kinase, is also achieved by an unknown nsy-5 mechanism and also by the mir–71 miRNA that is stabilized by nsy-4 and nsy-5 (Alqadah et al., 2013). Once AWC asymmetry is achieved by nsy-5 and nsy-4, maintenance of that asymmetry depends on olfactory signaling, transcriptional regulation including the zinc finger transcription factor die-1, and TGF-β signaling (Cochella et al., 2014; Hsieh et al., 2014).
The data revealed some striking similarities between how GJs are used in invertebrates to their functions in vertebrate development (Levin, 2002). In chick and frog embryos, the left and right sides of the embryos communicate with each other to correctly assign left-right identity before the onset of asymmetric gene expression. In both Xenopus and C. elegans, both over- and under-expression of GJ proteins lead to defects in laterality. The native pattern of communication mediated by GJs in both vertebrates and nematodes is between the Left and Right sides and involves a zone of junctional isolation (in this case, provided by an extracellular matrix layer). Moreover, the experiments by Chuang et al. revealed an asymmetry in how the left and right AWCs respond to nsy-5 expression, mirroring the differences in gap-junctional permeability that has been described on the left and right sides of the early frog embryo (Guthrie et al., 1988). Crucially, the discovery of this intrinsic bias in C. elegans shows that as in frog and chick, the communication via GJs does not initiate left–right information but functions as an intermediate step of the pathway.
GAP JUNCTION-MEDIATED SIGNALING IN HEAD PATTERNING
Long-Range Control of Brain Formation
The developing brain must coordinate its growth and morphogenesis. In Xenopus embryos, it was recently shown that brain size and patterning is in part regulated by endogenous gradients of transmembrane resting potential (Pai et al., 2015a). Experimentally modulating resting potential (by expressing hyperpolarizing or depolarizing channels) in neural cells, or even in distant (ventral) cells, could control cell proliferation and overall patterning in the nascent brain. This effect was mediated by gap junctional communication: blocking GJC either genetically or pharmacologically rescued hyperpolarization-triggered brain malformations, and prevented ventral hyperpolarization from inducing proliferative effects in the brain. The authors suggest that GJC is involved in coordinating growth across long distances in the embryo, allowing the developing brain to match its proliferative profile to the rest of the body.
Moreover, induction of specific voltage gradients via ion channel misexpression was shown to induce ectopic brain tissue in posterior regions (Pai et al., 2015a). Interestingly, brain markers are also induced in neighbors of cells that misexpress the brain-inducing channels. However, with time, these neighboring cells turn off the ectopic brain markers, leaving only the cells whose Vmem is continuously set to a brain-specific pattern to be able maintain brain fate. The authors speculate that the brain-inducing bioelectric state is spread non-cell-autonomously via GJs, but with progressive restriction of GJ communication as development proceeds and individual tissue compartments refine, cells that do not drive abnormal resting potentials with their own channels go back to their normal fates. This normalization also works in the opposite direction, often enabling neighbors to suppress aberrant functions in cells with unique resting potentials that can otherwise induce foci of ectopic eyes (Pai et al., 2012) or tumors (Chernet and Levin, 2013b).
Species-Specific Head Morphology During Planarian Regeneration
Planarian flatworms are complex creatures, with a true brain, bilateral symmetry, a complex behavioral repertoire, and many body organs (Sarnat and Netsky, 1985; Gentile et al., 2011). They also have the remarkable ability to regenerate completely from partial body fragments (Reddien and Sanchez Alvarado, 2004; Salo et al., 2009; Lobo et al., 2012). Their capacity for self-repair serves as a paradigm case of dynamic morphostasis and continuous remodeling toward a specific target morphology. While many details of molecular pathways required for correct stem cell differentiation and regenerative capacity are becoming clear (Handberg-Thorsager et al., 2008; Aboobaker, 2011), the mechanisms that determine the correct shape of the head, and cease growth and remodeling when that shape is achieved, are almost completely unknown (Lobo et al., 2012, 2014). Examining physiological inputs into this process, we recently asked two questions: (1) could modification of overall bioelectric network connectivity give rise to coherent patterning changes during regeneration, and (2) is it possible to obtain evolutionarily relevant patterns. The results suggested that shifting among different regions of planarian morphospace (Stone, 1997) is possible by physiological perturbation alone (Emmons-Bell et al., 2015).
When GJC was systemically disrupted in fragments of Girardia dorotocephala (GD), it induced the expected finer-grain Vmem regionalization among the endogenous bioelectric network (since GJC can normally act to establish isopotential cell fields). Remarkably, the resulting worms regenerated heads with an altered shape morphology that quantitatively resembles that of multiple other flatworm species (Emmons-Bell et al., 2015). This resemblance was more than skin deep: not only was the external shape of the head converted to the shapes of distant planarian relatives, but they also manifested different species-specific brain morphologies and stem cell distributions. The exact same treatment of a cohort of GD worms produced four types of worm heads in characteristic frequencies (proportional to their evolutionary distance from GD). It is not yet known whether this stochastic property is a consequence of the still relatively crude method of network topology perturbation (soaking in GJ blocker such as octanol), or whether it is an intrinsic aspect of the dynamics of this system. The ability to induce a different species’ head shape in a genetically wild-type worm suggests that the bioelectric network is a profound regulator of species-specific morphology. It remains to be seen whether changes in the dynamics of bioelectrical circuits have been widely exploited by evolution to explore variations of anatomical structure.
How to infer the large-scale outcomes (which kind of head, how many heads, etc.) from cell-level properties and signals? This question has been addressed for gene regulatory networks using dynamical state spaces built to describe transcriptional circuits have been used to map complex system behavior (Huang, 2011; Huang et al., 2009; Halley et al., 2012). More directly relevant to physiological networks, similar approaches have been used to understand global behaviors of electrical activity in neural networks during decision-making. Importantly, however, in planarian regeneration, as in the brain, circuit dynamics are not directly revealed from ion channel expression data but are complex and nonlinear. Such dynamics must be modeled quantitatively to understand their emergent properties (Cervera et al., 2015; Law and Levin, 2015). One possibility is that different anatomical outcomes correspond to specific attractors in the dynamical state space of the bioelectric network formed by the planarian body; in this paradigm, bioelectric perturbations can shift the system from a default (genome-specified) attractor to another nearby one (Fig. 4). We are currently working to computationally model this process, to quantitatively map stable attractors to underlying physiological details, and thus gain more control over the resulting shapes.
Figure 4.
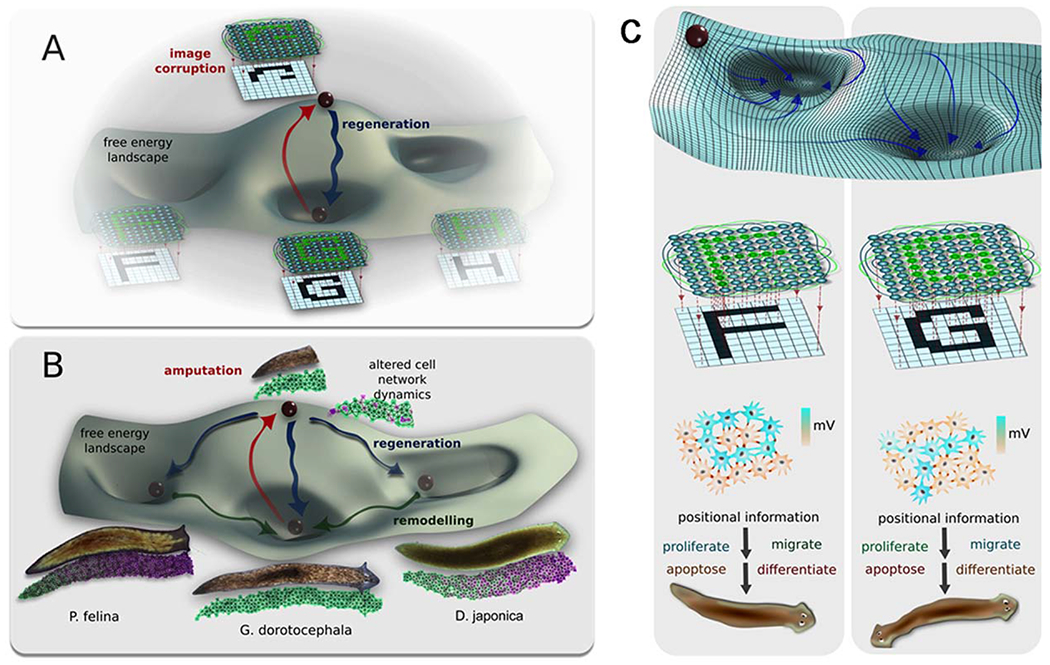
Morphogenetic memories visualized as attractors in GJ network state space. GJ dynamics may offer an opportunity to understand information processing, not only molecular biophysics, but also of pattern formation regulated by physiological cell–cell signaling. One way to visualize planarian regeneration is (A) as the function of a large network of electrically coupled cells. Some such networks have been shown (in computational neuroscience and artificial intelligence research) to have a planaria-like property of holographic memory storage: a trained network can recreate the entire pattern despite deletions of the pattern or of the network components. A well-accepted mathematical paradigm for understanding the global properties of such networks is as an energy landscape, with attractors corresponding to specific stable modes of the network. In our analogy, amputation raises the energy of the system, temporarily pulling it out of the attractor to which the system tends to return. One hypothesis is that these networks are responsible for storing the pattern of a normal planarian, and when damaged, issuing cell-level commands (differentiate, proliferate, and other instructions) that restore the anatomy (in parallel to how recall of complex geometric memories can be triggered by stimuli and induce goal-directed behavior in cognitive science studies of animal behavior). This hypothesis makes a prediction: that coherent changes in patterning will result from experimentally induced changes of the bioelectric network’s topology or dynamics. (B) It has been shown (Emmons-Bell et al., 2015) that altering the bioelectric connectivity in G. dorotocephala results in regeneration of one of four discrete head types. On this view, amputation of head and tail causes the system to move to an unable state from its basin of attraction. Partial interruption of gap-junction communication between cells (reduced connectivity and thus altered dynamics of the network) induces head wounds to regenerate (yellow arrows) new heads that resemble closely-related flatworm species that are regions of stability in the regeneration morphospace landscape (left to right: Schmidtea mediterranea, Dugesia japonica, and Philbertia felina) as well as heads of the original species in the center basin. The probability of regenerating a certain head shape is proportional to the evolutionary distance from Girardia dorotocephala. These states are non-permanent (shallow basins of attraction) and over time will remodel into their final morphological state (white dashed arrows) to the deepest and most stable basin of attraction of the original head shape. (C) The same process can be modeled as a neural-like network, with stable modes visualized as stable attractors (a.k.a. memories in neural nets), which lead to specific instructive signals regulating proliferation, migration, and differentiation that induces different but coherent patterning outcomes. Graphics by Alexis Pietak.
GAP JUNCTIONAL SIGNALING UNDERLIES ANATOMICAL PATTERN MEMORY
In order for a bisected worm to regenerate as two normal worms, the cells at the posterior-facing edge of the wound must make a tail, while those at the anterior-facing edge must make a head. This simple fact reveals that the adult stem cells which implement regeneration in each blastema are not locally controlled: those cells were direct neighbors until the scalpel separated them, and thus have identical positional information, and yet they form completely different structures. Local position is not sufficient for blastema cells to know what shape to build; rather, they must communicate with the remaining tissue to decide what anatomical structures must be formed. Searching for the mechanism of this long-range coordination, (Nogi and Levin, 2005) found that inhibition of GJC in the species Dugesia japonica for just 2 days resulted in subsequent regeneration of worm fragments forming heads at both ends (Nogi and Levin, 2005; Oviedo et al., 2010). These bipolar forms were viable, and could also be phenocopied by RNAi targeting 3 Innexins. By itself, this supported a role for GJ communication in anterior-posterior patterning in this species of flatworm. However, the real surprise came when the 2-headed worms were analyzed long after the GJ blocker (octanol) was gone (as shown by HPLC).
Weeks later, when these 2-headed animals had their heads and tails amputated again (in plain water, with no further perturbation), the same 2-headed phenotype resulted (Fig. 5), and this was repeated upon subsequent rounds of amputations (Oviedo et al., 2010). These data showed that a transient perturbation of physiological cell:cell communication via GJs could stably change the pattern to which the animal regenerates upon damage—its target morphology. This represents a clear example of physiological change permanently rewriting major anatomical features, despite a normal genome—a novel aspect of epigenetics in the original sense of the word (Noble, 2015). While chromatin modification processes may be involved, they are not a sufficient explanation for the effect, since the ectopic heads (tissue which might be suggested to have been epigenetically reprogrammed into a head state from its original tail identity) are discarded at each generation of cutting. What remains is a normal gut fragment, which has been reprogrammed to form 2 heads, not 1, upon future regeneration; the information about basic anatomical polarity and body organization must be stored in a distributed form throughout the animal.
Figure 5.
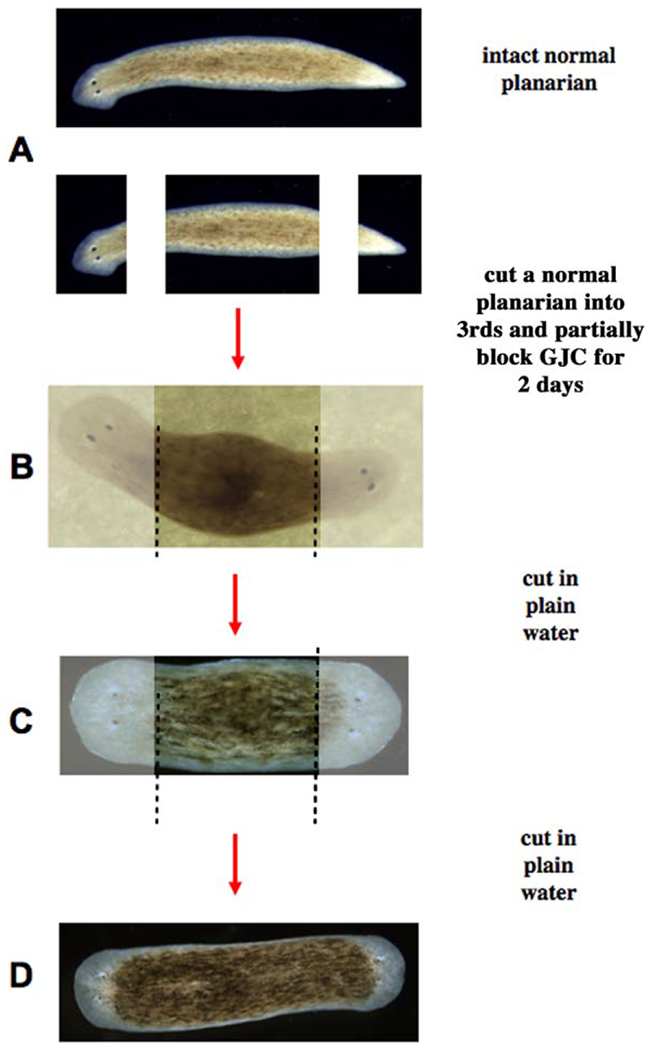
Stable inheritance of target morphology change after GJ network perturbation. A normal planarian has a head and tail, and regenerates each at the appropriate end of an amputated fragment (A). When cut into thirds, and the middle fragment is briefly exposed to octanol, which temporarily blocks long-range bioelectrical signaling between the wound and mature tissues, a 2-headed worm results (B). GJC, gap junctional communication. Remarkably, upon further rounds of cutting in plain water (long after the octanol has left the tissues, as confirmed by HPLC), the 2-headed form is recapitulated (C,D; images of 2-headed worms provided by Fallon Durant.). This change in the animal’s target morphology (the shape to which it regenerates upon damage) appears to be permanent, and persists across the animal’s normal reproductive mode (fissioning), despite the fact that the genomic sequence has not been altered. Chromatin modifications alone do not explain this, because the posterior wound cells, which could have been epigenetically reprogrammed to a head fate, are thrown away at each cut: the information encoding a bipolar 2-head animal is present even in the normal gut fragment—it is distributed throughout the body. We propose that this information is a kind of memory, encoded in electrical networks of somatic cells coupled by gap junctions, and is stored at the level of bioelectrical dynamics, not genetics.
GJS AS ELECTRICAL SYNAPSES IN BIOELECTRIC NETWORKS: FUNCTIONAL IMPLICATIONS
Synapses among cells are a key component of multi-cellularity, enabling cell–cell communication and information processing necessary for complex patterning and adaptive (regulative) physiology (Baluska and Mancuso, 2014). GJs are an especially important type of synapse because of the ability to regulate current based on voltage—this makes them similar to transistors or memristors (Jo et al., 2010; Pershin and Di Ventra, 2010), which are a basic unit of computation or logic gates. The central nervous system utilizes both chemical and electrical synaptic transmission. Chemical synapses use extracellular bursts of neurotransmitter release to relay digital information between adjacent cells. Electrical synapses use GJs to directly connect the interiors of the cells to one another, which results in a local and long-range relay of analog information through small molecules or changes in resting membrane potential (Pereda, 2014). These electrical synapses have been found to be particularly important in the imprinting of emotional memories, such as fear conditioning (Bissiere et al., 2011).
The main connexin in the mammalian brain is Connexin36, but there appear to be other connexins involved as well (Baker and Macagno, 2014). The regulation of connexin expression and the post-translational gating of the channels affects the strength of electrical synapses as does the nature of the connection formed by distinct connexin hemichannels. GJs that are formed by heterotypic channels comprised of different connexins have different conductivities than homotypic channels comprised of identical connexins (Palacios-Prado et al., 2014). Electrical synapses are bidirectional, but not necessarily equally conductive in both directions (Marder, 2009; Palacios-Prado, 2009). They are also not dependent on action potentials; therefore they are capable of sensing the simultaneous sub-threshold depolarizations of a population of connected cells. In neurons, this detection can result in synchronous firing or asynchronous firing due to changes in neuronal thresholds and frequency of firing (Saraga et al., 2006; Gutierrez and Marder, 2013). The synaptic strength of a neuron can be determined by these thresholds and is modulated by the “coupling potential” that is imparted on the neurons by the GJ conductance but also by the capacitance and resistance of the connected neurons (Pereda et al., 2013). One of the most important functional features implemented by these versatile electrical gating elements is that of plasticity: history-dependent conductive processes.
Plasticity of electrical synapses occurs when the strength of the electrical synapse is changed by any one of a number factors related to prior electrical activity (Postma et al., 2011). Neighboring inhibitory chemical synapses can change the coupling potential between cells by shunting away excitatory currents (O’Brien, 2014). Ion channel expression and activation in neurons can change the resistance of connected cells (Curti and Pereda, 2004). Neurotransmitters can also change the conductivity of the GJs themselves (Piccolino and Neyton, 1984). Dopamine is able to increase cAMP, activating cAMP-dependent protein kinase A (PKA) that phosphorylates sites on Cx36 and decreases its conductance (Urschel et al., 2006). Other neurotransmitters including nitric oxide, histamine, and noradrenaline also have a regulatory effect on GJ conductance (Hatton and Yang, 1996; Rörig and Sutor, 1996; Zsiros and Maccaferri, 2008). Glutamate from local glutamatergic synapses has a potentiating role on electrical synapses by increasing the availability of calcium that activates CaMKII, which then phosphorylates sites on Cx36 that are both unique to and the same as PKA (Alev et al., 2008). This binding further affects the plasticity of the GJ conductivity, usually resulting in an increase of coupling (Pereda et al., 2013). In this way, memory and computational circuits can be formed as physiological state changes alter GJ-dependent connectivity shaping subsequent intercellular signaling dynamics, resulting in feedback loops that can stabilize transient bioelectric states into stable cascades of downstream signaling pathways.
An important source of neuronal activity modulation in the brain is the astrocyte. These are specialized cells that support neuronal metabolism and housekeeping, provide a structural framework, and are capable of releasing a variety of signaling molecules called gliotransmitters including glutamate, D-serine, and ATP to affect neuronal spiking thresholds (Hamilton and Attwell, 2010). Astrocytes have many processes and each one can connect to a large number of neurons. Although astrocytes do not form GJs with neurons, they do form GJs between themselves. These GJs allow the passage of calcium waves that are induced in response to patterns of neuronal activity, which are conveyed to astrocytes via glutamate (Wade et al., 2011). The propagation of these calcium waves through GJs is important for the coordination of neuronal groups that are not directly linked to one another, as these waves are capable of influencing any neuron that is coupled to the astrocytic network through which it is propagating (Pereira and Furlan, 2010). The coordination of information processing in astrocytes via GJ network plasticity is thought to underlie important aspects of cognitive information-processing in the brain (Verwey and Edwards, 2010; Stehberg et al., 2012). Since many of the same mechanisms (bioelectric signaling, calcium waves, cAMP, etc.) occur in numerous other somatic cell types, it is possible that some of the patterning plasticity observed during regulative development and regeneration could be implemented by brain-like circuits of GJs that stabilize altered downstream signaling events as a function of prior physiological state.
CONCLUSION
What are the GJ-Permeable Signals?
What sorts of signals endogenously traverse GJs during patterning? In general, a variety of small molecules and metabolites are thought to permeate GJs, including cAMP (Burnside and Collas, 2002; Webb et al., 2002; Bedner et al., 2003), ATP (Bao et al., 2004; Pearson et al., 2005), cGAMP (Chen et al., 2016), Ca++ (Toyofuku et al., 1998; Blomstrand et al., 1999; Paemeleire et al., 2000), serotonin (Wolszon et al., 1994; Fukumoto et al., 2005b; Gairhe et al., 2012; Hou et al., 2013), and histamine (Chaturvedi et al., 2014). These are in general impossible to watch traversing GJ in situ, as these molecules are too small to be labeled (fluorescently) without radically altering their properties. However, in addition to small molecule metabolites, several recent reports have demonstrated that molecules much larger than the normal ≈1 kDa size cutoff can penetrate through GJs under some circumstances (Brooks and Woodruff, 2004; Valiunas et al., 2005). These include siRNA’s (Wolvetang et al., 2007; Katakowski et al., 2010; Lim et al., 2011; Rimkute et al., 2016) and even the protein calmodulin (Brooks and Woodruff, 2004; Woodruff, 2005); one possibility is that the crucial parameter is shape, not overall size, and that long, thin molecules may be able to traverse GJ channels. The alignment of such molecules to facilitate GJC-mediated transfer could be provided by an endogenous electric field (Woodruff, 2005). Understanding the full range of endogenous permeant signals is an important area for future investigation.
Junctional selectivity can result in radically different permeabilities of GJs to different types of molecules (Bevans et al., 1998; Goldberg et al., 1999; Nicholson et al., 2000; Goldberg et al., 2002). This may have important consequences for the morphogenetic system, and means that studies of this process in vivo may be strongly dependent upon the experimental probe used. For example, at 7.5 days, the mouse embryo was found to be subdivided into at least nine GJC compartments with respect to Lucifer Yellow (LY) transfer, but only two domains with respect to ionic coupling (Kalimi and Lo, 1989). In the loach (Misgurnus fossilis), LY, fluorescein, and DAPI showed consistent differences in their ability to transfer between tissues during mesoderm induction and patterning (Bozhkova and Rozanova, 2000). Moreover, the chemical selectivity of the GJs connecting early embryonic cells changes appreciably during loach development (Bozhkova, 1998), suggesting that embryonic patterning can utilize regulation of not only the amount of GJC but also of the various types of molecules being passed through the GJs. Significant advances can be expected to result from future development of versatile fluorescent methods to reveal patterns of distinct GJ paths in vivo.
GJC and Bioelectricity: Two Key Components of Computational Networks
In addition to chemical metabolites, one of the most important signals propagated through GJ paths is current. This allows GJs to demarcate isopotential compartments of cells with similar Vmem (Sherman and Rinzel, 1991). This is thought to underlie normalization of aberrant founder cells in the production of ectopic brain tissue (Pai et al., 2015a) or tumors (Chernet et al., 2015). GJs and resting potential are bidirectionally linked, since Vmem can regulate GJ opening, while GJs regulate the sharing of Vmem among neighboring cells, but are not interchangeable. Genetic crosses have revealed that in the zebrafish tail, connexins and ion channels have distinct roles (Hoptak-Solga et al., 2008; Perathoner et al., 2014). And in the case of voltage control of nerve sprouting (Blackiston et al., 2015), depolarization cannot induce the hyperinnervation phenotype if GJ is shut down. Indeed, GJs allow multiple cells to act as one in response to electric fields (Cooper, 1984; Tsutsui et al., 2014) and alter their bioelectric dynamics (Baigent et al., 1997; Donnell et al., 2009). One of the best recent linkages between physiology and transcriptional regulation was characterized in the highly GJ-coupled heart tissue, in which Wnt1 controls the bioelectrical gradient via regulation of the L-type calcium channel (Panakova et al., 2010).
Thus, one mechanism by which GJ activity regulates pattern formation is by shaping endogenous bioelectric gradients. The importance of these gradients have been confirmed by numerous functional studies implicating spatiotemporal Vmem differences as an instructive parameter for cell behavior and large-scale patterning (Jaffe, 1981; Nuccitelli, 2003; McCaig et al., 2005; Funk, 2013; Levin, 2014b). Ion channel-mediated changes in Vmem not only affects individual cell behaviors such as proliferation, differentiation, apoptosis, and migration (Sundelacruz et al., 2009), but also determines large-scale parameters such as organ size, shape, and axial patterning of the entire body (Beane et al., 2011; Perathoner et al., 2014). In a range of vertebrate and invertebrate model systems, Vmem regulates the formation of the brain, eye, wing, and face, and controls patterning along the anterior–posterior and left–right axes during embryonic development (Levin et al., 2002; Dahal et al., 2012; Pai et al., 2015a). Moreover, experimental control of bioelectric gradients has enabled induction of regenerative ability in non-regenerative contexts (Tseng et al., 2010; Leppik et al., 2015), induced reprogramming of gut tissue into complete eyes (Pai et al., 2012), and normalized tumors (Chernet and Levin, 2013b). Electrical synapses (GJs) and neurotransmitters like serotonin are a key component of several patterning systems, having been implicated in embryonic left–right asymmetry, bone patterning, tumor suppression, and brain size control (Levin and Mercola, 1998b; Iovine et al., 2005; Levin, 2007; Chernet et al., 2015; Pai et al., 2015a). As in the brain, these elements often work together, such as the bioelectrically controlled movement of serotonin through GJs during left–right patterning and control of nerve growth (Levin et al., 2006; Blackiston et al., 2015).
In addition to the known downstream targets (Pai et al., 2015b) of the bioelectric signals propagated and limited by GJ paths, specific molecular endpoints for GJ include NFATc1 downstream of Cx37 signaling (Kanady et al., 2015), semaphorin downstream of Cx43 function (Ton and Iovine, 2012), and numerous targets of SP1 and SP3 transcription factors (Stains et al., 2003; Stains and Civitelli, 2005). Because so many downstream targets are impacted by GJC, it is a kind of master regulator node, like Ca++ (Slusarski and Pelegri, 2007) signaling or the RAS gene (Jindal et al., 2015). Thus, one of the exciting aspects of GJC function, as discussed above in the context of selecting alternate head morphologies in planaria, is the ability to regulate large-scale system-wide properties by targeting a single element GJC (or even individual connexins). An interesting recent example is the demonstration that decreasing Connexin36 GJ coupling compensates for overactive KATP channels to restore insulin secretion and prevent hyperglycemia in a mouse model of neonatal diabetes (Nguyen et al., 2014). GJs will surely play an increasing role in the understanding and management of circuit disorders, in the fields of birth defects and cancer (Huang et al., 2009).
Next Steps: Finer-Scale Reductive Studies
Confocal microscopy uncaging and FRET methodologies will greatly facilitate the study of endogenous GJC paths in embryos (Bedner et al., 2003; Braet et al., 2003; Cannell et al., 2004; Dakin et al., 2005; Di et al., 2005). Key future research areas include the characterization of factors which set up patterns of differential GJC in various embryonic tissues (at the transcriptional level, as well as at the level of controlling GJC states, such as by endogenous patterns of pH and voltage gradients (Ek-Vitorin et al., 1996; Morley et al., 1997; Calero et al., 1998; Francis et al., 1999; Gu et al., 2000), and the mapping of paths which exist in and between different tissues to molecules of various charges and sizes.
Experiments in mouse (and other systems) must, of necessity, be performed in the context of multiple connexin knock-outs, since it is becoming increasingly clear that due to compensation and redundancy, single loss of GJ genes can obscure important phenotypes (Simon et al., 2004). Indeed, knock-in of dominant negative mutants with different specificities for endogenous connexin families is likely to reveal many important and novel roles (Paul et al., 1995; Fiorini et al., 2004; Beahm et al., 2006). Likewise, more sophisticated technologies allowing expression of dominant negative mutants restricted in space and time during development will allow the circumvention of embryonic lethal phenotypes and likely to lead to the discovery of novel patterning mechanisms (Becker et al., 1992; Bakirtzis et al., 2003). While we now have some understanding of downstream transcriptional changes that occur when gap junctional signaling is perturbed (Kim et al., 2005), next generation sequencing analysis of GJC-inhibited tissues may lead to the discovery of proximal early response genes to GJC-permeable morphogens. Perhaps the most impactful tool would be the ability to selectively close or open GJ channels, in the way that optogenetics currently allows for ion channel function (Bernstein et al., 2012).
Next Steps: Systems-Level Integration
Perhaps even more important than increasing the molecular resolution with which we understand GJ function at the cellular level, is the converse task of synthesizing reductive data into a systems view of cellular networks coupled by dynamic GJC paths. Long-range GJ-mediated signaling, and feedback loops such as the one implemented by calmodulin, which regulates GJC (Peracchia et al., 1983; Burr et al., 2005; Dodd et al., 2008) but is itself a GJ-permeable signal (Brooks and Woodruff, 2004; Woodruff, 2005), ensure regulatory modes that cannot be captured via simple pathway models. Especially important is the investigation of GJs in reducing the impact of physiological variability and noise in individual cells to facilitate robustness (Hallett, 1989), and their converse roles in sharpening cell responses to weak stimuli (Cooper, 1984). Because of the inherent complexity and nonlinearity of the behavior of such networks, the first task is systems-level modeling. Theoretical analyses of GJC signaling have begun via mathematical models (Cooper, 1984; Cooper et al., 1989; Vogel and Weingart, 1998; Nygren and Giles, 2000; Hofer et al., 2002; Vogel and Weingart, 2002; Fortier and Bagna, 2006; Cervera et al., 2015), and in future work it will be very important to apply these to the understanding of specific patterning processes in embryonic and regenerative morphogenesis (Esser et al., 2006; Zhang and Levin, 2009). Such models, especially as they become integrated with bioelectric and transcriptional networks, will become an essential tool for understanding endogenous pattern formation and developing interventions to control complex remodeling events for regenerative medicine and bioengineering applications.
A Speculative Hypothesis: Somatic Memories
Beyond modeling the biophysics of GJ-coupled cells, the field will truly mature when it also develops conceptual tools to explain information and computation propagating through GJ-coupled cell networks (Friston et al., 2015; Pezzulo and Levin, 2015). GJs are known to be important for memory and computation in the brain (Wang and Belousov, 2011; Wu et al., 2011; Dere and Zlomuzica, 2012), and the ability of most general anesthetics to serve as GJ uncoupling agents have motivated models of cognition and consciousness based on GJ-mediated integration across the brain (Mantz, 1992; Juszczak and Swiergiel, 2009). Interestingly, recent work has applied the same types of Hebbian plasticity concepts to understand the function of the heart (Chakravarthy and Ghosh, 1997; Zoghi, 2004) and pancreas (Goel and Mehta, 2013). We have recently extended the parallelism between brain and non-neural tissues’ use of GJ-dependent synapses by proposing that target morphologies for regenerating systems are encoded via a memory-like mechanism within somatic tissues (Friston et al., 2015; Pezzulo and Levin, 2015). Because of the molecular and functional similarity in the use of GJs in the brain and in non-neural patterning tissues (Fig. 3), it is possible that circuits that guide self-limiting, flexible remodeling and regeneration programs are implementing true memories. The central involvement of electrical synapses and the holographic-like nature of patterning information that can be stably re-written (e.g., permanently 2-headed planaria) suggest models in which the target morphology is actually stored (encoded) within the real-time current dynamics, perhaps akin to storage of spatial memory in neural networks or similar proposed processes of memory in non-neural tissues (McConnell et al., 1959; Turner et al., 2002; Zoghi, 2004; Levin, 2011; Baluska and Mancuso, 2012; Levin, 2012b; Shomrat and Levin, 2013). This view makes two major predictions, currently being addressed in our lab. First, it suggests that models taken from cognitive neuroscience for understanding the system-wide dynamics of neural networks (Balduzzi and Tononi, 2009; Friston, 2010; Ebner and Hameroff, 2011; Friston, 2013; Hoel et al., 2013; Pezzulo et al., 2015) may be appropriate formalisms for efficient prediction and control of morphogenesis.
Second, it suggests that patterning could be controlled top-down, in a complementary approach to today’s focus on bottom-up (molecular-level) interventions. If GJ-coupled tissues are indeed information processing agents as they are in the brain, then it may be possible to efficiently exploit the plasticity and Hebbian-like learning capabilities facilitated by connexins in non-neural tissues by literally training them to desired outcomes: providing positive and negative reinforcement for collections of cells undergoing morphogenesis to encourage specific types of growth patterns. As with training in cognitive animal models, rewarding for final outcome (as opposed to directly regulating molecular signaling at each point in the tissue) may allow bioengineers to capitalize on the inherent modularity and plasticity of pattern formation (Sullivan et al., 2016). This may enable the induction of desired anatomical patterning changes without facing head-on the complexity explosion that stymies attempts to construct complex organs such as limbs directly.
Training non-neural tissues and treating them like “neural” networks with plasticity and memory could be feasible as shown by recent studies that heart and pancreas cells are capable of memory. The repolarization of the ventricles in the heart, referred to as the T-wave, can be inverted using atrial pacing. Depending on the duration and frequency of that pacing, the inversion strength and duration of inversions after pacing are increased, displaying a Hebbian-like memory (Rosenbaum et al., 1982; Chakravarthy and Ghosh, 1997; Zoghi, 2004). Networks of pancreatic islet beta-cells release insulin due to calcium waves that travel through GJs, and display a loss in GJC in response to heightened levels of glucose that can be modeled using learning theories also based on Hebbian-like memory (Benninger et al., 2008; Goel and Mehta, 2013). These data suggest that GJ-based plasticity outside the brain could be exploited to offload the computational complexity of micromanaging pattern of physiology onto the cells themselves (as occurs during reward circuit shaping in behavioral training). A whole new paradigm for medicine that incorporates tissue training could arise if these two important tissues can be physiologically entrained to correct certain heart arrhythmias and insulin secretion disorders.
Looking to neuroscience for examples of how neural networks are trained can inform new strategies to train non-neural cellular networks for desired GJC. A number of neuronal plasticity studies grow neurons on microelectrode arrays (MEAs) that can then be used to monitor the patterns of firing neurons and to also deliver electrical impulses that can stimulate the firing of certain neurons in the network (DeMarse and Dockendorf, 2005; le Feber et al., 2008; Franke et al., 2012; Pimashkin et al., 2013). Cardiomyocytes, pancreatic islet cells, and fibroblasts can all be monitored for electrical activity using MEAs (Parak et al., 1999; Bornat et al., 2010; Pfeiffer et al., 2011; Spira and Hai, 2013; Schönecker et al., 2014). However, the appropriate method for training GJ connectivity in non-neural networks might differ from neuronal networks.
Adaptive learning in neural networks in vitro requires an appropriate stimulation and feedback mechanism. In two studies, which utilized extracellular microelectrode arrays, low frequency stimulation was delivered to a culture of dissociated rat cortical neurons or murine hippocampal neurons via a stimulation electrode, and the spiking frequency was read on the other recording electrodes. Whenever the output at a specific recording electrode was over a certain threshold that considers previous output (response-to-stimulus ratio), then the next recurrence of the low frequency stimulation at the stimulation electrode was delayed (Shahaf and Marom, 2001; Pimashkin et al., 2013). This strategy for training neuronal networks has shown more promise than open-looped strategies, since it results in more space exploration and greater changes in connectivity (le Feber et al., 2010). Indeed, closing the loop by using embodied networks that are capable of interpreting spiking outputs as instructions for robotic movement and in turn take sensory information from those robots and translate them into changes in stimulation patterns is showing great promise as way to demonstrate neuronal plasticity (Bakkum et al., 2004, 2008; Chao et al., 2008). Linking spiking outputs to flight simulator software controls with one stimulating electrode for pitch and the other stimulating electrode for roll can be used to train neuronal networks to fly a plane in the simulator and be able to correct for changes in headwind (DeMarse and Dockendorf, 2005). New optogenetic stimulation of action potentials allows for even better time resolution of output spiking due to the lack of an artifact that occurs when using electrode stimulation (Dranias et al., 2013).
Adapting these neuronal training techniques to non-neural GJs can be achieved using electrodes and optogenetics to change membrane potentials and thus affect the GJC between those and surrounding cells. While the details could be connexin- and cell-specific, one class of training strategies could deliver dopamine or glutamate as ways to provide GJ-modulatory feedback to the network. Microfluidics can be used to implement stimulation and also to directly measure the GJC between cells (Bathany et al., 2012). Real-time monitoring of calcium wave patterns using transgenic cell lines could also provide better measurement of output (Shigetomi et al., 2010).
Thus, one way to control the patterning outcome of GJ-coupled cell networks may be to modify their information content, whether by rewriting shape memories directly (via optogenetic-like approaches), or providing stimuli that exploit learning. Whether the neural computation analogy turns out to be the best way of understanding GJ dynamics, it is clear that GJs are a central hub for cellular information. Cells rely on GJ-permeable signals to regulate their individual activities toward large-scale construction, remodeling, and repair of complex anatomical structures. Thus, gaining predictive control over GJ dynamics is likely to be at the forefront of future efforts to exploit the ability of biological systems to regulate not just gene expression and physiology, but three-dimensional structure. The implications will be not only in fields like birth defects, regenerative medicine, and cancer, but are likely to impact evolutionary biology, unconventional computation, and the design of robust hybrid technologies. The technological revolution precipitated by the introduction of transistors in electronics was driven not only by their physical properties, but also by the appreciation of their unique computational capabilities. GJs support similar functions at the cellular level, with similar implications for computational capabilities of GJ-coupled networks (Scarle, 2008; Simon, 2009). Thus, it may not be unreasonable to predict that fully understanding and making use of GJ signaling dynamics in biological tissues may lead to the same scale of revolutionary advances in basic biology and applied biomedicine.
ACKNOWLEDGMENTS
We thank Alexis Pietak, Alberto Pereda, Jose Ek Vitorin, and the members of the Levin lab for many useful discussions about the role of GJs in pattern regulation.
Contract grant sponsor: NIH; contract grant numbers: HD081326, AR055993, AR061988.
Contract grant sponsor: NSF; contract grant number: CBET-0939511.
Contract grant sponsor: The G. Harold and Leila Y. Mathers Charitable Foundation.
Contract grant sponsor: The W. M. KECK Foundation.
Contract grant sponsor: Templeton World Charity Foundation; contract grant number: TWCF0089/AB55.
Footnotes
The authors declare no conflicts or competing interests.
REFERENCES
- Aboobaker AA. 2011. Planarian stem cells: A simple paradigm for regeneration. Trends Cell Biol 21:304–311. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, et al. 2006. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 133:1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahir BK, Pratten MK. 2014. Structure and function of gap junction proteins: Role of gap junction proteins in embryonic heart development. Int J Dev Biol 58:649–662. [DOI] [PubMed] [Google Scholar]
- Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. 2000. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest 105:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alev C, Urschel S, Sonntag S, Zoidl G. 2008. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A 105:20964–20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen F, Tickle C, Warner A. 1990. The role of gap junctions in patterning of the chick limb bud. Development 108:623–634. [DOI] [PubMed] [Google Scholar]
- Alqadah A, Hsieh YW, Chuang CF. 2013. microRNA function in left-right neuronal asymmetry: Perspectives from C. elegans. Front Cell Neurosci 7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanaviciute I, Rysevaite K, Liutkevicius V, Marandykina A, Rimkute L, Sveikatiene R, Uloza V, et al. 2014. Long-distance communication between laryngeal carcinoma cells. PloS One 9:e99196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Eckardt D, Riquelme MA, Willecke K, Saez JC. 2003. Presence and importance of connexin43 during myogenesis. Cell Commun Adhes 10:451–456. [DOI] [PubMed] [Google Scholar]
- Araya R, Eckardt D, Maxeiner S, Kruger O, Theis M, Willecke K, et al. 2005. Expression of connexins during differentiation and regeneration of skeletal muscle: Functional relevance of connexin43. J Cell Sci 118:27–37. [DOI] [PubMed] [Google Scholar]
- Asai R, Taguchi E, Kume Y, Saito M, Kondo S. 1999. Zebrafish leopard gene as a component of the putative reaction-diffusion system. Mech Dev 89:87–92. [DOI] [PubMed] [Google Scholar]
- Aslanidi KB, Boitsova L, Chailakhyan LM, Kublik LN, Marachova II, Potapova TV, Vinogradova TA. 1991. Energetic cooperation via ion-permeable junctions in mixed animal cell cultures. FEBS Lett 283:295–297. [DOI] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. 2008. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev 125:353–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. 2010. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol 346:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent S, Stark J, Warner A. 1997. Modelling the effect of gap junction nonlinearities in systems of coupled cells. J Theor Biol 186:223–239. [Google Scholar]
- Baker MW, Macagno ER. 2014. Control of neuronal morphology and connectivity: Emerging developmental roles for gap junctional proteins. FEBS Lett 588:1470–1479. [DOI] [PubMed] [Google Scholar]
- Bakirtzis G, Jamieson S, Aasen T, Bryson S, Forrow S, Tetley L, Finbow M, et al. 2003. The effects of a mutant connexin 26 on epidermal differentiation. Cell Commun Adhes 10:359–364. [DOI] [PubMed] [Google Scholar]
- Bakkum DJ, Shkolnik AC, Ben-Ary G. 2004. Removing some ’A’from AI: Embodied cultured networks. In: Iida F, Pfeifer R, Steels L, Kuniyoshi Y, editors. Embodied Artificial Intelligence. New York: Springer. [Google Scholar]
- Bakkum DJ, Chao ZC, Potter SM. 2008. Spatio-temporal electrical stimuli shape behavior of an embodied cortical network in a goal-directed learning task. J Neural Eng 5:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi D, Tononi G. 2009. Qualia: The geometry of integrated information. PLoS Comput Biol 5:e1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Mancuso S. 2012. Ion channels in plants: From bioelectricity, via signaling, to behavioral actions. Plant Signal Behav 8:e23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Mancuso S. 2014. Synaptic view of eukaryotic cell. Int J Gen Syst 43:740–756. [Google Scholar]
- Banerji R, Eble DM, Iovine KM, Skibbens RV. 2016. Esco2 regulates cx43 expression during skeletal regeneration in the zebrafish fin. Dev Dyn 245:7–21. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Underhill TM, Naus CC. 1999. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev Genet 24:69–81. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. 2004. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68. [DOI] [PubMed] [Google Scholar]
- Bates E. 2015. Ion channels in development and cancer. Annu Rev Cell Dev Biol 31:231–247. [DOI] [PubMed] [Google Scholar]
- Bathany C, Beahm DL, Besch S, Sachs F, Hua SZ. 2012. A microfluidic platform for measuring electrical activity across cells. Biomicrofluidics 6:34121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Hoch M. 2001. Gastrointestinal development in the Drosophila embryo requires the activity of innexin gap junction channel proteins. Cell Commun Adhes 8:307–310. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. 2002. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci 115:1859–1867. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Martini J, Eckardt F, Hoch M. 2004. Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo. Mol Biol Cell 15:2992–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, et al. 2006. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem 281:7994–8009. [DOI] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Adams DS, Levin M. 2011. A Chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol 18:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DL, Leclerc-David C, Warner A. 1992. The relationship of gap junctions and compaction in the preimplantation mouse embryo. Dev Suppl 113–118. [PubMed] [Google Scholar]
- Becker DL, Evans WH, Green CR, Warner A. 1995. Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J Cell Sci 108:1455–1467. [DOI] [PubMed] [Google Scholar]
- Becker DL, McGonnell I, Makarenkova HP, Patel K, Tickle C, Lorimer J, Green CR. 1999. Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev Gen 24:33–42. [DOI] [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, Willecke K, Harz H. 2003. A method to determine the relative cAMP permeability of connexin channels. Exp Cell Res 291:25–35. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi D, Shilo BZ, Barkai N. 2011. Scaling of morphogen gradients. Curr Opin Genet Dev 21:704–710. [DOI] [PubMed] [Google Scholar]
- Bennett MV. 1997. Gap junctions as electrical synapses. J Neurocytol 26:349–366. [DOI] [PubMed] [Google Scholar]
- Benninger RKP, Zhang M, Head WS, Satin LS. 2008. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 95:5048–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JG, Garrity PA, Boyden ES. 2012. Optogenetics and thermogenetics: Technologies for controlling the activity of targeted cells within intact neural circuits. Curr Opin Neurobiol 22:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans CG, Kordel M, Rhee SK, Harris AL. 1998. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273:2808–2816. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS. 2011. Electrical synapses control hippocampal contributions to fear learning and memory. Science 331:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. 2011. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Models Mech 4:67–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, Anderson GM, Rahman N, Bieck C, Levin M. 2015. A novel method for inducing nerve growth via modulation of host resting potential: Gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics 12:170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennerhassett MG, Caveney S. 1984. Separation of developmental compartments by a cell type with reduced junctional permeability. Nature 309:361–364. [Google Scholar]
- Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. 1999. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience 92:255–265. [DOI] [PubMed] [Google Scholar]
- Bornat Y, Raoux M, Boutaib Y, Morin FO. 2010. Detection of electrical activity of pancreatic beta-cells using microelectrode arrays. Proceedings 5th IEEE Int. Symposium on Electronic Design, Test & Applications. [Google Scholar]
- Bozhkova VP. 1998. The specificity of gap junctional channels in early fish embryos and its significance for pattern formation in development. Membr Cell Biol 11:803–815. [PubMed] [Google Scholar]
- Bozhkova VP, Rozanova NV. 2000. Local changes in gap junctional permeability accompany regionalization of the mesoderm in early fish (loach, Misgurnus fossilis) embryos. Membr Cell Biol 14:189–198. [PubMed] [Google Scholar]
- Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. 2003. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33:37–48. [DOI] [PubMed] [Google Scholar]
- Britz-Cunningham S, Shah M, Zuppan C, Fletcher W. 1995. Mutations of the connexin-43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med 332:1323–1329. [DOI] [PubMed] [Google Scholar]
- Brooks RA, Woodruff RI. 2004. Calmodulin transmitted through gap junctions stimulates endocytic incorporation of yolk precursors in insect oocytes. Dev Biol 271:339–349. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Giaume C. 1999. Connexins and information transfer through glia. Adv Exp Med Biol 468:321–337. [DOI] [PubMed] [Google Scholar]
- Bullara D, Decker DY. 2015. Pigment cell movement is not required for generation of Turing patterns in zebrafish skin. Nat Commun 6:6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside AS, Collas P. 2002. Induction of Oct-3/4 expression in somatic cells by gap junction-mediated cAMP signaling from blastomeres. Eur J Cell Biol 81:585–591. [DOI] [PubMed] [Google Scholar]
- Burr GS, Mitchell CK, Keflemariam YJ, Heidelberger R, O’Brien J. 2005. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem Biophys Res Commun 335:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Cheng A, Luo Y, Lu C, Mattson MP, Rao MS, Furukawa K. 2004. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem 88:212–226. [DOI] [PubMed] [Google Scholar]
- Calero G, Kanemitsu M, Taffet SM, Lau AF, Delmar M. 1998. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circ Res 82:929–935. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Jacobs MD, Donaldson PJ, Soeller C. 2004. Probing microscopic diffusion by 2-photon flash photolysis: Measurement of isotropic and anisotropic diffusion in lens fiber cells. Microsc Res Tech 63:50–57. [DOI] [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, et al. 2011. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera J, Manzanares JA, Mafe S. 2015. Electrical coupling in ensembles of nonexcitable cells: Modeling the spatial map of single cell potentials. J Phys Chem B 119:2968–2978. [DOI] [PubMed] [Google Scholar]
- Chakravarthy SV, Ghosh J. 1997. On Hebbian-like adaptation in heart muscle: A proposal for ’cardiac Memory’. Biol Cybern 76:207–215. [DOI] [PubMed] [Google Scholar]
- Chang Q, Tang W, Kim Y, Lin X. 2014. Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing. Neurobiol Dis 73:418–427. [DOI] [PubMed] [Google Scholar]
- Chao ZC, Bakkum DJ, Potter SM. 2008. Shaping embodied neural networks for adaptive goal-directed behavior. PLoS Comput Biol 4:e1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi R, Reddig K, Li HS. 2014. Long-distance mechanism of neurotransmitter recycling mediated by glial network facilitates visual function in Drosophila. Proc Natl Acad Sci U S A 111:2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob S, et al. 2016. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet B, Levin M. 2013a. Endogenous voltage potentials and the microenvironment: Bioelectric signals that reveal, induce and normalize cancer. J Clin Exp Oncol Suppl 1:S1–002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Levin M. 2013b. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis Models Mech 6:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Levin M. 2014. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget 5:3287–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Fields C, Levin M. 2015. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front Physiol 5:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Vanhoven MK, Fetter RD, Verselis VK, Bargmann CI. 2007. An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell 129:787–799. [DOI] [PubMed] [Google Scholar]
- Chuang-Tseng MP, Chuang HH, Sandri C, Akert K. 1982. Gap junctions and impulse propagation in embryonic epithelium of Amphibia. A freeze-etching study. Cell Tissue Res 225:249–258. [DOI] [PubMed] [Google Scholar]
- Civitelli R. 2008. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys 473:188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Haydon PG. 2014. Connexin 30 controls the extension of astrocytic processes into the synaptic cleft through an unconventional non-channel function. Neurosci Bull 30:1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella L, Tursun B, Hsieh YW, Galindo S. 2014. Two distinct types of neuronal asymmetries are controlled by the Caenorhabditis elegans zinc finger transcription factor die-1. Genes Dev 28:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CN, Kosher RA. 1991. A gradient of gap junctional communication along the anterior-posterior axis of the developing chick limb bud. Dev Biol 148:529–535. [DOI] [PubMed] [Google Scholar]
- Cogliati B, Mennecier G, Willebrords J, Da Silva TC, Maes M, Pereira IV, Crespo Yanguas S, et al. 2016. Connexins, pannexins, and their channels in fibroproliferative diseases. J Membr Biol 249:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MS. 1984. Gap junctions increase the sensitivity of tissue cells to exogenous electric fields. J Theor Biol 111:123–130. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Miller JP, Fraser SE. 1989. Electrophoretic repatterning of charged cytoplasmic molecules within tissues coupled by gap junctions by externally applied electric fields. Dev Biol 132:179–188. [DOI] [PubMed] [Google Scholar]
- Curti S, Pereda AE. 2004. Voltage-dependent enhancement of electrical coupling by a subthreshold sodium current. J Neurosci 24:3999–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal GR, Rawson J, Gassaway B, Kwok B, Tong Y, Ptacek LJ, Bates E. 2012. An inwardly rectifying K+ channel is required for patterning. Development 139:3653–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin K, Zhao Y, Li WH. 2005. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat Methods 2:55–62. [DOI] [PubMed] [Google Scholar]
- Dang X, Doble BW, Kardami E. 2003. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 242:35–38. [PubMed] [Google Scholar]
- Dang X, Jeyaraman M, Kardami E. 2006. Regulation of connexin-43-mediated growth inhibition by a phosphory-latable amino-acid is independent of gap junction-forming ability. Mol Cell Biochem 289:201–207. [DOI] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. 2006. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol 4:e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat SW, Tertoolen LG, Dorresteijn AW, van den Biggelaar JA. 1980. Intercellular communication patterns are involved in cell determination in early molluscan development. Nature 287:546–548. [DOI] [PubMed] [Google Scholar]
- Debeer P, Van Esch H, Huysmans C, Pijkels E, De Smet L, Van de Ven W, Devriendt K, Fryns JP. 2005. Novel GJA1 mutations in patients with oculo-dento-digital dysplasia (ODDD). Eur J Med Genet 48:377–387. [DOI] [PubMed] [Google Scholar]
- Defamie N, Chepied A, Mesnil M. 2014. Connexins, gap junctions and tissue invasion. FEBS Lett 588:1331–1338. [DOI] [PubMed] [Google Scholar]
- DeMarse TB, Dockendorf KP. 2005. Adaptive flight control with living neuronal networks on microelectrode arrays. In: Proceedings of the 2005 IEEE International Joint Conference on Neural Networks. [Google Scholar]
- Dere E, Zlomuzica A. 2012. The role of gap junctions in the brain in health and disease. Neurosci Biobehav Rev 36:206–217. [DOI] [PubMed] [Google Scholar]
- Di WL, Gu Y, Common JE, Aasen T, O’Toole EA, Kelsell DP, Zicha D. 2005. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci 118:1505–1514. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Hertig G, Lechner H, Worsdorfer P, Wulf V, Dicke N, Eckert D, et al. 2009. Loss of connexin43-mediated gap junctional coupling in the mesenchyme of limb buds leads to altered expression of morphogens in mice. Hum Mol Genet 18:2899–2911. [DOI] [PubMed] [Google Scholar]
- Dodd R, Peracchia C, Stolady D, Torok K. 2008. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J Biol Chem 283:26911–26920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnell P, Baigent SA, Banaji M. 2009. Monotone dynamics of two cells dynamically coupled by a voltage-dependent gap junction. J Theor Biol 261:120–125. [DOI] [PubMed] [Google Scholar]
- Dranias MR, Ju H, Rajaram E. 2013. Short-term memory in networks of dissociated cortical neurons. J Neurosci 33:1940–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyce PW, Li D, Barr KJ, Kidder GM. 2014. Connexin43 is required for the maintenance of multipotency in skin-derived stem cells. Stem Cells Dev 23:1636–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner M, Hameroff S. 2011. Lateral information processing by spiking neurons: A theoretical model of the neural correlate of consciousness. Comput Intell Neurosci 2011:247879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. 1996. pH regulation of connexin43: Molecular analysis of the gating particle. Biophys J 71:1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saghir JA, El-Habre ET, El-Sabban ME, Talhouk RS. 2011. Connexins: A junctional crossroad to breast cancer. Int J Dev Biol 55:773–780. [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein R, Hulser D, et al. 1995. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 129:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias L, Kriegstein AR. 2008. Gap junctions: Multifaceted regulators of embryonic cortical development. Trends Neurosci 31:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons-Bell M, Durant F, Hammelman J, Bessonov N, Volpert V, Morokuma J, Pinet K, et al. 2015. Gap junctional blockade stochastically induces different species-specific head anatomies in genetically wild-type girardia dorotocephala flatworms. Int J Mol Sci 16:27865–27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser AT, Smith KC, Weaver JC, Levin M. 2006. Mathematical model of morphogen electrophoresis through gap junctions. Dev Dyn 235:2144–2159. [DOI] [PubMed] [Google Scholar]
- Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, Chin AJ, et al. 1997. Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development 124:1281–1292. [DOI] [PubMed] [Google Scholar]
- Fan RJ, Marin-Burgin A, French KA, Otto Friesen W. 2005. A dye mixture (Neurobiotin and Alexa 488) reveals extensive dye-coupling among neurons in leeches; physiology confirms the connections. J Comp Physiol a Neuroethol Sens Neural Behav Physiol 191:1157–1171. [DOI] [PubMed] [Google Scholar]
- Fiorini C, Mograbi B, Cronier L, Bourget I, Decrouy X, Nebout M, Ferrua B, et al. 2004. Dominant negative effect of connexin33 on gap junctional communication is mediated by connexin43 sequestration. J Cell Sci 117:4665–4672. [DOI] [PubMed] [Google Scholar]
- Fortier PA, Bagna M. 2006. Estimating conductances of dual-recorded neurons within a network of coupled cells. J Theor Biol 240:501–510. [DOI] [PubMed] [Google Scholar]
- Francis D, Stergiopoulos K, Ek-Vitorin JF, Cao FL, Taffet SM, Delmar M. 1999. Connexin diversity and gap junction regulation by pHi. Dev Gen 24:123–136. [DOI] [PubMed] [Google Scholar]
- Franke F, Jäckel D, Dragas J, Mäller J. 2012. High-density microelectrode array recordings and real-time spike sorting for closed-loop experiments: An emerging technology to study neural plasticity. Front Neural Circuits 6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. 2010. Is the free-energy principle neurocentric? Nat Rev Neurosci 11:127–138. [DOI] [PubMed] [Google Scholar]
- Friston K. 2013. Active inference and free energy. Behav Brain Sci 36:212–213. [DOI] [PubMed] [Google Scholar]
- Friston K, Levin M, Sengupta B, Pezzulo G. 2015. Knowing one’s place: A free-energy approach to pattern regulation. J R Soc Interface 12:20141383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. 2005a. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci 27:349–363. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. 2005b. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol 15:794–803. [DOI] [PubMed] [Google Scholar]
- Funk R. 2013. Ion gradients in tissue and organ biology. Biol Syst 2:105. [Google Scholar]
- Gairhe S, Bauer NN, Gebb SA, McMurtry IF. 2012. Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am J Physiol Lung Cell Mol Physiol 303:L767–L777. [DOI] [PubMed] [Google Scholar]
- Gentile L, Cebria F, Bartscherer K. 2011. The planarian flatworm: An in vivo model for stem cell biology and nervous system regeneration. Dis Models Mech 4:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Forbes A, Tazuke SI, Fuller MT, Lehmann R. 2003. Germ line stem cell differentiation in Drosophila requires gap junctions and proceeds via an intermediate state. Development 130:6625–6634. [DOI] [PubMed] [Google Scholar]
- Goel P, Mehta A. 2013. Learning theories reveal loss of pancreatic electrical connectivity in diabetes as an adaptive response. PLoS One 8:e70366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ. 1999. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1:457–459. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Moreno AP, Lampe PD. 2002. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem 277:36725–36730. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Valiunas V, Brink PR. 2004. Selective permeability of gap junction channels. Biochim Biophys Acta 1662:96–101. [DOI] [PubMed] [Google Scholar]
- Govindan J, Iovine KM. 2014. Hapln1a is required for connexin43-dependent growth and patterning in the regenerating fin skeleton. PLoS ONE 9:e88574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grek CL, Rhett JM, Ghatnekar GS. 2014. Cardiac to cancer: Connecting connexins to clinical opportunity. FEBS Lett 588:1349–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Ek-Vitorin JF, Taffet SM, Delmar M. 2000. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: A model for synergistic interactions among connexins. Circ Res 86:E98–E103. [PubMed] [Google Scholar]
- Gu H, Smith FC, Taffet SM, Delmar M. 2003a. High incidence of cardiac malformations in connexin40-deficient mice. Circ Res 93:201–206. [DOI] [PubMed] [Google Scholar]
- Gu S, Yu XS, Yin X, Jiang JX. 2003b. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest Ophthalmol Vis Sci 44:2103–2111. [DOI] [PubMed] [Google Scholar]
- Guthrie S 1984. Patterns of junctional communication in the early amphibian embryo. Nature 311:149–151. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Gilula N. 1989. Gap junctional communication and development. Trends Neurosci 12:12–16. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Turin L, Warner A. 1988. Patterns of junctional communication during development of the early amphibian embryo. Development 103:769–783. [DOI] [PubMed] [Google Scholar]
- Gutierrez GJ, Marder E. 2013. Rectifying electrical synapses can affect the influence of synaptic modulation on output pattern robustness. J Neurosci 33:13238–13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett MB. 1989. The unpredictability of cellular behavior: Trivial or of fundamental importance to cell biology? Perspect Biol Med 33:110–119. [DOI] [PubMed] [Google Scholar]
- Halley JD, Smith-Miles K, Winkler DA, Kalkan T, Huang S, Smith A. 2012. Self-organizing circuitry and emergent computation in mouse embryonic stem cells. Stem Cell Res 8:324–333. [DOI] [PubMed] [Google Scholar]
- Hamada H, Watanabe M, Lau HE, Nishida T, Hasegawa T, Parichy DM, Kondo S. 2014. Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development 141:1418–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. 2010. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11:227–238. [DOI] [PubMed] [Google Scholar]
- Handberg-Thorsager M, Fernandez E, Salo E. 2008. Stem cells and regeneration in planarians. Front Biosci 13:6374–6394. [DOI] [PubMed] [Google Scholar]
- Hatler JM, Essner JJ, Johnson RG. 2009. A gap junction connexin is required in the vertebrate left-right organizer. Dev Biol 336:183–191. [DOI] [PubMed] [Google Scholar]
- Hatton GI, Yang QZ. 1996. Synaptically released histamine increases dye coupling among vasopressinergic neurons of the supraoptic nucleus: Mediation by H1 receptors and cyclic nucleotides. J Neurosci 16:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann P, Grummer R, Schirrmacher K, Rook M, Traub O, Winterhager E. 1999. Transfection with different connexin genes alters growth and differentiation of human choriocarcinoma cells. Exp Cell Res 246:480–490. [DOI] [PubMed] [Google Scholar]
- Hironaka K, Morishita Y. 2012. Encoding and decoding of positional information in morphogen-dependent patterning. Curr Opin Genet Dev 22:553–561. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Burt JM, Hirschi KD, Dai C. 2003. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93:429–437. [DOI] [PubMed] [Google Scholar]
- Hitomi M, Deleyrolle LP, Mulkearns-Hubert EE, Jarrar A, Li M, Sinyuk M, Otvos B, et al. 2015. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep 11:1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoel EP, Albantakis L, Tononi G. 2013. Quantifying causal emergence shows that macro can beat micro. Proc Natl Acad Sci U S A 110:19790–19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Venance L, Giaume C. 2002. Control and plasticity of intercellular calcium waves in astrocytes: A modeling approach. J Neurosci 22:4850–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptak-Solga AD, Klein KA, DeRosa AM, White TW, Iovine KM. 2007. Zebrafish short fin mutations in connexin43 lead to aberrant gap junctional intercellular communication. FEBS Lett 581:3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptak-Solga AD, Nielsen S, Jain I, Thummel R, Hyde DR, Iovine MK. 2008. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev Biol 317:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Li Y, Paul DL. 2013. A novel, highly sensitive method for assessing gap junctional coupling. J Neurosci Methods 220:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YW, Alqadah A, Chuang CF. 2014. Asymmetric neural development in the Caenorhabditis elegans olfactory system. Genesis 52:544–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. 2011. On the intrinsic inevitability of cancer: From foetal to fatal attraction. Semin Cancer Biol 21:183–199. [DOI] [PubMed] [Google Scholar]
- Huang S, Ernberg I, Kauffman S. 2009. Cancer attractors: A systems view of tumors from a gene network dynamics and developmental perspective. Semin Cell Dev Biol 20:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. 1998a. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol 143:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GY, Wessels A, Smith BR, Linask KK, Ewart JL, Lo CW. 1998b. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev Biol 198:32–44. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yamanaka H, Kondo S. 2012. Pigment pattern formation by contact-dependent depolarization. Science 335:677. [DOI] [PubMed] [Google Scholar]
- Iovine KM, Higgins EP, Hindes A, Coblitz B, Johnson SL. 2005. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev Biol 278:208–219. [DOI] [PubMed] [Google Scholar]
- Irion U, Frohnhöfer H, Krauss J, Champollion T, Maischein HM, Geiger-Rudolph S, Weiler C, et al. 2014. Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. eLife 3:e05125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL, Kurachi Y, Okada N, et al. 2006. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: Implications for the regulation of melanosome movement. PLoS Genet 2:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. 1981. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond Ser B, Biol Sci 295:553–566. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. 2008. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol 214:345–353. [DOI] [PubMed] [Google Scholar]
- Jindal GA, Goyal Y, Burdine RD, Rauen KA, Shvartsman SY. 2015. RASopathies: Unraveling mechanisms with animal models. Dis Models Mech 8:1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SH, Chang T, Ebong I, Bhadviya BB, Mazumder P, Lu W. 2010. Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett 10:1297–1301. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Swiergiel AH. 2009. Properties of gap junction blockers and their behavioural, cognitive and electro-physiological effects: Animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry 33:181–198. [DOI] [PubMed] [Google Scholar]
- Kalimi GH, Lo CW. 1989. Gap junctional communication in the extraembryonic tissues of the gastrulating mouse embryo. J Cell Biol 109:3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. 2011. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol 354:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JD, Munger SJ, Witte MH, Simon AM. 2015. Combining Foxc2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling. Dev Biol 405:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandouz M, Batist G. 2010. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin Ther Targets 14:681–692. [DOI] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. 2010. Functional microRNA is transferred between glioma cells. Cancer Res 70:8259–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Cho SW, Lee MJ, Hwang HJ, Lee JM, Lee SI, Muramatsu T, et al. 2005. Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res 320:409–415. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Kim JS, Hagendorff A, Thonnissen E, Kruger O, Lamers WH, Willecke K. 2000. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ Res 87:399–405. [DOI] [PubMed] [Google Scholar]
- Kondo S 2002. The reaction-diffusion system: A mechanism for autonomous pattern formation in the animal skin. Genes Cells 7:535–541. [DOI] [PubMed] [Google Scholar]
- Kondo S, Asai R. 1995. A reaction-diffusion wave on the skin of the marine angelfish Pomacanthus. Nature 376:765–768. [DOI] [PubMed] [Google Scholar]
- Kotini M, Mayor R. 2015. Connexins in migration during development and cancer. Dev Biol 401:143–151. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. 2000. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development 127:3501–3512. [DOI] [PubMed] [Google Scholar]
- Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. 2006. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci U S A 103:10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R, Levin M. 2015. Bioelectric memory: Modeling resting potential bistability in amphibian embryos and mammalian cells. Theor Biol Med Model 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Feber J, Stegenga J, Rutten W. 2008. Do external stimuli, applied to train cultured cortical networks, disturb the balance between activity and connectivity? Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Annual Conference on IEEE Engineering in Medicine and Biology Society, 2008:5081–5084. [DOI] [PubMed] [Google Scholar]
- le Feber J, Stegenga J, Rutten WLC. 2010. The effect of slow electrical stimuli to achieve learning in cultured networks of rat cortical neurons. PLoS ONE 5:e08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. 2000. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 151:931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Lechner H, Loer B, Knieps M, Herrmann S, Famulok M, Bauer R, et al. 2006. Heteromerization of innexin gap junction proteins regulates epithelial tissue organization in drosophila. Mol Biol Cell 17:1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithe E, Sirnes S, Omori Y, Rivedal E. 2006. Downregulation of gap junctions in cancer cells. Crit Rev Oncog 12:225–256. [DOI] [PubMed] [Google Scholar]
- Leppik LP, Froemel D, Slavici A, Ovadia ZN, Hudak L, Henrich D, Marzi I, et al. 2015. Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci Rep 5:18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M 1998. Left-right asymmetry and the chick embryo. Semin Cell Dev Biol 9:67–76. [DOI] [PubMed] [Google Scholar]
- Levin M 2002. Isolation and community: A review of the role of gap-junctional communication in embryonic patterning. J Membr Biol 185:177–192. [DOI] [PubMed] [Google Scholar]
- Levin M 2007. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol 94:186–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M 2011. The wisdom of the body: Future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regener Med 6:667–673. [DOI] [PubMed] [Google Scholar]
- Levin M 2012a. Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays 34:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M 2012b. Morphogenetic fields in embryogenesis, regeneration, and cancer: Non-local control of complex patterning. Bio Systems 109:243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M 2014a. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol 592:2295–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M 2014b. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell 25:3835–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1998a. The compulsion of chirality: Toward an understanding of left-right asymmetry. Genes Dev 12:763–769. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1998b. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol 203:90–105. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 1999. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 126:4703–4714. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. 2000. Expression of connexin 30 in Xenopus embryos and its involvement in hatching gland function. Dev Dyn 219:96–101. [DOI] [PubMed] [Google Scholar]
- Levin M, Nascone N. 1997. Two molecular models of initial left-right asymmetry generation. Med Hypotheses 49:429–435. [DOI] [PubMed] [Google Scholar]
- Levin M, Stevenson CG. 2012. Regulation of cell behavior and tissue patterning by bioelectrical signals: Challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng 14:295–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. 2006. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci 28:171–185. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. 2002. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell 111:77–89. [DOI] [PubMed] [Google Scholar]
- Li WE, Waldo K, Linask KL, Chen T, Wessels A, Parmacek MS, Kirby ML, et al. 2002. An essential role for connexin43 gap junctions in mouse coronary artery development. Development 129:2031–2042. [DOI] [PubMed] [Google Scholar]
- Li S, He H, Zhang G, Wang F, Zhang P, Tan Y. 2015. Connexin43-containing gap junctions potentiate extracellular Ca-induced odontoblastic differentiation of human dental pulp stem cells via Erk1/2. Exp Cell Res 338:1–9. [DOI] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, et al. 2011. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 71:1550–1560. [DOI] [PubMed] [Google Scholar]
- Lo CW. 1996. The role of gap junction membrane channels in development. J Bioenerg Biomembr 28:379–385. [DOI] [PubMed] [Google Scholar]
- Lo CW, Gilula NB. 1979. Gap junctional communication in the post-implantation mouse embryo. Cell 18:411–422. [DOI] [PubMed] [Google Scholar]
- Lo CW, Waldo KL, Kirby ML. 1999. Gap junction communication and the modulation of cardiac neural crest cells. Trends Cardiovasc Med 9:63–69. [DOI] [PubMed] [Google Scholar]
- Lo CW, Cohen MF, Huang GY, Lazatin BO, Patel N, Sullivan R, Pauken C, et al. 1997. Cx43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev Gen 20:119–132. [DOI] [PubMed] [Google Scholar]
- Lobikin M, Chernet B, Lobo D, Levin M. 2012. Resting potential, oncogene-induced tumorigenesis, and metastasis: The bioelectric basis of cancer in vivo. Phys Biol 9:065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo D, Beane WS, Levin M. 2012. Modeling planarian regeneration: A primer for reverse-engineering the worm. PLoS Comput Biol 8:e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo D, Solano M, Bubenik GA, Levin M. 2014. A linear-encoding model explains the variability of the target morphology in regeneration. J R Soc Interface 11:20130918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loch-Caruso R, Trosko JE. 1985. Inhibited intercellular communication as a mechanistic link between teratogenesis and carcinogenesis. Crit Rev Toxicol 16:157–183. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR. 1969. Transfer of information through cell junctions and growth control. Proc Can Cancer Conf 8:162–170. [PubMed] [Google Scholar]
- Loewenstein WR. 1979. Junctional intercellular communication and the control of growth. Biochim Biophys Acta 560:1–65. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR. 1980. Junctional cell-to-cell communication and growth control. Ann N Y Acad Sci 339:39–45. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR, Kanno Y. 1966. Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature 209:1248–1249. [DOI] [PubMed] [Google Scholar]
- Maeda S, Tsukihara T. 2011. Structure of the gap junction channel and its implications for its biological functions. Cell Mol Life Sci 68:1115–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini MV, Calabro JM, Soto AM, Sonnenschein C. 2005. Stromal regulation of neoplastic development: Age-dependent normalization of neoplastic mammary cells by mammary stroma. Am J Pathol 167:1405–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J 1992. Effects of intravenous anesthetics on neurons of the central nervous system: Mechanisms of cellular and molecular action. Ann Fr Anesth Reanim 11:540–557. [DOI] [PubMed] [Google Scholar]
- Marder E. 2009. Electrical synapses: Rectification demystified. Curr Biol 19:R34–R35. [DOI] [PubMed] [Google Scholar]
- Marins M, Xavier AL, Viana NB, Fortes FS, Froes MM, Menezes JR. 2009. Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev Neurobiol 69:715–730. [DOI] [PubMed] [Google Scholar]
- Maximino C, Puty B, Oliveira MKR, Herculano AM. 2013. Behavioral and neurochemical changes in the zebrafish leopard strain. Genes Brain Behav 12:576–582. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. 2005. Controlling cell behavior electrically: Current views and future potential. Physiol Rev 85:943–978. [DOI] [PubMed] [Google Scholar]
- McConnell JV, Jacobson AL, Kimble DP. 1959. The effects of regeneration upon retention of a conditioned response in the planarian. J Compar Physiol Psychol 52:1–5. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Hotz-Wagenblatt A, Rose B, Shalloway D, Loewenstein WR. 1991. Incorporation of the gene for a cell-cell channel protein into transformed cells leads to normalization of growth. J Membr Biol 124:207–225. [DOI] [PubMed] [Google Scholar]
- Melloy PG, Kusnierczyk MK, Meyer RA, Lo CW, Desmond ME. 2005. Overexpression of connexin43 alters the mutant phenotype of midgestational wnt-1 null mice resulting in recovery of the midbrain and cerebellum. Anat Rec a Discov Mol Cell Evol Biol 283:224–238. [DOI] [PubMed] [Google Scholar]
- Mendoza-Naranjo A, Saez PJ, Johansson CC, Ramirez M, Mandakovic D, Pereda C, Lopez MN, et al. 2007. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J Immunol 178:6949–6957. [DOI] [PubMed] [Google Scholar]
- Merrifield PA, Laird DW. 2015. Connexins in skeletal muscle development and disease. Semin Cell Dev Biol 50:67–73. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. 2005. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta 1719:125–145. [DOI] [PubMed] [Google Scholar]
- Moreno E 2008. Is cell competition relevant to cancer? Nat Rev Cancer 8:141–147. [DOI] [PubMed] [Google Scholar]
- Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M. 1997. Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol 8:939–951. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. 2008. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem 21:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagajski D, Guthrie S, Ford C, Warner A. 1989. The correlation between patterns of dye transfer through gap junctions and future developmental fate in Xenopus. Development 105:747–752. [Google Scholar]
- Nguyen LM, Pozzoli M, Hraha TH, Benninger RK. 2014. Decreasing cx36 gap junction coupling compensates for overactive KATP channels to restore insulin secretion and prevent hyperglycemia in a mouse model of neonatal diabetes. Diabetes 63:1685–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. 2000. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res 33:369–378. [DOI] [PubMed] [Google Scholar]
- Nishii K, Kumai M, Shibata Y. 2001. Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med 11:213–218. [DOI] [PubMed] [Google Scholar]
- Noble D 2015. Conrad Waddington and the origin of epigenetics. J Exp Biol 218:816–818. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. 2005. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol 287:314–335. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R 2003. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry 106:375–383. [DOI] [PubMed] [Google Scholar]
- Nygren A, Giles WR. 2000. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular. Ann Biomed Eng 28:951–957. [DOI] [PubMed] [Google Scholar]
- O’Brien J 2014. The ever-changing electrical synapse. Curr Opin Neurobiol 29:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SK, Shin JO, Baek JI, Lee J, Bae JW, Ankamerddy H, Kim MJ, et al. 2015. Pannexin 3 is required for normal progression of skeletal development in vertebrates. FASEB J 29:4473–4484. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Christian JL, Moon RT. 1991. Effect of wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science 252:1173–1176. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Moon RT. Distinct effects of ectopic expression of Wnt-1, activin B, and bFGF on gap junctional permeability in 32-cell Xenopus embryos. Dev Biol 151:204–212. [DOI] [PubMed] [Google Scholar]
- Omori Y, Yamasaki H. 1998. Mutated connexin43 proteins inhibit rat glioma cell growth suppression mediated by wild-type connexin43 in a dominant-negative manner. Int J Cancer 78:446–453. [DOI] [PubMed] [Google Scholar]
- Omori Y, Duflot-Dancer A, Mesnil M, Yamasaki H. 1998. Role of connexin (gap junction) genes in cell growth control. Toxicol Lett 96-97:105–110. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. 2007. smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development 134:3121–3131. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, et al. 2010. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 339:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paemeleire K, Martin PE, Coleman SL, Fogarty KE, Carrington WA, Leybaert L, Tuft RA, et al. 2000. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol Biol Cell 11:1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. 2012. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 139:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Lemire JM, Pare JF, Lin G, Chen Y, Levin M. 2015a. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J Neurosci 35:4366–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Martyniuk CJ, Echeverri K, Sundelacruz S, Kaplan DL, Levin M. 2015b. Genome-wide analysis reveals conserved transcriptional responses downstream of resting potential change in Xenopus embryos, axolotl regeneration, and human mesenchymal cell differentiation. Regeneration 3:3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N 2009. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci U S A 106:14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Huetteroth W, Pereda AE. 2014. Hemichannel composition and electrical synaptic transmission: Molecular diversity and its implications for electrical rectification. Front Cell Neurosci 8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Werdich AA, Macrae CA. 2010. Wnt11 patterns a myocardial electrical gradient through regulation of the L-type Ca(2+) channel. Nature 466:874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraguassu-Braga FH, Borojevic R, Bouzas LF, Barcinski MA, Bonomo A. 2003. Bone marrow stroma inhibits proliferation and apoptosis in leukemic cells through gap junction-mediated cell communication. Cell Death Differ 10:1101–1108. [DOI] [PubMed] [Google Scholar]
- Parak WJ, Domke J, George M, Kardinal A, Radmacher M, Gaub HE, de Roos ADG, et al. 1999. Electrically excitable normal rat kidney fibroblasts: A new model system for cell-semiconductor hybrids. Biophys J 76:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D, Yu K, Bruzzone R, Gimlich R, Goodenough D. 1995. Expression of a dominant negative inhibitor of intercellular communication in the early Xenopus embryo causes delamination and extrusion of cells. Development 121:371–381. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. 2005. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46:731–744. [DOI] [PubMed] [Google Scholar]
- Peracchia C 2004. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta 1662:61–80. [DOI] [PubMed] [Google Scholar]
- Peracchia C, Bernardini G, Peracchia LL. 1983. Is calmodulin involved in the regulation of gap junction permeability? Pflugers Arch 399:152–154. [DOI] [PubMed] [Google Scholar]
- Perathoner S, Daane JM, Henrion U, Seebohm G, Higdon CW, Johnson SL, Nusslein-Volhard C, et al. 2014. Bioelectric signaling regulates size in zebrafish fins. PLoS Genet 10:e1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE. 2014. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci 15:250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. 2013. Gap junction-mediated electrical transmission: Regulatory mechanisms and plasticity. Biochim Biophys Acta 1828:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A, Furlan F. 2010. Astrocytes and human cognition: Modeling information integration and modulation of neuronal activity. Prog Neurobiol 92:405–420. [DOI] [PubMed] [Google Scholar]
- Pershin YV, Di Ventra M. 2010. Experimental demonstration of associative memory with memristive neural networks. Neural Netw 23:881–886. [DOI] [PubMed] [Google Scholar]
- Pezzulo G, Levin M. 2015. Re-membering the body: Applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr Biol (Camb) 7:1487–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo G, Rigoli F, Friston K. 2015. Active Inference, homeostatic regulation and adaptive behavioural control. Prog Neurobiol 134:17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Kraushaar U, Düfer M, Schönecker S, Haspel D, Günther E, Drews G, et al. 2011. Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays. Pflügers Arch-Eur J Physiol 462:835–840. [DOI] [PubMed] [Google Scholar]
- Phelan P 2005. Innexins: Members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711:225–245. [DOI] [PubMed] [Google Scholar]
- Piccolino M, Neyton J. 1984. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3’:5’-monophosphate in horizontal cells of turtle retina. J Neurosci 4:2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimashkin A, Gladkov A, Mukhina I, Kazantsev V. 2013. Adaptive enhancement of learning protocol in hippocampal cultured networks grown on multielectrode arrays. Front Neural Circuits 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts JD, Finbow ME, Kam E. 1988. Junctional communication and cellular differentiation. Br J Cancer Suppl 9:52–57. [PMC free article] [PubMed] [Google Scholar]
- Pizard A, Burgon PG, Paul DL, Bruneau BG, Seidman CE, Seidman JG. 2005. Connexin 40, a target of transcription factor tbx5, patterns wrist, digits, and sternum. Mol Cell Biol 25:5073–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, Dallapiccola B. 2004. A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype. Hum Mutat 23:286. [DOI] [PubMed] [Google Scholar]
- Postma F, Liu CH, Dietsche C. 2011. Electrical synapses formed by connexin36 regulate inhibition-and experience-dependent plasticity. Proc Natl Acad Sci U S A 108:13770–13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter VR. 1980. Initiation and promotion in cancer formation: The importance of studies on intercellular communication. Yale J Biol Med 53:367–384. [PMC free article] [PubMed] [Google Scholar]
- Ramsdell AF, Yost HJ. 1998. Molecular mechanisms of vertebrate left-right development. Trends Genet 14:459–465. [DOI] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kilkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, et al. 1995. Cardiac malformation in neonatal mice lacking connexin43. Science 267:1831–1834. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sanchez Alvarado A. 2004. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20:725–757. [DOI] [PubMed] [Google Scholar]
- Rela L, Szczupak L. 2004. Gap junctions: Their importance for the dynamics of neural circuits. Mol Neurobiol 30:341–357. [DOI] [PubMed] [Google Scholar]
- Richard M, Hoch M. 2015. Drosophila eye size is determined by Innexin 2-dependent Decapentaplegic signalling. Dev Biol 408:26–40. [DOI] [PubMed] [Google Scholar]
- Rimkute L, Jotautis V, Marandykina A, Sveikatiene R, Antanaviciute I, Skeberdis VA. 2016. The role of neural connexins in HeLa cell mobility and intercellular communication through tunneling tubes. BMC Cell Biol 17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Hampson EC, Munro MN, Vaney DI. 1993. Unidirectional coupling of gap junctions between neuroglia. Science 262:1072–1074. [DOI] [PubMed] [Google Scholar]
- Rogers KW, Schier AF. 2011. Morphogen gradients: From generation to interpretation. Annu Rev Cell Dev Biol 27:377–407. [DOI] [PubMed] [Google Scholar]
- Rörig B, Sutor B. 1996. Nitric oxide-stimulated increase in intracellular cGMP modulates gap junction coupling in rat neocortex. Neuroreport 31:569–572. [DOI] [PubMed] [Google Scholar]
- Rose B, Mehta PP, Loewenstein WR. 1993. Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis 14:1073–1075. [DOI] [PubMed] [Google Scholar]
- Rosenbaum MB, Blanco HH, Elizari MV, Lázzari JO, Davidenko JM. 1982. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol 50:213–222. [DOI] [PubMed] [Google Scholar]
- Rubin H 1985. Cancer as a dynamic developmental disorder. Cancer Res 45:2935–2942. [PubMed] [Google Scholar]
- Salo E, Abril JF, Adell T, Cebria F, Eckelt K, Fernandez-Taboada E, Handberg-Thorsager M, et al. 2009. Planarian regeneration: Achievements and future directions after 20 years of research. Int J Dev Biol 53:1317–1327. [DOI] [PubMed] [Google Scholar]
- Saraga F, Ng L, Skinner FK. 2006. Distal gap junctions and active dendrites can tune network dynamics. J Neurophysiol 95:1669–1682. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Netsky MG. 1985. The brain of the planarian as the ancestor of the human brain. Can J Neurol Sci 2:296–302. [DOI] [PubMed] [Google Scholar]
- Scarle S 2008. Implications of the Turing completeness of reaction-diffusion models. Comput Biol Chem 33:253–260. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. 2007. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann Y 1991. An hypothesis: Phosphorylation fields as the source of positional information and cell differentiation–(cAMP, ATP) as the universal morphogenetic Turing couple. Prog Biophys Mol Biol 56:79–105. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y 2005. Induction and the Turing-Child field in development. Prog Biophys Mol Biol 89:36–92. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y 2008. The Turing-Child energy field as a driver of early mammalian development. Prog Biophys Mol Biol 98:107–117. [DOI] [PubMed] [Google Scholar]
- Schönecker S, Kraushaar U, Düfer M, Sahr A, Härdtner C, Guenther E, Walther R, et al. 2014. Long-term culture and functionality of pancreatic islets monitored using microelectrode arrays. Integr Biol 6:540–544. [DOI] [PubMed] [Google Scholar]
- Schumacher JA, Hsieh YW, Chen S, Pirri JK. 2012. Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans. Development 139:4191–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahaf G, Marom S. 2001. Learning in networks of cortical neurons. J Neurosci 21:8782–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM. 2013. Long-distance relationships: Do membrane nanotubes regulate cell-cell communication and disease progression? Mol Biol Cell 24:1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman A, Rinzel J. 1991. Model for synchronization of pancreatic beta-cells by gap junction coupling. Biophys J 59:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Sofroniew MV. 2010. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 13:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomrat T, Levin M. 2013. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. J Exp Biol 216:3799–3810. [DOI] [PubMed] [Google Scholar]
- Simon S 2009. Implications of the Turing completeness of reaction-diffusion models, informed by GPGPU simulations on an XBox 360: Cardiac arrhythmias, re-entry and the Halting problem. Comput Biol Chem 33:253–260. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR, Dones JA, Jackson CL, Chen H. 2004. Heart and head defects in mice lacking pairs of connexins. Dev Biol 265:369–383. [DOI] [PubMed] [Google Scholar]
- Sims K, Eble DM, Iovine KM. 2009. Connexin43 regulates joint location in zebrafish fins. Dev Biol 327:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Crespin S, Mesnil M. 2012. Opposing roles of connexin43 in glioma progression. Biochim Biophys Acta 1818:2058–2067. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Pelegri F. 2007. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol 307:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smendziuk CM, Messenberg A, Vogl AW, Tanentzapf G. 2015. Bi-directional gap junction-mediated soma-germline communication is essential for spermatogenesis. Development 142:2598–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Willecke K. 2004. Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. 2014. Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett 588:1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C. 2011. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. BioEssays 33:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Petzoldt A, Suzanne M, Noselli S. 2007. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev 17:351–358. [DOI] [PubMed] [Google Scholar]
- Spira ME, Hai A. 2013. Multi-electrode array technologies for neuroscience and cardiology. Nat Nanotechnol 8:83–94. [DOI] [PubMed] [Google Scholar]
- Stains JP, Civitelli R. 2005. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell 16:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. 2003. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem 278:24377–24387. [DOI] [PubMed] [Google Scholar]
- Starich TA, Hall DH, Greenstein D. 2014. Two classes of gap junction channels mediate soma-germline interactions essential for germline proliferation and gametogenesis in caenorhabditis elegans. Genetics 198:1127–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Miller A, Nguyen RL, Hall DH, Shaw JE. 2003. The caenorhabditis elegans innexin INX-3 is localized to gap junctions and is essential for embryonic development. Dev Biol 256:403–417. [DOI] [PubMed] [Google Scholar]
- Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverria C, Orellana JA, Bultynck G, et al. 2012. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. Faseb J 26:3649–3657. [DOI] [PubMed] [Google Scholar]
- Stewart A, Cachat J, Gaikwad S, Robinson KSL, Gebhardt M, Kalueff AV. 2013. Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem Int 62:893–902. [DOI] [PubMed] [Google Scholar]
- Steyn-Ross ML, Steyn-Ross DA, Wilson MT, Sleigh JW. 2007. Gap junctions mediate large-scale Turing structures in a mean-field cortex driven by subcortical noise. Phys Rev E 76:011916. [DOI] [PubMed] [Google Scholar]
- Stoletov K, Strnadel J, Zardouzian E, Momiyama M, Park FD, Kelber JA, Pizzo DP, et al. 2013. Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci 126:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JR. 1997. The spirit of D’arcy Thompson dwells in empirical morphospace. Math Biosci 142:13–30. [DOI] [PubMed] [Google Scholar]
- Sullivan KG, Emmons-Bell M, Levin M. 2016. Physiological inputs regulate species-specific anatomy during embryogenesis and regeneration. Commun Integr Biol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Huang GY, Meyer RA, Wessels A, Linask KK, Lo CW. 1998. Heart malformations in transgenic mice exhibiting dominant negative inhibition of gap junctional communication in neural crest cells. Dev Biol 204:224–234. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. 2009. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep 5:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hagerty T. 2005. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta 1719:59–68. [DOI] [PubMed] [Google Scholar]
- Takagi H, Kaneko K. 2005. Dynamical systems basis of metamorphosis: Diversity and plasticity of cellular states in reaction diffusion network. J Theor Biol 234:173–186. [DOI] [PubMed] [Google Scholar]
- Tarin D 2012. Clinical and biological implications of the tumor microenvironment. Cancer Microenviron 5:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. 1997. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol 7:713–716. [DOI] [PubMed] [Google Scholar]
- Ton QV, Iovine KM. 2012. Semaphorin3d mediates Cx43-dependent phenotypes during fin regeneration. Dev Biol 366:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton QV, Iovine KM. 2013. Identification of an evx1-dependent joint-formation pathway during fin regeneration. PLoS ONE 8:e81240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. 1998. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem 273:1519–1528. [DOI] [PubMed] [Google Scholar]
- Trosko JE. 2005. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed Pharmacother 59 Suppl 2:S326–S331. [DOI] [PubMed] [Google Scholar]
- Trosko JE. 2007. Gap junctional intercellular communication as a biological “Rosetta stone” in understanding, in a systems biological manner, stem cell behavior, mechanisms of epigenetic toxicology, chemoprevention and chemotherapy. J Membr Biol 218:93–100. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. 2010. Induction of vertebrate regeneration by a transient sodium current. J Neurosci 30:13192–13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Jinno Y, Tomita A, Okamura Y. 2014. Rapid evaluation of a protein-based voltage probe using a field-induced membrane potential change. Biochim Biophys Acta 1838:1730–1737. [DOI] [PubMed] [Google Scholar]
- Turin L, Warner AE. 1980. Intracellular pH in early Xenopus embryos: Its effect on current flow between blastomeres. J Physiol 300:489–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. 1953. The chemical basis of morphogenesis. Philos Trans R Soc B: Biol Sci 237:37–72. [Google Scholar]
- Turner CH, Robling AG, Duncan RL, Burr DB. 2002. Do bone cells behave like a neuronal network? Calcif Tissue Int 70:435–442. [DOI] [PubMed] [Google Scholar]
- Umino Y, Saito T. 2002. Spatial and temporal patterns of distribution of the gap junctional protein connexin43 during retinal regeneration of adult newt. J Comp Neurol 454:255–262. [DOI] [PubMed] [Google Scholar]
- Urschel S, Höher T, Schubert T, Alev C, Söhl G. 2006. Protein kinase A-mediated phosphorylation of connexin36 in mouse retina results in decreased gap junctional communication between AII amacrine cells. J Biol Chem 281:33163–33171. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, et al. 2005. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. 1998. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci 111:1741–1749. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. 2009. Perspectives and open problems in the early phases of left-right patterning. Semin Cell Dev Biol 20:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Lemire JM, Levin M. 2012. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis Models Mech 6:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Blackiston DJ, Rea AC, Dore TM, Levin M. 2014. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. Int J Dev Biol 58:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHoven MK, Huang SLB, Albin SD, Bargmann CI. 2006. The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron 1:291–302. [DOI] [PubMed] [Google Scholar]
- Verwey LJ, Edwards TM. 2010. Gap junctions and memory: An investigation using a single trial discrimination avoidance task for the neonate chick. Neurobiol Learn Mem 93:189–195. [DOI] [PubMed] [Google Scholar]
- Vink MJ, Suadicani SO, Vieira DM, Urban-Maldonado M, Gao Y, Fishman GI, Spray DC. 2004. Alterations of intercellular communication in neonatal cardiac myocytes from connexin43 null mice. Cardiovasc Res 62:397–406. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Leybaert L, Bultynck G, Himpens B, Vanhaecke T, Rogiers V. 2012. Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta 1818:2002–2008. [DOI] [PubMed] [Google Scholar]
- Vogel R, Weingart R. 1998. Mathematical model of vertebrate gap junctions derived from electrical measurements on homotypic and heterotypic channels. J Physiol 510:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel R, Weingart R. 2002. The electrophysiology of gap junctions and gap junction channels and their mathematical modelling. Biol Cell 94:501–510. [DOI] [PubMed] [Google Scholar]
- Wade JJ, McDaid LJ, Harkin J, Crunelli V, Kelso JA. 2011. Bidirectional coupling between astrocytes and neurons mediates learning and dynamic coordination in the brain: A multiple modeling approach. PLoS One 6:e29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. 1999. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev Biol 208:307–323. [DOI] [PubMed] [Google Scholar]
- Waller BR 3rd, McQuinn T, Phelps AL, Markwald RR, Lo CW, Thompson RP, Wessels A. 2000. Conotruncal anomalies in the trisomy 16 mouse: An immunohisto-chemical analysis with emphasis on the involvement of the neural crest. Anat Rec 260:279–293. [DOI] [PubMed] [Google Scholar]
- Wang Y, Belousov AB. 2011. Deletion of neuronal gap junction protein connexin 36 impairs hippocampal LTP. Neurosci Lett 502:30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. 2010. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci U S A 107:17194–17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner AE. 1985. The role of gap junctions in amphibian development. J Embryol Exp Morphol 89 Suppl:365–380. [PubMed] [Google Scholar]
- Warner A 1999. Interactions between growth factors and gap junctional communication in developing systems. Novartis Found Symp 219:60–72. [DOI] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB. 1984. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature 311:127–131. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kondo S. 2014. Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet 31:88–96. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N. 2006. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep 7:893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. 2002. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase a in mammalian oocytes. Dev Biol 246:441–454. [DOI] [PubMed] [Google Scholar]
- Weir MP, Lo CW. 1982. Gap junctional communication compartments in the Drosophila wing disk. Proc Natl Acad Sci U S A 79:3232–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MP, Lo CW. 1984. Gap-junctional communication compartments in the Drosophila wing imaginal disk. Dev Biol 102:130–146. [DOI] [PubMed] [Google Scholar]
- White TW. 2002. Unique and redundant connexin contributions to lens development. Science 295:319–320. [DOI] [PubMed] [Google Scholar]
- Wittig D, Wang X, Walter C, Gerdes HH, Funk RH, Roehlecke C. 2012. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PloS One 7:e33195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolszon LR, Gao WQ, Passani MB, Macagno ER. 1994. Growth cone “collapse” in vivo: Are inhibitory interactions mediated by gap junctions? J Neurosci 14:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvetang EJ, Pera MF, Zuckerman KS. 2007. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun 363:610–615. [DOI] [PubMed] [Google Scholar]
- Wong RC, Pera MF, Pebay A. 2008. Role of gap junctions in embryonic and somatic stem cells. Stem Cell Rev 4:283–292. [DOI] [PubMed] [Google Scholar]
- Woodruff RI. 2005. Calmodulin transit via gap junctions is reduced in the absence of an electric field. J Insect Physiol 51:843–852. [DOI] [PubMed] [Google Scholar]
- Wu CL, Shih MF, Lai JS, Yang HT, Turner GC, Chen L, Chiang AS. 2011. Heterotypic gap junctions between two neurons in the drosophila brain are critical for memory. Curr Biol 21:848–854. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield S. 1997. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Compar Neurol 383:512–528. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, et al. 2001. N-cadherin and Cx43alpha1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adhes 8:321–324. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yoshimoto E, Kondo S. 2007. Pattern regulation in the stripe of zebrafish suggests an underlying dynamic and autonomous mechanism. Proc Natl Acad Sci U S A 104:4790–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Enomoto T, Martel N. 1984. Intercellular communication, cell differentiation and tumour promotion. IARC Sci Publ 56:217–238. [PubMed] [Google Scholar]
- Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V. 1995. Intercellular communication and carcinogenesis. MutatRes 333:181–188. [DOI] [PubMed] [Google Scholar]
- Yano T, Fujimoto E, Hagiwara H, Sato H, Yamasaki H, Negishi E, Ueno K. 2006. Connexin 32 as an anti-invasive and anti-metastatic gene in renal cell carcinoma. Biol Pharm Bull 29:1991–1994. [DOI] [PubMed] [Google Scholar]
- Zahler S, Hoffmann A, Gloe T, Pohl U. 2003. Gap-junctional coupling between neutrophils and endothelial cells: A novel modulator of transendothelial migration. J Leukoc Biol 73:118–126. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. 2009. Particle tracking model of electrophoretic morphogen movement reveals stochastic dynamics of embryonic gradient. Dev Dyn 238:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Green C, Stott NS. 2002. Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J Cell Physiol 193:233–243. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Hu Y, Wang BJ, Lin ZX, Naus CC, Nicholson BJ. 2003. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis 25:473–482. [DOI] [PubMed] [Google Scholar]
- Zoghi M 2004. Cardiac memory: Do the heart and the brain remember the same? J Interv Cardiac Electrophysiol 11:177–182. [DOI] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. 2008. Noradrenergic modulation of electrical coupling in GABAergic networks of the hippocampus. J Neurosci 28:1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]


